Abstract
This study aimed to investigate the protective effects of Schisandrin C during diabetic nephropathy (DN) treatment. After DN induction, mice were treated with Schisandrin C, and diabetic metabolic parameters and renal function-associated factors were measured. Renal structural damage was evaluated by hematoxylin and eosin (HE) and Masson’s trichrome staining. Macrophage polarization and macrophage-mediated inflammatory factors were detected in the kidneys by immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA), respectively. The Swiprosin-1/interferon (IFN)-γ-Rβ pathway was evaluated by western blot (WB) analysis. The preliminary effects of Schisandrin C in high-glucose-stimulated macrophages from DN mice were verified by flow cytometry, ELISA, and WB analyses. These results indicated that Schisandrin C significantly regulated physiological parameters in DN. Renal structural damage was mitigated by Schisandrin C. In Schisandrin-C-treated groups, the expression levels of CD86, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β decreased, whereas CD206, IL-10, and transforming growth factor (TGF)-β expression levels increased. In vitro experiments indicated that among CD86+ cells, TNF-α, IL-6, and IL-1β expression levels significantly decreased, whereas among CD206+ cells, IL-10 and TGF-β expression increased following Schisandrin-C-treatment. Finally, Schisandrin C inhibited the expression of Swiprosin-1, IFN-γ-Rβ, phospho-Janus kinase 2 (p-JAK2), phospho-signal transducer and activator of transcription 1 (p-STAT1), and p-STAT3, in both DN model mice and high-glucose-stimulated RAW264.7 cells. The present study indicated a novel use for Schisandrin C to suppress DN progression, by promoting M1 to M2 macrophage polarization. Schisandrin C exerted protective effects against DN by regulating the polarization-dependent Swiprosin-1/IFN-γ-Rβ signaling pathway in macrophages.
Keywords: Diabetic nephropathy, macrophage polarization, inflammatory factors, Schisandrin C, Swiprosin-1/IFN-γ-Rβ
Introduction
According to the International Diabetes Federation, 463 million people have diabetes mellitus (DM) worldwide, and DM is expected to affect 578 million people by 2030 [1]. Diabetic nephropathy (DN) is a primary complication of DM, and current treatment options are unsatisfactory. DN is a critical factor in the induction of end-stage renal disease, which imposes a high societal burden [2]. Clinical characteristics of end-stage renal disease include persistent albuminuria and a progressively declining glomerular filtration rate [3]. Increasing evidence has shown that multiple mechanisms may contribute to the development and outcomes of DN, including inflammation, hyperglycemia, protein kinase C levels, advanced glycation end products, poly (ADP-ribose) polymerase activation, and oxidative stress [4]. During the last decade, macrophages have become a focal point for studies of DN pathogenesis. Macrophages are central mediators of the inflammatory response, directly interacting with kidney cells to induce cell proliferation and extracellular matrix production [5,6]. In a variety of human kidney diseases, macrophages have been shown to accumulate in both glomeruli and interstitial tissues, and macrophage accumulation correlates closely with the degree of progressive injury and fibrosis in DN [7,8]. Thus, macrophage inhibition could represent a novel therapeutic approach, which may be able to prevent DN progression.
Currently available therapies, such as glycemic and blood pressure control, can slow but not prevent DN development [9]. Thus, the identification of novel and effective therapeutic approaches that are capable of preventing DN development in DM patients is crucial. Traditional Chinese medicine has demonstrated good clinical results for kidney disease management, including medicines derived from Schisandra fruits (Wuweizi, in Chinese), including the dried, ripe fruits of Schisandra chinensis (Turcz.) Bail (Bei Wuweizi, in Chinese) or Schisandra sphenanthera, Rehd. et Wils. (Nan Wuweizi, in Chinese). Schisandra fruits have been widely used in Asian countries for the treatment of liver and kidney diseases [10] because they exert a wide array of positive effects, including detoxifying, anti-inflammatory, antioxidative, and anticancer activities [11]. Schisandra fruit extracts also exert positive effects on DN [12]. Schisandrin C (SC) (Figure 1), an active constituent found in Schisandra fruits, has been associated with the significant pharmacological activities observed for the Schisandra fruit extracts. Intensive studies of SC bioactivity have indicated that SC exhibits anti-oxidative and anti-inflammation characteristics, which exert protective effects in various tissues [13-15]. However, no reports have examined the renoprotective effects of SC in DN patients. We hypothesized that SC may serve as the active ingredient in Schisandra fruits, which may mediate the treatment effects observed for Schisandra fruits in DN. Thus, in this study, we investigated whether SC can be used to suppress DN in streptozotocin (STZ)-induced DN model mice and explored the potential molecular mechanisms associated with this suppression.
Figure 1.
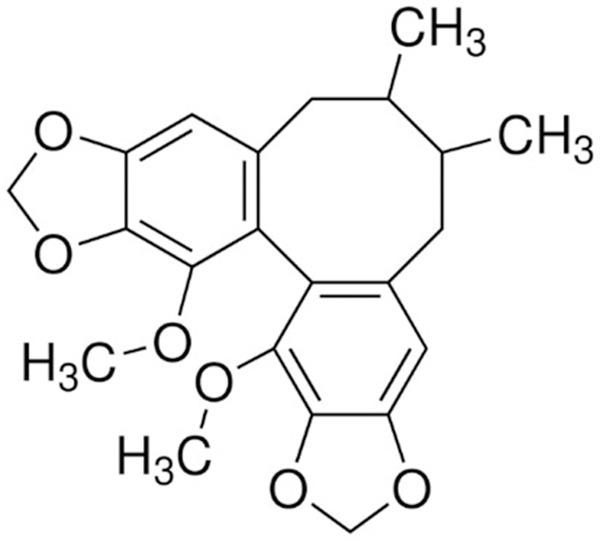
The chemical structure of Schisandrin C. Molecular formula: C22H24O6. Molecular weight: 384.42 g/mol.
Materials and methods
Animal procedures
All experiments were performed in C57/BL6 mice (20 ± 2 g, female), which were kindly provided by Beijing Vital River Laboratory Animal Technologies Co. Ltd (Beijing, China). All animals were housed under specific pathogen-free conditions, and allowed to acclimate for 1 week. DN model mice were established, as previously described [16]. Briefly, C57/BL6 mice were fed with a high-fat diet for 1 week, followed by daily intraperitoneal injections of STZ (40 mg/kg) for five days. After 2 weeks, fasting blood glucose (FBG) levels were detected. Mice with FBS values ≥ 11.1 mM were considered to represent successfully induced diabetic model mice. Subsequently, the diabetic model mice were divided into 4 groups (n = 6 for each group), including the control Model group, low-dose (100 mg/kg/d) and high-dose SC (200 mg/kg/d) treatment groups, and a metformin treatment group (195 mg/kg/d). Mice in the Control group were sham-injected with sodium citrate buffer and provided with a normal, standard, balanced diet. The mice in the treatment groups were administered SC or metformin, intragastrically, whereas the mice in the control Model group were treated with an equal volume of saline. After 8 weeks, mice body weights and FBG levels were measured. Subsequently, all mice were housed in individual, metabolic cages for 24 h, to detect 24-h food consumption, 24-h water intake, and 24-h urinary excretion amounts. Urine proteinuria was determined by a Bradford assay [17]. Mice were euthanized by thiopental overdose (200 mg/kg, i.p.), and blood was collected to measure serum creatinine (Scr), blood urea nitrogen (BUN), and serum insulin levels, using colorimetric kits (Jiancheng, Nanjing, China). Subsequently, the kidneys were rapidly dissected and weighed. The left kidneys were snap-frozen for enzyme-linked immunosorbent assay (ELISA) and western blot (WB) analyses, whereas the right kidneys were fixed in 10% paraformaldehyde for further pathological analysis. All animal-related protocols were approved by the Animal Ethics Committee on the Care and Use of Laboratory Animals of Tianjin Medical University (Tianjin, China).
Renal histological and immunohistochemistry analysis
The right kidneys were dehydrated, embedded in paraffin, and sectioned into 5-µm-thick sections. Sections were stained with hematoxylin and eosin (HE) and Masson’s trichrome staining kits, according to the manufacturer’s instructions. Each stained section was observed under a microscope (400×; Nikon, Tokyo, Japan), in a blinded fashion, to evaluate histopathological damage. Immunohistochemistry (IHC) analysis was used to determine the CD86 and CD206 expression levels in paraffin-embedded kidney tissue sections. Briefly, sections were pretreated by microwave for 20 min, in 10 mmol/L sodium citrate buffer (pH 6.0), to perform antigen retrieval, and were then incubated with a blocking agent. Subsequently, kidney sections were stained with primary antibodies, overnight at 4°C. Sections were then incubated with secondary antibody. Finally, the sections were incubated with VECTASTAIN@ABC reagent (Vector Laboratories, Burlingame, CA, USA), and color development was achieved using 3, 3’-diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, USA). Positive staining (dark brown) was quantified using computerized morphometry (Image Pro-Plus 6.0 software, Bethesda, MD). The immunohistochemical assessments were performed by 2 investigators, in a double-blinded manner, at 400× magnification.
Cell culture and treatment
RAW264.7 cells were kindly provided by Shanghai Institutes for Biological Sciences (Shanghai, China). Subsequently, the cells were cultured (37°C, 5% CO2), in RPMI 1640 media [10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S)]. After RAW264.7 cells were incubated in the presence of 100 ng/ml phorbol-12-myristate-13-acetate (PMA), the cells differentiated into macrophages. The differentiated cells were pre-treated with 30 mM D-glucose, for 12 h, as previously described [18], followed by SC treatment (20, 40, or 80 µM) for 48 h. Finally, ELISA was used to measure the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, IL-10, and transforming growth factor (TGF)-β in the cell supernatant. Macrophages were washed twice with phosphate-buffered saline (PBS), followed by measurement using flow cytometry. In addition, the expression levels of proteins in macrophages were analyzed by WB analysis.
Flow cytometry analysis
Macrophages were stained for 20 min on ice, using a specific antibody against F4/80-APC (BD Biosciences, San Jose, CA), as a general marker. A fluorescein isothiocyanate (FITC)-conjugated antibody against CD86 and an R-phyco-ethrin (PE)- and Cy5-conjugated antibody against CD206 were used to stain M1 and M2 macrophages, respectively. All antibodies were prepared in fluorescence-associated cell sorting (FACS) buffer. For immunophenotypic analyses, cell suspensions (2×106 cells/ml) were incubated with 10% goat serum, in the dark (4°C, 15 min) and then incubated with antibodies for 30 min. Flow cytometry was performed on a BD LSR II (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, San Carlos, CA). Each experiment was performed in triplicate.
Enzyme-linked immunosorbent assays
After treatment with different concentrations of SC, TNF-α, IL-6, IL-1β, IL-10, and TGF-β contents in kidney tissues were measured using commercial assay kits (mlbio, Shanghai, China), according to the manufacturer’s protocols.
Protein analysis
Kidney tissues and harvested cells were lysed, on ice, with RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China). The lysates were collected and centrifuged (12,000×g, 15 min, 4°C), and protein concentrations were detected using a bicinchoninic acid (BCA) assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Protein samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Non-specific binding was blocked in Tris-buffered saline containing 0.1% Tween-20 (TBS-T), with 5% bovine serum albumin (BSA), for 1 h at room temperature, and incubated overnight at 4°C with the following primary antibodies (1:2,000): Swiprosin-1 [goat polyclonal antibody (pAb), ab24368], Janus kinase 2 [JAK2, rabbit monoclonal antibody (mAb), ab108596], phospho-JAK2 (rabbit mAb, ab32101), signal transducer and activator of transcription 1 (STAT1, mouse mAb, ab3987), phospho-STAT1 (mouse mAb, ab29045), STAT3 (mouse mAb, ab119352), phospho-STAT3 (rabbit mAb, ab76315), and IFN-γ (rabbit pAb, ab77246). All antibodies were obtained from Abcam (Cambridge, MA, USA). Anti-β-actin antibody was purchased from Wuhan Sanying Biotechnology (Wuhan, China). The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Boster Biotechnology, Wuhan, China), for 1 h at room temperature. The band density was visualized with an enhanced chemiluminescence (ECL) kit (Thermo Fisher, Waltham, Massachusetts, USA) and quantified using ImageJ software (NIH, Bethesda, USA).
Statistical analysis
All experiments were performed at least 3 times, and the values are presented as the mean ± standard deviation (SD). All data were assessed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). Unpaired Student’s t-tests were used for comparisons between two groups, and one-way analysis of variance (ANOVA), with post hoc, was used for multiple comparisons. Differences between groups with P < 0.05 were considered significant.
Results
Schisandrin C ameliorates kidney complications associated with diabetes in DN model mice
Hyperglycemia, polyphagia, polyuria, and polydipsia, accompanied by the loss of body weight, were initially observed in the DN model group, and reduced insulin secretion was also observed compared with the control group. However, SC was able to improve diabetic metabolic symptoms, in a dose-dependent manner (Figure 2A). Furthermore, high levels of Scr, BUN, and proteinuria were detected in the DN model group. SC notably ameliorated the pathological hallmarks of kidney damage (Figure 2B). To objectively observe and quantify kidney damage in DN model mice, the glomerular and fibrosis areas were evaluated in the HE- and Masson-stained sections. SC treatment significantly decreased the glomerular and fibrosis areas in DN model mice. HE staining also revealed glomerulosclerosis and glomerulus expansion, the diminution of the capillary lumen, diffuse mesangial matrix expansion, and peripheral capillaries with thick, stiff walls, in DN model mice. However, SC treatment significantly ameliorated these DM-induced histopathological alterations (Figure 2C). These in vivo data suggested the therapeutic effects of SC in diabetic model mice and the amelioration of kidney complications, which suggested that SC may serve as a potential agent for the development of novel DN therapies.
Figure 2.
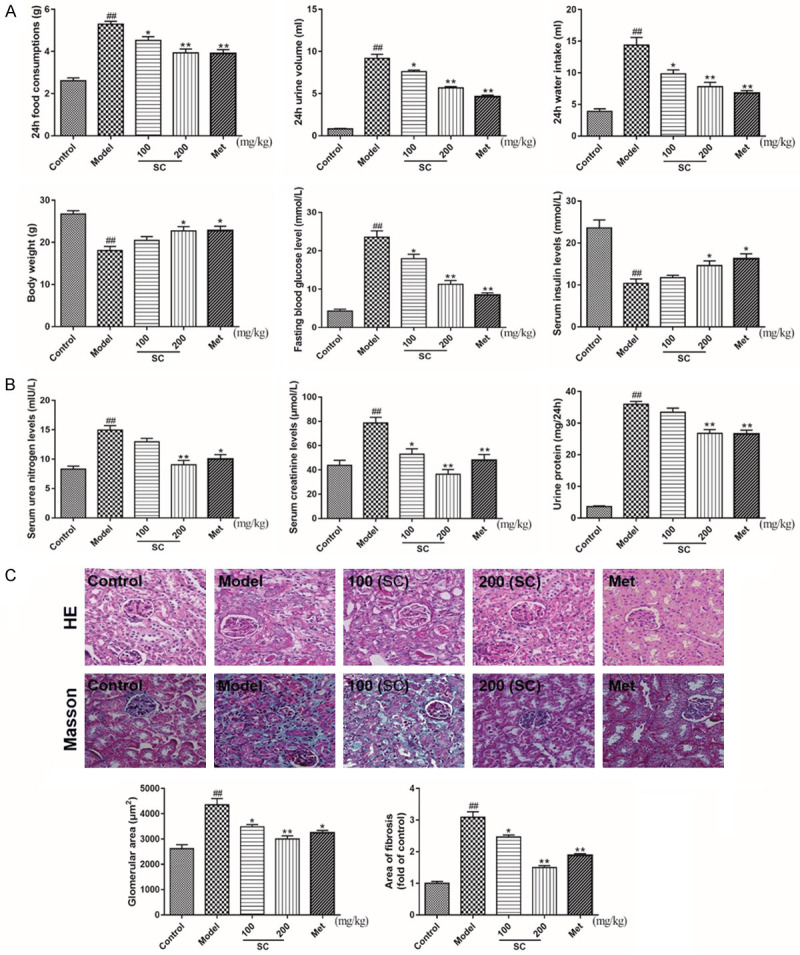
Schisandrin C ameliorates diabetic metabolic parameters and improves renal functional and structural damage in DN model mice. DN model mice were treated with saline and/or Schisandrin C (100 or 200 mg/kg/d) and/or Metformin (195 mg/kg/d), for 8 weeks. A. 24-h food consumption, 24-h urine volume, 24-h water intake, body weight, fasting blood glucose levels, and serum insulin levels were examined after 8 weeks. B. Serum urea nitrogen, serum creatinine, and urine protein contents were measured, using commercial assay kits. C. Representative examples of right kidneys from each experimental condition, after treatment. Histological analysis confirmed the pathological changes in diabetic renal tissues (400×). HE staining was used for the analysis of histological abnormalities. Masson’s trichrome staining was used for the detection of type IV collagen in kidney sections. Data are expressed as the mean ± S.D. ##P < 0.01 vs. control; **P < 0.01, *P < 0.05 vs. model.
Schisandrin C regulates macrophage polarization and macrophage-mediated inflammatory factors, in vivo
To further explore macrophage polarization, IHC staining for CD86 and CD206, as indicators for M1 and M2 macrophages, respectively, was performed, revealing the predominant expression of these proteins in the renal tissues of DN mice. As shown in Figure 3A, CD86 expression levels increased in the DN model group compared with the control group. In contrast, SC (100 and 200 mg/kg) treatment resulted in the prominent downregulation of CD86 levels. CD206 expression levels were significantly decreased in DN model mice compared with control mice. However, SC treatment at different concentrations resulted in the prominent upregulation of CD206 levels (Figure 3A). Inflammation-associated cytokines were measured in the left kidneys by ELISA. TNF-α, IL-6, and IL-1β levels were much higher in the DN model group than in the control group. Immediately after treatment with SC, the TNF-α, IL-6, and IL-1β levels were notably decreased compared with the DN model group (Figure 3B). Consistently, both IL-10 and TGF-β levels were markedly reduced in the DN model group relative to those in the control group and were significantly elevated by SC treatment, in a dose-dependent manner (Figure 3C). These results indicated that SC treatment was able to reverse DN progression, possibly by regulating macrophage polarization in the kidneys of DN model mice.
Figure 3.
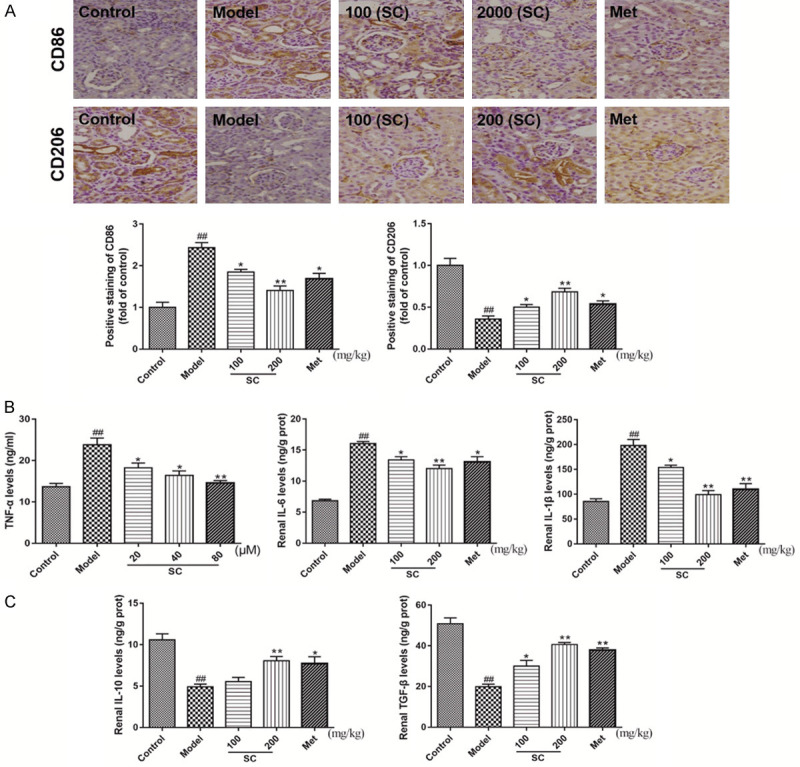
Schisandrin C promoted the polarization of M1 macrophages toward an M2 phenotype, suppressed pro-inflammatory cytokine secretion, and induced anti-inflammatory cytokine expression in the right kidneys of DN model mice. DN model mice were treated with saline, Schisandrin C (100 or 200 mg/kg/d), or Metformin (195 mg/kg/d), for 8 weeks. A. Immunohistochemistry was used to detect the polarized populations of M1 and M2 macrophages, by quantifying the positive staining (dark brown) against CD86 and CD206, respectively. B. TNF-α, IL-6, and IL-1β contents were measured, using commercial assay kits. C. IL-10 and TGF-β contents were measured, using commercial assay kits. Data are expressed as the mean ± S.D. ##P < 0.01 vs. control; **P < 0.01, *P < 0.05 vs. model.
Schisandrin C inhibits the activation of the Swiprosin-1/IFN-γ-Rβ axis, in vivo
As described above, SC treatment exhibited anti-inflammatory effects in the kidneys of DN model mice. Thus, certain molecules and signaling pathways associated with the regulation of macrophage polarization were examined. We initially measured the expression levels of Swiprosin-1/IFN-γ-Rβ-related proteins in the kidneys. WB analysis confirmed the upregulation of detectable Swiprosin-1 and IFN-γ-Rβ protein expression levels in DN model mice. Compared with the DN model group, the expression levels of these proteins were distinctly attenuated by SC treatment (Figure 4A). Furthermore, SC treatment significantly reduced the p-JAK2, p-STAT1, and p-STAT3 levels in renal tissues compared with those in the DN model group, in a dose-dependent manner (Figure 4B). Based on these results, we speculated that SC treatment might attenuate DN by promoting the polarization of M1 macrophages toward an M2 phenotype, via the Swiprosin-1/IFN-γ-Rβ signaling pathway. To validate this hypothesis, we examined pathological changes, in vitro.
Figure 4.
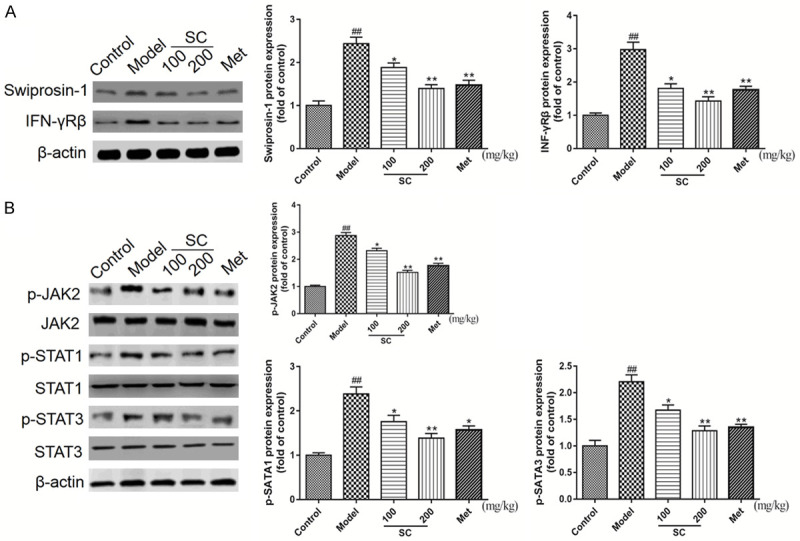
Schisandrin C downregulated the Swiprosin-1/IFN-γ-Rβ signaling pathway in the right kidneys of DN model mice. DN model mice were treated with saline, Schisandrin C (100 or 200 mg/kg/d), or Metformin (195 mg/kg/d), for 8 weeks. A. Swiprosin-1 and IFN-γRβ expression levels were analyzed by western blot analysis, using β-actin as a loading control. B. p-JAK2, p-STAT1, and p-STAT3 expression levels were analyzed by western blot analysis, using β-actin as a loading control. Representative blots are shown, with densitometry analysis. Original full-size blots are presented in Figure S1. Data are expressed as the mean ± S.D. ##P < 0.01 vs. control; **P < 0.01, *P < 0.05 vs. model.
Schisandrin C regulates macrophage polarization and macrophage-mediated inflammatory factors, in vitro
Having preliminarily demonstrated that SC affects macrophage polarization in the kidneys of DN model mice, we next verified the effects of SC on macrophage polarization, as determined by flow cytometry. M1 macrophages and M2 macrophages were identified as viable CD86+ and CD206+ cells, respectively, by flow cytometry. As shown in Figure 5A, M1 macrophages predominated among the total macrophage population after exposure to high-glucose alone. The proportion of M1 macrophages significantly decreased with increasing doses of SC during high-glucose exposure. Conversely, high-glucose exposure markedly decreased the proportion of M2 macrophages, whereas the proportion of M2 macrophages significantly increased, in a dose-dependent manner, with SC addition (Figure 5A). M1 macrophage-mediated inflammatory factors, such as TNF-α, IL-6, and IL-1β, were significantly increased after macrophage exposure to high-glucose conditions and were significantly decreased after SC treatment (Figure 5B). In contrast, M2 macrophage-mediated inflammatory factors, including IL-10 and TGF-β, were significantly decreased after macrophage exposure to high-glucose conditions, and SC treatment significantly increased IL-10 and TGF-β secretion during high-glucose exposure (Figure 5C). Taken together, these results suggest that SC treatment promoted the M1 to M2 macrophage polarization process.
Figure 5.
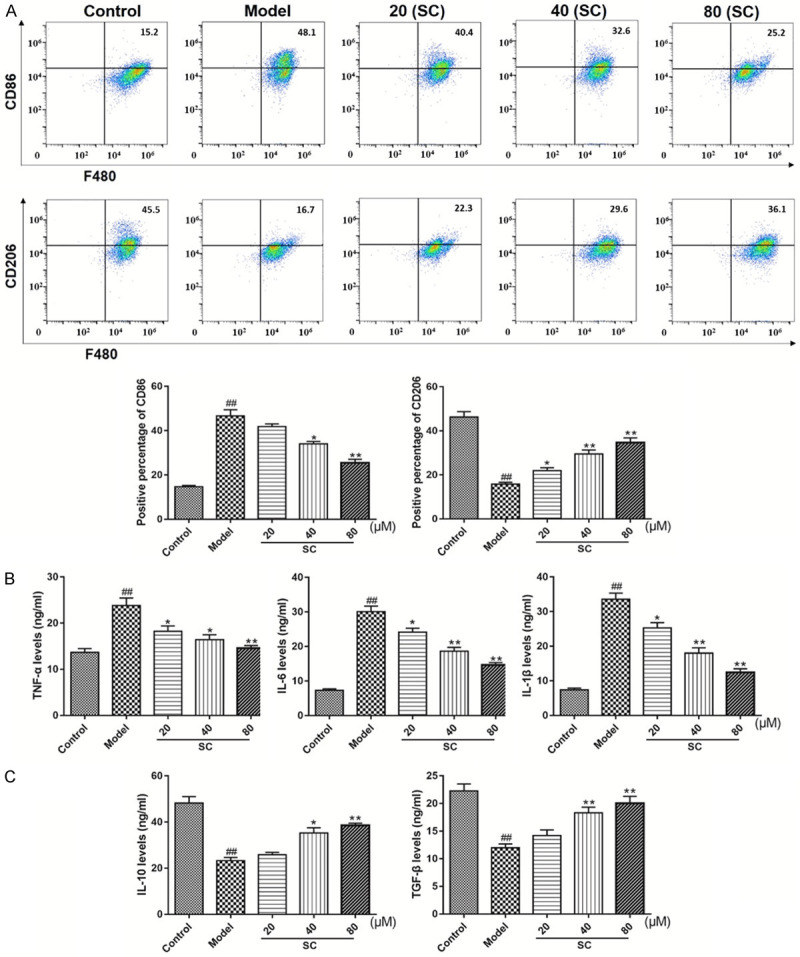
Schisandrin C promoted the polarization of M1 macrophages toward an M2 phenotype, suppressed pro-inflammatory cytokine secretion, and induced anti-inflammatory cytokine expressions in high-glucose-stimulated RAW264.7 cells. RAW264.7 cells were differentiated and pretreated with D-glucose (30 mM), for 12 h, and then treated with Schisandrin C (20-80 µM), for 48 h. A. Flow-cytometric analysis was applied to detect the polarized populations of M1 and M2 macrophages by quantifying CD86+ and CD206+ cells, respectively. B. TNF-α, IL-6, and IL-1β contents were measured, using commercial assay kits. C. IL-10 and TGF-β contents were measured, using commercial assay kits. Data are expressed as the mean ± S.D. ##P < 0.01 vs. control; **P < 0.01, *P < 0.05 vs. model.
Schisandrin C suppresses the activation of Swiprosin-1/IFN-γ-Rβ axis, in vitro
To further explore the protective mechanisms associated with SC treatment, WB analysis was performed, to detect Swiprosin-1/IFN-γ-Rβ pathway-related molecules in macrophages. High-glucose exposure markedly increased the Swiprosin-1 and IFN-γ-Rβ expression levels, whereas SC treatment distinctly alleviated these changes (Figure 6A). The results shown in Figure 6B also indicated that glutamate exposure increased the p-JAK2, p-STAT1, and p-STAT3 expression levels, which could be reduced by SC treatments, in a dose-dependent manner. These in vitro data further confirmed the protective effects of SC during the treatment of DN, which suggested that SC may have therapeutic effects on high-glucose-induced macrophage polarization and macrophage-mediated inflammatory factors, regulated by the Swiprosin-1/IFN-γ-Rβ signaling pathway.
Figure 6.
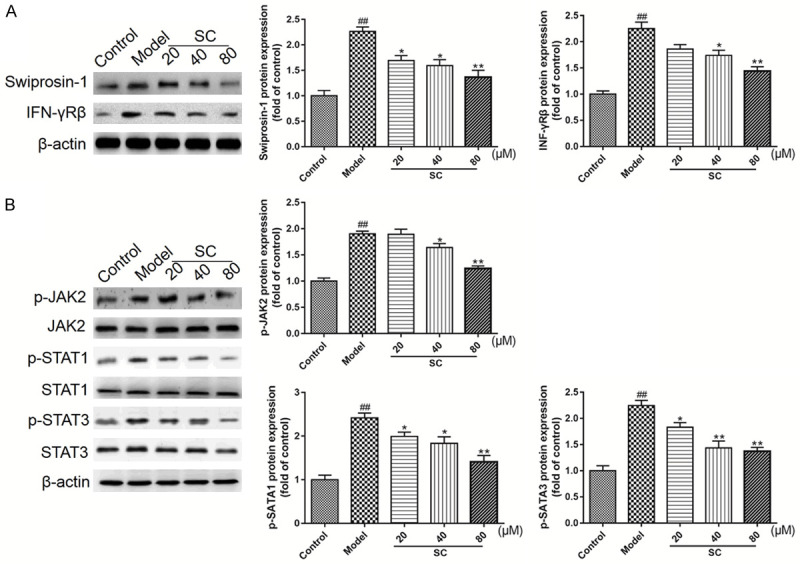
Schisandrin C downregulated the Swiprosin-1/IFN-γ-Rβ signaling pathway, in high-glucose-stimulated RAW264.7 cells. RAW264.7 cells were differentiated and pretreated with D-glucose (30 mM), for 12 h, and then treated with Schisandrin C (20-80 µM), for 48 h. A. Swiprosin-1 and IFN-γ-Rβ expression levels were analyzed by western blot analysis, using β-actin as a loading control. B. p-JAK2, p-STAT1, and p-STAT3 expression levels were analyzed by western blot analysis, using β-actin as a loading control. Representative blots are shown, with densitometry analysis. Original, full-size blots are presented in Figure S2. Data are expressed as the mean ± S.D. ##P < 0.01 vs. control; **P < 0.01, *P < 0.05 vs. model.
Discussion
DM has been the most common, global, and widespread metabolic disease during recent decades. Chronic hyperglycemia has been strongly associated with the dysfunction of various tissues, including the eyes, kidneys, heart, blood vessels, and nerves [19]. DN is a long-term DM complication that develops in approximately 30% of Type 1 DM and 10% of Type 2 DM patients, characterized by proteinuria and glomerulosclerosis [20,21]. Moreover, DN is functionally characterized by increased Scr and BUN levels [22,23]. Previous studies have demonstrated that mice injected with STZ exhibited the destruction of pancreatic β cells and reduced insulin secretory capacity [24]. Feeding mice a high-fat diet, followed by the injection of STZ, resulted in the establishment of an experimental DN model [25], which was the model used in our study. Consequently, our study showed that these DN model mice developed typical DM characteristics, including hyperglycemia, polyphagia, polydipsia, polyuria, and proteinuria, accompanied by the loss of body weight and reduced insulin synthesis. At the end of the experiment, BUN, Scr, and urine protein levels in DN model mice also significantly increased during a 24-h period, compared with those in control mice. Pathological section observations also indicated glomerular hypertrophy, mesangial matrix expansion, and accelerated glomerular tuft to Bowman’s capsule adhesion in diabetic renal samples. Thus, in the present study, renal protection associated with SC treatment was evaluated, to determine whether SC may serve as a novel therapeutic agent for DN. Our results revealed that SC treatment ultimately resulted in the amelioration of typical DM characteristics. Interestingly, SC treatments also markedly attenuated proteinuria, reduced serum BUN and Scr levels, and ameliorated glomerular histopathology, suggesting that SC could effectively prevent DN progression. Based on these findings, the current experiments further explored whether the protective effects of SC treatments against DN progression were associated with the regulation of macrophage polarization.
More recently, immune-inflammatory mechanisms have been reported to regulate the development and progression of DN. Accumulated evidence has implied that macrophages may play crucial roles during renal diseases, both in humans and in experimental models, and macrophages may directly stimulate renal cells [26]. Recent studies have also indicated that macrophage infiltration and pro-inflammatory cytokines could induce DN development and progression [27-29]. However, the inhibition of macrophage infiltration into the glomerular region significantly suppressed proteinuria [30]. In response to environmental changes, macrophages are well-known to exhibit the plastic ability to switch between 2 functional phenotypes. Classically activated M1 macrophages are primarily involved in inflammation and tissue damage, whereas the alternatively activated M2 macrophages mediate tissue repair and renal protection [31]. Previous studies have revealed that M1 macrophages release pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), which aggravate renal injury. In contrast, M2 macrophages secrete anti-inflammatory cytokines (IL-10 and TGF-β) and other factors associated with cell proliferation, tissue remodeling, and immune regulation [32,33]. In the current study, we investigated the effects of SC treatments on macrophage polarization, both in vitro and in vivo. The IHC results showed that M1 macrophages were significantly increased and M2 macrophages were significantly decreased in diabetic renal samples compared with normal control samples. However, SC treatment could significantly reduce the M1-polarized population and simultaneously increase the number of M2 macrophages. Consistent with these data, our flow cytometry results revealed that SC induced similar effects in high-glucose-stimulated RAW264.7 cells. Our further investigation showed that the expression of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) increased, whereas the expression of the anti-inflammatory cytokines (IL-10 and TGF-β) decreased dramatically, in both DN model mouse kidneys and high-glucose-stimulated RAW264.7 cells. We noted that SC treatment appeared to reduce pro-inflammatory cytokine secretion levels and promote the levels of anti-inflammatory cytokines. Our investigations demonstrated the potential anti-inflammatory effects of SC treatment in DN and suggested that the underlying mechanisms may be associated with the promotion of M1 macrophages toward an M2 phenotype in the renal tissues of DN model mice.
The JAK/STAT signaling pathway regulates many physiological activities. DN is perhaps the best-described renal disorder in which JAK/STAT activation is important [34]. Previous studies have demonstrated that the JAK/STAT pathway, especially the JAK2/STAT1/STAT3 signaling pathway, contributes to high-glucose-induced renal cell responses, such as leukocyte infiltration, cell growth, and fibrosis [35,36]. Furthermore, various strategies have revealed the specific effects of JAK2 inhibition, STAT1 antisense oligonucleotides, and STAT3 gene knockdown, which have been demonstrated to suppress the pathogenic influences of the JAK/STAT pathway on the progression of DN [37,38]. Recently, the activation of the JAK/STAT pathway was shown to be indispensable for the polarization of M1 macrophages [39]. Thus, the current study further explored whether the renal protective effects associated with SC treatment in DN were associated with the regulation of the JAK/STAT signaling pathway in macrophages. Our results indicated that SC treatment significantly decreased the p-JAK2, p-STAT1, and p-STAT3 levels in renal tissues of DN model mice, indicating that the protective effects of SC treatment against DN may involve the suppression of the JAK/STAT signaling pathway. In addition, the JAK/STAT signaling pathway is primarily regulated by the IFN-γ-R pathway. The IFN-γ-R complex is located within the intact lipid raft microdomain and controls signal transduction. Accumulating evidence has indicated that Swiprosin-1 affects the activation of the JAK2/STAT1/STAT3 pathway by regulating the expression of IFN-γ-Rβ in macrophages [40]. Notably, SC treatment effectively inhibited the expression levels of Swiprosin-1 and IFN-γ-Rβ in DN model mice. Finally, the effects of SC treatment on the regulation of the Swiprosin-1/IFN-γ-Rβ signaling pathway were also verified, in vivo. Therefore, we propose that SC treatment attenuates DN through the regulation of macrophage polarization, mediated by the inhibition of the Swiprosin-1/IFN-γ-Rβ signaling pathway.
In conclusion, to the best of our knowledge, the present study is the first study to demonstrate the potential role played by SC in the treatment of DN. Treatment with SC suppressed the structural and functional deterioration in the kidneys of DN model mice, and the underlying mechanism may be associated with the reduction of M1-polarized macrophages and pro-inflammatory cytokines levels, via the Swiprosin-1/IFN-γ-Rβ signaling pathway. These results are valuable for understanding the pharmacodynamic actions of SC during DN treatment. However, this mechanism requires additional elucidation and confirmation, in future studies.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 9th edition. 2019. [PubMed] [Google Scholar]
- 2.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez ML, DiStefano JK. The role of non-coding RNAs in diabetic nephropathy: potential applications as biomarkers for disease development and progression. Diabetes Res Clin Pract. 2013;99:1–11. doi: 10.1016/j.diabres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun. 2013;433:359–361. doi: 10.1016/j.bbrc.2013.02.120. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 2005;16:1537–1538. doi: 10.1681/ASN.2005040393. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology. 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol. 2014;10:325–346. doi: 10.1038/nrneph.2014.74. [DOI] [PubMed] [Google Scholar]
- 10.Hong M, Zhang Y, Li S, Tan HY, Wang N, Mu S, Hao X, Feng Y. A network pharmacology-based study on the hepatoprotective effect of fructus schisandrae. Molecules. 2017;22:1617. doi: 10.3390/molecules22101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szopa A, Ekiert R, Ekiert H. Current knowledge of schisandra chinensis turcz. Baill, as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem Rev. 2017;16:195–218. doi: 10.1007/s11101-016-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang D, Zhang M. Inhibition mechanism of compound ethanol extracts from Wuweizi on renal interstitial fibrosis in diabetic nephropathy model mice. J Tradit Chin Med. 2012;32:669–673. doi: 10.1016/s0254-6272(13)60090-4. [DOI] [PubMed] [Google Scholar]
- 13.Panossian A, Wikman G. Pharmacology of schisandra chinensis bail: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Park SJ, Park TG, Rajasekar S, Lee SJ, Choi YW. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int Immunopharmacol. 2013;17:415–426. doi: 10.1016/j.intimp.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Chun JN, Kim SY, Park EJ, Kwon EJ, Bae DJ, Kim IS, Kim HK, Park JK, Lee SW, Park HH, So I, Jeon JH. Schisandrin B suppresses TGFβ1-induced stress fiber formation by inhibiting myosin light chain phosphorylation. J Ethnopharmacol. 2014;152:364–71. doi: 10.1016/j.jep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H, Sun X, Liu R, Chen Z, Lin Z, Yang Y, Zhang M, Liu P, Quan S, Huang H. Gentiopicroside activates the bile acid receptor Gpbar1 to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol Res. 2020;151:1045–1059. doi: 10.1016/j.phrs.2019.104559. [DOI] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Tian J, Xian W, Xie T, Yang X. Pentraxin-3 attenuates renal damage in diabetic nephropathy by promoting M2 macrophage differentiation. Inflammation. 2015;38:1739–1747. doi: 10.1007/s10753-015-0151-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhang D, Dou M, Li Z, Zhang J, Zhao X. Dendrobium officinale Kimura et migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomed Pharmacother. 2016;84:1350–1358. doi: 10.1016/j.biopha.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 20.Stittcavanagh E, Macleod L, Kennedy CR. The podocyte in diabetic kidney disease. Sci World J. 2009;9:1127–1139. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213–219. doi: 10.1016/S0140-6736(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 22.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 23.Huang M, Liang Q, Li P, Xia J, Wang Y, Hu P, Jiang Z, He Y, Pang L, Han L, Wang Y, Luo G. Biomarkers for early diagnosis of type 2 diabetic nephropathy: a study based on an integrated biomarker system. Mol Biosyst. 2013;9:2134–2141. doi: 10.1039/c3mb25543c. [DOI] [PubMed] [Google Scholar]
- 24.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 25.Hwang HJ, Baek YM, Kim SW, Kumar GS, Cho EJ, Oh JY, Yun JW. Differential expression of kidney proteins in streptozotocin-induced diabetic rats in response to hypoglycemic fungal polysaccharides. J Microbiol Biotechnol. 2007;17:2005–2017. [PubMed] [Google Scholar]
- 26.Wilson HM, Walbaum D, Rees AJ. Macrophages and the kidney. Curr Opin Nephrol Hypertens. 2004;13:285–290. doi: 10.1097/00041552-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in sreptozotocin induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 28.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7:242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Wu H, Zhao CY, Panchapakesan U, Pollock C, Chadban SJ. Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int J Clin Exp Pathol. 2014;7:481–495. [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa D, Shikata K, Matsuda M, Okada S, Wada J, Yamaguchi S, Suzuki Y, Miyasaka M, Tojo S, Makino H. Preventive effect of sulphated colominic acid on P-selectin-dependent infiltration of macrophages in experimentally induced crescentic glomerulonephritis. Clin Exp Immunol. 2002;129:43–53. doi: 10.1046/j.1365-2249.2002.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng XM, Tang PM, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Dis. 2015;1:138–146. doi: 10.1159/000431214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 34.Brosius FC. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amiri F, Shaw S, Wang X, Tang J, Waller JL, Eaton DC, Marrero MB. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605–1616. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 36.Huang JS, Chuang LY, Guh JY, Huang YJ, Hsu MS. Antioxidants attenuate high glucose-induced hypertrophic growth in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:1072–1082. doi: 10.1152/ajprenal.00020.2007. [DOI] [PubMed] [Google Scholar]
- 37.Banes-Berceli AK, Shaw S, Ma G, Brands M, Eaton DC, Stern DM, Fulton D, Caldwell RW, Marrero MB. Effect of simvastatin on high glucose- and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol Renal Physiol. 2006;291:116–121. doi: 10.1152/ajprenal.00502.2005. [DOI] [PubMed] [Google Scholar]
- 38.Neria F, Castilla MA, Sanchez RF, Gonzalez Pacheco FR, Deudero JJ, Calabia O, Tejedor A, Manzarbeitia F, Ortiz A, Caramelo C. Inhibition of JAK2 protects renal endothelial and epithelial cells from oxidative stress and cyclosporin A toxicity. Kidney Int. 2009;75:227–234. doi: 10.1038/ki.2008.487. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Tu Y, Sun YM, Li Y, Wang RM, Cao Y, Li L, Zhang LC, Wang ZB. Swiprosin-1 deficiency impairs macrophage immune response of septic mice. JCI Insight. 2018;3:e95396. doi: 10.1172/jci.insight.95396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


