Abstract
The discovery of leptin was intrinsically associated with its ability to regulate body weight. However, the effects of leptin are more far-reaching and include profound glucose-lowering and anti-lipogenic effects, independent of leptin’s regulation of body weight. Regulation of glucose metabolism by leptin is mediated both centrally and via peripheral tissues and is influenced by the activation status of insulin signaling pathways. Ectopic fat accumulation is diminished by both central and peripheral leptin, an effect that is beneficial in obesity-associated disorders. The magnitude of leptin action depends upon the tissue, sex, and context being examined. Peripheral tissues that are of particular relevance include the endocrine pancreas, liver, skeletal muscle, adipose tissues, immune cells, and the cardiovascular system. As a result of its potent metabolic activity, leptin is used to control hyperglycemia in patients with lipodystrophy and is being explored as an adjunct to insulin in patients with type 1 diabetes. To fully understand the role of leptin in physiology and to maximize its therapeutic potential, the mechanisms of leptin action in these tissues needs to be further explored.
Keywords: leptin, glucose, lipid
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
-
-
Leptin lowers blood glucose and has anti-lipogenic effects.
-
-
Leptin regulates glucose and lipid metabolism centrally and peripherally.
-
-
Leptin’s peripheral target tissues include the endocrine pancreas, liver, skeletal muscle, adipose tissues, immune cells, and cardiovascular system.
Since its discovery approximately 25 years ago, leptin has been most extensively studied for its ability to decrease food intake by acting at the level of the central nervous system (CNS) (1). Despite initial disappointment of leptin not being a simple treatment for obesity (1), leptin has emerged as a powerful modulator of glucose, lipid, and protein metabolism, like the hormone insulin. The effects of leptin are complex since they often appear to be tissue- and sex-specific as well as context-dependent. Our objective in this review is to examine the tissue-specific effects of leptin on glucose and lipid metabolism. We focus on the involvement of the CNS, liver, skeletal muscle, adipose tissues, immune system, and the cardiovascular system.
Leptin
Leptin is a peptide hormone encoded by the Lepob gene and is mainly synthesized in white adipose tissue (2,3), although expression levels and rates of secretion differ between depots (4,5). Leptin concentration in plasma is typically proportional to fat mass (3), but the underlying mechanism is poorly understood. It was recently reported that the link between intracellular lipid content and leptin gene expression is likely the heterodimerization of nuclear receptors peroxisome proliferator-activated receptor γ and retinoid X receptor α (6). Intracellular glucose metabolites and circulating factors such as insulin stimulate leptin secretion (7,8), while leptin signaling in hypothalamic proopiomelanocortin (POMC) neurons inhibits leptin secretion during fasting (9). Importantly, leptin’s metabolic influence is contingent on the nutritional status of the animal, and therefore many of the leptin-induced changes described during leptin treatment of fasting animals are reduced or absent in fed controls (10). Plasma leptin concentrations have a circadian rhythm (highest during the night in humans) and are higher in women; in obesity, the rhythm is disrupted, and levels are elevated overall (hyperleptinemia) (11-13). Leptin is also synthesized in the gut, where it is released into its lumen and can evidently eventually arrive in the systemic circulation (14,15). Lastly, leptin expression is reportedly induced in skeletal muscle in response to exogenous leptin and to activation of the hexosamine biosynthetic pathway, which can be triggered by factors such as hyperglycemia and hyperlipidemia (7,16).
While this review focuses on mammals, mostly mice, rats, and humans, other animal models are also used to study leptin physiology. Leptin and white adipose tissue are found in various species, including humans, mice, rats, and fish (17-19). The ob/ob mouse (Lepob mouse), which does not have functional leptin, highlights the importance of leptin in energy balance and metabolism; it is characterized by severe obesity, hyperlipidemia, insulin resistance, and hyperglycemia depending on the genetic background (20,21). Zebrafish (Danio rerio) have two leptin genes (lepa and lepb) and one leptin receptor gene (22-24). Expression of leptin in zebrafish does not occur in adipose tissue, but it is found in other tissues, such as liver, gut, heart, pituitary gland, and ovary (22). Leptin receptor expression occurs in various tissues, including liver, heart, muscle, brain, ovary, and testes (23). Disruption of leptin signaling in zebrafish through leptin isoform A and/or leptin receptor knockdown affects embryonic development in various ways, including diminishing body size and brain differentiation (25). In adult zebrafish, leptin promotes an appetite-suppressing transcriptional profile in the brain (26), and lepa knockout adult zebrafish are obese (27). However, adult zebrafish with a mutation in the leptin receptor gene that leads to a premature stop codon in the cytoplasmic region have normal body weight (24), perhaps because this specific leptin receptor mutation is insufficient to alter energy balance. Interestingly, this leptin receptor mutation results in elevated whole-body glucose content at the larval stage, but better glucose tolerance that is associated with augmented insulin expression in adult zebrafish (24). In aggregate, these studies indicate that the role of leptin in zebrafish physiology depends on its stage of development, with leptin signaling in adulthood being important for maintaining normal body weight and restraining insulin expression. The fruit fly (Drosophila melanogaster) does not have white adipose tissue and therefore does not have leptin as an adipokine (17). However, fruit flies do have a leptin analog expressed in neurons, unpaired 1 (upd1), and Beshel et al. (28) reported that knockdown of upd1 causes obesity. Upd1 binds to the domeless receptor, which, similar to leptin receptors in mammals, activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (28,29). Moreover, human leptin binds to and activates the domeless receptor (30). Taken together, these findings indicate that leptin signaling prevents weight gain and alters glucose metabolism across greatly divergent species. Due to their affordability, zebrafish and drosophila are becoming increasingly more popular in physiological studies, and further study in these models may provide some novel insights regarding the role of leptin signaling in metabolism.
Leptin Signaling
We direct the reader to excellent reviews of leptin signaling pathways (eg, (31,32)). Briefly, there are various isoforms of the leptin receptor (LepR) that, with exception of the soluble leptin receptor, can be found at the plasma membrane (32). Different isoforms of LepR are the result of alternative splicing of messenger ribonucleic acid (mRNA) (32,33). In humans and mice, the soluble LepR is produced through protein cleavage, and in addition, mice have mRNA for soluble LepR (designated LepRe) (32-37). The soluble leptin receptor may increase the concentration of leptin in the circulation while decreasing leptin bioavailability (38-40).
Leptin action requires leptin binding to a receptor on the plasma membrane. Leptin receptor isoforms at the plasma membrane consist of the long isoform (LepRb) and multiple isoforms which have shorter intracellular domains compared to LepRb (32). In mice, the short isoforms of LepR are LepRa, LepRc, and LepRd (37, 41), while the short isoforms LepRa, LepRc, and LepRf have been detected in rats (42,43), and LepRa and LepRc have been found in humans (35,44). LepRs are expressed in many tissue/cell types throughout the body, and the hypothalamus of mice, rats, and humans is rich in LepRb (32,37,41,42,44-47). The majority of leptin’s actions appear to be mediated by LepRb, as demonstrated by the db/db mouse (Leprdb mouse), which lacks only LepRb and is characterized by severe obesity, hyperlipidemia, and insulin resistance comparable to that of the leptin deficient Lepob mouse model (20,37,48,49). The presence of fasting hyperglycemia in the Leprdb mouse depends on genetic strain, with the C57BLKS-Leprdb strain being particularly susceptible to diabetes (49). The cytoplasmic portion of LepRb associates with JAK2, which phosphorylates LepRb on specific tyrosine residues, allowing other mediators to be recruited to LepRb and phosphorylated by JAK2, such as STAT3 (48). Phosphorylated STAT3 dimerizes and translocates to the nucleus where it regulates the transcription of leptin target genes (48,50). Although JAK2/STAT3 is a prominent pathway of leptin-mediated regulation of body weight and glucose homeostasis (48,50), another JAK2-dependent, but STAT3-independent, pathway that has not yet been fully characterized is necessary for leptin’s full effects (51). Barnes et al (51) recently generated various LepRb deletion mutant mice using CRISPR/Cas9 and found that maximal obesity, hyperglycemia, hyperleptinemia, and hyperinsulinemia were obtained when proximal LepRb cytoplasmic regions, in addition to LepRb STAT3-binding sequences, were also deleted. The biological significance of the short isoforms of LepR is starting to be unravelled. In mice, knocking out LepRa results in lower fasting glycemia and better glucose tolerance on standard chow (52). These metabolic alterations are associated with elevated LepRb and LepRc mRNA levels in various tissues, although the expression of these leptin receptor splice variants was not affected in the hypothalamus (52). In hepatocytes, LepRa increases lipoprotein assembly through the mitogen activated protein kinase pathway (53). Hence, recent investigations have revealed the importance of pathways other than JAK2/STAT3 in mediating the metabolic effects of activated leptin receptors.
Obesity is associated with hyperleptinemia, and there is debate over whether hyperleptinemia induces leptin resistance (diminished leptin action at a given leptin concentration), or not. Recent studies provide evidence for the former (54) and the latter (55). Moreover, there is crosstalk between leptin and insulin signaling pathways and prolonged hyperleptinemia can cause insulin resistance (56,57). The effect of hyperleptinemia and leptin resistance on various tissues and the pathophysiology of various disorders are largely unexplored topics. This could be highly relevant to deciphering the mechanisms of leptin resistance associated with obesity in humans and could direct promising approaches to enhance leptin sensitivity and new weight loss therapies.
Except for treating lipodystrophy, therapeutic applications of leptin are still controversial due to apparently conflicting lines of evidence and the limitations of the widely used ob/ob model. Zhao et al (54) have shown that by genetically reducing the amount of functional leptin secreted by adipose tissue, mice are unexpectedly protected from high-fat diet-induced obesity. This finding suggests that protecting from hyperleptinemia may be important in preventing diet-induced obesity, which seems at odds with the paradigm that leptin decreases food intake and increases energy expenditure. Low leptin levels are associated with a greater capacity for weight loss in obese human subjects (58), which may indicate that high leptin levels result, in part, from leptin resistance. Thus, there is merit to the idea that lowering leptin levels could be therapeutically beneficial for weight gain prevention. However, the ob/ob model used by Zhao et al (54) comes with a number of important caveats relevant to energy metabolism and inflammation. The developmental consequences of reduced leptin in ob/ob mice include impaired proliferation and elongation in neurons of the arcuate nucleus (ARC), reduced projections to the dorsomedial nucleus (DMH), and reduced agouti-related protein (AgRP) and α-melanocyte-stimulating hormone projections to the paraventricular nucleus (59). These nuclei of the hypothalamus are essential sites of action for signals that affect feeding and energy expenditure. Furthermore, liver protease inhibitor levels such as serine protease inhibitor α1-antitrypsin can become disrupted in ob/ob mice and obese humans, driving inflammation in adipose tissue and liver, insulin resistance, and body weight gain (60). These studies show that models with constitutively lowered leptin suffer from developmental abnormalities and chronic inflammation, which may confound interpretations of leptin lowering therapy.
In contrast with Zhao et al (54), various groups have shown evidence that reducing leptin exacerbates the metabolic consequences of high-fat diet exposure. For example, weight gain resistant A/J mice had a 20-fold increase in leptin concentration in response to 14-week high-fat diet but weight gain susceptible C57BL/6 mice experienced only a 2-fold increase in leptin levels (61). This is not congruent with the idea that lower leptin during high-fat diet is favorable. Another study showed that lowering leptin levels can favor glucose intolerance, lipid metabolism dysregulation, and obesity in high-fat diet fed mice (62). These studies demonstrate that the suppression of leptin to prevent obesity and diabetes requires further study to better understand the mechanisms that underlie this paradoxical result.
Effects of Leptin on Glucose and Lipid Metabolism
Effects of peripherally administered leptin on metabolism in rodents
Leptin administered outside the CNS through various routes, namely intraperitoneal (i.p.), intravenous (i.v.), and subcutaneous (s.c.), reaches peripheral tissues and select regions of the CNS (63,64). Alterations in leptin levels in the systemic circulation modulate glucose and lipid metabolism. Indeed, the ability of leptin to lower the body weight of Lepob mice occurs alongside leptin-induced improved glycemic control (65,66). Moreover, a low leptin dose (1 mg kg−1 day−1) improves glycemic control without decreasing body weight in Lepob mice (65), and pair-feeding experiments indicate that improved glycemic control can be dissociated from leptin-induced decrease in food intake (67).
Peripherally administered leptin can alter glucose metabolism in various tissues. Although short-term (hours) administration (300 μg kg−1 h−1) of recombinant mouse leptin in rats does not affect basal (fasting) hepatic glucose production (68), leptin increased insulin sensitivity, specifically insulin-induced suppression of hepatic glucose production, and interestingly, while leptin had a robust additive effect on insulin-stimulated suppression of glycogenolysis, leptin blunted insulin-induced suppression of gluconeogenesis (68). In the same study, leptin did not alter insulin-stimulated glucose disposal (glucose uptake). At a lower peripheral infusion rate (180 μg kg−1 h−1) of recombinant mouse leptin in rats, similar effects on glycogenolysis and gluconeogenesis during hyperinsulinemia were found, but leptin did not change insulin-induced suppression of hepatic glucose production (69). In mice, short-term (hours) leptin administration (1 μg h−1) boosts hepatic glucose production as well as glucose uptake in skeletal muscle and brown adipose tissue (70). Prolonged (days) administration of recombinant mouse leptin (500 ± 200 μg kg−1 d−1) to rats also augments insulin-induced suppression of hepatic glucose production, and additionally, insulin-induced glycogenesis and glucose disposal are elevated (71). Leptin increases insulin sensitivity, specifically insulin-stimulated glucose utilization or glucose oxidation, in skeletal muscle and brown adipose tissue, but blunts it in white adipose tissue (72-74). Overall, these studies indicate that exogenous peripheral leptin can increase insulin sensitivity of glucose metabolism in the liver (glycogenolysis), skeletal muscle, and brown adipose tissue, but not in white adipose tissue.
It is important to note the impact of nutritional state on leptin’s effects. During fasting (24 h), but not the fed state, an i.p. injection of human or murine leptin significantly raises plasma glucose, insulin, and glucagon through sympathetic pathways in mice (75). Nevertheless, leptin-induced elevation of circulating glucose concentrations during the fasting state depends on circulating leptin concentrations. Perry et al (76) found that, in 48 h fasted rats with plasma leptin concentrations of ~30 pM, i.v. leptin infusion boosted circulating levels to ~60 pM and decreased plasma glucose concentrations, but when increased to supraphysiological levels (~1250 pM), circulating glucose was substantially elevated. Hence, the glycemic response to exogenous leptin depends on the phase of the fed-fasting cycle as well as the circulating leptin concentration that is achieved.
Lipid metabolism in various tissues is also modulated by systemic leptin administration. Systemic leptin increases lipolysis in white adipocytes (77), possibly via hormone-sensitive lipase (HSL) and nitric oxide (NO) synthase (78,79). Leptin also augments breakdown of triglycerides in skeletal muscle, oxidation of fatty acids in skeletal muscle and liver, and ketogenesis in the liver (80-82). Accordingly, leptin decreases the size of adipose tissue depots (71) and reduces lipid content in skeletal muscle as well as liver (81,83). Interestingly, the depletion of hepatic lipid induced by peripheral leptin administration can be mediated by Kupffer cells, which are resident macrophages in the liver (81). Leptin inhibits insulin-stimulated lipogenesis in white adipocytes (72) but enhances the inhibition of very low density lipoprotein (VLDL) synthesis in the liver by insulin (84). Overall, systemic leptin diminishes lipid accumulation in various tissues.
Leptin is a treatment for humans with lipodystrophy, a disorder with various causes that is characterized by diminished adipose tissue stores (85,86). A murine model of congenital generalized lipodystrophy was generated by expressing truncated nuclear sterol regulatory element-binding protein 1c in adipose tissues (nuclear sterol regulatory element-binding protein 1c is driven by the aP2 promoter) (87). These mice have low circulating levels of leptin, hyperglycemia, and hepatic steatosis, which are improved by peripheral and intracerebroventricular (i.c.v.) leptin administration (87,88). Lipodystrophy can also be induced in mice by dietary consumption of conjugated linoleic acid (89), and peripheral leptin treatment reduces hepatic lipid content, stimulates lipid oxidation, and increases insulin sensitivity (90,91). Hence, leptin improves glucose and lipid metabolism in rodent models of lipodystrophy.
Since leptin can inhibit insulin secretion (72,92), studies of leptin physiology have also been performed in models in which insulin is severely reduced (93) or completely absent (94) to define the insulin-independent actions of leptin. Peripheral administration of leptin in rodent models of insulin-deficient diabetes, induced for example by the pancreatic β cell-destroying toxin streptozotocin, improves glycemia, represses circulating lipids, and decreases hepatic lipid content (93,95). In addition, whole-body and hepatic insulin sensitivity are improved by leptin in rodent models of insulin-deficient diabetes (96,97). These effects usually occur independently of changes in body weight and thus are not merely secondary to the well-recognized weight-lowering effects of leptin. While the mechanisms for the glucose lowering effect of leptin therapy in insulin deficiency are still being elucidated, the effect is independent of increased insulin sensitivity (94) and involves decreased glucose production (93). However, these leptin mediated improvements in metabolism are associated with the risk of severe hypoglycemia (93). As well, while some aspects of metabolism in insulin-deficient rodents can be improved with physiological levels of leptin therapy, reduction of fasting blood glucose to normal levels requires pharmacological levels of leptin (98). This is in striking contrast to the glucose lowering effects of leptin in leptin-deficient ob/ob mice, which are highly sensitive to very low leptin doses (65). Hypoinsulinemia is associated with hypoleptinemia, and studies in insulin-deficient rodents indicate that leptin therapy is more effective as hypoinsulinemia or hypoleptinemia becomes more severe (99-102). Hence, leptin may be a potential adjuvant treatment for patients with insulin-deficient diabetes, but careful assessment of the risks for hypoglycemia will be needed.
Effects of peripherally administered leptin on metabolism in humans
The pivotal role of leptin in the regulation of body weight and metabolism in humans has been demonstrated in individuals who are leptin deficient or have impaired leptin signaling. Leptin deficiency is a term that encompasses patients with undetectable or lower than normal levels of leptin in the circulation as well as patients with elevated, but biologically inactive, circulating leptin (103-107). Congenital leptin deficiency, which results from various types of mutations in the leptin gene, and acquired leptin deficiency, which includes anorexia nervosa, have an overall low prevalence (103,106,108,109). Patients with congenital leptin deficiency are massively obese starting early in life and have impairments in immune and reproductive systems (104-106,110). With respect to metabolism, congenital leptin deficiency is associated with hyperinsulinemia, moderate dyslipidemia, and severe liver steatosis (111). Patients have also been identified with various types of mutations in the leptin receptor gene (reviewed in (112)). Dysfunction of the leptin receptor in humans results in similar characteristics to those observed with leptin deficiency, including obesity and dysregulated glucose metabolism (106,110,112-115). While congenital leptin deficiency is rare, numerous studies have reported improvements in metabolism following leptin replacement therapy. Leptin therapy results in significant decreases in body weight and fat mass (reviewed in (116)). Moreover, leptin therapy of congenital leptin deficiency leads to substantial (18%–85%) decreases in plasma insulin levels (117-119). In one patient with congenital leptin deficiency and type 2 diabetes, leptin therapy also decreased blood glucose levels from 131 mg/dL to 86 mg/dL after 18 months of therapy (120). Leptin therapy also improved insulin sensitivity in a patient with congenital leptin deficiency, reducing the HOMA-IR value from approximately 4.5 to 3.6 (121). In addition, the modest dyslipidemia (slightly elevated serum triglycerides and low density lipoprotein [LDL] cholesterol and low high density lipoprotein [HDL] cholesterol) was improved with the leptin therapy (117,119). Leptin replacement therapy for congenital leptin deficiency leads to rapid reversal of hepatic steatosis (121,122). Consistent with this, treatment of congenital leptin deficiency with acute leptin replacement therapy leads to a shift in the metabolome consistent with leptin therapy promoting lipolysis and fatty acid oxidation (123).
Human lipodystrophy is a collection of disorders characterized by loss of adipose tissue and forms with widespread loss of adipose tissue and reduced serum leptin levels. Subjects with congenital generalized lipodystrophy are the most hypoleptinemic with serum concentrations in the range of 0.05 to 3.7 ng/mL (124) compared to normal levels in individuals in the range of 7 ng/mL for males and 15 ng/mL for females (125); note that leptin levels are positively correlated with fat mass (126). Numerous studies of leptin replacement therapy in patients with lipodystrophy effectively raise leptin levels to greater than 10 ng/mL (reviewed in (127)). This increase in leptin is associated with reduced fasting blood glucose and insulin levels, reduced hemoglobin A1c, and lower serum triglycerides and cholesterol (total and LDL) as well as hepatic de novo lipogenesis (85,116,128-130). Metabolomics of blood suggest that leptin treatment in people with lipodystrophy elevates β oxidation of fatty acids and catabolism of amino acids (131). This agrees with the effect of acute leptin administration to healthy humans. Short-term (4 h) i.v. administration of leptin resulting in a ~20-fold increase in circulating leptin concentrations (similar to levels found in obese people), decreased insulin levels during the infusion and augmented systemic fatty acid oxidation as well as specifically in skeletal muscle (132). Interestingly, a proportion of the beneficial metabolic effects of leptin therapy in people with lipodystrophy occur independently of its hypophagic action (133). The success of leptin therapy to improve insulin sensitivity in leptin deficient people led to trials of methionyl leptin in obese patients with type 2 diabetes. However, neither low nor high dose therapy (raising leptin levels 3- or 150-fold, respectively) for 14 days led to weight loss-independent improvements in insulin sensitivity (134).
Leptin treatment may also benefit patients with anorexia nervosa, who have suboptimal body weight, are hypoleptinemic, and have greater lipid concentration in the circulation (135,136). In a study of adolescent girls with anorexia nervosa, leptin levels were in the range of 5 ng/mL compared to healthy controls that were closer to 17 ng/mL (137). Anorexia nervosa is a psychiatric disorder that causes individuals, mostly females, to chronically reduce their food intake (135). Clinical trials for the use of leptin to treat anorexia nervosa are warranted (138), but for leptin treatment to be successful in anorexia nervosa, leptin should diminish disturbances in metabolism while not further reducing body weight (135).
As previously mentioned, promising studies showing weight loss stimulated by leptin in rodent models have prompted clinical trials trying to replicate this finding in humans. Current evidence suggests that lean and obese individuals under no caloric restriction are not subject to the weight-reducing effects of leptin, but that the effects of leptin are significant under conditions of caloric restriction. In one clinical study that failed to demonstrate weight-reducing effects of leptin in humans, 0.3 mg kg−1 day−1 leptin was administered for 6 days in lean individuals, but this led to a large increase in circulating leptin, from 0.5 to 8 ng/mL in phosphate-buffered saline controls to 150 to 500 ng/mL in those receiving leptin (139). It should be noted that this measurement was made 2 h after subcutaneous leptin injection to capture maximum plasma leptin levels, and average leptin levels were thus likely much lower. Weekly 60 mg injections of long-acting pegylated leptin to obese humans for 8 weeks on a mildly hypoenergetic diet (140) also failed to induce any differences in energy expenditure, glucose metabolism, or body weight change relative to placebo. In a longer (24 weeks) study, a 0.3 mg kg−1 day−1 leptin dose was sufficient to moderately enhance weight loss in obese subjects under moderate caloric restriction (141). This finding has been replicated in obese individuals experiencing severe caloric restriction (142,143), which supports the idea that falling leptin levels are responsible for initiating energy conservation and certainly warrants further investigation of leptin as an adjuvant weight loss therapy under conditions where fat reduction may occur, such as caloric restriction, exercise, and even liposuction (144).
Based on the success of leptin therapy in normalizing metabolism in rodent models of type 1 diabetes, studies of leptin therapy in type 1 diabetes were initiated. In a small pilot study, patients with type 1 diabetes were given metreleptin therapy and followed for 20 weeks (145). The therapy increased plasma leptin levels modestly from 22 ng/mL to 49 ng/mL and did not significantly change hemoglobin A1c, fasting glucose, or plasma triglycerides. However, the total daily insulin dose was reduced by 50% at the end of study and a 6.6% decrease in body weight was achieved by the end of the therapy. While the leptin therapy did not improve glycemic control (aside from improved insulin sensitivity), it should be noted that, similar to the comparisons of leptin-induced weight loss in mice vs humans, this could be due to differences in the levels of leptin achieved in this study. The 2.1-fold increase in circulating leptin in the trial (145) is substantially less than the 13- to 15-fold increase used in most of the leptin therapy studies with rodent models of insulin-deficient diabetes (93-97). Due to the promising insulin-sensitizing effects of leptin and the discrepancies in leptin dosing in humans vs rodents, investigations into higher-dose leptin therapy for type 1 diabetes are warranted.
How peripheral leptin enters and communicates with the CNS
The mechanisms through which peripheral leptin enters the CNS have not been fully elucidated. Leptin from the periphery enters the CNS by transport through the blood-brain barrier, which consists of endothelial cells in blood vessels of the brain being tightly held together, and by transport through the barrier created by epithelial cells of the choroid plexus, a structure that produces cerebrospinal fluid (CSF) (146-149). Leptin can cross the blood-brain and blood-CSF barriers through a process involving LepRs in endothelial cells of microvessels and in epithelial cells of the choroid plexus, the latter of which transports leptin into CSF (43,150,151). LepR-mediated entry of leptin into the brain involves transcytosis (150,152,153). However, the extent of the importance of LepRs for leptin to enter the CNS is debatable and other receptors have been suggested to facilitate leptin transport (154). Circumventricular organs of the CNS, such as the median eminence in the hypothalamus, lack a blood-brain barrier due to their fenestrated capillaries, thus allowing free passage of leptin to select neurons of the mediobasal hypothalamus, particularly the ARC (150,151). Leptin entrance into the brain at the median eminence is controlled because the extent of fenestration of these capillaries is altered by hypothalamic neurons (155). In addition, some studies suggest an important role of tanycytes, specialized ependymoglial cells that line the third ventricle of the hypothalamus, in mediating leptin transport from the median eminence to hypothalamic neurons (151,156). However, their role is controversial because Yoo et al could not detect LepR in murine tanycytes (156,157). Dendrites of LepR-expressing ARC neurons also reach the median eminence, thereby giving these neurons immediate access to leptin leaving fenestrated capillaries, before leptin enters the brain per se (158). Binding of leptin to ARC neurons is increased during fasting (159), but the amount of leptin passing the blood-brain barrier may be decreased (160). In high-fat diet-induced obesity in mice, it has recently been reported that the ability of leptin to cross from the periphery to the CNS is not altered (151,161), in contrast to previous studies indicating that it is diminished (43).
Activation of STAT3 in various brain regions, namely the ARC, DMH, and ventromedial hypothalamus (VMH), and the nucleus of the solitary tract in the brainstem, is proportional to the amount of leptin administered peripherally in rodents (162,163). Notably, Faouzi et al (162) found differences in the kinetics of STAT3 activation in ARC and VMH/DMH by peripheral vs i.c.v. leptin, with i.c.v. leptin having a more immediate pan-activating effect. Hence, while administration of i.c.v. leptin is a reductionist approach to determine central leptin action, the results of such studies may not necessarily translate to the central effects caused by endogenous peripheral leptin. Peripheral leptin also communicates with the CNS via neural afferents. Murphy et al (164) reported that afferent neurons originating in white adipose tissue respond to leptin. In addition, knockout of LepR in vagal afferent neurons causes augmentations in food intake and body weight (165). Taken together, these findings indicate that leptin does not need to enter the CNS from the periphery to exert effects on the CNS.
Leptin’s Tissue-Specific Effects on Glucose and Lipid Metabolism
Effects of direct leptin action in the CNS
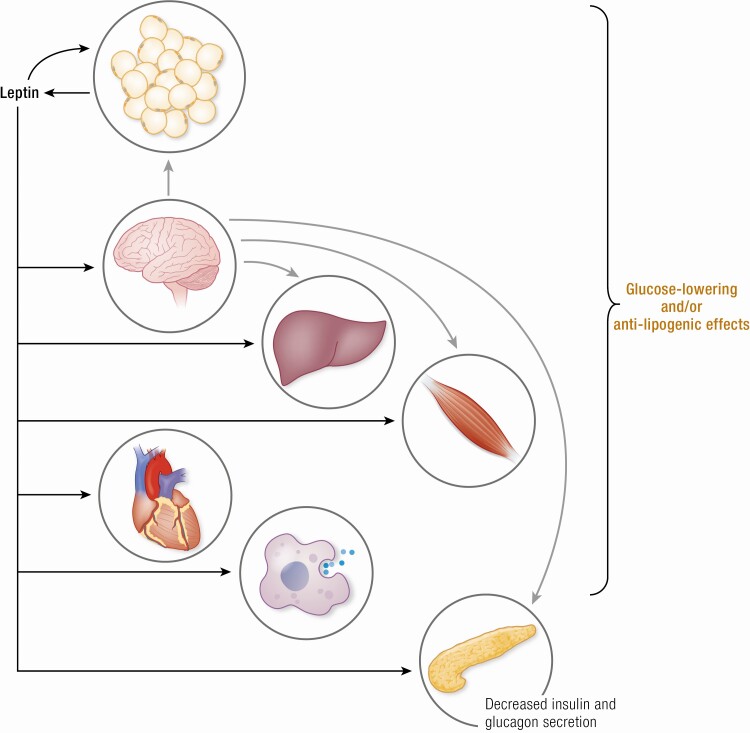
The CNS is currently considered the primary site of leptin action (Fig. 1) and various insightful reviews have been written about the mechanisms through which central leptin action regulates metabolism in the periphery (eg, (166-168)). Here we highlight recent reports and areas that we think require further investigation.
Figure 1.
Metabolic effects of direct leptin action in the central nervous system. Abbreviations: AgRP, agouti-related protein; POMC, proopiomelanocortin; SF1, steroidogenic factor-1.
The effects of peripheral vs i.c.v. administered exogenous leptin on metabolism have been compared. Since these effects generally appear to be similar (70,169,170) and the effects of i.c.v. leptin are potent, peripheral leptin may regulate peripheral metabolism predominantly through the CNS. Tamoxifen-inducible periphery-specific LepRb deficient mice do not have discernable changes in phenotype other than hyperleptinemia, which is caused by elevated bound leptin in the circulation (free leptin concentration in the circulation is not affected) (36). It is noteworthy that the hyperleptinemia did not result in an increase in hypothalamic phosphorylated STAT3, suggesting there was no change in bioactive leptin. The protein bound to circulating leptin is likely soluble LepR (36). Interestingly, while leptin secretion rate from white adipose tissue is elevated in mice with tamoxifen-inducible periphery-specific LepRb deletion, LepRe mRNA levels are not altered in white adipose tissue (36). This suggests that increases in LepRe mRNA levels in other tissues, LepRe translation, or LepR protein cleavage (thereby generating soluble LepR) may be necessary for the spike in circulating bound leptin among mice with peripheral LepRb deletion. It should be noted for this study that the level of LepRb inactivation is modest in several of the peripheral tissues examined, thereby limiting the conclusion as to the role of direct leptin signaling in the periphery. Central and peripheral leptin can communicate with the liver via autonomic nervous system efferents (171,172). Moreover, peripheral leptin increases white adipose tissue lipolysis while central leptin blunts white adipose tissue lipogenesis by activating the sympathetic nervous system (SNS) (173-175). Central leptin acts on skeletal muscle, brown adipose tissue, and heart via the SNS to modulate tissue glucose uptake (176-178), but peripheral leptin can also act directly on these tissues as discussed further below. I.c.v. leptin decreases circulating levels of the pancreatic hormones insulin and glucagon (179,180), a finding that is mirrored in ex vivo studies (92,181,182) (see later discussion of the endocrine pancreas). Although leptin has profound effects on metabolism in the periphery, the contribution of direct peripheral vs central leptin signaling to these outcomes in vivo remains to be clearly defined.
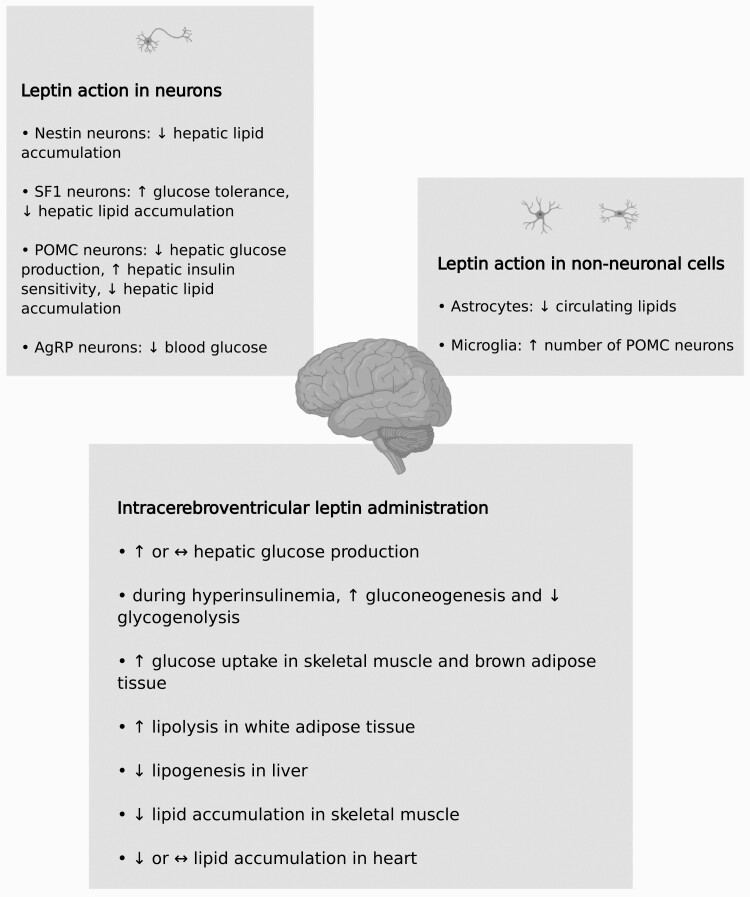
Short-term i.c.v. administration of leptin does not modify glucose production by the liver or glucose utilization, either in basal or hyperinsulinemic states, in rats (169). However, during hyperinsulinemia, gluconeogenesis increases with a proportional decrease in glycogenolysis (169). The effect on gluconeogenesis, but not the effect on glycogenolysis, requires the melanocortin pathway (69). The melanocortin pathway, in which melanocortin 4 receptors are stimulated by α-melanocyte-stimulating hormone and inhibited by AgRP secreted by neurons in the hypothalamus, mediates various effects of leptin (69). It also mediates the effect of peripherally administered leptin on gluconeogenesis (69). In mice, i.c.v. leptin administration (hours) elevates hepatic glucose production and glucose uptake in skeletal muscle as well as brown adipose tissue (70). Prolonged (days) i.c.v. leptin administration in rats also increases glucose uptake, glycogen content, and markers of insulin sensitivity in skeletal muscle (183). Hence, i.c.v. leptin increases insulin sensitivity of glucose metabolism in the liver (glycogenolysis) and skeletal muscle.
I.c.v. leptin also alters lipid metabolism in various tissues. Similar to what is observed with peripherally administered leptin (see previous discussion), i.c.v. leptin diminishes the size of adipose tissue depots and impedes ectopic fat accumulation (184). I.c.v. leptin stimulates white adipose tissue lipolysis, which is associated with augmented HSL gene expression (185). Recently, it was also found that i.c.v. leptin decreases lipogenesis in the liver (186). I.c.v. leptin also alters the peripheral activity of lipoprotein lipase (LPL), an enzyme that hydrolyzes triglycerides and facilitates fatty acid uptake into tissues (187,188); specifically, LPL activity is stimulated in skeletal muscle and blunted in white adipose tissue (187). I.c.v. leptin administration diminishes levels of triglycerides and free fatty acids in skeletal muscle (183). By way of the SNS (α-adrenergic receptor), central leptin activates adenosine monophosphate–activated protein kinase (AMPK) in skeletal muscle, which in turn restrains intramuscular acetyl coenzyme A carboxylase (189). Inhibition of acetyl coenzyme A carboxylase causes a shift towards fatty acid oxidation (190). However, not all studies have found that central leptin activates AMPK in skeletal muscle (191). I.c.v. leptin decreases (192) or does not change (193) triglyceride content in the heart. Interestingly, the effect of i.c.v. leptin on metabolism in the heart also depends on diet. I.c.v. leptin decreases fatty acid oxidation in the hearts of mice on a high-fat diet, while no effect is observed in mice fed a low fat diet (193). In various models of obesity and type 2 diabetes, i.c.v. leptin ameliorates glucose metabolism, lipid metabolism, insulin sensitivity, and energy balance, although not all of these parameters may be affected in a given model (194-196).
Effects of direct leptin action in specific regions of CNS
The central effects of leptin on the periphery emanate from specific regions in the CNS. Leptin receptors are expressed throughout the brain, including various locations in the hypothalamus and brainstem (197-199). Leptin signaling in the VMH augments glucose uptake in skeletal muscle, brown adipose tissue, and heart in mice and rats (176,177,200,201). Administration of leptin into the VMH stimulates glucose uptake in skeletal muscle via the SNS (β 2-adrenergic receptor), but intramuscular AMPK is not involved (177). Moreover, VMH leptin action increases insulin-induced reduction in hepatic glucose production and stimulation of glucose disposal (whole-body and skeletal muscle) (201). Buettner et al (175) found that leptin injected into the mediobasal hypothalamus decreases lipogenesis in white adipose tissue. Recently, it was demonstrated that leptin signaling in the dorsal vagal complex, located in the brainstem, stimulates secretion of VLDL (186). The ARC in the hypothalamus is crucial for leptin’s ability to maintain glucose homeostasis and for leptin’s effects on body weight (202). Glucose uptake by brown adipose tissue is also augmented following leptin injection in the ARC (200). Moreover, leptin’s stimulation of thermogenesis in brown adipose tissue is mediated by the hypothalamus (203,204). In summary, the central effects of leptin on energy balance, glucose metabolism, lipid metabolism, and insulin sensitivity are largely mediated by leptin signaling in the hypothalamus.
Effects of direct leptin action in specific neuronal populations in the CNS
The ablation or reconstitution of LepR, usually achieved with Cre-lox methodology in mice, has been used to investigate the role of leptin signaling in various types of neurons. To date, there is no Cre-expressing mouse that selectively targets all LepR-expressing neurons in the CNS. Synapsin1-Cre mice have been used to delete LepR in neurons throughout the CNS, with leptin signaling disrupted in the lateral hypothalamus and ventral premammillary nucleus of the hypothalamus (205), but not in ARC POMC and AgRP neurons (206). These mice do not have impairments in glucose metabolism and are resistant to obesity caused by high-fat diet (205). Nestin-Cre mice have also been used to inactivate LepR in neurons throughout the CNS (207); with Nestin-Cre mice, Cre-mediated recombination occurs centrally, including in the hypothalamus (207), as well as in the peripheral nervous system and some non-neuronal cells (208). LepR deficiency using Nestin-Cre mice causes severe obesity, hyperinsulinemia, hyperglycemia, and hepatic steatosis in male mice (207). Yet, the magnitude of these effects is less than what is observed in male Lepob mice (207). In contrast, the extent of hyperglycemia and hyperinsulinemia is similar between female mice with Nestin-specific LepR deficiency and female Lepob mice (207). It is important to note that in this study, while male and female mice with Nestin-specific LepR deletion were on a mixed genetic background, male and female Lepob mice were on a C57BL/6J background (207). Studies involving inactivation and reconstitution of LepR in steroidogenic factor-1 (SF1) neurons, which are found in the VMH, indicate that leptin signaling through SF1 neurons has a moderate effect on the regulation of body weight, glucose tolerance, and hepatic lipid content (209-211). POMC neurons, which are activated by leptin, and AgRP neurons, which are inhibited by leptin, are located in the ARC of the hypothalamus (202). Inducible and non-inducible LepR deletion as well as LepR reconstitution selectively in POMC neurons show that leptin signaling in POMC neurons (i) mediates basal and insulin-induced suppression of hepatic glucose production, resulting in improved glycemic control and (ii) moderately reduces body weight and diminishes circulating as well as hepatic lipids (9,212-214). Some of these effects are more pronounced in males (214). Neurons can release various neurotransmitters and widespread neurotransmitters in the CNS are γ-aminobutyric acid (GABA; inhibitory) and glutamate (excitatory) (215). While approximately half of POMC neurons are GABAergic, the majority of AgRP neurons are GABAergic (215). Vong et al (216) reported that inactivation of LepR in neurons capable of releasing GABA throughout the CNS, which includes AgRP neurons, results in a phenotype similar to that of Leprdb mice. Gonçalves et al (217) found that leptin signaling selectively in AgRP neurons of Leprdb mice improves glycemic control via the melanocortin pathway. GABA from AgRP neurons does not mediate this outcome, and obesity is mildly reduced (217). In another pivotal study, Xu et al. (218) found that inactivation of LepR in AgRP neurons using CRISPR-Cas9 results in obesity and hyperglycemia similar to Leprdb mice. Leptin also acts on AgRP neurons indirectly, by increasing release of GABA from the DMH (218). In aggregate, these studies indicate that leptin’s ability to lower blood glucose concentrations and maintain normal body weight occurs via leptin signaling in AgRP neurons, independently of GABA release by these neurons.
The role of specific neuronal populations in the control of lipid metabolism is less understood. A recent report demonstrated that leptin acts on AgRP and POMC neurons to activate sympathetic nerves innervating white and brown adipose tissues (219). Hence, AgRP and POMC neurons appear to be important first order neurons in the maintenance of peripheral lipid homeostasis by leptin.
Role of central leptin signaling in the beneficial effects of leptin in the context of insulin deficiency
Leptin delivered by various routes improves glucose and lipid metabolism in insulin-deficient diabetes (94,96,100,102,180,220-225). In insulin-deficient rodents, i.c.v. leptin decreases hepatic glucose production and increases glucose uptake in skeletal muscle, brown adipose tissue, heart, and brain (226). Insulin sensitivity is also augmented (227). I.c.v. leptin lowers blood glucose concentrations via leptin receptors in hypothalamic AgRP neurons in insulin-deficient mice (218). In contrast, inhibition of GABA release from leptin receptor-expressing neurons does not block i.c.v. leptin from normalizing blood glucose in insulin-deficient diabetes (228). Hence, while GABAergic neurons are important for the glucose-lowering effects of central leptin in insulin-deficient diabetes, GABA itself likely is not. As previously mentioned, the mechanisms of leptin action can depend on circulating insulin concentrations. In severe insulin deficiency, leptin signaling selectively in GABAergic and POMC neurons lowers blood glucose levels following i.c.v. leptin administration (229). Furthermore, Singha et al (230) recently reported that in severely insulin deficient mice, central leptin’s reduction of blood glucose levels is only partially blunted by LepR deletion in AgRP neurons. Furthermore, LepR deletion in neurons that express rat insulin II gene promoter (RIP) driven Cre, can reverse the leptin-mediated improvements in glycemic control and lipid metabolism (231). This reinforces the concept that leptin’s effects via the CNS are impacted substantially by pathological states such as diabetes and that the mechanisms through which central leptin improves glucose metabolism in insulin-deficient diabetes should be further investigated.
Peripherally administered leptin can improve metabolism in insulin-deficient diabetes via the CNS. Perry et al (99,101) found that i.v. leptin lowers concentrations of circulating glucose and lipids through inhibition of the hypothalamic-pituitary-adrenal axis in rats that are substantially insulin-deficient. Denroche et al (93) and Perry et al (101) reported that amelioration of glycemic control in insulin-deficient diabetes by s.c. leptin is accompanied by diminution of metabolites in the circulation and peripheral tissues, especially (i) lipids and glycerol in plasma; (ii) glucose, lipids, and glycerol in the liver; and (iii) lipids in skeletal muscle. Multiple pathways may be involved in the antidiabetic action of leptin and leptin action in the CNS may contribute to the antidiabetic effects of s.c. leptin in insulin-deficient diabetes (232). For example, s.c. leptin induces hypoglycemia after a prolonged fast (93) and leptin signaling in the parabrachial nucleus (brainstem) has been found to sustain hypoglycemia (233). Thus, it is possible that leptin-induced reductions in glucose could be mediated in part via brainstem pathways in addition to the previously described hypothalamic pathways. It is unclear, however, whether central leptin communication with the periphery is altered by insulin-deficient diabetes (222). Additional studies are needed to further understand the mechanisms through which s.c. leptin improves metabolism in insulin-deficient diabetes.
Effects of direct leptin action in non-neuronal cells in the CNS
In addition to neurons, the brain consists of several types of glial cells, including astrocytes and microglia, which interact with neurons. Recent studies indicate that leptin signaling in glial cells is important for homeostasis. I.c.v. leptin alters the morphology of astrocytes (234), which play an important role in brain homeostasis and maintenance of the blood-brain barrier. Inactivation of LepRb in astrocytes results in reduced circulating triglycerides, circulating leptin, leptin gene expression in white adipose tissue, and astrogliosis when mice are challenged with a high-fat diet (235). Moreover, uncoupling protein 1 gene expression is elevated in brown adipose tissue of mice with astrocyte-specific LepR knockout fed a high-fat diet (235). However, knocking out all LepR isoforms in astrocytes results in a different phenotype in high-fat feeding conditions; knockout mice are heavier, have elevated circulating leptin concentrations, and increased astrogliosis (236). To circumvent potential developmental effects of non-inducible Cre-lox methodology, mice with tamoxifen-inducible LepRb knockout in astrocytes were generated (237). These mice have mildly increased food intake following leptin administration as well as elevated circulating triglycerides (237,238), thereby indicating that leptin signaling in astrocytes of adult mice promotes energy balance and lipid homeostasis. Microglia act as resident immune cells of the brain and play an important role in synaptic pruning. LepR knockout in microglia causes morphological and functional changes in microglia, diminished count of POMC neurons, and mild obesity (239). Taken together, these studies suggest that leptin signaling in non-neuronal cells of the CNS maintains lipid homeostasis and energy balance.
Effects of direct leptin action in CNS on intracellular metabolism and pathways
It has been reported that activation/inhibition of POMC and AgRP neurons only partially explains the effect of leptin on energy balance and peripheral metabolism via these neurons (240) and that the intracellular mediator of leptin signaling phosphatidylinositol-3-kinase is important in certain types of neurons (241,242). The effect of leptin signaling on glucose and lipid metabolism may begin within CNS cells themselves. Leptin modulates glucose uptake by astrocytes (243), and central leptin promotes glucose sensing by neurons, a process that involves the conversion of glucose to lactate in astrocytes (244). Synthesis of lipids in astrocytes is also important for glucose metabolism in the brain and periphery (245), but whether leptin regulates lipid anabolism or catabolism centrally remains largely unexplored.
Effects of direct leptin action in the endocrine pancreas
Leptin directly modulates the secretion of hormones from the endocrine pancreas (Fig. 2). The islets of Langerhans in the pancreas are clusters of endocrine cells, predominantly β cells and α cells. While β cells secrete insulin, a hormone that stimulates glucose uptake, reduces hepatic glucose production, and increases lipid accumulation, α cells secrete the hormone glucagon, which reduces lipid accumulation and increases hepatic glucose production directly, but reduces hepatic glucose production via the brain (recently reviewed in (246,247)). Leptin decreases insulin secretion by islets from mice, rats, and humans as well as by β cell lines (92,181,247). The underlying mechanisms include activation and membrane translocation of KATP channels, which hyperpolarize the β cell membrane and thereby reduce insulin secretion (92,249). However, the role of β cell-specific LepR signaling in vivo is unclear, largely due to issues with the various Cre drivers (250). Deletion of LepRb from β cells and the hypothalamus using RIP-Cre mice results in hyperinsulinemia and glucose intolerance (251). However, these results are potentially confounded by Cre itself because RIP-Cre mice can be glucose intolerant (252). Subsequently, all isoforms of LepR were knocked out of β cells using pancreatic and duodenal homeobox 1-Cre mice (253), a Cre line that is also not specific for β cells (250). On standard chow, the knockout mice had better glucose tolerance and higher insulin secretion, but the opposite was found on a high-fat diet (253). The Ins1-Cre mouse is a more recent model that is β cell-specific, but it is also characterized by inefficient recombination in some cases (254). Glucose-stimulated insulin secretion from perfused islets of female mice with β cell-specific LepRb deletion, achieved with Ins1-Cre mice, was elevated and this is consistent with loss of leptin signaling leading to increased insulin secretion (255). However, it is noteworthy that β cell-specific restoration of LepRb signaling using Ins1-Cre mice in an otherwise LepRb null setting was not sufficient to reduce plasma insulin levels (256). Similarly, while leptin decreases glucagon secretion from islets (182), mice that have a partial deficiency in LepRb in α cells do not have an altered phenotype (255,257). The reasons for the discrepancies between ex vivo (islet) and in vivo (Cre-lox methodology in mice) studies are unclear but likely include poor efficiency of Cre-mediated recombination. Furthermore, islets express short isoforms of LepR, in addition to LepRb (182), but their individual contributions to β cell function in vivo remains unexplored. Leptin may also act directly on islet cells other than β or α cells to alter insulin and glucagon secretion. Interestingly, somatostatin-secreting δ cells in pancreatic islets also express LepR but the role of leptin in these cells is unknown (258). Lastly, it is possible that leptin robustly inhibits insulin secretion only in conditions of maximal insulin secretion in vivo, including hyperglycemia (259). In aggregate, studies indicate that leptin impedes secretion of insulin and glucagon by isolated islets, but additional investigations are necessary to clarify the in vivo role of leptin signaling in islet cells.
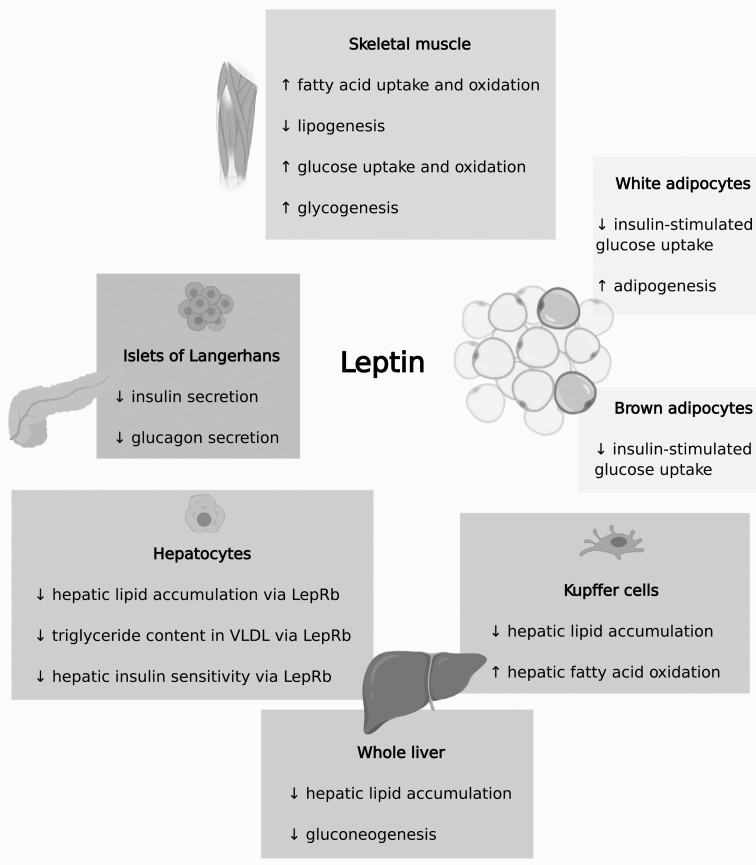
Figure 2.
Metabolic effects of direct leptin action in adipose tissues, endocrine pancreas, liver, and skeletal muscle. Abbreviations: LepRb, long isoform of the leptin receptor; VLDL, very low density lipoprotein.
Effects of direct leptin action in the liver
The liver receives instructions directly from circulating leptin for the regulation of lipid and glucose metabolism (Fig. 2). Exposure of isolated livers to leptin impedes gluconeogenesis via insulin receptor substrate-2 (260) and depletes triglyceride content in livers from rodents fed a standard chow but not in livers from high-fat diet-fed rodents (261). Accordingly, triglyceride levels in the liver and plasma are diminished following adenovirus-mediated restoration of functional LepR expression in the liver of fa/fa rats, which have a mutated LepR and therefore are obese and have hepatic steatosis (262). The liver is composed mainly of hepatocytes but also other cells that express LepR, such as Kupffer cells and hepatic stellate cells (262). Hepatocyte-specific LepR knockout in mice does not induce a robust phenotype (264), but Huynh et al (265) found that with aging, mice with hepatocyte-specific LepRb deficiency exhibit improved glucose tolerance and insulin-stimulated suppression of hepatic glucose production. Furthermore, mice with hepatocyte-specific LepRb deficiency have elevated hepatic triglyceride and cholesterol content, and enhanced hepatic activity of LPL and VLDL with greater triglyceride content (188,265). A recent report suggests that leptin promotes lipoprotein assembly from stored lipids in the liver but inhibits it from dietary sources in the intestine (53). As previously mentioned, leptin administration can also decrease triglyceride content and augment fatty acid oxidation in the liver by directly acting on Kupffer cells (81). Hence, direct leptin signaling in the liver promotes catabolism of lipids, similar to the effects of leptin on the liver via the CNS. However, in contrast to leptin’s action on the liver through the CNS, hepatic leptin signaling reduces hepatic insulin sensitivity of glucose metabolism.
Effects of direct leptin action in skeletal muscle
The metabolic effects of leptin on skeletal muscle have been reviewed elsewhere (166,266-268), and herein we will focus on recent developments (Fig. 2). The extent of the response to leptin in a given skeletal muscle, either via the CNS or directly, depends on the (i) predominant type of fibers in the skeletal muscle, with type 1 fibers being more responsive, and (ii) phase of the fed-fasted cycle (189,269-272). Leptin acts on skeletal muscle directly via LepRb and short LepR isoforms (270, 273-276). In skeletal muscle, leptin activates AMPK, raises fatty acid uptake, increases fatty acid oxidation, reduces triglyceride formation, and boosts thermogenesis (269-272,277,278). Analogous results have been obtained with various types of muscle cells (189,273,279,280) and some of these pathways are impaired in obesity (272,277). Moreover, Koo et al (274) recently found that elevation of fatty acid oxidation caused by leptin requires AMPK in the short-term and STAT3-mediated expression of fatty acid oxidation genes in the long-term. Various, but not all (281), studies show that exposure of skeletal muscle and muscle cells to leptin augments glucose uptake, glycogenesis, and glucose oxidation (275,282,283). In isolated skeletal muscle, C2C12 myotubes, and L6 muscle cells, leptin either does not lessen insulin sensitivity of glucose metabolism (269,283) or causes a short-term impairment (275,281). However, leptin inhibits insulin sensitivity of lipid metabolism in skeletal muscle (insulin promotes intramyocellular lipid accumulation) (269). In aggregate, studies indicate that direct leptin signaling in skeletal muscle has anti-lipogenic effects and promotes glucose disposal.
Effects of direct leptin action in adipose tissue
Adipocytes express LepR (284) and, being the main source of leptin (285), are exposed to high local leptin concentrations. In mice with LepR deficiency in peripheral tissues, the rate of leptin secretion by white adipose tissue is increased, suggesting that leptin exerts negative feedback on its secretion from adipocytes (36). Although the majority of leptin’s effects on adipose tissues in vivo are likely controlled indirectly via sympathetic activity (174) or other signals, which are reviewed elsewhere (3), multiple in vivo and in vitro models support a role for adipocyte leptin signaling in modulating glucose and lipid metabolism as well as adipogenesis (Fig. 2).
The intersection of leptin and insulin signaling pathways in adipocytes is an important determinant of leptin’s metabolic effects in adipocytes. It has been reported that in vitro, leptin increases lipolytic activity of white adipocytes from rodents (286) but not from humans (287). Most reports agree that leptin blunts the insulin response in adipocytes (288), resulting in reduced insulin-induced glucose uptake and lipogenesis in white adipocytes (287,289). In contrast, one study indicated insulin-sensitizing effects on lipogenesis in white adipocytes in vitro (72). In brown adipocytes, leptin signaling dampens insulin-stimulated glucose uptake via reduced insulin receptor kinase activity (290). Some cases demonstrating a lack of leptin action on adipocytes utilized supraphysiological leptin levels (291,292). This disparity highlights the importance of the bioactivity of leptin, dosing of leptin, and the biological context of the model in determining the direct effect of leptin on adipocyte lipid metabolism (293).
In vivo studies of preadipocytes or adipose tissue transplanted from Leprdb mice to mice with intact leptin signaling show that adipocytes in the implants shift over time to mirror the morphology of endogenous adipocytes despite being insensitive to leptin (294,295). This further suggests that the overall biological context is at least as important as leptin signaling directly at adipose tissue. Huan et al (284) found that knockdown of all LepR isoforms in white adipose tissue, using a LepR antisense sequence driven by the phosphoenolpyruvate carboxykinase promoter, results in obesity, glucose intolerance, insulin resistance, and ectopic fat accumulation. It is of note that phosphoenolpyruvate carboxykinase is expressed in cells other than adipocytes, such as cells of myeloid lineage (296), that express LepR and can modulate metabolism (see following discussion on the immune system). Using Cre-lox methodology, modest deficiency or reactivation of LepRb signaling in both mature white and brown adipocytes results in a mild phenotype (297). First, mice with adipocyte-specific LepR deficiency have lower plasma insulin concentrations during a glucose challenge; accordingly, plasma insulin concentrations are elevated during a glucose challenge in mice with adipocyte-specific LepR reconstitution (297). Second, in obesity, reconstitution of LepR in adipocytes improves blood glucose concentrations in an age-dependent manner (297). Third, adipocyte leptin signaling also mildly increases body weight in males (297). Fourth, surprisingly, the ability of leptin to lower blood glucose in insulin-deficient diabetes is faster in mice with adipocyte-specific LepR deficiency (297). It is possible that achieving greater recombination of LepR, knocking out LepR separately in white and brown adipocytes, or knocking out LepR in adipocytes at all stages of development (not just mature adipocytes) results in a more robust or different phenotype. Regarding the last possibility, a recent study demonstrated that leptin is beneficial in the maturation process of adipocytes (adipogenesis) (298), suggesting that inhibition of leptin signaling at earlier stages of adipocyte development may have a more detrimental effect on whole-body metabolism.
Recent studies in white adipose tissue continue to provide support for a role of leptin directly at adipocytes, where lipid metabolism is likely altered through inflammation mediated via NO synthases (299,300), altered HSL and adipose triglyceride lipase action in white adipose tissue (78,301), and mammalian target of rapamycin-mediated adipogenesis (298). Although the importance of these pathways in vivo needs to be further studied, lipase regulation by leptin in adipose tissue holds particular promise in diabetes. For example, suppression of lipolysis is crucial in i.v. leptin’s remarkable ability to restore normal blood glucose in streptozotocin diabetic rodents (101,302). Thus, the investigation of leptin’s direct role in adipose tissue maintenance of glucose and lipid metabolism in vivo, and the molecular mechanisms responsible, may provide valuable insights.
Effects of direct leptin action in the immune system
The immune system is broadly divided into the innate and adaptive immune systems. The innate immune system includes cells such as macrophages, neutrophils, and natural killer cells, while the adaptive immune system consists of T cells and B cells (303). Cells of the adult immune system mainly originate in the bone marrow, except for most tissue resident macrophages (304,305). Bone marrow is an important target for leptin (306), and there is evidence that leptin can promote or block immune responses (303,307). As a clinical example, leptin treatment of hypoleptinemic women with hypothalamic amenorrhea increases circulating CD4+ T cells (108). We refer the reader to multiple reviews on how leptin regulates immunology (eg, (303,307)); the focus of our current discussion will be on how leptin signaling through immune cells affects glucose and lipid metabolism (Fig. 3).
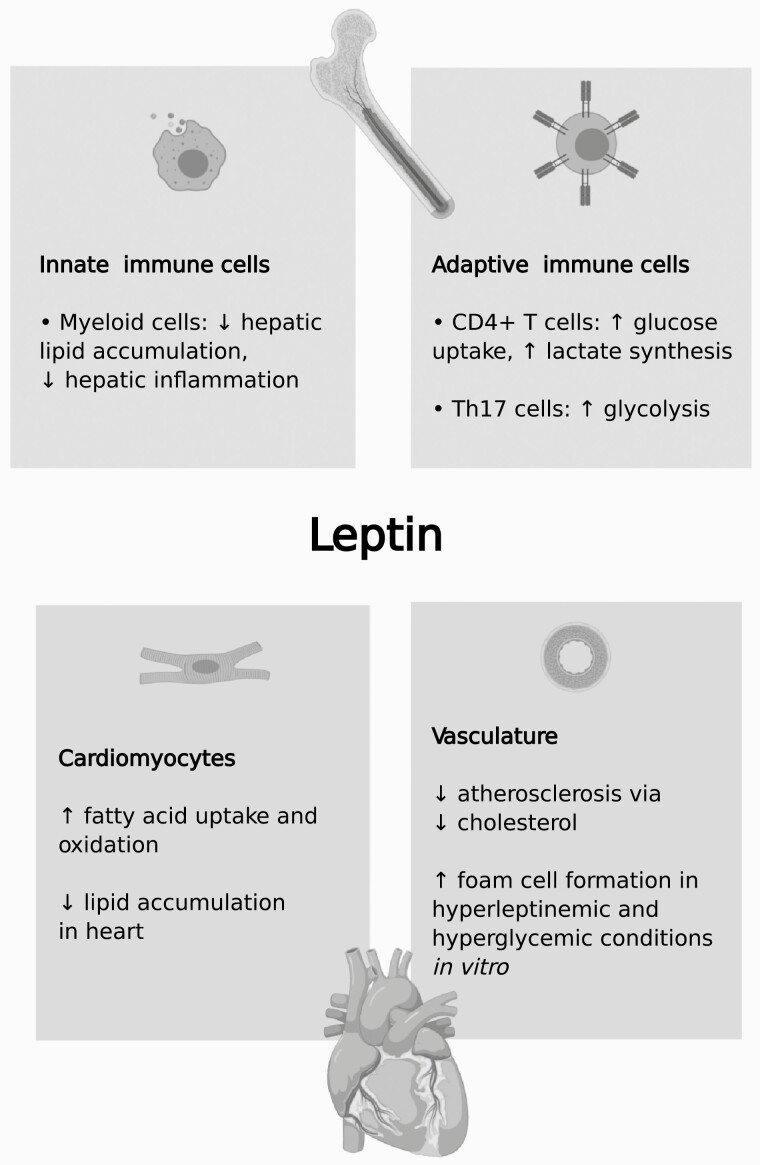
Figure 3.
Metabolic effects of direct leptin action in the cardiovascular and immune systems.
Immune cells can support homeostasis or impair it, depending on cell type, activation status, and disease stage (308). Macrophages are needed for appropriate adipose tissue remodeling in obesity (309). Conversely, recruitment of macrophage precursors from the circulation (monocytes) into tissues is a feature of obesity (308,310). Moreover, it is well established that inflammation in immune cells can disrupt glucose and lipid metabolism (311,312). It is likely that chronicity and type of inflammatory cytokines determine whether or not inflammation has an adverse effect on metabolism. The trigger for inflammation in immune cells can be metabolic, such as free fatty acids (313), or both metabolic and pathogenic, for example nucleotide-binding oligomerization domain 1 activation (314).
Bone marrow transplantation studies involving wild-type and Leprdb mice entail the ablation of immune cells followed by replenishment of the bone marrow, and consequently, cells of both the innate and adaptive immune system. In Leprdb mice, intact LepRb signaling in bone marrow-derived cells (ie, bone marrow from wild-type mice) reduces circulating leptin, while elevating circulating adiponectin (315). However, the reverse experiment, namely the transplantation of bone marrow from Leprdb mice into wild-type mice, does not alter these parameters (315). On a high-fat diet, the lack of LepRb in bone marrow-derived cells (ie, bone marrow from Leprdb mice) in wild-type mice reduces body weight, restrains adipose tissue inflammation, and ameliorates insulin sensitivity (316). No phenotypic differences were found in a similar experiment with a shorter high-fat diet regimen (317). Thus, results of bone marrow transplantation studies are inconsistent. Limiting factors of bone marrow transplantation studies include their invasiveness (315), different study designs, and likely their inability to substantially alter immune cell leptin signaling in tissues. For instance, the extent of leptin signaling in tissue resident macrophages may not be greatly affected by bone marrow transplantation because a proportion of tissue resident macrophages are not bone marrow–derived (304,305).
Another approach to investigating how leptin signaling in immune cells affects metabolism is to expose specific immune cells to leptin. The effect of leptin on cytokine expression in immune cells is not completely clear, perhaps due to different experimental designs, such as different leptin exposure durations, leptin concentrations, and species. Leptin stimulates proinflammatory cytokine release by natural killer cells acutely but not chronically (318). Classification of macrophages as pro-inflammatory (classically activated; M1) or anti-inflammatory (alternatively activated; M2) is associated with the cytokines they express (308,319). Macrophages from Lepob mice have elevated expression of proinflammatory cytokines (320), but it is unclear if this is due to the absence of leptin per se or due to the metabolic disturbances, such as obesity, resulting from blunted leptin signaling. In monocytes and macrophages, leptin stimulates (321,322) or does not alter (323,324) proinflammatory cytokine expression and secretion; others have found that leptin exposure causes a mixed M1/M2 phenotype (325). Moreover, recent investigations suggest that we may need to look beyond M1 and M2 cytokines. For example, insulin-resistant macrophages exhibit enhanced baseline glucose uptake and secretion of lactate (326); the latter can cause white adipose tissue browning (327). Hence, it would be of interest to determine if leptin affects glucose metabolism, especially glycolysis, in macrophages themselves.
The effects of leptin on whole-body metabolism via innate immune cells have also been studied in vivo using Cre-lox methodology. Cre-mediated recombination of LepR in myeloid cells, which includes macrophages, neutrophils, and microglia, has been achieved with LysM-Cre and Cx3cr1-Cre mice. Cx3cr1-Cre mice appear to induce more recombination in microglia than LysM-Cre mice (239). Myeloid-cell specific LepR deficient mice are, or show a trend toward being, moderately heavier (81,239,328), with effects more pronounced in male mice (239,328), or do not exhibit alterations in body weight (329,330). Although differences in blood glucose concentrations and glucose tolerance were not found (81,239,330), mice with deleted LepR in cells of myeloid lineage have been found to have elevated circulating leptin levels (330) and increased adipose tissue expression of the proinflammatory cytokine tumor necrosis factor-α (239). Studies that examined the liver of mice with myeloid cell-specific LepR deficiency consistently found abnormalities, namely hepatic triglyceride accumulation (81) and inflammation (328).
Macrophage leptin signaling also regulates cholesterol metabolism. Reverse cholesterol transport is the process whereby cholesterol picked up by macrophages is delivered to the liver via HDL and ultimately excreted from the body (331,332). Exposing J774.2 macrophages (mouse cell line) to leptin promotes cholesterol efflux by stimulating activity of HSL, which participates in the degradation of cholesterol esters (333). In hyperglycemic conditions, however, leptin favors intracellular accumulation of cholesterol (in esterified form) because the stimulatory effect on HSL is abolished and activity of acyl-CoA:cholesterol O-acyltransferase, which participates in the synthesis of cholesterol esters, is enhanced (334). Nevertheless, treating murine peritoneal macrophages or human monocytes/macrophages with leptin stimulates the accumulation of lipid (includes cholesteryl esters) in macrophages through various mechanisms, including elevated acyl-CoA:cholesterol O-acyltransferase activity and blunted efflux to HDL (335-337). Lipid-filled macrophages, known as foam cells, play a pathogenic role in atherosclerosis (see following discussion on the cardiovascular system). These in vitro studies suggest that exposure of macrophages to a hyperleptinemic and diabetic environment might contribute to foam cell formation.
Mice with T cell-specific LepR deficiency have been generated using LepRflox/flox mice crossed to lymphocyte protein tyrosine kinase-Cre mice, which target all T cells (338,339), and CD4-Cre mice, which express Cre only in CD4+ (helper) T cells (340,341). Mice with CD4+ T cell-specific LepR inactivation have normal body weight (342). CD4+ T cells from pan-T cell-specific LepR deficient mice have impaired glucose uptake, synthesis of lactate, and production of the proinflammatory cytokine interferon-γ (341,343). CD4+ T cells can differentiate into other T cell types, such as Th17 and Treg (341). Using T cells from CD4+ T cell-specific LepR deficient mice, it was found that Th17 cells, but not Treg cells, with inactivated LepR have a blunted rate of glycolysis (341). Hence, the effect of leptin signaling on intracellular T cell metabolism and, consequently, function depends on the cell type (343), but overall, leptin signaling appears to stimulate intracellular T cell glucose metabolism. T cells play a part in obesity-associated insulin resistance, but the involvement of T cell leptin signaling in this process is unknown (344). Taken together, studies indicate that leptin signaling in myeloid cells has a modest impact on whole-body lipid metabolism and energy balance, while the impact of T cell leptin signaling on whole-body metabolism has not been thoroughly described.
Effects of direct leptin action in the cardiovascular system
The direct effects of leptin on the heart and vasculature is of mounting interest because of the ramifications for cardiovascular health (Fig. 3). In in vivo studies of cardiomyocyte leptin signaling using tamoxifen-inducible Cre-lox methodology, cardiac function is worsened by LepR deletion (345-347). With respect to metabolism, leptin does not directly alter glucose oxidation in the heart (348). In vitro studies demonstrate that leptin enhances fatty acid uptake and oxidation by cardiomyocytes in an AMPK-dependent manner (278). In isolated heart, leptin contributes to fatty acid oxidation via a STAT3-NO-p38 mitogen activated protein kinase pathway, resulting in diminished accumulation of triglycerides (348,349). However, insulin blocks these effects (348). Consistent with direct anti-lipogenic effects of leptin on the heart, Hall et al (350) created mice that selectively express LepRb in cardiomyocytes on a Leprdb background and found that they had diminished triglyceride content in the heart.
The arterial wall consists of various cell types, including endothelial cells and smooth muscle cells (351). A principal function of the cardiovascular system is perfusion, a process that depends on arterial blood pressure. Arterial blood pressure is determined by cardiac output and, consequently, heart rate, as well as resistance to flow in blood vessels, which is increased by vascular smooth muscle contraction. Most reports indicate that central leptin signaling, with the exception of certain regions in the CNS (352), increases heart rate, blood pressure, and SNS activity, even in obesity and insulin-deficient diabetes (221,222,353-357). Leptin signaling in the periphery also increases blood pressure, through the carotid body (358) and, especially in females, through the leptin-aldosterone pathway (359,360). It has been known for decades that blood pressure modulates the function of blood vessels (361), and leptin-induced increases in blood pressure could be an indirect way through which leptin causes vascular endothelial cell dysfunction. Additionally, leptin can act directly on the vasculature. In vascular endothelial cells, leptin boosts production of NO, which can be a vasodilator, (362) and it increases production of detrimental reactive oxygen species (ROS) due to heightened fatty acid oxidation (363). Leptin increases the proliferation of vascular smooth muscle cells (364,365). Tamoxifen-inducible LepR deletion in vascular smooth muscle cells does not alter blood pressure or body weight, but arterial relaxation is elevated (366). Taken together, these studies indicate that leptin increases blood pressure, appears to increase vascular smooth muscle contraction, and increases ROS in vascular endothelial cells.
The role of leptin in the development and progression of atherosclerosis is still being defined and may occur through several distinct mechanisms. Atherosclerosis, defined as lipid accumulation in the intima of arterial walls, is characterized by disturbed lipid metabolism (351). Monocytes from the circulation cross the endothelial barrier and invade the intima, where they engulf LDL and become stationary lipid-laden macrophages (foam cells) (351). Oxidized LDL is especially pro-atherogenic (351) and LDL is oxidized perhaps due to increased ROS, which could be due to increased leptin signaling in endothelial cells (363). The atherosclerotic lesion also contains dysfunctional endothelial cells, vascular smooth cells, and T cells (351). Obesity, which is characterized by hyperleptinemia, is a risk factor for cardiovascular disease (367). In vivo studies using models of atherosclerosis, namely the apolipoprotein-E knockout (ApoE−/−) mouse and the LDL receptor knockout (LDLR−/−) mouse, suggest that deficiency in leptin signaling promotes atherosclerosis mainly by altering cholesterol metabolism. Deficiency in leptin signaling on a pro-atherogenic background (ApoE−/−;Leprdb mice and LDLR−/−;Lepob mice) accelerates development of atherosclerosis (368-370). Accordingly, leptin treatment of LDLR−/−;Lepob mice (371) or Ins2+/Akita;ApoE−/− mice, a model of type 1 diabetes and atherosclerosis, (372) mitigates the development of atherosclerosis. Plasma cholesterol is higher in ApoE−/−;Leprdb mice than ApoE−/− mice (368), leptin decreases plasma cholesterol in LDLR−/−;Lepob mice (371), and leptin simulates reverse cholesterol transport in vivo at the level of the liver through elevated expression of scavenger receptor class B type I, an HDL receptor (373). When plasma cholesterol concentrations are matched between LDLR−/− mice and LDLR−/−;Lepob mice by feeding LDLR−/− mice a high-fat diet and high cholesterol diet, while LDLR−/−;Lepob mice are on standard chow, LDLR−/−;Lepob mice have decreased atherosclerosis (374). Therefore, the anti-atherogenic effects of leptin appear to depend on its ability to lower circulating cholesterol. Bone marrow transplantation studies indicate that LepR signaling in immune cells does not alter development of atherosclerosis (375). Since atherosclerotic cardiovascular disease is a main cause of death (351) and the prevalence of obesity is high worldwide (376), it is worthwhile to explore the effect of leptin on the incidence of atherosclerosis.
Conclusions and Future Directions
Leptin has profound effects on glucose and lipid metabolism, at the whole-body and cellular levels. The effect of leptin on glucose metabolism can depend on whether leptin is acting centrally or directly on peripheral tissues and on whether the insulin signaling pathway is activated. Leptin therapy has therapeutic potential in insulin-dependent diabetes since it reduces insulin dose requirements and can independently improve hyperglycemia and other metabolic parameters. Central and peripheral leptin reduce ectopic fat accumulation and hence, leptin could also be used therapeutically to alleviate ectopic fat accumulation associated with obesity and insulin resistance (377). Leptin increases blood pressure, and tissue/cell-specific targeting of leptin action may be needed to maximize the beneficial metabolic effects of leptin while minimizing increases in this cardiovascular parameter. Although the relative importance of excessive leptin signaling vs leptin resistance has not been clarified in obesity and related disorders, differential leptin signaling across tissues may add another degree of complexity to the treatment of such disorders. Exciting work remains to further delineate the pathways and circuits throughout the body that result in leptin’s metabolic effects.
Acknowledgments
BioRender.com was used to make the graphical abstract and figures.
Financial Support: Research work on leptin was supported by a grant from the Canadian Institutes of Health Research to T.J.K.
Additional Information
Disclosure Summary: The authors have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Color versions of the figures are available upon request.
References
- 1. Flier JS. Starvation in the midst of plenty: reflections on the history and biology of insulin and leptin. Endocr Rev. 2019;40(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432. [DOI] [PubMed] [Google Scholar]
- 3. Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta. 2014;1842(3):414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47(6):913-917. [DOI] [PubMed] [Google Scholar]
- 5. Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (oblob) mice. FEBS Lett. 1995;368(3):488-490. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Dallner OS, Nakadai T, et al. A noncanonical PPARγ/RXRα-binding sequence regulates leptin expression in response to changes in adipose tissue mass. Proc Natl Acad Sci U S A. 2018;115(26):E6039-E6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393(6686):684-688. [DOI] [PubMed] [Google Scholar]
- 8. Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138(10):4463-4472. [DOI] [PubMed] [Google Scholar]
- 9. Caron A, Dungan Lemko HM, Castorena CM, et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. elife. 2018;7(03):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overton JM, Williams TD, Chambers JB, Rashotte ME. Central leptin infusion attenuates the cardiovascular and metabolic effects of fasting in rats. Hypertension. 2001;37(2 Pt 2):663-669. [DOI] [PubMed] [Google Scholar]
- 11. Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Licinio J, Negrão AB, Mantzoros C, et al. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83(11):4140-4147. [DOI] [PubMed] [Google Scholar]
- 13. Saad MF, Riad-Gabriel MG, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83(2):453-459. [DOI] [PubMed] [Google Scholar]
- 14. Mix H, Widjaja A, Jandl O, et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47(4):481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 2012;45(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Liu R, Liu L, et al. The effect of leptin on Lep expression is tissue-specific and nutritionally regulated. Nat Med. 1999;5(8):895-899. [DOI] [PubMed] [Google Scholar]
- 17. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129(10):4041-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moinat M, Deng C, Muzzin P, et al. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 1995;373(2):131-134. [DOI] [PubMed] [Google Scholar]
- 19. van der Spek R, Fliers E, la Fleur SE, Kalsbeek A. Daily gene expression rhythms in rat white adipose tissue do not differ between subcutaneous and intra-abdominal depots. Front Endocrinol (Lausanne). 2018;9:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10(2):131-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145(7):3258-3264. [DOI] [PubMed] [Google Scholar]
- 22. Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol. 2009;201(3):329-339. [DOI] [PubMed] [Google Scholar]
- 23. Liu Q, Chen Y, Copeland D, et al. Expression of leptin receptor gene in developing and adult zebrafish. Gen Comp Endocrinol. 2010;166(2):346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michel M, Page-McCaw PS, Chen W, Cone RD. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc Natl Acad Sci U S A. 2016;113(11):3084-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Q, Dalman M, Chen Y, et al. Knockdown of leptin A expression dramatically alters zebrafish development. Gen Comp Endocrinol. 2012;178(3):562-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahi EP, Brunel M, Tsakoumis E, Schmitz M. Transcriptional study of appetite regulating genes in the brain of zebrafish (Danio rerio) with impaired leptin signalling. Sci Rep. 2019;9(1):20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Audira G, Sarasamma S, Chen JR, et al. Zebrafish mutants carrying leptin a (lepa) gene deficiency display obesity, anxiety, less aggression and fear, and circadian rhythm and color preference dysregulation. Int. 2018;19(12):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beshel J, Dubnau J, Zhong Y. A leptin analog locally produced in the brain acts via a conserved neural circuit to modulate obesity-linked behaviors in Drosophila. Cell Metab. 2017;25(1):208-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown S, Hu N, Hombría JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11(21):1700-1705. [DOI] [PubMed] [Google Scholar]
- 30. Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allison MB, Myers MG Jr. 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223(1):T25-T35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wauman J, Zabeau L, Tavernier J. The leptin receptor complex: heavier than expected? Front Endocrinol (Lausanne). 2017;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peelman F, Zabeau L, Moharana K, Savvides SN, Tavernier J. 20 years of leptin: insights into signaling assemblies of the leptin receptor. J Endocrinol. 2014;223(1):T9-T23. [DOI] [PubMed] [Google Scholar]
- 34. Ge H, Huang L, Pourbahrami T, Li C. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. J Biol Chem. 2002;277(48):45898-45903. [DOI] [PubMed] [Google Scholar]
- 35. Chua SC Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45(2):264-270. [DOI] [PubMed] [Google Scholar]
- 36. Guo K, McMinn JE, Ludwig T, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148(8):3987-3997. [DOI] [PubMed] [Google Scholar]
- 37. Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632-635. [DOI] [PubMed] [Google Scholar]
- 38. Yang G, Ge H, Boucher A, Yu X, Li C. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol. 2004;18(6):1354-1362. [DOI] [PubMed] [Google Scholar]
- 39. Kratzsch J, Lammert A, Bottner A, et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab. 2002;87(10):4587-4594. [DOI] [PubMed] [Google Scholar]
- 40. Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276(9):6343-6349. [DOI] [PubMed] [Google Scholar]
- 41. Fei H, Okano HJ, Li C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94(13):7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor isoform in rat. FEBS Lett. 1996;392(2):87-90. [DOI] [PubMed] [Google Scholar]
- 43. Hileman SM, Pierroz DD, Masuzaki H, et al. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143(3):775-783. [DOI] [PubMed] [Google Scholar]
- 44. Burguera B, Couce ME, Long J, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71(3):187-195. [DOI] [PubMed] [Google Scholar]
- 45. Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun. 1997;232(2):383-387. [DOI] [PubMed] [Google Scholar]
- 46. De Matteis R, Dashtipour K, Ognibene A, Cinti S. Localization of leptin receptor splice variants in mouse peripheral tissues by immunohistochemistry. Proc Nutr Soc. 1998;57(3):441-448. [DOI] [PubMed] [Google Scholar]
- 47. Guerra B, Santana A, Fuentes T, et al. Leptin receptors in human skeletal muscle. J Appl Physiol (1985). 2007;102(5):1786-1792. [DOI] [PubMed] [Google Scholar]
- 48. Bates SH, Myers MG Jr. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14(10):447-452. [DOI] [PubMed] [Google Scholar]
- 49. Davis RC, Castellani LW, Hosseini M, et al. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes. 2010;59(7):1616-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4(1):49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barnes TM, Shah K, Allison MB, et al. Identification of the leptin receptor sequences crucial for the STAT3-independent control of metabolism. Mol Metab. 2020;32:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Z, Ceccarini G, Eisenstein M, Tan K, Friedman JM. Phenotypic effects of an induced mutation of the ObRa isoform of the leptin receptor. Mol Metab. 2013;2(4):364-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iqbal J, Mascareno E, Chua S, Hussain MM. Leptin-mediated differential regulation of microsomal triglyceride transfer protein in the intestine and liver affects plasma lipids. J Biol Chem. 2020;295(13):4101-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao S, Li N, Straub L, Zhang Z, Wang MY, Zhu Q, Kusminski CM, Elmquist JK, Scherer PE. Partial leptin deficiency confers resistance to diet-induced obesity in mice. Mol Metab. 2020;37:100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ottaway N, Mahbod P, Rivero B, et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab. 2015;21(6):877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balland E, Chen W, Tiganis T, Cowley MA. Persistent leptin signaling in the arcuate nucleus impairs hypothalamic insulin signaling and glucose homeostasis in obese mice. Neuroendocrinology. 2019;109(4):374-390. [DOI] [PubMed] [Google Scholar]
- 57. Balland E, Chen W, Dodd GT, et al. Leptin signaling in the arcuate nucleus reduces insulin’s capacity to suppress hepatic glucose production in obese mice. Cell Rep. 2019;26(2):346-355.e343. [DOI] [PubMed] [Google Scholar]
- 58. Verdich C, Toubro S, Buemann B, et al. Leptin levels are associated with fat oxidation and dietary-induced weight loss in obesity. Obes Res. 2001;9(8):452-461. [DOI] [PubMed] [Google Scholar]
- 59. Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108-110. [DOI] [PubMed] [Google Scholar]
- 60. Mansuy-Aubert V, Zhou QL, Xie X, et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17(4):534-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Surwit RS, Petro AE, Parekh P, Collins S. Low plasma leptin in response to dietary fat in diabetes- and obesity-prone mice. Diabetes. 1997;46(9):1516-1520. [DOI] [PubMed] [Google Scholar]
- 62. Begriche K, Lettéron P, Abbey-Toby A, et al. Partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice. Am J Physiol Endocrinol Metab. 2008;294(5):E939-E951. [DOI] [PubMed] [Google Scholar]
- 63. Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138(2):839-842. [DOI] [PubMed] [Google Scholar]
- 64. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci U S A. 1998;95(2):741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540-543. [DOI] [PubMed] [Google Scholar]
- 66. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543-546. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz MW, Baskin DG, Bukowski TR, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531-535. [DOI] [PubMed] [Google Scholar]
- 68. Rossetti L, Massillon D, Barzilai N, et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272(44):27758-27763. [DOI] [PubMed] [Google Scholar]
- 69. Gutiérrez-Juárez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279(48):49704-49715. [DOI] [PubMed] [Google Scholar]
- 70. Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389(6649):374-377. [DOI] [PubMed] [Google Scholar]
- 71. Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100(12):3105-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harris RB. Acute and chronic effects of leptin on glucose utilization in lean mice. Biochem Biophys Res Commun. 1998;245(2):502-509. [DOI] [PubMed] [Google Scholar]
- 73. Wang JL, Chinookoswong N, Scully S, Qi M, Shi ZQ. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology. 1999;140(5):2117-2124. [DOI] [PubMed] [Google Scholar]
- 74. Rouru J, Cusin I, Zakrzewska KE, Jeanrenaud B, Rohner-Jeanrenaud F. Effects of intravenously infused leptin on insulin sensitivity and on the expression of uncoupling proteins in brown adipose tissue. Endocrinology. 1999;140(8):3688-3692. [DOI] [PubMed] [Google Scholar]
- 75. Ahrén B, Havel PJ. Leptin increases circulating glucose, insulin and glucagon via sympathetic neural activation in fasted mice. Int J Obes Relat Metab Disord. 1999;23(6):660-665. [DOI] [PubMed] [Google Scholar]
- 76. Perry RJ, Wang Y, Cline GW, et al. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018;172(1-2):234-248.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frühbeck G, Aguado M, Gómez-Ambrosi J, Martínez JA. Lipolytic effect of in vivo leptin administration on adipocytes of lean and ob/ob mice, but not db/db mice. Biochem Biophys Res Commun. 1998;250(1):99-102. [DOI] [PubMed] [Google Scholar]
- 78. Takanashi M, Taira Y, Okazaki S, et al. Role of hormone-sensitive lipase in leptin-promoted fat loss and glucose lowering. J Atheroscler Thromb. 2017;24(11):1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Frühbeck G, Gómez-Ambrosi J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell Signal. 2001;13(11):827-833. [DOI] [PubMed] [Google Scholar]
- 80. Steinberg GR, Bonen A, Dyck DJ. Fatty acid oxidation and triacylglycerol hydrolysis are enhanced after chronic leptin treatment in rats. Am J Physiol Endocrinol Metab. 2002;282(3):E593-E600. [DOI] [PubMed] [Google Scholar]
- 81. Metlakunta A, Huang W, Stefanovic-Racic M, Dedousis N, Sipula I, O’Doherty RM. Kupffer cells facilitate the acute effects of leptin on hepatic lipid metabolism. Am J Physiol Endocrinol Metab. 2017;312(1):E11-E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147(3):1480-1487. [DOI] [PubMed] [Google Scholar]
- 83. Shimabukuro M, Koyama K, Chen G, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94(9):4637-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang W, Metlakunta A, Dedousis N, Ortmeyer HK, Stefanovic-Racic M, O’Doherty RM. Leptin augments the acute suppressive effects of insulin on hepatic very low-density lipoprotein production in rats. Endocrinology. 2009;150(5):2169-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570-578. [DOI] [PubMed] [Google Scholar]
- 86. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220-1234. [DOI] [PubMed] [Google Scholar]
- 87. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401(6748):73-76. [DOI] [PubMed] [Google Scholar]
- 88. Asilmaz E, Cohen P, Miyazaki M, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113(3):414-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, et al. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000;49(9):1534-1542. [DOI] [PubMed] [Google Scholar]
- 90. Nagao K, Inoue N, Ujino Y, et al. Effect of leptin infusion on insulin sensitivity and lipid metabolism in diet-induced lipodystrophy model mice. Lipids Health Dis. 2008;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bolze F, Bast A, Mocek S, et al. Treatment of diet-induced lipodystrophic C57BL/6J mice with long-acting PASylated leptin normalises insulin sensitivity and hepatic steatosis by promoting lipid utilisation. Diabetologia. 2016;59(9):2005-2012. [DOI] [PubMed] [Google Scholar]
- 92. Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46(6):1087-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Denroche HC, Kwon MM, Quong WL, et al. Leptin induces fasting hypoglycaemia in a mouse model of diabetes through the depletion of glycerol. Diabetologia. 2015;58(5):1100-1108. [DOI] [PubMed] [Google Scholar]
- 94. Neumann UH, Denroche HC, Mojibian M, Covey SD, Kieffer TJ. Insulin knockout mice have extended survival but volatile blood glucose levels on leptin therapy. Endocrinology. 2016;157(3):1007-1012. [DOI] [PubMed] [Google Scholar]
- 95. Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107(11):4813-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Denroche HC, Levi J, Wideman RD, et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60(5):1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48(7):1487-1492. [DOI] [PubMed] [Google Scholar]
- 98. German JP, Wisse BE, Thaler JP, et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59(7):1626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Perry RJ, Zhang XM, Zhang D, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20(7):759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Morton GJ, Meek TH, Matsen ME, Schwartz MW. Evidence against hypothalamic-pituitary-adrenal axis suppression in the antidiabetic action of leptin. J Clin Invest. 2015;125(12):4587-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perry RJ, Peng L, Abulizi A, Kennedy L, Cline GW, Shulman GI. Mechanism for leptin’s acute insulin-independent effect to reverse diabetic ketoacidosis. J Clin Invest. 2017;127(2): 657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zouhar P, Rakipovski G, Bokhari MH, et al. UCP1-independent glucose-lowering effect of leptin in type 1 diabetes: only in conditions of hypoleptinemia. Am J Physiol Endocrinol Metab. 2020;318(1):E72-E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Blüher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89(3):991s-997s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903-908. [DOI] [PubMed] [Google Scholar]
- 105. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213-215. [DOI] [PubMed] [Google Scholar]
- 106. Farooqi IS, O’Rahilly S. 20 years of leptin: human disorders of leptin action. J Endocrinol. 2014;223(1):T63-T70. [DOI] [PubMed] [Google Scholar]
- 107. Wabitsch M, Funcke JB, Lennerz B, et al. Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015;372(1):48-54. [DOI] [PubMed] [Google Scholar]
- 108. Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc Natl Acad Sci U S A. 2013;110(9):E818-E827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nunziata A, Borck G, Funcke JB, et al. Estimated prevalence of potentially damaging variants in the leptin gene. Mol Cell Pediatr. 2017;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kohlsdorf K, Nunziata A, Funcke JB, et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int J Obes (Lond). 2018;42(9):1602-1609. [DOI] [PubMed] [Google Scholar]
- 111. Funcke JB, von Schnurbein J, Lennerz B, et al. Monogenic forms of childhood obesity due to mutations in the leptin gene. Mol Cell Pediatr. 2014;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nunziata A, Funcke JB, Borck G, et al. Functional and phenotypic characteristics of human leptin receptor mutations. J Endocr Soc. 2019;3(1):27-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398-401. [DOI] [PubMed] [Google Scholar]
- 114. Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24(5):551-555. [DOI] [PubMed] [Google Scholar]
- 116. Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2015;64(1):146-156. [DOI] [PubMed] [Google Scholar]
- 117. Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Paz-Filho GJ, Babikian T, Asarnow R, et al. Leptin replacement improves cognitive development. PLoS One. 2008;3(8):e3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gibson WT, Farooqi IS, Moreau M, et al. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89(10):4821-4826. [DOI] [PubMed] [Google Scholar]
- 120. Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101(13):4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Frank-Podlech S, von Schnurbein J, Veit R, et al. Leptin replacement reestablishes brain insulin action in the hypothalamus in congenital leptin deficiency. Diabetes Care. 2018;41(4):907-910. [DOI] [PubMed] [Google Scholar]
- 122. von Schnurbein J, Heni M, Moss A, et al. Rapid improvement of hepatic steatosis after initiation of leptin substitution in a leptin-deficient girl. Horm Res Paediatr. 2013;79(5):310-317. [DOI] [PubMed] [Google Scholar]
- 123. Lawler K, Huang-Doran I, Sonoyama T, et al. Leptin-mediated changes in the human metabolome. J Clin Endocrinol Metab. 2020;105(8):2541-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395. [DOI] [PubMed] [Google Scholar]
- 125. Hickey MS, Israel RG, Gardiner SN, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59(1):1-6. [DOI] [PubMed] [Google Scholar]
- 126. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155-1161. [DOI] [PubMed] [Google Scholar]
- 127. Simha V. Metreleptin for metabolic disorders associated with generalized or partial lipodystrophy. Expert Rev Endocrinol Metab. 2014;9(3):205-212. [DOI] [PubMed] [Google Scholar]
- 128. Lebastchi J, Ajluni N, Neidert A, Oral EA. A report of three cases with acquired generalized lipodystrophy with distinct autoimmune conditions treated with metreleptin. J Clin Endocrinol Metab. 2015;100(11):3967-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Baykal AP, Parks EJ, Shamburek R, et al. Leptin decreases de novo lipogenesis in patients with lipodystrophy. JCI Insight. 2020;5(14):e137180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Muniyappa R, Abel BS, Asthana A, et al. Metreleptin therapy lowers plasma angiopoietin-like protein 3 in patients with generalized lipodystrophy. J Clin Lipidol. 2017;11(2):543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grewal S, Gubbi S, Fosam A, et al. Metabolomic analysis of the effects of leptin replacement therapy in patients with lipodystrophy. J Endocr Soc. 2020;4(1):bvz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wolsk E, Mygind H, Grondahl TS, Pedersen BK, van Hall G. The role of leptin in human lipid and glucose metabolism: the effects of acute recombinant human leptin infusion in young healthy males. Am J Clin Nutr. 2011;94(6):1533-1544. [DOI] [PubMed] [Google Scholar]
- 133. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60(5):1474-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schorr M, Miller KK. The endocrine manifestations of anorexia nervosa: mechanisms and management. Nat Rev Endocrinol. 2017;13(3):174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hussain AA, Hübel C, Hindborg M, et al. Increased lipid and lipoprotein concentrations in anorexia nervosa: a systematic review and meta-analysis. Int J Eat Disord. 2019;52(6):611-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Misra M, Miller KK, Kuo K, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(3):E373-E381. [DOI] [PubMed] [Google Scholar]
- 138. Hebebrand J, Milos G, Wabitsch M, et al. Clinical trials required to assess potential benefits and side effects of treatment of patients with anorexia nervosa with recombinant human leptin. Front Psychol. 2019;10:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes Res. 2001;9(8):462-469. [DOI] [PubMed] [Google Scholar]
- 140. Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26(4):504-509. [DOI] [PubMed] [Google Scholar]
- 141. Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568-1575. [DOI] [PubMed] [Google Scholar]
- 142. Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003;77(4):771-776. [DOI] [PubMed] [Google Scholar]
- 143. Fogteloo AJ, Pijl H, Frölich M, McCamish M, Meinders AE. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab. 2003;16(2):109-114. [PubMed] [Google Scholar]
- 144. Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83-T96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vasandani C, Clark GO, Adams-Huet B, Quittner C, Garg A. Efficacy and safety of metreleptin therapy in patients with type 1 diabetes: a pilot study. Diabetes Care. 2017;40(5):694-697. [DOI] [PubMed] [Google Scholar]
- 146. Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15(8):444-455. [DOI] [PubMed] [Google Scholar]
- 147. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood-brain and the blood-cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neuroscience. 2004;123(2):527-536. [DOI] [PubMed] [Google Scholar]
- 149. Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141(4):1434-1441. [DOI] [PubMed] [Google Scholar]
- 150. Di Spiezio A, Sandin ES, Dore R, et al. The LepR-mediated leptin transport across brain barriers controls food reward. Mol Metab. 2018;8:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Harrison L, Schriever SC, Feuchtinger A, et al. Fluorescent blood-brain barrier tracing shows intact leptin transport in obese mice. Int J Obes (Lond). 2019;43(6):1305-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tu H, Hsuchou H, Kastin AJ, Wu X, Pan W. Unique leptin trafficking by a tailless receptor. FASEB J. 2010;24(7):2281-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa-ObRd. J Cell Physiol. 2007;212(1):215-222. [DOI] [PubMed] [Google Scholar]
- 154. Gonzalez-Carter D, Goode AE, Fiammengo R, Dunlop IE, Dexter DT, Porter AE. Inhibition of leptin-ObR interaction does not prevent leptin translocation across a human blood-brain barrier model. J Neuroendocrinol. 2016;28(6):jne.12392. [DOI] [PubMed] [Google Scholar]
- 155. Jiang H, Gallet S, Klemm P, et al. MCH neurons regulate permeability of the median eminence barrier. Neuron. 2020;107(2):306-319.e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Balland E, Dam J, Langlet F, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19(2):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yoo S, Cha D, Kim DW, Hoang TV, Blackshaw S. Tanycyte-independent control of hypothalamic leptin signaling. Front Neurosci. 2019;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Djogo T, Robins SC, Schneider S, et al. Adult NG2-Glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 2016;23(5):797-810. [DOI] [PubMed] [Google Scholar]
- 159. Baskin DG, Breininger JF, Bonigut S, Miller MA. Leptin binding in the arcuate nucleus is increased during fasting. Brain Res. 1999;828(1-2):154-158. [DOI] [PubMed] [Google Scholar]
- 160. Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21(5):679-682. [DOI] [PubMed] [Google Scholar]
- 161. Kleinert M, Kotzbeck P, Altendorfer-Kroath T, Birngruber T, Tschöp MH, Clemmensen C. Time-resolved hypothalamic open flow micro-perfusion reveals normal leptin transport across the blood-brain barrier in leptin resistant mice. Mol Metab. 2018;13:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Faouzi M, Leshan R, Björnholm M, Hennessey T, Jones J, Münzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148(11):5414-5423. [DOI] [PubMed] [Google Scholar]
- 163. Maniscalco JW, Rinaman L. Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Physiol Regul Integr Comp Physiol. 2014;306(8):R576-R585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304(12):E1338-E1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3(6):595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. D’souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6(9): 1052-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci. 2018;19(3):153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Manceau R, Majeur D, Alquier T. Neuronal control of peripheral nutrient partitioning. Diabetologia. 2020;63(4):673-682. [DOI] [PubMed] [Google Scholar]
- 169. Liu L, Karkanias GB, Morales JC, et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273(47):31160-31167. [DOI] [PubMed] [Google Scholar]
- 170. Koch C, Augustine RA, Steger J, et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30(48):16180-16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tanida M, Yamamoto N, Morgan DA, Kurata Y, Shibamoto T, Rahmouni K. Leptin receptor signaling in the hypothalamus regulates hepatic autonomic nerve activity via phosphatidylinositol 3-kinase and AMP-activated protein kinase. J Neurosci. 2015;35(2):474-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Miyamoto L, Ebihara K, Kusakabe T, et al. Leptin activates hepatic 5’-AMP-activated protein kinase through sympathetic nervous system and α1-adrenergic receptor: a potential mechanism for improvement of fatty liver in lipodystrophy by leptin. J Biol Chem. 2012;287(48):40441-40447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007;416(2):193-197. [DOI] [PubMed] [Google Scholar]
- 174. Zeng W, Pirzgalska RM, Pereira MM, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84-94. [DOI] [PubMed] [Google Scholar]
- 175. Buettner C, Muse ED, Cheng A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48(2):287-291. [DOI] [PubMed] [Google Scholar]
- 177. Shiuchi T, Toda C, Okamoto S, et al. Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle beta2-adrenergic receptor but not by AMPK. Sci. 2017;7(1):15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48(9):1706-1712. [DOI] [PubMed] [Google Scholar]
- 179. Muzumdar R, Ma X, Yang X, et al. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB J. 2003;17(9):1130-1132. [DOI] [PubMed] [Google Scholar]
- 180. Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107(40):17391-17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kulkarni RN, Wang ZL, Wang RM, et al. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest. 1997;100(11):2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tudurí E, Marroquí L, Soriano S, et al. Inhibitory effects of leptin on pancreatic alpha-cell function. Diabetes. 2009;58(7):1616-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Burgos-Ramos E, Canelles S, Rodríguez A, et al. The increase in fiber size in male rat gastrocnemius after chronic central leptin infusion is related to activation of insulin signaling. Mol Cell Endocrinol. 2018;470:48-59. [DOI] [PubMed] [Google Scholar]
- 184. Gallardo N, Bonzón-Kulichenko E, Fernández-Agulló T, et al. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148(12):5604-5610. [DOI] [PubMed] [Google Scholar]
- 185. Tajima D, Masaki T, Hidaka S, Kakuma T, Sakata T, Yoshimatsu H. Acute central infusion of leptin modulates fatty acid mobilization by affecting lipolysis and mRNA expression for uncoupling proteins. Exp Biol Med (Maywood). 2005;230(3):200-206. [DOI] [PubMed] [Google Scholar]
- 186. Hackl MT, Fürnsinn C, Schuh CM, et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat Commun. 2019;10(1):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Arvaniti K, Richard D, Picard F, Deshaies Y. Lipid deposition in rats centrally infused with leptin in the presence or absence of corticosterone. Am J Physiol Endocrinol Metab. 2001;281(4):E809-E816. [DOI] [PubMed] [Google Scholar]
- 188. Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology. 2013;57(2):543-554. [DOI] [PubMed] [Google Scholar]
- 189. Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339-343. [DOI] [PubMed] [Google Scholar]
- 190. Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284(5):E863-E873. [DOI] [PubMed] [Google Scholar]
- 191. Laker RC, Henry BA, Wadley GD, Clarke IJ, Canny BJ, McConell GK. Central infusion of leptin does not increase AMPK signaling in skeletal muscle of sheep. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R511-R518. [DOI] [PubMed] [Google Scholar]
- 192. Mora C, Pintado C, Rubio B, et al. Central leptin regulates heart lipid content by selectively increasing PPAR β/δ expression. J Endocrinol. 2018;236(1):43-56. [DOI] [PubMed] [Google Scholar]
- 193. Keung W, Cadete VJ, Palaniyappan A, Jablonski A, Fischer M, Lopaschuk GD. Intracerebroventricular leptin administration differentially alters cardiac energy metabolism in mice fed a low-fat and high-fat diet. J Cardiovasc Pharmacol. 2011;57(1):103-113. [DOI] [PubMed] [Google Scholar]
- 194. Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54(11):3182-3189. [DOI] [PubMed] [Google Scholar]
- 195. Li X, Wu X, Camacho R, Schwartz GJ, LeRoith D. Intracerebroventricular leptin infusion improves glucose homeostasis in lean type 2 diabetic MKR mice via hepatic vagal and non-vagal mechanisms. PLoS One. 2011;6(2):e17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Keung W, Palaniyappan A, Lopaschuk GD. Chronic central leptin decreases food intake and improves glucose tolerance in diet-induced obese mice independent of hypothalamic malonyl CoA levels and skeletal muscle insulin sensitivity. Endocrinology. 2011;152(11):4127-4137. [DOI] [PubMed] [Google Scholar]
- 197. Leshan RL, Björnholm M, Münzberg H, Myers MG Jr. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring). 2006;14 Suppl (5):208S-212S. [DOI] [PubMed] [Google Scholar]
- 198. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98(5):1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Flak JN, Arble D, Pan W, et al. A leptin-regulated circuit controls glucose mobilization during noxious stimuli. J Clin Invest. 2017;127(8):3103-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Toda C, Shiuchi T, Lee S, et al. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58(12):2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Toda C, Shiuchi T, Kageyama H, et al. Extracellular signal-regulated kinase in the ventromedial hypothalamus mediates leptin-induced glucose uptake in red-type skeletal muscle. Diabetes. 2013;62(7):2295-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Coppari R, Ichinose M, Lee CE, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1(1):63-72. [DOI] [PubMed] [Google Scholar]
- 203. Rezai-Zadeh K, Yu S, Jiang Y, et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014;3(7):681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cakir I, Diaz-Martinez M, Lining Pan P, Welch EB, Patel S, Ghamari-Langroudi M. Leptin receptor signaling in Sim1-expressing neurons regulates body temperature and adaptive thermogenesis. Endocrinology. 2019;160(4):863-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Denroche HC, Glavas MM, Tudurí E, et al. Disrupted leptin signaling in the lateral hypothalamus and ventral premammillary nucleus alters insulin and glucagon secretion and protects against diet-induced obesity. Endocrinology. 2016;157(7):2671-2685. [DOI] [PubMed] [Google Scholar]
- 206. Ren H, Plum-Morschel L, Gutierrez-Juarez R, et al. Blunted refeeding response and increased locomotor activity in mice lacking FoxO1 in synapsin-Cre-expressing neurons. Diabetes. 2013;62(10):3373-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. do Carmo JM, da Silva AA, Gava FN, Moak SP, Dai X, Hall JE. Impact of leptin deficiency compared with neuronal-specific leptin receptor deletion on cardiometabolic regulation. Am J Physiol Regul Integr Comp Physiol. 2019;317(4):R552-R562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. B6.Cg-Tg(Nes-cre)1Kln/J. 2020. https://www.jax.org/strain/003771. Accessed June 19, 2020. [Google Scholar]
- 209. Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149(5): 2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191-203. [DOI] [PubMed] [Google Scholar]
- 211. Senn SS, Le Foll C, Whiting L, et al. Unsilencing of native LepRs in hypothalamic SF1 neurons does not rescue obese phenotype in LepR-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2019;317(3):R451-R460. [DOI] [PubMed] [Google Scholar]
- 212. Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983-991. [DOI] [PubMed] [Google Scholar]
- 213. Huo L, Gamber K, Greeley S, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9(6):537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Berglund ED, Vianna CR, Donato J Jr, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521(14):3287-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Gonçalves GH, Li W, Garcia AV, Figueiredo MS, Bjørbæk C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014;7(4):1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Xu J, Bartolome CL, Low CS, et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556(7702):505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wang P, Loh KH, Wu M, et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583(7818):839-844. [DOI] [PubMed] [Google Scholar]
- 220. da Silva AA, Spradley FT, Granger JP, Hall JE, do Carmo JM. Brain-mediated antidiabetic, anorexic, and cardiovascular actions of leptin require melanocortin-4 receptor signaling. J Neurophysiol. 2015;113(7):2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1275-R1282. [DOI] [PubMed] [Google Scholar]
- 222. Denroche HC, Kwon MM, Glavas MM, et al. The role of autonomic efferents and uncoupling protein 1 in the glucose-lowering effect of leptin therapy. Mol Metab. 2016;5(8):716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. da Silva AA, Hall JE, Moak SP, et al. Role of autonomic nervous system in chronic CNS-mediated antidiabetic action of leptin. Am J Physiol Endocrinol Metab. 2017;312(5):E420-E428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. D’souza AM, Johnson JD, Clee SM, Kieffer TJ. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Mol Metab. 2016;5(11):1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Neumann UH, Kwon MM, Baker RK, Kieffer TJ. Leptin contributes to the beneficial effects of insulin treatment in streptozotocin-diabetic male mice. Am J Physiol Endocrinol Metab. 2018;315(6):E1264-E1273. [DOI] [PubMed] [Google Scholar]
- 226. German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152(2):394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282(5):E1084-E1091. [DOI] [PubMed] [Google Scholar]
- 228. Xu Y, Chang JT, Myers MG Jr, Xu Y, Tong Q. Euglycemia restoration by central leptin in type 1 diabetes requires STAT3 signaling but not fast-acting neurotransmitter release. Diabetes. 2016;65(4):1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Fujikawa T, Berglund ED, Patel VR, et al. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18(3):431-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Singha AK, Yamaguchi J, Gonzalez NS, Ahmed N, Toney GM, Fujikawa T. Glucose-lowering by leptin in the absence of insulin does not fully rely on the central melanocortin system in male mice. Endocrinology. 2019;160(3):651-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Singha A, Palavicini JP, Pan M, et al. Leptin receptors in RIP-Cre25Mgn neurons mediate anti-dyslipidemia effects of leptin in insulin-deficient mice. Front Endocrinol. 2020;11:e588447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Meek TH, Matsen ME, Dorfman MD, et al. Leptin action in the ventromedial hypothalamic nucleus is sufficient, but not necessary, to normalize diabetic hyperglycemia. Endocrinology. 2013;154(9):3067-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Flak JN, Patterson CM, Garfield AS, et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci. 2014;17(12):1744-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. García-Cáceres C, Fuente-Martín E, Burgos-Ramos E, et al. Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology. 2011;152(5):1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Jayaram B, Pan W, Wang Y, et al. Astrocytic leptin-receptor knockout mice show partial rescue of leptin resistance in diet-induced obesity. J Appl Physiol (1985). 2013;114(6):734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W. Role of astrocytes in leptin signaling. J Mol Neurosci. 2015;56(4):829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kim JG, Suyama S, Koch M, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17(7):908-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Naranjo V, Contreras A, Merino B, et al. Specific deletion of the astrocyte leptin receptor induces changes in hippocampus glutamate metabolism, synaptic transmission and plasticity. Neuroscience. 2020;447:182-190. [DOI] [PubMed] [Google Scholar]
- 239. Gao Y, Vidal-Itriago A, Milanova I, et al. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol Metab. 2018;7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Üner AG, Keçik O, Quaresma PGF, et al. Role of POMC and AgRP neuronal activities on glycaemia in mice. Sci Rep. 2019;9(1):13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metab. 2016;5(8):669-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Huang Y, He Z, Gao Y, et al. Phosphoinositide 3-kinase is integral for the acute activity of leptin and insulin in male arcuate NPY/AgRP neurons. J Endocr Soc. 2018;2(6):518-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Fuente-Martín E, García-Cáceres C, Granado M, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012;122(11):3900-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Abraham MA, Rasti M, Bauer PV, Lam TKT. Leptin enhances hypothalamic lactate dehydrogenase A (LDHA)-dependent glucose sensing to lower glucose production in high-fat-fed rats. J Biol Chem. 2018;293(11):4159-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci U S A. 2017;114(5):1189-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Campbell JE, Drucker DJ. Islet α cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329-338. [DOI] [PubMed] [Google Scholar]
- 248. Chen J, Fu R, Cui Y, et al. LIM-homeodomain transcription factor Isl-1 mediates the effect of leptin on insulin secretion in mice. J Biol Chem. 2013;288(17):12395-12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wu Y, Fortin DA, Cochrane VA, Chen PC, Shyng SL. NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic β-cells. J Biol Chem. 2017;292(37):15512-15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Ramzy A, Tudurí E, Glavas MM, et al. AAV8 Ins1-Cre can produce efficient β-cell recombination but requires consideration of off-target effects. Sci. 2020;10(1):10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Covey SD, Wideman RD, McDonald C, et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4(4):291-302. [DOI] [PubMed] [Google Scholar]
- 252. Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281(5):2649-2653. [DOI] [PubMed] [Google Scholar]
- 253. Morioka T, Asilmaz E, Hu J, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117(10):2860-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Mosleh E, Ou K, Haemmerle MW, et al. Ins1-Cre and Ins1-CreER gene replacement alleles are susceptible to silencing by DNA hypermethylation. Endocrinology. 2020;161(8):bqaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Soedling H, Hodson DJ, Adrianssens AE, et al. Limited impact on glucose homeostasis of leptin receptor deletion from insulin- or proglucagon-expressing cells. Mol Metab. 2015;4(9):619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. D’Souza AM, Kieffer TJ. Restoration of Lepr in β cells of Lepr null mice does not prevent hyperinsulinemia and hyperglycemia. Mol Metab. 2017;6(6):585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tudurí E, Denroche HC, Kara JA, Asadi A, Fox JK, Kieffer TJ. Partial ablation of leptin signaling in mouse pancreatic α-cells does not alter either glucose or lipid homeostasis. Am J Physiol Endocrinol Metab. 2014;306(7):E748-E755. [DOI] [PubMed] [Google Scholar]
- 258. Lawlor N, George J, Bolisetty M, et al. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 2017;27(2):208-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Poitout V, Rouault C, Guerre-Millo M, Briaud I, Reach G. Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans. Endocrinology. 1998;139(3):822-826. [DOI] [PubMed] [Google Scholar]
- 260. Anderwald C, Müller G, Koca G, Fürnsinn C, Waldhäusl W, Roden M. Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol Endocrinol. 2002;16(7):1612-1628. [DOI] [PubMed] [Google Scholar]
- 261. Huang W, Dedousis N, Bhatt BA, O’Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279(21):21695-21700. [DOI] [PubMed] [Google Scholar]
- 262. Lee Y, Wang MY, Kakuma T, et al. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276(8):5629-5635. [DOI] [PubMed] [Google Scholar]
- 263. Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42(6):1339-1348. [DOI] [PubMed] [Google Scholar]
- 264. Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Huynh FK, Levi J, Denroche HC, et al. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes. 2010;59(12):3032-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig. 2012;3(2):115-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Minokoshi Y, Toda C, Okamoto S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J Endocrinol Metab. 2012;16(Suppl 3):S562-S568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Nicholson T, Church C, Baker DJ, Jones SW. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond). 2018;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Muoio DM, Dohm GL, Fiedorek FT Jr, Tapscott EB, Coleman RA, Dohn GL. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46(8):1360-1363. [DOI] [PubMed] [Google Scholar]
- 270. Dulloo AG, Stock MJ, Solinas G, Boss O, Montani JP, Seydoux J. Leptin directly stimulates thermogenesis in skeletal muscle. FEBS Lett. 2002;515(1-3):109-113. [DOI] [PubMed] [Google Scholar]
- 271. Solinas G, Summermatter S, Mainieri D, et al. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett. 2004;577(3):539-544. [DOI] [PubMed] [Google Scholar]
- 272. Janovská A, Hatzinikolas G, Staikopoulos V, McInerney J, Mano M, Wittert GA. AMPK and ACC phosphorylation: effect of leptin, muscle fibre type and obesity. Mol Cell Endocrinol. 2008;284(1-2):1-10. [DOI] [PubMed] [Google Scholar]
- 273. Akasaka Y, Tsunoda M, Ogata T, Ide T, Murakami K. Direct evidence for leptin-induced lipid oxidation independent of long-form leptin receptor. Biochim Biophys Acta. 2010;1801(10):1115-1122. [DOI] [PubMed] [Google Scholar]
- 274. Koo YD, Lee JS, Lee SA, et al. SUMO-specific protease 2 mediates leptin-induced fatty acid oxidation in skeletal muscle. Metabolism. 2019;95:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bates SH, Gardiner JV, Jones RB, Bloom SR, Bailey CJ. Acute stimulation of glucose uptake by leptin in l6 muscle cells. Horm Metab Res. 2002;34(3):111-115. [DOI] [PubMed] [Google Scholar]
- 276. Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Häring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40(11):1358-1362. [DOI] [PubMed] [Google Scholar]
- 277. Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283(1):E187-E192. [DOI] [PubMed] [Google Scholar]
- 278. Momken I, Chabowski A, Dirkx E, et al. A new leptin-mediated mechanism for stimulating fatty acid oxidation: a pivotal role for sarcolemmal FAT/CD36. Biochem J. 2017;474(1):149-162. [DOI] [PubMed] [Google Scholar]
- 279. Eguchi M, Shrivastava S, Lyakhovsky N, Kim W, Palanivel R, Sweeney G. Control of fatty acid metabolism by leptin in L6 rat myoblasts is regulated by hyperinsulinemia. J Endocrinol Invest. 2007;30(3):192-199. [DOI] [PubMed] [Google Scholar]
- 280. Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27(12):4317-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Sweeney G, Keen J, Somwar R, Konrad D, Garg R, Klip A. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in l6 rat skeletal muscle cells. Endocrinology. 2001;142(11):4806-4812. [DOI] [PubMed] [Google Scholar]
- 282. Ceddia RB, William WN Jr, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord. 1999;23(1):75-82. [DOI] [PubMed] [Google Scholar]
- 283. Berti L, Kellerer M, Capp E, Häring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia. 1997;40(5):606-609. [DOI] [PubMed] [Google Scholar]
- 284. Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem. 2003;278(46):45638-45650. [DOI] [PubMed] [Google Scholar]
- 285. Masuzaki H, Ogawa Y, Isse N, et al. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995;44(7):855-858. [DOI] [PubMed] [Google Scholar]
- 286. Frühbeck G, Aguado M, Martínez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun. 1997;240(3):590-594. [DOI] [PubMed] [Google Scholar]
- 287. Elimam A, Kamel A, Marcus C. In vitro effects of leptin on human adipocyte metabolism. Horm Res. 2002;58(2):88-93. [DOI] [PubMed] [Google Scholar]
- 288. Pérez C, Fernández-Galaz C, Fernández-Agulló T, et al. Leptin impairs insulin signaling in rat adipocytes. Diabetes. 2004;53(2):347-353. [DOI] [PubMed] [Google Scholar]
- 289. Müller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem. 1997;272(16):10585-10593. [DOI] [PubMed] [Google Scholar]
- 290. Kraus D, Fasshauer M, Ott V, et al. Leptin secretion and negative autocrine crosstalk with insulin in brown adipocytes. J Endocrinol. 2002;175(1):185-191. [DOI] [PubMed] [Google Scholar]
- 291. Ranganathan S, Ciaraldi TP, Henry RR, Mudaliar S, Kern PA. Lack of effect of leptin on glucose transport, lipoprotein lipase, and insulin action in adipose and muscle cells. Endocrinology. 1998;139(5):2509-2513. [DOI] [PubMed] [Google Scholar]
- 292. Mick G, Vanderbloomer T, Fu CL, McCormick K. Leptin does not affect adipocyte glucose metabolism: studies in fresh and cultured adipocytes. Metabolism. 1998;47(11):1360-1365. [DOI] [PubMed] [Google Scholar]
- 293. Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1(8):754-764. [DOI] [PubMed] [Google Scholar]
- 294. Meade CJ, Ashwell M, Sowter C. Is genetically transmitted obesity due to an adipose tissue defect? Proc R Soc Lond B Biol Sci. 1979;205(1160):395-410. [DOI] [PubMed] [Google Scholar]
- 295. Guo K, Mogen J, Struzzi S, Zhang Y. Preadipocyte transplantation: an in vivo study of direct leptin signaling on adipocyte morphogenesis and cell size. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1339-R1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Ko CW, Counihan D, Wu J, Hatzoglou M, Puchowicz MA, Croniger CM. Macrophages with a deletion of the phosphoenolpyruvate carboxykinase 1 (Pck1) gene have a more proinflammatory phenotype. J Biol Chem. 2018;293(9): 3399-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Pereira S, O’Dwyer SM, Webber TD, et al. Metabolic effects of leptin receptor knockdown or reconstitution in adipose tissues. Sci Rep. 2019;9(1):3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Palhinha L, Liechocki S, Hottz ED, et al. Leptin induces proadipogenic and proinflammatory signaling in adipocytes. Front Endocrinol (Lausanne). 2019;10:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Gupta A, Beg M, Kumar D, et al. Chronic hyper-leptinemia induces insulin signaling disruption in adipocytes: Implications of NOS2. Free Radic Biol Med. 2017;112:93-108. [DOI] [PubMed] [Google Scholar]
- 300. Becerril S, Rodríguez A, Catalán V, et al. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. Int J Obes (Lond). 2018;42(8):1458-1470. [DOI] [PubMed] [Google Scholar]
- 301. Koltes DA, Spurlock ME, Spurlock DM. Adipose triglyceride lipase protein abundance and translocation to the lipid droplet increase during leptin-induced lipolysis in bovine adipocytes. Domest Anim Endocrinol. 2017;61:62-76. [DOI] [PubMed] [Google Scholar]
- 302. Oberlin D, Buettner C. How does leptin restore euglycemia in insulin-deficient diabetes? J Clin Invest. 2017;127(2):450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Abella V, Scotece M, Conde J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100-109. [DOI] [PubMed] [Google Scholar]
- 304. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392-404. [DOI] [PubMed] [Google Scholar]
- 305. Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737-748. [DOI] [PubMed] [Google Scholar]
- 306. Ceccarini G, Flavell RR, Butelman ER, et al. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10(2):148-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371-379. [DOI] [PubMed] [Google Scholar]
- 308. McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127(1):5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20(13):3149-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Morinaga H, Mayoral R, Heinrichsdorff J, et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64(4):1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191-198. [DOI] [PubMed] [Google Scholar]
- 312. Perry RJ, Camporez JG, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25(10): 2062-2068. [DOI] [PubMed] [Google Scholar]
- 314. Rivers SL, Klip A, Giacca A. NOD1: an interface between innate immunity and insulin resistance. Endocrinology. 2019;160(5):1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Gove ME, Sherry CL, Pini M, Fantuzzi G. Generation of leptin receptor bone marrow chimeras: recovery from irradiation, immune cellularity, cytokine expression, and metabolic parameters. Obesity (Silver Spring). 2010;18(12):2274-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Dib LH, Ortega MT, Fleming SD, Chapes SK, Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Gutierrez DA, Hasty AH. Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. J Endocrinol. 2012;212(3):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Wrann CD, Laue T, Hübner L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012;302(1):E108-E116. [DOI] [PubMed] [Google Scholar]
- 319. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Lee FY, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Physiol. 1999;276(2):C386-C394. [DOI] [PubMed] [Google Scholar]
- 321. Lee SM, Choi HJ, Oh CH, Oh JW, Han JS. Leptin increases TNF-α expression and production through phospholipase D1 in Raw 264.7 cells. PLoS One. 2014;9(7):e102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Tsiotra PC, Boutati E, Dimitriadis G, Raptis SA. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res Int. 2013;2013:487081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Vaughan T, Li L. Molecular mechanism underlying the inflammatory complication of leptin in macrophages. Mol Immunol. 2010;47(15):2515-2518. [DOI] [PubMed] [Google Scholar]
- 324. Flatow EA, Komegae EN, Fonseca MT, et al. Elucidating the role of leptin in systemic inflammation: a study targeting physiological leptin levels in rats and their macrophages. Am J Physiol Regul Integr Comp Physiol. 2017;313(5):R572-R582. [DOI] [PubMed] [Google Scholar]
- 325. Acedo SC, Gambero S, Cunha FG, Lorand-Metze I, Gambero A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell Dev Biol Anim. 2013;49(6):473-478. [DOI] [PubMed] [Google Scholar]
- 326. Ieronymaki E, Theodorakis EM, Lyroni K, et al. Insulin resistance in macrophages alters their metabolism and promotes an M2-like phenotype. J Immunol. 2019;202(6):1786-1797. [DOI] [PubMed] [Google Scholar]
- 327. Carrière A, Jeanson Y, Berger-Müller S, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63(10):3253-3265. [DOI] [PubMed] [Google Scholar]
- 328. Scheller EL, Song J, Dishowitz MI, Hankenson KD, Krebsbach PH. A potential role for the myeloid lineage in leptin-regulated bone metabolism. Horm Metab Res. 2012;44(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Fischer J, Gutièrrez S, Ganesan R, et al. Leptin signaling impairs macrophage defenses against Salmonella Typhimurium. Proc Natl Acad Sci U S A. 2019;116(33):16551-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Mancuso P, Curtis JL, Freeman CM, Peters-Golden M, Weinberg JB, Myers MG Jr. Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of Streptococcus pneumoniae and alveolar macrophage bactericidal function. Am J Physiol Lung Cell Mol Physiol. 2018;315(1):L78-L86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Annema W, Tietge UJ. Regulation of reverse cholesterol transport - a comprehensive appraisal of available animal studies. Nutr Metab (Lond). 2012;9(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124(10):1505-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. O’Rourke L, Yeaman SJ, Shepherd PR. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes. 2001;50(5):955-961. [DOI] [PubMed] [Google Scholar]
- 334. O’Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277(45):42557-42562. [DOI] [PubMed] [Google Scholar]
- 335. Maya-Monteiro CM, Almeida PE, D’Avila H, et al. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J Biol Chem. 2008;283(4):2203-2210. [DOI] [PubMed] [Google Scholar]
- 336. Hongo S, Watanabe T, Arita S, et al. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am J Physiol Endocrinol Metab. 2009;297(2):E474-E482. [DOI] [PubMed] [Google Scholar]
- 337. Cabrero A, Cubero M, Llaverías G, et al. Leptin down-regulates peroxisome proliferator-activated receptor gamma (PPAR-gamma) mRNA levels in primary human monocyte-derived macrophages. Mol Cell Biochem. 2005;275(1-2):173-179. [DOI] [PubMed] [Google Scholar]
- 338. B6.Cg-Tg(Lck-cre)548Jxm/J. 2020. https://www.jax.org/strain/003802. Accessed June 19, 2020. [Google Scholar]
- 339. B6.Cg-Tg(Lck-icre)3779Nik/J. 2020. https://www.jax.org/strain/012837. Accessed June 19, 2020. [Google Scholar]
- 340. Germain RN. T-cell development and the CD4-CD8 lineage decision. Nature Rev Immunol. 2002;2(5):309-322. [DOI] [PubMed] [Google Scholar]
- 341. Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192(1):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Reis BS, Lee K, Fanok MH, et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J Immunol. 2015;194(11):5253-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Gerriets VA, Danzaki K, Kishton RJ, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46(8):1970-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. McGaffin KR, Witham WG, Yester KA, et al. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011;89(1):60-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Witham W, Yester K, O’Donnell CP, McGaffin KR. Restoration of glucose metabolism in leptin-resistant mouse hearts after acute myocardial infarction through the activation of survival kinase pathways. J Mol Cell Cardiol. 2012;53(1):91-100. [DOI] [PubMed] [Google Scholar]
- 347. Hall ME, Smith G, Hall JE, Stec DE. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am J Physiol Regul Integr Comp Physiol. 2012;303(12):R1241-R1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem. 2002;277(33):29424-29430. [DOI] [PubMed] [Google Scholar]
- 349. Sharma V, Mustafa S, Patel N, Wambolt R, Allard MF, McNeill JH. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur J Pharmacol. 2009;617(1-3):113-117. [DOI] [PubMed] [Google Scholar]
- 350. Hall ME, Maready MW, Hall JE, Stec DE. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab. 2014;307(3):E316-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. [DOI] [PubMed] [Google Scholar]
- 352. Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol. 2012;24(3):504-510. [DOI] [PubMed] [Google Scholar]
- 353. Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens. 2011;29(4):758-762. [DOI] [PubMed] [Google Scholar]
- 354. Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46(12):2040-2043. [DOI] [PubMed] [Google Scholar]
- 355. Zheng H, Liu X, Li Y, Patel KP. A hypothalamic leptin-glutamate interaction in the regulation of sympathetic nerve activity. Neural Plast. 2017;2017:2361675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic-vagal balance and baroreflex sensitivity in diabetic rats. Am J Physiol Heart Circ Physiol. 2008;295(5):H1974-H1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17(4):599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Shin MK, Eraso CC, Mu YP, et al. Leptin induces hypertension acting on transient receptor potential melastatin 7 channel in the carotid body. Circ Res. 2019;125(11):989-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Huby AC, Otvos L Jr, Belin de Chantemèle EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67(5):1020-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Faulkner JL, Belin de Chantemèle EJ. Leptin and aldosterone. Vitam Horm. 2019;109:265-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Hüttner I, Boutet M, Rona G, More RH. Studies on protein passage through arterial endothelium. 3. Effect of blood pressure levels on the passage of fine structural protein tracers through rat arterial endothelium. Lab Invest. 1973;29(5):536-546. [PubMed] [Google Scholar]
- 362. Vecchione C, Maffei A, Colella S, et al. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51(1):168-173. [DOI] [PubMed] [Google Scholar]
- 363. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276(27):25096-25100. [DOI] [PubMed] [Google Scholar]
- 364. Xie X, Li S, Zhu Y, et al. Egr-1 mediates leptin-induced PPARγ reduction and proliferation of pulmonary artery smooth muscle cells. Mol Biol Cell. 2018;29(3):356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Schroeter MR, Leifheit-Nestler M, Hubert A, et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc Res. 2013;99(3):555-565. [DOI] [PubMed] [Google Scholar]
- 366. Ryan MJ, Coleman TT, Sasser JM, Pittman KM, Hankins MW, Stec DE. Vascular smooth muscle-specific deletion of the leptin receptor attenuates leptin-induced alterations in vascular relaxation. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R960-R967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968-977. [DOI] [PubMed] [Google Scholar]
- 368. Wu KK, Wu TJ, Chin J, et al. Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis. 2005;181(2):251-259. [DOI] [PubMed] [Google Scholar]
- 369. Hasty AH, Shimano H, Osuga J, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276(40):37402-37408. [DOI] [PubMed] [Google Scholar]
- 370. Mertens A, Verhamme P, Bielicki JK, et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107(12):1640-1646. [DOI] [PubMed] [Google Scholar]
- 371. Hoffmann A, Ebert T, Klöting N, et al. Leptin dose-dependently decreases atherosclerosis by attenuation of hypercholesterolemia and induction of adiponectin. Biochim Biophys Acta. 2016;1862(1):113-120. [DOI] [PubMed] [Google Scholar]
- 372. Jun JY, Ma Z, Pyla R, Segar L. Leptin treatment inhibits the progression of atherosclerosis by attenuating hypercholesterolemia in type 1 diabetic Ins2(+/Akita):apoE(-/-) mice. Atherosclerosis. 2012;225(2):341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Lundåsen T, Liao W, Angelin B, Rudling M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J Biol Chem. 2003;278(44):43224-43228. [DOI] [PubMed] [Google Scholar]
- 374. Taleb S, Herbin O, Ait-Oufella H, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(12):2691-2698. [DOI] [PubMed] [Google Scholar]
- 375. Surmi BK, Atkinson RD, Gruen ML, Coenen KR, Hasty AH. The role of macrophage leptin receptor in aortic root lesion formation. Am J Physiol Endocrinol Metab. 2008;294(3):E488-E495. [DOI] [PubMed] [Google Scholar]
- 376. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201-229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Color versions of the figures are available upon request.