Abstract
R848 is an imidazoquinoline compound that is a specific activator of toll-like receptor (TLR) 7/8 and is often used in immunological research in mammals and teleosts. However, the immune responses initiated by R848 through the TLR7/8 pathway in response to bacterial infection remain largely unexplored in teleosts. In the current study, we investigated the antibacterial response and the participating signaling pathway initiated by R848 in golden pompano (Trachinotus ovatus). We found that R848 could stimulate the proliferation of head kidney lymphocytes (HKLs) in a dose-dependent manner, enhance the survival rate of HKLs, and inhibit the replication of bacteria in vivo. However, these effects induced by R848 were significantly reduced when chloroquine (CQ) was used to blocked endosomal acidification. Additionally, an in vivo study showed that R848 strengthened the antibacterial immunity of fish through a TLR7/8 and Myd88-dependent signaling pathway. A cellular experiment showed that Pepinh-MYD (a Myd88 inhibitor) significantly reduced the R848-mediated proliferation and survival of HKLs. Luciferase activity analysis showed that R848 enhanced the nuclear factor kappa B (NF-κB) activity, whereas this activity was reduced when CQ and Pepinh-MYD were present. Additionally, when an NF-κB inhibitor was present, the R848-mediated pro-proliferative and pro-survival effects on HKLs were significantly diminished. An in vivo study showed that knockdown of TLR7, TLR8, and Myd88 expression in golden pompano via siRNA following injection of R848 resulted in increased bacterial dissemination and colonization in fish tissues compared to that of fish injection of R848 alone, suggesting that R848-induced antibacterial immunity was significantly reduced. In conclusion, these results indicate that R848 plays an essential role in the antibacterial immunity of golden pompano via the TLR7/8-Myd88-NF-κB- signaling pathway.
Keywords: R848, TLR7/8, antibacterial immune response, Myd88, NF-κB
Introduction
R848 (Resiquimod) is an immune response modifier that is also known for its potential antiviral activity (1, 2). Clinical studies have shown that this low-molecular-weight compound can be successfully used in the treatment of infectious viral diseases caused by hepatitis C virus or the herpes simplex virus (1–4). R848 affects antiviral activity by reducing viral shedding and viral reoccurrence (1–4). R848 is recognized by toll-like receptor (TLR) 7 and TLR8, synergistically activates immune cells, and induces an environment of T helper (Th) 1 immune responses (5–8). It acts as a selective activating ligand for both TLR7 and TLR8 in humans but only TLR7 in mice (9, 10). R848 activates TLR7/TLR8 in a Myd88-dependent signaling pathway, then activates transcription factors NF-κB (nuclear factor kappa B) and JNK (c-Jun NH2-terminal kinase) and ultimately induces the production of Th1 cytokines, including IFN I (type I interferon), IL (interleukin)-6, IL-12, IFN-γ, TNF-α (tumor necrosis factor-α), and inhibits the expression and secretion of the Th2 cytokine IL-4 (11–14). In addition, R848 activates pDCs (plasmacytoid dendritic cells), monocytes, and macrophages through TLR7/8 to secrete a variety of cytokines that mediate innate and acquired immunity (3–6).
TLR7 and TLR8 are highly conserved in vertebrates, exhibiting similar structures, including 13-15 LRR (leucine rich repeat) domains among mammals. Previous studies found that the loci of both TLR7 and TLR8 are located on the same chromosome in fish and mammals (15–17). TLR7 and TLR8 are expressed in endosomal compartments but not on the cell surface, such as the ER (endoplasmic reticulum), endolysosomes, lysosomes, and endosomes (18, 19). TLR7 and TLR8 are responsible for recognizing viruses, ssRNA (synthetic single-stranded RNA), and some imidazoquinoline compounds, such as R848 and R837, and inducing antiviral immune responses (20, 21). In addition, recent studies show that TLR7 and TLR8 can also recognize bacteria leading to antibacterial immunity (22). To date, TLR7 has been identified in many teleosts, including zebrafish (Danio rerio) (23), Japanese flounder (Paralichthys olivaceus) (24), channel catfish (Ictalurus punctatus) (25), common carp (Cyprinus carpio) (26), rainbow trout (Oncorhynchus mykiss) (15), and sea bream (Sparus aurata) (27). TLR8 has been identified in zebrafish (23), channel catfish (25), rainbow trout (15), sea bream (27), Atlantic salmon (Salmo salar) (28), and many other species as well. In some of the fish listed above, TLR7 and TLR8 have been shown to respond to viruses or ssRNA (29–31). Therefore, TLR7 and TLR8 play important roles in host immunity to viral infections in fish. As an agonist of TLR7/TLR8, R848 has been reported to induce immune responses in many mammals (32, 33). However, little is known about the immune function induced by R848 and associated working mechanism in teleosts, especially the antibacterial immunity.
Golden pompano (Trachinotus ovatus) is an economically important fish with desirable flavor and high nutritive value and is also the primary species raised in offshore cages in China (34). In this study, golden pompano was used as a model, and the antibacterial responses and participating signaling pathways induced by R848 were examined.
Materials and Methods
Fish
Golden pompano (body weight 17.2 ± 3.5 g) was purchased from the Haifeng Aquatic Product Company (Hainan Province, China) and kept in indoor aquaria with a flow-through seawater system at 26°C for 1 week before conducting experiments. Animals were anaesthetized with MS-222 before any procedure. Before performing experiments, fish were randomly examined to assure they were free of bacterial infections by plate counting. All animal care in the current study was performed according to the guidelines and regulations and approved by the Animal Care and Use Committee of Hainan University (no. HNU190402, approval date: 3 April 2019).
Cell Lines
Golden pompano snout (GPS) cells were cultured in L-15 medium (Leibovitz’s L15, Gibco, USA) supplemented with 10% FBS (fetal bovine serum), 1% Pen Strept (penicillin-streptomycin), 0.5% HEPES (1 M), and 1% NaCl in a humidified incubator at 26°C as previously described (35).
R848 and Edwardsiella tarda Infection
To study the antibacterial immunity induced by R848 (InvivoGen, USA) in T. ovatus, fish were separated into four groups with 15 fish in each group. Prior to the formal experiment, one preliminary experiment was performed to confirm the best dosage of R848 on golden pompano. Three different dosages (5, 10, and 15 μg per fish) of R848 advised by previous studies were injected intramuscularly (i.m.) into fish, and the result showed that 15 μg of R848 was the most suitable dose (36–38). In the formal experiment, fish were injected i.m. with 100 μl R848 (150 μg/ml), and 100 μl chloroquine (CQ, blocks endosomal acidification) (InvivoGen, USA) (25 μg/ml), 50 μl R848 (300 μg/ml) plus 50 μl CQ (50 μg/ml), and 100 μl PBS (as control), respectively. At 24 h post-stimulation, the fish were injected intraperitoneally (i.p.) with 100 μl of E. tarda (5 × 106 CFU/ml) PBMM02142, which was isolated from one diseased T. ovatus that was suffered from skin lesions in the Hainan Province of China and saved in our lab, which had been described by Sun et al. (39). At 6, 12, and 24 h post-infection (hpi), the liver, spleen, and kidney were obtained aseptically from five fish randomly selected from each group. The amount of E. tarda in these three tissues was counted by the spread plate method. To analyze whether R848 has the bactericidal ability, in vitro experiments were conducted. Specifically, R848 was resuspended in PBS at different doses (0, 40, 60, 80, 120, 160, and 320 μg/ml), and 20 μl R848 was incubated with 180 μl of E. tarda (104 CFU/ml) for 3 h at 28°C. Then, the number of bacterial colonies was determined by the spread plate method.
Cell Counting Kit-8 Assay
To evaluate the impact of R848 on head kidney lymphocytes (HKLs) proliferation, HKLs were extracted from three T. ovatus (460 ± 54 g) as described previously (40). Subsequently, the HKLs (5 × 105 cells/well) were seeded in a 96-well cell culture plate. Then, the cells were incubated with different doses of R848 (0, 0.175, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg/ml) at 26°C for 12 h. The proliferation of HKLs induced by R848 was measured using the Cell Counting Kit-8 (CCK8, Biosharp, China) following the manual. Additionally, HKLs were treated with CQ (final concentration of 5 μg/ml) for 1 h and then treated with R848 (final concentration of 8 μg/ml) at 26°C for 12 h. Afterwards, the proliferation of the HKLs was measured with CCK8 as described above. To examine the impact of Myd88 on the proliferation of cells induced by R848, 50 μM Pepinh-MYD (known to be a Myd88 inhibitory peptide) or 50 μM Pepinh-Control (a control peptide) (InvivoGen, USA) was mixed with HKLs for 6 h at 26°C. Subsequently, the cells were incubated with R848 (final concentration of 8 μg/ml) and the proliferation of the cells was determined as described above. Furthermore, to estimate the impact of NF-κB inactivation on the proliferation of cells induced by R848, 1 μM BAY-11-7082 (Beyotime, China), an IκB-α phosphorylation inhibitor that prevents NF-κB activation, was added to cells and incubated for 1 h at 26°C. Then, R848 (final concentration of 8 μg/ml) was added to the cells, and the proliferation of the cells was detected as described above. All assays were independently performed three times.
Apoptosis Assay
To evaluate the effect of R848 on HKLs apoptosis, freshly isolated HKLs cultured in 6-well plates (106 cells/well) were treated with CQ (final concentration of 5 μg/ml) for 1 h and then treated with R848 (final concentration of 8 μg/ml) for 24 h; the survival of HKLs was evaluated with the Annexin V-FITC/PI Apoptosis Detection Kit I (BD Pharmingen™, USA) and flow cytometry analysis performed on a Guava easyCyte™ flow cytometer (EMD Millipore Corp., USA). Final data was analyzed with guavaSoft 3.1.1. To examine the requirement for Myd88 in R848-induced HKL apoptosis, 50 μM Pepinh-MYD or Pepinh-Control was incubated with HKLs for 6 h at 26°C. After the incubation, the cells were treated with R848 (final concentration of 8 μg/ml), and flow cytometry was used as described above to detect cell survival. To analyze the impact of NF-κB activation on R848-induced apoptosis in HKLs, 1 μM BAY-11-7082 was added to cells for 1 h at 26°C. Then, the cells were treated with R848 (final concentration of 8 μg/ml). The experiments were repeated in triplicate.
Luciferase Reporter Assay
To examine the activation of NF-κB, a dual-luciferase reporter assay was performed. GPS cells (1 × 106 cells/well) were cultured in a 24-well plate. Then, 0.3 μg of pGL4.32 (luc2P/NF-κB-RE/Hygro) vector (Promega, USA), which is an NF-κB–specific firefly luciferase reporter vector, and 0.3 μg of pRL-CMV (a control vector) were co-transfected into cells with medium containing 5% FBS using FuGENE® 6 Transfection Reagent Limited Use Label License (Promega, USA) following the manual. At 12 h post-transfection, the GPS cells were cultured in complete medium containing antibiotics and 10% FBS. To evaluate the impact of R848 on the activation of NF-κB, transfected cells were treated with R848 (final concentration of 8 μg/ml), CQ (final concentration of 5 μg/ml), R848 (final concentration of 8 μg/ml) plus CQ (final concentration of 5 μg/ml) or PBS (control) and incubated for 24 h at 26°C. Then, the firefly and Renilla luciferase activities of cell lysates were measured with the Dual-Luciferase Reporter Assay Kit (Vazyme, China) according to the manual of GloMax® Discover (Promega, USA). To detect the necessity effect of TLR7 and TLR8 on the NF-κB activation induced by R848, in vitro knockdown of TLR7 and TLR8 expression was carried out with small interfering RNA (siRNA) technology using the T7 RiboMAX™ Express RNAi System (Promega, USA) as we previously described (41). Briefly, siTLR7 was synthesized using siTLR7-P1/siTLR7-P2 and siTLR7-P3/siTLR7-P4 (Table S1), similar to siTroTLR8 and siRNA-C (control siRNA). GPS cells (1 × 106 cells/well) were seeded in a 24-well plate and co-transfected with pGL4.32, pRL-CMV, and siRNA (siTLR7, siTLR8, siTLR7 plus siTLR8, siRNA-C, or PBS). A 12 h post-transfection, the cells were treated with R848 (final concentration of 8 μg/ml) for 24 h and then evaluated with the dual-luciferase reporter assay described above. To examine the requirement for Myd88 in the NF-κB activity induced by R848, 50 μM Pepinh-MYD or Pepinh-Control was incubated with transfected cells, which were co-transfected with pGL4.32 and pRL-CMV, for 6 h at 26°C. Subsequently, the cells were treated with R848 (final concentration of 8 μg/ml) for 24 h and then evaluated with the dual-luciferase reporter assay. Each experiment was conducted independently three times.
TLR7/8 Knockdown and Edwardsiella tarda Infection In Vivo
To evaluate the effects of TLR7 and TLR8 on antibacterial immunity induced by R848 in golden pompano, 75 fish were divided into five groups (15 fish/group) and intramuscularly injected with 15 μg of siRNA-C, siTLR7, siTLR8, siTLR7 plus siTLR8, or PBS. Twelve hours after the stimulation, the fish were treated with 15 μg of R848 via intramuscular injection, and another 15 fish were injected with PBS as a control. Then, 24 h later, all the fish were injected intraperitoneally with 100 μl of E. tarda (5 × 106 CFU/ml). At 6, 12, and 24 hpi, the bacterial loads in the liver, spleen, and kidney of five fish per group at each time point were counted by plate counting.
Myd88 Knockdown and Edwardsiella tarda Infection In Vivo
To examine whether the antibacterial effects of R848 were mediated via Myd88, 60 fish were included in this experiment. siMyd88 was synthesized using siMyd88-P1/siMyd88-P2 and siMyd88-P3/siMyd88-P4 (Table S1) as described above. Forty-five fish were divided into three groups (15 fish/group) and intramuscularly injected with 15 μg of siRNA-C, 15 μg of siMyd88, or PBS. Twelve hours after the stimulation, some fish were treated with 100 μl R848 (150 μg/ml) via intramuscular injection, and another 15 fish were injected with PBS as a control. Then, 24 h later, all the fish were injected intraperitoneally with 100 μl of E. tarda (5 × 106 CFU/ml). At 6, 12, and 24 hpi, the liver, spleen, and kidney of five fish per group were obtained aseptically. The bacterial loads in these three tissues were counted by the spread plate method as described above.
Quantitative Real-Time Reverse Transcriptase-PCR (qRT-PCR) Analysis of the Expression of Immune Genes
To further investigate the effects of TLR7/8 and Myd88 on R848-induced immunity, adaptor genes and some inflammatory genes, including TLR7, TLR8, Myd88, IFN-γ (interferon γ), IRF3 (interferon regulating factor 3), TNF-α (tumor necrosis factor α), interleukin-10 (IL-10), CCL4 [chemokine (C-C motif) ligand 4], and BLyS (B lymphocyte stimulating factor), were examined (Table S1). Thirty golden pompanos were injected intramuscularly with siRNA-C, siTLR7, siTLR8, siTLR7 plus siTLR8, siMyd88, or PBS (five fish/group). At 12 h post-administration, some fish were treated with 100 μl R848 (150 μg/ml) via intramuscular injection, while another five fish were injected with PBS as a control. Twenty-four hours later, the head kidney was harvested from each fish. Total RNA extraction and cDNA synthesis were performed as described in our previous study (34). qRT-PCR was carried out using the Eastep® qPCR Master Mix Kit (Promega, Madison, USA) according to the manufacturer’s protocol using the primers listed in Table S1. The mRNA levels of target genes were determined by the 2-ΔΔCt method with beta-2-microglobulin (B2M) used as the housekeeping gene according to our previous study (42).
Statistical Analysis
GraphPad Prism software (version 8, La Jolla CA, USA) was used to analyze data. All quantitative data were analyzed by a t-test. Differences were considered significant with a P-value less than 0.05.
Results
Antibacterial Activity of R848 in Golden Pompano
To examine whether R848 has antibacterial ability, different doses of R848 were incubated with E. tarda. The results showed that the number of bacteria incubated with R848 was similar to that incubated with the control, suggesting that R848 did not have direct bactericidal effects (data not shown). To examine whether R848 can induce antibacterial immunity in golden pompano, fish were injected with R848 with or without CQ prior to infection with E. tarda. At 6, 12, and 24 hpi, the bacterial loads in the liver, spleen, and kidney were counted. The results revealed that in all the examined tissues, E. tarda infection was extremely significantly decreased in the fish treated with R848 compared to the fish in the control group at each sampling time point. In contrast, the bacterial loads in the fish administered CQ alone or R848 and CQ together were not significantly different from those in the fish in the control group but were significantly higher than those in the R848-treated fish (Figure 1).
Figure 1.
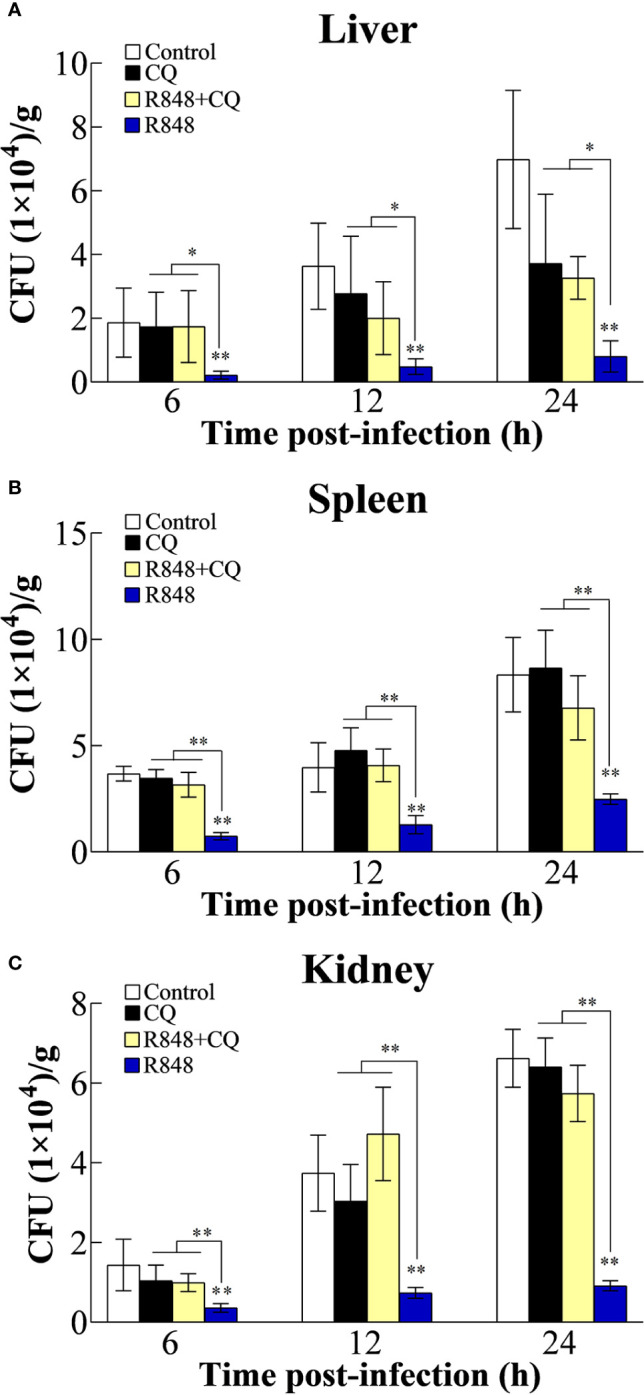
Effect of R848 and chloroquine (CQ) on bacterial infection. Golden pompano administered with R848, CQ, R848 plus CQ, or PBS (control) were infected with Edwardsiella tarda, and the amount of bacteria in the liver (A), spleen (B), and kidney (C) were determined at different time points. All data are shown as the means ± SD (N = 5), and the statistical significance is indicated. *P < 0.05, **P < 0.01.
Effects of R848 on the Proliferation and Apoptosis of Lymphocytes
Effect on Proliferation
To evaluate whether lymphocyte proliferation can be enhanced by R848, different doses of R848 were used to treat HKLs derived from golden pompano. CCK8 assay results showed that 0.25–32 μg/ml R848 significantly promoted HKL proliferation (Figure 2A). Once CQ was added, the promotive effect of R848 disappeared (Figure 2B).
Figure 2.
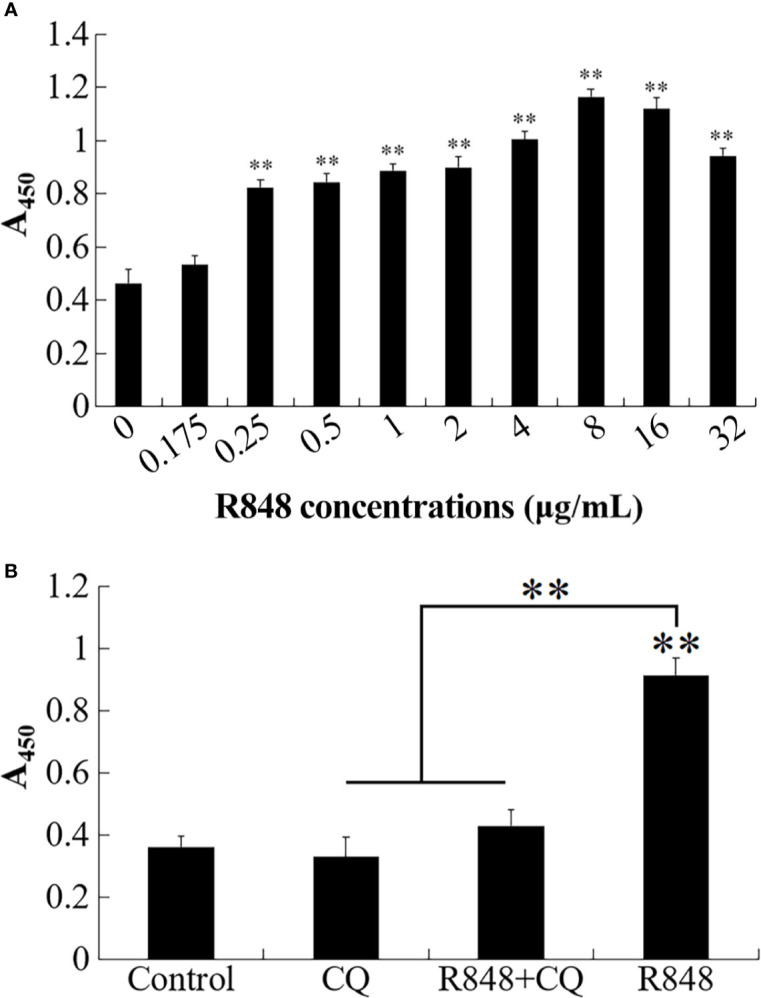
Effect of R848 on the proliferation of HKLs. (A) Golden pompano head kidney lymphocytes (HKLs) were treated with different concentrations of R848, and then the proliferation of HKLs was examined by CCK8 assay. (B) Golden pompano HKLs were treated with or without (control) 5 µg/ml of chloroquine (CQ), 8 µg/ml R848 plus 5 µg/ml of CQ, or 8 µg/ml R848, and then examined for proliferation by CCK8 assay. Data are presented as means ± SEM (N = 3). N, the number of times the experiment was performed. **P < 0.01.
Effect on Apoptosis
To detect whether R848 can induce HKL apoptosis, an Annexin V-FITC/PI staining assay was performed. After R848 monotherapy or combination treatment with R848 and CQ for 24 h, cellular apoptosis was evaluated. The results suggested that the number of apoptotic cells was significantly lower with R848 monotherapy (32.52%) than with control treatment. However, treatment with R848 and CQ together resulted in a significantly higher number of apoptotic cells (45.48%) than treatment with R848 alone, but there was no significant difference compared with control treatment (49.55%) (Figure 3).
Figure 3.
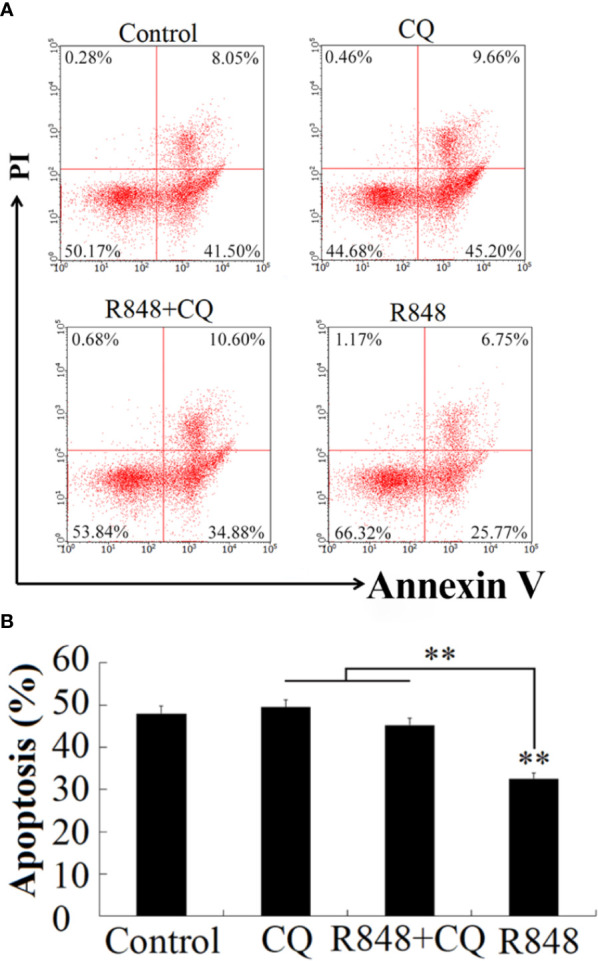
Effect of R848 and chloroquine (CQ) on the apoptosis of HKLs. (A) Golden pompano HKLs treated with or without (control) 8 µg/ml R848, 5 µg/ml CQ, or 8 µg/ml R848 plus 5 µg/ml CQ, and then the apoptosis rates of these cells were determined by the flow cytometry. (B) The percentages of apoptotic cells are represented with statistic bar charts. Data are presented as means ± SD (N = 3). N, the number of times the experiment was performed. **P < 0.01.
Myd88-Dependent Immune Responses Induced by R848
To explore whether Myd88 is involved in R848-induced immunity, the effect of Pepinh-MYD (Myd88 inhibitor) on the proliferation of HKLs induced by R848 was determined. The results showed that the proliferation of HKLs treated with R848 and Pepinh-MYD together was significantly decreased compared with that of cells stimulated with R848 with or without Pepinh-Control, whereas there was no significant difference between the cells administered R848 and those administered R848 plus Pepinh-Control. In contrast, cells stimulated with Pepinh-MYD or R848 plus Pepinh-MYD showed no significant differences from control cells (Figure 4A). In line with these results, Annexin V-FITC/PI staining revealed that R848 significantly inhibited apoptosis in HKLs compared to PBS (control), but the effect was weakened when Pepinh-MYD but not Pepinh-Control was present (Figures 4B, C).
Figure 4.
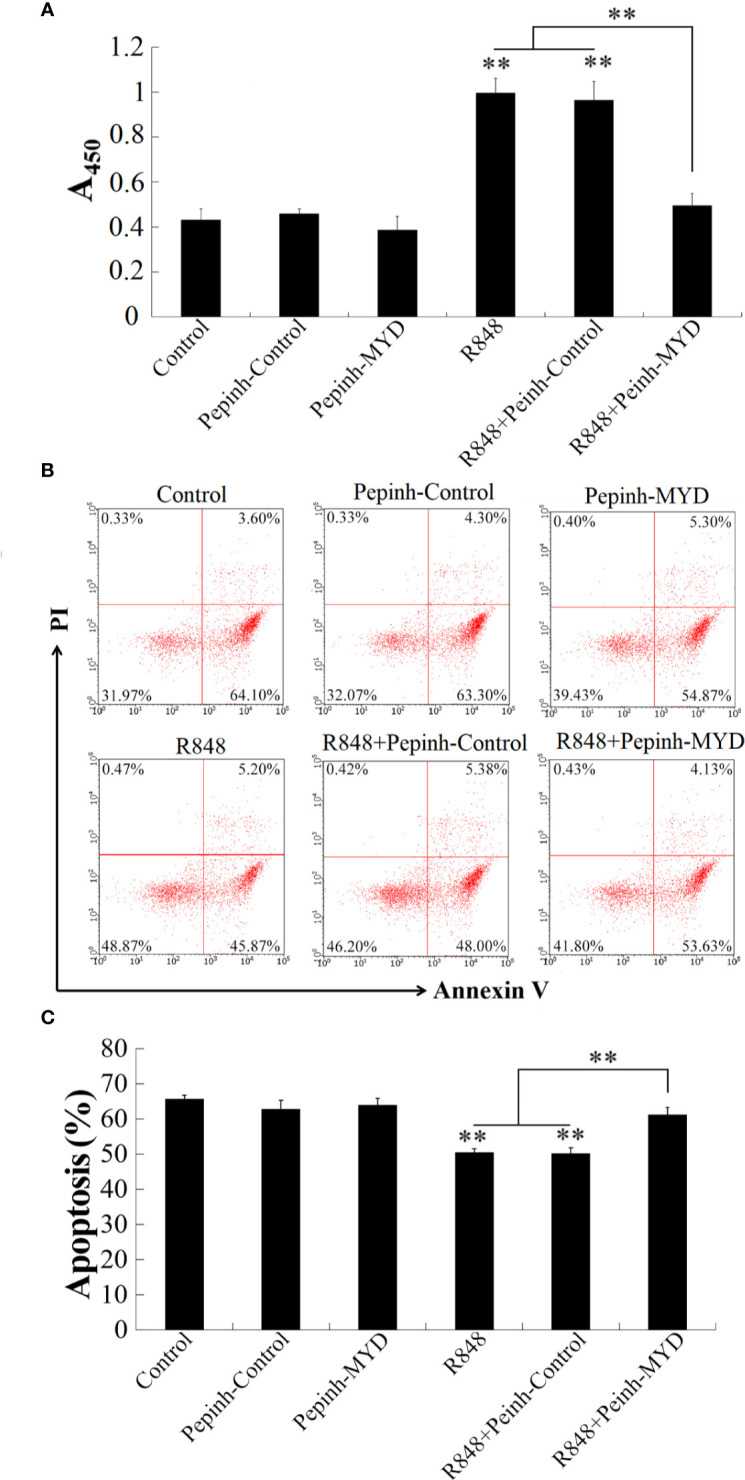
R848-induced cellular activity through Myd88-signaling pathway. Golden pompano HKLs were treated with PBS (control), R848, Pepinh-Control, Pepinh-MYD, R848 plus Pepinh-Control, or R848 plus Pepinh-MYD. The cells were then determined for proliferation (A), apoptosis (B), and statistic bar chart of the apoptosis percentage (C). Data in are presented as means ± SEM (N = 3), and (C) are presented as means ± SD (N = 3). **P < 0.01. Data in (B) are one representative of three independent experiments.
NF-κB Activity Induced by R848 and Its TLR7/8 and Myd88 Dependence
To detect the NF-κB activity induced by R848, GPS cells were transfected with NF-κB-RE-luc2P, and then the cells were stimulated with R848 with or without CQ. The results demonstrated that the luciferase activity of the cells stimulated with R848 was significantly increased (4.9-fold) compared to that of the control cells, whereas the luciferase activity of the cells stimulated with R848 and CQ together was not significantly different from that of the control cells (Figure 5A). When TLR7 and TLR8 expression was knocked down by transfection with siTLR7 and siTLR8, respectively, luciferase activity was significantly decreased compared with that stimulated with R848 alone or R848 plus siRNA-C. Furthermore, when siTLR7 and siTLR8 were transfected together, the decrease in luciferase activity was enhanced (Figure 5B). When GPS cells were treated with R848 and Pepinh-MYD, significantly decreased luciferase activity was observed compared with that observed when cells were administered R848 alone or R848 plus Pepinh-Control (Figure 5C).
Figure 5.
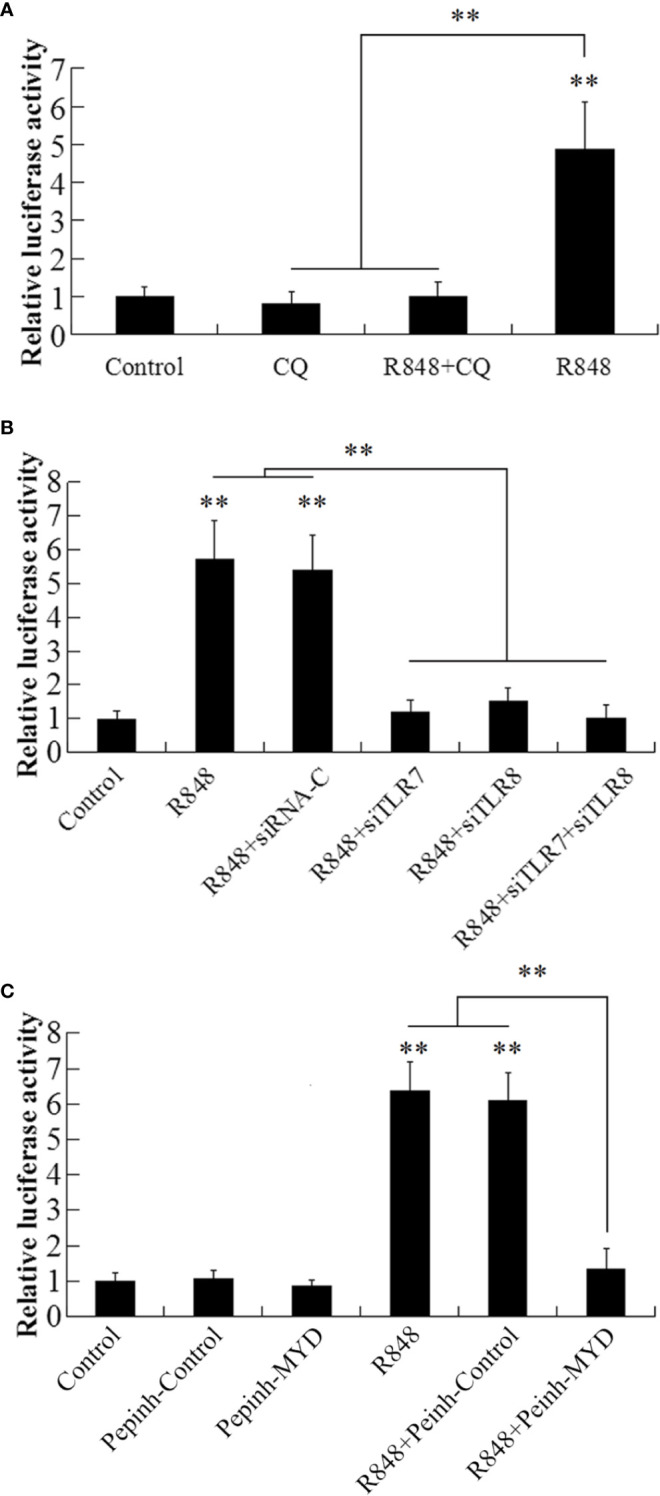
Effect of R848 on NF-κB activity. GPS cells containing NF-κB-RE-luc2P reporter were treated with or without (control) R848, chloroquine (CQ), or R848 plus CQ and then analyzed for luciferase activity (A). GPS cells containing NF-κB-RE-luc2P reporter were transfected with siTLR7, siTLR8, siTLR7 plus siTLR8, siRNA-C, or PBS (control) and then treated with R848. The luciferase activity in the cells was then determined (B). GPS cells containing NF-κB-RE-luc2P reporter were treated with R848 in the presence or absence of Pepinh-Control or Pepinh-MYD; the control cells were treated with PBS. The luciferase activity in the cells was then determined (C). Data are expressed as means ± SEM (N = 3). N, the number of times the experiment was performed. **P < 0.01.
NF-κB-Dependent Signaling in R848-Induced Immune Response
To further detect the immune response induced by R848 whether relying on NF-κB, HKLs were incubated with R848 with or without BAY-11-7082 (NF-κB inhibitor), and then cell proliferation and apoptosis were examined. The results showed that in the cells treated with R848 and BAY-11-7082 together, the effects of R848 were significantly reduced compared with those in cells treated with R848 alone (Figure 6A). Correspondingly, Annexin V-FITC/PI staining revealed that apoptosis was significantly reduced in HKLs treated with R848 (26.01%) compared to control cells (44.48%). However, the apoptosis rate of HKLs stimulated with R848 and BAY-11-7082 together demonstrated a significant increase compared with that of the cells stimulated with R848 alone but was comparable to that of the control cells (Figures 6B, C).
Figure 6.
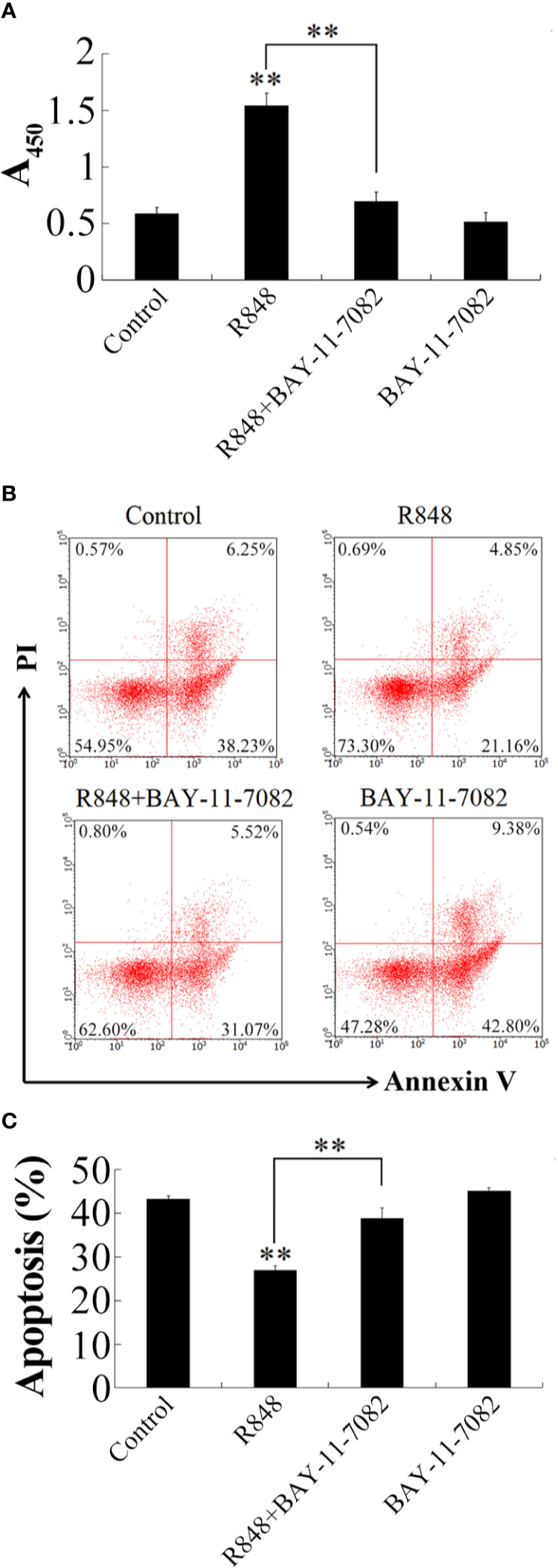
Effect of BAY-11-7082 on R848-induced cellular activity. (A) Golden pompano HKLs were treated with PBS (control), R848, BAY-11-7082, or R848 plus BAY-11-7082. The cells were then determined for proliferation (A), apoptosis (B), and statistic bar chart of the apoptosis percentage (C). Data in (A) are presented as means ± SEM (N = 3), and (C) are presented as means ± SD (N = 3). **P < 0.01. Data in (B) are one representative of three independent experiments.
In Vivo Antibacterial Effect of R848 Mediated Through the TLR7/8-Myd88-Dependent Pathway
Effects of TLR7/8 and Myd88 Knockdown on Bacterial Infection
To further analyze the antibacterial effect induced by R848 in vivo and the associated working mechanism, the expression of TLR7/8 and Myd88 was knocked down in golden pompano via siRNA technology. For this purpose, golden pompano fish were administered synthesized siRNAs (siTLR7, siTLR8, siTLR7+siTLR8, or siRNA-C) and then stimulated with R848. Our in vivo experiments showed that at 6, 12, and 24 hpi, the bacterial amounts in the liver, spleen, and kidney of the fish stimulated with R848 or R848 plus siRNA-C were significantly decreased compared with those of the fish stimulated with PBS (control) or R848 with preinjection of siTLR7, siTLR8, or siTLR7+siTLR8 (Figure 7). To further evaluate the significance of Myd88 in the antibacterial response induced by R848, Myd88 expression was knocked down in vivo via siRNA technology. For this purpose, golden pompano fish were administered synthesized siRNA (siMyd88 or siRNA-C) and R848. The results suggested that at 6, 12, and 24 hpi, significantly lower numbers of bacterial colonies were found in the liver, spleen, and kidney of fish administered R848 or R848 with preinjection of siRNA-C than those of fish treated with R848 and preinjection of siMyd88 (Figure 8).
Figure 7.
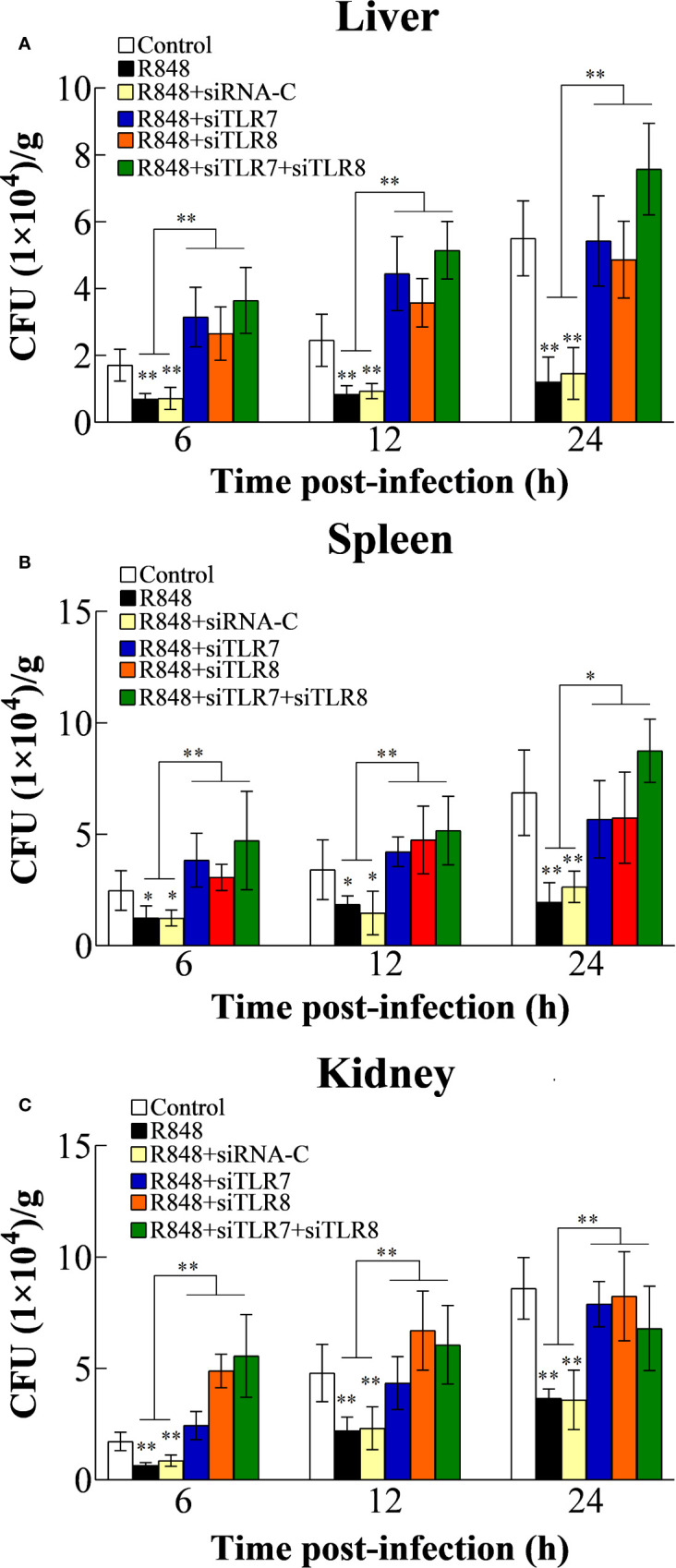
Effect of R848 on bacterial infection through TLR7/8-signaling pathway. Golden pompano administered with R848, R848 plus siRNA-C, R848 plus siTLR7, R848 plus siTLR8, R848 plus siTLR7 and siTLR8, or PBS (control) were infected with Edwardsiella tarda, and the amount of bacteria in the liver (A), spleen (B), and kidney (C) were determined at different time points. All data are shown as the means ± SD (N = 5), and the statistical significance is indicated. **P < 0.01.
Figure 8.

Effect of R848 on bacterial infection through Myd88-signaling pathway. Golden pompano administered with R848, R848 plus siRNA-C, R848 plus siMyd88, or PBS (control) were infected with Edwardsiella tarda, and the amount of bacteria in the liver (A), spleen (B), and kidney (C) were determined at different time points. All data are shown as the means ± SD (N = 5), and the statistical significance is indicated. *P < 0.05, **P < 0.01.
Expression of Immune Genes
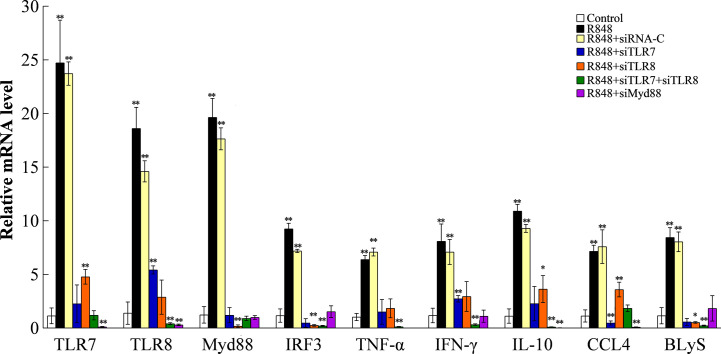
To understand the working mechanism of R848 in vivo, after knocking down the expression of TLR7/8 and Myd88 as described above, we examined the expression of immune-related genes. qRT-PCR was carried out to analyze the expression of the TLR7, TLR8, Myd88, IFN-γ, IRF3, TNF-α, IL-10, CCL4, and BLyS genes, with B2M as an internal control. The results showed that R848 significantly increased the expression of all the examined immune-related genes compared to control treatment. R848 plus siRNA-C was comparable to R848. In contrast, after knockdown of TLR7, TLR8, or Myd88 expression, the expression of all the examined immune-related genes were significantly reduced compared with that in the R848 group (Figure 9). The effects induced by R848 on the expression of immune-related genes were almost abolished after knockdown of TLR7, TLR8, or Myd88 expression.
Figure 9.
Effect of R848 on the expression of immune genes through TLR7/8-Myd88-signaling pathway. Expression of golden pompano TLR7/8 and Myd88 were knocked down in golden pompano via siRNA technology. Then, fish were treated with or without (control) R848. The expression profiles of immune genes were investigated in head kidney by qRT-PCR. The internal reference gene was B2M. Values are shown as means ± SD (N=5), and the statistical significance is indicated. *P < 0.05, **P < 0.01.
Discussion
R848 is a low-molecular-weight imidazoquinoline compound that can be recognized by TLR7 and TLR8 (1). As effective agents to treat cancer and infectious diseases and to improve immune responses, agonists of TLR7 and TLR8 have attracted great attention. At present, R848 is mainly used in the treatment of infectious viral diseases and tumors (43–45). However, there are few reports on its antibacterial activity in fish. In the current study, we found that R848 could significantly reduce the bacterial loads in the liver, kidney, and spleen of T. ovatus, which indicated that R848 induced effective immune responses in lower vertebrates. This study sought to evaluate the mechanism involving R848 and TLR7/8 in fish.
As an analog of imiquimod (R837), R848 is the first imidazoquinoline recognized by both TLR7 and TLR8. The immune responses induced by R848 and R837 are similar; however, the immune effects elicited by R848 are 50–100 times greater than those elicited by R837 (46–48). Many studies have shown that R848 elicits effective immune responses against viral infection (43, 46–49). Some studies have reported that it also has antibacterial properties (44, 50). In fish, studies on R848 have focused on its immunostimulatory and antiviral effects, but its antibacterial effects and working mechanism are still unknown (15, 37, 51–54). In this study, we found that after golden pompano administered with R848, bacterial loads in the liver, spleen, and kidney after E. tarda infection were significantly reduced; however, R848 did not have direct antimicrobial activity. These results suggested that R848 induced the immune responses against bacteria in golden pompano.
In mammals, R848 is recognized by human TLR7/8 and mouse TLR7 and then activates many immune cells, such as pDCs, macrophages, and monocytes, leading to the secretion of IFN I, IL-8, IL-12, TNF-α, and so on (8, 16, 55). Previous studies have shown that R848 induces the activation of CD4+ Th1 cells and CD8+ cytotoxic T lymphocytes (8, 55), promotes the proliferation of B cells and inhibits their apoptosis (21, 56, 57). Recently, R848 was demonstrated to enhance human B cell differentiation induced by IL-21 and sCD40L (58). In mice, R848 augments the proliferation of the bone marrow CD11c+ B lymphocyte population (59). R848 increases the proliferation of lymph node cells and promotes B cell activation (60). Consistent with these observations, R848 was found to increase the production of BlyS, which could promote the proliferation, differentiation, and survival of B cells (61). In addition, R848 was found to induce a cytotoxic T lymphocyte (CTL) response and maturation of monocytes (45). In line with this, we found that R848 promoted HKLs proliferation and reduced HKLs apoptosis. Further studies showed that when TLR signaling was blocked with CQ (62, 63), the effects induced by R848 were significantly reduced, suggesting that R848 may act through a TLR signaling pathway.
TLR7 and TLR8 in golden pompano were reported to exhibit the typical TLR domain architecture, including a TIR domain located in the cytoplasmic region (64). When TLRs recognize PAMPs, the TLR conformation changes, and then adaptor molecules, including Myd88, are recruited (65, 66). Previous studies have shown that Myd88 is required for activation of the TLR7/8 pathway. The TIR domains of Myd88 and TLR7/8 interact and then recruit interleukin receptor-associated kinases (IRAKs), including IRAK1, IRAK2, IRAK4, and IRAK-M (19). Once IRAK4 is phosphorylated, it dissociates from Myd88 molecules and interacts with TRAF6 to activate the NF-κB pathway and induce the secretion of proinflammatory cytokines (14, 21). Studies have demonstrated that the immune responses induced by R848 are abolished in TLR7- and Myd88-deficient mice and that the production of inflammatory cytokines is inhibited, suggesting that R848 functions through the TLR7/TLR8 Myd88-dependent signaling pathway (21, 67, 68). In this study, our results showed that when Myd88 activation was blocked by Pepinh-MYD, the proliferation and apoptosis of HKLs were comparable to those of control-treated cells, which demonstrated that Pepinh-MYD significantly reduced the effects of R848, suggesting that Myd88 is required in this immune response in teleosts.
Previous studies have shown that Toll-like receptor ligands promote multiple myeloma cell growth and survival via activation of NF-κB (69). NF-κB is also known to play key roles in the inflammatory response and immune response. Normally, NF-κB binds to members of an inhibitory protein family, mainly in the form of the p65/P50/I-κB complex (70, 71). NF-κB activation is induced by various stimuli, such as bacteria, viruses, and cytokines (72–75). TLR-NF-κB signal transduction transmits activation signals to cells through a series of adaptor proteins, thus activating NF-κB to regulate the expression of related genes, especially those encoding inflammatory cytokines. In vitro transfection experiments previously showed that R848 induces the activation of NF-κB in HEK293 cells in a dose-dependent manner (21). Consistent with this, many studies have shown that NF-κB is needed for the production of cytokines in mammalian cells treated with R848 (76, 77). In line with these findings, our results demonstrated that R848 could significantly induce the activation of NF-κB, but when CQ was added, NF-κB activation disappeared. Further studies showed that when TLR7 and TLR8 expression was knocked down in cells, the activation of NF-κB induced by R848 was significantly reduced. When Myd88 was inhibited by Pepinh-MYD, R848-induced NF-κB activation was significantly decreased. Our results indicate that R848 induces the activation of NF-κB through the TLR7/8-Myd88 signaling pathway. In addition, our results showed that NF-κB plays important roles in the immune effects induced by R848. When HKLs were treated with BAY-11-7082, which was used to suppress the activation of NF-κB, the effects of increased cell proliferation and decreased apoptosis induced by R848 were significantly reduced. It is well known that in vivo animal models are very useful approaches to study immune responses. In line with our in vitro studies, when TLR7, TLR8, and Myd88 expression was knocked down by siRNA following injection of R848 into golden pompano, bacterial invasion in the liver, spleen, and kidney in the context of E. tarda infection was enhanced compared to when R848 treatment was administered alone, suggesting that R848-induced antibacterial immunity was significantly reduced. These results further support the conclusion that R848 plays essential roles in the antibacterial immunity of golden pompano via the TLR7/8 Myd88-dependent signaling pathway. Previous studies have shown that TLR7/TLR8 recognize R848 and then activate NF-κB via a Myd88-dependent signaling pathway, ultimately inducing the secretion of a variety of cytokines, such as IFN-γ, TNF-α, and BlyS, that mediate innate and acquired immunity (61, 67, 68). In line with these findings, our results showed that R848 significantly induced the expression of IFN-γ, TNF-α, IL-10, CCL4, and BlyS. In contrast, when TLR7, TLR8, and Myd88 expression was knocked down by siRNA, this increased expression was reduced, suggesting that TLR7, TLR8, and Myd88 are required for the R848-induced effects.
In conclusion, we illustrated that R848 is an immunostimulatory molecule using golden pompano as a model and that R848 enhanced the resistance to bacterial infection and induced immune responses. Moreover, the antibacterial effects of R848 might be mediated via the TLR7/8-Myd88-NF-κB signaling pathway.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Hainan University.
Author Contributions
YZ, XC, and YS designed the research. ZC, JL, and HL analyzed the data. YW and ZZ supervised the study. YZ and XC performed the research, analyzed the data, and wrote the manuscript with support from all authors. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (No. 41666006 and No. 42066007), and the Nanhai Famous Youth Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank South China Agricultural University for providing the GPS cells.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.617522/full#supplementary-material
References
- 1. Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect (2008) 21:69–87. 10.1358/dnp.2008.21.2.1188193 [DOI] [PubMed] [Google Scholar]
- 2. Grela F, Aumeunier A, Bardel E, Van LP, Bourgeois E, Vanoirbeek J, et al. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-gamma. J Immunol (2011) 186:284–90. 10.4049/jimmunol.1001348 [DOI] [PubMed] [Google Scholar]
- 3. Boghdadi G, Hammad N, Amer A, Sammour S, Sorour S. R848, a Toll-like receptors 7 and 8 agonist, a potential therapy for allergic rhinitis patients. Inflammation Allergy Drug Targ (2014) 13:144–9. 10.2174/1871528113666140429111658 [DOI] [PubMed] [Google Scholar]
- 4. Siebeneicher S, Reuter S, Krause M, Wangorsch A, Maxeiner J, Wolfheimer S, et al. Epicutaneous immune modulation with Bet v 1 plus R848 suppresses allergic asthma in a murine model. Allergy (2014) 69:328–37. 10.1111/all.12326 [DOI] [PubMed] [Google Scholar]
- 5. Bernstein DI, Harrison CJ, Tomai MA, Miller RL. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J Infect Dis (2001) 183:844–9. 10.1086/319262 [DOI] [PubMed] [Google Scholar]
- 6. Brugnolo F, Sampognaro S, Liotta F, Cosmi L, Annunziato F, Manuelli C, et al. The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-gamma-producing cells. J Allergy Clin Immunol (2003) 111:380–8. 10.1067/mai.2003.102 [DOI] [PubMed] [Google Scholar]
- 7. Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine (2005) 23:5263–70. 10.1016/j.vaccine.2005.06.024 [DOI] [PubMed] [Google Scholar]
- 8. Wu JJ, Huang DB, Tyring SK. Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses. Antiviral Res (2004) 64:79–83. 10.1016/j.antiviral.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 9. Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol (2006) 177:6584–7. 10.4049/jimmunol.177.10.6584 [DOI] [PubMed] [Google Scholar]
- 10. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science (2004) 303:1526–9. 10.1126/science.1093620 [DOI] [PubMed] [Google Scholar]
- 11. Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett (2003) 85:85–95. 10.1016/s0165-2478(02)00228-6 [DOI] [PubMed] [Google Scholar]
- 12. Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol (2003) 33:827–33. 10.1002/eji.200323797 [DOI] [PubMed] [Google Scholar]
- 13. Sauder DN, Smith MH, Senta-McMillian T, Soria I, Meng TC. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob Agents Chemother (2003) 47:3846–52. 10.1128/aac.47.12.3846-3852.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, et al. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol (1999) 191:10–9. 10.1006/cimm.1998.1406 [DOI] [PubMed] [Google Scholar]
- 15. Palti Y, Gahr SA, Purcell MK, Hadidi S, Rexroad CE3, Wiens GD. Identification, characterization and genetic mapping of TLR7, TLR8a1 and TLR8a2 genes in rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol (2010) 34:219–33. 10.1016/j.dci.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 16. Temperley ND, Berlin S, Paton IR, Griffin DK, Burt DW. Evolution of the chicken Toll-like receptor gene family: a story of gene gain and gene loss. BMC Genomics (2008) 9:62. 10.1186/1471-2164-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avunje S, Oh MJ, Jung SJ. Impaired TLR2 and TLR7 response in olive flounder infected with viral haemorrhagic septicaemia virus at host susceptible 15°C but high at non-susceptible 20 °C. Fish Shellfish Immunol (2013) 34:1236–43. 10.1016/j.fsi.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 18. Akira S, Uematsu S O, Takeuchi O. Pathogen recognition and innate immunity. Cell (2006) 124:783–801. 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 19. Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity (2010) 32:305–15. 10.1016/j.immuni.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 20. Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol (2005) 174:1259–68. 10.4049/jimmunol.174.3.1259 [DOI] [PubMed] [Google Scholar]
- 21. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol (2002) 3:196–200. 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 22. Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol (2009) 183:5279–92. 10.4049/jimmunol.0901390 [DOI] [PubMed] [Google Scholar]
- 23. Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A (2005) 102:9577–82. 10.1073/pnas.0502272102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avunje S, Kim WS, Park CS, Oh MJ, Jung SJ. Toll-like receptors and interferon associated immune factors in viral haemorrhagic septicaemia virus-infected olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol (2011) 31:407–14. 10.1016/j.fsi.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 25. Quiniou SM, Boudinot P, Bengtén E. Comprehensive survey and genomic characterization of Toll-like receptors (TLRs) in channel catfish, Ictalurus punctatus: identification of novel fish TLRs. Immunogenetics (2013) 65:511–30. 10.1007/s00251-013-0694-9 [DOI] [PubMed] [Google Scholar]
- 26. Tanekhy M, Kono T, Sakai M. Cloning, characterization, and expression analysis of Toll-like receptor-7 cDNA from common carp, Cyprinus carpio L. Comp Biochem Physiol Part D Genomics Proteomics (2010) 5:245–55. 10.1016/j.cbd.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 27. Pietretti D, Wiegertjes GF. Ligand specificities of Toll-like receptors in fish: indications from infection studies. Dev Comp Immunol (2014) 43:205–22. 10.1016/j.dci.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 28. Skjaeveland I, Iliev DB, Strandskog G, Jørgensen JB. Identification and characterization of TLR8 and MyD88 homologs in Atlantic salmon (Salmo salar). Dev Comp Immunol (2009) 33:1011–7. 10.1016/j.dci.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 29. McBeath AJ, Snow M, Secombes CJ, Ellis AE, Collet B. Expression kinetics of interferon and interferon-induced genes in Atlantic salmon (Salmo salar) following infection with infectious pancreatic necrosis virus and infectious salmon anaemia virus. Fish Shellfish Immunol (2007) 22:230–41. 10.1016/j.fsi.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 30. Skjesol A, Skjæveland I, Elnæs M, Timmerhaus G, Fredriksen BN, Jørgensen SM, et al. IPNV with high and low virulence: host immune responses and viral mutations during infection. Virol J (2011) 8:396. 10.1186/1743-422X-8-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su J, Su J, Shang X, Wan Q, Chen X, Rao Y. SNP detection of TLR8 gene, association study with susceptibility/resistance to GCRV and regulation on mRNA expression in grass carp, Ctenopharyngodon idella. Fish Shellfish Immunol (2015) 43:1–12. 10.1016/j.fsi.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 32. Purcell MK, Smith KD, Hood L, Winton JR, Roach JC. Conservation of Toll-Like Receptor Signaling Pathways in Teleost Fish. Comp Biochem Physiol Part D Genomics Proteomics (2006) 1:77–88. 10.1016/j.cbd.2005.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Svingerud T, Solstad T, Sun B, Nyrud ML, Kileng O, Greiner-Tollersrud L, et al. Atlantic salmon type I IFN subtypes show differences in antiviral activity and cell-dependent expression: evidence for high IFNb/IFNc-producing cells in fish lymphoid tissues. J Immunol (2012) 189:5912–23. 10.4049/jimmunol.1201188 [DOI] [PubMed] [Google Scholar]
- 34. Cao Z, He M, Chen X, Wang S, Cai Y, Xie Z, et al. Identification, polymorphism and expression of MHC class Iα in golden pompano, Trachinotus ovatus. Fish Shellfish Immunol (2017) 67:55–65. 10.1016/j.fsi.2017.05.058 [DOI] [PubMed] [Google Scholar]
- 35. Yu Y, Wei S, Wang Z, Huang X, Huang Y, Cai J, et al. Establishment of a new cell line from the snout tissue of golden pompano Trachinotus ovatus, and its application in virus susceptibility. J Fish Biol (2016) 88:2251–62. 10.1111/jfb.12986 [DOI] [PubMed] [Google Scholar]
- 36. Zhou ZX, Sun L. Immune effects of R848: evidences that suggest an essential role of TLR7/8- induced, Myd88- and NF-κB-dependent signaling in the antiviral immunity of Japanese flounder (Paralichthys olivaceus). Dev Comp Immunol (2015) 49:113–20. 10.1016/j.dci.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 37. Li XP, Sun L. TLR7 is required for optimal immune defense against bacterial infection in tongue sole (Cynoglossus semilaevis). Fish Shellfish Immunol (2015) 47(1):93–9. 10.1016/j.fsi.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 38. Svingerud T, Solstad T, Sun B, Nyrud ML, Kileng Ø, Greiner-Tollersrud L, et al. Atlantic salmon type I IFN subtypes show differences in antiviral activity and cell-dependent expression: evidence for high IFNb/IFNc-producing cells in fish lymphoid tissues. J Immunol (2012) 189:5912–23. 10.4049/jimmunol.1201188 [DOI] [PubMed] [Google Scholar]
- 39. Sun B, Lei Y, Cao Z, Zhou Y, Sun Y, Wu Y, et al. TroCCL4, a CC chemokine of Trachinotus ovatus, is involved in the antimicrobial immune response. Fish Shellfish Immunol (2019) 86:525–35. 10.1016/j.fsi.2018.11.080 [DOI] [PubMed] [Google Scholar]
- 40. Sun Y, Sun L. CsBAFF, a Teleost B Cell Activating Factor, Promotes Pathogen-Induced Innate Immunity and Vaccine-Induced Adaptive Immunity. PLoS One (2015) 10:e0136015. 10.1371/journal.pone.0136015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Y, Zhou Y, Yang X, Cao Z, Chen X, Qin Q, et al. Insulin-like growth factor binding protein 3 gene of golden pompano (TroIGFBP3) promotes antimicrobial immune defense. Fish Shellfish Immunol (2020) 103:47–57. 10.1016/j.fsi.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 42. Chen XJ, Zhang XQ, Sun Y, Tu ZG, Cao ZJ, Wang SF, et al. Assessment of internal controls for data normalization of gene expression after different bacterial stimulation by quantitative real-time PCR in golden pompano (Trachinotus blochii). J Oceanol Immunol (2020) 38:480–9. 10.1007/s00343-019-9053-5 [DOI] [Google Scholar]
- 43. Vanwalscappel B, Tada T, Landau NR. Toll-like receptor agonist R848 blocks Zika virus replication by inducing the antiviral protein viperin. Virology (2018) 522:199–208. 10.1016/j.virol.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Périanin A, Rolas L. Methods and Pharmaceutical Compositions for The Treatment of Bacterial Infections in Patients Suffering From Cirrhosis. In: US patent BIO15052-US-PCT. N◦ 10, 201,536. Munich: European Patent Office; (2019). [Google Scholar]
- 45. Ding Y, Liu J, Lu S, Igweze J, Xu W, Kuang D, et al. Self-assembling peptide for co-delivery of HIV-1 CD8+ T cells epitope and Toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response. J Control Release (2016) 236:22–30. 10.1016/j.jconrel.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 46. Shimizu A, Kato M, Ishikawa O. Bowenoid papulosis successfully treated with imiquimod 5% cream. J Dermatol (2014) 41:545–6. 10.1111/1346-8138.12510 [DOI] [PubMed] [Google Scholar]
- 47. van Egmond S, Hoedemaker C, Sinclair R. Successful treatment of perianal Bowen’s disease with imiquimod. Int J Dermatol (2007) 46:318–9. 10.1111/j.1365-4632.2007.03200.x [DOI] [PubMed] [Google Scholar]
- 48. Haas F, Yamauchi K, Murat M, Bernasconi M, Yamanaka N, Speck RF, et al. Activation of NF-κB via endosomal Toll-like receptor 7 (TLR7) or TLR9 suppresses murine herpesvirus 68 reactivation. J Virol (2014) 88:10002–12. 10.1128/JVI.01486-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Makris S, Johansson C. R848 or influenza virus can induce potent innate immune responses in the lungs of neonatal mice. Mucosal Immunol (2020), 1–10. 10.1038/s41385-020-0314-6 [DOI] [PMC free article] [PubMed]
- 50. Abt MC, Buffie CG, Sušac B, Becattini S, Carter RA, Leiner I, et al. TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci Transl Med (2016) 8:327–25. 10.1126/scitranslmed.aad6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liang Y, Wang Y, He L, He J, Peng W, Zhou L, et al. Unc93b1 is essential for cytokine activation of five PAMPs in the orange-spotted grouper (Epinephelus coioides). Dev Comp Immunol (2018) 81:8–18. 10.1016/j.dci.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 52. Lu R, Groer C, Kleindl PA, Moulder KR, Huang A, Hunt JR, et al. Formulation and preclinical evaluation of a toll-like receptor 7/8 agonist as an anti-tumoral immunomodulator. J Control Release (2019) 306:165–76. 10.1016/j.jconrel.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, Liu M, Wu Y, Yoon S, Alnabulsi A, Liu F, et al. Immune-modulation of two BATF3 paralogues in rainbow trout Oncorhynchus mykiss. Mol Immunol (2018) 99:104–14. 10.1016/j.molimm.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 54. Yang L, Li X, Jiang S, Qiu L, Zhou F, Liu W, et al. Characterization of Argonaute2 gene from black tiger shrimp (Penaeus monodon) and its responses to immune challenges. Fish Shellfish Immunol (2014) 36:261–9. 10.1016/j.fsi.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 55. Tomai MA, Gibson SJ, Imbertson LM, Miller RL, Myhre PE, Reiter MJ, et al. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res (1995) 28:253–64. 10.1016/0166-3542(95)00054-p [DOI] [PubMed] [Google Scholar]
- 56. Bishop GA, Hsing Y, Hostager BS, Jalukar SV, Ramirez LM, Tomai MA. Molecular mechanisms of B lymphocyte activation by the immune response modifier R-848. J Immunol (2000) 165:5552–7. 10.4049/jimmunol.165.10.5552 [DOI] [PubMed] [Google Scholar]
- 57. Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA. The immune response modifier resiquimod mimics CD40-induced B cell activation. Cell Immunol (2001) 208:9–17. 10.1006/cimm.2001.1769 [DOI] [PubMed] [Google Scholar]
- 58. Auladell M, Nguyen TH, Garcillán B, Mackay F, Kedzierska K, Fox A. Distinguishing naive- from memory-derived human B cells during acute responses. Clin Transl Immunol (2019) 8:e01090. 10.1002/cti2.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dungan M, Carrithers MD. Regulation of expansion of CD11c+ B cells and anti-viral immunity by epithelial V-like antigen. Immunobiology (2020) 225:151883. 10.1016/j.imbio.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 60. Holbrook BC, Aycock ST, Machiele E, Clemens E, Gries D, Jorgensen MJ, et al. An R848 adjuvanted influenza vaccine promotes early activation of B cells in the draining lymph nodes of non-human primate neonates. Immunology (2018) 153:357–67. 10.1111/imm.12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu H, Liu Y, Han J, Yang Z, Sheng W, Dai H, et al. TLR7 regulates dendritic cell-dependent B-cell responses through BlyS in immune thrombocytopenic purpura. Eur J Haematol (2011) 86:67–74. 10.1111/j.1600-0609.2010.01534.x [DOI] [PubMed] [Google Scholar]
- 62. Weiss G, Maaetoft-Udsen K, Stifter SA, Hertzog P, Goriely S, Thomsen AR, et al. MyD88 drives the IFN-β response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J Immunol (2012) 189:2860–8. 10.4049/jimmunol.1103491 [DOI] [PubMed] [Google Scholar]
- 63. Wermuth PJ, Jimenez SA. Gadolinium compounds signaling through TLR4 and TLR7 in normal human macrophages: establishment of a proinflammatory phenotype and implications for the pathogenesis of nephrogenic systemic fibrosis. J Immunol (2012) 189:318–27. 10.4049/jimmunol.1103099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei Y, Hu S, Sun B, Zhang Q, Qiao G, Wang Z, et al. Molecular cloning and expression analysis of toll-like receptor genes (TLR7, TLR8 and TLR9) of golden pompano (Trachinotus ovatus). Fish Shellfish Immunol (2017) 63:270–6. 10.1016/j.fsi.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 65. Sawaki J, Tsutsui H, Hayashi N, Yasuda K, Akira S, Tanizawa T, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol (2007) 19:311–20. 10.1093/intimm/dxl148 [DOI] [PubMed] [Google Scholar]
- 66. Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of γδ T cell responses by TLR ligands. Cell Mol Life Sci (2011) 68:2357–70. 10.1007/s00018-011-0699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou CX, Li D, Chen YL, Lu ZJ, Sun P, Cao MY, et al. Resiquimod and polyinosinic-polycytidylic acid formulation with aluminum hydroxide as an adjuvant for foot-and-mouth disease vaccine. BMC Vet Res (2014) 10:2. 10.1186/1746-6148-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kang JY, Lee JO. Structural biology of the Toll-like receptor family. Annu Rev Biochem (2011) 80:917–41. 10.1146/annurev-biochem-052909-141507 [DOI] [PubMed] [Google Scholar]
- 69. Xu Y, Zhao Y, Huang H, Chen G, Wu X, Wang Y, et al. Expression and function of toll-like receptors in multiple myeloma patients: toll-like receptor ligands promote multiple myeloma cell growth and survival via activation of nuclear factor-kappaB. Br J Haematol (2010) 150:543–53. 10.1111/j.1365-2141.2010.08284.x [DOI] [PubMed] [Google Scholar]
- 70. Lu ZY, Yu SP, Wei JF, Wei L. Age-related neural degeneration in nuclear-factor kappaB p50 knockout mice. Neuroscience (2006) 139:965–78. 10.1016/j.neuroscience.2005.12.062 [DOI] [PubMed] [Google Scholar]
- 71. Reise Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol (2004) 16:27–34. 10.1016/j.smim.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 72. Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol (2003) 171:4304–10. 10.4049/jimmunol.171.8.4304 [DOI] [PubMed] [Google Scholar]
- 73. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science (2003) 301:640–3. 10.1126/science.1087262 [DOI] [PubMed] [Google Scholar]
- 74. Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol (2003) 4:161–7. 10.1038/ni886 [DOI] [PubMed] [Google Scholar]
- 75. Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol (2002) 169:6668–72. 10.4049/jimmunol.169.12.6668 [DOI] [PubMed] [Google Scholar]
- 76. Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol (2007) 247(2):72–84. 10.1016/j.cellimm.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 77. Miyamoto R, Ito T, Nomura S, Amakawa R, Amuro H, Katashiba Y, et al. Inhibitor of IkappaB kinase activity, BAY 11-7082, interferes with interferon regulatory factor 7 nuclear translocation and type I interferon production by plasmacytoid dendritic cells. Arthritis Res Ther (2010) 12:R87. 10.1186/ar3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.