Abstract
There are no effective treatments available to halt or reverse the progression of age-related cognitive decline and Alzheimer’s disease. Thus, there is an urgent need to understand the underlying mechanisms of disease etiology and progression to identify novel therapeutic targets. Age-related changes to the vasculature, particularly increases in stiffness of the large elastic arteries, are now recognized as important contributors to brain aging. There is a growing body of evidence for an association between greater large artery stiffness and cognitive impairment among both healthy older adults and patients with Alzheimer’s disease. However, studies in humans are limited to only correlative evidence, whereas animal models allow researchers to explore the causative mechanisms linking arterial stiffness to neurocognitive dysfunction and disease. Recently, several rodent models of direct modulation of large artery stiffness and the consequent effects on the brain have been reported. Common outcomes among these models have emerged, including evidence that greater large artery stiffness causes cerebrovascular dysfunction associated with increased oxidative stress and inflammatory signaling. The purpose of this mini-review is to highlight the recent findings associating large artery stiffness with deleterious brain outcomes, with a specific focus on causative evidence obtained from animal models. We will also discuss the gaps in knowledge that remain in our understanding of how large artery stiffness affects brain function and disease outcomes.
Keywords: aging, cerebrovascular, cognitive dysfunction, endothelial function, vascular stiffness
INTRODUCTION
In the last 20 years, the public health risk posed by cognitive decline and age-related dementias has increased dramatically. From 2000 to 2017, the number of people in the United States who died from dementia more than tripled, from over 83,000 in 2000 to over 260,000 in 2017 (1). Age-related dementias, notably Alzheimer’s disease (AD), are chronic neurodegenerative disorders that result in memory deficits and difficulty with executive function. Although there are therapies to slow the progression of cognitive decline in AD, such as cholinesterase inhibitors (2), there are no therapies available to prevent the onset or reverse the progression of cognitive decline or age-related dementias. It is, therefore, critical to investigate the contributors to AD and cognitive decline to gain insight into potential avenues for intervention.
The age-related increase in large artery stiffness has recently emerged as a potential predictor of cognitive decline (3). The correlations between arterial stiffness and cerebral pathologies, including AD, in human subjects have been reported repeatedly, but the mechanisms underlying these correlations are not fully understood. Animal models of large artery stiffness can be used to explore the pathogenesis of cognitive decline by examining molecular mechanisms in the brain that are too invasive to study in humans. Rodent models are also ideal for measuring outcomes across the lifespan and/or performing lifelong interventions as these studies are time and cost prohibitive in humans. In sum, animal models of altered large artery stiffness are tools for discovering causation and understanding the molecular mechanisms underlying the association of large artery stiffness and brain outcomes.
The purpose of this mini-review is to describe the findings from recent literature that detail the importance of large artery stiffness on the progression of cognitive decline. We will first briefly describe the scientific premise and results from studies in humans. Then, we will summarize the recent results of studies with rodent models of modulated large artery stiffness and the effects on the brain. We will also briefly describe rodent models associated with increased arterial stiffness, such as hypertensive models, and describe future research directions. Our goal is to leave the reader with an understanding of the importance of developing animal models of large artery stiffness, what these models have demonstrated thus far, and what gaps in knowledge remain related to large artery stiffness and the brain.
OVERVIEW OF LARGE ARTERY STIFFNESS
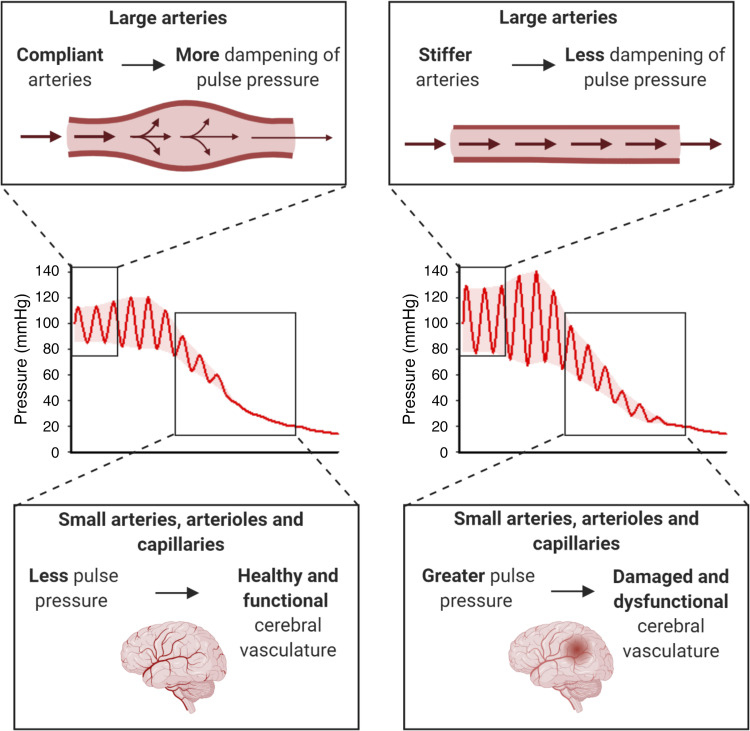
A universal feature of cardiovascular aging is the increase in stiffness of the large elastic arteries, namely the aorta and carotid arteries. With age, a decrease in elastin protein, increase in elastin fragmentation, and increase in collagen crosslink formation contribute to the onset of large artery stiffening (4). The increase in large artery stiffness translates to a decrease in the ability of these arteries to dampen pulse pressure, causing a higher pulsatile pressure to be transmitted to the microvasculature. Small arteries, arterioles, and capillaries are thin-walled and not equipped to handle high-pulsatile pressure, and as a result increases in large artery stiffness dramatically increase the risk for microvascular damage (Fig. 1) (5). More detailed descriptions of the causes and consequences of large artery stiffness can be found in recent reviews by Chirinos et al. (6) and Cooper and Mitchell (5).
Figure 1.
Hypothesized mechanism for cerebrovascular impairment caused by greater large artery stiffness. Top: the distension of the arterial wall dampens the pulse wave as it travels through the large arteries. When large arteries become less compliant (more stiff), as with advancing age, this dampening of pulse pressure is reduced. Middle: the highest mean blood pressure and widest pulse pressure are found in the large elastic and muscular arteries. Moving through the arterial tree, the mean blood pressure and pulse pressure are diminished to the extent that the pressure is constant (not pulsatile) in capillaries. However, with increased stiffness of the large arteries, the pulse pressure is widened throughout the arterial tree, including greater pulse pressure in small arteries, arterioles, and capillaries. Bottom: the small arteries, arterioles, and capillaries in the brain are thin-walled and do not have the structure to cope with the increased pulse pressure that results from greater large artery stiffness. This increased pulse pressure in the cerebral circulation potentially leads to cerebrovascular damage and dysfunction. Created with Biorender with permission.
The brain is highly vulnerable to the effects of increased large artery stiffness. This susceptibility of the brain arises from its high-blood flow as well as the need for tight blood pressure regulation (4, 7). As such, it is hypothesized that greater large artery stiffness causes damage and dysfunction to the cerebral small arteries and microvasculature. The cerebrovascular impairment is characterized by reduced cerebral blood flow and increased blood-brain barrier permeability, and can lead to neuroinflammation, neurodegeneration, and cognitive decline. This hypothesis is currently supported by evidence from the studies of human subjects and rodent models.
HUMAN STUDIES OF LARGE ARTERY STIFFNESS AND BRAIN OUTCOMES
Evidence from human studies supports the association between arterial stiffness and cognitive decline later in life. Aortic pulse wave velocity, the gold standard measure of aortic stiffness in humans, is a strong predictor of chronic vascular diseases and all-cause mortality (8). Greater aortic pulse wave velocity is predictive of the onset of mild cognitive impairment and for conversion from mild cognitive impairment to clinical dementia (9, 10). Greater aortic stiffness is also associated with reduced cerebral white matter microstructural integrity (11) and with greater amyloid-β plaque deposition, a hallmark of AD pathology (12, 13). Previous reviews have more thoroughly summarized the associations between arterial stiffness with cognitive impairment and AD (14–16).
Studies in humans have also associated greater large artery stiffness with cerebrovascular dysfunction. Increased aortic pulse wave velocity is associated with a decrease in global brain blood flow in cognitively healthy older adults, and this relation is greatest among carriers of the APOE ε4 genotype (18). In contrast, in patients with mild cognitive impairment, global brain blood flow is not correlated with aortic pulse wave velocity, perhaps as the relation is masked by other disease mechanisms (e.g., amyloid deposition) (18). Less clear is the influence on cerebrovascular reactivity, an indicator of the potential to increase blood flow to working brain regions, as reactivity has been both positively and negatively associated with greater arterial stiffness (18, 19). Taken together, these results suggest that large artery stiffness is associated with cerebrovascular and cognitive impairments.
DIRECT MODULATION OF ARTERIAL STIFFNESS IN RODENTS
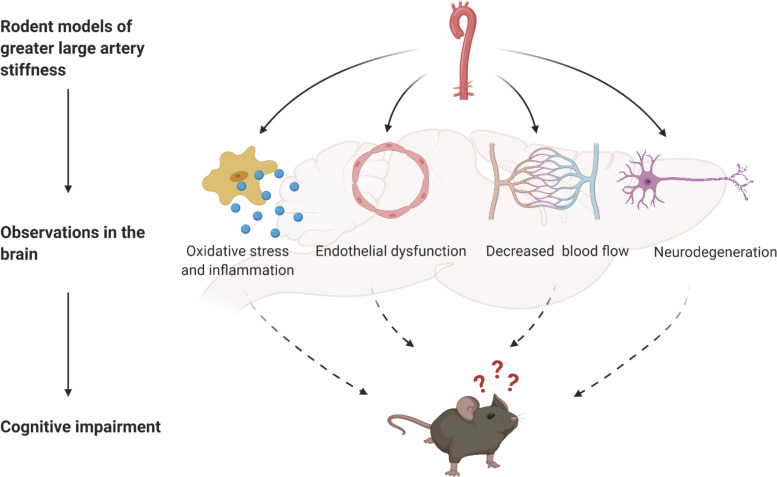
Similar to humans, aging in rodents is marked by increases in arterial stiffness and declines in cognitive function (20, 21). However, also similar to the study of human subjects, the observational study of rodents is confounded by age-related changes that are independent of large artery stiffening. Thus, establishing approaches to modify large artery stiffness in rodents is important for examining mechanisms and testing interventions. To date, the methods used to modulate large artery stiffness and examine brain outcomes in rodents include transgenic manipulation (Eln and Fbn1), induced carotid calcification, and aged and diabetic rodents treated with collagen cross-link breakers. Each model has its own strengths and limitations (summarized in Table 1), and rodent models in general are limited as they do not naturally develop cardiovascular or neurodegenerative diseases, due to differences from humans in lipid metabolism, amyloid-β production, or other factors. However, the commonalties found between these models provide further support for the mechanisms underlying the association of large artery stiffness and deleterious brain outcomes (Fig. 2), and future studies can combine these with transgenic model that more closely mimic human neurodegenerative diseases.
Table 1.
Summary of models of large artery stiffness and findings in the brain
| Model | Benefits | Drawbacks | Key Findings | |
|---|---|---|---|---|
| Humans | Human cross-sectional and prospective studies Refs 9–13, 18, 19 | Directly applicable to aging and disease populations, including AD | Only correlative evidence | Greater large artery stiffness is correlated with:
|
| Rodent models: direct modulation of arterial stiffness | Elastin haploinsufficient: Heterozygote Eln deletion (full knockout of Eln not viable). Refs 35–37 |
|
|
Greater large artery stiffness leads to:
|
| Fibrillin-1 mutation: Heterozygote mutation of fribrillin-1, Fbn1C1039G/+. A model of Marfan Syndrome. Ref 39 | Targeted increase in arterial extracellular matrix stiffness |
|
Greater large artery stiffness leads to:
|
|
| Carotid artery calcification: Mice studied 2–3 wk after application of calcium chloride to the carotid artery. Refs 28, 42, 43 |
|
An acute, dramatic increase in arterial stiffness that does not model the gradual process of arterial stiffening with human aging. | Increased carotid artery stiffness leads to:
|
|
| Cross-link breakers: ALT-711 (Alagebrium) treatment typically for 4–8 wk. Ref 46 | Reduces large artery stiffness (unlike other rodent models of increased stiffness) |
|
Reduced large artery stiffness leads to:
|
|
| Rodent model associated with greater pulse pressure | Transverse aortic constriction (TAC): Constriction resulting from suture tied around the aortic arch, usually between the right and left carotid artery branches. Refs 17, 22, 50 |
|
|
Increased carotid artery pulse pressure leads to:
|
Aβ, amyloid-β; AD, Alzheimer’s disease; BBB, blood-brain barrier; MCI, mild cognitive impairment.
Figure 2.
Summary of findings from studies of rodent models of greater large artery stiffness and brain-related outcomes. Rodent models of greater large artery stiffness include transgenic mice (Eln and Fbn1) and induced carotid calcification in mice. In these models, the greater large artery stiffness results in greater cerebral oxidative stress and inflammation, cerebral artery endothelial dysfunction, impaired cerebral blood flow, and neurodegeneration. Increased large artery stiffness also leads to cognitive impairment in mice, and it is assumed that this cognitive impairment results in the cerebral vascular and cellular changes. Created with Biorender with permission.
Elastin Haploinsufficient Mouse
The heterozygote knockout of the elastin gene (Eln+/− mice) results in young mice with lower aortic elastin content and greater aortic stiffness compared with wild-type littermates (32–34). This reduced elastin content and increased stiffness is primarily observed in large arteries and is not present in cerebral arteries. The Eln+/− mice have elevated systolic blood pressure and pulse pressure, an expected consequence of increased large artery stiffness (35). Importantly, the magnitude of the difference for aortic stiffness and systolic blood pressure between Eln+/− and Eln+/+ mice is similar in magnitude to the difference between old and young wild-type mice (23, 35). Thus, the Eln+/− mouse model can be used to examine the effects of elevated large artery stiffness, in a magnitude similar to aging but without many of the confounding changes associated with old age.
Results from the studies of the Eln model establish mechanistic connections between greater large artery stiffness and cerebrovascular dysfunction. Eln+/− mice have impaired cerebral artery endothelial function caused by reduced nitric oxide bioavailability and greater oxidative stress assessed ex vivo in isolated, pressurized arteries (35). Isolated cerebral arteries from Eln+/− mice also have a greater vasoconstrictor response to ex vivo administration of angiotensin II compared with cerebral arteries from Eln+/+ mice (36). Furthermore, perfusion of the cerebral cortex is lower in Eln+/− mice compared with Eln+/+ mice as assessed by arterial spin labeling MRI (37). In summary, the results from the Eln model support an effect of increased large artery stiffness on cerebrovascular dysfunction. The effects of Eln+/− on other brain outcomes, such as neuroinflammation and cognitive function, have not been reported.
Fibrillin-1 Haploinsufficient Mouse
Fibrillin-1 is a component of microfibrils in the extracellular matrix that associates with elastin lamina in the vasculature. In mice, heterozygote mutation of fribrillin-1, Fbn1C1039G/+, leads to increased aortic stiffness. However, the study of Fbn1C1039G/+ mice as model for isolated large artery stiffness is limited, as these mice were established to model Marfan syndrome and are complicated by the increased risk for aortic aneurysm and dissection (38). Fbn1C1039G/+ mice have greater cerebral artery superoxide production compared with wild-type control mice (39). The source of this superoxide is unknown, although superoxide production by NADPH oxidase 4 (NOX4) does not appear to play a role (39). Fbn1C1039G/+ mice also have increased cerebral artery wall thickness, but no changes to cerebral artery stiffness (39). It is unclear if this remodeling is a direct effect of the Fbn1 mutation within the cerebral vascular cells or if this remodeling is in response to the altered cerebral hemodynamics due to increased large artery stiffness. Thus, results from the Fbn1 model are similar to that of the Eln model, indicating that greater large artery stiffness leads to greater cerebral artery oxidative stress.
Carotid Artery Calcification
Calcification of the vasculature is a potential contributor to increased large artery stiffness with aging (31, 40, 41). To model increased calcification, calcium chloride can be directly applied to the adventitia of the carotid artery, and this results in greater arterial stiffness in mice (42). The advantages of this procedure are that it can be induced in an adult mouse, studied in a short time frame (2–3 wk), and does not change systemic blood pressure (42). Manipulating one carotid artery gives researchers a distinct advantage over transgenic models that are potentially confounded by effects of the genetic manipulation on non-large arteries. In addition, the brain-related outcomes have been studied more extensively in this carotid artery calcification model compared with the transgenic models.
Carotid calcification appears to impact the cerebral vasculature, neuroinflammation, and cognitive function. Carotid artery calcification results in increased blood flow pulsatility in medium-sized cerebral arteries (42); thus, supporting the concept of increased pulse pressure in the brain as a mechanism for the deleterious effects of large artery stiffness. Two weeks following carotid artery calcification, there is a decrease in resting cerebral blood flow and an impaired blood flow response to neuronal activity or endothelium-dependent stimuli (28). This reduced blood flow coincides with the presence of fewer blood vessels in the brain after carotid artery calcification surgery, as well as increased blood brain barrier permeability in the hippocampus (28). Furthermore, there is increased microglia and astrocyte activation in the hippocampus that is driven by increased oxidative stress after carotid calcification (43). Importantly, carotid artery calcification also leads to greater neurodegeneration and impaired memory (28, 43). These studies demonstrate that increased stiffness of the carotid arteries alone is sufficient to cause cerebrovascular dysfunction, neuroinflammation, and impaired memory.
Crosslink Breaker Treatment in Aged and Diabetic Rodents
The transgenic and carotid artery calcification models described above demonstrate the effects of increasing stiffness of the large arteries, but it is valuable to know the effects of reducing stiffness as well. Aging and diabetes lead to increased cross linking of collagen, and this can be reversed by treatment with collagen cross-link breakers, such as ALT-711 (44). ALT-711 reduces arterial stiffness in aged and diabetic rodents (30, 45). The effects of ALT-711 on cardiac and peripheral vascular outcomes have been studied extensively (24, 27); yet, there are only limited reports of the effect of ALT-711 on the brain. ALT-711 treatment results in preserved working memory, without modulating neurodegeneration, in a streptozotocin (STZ)-induced diabetic rat model (46). However, studies with compounds that alter collagen cross links are confounded by the potential direct effects on nonvascular cells in the brain (47). There is a need for methods to reduce large artery stiffness in a more controlled manner in aged rodents.
RODENT MODELS OF HYPERTENSION
Rodent models of hypertension are associated with greater large artery stiffness and/or cerebral artery pulse pressure (22, 26). Although these models lack direct cause-and-effect evidence for the effects of large artery stiffness, they are studied more extensively for brain outcomes than the models described in DIRECT MODULATION OF ARTERIAL STIFFNESS IN RODENTS. The hypertensive models of angiotensin II infusion in mice and the spontaneously hypertensive stroke-prone rat are associated with cognitive impairment and increased blood brain barrier permeability (48, 49). A limitation of these hypertensive models is that the increases in blood pressure are often extreme. Related to the effects of large artery stiffness, a more relevant model is transverse aortic constriction (TAC) as it increases arterial pulse pressure. TAC in mice leads to impaired memory, reduced cerebral perfusion, cerebral artery endothelial dysfunction, increased cerebral microbleeds, and increased blood brain barrier permeability (22). Importantly, when TAC is performed in transgenic mice with greater aberrant amyloid-β production, these vascular changes result in greater amyloid-β deposition (17, 22). Mechanisms involved in these cerebrovascular and cognitive impairments after TAC appear similar to those in models of large artery stiffness, with an increase in inflammatory and oxidative stress markers found in microvessels and brain tissue, as well as reduction in the gene expression of tight junction proteins (50). Thus, these hypertensive rodent models demonstrate that increased pulse pressure, as occurs with large artery stiffening, leads to cerebrovascular dysfunction, cognitive impairment, and increased AD-related pathology.
FUTURE DIRECTIONS
Although rodent models have provided proof-of-concept evidence for the association between increased large artery stiffness and deleterious effects on the brain, there remain significant gaps in our understanding of the related mechanisms. The continued study of available rodent models of greater large artery stiffness, and identifying the commonalities among these models, is crucial. It is also important to test the effects on the brain of reducing/preventing age-related increases in large artery stiffness. This could be accomplished with existing pharmacologic approaches, such as ALT-711 and pyridoxamine, or by novel interventions that more directly modulate large artery stiffness, such as perturbation of the vascular smooth muscle cell cytoskeleton (51). In addition, proof-of-concept evidence to support the associations between increased large artery stiffness and Alzheimer’s disease is still needed. Acquiring this evidence is hampered by the fact that mice do not naturally accumulate amyloid-β. Thus, an examination of combined rodent models of large artery stiffness and transgenic AD will be needed. For these studies, newer transgenic mouse models that more reasonably replicate the slow accumulation of Aβ in late-onset AD should be considered. Lastly, more attention is needed for the potential sex differences in these mechanisms, as well as the possibility for the influence of background strain in rodent studies (29). Two-thirds of patients with AD are women, highlighting the importance of understanding the mechanisms for cognitive decline and neurodegenerative disease in aging (postmenopausal) females. Estrogen is neuroprotective in premenopausal women, and the menopausal transition is associated with estrogen deficiency, greater arterial stiffness, and AD-related changes in brain, including a shift in brain metabolic processes (25, 52). Unfortunately, there is a lack of research in both humans and animals on the interaction of arterial stiffness, brain outcomes, and estrogen/menopause. Thus, continued study of rodent models is needed on multiple fronts to more fully understand the impact of large artery stiffness on the brain.
CONCLUSIONS
The strong associations between age, cardiovascular diseases, and dementia underscore the potential deleterious effects of vascular aging on the brain. There is overwhelming evidence that greater large artery stiffness relates to cognitive impairment among healthy older adults and patients with AD. However, studies in humans are limited to only correlative evidence whereas animal models allow researchers to explore the causative mechanisms linking arterial stiffness and neurocognitive dysfunction and diseases. There are multiple rodent models of direct modulation of large artery stiffness, each with their own strengths and limitations. Common outcomes in the brain have emerged among these models, including cerebrovascular dysfunction and increased oxidative stress. In addition, there is evidence for increased neuroinflammation, neurodegeneration, and cognitive impairments in mice with increased large artery stiffness. Key gaps in knowledge that remain are identifying the direct influence of greater large artery stiffness on AD-related pathology and determining the effects of preventing age-related increases in large artery stiffness on the brain. After these mechanisms have been solidified, the ultimate goal is to use these findings to identify and/or develop interventions to delay the onset or slow the progression of age-related cognitive decline and AD.
GRANTS
This work was supported by the National Institutes of Health Grant R01 AG064016 and Alzheimer’s Association Grant AARG-20-675709.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R.W., E.H.R., and A.E.W. conceived and designed research; N.R.W., E.H.R., and A.E.W. prepared figures; N.R.W., E.H.R., and A.E.W. drafted manuscript; N.R.W., E.H.R., and A.E.W. edited and revised manuscript; N.R.W., E.H.R., and A.E.W. approved final version of manuscript.
REFERENCES
- 1.Kramarow EA, Tejada-Vera B. Dementia mortality in the United States 2000–2017. Natl Vital Stat Rep 68: 1–29, 2019. [PubMed] [Google Scholar]
- 2.Cummings JL, Cole G. Alzheimer disease. JAMA 287: 2335–2338, 2002. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A Slowing arterial aging: how far have we progressed? J Hypertens 25: 509–510, 2007. doi: 10.1097/HJH.0b013e328017f71c. [DOI] [PubMed] [Google Scholar]
- 4.Thorin-Trescases N, Thorin E. Lifelong cyclic mechanical strain promotes large elastic artery stiffening: increased pulse pressure and old age-related organ failure. Can J Cardiol 32: 624–633, 2016. doi: 10.1016/j.cjca.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LL, Mitchell GF. Aortic stiffness, cerebrovascular dysfunction, and memory. Pulse (Basel ) 4: 69–77, 2016. doi: 10.1159/000448176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone J, Johnstone DM, Mitrofanis J, O'Rourke M. The mechanical cause of age-related dementia (Alzheimer’s disease): the brain is destroyed by the pulse. J Alzheimers Dis 44: 355–373, 2015. doi: 10.3233/JAD-141884. [DOI] [PubMed] [Google Scholar]
- 8.Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc 7: e007621, 2018.doi: 10.1161/JAHA.117.007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouch L, Cestac P, Sallerin B, Andrieu S, Bailly H, Beunardeau M, Cohen A, Dubail D, Hernandorena I, Seux ML, Vidal JS, Hanon O. Pulse wave velocity is associated with greater risk of dementia in mild cognitive impairment patients. Hypertension 72: 1109–1116, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11443. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Palta P, Meyer ML, Kucharska-Newton A, Pence BW, Aiello AE, Power MC, Walker KA, Sharrett AR, Tanaka H, Jack CR, Mosley TH, Reid RI, Reyes DA, Heiss G. Aortic stiffness and white matter microstructural integrity assessed by diffusion tensor imaging: the ARIC-NCS. J Am Heart Assoc 9: e014868, 2020.doi: 10.1161/JAHA.119.014868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol 71: 562–568, 2014. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, Wong D, Zhou Y, Knopman D, Mosley TH, Gottesman RF. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET study. Neurology 90: e1248–e1256, 2018. doi: 10.1212/WNL.0000000000005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Bueno C, Cunha PG, Martinez-Vizcaino V, Pozuelo-Carrascosa DP, Visier-Alfonso ME, Jimenez-Lopez E, Cavero-Redondo I. Arterial stiffness and cognition among adults: a systematic review and meta-analysis of observational and longitudinal studies. J Am Heart Assoc 9: e014621, 2020. doi: 10.1161/JAHA.119.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J Neurochem 144: 527–548, 2018. doi: 10.1111/jnc.14235. [DOI] [PubMed] [Google Scholar]
- 16.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 53: 121–130, 2015. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, Passarelli F, Landolfi A, Carullo P, Lembo GB. Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging 30: 222–228, 2009. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, Nair S, Pechman KR, Rane S, Davis LT, Gifford KA, Hohman TJ, Bell SP, Wang TJ, Beckman JA, Carr JJ. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 138: 1951–1962, 2018. doi: 10.1161/CIRCULATIONAHA.118.032410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuBose LE, Boles Ponto LL, Moser DJ, Harlynn E, Reierson L, Pierce GL. Higher aortic stiffness is associated with lower global cerebrovascular reserve among older humans. Hypertension 72: 476–482, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes CA Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93: 74–104, 1979. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 21.Henson GD, Walker AE, Reihl KD, Donato AJ, Lesniewski LA. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol Rep 2: e00268, 2014. doi: 10.1002/phy2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Montgolfier O, Pincon A, Pouliot P, Gillis MA, Bishop J, Sled JG, Villeneuve L, Ferland G, Levy BI, Lesage F, Thorin-Trescases N, Thorin E. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension 73: 217–228, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12048. [DOI] [PubMed] [Google Scholar]
- 23.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX, Degroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 11: 191–195, 2005. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Moreau KL, Hildreth KL, Klawitter J, Blatchford P, Kohrt WM. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 42: 1699–1714, 2020. doi: 10.1007/s11357-020-00236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leloup AJA, De Moudt S, Van Hove CE, Dugaucquier L, Vermeulen Z, Segers VFM, De Keulenaer GW, Fransen P. Short-term angiotensin ii treatment affects large artery biomechanics and function in the absence of small artery alterations in mice. Front Physiol 9: 582, 2018. doi: 10.3389/fphys.2018.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 104: 1464–1470, 2001. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 28.Muhire G, Iulita MF, Vallerand D, Youwakim J, Gratuze M, Petry FR, Planel E, Ferland G, Girouard H. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J Am Heart Assoc 8: e011630, 2019. doi: 10.1161/JAHA.118.011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuner SM, Heuer SE, Huentelman MJ, O'Connell KMS, Kaczorowski CC. Harnessing genetic complexity to enhance translatability of Alzheimer's disease mouse models: a path toward precision medicine. Neuron 101: 399–411.e5, 2019. doi: 10.1016/j.neuron.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DE. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol 47: 565–572, 2012. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117: 2938–2948, 2008. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP, Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res 11: 97–112, 2008. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 35.Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA, Donato AJ. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol 593: 1931–1943, 2015. doi: 10.1113/jphysiol.2014.285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker AE, Kronquist EK, Chinen KT, Reihl KD, Li DY, Lesniewski LA, Donato AJ. Cerebral and skeletal muscle feed artery vasoconstrictor responses in a mouse model with greater large elastic artery stiffness. Exp Physiol 104: 434–442, 2019. doi: 10.1113/EP087453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knutsen RH, Beeman SC, Broekelmann TJ, Liu D, Tsang KM, Kovacs A, Ye L, Danback JR, Watson A, Wardlaw A, Wagenseil JE, Garbow JR, Shoykhet M, Kozel BA. Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am J Physiol Heart Circ Physiol 315: H18–H32, 2018. doi: 10.1152/ajpheart.00683.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung AW, Au Yeung K, Sandor GG, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res 101: 512–522, 2007. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 39.Onetti Y, Meirelles T, Dantas AP, Schroder K, Vila E, Egea G, Jimenez-Altayó F. NADPH oxidase 4 attenuates cerebral artery changes during the progression of Marfan syndrome. Am J Physiol Heart Circ Physiol 310: H1081–1090, 2016. doi: 10.1152/ajpheart.00770.2015. [DOI] [PubMed] [Google Scholar]
- 40.Bhatt SP, Cole AG, Wells JM, Nath H, Watts JR, Cockcroft JR, Dransfield MT. Determinants of arterial stiffness in COPD. BMC Pulm Med 14: 1, 2014. doi: 10.1186/1471-2466-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Fujiyoshi A, Willcox B, Choo J, Vishnu A, Hisamatsu T, Ahuja V, Takashima N, Barinas-Mitchell E, Kadota A, Evans RW, Miura K, Edmundowicz D, Masaki K, Shin C, Kuller LH, Ueshima H, Sekikawa A, Ejs G. Increased aortic calcification is associated with arterial stiffness progression in multiethnic middle-aged men. Hypertension 69: 102–108, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadekova N, Vallerand D, Guevara E, Lesage F, Girouard H. Carotid calcification in mice: a new model to study the effects of arterial stiffness on the brain. J Am Heart Assoc 2: e000224, 2013.doi: 10.1161/JAHA.113.000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadekova N, Iulita MF, Vallerand D, Muhire G, Bourmoum M, Claing A, Girouard H. Arterial stiffness induced by carotid calcification leads to cerebral gliosis mediated by oxidative stress. J Hypertens 36: 286–298, 2018. doi: 10.1097/HJH.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 44.Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Da K. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens 25: 577–583, 2007. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]
- 45.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci U S A 95: 4630–4634, 1998. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakaria MN, El-Bassossy HM, Barakat W. Targeting AGEs signaling ameliorates central nervous system diabetic complications in rats. Adv Pharmacol Sci 2015: 1–9, 2015. 346259, doi: 10.1155/2015/346259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging 32: 763–777, 2011. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 43: 1115–1122, 2012. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner A, Minnerup J, Soria G, Planas AM. Structural and functional brain alterations in a murine model of angiotensin II-induced hypertension. J Neurochem 140: 509–521, 2017. doi: 10.1111/jnc.13905. [DOI] [PubMed] [Google Scholar]
- 50.de Montgolfier O, Pouliot P, Gillis MA, Ferland G, Lesage F, Thorin-Trescases N, Thorin E. Systolic hypertension-induced neurovascular unit disruption magnifies vascular cognitive impairment in middle-age atherosclerotic LDLr(−/−):hApoB(+/+) mice. Geroscience 41: 511–532, 2019. doi: 10.1007/s11357-019-00070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholson CJ, Singh K, Saphirstein RJ, Gao YZ, Li Q, Chiu JG, Leavis P, Verwoert GC, Mitchell GF, AortaGen C, Porter T, Morgan KG. Reversal of aging-induced increases in aortic stiffness by targeting cytoskeletal protein-protein interfaces. J Am Heart Assoc 7: e008926, 2018. doi: 10.1161/JAHA.118.008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, Etingin O, Henchcliffe C, Brinton RD, Mosconi L. Sex and gender driven modifiers of Alzheimer's: the role for estrogenic control across age, race, medical, and lifestyle risks. Front Aging Neurosci 11: 315, 2019. doi: 10.3389/fnagi.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]