Abstract
Evaluation of circulating tumor cells (CTCs) has demonstrated clinical validity as a prognostic tool based on enumeration, but since the introduction of this tool to the clinic in 2004, further clinical utility and widespread adoption have been limited. However, immense efforts have been undertaken to further the understanding of the mechanisms behind the biology and kinetics of these rare cells, and progress continues toward better applicability in the clinic. This review describes recent advances within the field, with a particular focus on understanding the biological significance of CTCs, and summarizes emerging methods for identifying, isolating, and interrogating the cells that may provide technical advantages allowing for the discovery of more specific clinical applications. Included is an atlas of high-definition images of CTCs from various cancer types, including uncommon CTCs captured only by broadly inclusive nonenrichment techniques.
Keywords: biomarkers, metastasis, liquid biopsy, precision medicine, fluid biopsy, circulating tumor microemboli
INTRODUCTION
Improvements in cancer prevention and control as well as changes in medical practice have contributed to cancer death rates decreasing by 22% over the last two decades. However, cancer still remains the second-leading cause of death in the United States, resulting in more than half a million deaths and 1.6 million newly diagnosed patients yearly (1). Cancer is a heterogeneous disease, with each type linked to its own prognostic outcome. Each type can often be further subdivided into a subtype that has a specific phenotype with targets, like hormone receptors or growth factors, that are expressed and allow for specialized targeted therapy (2). However, the clinical experience in both leukemias and solid tumors of the emergence of resistance, as well as many studies describing discordance between the primary site and metastatic lesions, indicates that these biomarker expressions are not static, but dynamic (3–5). The dynamic phenotype of neoplastic cells poses a challenge for oncologists trying to optimize treatment. Ideally, each time the cancer progresses and a therapy change is considered, a fresh biopsy of tumor tissue would be obtained to be certain that it contains the target(s) against which the next round of therapy could be aimed. However, unlike in the leukemias, for which repeat bone marrow biopsies are frequently obtained when disease seems to change its behavior, in solid tumors repeat biopsies are clinically unfeasible and pose risks for patients. A possible solution to the dilemma is to utilize ever-improving technological breakthroughs to obtain small portions of solid tumors that are easily accessible via blood draw. Thus, interest in the concept of the fluid biopsy is growing. A further advantage of this approach, especially in a patient with multiple metastatic sites, is that the circulating component likely represents cells from multiple locations and may thus provide a more informative sample than a single biopsy of a single metastasis. After dissociation from a solid tumor mass, circulating tumor cells (CTCs) travel through the vasculature as single cells or aggregates and contribute to forming a distant metastasis. Although not every CTC represents a potential future metastasis, many distant metastases are considered to be established by hematogenous spread of these cells, rather than by lymphatic or direct intracavitary spread, which likely occurs by a different mechanism. These rare cells therefore provide a rich source of tissue material and may eventually be analyzed by pathologists alongside other cancer cells recovered from body fluids such as pleural and peritoneal fluid. This review discusses what is known regarding the nature of CTCs and the mechanisms of CTC circulation and focuses on the challenges, opportunities, and new insights. Established and novel methods for CTC identification and characterization are discussed, along with these methods’ implications for CTC research and potential translation to clinical practice.
CIRCULATING TUMOR CELLS: BACKGROUND
CTCs are surrogates of a tumor in the bloodstream. They were first described nearly 150 years ago by Thomas Ashworth (6), who noticed cells with an unusual morphology in the blood of a patient who had died from cancer. Ashworth considered these cells’ possible tumor origin because of morphologic features in common with the solid tumor tissue he had found elsewhere in the patient’s body (6). CTCs arise from the tumor, pass through various intervening structures either actively or passively, and reach the lumen of nearby vasculature. Only approximately 0.0000001% of all tumor cells will reach the bloodstream, according to estimations by Fischer (7). CTCs circulate alongside normal blood cells in the vasculature, where they account for such a tiny percentage of nucleated cells that they are virtually unrecognizable on routine peripheral blood smears and are extremely challenging to detect, even using sophisticated instrumentation. If CTCs are present in a peripheral blood draw, they account for only a fraction of 0.0001% of all nucleated cells (1–10 cells/mL) (8).
Sensitivity of CTC detection in patient blood is highly dependent on the method of detection that is used, as different methods likely detect different subpopulations of these cells. Because the true number of CTCs in patients is unknowable, approximations of sensitivity for each method are generally determined by spiking experiments using a known number of cells from a cancer cell line spiked into healthy donor blood. For the CELLSEARCH® system (Veridex, Warren, NJ), the only FDA-approved CTC detection system, sensitivity of ≥85% has been reported (9).With other methods, like microchips or a platform that considers all nucleated cells, such as the high-definition single-cell analysis (HD-SCA) platform (Epic Sciences, San Diego, CA), sensitivity can be as high as 99.9% (10, 11).
Regarding specificity, healthy people and the limited sets of people with benign disease thus far studied rarely have CTCs (0.3% of samples show ≥2 CTCs/7.5 mL blood sample). Like sensitivity, specificity varies with the method used. Specificity for CTC detection can be as high as 99.7%, as reported using the CELLSEARCH® system in 145 healthy women and 199 women with benign breast disease (9), or even up to 100% with a microchip platform (acquired from 20 healthy subjects, although none with benign disease were studied in this publication) (11).
CTC prevalence in patients who harbor malignant disease differs with carcinoma type and stage. In early breast cancer, CTCs are detected in 18–30% of patients compared with detection rates of ~70% in patients with metastatic disease (12). A study using CELLSEARCH® and blood samples of metastatic patients with colon, breast, rectal, gastric, ovarian, and prostate cancer resulted in 54% of total samples with detected CTCs, including 71% CTC-positive samples for breast cancer, 64% for colon cancer, 33% for gastric cancer, 66% for rectal cancer, 60% for ovarian cancer, and 20% for prostate cancer (13). Another study showed similar results for CTC occurrence in breast cancer, with a detection rate of ~70% in metastatic breast cancer patients in a large cohort (14).
The prognostic potential of CTCs has been discussed since the 1960s, when they were reported to be associated with decreased overall survival (OS) (15). However, other CTC studies between 1955 and 1962 resulted in varying incidences of CTCs in the peripheral blood, and this disparity led to an official statement by the World Health Organization (WHO) in 1963 that CTCs were not yet established as a detection or diagnostic method and needed further studies before clinicians should draw conclusions (16, 17). This and other interesting developments in CTC history over the last 150 years are summarized in Table 1.
Table 1.
Overview of some milestones in CTC research history
| Year | Milestone | Reference(s) |
|---|---|---|
| 1841 | Langenbeck performed a microscopic study of cancer patient blood. He reasoned that cancer cells are carried by blood. | 163 |
| 1869 | Ashworth observed cells in the blood that were similar in appearance to those in the primary tumor of a woman with metastatic breast cancer. | 6 |
| 1889 | Paget developed his seed-and-soil theory: Metastasis depends on the interaction between a CTC (seed) and an organ-specific microenvironment (soil). | 96 |
| 1955 | Engell used saponin to lyse red blood cells and produced white blood cell concentrates with CTCs. He found 50% positive samples for advanced carcinoma samples. | 164 |
| 1959 | Seal developed a floatation method with silicone oil (45% of samples contained CTCs). | 165 |
| 1960 | Alexander & Spriggs studied white blood cell concentrates by the dextran sedimentation technique. Slides were prepared and analyzed in bright field. CTCs were compared with other cell types in the blood. | 166 |
| 1962 | The first long-term follow-up study with 99 patients (with colon and breast cancer) found that overall survival correlated with the absence of CTCs. | 15 |
| 1963 | The WHO warned that CTCs are unreliable and that more studies would be needed before “drawing any conclusions” for clinical decision making. | 16 |
| 1975 | A review of CTCs by Salsbury summarized results of CTC research and the role of CTCs in metastasis. The review pointed out varying results of correlations of CTC occurrence with overall survival, drawing the conclusion that CTCs are not a death sentence. | 16 |
| 1987 | CTCs were isolated through buffy coat, and their presence was correlated with metastasis/micrometastasis or disseminated single tumor cells. The method used was Ficoll density gradient plus cytokeratin stain. | 167, 168 |
| 1993 | CTCs were detected using immunobead-PCR: Dynabeads® labeled with Ber-Ep4 (anti-EpCAM). | 169 |
| 1998 | The first trials of DAPI-positive, cytokeratin-positive, CD45-negative cell isolation with flow cytometry occurred. | 170 |
| 2000 | ISET® (isolation by the size of epithelial tumor cells) was developed. | 171 |
| 2001 | Terstappen combined EpCAM detection of CTCs with flow cytometry. | 152 |
| 2004 | The FAST system (a fiber-optic array scanning technology for direct CTC analysis) was developed. | 172 |
| 2004 | CELLSEARCH® was introduced (CTCs “represent a … ‘real-time’ biopsy”), with FDA approval obtained on January 21, 2004. | 173, p. 826 |
| 2007 | A microfluidic device to capture CTCs, the CTC-Chip, was introduced. | 11 |
| 2010 | HD-CTC (direct CTC analysis through digital microscopy scanning) was introduced. | 174 |
| 2014 | Yu et al. and Hodgkinson et al. developed CTC-derived explants to optimize studying the tumorigenicity of CTCs and individual drug sensitivity. | 137, 175 |
| 2015 | Vita-Assay® (functional capture of invasive CTCs by preferential binding of these cells to a mimicked tumor microenvironment) was introduced. | 176 |
Today, studies confirm the correlation of CTC presence with decreased OS or decreased progression-free survival (PFS) in many cancer types, including breast, colon, lung, ovarian, and prostate cancers (8, 18). The National Comprehensive Cancer Network even defined a new category of disease for CTC-positive breast cancer patients in its 2015 clinical practice guidelines on breast cancer (19).
CTCs have been demonstrated in various cancer types, and numerous studies show an association of CTC presence with poor prognosis for patients with melanoma (tumors derived from melanocytes) (20) and sarcoma (tumors of mesenchymal origin) (21).However, the best-developed applications and methodologies are those for carcinomas, and we focus on this area in this review.
The concept of a fluid biopsy is not limited to evaluation of circulating cells derived from tumor. Other components of tumor-produced material, or tumor-associated material, are often discussed under this broad umbrella. For example, a lot of exciting work is currently being done in evaluation of isolation of circulating tumor DNA (ctDNA) (22), exosomes, and platelets (23). Platelets accompany and interact with CTCs through direct signaling in the bloodstream (24). Exosomes are membrane-bound vesicles and are part of the signaling process for intercellular molecular communication. Tumor-derived exosomes carry DNA that reflects the genome and mutational status of the tumor and therefore have potential as biomarkers for cancer and metastasis detection or monitoring (25). Additionally, fragments of ctDNA may be released to circulate freely in the bloodstream by apoptotic or necrotic CTCs, primary tumor cells, or cells from a metastatic site (22). However, white blood cells (WBCs) also release DNA and therefore contribute to the bulk of DNA detected in the bloodstream, which makes the specific detection of ctDNA challenging. Ultrasensitive sequencing techniques allow mutation identification with 96% specificity in late-stage disease, but very low concentrations of ctDNA in the total circulating DNA fraction lowered detections in stage I non–small cell lung cancer (NSCLC) to 50% (26). Specificity is also a challenge, but ctDNA is nonetheless another biomarker that is obtainable from a blood sample, providing information about genomic aberrations affecting the efficiency of targeted drugs or revealing the effect of chemotherapeutics on the genome (22).
PHYSICAL PROPERTIES OF CIRCULATING TUMOR CELLS
Diameters of Circulating Tumor Cells
CTCs can derive from many different carcinoma types, and their size varies depending on the tissue of origin or possibly other factors such as changes in protein expression. Small cell lung cancer cells might reach a diameter of only 10 μm, whereas breast cancer CTCs might have a diameter of up to 70 μm, and prostate CTCs can occasionally be larger than 100 μm (27). The surrounding WBCs—erythrocytes (~8-μm diameter), granulocytes (~15-μm diameter), lymphocytes (6–18-μm diameter), and monocytes (12–20-μm diameter)—are generally smaller (28).
Deformability of Circulating Tumor Cells
Deformability (the ability to change shape under the application of stress) can be measured using a microfluidic optical stretcher, which is a two-beam laser trap that deforms single cells in suspension by optically induced surface forces. A mixture of cell line CTCs can be sorted according to whether they derive from benign (MCF-10), nonmotile/nonmetastatic (MCF-7), or highly metastatic (MDAMB-231) cells on the basis of varying optical deformability, with cells derived from highly metastatic tumors showing increased deformability (29). The difference in deformability results from various factors like extracellular matrix (ECM) signaling, chemotherapy, or DNA rearrangements that may cause a shift in protein expression. This shift transforms the structure of the cytoskeleton in cancer cells, causing their shape, motility, and deformability to differ as well (30). The nucleus of a cancer cell is often enlarged, which causes the cell to be more rigid than a WBC. In 2016 Park et al. (31) enriched CTCs from the blood of prostate cancer patients on the basis of their lower deformability relative to blood cells, with a 25-times-higher yield relative to enrichment by surface epithelial marker positivity using CELLSEARCH®. In cancer cell lines, in contrast, higher deformability has been linked to higher metastatic potential of the tumors from which the cell lines were derived (29, 32), and in studies with patient-derived CTCs, the comparison of benign epithelial cells with CTCs demonstrated higher deformability of CTCs (33). Therefore, the literature seems to conclude that CTCs are less deformable than WBCs but show increasing deformability as they increase in metastatic potential (34).
Polarity and Electrical Charge of Circulating Tumor Cells
As a result of changes in shape, nuclear size, and cytoplasm as well as protein expression changes during mobilization and/or activation of survival mechanisms in circulation, the ratio of suspensoid polarizable particles (e.g., proteins, nucleic acids, and peptides) to their solvent is different in CTCs than in WBCs and benign epithelial cells. This altered ratio becomes an important and very useful dielectric property (35). One method to exploit electrical properties is dielectrophoresis (DEP), in which a nonuniform electrical field separates cells by either pulling them away or moving them toward the electromagnetic field, depending on the cells’ own electrical charge (35). Gascoyne & Shim (36) demonstrated a lack of overlap in dielectric properties of cancer cells from cell lines versus blood cells so that DEP can be used for CTC enrichment. The isolated cells were transferred to cell culture medium and demonstrated viability. Proof of concept in multiple cancers was shown in a study with patient samples from NSCLC, breast cancer, and prostate cancer; dielectric properties were successfully used to enrich CTCs (37).
Density of Circulating Tumor Cells
Other normal constituents of whole blood provide a convenient internal control against which to measure various properties of CTCs. A predominant cell type in whole blood is red blood cells (RBCs). Their cytoplasm contains hemoglobin, a protein bound to iron atoms, which causes RBCs to have higher density (weight per volume) than WBCs or CTCs (38). In 2012 Phillips et al. (39) compared densities (cellular dry mass density) of CTCs, RBCs, and leukocytes in an ovarian cancer patient. They reported not only 3.5–4.5-times lower densities of leukocytes and CTCs compared with RBC density but also a density overlap within the first two types of cells. Thus, CTCs would reside in the buffy coat in the clinical lab.
BIOLOGICAL PROPERTIES OF CIRCULATING TUMOR CELLS
Epithelial Adhesion Markers and Cytokeratins
In carcinoma patients, the most commonly used cell surface markers for the enrichment of CTCs from a blood sample is the epithelium-specific cell adhesion molecule (CAM) EpCAM, which contributes to cohesion within an intact epithelial structure. Cytokeratin (CK) is then often used to further confirm the epithelial nature of enriched or captured cells.
Tumor-Specific Markers
CTCs generally retain the protein expression profile of the tissue from which they are derived. This proteomic profile not only is epithelium specific but also, unless the tumor is extensively dedifferentiated, contains an organ-specific proteomic signature. In tissue biopsies this characteristic is exploited by using stains such as differential CKs (e.g., CK7, CK20) or organ system–specific stains like prostate-specific antigen (PSA), thyroid transcription factor 1 (TTF-1), and the homeobox protein CDX-2. Clinically actionable markers, such as receptor proteins that are used in tissue biopsies to guide therapy, can be found in CTCs as well. These markers and their expression levels are used to validate detection methods for CTCs, and their potential to add prognostic information over and above current standard tumor markers from serum or tissue is being investigated. Studies are currently evaluating the comparability of marker expression in CTCs with the primary tumor or metastatic lesion (40). For example, the expression of receptors such as HER2 in metastatic breast cancer (41) and EGFR in colorectal cancer patients (42) can exhibit disparities between CTCs and the primary tumor. Such disparities may reflect an evolution of disease or may represent the true heterogeneity of a tumor that underwent only limited sampling. Today’s knowledge about proteomic profiles of CTCs is insufficient to guide therapeutic decisions, and thus only a few early trials are testing therapy alteration based on marker levels in CTCs, such as HER2 expression in metastatic breast cancer CTCs (43–45).
MORPHOLOGIC HETEROGENEITY OF CIRCULATING TUMOR CELLS
Because different methodologies capture varying subsets of CTCs, it is difficult to fully characterize the breadth of the morphologic heterogeneity of these cells. Techniques capable of detecting the widest spectrum of possible CTCs have the most potential to capture more of this morphologic heterogeneity (46, 47). With the HD-SCA platform, for example, all nucleated cells from a blood sample are preserved for analysis. “No cell gets left behind,” and from each candidate cell, images are generated from three or four fluorescent channels, each devoted to a separate protein.
Heterogeneity of Circulating Tumor Cells and Circulating Tumor Cell Candidates Within Single Samples and Within Single Cancer Types
CTCs are not uniform, even within a single sample or within a single cancer type. Under investigation are not only traditional CK-positive, CD45-negative cells with a strong DAPI signal (HD-CTCs), but also other, less-understood cells that are captured by detection systems and that morphologically or immunophenotypically differ from cells of the hematopoietic lineage (see Figure 1). Conventional HD-CTCs show a wide variety of shapes and sizes, with a distinct cytoplasmic CK signal surrounding a nucleus that is larger than the surrounding WBC nuclei. Other categories of cells captured and annotated with the HD-SCA platform, and described as well by other investigators, are CTCs with low or no CK signal (CTC-LowCK), CTCs with a nucleus that does not differ in size from WBC nuclei (CTC-Small), and CTCs that show apoptotic characteristics such as a disrupted nucleus and/or CK stain and that are theorized to be a source of tumor DNA [CTC–cell free DNA (cfDNA) producing] (48). CTC-LowCKs show an enlarged, irregularly shaped nucleus. The loss of CK signal in these cells may be due to a transition to a mesenchymal phenotype, which would imply that these cells, generally not identified with EpCAM-based methods, may be more invasive and thus even more important for the prediction of disease progression than their epithelium-like counterparts (49). Preliminary data in breast cancer have also shown that these cells indeed frequently have an aberrant copy number variation (CNV) profile, and further investigations using single-cell next-generation sequencing (NGS) will give more insight into this cell category. Alternative possibilities include stripped cells with absent cytoplasm resulting from sample preparation effects. Such cells may also represent an entirely different cell type, for example, immature megakaryocytes. The next category of cells that are of potential interest are those that appear apoptotic (labeled in Figure 1 as CTC-cfDNA producing). Tracking of these cells potentially allows for evaluation of the extent of dying CTCs in the blood, with potential treatment response implications. Another category of small but CK-positive and CD45-negative cells is confounding to many groups, particularly because in tissue biopsies of cancer, tumor cells are virtually always larger than any surrounding benign cells.
Figure 1.
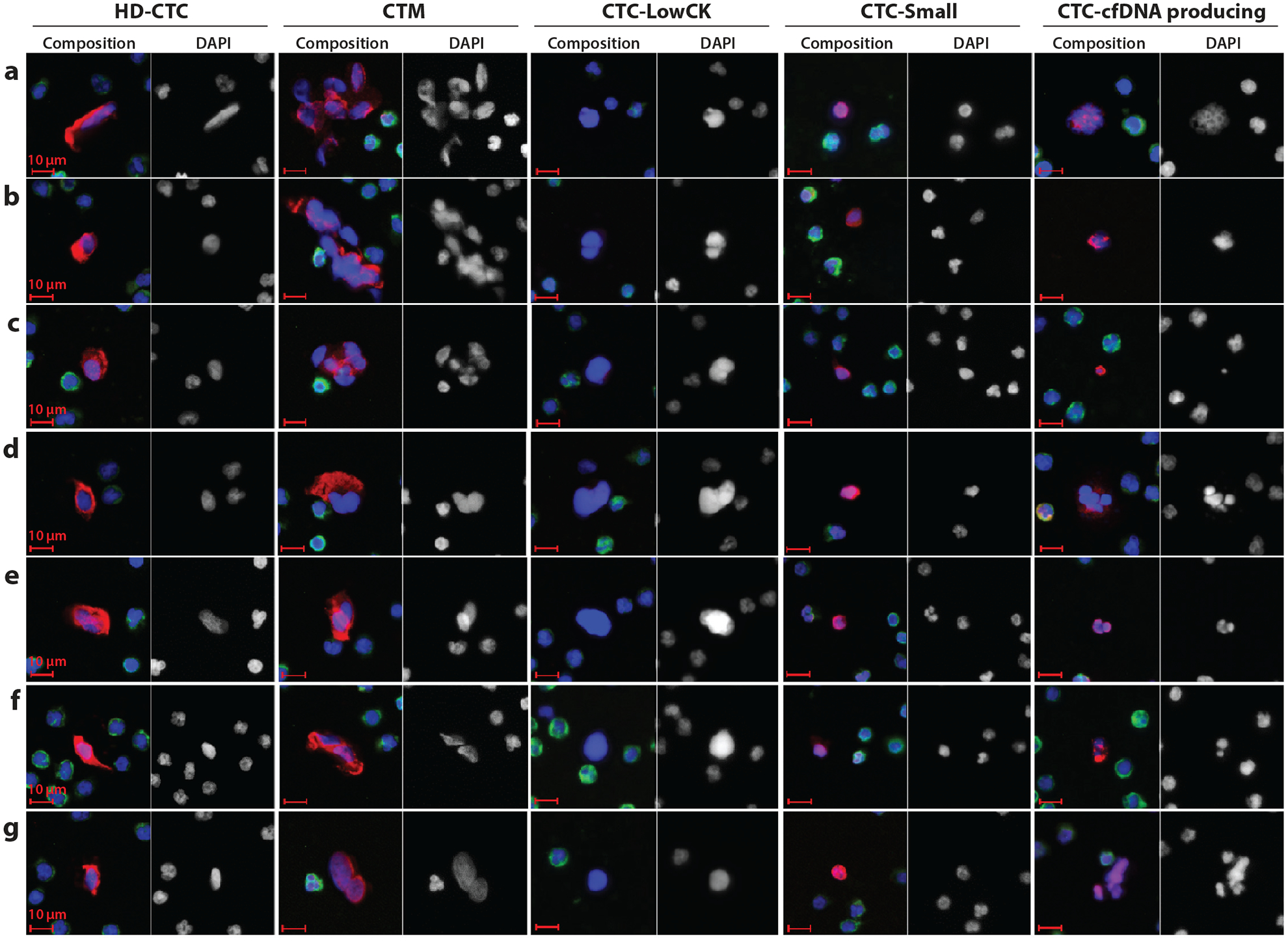
Candidate cells of circulating tumor cell (CTC) detection by the high-definition single-cell analysis (HD-SCA) approach of Kuhn and colleagues (J.-A. Thiele, P. Pitule, P. Ostašov, V. Liška, M. Králíčková, et al., unpublished data). The images represent the pleomorphic population of candidate cells found in the blood (before surgery) of metastatic colorectal cancer patients; these cells are stained with DAPI (blue), CK-pan mix (red), and CD45 (green). The composite image and the DAPI image are displayed for each cell type. Cell type abbreviations (from left to right): CTCs detected by the HD-SCA platform (HD-CTCs), CK-positive and CD45-negative cells with a nucleus distinct from WBC nuclei; circulating tumor microemboli (CTM), HD-CTC clusters (at least two or more HD-CTCs); CTC-LowCK, cells with a nuclear shape different from that of WBCs but CK negative and CD45 negative; CTC-Small, CK-positive and CD45-negative cells with a small (WBC-like) nucleus; CTC-cfDNA producing, CTCs undergoing apoptosis with irregular nuclear or cytoplasmic condensation and a possible source of circulating tumor DNA.
Heterogeneity of Circulating Tumor Cells in Cancer Types
Finally, comparison of intertumor morphologic features of CTCs may be studied as well. Park et al. (50) observed, in prostate cancer patients, small CTCs only ~8 μm in diameter (similar in size to leukocytes) but with an elongated shape (50). These findings concur with those from another study that demonstrated that CTCs of prostate and colorectal cancer are smaller in size than breast cancer cells (51). Figure 2 shows some differences in CTC morphology in four cancer types. Thorough and extensive comparisons of the morphologic features of CTCs from various tumor types, with attention to the grade(s) and specific histologic features of the tumors from which they have arisen, are lacking, but such comparisons may emerge as techniques for isolation and visualization of these rare cells become more available.
Figure 2.
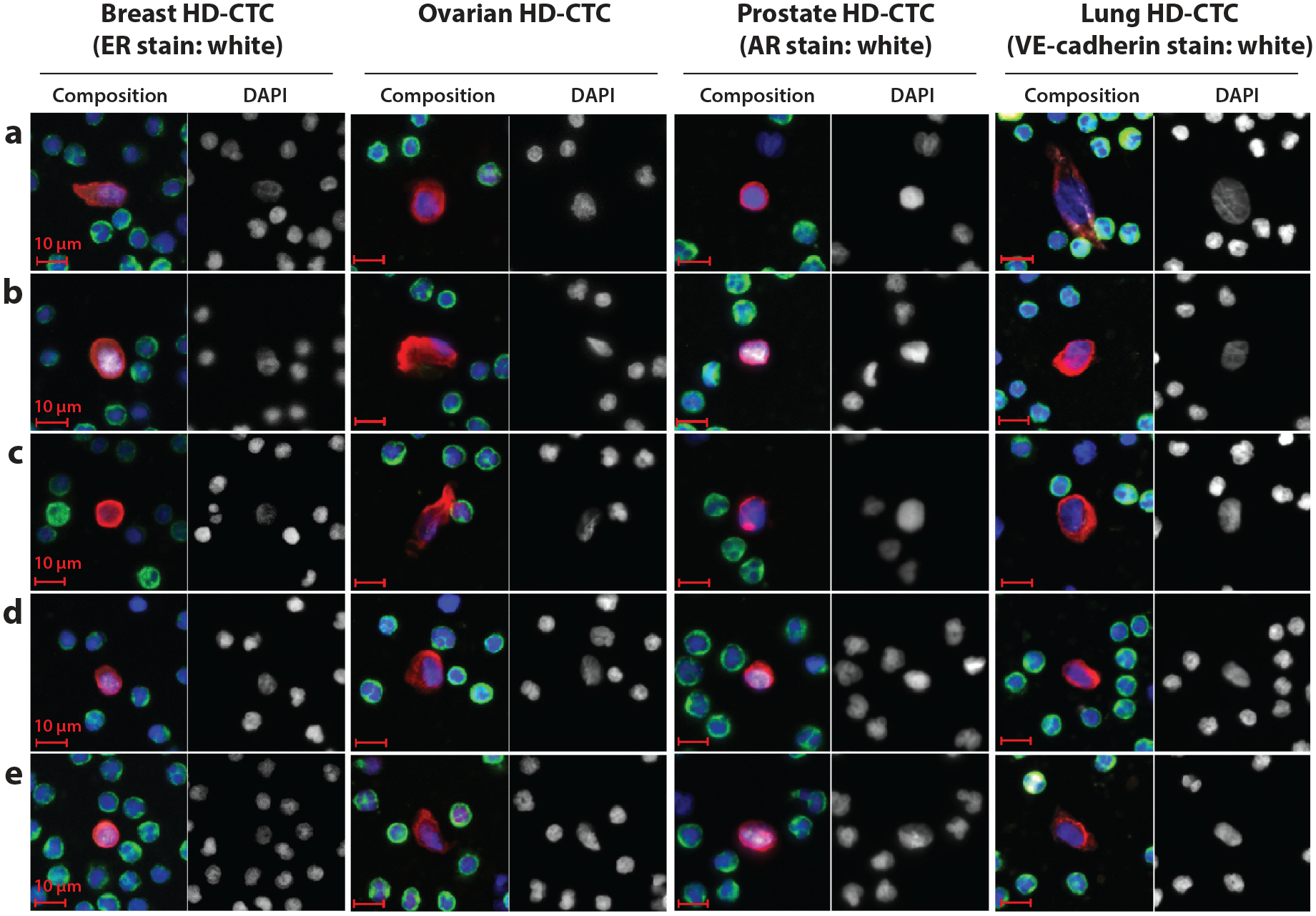
Circulating tumor cells (CTCs) detected by the HD-SCA platform (HD)-CTCs in four different cancer types (C. Ruiz Velasco, L. Welter, N.A. Carlsson, P. Malihi, P. Kuhn, et al., unpublished data). Although this figure does not thoroughly classify CTC morphology differences by cancer type, these images demonstrate some subtle but recognizable differences. In breast HD-CTCs, the endoplasmic reticulum shows a very distinct pattern (b), and these CTCs are more round in shape than the HD-CTCs of other cancer types. For ovarian HD-CTCs, variation in shape is high. Prostate HD-CTCs are round in shape (similar to breast HD-CTC shape) and are often not much bigger than white blood cells (results are concordant with Reference 50). Lung HD-CTCs show more elliptical shapes and varying sizes compared with the other three HD-CTC types shown here.
KINETICS OF CIRCULATING TUMOR CELLS
There are many theories about the origination of CTCs. What mechanisms are in play as these anchorage-dependent tumor cells leave the tumor to face harsh hemodynamic stress, immune response, a CTC-hostile microenvironment, and probable death (52)? How do they get into the bloodstream, how do they get out, and how long do they circulate? Experimental evidence has been collected over the years, and although mysteries remain, some progress can be reported (Figure 3).
Figure 3.
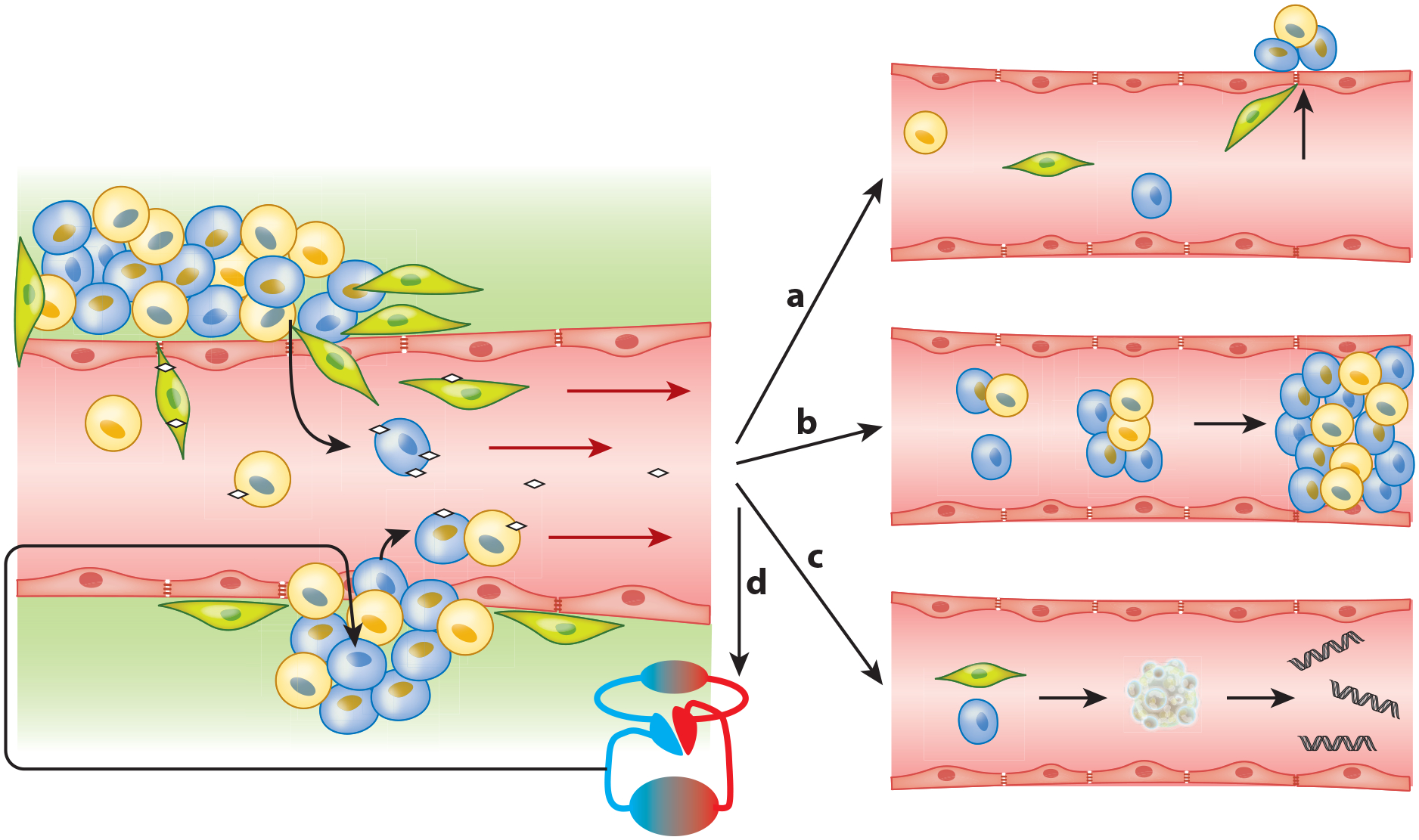
Proposed circulating tumor cell (CTC) dissemination into the vasculature and subsequent prospects. (Left) Epithelial heterogeneous tumors shedding tumor cells into the bloodstream. Such shedding can be passive (bottom) as parts of the tumor (fragile vessels) break off, thereby shedding epithelial CTCs (blue and yellow) into the blood, sometimes even as circulating tumor microemboli (CTM). Alternatively, shedding may be active (top) as cells undergo epithelial-to-mesenchymal transition (EMT) (green, EMT-CTCs), which may happen partially or completely and is often induced by platelet interaction (little white diamonds) (24). As these EMT-CTCs actively penetrate into the blood vessel, some epithelial cells may passively follow (73), driven by the bloodstream-induced low pressure. Once within the vasculature, a CTC has multiple fate options. (Right) (a) Cells that underwent partial or full EMT may reverse this process and start mesenchymal-to-epithelial transition as part of metastasis formation (73). However only 0.01% of CTCs are likely to form a metastasis at a distant site (92). (b) CTM and CTCs in the vasculature may get stuck in blood vessels and are suspected to have a role in causing venous thromboembolism (83). Additionally, CTM may initiate metastatic growth after lodging in a distal capillary (74, 87). (c) CTCs (epithelial or mesenchymal) may undergo apoptosis, which releases circulating tumor–derived DNA into the bloodstream. (d) After survival in the vasculature, CTCs may self-seed. This process is often observed and is supported by the CTC-friendly microenvironment of the primary site (95, 97).
Passive Shedding of Circulating Tumor Cells
Many research groups have investigated the existence of passive tumor cell shedding into the bloodstream (53, 54). Even with complex experimental setups, distinguishing between passive shedding and active intravasation is difficult. Therefore, the theory of passive shedding as a frequent mechanism producing CTCs is supported only by circumstantial evidence. Pathologists routinely observe microscopic invasion of small blood vessels on histologic slides. Such invasion appears to occur via tongues or clusters of contiguous tumor cells growing directly into blood vessels. This commonly observed phenomenon suffers both from being a rather gross observation in terms of individual cell behavior and from being a moment frozen in time at the point of tissue fixation, and abstracting more detailed mechanistic information is difficult. Abundant mosaic blood vessels (endothelial and tumor cells constituting the luminal surface) in various tumors indicate an erosion type mechanism. A study of colorectal carcinoma showed that 13.4% of the 367 vessels analyzed were mosaic (55).
Active Migration into Blood Vessels
Possible more active mechanisms of vascular intravasation include macrophage interactions with tumor cells; such interactions may induce tumor cell movement along collagen fibers toward blood vessels. Tumor cells and macrophages collaborate in a paracrine loop in which the cancer cells express the epidermal growth factor receptor (EGFR) and secrete colony-stimulating factor 1 (CSF-1), which attracts macrophages, whereas macrophages secrete epidermal growth factor (EGF), which binds to the corresponding receptor of the cancer cell. The collagen fibers along which the macrophages and the attracted tumor cells are moving can be envisioned as a spider web, with the center of this web being the blood vessel. Growth factor and nutrient gradients are directing the tumor cells toward the vasculature (56). This movement of tumor cells along collagen fibers is often described as occurring through a crawler mechanism (57).
The invasive movement of epithelial cancer cells away from their collective and through stromal tissue and vascular walls is an area of extensive research. One of the main theories regarding the mechanisms for such invasion is epithelial-to-mesenchymal transition (EMT). EMT is frequently theorized to help explain active intravasation mechanisms of tumor cells into the vasculature. In this process, epithelial tumor cells lose their characteristic cell-cell contacts and apical-basal polarity to gain elongated morphology with increased mobility and invasive properties (58). Activation of this process, which leads to a more invasive cell type, is thought to be induced by the microenvironment of the tumor (59). EMT-triggering signals include transforming growth factor β (TGF-β) secretion by platelets (24), integrin-macrophage interactions through EGF supply (as mentioned above) (56), secretion of proinflammatory cytokines by fibroblasts (60), and hypoxia (the tumor outgrowing its blood supply) (61), in addition to others (62, 63).
As a result of the EMT-triggering signals, transcription factors like snail family transcriptional repressor 1 (SNAIL), snail family transcriptional repressor 2 (SLUG), and forkhead transcription factor 2 (FOXC2) are activated (58). A series of signaling networks are induced and cause, for example, the loss of the CAM E-cadherin. E-cadherin downregulation triggers the so-called cadherin switch and enhances expression of N-cadherin, a promoter of motility and invasion (64). CK expression is also reduced, whereas expression of vimentin and many more key regulators of EMT—like the helix-loop-helix protein Twist, E-box-binding repressors Zeb1 and Zeb2, β-catenin, Rac1, Akt2, and the cytoskeletal regulator integrin—increases (65–67). All these factors finally cause a redesign of the cytoskeleton, mainly by a switch from a CK-rich filament network to one composed mainly of vimentin (68). Adherent junctions are destabilized, polarity is lost, and filo- and lamellipodia for migration form. The cell finally reaches the mesenchymal, mobile, and protected phenotype, which allows for invasion through stroma and possibly intravasation and survival in the bloodstream (66, 68). All these factors are being investigated as markers for determining the cell state and invasive character of isolated CTCs.
Diagnostic pathologists recognize tumor types that have characteristics of EMT. Certain tumors are known to have lost some of their cohesive epithelial nature. This loss can be immunohistochemically demonstrated; one example is the loss of E-cadherin staining in lobular breast cancer and certain diffusely infiltrating gastric cancers. Within an individual tumor, however, histologic identification of specific cells or areas that may be undergoing EMT at the invading front of a tumor is challenging from a pathologic standpoint and has not historically been pursued. These cells usually do not display a typical spindle-shape mesenchymal phenotype and for that reason are rarely observed in the clinical histopathologic environment (69). However, in 2014 Ueno et al. (70) developed an approach to visibly detect and evaluate EMT potential in colorectal cancer histologically. They developed the HistologyEMT system by using poorly differentiated clusters, desmoplastic reaction, and stroma maturity to analyze EMT at the leading edge of the primary tumor and showed that this method has significantly higher prognostic value than the current standard of TNM staging for disease-free survival (70). Recent papers evaluated the leading invasive edge of various cancers by immunostain panels and describe an immunohistochemically recognizable diminution in the staining intensity of some surface epithelial markers with increased vimentin or other mesenchymal marker positivity in cells at the leading invasive edge relative to those within the central tumor mass. In fact, the plasticity of cells at the leading invasive edge has been proposed to be an inherent quality that may be a better indicator of invasive and metastatic potential than overall tumor differentiation (71, 72).
EMT may also be secondarily involved in the passive shedding of tumor cells. Tsuji et al. (73) found evidence for a synergy between cells undergoing EMT and cancer cells that do not (non-EMT cells). The cells undergoing EMT degraded the surrounding matrix, enabling invasion and intravasation of non-EMT cells.
Even though EMT has been theorized as a leading mechanism of metastasis, this theory has also been debated (73) and recently even challenged. Aceto et al. (74) revealed that EMT is not necessarily responsible for the mobility of tumor cells. In addition, recent mouse model experiments in which EMT regulators were downregulated showed that EMT was dispensable for metastasis but contributed immensely to chemotherapy resistance (75, 76).
Survival of Circulating Tumor Cells in the Vasculature
After intravasation, CTCs are confronted with multiple stress factors. The sheer force generated by the bloodstream is quite different from the forces exerted on cells in their tissue of origin. In addition, epithelial cells that lose their cell-cell and cell-matrix interactions usually undergo anoikis (77). CTCs may also undergo anoikis and release cfDNA (22). Studies of kinetics suggest a short survival time in the bloodstream. A mouse model experiment with renal cell carcinoma cell lines demonstrated total cell numbers shed (as directly measured from the renal vein of the kidney) to be as high as 6 million cells/(day·g), with approximately 89% of these cells being nonviable immediately after being shed and a predominant subset of the nonviable cells being apoptotic (78). The viable cells usually have a short lifetime. Meng et al. (79) measured, in breast cancer patients, a CTC half-life of 2.4 h, with a maximum survival of 24 h after primary tumor resection. Rossi et al. (80) recently detected and distinguished between spontaneous and drug-induced apoptosis of CTCs in breast, renal, and colorectal cancer patients. Using M30, a biomarker for apoptosis in epithelial cells, Rossi et al. detected a fraction of 50–80% M30-positive CTCs undergoing spontaneous apoptosis.
Circulating Tumor Cell Aggregates and Their Possible Contribution to Venous Thromboembolism
CTCs are not found just as single cells within the vasculature. They can often be detected as aggregates, so-called circulating tumor microemboli (CTM), which are composed of two or more cancer cells (81). Studies on mouse models suggest that, rather than CTCs forming clusters after intravasation, the aggregates of cells enter the vasculature as groups after dissociating from a solid tumor mass (74). This process has also been associated with overexpression of vascular epidermal growth factor A (VEGF-A) by tumor cells; VEGF-A may induce the release of CTM into the vasculature (82).CTM are theorized to play an important role in both tumor metastasis and venous thromboembolism (VTE), which are the two main causes of mortality in cancer patients (83). Cho et al. (81) detected CTM in 54% of breast cancer patients, in 52.5% of NSCLC patients, and in 73% of prostate cancer patients (all stage IV patients). Frequently, both CTM and CTCs were physically associated with other cell types such as fibroblasts (84), leukocytes (85), endothelial cells (86), and platelets (24). Experimental evidence has supported the view that CTM may be particularly ominous; as early as the 1970s, CTM were shown to have a higher metastatic potential in mouse model experiments comparing tumor growth from injection of single cells with tumor growth from injection of clusters (87), an observation recently confirmed by Hou et al. (88).
During circulation through blood vessels, CTCs and most notably CTM may contribute to VTE (83), but the mechanisms are poorly understood. The expression of the coagulation cascade member tissue factor (TF) by CTCs may play a role. TF is a trigger of coagulation and can be regulated by oncogenes, tumor suppressors, or growth factors in cancers (89). In 2014 Phillips et al. (90) demonstrated, through a dynamic simulation of CTCs and CTM under flow within the vasculature, that CTM may cause a regional elevation of thrombin levels that is enabled by reduced flow speed caused by vessel junctures. CTCs and CTM have thus been suggested as mobile sources of thrombin, but their detailed role in VTE remains ill defined (91). Given cell counts of up to 50 within one CTM, another theory for VTE could be based on a pure size issue, with the cell mass clogging a small blood vessel, as displayed in Figure 3b (74).
Establishment of Metastasis
The last step of metastasis is the actual occupation of distant areas of preexisting normal tissue, and this development requires exit from the bloodstream and the establishment of a stable proliferative state in a new location. This process is highly complex and inefficient. In animal models only 0.01% of all tumor cells entering the bloodstream were able to extravasate into tissue and form a metastasis (92). Other potential outcomes are anoikis, destruction by immune cells, and transition to a dormant nonproliferating state (93). Whether extravasation can take place is dependent on the environment that CTCs find during their travel and on the inherent metastatic potential of the CTCs. If a metastable CTC arrives by arrest in a small capillary with subsequent ischemic capillary wall destruction, by extravasation through the capillary endothelium, or by extravasation across the wall of a larger vessel, it may encounter a favorable microenvironment known as the metastatic niche. Some of the factors defining the metastatic niche are stromal cells and ECM proteins that support survival and self-renewal (52). Duda and colleagues (94) demonstrated in mouse models that CTCs may even bring their own “soil” from the primary site. They observed CTCs that were embedded, during circulation, in stromal components, like fibroblasts, macrophages, and endothelial cells, which provided the CTCs with survival and growth advantages. Alternatively, favorable environments may already be present within an existing tumor or metastatic site that a CTC might encounter (95). Therefore, Paget’s seed-and-soil theory (96) cannot be seen as an unidirectional process, but rather more as an encounter between a circulating cell and a suitable environment (97).
Finally, because well-established metastases almost always strongly resemble the primaries from which they arose, EMT as a mechanism of circulatory spread must be an evanescent state and requires a reversal step to explain the fully expressed epithelial features recognized by pathologists in established metastases. The cells somehow have to achieve a recognizably epithelial state again. The theory that CTCs undergo a reverse process, the mesenchymal-to-epithelial transition (MET), has been proposed (58). In 2016 this potential reverse transition was further examined, and adenosine 3′,5′-monophosphate (cAMP) and its main effector, protein kinase A (PKA), were proposed to play key roles (98). MET involves activation of extracellular proteases that dissolve membrane proteins, as well as reactivation of CAMs, integrins, E-cadherin, and β-catenin (99), as portrayed in Figure 3b.
OTHER EMERGING CONCEPTS
Spreaders and Sponges
The journey of a CTC may not be a one-way trip from a primary tumor to metastatic niche (100, 101).Newton and colleagues (102) developed a mathematical (Markov chain) model, attempting to shed light on the complex circulation pathways of CTCs. They described a pattern of metastasis best explained by three pathways: self-seeding of the primary tumor, reseeding of the primary tumor from a metastatic site, and self-seeding of metastatic tumors. Using a large autopsy data set from lung cancer patients, they were able to classify metastatic sites into two distinct categories. If a metastatic site appeared to absorb more metastatic potential than it gave rise to, then it was classified as a sponge. If the site was observed to spread more to other sites, then it was classified as a spreader. It was determined from the model that an important metastatic site, and the most important sponge, was the group of regional lymph nodes. Although the model is still being developed, it is able to illustrate how cancer progression is a multidirectional process (102). In the future, these types of models could potentially be utilized for prediction of metastatic sites that will inform clinicians when and how to adjust treatment.
Dormancy
How can patients go for many years with no evidence of disease and yet relapse eventually with the same cancer? Moreover, patients can have evidence of CTCs even years after surgery without disease recurrence (79). These observations suggest that there is a condition of CTCs in which extravasation, or growth of extravasated tumor cells into metastases, is temporarily stopped. This state is known as dormancy and is proposed as a mechanism often utilized by cells to adapt to new microenvironments (103). Dormancy is defined as a nondividing phenotype. Ki-67, a nuclear protein for cell proliferation, is absent or downregulated in CTCs that are considered dormant (104). Studies have shown that, even up to 22 years after mastectomy, dormant CTCs can be found in patient blood (79).The time from dormancy to metastatic progression has been referred to as the latent niche (105). Whether dormancy (Ki-67-negative CTCs) could be related to future relapse has not been fully established. However, authors of a study of breast cancer patients undergoing adjuvant chemotherapy observed that Ki-67-negative CTCs may be resistant to therapy (106). A better understanding of this process and identification of dormant cells would enable the targeting of these cells, which may represent a type of minimal residual disease.
TECHNIQUES: METHODS FOR CAPTURE AND CHARACTERIZATION OF CIRCULATING TUMOR CELLS
Today’s CTC technologies are pushing past mere identification and enumeration and are attempting to better understand the molecular features of these rare cells in a two-stage process. The first stage is a detection or capture stage, often utilizing some type of enrichment component that increases CTC concentration and/or depletes surrounding normal blood cells. In the second stage, either a CTC-enriched population of cells or a pure population of individually retrieved CTCs is further characterized by various molecular techniques.
To date, detection methods have been developed to target specific biological and physiological properties of CTCs. Initial specificity, efficiency, and purity measures are usually obtained by spiking cancer cell line cells into healthy donor blood followed by further validation with clinical specimens. Multiple methods are available and have been discussed at length elsewhere (107–109).Here, we provide a brief overview of different approaches for CTC detection (see Figure 4), including established methods as well as novel techniques that are under development.
Figure 4.
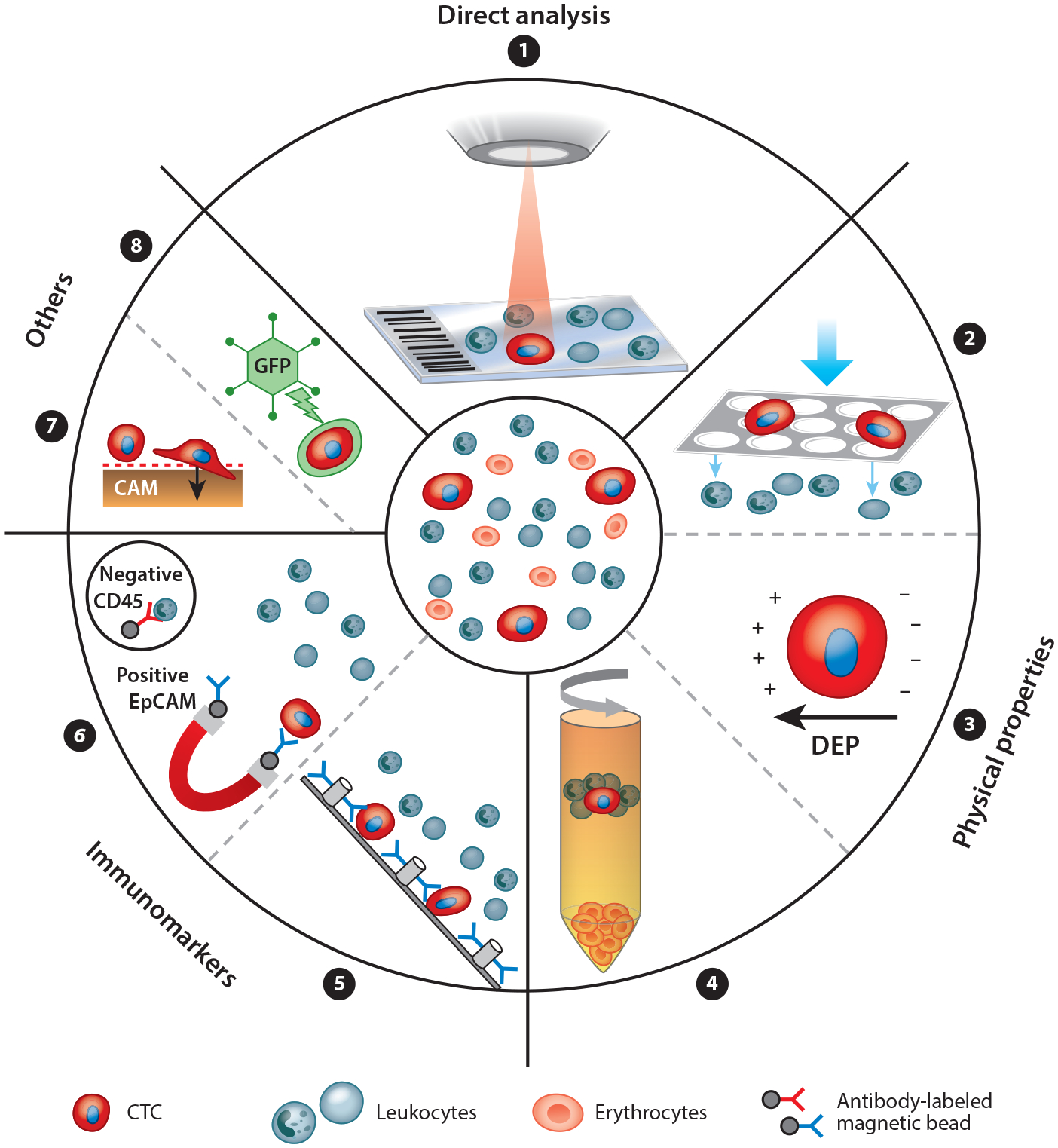
The principles of circulating tumor cell (CTC) enrichment. CTCs can be detected in many ways. A nonenrichment approach involves the direct analysis, by microscopy, of all peripheral blood mononuclear cells of a patient sample (➊). The enrichment approaches are divided into label dependent (addressing protein expression) and non–label dependent (enrichment by physical properties of CTCs) approaches. Positive or negative enrichment by physical properties (➋➌➍) is possible, for example, using filtration by size (e.g., ISET®) (➋), using dielectrophoretic properties (DEPs) (➌), or using centrifugation through a Ficoll density gradient (➍). Enrichment through immunomarker positivity (e.g., for epithelial markers like EpCAM or mesenchymal markers like N-cadherin) is a common and well developed method (➎➏). Label-dependent techniques have been optimized by many microdevices like the CTC-Chip by using antibody-labeled microposts (➎). Alternatively, negative enrichment may be performed by depletion of leukocytes using antibodies against CD45 (➏). Some other approaches on the market use specific CTC functions like protein secretion (EPISPOT) or invasive behavior on a cell adhesion matrix (CAM) (➐). DNA targets can be detected within CTCs through aptamer technology or through oncolytic green fluorescent protein (GFP)–containing, telomerase-specific adenoviruses (➑).
Positive Detection Strategies
CTC enrichment can be performed by utilizing EpCAM as a positive detection strategy. All EpCAM-based technologies focus on this cell adhesion protein expressed on the surfaces of epithelium-derived CTCs. The detection step is based on antibody-labeled beads, columns, microposts, or other devices to isolate CTCs.
A commonly recognized method for CTC detection is CELLSEARCH®. An automated system for enumeration of CTCs, it uses immunomagnetic capturing of cells that express EpCAM on the cell surface; in this system, a CTC is defined as a nucleated DAPI-positive, CK-positive, and CD45-negative cell (110). CELLSEARCH® is the only FDA-approved CTC detection method. Enumeration of CTCs has been proven to be a prognostic marker for metastatic breast, colorectal, and prostate cancers (8). CELLSEARCH® has opened the door for CTCs as potentially valuable clinical biomarkers. However, the mere enumeration of CTCs has proven insufficiently informative to engender widespread clinical adoption.
Other approaches include microfluidic platforms like the CTC-Chip. CTCs interact with EpCAM-coated microposts under laminar flow conditions (11). The process involves a one-step setup and is very gentle with regard to cell handling. Over time, the CTC-Chip developed into the Herringbone Chip after undergoing multiple improvements (111). The addition of a microvortex within the device has optimized antibody affinity. Moreover, changing the shape from sharp grooves to wavy corners has enhanced the EpCAM-coated surface. These adjustments have boosted the purity of CTCs (meaning an absence of nonspecifically captured WBCs) from 25.7% to 39% while retaining an efficiency of 85% cell recovery (112). These microfluidic chips are a cheap and fast method for CTC capture, but as long as they depend on EpCAM detection, they face the same limitations as CELLSEARCH®.
Following the development of coated microposts, nanostructured substrates were also developed. Silicon nanopillars coated with EpCAM antibodies are combined with micromixing abilities. This technique achieves approximately 95% efficiency but processes only 1 mL/h of blood. This system can detect CTCs in 20 of 26 patients compared with detection in 8 of 26 patients with CELLSEARCH® (113).
Another innovation is the MagSweeper® (Illumina, San Diego, CA) (114), in which blood samples are diluted and prelabeled with EpCAM-coated magnetic particles that can subsequently be captured by a magnetic rod sweeping through the sample. The magnetic rod then switches to a washing well, and in a buffer solution an external magnetic field causes the release of labeled cells, which remain viable and unaffected and are therefore transferable to cell culture (115).
The CellCollector® (GILUPI GmbH, Potsdam, Germany) is an inventive device that captures CTCs on the basis of EpCAM surface expression in vivo. Antibodies are coated on a gold-plated nanoguidewire that is inserted into the patient’s cubital vein for 30 min through a venous cannula (116). Thereafter, adherent CTCs can be immunocytochemically stained and evaluated. Treatment response in lung cancer may be associated with decreasing CTC counts, and mutational analysis of captured CTCs was possible (117). This method overcomes blood volume limitations but is a rather unpleasant and more invasive procedure for the patient than a blood draw.
All the methods mentioned above are limited by their reliance on enrichment for EpCAM-positive cells, and thus only the EpCAM-positive subpopulation of CTCs are detected through these methods. CTCs that have undergone EMT may downregulate their epithelial markers like EpCAM and E-cadherin, and these cells would thus be invisible to these methodologies (118).
A somewhat broader capturing approach utilizing immunoaffinity is offered by AdnaGen (QI-AGEN, Hilden, Germany).With prelabeled magnetic beads and its combination-of-combination principle, the AdnaTest® offers a variety of capturing antibodies (EpCAM, HER2,MUC1).However, even if the limitation might not be as stringent as EpCAM-only-based techniques, it is still a constraint on a defined subpopulation of CTCs that is being captured. An additional drawback of the AdnaTest® is the lysis of captured CTCs, which allows for bulk PCR analysis of cancer-specific biomarkers but negates the possibility of analyzing single cells by methods such as single-cell NGS.
Detection Based on Differential Physical Properties
Alternative methods include density gradient centrifugation like OncoQuick® (centrifugation only; Greiner Bio-One GmbH, Frickenhausen, Germany) (119), filtration systems isolating CTCs by size [the most famous example is ISET® (isolation by size of epithelial tumor cells, RARECELLS US Inc., Austin, TX)] (120), and microdevices using size and deformability like the ClearCell® FX (Clearbridge BioMedics, Singapore), which shows approximately 80% efficiency (121). Alternatively, CTCs can be enriched by electrical charge, wherein electrodes create spherical dielectrophoretic cages to detect rare cells and sort them by morphologic parameters.
A label-freemethod using deformability in combination with microfluidic ratchets was recently used to capture CTCs with a 25-times-greater yield than that of CELLSEARCH®. In addition, the captured cells are viable and obtainable for downstream analysis (31).
Another platform consists of a spiral microchannel using Dean drag forces to separate larger cells (CTCs) by strong inertial lift forces. Dean flow fractionation is able to recover 85% of CTCs in spiked blood samples and isolates CTCs in a completely antibody-independent manner at a rate of 3 mL/h. However, only a low purity of ~10% could be achieved. Validation with clinical samples of metastatic lung cancer patients showed a 100% detection rate (122).
Negative Detection Strategies: Leukocyte Depletion
The biantibody leukocyte depletion kit EasySep® (STEMCELL Technologies, Vancouver, Canada) allows CTC enrichment through leukocyte depletion by using CD45-labeled magnetic beads (123). Leukocyte depletion methods have been reported to result in lower purities than purities obtained by using positive CTC selection (109) and are therefore often combined with other enrichment techniques. However, after depletion methods of enrichment, CTCs are viable and can be used for further experiments (124).
Combined Methods
The approaches described above often do not alone obtain the desired purity or specificity; therefore, a combination of methods can be utilized for detection of CTCs. Negative selection by CD45 sorting can, for example, be combined with a functional-type assay (107). Such a technology, EPISPOT, detects protein secretion of viable CTCs through labeled antibodies on the bottom of the culture dish (125).Through this method, researchers were able to classify metastatic breast cancer patients into high- and low-risk groups. In combination with the CELLSEARCH® method, reported data were a strong predictor for OS (126). Another combinational platform is maintrac (Simfo GmbH, Bayreuth, Germany), which consists of RBC lysis, centrifugation, and detection through EpCAM-labeled antibodies (127). Another example is CanPatrol® (SurExam Biotech Ltd., Guangzhou, China), which involves CTC enrichment by filtration followed by RNA in situ hybridization. For example, by using EMT-related markers like Twist and vimentin, recovery rates of 80% were reported (128).
A combination strategy consisting of microfiltration and a telomerase-selective adenovirus takes advantage of the enhanced telomerase activity of tumor cells. Experiments were executed without filtration, but the viruses were not specific for cancer cells, and therefore filtration by size was included as an enrichment step. With this strategy, sensitivity was between 75% and 93%, but false-positive cells were detected as well (129). This method needs further investigation before any statement about clinical value can be made.
Another method that is still in its infancy is aptamer-based CTC detection. Aptamers are small nucleotide sequences that can be designed against any molecular target and can bind to it with high specificity and affinity. Zamay at al. (130) were able to select and identify DNA aptamers for lung cancer cells from surgical excision tissue. These aptamers were then used to identify CTCs from patient blood plated onto slides after RBC lysis. Of 52 primary lung cancer patients, 44 were positive for CTCs, and of that subset, 15 of those had less than 2 cells/3 mL.
ScreenCell® (ScreenCell SA, Paris, France) is a newer filtration device with choices of pore sizes (8 or 6.5 μm) and buffers for either fixation or cell culture. It is possible to filter CTCs and directly culture them for further experiments. Recovery of cell line samples has been demonstrated at approximately 90%, with more than 85% of the sample being viable cells (131), suggesting that ScreenCell® may be a useful combination method by which to obtain living CTC cultures or xenograft models.
A big step toward automation was achieved by the fully automated DEPArray™ (Silicon Biosystems, San Diego, CA) instrument, which detects, quantifies, and recovers CTCs for downstream analysis from CTC-enriched blood samples. This method needs a CTC enrichment step like CELLSEARCH® before further analysis, but subsequently everything is automated, with minimal hands-on requirements. The enriched cell solution is pipetted into a chip and is loaded to the DEPArray™ instrument. Within the chip are various microelectrodes, which produce electric cages to trap (up to 40,000) single cells. Once the cells are trapped in individual cages, they can be identified and selected on the basis of fluorescent markers. Single cells can be moved within the chip by electrical forces and recovered in a tube for further genomic analysis (132, 133). Fernandez et al. (132) reported CTC subclones in metastatic breast cancer patients; some patients showed genomic concordance with the primary tumor, and some did not. These highly automated techniques could therefore be a useful tool to monitor disease progression and tumor heterogeneity.
Nonenrichment Strategies for Detection: Direct Analysis
The HD-SCA assay developed by Kuhn and colleagues (10) is under commercialization by Epic Sciences (San Diego, CA). This technique uses amore holistic approach to CTC detection. Peripheral blood is drawn, and within 48 h, RBC lysis is performed, and peripheral blood mononuclear cells (PBMCs) are plated on customized glass slides and stained for HD-CTC identification with antibodies against a CK-pan mix, CD45, and a nuclei counterstain with DAPI. For image analysis, an automated system is used, and custom-made software generates a report that presents the candidate cells to a hematopathologist-trained technical analyst for analysis and interpretation. This approach not only allows nucleated CK-positive, CD45-negative cells to be reported in CTC analysis, but also records all cells of interest as shown in Figure 1. No subpopulation is selected for, as in other methods described, which use surface markers or other limited properties for detection. All cells of the PBMC fraction of each patient are recorded and imaged, and no image is discarded. In a study of 28 small cell lung cancer patients, Kuhn and colleagues (134) detected numbers as low as 0.3 CTCs/mL at study enrollment. This number increased to an average of 13.4 CTCs/mL, with higher CTC counts in follow-up samples. This platform recently underwent major enhancement by integrating NGS at the single-cell level. Any CTC detected in a patient sample can be picked from its slide and can undergo CNV analysis or mutation screening to uncover the clonal relationships within and between CTC populations and the primary tumor. A drawback of this direct-analysis platform is the fixation of all cells such that no viable CTCs can be obtained for further studies.
DOWNSTREAM CHARACTERIZATION OF CIRCULATING TUMOR CELLS
Establishment of Circulating Tumor Cell Cultures and Xenograft Models
One highly attractive downstream option for further investigation of CTCs after they are identified is the culturing of viable CTCs. Due to the scarcity of CTCs, this approach cannot be implemented for all patient samples. For example, in cases of prostate cancer, at least 100 cells are required for successful culture of CTCs (135). Once a CTC cell line is established, it can either be used for direct testing of drug sensitivity (47, 136) or be implanted into immunosuppressed mice to create xenograft models and thus permit further drug testing and genetic profiling studies (137). However, either option limits the ability to study fundamental heterogeneity, as only the most robust cells are likely to survive the process of ex vivo engraftment. Moreover, the cells will have been removed from their host environment and immune system, which may alter their nature, precluding meaningful conclusions regarding disease evolution or treatment resistance (138).
Molecular Characterization of Circulating Tumor Cells
The recent prevailing aim is characterization of the DNA content of single CTCs. Over the past decade, NGS methods have immensely increased our knowledge about cancer genotypes and their derived CTCs(139, 140).Performing any single-cell method at the DNA level first requires whole-genome amplification (WGA) to increase the minute amount of 6.6 pg total DNA/cell before further analysis (42). This process increases copy numbers of the entire genome but increases the risk of bias and thus false findings (141). Therefore, studies have to include a comparison of CTCs to normal cells or in some other way demonstrate fidelity during amplification. After WGA the genome can be analyzed in multiple ways: mutation analysis of therapeutic target genes (42, 142), massive parallel sequencing for the detection of new druggable mutations (143), or CNV detection through NGS(144).Mutations in KRAS, for example, can blockEGFR-targeted therapy efficiency in colorectal cancer. Studies revealed high intrapatient and interpatient heterogeneity of KRAS mutations (143). One theory is that CTCs carrying the mutation may escape therapy and then cause relapse or disease progression. The ability to detect and identify these cells early could lead to adjustments in precision medicine (22).
RNA-based CTC profiling also offers promise. Gene expression studies at the RNA level may reveal important information about tumor heterogeneity. Popular techniques are fluorescence in situ hybridization (FISH) (145), reverse transcription PCR followed by other PCR techniques (like quantitative real-time PCR) (42), and microarray mRNA sequencing (115).Using quantitative real-time PCR, Steinestel et al. (146) investigated the two major mutations—the AR-V7 and AR point mutations—in the androgen receptor gene (AR) in advanced prostate cancer CTCs in the context of probable drug resistance. Patients underwent a molecularly uninformed therapy switch with a response rate of 38%. Steinestel and colleagues then discovered in retrospect that 71.4% of the responders had had CTCs that showed a matching molecular profile for the therapy switch. The overall calculated benefits of known molecular profiles of CTC for the success of a therapy switch were estimated to be ~27%.
Finally, CTCs can be interrogated at the protein level. The most common approach by which to attain expression data from CTCs is protein detection through direct antibody-target binding. This method has become standard and is integrated within many techniques like CELLSEARCH® (41). These techniques usually evaluate the hormone status of patients and compare protein expression levels of the tissue sample with CTC expression rates. Examples of proteins of interest are HER2 in breast cancer (41, 147) and PSMA in prostate cancer (148). Studies are still ongoing to determine whether patients can benefit from a therapy switch based on CTC marker expression. Techniques using antibody-target binding include immunohistochemistry approaches using either labeled fluorophores or magnetic particles with subsequent microscopy (10, 149) and enzyme-linked immunosorbent assay (150). Improved high-throughput methods include protein microarrays (which have a device surface covered with capture antibodies) and mass spectrometry, both of which offer high sensitivity and specificity (151). Additionally, nanotechnology can be used to detect protein expression in CTCs through labeled nanoparticles or nanowires (116).
CLINICAL IMPLICATIONS
Using these new technologies to fully interrogate CTCs could lead to many possible clinical applications. Studies demonstrating the predictive value of CTCs for OS or PFS in cancer patients have been copious over the last decade (79, 110, 150, 152). However, CTCs carry much greater promise than has yet been realized, and within the last three years the focus in CTC research has shifted to studies focusing on the transfer of research results to clinical practice (138).
Therapeutic Target Discovery
CTCs may allow for the detection of known therapeutic targets in situations in which tissue is limited. An excellent example is lung cancer. In NSCLC the diagnostic test for crizotinib treatment effectiveness in rearranged anaplastic lymphoma kinase (ALK) patients is currently performed on tumor biopsy samples. To determine whether CTCs could provide the same diagnostic information—which could be especially useful when biopsy tissue is limited, as it so often is for fine-needle lung biopsies—Pailler et al. (153) designed a platform for CTCs, using a filter-adapted FISH method in combination with the FDA-approved companion diagnostic FISH probe. They used a NSCLC cohort of 32 patients and compared results from CTCs with those from tumor biopsies. This study produced concordant results of CTCs with biopsy examinations for ALK rearrangements. All ALK-positive patients had CTCs with ALK rearrangements, and there was only one ALK rearrangement in CTCs of the ALK-negative patients. Interestingly, ALK-rearranged CTCs were positive for EMT markers like vimentin and N-cadherin but had no CK; the authors suggest that these cells thus showed a more mesenchymal phenotype, which implies a higher metastatic potential. The group later showed the system to be effective with c-ros oncogene 1 as well (154).
Disease and Treatment Monitoring
CTCs may provide markers for treatment sensitivity. In 62 prostate cancer patients, men with AR-V7-positive CTCs had shorter PFS and OS than did AR-V7-negative patients, which shows that AR-V7-positive CTCs predict resistance to enzalutamide and abiraterone (155). One year after this study, the group investigated the same marker in correlation with taxane treatment and observed that taxane is more effective in men with AR-V7-positive CTCs and shows an efficiency comparable to that of enzalutamide and abiraterone in AR-V7-negative patients (156).
CTCs may predict treatment response. For example, Wallwiener et al. (157) demonstrated that in metastatic breast cancer a continuously high CTC level after one cycle of chemotherapy predicts shorter OS and PFS and that CTCs therefore may be useful in adjusting systemic therapies. The SWOG S0500 trial confirmed CTC counts to be a predictive marker for PFS and OS but failed to show improved survival after a therapy switch based on CTC counts (158). The Wallwiener group stated that these results could also indicate that there is a need for more effective treatment for this subpopulation of patients (157).
CTCs may allow us to recognize heterogeneity and better investigate therapy resistance mechanisms and to even identify novel therapeutic targets. NGS has revealed that tumor heterogeneity is dynamic, with biomarkers changing during disease progression (4). CTCs may provide an even more precise representation of the mutation spectrum of the primary tumor. This theory is supported by findings of Heitzer et al. (143), who investigated concordance between the primary tumor, the metastasis, and CTCs for KRAS. Driver mutations like those in the genes encoding KRAS, APC, and PIK3CA were found mostly in metastases and the corresponding CTCs, but some mutations appeared only in CTCs. This theory could potentially explain some cases of treatment resistance seen in, for example, hormone receptor–positive patients who show no benefit from endocrine treatment. Changes in estrogen receptor and/or progesterone receptor expression between solid tumor sites and CTCs may explain the therapy failure (3). CTCs that derive from an overall marker-positive site may in fact be heterogeneous, and the important subpopulations that survive treatment and cause disease progression or recurrence may for this reason be found among the CTCs.
Prediction of Risk of Relapse
CTCs may be prognostic of relapse. A large multicenter study was conducted with 2,026 early breast cancer patients by using the CELLSEARCH® system. The worst prognosis was shown for patients with ≥ 5 CTCs/30mL of blood, and the detection of CTCs before and after chemotherapy was linked to an increased risk of relapse (12). In addition to this breast cancer study, studies of prostate cancer (159) and bladder cancer (160) have shown a predictive correlation of CTC counts with metastatic relapse.
Technologies incorporating downstream analysis and characterization of CTCs after detection can be applied to determine the metastatic potential of CTCs by characterizing mutations, CNVs, or protein expression changes in one or more of the factors involved in the metastatic cascade. Baccelli and colleagues (124) characterized the metastatic potential of CTC subpopulations. The presence of a group of EpCAM-positive, CD44-positive, CD47-positive, and MET-positive CTCs was correlated with patient survival and metastasis. This identification of metastasis-initiating CTCs and the markers used could play a key role in prognosis of relapse or metastasis. Given that CTCs can be detected before metastasis, their analysis may allow for more precise staging of early-stage patients and thus function as a prognostic tool for therapy decision making—one that can easily, reproducibly, and repeatedly be obtained through a fluid biopsy from cancer patients (161).
Treatment Vehicles
Finally, at the very cutting edge, CTCs have been proposed as treatment vehicles in a potential Trojan horse role. Targeting CTCs with genetically modified platelets that express TRAIL (tumor necrosis factor–related apoptosis-inducing ligand) could induce apoptosis in tumor cells (162). Due to platelet interactions in the bloodstream, CTCs would be the first target of these modified platelets, as successfully shown in vitro and in mouse models. This strategy could be a next step toward slowing down the metastatic cascade (162).
CTC:
circulating tumor cell
HD-SCA:
high-definition single-cell analysis
OS:
overall survival
PFS:
progression-free survival
ctDNA:
circulating tumor DNA
NSCLC:
non–small cell lung cancer
NGS:
next-generation sequencing
CTM:
circulating tumormicroemboli
VTE:
venous thromboembolism
ACKNOWLEDGMENTS
This work was supported by National Sustainability Program I LO1503 provided by the Ministry of Education, Youth, and Sports of the Czech Republic. We thank Michaela Brychtová, MUDr., PhD, for preparing Figure 3.
Footnotes
DISCLOSURE STATEMENT
P.K. is a shareholder of and consultant to Epic Sciences.
LITERATURE CITED
- 1.Siegel RL, Miller KD, Jemal A. 2015. Cancer statistics, 2015. CA Cancer J. Clin 65(1):5–29 [DOI] [PubMed] [Google Scholar]
- 2.Burrell RA, McGranahan N, Bartek J, Swanton C. 2013. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501(7467):338–45 [DOI] [PubMed] [Google Scholar]
- 3.Babayan A, Hannemann J, Spötter J, Müller V, Pantel K, Joosse SA. 2013. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLOS ONE 8(9):e75038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Guan Y, Sun Y, Ai D, Guo Q. 2016. Tumor heterogeneity and circulating tumor cells. Cancer Lett. 374(2):216–23 [DOI] [PubMed] [Google Scholar]
- 5.de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, et al. 2014. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346(6206):251–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashworth TR. 1869. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med. J. Aust 14:146–47 [Google Scholar]
- 7.Fischer AH. 2009. Circulating tumor cells: Seeing is believing. Arch. Pathol. Lab. Med 133(9):1367–69 [DOI] [PubMed] [Google Scholar]
- 8.Miller MC, Doyle GV, Terstappen LWMM. 2010. Significance of circulating tumor cells detected by the Cell Search system in patients with metastatic breast colorectal and prostate cancer. J. Oncol 2010:617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, et al. 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res 10(20):6897–904 [DOI] [PubMed] [Google Scholar]
- 10.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, et al. 2012. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys. Biol 9(1):016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, et al. 2007. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450(7173):1235–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, et al. 2014. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst 106(5):dju066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, et al. 2005. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytom. B Clin. Cytom 68(1):25–30 [DOI] [PubMed] [Google Scholar]
- 14.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, et al. 2007. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res 13(3):920–28 [DOI] [PubMed] [Google Scholar]
- 15.Drye JC, Rumage WT, Anderson D. 1962. Prognostic import of circulating cancer cells after curative surgery: a long time follow up study. Ann. Surg 155:733–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salsbury AJ. 1975. The significance of the circulating cancer cell. Cancer Treat. Rev 2(1):55–72 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organ. (WHO). 1963. Cancer control: first report of an expert committee Tech. Rep 251, WHO [Google Scholar]
- 18.Bao H, Burke PA, Huang J, Chen X, Brohawn PZ, et al. 2013. Circulating tumor cells: application as a biomarker for molecular characterization and predictor of survival in an all-comer solid tumor Phase I clinical study. PLOS ONE 8(8):e58557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, et al. 2015. Breast Cancer Version 2.2015: clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw 13(4):448–75 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz C, Li J, Luttgen MS, Kolatkar A, Kendall JT, et al. 2015. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys. Biol 12(1):016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Asatrian G, Dry SM, James AW. 2015. Circulating tumor cells in sarcomas: a brief review. Med. Oncol 32(1):430. [DOI] [PubMed] [Google Scholar]
- 22.Alix-Panabières C, Pantel K. 2016. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6:479–91 [DOI] [PubMed] [Google Scholar]
- 23.Pantel K, Alix-Panabières C. 2016. Liquid biopsy: potential and challenges. Mol. Oncol 10(3):371–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD. 2015. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the Association for Molecular Pathology. J. Mol. Diagn 17(3):209–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman AM, Bratman SV, To J, Wynne JF, Eclov NCW, et al. 2014. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med 20(5):548–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harouaka RA, Nisic M, Zheng S-Y. 2013. Circulating tumor cell enrichment based on physical properties. J. Lab. Autom 18(6):455–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro HM, Schildkraut ER, Curbelo R, Laird CW, Turner B, Hirschfeld T. 1976. Combined blood cell counting and classification with fluorochrome stains and flow instrumentation. J. Histochem. Cytochem 24(1):396–401 [DOI] [PubMed] [Google Scholar]
- 29.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, et al. 2005. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J 88(5):3689–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suresh S 2007. Biomechanics and biophysics of cancer cells. Acta Biomater. 3(4):413–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park ES, Jin C, Guo Q, Ang RR, Duffy SP, et al. 2016. Continuous flow deformability-based separation of circulating tumor cells using microfluidic ratchets. Small 12(14):1909–19 [DOI] [PubMed] [Google Scholar]
- 32.Coughlin MF, Bielenberg DR, Lenormand G, Marinkovic M, Waghorne CG, et al. 2013. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin. Exp. Metastasis 30(3):237–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross SE, Jin Y-S, Rao J, Gimzewski JK. 2007. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol 2(12):780–83 [DOI] [PubMed] [Google Scholar]
- 34.Shaw Bagnall J, Byun S, Begum S, Miyamoto DT, Hecht VC, et al. 2015. Deformability of tumor cells versus blood cells. Sci. Rep 5:18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pethig R 2010. Dielectrophoresis: status of the theory, technology, and applications. Biomicrofluidics 4(2):022811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gascoyne P, Shim S. 2014. Isolation of circulating tumor cells by dielectrophoresis. Cancers 6(1):545–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis D, Gupta V, Garza M, Pace M, Wu W, et al. 2011. EpCAM-independent ApoStream™ technology isolates circulating tumor cells from blood of patients with various types of cancer. Mol. Cancer Ther 10(Suppl. 1):B20 (Abstr.) [Google Scholar]
- 38.Ponder E 1942. The relation between red blood cell density and corpuscular hemoglobin concentration. J. Biol. Chem 144:333–38 [Google Scholar]
- 39.Phillips KG, Velasco CR, Li J, Kolatkar A, Luttgen M, et al. 2012. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front. Oncol 2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadal R, Lorente JA, Rosell R, Serrano MJ. 2013. Relevance ofmolecular characterization of circulating tumor cells in breast cancer in the era of targeted therapies. Expert Rev. Mol. Diagn 13(3):295–307 [DOI] [PubMed] [Google Scholar]
- 41.Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, et al. 2010. Her2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat 124(2):403–12 [DOI] [PubMed] [Google Scholar]
- 42.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, et al. 2013. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem 59(1):252–60 [DOI] [PubMed] [Google Scholar]
- 43.Schramm A, Friedl TWP, Schochter F, Scholz C, de Gregorio N, et al. 2016. Therapeutic intervention based on circulating tumor cell phenotype in metastatic breast cancer: concept of the DETECT study program. Arch. Gynecol. Obstet 293(2):271–81 [DOI] [PubMed] [Google Scholar]
- 44.Gazzaniga P, Raimondi C, Gradilone A, Cortesi E, Naso G. 2014. Clinical utility of circulating tumor cell counting through CellSearch® : the dilemma of a concept suspended in limbo. OncoTargets Ther. 7:619–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda T, Hayashi N, Iguchi T, Ito S, Eguchi H, Mimori K. 2016. Clinical and biological significance of circulating tumor cells in cancer. Mol. Oncol 10(3):408–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrinucci D, Bethel K, Bruce RH, Curry DN, Hsieh B, et al. 2007. Case study of the morphologic variation of circulating tumor cells. Hum. Pathol 38(3):514–19 [DOI] [PubMed] [Google Scholar]
- 47.Kolostova K, Spicka J, Matkowski R, Bobek V. 2015. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am. J. Transl. Res 7(7):1203–13 [PMC free article] [PubMed] [Google Scholar]
- 48.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, et al. 2001. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61(4):1659–65 [PubMed] [Google Scholar]
- 49.Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH,Dive C. 2014. Molecular analysis of circulating tum our cells—biology and biomarkers. Nat. Rev. Clin. Oncol 11(3):129–44 [DOI] [PubMed] [Google Scholar]
- 50.Park S, Ang RR, Duffy SP, Bazov J, Chi KN, et al. 2014. Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. PLOS ONE 9(1):e85264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ligthart ST, Coumans FAW, Bidard F-C, Simkens LHJ, Punt CJA, et al. 2013. Circulating tumor cells count and morphological features in breast, colorectal and prostate cancer. PLOS ONE 8(6):e67148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantel K, Speicher MR. 2016. The biology of circulating tumor cells. Oncogene 35(10):1216–24 [DOI] [PubMed] [Google Scholar]
- 53.Cavallaro U, Christofori G. 2001. Cell adhesion in tumor invasion and metastasis: Loss of the glue is not enough. Biochim. Biophys. Acta 1552(1):39–45 [DOI] [PubMed] [Google Scholar]
- 54.Liotta LA, Kleinerman J, Saidel GM. 1974. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 34(5):997–1004 [PubMed] [Google Scholar]
- 55.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. 2000. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. PNAS 97(26):14608–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Condeelis J, Pollard JW. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–66 [DOI] [PubMed] [Google Scholar]
- 57.Bockhorn M, Jain RK,Munn LL. 2007. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 8(5):444–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalluri R, Weinberg RA. 2009. The basics of epithelial-mesenchymal transition. J. Clin. Investig 119(6):1420–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietilä M, Ivaska J,Mani SA. 2016. Whom to blame for metastasis, the epithelial-mesenchymal transition or the tumor microenvironment? Cancer Lett. 380(1):359–68 [DOI] [PubMed] [Google Scholar]
- 60.Laberge R-M, Awad P, Campisi J, Desprez P-Y. 2012. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 5(1):39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z-J, Semenza GL, Zhang H-F. 2015. Hypoxia-inducible factor 1 and breast cancer metastasis. J. Zhejiang Univ. Sci. B 16(1):32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bacigalupo ML, Manzi M, Espelt MV, Gentilini LD, Compagno D, et al. 2015. Galectin-1 triggers epithelial-mesenchymal transition in human hepatocellular carcinoma cells. J. Cell. Physiol 230(6):1298–309 [DOI] [PubMed] [Google Scholar]
- 63.Mamuya FA, Duncan MK. 2012. αV integrins and TGF-β-induced EMT: a circle of regulation. J. Cell. Mol. Med 16(3):445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. 2004. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci 1014:155–63 [DOI] [PubMed] [Google Scholar]
- 65.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. 2013. Cancer invasion and metastasis: molecular and cellular perspective In Metastatic Cancer: Clinical and Biological Perspectives, ed. Jandial R. Austin: Landes Biosci. [Google Scholar]
- 66.Chang JT, Mani SA. 2013. Sheep, wolf, or werewolf: cancer stem cells and the epithelial-to-mesenchymal transition. Cancer Lett. 341(1):16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibue T, Brooks MW, Weinberg RA. 2013. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24(4):481–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sleeman JP, Thiery JP. 2011. SnapShot: the epithelial-mesenchymal transition. Cell 145(1):162.e1. [DOI] [PubMed] [Google Scholar]
- 69.Clark AG, Vignjevic DM. 2015. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol 36:13–22 [DOI] [PubMed] [Google Scholar]
- 70.Ueno H, Shinto E, Kajiwara Y, Fukazawa S, Shimazaki H, et al. 2014. Prognostic impact of histological categorisation of epithelial-mesenchymal transition in colorectal cancer. Br. J. Cancer 111(11):2082–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, et al. 2015. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol 30(Suppl. 1):78–84 [DOI] [PubMed] [Google Scholar]
- 72.Stewart CJR, McCluggage WG. 2013. Epithelial-mesenchymal transition in carcinomas of the female genital tract. Histopathology 62(1):31–43 [DOI] [PubMed] [Google Scholar]
- 73.Tsuji T, Ibaragi S, Hu G-F. 2009. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 69(18):7135–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, et al. 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer KR, Durrans A, Lee S, Sheng J, Li F, et al. 2015. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527(7579):472–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, et al. 2015. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527(7579):525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frisch SM, Francis H. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol 124(4):619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bockhorn M, Roberge S, Sousa C, Jain RK, Munn LL. 2004. Differential gene expression in metastasizing cells shed from kidney tumors. Cancer Res. 64(7):2469–73 [DOI] [PubMed] [Google Scholar]
- 79.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, et al. 2004. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res 10:8152–62 [DOI] [PubMed] [Google Scholar]
- 80.Rossi E, Basso U, Celadin R, Zilio F, Pucciarelli S, et al. 2010. M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin. Cancer Res 16(21):5233–43 [DOI] [PubMed] [Google Scholar]
- 81.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, et al. 2012. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys. Biol 9(1):016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, et al. 2009. Circulating tumour tissue fragments in patientswith pulmonary metastasis of clear cell renal cell carcinoma. J. Pathol 219(3):287–93 [DOI] [PubMed] [Google Scholar]
- 83.Khorana AA, Francis CW, Culakova E,Kuderer NM, Lyman GH. 2007. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost 5(3):632–34 [DOI] [PubMed] [Google Scholar]
- 84.Shiao SL, Chu GC-Y, Chung LWK. 2016. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 380(1):340–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, Qiao X, Shi H, Han X, Liu W, et al. 2016. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumour Biol. 37(4):5397–404 [DOI] [PubMed] [Google Scholar]
- 86.Yadav A, Kumar B, Yu J-G, Old M, Teknos TN, Kumar P. 2015. Tumor-associated endothelial cells promote tumor metastasis by chaperoning circulating tumor cells and protecting them from anoikis. PLOS ONE 10(10):e0141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liotta LA, Saidel MG, Kleinerman J. 1976. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 36(3):889–94 [PubMed] [Google Scholar]
- 88.Hou J-M, Krebs MG, Lancashire L, Sloane R, Backen A, et al. 2012. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol 30(5):525–32 [DOI] [PubMed] [Google Scholar]
- 89.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, et al. 2005. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105(4):1734–41 [DOI] [PubMed] [Google Scholar]
- 90.Phillips KG, Lee AM, Tormoen GW, Rigg RA, Kolatkar A, et al. 2015. The thrombotic potential of circulating tumor microemboli: computational modeling of circulating tumor cell–induced coagulation. Am. J. Physiol. Cell Physiol 308(3):C229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitrugno A, Tormoen GW, Kuhn P, McCarty OJT. 2016. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 30(1):11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhe X, Cher ML, Bonfil RD. 2011. Circulating tumor cells: finding the needle in the haystack. Am. J. Cancer Res 1(6):740–51 [PMC free article] [PubMed] [Google Scholar]
- 93.Xu L, Shamash J, Lu Y-J. 2015. Circulating tumor cells: a window to understand cancer metastasis, monitor and fight against cancers. J. Cancer Res. Updat 4(1):13–29 [Google Scholar]
- 94.Duda DG, Duyverman AMMJ, Kohno M, Snuderl M, Steller EJA, et al. 2010. Malignant cells facilitate lung metastasis by bringing their own soil. PNAS 107(50):21677–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norton L, Massagué J. 2006. Is cancer a disease of self-seeding? Nat. Med 12(8):875–78 [DOI] [PubMed] [Google Scholar]
- 96.Paget S 1989. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 8(2):98–101 [PubMed] [Google Scholar]
- 97.Comen E, Norton L, Massagué J. 2011. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol 8(6):369–77 [DOI] [PubMed] [Google Scholar]
- 98.Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, et al. 2016. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351(6277):aad3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao D, Dai C, Peng S. 2011. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res 9(12):1608–20 [DOI] [PubMed] [Google Scholar]
- 100.Massagué J, Obenauf AC. 2016. Metastatic colonization by circulating tumour cells. Nature 529(7586):298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, et al. 2009. Tumor self-seeding by circulating cancer cells. Cell 139(7):1315–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newton PK, Mason J, Bethel K, Bazhenova L, Nieva J, et al. 2013. Spreaders and sponges define metastasis in lung cancer: a Markov chain Monte Carlo mathematical model. Cancer Res. 73(9):2760–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science 331(6024):1559–64 [DOI] [PubMed] [Google Scholar]
- 104.Spiliotaki M, Mavroudis D, Kapranou K, Markomanolaki H, Kallergi G, et al. 2014. Evaluation of proliferation and apoptosis markers in circulating tumor cells of women with early breast cancer who are candidates for tumor dormancy. Breast Cancer Res. 16(6):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJA. 2009. A “latent niche” mechanism for tumor initiation. PNAS 106(28):11617–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeh AC, Ramaswamy S. 2015. Mechanisms of cancer cell dormancy—another hallmark of cancer? Cancer Res. 75(23):5014–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joosse SA, Gorges TM, Pantel K. 2015. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med 7(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alix-Panabières C, Pantel K. 2014. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14(9):623–31 [DOI] [PubMed] [Google Scholar]
- 109.Harouaka R, Kang Z, Zheng S-Y, Cao L. 2014. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol. Ther 141(2):209–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, et al. 2005. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol 23(7):1420–30 [DOI] [PubMed] [Google Scholar]
- 111.Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, et al. 2010. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. PNAS 107(43):18392–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S, Thomas A, Lee E, Yang S, Cheng X, Liu Y. 2016. Highly efficient and selective isolation of rare tumor cells using a microfluidic chip with wavy-herringbone micro-patterned surfaces. Analyst 141:2228–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang S, Liu K, Liu J, Yu ZT-F, Xu X, et al. 2011. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed 50(13):3084–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh K-H, et al. 2009. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. PNAS 106(10):3970–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cann GM, Gulzar ZG, Cooper S, Li R, Luo S, et al. 2012. mRNA-Seqof single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLOS ONE 7(11):e49144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saucedo-Zeni N, Mewes S,Niestroj R,Gasiorowski L, Murawa D, et al. 2012. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol 41(4):1241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorges TM, Penkalla N, Schalk T, Joosse SA, Riethdorf S, et al. 2016. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin. Cancer Res 22(9):2197–206 [DOI] [PubMed] [Google Scholar]
- 118.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, et al. 2012. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baker MK, Mikhitarian K, Osta W, Callahan K, Hoda R, et al. 2003. Molecular detection of breast cancer cells in the peripheral blood of advanced-stage breast cancer patients using multimarker real-time reverse transcription-polymerase chain reaction and a novel porous barrier density gradient centrifugation technology. Clin. Cancer Res 9(13):4865–71 [PubMed] [Google Scholar]
- 120.Chinen LTD, de Carvalho FM, Rocha BMM, Aguiar CM, Abdallah EA, et al. 2013. Cytokeratin-based CTC counting unrelated to clinical follow up. J. Thorac. Dis 5(5):593–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan SJ, Yobas L, Lee GYH, Ong CN, Lim CT. 2009. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 11(4):883–92 [DOI] [PubMed] [Google Scholar]
- 122.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, et al. 2013. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep 3:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin J,Wang Y, Yin H, Chen W, Jin G, et al. 2015. Circulating tumor cells enriched by the depletion of leukocytes with bi-antibodies in non–small cell lung cancer: potential clinical application. PLOS ONE 10(8):e0137076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, et al. 2013. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol 31(6):539–44 [DOI] [PubMed] [Google Scholar]
- 125.Alix-Panabières C 2012. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients In Minimal Residual Disease and Circulating Tumor Cells in Breast Cancer. Recent Results in Cancer Research, Volume 195, ed. Ignatiadis M, Sotiriou C, Pantel K, pp. 69–76. Berlin/Heidelberg, Ger.: Springer [Google Scholar]
- 126.Ramirez J-M, Fehm T, Orsini M, Cayrefourcq L, Maudelonde T, et al. 2014. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin. Chem 60(1):214–21 [DOI] [PubMed] [Google Scholar]
- 127.Pizon M, Zimon D, Carl S, Pachmann U, Pachmann K, Camara O. 2013. Heterogeneity of circulating epithelial tumour cells from individual patients with respect to expression profiles and clonal growth (sphere formation) in breast cancer. ecancermedicalscience 7:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu S, Liu S, Liu Z, Huang J, Pu X, et al. 2015. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLOS ONE 10(4):e0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma Y, Hao S, Wang S, Zhao Y, Lim B, et al. 2015. A combinatory strategy for detection of live CTCs using microfiltration and a new telomerase-selective adenovirus. Mol. Cancer Ther 14(3):835–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zamay GS, Kolovskaya OS, Zamay TN, Glazyrin YE, Krat AV, et al. 2015. Aptamers selected to postoperative lung adenocarcinoma detect circulating tumor cells in human blood. Mol. Ther 23(9):1486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Desitter I, Guerrouahen BS, Benali-Furet N,Wechsler J, Jänne PA, et al. 2011. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 31(2):427–41 [PubMed] [Google Scholar]
- 132.Fernandez SV, Bingham C, Fittipaldi P, Austin L, Palazzo J, et al. 2014. TP53 mutations detected in circulating tumor cells present in the blood of metastatic triple negative breast cancer patients. Breast Cancer Res. 16(5):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fabbri F, Carloni S, Zoli W, Ulivi P, Gallerani G, et al. 2013. Detection and recovery of circulating colon cancer cells using a dielectrophoresis-based device: KRAS mutation status in pure CTCs. Cancer Lett. 335(1):225–31 [DOI] [PubMed] [Google Scholar]
- 134.Nieva J, Wendel M, Luttgen MS, Marrinucci D, Bazhenova L, et al. 2012. High-definition imaging of circulating tumor cells and associated cellular events in non–small cell lung cancer patients: a longitudinal analysis. Phys. Biol 9(1):016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams ES, Rodriguez-Bravo V, Chippada-Venkata U, De Ia Iglesia–Vicente J, Gong Y, et al. 2015. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J. Vis. Exp 104:e53182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, et al. 2015. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget 6(17):15578–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu M, Bardia A, Aceto N, Bersani F, Madden MW, et al. 2014. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345(6193):216–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hart CD, Galardi F, Pestrin M, De Luca F, Risi E, Di Leo A. 2016. Using CTCs for pharmacogenomics analysis. Pharmacol. Res 106:92–100 [DOI] [PubMed] [Google Scholar]
- 139.Metzker ML. 2010. Sequencing technologies—the next generation. Nat. Rev. Genet 11(1):31–46 [DOI] [PubMed] [Google Scholar]
- 140.Baslan T, Hicks J. 2014. Single cell sequencing approaches for complex biological systems. Curr. Opin. Genet. Dev 26:59–65 [DOI] [PubMed] [Google Scholar]
- 141.Dean FB,Hosono S, Fang L, Wu X, Faruqi AF, et al. 2002. Comprehensive human genome amplification using multiple displacement amplification. PNAS 99(8):5261–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Polzer B, Medoro G, Pasch S, Fontana F, Zorzino L, et al. 2014. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol. Med 6(11):1371–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heitzer E, Auer M,Gasch C, Pichler M,Ulz P, et al. 2013. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73(10):2965–75 [DOI] [PubMed] [Google Scholar]
- 144.Dago AE, Stepansky A, Carlsson A, Luttgen M, Kendall J, et al. 2014. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLOS ONE 9(8):e101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gasch C, Plummer PN, Jovanovic L, McInnes LM, Wescott D, et al. 2015. Heterogeneity of miR-10b expression in circulating tumor cells. Sci. Rep 5:15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steinestel J, Luedeke M, Arndt A, Schnoeller TJ, Lennerz JK, et al. 2016. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, et al. 2010. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res 16(9):2634–45 [DOI] [PubMed] [Google Scholar]
- 148.Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, et al. 2012. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2(11):995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kasimir-Bauer S, Bittner A-K, König L, Reiter K, Keller T, et al. 2016. Does primary neoadjuvant systemic therapy eradicateminimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res. 18(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fehm T, Becker S, Duerr-Stoerzer S, Sotlar K, Mueller V, et al. 2007. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res. 9(5):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cho WCS. 2014. Emerging techniques in molecular detection of circulating tumor cells. Expert Rev. Mol. Diagn 14(2):131–34 [DOI] [PubMed] [Google Scholar]
- 152.Moreno JG, O’Hara SM, Gross S, Doyle G, Fritsche H, et al. 2001. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology 58(3):386–92 [DOI] [PubMed] [Google Scholar]
- 153.Pailler E, Adam J, Barthélémy A, Oulhen M, Auger N, et al. 2013. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol 31(18):2273–81 [DOI] [PubMed] [Google Scholar]
- 154.Pailler E, Auger N, Lindsay CR, Vielh P, Islas-Morris-Hernandez A, et al. 2015. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann. Oncol 26(7):1408–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Antonarakis ES, Lu C, Wang H, Luber B,Nakazawa M, et al. 2014. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med 371(11):1028–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Antonarakis ES, Lu C, Luber B,Wang H, Chen Y, et al. 2015. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 1(5):582–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, et al. 2014. Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer 14:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, et al. 2014. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol 32(31):3483–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thalgott M, Rack B, Horn T, Heck MM, Eiber M, et al. 2015. Detection of circulating tumor cells in locally advanced high-risk prostate cancer during neoadjuvant chemotherapy and radical prostatectomy. Anticancer Res. 35(10):5679–85 [PubMed] [Google Scholar]
- 160.Gazzaniga P, de Berardinis E, Raimondi C, Gradilone A, Busetto GM, et al. 2014. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int. J. Cancer 135(8):1978–82 [DOI] [PubMed] [Google Scholar]
- 161.He W, Wang H, Hartmann LC, Cheng J-X, Low PS. 2007. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. PNAS 104(28):11760–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li J, Sharkey CC, Wun B, Liesveld J, King MR. 2016. Genetic engineering of platelets to neutralize circulating tumor cells. J. Control. Release 228:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Langenbeck B 1841. On the development of cancer in the veins. Edinb. Med. Surg. J 55:251–53 [Google Scholar]
- 164.Engell HC. 1955. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir. Scand. Suppl 201:1–70 [PubMed] [Google Scholar]
- 165.Seal SH. 1959. Silicone flotation: a simple quantitative method for the isolation of free-floating cancer cells from the blood. Cancer 12(3):590–95 [DOI] [PubMed] [Google Scholar]
- 166.Alexander RF, Spriggs AI. 1960. The differential diagnosis of tumour cells in circulating blood. J. Clin. Pathol 13:414–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song MJ, Kornatowski G, Parsons DF, King MV. 1987. Detection and characterization of circulating rat mammary tumor cells in buffy coat and correlation with metastasis. Cancer Investig. 5(5):429–41 [DOI] [PubMed] [Google Scholar]
- 168.Schlimok G, Funke I,Holzmann B,Göttlinger G, Schmidt G, et al. 1987. Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17–1A monoclonal antibodies. PNAS 84(23):8672–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hardingham JE, Kotasek D, Farmer B, Butler RN, Mi JX, et al. 1993. Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer Res. 53(15):3455–58 [PubMed] [Google Scholar]
- 170.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, et al. 1998. Detection and characterization of carcinoma cells in the blood. PNAS 95(8):4589–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vona G, Sabile A, Louha M, Sitruk V, Romana S, et al. 2000. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am. J. Pathol 156(1):57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, et al. 2004. A rare-cell detector for cancer. PNAS 101(29):10501–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O’Hara SM, Moreno JG, Zweitzig DR, Gross S, Gomella LG, Terstappen LWMM. 2004. Multigene reverse transcription–PCR profiling of circulating tumor cells in hormone-refractory prostate cancer. Clin. Chem 50(5):826–35 [DOI] [PubMed] [Google Scholar]
- 174.Marrinucci D, Bethel K, Lazar D, Fisher J, Huynh E, et al. 2010. Cytomorphology of circulating colorectal tumor cells: a small case series. J. Oncol 2010:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hodgkinson CL,Morrow CJ, Li Y, Metcalf RL, Rothwell DG, et al. 2014. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med 20(8):897–903 [DOI] [PubMed] [Google Scholar]
- 176.Tulley S, Zhao Q, Dong H, Pearl ML, Chen W-T. 2016. Vita-AssayTM method of enrichment and identification of circulating cancer cells/circulating tumor cells (CTCs). Methods Mol. Biol 1406:107–19 [DOI] [PubMed] [Google Scholar]


