Abstract
Study Objectives:
The aim was to assess the relationship between continuous positive airway pressure (CPAP) therapy and cognitive function in patients with mild cognitive impairment (MCI) and obstructive sleep apnea (OSA).
Methods:
This was a retrospective chart review of patients with MCI and OSA. CPAP therapy compliance was defined as average use of CPAP therapy for at least 4 hours per night. Kaplan-Meier estimates, log-rank tests, and Cox proportional hazards regression were done to compare the compliance groups in terms of progression to dementia, defined as a Clinical Dementia Rating of 1 or greater. Linear mixed models were used to assess the relationships between CPAP therapy compliance and neurological cognitive function outcomes over time.
Results:
Ninety-six patients were included with mean age at MCI diagnosis of 70.4 years, mean apnea-hypopnea index of 25.9 events/h, and mean duration of neurology follow-up of 2.8 years. Forty-two were CPAP compliant, 30 were noncompliant, and 24 had no CPAP use. No overall difference between the groups was detected for progression to dementia (P = .928, log-rank test). Patients with amnestic MCI had better CPAP use (P = .016) and shorter progression time to dementia (P = .042), but this difference was not significant after adjusting for age, education, and race (P = .32).
Conclusions:
CPAP use in patients with MCI and OSA was not associated with delay in progression to dementia or cognitive decline.
Citation:
Skiba V, Novikova M, Suneja A, McLellan B, Schultz L. Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med. 2020;16(6):863–870.
Keywords: continuous positive airway pressure, dementia, mild cognitive impairment, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Studies show a relationship between obstructive sleep apnea (OSA) and cognitive decline, including dementia and mild cognitive impairment (MCI). However, there are few studies that show beneficial effects of continuous positive airway pressure (CPAP) therapy in patients with cognitive impairment.
Study Impact: In our retrospective chart review study, CPAP use in patients with MCI and OSA was not associated with delay in progression to dementia or cognitive decline. Further prospective studies of patients with MCI and OSA treated with CPAP therapy for a longer duration and larger sample size are needed and may need stricter definitions of CPAP adherence.
INTRODUCTION
Obstructive sleep apnea (OSA) is thought to be a risk factor for the development of dementia, and treating OSA is a potential target for preventing dementia. The prevalence of OSA in the general population is estimated to be 5–10%, and up to 50% in patients with Alzheimer disease (AD).1–3 OSA is thought to increase risk of cognitive decline through hypertension, inflammation, oxidative stress, impaired cerebral perfusion, and endothelial dysfunction. OSA can lead to white matter changes and increase gray matter atrophy. Cerebral hypoperfusion and hypoxia can upregulate expression of the amyloid precursor protein, decrease α-β clearance, and upregulate τ phosphorylation.4–7 Patients with OSA have deficits in attention and concentration, short- and long-term memory, executive function, and problem-solving.8 OSA is also associated with development of AD, with a risk ratio of 1.5–2 over 15 years.9
Continuous positive airway pressure (CPAP) therapy is the gold-standard treatment for OSA and has been shown to improve mortality with long-term use.10 In some studies, CPAP therapy has been shown to increase network connectivity and reverse white matter abnormalities.11–13 In healthy patients, CPAP therapy has been shown to improve executive and frontal lobe function.14,15 CPAP therapy patients with AD have been looked at in a number studies of short duration and limited number of patients and has been found to improve executive function and psychomotor speed and slow decline.16–18
Mild cognitive impairment (MCI) is a heterogeneous state of impaired cognition that does not meet criteria for dementia but places patients at high risk of progression to dementia.19,20 Patients with MCI can be categorized as amnestic MCI and nonamnestic MCI. Memory loss is the predominant feature in amnestic MCI, and classically, it has been associated with increased risk to further conversion to AD. In patients with nonamnestic MCI, cognitive impairments are present in other domains than memory, such as deficits in attention, executive function, and mental flexibility (functions of fronto-subcortical networks). Nonamnestic MCI causes are either due to nonneurodegenerative conditions such as depression, medication side effects, or due to other brain conditions, such as Lewy body disease and fronto-temporal dementia. Up to 20% of patients with MCI revert to normal, more often in those who are younger, have less severe deficits, and have nonamnestic MCI.19 At this time, there are no US Food and Drug Administration–approved treatments for MCI. In addition to regular exercise, interventions to address cognitive, behavioral, and neuropsychiatric symptoms are recommended.20
At the time of the manuscript preparation, we found 1 pilot clinical trial in which 54 patients with MCI were treated with CPAP therapy and followed over 1 year. Patients using CPAP therapy had increases in psychomotor/cognitive processing and a trend of improving on the Clinical Dementia Rating (CDR) scale.21
Our aim, through a chart review, was to look at a large number of patients with MCI followed over several years and assess the relationship between CPAP use and cognitive function over time.
METHODS
Study population and design
This was a retrospective chart review. Patients over 18 years old were identified within a large, urban, tertiary health center as having MCI and OSA based on an electronic health records search. These patients were followed in the behavioral neurology clinic between 1 November 2011 and 1 December 2017 and were also followed in the sleep medicine clinic for OSA. Institutional review board approval was obtained prior to initiation of the study. Charts from each clinic were blindly reviewed as to their status in the other clinic. Patients with MCI were selected from the behavioral neurology clinic. Patients not attending the sleep medicine clinic were excluded, along with patients whose OSA treatment involved surgical options or an oral appliance.
CDR scores were reviewed for each of the patients. This global assessment instrument that yields global and sum of boxes scores is used in clinical and research settings to stage dementia severity. A global CDR score of 0 is considered normal, 0.5 is considered MCI, and 1 is considered mild dementia. The CDR sum of boxes offers increased sensitivity in tracking changes within and between stages of dementia severity. Annual Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores were also reviewed; this is a neuropsychological battery that is a standardized and validated measure for the assessment of AD. Our modified CERAD included the following subtests: orientation, clock drawing, verbal fluency, phonemic fluency, Boston naming, word list learning, recall and recognition, constructional praxis learning and recall, simple and complex visuospatial praxis (Rey–Osterrieth complex figure test), simple calculations, and Trail Making Test part A and part B. In addition, Epworth Sleepiness Scale (ESS) scores from the sleep visits were collected.
OSA was diagnosed either by an attended polysomnogram (full-night diagnostic or a split-night study) or home sleep apnea testing. OSA severity was defined by the apnea-hypopnea index (AHI) on the initial sleep study, with mild OSA as an AHI 5–14.9 events/h, moderate OSA as an AHI 15–29.9 events/h, and severe OSA as an AHI >30 events/h. CPAP therapy settings and compliance were recorded. CPAP adherence data were obtained by chart review of the electronic health record, cloud-based CPAP downloads, and downloads on file by the durable medical equipment company.
It has become standard in the sleep clinics to collect the percentage of nights CPAP therapy was used for 4 or more hours in the past 30 days, partly due to most insurers requiring use of CPAP for 4 hours or more on at least 70% of the nights in a 30-day period. This was our aim initially to use this cutoff to define compliance; however, many of the older encounters included only the average use in the past 30 days. Therefore, compliance was defined as average use of 4 hours or more, noncompliance was defined as having an average CPAP use of less than 4 hours, and no CPAP was defined as CPAP therapy never started.
Primary and secondary outcomes
The primary aim was to determine if treatment of OSA with CPAP therapy in patients with MCI was associated with delay in progression to dementia, based on a CDR score of 1. Secondary outcomes included the following: (1) to determine if treatment of OSA with CPAP therapy in patients with MCI was associated with slowing down the decline of cognitive function based on neuropsychological testing, (2) to determine if there was a difference in outcomes by CPAP compliance within patients with nonamnestic MCI and amnestic MCI, (3) to determine if there was a difference in outcomes based on severity of OSA, (4) and to determine if there was a difference in outcomes based on CPAP compliance within the patients with moderate and severe OSA. We reviewed the CERAD subtests and focused on the Trail Making Test part A to measure attention and Trail Making Test part B to measure executive functioning.
The study was reviewed and approved by the Henry Ford Health System Institutional Review Board, number 10996.
Statistical analysis
Patient demographic, past medical history, treatment, and initial AHI and ESS information were compared among the 3 CPAP compliance groups using chi-square and Fisher’s exact tests for the binary and categorical measurements, and analysis of variance and Kruskal-Wallis tests for the continuous measurements. For progression to dementia, Kaplan-Meier estimates and log-rank tests were done to compare the compliance groups. Time to progression was defined as the time from initial diagnosis of MCI to diagnosis of dementia or last neurology follow-up for those patients without a dementia diagnosis. Cox proportional hazard regression models were done to adjust for any demographic differences between the CPAP compliance groups and other factors that may influence the development of dementia. Linear mixed models using structural equation methods were used to assess the relationships between CPAP compliance and neurological cognitive function outcomes over time. This method allows for each patient to have a random starting point (intercept) and slope of cognitive decline by taking into account the varying amounts of data available per patient as well as the correlations among multiple measurements within the same patient. The inclusion of interactions between time and compliance was incorporated into the models and used to test whether changes in cognitive functioning over time differ by CPAP compliance. z scores computed and adjusted for age, sex and education were used for the Trail A and Trail B cognitive function measures.22 For the cognitive function measure of CDR sum of boxes, age, sex, and education were adjusted for in the linear mixed models. Similar analyses were done to compare the patients with nonamnestic and amnestic MCI and severity of OSA. Associations between ESS and the neurological cognitive function outcomes were assessed using correlation coefficients with P values that took into account multiple measurements over time within the same patient.
RESULTS
Patient selection
There were 158 patients who met the inclusion criteria. Of these, 62 (39%) did not have a follow-up visit with neurology and the remaining 96 patients were analyzed. Based on the compliance definitions in the Methods section, 42 (44%) patients were compliant, 30 (31%) were not compliant, and 24 (25%) had no CPAP use. For the entire sample, the median of the average CPAP use was 3.88 hours, with a range from 0 to 12 hours per night.
Of the 96 patients, the mean age at MCI diagnosis was 70.4 years, with a range from 40 to 92 years; 63 (66%) were male; and 63 (66%) were Caucasian. Of the 66 patients with education information, 5 (8%) had less than a high school education, 19 (29%) had a high school education, 13 (20%) had some college education, and 29 (44%) had a 4-year college education or higher. Hypertension (n = 64, 67%), obesity (n = 41, 43%), mood or anxiety disorder (n = 30, 31%), and diabetes (n = 27, 28%) were the most common comorbidities in this population. The mean AHI was 25.9 events/h (SD = 16.8, range 4 to 84.88) and the mean ESS was 8.4 (SD = 5.4; range: 0–20). The median Trail A time was 51 seconds (range: 20–157), the median Trail B time was 137 seconds (range: 45 to ≥300), and the median CDR sum of boxes was 1, with a range from 0 to 5. Most patients were treated with either a single CPAP pressure or an autotitrating range of CPAP pressures, and some were treated with bilevel positive airway pressure therapy. At the time of MCI diagnosis, 79 (82%) patients were not taking medication for their MCI and 47 (49%) had amnestic type MCI. The only significant difference between the 3 compliance groups was for chronic kidney disease (P = .03), with chronic kidney disease rates of 29% for patients with no CPAP use, 3% for patients with CPAP noncompliance, and 14% for patients with CPAP compliance. No other group comparisons were significant (Table 1). Patients were followed for an average of 2.8 years (SD = 1.9; range: 50 days to 10.4 years).
Table 1.
Demographic, comorbidity, and follow-up comparisons among the 3 CPAP-use groups.
| Response | No CPAP (n = 24) | Noncompliant (n = 30) | Compliant (n = 42) | P |
|---|---|---|---|---|
| Age at MCI, years | .804a | |||
| Mean (SD) | 70.1 (11.3) | 71.4 (7.7) | 70.0 (9.4) | |
| Range | 40–92 | 53–85 | 44–90 | |
| Median (IQR) | 70.5 (12) | 71.5 (10) | 69 (15) | |
| Sex, n (%) | .257b | |||
| Male | 13 (54) | 19 (63) | 31 (74) | |
| Female | 11 (46) | 11 (37) | 11 (26) | |
| Race, n (%) | .074c | |||
| Caucasian | 15 (63) | 15 (50) | 33 (79) | |
| African American | 6 (25) | 10 (33) | 7 (17) | |
| Other | 2 (8) | 4 (13) | 0 (0) | |
| Unknown | 1 (4) | 1 (3) | 2 (5) | |
| Education, n (%) | .527c | |||
| Less than high school | 1 (6) | 2 (10) | 2 (7) | |
| High school | 7 (41) | 7 (35) | 5 (17) | |
| Some college | 4 (24) | 3 (15) | 6 (21) | |
| College degree or more | 5 (29) | 8 (40) | 16 (55) | |
| BMI, kg/m2 | .786a | |||
| Mean (SD) | 30.1 (6.3) | 30.0 (7.2) | 29.1 (6.2) | |
| Range | 20.43–44 | 22–53 | 18.8–50 | |
| BMI category, n (%) | .940b | |||
| Normal | 5 (21) | 8 (27) | 10 (24) | |
| Overweight | 7 (29) | 10 (33) | 15 (36) | |
| Obese | 12 (50) | 12 (40) | 17 (40) | |
| Medication, n (%) | .261c | |||
| No | 22 (92) | 22 (73) | 35 (83) | |
| Yes, already taking | 1 (4) | 1 (3) | 3 (7) | |
| Yes, starting | 1 (4) | 7 (23) | 4 (10) | |
| AHI, events/h | .052d | |||
| Mean (SD) | 19.4 (14.0) | 28.0 (20.1) | 28.2 (15.0) | |
| Range | 4–61.98 | 6.01–84.88 | 5.96–64 | |
| ESS | .986d | |||
| Mean (SD) | 8.5 (5.9) | 8.2 (5.0) | 8.5 (5.6) | |
| Range | 0–20 | 0–18 | 0–20 | |
| Neurology follow-up, years | .819d | |||
| Mean (SD) | 2.7 (2.2) | 2.8 (1.9) | 2.8 (1.8) | |
| Range | 0.14–10.4 | 0.74–9.9 | 0.15–8.4 | |
| Median (IQR) | 2.9 (1.5) | 2.7 (1.5) | 2.6 (1.5) | |
| Trail A, seconds | .132d | |||
| Mean (SD) | 46.5 (15.2) | 60.1 (21.5) | 57.8 (34.4) | |
| Range | 24–78 | 27–116 | 20–157 | |
| Median (IQR) | 43.5 (13) | 58.5 (19) | 50.5 (38) | |
| Trail B,e seconds | .381d | |||
| Mean (SD) | 164.9 (104) | 193.9 (98.6) | 159.8 (92) | |
| Range | 49–300 | 62–300 | 45–300 | |
| Median (IQR) | 137 (270) | 192 (199) | 127 (188.5) | |
| CDR sum of boxes | .861d | |||
| Mean (SD) | 1.54 (0.97) | 1.4 (0.95) | 1.61 (1.77) | |
| Range | 0.5–4 | 0–4.5 | 0.5–5 | |
| Median (IQR) | 1.5 (1) | 1(1) | 1 (1.25) |
There were no statistical differences between the compliance groups in terms of baseline demographics, sleep apnea severity, ESS, or behavioral scales at time of MCI diagnosis. aAnalysis of variance. bChi-square test. cFisher's exact test. dKruskal-Wallis test. eTrail B times >300 minutes were truncated at 300 minutes. AHI = apnea-hypopnea index; BMI = body mass index; CDR = Clinical Dementia Rating; CPAP = continuous positive airway pressure; ESS = Epworth Sleepiness Scale; IQR = interquartile range; MCI = mild cognitive impairment.
Progression to dementia
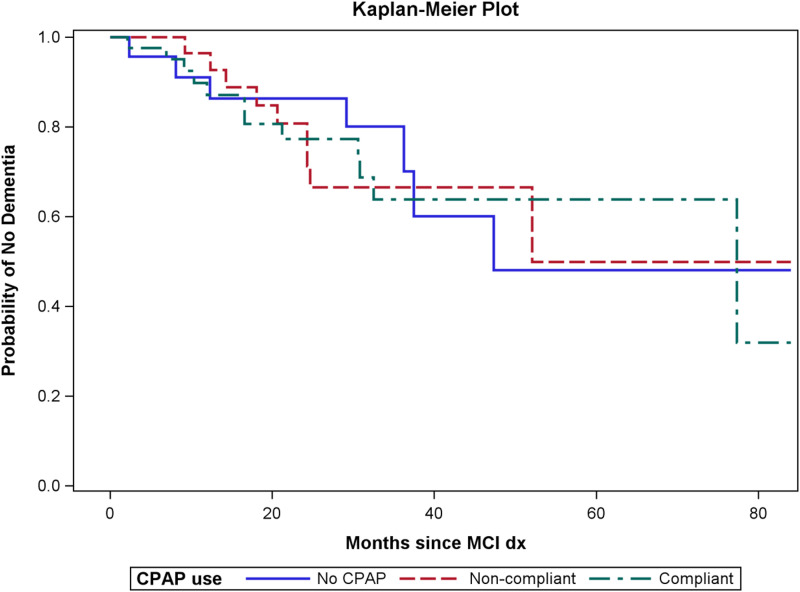
There were 28 patients who progressed to dementia during their follow-up visits with neurology. The median time to progression was 47.3 months for patients with no CPAP use, 52.1 months for patients with CPAP use but not compliant, and 77.3 months for patients with compliant CPAP use (the median time to progression is the time that corresponds with the “survival” curve dropping below 50%). Figure 1 contains the Kaplan-Meier estimates for the 3 groups. No overall difference was detected between the 3 groups for progression to dementia (P = .993, log-rank test). The overall difference was also not significant after adjusting for age at MCI, education, and race (P = .94, Cox regression).
Figure 1. Time to progression to dementia by compliance group.
There was no difference in time to progression to dementia between the 3 compliance groups (P = .993). CPAP = continuous positive airway pressure; dx = diagnosis; MCI = mild cognitive impairment.
Time to progression to dementia was also compared for patients with and without any CPAP use. The median time to progression was 47.3 months for patients with no CPAP use and 77.3 months for patients with any CPAP use (P = .915, log-rank test, and P = .765, Cox regression). Additionally, the difference in time to progression for patients with compliant use compared with patients without any CPAP or noncompliant CPAP use was not significant (P = .923, log-rank test, and P = .997, Cox regression).
Decline in cognitive function
Estimated regression models for Trail A, Trail B, and CDR sum of boxes for each CPAP group were computed and compared to assess the linear relationship between the cognitive function scores over time. None of the pairwise comparisons of the 3 CPAP use regression lines were significant for any of the 3 scores. Additional analyses were done combining the compliant- and noncompliant-CPAP-use groups and comparing with the no-CPAP-use group, as well as comparing the compliant group with the combined noncompliant and no-CPAP-use groups. None of the additional comparisons were significant.
Progression to dementia in patients with amnestic and nonamnestic MCI
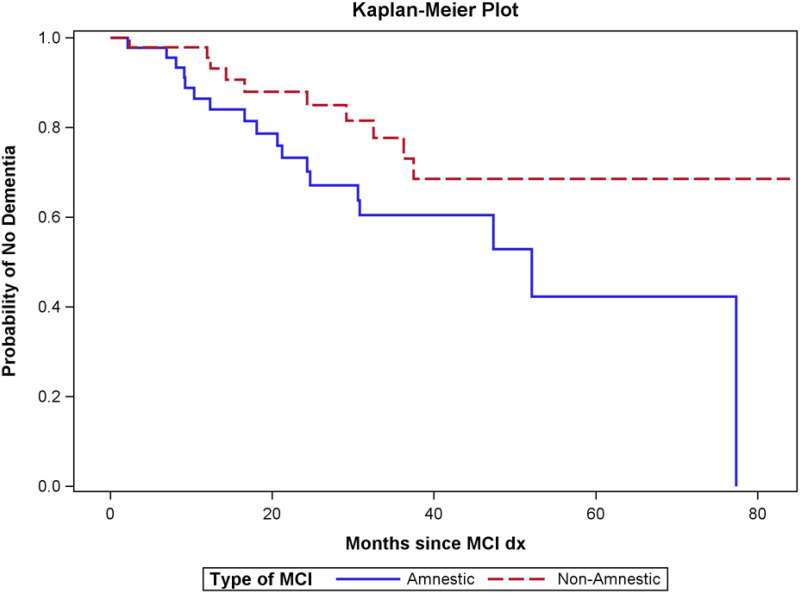
There was a significant association between time to progression to dementia and type of MCI (amnestic vs nonamnestic), with patients with amnestic MCI having shorter progression time to dementia (median time to dementia: 52.1 vs >84 months, P = .042; Figure 2). However, after adjusting for age at MCI, education, and race, this difference was no longer significant (P = .32 Cox regression). In addition, the association between amnestic and CPAP use was significant (Table 2) (P = .016). Patients with amnestic MCI had higher rates of CPAP use (both compliant and noncompliant) compared with patients with nonamnestic MCI. When analyses were done within the amnestic and nonamnestic groups to assess the relationship between CPAP use and progression to dementia, no significant differences were observed (amnestic MCI: P = .629, log-rank test, and P = .72, Cox regression; nonamnestic MCI: P = .694, log-rank test, and P = .754, Cox regression).
Figure 2. Time to progression to dementia comparing patients with amnestic and nonamnestic MCI.
Patients with amnestic MCI progressed to dementia faster than those with nonamnestic MCI (P = .042). dx = diagnosis; MCI = mild cognitive impairment.
Table 2.
Association between amnestic/nonamnestic and CPAP use.
| CPAP Use | Amnestic (n = 47) | Nonamnestic (n = 49) |
|---|---|---|
| No use | 6 (13) | 18 (38) |
| Noncompliant | 19 (40) | 11 (22) |
| Compliant | 22 (47) | 20 (41) |
| P | .016 (chi-square test) | |
Values are n (%). Patients with amnestic mild cognitive impairment had greater CPAP use compared with those with nonamnestic mild cognitive impairment. CPAP = continuous positive airway pressure.
Impact of CPAP compliance
In order to see if higher CPAP use led to improved outcomes, a more strict compliance criterion of an average use of 6 hours or more was used. Of the 96 patients, 24 had no CPAP use, 48 had between 0 and 6 hours of CPAP use, and 24 had 6 hours or more of CPAP use. No significant differences between the 3 CPAP groups for progression to dementia were observed. Comparisons of the regression models for Trail A, Trail B, and CDR sum of boxes by CPAP use group also showed no significant differences.
Impact of ESS
None of the associations between ESS and the cognitive function outcomes (Trail A and B and CDR sum of boxes) were significant (Trail A: r = .174, P = .163; Trail B: r = .05, P = .654; and CDR sum of boxes: r = .163, P = .075).
Impact of OSA severity
To assess the relationship of OSA severity and progression to dementia and cognitive decline, patients were divided into 3 groups based on their AHI values. There were 29 (30%) patients classified as having mild OSA (AHI 5–14.9 events/h), 34 (35%) classified as moderate OSA (AHI 15–29.9 events/h), and 33 (34%) classified as severe OSA (AHI ≥30 events/h). The overall difference between the 3 OSA severity groups for time to progression to dementia was not significant (P = .177, log-rank test, and P = .307, Cox regression). Also, no differences in the regression models for cognitive function scores were observed between the 3 OSA groups.
Additional analyses were done assessing the relationship between CPAP use and progression to dementia and cognitive function scores among patients with only moderate or severe OSA. No difference between the 3 CPAP compliance groups was seen for time to progression of dementia (P = .897, log-rank test, and P = .718, Cox regression). Also, no differences in the regression models between the 3 groups were seen for the cognitive function measures.
DISCUSSION
This study retrospectively reviewed 96 patients with MCI and OSA who were followed for an average of 2.8 years. During this period, 28 patients progressed to dementia, and although the median time for progression was longer for those compliant with CPAP therapy (77.3 months vs 52.1 months for noncompliant vs 47.3 months for those not on CPAP therapy), this difference was not significant. We did not find a difference in the rate of decline in cognitive domains including Trail A, Trail B, and CDR sum of boxes.
Since we initiated this study, Richards et al21 published a series of 54 patients with MCI followed over 1 year treated with CPAP therapy. Those who were compliant had significant increases in psychomotor/cognitive processing only and showed trends to improvement in CDR, memory, and attention. This study is limited by its smaller sample size and shorter duration of follow-up.
As would be expected, we found that the patients with amnestic MCI progressed to dementia faster that those with nonamnestic MCI, but this difference was not significant after adjusting for age, education, and race. Amnestic MCI is believed to be a precursor to AD, whereas nonamnestic MCI is believed to have varying etiologies that may potentially be reversible. However, no significant differences were found when analyses were done within the amnestic and nonamnestic groups to assess the relationship between CPAP use and progression to dementia. Interestingly, those with amnestic MCI had significantly better CPAP therapy adherence than those with nonamnestic MCI. A recent meta-analysis found that 10.7–38% of patients with cognitive impairment have poor medication adherence, and intact memory was a significant predictor of medication adherence, but associations with executive functioning were unclear due to discordant study results.23 It is unclear why patients with amnestic MCI were more compliant with CPAP use. We could hypothesize that patients with amnestic MCI (or their family members) were more worried about their progression to clinical dementia due to AD and thus were motivated to try any interventions to slow down such progression. Also, it is possible that family members of patients with amnestic MCI are more involved in the medical care as the cognitive deficits may preclude a patient from being an active participant in his or her own medical care. Thus, family members could be reminding and assisting in using CPAP therapy nightly. The patients with nonamnestic MCI had various etiologies that may have affected their executive functioning and motivation to treat OSA. It is plausible that this group may have greater benefits from treatment of OSA, but the lower compliance prevents the benefits from being fully realized. However, no significant differences were found when analyses were done within the amnestic and nonamnestic groups to assess the relationship between CPAP use and progression to dementia.
Similar to other studies looking at CPAP compliance,15,24 only about half of the patients were complaint with CPAP, as defined by an average use of at least 4 hours, and the average CPAP use was 3.9 hours per night. It is possible that this “compliance” cutoff, while used by many insurance companies to qualify patients for ongoing CPAP coverage, is not sufficient to provide physiological benefits. Several studies do, in fact, show that there is a linear relationship between duration of CPAP use and cardiovascular risk reduction.25,26 We examined the relationship between longer CPAP use of more than 6 hours per night and progression to dementia or changes on neuropsychological testing and did not find a statistical difference compared with shorter CPAP use. However, there were only 24 patients (25%) who achieved this use.
Some studies suggest that greater severity of OSA leads to more significant consequences.27 We compared patients with all 3 OSA severities with each other in terms of progression to dementia and changes in cognitive function. We did not find any differences, other than a trend toward faster decline in Trail A and CDR sum of boxes in patients with severe compared with those with mild OSA. This is similar to the findings by Kushida et al14 who did not find any differences in cognitive improvement except for patients with severe OSA only on the Sustained Working Memory Test-Overall Mid-Day Index.
Limitations
Our study has several limitations. This is a retrospective review rather than a randomized trial, which may introduce bias into overall compliance with medical treatment. Using medical records and not a defined follow-up protocol from a prospective study, patients had varying durations and amounts of data available for analyses, with 39% of potentially eligible patients having no neurology follow-up data available. When comparing the patients included in this study with those who were excluded, the only significant differences detected were for sex. with a higher percentage of males included (66% vs 48%, P = .032), and the distribution of education, with patients included in the study having higher educational levels (P = .04). It is also possible that the patients who were not followed up actually improved in their cognition and reverted from MCI to normal and believed that they no longer needed to follow up with their neurologist. Not including these patients may lead to false-negative findings.
We also included all adult patients, and since patients with younger onset of cognitive decline tend to have a stronger genetic influence, they may not be affected by the intervention to the same extent as those with an older onset. There were 23 patients under the age of 65 and only one of them progressed to dementia during follow-up. Analyses were done for just those patients 65 or older and the findings were similar to those with the entire cohort, except for the univariate analyses comparing the time to progression between the amnestic and nonamnestic groups. The P value for this comparison was .08 instead of .042.
Using a behavioral neurology clinic sample can also introduce selection bias of the patients being analyzed. Many patients with MCI remain undiagnosed or may be managed by their primary care providers or general neurologists, and they may differ in symptom severity from those being referred to a subspecialty clinic.
There are several behavioral and neuropsychiatric factors that can affect nonamnestic MCI, such as depression, medication side effects, poor sleep patterns, and sleep–wake rhythm disorders. Our study did not address these potential contributing factors.
In our study there was also some variability in the types of testing used to diagnose OSA. Home sleep apnea testing leads to underestimation of the overall OSA severity compared with in-laboratory polysomnography; however, home sleep apnea testing is an accepted and widely used test both clinically and in research.
While our study examined a large number of patients, the 3 compliance groups were small and may have been too small to detect overall differences. In addition, the number of patients using CPAP therapy on most nights for most of the night was small, but perhaps this level of “compliance” is what is required to see the true physiological benefits. Even studies involving a large number of patients, such as 1,098 participants with OSA randomly assigned to active placebo or a control, often do not find significant improvements in cognitive function. Kushida et al14 found only small improvements in the executive and frontal lobe function but not in learning and memory function. Osorio et al15 followed 2,470 patients free of MCI or AD and found that those with OSA had earlier onset of MCI and AD; however, in only some of their subsets did CPAP use delay progression to MCI, and it did not delay progression to AD. The lack of demonstrable significant benefit may be due to the follow-up being too short, the presence of irreversible damage from years of OSA, or premorbid neurological changes.
Future directions
In conclusion, several studies show a relationship between OSA and cognitive decline; however, there are limited studies examining the beneficial effects of CPAP therapy on cognitive function in healthy patients and patients with AD. This is one of a small number of studies to our knowledge examining the effects of CPAP therapy on patients with MCI. Although none of the observed relationships of CPAP compliance with progression to dementia and cognitive decline were significant, there was a significant difference in CPAP adherence between the MCI subtypes. Further prospective studies of patients with MCI and OSA are needed to confirm these findings and to investigate other possible definitions of CPAP compliance. There also remains the broader question of whether OSA is an independent factor causing dementia, or whether OSA contributes to other processes and accelerates existing dementia pathology. Further community-based routine assessments of sleep and cognition are needed. We speculate that if CPAP therapy is beneficial in improving cognitive function or slowing down decline, the magnitude of this benefit may be small in the short term. Large, prospective studies with a longer duration of follow-up and a more strict definition of CPAP compliance are needed to assess the impact of CPAP therapy on cognition.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Henry Ford Hospital. Preliminary data have been presented in poster form at the American Academy of Neurology 70th Annual Meeting on 22 April 2018 and at the 33rd Annual Meeting of the Associated Professional Sleep Societies on 11 June 2019. The authors report no conflicts of interest.
ABBREVIATIONS
- AD
Alzheimer disease
- AHI
apnea-hypopnea index
- CDR
Clinical Dementia Rating
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- MCI
mild cognitive impairment
- OSA
obstructive sleep apnea
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Emamian F, Khazaie H, Tahmasian M, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. 10.3389/fnagi.2016.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. 10.1002/jnr.23634 [DOI] [PubMed] [Google Scholar]
- 5.Yun CH, Lee HY, Lee SK, et al. Amyloid burden in obstructive sleep apnea. J Alzheimers Dis. 2017;59(1):21–29. 10.3233/JAD-161047 [DOI] [PubMed] [Google Scholar]
- 6.He Y, Chen R, Wang J, et al. Neurocognitive impairment is correlated with oxidative stress in patients with moderate-to-severe obstructive sleep apnea hypopnea syndrome. Respir Med. 2016;120:25–30. 10.1016/j.rmed.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5):zsx011. [DOI] [PubMed] [Google Scholar]
- 8.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24(2):249–259. 10.1016/S0272-5231(03)00014-5 [DOI] [PubMed] [Google Scholar]
- 9.Lutsey PL, Misialek JR, Mosely TH, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(2):157–166. 10.1016/j.jalz.2017.06.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisan Q, Van Sloten T, Marques Vidal P, Haba Rubio J, Heinzer R, Empana JP. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the Sleep Heart Health Study. JAMA Otolaryngol Head Neck Surg. 2019;145(6):509–515. 10.1001/jamaoto.2019.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalmases M, Sole-Padulles C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214–1223. 10.1378/chest.15-0171 [DOI] [PubMed] [Google Scholar]
- 12.O’Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35(1):41–48. 10.5665/sleep.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465–1475. 10.5665/sleep.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2012;35(12):1593–1602. 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. 10.1212/WNL.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–2081. 10.1111/j.1532-5415.2008.01934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5(4):305–309. 10.5664/jcsm.27538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85(12):1405–1408. 10.1136/jnnp-2013-307544 [DOI] [PubMed] [Google Scholar]
- 19.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–772. 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen RC, Lopez O, Armstrong MJ. Practice guideline update summary: mild cognitive impairment. Neurology. 2018;90(3):126–135. 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards KC, Gooneratne N, Dicicco B, et al. CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc. 2019;67(3):558–564. 10.1111/jgs.15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirk SD, Mitchell MB, Shaughnessy LW, et al. A web-based normative calculator for Uniform Data Set (UDS) neuropsychological test battery. Alzheimers Res Ther. 2011;3(6):32–40. 10.1186/alzrt94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D, Lovell J, Weller C, et al. A systematic review of medication non-adherence in persons with dementia of cognitive impairment. PLoS One. 2017;12(2):e0170651. 10.1371/journal.pone.0170651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest. 2019;155(6):1272–1287. 10.1016/j.chest.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Bravata DM, Sico J, Vaz Fragoso CA, et al. Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease. J Am Heart Assoc. 2018;7(16):e008841. 10.1161/JAHA.118.008841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. 10.1016/S0140-6736(02)07445-7 [DOI] [PubMed] [Google Scholar]
- 27.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]