Visualization and isolation of FeRM from samples containing multiple species are commonly needed by researchers from different disciplines, such as environmental microbiology, environmental sciences, and geochemistry. However, no available method has been reported.
KEYWORDS: iron-reducing bacteria, extracellular electron transfer, fluorescent chemodosimeter, sediment
ABSTRACT
Iron-reducing microorganisms (FeRM) play key roles in many natural and engineering processes. Visualizing and isolating FeRM from multispecies samples are essential to understand the in situ location and geochemical role of FeRM. Here, we visualized FeRM by a “turn-on” Fe2+-specific fluorescent chemodosimeter (FSFC) with high sensitivity, selectivity, and stability. This FSFC could selectively identify and locate active FeRM from either pure culture, coculture of different bacteria, or sediment-containing samples. Fluorescent intensity of the FSFC could be used as an indicator of Fe2+ concentration in bacterial cultures. By combining the use of the FSFC with that of a single-cell sorter, we obtained three FSFC-labeled cells from an enriched consortium, and all of them were subsequently shown to be capable of iron reduction; two unlabeled cells were shown to have no iron-reducing capability, further confirming the feasibility of the FSFC.
IMPORTANCE Visualization and isolation of FeRM from samples containing multiple species are commonly needed by researchers from different disciplines, such as environmental microbiology, environmental sciences, and geochemistry. However, no available method has been reported. In this study, we provide a method to visualize FeRM and evaluate their activity even at the single-cell level. When this approach is combined with use of a single-cell sorter, FeRM can also be isolated from samples containing multiple species. This method can be used as a powerful tool to uncover the in situ or ex situ role of FeRM and their interactions with ambient microbes or chemicals.
INTRODUCTION
Iron minerals are widespread in anoxic subsurface environments and can be used as electron acceptors by many microorganisms (1). In natural environments, these iron-reducing microorganisms (FeRM) not only play a key role in the reduction of minerals and humic substances but also participate in the oxidation of sulfur compounds and organic matters (2–4). Moreover, FeRM are important in many engineered processes, such as wastewater treatment, bioremediation, and bioelectrochemical systems (5). The microbial iron-reducing process is an ancient form of respiration (1). However, many novel electron transfer strategies (e.g., bacterial nanowires and direct intercellular electron transfer) possessed by FeRM were recognized only recently (6, 7).
To explore the role of FeRM in various environments, a method to visualize FeRM would be helpful for research in environmental, microbiological, and earth sciences, as it could provide essential information, such as the location, amount, or even activity of FeRM. However, FeRM are phylogenetically rather ubiquitous, and thus, there is no 16S rRNA or functional gene-based assay to detect them so far. A phenanthroline-based spectrophotometric method has been used mostly to evaluate the capability of FeRM (8). However, this method is unable to identify, locate, or quantify FeRM from a multispecies consortium. Recently, some methods targeting cytochromes or extracellular electron transfer processes similar to iron reduction (including azo-dye reduction and tungsten trioxide reduction) have been reported (9–11). However, these methods are unsuitable for visualizing FeRM in a multispecies consortium, because (i) cytochrome proteins are commonly shared by FeRM and other bacteria, (ii) some FeRM do not reduce other extracellular electron acceptors (12), and (iii) it is hard to use such methods to locate FeRM in complex samples at the single-cell level.
A common characteristic of FeRM is that the ferrous phosphate or ferrous carbonate generated from iron reduction can be adsorbed by extracellular polymeric substances, thus creating a layer of accumulated Fe2+ on the cell surface of FeRM (13–15). This Fe2+ layer can be maintained by the reducing forces from outer membrane redox proteins, such as c-type cytochromes. Therefore, an Fe2+-selective fluorescent chemosensor may provide a convenient and sensitive tool to visualize FeRM in different environments. Several Fe2+-specific fluorescent probes have been developed for mammalian cells, but none of them has been tested in microorganisms (16–19). In contrast to intracellular Fe2+ detection in mammalian cells, there are several challenges for developing a fluorescent probe for FeRM. For example, the FeRM probe should be nonreactive to other microorganisms, as Fe2+ can also be adsorbed to their surfaces, especially in environments containing high concentration of Fe2+.
Once FeRM cells can be visualized with fluorescence, single-cell sorting techniques (e.g., microfluidic devices, laser tweezer, or laser ejection) can be used to isolate them and their partner microbes from where they are observed. Thus, both in situ and ex situ roles and the mechanisms of each targeted FeRM cell can be understood. Guided by this aim, we synthesized an oxygen-depleting Fe2+-specific fluorescent chemosensor (FSFC) which showed high sensitivity and selectivity to Fe2+. This FSFC also showed a good ability to visualize FeRM in pure-cultured and multispecies systems. Combined with the single-cell sorting technique, this probe could facilitate identification and isolation of FeRM from an enriched sediment consortium.
RESULTS AND DISCUSSION
Sensitivity, selectivity, and stability of FSFC.
FSFC was nonfluorescent (i.e., in the “off” state) in the absence of Fe2+ due to the heavy-atom effect of the tellurium atom on the naphthalimide fluorophore. Fe2+ can trigger the detelluration reaction of FSFC and cause a strong fluorescence (“on” state) (16). As evidenced by gas chromatography-mass spectrometry (GC-MS) (Fig. S3), the purity of the FSFC product was 94.2%. FSFC exhibited a very weak background fluorescence in the absence of Fe2+ (Fig. 1A). Upon the addition of Fe2+ from 0 to 2,000 μM, fluorescence emission increased accordingly. Figure 1B shows a linear relation between the fluorescence intensity (FI) and the logarithm of Fe2+ concentration. The theoretical limit of detection (LOD) was calculated to be 6.3 μM (based on the formula 3 × σ/m, where σ is the standard deviation of the response at the lowest tested concentration and m is the slope of the concentration-FI response) (20). Generally, the concentration of Fe2+ in practical environments varied from several to hundreds of micromolar units and could be up to several millimolar units in FeRM cultures (21, 22). Therefore, FSFC could be used as an alternative Fe2+ sensor or FeRM label for most environmental and experimental samples.
FIG 1.
Sensitivity, selectivity, and stability of FSFC in an Fe2+-containing solution. (A) Response of FSFC fluorescence spectra to different concentrations of Fe2+. (B) Relationship between the concentration of Fe2+ and the FI. The inset shows the linear relationship between FI and the logarithm of Fe2+ concentrations. (C) Selectivity of FSFC for Fe2+. Black bars indicate the fluorescence response of FSFC to deionized water (blank) and deionized water containing different metal cations (M+); red bars indicate the fluorescence response of FSFC to different cations combined with Fe2+. (D) Relative stability of FSFC and the traditional o-phenanthroline-based method.
Other metal ions in practical environments may affect the fluorescence response of FSFC to Fe2+. All tested metal ions (except for Fe2+) had no significant fluorescence response to FSFC individually (Fig. 1C). When coexisting with Fe2+, the metal ions K+, Na+, Ca2+, Mg2+, and Zn2+ had little effect on the Fe2+-FSFC fluorescence, while Cu2+, Mn2+, and Co2+ could affect the fluorescence to some extent. In typical natural environments, the concentrations of Co2+, Cu2+, and Mn2+ are generally several orders of magnitude lower than that of Fe2+ (21); e.g., the concentration of manganese was 2 orders of magnitude lower than that of iron (8 versus 800 μM) in the sediments of Yaquina Bay Estuary (21), indicating that the effects of other metal ions will be small for tests with natural environmental samples. It should be noted the effects of other metal ions on FSFC fluorescence may increase with their concentrations (Fig. S4). For some industrial wastewaters containing high concentrations of metal ions, the samples should be diluted or Fe2+ should be artificially elevated before using FSFC to visualize FeRM.
Figure 1D shows a stability comparison between FSFC fluorescence and the traditional o-phenanthroline method. The FI of FSFC remained stable within 5 h (deviation, <5%), while the signal of traditional phenanthroline-method increased by over 10% within 2 h. Therefore, the FSFC had better stability (within 5 h) than the phenanthroline method, which also means that FSFC has a particular advantage in studies needing long operation times or including large numbers of samples.
Fluorescence imaging of viable FeRM reducing soluble and solid Fe3+.
The iron-reducing capability of Shewanella and Geobacter species has been extensively demonstrated in previous studies (2, 6, 7, 15, 23, 24). Moreover, it has been reported that ferrous phosphate and ferrous carbonate aggregate on cellular surfaces during iron reduction by FeRM (15). Our results also showed that compared to non-iron-reducing microbes (non-FeRM) (0.1 to 0.3 μM/μg bacterial protein), much higher concentrations of Fe2+ accumulated on the cell surface of Shewanella decolorationis S12 (1.1 μM/μg bacterial protein) and Shewanella oneidensis MR-1 (1.2 μM/μg bacterial protein) when exposed to the same Fe2+-containing culture (Fig. S5), which further supported using the Fe2+ probe to identify FeRM. S. decolorationis S12 in iron-reducing medium displayed significant fluorescence, while cells grown aerobically (without Fe3+) had no fluorescence (Fig. 2), indicating that cell surface-adsorbed Fe2+ can selectively turn on the fluorescence of FSFC. Moreover, the FI on S12 cell surfaces increased with increasing Fe2+ concentration in the culture supernatant (Fig. 2B to D). By combining this technique with staining with propidium iodide (PI), a fluorescent dye targeting inactive bacteria (with impaired cellular membrane), it could be seen that FSFC labels only the active iron-reducing S12 cells (Fig. 3). This result demonstrated that FSFC selectively targeted the active iron-reducing strain S12 cells rather than the inactive or non-FeRM strain S12 cells, probably because the Fe3+-reducing activity of inactive cells was low and the Fe2+ accumulation layer could therefore not be maintained on the surfaces of such cells.
FIG 2.
Fluorescence response of FSFC to strain S12 using oxygen, soluble Fe3+, or solid Fe3+ as the electron acceptor. (A) S12 respiring with oxygen or at 0 h in Fe3+-reducing medium; (B to D) S12 respiring with soluble Fe3+ for 1, 3, and 5 h, respectively; (E to H) S12 respiring with solid Fe3+ for 0, 3, 5, and 7 days, respectively; (I and J) Fe2+ concentration and the corresponding FI of strain S12 with soluble Fe3+ and solid Fe3+, respectively.
FIG 3.
PI-FSFC costaining on strain S12. Fluorescence imaging of the iron-reducing strain S12 stained with PI for low-viability cells (A) (λex= 490 nm) and FSFC for iron-reducing cells (B) (λex = 445 nm). Panel C shows a merged image of panels A and B.
Considering that iron exists mainly as solids in natural environments, the reduction of poorly crystalline Fe(III) oxides by strain S12 was also investigated. Due to the low reducing capability of strain S12 on poorly crystalline Fe(III) oxides, almost no fluorescence was observed in the first 2 days (Fig. 2E and F). The results showed that FSFC had no fluorescence response to poorly crystalline Fe(III) oxides. Over the next 5 days, fluorescence on S12 cells gradually increased with the increase in Fe2+ concentration (Fig. 2G and H). S12 cells grown with poorly crystalline Fe(III) oxides had much lower FI than those grown with ferric citrate, which corresponded to the different reduction rates of strain S12 with the two forms of Fe3+. In the system with either soluble Fe3+ or poorly crystalline Fe(III) oxides, the FI of the cells showed a linear relationship to the ambient Fe2+ concentration (Fig. 2I and J). We also tested the performance of FSFC with S. oneidensis MR-1 reducing ferric citrate or poorly crystalline Fe(III) oxides, which showed results consistent with those for S. decolorationis S12 (Fig. S6).
Geobacter has extracellular electron transfer pathways different from those of Shewanella (5, 7). When ferric citrate was used as the electron acceptor, the FI of Geobacter sulfurreducens PCA cells increased with the Fe2+ concentration, which was similar to what was observed with the two Shewanella species. However, when reducing poorly crystalline Fe(III) oxides, only G. sulfurreducens PCA cells attached to the poorly crystalline Fe(III) oxides showed fluorescence, while planktonic cells showed weak or no fluorescence (Fig. S7). The different fluorescence levels between Shewanella and Geobacter when reducing poorly crystalline Fe(III) oxides may be explained by their extracellular electron transfer pathways: Shewanella can secrete soluble electron mediators to dissolve and reduce poorly crystalline Fe(III) oxides without attaching to the particles, while G. sulfurreducens PCA can reduce poorly crystalline Fe(III) oxides only via outer membrane cytochrome c or electrically conductive pili (e-pili) after attaching to the particles (5–7). These results demonstrated that FSFC can visualize FeRM reducing either dissolved ferric citrate or solid poorly crystalline Fe(III) oxides. Moreover, the FI on the bacterial surface can be considered an indicator of the Fe2+ concentration in the pure cultures reducing ferric citrate. However, it should be noted that the FI of different G. sulfurreducens PCA cells on the aggregates of poorly crystalline Fe(III) oxides varied largely (Fig. S7), indicating their different physiological statuses at the single-cell level.
Evaluating the iron-reducing capability of different bacteria.
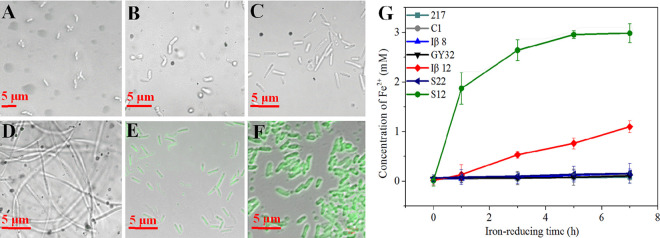
In addition to iron-reducing capability, bacteria from different genera usually have different shapes, surface properties, and metabolites that may affect the fluorescence of FSFC. To further analyze the selectivity of FSFC, we used FSFC to test in a blinded fashion five bacterial samples (five bacteria newly isolated from sediment with unknown iron-reducing performance) (Table 1), with S. decolorationis S12 and S. oneidensis MR-1 as positive controls (capable of iron reduction) and the ccmA mutant strain S22 (deficient in producing mature c-type cytochromes) (25), S. oneidensis MR-1 without an electron donor, Massilia rivuli FT92W, and Duganella lactea FT50W as negative controls (incapable of iron reduction) (26). As expected, S. decolorationis S12 and S. oneidensis MR-1 showed fluorescence, while the negative controls showed no fluorescence (Fig. 4; Fig. S8). Among the five blind bacterial samples, only Paenibacillus motobuensis Iβ12 had fluorescence, but the FI was lower than that of S. decolorationis S12. The other bacteria had no fluorescence (Fig. 4A to G). Similarly, the traditional o-phenanthroline method showed that only P. motobuensis Iβ12 had iron-reducing capability and its iron-reducing rate is much lower than that of S. decolorationis S12 (0.14 versus 0.58 mM/h). The results indicated that FSFC could be used as a simple and visualizing method to identify and evaluate the iron-reducing capability of different bacteria.
TABLE 1.
Bacterial strains
| Strain | Gram staining | Cell shape | Iron reduction |
|---|---|---|---|
| S. decolorationis S12 | Negative | Rod | Yes |
| S. decolorationis S22 | Negative | Rod | No |
| S. oneidensis MR-1 | Negative | Rod | Yes |
| G. sulfurreducens PCA | Negative | Rod | Yes |
| M. rivuli FT92W | Negative | Rod | No |
| D. lactea FT50W | Negative | Rod | No |
| Ciceribacter sp. strain F217 | Negative | Rod | Unknown |
| S. hydrophobicum C1 | Negative | Rod | Unknown |
| Bacillus sp. strain Iβ8 | Positive | Rod | Unknown |
| L. varians GY32 | Positive | Filament | Unknown |
| P. motobuensis Iβ12 | Positive | Rod | Unknown |
FIG 4.
(A through F) Fluorescence images of FSFC in different bacterial cultures containing ferric citrate. (A) Ciceribacter sp. strain F217; (B) S. hydrophobicum C1; (C) Bacillus strain Iβ8; (D) L. varians GY32; (E) P. motobuensis Iβ12; (F) S. decolorationis S12. Bars, 5 μm. (G) Iron reduction of different strains.
Visualizing FeRM from bacterial cocultures.
Coculture of FeRM and bacteria with other functions is an important way to understand the interaction between FeRM and other bacteria. In such coculture systems, one possible problem challenging FSFC is that the Fe(II) generated by FeRM may be adsorbed to non-FeRM and render the latter fluorescent. To test whether FSFC can identify FeRM in coculture systems, we cocultured a filamentous non-FeRM, Lysinibacillus varians GY32, and S. decolorationis S12 using lactate as the electron donor. As shown in Fig. 5A, the rod-shaped strain S12 showed strong fluorescence, while the filamentous bacterium L. varians GY32 had no fluorescence in the same iron-reducing culture. This suggests that FSFC can selectively visualize the FeRM in microbial samples containing FeRM and non-FeRM. The result was consistent with the observation that the amount of Fe2+ accumulated on the surfaces of non-FeRM is much less even in the same Fe2+-containing environment (Fig. S5). By combining this approach with flow cytometry, we could separate the iron-reducing bacterium S. decolorationis S12 from a coculture of two rod-shaped bacteria (S. decolorationis S12 and Sphingobium hydrophobicum C1) (Fig. S9) by its fluorescence, suggesting potential application of FSFC for detecting FeRM with properly controlled flow cytometry or other microfluidic techniques. However, the bacterial samples for microfluid- or microdroplet-based techniques must be simple and well separated. The pretreatment of most environmental samples which contain aggregates or filamentous bacteria will be challenging for such microfluidic techniques.
FIG 5.
Fluorescence images of S. decolorationis S12 and L. varians GY32 coculture. (A) Fluorescence-mode image of the coculture (Fe2+ concentration, 2.3 mM), magnified from the area indicated with a red rectangle in the inset; (B) light-fluorescence merged image of the coculture in liquid medium (Fe2+concentration, 2.3 mM), magnified from the area indicated with a red rectangle in the inset; (C and D) light-fluorescence merged images of the sediments with and without coculture, respectively (Fe2+ concentration, 1.9 mM).
To evaluate the feasibility of FSFC in more complex environments, FSFC was used to coculture L. varians GY32 and S. decolorationis S12 in sterilized sediment containing ferric citrate. In sediments without coculture, only a minority of particles showed fluorescence, probably due to the inherent Fe2+ on those sediment particles, and no bacterium-like particles showed fluorescence (Fig. 5C). The results showed that FSFC had little background fluorescence in sediments and that the nonviable (sterilized) microorganisms in sediment could not trigger the fluorescence of FSFC. In the coculture system, the short-rod strain S12 showed significant fluorescence, while the filamentous L. varians GY32 bacteria had no fluorescence, indicating the feasibility of FSFC for visualizing FeRM in sediment-containing environments. However, it should be noted that a minor portion of particles in sediments also had a fluorescent response to FSFC, probably because some particles can absorb the Fe2+ generated by FeRM. A proper dilution or filter could be used to remove such particles from the sediment samples.
Visualizing and isolating single-cell FeRM from a multispecies consortium.
In addition to visualizing FeRM, isolating FeRM from multispecies samples is a general and important need for understanding iron-associated biogeochemical processes (27). The selective fluorescence of FSFC for FeRM provides the possibility of isolating single FeRM cells from multispecies samples with a single-cell-isolating platform. Figure S10 shows that S. decolorationis S12 can be distinguished and isolated from the coculture containing wild strain S12 and mutant strain S22 by combining FSFC with a laser-based single-cell sorter. The laser power used to eject the single microbial cell (<1 μJ) with this platform was 3 orders of magnitude lower than the power level that may impair cell viability (several millijoules) (28).
We combined FSFC and PI to label the biofilms in an enriched iron-reducing reactor. Confocal laser scanning microscopy (CLSM) showed that the active FeRM cells were mainly located at the outer layer of the biofilms, while cells in the inner (bottom) layer of the biofilms showed low activity and little FSFC fluorescence (Fig. 6A). This activity profile was similar to that of the biofilms respiring with nitrate or azo dyes as electron acceptors (29), indicating that the Fe3+ was inaccessible to the inner biofilm layers and, thus, that only the outer-layer biofilm cells can reduce Fe3+ and maintain high activity. Microbial community analysis showed that the diversity of the enriched biofilm consortium was significantly decreased compared to that of the initial community (Fig. S11). Gram-positive bacteria were dominant in the enriched consortium. After addition of FSFC to the suspended biofilm consortium, both fluorescent bacteria and nonfluorescent bacteria were observed (Fig. S11). Seven single cells with fluorescence and six single cells without fluorescence were isolated from the enriched consortium using the single-cell sorter (Fig. 6). Three of the isolated fluorescent single cells (named S1, S2, and S3) were successfully cultivated, and all of them could use acetate as an electron donor to reduce ferric citrate (Fig. 6F). The 16S rRNA genes of the isolated FeRM S1 (GenBank accession number MT947627) and S2 (GenBank accession number MT947628) were close to those of Lysinibacillus fusiformis NBRC15717 (similarity, 99.84%) and Lysinibacillus pakistanensis NCCP-54 (similarity, 100%), respectively. S3 (GenBank accession number MT947629) was close to Paenibacillus glucanolyticus NBRC 15330 (similarity, 99.25%). Lysinibacillus commonly exists in various environments, such as sediment and wastewater (30–32). Although the ability of several Lysinibacillus strains to use electrodes as electron acceptors has been reported (31, 32), our results present evidence that the genus Lysinibacillus can reduce iron. Paenibacillus is also a common Gram-positive bacterial genus, and several species in this genus have been demonstrated to reduce iron (33, 34). On the other hand, two of the nonfluorescent single cells with 16S rRNA genes similar to those of Bacillus terrae RA99 (similarity, 99.28%; GenBank accession number MT947630) and Paenibacillus barengoltzii NBRC 101215 (similarity, 99.58%; GenBank accession number MT947631) were successfully cultivated and had no iron-reducing capacity (Fig. 6F). B. terrae was identified as a new aerobic species from rhizosphere soils, while P. barengoltzii NBRC 101215 is an aerobic bacterium that can degrade chitin (35, 36). The results also suggested that FSFC could be used as a novel and efficient method to isolate FeRM from different environments by being used in combination with single-cell isolation techniques.
FIG 6.
FSFC-based single-cell isolation and iron-reducing-capability test. (A) Vertical section view of enriched iron-reducing biofilm. Red indicates PI-stained cells, and green indicates FSFC-labeled cells. (B) Light-fluorescence merged image of the suspended iron-reducing biofilms. Cell 1 (nonfluorescent) and cell 2 (fluorescent) are two typically targeted cells to be isolated. The dark cross is a landmark on the glass slide. (C and D) Images before and after the laser ejection of cell 1 from the slide to a collecting pore containing PBS. (E and F) Images before and after the laser ejection of cell 2. (G) Iron reduction capabilities of the isolated bacteria.
This study reports a method that can visualize and isolate FeRM from bacterial cultures containing multiple species or even sediments. The FSFC has high sensitivity, selectivity, and stability to Fe2+ and low background fluorescence in both liquid and sediment environments. In pure cultures and in cocultures containing FeRM, FSFC could selectively visualize the active FeRM. By combining this approach with a single-cell sorting technique, targeted FeRM could be efficiently obtained from samples at the single-cell level. This novel method could be a powerful tool for obtaining novel FeRM and for acquiring a deeper understanding of the biogeochemical role of FeRM in different environments. It should be noted that the isolation and cultivation conditions should be modified according to the samples and aims. For example, although the method reported here can be used to visualize and isolate some anaerobic FeRM (e.g., Geobacter) at the single-cell level, exposure of the samples to air will decrease their culturability. For unknown microbial samples, the sorting laser power and cultivating medium should also be optimized.
MATERIALS AND METHODS
Synthesis of N-butyl-4-phenyltellanyl-1,8-naphthalimide.
The FSFC probe was selected due to its repeatable and simple synthesis method (Fig. S1) (16). First, 4-bromo-N-butyl-1,8-naphthalimide was synthesized (16, 37). In brief, 5.0 g 4-bromo-1,8-naphthalic anhydride and 3 ml n-butylamine were dissolved in 90 ml ethanol and refluxed in 82°C for 6 h. Then the mixture was filtered to obtain a wine-red solution. After evaporation with a rotary evaporator (90°C, 80 rpm, until all ethanol was evaporated), the crude product was purified by column chromatography (silica gel, ethyl acetate/petroleum ether ratio = 1:50) to get a pale yellow solid product (4.8 g). Second, N-butyl-4-phenyltellanyl-1,8-naphthalimide (the FSFC) was synthesized by a modified method based on a previous report (16). A 1.02-g portion of diphenyl ditelluride and 60 ml ethanol were added to a 150-ml three-neck flask flushed with nitrogen. The suspension was cooled to 0°C, and then 0.24 g sodium borohydride was dissolved in 12 ml ethanol and slowly added dropwise into the three-neck flask. After the red color faded, the reaction mixture was heated to reflux in 83°C. Then, a mixture of cuprous iodide (0.41 g, 2.2 mmol) and 4-bromo-N-butyl-1,8-naphthalimide (0.59 g, 1.8 mmol) were added. The mixture was stirred and refluxed for 30 min in a nitrogen atmosphere. After cooling to room temperature, the black mixture was filtered to remove insoluble materials. Then, the black filtrate was evaporated on a rotary evaporator. The residue was washed with ethanol and filtered again. After evaporation (90°C, 80 rpm, until all ethanol was evaporated), the residue was purified by column chromatography (silica gel; ethyl acetate/petroleum ether ratio = 1:125) to obtain a yellow solid product of FSFC (0.72 g). The final yellow product was dissolved in acetonitrile to get a 5 mM stock solution. It was further diluted by phosphate-buffered saline (PBS; 3.6 g/liter Na2HPO4·7H2O, 0.27 g/liter KH2PO4, 8 g/liter NaCl, and 0.2 g/liter KCl, pH 7.2) and stored in the dark before use.
Sensitivity and selectivity testing of FSFC.
Aqueous solutions of ferric citrate (FeC6H5O7·5H2O), ammonium iron (II) sulfate hexahydrate (H8FeN2O8S2·6H2O), MnCl2, ZnCl2, CaCl2, MgCl2, NiCl2·6H2O, CuCl2, Co(NO3)2·6H2O, CdCl2·2.5H2O, NaCl, and KCl were used for the selectivity and sensitivity tests of Fe3+, Fe2+, Mn2+, Zn2+, Ca2+, Mg2+, Ni2+, Cu2+, Co2+, Cd2+, Na+, and K+ respectively. Millipore water was used to prepare all aqueous solutions. For each experiment, fresh Fe2+ solution was prepared.
The sensitivity of FSFC to Fe2+ was tested with a PerkinElmer LS 45 fluorescence spectrometer. Typically, the sensitivity of FSFC was tested by incubating the FSFC (50 μM) with 0, 5 μM, 10 μM, 20 μM, 50 μM, 100 μM, 200 μM, 500 μM, 1,000 μM, and 2,000 μM Fe2+ (ammonium iron sulfate hexahydrate [H8FeN2O8S2·6H2O]) for 30 min. The reaction solution (3-ml final volume for each solution) was added to a quartz cell for fluorescence measurements with an excitation wavelength (λex) at 445 nm and an emission wavelength (λem) from 480 nm to 600 nm.
The selectivity of FSFC for Fe2+ was investigated by incubating 50 μM FSFC with 100 μM concentrations of various specified cations [Fe3+, Fe2+, Mn2+, Zn2+, Ca2+, Mg2+, Ni2+, Cu2+, Co2+, Cd2+, Na+, and K+ in ferric citrate (FeC6H5O7·5H2O), ammonium iron(II) sulfate hexahydrate (H8FeN2O8S2·6H2O), MnCl2, ZnCl2, CaCl2, MgCl2, NiCl2·6H2O, CuCl2, Co(NO3)2·6H2O, CdCl2·2.5H2O, NaCl, and KCl], respectively, for 30 min. The reaction solution (3-ml final volume for each solution) was added to a quartz cell for fluorescence measurements with an excitation wavelength (λex) of 445 nm and an emission wavelength (λem) of 530 nm.
Pure culture strains and growth conditions.
To test the selectivity of FSFC to FeRM, pure bacterial cultures, including three known FeRM (Shewanella decolorationis strain S12, S. oneidensis MR-1, and Geobacter sulfurreducens PCA) (5, 6, 25, 38), three non-FeRM (a ccmA-mutant S. decolorationis S22, Massilia rivuli FT92W, and Duganella lactea FT50W) incapable of iron reduction (25, 26), and five pure cultured bacteria newly isolated from sediments with unknown iron reduction capacity (Paenibacillus motobuensis Iβ12, Ciceribacter sp. strain F217, Sphingobium hydrophobicum C1, Bacillus strain Iβ8, and Lysinibacillus varians GY32) were used. Further information about these bacteria is listed in Table 1. All bacteria (except G. sulfurreducens PCA) were first grown aerobically in Luria-Bertani (LB) medium. The bacterial cells were washed with sterilized PBS twice and then inoculated to N2-flushed anaerobic lactate medium (LM, containing 2.0 g/liter lactate, 0.2 g/liter yeast extract, 12.8 g/liter Na2HPO4·7H2O, 3 g/liter KH2PO4, 0.5 g/liter NaCl, and 1.0 g/liter NH4Cl) with an initial optical density at 600 nm (OD600) of 0.1. A 3 mM concentration of ferric citrate or poorly crystalline Fe(III) oxides was used as the electron acceptor in the LM, unless otherwise stated. The cultures were grown at 33°C. G. sulfurreducens PCA (initial OD600 = 0.08) was anaerobically cultivated using freshwater medium containing acetate (10 mM) as the electron donor and ferric citrate (4 mM) or poorly crystalline Fe(III) oxides (4 mM) as the electron acceptor (6). The poorly crystalline Fe(III) oxides were prepared as previous reported (6). Meanwhile, the iron-reducing capability of these bacteria was tested by the traditional phenanthroline-based method (8).
Coculture grown in liquid medium and sediment.
Different coculture systems were used to test whether FSFC can distinguish the FeRM from non-FeRM in the same bacterial culture. The system included (i) coculture of S. decolorationis S12 and the non-FeRM L. varians GY32 in N2-flushed anaerobic LM solution with 3 mM ferric citrate for 8 h and (ii) inoculation of 10 ml of the coculture with 1 g of sterilized river sediment (obtained from Shijing River, Guangzhou, China) and cultivation for 8 h.
Fluorescence imaging.
The fluorescence imaging of the pure culture or coculture samples was carried out via confocal laser scanning microscopy (CLSM; LSM 700; Zeiss) after samples were stained with 50 μM FSFC for 15 min. FSFC concentrations higher than 0.5 mM may cause toxicity to bacteria (Fig. S2). A 10-μl portion of the stained cultures was applied dropwise onto a glass slide with a small piece of cover glass and then observed under the CLSM with an excitation wavelength (λex) of 445 nm for FSFC. Propidium iodide (PI; λex= 490 nm; Thermo Fisher) was used as a fluorescent indicator for evaluating the activity of the bacterial cells; only cells with low activities and impaired cell membranes can be stained by PI.
FSFC-based single-cell isolation.
An enriched iron-reducing biofilm consortium was used to test that whether FSFC can selectively label FeRM in a complex microbial community. This consortium was made by inoculating 1 g of sediment into 100 ml LM containing 5 mM ferric citrate in an anaerobic serum bottle. Six graphite plates (1 by 1 by 0.1 cm) were added to the culture for biofilm growth. Eighty percent of the enriched culture was replaced with fresh LM containing 5 mM ferric citrate every 2 weeks. After enrichment for 8 weeks, three of the graphite plates were retrieved and stained with FSFC and PI after a gentle wash in sterilized PBS. The stained biofilms were observed by CLSM. Biofilms on the other three graphite plates were scraped with a sterilized cotton swab. The resulting biofilm cells were suspended in 5 ml PBS and stained with 50 μM FSFC for 15 min. A 50-μl portion of the FSFC-stained samples was transferred to a glass slide designed for single-cell ejection and observed in fluorescence mode of a single-cell precision sorter (PRECI SCS; Hooke Instruments). The selected cells (with or without fluorescence) on the slides were ejected by a laser beam controlled by PRECI SCS software. Seven bacterial cells with fluorescence and 6 bacterial cells without fluorescence were ejected from the slide into a collector containing sterilized PBS by a low-power laser (0.5 to 1 μJ, varied according to the cell shape and adsorption force on the slide surface). The collected single bacteria were then anaerobically cultivated in LB medium containing 2 mM ferric citrate. The bacteria were then cultivated in the same freshwater medium used for G. sulfurreducens PCA with ferric citrate as the sole electron acceptor and acetate as the electron donor.
Data availability.
Newly determined sequences were deposited in GenBank under accession numbers MT947627 to MT947631.
Supplementary Material
ACKNOWLEDGMENTS
We thank Li Zhuang in Jinan University for her donation of Geobacter sulfurreducens PCA.
This work was supported by the National Natural Science Foundation of China (91851202, 31970110, 51678163), Guangdong Provincial Science and Technology Project (2016A030306021, 2019B110205004), GDAS’ Special Project of Science and Technology Development (2019GDASYL-0301002 and 2018GDASCX-0102), Guangdong technological innovation strategy of special funds key areas of research and development program (2018B020205003), Open Project of State Key Laboratory of Applied Microbiology Southern China (SKLAM001-2018).
We declare no competing financial interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lloyd JR. 2003. Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425. doi: 10.1016/S0168-6445(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 2.Lovley DR, Anderson RT. 2000. Influence of dissimilatory metal reduction on fate of organic and metal contaminants in the subsurface. Hydrogeol J 8:77–88. doi: 10.1007/PL00010974. [DOI] [Google Scholar]
- 3.Byrne JM, Klueglein N, Pearce C, Rosso KM, Appel E, Kappler A. 2015. Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria. Science 347:1473–1476. doi: 10.1126/science.aaa4834. [DOI] [PubMed] [Google Scholar]
- 4.Yun J, Malvankar NS, Ueki T, Lovley DR. 2016. Functional environmental proteomics: elucidating the role of a c-type cytochrome abundant during uranium bioremediation. ISME J 10:310–320. doi: 10.1038/ismej.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan BE, Rossi R, Ragab A, Saikaly PE. 2019. Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol 17:307–319. doi: 10.1038/s41579-019-0173-x. [DOI] [PubMed] [Google Scholar]
- 6.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Xu M, Guo J, Sun G. 2012. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem 47:1707–1714. doi: 10.1016/j.procbio.2012.07.032. [DOI] [Google Scholar]
- 8.Fortune WB, Mellon MG. 1938. Determination of iron with o-phenanthroline: a spectrophotometric study. Ind Eng Chem Anal Ed 10:60–64. doi: 10.1021/ac50118a004. [DOI] [Google Scholar]
- 9.Zhou S, Wen J, Chen J, Lu Q. 2015. Rapid measurement of microbial extracellular respiration ability using a high-throughput colorimetric assay. Environ Sci Technol Lett 2:26–30. doi: 10.1021/ez500405t. [DOI] [Google Scholar]
- 10.Xiao X, Liu Q, Li T, Zhang F, Li W, Zhou X, Xu M, Li Q, Yu H. 2017. A high-throughput dye-reducing photometric assay for evaluating microbial exoelectrogenic ability. Bioresour Technol 241:743–749. doi: 10.1016/j.biortech.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Cheng Y, Zhang F, Li B, Mu Y, Li W, Yu H. 2016. Rapid detection and enumeration of exoelectrogenic bacteria in lake sediments and a wastewater treatment plant using a coupled WO3 nanoclusters and most probable number method. Environ Sci Technol Lett 3:133–137. doi: 10.1021/acs.estlett.6b00112. [DOI] [Google Scholar]
- 12.Richter H, Lanthier M, Nevin KP, Lovley DR. 2007. Lack of electricity production by pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl Environ Microbiol 73:5347–5353. doi: 10.1128/AEM.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luef B, Fakra SC, Csencsits R, Wrighton KC, Williams KH, Wilkins MJ, Downing KH, Long PE, Comolli LR, Banfield JF. 2013. Iron-reducing bacteria accumulate ferric oxyhydroxide nanoparticle aggregates that may support planktonic growth. ISME J 7:338–350. doi: 10.1038/ismej.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly SE, Watkins J, Furukawa Y. 2005. Secondary mineral formation associated with respiration of nontronite, NAu-1 by iron reducing bacteria. Geochem Trans 6:67–76. doi: 10.1186/1467-4866-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peretyazhko TS, Zachara JM, Kennedy DW, Fredrickson JK, Arey BW, McKinley JP, Wang CM, Dohnalkova AC, Xia Y. 2010. Ferrous phosphate surface precipitates resulting from the reduction of intragrain 6-line ferrihydrite by Shewanella oneidensis MR-1. Geochim Cosmochim Acta 74:3751–3767. doi: 10.1016/j.gca.2010.04.008. [DOI] [Google Scholar]
- 16.Qu ZJ, Li P, Zhang XX, Han KL. 2016. A turn-on fluorescent chemodosimeter based on detelluration for detecting ferrous iron (Fe2+) in living cells. J Mater Chem B 4:887–892. doi: 10.1039/C5TB02090E. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama T, Okuda K, Nagasawa H. 2013. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem Sci 4:1250–1256. doi: 10.1039/c2sc21649c. [DOI] [Google Scholar]
- 18.Hirayama T, Tsuboi H, Niwa M, Miki A, Kadota S, Ikeshita Y, Okuda K, Hideko N. 2017. A universal fluorogenic switch for Fe(II) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells. Chem Sci 8:4858–4866. doi: 10.1039/C6SC05457A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Wang Y, Liu R, Zhang Y, Tang J, Yang E, Zhang D, Zhao Y, Ye Y. 2019. A novel ICT-based two photon and NIR fluorescent probe for labile Fe2+ detection and cell imaging in living cells. Sensor Actuat B Chem 288:217–224. doi: 10.1016/j.snb.2019.02.123. [DOI] [Google Scholar]
- 20.Li N, Than A, Sun C, Tian J, Chen J, Pu K, Dong X, Chen P. 2016. Monitoring dynamic cellular redox homeostasis using fluorescence-switchable graphene quantum dots. ACS Nano 10:11475–11482. doi: 10.1021/acsnano.6b07237. [DOI] [PubMed] [Google Scholar]
- 21.Ryckelynck N, Stecher HA, Reimers CE. 2005. Understanding the anodic mechanism of a seafloor fuel cell: interactions between geochemistry and microbial activity. Biogeochemistry 76:113–139. doi: 10.1007/s10533-005-2671-3. [DOI] [Google Scholar]
- 22.Nicolaidou A, Nott JA. 1998. Metals in sediment, seagrass and gastropods near a nickel smelter in Greece: possible interactions. Mar Pollut Bull 36:360–365. doi: 10.1016/S0025-326X(97)00195-1. [DOI] [Google Scholar]
- 23.Yang Y, Sun G, Guo J, Xu M. 2011. Differential biofilms characteristics of Shewanella decolorationis microbial fuel cells under open and closed circuit conditions. Bioresour Technol 102:7093–7098. doi: 10.1016/j.biortech.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Li E, Li J, Liu F, Yang X, Xu M. 2019. Effects of flavin-goethite interaction on goethite reduction by Shewanella decolorationis S12. Front Microbiol 10:1623. doi: 10.3389/fmicb.2019.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Xu M, Wei J, Sun G. 2010. Two different electron transfer pathways may involve in azoreduction in Shewanella decolorationis S12. Appl Microbiol Biotechnol 86:743–751. doi: 10.1007/s00253-009-2376-y. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Deng T, Liu F, Wang Y, Yang X, Xu M. 2020. Duganella lactea sp. nov., Duganella guangzhouensis sp. nov., Duganella flavida sp. nov. and Massilia rivuli sp. nov., isolated from a subtropical stream in PR China and proposal to reclassify Duganella ginsengisoli as Massilia ginsengisoli comb. nov. Int J Syst Evol Microbiol 70:4822–4830. doi: 10.1099/ijsem.0.004355. [DOI] [PubMed] [Google Scholar]
- 27.Hori T, Aoyagi T, Itoh H, Narihiro T, Oikawa A, Suzuki K, Ogata A, Friedrich MW, Conrad R, Kamagata Y. 2015. Isolation of microorganisms involved in reduction of crystalline iron(III) oxides in natural environments. Front Microbiol 6:386. doi: 10.3389/fmicb.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Ji Y, Wharfe ES, Meadows RS, March P, Goodacre R, Xu J, Huang WE. 2013. Raman activated cell ejection for isolation of single cells. Anal Chem 85:10697–10701. doi: 10.1021/ac403107p. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Xiang Y, Sun G, Wu W, Xu M. 2015. Electron acceptor-dependent respiratory and physiological stratifications in biofilms. Environ Sci Technol 49:196–202. doi: 10.1021/es504546g. [DOI] [PubMed] [Google Scholar]
- 30.Uma Vanitha M, Natarajan M, Sridhar H, Umamaheswari S. 2017. Microbial fuel cell characterisation and evaluation of Lysinibacillus macroides MFC02 electrigenic capability. World J Microbiol Biotechnol 33:91. doi: 10.1007/s11274-017-2252-3. [DOI] [PubMed] [Google Scholar]
- 31.Azhar ATS, Nabila ATA, Nurshuhaila MS, Zaidi E, Azim MAM, Farhana SMS. 2016. Assessment and comparison of electrokinetic and electrokinetic-bioremediation techniques for mercury contaminated soil. IOP Conf Ser Mat Sci Engin 160:12077–12085. doi: 10.1088/1757-899X/160/1/012077. [DOI] [Google Scholar]
- 32.He H, Yuan S, Tong Z, Huang Y, Lin Z, Yu H. 2014. Characterization of a new electrochemically active bacterium, Lysinibacillus sphaericus D-8, isolated with a WO3 nanocluster probe. Process Biochem 49:290–294. doi: 10.1016/j.procbio.2013.11.008. [DOI] [Google Scholar]
- 33.Ahmed B, Cao B, McLean JS, Ica T, Dohnalkova A, Istanbullu O, Paksoy A, Fredrickson JK, Beyenal H. 2012. Fe(III) reduction and U(VI) immobilization by Paenibacillus sp. strain 300A, isolated from Hanford 300A subsurface sediments. Appl Environ Microbiol 78:8001–8009. doi: 10.1128/AEM.01844-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y, Chen F, Li Y, Wei S, Wang G. 2015. Paenibacillus ferrarius sp. nov., isolated from iron mineral soil. Int J Syst Evol Microbiol 65:165–170. doi: 10.1099/ijs.0.063552-0. [DOI] [PubMed] [Google Scholar]
- 35.Diez-Mendez A, Rivas R, Mateos PF, Martinez-Molina E, Julio Santin P, Antonio Sanchez-Rodriguez J, Velazquez E. 2017. Bacillus terrae sp. nov. isolated from Cistus ladanifer rhizosphere soil. Int J Syst Evol Microbiol 67:1478–1481. doi: 10.1099/ijsem.0.001742. [DOI] [PubMed] [Google Scholar]
- 36.Osman S, Satomi M, Venkateswaran K. 2006. Paenibacillus pasadenensis sp. nov. and Paenibacillus barengoltzii sp. nov., isolated from a spacecraft assembly facility. Int J Syst Evol Microbiol 56:1509–1514. doi: 10.1099/ijs.0.64085-0. [DOI] [PubMed] [Google Scholar]
- 37.Ren J, Wu Z, Zhou Y, Li Y, Xu Z. 2011. Colorimetric fluoride sensor based on 1,8-naphthalimide derivatives. Dyes Pigments 91:442–445. doi: 10.1016/j.dyepig.2011.04.012. [DOI] [Google Scholar]
- 38.Xu M, Guo J, Kong X, Chen X, Sun G. 2007. Fe(III)-enhanced azo reduction by Shewanella decolorationis S12. Appl Microbiol Biotechnol 74:1342–1349. doi: 10.1007/s00253-006-0773-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Newly determined sequences were deposited in GenBank under accession numbers MT947627 to MT947631.