Abstract
Purpose
To investigate the associations between concentrations of Aβ40 and Aβ42 and vascular cognitive impairment (VCI) in cerebral small vessel disease (CSVD) patients and evaluate the value of combination of levels of Aβ40 or Aβ42 and the total CSVD score in predicting VCI.
Patients and Methods
A total of 199 CSVD patients were divided into VCI group and non-VCI group according to the criteria of VCI. Demographic data, MRI markers of CSVD, blood pressure, vascular risk factors, laboratory markers, and serum Aβ40 and Aβ42 concentration were collected. Univariate analysis was performed with the Student’s t-test, Mann–Whitney U-test or Chi-square test. Variables with P<0.10 in univariate analysis were then included in multivariate analysis that used a backward stepwise logistic regression model. The predictive values were assessed with receiver operating characteristic (ROC) curve.
Results
VCI was determined in 112 CSVD patients (56.3%). Hyperlipidemia (OR: 1.618, 95% CI: 1.265–3.049), the total CSVD score (OR: 1.414, 95% CI: 1.213–2.278) and serum Aβ42 concentration (OR: 1.401, 95% CI: 1.212–1.946) were independent risk factors for VCI in CSVD patients with adjustment for age, education years, diabetes and fasting blood-glucose (FBG). The area under curves (AUCs) were 0.640 (SE: 0.040, 95% CI: 0.563–0.718), 0.733 (SE: 0.035, 95% CI: 0.664–0.802) and 0.827 (SE: 0.030, 95% CI: 0.768–0.887), respectively, for the total CSVD score, serum Aβ42 concentration and their combination applied in predicting VCI in CSVD patients. Z test demonstrated that the AUC of combination prediction was significantly higher than individual prediction (0.827 vs 0.640, Z=3.740, P<0.001; 0.827 vs 0.733, Z=2.039, P=0.021).
Conclusion
Combination of Aβ42 and total CSVD score could significantly elevate the predictive value of cognitive impairment in CSVD patients.
Keywords: Aβ40, Aβ42, vascular cognitive impairment, cerebral small vessel disease, total cerebral small vessel disease score
Introduction
Cerebral small vessel disease (CSVD) is a syndrome induced by intracranial small vessel lesions, which manifests as a series of changes in neuropathology, neuroimaging, cognitive function, sphincter dysfunctions, psychiatric disorders, and gait disorder, etc.1,2 Its typical brain magnetic resonance imaging (MRI) markers mainly include white matter hyperintensities (WMH), cerebral microbleeds (CMBs), lacunar infarction (LI) and enlarged perivascular spaces (EPVS).1,2 CSVD is one of the most common causes of vascular cognitive impairment (VCI) and can lead to vascular dementia (VaD) in severe cases.3 Patients with a first-ever lacunar infarction present cognitive impairment (mainly executive dysfunction) in more than half of the cases and more than 55% of the patients meet the criteria for mild cognitive impairment of the vascular type.4 VaD is the end-stage of cognitive impairment and its therapeutic outcome is poor. VCI is the early stage of VaD and can be prevented, and moreover, its progression can be slowed or even reversed.5,6
The amyloid β (Aβ) is derived from a proteolytic processing of the amyloid precursor protein through the activation of β- and γ-secretase.7 The Aβ mainly includes Aβ40 and Aβ42 isoforms, and most of the Aβ deposited in the brain is Aβ42.8 Investigations on association between Aβ levels and Alzheimer’s disease (AD) have reported contradictory results, with some reporting decreased Aβ40 and Aβ42 levels in mild cognitive impairment and AD whereas others have reported elevated levels in AD.
Most CSVD patients have two or more MRI markers at the same time, and cognitive impairment is usually the interaction result of multiple lesions.9 Recent studies have employed the total MRI cerebral small vessel disease score to evaluate the severity of CSVD.10 This score is more precise due to synthesizing the type and amount of MRI markers.11 Moreover, it has been demonstrated to have a significant association with cognitive performance.12–14 In this paper, the associations between concentrations of Aβ40 and Aβ42 and VCI in CSVD patients were analyzed, and the value of combination of the total CSVD score and concentrations of Aβ40 or Aβ42 in predicting VCI was evaluated.
Patients and Methods
Patients
Two-hundred and five consecutive CSVD patients were recruited at Department of Neurology, Tianjin Teda Hospital between July 2017 and June 2020. All patients had brain MRI markers of CSVD (WMH, CMBs, LI and EPVS) and were clinically diagnosed with CSVD according to the criteria of Trial of Org 10,172 in Acute Stroke Treatment.15 Exclusion criteria included: 1) extra-intracranial large artery stenosis ≥50%; 2) cortical nonlacunar infarcts, subcortical nonlacunar infarcts or cardio embolic source infarcts; 3) cerebral hemorrhage; 4) presence of other diseases which could affect cognitive function, such as Alzheimer’s disease (AD), Parkinson’s disease, traumatic brain injury and congenital mental retardation; 5) presence of mental diseases such as serious anxiety, depression or schizophrenia; 6) difficulties in evaluation of cognitive function due to hearing and visual impairments. This study was performed in accordance with the Declaration of Helsinki and permitted by the Ethics Committee of Tianjin Teda Hospital. Informed consent was provided by patients or family members.
Patient Characteristics
The following variables were collected: 1) demographic data (age, sex, years of education); 1) MRI markers of CSVD (WMH, CMBs, LI and EPVS); 3) blood pressure (systolic blood pressure and diastolic blood pressure); 4) vascular risk factors (drinking, smoking, body mass index [BMI], hypertension, hyperlipidemia, atrial fibrillation and diabetes); and 5) laboratory markers at admission (fasting blood-glucose[FBG], triacylglycerol [TG], total cholesterol [TC], high density lipoprotein cholesterol [HDL-C] and low density lipoprotein cholesterol [LDL-C)].
Neuropsychological Assessment
Two neurologists performed neuropsychological assessment with Montreal Cognitive Assessment (MoCA) and clinical dementia rating (CDR) after unified training. VCI was diagnosed according to the criteria proposed by Geriatric Neurology Group, Chinese Society of Geriatrics and Clinical Practice Guideline for Cognitive Impairment of Cerebral Small Vessel Disease Writing Group: 1) patients or their caregivers reported a decline in subjective cognitive function that affected or did not affect life independence; 2) neuropsychological tests indicated cognitive impairment in one or more cognitive domain, and the judgment basis was MoCA score < 26 points and CDR score ≥ 0.5 points; and 3) cognitive impairment was associated with CSVD according to imaging features and medical history.16 According to the criteria of VCI, all CSVD patients were divided into VCI group and non-VCI group.
Determination of the Total CSVD Score
The total CSVD score was calculated through counting the presence of each of the four MRI markers of CSVD, with 1 point applied for the presence of each of WMH, CMBs, LI and EPVS. The presence of CMBs or LI was defined as the presence of one or more foci; the presence of WMH was defined as either irregular periventricular hyperintensity extending into the deep white matter (Fazekas score 3) and/or (early) confluent deep WMH (Fazekas score 2 or 3); and the presence of moderate to severe basal ganglia EPVS was counted if it was grade 2–4. Therefore, the total CSVD score ranged from 0 to 4. Two radiologists determined the total CSVD score of each patient without knowing the results of neuropsychological assessment, and they should discuss and reach an agreement in case of disagreement.
Detection of Aβ40 and Aβ42 Concentrations
Fasting venous blood samples were collected for all patients on admission, and centrifuged for 15 minutes at 3500 r/min. The serum was then immediately frozen and stored at −80°C. The concentrations of Aβ40 and Aβ42 were detected using commercially available enzyme-linked immunosorbent assay kits (Thermo Fisher Scientific, Waltham, MA, USA). Detection was performed in an independent laboratory, and testers were blinded to MRI and clinical data.
Statistical Analysis
Statistical analysis was conducted using the SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and significance was set at P<0.05. Quantitative data were assessed with Kolmogorov–Smirnov test for their distribution. Data with normal distribution were described with mean ± standard deviation, and data without normal distribution were described with median (M) and interquartile range (IQR). Qualitative data were described with percentages or ratios (%). Univariate analysis was performed with the Student’s t-test, Mann–Whitney U-test or Chi-square test. Variables with P<0.10 in univariate analysis were then included in multivariate analysis that used a backward stepwise logistic regression model. The predictive values of the total CSVD score, Aβ concentrations and their combination were assessed with receiver operating characteristic (ROC) curve. Z test was employed to compare area under curve (AUC).
Results
General Data
A total of 225 CSVD patients were recruited during the study period. 17 cases were excluded because the quality of their MRI images was unqualified, and 9 cases were excluded because they could not cooperate to complete the neuropsychological tests. Finally, 199 cases were included in this analysis. They included 107 males (53.8%) and 92 females (46.2%) with a mean age of (69.03±7.79) years old. Among them, 146 cases (73.4%) demonstrated LI, 94 cases (47.2%) demonstrated WMH, 83 cases (41.7%) demonstrated EPVS, and 65 cases (32.7%) demonstrated CMBs. Thirty-one cases (15.6%) had 0 point of the total cerebral small vessel disease score, 44 cases (22.1%) had 1 point, 37 cases (18.6%) had 2 points, 53 cases (26.6%) had 3 points, and 34 cases (17.1%) had 4 points. VCI was determined in 112 cases (56.3%).
Analysis of Risk Factors for VCI
According to univariate analysis (Table 1), age, diabetes, hyperlipidemia, FBG, the total CSVD score and serum Aβ42 concentration were significantly different between VCI group and non-VCI group (P<0.05), and the other variables were not (P>0.05). Multivariate analysis was performed with the occurrence of VCI or not as the dependent variable (occurrence =1, not occurrence =0) and the variables with P<0.1 in univariate analysis as the independent variables including age, education years, diabetes, hyperlipidemia, FBG, the total CSVD score and serum Aβ42 concentration. The results demonstrated that hyperlipidemia (OR: 1.618, 95% CI: 1.265–3.049), the total CSVD score (OR: 1.414, 95% CI: 1.213–2.278) and serum Aβ42 concentration (OR: 1.401, 95% CI: 1.212–1.946) were independent risk factors for VCI in CSVD patients with adjustment for age, education years, diabetes and FBG.
Table 1.
Results of Univariate Analysis Between VCI Group and Non-VCI Group
| Variables | All CSVD Patients (n=199) | VCI Group (n1=112) | Non-VCI Group (n2=87) | t/Z/Χ2 | P |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age (years old, mean±standard) | 69.03±7.79 | 70.08±7.98 | 67.68±7.54 | 2.171 | 0.034 |
| Male (n, %) | 107 (53.8%) | 62 (55.4%) | 45 (51.7%) | 0.260 | 0.610 |
| Education years (years, mean±standard) | 6.78±3.49 | 6.37±3.32 | 7.31±3.70 | −1.859 | 0.072 |
| Vascular risk factors (n, %) | |||||
| Drinking | 67 (33.7%) | 41(36.6%) | 26 (29.9%) | 0.991 | 0.320 |
| Smoking | 78 (39.2%) | 47 (42.0%) | 31 (35.6%) | 0.824 | 0.364 |
| Hypertension | 114 (57.3%) | 67 (59.8%) | 47 (54.0%) | 0.673 | 0.412 |
| Hyperlipidemia | 60 (30.2%) | 41 (36.6%) | 19 (21.8%) | 5.071 | 0.024 |
| Atrial fibrillation | 22 (11.1%) | 12 (10.7%) | 10 (11.5%) | 0.030 | 0.862 |
| Diabetes | 75 (37.7%) | 50 (44.6%) | 25 (28.7%) | 5.276 | 0.022 |
| Blood pressure (mmHg, mean±standard) | |||||
| Systolic blood pressure | 143.72±15.14 | 144.26±15.18 | 143.02±15.09 | 0.574 | 0.549 |
| Diastolic blood pressure | 83.92±10.95 | 84.18±10.41 | 83.59±11.64 | 0.371 | 0.694 |
| Laboratory markers (mmol/L, mean±standard) |
|||||
| TC | 4.13±1.10 | 4.17±1.19 | 4.08±0.99 | 0.582 | 0.547 |
| TG | 1.56±0.84 | 1.62±0.90 | 1.48±0.76 | 1.189 | 0.258 |
| LDL-C | 2.57±1.06 | 2.63±1.14 | 2.48±0.95 | 1.012 | 0.305 |
| HDL-C | 1.23±0.44 | 1.19±0.42 | 1.29±0.47 | −1.559 | 0.176 |
| FBG | 6.85±2.81 | 7.30±2.85 | 6.26±2.76 | 2.599 | 0.011 |
| MRI markers of CSVD (n, %) | |||||
| LI | 146 (73.4%) | 85 (75.9%) | 61 (70.1%) | 0.837 | 0.360 |
| WMH | 94 (47.2%) | 57 (50.9%) | 37 (42.5%) | 1.374 | 0.241 |
| CMBs | 65 (32.7%) | 34 (30.4%) | 31 (35.6%) | 0.619 | 0.431 |
| EPVS | 83 (41.7%) | 44 (39.3%) | 39 (44.8%) | 0.619 | 0.432 |
| Aβ40 (pg/mL, mean±standard) | 19.82±8.94 | 20.47±9.05 | 18.98±8.79 | 1.171 | 0.262 |
| Aβ42 (pg/mL, mean±standard) | 14.81±7.80 | 16.38±8.56 | 12.79±6.82 | 3.293 | 0.002 |
| Total CSVD score (M, IQR) | 2 (2) | 3 (2) | 1 (2) | −3.470 | 0.001 |
| Distribution of total CSVD score (n, %) | 12.710 | 0.013 | |||
| 0 point | 31 (15.6%) | 12 (10.7%) | 19 (21.8%) | ||
| 1 point | 44 (22.1%) | 19 (17.0%) | 25 (28.7%) | ||
| 2 points | 37 (18.6%) | 21 (18.8%) | 16 (18.4%) | ||
| 3 points | 53 (26.6%) | 36 (32.1%) | 17 (19.5%) | ||
| 4 points | 34 (17.1%) | 24 (21.4%) | 10 (11.5%) |
Analysis of Predictive Value
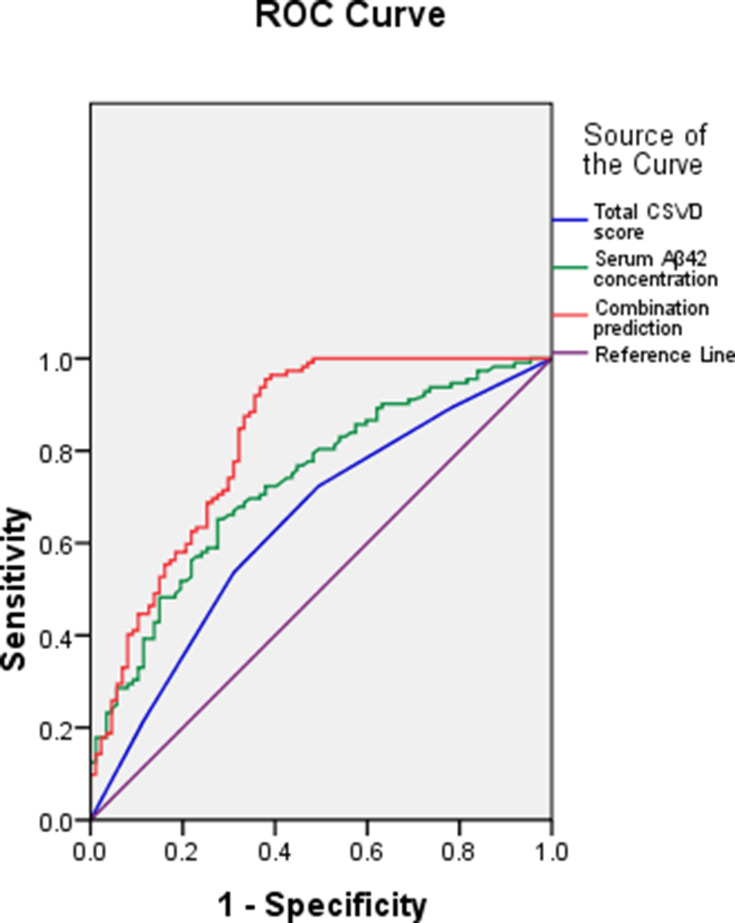
The predictive values of the total CSVD score, serum Aβ42 concentration and their combination were evaluated with ROC curves. Their AUCs were 0.640 (SE: 0.040, 95% CI: 0.563–0.718), 0.733 (SE: 0.035, 95% CI: 0.664–0.802) and 0.827 (SE: 0.030, 95% CI: 0.768–0.887), respectively (Figure 1). According to Z test, the AUC of combined prediction was significantly higher than individual prediction (0.827 vs 0.640, Z=3.740, P<0.001; 0.827 vs 0.733, Z=2.039, P=0.021). Their clinical utility indexes were demonstrated in Table 2. The positive predictive value was 69.0%, 75.3% and 82.9%, respectively for the total CSVD score, serum Aβ42 concentration and their combination, and the negative predictive value was 53.6%, 61.8% and 77.3%, respectively.
Figure 1.
ROC curves of the total CSVD score, serum Aβ42 concentration and their combination.
Table 2.
Clinical Utility Indexes of the Total CSVD Score, Aβ42 Concentration and Their Combination for Predicting VCI in CSVD Patients
| Best Cut-Off | Sensitivity | Specificity | Accuracy | False Positive Rate | False Negative Rate | Positive Predictive Value | Negative Predictive Value | Youden Index | |
|---|---|---|---|---|---|---|---|---|---|
| Total CSVD score | 2 | 72.3% | 50.6% | 60.3% | 31.0% | 46.4% | 69.0% | 53.6% | 0.23 |
| Serum Aβ42 concentration (pg/mL) | 12.53 | 65.2% | 72.4% | 68.3% | 24.7% | 38.2% | 75.3% | 61.8% | 0.38 |
| Combination prediction | 82.1% | 78.2% | 80.4% | 17.1% | 22.7% | 82.9% | 77.3% | 0.60 |
Discussion
In our study, serum Aβ42 levels were higher in CSVD patients with VCI than without VCI, and Aβ40 levels were not statistically different. Moreover, multivariate analysis showed that the total CSVD score and serum Aβ42 levels were independently associated with VCI in CSVD patients.
Previous studies have shown that elevated levels of Aβ40 and Aβ42 are associated with WMH and LI.17–20 This may be involved in multiple mechanisms. Firstly, excessive Aβ results in deposition in the adventitia and media of arteries and cortical arterioles, which can be further promoted by Apoe4 isoform that contributes to the transformation of Aβ oligomers from β sheet.21 Secondly, fibrillary deposition of Aβ in the vessel wall can lead to loss of vascular smooth muscle tone, obliteration of the vessel lumina and endothelial damage, which is responsible for cerebrovascular damage and hypoperfusion.20 Thirdly, hypoperfusion or decreased cerebral blood flow may promote the production of Aβ through upregulating the expression of amyloid-β protein precursor in endothelial cells and thereby results in its secretion in the peripheral circulation.22 Fourthly, soluble Aβ may enhance endothelin-1 induced vasoconstriction and thereby influence cerebral vasoreactivity. This alteration can influence brain regions, which limits collateral circulation leading to WMH and LI.23 In addition to WMH and LI, Aβ levels have been reported an association with CMBs.24
With respect to cognition, it has been shown that elevated Aβ levels may induce cortical thinning and deceased grey matter density in temporal lobes, which can cause cognitive dysfunction via disrupting important cognitive networks.25,26 However, investigations on association between Aβ levels and AD have yielded controversial results. Llado-Saz et al indicated that the elevated Aβ40 level was correlated with decreased information processing speed, poor memory and cognitive decline among cognitively normal adults.27 Hilal et al investigated plasma Aβ levels in two independent cohorts of the Rotterdam Study.24 The results showed that elevated Aβ40 levels, Aβ42 levels and Aβ40/Aβ42 ratio were related to increasing microbleeds and lacunar counts. Moreover, elevated Aβ40 levels and Aβ40/Aβ42 ratio were significantly correlated with large volumes of WMH. For cognition, elevated Aβ40 levels and Aβ40/Aβ42 ratio were associated with poor performance on cognitive test. Mayeux et al detected plasma Aβ40 and Aβ42 levels of 530 individuals participating in an epidemiologic study on association between aging and dementia at different time points.28 Their results demonstrated that plasma Aβ40 and Aβ42 levels increased with age. Plasma Aβ40 and Aβ42 levels were elevated in some patients before and during the early stages of AD but decline thereafter. Seppälä et al demonstrated that the low plasma Aβ42 level was associated with cognitive decline in a population-based cohort including 52 cognitively impaired and 217 controls.29 Chouraki et al investigated plasma Aβ40 and Aβ42 levels in a community-based cohort under ongoing surveillance for AD (the Framingham cohort).17 The results demonstrated that decreased plasma Aβ42 levels and Aβ42/Aβ40 ratio were correlated with risk of dementia and AD, but plasma Aβ40 levels were not. Hansson et al investigated plasma Aβ40 and Aβ42 levels in two independent cohorts of patients with mild cognitive impairment (MCI) and age-matched controls.30 The results showed that decreased Aβ42 levels in CSF were correlated with risk of incident AD. The inconsistency between our results and some previous results might be associated with different participants and different timing of Aβ detection.
Multiple MRI markers of CSVD have been confirmed to have a significant correlation with cognitive impairment.12–14 WMH and LI are associated with executive dysfunction, working memory disturbances and decreased information processing speed,31–36 and CMBs are associated with decreased information processing speed and executive dysfunction.37,38 The total CSVD score is a new scoring method proposed in recent years. It can be used to evaluate the total burden of CSVD through synthesizing the type and amount of MRI markers.11 The total CSVD score can assess the impact of CSVD on cognitive function more precisely compared with individual MRI marker of CSVD. In the general population, community-dwelling older people, memory clinic population, first lacunar infarction patients and hypertension patients, the total CSVD score is associated with cognition.9,39,40 Moreover, it could be used to predict the cognitive decline in hypertension patients over 4 years, especially in executive function.41
In this study, we further evaluated the value of the total CSVD score, serum Aβ42 levels and their combination in predicting VCI in CSVD patients. The AUC of the total CSVD score in predicting VCI in CSVD patients was 0.640 with the best cut-off of 2, and serum Aβ42 levels was 0.733 with the best cut-off of 12.53 pg/mL. The AUC of their combination was 0.827 with a sensitivity of 82.1% and a specificity of 78.2%, significantly higher than individual prediction. Therefore, their combination could be applied in prediction of VCI in CSVD patients.
The limitation of the study is that the potential role of cerebral atrophy in the cognitive impairment of patients in the study sample has not been evaluated and it is a fact of recent interest in the study of cognitive impairment in small vessel cerebrovascular disease.42
Conclusion
The total CSVD score and serum Aβ42 concentration were independent risk factors of VCI in CSVD patients. Combination of Aβ42 and total CSVD score could elevate the predictive value of cognitive impairment in CSVD patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; agreed on the journal to which the article will be submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Rost NS, Etherton M. Cerebral small vessel disease. Continuum (Minneap Minn). 2020;26(2):332–352. doi: 10.1212/CON.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 2.Cannistraro RJ, Badi M, Eidelman BH, et al. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Østergaard L, Engedal TS, Moreton F, et al. Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36(2):302–325. doi: 10.1177/0271678X15606723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grau-Olivares M, Arboix A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: a forerunner of vascular dementia? Expert Rev Neurother. 2009;9(8):1201–1217. doi: 10.1586/ern.09.73. [DOI] [PubMed] [Google Scholar]
- 5.Luo H, Han G, Wang J, et al. Common aging signature in the peripheral blood of vascular dementia and Alzheimer’s disease. Mol Neurobiol. 2016;53(6):3596–3605. doi: 10.1007/s12035-015-9288-x. [DOI] [PubMed] [Google Scholar]
- 6.Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018;33(8):500–507. doi: 10.1177/1533317518791401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 8.Gravina SA, Ho L, Eckman CB, et al. Amyloid beta protein (Abeta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43). J Biol Chem. 1995;270(13):7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- 9.Del Brutto VJ, Ortiz JG, Del Brutto OH, et al. Total cerebral small vessel disease score and cognitive performance in community-dwelling older adults. Results from the Atahualpa project. Int J Geriatr Psychiatry. 2018;33(2):325–331. doi: 10.1002/gps.4747. [DOI] [PubMed] [Google Scholar]
- 10.Lau KK, Li L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology. 2017;88(24):2260–2267. doi: 10.1212/WNL.0000000000004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staals J, Makin SD, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83(14):1228–1234. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huijts M, Duits A, van Oostenbrugge RJ, et al. Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever lacunar stroke and hypertensive patients. Front Aging Neurosci. 2013;5:72. doi: 10.3389/fnagi.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36(10):2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Li S, Li W, et al. MRI lesion load of cerebral small vessel disease and cognitive impairment in patients with CADASIL. Front Neurol. 2018;9:862. doi: 10.3389/fneur.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melkas S, Putaala J, Oksala NK, et al. Small-vessel disease relates to poor poststroke survival in a 12-year follow-up. Neurology. 2011;76(8):734–739. doi: 10.1212/WNL.0b013e31820db666 [DOI] [PubMed] [Google Scholar]
- 16.Geriatric Neurology Group, Chinese Society of Geriatrics; Clinical Practice Guideline for Cognitive Impairment of Cerebral Small Vessel Disease Writing Group. Clinical practice guideline for cognitive impairment of cerebral small vessel disease of China. Chin J Geriatr. 2019;38(4):345–354. doi: 10.3760/cma.j.issn.0254-9026.2019.04.001. [DOI] [Google Scholar]
- 17.Chouraki V, Beiser A, Younkin L, et al. Plasma amyloid-β and risk of Alzheimer’s disease in the Framingham Heart study. Alzheimers Dement. 2015;11(3):249–257.e1. doi: 10.1016/j.jalz.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toledo JB, Vanderstichele H, Figurski M, et al. Alzheimer’s disease neuroimaging initiative. factors affecting Aβ plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122(4):401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66(1):23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk EJ, Prins ND, Vermeer SE, et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55(4):570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun ME, Burgermeister P, Phinney AL, et al. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci U S A. 1999;96(24):14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett SA, Pappas BA, Stevens WD, et al. Cleavage of amyloid precursor protein elicited by chronic cerebral hypoperfusion. Neurobiol Aging. 2000;21(2):207–214. doi: 10.1016/s0197-4580(00)00131-7. [DOI] [PubMed] [Google Scholar]
- 23.Paris D, Humphrey J, Quadros A, et al. Vasoactive effects of A beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer’s disease: role of inflammation. Neurol Res. 2003;25(6):642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- 24.Hilal S, Akoudad S, van Duijn CM, et al. Plasma amyloid-β levels, cerebral small vessel disease, and cognition: the Rotterdam study. J Alzheimers Dis. 2017;60(3):977–987. doi: 10.3233/JAD-170458. [DOI] [PubMed] [Google Scholar]
- 25.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czirr E, Cottrell BA, Leuchtenberger S, et al. Independent generation of Abeta42 and Abeta38 peptide species by gamma-secretase. J Biol Chem. 2008;283(25):17049–17054. doi: 10.1074/jbc.M802912200. [DOI] [PubMed] [Google Scholar]
- 27.Llado-Saz S, Atienza M, Cantero JL. Increased levels of plasma amyloid-beta are related to cortical thinning and cognitive decline in cognitively normal elderly subjects. Neurobiol Aging. 2015;36(10):2791–2797. doi: 10.1016/j.neurobiolaging.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Mayeux R, Honig LS, Tang MX, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 29.Seppälä TT, Herukka SK, Hänninen T, et al. Plasma A b42 and A b40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010;81(10):1123–1127. doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31(3):357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Ding X, Wu J, Zhou Z, et al. Specific locations within the white matter and cortex are involved in the cognitive impairments associated with periventricular white matter lesions (PWMLs). Behav Brain Res. 2015;289:9–18. doi: 10.1016/j.bbr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 33.Delano-Wood L, Bondi MW, Sacco J, et al. Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated white matter lesion pathology. J Int Neuropsychol Soc. 2009;15(6):906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ai Q, Pu YH, Sy C, et al. Impact of regional white matter lesions on cognitive function in subcortical vascular cognitive impairment. Neurol Res. 2014;36(5):434–443. doi: 10.1179/1743132814Y.0000000354. [DOI] [PubMed] [Google Scholar]
- 35.Blanco-Rojas L, Arboix A, Canovas D, et al. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC Neurol. 2013;13:203. doi: 10.1186/1471-2377-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benisty S, Gouw AA, Porcher R, et al.; LADIS Study group. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80(5):478–483. doi: 10.1136/jnnp.2008.160440. [DOI] [PubMed] [Google Scholar]
- 37.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam scan study. Neurology. 2012;78(5):326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 38.Gregoire SM, Scheffler G, Jäger HR, et al. Strictly lobar microbleeds are associated with executive impairment in patients with ischemic stroke or transient ischemic attack. Stroke. 2013;44(5):1267–1272. doi: 10.1161/STROKEAHA.111.000245. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Wang Y, Yuan Z, et al. Total cerebral small vessel disease burden is related to worse performance on the mini-mental state examination and incident dementia: a prospective 5-year follow-up. J Alzheimers Dis. 2019;69(1):253–262. doi: 10.3233/JAD-181135. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee G, Jang H, Kim HJ, et al. Total MRI small vessel disease burden correlates with cognitive performance, cortical atrophy, and network measures in a memory clinic population. J Alzheimers Dis. 2018;63(4):1485–1497. doi: 10.3233/JAD-170943. [DOI] [PubMed] [Google Scholar]
- 41.Uiterwijk R, van Oostenbrugge RJ, Huijts M, et al. Total cerebral small vessel disease MRI score is associated with cognitive decline in executive function in patients with hypertension. Front Aging Neurosci. 2016;8:301. doi: 10.3389/fnagi.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grau-Olivares M, Bartrés-Faz D, Arboix A, et al. Mild cognitive impairment after lacunar infarction: voxel-based morphometry and neuropsychological assessment. Cerebrovasc Dis. 2007;23(5–6):353–361. doi: 10.1159/000099134. [DOI] [PubMed] [Google Scholar]