Diffuse midline glioma (DMG), H3 K27M mutant (WHO grade IV) is listed as a separate CNS tumor entity since 2016 [5], after large sequencing efforts had discovered H3 K27M mutations frequently appearing in gliomas located in midline structures [11]. Over time, we and others have observed single cases of DMG with concomitant mutations within FGFR1 or BRAF [1, 2, 4, 6, 7, 9, 10, 12–14]. FGFR1 and BRAF mutations are typical hallmarks of low grade glioma, such as pilocytic astrocytoma, ganglioglioma, or dysembryoplastic neuroepithelial tumor [3, 8]. So, the parallel occurrence of H3 and FGFR1/BRAF mutations within a single tumor may complicate the diagnostic decision towards a low grade or a high grade glioma. This dilemma, which has direct clinical implications, is particularly evident, if only small biopsies are taken and low-grade histology may not be respresentative and hence may not mirror the biology of the neoplasm. On the other hand, the presence of a MAPK pathway alteration, such as FGFR1 or BRAF mutations, may open up additional possibilities of targeted therapies, independent of the tumor classification.
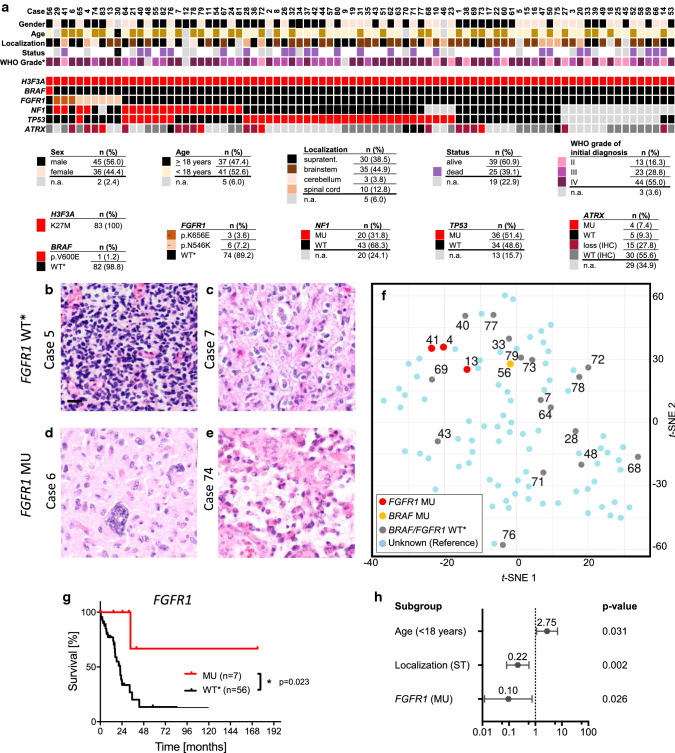
In order to learn more about the frequency and impact on such mutations, we analyzed a series of 83 DMG, H3F3A K27M mutant. Details on clinical characteristics of patients are listed in Fig. 1a and Supplementary Table 1, online resource. One case (1.2%) displayed a BRAF (p.V600E) mutation and 9/83 cases (10.8%) showed FGFR1 mutations (p.K656E or p.N546K). Mutations within NF1, TP53, and ATRX were detected in 31.8%, 51.4%, and 35.2%, respectively. TP53 mutations were significantly associated with FGFR1 wild type status (FGFR1 WT, p = 0.009, Χ2-test, Supplementary Fig. 1a, online resource).
Fig. 1.
Clinical, histological, and molecular parameters of H3F3A K27M mutated DMG with and without additional mutations in FGFR1. a Overview on all 83 analyzed cases with 12% of cases harboring BRAF or FGFR1 hotspot mutations. Percentages of characteristics refer to cases with known attribute only. Representative images of FGFR1 WT (b, c) and MU cases (d, e) demonstrate comparable histomorphology in both groups. T-SNE analysis of DMG reveals FGFR1 and BRAF MU cases to harbor similar DNA methylation profiles as FGFR1 and BRAF WT cases (f). FGFR1 MU cases showed a significantly better prognosis than FGFR1 WT cases (p = 0.023, g), and multivariate analyses confirmed significance of FGFR1 status independent of age and localization. WT* = wild type for respective hotspot, MU = mutant, n. a. = not available, *WHO grade of initial diagnosis
Similar to FGFR1 WT cases, cases with additional FGFR1 mutation displayed features of a diffusely growing glioma with increased cellularity and signs of anaplasia, such as increased cell pleomorphism, mitoses, or vessel proliferation (Fig. 1b-e). Furthermore, all analyzed FGFR1 MU cases (and the BRAF MU case) matched to the methylation class”DMG, H3 K27M mutant” (Supplementary Fig. 1b, online resource, Fig. 1f, Supplementary Table 1, online resource).
Higher age (≥ 18 years), supratentorial tumor localization and FGFR1 MU status were associated with a significantly better prognosis of patients (p = 0.038, p = 0.034, and p = 0.023, Fig. 1g and Supplementary Fig. 2a, b, online resource). In contrast, TP53 MU status was associated with a significantly worse prognosis of patients (p = 0.002, Supplementary Fig. 2c, online resource). Including the latter factors in a multivariate cox regression analyses showed localization and TP53 status as significant variables (Supplementary Fig. 2d, online resource). FGFR1 and TP53 mutations occurred almost mutually exclusive and hence did not represent independent variables (see also Supplementary Fig. 1a, online resource). Thus, we performed a multivariate analysis including the independent variables age, localization, and FGFR1 status only (Fig. 1h). In this context, FGFR1 MU status was significantly associated with a better overall survival, independently of patient age, and tumor localization (p = 0.026). Interestingly, the single patient (#56) with an accompanying BRAF p.V600E mutation remained alive at 24.5 months after initial diagnosis. However, the prognosis for such diffuse midline gliomas with dual H3F3A p.K27M and BRAF p.V600E mutations remains to be defined.
Together, our results suggest that RAS-MAPK-pathway signaling might play an important role in DMG with implications for diagnosis, prognosis, and therapy of respective patients.
Supplementary Information
Below is the link to the Supplementary Information.
Acknowledgements
We thank Anne Reichstein, Michael Ruiter, Janina Mielke, Celina Soltwedel, Matthias Dottermusch, and Michael Spohn for technical support. J.N. was supported by the Else-Kröner Fresenius Stiftung, the UKE Nachwuchsförderung and the Emmy-Noether program of the DFG. U.S. received further funding from the Werner Otto Stiftung and the Fördergemeinschaft Kinderkrebszentrum Hamburg.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Global DNA Methylation data have been deposited under GEO accession number GSE161944.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/9/2021
A Correction to this paper has been published: 10.1007/s00401-021-02273-8
Contributor Information
Ulrich Schüller, Email: u.schueller@uke.de.
Julia E. Neumann, Email: ju.neumann@uke.de
References
- 1.Dufour C, Perbet R, Leblond P, Vasseur R, Stechly L, Pierache A, et al. Identification of prognostic markers in diffuse midline gliomas H3K27M-mutant. Brain Pathol. 2020;30:179–190. doi: 10.1111/bpa.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, Nikbakht H, Gerges N, Fiset PO, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. 2014;46:462–466. doi: 10.1038/ng.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D, Huttler B, Jäger N, Korshunov A, Kool M, Warnatz HJ, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez GY, Oberheim Bush NA, Phillips JJ, Bouffard JP, Moshel YA, Jaeckle K, et al. Diffuse midline gliomas with subclonal H3F3A K27M mutation and mosaic H3.3 K27M mutant protein expression. Acta Neuropathol. 2017;134:961–963. doi: 10.1007/s00401-017-1780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis D, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger, et al. WHO classification of tumours of the central nervous system (Revised 4th edition) IARC: Lyon; 2016. [Google Scholar]
- 6.Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, et al. Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32:520–537. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen C, Colin I, Nanni-Metellus L, Padovani L, Maurage CA, Varlet P, et al. Evidence for BRAF V600E and H3F3A K27M double mutations in paediatric glial and glioneuronal tumours. Neuropathol Appl Neurobiol. 2015;41:403–408. doi: 10.1111/nan.12196. [DOI] [PubMed] [Google Scholar]
- 8.Pages M, Beccaria K, Boddaert N, Saffroy R, Besnard A, Castel D, et al. Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol. 2018;28:103–111. doi: 10.1111/bpa.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryall S, Krishnatry R, Arnoldo A, Buczkowicz P, Mistry M, Siddaway R, et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun. 2016;4:93. doi: 10.1186/s40478-016-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte JD, Buerki RA, La Pointe SL, Molinaro AM, Zhang Y, Villanueva-Meyer JE, et al. Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline glioma in adults. Neuro-Oncol Adv. 2020;2:vdaa142. doi: 10.1093/noajnl/vdaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 12.Sloan EA, Cooney T, Oberheim Bush NA, Buerki R, Taylor J, Clarke JL, et al. Recurrent non-canonical histone H3 mutations in spinal cord diffuse gliomas. Acta Neuropathol. 2019;138:877–881. doi: 10.1007/s00401-019-02072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3–K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;2016(26):569–580. doi: 10.1111/bpa.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vettermann F, Neumann J, Suchorska B, Bartenstein P, Giese A, Dorostkar MM, et al. K27M midline gliomas display malignant progression by imaging and histology. Neuropathol Appl Neurobiol. 2017;43:458–462. doi: 10.1111/nan.12371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Global DNA Methylation data have been deposited under GEO accession number GSE161944.