Graphic abstract
In order to examine bee pollen hydrolysates to assess their anticancer and antioxidant properties, hydrolysis of bee pollen was first performed using three different commercially available enzymes: Alcalase®, Neutrase®, and Flavourzyme®. The study used DPPH and ABTS assay to evaluate the antioxidant properties of the hydrolysates obtained from bee pollen. All of the tested hydrolysates demonstrated antioxidant activity, while hydrolysate based on Alcalase® offered a high value for IC50 and was therefore chosen for further separation into five sub-fractions via ultrafiltration. The greatest antioxidant activity was presented by the MW < 0.65 kDa fraction, which achieved an IC50 value of 0.39 ± 0.01 µg/mL in the DPPH assay and 1.52 ± 0.01 µg/mL for ABTS. Purification of the MW < 0.65 kDa fraction was completed using RP-HPLC, whereupon the three fractions from the original six which had the highest antioxidant activity underwent further examination through ESI-Q-TOF–MS/MS. These particular peptides had between 7 and 11 amino acid residues. In the case of the MW < 0.65 kDa fraction, testing was also carried out to determine the viability of lung cancer cell lines, represented by ChaGo-K1 cells. Analysis of the antiproliferative properties allowed in vitro assessment of the ChaGo-K1 cells’ viability following treatment using the MW < 0.65 kDa fraction. Flow-cytometry generated date which revealed that it was possible for the MW < 0.65 kDa fraction to induce apoptosis in the ChaGo-K1 cells in comparison to the results with cells which had not been treated.
Keywords: Apoptosis, Bee pollen, ChaGo-K-1, Human bronchogenic carcinoma cells, Protein hydrolysate
Introduction
Cancer causes a high proportion of deaths in developed nations. It involves cell division, which takes place physiologically within the body tissues. In healthy individuals, there is a balance between cell death and proliferation, whereby apoptosis serves to control both sides of this balance. Some changes in DNA can result in cancer, as the programming of cell death becomes disrupted. The process by which cells become cancerous is known as carcinogenesis, and involves changes at the genetic and cellular levels which result in cells becoming reprogrammed to divide uncontrollably to create malignant tumors which can then spread around the body (Fouad and Aanei 2017; Redza-Dutordoir and Averill-Bates 2016; Valko et al. 2007). Among the cancer types, lung cancer is one of the most common, and kills large numbers of people (Goodarzi et al. 2018). The development of lung carcinoma comes from a number of factors which can cause damage to DNA as well as epigenetic changes inside the lung cells. A majority of these factors are linked to respiration, with air pollutants and cigarette smoke responsible for most of the lung cancer cases in smokers and those otherwise exposed to smoke (Loomis et al. 2013). Air pollution is an increasingly significant concern today in urban areas, with the focus on PM2.5 particles, which are those pollutants with a diameter smaller than 2.5 μm, which are generally indicative of anthropogenic air pollution (Kurt et al. 2016; Huang et al. 2017; Li et al. 2018). In humans, their effect can be to cause epigenetic and micro-environmental changes in lung cancer conditions, thus having a long-term effect on people’s health (Cao et al. 2018).
Today, it appears that the diagnosis and treatment of cancer may be possible through the use of anticancer peptides. These peptides are superior to either chemicals or antibodies for a number of reasons, including their low toxicity and high target specificity. Furthermore, they can be produced with relative ease (Kurrikoff et al. 2019). This stands in contrast to the traditional cancer treatments such as radiation, chemotherapy, surgery, and hormonal treatments, which have unpleasant side effects and can be very expensive. When some anticancer drugs are used, they can damage normal cells via the mechanism of the oxidative stress-mediated effect (Yokoyama et al. 2017). Other cancers are able to resist many chemotherapeutic agents. To address these issues, the use of therapeutic peptides may prove effective in creating novel cancer treatments. It has been possible to design certain therapeutic peptides to target signal transduction pathways, tumor suppressor proteins, cell cycles, and transcription factors. There are peptides which have the potential to trigger cell death in certain types of cancer cells while leaving untransformed cells untouched, and these peptides are suitable candidates for further development to become treatments for cancer (Cui et al. 2019).
In preclinical cancer models, antioxidant peptides have been employed to support the overall effectiveness of cancer therapies. The biological and nutraceutical properties of these peptides offer health benefits as well as acting against cancer. A number of peptides offering antioxidant properties have been separated from natural food sources and undergone testing to determine their effect on cancer cells. Among those examined, peptides including β-lactoglobulin, α-/β-casomorphins, α-lactalbumin, caseinphosphopeptides, bovine serum albumin, and lactoferrin which all come from bovine milk were reported to be active against human cancer. A number of egg peptides such as lysozyme, avidin, cystatin, phosvitin, ovalbumin, ovomucin, and ovotransferrin are understood to offer anticancer properties (Lee and Paik 2019). Accordingly, it is important to study the anticancer qualities of naturally occurring peptides since these might be a useful resource in improving cancer therapies and providing better outcomes for patients.
Bees can also be excellent providers of peptides which offer anticancer and antioxidant properties. It has been found that peptides obtained from the honey, venom, propolis, and royal jelly of bees can trigger apoptotic cell death in the cancerous cells of a number of types, including bladder, prostate, renal, lymphoid, and lung cancers (Premratanachai and Chanchao 2014). Honey was shown to contain peptides and proteins which offered antioxidant capabilities through the scavenging of free radicals and its ferric reducing ability (Bocian et al. 2019). Moreover, melittin, which is a peptide derived from bee venom, has a number of anticancer effects both in animal model systems and in preclinical cell culture (Rady et al. 2017). However, despite these promising signs, few studies have sought to understand the activity of these bee pollen peptides and therefore their characterization and purification warrants further study. This study therefore examined the antioxidant and anticancer properties of hydrolysates derived from bee pollen through the use of commercially available proteases including Neutrase®, Alcalase®, and Flavourzyme®. The most suitable fraction obtained was then selected for further examination of its ability to induce apoptosis in lung cancer cells (ChaGo-K-1). Moreover, the most active of the peptides obtained from hydrolysates could be identified using electrospray (ESI)-Q-TOF–MS/MS.
Materials and methods
Materials and chemicals used
The Apis mellifera bee pollen used in this study was purchased from the Phatthanakit Bee Farm Limited Partnership (Chiang Mai, Thailand). Neutrase® (EC 3.4.24.28) came from Novozymes (Bagsværd, Denmark), while 2,20-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), bovine-serum albumin (BSA), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), fetal bovine serum (FBS), dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle Medium (DMEM), L-ascorbic acid, and trifluoric acetic acid (TFA) were all purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Alcalase® (EC 3.4.21.62) and Flavourzyme® (EC 3.4.11.1) were obtained from Brentag (Mülheim, Germany).
Amino acid content determination
The standard AOAC 994.12 and 988.15 acid hydrolysis techniques were employed for the purpose of evaluating the constituent amino acids in bee pollen (Sangtanoo et al. 2020). The approach requires that 25 mg of bee pollen first be dissolved into a test tube in 5 mL of 6 M HCl before heating to 110 °C for 24 h to release the amnio acids. Addition of the internal standard (10 µL of 2.5 mM L-amino-n-butyric acid in 0.1 M HCl) follows, whereupon the solution is then diluted using deionized water to a volume of 250 µL, and heated to 55 °C for 10 min. Analysis is then carried out using RP-HPLC. Elution is carried out using a Hypersil GOLD column C18 (4.6 mm × 150 mm, 3 µM) with a sodium acetate buffer (pH 4.90) and 60% (v/v) ACN at 0.3 mL/min. The amino acid content was determined by ALS Laboratory Group (Thailand) Co., Ltd., Suan Luang, Bangkok, Thailand.
Preparation of hydrolysates derived from bee pollen protein
A quantity of bee pollen (5 g/100 mL) underwent dispersal and pretreatment using 20 mM phosphate buffer (pH 7.2) prior to hydrolysis using different concentrations of Alcalase®, Flavourzyme®, and Neutrase® (10, 25, and 50 mg/mL) at a temperature of 50 °C for 4 h at pH 7.0 (or pH 8.0 in the case of Alcalase®) (Saisavoey et al. 2019). The eventual inactivation of the enzyme was achieved by heating to 80 °C for 20 min. The sample was then placed in a centrifuge for 15 min at 15,900 × g at a temperature of 4 °C, whereupon the supernatant was gathered and stored at − 20 °C until required.
Free radical scavenging activity determination
ABTS and DPPH assay were employed for the purpose of determining the free radical scavenging activity of the sample, in line with the approach proposed by Saisavoey et al. (2019). The ABTS assay required the solution to be prepared with 7 mM ABTS solution and 2.45 mM potassium persulphate in the ratio (v/v) of 1:1 under conditions of darkness for 12 h at room temperature. The mixture solution then underwent dilution to an absorbance at 734 nm of 0.7 ± 0.02 measured with a microplate reader. The ABTS solution, once active, was combined with the sample in the ratio of 1:30 (v/v) prior to incubation in darkness for 10 min at room temperature. Measurements were then taken at 734 nm. The DPPH assay required that 100 μM DPPH solution be combined with each of the samples in the ratio of 1:4 (v/v) before incubation in darkness for 15 min at room temperature for. In this case, the measurements were taken at 517 nm by microplate reader. The positive control for this experiment was ascorbic acid.
Calculation of the IC50 value
The radical scavenging percentage calculation is given below:
in which the absorbance of the control is given by Abs control (no sample), the absorbance of the sample which is tested is given by Abs sample, the sample absorbance is given by Abs background, and the deionized water absorbance is given by Abs blank. The IC50, or half maximal inhibitory concentration, was determined with the assistance of GraphPad Prism vs. 6.01 for Windows (GraphPad Software Inc., California, USA).
Determination of the protein
Determination of the protein was carried out in line with the approach previously explained (Bradford 1976). A solution of the venoms was prepared in water (1 mg/mL), and BSA (bovine serum albumin) at five concentrations varying between 0 and 20 μg/mL. This solution was employed as the standard.
Ultrafiltration used to separate protein hydrolysates
Ultrafiltration was used to separate the hydrolyzed bee pollen proteins using molecular weight cut-off membranes of 0.65, 3, 5, and 10 kDa. The resulted fractions were gathered, including > 10, 5–10, 3–5, 0.65–3 and < 0.65 kDa, and subsequently evaluated for their antioxidant capabilities (Saisavoey et al. 2020).
Purification via HPLC (high performance liquid chromatography)
RP-HPLC was employed to purify the fraction which had demonstrated the greatest antioxidant activity using a Luna 5U 250 × 4.6 mm column (Phenomenex, California, USA) which had a mobile phase A linear gradient of 0.1% TFA (v/v) and for mobile phase B, 70% ACN (v/v) in 0.05% TFA (v/v) under a flow rate equal to 0.7 mL/min. The chromatogram was evaluated in terms of the UV absorbance at 280 nm. Having identified the purified peak for collection, this was repeated more than 20 times without changing the conditions of the process. Centrifugal evaporation was used to concentrate the pooling fractions 4 °C and 5,000 rpm, whereupon the amino acid sequences were determined along with the extent of any free radical scavenging capability.
Determination of the amino acid sequences via electrospray (ESI)-Q-TOF–MS/MS
It was possible to determine the amino acid sequence of the bee pollen peptide along with an accurate value for the molecular mass using ESI-Q-TOF–MS/MS, having first calibrated the instrument to work with peptide chains with mass in the range of 50–25,000 m/z (Model Amazon SL, Bruker, Germany). Evaluation of all the ESI-Q-TOF–MS/MS data was carried out by de novo sequencing and mascot. The sequences were then compared to those in the NCBI database through the use of BLASTp.
Cell culture
This study used ChaGo-K1 cell lines which were supplied by the Institute of Biotechnology and Genetic Engineering of Chulalongkorn University. These ChaGo-K-1 cells were stored in CM-R (RPMI 1640 medium supplemented with 10% (v/v) FBS, 1000 U/mL penicillin, 1.7 mM streptomycin, and 2.7 μM Fungizone™). The ChaGo-K1 cells were then suspended in CM-R. The concentration was set at 105 cells per well for seeding at 200 μL/well in 96-well culture plates. Following incubation in a 5% carbon dioxide atmosphere for one night at a temperature of 37 °C, the media were used to supplement the MW < 0.65 kDa fraction at different concentrations before being cultured for a period of 72 h in the 5% carbon dioxide atmosphere at 37 °C. After this, 0.12 μM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was introduced to the cells, which were then incubated for a further 4 h, whereupon the culture medium was removed and 150 μL dimethylsufoxide was used in its place. Absorbance was then measured at 540 nm (A540) with a Multiskan™ FC microplate photometer (Thermo Fisher Scientific Inc., MA, USA). The calculation of the viable cell percentage in comparison to the control was performed as follows:
Measurement of apoptosis
The approach of Suttisuwan et al. (2019) was employed in exposing the ChaGo-K1 cells for periods of 24, 48, and 72 h to 1.37 µg/mL (matching the IC50 values) of the MW < 0.65 kDa fraction. The cells were then gathered and stained using Annexin V-FTIC along with propidium iodide (PI). The negative control used was a sample of untreated cells. The cells which had undergone treatment were then placed in flow-cytometry tubes before washing on two occasions in temperate phosphate-buffered saline and re-suspension in 100 μL Annexin V binding buffer (10 mM Hepes/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). In the next step, 5 μL of Annexin V-FITC and 5 μL of PI were introduced to each 100 μL of cell suspension. Following 15 min of incubation in darkness at room temperature, a further 400 μL of Annexin binding buffer was added and flow cytometry was performed for analysis purposes within the hour. A BD FACSAria (BD Biosciences, Piscataway, NJ, USA) was employed to measure the intensity of the signal while analysis was then carried out with BD FACSDiva (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The experiments were carried out in triplicate, and the data analysis techniques comprised ANOVA and least significant difference whereby the various means and differences were presented using SPSS software and a significance level of 5%.
Results and discussion
Bee pollen amino acids
Going back in history, bee pollen has long played a role in traditional medicine as well as serving as a food supplement. Bee pollen is the pollen which is collected by bees from flowers, and then mixed either with nectar or with the saliva of the bees. Both adult bees and bee larvae use it as a nutritional source. Its value comes from its contents, which include proteins, amino acids, lipids, carbohydrates, phenolic compounds, vitamins, fatty acids and other bio-elements. To collect pollen, traps are placed at the bee hive entrance so that the pellets of pollen will be removed from the legs of the bees as they enter the hive. The type and quality of pollen obtained will depend on the flowers the bees have visited, and the species of bee involved (Komosinska-Vassev et al. 2015; Kocot et al. 2018). In this particular research, the pollen came predominantly from Mimosa pigra flowers which is available throughout the year (Nascimento et al. 2015). Apis mellifera bees gathered the pollen in Chiang Mai, in the north of Thailand. The Mimosaceae family has been shown to be the source of pollen in the case of the Brazilian stingless bee (Melipona subnitida) (Sarić et al. 2009).
Identification of the bee pollen amino acids was presented in the form of % content (w/w) and can be observed in Table 1. The amino acid content, at 22.02%, matched the average protein content for been pollen of 22.7% according to Komosinska-Vassev et al. (2015). Among these amino acids, those found in the greatest quantities were aspartic acid (2.72%), proline (2.28%), and glutamic acid (2.26%). Making up a further 8.69% of the bee pollen tested are a number of essential amino acids (EAA) that cannot be synthesized by humans, namely leucine, histidine, isoleucine, threonine, lysine, methionine, valine, phenylalanine, tryptophan, and valine. In this bee pollen, the ration of EEA to amino acids as a whole was 0.4, which exceeds the minimum ratio advised by the FAO, WHO, and UNU which is 0.36 (Chen et al. 2017). It has also been shown that the bee pollen has high levels of hydrophobic amino acids, including cysteine, alanine, leucine, isoleucine, proline, methionine, phenylalanine, tyrosine, valine, and tryptophan, amounting to 46.5% of the overall amino acid content. A number of earlier research works have indicated the importance of hydrophobic amino acid (HAA) in antioxidant activity, while Saadi et al. (2015) suggested that protein hydrolysates with high levels of HAA may more readily either inhibit or scavenge free radicals. The findings therefore showed that bee pollen can provide amino acids of the quality required in producing anticancer and antioxidant peptides.
Table 1.
Amino acid content in proteins found in bee pollen
| Amino acid type | Amino acid | % (w/w)* |
|---|---|---|
| Hydrophilic | Arginine (Arg) | 1.32 ± 0.054 |
| Aspartic acid (Asp) | 2.72 ± 0.078 | |
| Glutamic acid (Gln) | 2.26 ± 0.048 | |
| Histidine (His) | 0.65 ± 0.003 | |
| Lysine (Lys) | 1.67 ± 0.059 | |
| Serine (Ser) | 1.23 ± 0.004 | |
| Threonine (Thr) | 1.02 ± 0.008 | |
| Hydrophobic | Alanine (Ala) | 1.27 ± 0.074 |
| Cysteine (Cys) | 0.50 ± 0.018 | |
| Glycine (Gly) | 0.91 ± 0.049 | |
| Isoleucine (Ile) | 0.88 ± 0.008 | |
| Leucine (Leu) | 1.76 ± 0.025 | |
| Methionine (Met) | 0.39 ± 0.053 | |
| Phenylalanine (Phe) | 0.98 ± 0.095 | |
| Proline (Pro) | 2.28 ± 0.089 | |
| Tryptophan (Trp) | 0.23 ± 0.048 | |
| Tyrosine (Tyr) | 0.84 ± 0.061 | |
| Valine (Val) | 1.11 ± 0.043 | |
| Total | 22.02 ± 0.084 | |
*The data shown take the form of mean ± S.D. from experiments conducted in triplicate
Preparation of bee pollen hydrolysates and their antioxidant activity
This research examined three proteases (Alcalase®, Flavourzyme®, and Neutrase®) which are readily available, to assess their performance at the concentrations of 10, 25, and 50 mg/mL, when producing protein hydrolysates from bee pollen. By breaking proteins down to form peptides of different sizes, useful hydrolysates can be produced, but their potential roles will be dependent upon the properties they have, which will be derived from the type of enzymes involved along with the precise environmental conditions in their production, such as time, pH, and temperature (Amarowicz 2008). It is then possible to assess the antioxidant capabilities of these bee pollen hydrolysates by evaluating the scavenging ability of the DPPH and ABTS radicals, as shown in Table 2. The samples which were hydrolyzed showed notable antioxidant capabilities in comparison to those samples which had not been hydrolyzed. This suggests that the process of enzymatic hydrolysis releases bioactive peptides from the protein molecule. These released peptides clearly have hydrogen donors capable of reacting with free radicals to form stable compounds which will thus cause the termination of the chain reaction involving the radicals. In comparing the scavenging capabilities of the different hydrolysates, the lowest IC50 values, indicating the strongest antioxidant activity, were obtained for the bee pollen hydrolyzed with the Alcalase® enzyme. Earlier work has revealed that Alcalase® hydrolysates derived from plants including rice, wheat, and barley offer greater antioxidant capability than hydrolysates obtained through other enzymes (Chalamaiah et al. 2018). Furthermore, Chalamaiah et al. (2018) noted that Alcalase® has been the focus of many studies seeking to create bioactive peptides from food proteins such as fish, eggs, milk, whey, soybeans, rice, and microalgae. Accordingly, high levels of antioxidant activity were found in Alcalase® hydrolysates at 25 and 50 mg/mL, leading to the selection of 25 mg/mL Alcalase® hydrolysate for further purification since both assays confirmed the statistical significance of its qualities.
Table 2.
The free radical scavenging capabilities in terms of IC50 values for ABTS and DPPH for the bee pollen hydrolysates produced using various enzyme types
| Enzyme | Free radical scavenging activity (IC50) (µg/mL)* | |||
|---|---|---|---|---|
| Control | 10 mg/mL | 25 mg/mL | 50 mg/mL | |
| ABTS assay | ||||
| Alcalase® | 27.99 ± 0.79f | 2.41 ± 0.06a | 3.64 ± 0.03ab | 2.81 ± 0.18a |
| Flavourzyme® | 35.46 ± 2.04g | 9.28 ± 0.25e | 5.67 ± 0.06bc | 6.56 ± 0.03cd |
| Neutrase® | 35.46 ± 2.04g | 8.51 ± 0.20de | 4.53 ± 0.07abc | 4.17 ± 0.16ab |
| DPPH assay | ||||
| Alcalase® | 50.55 ± 1.38f | 11.06 ± 0.30bc | 9.75 ± 0.03ab | 10.25 ± 0.13b |
| Flavourzyme® | 85.84 ± 0.67g | 14.50 ± 0.46d | 10.05 ± 0.13ab | 8.49 ± 0.25a |
| Neutrase® | 85.84 ± 0.67g | 16.60 ± 0.49e | 12.12 ± 0.29c | 9.77 ± 0.30ab |
*Results take the form of mean ± sem (n = 3). The different letters indicated as superscript show those items which have significant differences from others (p > 0.05)
Bee pollen hydrolysate ultrafiltration
The smaller peptide fractions which were deemed to be of the required molecular size, and which offered a high level of desirable bioactivity were separated using ultrafiltration. First of all, the 25 mg/mL Alcalase® hydrolysate underwent filtration for the 10, 5, 3, and 0.65 kDa MW cut-off, obtaining five fractions (> 10, 5–10, 3–5, 0.65–3 and < 0.65 kDa fractions) which were duly tested to determine the extent of their antioxidant capabilities through ABTS and DPPH assay. The findings are shown in Table 3, making clear that the greatest activity level occurred in the < 0.65 kDa fraction. The IC50 value which was determined showed that those peptides which had a low molecular weight had greater antioxidant activity than those which had a much higher molecular weight (Suttisuwan et al. 2019; Jeampakdee et al. 2020). Furthermore, the scavenging activity of the MW < 0.65 kDa fraction was shown to be greater in the case of 25-fold (DPPH) and 2.4-fold (ABTS) when compared to the 25 mg/mL Alcalase® hydrolysate fraction. Accordingly, it could be concluded that small peptide fractions offer significantly greater antioxidant capabilities than the hydrolysate taken as a whole. Moreover, these small peptides are also known to exhibit strong antioxidant activity in the context of in vitro assays involving cancer cells which have undergone high levels of oxidative stress (Wang et al. 2016). In the case of small peptides, their antioxidant activity can boost the cellular enzyme mechanisms, thereby preventing damage to the cells. As a result, the MW < 0.65 kDa fraction was chosen for further purification via the HPLC approach.
Table 3.
Radical scavenging activity of ABTS, and DPPH shown as IC50 values for the ultrafiltration fractions resulting from the 25 mg/mL Alcalase® bee pollen hydrolysate
| MWCO fraction | Free radical scavenging activity (IC50) (µg/mL)* | |
|---|---|---|
| ABTS | DPPH | |
| < 0.65 kDa | 1.52 ± 0.01a | 0.39 ± 0.01a |
| 0.65–3 kDa | 2.38 ± 0.07b | 0.52 ± 0.01a |
| 3–5 kDa | 4.35 ± 0.21c | 1.64 ± 0.02b |
| 5–10 kDa | 7.88 ± 0.15d | 2.02 ± 0.02c |
| > 10 kDa | 33.47 ± 0.06e | 11.66 ± 0.10d |
*Results take the form of mean ± sem (n = 3). The different letters indicated as superscript show those items which have significant differences from others (p > 0.05)
Purification and identification and of antioxidant peptides
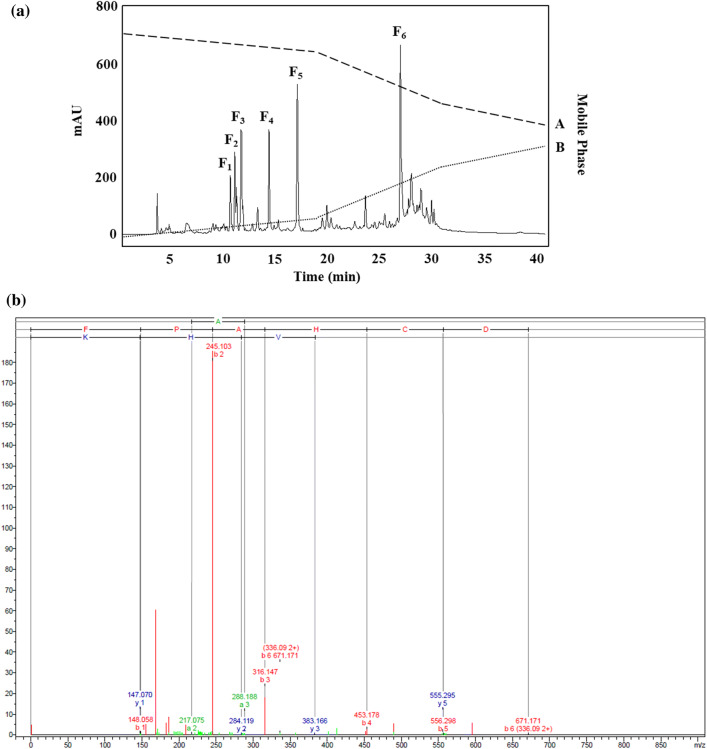
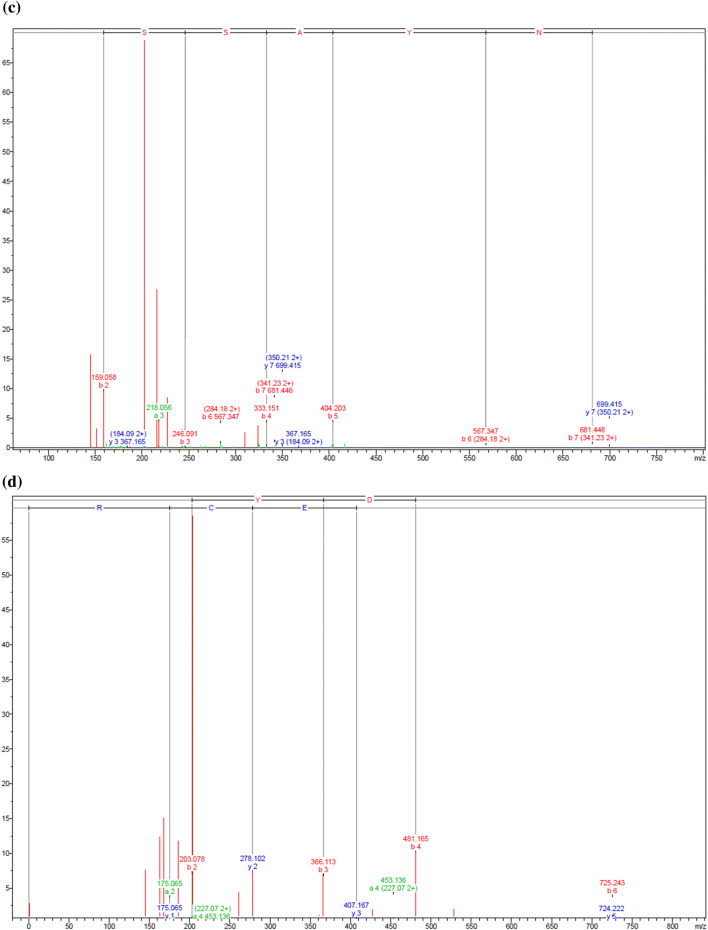
After purifying the MW < 0.65 kDa fraction through RP-HPLC, examination of the chromatogram profile indicated six peaks, as shown in Fig. 1a. These six peaks were recorded, and are considered to be associated with the F1-F6 fractions. The free radical scavenging activity assay provided findings indicating that the fractions designated as F3, F4, and F5 had IC50 values in the DPPH assay of 52.68 ± 1.07, 88.57 ± 2.46, and 89.84 ± 1.68 μg/mL respectively. These was no antioxidant activity shown by these fractions in the course of the ABTS radical scavenging activity assay, but it should be noted that DPPH acts as a free radical which receives radical electrons or hydrogen for the purpose of becoming stable, while in contrast, radical ABTS may be lowered if antioxidants act in combination with the ability to donate hydrogen or break chains. The way in which certain extracts can either quench or react with different radical types can also be explained through concepts such as radical stereoselectivity or extract solubility. These can come to the fore when certain different testing approaches are employed (Rival et al. 2011). For this reason, enhanced levels of activity in the DPPH assay does not indisputably confirm enhanced ability to scavenge ABTS radicals.
Fig. 1.
a Representation of the RP-HPLC chromatography of the MW < 0.65 kDa fraction derived from 25 mg/mL Alcalase® hydrolysate of bee pollen. Presents the mass fragmentation spectrum of the F3, F4, and F5 fractions derive using reversed-phase high-performance liquid chromatography (see Fig. 1). b FPAHCDGVHK, c TGSSAYN, and d MAYDEDWMECR
Having identified the F3, F4, and F5 fractions, ESI-Q-TOF–MS/MS and de novo amino acid sequencing were employed to obtain additional data which can then be checked using the NCBI database to determine the identity within the Mimosa genus (Fig. 1b, c). As Table 4 shows, it was possible to match the three amino acid sequences with M. pudica proteins. For instance, the F3 peptide (FPAHCDGVHK; Phe-Pro-Ala-His-Cys-Asp-Val-His-Lys; Fig. 1b) could be paired with cystathionine beta-lyase, which is an enzyme that can catalyze the direct precursor of methionine which is necessary in order to carry out the protein synthesis of S-adenosylmethionine (SAM). Reports also confirm that SAM is able to prevent the growth of gastric and colon cancer cells (Luo et al. 2010). Moreover, examination of the F4 (TGSSAYN; Thr-Gly-Ser-Ser-Ala-Tyr-Asn; Fig. 1c) and F5 (MAYDEDWMECR; Met-Ala-Tyr-Asp-Glu-Asp-Trp-Met-Glu-Cys-Arg; Fig. 1d) peptides indicated that they were apyrase, which is known to act against brain cancer tumors (Morrone et al. 2006). In addition, a certain quantity of HAA is found in the side chains of these peptides, which serve as the active site which plays a key role in facilitating the high levels of anticancer activity exhibited. It may also be the case according to this evidence that HAA residues within the sequence could further add to the anticancer properties.
Table 4.
Summary of the amino acid sequences of antioxidant peptides derived from the MW < 0.65 kDa fraction
| Fractions | Sequences | Origin protein from Mimosa pudica | Query cover | Mass (m/z) | Identity | Accession |
|---|---|---|---|---|---|---|
| F3 | FPAHCDGVHK | Plasma membrane intrinsic protein 2;5 | 40% | 1110.51 | 100% | BAD90701.1 |
| Plasma membrane intrinsic protein 2;4 | 40% | 1110.51 | 100% | BAD90700.1 | ||
| Plasma membrane intrinsic protein 2;1 | 40% | 1110.51 | 100% | BAD90697.1 | ||
| Cystathionine beta-lyase | 20% | 1110.51 | 100% | BAS32612.1 | ||
| F4 | TGSSAYN | Apyrase | 57% | 699.29 | 100% | AAC28339.1 |
| F5 | MAYDEDWMECR | Tonoplast intrinsic protein 1;2 | 36% | 1448.52 | 75% | BAD90703.1 |
| Apyrase | 45% | 1448.52 | 60% | BAK78979.1 | ||
| Actin isoform B | 54% | 1448.52 | 71% | BAA89214.1 |
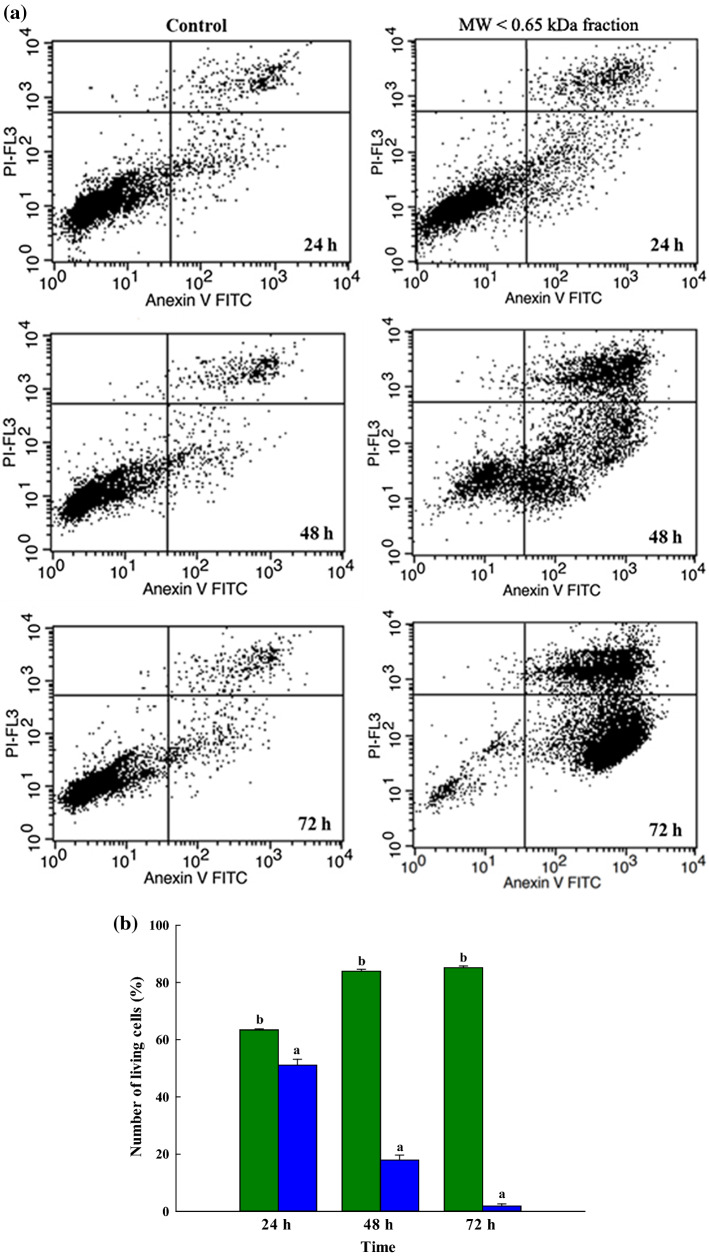
Apoptosis induction rates for ChaGo-K1 cells exposed to the MW < 0.65 kDa fraction
This research examined the effectiveness of peptides derived from bee pollen in the context of the in vitro growth of ChaGo-K1 over different time periods. Analysis was carried out via flow cytometry with Annexin V-FITC and PI staining in order to establish the nature of any cell changes in the living, apoptotic and necrosis cells during the course of the study. In order to quantify the early and late apoptotic stages, a double staining method was employed as the cells separated from those which were in the necrosis stage (Ortiz-Martinez et al 2017). Figure 2a shows that both early and late apoptotic activity can be increased when lung cancer cells are treated with the MW < 0.65 kDa fraction at the specific dose of 1.37 µg/mL which is suitable for the IC50 of ChaGo-K1. The eventual outcome of apoptosis or necrosis can be the death of the cell; apoptosis is a regulated type of cell death and is very common, whereas necrosis is unregulated and leads to a kind of cell death which stems from stresses placed upon the cell, whether internally or externally (Fink and Cookson 2005). To develop anticancer treatments, it is important to understand the process of apoptotic induction in lung cancer cells (Xu et al. 2009). In this case, it was found that apoptosis in the ChaGo-K1 cells was induced by the MW < 0.65 kDa fraction. Following 24, 48, and 72 h of incubation with exposure to the MW < 0.65 kDa fraction, in comparison to cells which had not been treated, it was shown that the number of living ChaGo-K1 cells had declined markedly, according to flow cytometry. The results shown in Fig. 2b, c confirm that the MW < 0.65 kDa fraction generated 22.28% apoptosis cells at 24 h (11.78% early apoptosis; 10.5% late apoptosis), 62.51% apoptosis at 48 h (33.82% early apoptosis; 28.69% late apoptosis), and 80.23% apoptosis at 72 h (45.15% early apoptosis; 35.08% late apoptosis). It was clear that the antiproliferative effects were influenced strongly by time. Meanwhile, necrosis cells were found to remain fewer than 1% during the treatments, although these results are not presented, and therefore the antiproliferative effects of the treatment focus on apoptosis induction. The findings demonstrate that the MW < 0.65 kDa fraction was able to induce apoptosis in ChaGo-K1 cells, and would serve as an important peptide with potential applications in treating lung carcinoma.
Fig. 2.
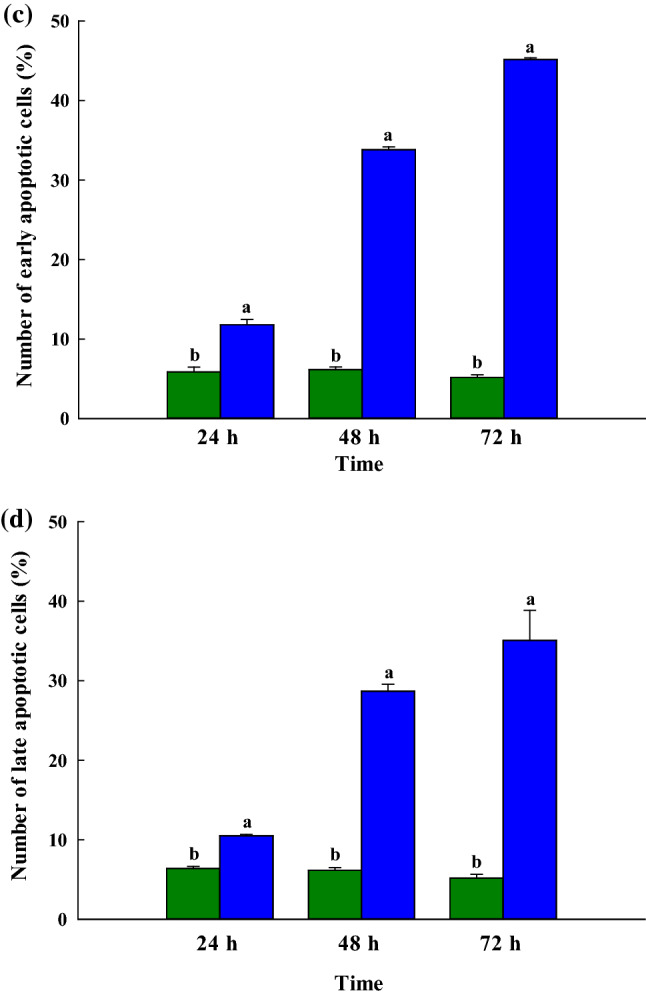
a Depiction of the Annexin V/PI double-staining assay of the ChaGo-K1 cells. Y indicates the PI-labeled population while X presents the FITC-labeled Annexin V positive cells. Presentation of the percentage of ChaGo-K1 cells categorized as b normal live cells, c early apoptotic cells, and d late apoptotic cells after 24 h, 48 h, or 72 h of treatment ([green color] control; [blue color] the MW < 0.65 kDa fraction). The findings are shown in the form of mean ± sem (n = 3). The various letters in superscript a, b show those items with statistically significant differences from each other (p > 0.05) (color figure online)
Conclusion
In this study, the antioxidant activity is achieved by bee pollen hydrolysates through the inhibition of DPPH and ABTS free radicals. Ultrafiltration and RP-HPLC were used to purify the target peptides which were obtained from the bee pollen protein hydrolysates using ultrafiltration and RP-HPLC. It was shown that these bee pollen protein hydrolysates offer selectively antiproliferative effects when targeting lung cancer cells through improving the apoptosis mechanism instead of necrosis in the case of trials with ChaGo-K1 cells. It can, however, be confirmed that bee pollen could be a useful source of bioactive peptides which have significant potential as agents offering antioxidant and anticancer properties which might be effectively harnessed for use as a pharmaceutical product for lung cancer treatment.
Acknowledgements
This study was completed with financial assistance from the Ratchadapisek Sompoch Fund for Postdoctoral Fellowship, Chulalongkorn University, the Annual Government Statement of Expenditure (GRB_BSS_79_57_61_11), and the Ratchadapisek Sompoch Endowment Fund (2019), Chulalongkorn University (762008) in recognition of the financial assistance offered which enabled this research study to be completed. The authors would also like to express their gratitude to the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, for the practical assistance offered and the facilities which were made available for the purposes of conducting the research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amarowicz R. Antioxidant activity of protein hydrolysates. Eur J Lipid Sci Technol. 2008;110:489–490. doi: 10.1002/ejlt.200800120. [DOI] [Google Scholar]
- Bocian A, Buczkowicz J, Jaromin M, Hus KK, Legáth J. An effective method of isolating honey proteins. Molecules. 2019 doi: 10.3390/molecules24132399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cao Q, Rui G, Liang Y. Study on PM2.5 pollution and the mortality due to lung cancer in China based on geographic weighted regression model. BMC Public Health. 2018;18(1):925. doi: 10.1186/s12889-018-5844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamaiah M, Yu W, Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang J, Liu W, Chen H. Physicochemical characterization, antioxidant and anticancer activities of proteins from four legume species. J Food Sci Technol. 2017;54(4):964–972. doi: 10.1007/s13197-016-2390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Han W, Zhang J, Zhang Z, Su X. Advances in the regulatory effects of bioactive peptides on metabolic signaling pathways in tumor cells. J Cancer. 2019;10(11):2425–2433. doi: 10.7150/jca.31359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–1036. [PMC free article] [PubMed] [Google Scholar]
- Goodarzi E, Sohrabivafa M, Adineh HA, Moayed L, Khazaei Z. Geographical distribution global incidence and mortality of lung cancer and its relationship with the Human Development Index (HDI); an ecology study in 2018. World Cancer Res J. 2018;6:e1354. doi: 10.32113/wcrj_20197_1354. [DOI] [Google Scholar]
- Huang F, Pan B, Wu J, Chen E, Chen L. Relationship between exposure to PM2.5 and lung cancer incidence and mortality: a meta-analysis. Oncotarget. 2017;8(26):43322–43331. doi: 10.18632/oncotarget.17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeampakdee P, Puthong S, Srimongkol P, Sangtanoo P, Saisavoey T, Karnchanatat A. The apoptotic and free radical-scavenging abilities of the protein hydrolysate obtained from chicken feather meal. Poult Sci. 2020;99(3):1693–1704. doi: 10.1016/j.psj.2019.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid Med Cell Longev. 2018;2018:7074209. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komosinska-Vassev K, Olczyk P, Kaźmierczak J, Mencner L, Olczyk K. Bee pollen: chemical composition and therapeutic application. Evid Based Complement Alternat Med. 2015;2015:297425. doi: 10.1155/2015/297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrikoff K, Aphkhazava D, Langel U. The future of peptides in cancer treatment. Curr Opin Pharmacol. 2019;47:27–32. doi: 10.1016/j.coph.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med. 2016;22(2):138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Paik HD. Anticancer and immunomodulatory activity of egg proteins and peptides: a review. Poult Sci. 2019;98(12):6505–6516. doi: 10.3382/ps/pez381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhou R, Zhang J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett. 2018;15(5):7506–7514. doi: 10.3892/ol.2018.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14(13):1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- Luo J, Li YN, Wang F, Zhang WM, Geng X. S-Adenosylmethionine inhibits the growth of cancer cells by reversing the hypomethylation status of c-myc and H-ras in human gastric cancer and colon cancer. Int J Biol Sci. 2010;6(7):784–795. doi: 10.7150/ijbs.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone FB, Oliveira DL, Gamermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AS, Marchini LC, Carvalho CAL, Araújo DFD, Silveira TA. Pollen spectrum of stingless bees honey (Hymenoptera: Apidae), Paraná State. Brazil J Entomol Zool Stud. 2015;3(2):290–296. [Google Scholar]
- Ortiz-Martinez M, de Mejia EG, García-Lara S, Aguilar O, Lopez-Castillo LM, Otero-Pappatheodorou JT. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J Funct Foods. 2017;34:36–48. doi: 10.1016/j.jff.2017.04.015. [DOI] [Google Scholar]
- Premratanachai P, Chanchao C. Review of the anticancer activities of bee products. Asian Pac J Trop Biomed. 2014;4(5):337–344. doi: 10.12980/APJTB.4.2014C1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Rival SG, Boeriu CG, Wichers HJ. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J Agric Food Chem. 2011;49(1):295–302. doi: 10.1021/jf0003911. [DOI] [PubMed] [Google Scholar]
- Saadi S, Saari N, Anwar F, Abdul Hamid A, Ghazali HM. Recent advances in food biopeptides: production, biological functionalities therapeutic applications. Biotechnol Adv. 2015;33(1):80–116. doi: 10.1016/j.biotechadv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Saisavoey T, Sangtanoo P, Reamtong O, Karnchanatat A. Free radical scavenging and anti-inflammatory potential of a protein hydrolysate derived from salmon bones on RAW 264.7 macrophage cells. J Sci Food Agric. 2019;99(11):5112–5121. doi: 10.1002/jsfa.9755. [DOI] [PubMed] [Google Scholar]
- Saisavoey T, Sangtanoo P, Chanchao C, Reamtong O, Karnchanatat A. Identification of novel anti-inflammatory peptides from bee pollen (Apis mellifera) hydrolysate in lipopolysaccharide-stimulated RAW264.7 macrophages. J Apic Res. 2020 doi: 10.1080/00218839.2020.1745434. [DOI] [Google Scholar]
- Sangtanoo P, Srimongkol P, Saisavoey T, Reamtong O, Karnchanatat A. Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculus nudus) in lipopolysaccharide-induced RAW264.7 macrophages. Food Funct. 2020;11(1):552–560. doi: 10.1039/c9fo02178g. [DOI] [PubMed] [Google Scholar]
- Sarić A, Balog T, Sobocanec S, Kusić B, Sverko V, Rusak G, Likić S, Bubalo D, Pinto B, Reali D, Marotti T. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem Toxicol. 2009;47(3):547–554. doi: 10.1016/j.fct.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Suttisuwan R, Phunpruch S, Saisavoey T, Sangtanoo P, Thongchul N, Karnchanatat A. Free radical scavenging properties and induction of apoptotic effects of FA fraction obtained after proteolysis of bioactive peptides from microalgae Synechococcus sp. VDW. Food Technol Biotechnol. 2019;57(3):358–368. doi: 10.17113/ftb.57.03.19.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Ding L, Yu Z, Zhang T, Ma S, Liu J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res Int. 2016;90:33–41. doi: 10.1016/j.foodres.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Xu CX, Jin H, Cho MH. Apoptosis and apoptosis-based therapy in lung cancer. Anticancer Agents Med Chem. 2009;9:952–957. doi: 10.2174/187152009789377682. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Sueyoshi Y, Ema M, Mori Y, Takaishi K, Hisatomi H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol Lett. 2017;14(5):6066–6070. doi: 10.3892/ol.2017.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]