Abstract
Background: Cumulative anticholinergic exposure, also known as anticholinergic burden, is associated with a variety of adverse outcomes. However, studies show that anticholinergic effects tend to be underestimated by prescribers, and anticholinergics are the most frequently prescribed potentially inappropriate medication in older patients. The grading systems and drugs included in existing scales to quantify anticholinergic burden differ considerably and do not adequately account for patients’ susceptibility to medications. Furthermore, their ability to link anticholinergic burden with adverse outcomes such as falls is unclear. This study aims to develop a prognostic model that predicts falls in older general practice patients, to assess the performance of several anticholinergic burden scales, and to quantify the added predictive value of anticholinergic symptoms in this context.
Methods: Data from two cluster-randomized controlled trials investigating medication optimization in older general practice patients in Germany will be used. One trial (RIME, n = 1,197) will be used for the model development and the other trial (PRIMUM, n = 502) will be used to externally validate the model. A priori, candidate predictors will be selected based on a literature search, predictor availability, and clinical reasoning. Candidate predictors will include socio-demographics (e.g. age, sex), morbidity (e.g. single conditions), medication (e.g. polypharmacy, anticholinergic burden as defined by scales), and well-being (e.g. quality of life, physical function). A prognostic model including sociodemographic and lifestyle-related factors, as well as variables on morbidity, medication, health status, and well-being, will be developed, whereby the prognostic value of extending the model to include additional patient-reported symptoms will be also assessed. Logistic regression will be used for the binary outcome, which will be defined as “no falls” vs. “≥1 fall” within six months of baseline, as reported in patient interviews.
Discussion: As the ability of different anticholinergic burden scales to predict falls in older patients is unclear, this study may provide insights into their relative importance as well as into the overall contribution of anticholinergic symptoms and other patient characteristics. The results may support general practitioners in their clinical decision-making and in prescribing fewer medications with anticholinergic properties.
Keywords: aged [MesH], anticholinergic burden, accidental falls [MeSH], general practice, prediction model, prognosis research, multimorbidity [MeSH], polypharmacy
Introduction
Medications with anticholinergic (ACh) properties are commonly used for a variety of indications (Jessen et al., 2010; Wawruch et al., 2012; Mate et al., 2015) and, along with sedatives, are the most frequently prescribed potentially inappropriate medications (PIM) in older adults (Hukins et al., 2019). Depending on patient population and setting, the prevalence of ACh drug use varies between 27% in community-based elderly patients (Ness et al., 2006) and up to 80% in more vulnerable populations such as older patients receiving home health care services or nursing home patients with dementia (Chatterjee et al., 2010; McNeely et al., 2013).
The terms “anticholinergic symptoms,” “anticholinergic side-effects,” “anticholinergic adverse effects” and “adverse drug reactions” have been used interchangeably to describe peripheral and central effects associated with anticholinergic drug use, including, for example dry mouth, dry skin, blurred vision, and drowsiness (Ness et al., 2006; Mintzer and Burns, 2016; Welsh et al., 2018). Moreover, anticholinergics have been associated with a variety of adverse outcomes, the most important of which include falls and quality of life, as well as cognitive and functional decline (Cardwell et al., 2015; Welsh et al., 2018). Falling, in particular, is a significant public health issue and represents the leading cause of disability and death in older age. It is further associated with an increase in mortality, morbidity, hospital admissions and costs for treatments (National Institute for Health and Care Excellence, 2013; Verma et al., 2016). The prevention of falls is therefore highly relevant and should be addressed by healthcare professionals involved in the care of older patients (National Institute for Health and Care Excellence, 2013). Gait and balance disorders, muscle weakness, and cognitive impairment all increase the risk of falling with advanced age (Deandrea et al., 2010). The high volume of ACh drug prescriptions raises concerns in terms of appropriateness and patient safety. This is especially true in older age, as it is associated with an increased likelihood of multiple chronic conditions (multimorbidity) and subsequent long-term medication use (polypharmacy), which may further lead to an accumulation of effects and result in substantial harm (Thomas and Brennan 2000; Hilmer et al., 2007a; Delafuente, 2008; Gnjidic et al., 2012; Kouladjian O’Donnell et al., 2017).
Salahudeen et al. have shown that older patients taking multiple ACh agents are 3.21 times more likely to experience ACh symptoms than those taking only one (Salahudeen et al., 2015b), underlining the potential risk of accumulating ACh effects, also known as ACh burden. However, ACh burden tends to be underestimated by general practitioners (GPs) (Magin et al., 2015). As a recent review of systematic reviews identified at least 18 different scales that have been developed and are used in a variety of clinical settings (Welsh et al., 2018), such underestimation may reflect the lack of an universal approach to quantifying ACh burden. These scales not only differ considerably in the way they are conducted and the (number of) included drugs, but also in their grading systems (Salahudeen et al., 2015a; Villalba-Moreno et al., 2016; Mayer et al., 2017; Welsh et al., 2018). For example, quetiapine is rated as having high or low activity, depending on the scale (Salahudeen et al., 2015a). Furthermore, the scales were developed based on expert opinions (Villalba-Moreno et al., 2016; Welsh et al., 2018) and with two exceptions (Hilmer et al., 2007b; Klamer et al., 2017), do not consider drug dose, or adequately account for patients’ individual characteristics such as sex, age, morbidity, and individual susceptibility to medications (Cardwell et al., 2015; Mayer et al., 2015).
The ability of scales to link ACh burden with numerous adverse outcomes has been inconsistently described in a variety of studies (Cardwell et al., 2015; Salahudeen et al., 2015a; Villalba-Moreno et al., 2016; Mayer et al., 2017; Welsh et al., 2018). A recent review of systematic reviews identified 62 original articles that focused mainly on the outcomes cognitive function, physical function, mortality, and delirium (Welsh et al., 2018). Little research has been undertaken to determine the predictive ability of ACh scales in relation to falls. The few studies that exist on this issue have generally involved vulnerable patient populations [e.g., from nursing homes and hospitals (Aizenberg et al., 2002; Landi et al., 2014; Vincentis et al., 2020)]. Findings from community-based settings or from general practice are not only scarce but also show inconsistent results (Marcum et al., 2015; Richardson et al., 2015; Zia et al., 2016). Furthermore, these trials, as well as ACh scales in general, were mainly conducted outside Germany (Carnahan et al., 2006; Hilmer et al., 2007b; Wilson et al., 2011; Dauphinot et al., 2014; Villalba-Moreno et al., 2016). It therefore remains unclear whether such scales are useful in clinical practice and whether they support clinical decision-making in the prescription of medications with ACh properties, for example in a German healthcare context.
The aims of this study are:
-
1.
To develop and validate a prognostic model that considers patients’ individual characteristics in predicting the risk of falls in older general practice patients.
-
2.
To assess the performance of several ACh burden scales in this context and to identify the one that best predict falls, and
-
3.
To quantify whether the addition of patient-reported ACh symptoms improves the model’s predictive performance.
Methods
Source of Data
Data from the international PROPERmed study (PROSPERO ID: CRD42018088129) will be used to develop the model. PROPERmed includes individual participant data from five cluster-randomized controlled trials (cRCTs) conducted in German and Dutch general practices that were combined to form a harmonized database for modelling purposes. cRCTs included in PROPERmed aimed to optimize medication in older chronically ill patients from general practices. Based on the core dataset that included variables on socio-demographics, well-being, morbidity, and medication, two prognostic models were developed that predict deterioration of health-related quality of life (González-González et al., 2020) and all-cause hospitalization (Meid et al., submitted). Details on the rationale and conduct of PROPERmed will be described elsewhere (González-González et al., re-submitted). To address the research question in our study, other variables in addition to PROPERmed’s core dataset are needed from the trials, which include variables on falls as well as on ACh symptoms (e.g., vertigo/dizziness, dry mouth). To avoid systematically missing variables, only trials that are able to provide the set of additional variables will be considered for our study. Therefore, we will only consider data from the two trials RIME (Thiem et al., 2020) and PRIMUM (Muth et al., 2018) for model development. Both cRCTs were conducted in German general practices and aimed to optimize medication in older chronically ill patients from this setting. The main characteristics of PRIMUM and RIME are provided in Table 1.
TABLE 1.
Main characteristics of the included trials.
| PRIMUM | RIME | |
|---|---|---|
| Title | PRIoritizing MUltimedication in Multimorbidity | Reduction of potentially inadequate medication in the Elderly |
| Trial registration | ISRCTN99526053, NCT01171339 | DRKS00003610 |
| Study region | Hesse (Germany) | Witten/Hanover (Germany) |
| Start-end | 2010–2012 | 2012–2014 |
| Design | 2-arm parallel CRT | 2-arm parallel CRT |
| Setting | General practices (N = 72) | General practices (N = 139) |
| Study population | N = 502 | N = 1,197 |
| ≥60 years | ≥70 years | |
| ≥3 chronic conditions | ≥6 chronic prescriptions | |
| ≥5 chronic prescriptions | ||
| Intervention | Structured medication review | Structured medication review |
| Data collection | 0, 6, and 9 months | 0, 6, and 12 months |
| No. of patients with ≥1 fall at 6-month follow-up | 75 | 202 |
CRT, cluster-randomized trial.
Participants
Participants in both trials were older patients from German general practices. In PRIMUM, 502 participants aged ≥60 years with ≥3 chronic conditions and ≥5 chronic prescriptions were included. RIME included 1,197 patients aged ≥70 years with ≥6 chronic prescriptions. To take part in the trials, participants had to provide written informed consent and be capable of participating in telephone interviews and understanding the provided information. Patients with dementia or cognitive impairment and patients with a life expectancy of less than six months (RIME) or 12 months (PRIMUM) were excluded from participation.
Outcome
Based on the data provided in patient interviews, the study outcome of interest will be a binary indicator defined as “no falls” vs. “≥1 fall” within six months of baseline as reported in patient interviews. Patient interviews were conducted on the telephone by trained staff, whereby information concerning falls was gathered by asking the following question: “In the past six months, have you slipped, stumbled, or fallen, such that you lost your balance and landed in a lower position or on the ground?”
Predictors
Research has identified numerous predictors for falls in the elderly such as socio-demographics (e.g. age, sex), physiological, and morbidity-related factors (e.g., gait and balance problems, dementia, depression), environmental factors (e.g., home, footwear), and medication-related factors (Deandrea et al., 2010; Ambrose et al., 2013; Zia et al., 2015; Sousa et al., 2017). Rather than include such a wide range of variables, we will follow recommendations on variable selection in prediction models that they should be clearly defined, available in medical practice, and measured in a reproducible and standardized way to improve the applicability and predictive ability of the model in new individuals (Moons et al., 2012b; Hendriksen et al., 2013; Harrell 2019). If the number of outcomes in the dataset is limited, the authors further recommend restricting the number of predictors in order to avoid overfitting the model to the data and erroneously including predictors in the model (Hendriksen et al., 2013; Moons et al., 2012b). The candidate predictors considered for our model will therefore be predefined based on a) a literature review, b) predictor availability in the included trials (RIME and PRIMUM), and c) clinical reasoning. The full list of candidate predictors and how they were measured is provided in Table 2. All predictor information was collected at baseline and includes sociodemographic and lifestyle-related factors, variables on morbidity, health status, well-being, symptoms and medication. The analyses will only consider medication with systemic effect. In accordance with Brueckle et al. (Brueckle et al., 2020), the following symptoms available in the RIME trial are considered to be anticholinergic drug reactions and will be included as potential predictors in our analyses: dizziness/vertigo, stomach pain, problems urinating, dry mouth, itching, constipation, drowsiness/fatigue. ACh burden, in particular, will be measured using five differenct scales/equations with the aim of comparing their ability to predict falls. An overview of ACh scales’ characteristics is presented in Table 3. For our study, we have selected the scales that were found to be frequently used in the review of systematic reviews examining the association between ACh burden and patient-relevant outcomes published by Welsh et al. (2018): the Anticholinergic Drug Scale (ADS) (Carnahan et al., 2006), the Anticholinergic Risk Scale (ARS) (Rudolph et al., 2008) and the Drug-Burden-Index (DBI) (Hilmer et al., 2007b). We will further include the Muscarinic Acetylcholinergic Receptor ANTagonist Exposure Scale (MARANTE) (Klamer et al., 2017), as this scale combines potency with the dosage spectrum as well as the German Anticholinergic Burden Score (Ger-ABS) (Kiesel et al., 2018), as it is the only scale that was explicitly developed for the German drug market. In the two studies RIME and PRIMUM, prescribed drugs were coded based on the Anatomic Therapeutic Chemical (ATC) classification. For our analyses, ACh burden will be calculated in accordance with the scales’ definitions, and respective ATC codes for the German drug market will be identified accordingly. For the calculation of the DBI, the minimum recommended daily dose as listed by the US Food and Drug Administration (FDA) is required (Kouladjian et al., 2014) As minimum recommended daily doses vary between countries, we will use a version of the DBI that was adapted for the German drug market in the COFRAIL-study by Thürmann et al. (German Innovation Funds No. 01VSF17053). Candidate predictors collected as continuous measurements will be kept continuous in the analyses. We will assume continuous candidate predictors to be linearly associated with the outcome with the exception of ACh variables, for which research has shown that high ACh load appears to reach a plateau, indicating a non-linear relationship (Kersten et al., 2013; Mayer et al., 2015).
TABLE 2.
Candidate predictors available in the development dataset.
| Group | Type of predictor | Candidate predictor | Data collection | Data type | Measurement unit |
|---|---|---|---|---|---|
| A | Sociodemographic and lifestyle-related | Intervention status | Registration form | Cat | Intervention, control |
| Age | Registration form | Cont | Years | ||
| Sex | Registration form | Cat | Male, female | ||
| Living situation | Patient interview | Cat | Home, institutionalized | ||
| Educational | CRF | Cat | Low, medium, high | ||
| Smoking | Patient interview | Cat | Smoker, ex-smoker, non-smoker | ||
| B | Morbidity-related | Hypertension | Patient interview | Bin | No, yes |
| Diabetes mellitus | Patient interview | Bin | No, yes | ||
| Coronary heart disease | Patient interview | Bin | No, yes | ||
| Osteoarthritis | Patient interview | Bin | No, yes | ||
| COPD/asthma | Patient interview | Bin | No, yes | ||
| Vision problems | Patient interview | Bin | No, yes | ||
| Hearing problems | Patient interview | Bin | No, yes | ||
| Cancer | Patient interview | Bin | No, yes | ||
| Heart failure | Patient interview | Bin | No, yes | ||
| Cerebrovascular disease | Patient interview | Bin | No, yes | ||
| Osteoporosis | Patient interview | Bin | No, yes | ||
| Depression | Patient interview | Bin | No, yes | ||
| Rheumatoid/seropositive arthritis | Patient interview | Bin | No, yes | ||
| Atherosclerosis/peripheral vascular disease | Patient interview | Bin | No, yes | ||
| Parkinsonism | Patient interview | Bin | No, yes | ||
| HIV/AIDS | Patient interview | Bin | No, yes | ||
| Lipid disorder | Patient interview | Bin | No, yes | ||
| Gout | Patient interview | Bin | No, yes | ||
| Thyroid disorders | Patient interview | Bin | No, yes | ||
| Gastric or duodenal ulcer | Patient interview | Bin | No, yes | ||
| Liver disorder | Patient interview | Bin | No, yes | ||
| Urinary disease | Patient interview | Bin | No, yes | ||
| Anemia | Patient interview | Bin | No, yes | ||
| No. of chronic conditions | Patient interview | Cont | No, yes | ||
| C | Health status and well-being related | Pain | Patient interview | Bin | No, yes |
| Quality of life (EuroQol Group 1990) | Patient interview | Cont | Score | ||
| Functional status (Saliba et al., 2001) | Patient interview | Cont | Score | ||
| Cognitive function | Patient interview | Cont | Score | ||
| All-cause hospital admissions | Patient interview | Bin | No, yes | ||
| History of falls | Patient interview | Cat | ≤1 fall, ≥ 2 falls | ||
| D | Medication related | No. of drugs | Patient interview | Cont | Frequency |
| ACh burden (Carnahan et al., 2006; Hilmer et al., 2007b; Rudolph et al., 2008; Klamer et al., 2017; Kiesel et al., 2018) | Calculated using medication data from patient interview | Cont | Weighted index | ||
| E | Symptoms | No. of symptoms | Patient interview | Cont | Frequency |
| Dizziness/vertigo | Patient interview | Bin | No, yes | ||
| Problems urinating | Patient interview | Bin | No, yes | ||
| Stomach pain | Patient interview | Bin | No, yes | ||
| Patient interview | Bin | No, yes | |||
| Drowsiness/fatigue a | Patient interview | Bin | No, yes | ||
| Dry mouth a | Patient interview | Bin | No, yes | ||
| Itching a | Patient interview | Bin | No, yes | ||
| Constipation a | Patient interview | Bin | No, yes |
ACh, anticholinergic; Bin., binary; Cat., categorial; Cont., continuous; COPD, chronic obstructive pulmonary disease; CRF, case report form; HIV/AIDS, human immunodeficiency virus/acquired immune deficiency syndrome; No, number.
Symptoms only available in the development dataset (RIME).
TABLE 3.
Overview of anticholinergic scales’ characteristics.
| Study (year) | Scale | Country | No of included drugs | Grading system | Dosage considered |
|---|---|---|---|---|---|
| Carnahan et al. (2006) | Anticholinergic drug scale (ADS) | USA | 117 | Scores: 0–3 | No |
| Klamer et al. (2017) | Muscarinic acetylcholinergic receptor ANTagonist exposure scale (MARANTE) | Belgium | 41 | Equation | Yes |
| Hilmer et al. (2007b) | Drug-burden-index (DBI) | Australia | 128 | Equation | Yes |
| Rudolph et al. (2008) | Anticholinergic risk scale (ARS) | USA | 49 | Scores: 0–3 | No |
| Kiesel et al. (2018) | German anticholinergic burden score (Ger-ABS) | Germany | 151 | Scores: 0–3 | No |
Sample Size
When designing a prediction model study, Riley et al. (2019) recommend that the minimum sample size is calculated to meet certain criteria and minimize overfitting. As we will retrospectively analyze individual participant data, the sample size and prevalence are already given by the included trials. The pmsampsize package in R provided by the authors will be used to calculate whether our sample size meets the suggested criteria, and, in view of the number of predictors, to determine the level of expected overfitting of our model (Ensor et al., 2019). We will use data from the RIME study for model development because of its larger sample size (n = 1,197 vs. n = 502) and larger number of events (n = 202 vs. n = 75) compared to PRIMUM.
Missing Data
If a predictor is not present in either of the included studies, it will be considered systematically missing for that study. For practical reasons, systematically missing variables will not be imputed and will therefore not be considered candidate predictors in model development.
Because the exclusion of participants with missing values from analyses (as in complete case analyses) reduces the effective sample size and may lead to biased estimates, we will follow recommendations on dealing with the missing values that inevitably occur in predictors and use multiple imputation (MI) techniques to impute partially missing data (Moons et al., 2012b; Harrell 2015). In the first stage of MI, multiple datasets will be created and the missing values imputed based on the observed data and on the assumption that data are missing at random. The imputation will then be repeated multiple times to properly account for variability in the imputation process. As an approximation, the number of imputed datasets (m) will correspond to the percentage of incomplete observations (White et al., 2011). The analyses will be performed for each dataset, resulting in m analysis results. The last step of MI is a pooling step, whereby overall estimates will be obtained using Rubin’s rules (White et al., 2011; Harrell 2015). In line with the reporting suggestions of Sterne et al., we will compare results from complete cases compared with results based on MI in order to be able to detect and understand differences between them (Sterne et al., 2009).
Statistical Analysis Methods
All statistical analyses will be conducted using R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Model Development, Performance and Internal Validation
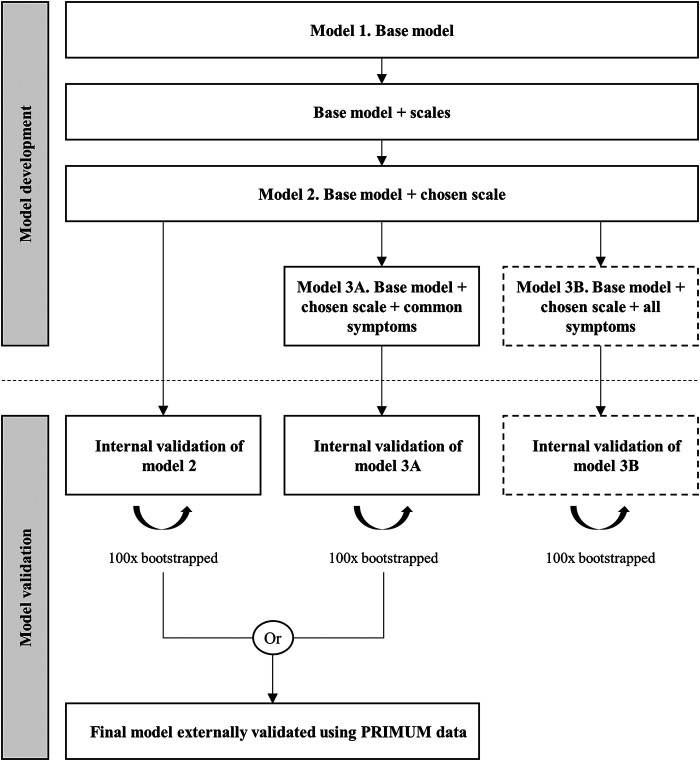
The model will be built as follows (see Figure 1).
FIGURE 1.
Model development and validation process.
Model 1. Base Model
First, multivariable logistic regression will be used to develop a base model that employs data from the RIME trial to predict falls within six months of baseline. To find the best combination of candidate predictors (groups A to C), the multivariable fractional polynomial (MFP) approach will be used. The MFP approach is a procedure that builds the model, while simultaneously determining a suitable functional form for continuous variables. It starts with a full model that includes all candidate predictors and removes the least significant variables via backward elimination. The choice of predictors for elimination will be based on Akaike’s Information Criterion (AIC) (Sauerbrei et al., 2020).
Model 2. Base Model + Scale
Secondly, we will add variables on ACh burden (group D) to the base model. Here, we aim to compare different models for each of the four scales and one equation that measure ACh burden (Carnahan et al., 2006; Hilmer et al., 2007b; Rudolph et al., 2008; Klamer et al., 2017; Kiesel et al., 2018). We will assess the apparent performance of the models for discrimination using the c-statistic [equal to the area under the receiver operating characteristic curve (ROC)] and the model fit using AIC. The preferred model will be the one with the lowest AIC and the highest c-statistic. A c-statistic close to one indicates excellent discrimination and 0.5 indicates that the model cannot discriminate between individuals that have the outcome and those that do not (Steyerberg and Vergouwe 2014).
Model 3. Base Model + Chosen Scale + Symptoms
In a third step, we will extend the model by adding symptom variables (group E) and quantify whether adding these variables to the developed model is beneficial. The development and validation datasets have three symptoms in common: dizziness/vertigo, problems urinating, and stomach pain. The development dataset includes four further symptoms: drowsiness/fatigue, dry mouth, itching, and constipation (see Table 2). We will therefore develop two different models at this stage: model 3A (Base model + chosen scale + common symptoms), which will be considered for external validation, and model 3B (Base model + chosen scale + all symptoms), which will be developed to conduct an exploratory assessment of whether the additional variables considerably improve predictive performance.
Prior to comparing performance, models 2, 3A, and 3B will be internally validated using bootstrapping. This approach includes: 1) generating a bootstrap sample, 2) developing the model using that bootstrap sample, 3) determining the predictive performance of the model in the bootstrap and the original samples, 4) calculating optimism as the difference in performance between bootstrap and the original samples for each performance measure, 5) repeating steps 2 to 4, 100 times, and 6) averaging the estimates of optimism and subtracting average optimism from the apparent performance of the original model in the original dataset to obtain optimism-adjusted performance estimates (Moons et al., 2015). If necessary, we will shrink coefficients to correct for overfitting.
After internal validation, we will compare the performance and discriminative ability of the models before and after adding the variables on anticholinergic symptoms (model 2 vs. model 3A). In case of high collinearity between the predictors, we will assume that adding symptoms will not improve model performance (Harrell 2015). We will compare the c-statistics of the models and determine the change in AIC (Moons et al., 2012a; Cook 2018). The model with the higher c-statistic and lower AIC will be selected for external validation. Because the validation dataset does not include all symptoms contained in model 3B (base model + chosen scale + all symptoms), this model will not be considered for external validation. However, the use of internal validation to compare the performance of models 3A and 3B may indicate whether the additional symptoms considerably improve predictive performance.
External Validation
Using the PRIMUM dataset, we will externally validate the final model and assess discriminative ability by estimating the observed/expected ratio (comparing the predicted number of falls from our final model with the observed number of falls in PRIMUM) and the c-statistic. We will further calculate the calibration slope, calibration-in-the-large, and produce a calibration plot (Steyerberg et al., 2010).
Anticipated Results
Our results are expected to contribute to the ongoing discussion regarding the use of ACh scales in clinical practice. Analyses may identify differences in the scales’ ability of linking ACh burden with falls for older general practice patients, indicating their usefulness in clinical practice in a German health care context. Patients’ individual characteristics and patient-reported symptoms may reflect clinically relevant and feasible indicators to identify patients from a heterogeneous population that might be at high risk of experiencing falls.
Strengths
As one of the limitations of ACh scales’ is their failure to take into account individual patient characteristics, patient-reported symptoms may function as a surrogate for patient susceptibility to such medications and may therefore help clinicians identify patients at high risk of falls. Furthermore, a prognostic model tends to perform optimistically when using the data from which it was derived. It is therefore necessary to assess its accuracy by externally validating it using a separate dataset (Moons et al., 2015). External validation will enable to demonstrate its predictive value and generalizability in a similar population but with different individuals (Steyerberg and Vergouwe, 2014).
Limitations
One limitation is the risk of recall-bias in the patients’ ability to self-report falls (Gillespie et al., 2012). A verification of patient-reported falls in electronic medical records (EMR) was not possible in the studies because are not systematically documented in EMRs. The eligibility criteria and availability of data will not allow us to include some of the known risk factors for falls in the elderly such as dementia, muscle strength, or gait problems (Deandrea et al., 2010; Ambrose et al., 2013; Sousa et al., 2017). It will also prevents us from considering the known increased risk of inter-individual variability in older age that results from, for example, age-related pharmacokinetic and pharmacodynamic changes and genetic variation in metabolism (Nishtala et al., 2016). Furthermore, we are not able to prove the causality of the symptoms, as they may have been caused by factors other than a (anticholinergic drug) reaction to the medication. Nevertheless, as patients’ susceptibility varies considerably, we believe that taking patient-reported symptoms into consideration will provide additional decision support to the mere calculation of anticholinergic load (Kersten and Wyller, 2014). As external validation will be carried out retrospectively using an existing dataset, the 75 events from PRIMUM will not meet the suggested minimum of 100 events required to externally validate a prognostic model (Collins et al., 2016). In consequence, we expect estimates of performance measures to have relatively wide 95% confidence intervals. In view of this limitation, we will carefully discuss and interpret our findings.
Discussion
Literature on ACh scales’ ability to predict falls is scarce. This study may therefore provide insights into the issue by comparing the measurements provided by several scales with the number of falls and determining whether the inclusion of patient-reported symptoms is beneficial in this context. Our results may therefore support GPs with their clinical decision-making in reducing medications with ACh properties in older patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to dinh@allgemeinmedizin.uni-frankfurt.de.
Ethics Statement
The studies involving human participants were reviewed and approved. The Ethics Commission of the Medical Faculty of the Johann Wolfgang Goethe University, Frankfurt/Main approved the PRIMUM study (date: 20/05/2010, reference: E46/10). The Ethics Commission of the University Witten/Herdecke approved the RIME study (date: 28.02.2012, reference: 147/2011). The Ethics Commission of the Medical Faculty of the Goethe University, Frankfurt/Main confirmed that no extra vote was necessary for the anonymous use of data within the PROPERmed study (13/07/2017) because included trials were approved by their ethics commissions separately. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TD, PG and CM initiated and designed the study. TD drafted the manuscript. AG-G, AM, JB, H-JT, HR, KS, M-SB, MA, ND-B, PE, SH, UT, and WH provided methodological guidance. All authors contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
PROPERmed was funded by the German Innovation Fund (grant number 01VSF16018). RIME was funded by the German Federal Ministry of Education and Research (grant number 01ET1005A). PRIMUM was funded by the German Federal Ministry of Education and Research (grant number 01GK0702). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
TD, AG-G, AM, HR, JB, H-JT, PE, FG, MA, WH, and CM report grants from the German Innovation Fund during the conduct of the PROPERmed study (grant number 01VSF16018). KS reports grants from NIHR School for Primary Care Research during the conduct of the study. KS is funded by the National Institute for Health Research School for Primary Care (NIHR SPCR Launching Fellowship). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. UT reports grants from the German Federal Ministry of Research and Eductation during the conduct of the RIME study (grant number 01ET1005A). UT has received travel expenses from the German Society of Internal Medicine and the Goethe-University Frankfurt/Main for scientific meetings. UT has received travel expenses and an honorarium for a lecture from the Foundation for Quality and Efficiency in Health Care (IQWIG, Germany) and for a symposium from the BANSS Foundation (Biedenkopf, Germany).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank members of the original study groups of trials included in the PROPERmed IPD-MA: Wendy den Elzen, Wilbert van den Hout, Anne van Houwelingen, Margot Heijmans, Theo Stijnen for the ISCOPE study; Donna Bosch-Lenders, Prof. André Knotterus, Jelle Stoffers and Bjorn Winkens for the PIL study; Prof. Greiner, Prof. Eva Hummers, Prof. Ulrike Junius-Walker, Prof. Petra Thürmann and Prof. Stefan Wilm for the RIME study. The authors thank Phillip Elliot from Goethe-University for conducting a language review.
Abbreviations
ACh, anticholinergic; ADS, anticholinergic drug scale; AIC, akaike’s information criterion; ARS, anticholiergic risk scale; ATC, anatomic therapeutic chemical; cRCT, cluster-randomized controlled trials; DBI, drug burden index; EMR, electronic medical record; FDA, food and drug administration; Ger-ABS, german anticholinergic burden score; GP, general practitioner; MI, multiple imputation; MFP, multivariable fractional polynomial; PIM, potentially inappropriate medication; PRIMUM, prioritizing multimedication in multimorbidity; RIME, reduction of potentially inadequate medication in the elderly; ROC, receiver operating characteristic
References
- Aizenberg D., Sigler M., Abraham W., Barak Y. (2002). Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int. Psychogeriatr. 14 (3), 307–310. 10.1017/s1041610202008505 [DOI] [PubMed] [Google Scholar]
- Ambrose A. F., Paul G., Jeffrey M. H. (2013). Risk factors for falls among older adults: a review of the literature. Maturitas 75 (1), 51–61. 10.1016/j.maturitas.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Brueckle M.-S., Thomas E. T., Seide S. E., Pilz M., Gonzalez-Gonzalez A. I., Sophia Nguyen T., et al. (2020). Adverse drug reactions associated with amitriptyline—protocol for a systematic multiple-indication review and meta-analysis. Syst. Rev. 9 (1), e011613 10.1186/s13643-020-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell K., Hughes C. M., Ryan. C. (2015). The association between anticholinergic medication burden and health related outcomes in the ‘oldest old': a systematic review of the literature. Drugs Aging 32 (10), 835–848. 10.1007/s40266-015-0310-9 [DOI] [PubMed] [Google Scholar]
- Carnahan R. M., Lund B. C., Perry P. J., Pollock B. G., Culp K. R. (2006). The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J. Clin. Pharmacol. 46 (12), 1481–1486. 10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Mehta S., Sherer J. T., Aparasu R. R. (2010). Prevalence and predictors of anticholinergic medication use in elderly nursing home residents with dementia: analysis of data from the 2004 National Nursing Home Survey. Drugs Aging 27 (12), 987–997. 10.2165/11584430-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Collins G. S., Ogundimu E. O., Altman. D. G. (2016). Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat. Med. 35 (2), 214–226. 10.1002/sim.6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. R. (2018). Quantifying the added value of new biomarkers: how and how not. Diagn. Progn. Res. 2, 14 10.1186/s41512-018-0037-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinot V., Faure R., Omrani S., Goutelle S., Bourguignon L., Krolak-Salmon P., et al. (2014). Exposure to anticholinergic and sedative drugs, risk of falls, and mortality: an elderly inpatient, multicenter cohort. J. Clin. Psychopharmacol. 34 (5), 565–570. 10.1097/JCP.0000000000000195 [DOI] [PubMed] [Google Scholar]
- Deandrea S., Lucenteforte E., Bravi F., Foschi R., La Vecchia C., Negri E. (2010). Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology 21 (5), 658–668. 10.1097/EDE.0b013e3181e89905 [DOI] [PubMed] [Google Scholar]
- Delafuente J. C. (2008). Pharmacokinetic and pharmacodynamic alterations in the geriatric patient. Consult. Pharm. 23 (4), 324–334. 10.4140/tcp.n.2008.324 [DOI] [PubMed] [Google Scholar]
- Ensor J., Martin E. C., Riley R. D. (2019). Pmsampsize: calculates the minimum sample size required for developing a multivariable prediction model. Available at: https://cran.r-project.org/web/packages/pmsampsize/index.html (Accessed April 07, 2020).
- EuroQol Group (1990). EuroQol—a new facility for the measurement of health-related quality of life. Health Pol. 16 (3), 199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- Gillespie L. D., Clare Robertson M., Gillespie W. J. (2012). Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 9, CD007146 10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjidic D., Hilmer S. N., Blyth F. M., Naganathan V., Waite L., Seibel M. J., et al. (2012). Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 65 (9), 989–995. 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- González-González A. I., Meid A. D., Dinh T. S., Blom J. W., Akker M., JmElders P., et al. (2020). A prognostic model predicted deterioration in health-related quality of life in older patients with multimorbidity and polypharmacy. J. Clin. Epidemiol. 10.1016/j.jclinepi.2020.10.006 [DOI] [PubMed] [Google Scholar]
- Harrell F. E. (2015). Regression Model Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd Edition Cham Heidelberg, London: Springer. [Google Scholar]
- Harrell F. E. (2019). Statistically efficient ways to quantify added predictive value of new measurements. Available at: https://www.fharrell.com/post/addvalue/ (Accessed February 24, 2020).
- Hendriksen J. M., Geersing G. J., Moons K. G., de Groot J. A. (2013). Diagnostic and prognostic prediction models. J. Thromb. Haemostasis 11 (Suppl 1), 129–141. 10.1111/jth.12262 [DOI] [PubMed] [Google Scholar]
- Hilmer S. N., McLachlan A. J., Le Couteur D. G. (2007a). Clinical pharmacology in the geriatric patient. Fundam. Clin. Pharmacol. 21 (3), 217–230. 10.1111/j.1472-8206.2007.00473.x [DOI] [PubMed] [Google Scholar]
- Hilmer S. N., Mager D. E., Simonsick E. M., Cao Y., Ling S. M., Windham B. G., et al. (2007b). A drug burden index to define the functional burden of medications in older people. Arch. Intern. Med. 167 (8), 781–787. 10.1001/archinte.167.8.781 [DOI] [PubMed] [Google Scholar]
- Hukins D., Macleod U., Boland J. W. (2019). Identifying potentially inappropriate prescribing in older people with dementia: a systematic review. Eur. J. Clin. Pharmacol. 75 (4), 467–481. 10.1007/s00228-018-02612-x [DOI] [PubMed] [Google Scholar]
- Jessen F., Hanna K., Daerr M., Bickel H., Pentzek M., Riedel-Heller S., et al. (2010). Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur. Arch. Psychiatr. Clin. Neurosci. 260 (S2), S111–S115. 10.1007/s00406-010-0156-4 [DOI] [PubMed] [Google Scholar]
- Kersten H., Wyller T. B. (2014). Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin. Pharmacol. Toxicol. 114 (2), 151–159. 10.1111/bcpt.12140 [DOI] [PubMed] [Google Scholar]
- Kersten H., Molden E., Willumsen T., Engedal K., Wyller T. B. (2013). Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from 21 Norwegian nursing homes. Br. J. Clin. Pharmacol. 75 (3), 842–849. 10.1111/j.1365-2125.2012.04411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel E. K., Marina Hopf Y., Michael D. (2018). An anticholinergic burden score for German prescribers: score development. BMC Geriatr. 18 (1), 239 10.1186/s12877-018-0929-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer T. T., Wauters M., Azermai M., Durán C., Christiaens T., Elseviers M., Robert Vander S. (2017). A novel scale linking potency and dosage to estimate anticholinergic exposure in older adults: the muscarinic acetylcholinergic receptor ANTagonist exposure scale. Basic Clin. Pharmacol. Toxicol. 120 (6), 582–590. 10.1111/bcpt.12699 [DOI] [PubMed] [Google Scholar]
- Kouladjian L., Gnjidic D., Chen T. F., Mangoni A. A., Hilmer S. N. (2014). Drug burden index in older adults: theoretical and practical issues. Clin. Interv. Aging 9, 1503–1515. 10.2147/CIA.S66660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouladjian O’Donnell L., Gnjidic D., Nahas R., Simon Bell J., Hilmer S. N. (2017). Anticholinergic burden: considerations for older adults. J. Pharm. Pract. Res. 47 (1), 67–77. 10.1002/jppr.1303 [DOI] [Google Scholar]
- Landi F., Dell’Aquila G., Collamati A., Martone A. M., Zuliani G., Gasperini B., et al. (2014). Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J. Am. Med. Dir. Assoc. 15 (11), 825–829. 10.1016/j.jamda.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Magin P., Goode S., Pond D. (2015). GPs, medications and older people: a qualitative study of general practitioners’ approaches to potentially inappropriate medications in older people. Australas. J. Ageing 34 (2), 134–139. 10.1111/ajag.12150 [DOI] [PubMed] [Google Scholar]
- Marcum Z. A., Perera S., Thorpe J. M., Switzer G. E., Gray S. L., Castle N. G., Strotmeyer E. S., et al. (2015). Anticholinergic use and recurrent falls in community-dwelling older adults: findings from the health ABC study. Ann. Pharmacother. 49 (11), 1214–1221. 10.1177/1060028015596998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate K. E., Kerr K. P., Pond D., Williams E. J., Marley J., Disler P., Henry B., Parker M. (2015). Impact of multiple low-level anticholinergic medications on anticholinergic load of community-dwelling elderly with and without dementia. Drugs Aging 32 (2), 159–167. 10.1007/s40266-014-0230-0 [DOI] [PubMed] [Google Scholar]
- Mayer T., Haefeli W. E., Seidling H. M. (2015). Different methods, different results—how do available methods link a patient's anticholinergic load with adverse outcomes? Eur. J. Clin. Pharmacol. 71 (11), 1299–1314. 10.1007/s00228-015-1932-x [DOI] [PubMed] [Google Scholar]
- Mayer T., Meid A. D., Saum K. U., Brenner H., Ben S., Seidling H. M., Haefeli W. E. (2017). Comparison of nine instruments to calculate anticholinergic load in a large cohort of older outpatients: association with cognitive and functional decline, falls, and use of laxatives. Am. J. Geriatr. Psychiatr. 25 (5), 531–540. 10.1016/j.jagp.2017.01.009 [DOI] [PubMed] [Google Scholar]
- McNeely S. S., Bhattacharya R., Aparasu R. R. (2013). Prevalence of anticholinergic use among older home health patients. J. Clin. Nurs. 22 (1-2), 285–288. 10.1111/j.1365-2702.2012.04258.x [DOI] [PubMed] [Google Scholar]
- Mintzer J., Burns A. (2016). Anticholinergic side-effects of drugs in elderly people. J. R. Soc. Med. 93 (9), 457–462. 10.1177/014107680009300903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons K. G., Pascal Kengne A., Grobbee D. E., Royston P., Vergouwe Y., Altman D. G., (2012a). Risk prediction models: II. External validation, model updating, and impact assessment. Heart 98 (9), 691–698. 10.1136/heartjnl-2011-301247 [DOI] [PubMed] [Google Scholar]
- Moons K. G., Pascal Kengne A., Woodward M., Royston P., Vergouwe Y., Altman D. G., et al. (2012b). Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 98 (9), 683–690. 10.1136/heartjnl-2011-301246 [DOI] [PubMed] [Google Scholar]
- Moons K. G. M., Altman D. G., Reitsma J. B., Ionnidis J. P. A., Macaskill P., Steyerberg E. W., et al. (2015). Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann. Intern. Med. 162 (1), 55–63. 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- Muth C., Uhlmann L., Haefeli W. E., Rochon J., van den Akker M., et al. (2018). Effectiveness of a complex intervention on prioritising multimedication in multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 8 (2), e017740 10.1136/bmjopen-2017-017740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2013). Falls: assessment and prevention of falls in older people. Available at: https://www.ncbi.nlm.nih.gov/books/NBK258885/pdf/Bookshelf_NBK258885.pdf (Accessed April 14, 2020). [PubMed]
- Ness Jose., Hoth A., Barnett M. J., Shorr R. I., Kaboli P. J. (2006). Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am. J. Geriatr. Pharmacother. 4 (1), 42–51. 10.1016/j.amjopharm.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Nishtala P. S., Saji Salahudeen M., Hilmer S. N. (2016). Anticholinergics: theoretical and clinical overview. Expet Opin. Drug Saf. 15 (6), 753–768. 10.1517/14740338.2016.1165664 [DOI] [PubMed] [Google Scholar]
- Richardson K., Bennett K., Maidment I. D., Fox C., Smithard D., Kenny R. A. (2015). Use of medications with anticholinergic activity and self-reported injurious falls in older community-dwelling adults. J. Am. Geriatr. Soc. 63 (8), 1561–1569. 10.1111/jgs.13543 [DOI] [PubMed] [Google Scholar]
- Riley R. D., Ie Snell K., Ensor J., Burke D. L., Harrell F. E., Moons K. G. M., et al. (2019). Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat. Med. 38 (7), 1276–1296. 10.1002/sim.7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph J. L., Salow M. J., McGlinchey R. E. (2008). The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch. Intern. Med. 168 (5), 508–513. 10.1001/archinternmed.2007.106 [DOI] [PubMed] [Google Scholar]
- Salahudeen M. S., Duffull S. B., Nishtala P. S. (2015a). Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 15, 31 10.1186/s12877-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen M. S., Nishtala P. S., Duffull S. B. (2015b). The influence of patient characteristics on anticholinergic events in older people. Dement. Geriatr. Cogn. Dis. Extra. 5 (3), 530–541. 10.1159/000441718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba D., Elliott M., Rubenstein L. Z., Solomon D. H., Young R. T., Kamberg C. J., et al. (2001). The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J. Am. Geriatr. Soc. 49 (12), 1691–1699. 10.1046/j.1532-5415.2001.49281.x [DOI] [PubMed] [Google Scholar]
- Sauerbrei W., Perperoglou A., Schmid M., Abrahamowicz M., Becher H., Binder H., et al. (2020). State of the art in selection of variables and functional forms in multivariable analysis-outstanding issues. Diagn. Progn. Res. 4, 3 10.1186/s41512-020-00074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa L. M., Marques-Vieira C. M., Caldevilla M. N., Henriques C. M., Severino S. S., Caldeira S. M. (2017). Risk for falls among community-dwelling older people: systematic literature review. Rev. Gaucha Enferm 37 (4), e55030 10.1590/1983-1447.2016.04.55030 [DOI] [PubMed] [Google Scholar]
- Sterne J. A., White I. R., John B. C., Royston P., Kenward M. G., Wood A. M., et al. (2009). Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338, b2393 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E. W., Vickers A. J., Cook N. R., Gerds T., Gonen M., Obuchowski N., et al. (2010). Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21 (1), 128–138. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E. W., Vergouwe Y. (2014). Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur. Heart J. 35 (29), 1925–1931. 10.1093/eurheartj/ehu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiem U., Wilm S., Greiner W., Rudolf H., Trampisch H. J., Müller C., et al. (2020). Reduction of potentially inappropriate medication in the elderly: design of a cluster-randomised controlled trial in German primary care practices (RIME). Ther. Adv. Drug Saf. 12, 2042098620918459 10.1177/2042098620918459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. J., Brennan T. A. (2000). Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 320 (7237), 741–744. 10.1136/bmj.320.7237.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. K., Willetts J. L., Corns H. L., HelenMarucci-Wellman R., Lombardi D. A., Courtney T. K., et al. (2016). Falls and fall-related injuries among community-dwelling adults in the United States. PLoS One 11 (3), e0150939 10.1371/journal.pone.0150939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Moreno A. M., Angela M., Rocío Alfaro-Lara E., Concepción Pérez-Guerrero M., Maria Dolores N.-M., Santos-Ramos B. (2016). Systematic review on the use of anticholinergic scales in poly pathological patients. Arch. Gerontol. Geriatr. 62, 1–8. 10.1016/j.archger.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Vincentis A. de., Gallo Paolo., Finamore P., Pedone C., Costanzo L., Pasina L., et al. (2020). Potentially inappropriate medications, drug-drug interactions, and anticholinergic burden in elderly hospitalized patients: does an association exist with post-discharge health outcomes? Drugs Aging 37, 585–593. 10.1007/s40266-020-00767-w [DOI] [PubMed] [Google Scholar]
- Wawruch M., Macugova A., Kostkova L., Luha J., Dukat A., Murin J., et al. (2012). The use of medications with anticholinergic properties and risk factors for their use in hospitalised elderly patients. Pharmacoepidemiol. Drug Saf. 21 (2), 170–176. 10.1002/pds.2169 [DOI] [PubMed] [Google Scholar]
- Welsh T. J., van der Wardt V., Ojo G., Gordon A. L., Gladman J. R. F. (2018). Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging 35 (6), 523–538. 10.1007/s40266-018-0549-z [DOI] [PubMed] [Google Scholar]
- White I. R., Royston P., Wood A. M. (2011). Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30 (4), 377–399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- Wilson N. M., Hilmer S. N., March L. M., Cameron I. D., Lord S. R., Seibel M. J., et al. (2011). Associations between drug burden index and falls in older people in residential aged care. J. Am. Geriatr. Soc. 59 (5), 875–880. 10.1111/j.1532-5415.2011.03386.x [DOI] [PubMed] [Google Scholar]
- Zia A., Kamaruzzaman S., Myint P. K., Tan M. P. (2016). Anticholinergic burden is associated with recurrent and injurious falls in older individuals. Maturitas 84, 32–37. 10.1016/j.maturitas.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Zia A., Kamaruzzaman S., Tan M. P. (2015). Polypharmacy and falls in older people: balancing evidence-based medicine against falls risk. Postgrad. Med. 127 (3), 330–337. 10.1080/00325481.2014.996112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to dinh@allgemeinmedizin.uni-frankfurt.de.