Summary
Background
Positive airway pressure (PAP) has become a prominent treatment for children with sleep-disordered breathing. However, there are no large-scale studies to clarify whether PAP is well tolerated in children, and which factors are associated with better adherence to PAP therapy. In this study, we aimed to clarify adherence patterns of PAP therapy in a large paediatric population.
Methods
We did a cross-sectional big-data analysis in children from Oct 1, 2014, to Aug 1, 2018, using existing data derived from PAP devices uploaded nightly in the AirView cloud database. The AirView database is a usage tracking system available to all patients who are assigned PAP therapy, which requires consent from the patient or parent or guardian. All patients older than 4 years and younger than 18 years who used continuous or automated PAP devices were evaluated. Only patients living in the USA and enrolled with a single insurance company were included. If patients were participating in an engagement programme, programme onset must have been within 7 days of therapy onset. Our primary outcome was the proportion of patients who used PAP continuously over 90 days. The primary outcome was assessed in all patients who met the age inclusion criterion and had reliable age data available. Data on missing PAP use were imputed as zero, but data on other metrics were not imputed and excluded from analysis.
Findings
We used data recorded from Oct 1, 2014, to Aug 1, 2018. Of 40 140 children screened, 36 058 (89·8%) were US residents and 20 553 (90·1%) of them met the eligibility criteria and had accessible data (mean age 13·0 years [SD 3·7]). On the basis of 90 days of monitoring data, 12 699 (61·8%) patients continuously used PAP. Factors significantly associated with adherence included age group, residual apnoea–hypopnoea index, use and onset of patient engagement programmes, PAP pressure, and nightly median PAP mask leak, all over the 90-day study period.
Interpretation
To our knowledge, our study represents the largest analysis of children using PAP therapy to date. The findings suggest that adherence to PAP therapy is lower than in previous reports from adults. However, numerous actionable factors were associated with improvements in adherence and should be used strategically in clinical decision making to improve PAP adherence in children.
Funding
ResMed.
Introduction
Obstructive sleep apnoea is a highly prevalent condition in children, with a reported overall prevalence of 2–3%.1 Adenotonsillar hypertrophy is the main contributor to the pathogenesis of paediatric obstructive sleep apnoea.2 Therefore, adenotonsillectomy is the first-line therapy for children with obstructive sleep apnoea.3 However, this approach is not always effective, particularly in older children (>7 years), children with obesity, or those with very severe obstructive sleep apnoea.4 Nevertheless, increased awareness of the complications of untreated obstructive sleep apnoea (including cardiovascular dysfunction,5 systemic inflammation,6,7 insulin resistance and metabolic disease,8 and reduced quality of life)9 mean that, over time, an increasing number of adenotonsillectomies are being done.10
Childhood obesity is now second to adenotonsillar hypertrophy as the most important contributor to paediatric obstructive sleep apnoea. With childhood obesity prevalence ranging from 7% to 22% in various high-income countries,11,12 there has been an associated marked increase in the prevalence of paediatric obstructive sleep apnoea.13,14 Further, at any level of obstructive sleep apnoea severity, the magnitude of adenotonsillar hypertrophy has low relevance in children with obesity,15 potentially complicating standard treatment approaches. When adenotonsillectomy is contraindicated (particularly in children with morbid obesity) or when obstructive sleep apnoea is refractory to surgery, positive airway pressure (PAP) becomes the mainstay for therapy. With the increasing prevalence of childhood obesity, it is also anticipated that an increasing number of children will require PAP therapy to treat obstructive sleep apnoea effectively.
Evidence supporting the efficacy of PAP therapy in children comes only from studies with small sample sizes.16–24 Although PAP has been shown to be an efficacious therapy in paediatric obstructive sleep apnoea, adherence is often suboptimal and is poorly understood.23 In this study, we used big data to evaluate adherence in US children who were prescribed PAP therapy. Additionally, we investigated whether actionable clinical factors (ie, PAP nightly use, mask leak, residual obstructive sleep apnoea persisting despite therapy, and use of a patient engagement programme) would be predictive of PAP adherence. The main goal was to identify strategies to optimise obstructive sleep apnoea treatment by improving PAP adherence in children.
Methods
Study design and participants
This retrospective cross-sectional analysis of the AirView database (ResMed; San Diego, CA, USA) was reviewed by a central Institutional Review Board and deemed exempt from ethical oversight as per Department of Health and Human Services regulations.25 The data analysed were those that met the following criteria: all available data collected from Oct 1, 2014 to Aug 1, 2018; appropriate age of the patient (>4 to <18 years) at time of activation; onset of patient engagement programme within 7 days of therapy onset; and use of specific PAP devices (AirSense 10 or AirCurve 10; ResMed).
The first day of therapy was taken as the AirView setup date, which is the day when the health-care provider registered the patient’s device in the database. Only patients who used continuous positive airway pressure (CPAP) or automatic positive airway pressure (APAP) were included. Bilevel modes of PAP were excluded because they are typically used in children to treat sleep-related hypoventilation or neuromuscular disease, rather than obstructive sleep apnoea only.
Only patients enrolled with one company for durable medical equipment were included to eliminate duplicates in the cohort. Enrolment with multiple companies could have occurred if patients moved to a different state or if they changed insurance companies, and could result in one individual being provided with multiple devices; such duplicate patients were excluded. Patients enrolled in military health-care plans were also excluded due to a high probability that these were adults misclassified as children.
AirView is a password-protected cloud technology compliant with the Health Insurance Portability and Accountability Act. Following written informed consent or digital consent obtained by the manufacturing company from the patient or their parent or guardian, data derived from PAP devices are recorded nightly and are automatically uploaded to AirView on a daily basis to help clinicians and companies that produce durable medical equipment to remotely monitor patient adherence.
Data collected include nightly PAP use (total duration and time of use), efficacy of PAP use including residual apnoea–hypopnoea index (rAHI), PAP pressure (cm H2O), and mask leak (L/min). The rAHI is calculated through machine detection of apnoeas and hypopnoeas during PAP device use. The total number of apnoeas and hypopnoeas is then averaged per hour of device use. The patient engagement programme (myAir; ResMed) is designed for patients and provides real-time daily feedback to the patient about their PAP use, while also providing them with coaching on the basis of the data collected. Interested patients sign up themselves if they choose to opt in, but parents and guardians will often sign up on behalf of their young children.
Patients can access information by logging onto the proprietary website, or via a smartphone app. Real-time feedback provided to patients and families includes a score that incorporates use time and duration, mask seal indicating leak level, respiratory events per hour of use, and the number of times the mask was put on and taken off. Patients also receive constructive suggestions on the basis of mask leak and rAHI data. Personalised coaching messages are sent via email or through the smartphone app. These messages are designed to enhance self-management, recognise success through awards, and allow patients to identify and resolve basic treatment issues on their own. The platform provides feedback with an overall goal to improve PAP adherence (appendix p 3).
Outcomes
The primary outcome was the proportion of patients who continued to use PAP therapy over 90 days—ie, not having terminated their therapy due to 30 consecutive nights of non-use during the first 90 days of data collection, which was considered to be evidence of ineffective therapy due to therapy termination. Thus, the primary outcome was met in the absence of therapy termination.
Additionally, as a secondary outcome, we evaluated the proportion of patients who met the Centers for Medicare and Medicaid Services (CMS) criteria for adherence (ie, PAP use for 4 h or more per night on at least 70% of the nights since therapy onset during a consecutive 30-day period during the initial 90 days of use).26 These CMS criteria were originally intended for adult patients using PAP devices. Secondary outcomes included days to achieve CMS adherence; proportion of adherent days (≥4 h per night); average use per session (h) defined as one night of PAP use; and average daily use.
To evaluate whether age was predictive of the primary outcome, we split the cohort into four prespecified groups by age (>4 to <6 years, 6 to <12 years, 12 to <15 years, and 15 to <18 years). These age groups were chosen arbitrarily to reflect different school-age groups, namely preschool, primary school, middle school, and high school.
In addition to age, we assessed whether other factors were predictive of PAP adherence, including rAHI over 90 days, onset of the patient engagement programme, overall pressure settings over 90 days as measured by the 95th percentile pressure, and average nightly median leak over 90 days.
Statistical analysis
All data that met the inclusion criteria were de-identified before the analysis of the outcomes. Missing data on CPAP use were imputed as zero, whereas missing data in therapy metrics, such as pressure settings, reported pressure, and mask leak, were not imputed and not included in the calculations of means, SDs, and proportions. When calculating summary statistics of rAHI, use sessions shorter than 1 h were removed. Statistical hypothesis testing to compare the distribution from different age groups was done using the χ2 test for categorical variables or ANOVA test for continuous variables. A multivariate Cox proportional hazard model was created controlling for all significant predictors. Proportional assumptions were tested in the study.
Non-parametric Kaplan-Meier estimates were used to determine the length of time during which patients were self-administering therapy (ie, adherence to therapy). Patients who did not terminate therapy before Aug 1, 2018, were right-censored. A log-rank test was used to determine the significance of adherence predictors. Survival curves for the first 90-day window were calculated with non-survival meaning 30 consecutive nights of nonuse, with day 1 of non-use corresponding to the date of drop-off. All statistical calculations were done using R statistical software (version 1.0.153).
Role of the funding source
Representatives from the study sponsor were involved in the study design, collection, analysis and interpretation of data, writing of the report, and in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
40 140 children were screened and were younger than 18 years, of whom 36 058 (89·8%) were living in the USA. Of the screened participants, 22 785 (56·8%) were enrolled in a non-military health plan, but only 20 533 (51·2%) were of the required age and had reliable data available and were thus included in the analyses (figure 1). Mean patient age was 13·0 years (SD 3·7), and the largest age group was 15 to less than 18 years (7949 [38·7%] of all patients who met the eligibility criteria; table 1). The average minimum PAP pressure was 6·8 cm H2O (SD 2·6) and the average maximum pressure was 10·4 cm H2O (4·4). 4109 (20·0%) of patients who had reliable age data chose to enrol in the patient engagement programme during the study period (table 1).
Figure 1: Patient selection from the AirView database.
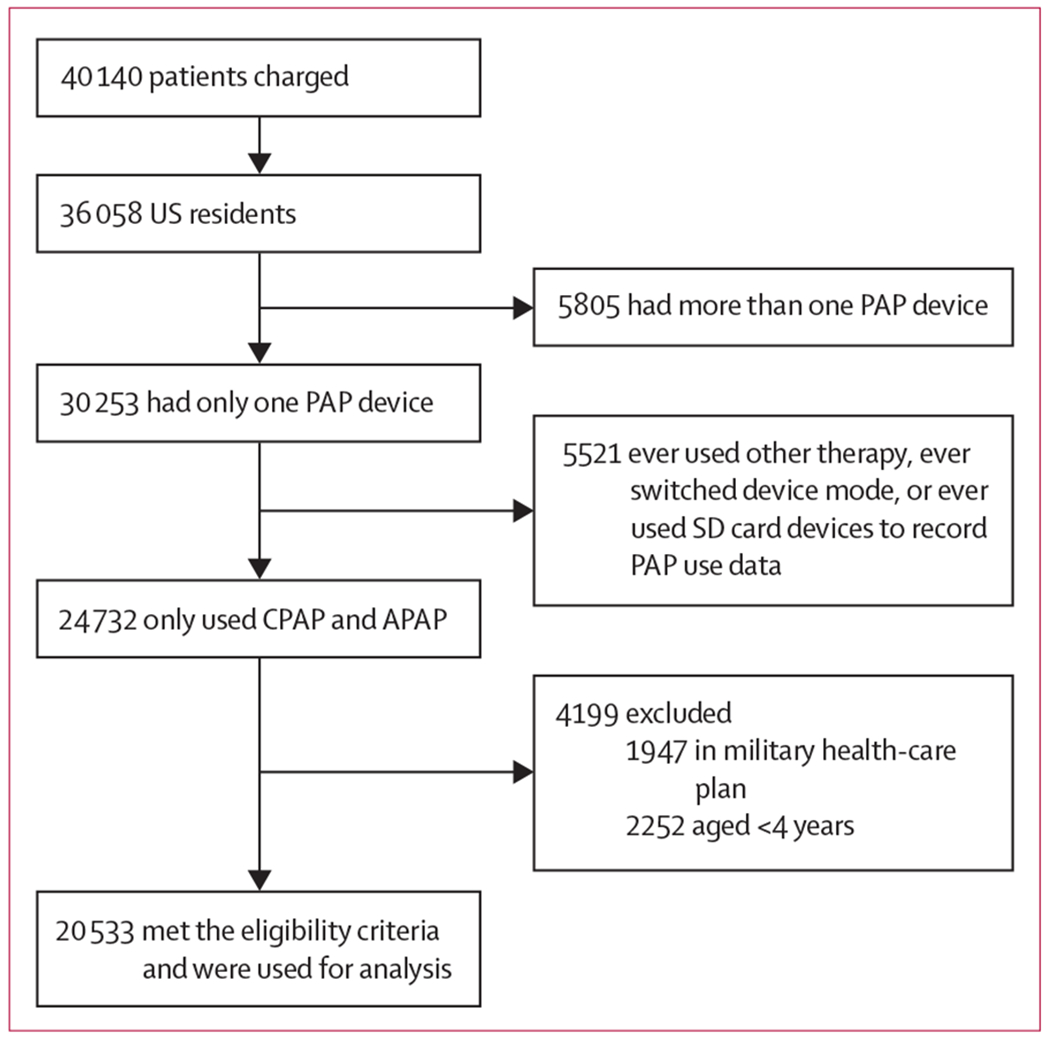
APAP=automated PAP. CPAP=continuous PAP. PAP=positive airway pressure. SD card=Secure Digital card.
Table 1:
Summary patient data extracted from the AirView database
| Patients (n=20 553) | |
|---|---|
| Age, years | 13·0 (3·7) |
| Age group | |
| >4 to <6 years | 1052 (5·1%) |
| 6 to <12 years | 6407 (31·2%) |
| 12 to <15 years | 5125 (25·0%) |
| 15 to <18 years | 7949 (38·7%) |
| Patient engagement programme users | 4109 (20·0%) |
| Minimum pressure setting, cm H2O | 6·8 (2·6) |
| Maximum pressure setting, cm H2O | 10·4 (4·4) |
| PAP use and adherence | |
| Patients actively using PAP over 90 days | 12 699 (61·8%) |
| Patients meeting CMS adherence criteria | 9504 (46·3%) |
| Proportion of days with ≥4 h use | 45·1 (33·3%) |
| Use per session, h | 5·2 (2·6) |
| Daily use across all days, h | 3·9 (2·8) |
Data are mean (SD), or number (%). CMS=Centers for Medicare and Medicaid Services. PAP=positive airway pressure.
Over the first 90 days of monitoring data, PAP therapy was active in 12 699 (61·8%) of analysed patients and was not terminated according to our definition. According to CMS-defined adherence criteria, 9504 (46·3%) patients met the criteria of 4 or more h of use for at least 70% of the nights for 30 consecutive nights during the first 90 days (table 1). Geographical representation of adherence showed relatively consistent adherence patterns across all states (appendix p 1). Overall, mean average daily use was 3·9 h (SD 2·8), with a mean average use per session of 5·2 h (2·6; table 1). The proportion of days adherent (days with PAP use ≥4 h) was 45·1% (SD 33·3). When investigating adherence across the predefined age groups, the group aged 15 to less than 18 years had the least number of patients meeting CMS adherence criteria, whereas those aged 6 to less than 12 years were most likely to meet them (table 2). Average use per session was also highest in the age group of 6 to less than 12 years, whereas the group aged 15 to less than 18 years had the lowest use per session (table 2).
Table 2:
PAP adherence in subgroups based on patient age
| >4 to <6 years | 6 to <12 years | 12 to <15 years | 15 to <18 years | p value | |
|---|---|---|---|---|---|
| Patients actively using PAP over 90 days | 587 (55·8%) | 4408 (68·8%) | 3213 (62·7%) | 4491 (56·5%) | 0·0015 |
| Patients meeting CMS adherence criteria | 483 (45·9%) | 3577 (55·8%) | 2320 (45·3%) | 3124 (39·3%) | <0·0001 |
| Patients terminating PAP over 90 days | 465 (44·2%) | 1999 (31·2%) | 1912 (37·3%) | 3458 (43·5%) | <0·0001 |
| Days of active PAP use | 22 (6–45) | 35 (12·5–59) | 33 (13–60) | 29 (9–55) | <0·0001 |
| Proportion of days with ≥4 h use | 45·5 (35·8) | 52·4 (32·7) | 44·2 (32·9) | 39·7 (32·7) | <0·0001 |
| Use per session, h | 5·3 (3·1) | 5·9 (2·7) | 5·0 (2·5) | 4·6 (2·4) | <0·0001 |
| Daily use across all days, h | 4·1 (3·1) | 4·6 (2·9) | 3·8 (2·7) | 3·3 (2·5) | <0·0001 |
Data are mean (SD), number (%), or median (IQR). CMS=Centers for Medicare and Medicaid Services. PAP=positive airway pressure.
Survival curve analysis (figure 2, table 2), showing the proportion of patients continuously using PAP therapy over the 90-day period indicated that variations in PAP use between the four age groups became evident early during treatment follow-up (within the first 30 days). Significantly more patients enrolled in the patient engagement programme used PAP over the 90-day period than those who were not enrolled. Median PAP use in those patients who were enrolled was 45 days (95% CI 42–48), and median PAP use in patients who were not enrolled was 29 days (23–69). 3249 (79·1%) of 4109 users of the patient engagement programme remained on PAP therapy, compared with 2363 (57·5%) of the 16 424 participants not enrolled in the programme (p<0·0001; figure 3). This effect was consistent across each age group (figure 3).
Figure 2: PAP use across age-based patient subgroups over 90 days of monitoring data.
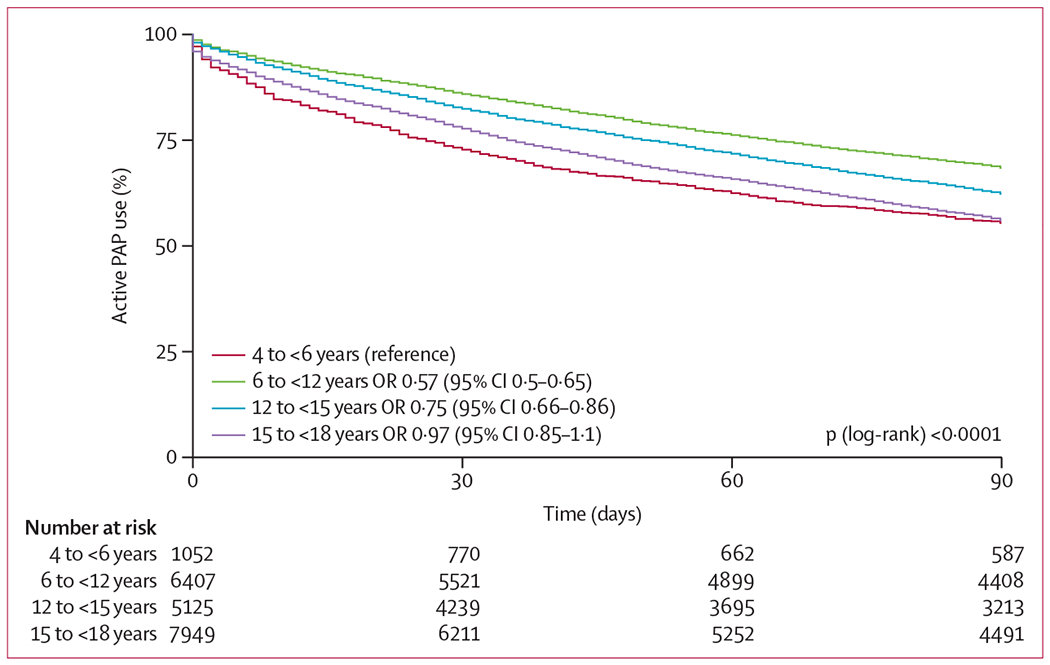
The number at risk reflects the number of remaining active PAP users at each time period. PAP=positive airway pressure. OR=odds ratio.
Figure 3: Effect of a patient engagement programme on PAP use in the entire cohort (A) and age-based patient subgroups (B).
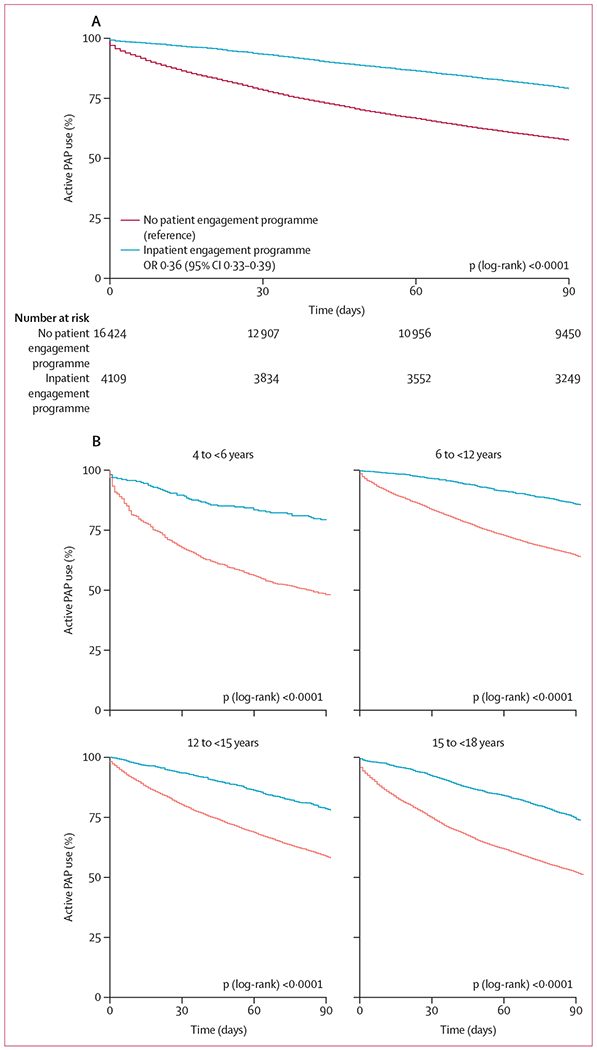
The number at risk reflects the number of remaining active PAP users at each time period. OR=odds ratio.
Conversely, 860 (20·9%) patients enrolled in an engagement programme terminated therapy, as did 6974 (42·5%) patients not enrolled in a programme. Additionally, the presence of residual sleep apnoea had marked effects on PAP use: patients with a rAHI of at least five events per h had the lowest reported PAP use (1822 [55·6%], p<0·0001; appendix p 2). Average 95th percentile pressure level over 90 days also influenced PAP use, with the lowest 95th percentile pressure range (4 to <6 cm H2O) being associated with the lowest PAP use (1609 [52·0%], p<0·0001; appendix p 2). Finally, median mask leak averaged over 90 days was also significantly associated with PAP use, which was lowest when average median leak exceeded 24 L/min (282 [43·7%], p<0·0001; appendix p 2). Using multivariate Cox regression modelling, patient age, patient engagement programme activation status, mask leak, rAHI, and 95th percentile pressure and median leak remained significantly associated with PAP use at the end of the 90-day period (figure 4). Patient engagement programme activation showed the greatest association with improved PAP use compared with other variables, whereas increased median mask leak was most likely to curb use or to lead to therapy termination.
Figure 4: Multivariate Cox regression model assessing the influence of known risk factors on PAP therapy termination.
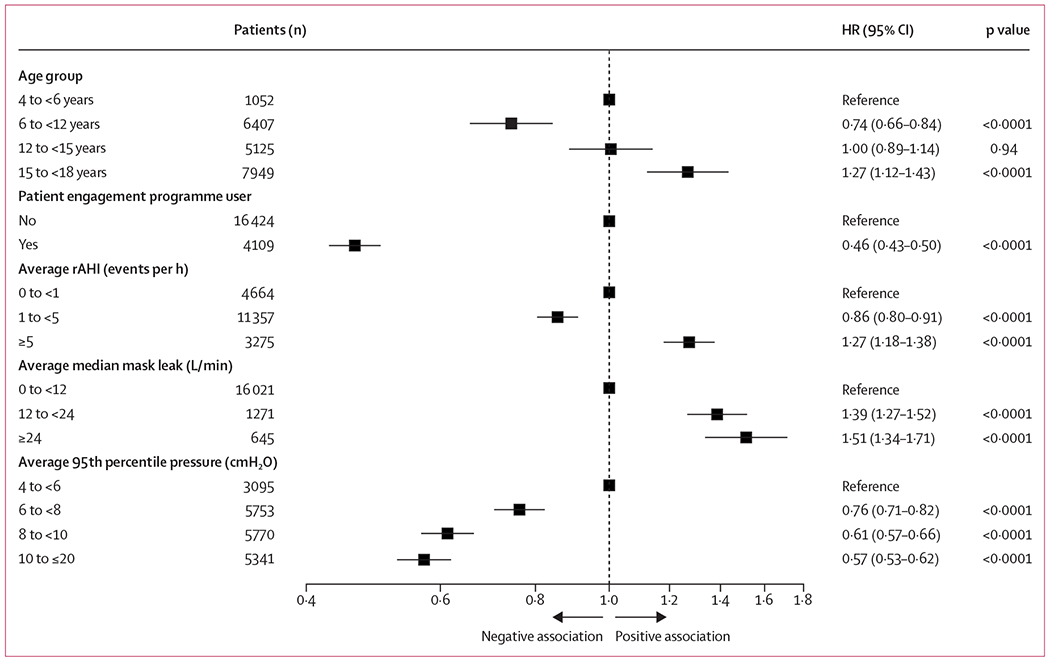
The total number of events was 5912. HR=hazard ratio. PAP=positive airway pressure. rAHI=residual apnoea–hypopnoea index.
Discussion
In our study, we report real-world data describing adherence to PAP therapy in a paediatric population. To our knowledge, this is the largest analysis of PAP adherence in paediatric patients with obstructive sleep apnoea to date. We found that most children were actively using PAP therapy; 61·8% of the cohort were still using their device after 90 days and did not have therapy terminated due to 30 consecutive nights of non-use.However, only 46·3% of the cohort met CMS criteria for adherence at 90 days. This adherence compares favourably with other chronic medical therapies in children,27 but is worse than reports from studies using similar methods to measure adherence in adults.28
We have also identified potential modifiable factors that could be used as therapeutic targets to optimise adherence in future studies of paediatric obstructive sleep apnoea. Moreover, the identification of groups most likely to struggle with PAP use could also help to guide clinical decision making. For example, our data suggest that young children aged more than 4 to less than 6 years and teenagers aged 15 to less than 18 years might need closer attention and support than other age groups, meaning that age-specific behavioural interventions could be needed.
Additionally, increased rAHI resulted in poor adherence. High rAHI might be indicative of subtherapeutic pressures leading to suboptimal therapy, reducing an individual’s motivation to use the PAP device. Our finding that patients using higher PAP pressures had better adherence potentially reflects better pressure delivery and symptom resolution (although use of increased pressure could also be due to increased obstructive sleep apnoea severity). Mask fit as denoted by mask leak was also highly predictive of patient adherence. Finally, we observed that a patient engagement programme was associated with a substantial beneficial effect on PAP use in paediatric patients. This finding is consistent with adult studies,29 but was somewhat surprising, given that all age groups appeared to benefit from technologies developed for adult patients.
In reviewing the literature regarding strategies to improve adherence to PAP therapy, we note that most studies have been done in adults rather than in children. Paediatric studies are small, with sample sizes ranging from 29 to 140 patients.16–24 Moreover, reported PAP adherence is highly variable (49–70% of patients), perhaps as a consequence of the small study populations. Additionally, these studies did not always identify factors such as age, severity of obstructive sleep apnoea, mask interface, and therapeutic pressure as predictors of adherence. A European study evaluated adolescents versus primary-school children and, similar to our findings, showed that adolescents had worse PAP adherence.24
Additional demographic factors, including race and maternal education, have been previously reported to be significantly associated with PAP adherence in children,17 but data on these parameters were not available in our study because of privacy issues.
Another limitation of existing paediatric PAP literature is the considerable heterogeneity in clinical practice across different centres, including access to multidisciplinary approaches that might involve child psychologists. These differences mean that such studies might lack generalisability. The inclusion of a large dataset from across the USA is an important positive feature of our study. For this preliminary analysis, we did not include data for children outside the USA to try and ensure that our population was relatively homogeneous. However, a more global population will be included in future studies, allowing evaluation and comparison of PAP adherence for different devices and countries.
Our findings support monitoring of treatment effectiveness parameters collected by PAP devices as an important strategy to identify methods to improve adherence. It should be acknowledged that we are unclear about how accurate PAP devices are at assessing sleep-disordered breathing in children. In theory, thresholds for mask leak and rAHI detection in small children are different from those in adults. Regardless, we clearly advocate for further study of paediatric obstructive sleep apnoea management and highlight the importance of technological advancement with paediatric patients in mind. Although we accept that adherence in children with obstructive sleep apnoea appears lower than that reported in adults, we would argue that the potential therapeutic effect of treating children with obstructive sleep apnoea could be substantial, given the vulnerability of the developing brain and the potential for primary prevention of adult cardiometabolic disease.30
The optimal definition of PAP adherence in children is not yet clear. Given that total sleep time is longer in children than in adults, it is possible that more hours of PAP use are required to optimise health outcomes in children than in adults. Furthermore, the usefulness of the CMS criteria used in our study for defining adherence in children is also unknown. Presumably, external factors such as travel (eg, summer vacation) or occurrence of a respiratory illness, could contribute to 30 days of non-use. Published studies and clinical experience both suggest that some children require time to achieve motivation for adherence (ie, there is a learning curve).31 Ultimately, decisions regarding optimal PAP use will need to be data-driven on the basis of robust health outcome measures.
Despite the strengths of our study, we acknowledge several important limitations related to confounding factors and the observational nature of our data. First, there is insufficient detail about participant characteristics, including diagnostic data and demographic information, such as socioeconomic status. We were also unaware of whether patients underwent additional interventions to manage obstructive sleep apnoea (eg, adenotonsillectomy or weight loss) during the study period, and whether such interventions resulted in interruption or termination of PAP therapy. Thus, our estimates should be considered conservative, given that some patients who did not adhere to PAP might have received alternative therapy. Second, we did not do a randomised trial and thus we cannot conclude with certainty that the patient engagement programme was directly responsible for improved PAP use. Instead, the association identified provides a hypothesis and rationale for future randomised controlled trials. In theory, the patients and families using the patient engagement programme might be generally more motivated or better educated than other groups, and thus use PAP better.
We acknowledge that the patient engagement programme was designed for the specific PAP devices and we cannot determine whether the specific patient engagement programme we investigated is applicable to all currently available PAP devices. Thus, further work would be required to determine whether other platforms that support patient engagement would also contribute to improved adherence. Third, study data were obtained only from patients who used CPAP and APAP devices.Patients using bilevel modes were excluded because the primary use of bilevel PAP in children is for supportive ventilation rather than treating obstructive sleep apnoea.
Therefore, our findings only apply to the devices and population studied. Another important point to note is that we might have included patients using CPAP or APAP for indications not related to obstructive sleep apnoea, such as upper airway resistance syndromes or hypoventilation. Furthermore, because we used a single database, conclusions cannot be drawn about other patient groups who were not included. For example, we were unable to review data from PAP devices from other manufacturers. Because our ethics approval was limited to the USA, we support studies that examine important issues in children with sleep apnoea globally.32 However, in the USA, we view our cohort as highly generalisable, given the geographical variability and the large sample of unselected participants in our analyses. Nevertheless, there are a group of patients who have neither adequate health-care access nor access to diagnostic sleep testing or to PAP therapy, who would not have been included in our analysis. Therefore, we would advocate for further study investigating how best to deliver PAP treatment for all patients who need it.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published in English from inception to Aug 20, 2019, using the search terms “OSA”, “positive airway pressure”, and “adherence”, for data on children younger than 18 years. Currently available evidence examining the efficacy of positive airway pressure (PAP) devices for the treatment of paediatric sleep-disordered breathing is restricted to single-centre studies with small population sizes (29 to 140 patients per study). Although these studies have evaluated factors associated with improvements in adherence to PAP therapy, the heterogeneity of both the populations and clinical practices across various paediatric sleep clinics, combined with the small population size, limit the generalisability of these findings.
Added value of this study
Using a big-data approach, we integrated data from across the USA, included a large cohort of children, and critically examined adherence to PAP. We were able to determine several factors that were significantly associated with PAP adherence, including use of patient engagement tools, mask leak, and age. Our study provides real-world data on PAP use in children to describe actual adherence patterns of PAP use nationwide.
Implications of all the available evidence
Our analysis documented PAP adherence in children that was lower than that in previous reports from adults. However, several modifiable factors were found to be associated with improvements in adherence. Future well designed, randomised controlled trials should evaluate the ability of interventions to modify these factors to improve adherence patterns during the use of PAP therapy in children.
Acknowledgments
Medical writing assistance for editing was provided by Nicola Ryan, independent medical writer, funded by ResMed.
Declaration of interests
RB has received consulting and speaker’s fees from Jazz Pharmaceuticals. AVB, YY, CMN, and JA are all employees of ResMed. J-LDP is supported by the French National Research Agency in the framework of the Investissements d’avenir programme (ANR-15-IDEX-02), and his department has received research support from Philips Respironics, Fisher and Paykel, and ResMed. PAC has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding; has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies; is a consultant to Zephyr Sleep Technologies and Narval; and has a pecuniary interest in SomnoMed related to a 2004 role in research and development (2004). HW has received consulting and speaker’s fees from ResMed and Inspire Medical. AM relinquished all outside personal income as an officer of the American Thoracic Society in 2012. ResMed gave a philanthropic donation to University of California San Diego (La Jolla, CA, USA), but AM receives no personal income from ResMed.
Footnotes
References
- 1.Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest 1995; 107: 963–66. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep 2004; 27: 997–1019. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012; 130: 576–84. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010; 182: 676–83. [DOI] [PubMed] [Google Scholar]
- 5.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 2008; 51: 84–91. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee R, Kheirandish-Gozal L, Kaditis AG, Verhulst SL, Gozal D. C-reactive protein as a potential biomarker of residual obstructive sleep apnea following adenotonsillectomy in children. Sleep 2016; 39: 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye J, Liu H, Li P, et al. CD4(+)T-lymphocyte subsets in nonobese children with obstructive sleep apnea syndrome. Pediatr Res 2015; 78: 165–73. [DOI] [PubMed] [Google Scholar]
- 8.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008; 177: 1142–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur Respir J 2017; 50: 1700985. [DOI] [PubMed] [Google Scholar]
- 10.Erickson BK, Larson DR, St Sauver JL, Meverden RA, Orvidas LJ. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970–2005. Otolaryngol Head Neck Surg 2009; 140 : 894–901. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 2006; 1: 11–25. [DOI] [PubMed] [Google Scholar]
- 12.Lobstein T, Jackson-Leach R. Child overweight and obesity in the USA: prevalence rates according to IOTF definitions. Int J Pediatr Obes 2007; 2: 62–64. [DOI] [PubMed] [Google Scholar]
- 13.Rudnick EF, Walsh JS, Hampton MC, Mitchell RB. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg 2007; 137: 878–82. [DOI] [PubMed] [Google Scholar]
- 14.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev 2006; 7: 247–59. [DOI] [PubMed] [Google Scholar]
- 15.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest 2009; 136: 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics 2006; 117: e442–51. [DOI] [PubMed] [Google Scholar]
- 17.DiFeo N, Meltzer LJ, Beck SE, et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med 2012; 8: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machaalani R, Evans CA, Waters KA. Objective adherence to positive airway pressure therapy in an Australian paediatric cohort. Sleep Breath 2016; 20: 1327–36. [DOI] [PubMed] [Google Scholar]
- 19.Nixon GM, Mihai R, Verginis N, Davey MJ. Patterns of continuous positive airway pressure adherence during the first 3 months of treatment in children. J Pediatr 2011; 159: 802–07 [DOI] [PubMed] [Google Scholar]
- 20.Puri P, Ross KR, Mehra R, et al. Pediatric positive airway pressure adherence in obstructive sleep apnea enhanced by family member positive airway pressure usage. J Clin Sleep Med 2016; 12: 959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez A, Khirani S, Aloui S, et al. Continuous positive airway pressure and noninvasive ventilation adherence in children. Sleep Med 2013; 14: 1290–94. [DOI] [PubMed] [Google Scholar]
- 22.Uong EC, Epperson M, Bathon SA, Jeffe DB. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics 2007; 120: e1203–11. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins SM, Jensen EL, Simon SL, Friedman NR. Correlates of Pediatric CPAP Adherence. J Clin Sleep Med 2016; 12: 879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perriol MP, Jullian-Desayes I, Joyeux-Faure M, et al. Long-term adherence to ambulatory initiated continuous positive airway pressure in non-syndromic OSA children. Sleep Breath 2019; 23: 575–78. [DOI] [PubMed] [Google Scholar]
- 25.Office for Human Research Protections. 45 CFR 46. 2016. https://www.govinfo.gov/content/pkg/CFR-2016-title45-vol1/pdf/CFR-2016-title45-vol1-part46.pdf (accessed Dec 6, 2019).
- 26.Centers for Medicare & Medicaid Services. Decision memo for continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA) (CAG-00093N). 2001. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=19&fromdb=true (accessed Jan 18, 2019).
- 27.Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child 2010; 95: 717–23. [DOI] [PubMed] [Google Scholar]
- 28.Cistulli PA, Armitstead J, Pepin JL, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med 2019; 59: 114–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018; 153: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker DJ. Fetal origins of coronary heart disease. BMJ 1995; 311: 171–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang EK, Xanthopoulos MS, Kim JY, et al. Adherence to positive airway pressure for the treatment of obstructive sleep apnea in children with developmental disabilities. J Clin Sleep Med 2019; 15: 915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7: 687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


