ABSTRACT
The influence of diet on the gut microbiota is an emerging research area with significant impact on human health and disease. However, the effects of beef, the most consumed red meat in the United States, on gut microbial profile are not well studied. Following Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols, the objective of this systematic review was to conduct a rigorous and thorough review of the current scientific literature regarding the effects of beef protein and the resulting bioactivity of beef protein and amino acids on the gut microbiota, with the goal of identifying gaps in the literature and guiding future research priorities. Utilizing MEDLINE Complete, PubMed, ScienceDirect, Scopus, and Google Scholar databases, we conducted searches including terms and combinations of the following: animal protein, amino acid, beef, bioactive compounds, diet, health, microbiome, peptide, processed beef, and protein. We identified 131 articles, from which 15 were included in our review. The effects of beef on mouse and rat models were mostly consistent for the bacterial phylum level. Short-term (1–4-wk) beef intakes had little to no effect on microbial profiles in humans. Most studies utilized high beef feeding (240–380 g/d), and no study examined recommended amounts of protein [∼3.71 oz/d (105 g/d) meats, poultry, and eggs, or ∼26 oz/week (737 g/wk) from these food sources] according to US dietary guidelines. Additionally, the majority of animal and human studies with adverse findings examined the impact of beef in the context of a diet high in fat or sugar. In conclusion, an extensive gap exists in the literature regarding beef and the microbiota. More studies are necessary to elucidate the role of the microbiota following the consumption of beef, especially in interaction with other dietary compounds, and how beef preparation, processing, and cooking methods differentially influence the biological effects of beef on human health.
Keywords: beef, beef protein, health, microbiota, processed meat, protein, red meat
Introduction
The United States is a leading nation in the consumption of red meat, with 2017 estimates indicating 49.5 kg (109 lb) per capita, which is expected to increase to 50.8 kg (112 lb) by 2027 (1). A recent NHANES evaluation of trends in meat consumption from 1999 to 2016 reported beef as the most abundantly consumed type of red meat in the United States (2). Given the increasing health concerns about red meat consumption (3), it is crucial to understand how the biological functions of the nutrients in red meat, particularly beef, vary and differentially impact health since the nutritional profile of meat varies by the type of meat, for example, white or red meat, processed or unprocessed meat (see Table 1 for defined terminology) (4).
TABLE 1.
Relevant terms related to beef
| Term | Definition |
|---|---|
| Meat | The flesh of an animal as food |
| Processed meat | Meat that has been preserved by methods other than freezing, such as salting, smoking, marinating, air-drying, or heating (e.g., ham, bacon, sausages, hamburgers, salami, corned beef, and tinned meat) |
| Red meat | All types of mammalian muscle meat, such as beef, veal, pork, and lamb (fresh, minced, and frozen) |
| Beef | The flesh of a cow, bull, or ox, used as food |
| Protein | Any of a class of nitrogenous organic compounds that consist of large molecules composed of ≥1 long chains of amino acids and are an essential part of all living organisms, especially as structural components of body tissues such as muscle |
| Peptide | A compound consisting of ≥2 amino acids linked in a chain |
| Amino acid | A simple organic compound containing both a carboxyl (–COOH) and an amino (–NH2) group |
| Protein-derived bioactive compounds | Intermediates of proteolysis or amino acid sequences which exert a beneficial effect on body function and/or positively impact human health, beyond its known nutritional value |
Protein exerts nutritional, functional, and biological properties and plays an essential role in human health. A comprehensive assessment of dietary protein quality includes amino acid composition, digestibility, rate of protein digestion, and potential for generation of biologically active peptides (i.e., bioactive peptides) (5). Upon consumption, animal protein provides all 9 essential amino acids required by the human body. Distinctively, red meat is also a source of heme iron, which has higher bioavailability (i.e., meaning it is more absorbable) compared with nonheme iron found in plants (6), in addition to vitamins, especially B vitamins, and minerals, such as copper, manganese, zinc, and iron (7).
Moreover, recent research suggests that sources of dietary protein (animal compared with plant) and their associated nutrients can differently influence the gut microbiota (8–10), which is recognized as an important mediator between food and host (11) that can instigate or prevent chronic and metabolic diseases, including cancer and cardiovascular disease (12, 13). The human intestinal tract houses 10 trillion microorganisms, including bacteria, viruses, fungi, and protozoa. Of these, the intestinal bacterial profile, known as the “gut microbiota,” is of significant interest, given its role in human disease (14, 15). Differentiation of the microbiota—or specific changes in particular microbes or groups of microbes—alters gut homeostasis. Gut dysbiosis is characterized by adverse disruptions in microbial profile (16), which increase systemic inflammation through leaks of inflammatory substances from the gut (such as bacterial LPSs). Inflammation further contributes to increased risk of metabolic and chronic diseases, such as obesity, diabetes, and cancer (14, 17).
There are 5 major bacterial phyla in the human digestive tract, including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia. Firmicutes (Gram-positive) and Bacteroidetes (Gram-negative) constitute the majority, making up ∼65% of total bacteria (18). Resident microorganisms of the human gut vary among individuals based on several factors, including mode of birth, sex, age, health, body weight, diet, physical activity, and medicinal history, particularly the use of antibiotics (19). Changes in diet rapidly alter colonic microflora, indicating that diet can strongly influence microbiota, even more than host genetics (20, 21). Because these bacteria are primarily responsible for breaking down indigestible starches, several published studies have focused on the microbiota's response to dietary carbohydrate (22, 23). Additionally, human studies have also focused on the response of the microbiota to quantity and quality of dietary fat intake (24, 25). However, less is known regarding the microbiota's response to and digestion of protein.
Protein and the microbiota
Colonic microbes present considerable proteolytic power. Metabolic activity of the gut microbial community is perhaps even more efficacious than that which occurs in the small intestine under enzymatic control of the host (26, 27). In the gastrointestinal tract, proteins are first hydrolyzed by peptidases to polypeptides and further to sequences of amino acids—tripeptides, dipeptides, and single amino acids. Bacterial proteases can generate small peptides and single amino acids that can be fermented to produce SCFAs, including acetate, propionate, and n-butyrate, as well as derivatives of branched-chain amino acids, branched-chain fatty acids, which include isobutyrate, isovalerate, and 2-methylbutyrate (28, 29). The amino acids Arg, Asp, Gly, Phe, Pro, Ser, Thr, and Trp more likely undergo bacterial, rather than host, digestion (30). Although digestion and absorption of dietary protein is efficient in healthy humans, ∼10% reaches the large intestine and is available for bacterial fermentation (31, 32). Early human fecal culture–based microbiology techniques identified Bacteroides and Propionibacterium as two dominant proteolytic genera (28). Other proposed important genera include Bifidobacterium, Clostridium, and Streptococcus (28, 33).
Many factors can influence the proportion of dietary protein that reaches and is digested by bacteria in the large intestine. However, protein modifications during cooking, as well as interactions with other dietary nutrients, can alter protein bioavailability (34). Additionally, microbial enzymes use different cleavage sites than digestive enzymes and thus produce different peptides with different biological activity (35).
Interestingly, recent studies indicate that bacterial presence in the human digestive tract might mediate the production of toxic compounds from proteins, such as the production of trimethylamine from l-carnitine via microbial metabolism (36). Thus, the interactions of colonic microflora and dietary protein have prompted significant interest in their implications for human health since the microbial profile is impacted by diet, and the activity of microbial enzymes impacts the production of protein-derived bioactive compounds.
Dietary protein from beef
Protein-derived bioactive compounds are usually low-molecular-weight peptides (<5 kDa), which are either intermediate products of proteolysis (protein degradation), or amino acid sequences within the protein that, upon isolation, exert a beneficial effect on body function with potential positive impacts on human health, beyond any known nutritional value (37). There are 3 ways in which bioactive peptides can be generated: 1) during digestion via digestive enzymes; 2) during digestion via microbial enzymes; and 3) during food processing or ripening using purified or microbial enzymes (38, 39). Both digestion site and responsible enzyme result in various peptides, thus altering the bioactivity (35). Processes that generate peptides, and limitations of and opportunities within these processes, have been extensively reviewed (40). Although protein requirements are well established—the RDA for protein is 0.8 g/kg body weight/d, with an intake of ≤2.5 g/kg body weight/d being acceptable (41)—there are no federal/medical recommended values for protein-derived bioactive compounds, and little information regarding requirements and relational health benefits.
Protein-derived peptides have various bioactive properties, including antihypertensive, mineral-binding, antimicrobial, immunomodulating, cell-modulating, anticarcinogenic, anti-inflammatory, and cholesterol-lowering properties (40, 42–45). Widely studied, the role of biologically active peptides from dietary protein sources, such as milk (46), fish (47), seaweed (43), and soybeans (48), in human health is established; however, that of bioactive compounds from protein-rich foods, including beef, is less known.
Muscle (meat)-derived bioactive peptides and amino acids include anserine (β-Ala-1-methyl-His), carnosine (β-Ala-His), l-carnitine (β-hydroxy-γ-trimethylaminobutyric acid), glutathione (γ-Glu-Cys-Gly), and taurine (49–51). Of these, the histidine-containing dipeptides, carnosine (β-Ala-His) and anserine (β-Ala-1-methyl-His), are the major dipeptides present in mammalian skeletal muscle (49). A limited number of studies exist on the presence of amino acids and peptides present in beef, and those that do present incomplete information and inconsistent findings. Onlyone study on beef (52) has sought to determine all (proteinogenic and nonproteinogenic) amino acids and small peptide content in beef, and reported glutamine to be the most abundant amino acid, followed by taurine, alanine, glutamate, and β-alanine (52). Postmortem protein degradation results in the production of polypeptide fragments, which can be further hydrolyzed via peptidyl peptidases and aminopeptidases to generate smaller peptides and individual amino acids (53). Concentrations of these bioactive dipeptides are lower in cooked beef meat compared with fresh muscle (54, 55); however, cooked beef is still a substantial source of carnosine and anserine (55).
It is critical to understand how individual strains of the gut microbiota respond, as well as how they interact with one another as functional groups when exposed to carbohydrate, fat, and protein, and how, in turn, these changes impact the production of their enzymatic products. Therefore, through this systematic review, we conducted a rigorous and thorough review of the current scientific literature regarding the effects of beef consumption on gut microbiota; beef was chosen because it is the most abundantly consumed red meat in the United States. We examined the bioactivity of beef proteins and amino acids on gut microbiota and identified gaps within the literature to guide future research priorities.
Methods
This systematic review referred and followed checklists (Supplemental Table 1) provided by the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (56). The literature search identified animal and human studies published from database inception to July 31, 2019. We collected peer-reviewed, English-written articles from: 1) MEDLINE Complete, 2) PubMed, 3) ScienceDirect, 4) Scopus, and 5) Google Scholar. Search terms included combinations of the following: animal protein, amino acid, beef, bioactives, diet, health, microbiome, peptide, processed beef, and protein. We retrieved and reviewed relevant articles, and from those we closely analyzed references to identify any missed articles. Articles containing a “red meat” descriptor, where the study specified the red meat was composed of beef as the primary protein source, were included in this systematic review (Figure 1). Articles that did not include beef as a food type were excluded. Three independent reviewers determined article eligibility.
FIGURE 1.
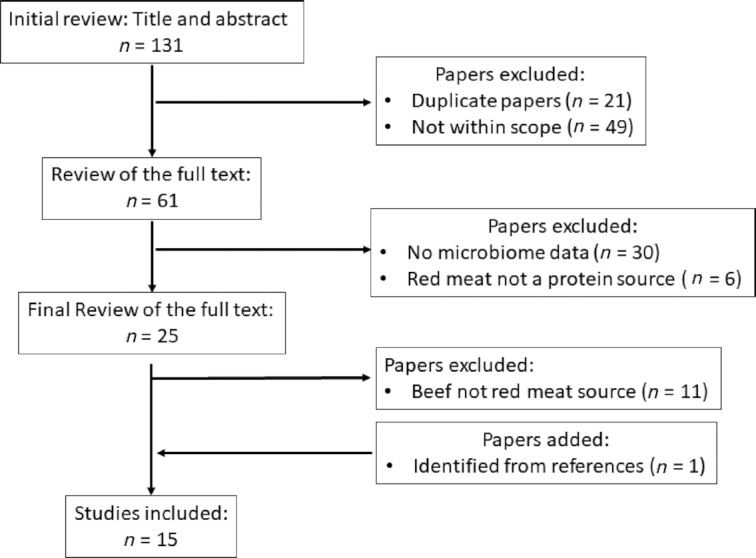
PRISMA flow diagram representing overview of literature selection process for inclusion in systematic review. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
Overall, there has been a minimal number of studies that have examined the role of beef protein on the microbiota. Given our criteria, our search yielded a total of 15 eligible articles and included 10 animal and 5 human studies.
Beef protein and gut microbiota: animal studies
The effects of beef on mouse and rat models are consistent for bacterial phylum level (Table 2), with increases in the relative abundance of Proteobacteria (10, 57), and Firmicutes (10, 57, 58) and decreases in Bacteroidetes (10, 57). The lactic acid–producing genus, Lactobacillus, increased when just beef extract was fed (10, 58). Our literature search also indicates that beef feeding increases Proteobacteria both in mice and pig models (59). See Table 3 for findings from pig studies. Beef consumption showed a mixed impact on some of the SCFA-producing bacterial genera. For example, beef increased the genera Clostridium (57, 60) and Blautia (58, 61), whereas Bifidobacterium (62) and Akkermansia (61, 62) decreased. All mouse and rat studies were conducted on males, emphasizing the need for further studies on female animal models.
TABLE 2.
Effects of beef feeding on the microbiota of male mice and rats1
| Impact on microbial community | |||||||
|---|---|---|---|---|---|---|---|
| Animal model | n | Beef diet | Other diet | Duration, d | Increased | Decreased | Reference |
| C57BL/6 mice | 40 | Freshly prepared steamed beef | Nonpurified diet | 56 | ↑Firmicutes (P)↑Oscillospira (G)↑Clostridium (G)↑Proteobacteria (P)↑Escherichia (G) | ↓Bacteroidetes (P)↓Prevotella (G) | 57 |
| C57BL/6 mice | 60 | LFB (12% kcal), and HFB (60% kcal) | Casein | 84 | Changes in LFB:Verrucomicrobia (P) ↑Akkermansia (G)Deferribacteres (P) ↑Deferribacteraceae (F) ↑Mucispirillum (G)Proteobacteria (P) ↑Desulfovibrionaceae (F)Bacteroidetes(P) ↑Bacteroidaceae (F)Changes in HFB:Deferribacteres (P) ↑Mucispirillum (G)Proteobacteria (P) ↑Escherichia (G) ↑Shigella (G)Tenericutes (P) ↑Mollicutes (C)Firmicutes (P) ↑Blautia (G) ↑Romboutsia (G) ↑Oscillibacter (G)Bacteroidetes (P) ↑Odoribacter (G) | Changes in HFB:Verrucomicrobia (P)*↓Akkermansia (G)Firmicutes (P)*↓Anaerotruncus (G) *↓Butyricicoccus (G) *↓Lactobacillus (G) | 61 |
| Sprague Dawley rats | 32 | Proteins were extracted from beef muscle, cooked at 72°C, freeze-dried, and ground into powder | Casein | 90 | Fusobacteria (P) ↑Fusobacterium (G)↑Bacteroidetes (P) | ↓ Firmicutes (P) | 83 |
| Sprague Dawley rats | 55 | Proteins were extracted from beef muscle, cooked at 72°C, freeze-dried, and ground into powder | Casein | 14 | *↑Firmicutes (P)*↑Allobaculum (G)*↑Blautia (G)*↑Lactobacillus (G) | Bacteroidetes (P)*↓Bacteroides (G)Firmicutes (P)*↓Lachnospiraceae (F) | 58 |
| Sprague Dawley rats | 66 | Proteins were extracted from beef muscle, cooked at 72°C, freeze-dried, and ground into powder | Casein | 90 | ↑Firmicutes (P)↑Lactobacillus (G)↑Proteobacteria (P) | Firmicutes (P)*↓Roseburia (G)↓Bacteroidetes (P) *↓Alloprevotella (G) | 10 |
| SPF Wistar rats | 60 | Powdered beef: a lean cut of beef was chosen, minced, and dehydrated in a drying oven, with air circulating at 105°C, for 3–4 h. The dried meat was then ground to a fine “flour” | Casein | 60 | Firmicutes (P)*↑Clostridium (G) *↑Enterococcus spp. (G)Proteobacteria (P) *↑Enterobacteriaceae (F)Bacteroidetes (P) *↑Bacteroides (G) | Firmicutes (P)*↓Lactobacillus (G) | 60 |
| BALB/c mice | 32 | Cooked red meat at a concentration of 30 g/100 g diet, and cooked red meat at a concentration of 30 g/100 g diet mixed with high-amylose maize starch at a concentration of 10 g/100 g diet | Casein | 12 | ↑Bacteroides—Prevotella groupProteobacteria (P) ↑Escherichia coli | Verrucomicrobia (P) ↓Akkermansia muciniphila Actinobacteria (P) ↓Bifidobacterium spp.Bacteroidetes (P) ↓Parabacteroides distasonis Firmicutes (P) ↓Clostridium leptum ↓Faecalibacterium prauznitzii ↓Ruminococcus gnavus ↓Enterococcus spp. (G) | 62 |
C, class; F, family; G, genus; HFB, high-fat beef; LFB, low-fat beef; O, order; P, phylum. Arrows indicate changes reported: ↑, increased; ↓, decreased. Taxa (phyla) without arrows are listed for clarification. *Significant change, P < 0.05. Arrows without * indicate changes based on relative abundance.
TABLE 3.
Effects of beef feeding on the microbiota of pigs1
| Impact on microbial community | |||||||
|---|---|---|---|---|---|---|---|
| Animal model | n (sex) | Beef | Other diet | Duration, wk | Increase | Decrease | Reference |
| Pigs (German Landrace × Large White × Piétrain) | 45 (F) | Cooked lean beef | Lupin protein isolate as source of a plant protein and casein | 4 | ↑Proteobacteria (P) | ↓Actinobacteria (P) | 59 |
| Pigs (Large White) | 20 (M) | Beef steak (trimmed of fat, cooked, minced, and dried) | Arabinoxylan + beef | 4 | Firmicutes (P) ↑Ruminococcus*(G)↑Clostridium* (G) | Bacteroidetes (P) ↓Prevotella ruminicola* ↓Prevotella disiens *Firmicutes (P) ↓Faecalibacterium* (G) | 96 |
G, genus; P, phylum. Arrows indicate changes reported based on relative abundance: ↑, increased; ↓, decreased. *Significant change, P < 0.05. Taxon (phylum) listed without arrow for clarification.
Beef protein and gut microbiota: human studies
Of the 5 human studies that were relevant to our topic of beef protein and gut microbiota in humans, a considerable variation existed on the influence that beef has on the gut microbiota (Table 4); most likely this is a result of differences in objectives, study design, intervention duration, subject characteristics, beef portion, and diet composition. For example, subjects ranged from breastfed infants (63) to endurance athletes (64). Consistently, short-term (1–4-wk) beef intakes had little to no effect on microbial profile (65–67). Most studies utilized high beef feeding (240–380 g/d) (65–67), and no study examined recommended amounts of protein according to Dietary Guidelines for Americans (68).
TABLE 4.
Effects of beef feeding on the microbiota of humans1
| Impact on microbial community | |||||||
|---|---|---|---|---|---|---|---|
| n (M/F) | Participants’ age | Beef | Other diet | Duration | Increase | Decrease | Reference |
| 23 (17/6) | 62.4 y | HRM (300 g/d), and HRM + HAMSB | Crossover design | 4 wk/diet | HRM:NCŦHAMSB:Firmicutes (P)*↑Ruminococcus bromii*↑Clostridium coccoides group*↑Clostridium leptum group*↑Lactobacillus spp.Bacteroidetes (P) *↑Parabacteroides distasonis | HRM:NCŦHAMSB:Proteobacteria (P) *↓Escherichia coli Firmicutes (P) *↓Ruminococcus gnavus*↓Ruminococcus torques | 66 |
| 27 (18/9) | 40–85 y | PRM (240 g/ d), and RM (240 g) | No meat | 1 wk | PRM:NCRM:NC | PRM:Actinobacteria (P) ↓Bifidobacterium(G)RM:NC | 67 |
| 45 (N/A) | 5 mo | Pureed red meat (71 g/ d) | Fortified cereal | 20 wk | ↑Firmicutes (P)*↑Clostridium group XIVa | ↓Proteobacteria (P)↓Enterobacteriaceae (F) | 63 |
| Iron-fortified cereal | 20 wk | *↑Actinobacteria (P)Firmicutes (P)*↑Bacilli (C) | *↓Bacteroidetes (P) | ||||
| 10 (10/0) | N/A | High red meat (380 g) | No meat | 4 wk/diet | Actinobacteria (P)*↑Bifidobacterium adolescentis Bacteroidetes (P)*↑Bacteroides (G) ↑Bacteroides fragilis | — | 65 |
| 18 (18/0) | 18–45 y | Beef hydrolysate (10 g) + whey isolate (10 g) | Carbohydrate | 10 wk | ↑Bacteroidetes (P)↑Bacteroides (G) | Firmicutes (P)*↓Bacilli(C)*↓Lactobacillales | 64 |
| Actinobacteria (P)*↓Bifidobacterium longumProteobacteria(P) ↓Citrobacter(G)*↓Synergistetes(P)*↓Synergistia(C)*↓Synergistales(O)Firmicutes(P)*↓Blautia(G)*↓Coprococcus(G)*↓Lachnospiraceae(F)*↓Roseburia(G) | |||||||
C, class; F, family; G, genus; HAMSB, high-lean red meat + butyrylated high-amylose maize starch; HRM, high-lean red meat; NCŦ, no changes between baseline and following 4 wk of HRM; O, order; P, phylum; PRM, processed red meat; RM, red meat. Arrows indicate reported changes: ↑, increased; ↓, decreased. Taxa (phyla) without arrows are listed for clarification. *Significant change, P < 0.05. Arrows without * indicate changes based on relative abundance.
Discussion
Animal meat is a complete source of dietary protein, composed of all essential amino acids, and is also a source of fatty acids, vitamins, and minerals (69). Furthermore, animal protein promotes satiety and enhances energy expenditure and fat loss compared with plant proteins (70). Observational studies have reported red meat consumption as a risk factor for cardiovascular disease and other metabolic diseases, such as type 2 diabetes mellitus, although recent studies indicate that this link might only exist with the consumption of processed red meat (3). Respective of the controversial health findings, the majority of studies included in this review examined the impact of excessive intakes of beef in the context of a diet high in fat or sugar. This type of diet, sometimes identified as the Western diet or standard American diet, is independently associated with increased chronic noncommunicable disease risk (71), and is often associated with a high intake of refined and processed foods, including processed meats and simple (added) sugars and fat and low intake of fruits, vegetables, and whole grains. Furthermore, a recent clinical investigation reported that ultraprocessed foods increase weight gain independent of energy intake (72), highlighting the need to better understand the association of unprocessed and processed red meat with chronic disease risk (73, 74), particularly regarding the role of gut microbiota and related metabolites in mediating effects of lean beef or beef proteins.
In conjunction with a diet high in fat or sugar, beef protein can adversely affect health, microbial composition, and the gut barrier when compared with casein or white meat protein (61, 75), for example, by increasing numbers of the Proteobacteria (75), which are associated with dysbiosis (76). However, changes in bacterial composition in response to high-fat + beef and high-sucrose + beef feedings are comparable with those reported in Westernized diet feedings in animal studies, with increases in Desulfovibrionaceae and decreases in Lactobacillaceae (77, 78). Interestingly, high-fat diet–related decreases in Bacteroidetes and increases in both Firmicutes and Proteobacteria are independent of obesity (78), implying the influence of diet composition, particularly fat, in the microbial response. Moreover, there were no significant differences in serum indicators of health or microbial profile when various protein sources (beef, casein, soy) were consumed in conjunction with a low-fat diet (61).
Gram-negative bacteria, such as Bacteroidetes, produce LPS, (79, 80), an endotoxin that drives systemic inflammation and metabolic endotoxemia by upregulating proinflammatory cytokines if leaked into circulation from the intestine (81, 82). Few animal studies reported decreased relative abundance of Bacteroidetes in response to beef feeding compared with a nonpurified diet (57) or casein diet (10, 58). Additionally, LPS-binding protein, an indicator of gut barrier damage, was highest in the casein group when compared with other low-fat groups (61). Therefore, high-fat diets could be of greater concern than dietary protein sources for adversely impacting microbial profile.
In animal studies, beef feeding increased the relative abundance of Firmicutes (10, 57, 58), while decreasing Bacteroidetes (10, 57). In other words, red meat can increase the Firmicutes/Bacteroidetes ratio, which is often associated with increased BMI in human subjects (84, 85). Related findings were variable in the few human studies we identified in our literature search, with contradictory changes in the relative abundance of Firmicutes being reported (63, 64). Additionally, bacterial cultures following high-beef feeding (380 g/d) in humans indicated significant increases in Bacteroides (65), a genus of the Bacteroidetes phylum. According to the 2015–2020 Dietary Guidelines for Americans, 26 oz/wk (737 g/wk), or ∼3.71 oz/d (105 g/d), from protein foods, including meats, poultry, and eggs, is recommended. Assuming that 1 oz (28.35 g) of meat contains ∼7 g protein, this equates to ∼26 g protein/d from this food type (68); however, protein recommendations can vary according to need, and thus more protein could be consumed from this food group to meet upper protein recommendations (2.5 g/kg/d) based on body weight, for example (41). Gaps between protein recommendations and amounts used in human studies denote a need for evaluating the impact of protein quality and quantity on human microbiota, especially in populations with elevated protein intakes, such as athletes (86).
Food processing, preparation, and storage affect the nutritional, functional, and biological properties and digestibility of protein. In vitro studies of human fecal batch cultures indicate that meat type and cooking method both impact microbial profile (87). Additionally, adverse findings associated with excessive beef intake include those related directly to cooking at high temperatures, which can result in the production of polycyclic aromatic hydrocarbons and heterocyclic amines (88), N-nitroso compounds generated as microbial byproducts, or the presence of the nonhuman sialic acid N-glycolylneuraminic acid (89).
Moreover, mechanisms linking beef and the development of chronic noncommunicable diseases are not fully understood but could be related to micronutrients because iron and zinc have reportedly influenced microbial profile (63). For example, several studies have examined the effect of heme iron on health and microbial composition; however, these cannot be translated directly to beef feeding (90). Additionally, even though iron content can be standardized when comparing protein diets, animal and plant proteins comprise different types of iron (heme and nonheme, respectively), which can differentially impact microbial profile. Accordingly, heme studies and animal protein studies have conflicting results—elevated heme intake results in decreased Lactobacillus and increased Proteobacteria (91), whereas proteins extracted from beef have the opposite effect on Lactobacillus (10, 58). Lactobacillus is considered a key player in host energy metabolism (92, 93) and in reducing inflammation by positively shifting the gut microbial profile and protecting the gut barrier (82, 94, 95). These findings
emphasize the impact of individual components of beef (i.e., heme compared with proteins extracted from beef) compared with beef as a whole on microbial profiles. Animal studies indicate significant decreases in Lactobacillus with high-fat beef feeding compared with casein (61), and beef feeding compared with chicken (75). A systematic review of adverse compounds associated with red meat suggests that better designed and controlled studies that use relevant concentrations of meat or meat-derived compounds in conjunction with diets representative of human diets are needed (97). Furthermore, approaches to prevent the toxicity associated with red meat consumption have been explored, for example, by increasing calcium in the diet, altering meat processing, or adding vitamin E (98). As these studies evolve, future microbiome and microbiota studies need to include the effects of these combinations, processing methods, and additives in regard to beef consumption.
Future Perspectives
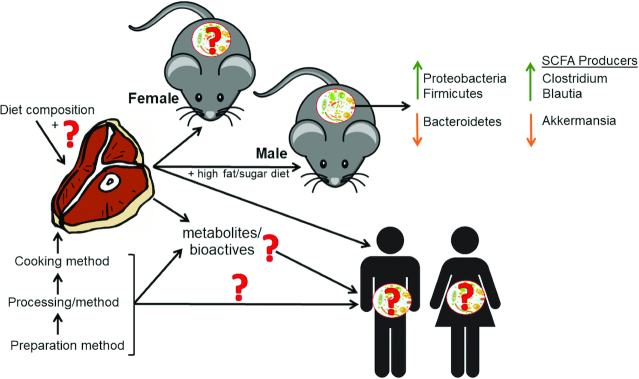
More studies are necessary to elucidate the role of the gut microbiota following the consumption of beef, especially in interaction with other food compounds, particularly fat and carbohydrate (Figure 2). Studies are needed in both animals and humans to understand how different beef preparation, processing, and cooking methods differentially influence the biological effects of beef. Further, animal protein sources vary in composition, for example, by fatty acid profile, which is largely influenced by the diet of the animals (99) and is an important consideration for studies comparing microbial changes and chronic disease risk in response to diet (100). These diets must be standardized in fatty acid profile and micronutrients, for example. Moreover, several techniques have been utilized to understand microbial digestion of dietary protein and the resulting bioactive compounds; however, there is a need to develop more cost-effective and appropriate techniques because there are many factors that influence this process, including the presence of other macro- and micronutrients (40).
FIGURE 2.
Extensive gaps exist in the literature regarding beef, its related bioactive compounds, and effects on gut microbiota. Various preparations, processing and cooking methods, and temperatures are utilized in the examined literature, which likely impact the bioactivity of beef. Research subjects varied in age and models used from rodents to human subjects. The effects of beef on the male mice and rats were mostly consistent, indicating increased Proteobacteria and Firmicutes and decreased Bacteroidetes. Findings regarding effects on SCFA-producing bacterial genera were discrepant, with increases in Clostridium and Blautia and decreases in Akkermansia. Short-term (1–4-wk) beef intakes had little to no effect on microbial profiles in humans. Most studies with adverse findings (animal and human studies) examined the impact of red meat or excessive intakes of red meat in the context of a diet high in fat or sugar.
Additionally, microbiome-related metabolomics studies are needed to determine the impact of beef consumption on changes in gut microbiota and how these correlate with host physiology and health. Moreover, how these diets affect metabolism in a tissue-specific manner is not well studied in animal models. Further mechanistic studies investigating the role of beef-associated bioactive compounds, in the context of various diets, are needed. For example, l-carnitine has been shown to ameliorate the negative influence of high-fat diets on the lipid profile in rats (101) but has also been shown to increase the production of toxic byproducts (36). However, the metabolism of these protein-related compounds largely depends on the colonic microbiota composition and its metabolic influence; therefore, paired analyses of protein-derived bioactive compounds and microbially related metabolites should be considered.
SCFAs are the end-products of carbohydrate and protein microbial digestion in the large intestine, which can positively impact health (102). Production of SCFAs varies by microbiota profile—bacteria produce different SCFAs based on substrate preference—and also by the undigested substrate that enters into the large intestine (103–105). To our knowledge, only 2 animal studies (by the same research group) have compared different protein extracts from different sources on SCFA production in conjunction with changes in microbial composition (58, 83). Each reported higher SCFA production following consumption of soy protein compared with protein extracted from chicken and beef (58, 83); however, this could be due to the limited bioavailability of soy compared with meat protein (70). Several animal studies have demonstrated that resistant starch, in addition to red meat, decreased the risk of colon cancer (106–108). A similar human study by the same group reported increased SCFA production and changes in microbial profile following a high-beef (300 g/d) + butyrylated high-amylose maize starch diet for 4 wk (66). Interestingly, pureed beef as a complementary food in otherwise breastfed infants increased fecal SCFA-producing bacteria (63), which could have implications in the healthy development of the infant microbiota, nutrient metabolism, and immune system development (109). Future studies are needed to investigate the long-term combined effects of starch and beef proteins in recommended amounts on microbial composition and respective SCFA production, and, further, to investigate the impact of this combination in overweight individuals or in the context of a high-fat/high-sucrose diet.
Conclusions
In conclusion, when consumed at higher than recommended levels as part of a diet high in sugar or fat, beef has adverse consequences for the gut microbiota. Human studies indicate minimal changes in the gut microbial profile in response to short-term (1–4-wk) beef feeding. Future research is needed to: 1) elucidate changes in the microbiota in response to the consumption of beef, itself, in recommended and excessive amounts according to dietary guidelines, and in combination with other nutrients; 2) conduct microbiome-related metabolomics studies to understand how changes in microbiota correlate with host physiology; 3) investigate the role of beef-derived bioactive compounds in the context of various diets; 4) determine if other nutrients, such as complex carbohydrates, can ameliorate some negative effects of increased beef consumption as part of high-fat and/or high-sucrose diets; 5) identify alterations in SCFA production with beef consumption; and 6) develop more cost-effective and appropriate techniques to understand microbial digestion of dietary protein, including food types such as beef.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the National Cattleman's Beef Association, a contractor of The Beef Checkoff, for funding this work. We also thank Cynthia Henry for her guidance as well as support from the College of Human Sciences and the Obesity Research Institute.
The authors’ responsibilities were as follows—KA-S and NM-M: designed the study; KA-S, TI, and PJ: conducted the literature search and collected data; KA-S: wrote the initial draft, which was modified after feedback from all coauthors; NM-M: had primary responsibility for content; and all authors: read and approved the final manuscript.
Notes
KA-S, TI, and PJ were funded in the writing of this review by the National Cattleman's Beef Association (NCBA), a contractor of The Beef Checkoff (principal investigator: NM-M). The NCBA approved our proposed outline for this systematic review, but had no role in the writing or editing of this manuscript or the report on findings or discussion.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Contributor Information
Kembra Albracht-Schulte, Department of Nutritional Sciences and Obesity Research Institute, Texas Tech University, Lubbock, TX, USA.
Tariful Islam, Department of Nutritional Sciences and Obesity Research Institute, Texas Tech University, Lubbock, TX, USA.
Paige Johnson, Department of Nutritional Sciences and Obesity Research Institute, Texas Tech University, Lubbock, TX, USA.
Naima Moustaid-Moussa, Department of Nutritional Sciences and Obesity Research Institute, Texas Tech University, Lubbock, TX, USA.
References
- 1. USDA USDA agricultural projections to 2027. Washington (DC): United States Department of Agriculture; 2018. [Google Scholar]
- 2. Zeng L, Ruan M, Liu J, Wilde P, Naumova EN, Mozaffarian D, Zhang FF. Trends in processed meat, unprocessed red meat, poultry, and fish consumption in the United States, 1999–2016. J Acad Nutr Diet. 2019;119(7):1085–98.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125(5-6):507–25. [DOI] [PubMed] [Google Scholar]
- 5. Millward DJ, Layman DK, Tome D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87(5):1576S. [DOI] [PubMed] [Google Scholar]
- 6. Biesalski HK Meat as a component of a healthy diet – are there any risks or benefits if meat is avoided in the diet?. Meat Sci. 2005;70(3):509–24. [DOI] [PubMed] [Google Scholar]
- 7. Friedman M Nutritional value of proteins from different food sources. a review. J Agric Food Chem. 1996;44(1):6–29. [Google Scholar]
- 8. Lang JM, Pan C, Cantor RM, Tang WHW, Garcia-Garcia JC, Kurtz I, Hazen SL, Bergeron N, Krauss RM, Lusis AJ. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio. [Internet]2018;9(6). doi:10.1128/mBio.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reese AT, Pereira FC, Schintlmeister A, Berry D, Wagner M, Hale LP, Wu A, Jiang S, Durand HK, Zhou Xet al. Microbial nitrogen limitation in the mammalian large intestine. Nat Microbiol. 2018;3(12):1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep. 2015;5:15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6(2):320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao Het al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Logan AC, Jacka FN, Prescott SL. Immune-microbiota interactions: dysbiosis as a global health issue. Curr Allergy Asthma Rep. 2016;16(2):13. [DOI] [PubMed] [Google Scholar]
- 17. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana Det al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costea PI, Hildebrand F, Arumugam M, Backhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich SD, Fraser CM, Hattori Met al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MCet al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MAet al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SSet al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio. [Internet]2017;8(5). doi:10.1128/mBio.01343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Darko KO, Huang Y, He C, Yang H, He S, Li J, Li J, Hocher B, Yin Y. Resistant starch regulates gut microbiota: structure, biochemistry and cell signalling. Cell Physiol Biochem. 2017;42(1):306–18. [DOI] [PubMed] [Google Scholar]
- 24. Fava F, Gitau R, Griffin B, Gibson G, Tuohy K, Lovegrove J. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int J Obes. 2013;37(2):216–23. [DOI] [PubMed] [Google Scholar]
- 25. Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25(3):270–80. [DOI] [PubMed] [Google Scholar]
- 26. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. [DOI] [PubMed] [Google Scholar]
- 27. Gibson SA, McFarlan C, Hay S, MacFarlane GT. Significance of microflora in proteolysis in the colon. Appl Environ Microbiol. 1989;55(3):679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132(6):1647–56. [DOI] [PubMed] [Google Scholar]
- 29. Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997;3(5):327–37. [DOI] [PubMed] [Google Scholar]
- 30. Rowan AM, Moughan PJ, Wilson MN, Maher K, Tasman-Jones C. Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man. Br J Nutr. 1994;71(1):29–42. [DOI] [PubMed] [Google Scholar]
- 31. Chacko A, Cummings JH. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988;29(6):809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gibson JA, Sladen GE, Dawson AM. Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br J Nutr. 1976;35(1):61–5. [DOI] [PubMed] [Google Scholar]
- 33. Van der Meulen R, Camu N, Van Vooren T, Heymans C, De Vuyst L. In vitro kinetic analysis of carbohydrate and aromatic amino acid metabolism of different members of the human colon. Int J Food Microbiol. 2008;124(1):27–33. [DOI] [PubMed] [Google Scholar]
- 34. Tuohy KM, Hinton DJ, Davies SJ, Crabbe MJ, Gibson GR, Ames JM. Metabolism of Maillard reaction products by the human gut microbiota—implications for health. Mol Nutr Food Res. 2006;50(9):847–57. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto N Antihypertensive peptides derived from food proteins. Biopolymers. 1997;43(2):129–34. [DOI] [PubMed] [Google Scholar]
- 36. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des. 2003;9(16):1309–23. [DOI] [PubMed] [Google Scholar]
- 38. Schrezenmeir J, Korhonen H, Williams CM, Gill HS, Shah NP. Foreword. Br J Nutr. 2000;84(1):1.10961153 [Google Scholar]
- 39. Daliri EB, Oh DH, Lee BH. Bioactive peptides. Foods [Internet]. 2017;6(5). doi:10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chakrabarti S, Guha S, Majumder K. Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018;10(11):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): National Academies Press; 2005. [Google Scholar]
- 42. Möller NP, Scholz-Ahrens KE, Roos N, Schrezenmeir J. Bioactive peptides and proteins from foods: indication for health effects. Eur J Nutr. 2008;47(4):171–82. [DOI] [PubMed] [Google Scholar]
- 43. Admassu H, Gasmalla MAA, Yang R, Zhao W. Bioactive peptides derived from seaweed protein and their health benefits: antihypertensive, antioxidant, and antidiabetic properties. J Food Sci. 2018;83(1):6–16. [DOI] [PubMed] [Google Scholar]
- 44. Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rutherfurd-Markwick KJ Food proteins as a source of bioactive peptides with diverse functions. Br J Nutr. 2012;108(Suppl 2):S149–57. [DOI] [PubMed] [Google Scholar]
- 46. Giromini C, Cheli F, Rebucci R, Baldi A. Invited review: dairy proteins and bioactive peptides: modeling digestion and the intestinal barrier. J Dairy Sci. 2019;102(2):929–42. [DOI] [PubMed] [Google Scholar]
- 47. Halim NRA, Yusof HM, Sarbon NM. Functional and bioactive properties of fish protein hydrolysates and peptides: a comprehensive review. Trends Food Sci Tech. 2016;51:24–33. [Google Scholar]
- 48. Agyei D Bioactive proteins and peptides from soybeans. Recent Pat Food Nutr Agric. 2015;7(2):100–7. [DOI] [PubMed] [Google Scholar]
- 49. Boldyrev AA, Severin SE. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul. 1990;30:175–94. [DOI] [PubMed] [Google Scholar]
- 50. Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient?. Curr Opin Clin Nutr Metab Care. 2006;9(6):728–33. [DOI] [PubMed] [Google Scholar]
- 51. Rakowska R, Sadowska A, Waszkiewicz-Robak B. Influence of pre- and post-slaughter factors on the reduced glutathione content of beef muscles. Meat Sci. 2017;124:48–53. [DOI] [PubMed] [Google Scholar]
- 52. Wu G, Cross HR, Gehring KB, Savell JW, Arnold AN, McNeill SH. Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts. J Anim Sci. 2016;94(6):2603–13. [DOI] [PubMed] [Google Scholar]
- 53. Etherington DJ Enzymes in food processing. London: Blackie; 1991. [Google Scholar]
- 54. Purchas RW, Rutherfurd SM, Pearce PD, Vather R, Wilkinson BH. Cooking temperature effects on the forms of iron and levels of several other compounds in beef semitendinosus muscle. Meat Sci. 2004;68(2):201–7. [DOI] [PubMed] [Google Scholar]
- 55. Bauchart C, Rémond D, Chambon C, Patureau Mirand P, Savary-Auzeloux I, Reynès C, Morzel M. Small peptides (<5kDa) found in ready-to-eat beef meat. Meat Sci. 2006;74(4):658–66. [DOI] [PubMed] [Google Scholar]
- 56. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 57. Zhang Z, Li D, Tang R. Changes in mouse gut microbial community in response to the different types of commonly consumed meat. Microorganisms. 2019;7(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu Y, Lin X, Li H, Li Y, Shi X, Zhao F, Xu X, Li C, Zhou G. Intake of meat proteins substantially increased the relative abundance of genus Lactobacillus in rat feces. PLoS One. 2016;11(4):e0152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schutkowski A, König B, Kluge H, Hirche F, Henze A, Schwerdtle T, Lorkowski S, Dawczynski C, Gabel A, Große Iet al. Metabolic footprint and intestinal microbial changes in response to dietary proteins in a pig model. J Nutr Biochem. 2019;67:149–60. [DOI] [PubMed] [Google Scholar]
- 60. Bedani R, Pauly-Silveira ND, Roselino MN, de Valdez GF, Rossi EA. Effect of fermented soy product on the fecal microbiota of rats fed on a beef-based animal diet. J Sci Food Agric. 2010;90(2):233–8. [DOI] [PubMed] [Google Scholar]
- 61. Ijaz MU, Ahmed MI, Zou X, Hussain M, Zhang M, Zhao F, Xu X, Zhou G, Li C. Beef, casein, and soy proteins differentially affect lipid metabolism, triglycerides accumulation and gut microbiota of high-fat diet-fed C57BL/6J mice. Front Microbiol. 2018;9:2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Le Leu RK, Young GP, Hu Y, Winter J, Conlon MA. Dietary red meat aggravates dextran sulfate sodium-induced colitis in mice whereas resistant starch attenuates inflammation. Dig Dis Sci. 2013;58(12):3475–82. [DOI] [PubMed] [Google Scholar]
- 63. Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr. 2013;163(2):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moreno-Perez D, Bressa C, Bailen M, Hamed-Bousdar S, Naclerio F, Carmona M, Perez M, Gonzalez-Soltero R, Montalvo-Lominchar MG, Carabana Cet al. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients. 2018;10(3):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hentges DJ, Maier BR, Burton GC, Flynn MA, Tsutakawa RK. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977;37(2):568–71. [PubMed] [Google Scholar]
- 66. Le Leu RK, Winter JM, Christophersen CT, Young GP, Humphreys KJ, Hu Y, Gratz SW, Miller RB, Topping DL, Bird ARet al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: a randomised clinical trial. Br J Nutr. 2015;114(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, Glen RC, Goodman JM, Pollock JR, Bingham SA. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28(3):685–90. [DOI] [PubMed] [Google Scholar]
- 68. US Department of Health and Human Services and US Department of Agriculture 2015–2020 dietary guidelines for Americans. 8th ed[Internet] 2015. Retrieved 2020 Feb 1 from: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/. [Google Scholar]
- 69. Pereira PM, Vicente AF. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93(3):586–92. [DOI] [PubMed] [Google Scholar]
- 70. Gilbert JA, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B16–31. [DOI] [PubMed] [Google Scholar]
- 71. Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. [Internet]2019;11(7). doi:10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey Vet al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klurfeld DM Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015;109:86–95. [DOI] [PubMed] [Google Scholar]
- 74. Binnie MA, Barlow K, Johnson V, Harrison C. Red meats: time for a paradigm shift in dietary advice. Meat Sci. 2014;98(3):445–51. [DOI] [PubMed] [Google Scholar]
- 75. Van Hecke T, De Vrieze J, Boon N, De Vos WH, Vossen E, De Smet S. Combined consumption of beef-based cooked mince and sucrose stimulates oxidative stress, cardiac hypertrophy, and colonic outgrowth of Desulfovibrionaceae in rats. Mol Nutr Food Res. 2019;63(2):e1800962. [DOI] [PubMed] [Google Scholar]
- 76. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. [DOI] [PubMed] [Google Scholar]
- 77. Tachon S, Lee B, Marco ML. Diet alters probiotic Lactobacillus persistence and function in the intestine. Environ Microbiol. 2014;16(9):2915–26. [DOI] [PubMed] [Google Scholar]
- 78. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weiss J Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans. 2003;31(4):785–90. [DOI] [PubMed] [Google Scholar]
- 80. Lindberg AA, Weintraub A, Zahringer U, Rietschel ET. Structure-activity relationships in lipopolysaccharides of Bacteroides fragilis. Rev Infect Dis. 1990;12(Suppl 2):S133–41. [DOI] [PubMed] [Google Scholar]
- 81. Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8(3):946–52. [DOI] [PubMed] [Google Scholar]
- 82. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
- 83. Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, Zhou G. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach Set al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tseng C-H, Wu C-Y. The gut microbiome in obesity. J Formos Med Assoc. 2019;118:S3–9. [DOI] [PubMed] [Google Scholar]
- 86. Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine joint position statement: nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–68. [DOI] [PubMed] [Google Scholar]
- 87. Shen Q, Chen YA, Tuohy KM. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16(6):572–7. [DOI] [PubMed] [Google Scholar]
- 88. Abid Z, Cross AJ, Sinha R. Meat, dairy, and cancer. Am J Clin Nutr. 2014;100(Suppl 1):386S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SLet al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015;112(2):542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63(10):2358–60. [PubMed] [Google Scholar]
- 91. Constante M, Fragoso G, Calvé A, Samba-Mondonga M, Santos MM. Dietary heme induces gut dysbiosis, aggravates colitis, and potentiates the development of adenomas in mice. Front Microbiol. 2017;8(1809):1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marco ML, de Vries MC, Wels M, Molenaar D, Mangell P, Ahrne S, de Vos WM, Vaughan EE, Kleerebezem M. Convergence in probiotic Lactobacillus gut-adaptive responses in humans and mice. ISME J. 2010;4(11):1481–4. [DOI] [PubMed] [Google Scholar]
- 93. Arora T, Anastasovska J, Gibson G, Tuohy K, Sharma RK, Bell J, Frost G. Effect of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in diet-induced obese mice. Br J Nutr. 2012;108(8):1382–9. [DOI] [PubMed] [Google Scholar]
- 94. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo Cet al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 95. Wang W, Li Q, Chai W, Sun C, Zhang T, Zhao C, Yuan Y, Wang X, Liu H, Ye H. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci Nutr. 2019;7(8):2636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Williams BA, Zhang D, Lisle AT, Mikkelsen D, McSweeney CS, Kang S, Bryden WL, Gidley MJ. Soluble arabinoxylan enhances large intestinal microbial health biomarkers in pigs fed a red meat-containing diet. Nutrition. 2016;32(4):491–7. [DOI] [PubMed] [Google Scholar]
- 97. Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: a systematic review of experimental results. Exp Biol Med (Maywood). 2017;242(8):813–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Corpet DE Red meat and colon cancer: should we become vegetarians, or can we make meat safer?. Meat Sci. 2011;89(3):310–6. [DOI] [PubMed] [Google Scholar]
- 99. Smet SD, Raes K, Demeyer D. Meat fatty acid composition as affected by fatness and genetic factors: a review. Anim Res. 2004;53(2):81–98. [Google Scholar]
- 100. McNeill SH, Harris KB, Field TG, Van Elswyk ME. The evolution of lean beef: identifying lean beef in today's U.S. marketplace. Meat Sci. 2012;90(1):1–8. [DOI] [PubMed] [Google Scholar]
- 101. Amin KA, Nagy MA. Effect of carnitine and herbal mixture extract on obesity induced by high fat diet in rats. Diabetol Metab Syndr. [Internet]2009;1(1):17 doi:10.1186/1758-5996-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Adolph TE, Mayr L, Grabherr F, Schwarzler J, Tilg H. Pancreas-microbiota cross talk in health and disease. Annu Rev Nutr. 2019;39:249–66. [DOI] [PubMed] [Google Scholar]
- 103. Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling Met al. Resistant starch: promise for improving human health. Adv Nutr. 2013;4(6):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. van Hylckama Vlieg JE, Veiga P, Zhang C, Derrien M, Zhao L. Impact of microbial transformation of food on health – from fermented foods to fermentation in the gastro-intestinal tract. Curr Opin Biotechnol. 2011;22(2):211–9. [DOI] [PubMed] [Google Scholar]
- 105. Rist VT, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. 2013;7(7):1067–78. [DOI] [PubMed] [Google Scholar]
- 106. Winter J, Nyskohus L, Young GP, Hu Y, Conlon MA, Bird AR, Topping DL, Le Leu RK. Inhibition by resistant starch of red meat-induced promutagenic adducts in mouse colon. Cancer Prev Res. 2011;4(11):1920–8. [DOI] [PubMed] [Google Scholar]
- 107. Toden S, Bird AR, Topping DL, Conlon MA. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer Biol Ther. 2007;6(2):253–8. [DOI] [PubMed] [Google Scholar]
- 108. O'Callaghan NJ, Toden S, Bird AR, Topping DL, Fenech M, Conlon MA. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin Nutr. 2012;31(1):60–4. [DOI] [PubMed] [Google Scholar]
- 109. Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19(9):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.