Bisbenzylisoquinoline (BBIQ) alkaloids are a diverse group of natural products that demonstrate a range of biological activities. In this study, the in vitro antiplasmodial activity of three BBIQ alkaloids (cycleanine [compound 1], isochondodendrine [compound 2], and 2′-norcocsuline [compound 3]) isolated from the Triclisia subcordata Oliv. medicinal plant traditionally used for the treatment of malaria in Nigeria are studied alongside two semisynthetic analogues (compounds 4 and 5) of cycleanine.
KEYWORDS: malaria, Plasmodium falciparum, Plasmodium berghei, bisbenzylisoquinoline alkaloids, cycleanine, metabolism, in vivo activity, antimalarial agents, drug metabolism
ABSTRACT
Bisbenzylisoquinoline (BBIQ) alkaloids are a diverse group of natural products that demonstrate a range of biological activities. In this study, the in vitro antiplasmodial activity of three BBIQ alkaloids (cycleanine [compound 1], isochondodendrine [compound 2], and 2′-norcocsuline [compound 3]) isolated from the Triclisia subcordata Oliv. medicinal plant traditionally used for the treatment of malaria in Nigeria are studied alongside two semisynthetic analogues (compounds 4 and 5) of cycleanine. The antiproliferative effects against a chloroquine-resistant Plasmodium falciparum strain were determined using a SYBR green 1 fluorescence assay. The in vivo antimalarial activity of cycleanine is then investigated in suppressive, prophylactic, and curative murine malaria models after infection with a chloroquine-sensitive Plasmodium berghei strain. BBIQ alkaloids (compounds 1 to 5) exerted in vitro antiplasmodial activities with 50% inhibitory concentration (IC50) at low micromolar concentrations and the two semisynthetic cycleanine analogues showed an improved potency and selectivity compared to those of cycleanine. At oral doses of 25 and 50 mg/kg body weight of infected mice, cycleanine suppressed the levels of parasitemia and increased mean survival times significantly compared to those of the control groups. The metabolites and metabolic pathways of cycleanine were also studied using high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Twelve novel metabolites were detected in rats after intragastric administration of cycleanine. The metabolic pathways of cycleanine were demonstrated to involve hydroxylation, dehydrogenation, and demethylation. Overall, these in vitro and in vivo results provide a basis for the future evaluation of cycleanine and its analogues as leads for further development.
TEXT
In 2018, the World Health Organization (WHO) report estimated a global burden of 228 million cases accounting for 405,000 deaths (1). The majority of this burden fell on the WHO Africa Region, where malaria, particularly that caused by the most virulent etiological agent Plasmodium falciparum, exerts an immense economic impact. While malaria cases and mortality figures continue to fall (1, 2), the development and spread of resistance to available chemotherapeutic agents poses a significant threat to malaria treatment and management (3). Natural products of plant origin have traditionally provided good sources for discovery of drug leads or novel compounds in modern drug research (4, 5). For example, artemisinin isolated from Artemisia annua, sweet wormwood, a traditional Chinese medicine, together with a series of its semisynthetic derivatives, has become the first-line therapy for P. falciparum malaria (6, 7). However, due to the development of artemisinin drug resistance (8), novel therapies are still urgently needed.
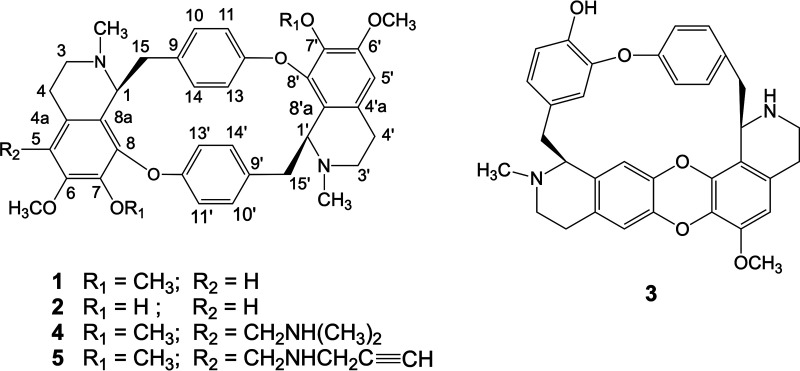
Bisbenzylisoquinoline (BBIQ) alkaloids are a diverse group of natural products consisting of two benzylisoquinoline groups (9). BBIQ alkaloids are primarily found in the Berberidaceae, Lauraceae, Menispermaceae, and Ranunculaceae plant families. These alkaloids possess a variety of biological activities, which include antimalarial activities (9, 10). For example, BBIQ alkaloids isolated and identified from Triclisia species of the Menispermaceae family have antiproliferative activities (10). In Nigeria, the root of Triclisia subcordata Oliv. is traditionally used for the treatment of a range of diseases, including malaria (11, 12). The bioactive components of T. subcordata are the BBIQ alkaloids cycleanine (compound 1), isochondodendrine (compound 2) and 2′-norcocsuline (compound 3) (Fig. 1) and have previously been isolated and characterized by our group (13, 14). We have also produced synthetic analogues of cycleanine (compounds 4 and 5) (Fig. 1) (15). The three naturally occurring BBIQ alkaloids, cycleanine (16–18), isochondodendrine (18, 19), and 2′-norcocsuline (16, 20) have been reported to possess antiplasmodial effects against chloroquine-sensitive and chloroquine-resistant P. falciparum strains. Despite the promising in vitro biological activity of these natural BBIQ alkaloids, the in vivo antimalarial activity of BBIQ alkaloids has not been evaluated, nor has their potential in vivo metabolism. Here, we assess the in vivo antimalarial activity and metabolism of cycleanine (compound 1). The effect of cycleanine analogues (compounds 4 and 5) on antiplasmodial potency and selectivity is also investigated.
FIG 1.
Chemical structure of bisbenzylisoquinoline (BBIQ) alkaloids. Cycleanine (compound 1), isochondodendrine (compound 2), and 2′-norcocsuline (compound 3) from T. subcordata and two novel semisynthetic analogues (compounds 4 and 5) of cycleanine.
This study sets out an evaluation of the in vitro antimalarial activities of BBIQ alkaloid compounds 1 to 3 compared to those of two semisynthetic BBIQ alkaloids (compounds 4 and 5) derived by a modification of cycleanine at the C-5 position by introducing additional secondary or tertiary amine moieties in an attempt to increase potential solubility and potency (15). The most abundant BBIQ alkaloid in T. subcordata extract is cycleanine; this was therefore used to establish in vivo antimalarial activity in a murine malaria model. In addition, the metabolites and metabolic pathways of cycleanine were analyzed after intragastric administration in rats to help understand how cycleanine is eliminated in vivo to guide future optimization of cycleanine for antimalarial development.
RESULTS
The semisynthetic derivatives of cycleanine have improved in vitro antiplasmodial activity and selectivity.
The in vitro antiplasmodial activities of the five BBIQ alkaloids (compounds 1 to 5), as well as that of a chloroquine control, were determined against intraerythrocytic stages of the P. falciparum Dd2 chloroquine-resistant strain using a malaria SYBR green I fluorescence assay. These data are provided in Table 1 (see Fig. S1 in the supplemental material) as 50% inhibitory concentration (IC50) values (mean ± standard deviation [SD] for n = 3 independent biological repeats). While the data for chloroquine in Dd2 are comparable to those of the chloroquine-resistant strain W2, the activities of cycleanine, isochondodendrine, and 2′-norcocsuline are significantly lower in Dd2 than that reported in W2, and certainly lower than that in the chloroquine-sensitive strain D6. The semisynthetic products 4 and 5 are relatively more potent than compound 1 to 3 in Dd2, with the most potent, compound 4, being some 25.2-fold more potent than its natural precursor, cycleanine (compound 1).
TABLE 1.
Cytotoxicity data for BBIQ alkaloids
| BBIQ alkaloid (compound no.) | IC50 (μM) against P. falciparum straina,d: |
CC50 (μM) against cancer cell line(s): |
SIf |
||||
|---|---|---|---|---|---|---|---|
| Dd2 | W2 | D6 | KBb or HCTc | HOEe | KB/W2 | HOE/Dd2 | |
| Cycleanine (1) | 17.7 ± 2.0 | 0.25b; 4.5c | 0.07b | >33.7b; 531 (HCT)c | 35.0 ± 0.1 | >133 | 2.0 |
| Isochondodendrine (2) | 6.1 ± 1.3 | 0.2c | NDd | 29 (HCT)c | 10.5 ± 1.2 | 116 | 1.7 |
| 2′-Norcocsuline (3) | 7.0 ± 1.6 | 0.28b | 0.048b | 3.8b | 8.0 ± 0.2 | 14 | 1.1 |
| 5-[(Dimethylamino)methyl]cycleanine (4) | 0.7 ± 0.1 | ND | ND | ND | 10.0 ± 0.2 | ND | 14.3 |
| 5-[(Propargylamino)methyl]cycleanine (5) | 1.8 ± 0.2 | ND | ND | ND | 32.0 ± 1.6 | ND | 17.8 |
| Chloroquine | 0.18 ± 0.03 | 0.135b | 0.006b | 33.7b | ND | 250 | ND |
In vitro 50% inhibitory concentration (IC50) values of BBIQ alkaloids (compounds 1 to 5) against P. falciparum chloroquine-resistant strains (Dd2 and W2 strains) and the chloroquine-sensitive strain (D6). IC50 values are expressed as mean ± SD for n = 3 independent biological repeats.
IC50 data against P. falciparum W2 and D6 strains and 50% cytotoxic concentration (CC50) values for human oral epidermoid carcinoma (KB) cells were sourced from a previous report (16).
IC50 data against chloroquine-resistant P. falciparum strain W2 and CC50 values for HCT-116 human colon carcinoma cells were sourced from a previous report (18).
ND, not determined.
CC50 data for human ovarian epithelial (HOE) cells. Data in this column for compounds 1 to 5 were sourced from our previous reports (13, 15).
This selectivity index (SI) was calculated as CC50 /IC50 against P. falciparum.
Data from cytotoxicity studies of BBIQ alkaloids 1 to 3 in human oral epidermoid carcinoma (KB) or HCT-116 human colon carcinoma cells suggest low to moderate selectivity, with a selectivity index (SI) of 14 to >133. Fifty percent cytotoxic concentration (CC50) data for all five compounds are available from human ovarian epithelial (HOE) cells (Table 1). These data reinforce the findings of low selectivity, albeit improved in semisynthetic products 4 and 5.
In vivo antimalarial activity of cycleanine (compound 1).
The isolation of the abundant cycleanine (compound 1) in T. subcordata root enabled us to investigate its toxicity in healthy mice and efficacy in murine malaria models after infection with Plasmodium berghei. The acute median lethal dose (LD50) of cycleanine after 24-h oral administration was determined to be 4.5 g/kg in mice, indicating a good safety profile. The malaria-suppressive activity of cycleanine using two oral doses (25 and 50 mg/kg of body weight/day) following P. berghei infection was demonstrated through a significant suppression of parasitemia and an increased mean survival time (MST) compared to those of untreated controls (Table 2). In particular, the higher dose (50 mg/kg/day) showed efficacy, both in terms of suppression of parasitemia and in MST, comparable to that for chloroquine at a dose of 5 mg/kg/day. The prophylactic activity of cycleanine, with the same 25 and 50 mg/kg dosing regimen during P. berghei infection in mice, was also demonstrated (Table 3). At the higher dose (50 mg/kg), cycleanine showed a suppression of parasitemia by 59.0%, only slightly less than that of 76.2% using the prophylactic pyrimethamine control at a dose of 1.2 mg/kg /day.
TABLE 2.
Suppressive activity of cycleanine during early Plasmodium berghei infection of mice
| Treatment | Dose (mg/kg) per day | Parasitemia after infection for 96 h (%)a | Suppression of parasitemia at 96 h (%)a | MST (days)a |
|---|---|---|---|---|
| Untreated control | 28.3 ± 1.8 | 12.5 ± 0.3 | ||
| Cycleanine | 25 | 15.7 ± 1.8b | 44.7 | 24.7 ± 1.1b |
| 50 | 3.8 ± 0.7b | 86.5 | 28.2 ± 0.9b | |
| Chloroquine | 5 | 2.0 ± 0.8b | 94.0 | 30.0 ± 0.0b |
Values are expressed as mean ± standard error of the mean (SEM) (n = 6 in each group).
Significant relative to untreated control; P < 0.001.
TABLE 3.
Prophylactic activity of cycleanine in Plasmodium berghei infection of mice
| Treatment | Dose (mg/kg) per day | Parasitemia level after infection for 72 h (%)a | Suppression of parasitemia level after infection for 72 h (%)a | MST (days)a |
|---|---|---|---|---|
| Untreated control | 20.3 ± 0.8 | 12.7 ± 0.3 | ||
| Cycleanine | 25 | 11.5 ± 0.9b | 43.4 | 23.0 ± 0.6b |
| 50 | 8.3 ± 1.0b | 59.0 | 24.5 ± 0.6b | |
| Pyrimethamine | 1.2 | 4.8 ± 1.1b | 76.2 | 29.8 ± 0.2b |
Values are expressed as mean ± SEM (n = 6 in each group).
Significant relative to control; P < 0.001.
The curative activity and MST of mice after initial P. berghei infection and subsequent treatment with cycleanine (compound 1) were determined. After infection of mice for 3 days, cycleanine was administered at both doses of 25 and 50 mg/kg and mice showed decreasing parasitemia in a dose-dependent and time-dependent manner from day 3 to day 7 (Fig. 2). The speed of killing of P. berghei parasites by chloroquine was much faster than that with cycleanine. Chloroquine reached 0% parasitemia after 5 days, while at that time cycleanine at doses of 25 and 50 mg/kg had remaining levels of 13.3 and 10.5%, respectively (Fig. 2). In this curative model, the MST of mice at doses of 25 and 50 mg/kg were 21 and 25 days, respectively, which were significantly longer than that of the control (12 days). However, they were both shorter than that of mice treated with chloroquine (30 days) (see Table S1 in the supplemental material).
FIG 2.
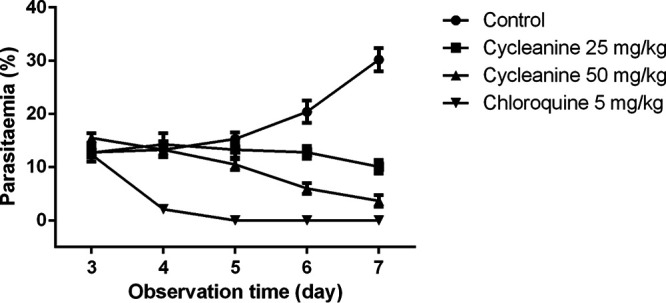
The curative activity of mice treated with cycleanine (compound 1) during established P. berghei infection. After infection of mice for 3 days, cycleanine was administered at both doses of 25 and 50 mg/kg, while water and chloroquine at 5 mg/ml were administered as negative and positive controls, respectively. The parasitemia levels were monitored for a total duration of 4 days (from day 3 to day 7).
In vivo metabolism of cycleanine.
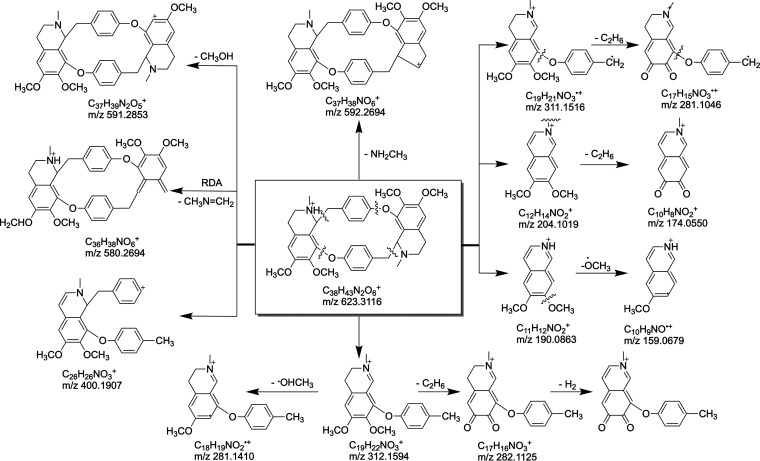
In order to explore the in vivo metabolism of cycleanine, the plasma and urine of Wistar rats following an oral dose of 120 mg/kg body weight/day over a 24-h period were analyzed for cycleanine metabolites. Samples from urine and plasma were prepared and submitted to high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry (HPLC-ESI-MS/MS) analysis. The peak at the retention time of 9.7 min was cycleanine (M0) with the protonated molecular ion m/z of 623.3116 [M+H]+ (elemental composition C18H43N2O6) in the positive ion mode spectrum (Table 4 and Fig. 3; see also Fig. S2 in the supplemental material). In MS/MS, the quasimolecular ion loses a neutral molecular NH2CH3 fragment to generate an ion m/z of 592.2694 also by symmetric cleavage, and breaking C-O and C-C bonds to produce a fragment ion m/z of 312.1594, which can also lose C2H6 to produce a fragment ion m/z of 282.1125. After another C-O and C-C bond cleavage and the subsequent loss of CH3 and OCH3, fragment ions m/z 204.101, 190.0863, and 159.0679 were generated. A fragment ion m/z of 400.1907 was also generated by simultaneous C-O bond cleavage and C-C bond cleavage adjacent to the N atom (Fig. 3 and Fig. S2).
TABLE 4.
HPLC-QTOF-MS retention times and mass spectrometric data of cycleanine and its metabolites
| Prototype or metabolite | t (min) | Measured [M+H]+ m/z | Δppm | Formula | MS/MS fragment(s) | Metabolic pathway(s) | Plasma | Urine |
|---|---|---|---|---|---|---|---|---|
| M0 | 9.9 | 623.3125 | 1.41 | C38H43N2O6 | 592.2696, 400.1895, 312.1583, 311.1508, 281.1165, 204.1011, 190.0857, 174.0911, 159.1038 | Parent | + | + |
| M1 | 7.2 | 639.3075 | 1.36 | C38H43N2O7 | 592.2472, 416.1838, 310.1422, 220.0964, 204.1046, 190.0815, 175.0955, 157.0901 | Hydroxylation | − | + |
| M2 | 7.9 | 639.3084 | 2.79 | C38H43N2O7 | 621.2977, 416.1864, 400.1917, 327.1469, 312.1361, 220.0964, 206.0780, 175.0988 | Hydroxylation | + | + |
| M3 | 8.1 | 625.2911 | 0.84 | C37H41N2O7 | 607.2784, 425.1379, 312.1591, 298.1434, 204.0999, 190.0854, 176.0691, 159.1033 | Demethylation and hydroxylation | − | + |
| M4 | 9.6 | 609.2956 | 0.96 | C37H41N2O6 | 593.2750, 427.1577, 357.1449, 312.1580, 298.1435, 204.1020, 190.0850, 176.0704, 145.0880 | Demethylation | − | + |
| M5 | 10.1 | 595.2799 | 0.73 | C36H39N2O6 | 578.2505, 284.1282, 176.0703, 145.0879 | Didemethylation | − | + |
| M6 | 10.4 | 637.2918 | 1.12 | C38H41N2O7 | 328.1553, 309.1381, 202.0855, 188.0656, 157.0879 | Dehydrogenation and hydroxylation | + | + |
| M7 | 11.1 | 653.2855 | 0.38 | C38H41N2O8 | 635.2754, 326.1384, 309.1381, 202.0855, 188.0656, 157.0879 | Dehydrogenation and dihydroxylation | + | − |
| M8 | 12.1 | 653.2868 | 1.23 | C38H41N2O8 | 592.2459, 310.1420, 293.1154, 281.1163, 269.1169, 204.1031, 190.0884 | Dehydrogenation and dihydroxylation | − | + |
| M9 | 13 | 653.2856 | 0.27 | C38H41N2O8 | 635.2701, 400.1881, 326.1380, 310.1427, 202.0855, 173.0820, 157.0881 | Dehydrogenation and dihydroxylation | − | + |
| M10 | 13.4 | 621.2966 | 0.97 | C38H41N2O6 | 591.2467, 400.1893, 398.1739, 312.1572, 310.1435, 204.1013, 202.0860, 190.0863, 188.0725, 159.1028, 157.0883 | Dehydrogenation | + | − |
| M11 | 13.6 | 653.2859 | 0.73 | C38H41N2O8 | 413.1375, 324.1595, 309.1345, 281.1158, 204.1015, 159.1021 | Dehydrogenation and dihydroxylation | + | + |
| M12 | 14.1 | 637.2919 | 1.61 | C38H41N2O7 | 594.2486, 414.1684, 326.1381, 312.1237, 281.1159, 218.0824, 204.1013, 190.0874, 173.0830 | Dehydrogenation and hydroxylation | + | + |
FIG 3.
Possible fragmentation pattern of cycleanine. For analysis of fragment ions, see the text.
Twelve peaks on LC-MS/MS chromatograms relevant to cycleanine were detected in either urine or plasma samples (Table 4 and Fig. S3 in the supplemental material). The original form of cycleanine and 11 metabolites were found from the urine of rats, which were presumed to be hydroxylation (M1 and M2), demethylation and hydroxylation (M3), monodemethylation (M4), didemethylation (M5), dehydrogenation and hydroxylation (M6 and M12), and dehydrogenation and dihydroxylation (M7) metabolites, and the isomeric metabolites of M7 (M8, M9, and M11). From the cycleanine-containing plasma of rats, the original form cycleanine (M0) and five metabolites were found, which were presumed to be hydroxylation (M2 and M10), dehydrogenation and hydroxylation (M6 and M12), and dehydrogenation and dihydroxylation (M7) metabolites. Among them, the prototype (M0), hydroxylation (M1), and dehydrogenation and hydroxylation (M6, M12) metabolites were detected in both rat urine and plasma (Table 4 and supplemental material). Therefore, the metabolic pathway of cycleanine in rats involves hydroxylation, dehydrogenation, and demethylation or their combination, which are the main means of biotransformation of cycleanine to generate a large number of metabolites (M1 to M12) (see Fig. S5 in the supplemental material).
DISCUSSION
Natural products (e.g., artemisinin and quinine) have demonstrated their potential as a source of antimalarial drugs. Previously, a number of BBIQ alkaloids were demonstrated to have in vitro antiplasmodial activities (16). Cycleanine had antiplasmodial effects with an IC50 of 70 nM (16) (or 80 nM [17]) against P. falciparum chloroquine-sensitive clone D6 (or 3D7) and an IC50 of 4.5 μM against a chloroquine-resistant strain (18). Isochondodendrine showed a low IC50 of 0.2 μM against a chloroquine-resistant strain (18, 19). 2′-Norcocsuline also showed potent in vitro antiplasmodial activity, with IC50 values of 48 and 248 nM against chloroquine-sensitive clone D6 (3D7) and chloroquine-resistant clone W2 (16, 20), respectively (Table 1). Our results against P. falciparum chloroquine-resistant strain Dd2 also confirmed the in vitro antimalarial activity of these compounds but with slightly higher IC50 values (Table 1) compared to the corresponding values reported in literature. Isochododendrine is a structurally demethylated analogue of cycleanine, and showed a greater potency than cycleanine in the chloroquine-resistant W2 strain and the Dd2 strain in this study (Table 1). This indicated that the increase of the hydrophilicity of cycleanine could improve its antiplasmodial activity. The SI values of all three BBIQ alkaloids ranged from 14 to 133 based on the KB or HTC-116 cells and the W2 strain, which were much greater than those based on HOE cells and the Dd2 strain. The discrepancy might be due to the different methodologies (16) used to determine IC50 or the different mammalian cancer cells or P. falciparum clones used. The semisynthetic analogues of cycleanine (compounds 4 and 5) produced by chemical modification of cycleanine through introduction of dimethylamino and (mono)alkynylamino groups, respectively, at the C-5 position exhibited increase in antiplasmodial potency and SI relative to cycleanine. The presence of a dimethylamino group in compound 4 could also increase the water solubility of the parent compound as is often found in the modification of other natural products, such as camptothecin (21) and thymoquinone (22). Compound 5, with a unique aminoalkynyl group, was used as a chemical probe for exploring the mechanism of action (e.g., cellular uptake) of cycleanine in cancer cells using click chemistry (15), and can also be utilized for identification of the molecular target of cycleanine in parasite-infected blood cells using a chemoproteomic approach (23). By changing the amino substitution groups, additional analogues of cycleanine with a variety of diverse structures can be synthesized for in vitro antiplasmodial evaluation.
To further confirm and validate the efficacy of cycleanine (compound 1) in vivo, its safety in healthy mice and efficacy in a murine malaria model was investigated. The LD50 (4.5 g/kg) of cycleanine indicated that cycleanine has a good safety profile, in agreement with the LD50 of 1.1 g/kg found previously in mice (24). Using suppression, prophylactic, and curative murine malaria models after infection with P. berghei (25), cycleanine showed a similar or closer effect at an oral dose of 50 mg/kg to their positive controls (chloroquine [5 mg/kg] and pyrimethamine [1.2 mg/kg]). At least, a much higher dose of cycleanine was needed to achieve the effects of these positive controls, indicating a mild efficacy in vivo. However, its low toxicity profile could allow increase of the oral dose (e.g., 100 mg/kg/day), which is expected to improve its efficacy. In the curative model, the slower effect of cycleanine comparing to chloroquine might be due to the metabolism of cycleanine to various metabolites. The in vivo antimalarial activity of cycleanine was consistent with its in vitro antiplasmodial activity. To our knowledge, this is the first demonstration of the in vivo antimalarial efficacy of a BBIQ alkaloid, cycleanine. Overall, three alkaloids (compounds 1 to 3) of T. subcordata could contribute to the antimalarial effects of this medicinal plant used in Nigeria for the treatment of malaria. BBIQ alkaloids of Triclisia gilletii (De Wild) Staner were also reported to be attributed to the in vitro and in vivo antimalarial activity of its plant extract (26).
Study on the metabolism of drugs can help to further understand their pharmacokinetics, efficacy, and safety (27). For example, metabolites of piperaquine were shown to have stronger antiplasmodial activity (28). However, there have been only a few in vivo metabolism studies of BBIQ alkaloids. Previously, in vitro metabolites of a BBIQ alkaloid, isoliensinine, from dog hepatic microsomes were identified as 2′-N-desmethylisoliensinine, 2-N-desmethyl-isoliensinine, and 2′-N-6-O-didesmethylisoliensinine (29). The study of the pharmacokinetics and metabolism of another BBIQ alkaloid, neferine, indicated that it was partially converted to liensinine, desmethyl-liensinine, isoliensinine, and desmethyl-isoliensinine by CYP2D6 (30). Tetrandrine was found to be initially biotransformed to a quinone methide-derived metabolite mediated by CYP3A enzymes, which was then trapped by a glutathione molecule to form a glutathione conjugate in mice (31). Metabolism of isotetrandrine in vitro by the rat hepatic system produced a major metabolite, N-desmethyl isotetrandrine (16%), and three minor oxidized metabolites, oxo-isotetrandrine (7%), hydroxy-isotetrandrine (6%), and oxohydroxy-isotetrandrine (7%), via N-demethylation and isoquinoline ring oxidation (32).
Our identification of 12 new metabolites of cycleanine in both plasma and urine in rats using LC-MS/MS has indicated that there are various metabolic pathways of cycleanine. These metabolites of cycleanine found in rats are also likely generated in mice after the same route of oral administration, and therefore they could contribute to its in vivo antimalarial efficacy found in the murine malarial model and its toxicity finding in healthy mice. Hydroxylation and demethylation of cycleanine were the common pathways consistent with those found in isoliensinine, neferine, and isotetrandrine described above. Preparation of these metabolites through chemical synthesis (33) or in vitro biotransformation using hepatic microsomes and/or P450 enzymes (34, 35) is possible and necessary to evaluate their potency and toxicity. Such information can be used to further guide the chemical design and modification of cycleanine to improve its potency and pharmacokinetics and to increase metabolic stability (36). Further work is necessary and ongoing in our laboratory to determine the in vivo antimalarial effects of BBIQ alkaloids (compounds 2 and 3) and semisynthetic derivatives (compounds 4 and 5) and the in vitro and/or in vivo antimalarial activity of the metabolites of cycleanine. Novel active drugs, particularly those with a wide safety margin, are required to help alleviate malaria morbidity and mortality and to contribute to the global control of malaria and infectious diseases.
MATERIALS AND METHODS
Chemicals.
Chloroquine and pyrimethamine were sourced from Sigma-Aldrich. Cycleanine (compound 1) (13), and two minor alkaloids, isochondodendrine (compound 2) and 2′-norcocsuline (compound 3), were isolated from Triclisia subcordata (14). Compounds 4 and 5 (Fig. 1) were previously prepared from cycleanine (compound 1) (15).
In vitro antiplasmodial activity.
The evaluation of in vitro antiplasmodial activity of the alkaloids (compounds 1 to 3) and semisynthetic analogues (compounds 4 and 5) were performed on the intraerythrocytic P. falciparum Dd2 strain (chloroquine-resistant strain) using a SYBR green1 fluorescence dye assay as described (22, 37, 38). Compounds 1 to 5 were prepared in dimethyl sulfoxide (DMSO) with no greater than 1% of the total solvent concentration in any assay. Normalized fluorescence signals were measured against controls with 1% DMSO (100% growth) and after exposure to a supralethal concentration (10 μM) of chloroquine (0% growth). Determination of the 50% inhibitory concentration (IC50) was performed from a log concentration versus the mean normalized fluorescence signal curve using GraphPad Prism software (v5.0). Each biological replicate consisted of three technical repeats, with three independent biological replicates performed.
Evaluation of the in vivo antimalarial activity of cycleanine.
(i) Malaria parasite. Chloroquine-sensitive strains of P. berghei were sourced from the National Institute of Medical Research (NIMER), Yaba Lagos, Nigeria, and maintained by subpassage in mice.
(ii) Parasite inoculation. Each mouse was inoculated intraperitoneally with about 1 × 107 P. berghei parasitized erythrocytes in 0.2 ml of infected blood (5 × 107 P. berghei erythrocytes/ml) according to published procedure (39).
(iii) Experimental animals. Female and male Swiss albino mice (18 to 25 g) were obtained from the University of Uyo’s animal house. Before use, mice were kept in cages and acclimatized for 10 days. All mice were kept in cross-ventilated rooms at room temperature. The care and use of mice were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). This investigation was approved by the University of Uyo’s Animal Ethics Committee.
(iv) Determination of median lethal dose (LD50) of cycleanine. The median lethal dose (LD50) of cycleanine was determined using albino mice by the intraperitoneal (i.p.) route (40). Different doses of cycleanine (10 to 5,000 mg/kg) were intraperitoneally administered to groups of three mice each. The mice were monitored for manifestation of physical signs of toxicity, including decrease of motor activity, writhing, decrease of body/limb tone, weakness, and death. The number of deaths in each group within 24 h was recorded. The LD50 value was calculated as the geometrical means of the minimum dose producing 100% mortality and the maximum dose producing 0%.
(v) Drug administration. Cycleanine, chloroquine, and pyrimethamine were prepared in water and administered orally with the aid of a stainless metallic feeding cannula.
(vi) Suppressive activity of cycleanine. The schizontocidal activity of cycleanine and chloroquine against early P. berghei infection in mice was measured according to an established protocol (25, 41, 42). On the first day, 20 to 24 mice were infected with the parasite and randomly separated into four groups. The mice in groups 1 and 2 were given 25 and 50 mg/kg of cycleanine, respectively, those in group 3 were given 5 mg/kg of chloroquine (positive control), and those in group 4 were given distilled water (10 ml/kg, negative control) for four consecutive days. Thin films were made from the tail blood on the fifth day. Parasitized erythrocytes were counted in stained films (Giemsa stain) under a microscope. The average suppression of parasitemia (%) was calculated as follows:
The MST (in days) of the mice in each group was determined over a period of 30 days.
(vii) Prophylactic activity of cycleanine. The prophylactic activity of cycleanine was evaluated using a previously described method (42, 43). The mice were randomly divided into four groups of six mice per group. Groups 1 and 2 were given 25 and 50 mg/kg of cycleanine, respectively, group 3 was given 1.2 mg/kg of pyrimethamine (positive control), and group 4 was given 10 ml/kg of distilled water (negative control). Administration of cycleanine or the drug continued for three consecutive days. On the fourth day, the mice were inoculated with P. berghei. The parasitemia level was evaluated by blood smears after 3 days. The survival times (days) of the mice were recorded over a period of 30 days, and MST were calculated.
(viii) Curative activity of cycleanine. The curative activity of cycleanine was assessed according to a method described previously (42, 44). P. berghei was injected intraperitoneally into another 24 mice on the first day. Three days later, the mice were also separated into four groups of six mice per group. Groups 1 and 2 were administered different doses of cycleanine, 25 and 50 mg/kg, respectively, group 3 was given 5 mg/kg chloroquine (positive control), and group 4 was given 10 ml/kg distilled water (negative control). Cycleanine and chloroquine were given once a day for 5 days. Mouse tail blood samples were collected on each day, and Giemsa-stained thin smears were prepared to determine the parasitemia level. The MST of the mice in each group was determined over a period of 30 days.
Metabolism of cycleanine in rats.
(i) High-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry. Analysis of cycleanine metabolites was performed through high-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry (HPLC-QTOF-MS/MS) system that consists of an Agilent 1260 HPLC coupled with an 6530 QTOF mass spectrometer with dual Agilent Jet Stream electrospray ionization source (Agilent Technologies, CA). The mass spectra were recorded in positive auto MS/MS mode, and the parameters were set as follows: temperature of drying and sheath gas, 300°C and 350°C; skimmer, 75 V; capillary voltage, 4,000 V; fragmentor, 110 V; nozzle voltage, 1,000 V; collision energy, 50 eV; pressure of nebulizer, 35 lb/in2; and flow rate of the drying and sheath gas, 5 and 11 liter/min, respectively. The QTOF mass spectra were recorded in high-resolution mode. The range of mass-to-charge ratio (m/z) scanning was set between 100 and 1,200. Samples (5 μl) were loaded onto an Agilent Poroshell 120 EC-C18 column (100 × 2.1 mm, 2.7 μm) at 35°C. The mobile phase consisted of water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B) at a flow rate of 0.35 ml/min. Gradient separation was achieved by changing the proportion of the solvent B mobile phase as follows: 0 to 2 min, 10% B; 2.1 to 5 min, 18% to 20% B; 30 to 45 min, 70% to 90% B; and 45 to 50 min, 10% B. MassHunter WorkStation software (Agilent Technologies) was utilized for the system operation and data analysis.
(ii) In vivo experiments. In vivo animal experiments were approved by the Animal Ethics Committee of Shanghai Institute of Materia Medica, and performed according to procedures approved by the Institutional Animal Care and Use Committee of Shanghai Institute of Materia Medica, Chinese Academy of Science. Male Wistar rats were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were given free access to water and a standard diet under controlled humidity (45% to 55%) and temperature (20°C to 24°C), except in the overnight fasting period before administration of cycleanine. The rats were adapted to the environment for a week.
Cycleanine (compound 1) was suspended in 0.4% carboxymethyl cellulose sodium (CMC-Na) and was formulated at 12 mg/ml for intragastric administration to Wistar rats (male, 220 ± 10 g, fasted for 12 h prior to administration) at a dose of 120 mg/kg body weight. Three rats were used for blood collection through the orbital vein using cannulation at 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h postdose after anaesthetization with isoflurane. The plasma samples were separated from blood by centrifugation at 12,000 rpm and 4°C for 10 min. Another three rats were placed in the metabolism cages, and urine samples were collected into tubes from 0 to 24 h after oral administration of cycleanine. All samples were stored in a −80°C freezer before analysis. Aliquots of 1.2 ml of plasma or urine samples were mixed with 3 times the volume of acetonitrile to precipitate proteins. After centrifugation at 14,000 rpm for 10 min, the supernatant was collected and evaporated under vacuum. The residue was reconstituted in 200 μl methanol, and 5 μl of each sample was injected for HPLC-QTOF-MS/MS analysis.
Statistical analysis.
Data were expressed as mean ± standard error of the mean (SEM) for in vivo antimalarial experiments. Data were subjected to GraphPad Prism software analysis. Results were analyzed using one-way analysis of variance (ANOVA) followed by a post hoc Tukey multiple-comparison test. The difference between the mean of the experimental and control groups was considered significant at a P value of <0.05 (ANOVA).
Conclusions.
Three BBIQ alkaloids of T. subcordata, cycleanine (compound 1), isochondodendrine (compound 2), and 2′-norcocsuline (compound 3) and two semisynthetic analogues (compounds 4 and 5) of cycleanine were demonstrated to exert significant in vitro antiplasmodial activities against P. falciparum. Cycleanine (compound 1) was further demonstrated to have safety and efficacy in the treatment of mice infected with P. berghei. Cycleanine was transformed to various metabolites in rats after oral delivery. The findings from this study support the use of T. subcordata as an antimalarial agent in traditional medicine. BBIQ alkaloids could be exploited in novel drug development in search of antimalarial agents/drugs that are urgently needed to challenge resistant plasmodium species that currently present a significant threat to human life.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by Nigerian ETF and NDDC and by BBSRC (HVfCP business interaction voucher).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2019. World malaria report 2019. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/rest/bitstreams/1262394/retrieve. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle K, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, Se Y, Chann S, Ittiverakul M, Sia-Ngam P, Kuntawunginn W, Arsanok M, Buathong N, Chaorattanakawee S, Gosi P, Ta-Aksorn W, Chanarat N, Sundrakes S, Kong N, Heng TK, Nou S, Teja-Isavadharm P, Pichyangkul S, Phann ST, Balasubramanian S, Juliano JJ, Meshnick SR, Chour CM, Prom S, Lanteri CA, Lon C, Saunders DL. 2015. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis 15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Newman DJ. 2013. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Alvaro E, Hong WD, Nixon GL, O'Neill PM, Calderon F. 2016. Antimalarial chemotherapy: natural product inspired development of preclinical and clinical candidates with diverse mechanisms of action. J Med Chem 59:5587–5603. doi: 10.1021/acs.jmedchem.5b01485. [DOI] [PubMed] [Google Scholar]
- 6.Bridgford JL, Xie SC, Cobbold SA, Pasaje CFA, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, Spillman NJ, Tilley L. 2018. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun 9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu Y 2016. Artemisinin—a gift from traditional Chinese medicine to the world (Nobel Lecture). Angew Chem Int Ed Engl 55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 8.Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, Zhu G, Tang J, Liu Y, Wang W, Cao Y, Xu S, Gu Y, Li J, Zhang C, Gao Q, Menard D, Pain A, Yang H, Zhang Q, Cao J. 2017. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med 376:991–993. doi: 10.1056/NEJMc1612765. [DOI] [PubMed] [Google Scholar]
- 9.Schiff PL 1997. Bisbenzylisoquinoline alkaloids. J Nat Prod 60:934–953. doi: 10.1021/np9700174. [DOI] [Google Scholar]
- 10.Weber C, Opatz T. 2019. Bisbenzylisoquinoline alkaloids. Alkaloids Chem Biol 81:1–114. doi: 10.1016/bs.alkal.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sofowora LA 1982. Medicinal plants and traditional medicine in Africa. John Wiley and Sons Limited, Chichester, United Kingdom. [Google Scholar]
- 12.Asuzu IU, Anaga AO. 1996. The antiulcer effect of the methanolic extract of Triclisia subcordata leaves in rats. J Herbs Spices Med Plants 3:45–53. doi: 10.1300/J044v03n03_07. [DOI] [Google Scholar]
- 13.Uche FI, Drijfhout FP, McCullagh J, Richardson A, Li WW. 2016. Cytotoxicity effects and apoptosis induction by bisbenzylisoquinoline alkaloids from Triclisia subcordata. Phytother Res 30:1533–1539. doi: 10.1002/ptr.5660. [DOI] [PubMed] [Google Scholar]
- 14.Uche FI, Abed MN, Abdullah MI, Drijfhout FP, McCullagh J, Claridge TWD, Richardson A, Li WW. 2017. Isochondodendrine and 2′-norcocsuline: additional alkaloids from Triclisia subcordata, induced cytotoxicity and apoptosis in ovarian cancer cell lines. RSC Adv 7:44154–44161. doi: 10.1039/C7RA08032H. [DOI] [Google Scholar]
- 15.Uche FI, McCullagh J, Claridge TWD, Richardson A, Li WW. 2018. Synthesis of (aminoalkyl)cycleanine analogues: cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells. Bioorg Med Chem Lett 28:1652–1656. doi: 10.1016/j.bmcl.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Angerhofer CK, Guinaudeau H, Wongpanich V, Pezzuto JM, Cordell GA. 1999. Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62:59–66. doi: 10.1021/np980144f. [DOI] [PubMed] [Google Scholar]
- 17.Fadaeinasab M, Taha H, Fauzi PN, Ali HM, Widyawaruyanti A. 2015. Anti-malarial activity of isoquinoline alkaloids from the stem bark of Actinodaphne macrophylla. Nat Prod Commun 10:1541–1542. [PubMed] [Google Scholar]
- 18.Otshudi AL, Apers S, Pieters L, Claeys M, Pannecouque C, De Clercq E, Van Zeebroeck A, Lauwers S, Frederich M, Foriers A. 2005. Biologically active bisbenzylisoquinoline alkaloids from the root bark of Epinetrum villosum. J Ethnopharmacol 102:89–94. doi: 10.1016/j.jep.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Mambu L, Martin MT, Razafimahefa D, Ramanitrahasimbola D, Rasoanaivo P, Frappier F. 2000. Spectral characterisation and antiplasmodial activity of bisbenzylisoquinolines from Isolona ghesquiereina. Planta Med 66:537–540. doi: 10.1055/s-2000-8610. [DOI] [PubMed] [Google Scholar]
- 20.Guinaudeau H, Bohlke M, Lin LZ, Angerhofer CK, Cordell GA, Ruangrungsi N. 1997. (+)-Angchibangkine, a new type of bisbenzylisoquinoline alkaloid, and other dimers from Pachygone dasycarpa. J Nat Prod 60:258–260. doi: 10.1021/np960568e. [DOI] [PubMed] [Google Scholar]
- 21.Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK. 1991. Synthesis of water-soluble (aminoalkyl)camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem 34:98–107. doi: 10.1021/jm00105a017. [DOI] [PubMed] [Google Scholar]
- 22.Johnson-Ajinwo OR, Ullah I, Mbye H, Richardson A, Horrocks P, Li WW. 2018. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg Med Chem Lett 28:1219–1222. doi: 10.1016/j.bmcl.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Drewes G, Knapp S. 2018. Chemoproteomics and chemical probes for target discovery. Trends Biotechnol 36:1275–1286. doi: 10.1016/j.tibtech.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Wambebe C, Gamaniel K, Akah P, Kapu S, Samson A, Orisadipe A, Okogun J. 1997. Central and uterotonic effects of cycleanine. Indian J Pharmcol 29:366–372. [Google Scholar]
- 25.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 26.Kikueta CM, Kambu OK, Mbenza AP, Mavinga ST, Mbamu BM, Cos P, Maes L, Apers S, Pieters L, Cimanga RK. 2013. In vitro and in vivo antimalarial activity and cytotoxicity of extracts and fractions from the leaves, root-bark and stem-bark of Triclisia gilletii. J Ethnopharmacol 149:438–442. doi: 10.1016/j.jep.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Yisimayili Z, Guo X, Liu H, Xu Z, Abdulla R, Akber Aisa H, Huang C. 2019. Metabolic profiling analysis of corilagin in vivo and in vitro using high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal 165:251–260. doi: 10.1016/j.jpba.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Zhou H, Cai T, Yang A, Zang M, Xing J. 2018. Metabolism of piperaquine to its antiplasmodial metabolites and their pharmacokinetic profiles in healthy volunteers. Antimicrob Agents Chemother 62:e00260-18. doi: 10.1128/AAC.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Li L, Jiang H, Zeng S. 2012. Identification of three new N-demethylated and O-demethylated bisbenzylisoquinoline alkaloid metabolites of isoliensinine from dog hepatic microsomes. Molecules 17:11712–11720. doi: 10.3390/molecules171011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Bai Y, Zhao L, Hu T, Hu B, Wang J, Xiang J. 2007. Pharmacokinetics and metabolism of neferine in rats after a single oral administration. Biopharm Drug Dispos 28:361–372. doi: 10.1002/bdd.556. [DOI] [PubMed] [Google Scholar]
- 31.Tian Y, Shen S, Jiang Y, Shen Q, Zeng S, Zheng J. 2016. CYP3A5 mediates bioactivation and cytotoxicity of tetrandrine. Arch Toxicol 90:1737–1748. doi: 10.1007/s00204-015-1584-8. [DOI] [PubMed] [Google Scholar]
- 32.Wu WN, McKown LA, Gopaul VS. 2004. In-vitro metabolism of isotetrandrine, a bisbenzylisoquinoline alkaloid, in rat hepatic S9 fraction by high-performance liquid chromatography-atmospheric pressure ionization mass spectrometry. J Pharm Pharmacol 56:749–755. doi: 10.1211/0022357023547. [DOI] [PubMed] [Google Scholar]
- 33.Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW. 2016. The medicinal chemist's toolbox for late stage functionalization of drug-like molecules. Chem Soc Rev 45:546–576. doi: 10.1039/c5cs00628g. [DOI] [PubMed] [Google Scholar]
- 34.Ren X, Yorke JA, Taylor E, Zhang T, Zhou W, Wong LL. 2015. Drug oxidation by cytochrome P450BM3: metabolite synthesis and discovering new P450 reaction types. Chemistry 21:15039–15047. doi: 10.1002/chem.201502020. [DOI] [PubMed] [Google Scholar]
- 35.Cusack KP, Koolman HF, Lange UE, Peltier HM, Piel I, Vasudevan A. 2013. Emerging technologies for metabolite generation and structural diversification. Bioorg Med Chem Lett 23:5471–5483. doi: 10.1016/j.bmcl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Thompson TN 2001. Optimization of metabolic stability as a goal of modern drug design. Med Res Rev 21:412–449. doi: 10.1002/med.1017. [DOI] [PubMed] [Google Scholar]
- 37.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/aac.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldulaimi O, Uche FI, Hameed H, Mbye H, Ullah I, Drijfhout F, Claridge TDW, Horrocks P, Li WW. 2017. A characterization of the antimalarial activity of the bark of Cylicodiscus gabunensis Harms. J Ethnopharmacol 198:221–225. doi: 10.1016/j.jep.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Odetola A, Basir O. 1980. Evaluation of antimalarial properties of some Nigerian medicinal plants, p 275–283. In Sofowora A (ed), Proceedings of African Bioscience Network, Federal Ministry of Science and Technology. University of Ife Organized Workshop Nigerian Society of Pharmacology and Drug Research and Production Unit, Ife, Nigeria. [Google Scholar]
- 40.Lorke D 1983. A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 41.Knight DJ, Peters W. 1980. The antimalarial activity of N-benzyloxydihydrotriazines. I. The activity of clociguanil (BRL 50216) against rodent malaria, and studies on its mode of action. Ann Trop Med Parasitol 74:393–404. doi: 10.1080/00034983.1980.11687360. [DOI] [PubMed] [Google Scholar]
- 42.Okokon JE, Antia BS, Mohanakrishnan D, Sahal D. 2017. Antimalarial and antiplasmodial activity of husk extract and fractions of Zea mays. Pharm Biol 55:1394–1400. doi: 10.1080/13880209.2017.1302966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters W 1965. Drug resistance in Plasmodium berghei Vincke and Lips, 1948. I. Chloroquine resistance. Exp Parasitol 17:80–89. doi: 10.1016/0014-4894(65)90012-3. [DOI] [PubMed] [Google Scholar]
- 44.Ryley JF, Peters W. 1970. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol 64:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.