ABSTRACT
Use of high-fat, ketogenic diets (KDs) to support physical performance has grown in popularity over recent years. While these diets enhance fat and reduce carbohydrate oxidation during exercise, the impact of a KD on physical performance remains controversial. The objective of this work was to assess the effect of KDs on physical performance compared with mixed macronutrient diets [control (CON)]. A systematic review of the literature was conducted using PubMed and Cochrane Library databases. Randomized and nonrandomized studies were included if participants were healthy (free of chronic disease), nonobese [BMI (kg/m2) <30], trained or untrained men or women consuming KD (<50 g carbohydrate/d or serum or whole-blood β-hydroxybutyrate >0.5 mmol/L) compared with CON (fat, 12–38% of total energy intake) diets for ≥14 d, followed by a physical performance test. Seventeen studies (10 parallel, 7 crossover) with 29 performance (13 endurance, 16 power or strength) outcomes were identified. Of the 13 endurance-type performance outcomes, 3 (1 time trial, 2 time-to-exhaustion) reported lower and 10 (4 time trials, 6 time-to-exhaustion) reported no difference in performance between the KD compared with CON. Of the 16 power or strength performance outcomes, 3 (1 power, 2 strength) reported lower, 11 (4 power, 7 strength) no difference, and 2 (power) enhanced performance in the KD compared with the CON. Risk of bias identified some concern of bias primarily due to studies allowing participants to self-select diet intervention groups and the inability to blind participants to the study intervention. Overall, the majority of null results across studies suggest that a KD does not have a positive or negative impact on physical performance compared with a CON diet. However, discordant results between studies may be due to multiple factors, such as the duration consuming study diets, training status, performance test, and sex differences, which will be discussed in this systematic review.
Keywords: ketogenic diet, ketosis, endurance exercise, strength and power, carbohydrate
Introduction
Carbohydrate stored in liver and muscle as glycogen is a readily available energy source used to sustain prolonged physical performance and is the primary fuel for higher-intensity exercise [>80% maximal oxygen uptake (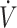 O2max)] (1, 2). To ensure adequate glycogen content, high-carbohydrate diets (≥6 g · kg−1 · d−1) are typically recommended to fuel skeletal muscle during training and competition (3, 4). Even with high carbohydrate intake, glycogen storage capacity is limited to ∼100 g (∼400 kcal) in the liver and ∼300–700 g (∼1200–2800 kcal) in skeletal muscle (5). Glycogen stores can be depleted during a prolonged exercise bout, resulting in fatigue and reduced physical performance (5, 6). Limited capacity to store carbohydrate has resulted in much interest in dietary interventions that spare glycogen use during exercise (7).
O2max)] (1, 2). To ensure adequate glycogen content, high-carbohydrate diets (≥6 g · kg−1 · d−1) are typically recommended to fuel skeletal muscle during training and competition (3, 4). Even with high carbohydrate intake, glycogen storage capacity is limited to ∼100 g (∼400 kcal) in the liver and ∼300–700 g (∼1200–2800 kcal) in skeletal muscle (5). Glycogen stores can be depleted during a prolonged exercise bout, resulting in fatigue and reduced physical performance (5, 6). Limited capacity to store carbohydrate has resulted in much interest in dietary interventions that spare glycogen use during exercise (7).
High-fat, ketogenic diets (KDs; <50 g carbohydrate/d, ∼60–80% kcal from fat, ∼1.2 g protein · kg body mass−1 · d−1) have recently re-emerged as a potential intervention to “spare” glycogen during exercise (8, 9). Consuming a KD results in reduced total carbohydrate oxidation and a 2–3-fold increase in whole-body fat oxidation and lipolysis during steady-state aerobic exercise (10, 11). Within even lean (7–14% body fat) individuals, fat makes up a large energy reserve (∼30,000 kcal), which, if optimized for mobilization, would result in boundless fuel to support physical performance (8). Given the abundance of energy stored as fat in muscle and adipose tissue, and the propensity for a KD to support fat oxidation and slow declines in glycogen (11, 12), consuming a KD has potential ergogenic effects.
Despite considerable research showing a clear increase in fat oxidation and decrease in carbohydrate oxidation following a KD (13), its efficacy to enhance physical performance remains controversial (7, 14). Investigations have yielded conflicting results, reporting no change (15, 16), decrements (17), or improvements in physical performance (18) following a KD. Potential decrements in performance have been attributed to severe carbohydrate restriction (14). Although a KD reduces the rate of decline in glycogen during exercise, this may be due to lower glycogen content at the onset of exercise (11, 12). Lower muscle glycogen is associated with impaired performance of higher intensity (≥80% 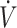 O2max) and potentially long-duration (>3 h) events (1, 2). To avoid negative effects of lower glycogen content, it has been proposed that a KD should be followed for longer durations (>4 wk) to allow for metabolic adaptations supporting enhanced fat metabolism and oxidation to fully occur (8, 19). Additional factors that may contribute to discordant results across studies are participant training status, as highly trained individuals have a higher capacity to oxidize fat (20–22), and performance test, with higher-intensity events relying on glycogen stores for fuel (1). Variability in study design may thus contribute to conflicting results, making it difficult to provide concrete recommendations on the use of a KD to enhance physical performance based on results from any individual study. Therefore, the objective of this systematic review was to aggregate results from multiple investigations to characterize the overall effects of a KD on physical performance and discuss variables that might contribute discordant results between studies.
O2max) and potentially long-duration (>3 h) events (1, 2). To avoid negative effects of lower glycogen content, it has been proposed that a KD should be followed for longer durations (>4 wk) to allow for metabolic adaptations supporting enhanced fat metabolism and oxidation to fully occur (8, 19). Additional factors that may contribute to discordant results across studies are participant training status, as highly trained individuals have a higher capacity to oxidize fat (20–22), and performance test, with higher-intensity events relying on glycogen stores for fuel (1). Variability in study design may thus contribute to conflicting results, making it difficult to provide concrete recommendations on the use of a KD to enhance physical performance based on results from any individual study. Therefore, the objective of this systematic review was to aggregate results from multiple investigations to characterize the overall effects of a KD on physical performance and discuss variables that might contribute discordant results between studies.
Methods
Literature search strategy
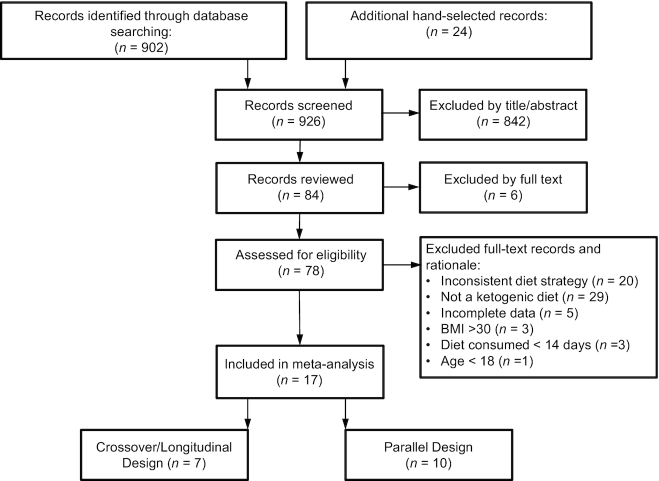
Abstracts of publications identified in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and the Cochrane Library (https://www.cochranelibrary.com/) were reviewed for relevance using the Abstrackr citation program [http://abstrackr.cebm.brown.edu (23)] by 2 researchers (NEM and CTC). A search of all identified terms took place on 15 March 2019 and was not restricted by publication date. Exact search terms are recorded in Supplemental Table 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) search strategy and further reference narrowing is described in Figure 1 (24). Reference lists from these publications were hand-searched for relevant reports missed in the database search. Additional relevant manuscripts were also identified in subsequent searches (17 April 2020) outside of the initial search. Relevant studies that were published after the initial searches were assessed for inclusion in the current analysis. There were no language restrictions, although English search terms were used. Full-text publications were reviewed independently by 2 researchers (NEM and CTC) for physical performance outcomes and KD use. Discrepancies between researchers were assessed by a third investigator (LMM). The final 17 publications were reviewed by all involved researchers. Search strategy details can be found at the University of York Centre for Reviews and Dissemination (PROSPERO) website (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019137926).
FIGURE 1.
PRISMA search strategy diagram. Full-text exclusions listed as having an inconsistent diet strategy contained confounding supplementation, nonketogenic diet (carbohydrate intake >50 g/d), or ketosis unconfirmed with βHB measurement, no mixed macronutrient control group, test diet lasting <14 d, or carbohydrate restoration. βHB, β-hydroxybuterate; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Inclusion criteria
Randomized and nonrandomized crossover/longitudinal or parallel-controlled trials assessing the impact of consuming high-fat, KDs and/or mixed macronutrient control diets (CON) in healthy (without chronic disease), nonobese [BMI (kg/m2) <30], trained or untrained men or women aged >18 y were included in the current analysis. Dietary carbohydrate must have been <50 g/d or ketosis confirmed by circulating β-hydroxybutyrate (βHB) ≥0.5 mmol/L. Diet must have been consumed for ≥14 d.
Exclusion criteria
Studies examining effects of a high-fat diet on obese (BMI >30) or infirm populations (diabetics, epileptics, etc.), children/adolescents (<18 y), and animal models were excluded. Cross-sectional studies were excluded. Studies were excluded if the KD intervention was >50 g carbohydrate/d or ketosis was not confirmed using circulating βHB >0.5 mmol/L, administered for <14 d, replenished with carbohydrate prior to exercise, or had no corresponding control. Studies were excluded if dietary intake was not reported or ketosis was not confirmed. Studies comparing KD with CON for outcomes other than physical performance were excluded from the current review. If a confounding variable, such as a dietary supplement, was provided to 1 treatment but not the other, the study was excluded. If study interventions involved supplements or meal replacements and not focused on whole-diet modifications, they were excluded. If data were missing from a manuscript, the corresponding authors were contacted.
Bias and limitations
A bias analysis was performed by LMM in accordance with PRISMA guidelines recommended by Sterne et al. (25). Ratings including low, unclear, or high risk of selection, performance, attrition, and reporting bias were assigned to each study.
Data extraction
Data were extracted from 17 articles determined to meet the inclusion and exclusion criteria of the current analysis. Studies were identified as being crossover/longitudinal (12, 26–31) or parallel (15–18, 32–37) designs. Sex, training status, age, weight, and 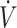 O2max were extracted to provide volunteer descriptive characteristics. Physical performance outcomes were extracted. Performance data were not included if multiple outcome variables were derived from a single performance test. Dietary fat, carbohydrate, and protein intake data were extracted and presented as percentage energy intake and grams consumed per day in the current analysis. Data that were not reported numerically were generated from provided figures by digitally measuring the height of histogram bars and calculating relative to measured y-axis units (38). Aggregated data are presented as means ± SDs.
O2max were extracted to provide volunteer descriptive characteristics. Physical performance outcomes were extracted. Performance data were not included if multiple outcome variables were derived from a single performance test. Dietary fat, carbohydrate, and protein intake data were extracted and presented as percentage energy intake and grams consumed per day in the current analysis. Data that were not reported numerically were generated from provided figures by digitally measuring the height of histogram bars and calculating relative to measured y-axis units (38). Aggregated data are presented as means ± SDs.
Results
Study characteristics
From our literature searches, 926 studies were identified to be screened for inclusion (Figure 1). Of these studies, 17 (10 parallel and 7 crossover/longitudinal) met the inclusion criteria for the current review. Within these 17 studies, 327 individuals participated in the parallel (209; 181 men, 28 women) and crossover (69; 40 men, 29 women) studies (Table 1). Mean dietary intervention duration was 58 ± 27 d (range: 21–84 d) for parallel studies and 43 ± 24 d (range: 21–84 d) for crossover and longitudinal studies. Mean macronutrient intake during parallel studies for the KD was 204 ± 79 g fat/d (72%), 36 ± 9 g carbohydrate/d (6%), and 131 ± 28 g protein/d (22%), and for CON was 69 ± 15 g fat/d (26%), 357 ± 131 g carbohydrate/d (56%), and 105 ± 24 g protein/d (18%) (Table 2). Mean macronutrient intake during crossover/longitudinal studies for the KD was 217 ± 64 g fat/d (73%), 32 ± 10 g carbohydrate/d (5%), and 126 ± 34 g protein/d (22%), and for CON was 100 ± 21 g fat/d (33%), 327 ± 79 g carbohydrate/d (49%), and 124 ± 16 g protein/d (18%).
TABLE 1.
Characteristics of healthy participants and duration of studies included in the systematic review of performance following consumption of ketogenic versus mixed macronutrient control diets by parallel and crossover/longitudinal study designs1
| First author (ref) | Study duration, d | n | Sex | Training status | Age, y | Weight, kg |
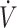 O2max, mL · kg−1 · min−1 O2max, mL · kg−1 · min−1
|
|---|---|---|---|---|---|---|---|
| Parallel study design | |||||||
| Burke et al. (16) | 21 | KD: 8 CON: 10 | M | Elite race walkers | 28 25 | 67 64 | 66 62 |
| Burke et al. (37) | 25 | KD: 10 CON: 8 | 9 M,1 F 5 M, 3 F | Elite race walkers | 29 26 | 67 63 | 61 58 |
| Cipryan et al. (15) | 28 | KD: 8 CON: 9 | M | Moderately trained | 24 24 | 83 84 | 52 52 |
| Dostal et al. (33) | 84 | KD: 12 CON: 12 | 3 M, 9 F 4 M, 8 F | Recreationally active | 25 24 | 67 73 | 46 44 |
| Fleming et al. (17) | 42 | KD: 12 CON: 8 | M | Recreationally active | 36 35 | 79 85 | 44 45 |
| Kephart et al. (35) | 84 | KD: 7 CON: 5 | 5 M, 2 F 4 M, 1 F | CrossFit trainees | 32 29 | 83 77 | NR NR |
| LaFountain et al. (36) | 84 | KD: 15 CON: 14 | 13 M, 2 F 12 M, 2 F | Army ROTC, reservist, Air Force enlisted, National Guard, military veterans | 27 25 | 86 80 | NR NR |
| McSwiney et al. (18) | 84 | KD: 9 CON: 11 | M | Endurance trained | 34 32 | 86 77 | 54 53 |
| Vargas-Molina et al. (34) | 56 | KD: 10 CON: 11 | F | Resistance trained | 27 28 | 62 63 | NR |
| Wilson et al. (32) | 70 | KD: 15 CON: 15 | M | Resistance trained | 24 21 | 80 78 | NR |
| Crossover and longitudinal study design | |||||||
| Greene et al. (26) | 84 | 12 | 7 M, 5 F | Olympic weight lifters | 35 | 78 | NR |
| Heatherly et al. (27) | 21 | 8 | M | Runners | 40 | NR | 49 |
| Phinney et al. (12) | 28 | 5 | M | Cyclists | 22 | 73 | 65 |
| Prins et al. (28) | 42 | 7 | M | Trained runners | 36 | 69 | 61 |
| Shaw et al., (29) | 31 | 8 | M | Endurance trained | 30 | 73 | 59 |
| Sjodin et al. (30) | 28 | 24 | F | Untrained | 24 | 62 | 44 |
| Zinn et al. (31) | 70 | 5 | 1 M, 4 F | Endurance trained | 51 | 65 | 47 |
Values are means. CON, mixed macronutrient control diet; KD, ketogenic diet; NR, not reported; ref, reference; 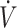 O2max, maximal oxygen uptake.
O2max, maximal oxygen uptake.
TABLE 2.
Mean daily macronutrient consumption on a KD versus CON diet and βHB concentration in healthy subjects included in the systematic review by parallel and crossover/longitudinal study designs1
| KD | CON | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βHB, mmol/L | Fat | CHO | PRO | Fat | CHO | PRO | |||||||
| First author (ref) | g/d | %E | g/d | %E | g/d | %E | g/d | %E | g/d | %E | g/d | %E | |
| Parallel studies | |||||||||||||
| Burke et al. (16) | 1.922 | 312 | 78 | 33 | 4 | 144 | 18 | 77 | 20 | 549 | 63 | 138 | 17 |
| Burke et al. (37) | 0.802 | 326 | 78 | 35 | 4 | 144 | 16 | 69 | 18 | 534 | 65 | 127 | 15 |
| Cipryan et al. (15) | 0.402 | 120 | 62 | 35 | 8 | 128 | 30 | 92 | 36 | 270 | 47 | 90 | 17 |
| Dostal et al. (33) | 0.442 | 149 | 69 | 40 | 8 | 113 | 23 | 67 | 45 | 201 | 34 | 82 | 21 |
| Fleming et al. (17) | 0.293 | 158 | 61 | 47 | 8 | 181 | 31 | 50 | 25 | 268 | 59 | 73 | 16 |
| Kephart et al. (35) | 1.562 | 170 | 79 | 15 | 3 | 89 | 18 | NR | NR | NR | NR | NR | NR |
| LaFountain et al. (36) | 1.002 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| McSwiney et al. (18) | 0.503 | 259 | 77 | 45 | 6 | 128 | 17 | 59 | 20 | 430 | 65 | 99 | 15 |
| Vargas-Molina et al. (34) | NR | 122 | 64 | 39 | 9 | 115 | 27 | 51 | 23 | 282 | 57 | 97 | 20 |
| Wilson et al. (32) | 1.402 | 217 | 75 | 31 | 5 | 134 | 20 | 83 | 25 | 318 | 55 | 132 | 20 |
| Crossover studies | |||||||||||||
| Greene et al. (26) | 0.402 | 159 | 69 | 42 | 8 | 118 | 23 | 76 | 33 | 230 | 45 | 113 | 12 |
| Heatherly et al. (27) | 0.702 | 134 | 64 | 33 | 7 | 90 | 29 | 119 | 38 | 303 | 43 | 134 | 19 |
| Phinney et al. (12) | 1.282 | 290 | 84 | 17 | 2 | 107 | 14 | 96 | 28 | 432 | 56 | 128 | 16 |
| Prins et al. (28) | 0.552 | 226 | 69 | 43 | 6 | 184 | 25 | 89 | 28 | 402 | 56 | 106 | 15 |
| Shaw et al. (29) | 0.942 | 284 | 78 | 33 | 4 | 147 | 18 | 132 | 38 | 336 | 43 | 149 | 19 |
| Sjodin et al. (30) | 1.323 | 206 | 76 | 24 | 4 | 111 | 19 | 88 | 33 | 259 | 44 | 115 | 20 |
| Zinn et al. (31) | 0.5 – 4.22,4 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Values are means in grams per day or % of total calorie intake. CHO, carbohydrate; CON, mixed macronutrient control diet; KD, ketogenic diet; NR, not reported; PRO, protein; ref, reference; βHB, β-hydroxybutyrate; %E, percentage of energy.
βHB measured in whole blood.
βHB measured in serum, presented for KD only.
Data presented as range.
Performance outcomes
In 11 studies assessing endurance-type performance, 13 performance outcomes were included in this systematic review (Table 3). Endurance-type performance outcomes were split into high or low 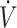 O2max, dichotomized by the median (53 mL · kg−1 · min−1) value across studies. There were 7 performance outcomes in high-
O2max, dichotomized by the median (53 mL · kg−1 · min−1) value across studies. There were 7 performance outcomes in high-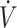 O2max (≥53 mL · kg−1 · min−1) studies: 6 (3 time trials, 3 time-to-exhaustion) reported no difference and 1 (time trial) reported lower physical performance when consuming a KD compared with CON. There were 6 performance outcomes in low-
O2max (≥53 mL · kg−1 · min−1) studies: 6 (3 time trials, 3 time-to-exhaustion) reported no difference and 1 (time trial) reported lower physical performance when consuming a KD compared with CON. There were 6 performance outcomes in low-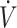 O2max (<53 mL · kg−1 · min−1) studies: 4 (1 time trials, 3 time-to-exhaustion) reported no difference and 2 (time-to-exhaustion) reported lower physical performance when consuming the KD compared with CON.
O2max (<53 mL · kg−1 · min−1) studies: 4 (1 time trials, 3 time-to-exhaustion) reported no difference and 2 (time-to-exhaustion) reported lower physical performance when consuming the KD compared with CON.
TABLE 3.
Study design, duration, and endurance-type performance measurement outcome in healthy subjects in systematic reviews split by training status1
| First author (ref) | Study design | Duration, d | Performance measure | Performance outcome2 |
|---|---|---|---|---|
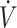 O2max ≥53 mL · kg−1 · min−1 O2max ≥53 mL · kg−1 · min−1
|
||||
| Burke et al. (16) | Parallel NRCT | 21 | TT, race walk (10 km) | ↔ |
| Burke et al. (37) | Parallel NRCT | 25 | TT, race walk (10 km) | ↓ |
| McSwiney et al. (18) | Parallel NRCT | 84 | TT, cycle ergometer (100 km) | ↔ |
| Phinney et al. (12) | Longitudinal | 28 | TTE, cycle ergometer (60–65% 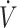 O2max) O2max) |
↔ |
| Prins et al. (28) | Crossover RCT | 42 | TT, treadmill (5 km) | ↔ |
| TTE, cycle ergometer (graded intensity) | ↔ | |||
| Shaw et al. (29) | Crossover RCT | 31 | TTE, treadmill (70% 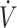 O2max) O2max) |
↔ |
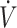 O2max <53 mL · kg−1 · min−1 O2max <53 mL · kg−1 · min−1
|
||||
| Cipryan et al. (15) | Parallel BRCT | 28 | TTE, treadmill (100% 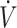 O2max) O2max) |
↔ |
| Dostal et al. (33) | Parallel NRCT | 84 | TTE, treadmill (graded test) | ↔ |
| TTE, shuttle run (30–15 intermittent fitness test) | ↔ | |||
| Heatherly et al. (27) | Longitudinal | 21 | TT, treadmill (5 km) | ↔ |
| Sjodin et al. (30) | Crossover RCT | 28 | TTE, cycle ergometer (graded intensity) | ↓ |
| Zinn et al. (31) | Longitudinal | 70 | TTE, cycle ergometer (graded intensity) | ↓ |
1BRCT, block randomized controlled trial; CON, mixed macronutrient control diet; KD, ketogenic diet; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial; ref, reference; TT, time trial; TTE, time-to-exhaustion; 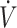 O2max, maximal oxygen uptake.
O2max, maximal oxygen uptake.
Physical performance significantly lower (↓), higher (↑), or not different (↔) following a KD compared with CON.
In 7 studies assessing power or strength performance, 16 performance outcomes were included in this systematic review (Table 4). There were 7 assessments of lower-body power, with 4 reporting no difference, 2 reporting enhanced, and 1 reporting lower physical performance when consuming a KD compared with CON. There were 9 assessments of 1-repetition-maximum, with 7 reporting no difference and 2 reporting lower physical performance when consuming a KD compared with CON.
TABLE 4.
Study design, duration, and lower-body power or strength performance measurement in healthy subjects in systematic reviews1
| First author (ref) | Study design | Duration, d | Performance measure | Performance outcome2 |
|---|---|---|---|---|
| Fleming et al. (17) | Parallel NRCT | 42 | Power, Wingate (30 s) | ↓ |
| Greene et al. (26) | Crossover RCT | 84 | Olympic lift (1 RM) | ↔ |
| Kephart et al. (35) | Parallel NRCT | 84 | Back squat (1 RM) | ↔ |
| Power clean (1 RM) | ↔ | |||
| LaFountain et al. (36) | Parallel NRCT | 84 | Squat (1 RM) | ↔ |
| Vertical jump (W/body mass) | ↔ | |||
| Sprint intervals (W) | ↔ | |||
| Bench press (1 RM) | ↔ | |||
| McSwiney et al. (18) | Parallel NRCT | 84 | Power, cycle ergometer, SS sprint | ↑ |
| Power, cycle ergometer, CPT | ↑ | |||
| Vargas-Molina et al. (34) | Parallel RCT | 56 | Vertical jump (cm) | ↔ |
| Back squat (1 RM) | ↓ | |||
| Bench press (1 RM) | ↓ | |||
| Wilson et al. (32) | Parallel RCT | 70 | Back squat (1 RM) | ↔ |
| Wingate (W) | ↔ | |||
| Bench press (1 RM) | ↔ |
CON, mixed macronutrient control diet; CPT, critical power test; KD, ketogenic diet; NRCT, nonrandomized controlled trial; RCT, randomized controlled trial; ref, reference; RM, repetition maximum; SS, 6 second; W, Watts.
Physical performance significantly lower (↓), higher (↑), or not different (↔) following a KD compared with CON.
Risk of bias
A risk-of-bias assessment for the individual studies included in this systemic review is reported in Supplemental Table 2. Overall, risk of bias identified some concern of bias primarily due to studies allowing for participants to self-select diet intervention group, use of longitudinal study design, and inability to blind participants to the study intervention. Self-selection of diets was stated to improve compliance with diet and mimic how individuals would choose dietary intake under “real world” conditions. Although allowing participants to self-select diets might improve compliance, this also introduces selection bias, which may influence physical performance outcomes. Participant blinding when feeding intervention diets of varying macronutrient content, particularly one as restrictive as a KD, is difficult. This results in participants and study staff being unblinded. Inability to blind participants to the diet intervention may impact physical performance outcomes. Although there is overall some concern of bias, these concerns were present regardless of whether a study reported positive, negative, or null effects of a KD on performance compared with CON.
Discussion
Duration consuming KDs
There is much debate regarding whether a KD needs to be consumed for a specific length of time before effects on physical performance could be expected (39, 40). Sherrier and Li (39) recently noted that assessing the impact of a KD on physical performance from short-term (1–7 d) crossover studies has resulted in confusion and misinterpretation due to insufficient time for metabolic adaptations to translate to performance improvements. Thorough descriptions of metabolic adaptions to a KD have been reported elsewhere (39, 41, 42). In brief, restricted carbohydrate intake (<50 g/d) with a KD increases mobilization and availability of free fatty acids to be oxidized for energy. Alterations in fatty acid oxidation are likely the result of an upregulation in transcription factors peroxisome proliferator activated receptors (PPARs), which increase the expression of fatty acid translocase (CD36/FAT), carnitine palmitoyl transferase 1a (CPT1a), and hydroxyacyl-CoA dehydrogenase (HADHA) to facilitate increases in fat oxidation (43–49). Increased oxidation of fatty acids results in increased production of ketone bodies acetoacetate (AcAc), acetone, and βHB. Ketone bodies are converted by ketolytic enzymes [β-hydroxybutyrate dehydrogenase (BDH), acetoacetyl-CoA thiolase (ACAT), and succinyl-CoA/3-ketoacid CoA transferase] to enter the tricarboxylic acid (TCA) cycle where they can be used as an alternative fuel source (39, 41, 42). Indeed, previous studies assessing the impact of short-term (<7 d) consumption of a KD have observed decrements in time trial (50) and time-to-exhaustion (51) performance compared with CON. Prins et al. (28) highlighted the need for prolonged consumption of a KD to avoid potential negative effects on physical performance at initiation of the diet. This study assessed time-course changes in performance, with recreational distance runners completing a 5-km time trial on day 4, 14, 28, and 42 of consuming a KD or CON (28). On study day 4, time-to-completion was slower in the KD compared with the CON group. However, by day 14, there was no difference in 5-km time trial performance when consuming a KD or CON. Similarly, McSwiney et al. (18) noted a drop in perceived energy level and performance during the first 7–10 d of endurance-trained athletes consuming a KD. Time-course change in physical performance may reflect metabolic adaptations that occur when consuming a KD.
Although an adequate adaptation time may be required when starting a KD (40), extended consumption does not appear to independently result in enhanced physical performance. Duration of diet consumption in the studies included in the current systematic review ranged from 21 to 84 d. Separating studies into tertiles, KD consumption of 21–31 d resulted in lower (30, 37) or no difference (12, 15, 16, 27) in physical performance compared with CON. Studies feeding KDs for 42–70 d resulted in lower (17, 31, 34) or no difference (28, 32) in physical performance compared with CON. When KD consumption was 84 d, physical performance was either not different (26, 33, 35, 36) or enhanced (18) compared with CON. Although only 1 study reported performance enhancement, these latter data may suggest that 84 d of consuming a KD is required for metabolic adaptations to abate negative effects on physical performance. In agreement with these observations, McSwiney et al. (18) reported a “lag” in performance with the KD during the first 28–42 d of their 84-d study. However, there remains a need to better understand time-course changes in performance when consuming a KD. Future investigations should use the approach by Prins et al. (28), assessing change in physical performance over time. Additionally, discordant null or negative results across more moderate-duration (21–70 d) studies suggest other variables beyond diet duration influence performance impacts of a KD. There are several factors, such as training status, performance test type and intensity, and sex differences, that likely contribute to discrepancies across studies.
Training status
Without diet modification, exercise training alone enhances fat oxidation (52, 53). Exercise training increases maximal oxygen uptake and whole-body lipolysis, increasing the ability and availability to oxidize free fatty acid for fuel (22). Within skeletal muscle, training results in greater lipid storage, mitochondrial area, and proportion of lipid stores in contact with mitochondria allowing for enhanced fat oxidation (52, 53). Adaptations in fatty acid metabolism occur concomitantly with an increased capacity to store carbohydrate as glycogen within skeletal muscle (54). Despite greater glycogen availability, training adaptations result in increased rates of fat oxidation and decreased rates of total carbohydrate and muscle glycogen oxidation (55). Such improvements in fatty acid utilization likely reflect enhanced metabolic flexibility, the ability to shift substrate oxidation based on metabolic demand, with training (56).
Greater efficiency to utilize fat for energy in trained individuals may result in more favorable performance outcomes when consuming a KD compared with untrained individuals. Due to variations in training status and type (i.e., resistance vs. endurance), it is not feasible to completely isolate the effects of training status across all 17 studies identified in this systematic review. Previous regression analysis has reported that higher 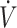 O2max (i.e., aerobic capacity) is associated with higher rates of fat oxidation (20, 21). As such, we used high or low
O2max (i.e., aerobic capacity) is associated with higher rates of fat oxidation (20, 21). As such, we used high or low 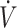 O2max, dichotomized by the median (53 mL · kg−1 · min−1) value, in the 11 studies assessing endurance-type performance (time trial or time-to-exhaustion) to gain a better understanding of whether higher
O2max, dichotomized by the median (53 mL · kg−1 · min−1) value, in the 11 studies assessing endurance-type performance (time trial or time-to-exhaustion) to gain a better understanding of whether higher 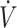 O2max resulted in enhanced physical performance with a KD compared with CON. In studies using high
O2max resulted in enhanced physical performance with a KD compared with CON. In studies using high 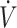 O2max (≥53 mL · kg−1 · min−1), participants maintained performance (12, 16, 18, 28, 29) with the KD except for 1 study (37), where physical performance was lower with a KD compared with CON. Specifically, Burke et al. (37) reported a 3.3% decline in 10-km time trial performance in elite race walkers consuming a KD for 25 d, while consuming a high-carbohydrate diet showed a 4.8% performance improvement compared with baseline. Reductions in time trial performance were stated to be the result of lower exercise economy, defined as greater oxygen cost for a given speed or power output, when consuming a KD (37). Although performance was maintained, this observation of lower exercise economy while consuming a KD is corroborated in other investigations (16, 29). Despite preservation of performance, Burke et al. (16) reported that 10-km time trial performance in elite race walkers was improved by 6.6% when consuming a high-carbohydrate diet compared with a 1.6% decline when consuming a KD for 21 d. Although the performance decline in KD was not significant, the gap in performance between KD and CON was similar (∼8%) in both studies conducted by Burke et al. (16, 37). Additionally, Shaw et al. (29) observed that, while physical performance was maintained despite reduced exercise economy, variability doubled in time-to-exhaustion when endurance-trained individuals consumed a KD compared with CON. Individual variance with a KD was the result of participants’ respiratory exchange ratio (RER) at
O2max (≥53 mL · kg−1 · min−1), participants maintained performance (12, 16, 18, 28, 29) with the KD except for 1 study (37), where physical performance was lower with a KD compared with CON. Specifically, Burke et al. (37) reported a 3.3% decline in 10-km time trial performance in elite race walkers consuming a KD for 25 d, while consuming a high-carbohydrate diet showed a 4.8% performance improvement compared with baseline. Reductions in time trial performance were stated to be the result of lower exercise economy, defined as greater oxygen cost for a given speed or power output, when consuming a KD (37). Although performance was maintained, this observation of lower exercise economy while consuming a KD is corroborated in other investigations (16, 29). Despite preservation of performance, Burke et al. (16) reported that 10-km time trial performance in elite race walkers was improved by 6.6% when consuming a high-carbohydrate diet compared with a 1.6% decline when consuming a KD for 21 d. Although the performance decline in KD was not significant, the gap in performance between KD and CON was similar (∼8%) in both studies conducted by Burke et al. (16, 37). Additionally, Shaw et al. (29) observed that, while physical performance was maintained despite reduced exercise economy, variability doubled in time-to-exhaustion when endurance-trained individuals consumed a KD compared with CON. Individual variance with a KD was the result of participants’ respiratory exchange ratio (RER) at 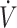 O2max (29). When RER was >1.0, indicating preservation of carbohydrate oxidation at higher exercise intensities, participants maintained physical performance; when <1.0, performance declined after 31 d of a KD (29). These findings suggested that even in more trained participants, there is variance in how individuals respond to KDs. This indicates that individuals interested in a KD to support physical performance should test the diet, potentially using RER at
O2max (29). When RER was >1.0, indicating preservation of carbohydrate oxidation at higher exercise intensities, participants maintained physical performance; when <1.0, performance declined after 31 d of a KD (29). These findings suggested that even in more trained participants, there is variance in how individuals respond to KDs. This indicates that individuals interested in a KD to support physical performance should test the diet, potentially using RER at 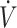 O2max as a mark, well before competition. Although exercise economy was reduced in these studies (16, 29, 37), duration of KD consumption was only 21–31 d. Whether lower exercise economy persists if a KD was followed for ≥84 d remains unknown. Regardless, although overall performance with high
O2max as a mark, well before competition. Although exercise economy was reduced in these studies (16, 29, 37), duration of KD consumption was only 21–31 d. Whether lower exercise economy persists if a KD was followed for ≥84 d remains unknown. Regardless, although overall performance with high 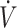 O2max (≥53 mL · kg−1 · min−1) was maintained, there is some individual variance with KDs potentially resulting in negative effects on physical performance that should be considered.
O2max (≥53 mL · kg−1 · min−1) was maintained, there is some individual variance with KDs potentially resulting in negative effects on physical performance that should be considered.
In studies where participants had a low 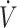 O2max (<53 mL · kg−1 · min−1), endurance-type performance was either maintained (15, 27, 33) or declined (30, 31) following a KD compared with CON. Discrepancies between maintenance and decline in performance may be attributed to training adaptations during the diet interventions. Studies reporting increases in
O2max (<53 mL · kg−1 · min−1), endurance-type performance was either maintained (15, 27, 33) or declined (30, 31) following a KD compared with CON. Discrepancies between maintenance and decline in performance may be attributed to training adaptations during the diet interventions. Studies reporting increases in 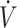 O2max from baseline to post-KD showed maintenance in physical performance (15, 33). When
O2max from baseline to post-KD showed maintenance in physical performance (15, 33). When 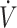 O2max was maintained or slightly declined from baseline to post-KD, physical performance declined (30, 31). This suggests that some level of exercise training may be needed when consuming a KD diet to ensure physical performance is maintained. While endurance-type performance appears to be maintained, following a KD does not appear to result in an overall performance enhancement. Future investigation may be warranted to assess the impact of training status and/or
O2max was maintained or slightly declined from baseline to post-KD, physical performance declined (30, 31). This suggests that some level of exercise training may be needed when consuming a KD diet to ensure physical performance is maintained. While endurance-type performance appears to be maintained, following a KD does not appear to result in an overall performance enhancement. Future investigation may be warranted to assess the impact of training status and/or 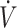 O2max and RER on physical performance response to a KD compared with CON.
O2max and RER on physical performance response to a KD compared with CON.
Performance test type and intensity
Fat is a major energy source during low to moderate submaximal (45–65% 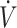 O2max) endurance exercise (57, 58). Sustained (20 mo) consumption of a KD can enhance maximal fat oxidation to 1.5 g/min at 70%
O2max) endurance exercise (57, 58). Sustained (20 mo) consumption of a KD can enhance maximal fat oxidation to 1.5 g/min at 70% 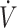 O2max, replacing carbohydrate as the primary energy source (10). This has resulted in the utility of a KD to be considered in the context of prolonged endurance performance (40). Conversely, as described above, exercise economy has been reported to decline when consuming a KD (16, 29, 37), with reductions being most prevalent during higher (>70%
O2max, replacing carbohydrate as the primary energy source (10). This has resulted in the utility of a KD to be considered in the context of prolonged endurance performance (40). Conversely, as described above, exercise economy has been reported to decline when consuming a KD (16, 29, 37), with reductions being most prevalent during higher (>70% 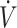 O2max) exercise intensities (29). These findings have led to the assertion that a KD may impair high-intensity exercise performance (16, 29, 37). Potential negative effects during higher-intensity exercise have been speculated to be the result of lower glycogenolysis due to reduced glycogen stores and impaired pyruvate dehydrogenase (PDH) activity (29, 40). Importantly, reductions in PDH activity have only been observed following a high-fat, non-KD (>50 g carbohydrate/d) with carbohydrate feeding during or glycogen restoration prior to exercise (49, 59). Relatively little work has been conducted on alterations of enzymatic activity and molecular pathways in humans following a KD. It is presently unknown if PDH activity is lower following a KD, and if lower activity is the result of an impairment, lower glycogen stores, or cellular adaptation.
O2max) exercise intensities (29). These findings have led to the assertion that a KD may impair high-intensity exercise performance (16, 29, 37). Potential negative effects during higher-intensity exercise have been speculated to be the result of lower glycogenolysis due to reduced glycogen stores and impaired pyruvate dehydrogenase (PDH) activity (29, 40). Importantly, reductions in PDH activity have only been observed following a high-fat, non-KD (>50 g carbohydrate/d) with carbohydrate feeding during or glycogen restoration prior to exercise (49, 59). Relatively little work has been conducted on alterations of enzymatic activity and molecular pathways in humans following a KD. It is presently unknown if PDH activity is lower following a KD, and if lower activity is the result of an impairment, lower glycogen stores, or cellular adaptation.
With regard to muscle glycogen content, recent cross-sectional findings by Volek et al. (10) have questioned if lower muscle glycogen content and glycogenolysis are present with a prolonged (9–36 mo) KD. Specifically, resting muscle glycogen content was not different, and rate of glycogen decline was similar during treadmill running at 65% 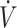 O2max in elite ultra-endurance athletes habitually consuming either KDs or high-carbohydrate diets (10). However, results in muscle glycogen conflict with indirect calorimetry measurements of lower carbohydrate oxidation during exercise in KD compared with the high-carbohydrate athletes (10). As KD athletes performed exercise 90 min after consuming only 4 g of carbohydrate, lower carbohydrate oxidation rates would be reflective of lower rates of endogenous (i.e., glycogenolysis) carbohydrate oxidation. Additionally, Phinney et al. (12) and Webster et al. (11) reported lower resting muscle glycogen concentrations and glycogenolysis in well-trained cyclists during exercise following 6 wk and 8 mo, respectively, of a KD. Lower muscle glycogen results from severe carbohydrate restriction and lower endogenous glucose production due to reductions in hepatic glycolysis, while gluconeogenesis was maintained when consuming a KD (11). Despite carbohydrate intake restricted to <50 g/d and lower gluconeogenesis, under resting conditions glycogen content was only ∼50% lower with a KD compared with CON, with no differences postexercise (11, 12). These data suggest that, although lower, some degree of gluconeogenesis allows for glycogen resynthesis during exercise recovery. Additionally, these data may indicate that lower glycogenolysis during exercise is the result of initiating exercise with lower glycogen content, rather than impairment of glycolytic flux with a KD compared with CON.
O2max in elite ultra-endurance athletes habitually consuming either KDs or high-carbohydrate diets (10). However, results in muscle glycogen conflict with indirect calorimetry measurements of lower carbohydrate oxidation during exercise in KD compared with the high-carbohydrate athletes (10). As KD athletes performed exercise 90 min after consuming only 4 g of carbohydrate, lower carbohydrate oxidation rates would be reflective of lower rates of endogenous (i.e., glycogenolysis) carbohydrate oxidation. Additionally, Phinney et al. (12) and Webster et al. (11) reported lower resting muscle glycogen concentrations and glycogenolysis in well-trained cyclists during exercise following 6 wk and 8 mo, respectively, of a KD. Lower muscle glycogen results from severe carbohydrate restriction and lower endogenous glucose production due to reductions in hepatic glycolysis, while gluconeogenesis was maintained when consuming a KD (11). Despite carbohydrate intake restricted to <50 g/d and lower gluconeogenesis, under resting conditions glycogen content was only ∼50% lower with a KD compared with CON, with no differences postexercise (11, 12). These data suggest that, although lower, some degree of gluconeogenesis allows for glycogen resynthesis during exercise recovery. Additionally, these data may indicate that lower glycogenolysis during exercise is the result of initiating exercise with lower glycogen content, rather than impairment of glycolytic flux with a KD compared with CON.
The ability to maintain some level of muscle glycogen may indicate sufficient stores are available to support higher-intensity physical performance following a KD. In agreement with this, McSwiney et al. (18) reported enhanced peak and maintenance of average power output during sprint performance in endurance-trained athletes following 84 d of a KD compared with CON. Similarly, Dostal et al. (33) reported no impairment of high-intensity continuous or intermittent exercise lasting 25 min in recreationally trained individuals following 84 d of a KD compared with CON. Other studies assessing higher-intensity performance using Wingate (32), sprint test (36), or time-to-exhaustion (70–100% 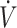 O2max) also reported no difference (12, 15, 33) with KDs compared with CON. However, some studies have reported lower physical performance on Wingate (17) and time-to-exhaustion (30, 31) with KDs compared with CON. In these studies, participants were categorized in this systematic review to have low
O2max) also reported no difference (12, 15, 33) with KDs compared with CON. However, some studies have reported lower physical performance on Wingate (17) and time-to-exhaustion (30, 31) with KDs compared with CON. In these studies, participants were categorized in this systematic review to have low 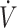 O2max (<53 mL · kg−1 · min−1), which did not change during the course of the diet intervention. As described in the previous section, lower training status and lack of training adaptation during the intervention may have contributed to the negative effects of the KD on performance. Time trial performance, which is generally more moderate in intensity, was no different (18, 27, 28, 60) with a KD compared with CON, except for Burke et al. (37), which is described above. Overall, these data suggest moderate- to high-intensity exercise performance can be maintained, although training status should be considered when initiating a KD. Although performance may be maintained, overall, results from these studies do not suggest enhancement in physical performance.
O2max (<53 mL · kg−1 · min−1), which did not change during the course of the diet intervention. As described in the previous section, lower training status and lack of training adaptation during the intervention may have contributed to the negative effects of the KD on performance. Time trial performance, which is generally more moderate in intensity, was no different (18, 27, 28, 60) with a KD compared with CON, except for Burke et al. (37), which is described above. Overall, these data suggest moderate- to high-intensity exercise performance can be maintained, although training status should be considered when initiating a KD. Although performance may be maintained, overall, results from these studies do not suggest enhancement in physical performance.
While KDs have historically been associated with endurance-type performance, recent studies have begun assessing their impact on lower-body power using vertical jump tests and upper- and lower-body strength. The majority of studies showed no difference (26, 32, 35, 36) with a KD compared with CON. Vargas-Molina et al. (34) was the only study to report lower squat and bench-press maximum lift performance in strength-trained women following 56 d of a KD compared with CON. This investigation included a controlled resistance-training program designed to increase muscle size and strength. All participants, regardless of KD or CON, increased bench-press and squat strength compared with baseline; however, strength gains were greater in the CON compared with the KD group. These findings suggest that increases in strength can be achieved on a KD, but these gains may be blunted compared with CON in women. To date, this is the only study assessing the impact of a KD on measurements of strength in women, so further investigation is warranted. Overall, data from these studies indicate there is no negative or positive effect of a KD on lower-body power measured by vertical jump or upper- and lower-body strength compared with CON.
Sex differences
Sex is a primary factor regulating substrate oxidation (20, 21). Women have higher rates of fat oxidation and lower rates of carbohydrate oxidation during aerobic exercise compared with men when consuming mixed macronutrient diets (61). When comparing the response in substrate oxidation between men and women consuming a KD, fat oxidation was higher during exercise at 35–80% 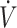 O2max in men, while women had higher rates of fat oxidation when exercise was >80%
O2max in men, while women had higher rates of fat oxidation when exercise was >80% 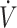 O2max with a KD compared with CON (62). Whether differences in substrate oxidation indicate physical performance responses to a KD compared with CON vary between men and women has not been determined. Although sex difference responses in physical performance following a KD have not specifically been tested, in 3 of the 5 studies that reported lower physical performance participants were all (30, 34) or primarily (31) women. Adherence to a KD has been reported to lower dietary iron intake and mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration decreased, although serum ferritin was unchanged (63). Additionally, KDs have been reported to result in higher circulating IL-6 concentrations postexercise compared with CON (64). Elevations in IL-6 result in increased circulating hepcidin concentrations, which inhibits iron transport (65). Poor iron status reduces oxygen-carrying capacity of RBCs, leading to declines in physical performance (66, 67). As women are biologically susceptible to lower iron status, lower iron intake with a KD may impact women more so than men. It is important to state that iron status was not assessed in the female participants in these KD studies (30, 31, 34). Additionally, participants in Sjodin et al. (30) and Zinn et al. (31) were categorized as having low
O2max with a KD compared with CON (62). Whether differences in substrate oxidation indicate physical performance responses to a KD compared with CON vary between men and women has not been determined. Although sex difference responses in physical performance following a KD have not specifically been tested, in 3 of the 5 studies that reported lower physical performance participants were all (30, 34) or primarily (31) women. Adherence to a KD has been reported to lower dietary iron intake and mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration decreased, although serum ferritin was unchanged (63). Additionally, KDs have been reported to result in higher circulating IL-6 concentrations postexercise compared with CON (64). Elevations in IL-6 result in increased circulating hepcidin concentrations, which inhibits iron transport (65). Poor iron status reduces oxygen-carrying capacity of RBCs, leading to declines in physical performance (66, 67). As women are biologically susceptible to lower iron status, lower iron intake with a KD may impact women more so than men. It is important to state that iron status was not assessed in the female participants in these KD studies (30, 31, 34). Additionally, participants in Sjodin et al. (30) and Zinn et al. (31) were categorized as having low 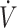 O2max (<53 mL · kg−1 · min−1) in the current systematic review, with no adaptations in
O2max (<53 mL · kg−1 · min−1) in the current systematic review, with no adaptations in 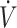 O2max from baseline to post-KD. On the contrary, work by Dostal et al. (33), which included primarily female participants with low
O2max from baseline to post-KD. On the contrary, work by Dostal et al. (33), which included primarily female participants with low 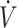 O2max (<53 mL · kg−1 · min−1), but who increased
O2max (<53 mL · kg−1 · min−1), but who increased 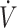 O2max during the 84-d KD intervention, were able to maintain physical performance. These data suggest that females can maintain physical performance following a KD. It is possible that negative effects in Sjodin et al. (30) and Zinn et al. (31) were the result of lower training status and lack of a training response when consuming a KD and not sex. Presently, it has not been determined if KDs result in poorer iron status in females. Future investigation is needed to determine if a KD negatively impacts iron status differently between men and women, and what impact this may have on physical performance. At a minimum, individuals who wish to follow a KD should be educated on both the risks of low iron status and nutritional strategies to avoid it, whether by consumption of iron-rich foods, or by inclusion of an iron supplement or multivitamin while on a KD.
O2max during the 84-d KD intervention, were able to maintain physical performance. These data suggest that females can maintain physical performance following a KD. It is possible that negative effects in Sjodin et al. (30) and Zinn et al. (31) were the result of lower training status and lack of a training response when consuming a KD and not sex. Presently, it has not been determined if KDs result in poorer iron status in females. Future investigation is needed to determine if a KD negatively impacts iron status differently between men and women, and what impact this may have on physical performance. At a minimum, individuals who wish to follow a KD should be educated on both the risks of low iron status and nutritional strategies to avoid it, whether by consumption of iron-rich foods, or by inclusion of an iron supplement or multivitamin while on a KD.
Body composition
In addition to metabolic adaptations, KDs have been suggested to have beneficial effects on reducing body and fat mass (57, 68). Results from 9 studies that measured changes in body composition in the current systematic review appear to corroborate this statement (Table 5). Following consumption of a KD, body mass, fat mass, and fat-free mass declined, with comparatively little change observed when consuming CON diets. Reductions in body mass came primarily from fat mass, with a relatively small contribution from fat-free mass. Minimal loss in muscle mass may be attributed to protein intake being consumed at twice the RDA (1.6 g · kg−1 · d−1), which has previously been shown to minimize declines in fat-free mass during weight loss due to maintenance of nitrogen balance (69). It was not the intention of these studies for participants to consume hypocaloric diets on a KD compared with CON. Regardless, the observed reductions in body and fat mass while consuming a KD may suggest some benefit to athletes who compete in sports with weight classes or have an aesthetic component. However, disparities in changes in body mass between a KD and CON make it difficult to determine impact on physical performance. Our group previously reported that severity of negative energy balance and change in body mass is associated with declines in physical performance following military operations (70). Whether reductions in body mass impaired any potential performance gains while consuming a KD is unclear. Future investigation is needed, tightly controlling dietary intake to ensure similar changes in body mass between KD and CON diets to remove this potential confounding variable.
TABLE 5.
Change in body composition following a KD versus CON diet in healthy subjects1
| KD | CON | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author (ref) | Duration, d | Body mass, kg | Fat mass, kg | Fat-free mass, kg | Protein, g · kg−1 · d−1 | Body mass, kg | Fat mass, kg | Fat-free mass, kg | Protein, g · kg−1 · d−1 |
| Dostal et al. (33) | 84 | −3.6 | −2.9 | −0.72 | 1.69 | −0.9 | −0.4 | −0.52 | 1.13 |
| Greene et al. (26) | 84 | −1.9 | 0 | −1.8 | 1.55 | 1.5 | 1 | 0.4 | 1.43 |
| Heatherly et al. (27) | 21 | −2.1 | −2.2 | −0.1 | 1.13 | NR | NR | NR | 1.64 |
| Kephart et al. (35) | 84 | −3 | −2.5 | −0.5 | 1.08 | −0.3 | −0.3 | 0 | NR |
| LaFountain et al. (36) | 84 | −7.7 | −5.9 | −1.4 | NR | 0.1 | −0.6 | 0.8 | NR |
| McSwiney et al. (18) | 84 | −5.9 | −4.6 | 0.3 | 1.49 | −0.8 | −0.5 | 0.1 | 1.30 |
| Prins et al. (28) | 42 | 0.5 | −0.2 | 0.4 | 2.66 | 0.1 | 0.5 | −0.4 | 1.55 |
| Vargas-Molina et al. (34) | 56 | −2.2 | −1.1 | −0.7 | 1.86 | 0.8 | 0.4 | 0.7 | 1.55 |
| Wilson et al. (32) | 70 | −2.6 | −4.2 | 1.5 | 1.67 | 0.6 | −2.2 | 2.7 | 1.69 |
Values are means. Change calculated at post minus pre diet intervention. CON, mixed macronutrient control diet; KD, ketogenic diet; NR, not reported; ref, reference.
Data not presented in original manuscript.
Conclusions
In conclusion, the overall evidence of the 17 studies included in this systematic review suggests that physical performance can be maintained when consuming a KD compared with CON. However, the current evidence does not support an ergogenic effect of consuming a KD. Despite renewed interest by the athletic and scientific community, there remains relatively little research in the area on the impact of a KD on physical performance. Additionally, the variance in study design to include population training status, duration of consuming a KD, performance outcome type and intensity, and sex may contribute to discrepancies in outcomes across studies. Although evidence is limited, shorter study duration, training status/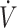 O2max, and potentially sex differences may be possible factors that account for lower physical performance when consuming KDs compared with CON diets. Future investigations should consider assessing time-course changes in physical performance, and powering sample sizes to examine the effects of training status and/or sex on physical performance following a KD compared with CON.
O2max, and potentially sex differences may be possible factors that account for lower physical performance when consuming KDs compared with CON diets. Future investigations should consider assessing time-course changes in physical performance, and powering sample sizes to examine the effects of training status and/or sex on physical performance following a KD compared with CON.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Dr Andrew Young for his critical review of this manuscript, as well as the subjects and authors of the papers included in this systematic review. The authors’ responsibilities were as follows—LMM: developed the research question and finalized paper inclusion; NEM and CTC: performed systematic review and extracted the data; NEM, CTC, and LMM: interpreted results, prepared tables and figures, and drafted and finalized the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by the US Army Medical Research and Development Command.
Author disclosures: The authors report no conflicts of interest. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: CON, control (mixed macronutrient diet); KD, ketogenic diet; PDH, pyruvate dehydrogenase; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RER, respiratory exchange ratio;  O2max, maximal oxygen uptake; βHB, β-hydroxybutyrate.
O2max, maximal oxygen uptake; βHB, β-hydroxybutyrate.
Contributor Information
Nancy E Murphy, Military Nutrition Division, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
Christopher T Carrigan, Military Nutrition Division, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
Lee M Margolis, Military Nutrition Division, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
References
- 1. Hearris MA, Hammond KM, Seaborne RA, Stocks B, Shepherd SO, Philp A, Sharples AP, Morton JP, Louis JB. Graded reductions in preexercise muscle glycogen impair exercise capacity but do not augment skeletal muscle cell signaling: implications for CHO periodization. J Appl Physiol. 2019;126(6):1587–97. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien MJ, Viguie CA, Mazzeo RS, Brooks GA. Carbohydrate dependence during marathon running. Med Sci Sports Exerc. 1993;25(9):1009–17. [PubMed] [Google Scholar]
- 3. Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL, Morton JP. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018;48(5):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine Joint Position Statement: nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–68. [DOI] [PubMed] [Google Scholar]
- 5. Knuiman P, Hopman MT, Mensink M. Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr Metab (Lond). 2015;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2):140–50. [DOI] [PubMed] [Google Scholar]
- 7. Yeo WK, Carey AL, Burke L, Spriet LL, Hawley JA. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. 2011;36(1):12–22. [DOI] [PubMed] [Google Scholar]
- 8. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13–20. [DOI] [PubMed] [Google Scholar]
- 9. Burke LM. Re-examining high-fat diets for sports performance: did we call the “nail in the coffin” too soon?. Sports Med. 2015;45(Suppl 1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresh CMet al. . Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65(3):100–10. [DOI] [PubMed] [Google Scholar]
- 11. Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA, Smith JA. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J Physiol. 2016;594(15):4389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32(8):769–76. [DOI] [PubMed] [Google Scholar]
- 13. Purdom T, Kravitz L, Dokladny K, Mermier C. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr. 2018;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(Suppl 1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cipryan L, Plews DJ, Ferretti A, Maffetone PB, Laursen PB. Effects of a 4-week very low-carbohydrate diet on high-intensity interval training responses. J Sports Sci Med. 2018;17(2):259–68. [PMC free article] [PubMed] [Google Scholar]
- 16. Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Strobel N, Sharma APet al. . Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleming J, Sharman MJ, Avery NG, Love DM, Gomez AL, Scheett TP, Kraemer WJ, Volek JS. Endurance capacity and high-intensity exercise performance responses to a high fat diet. Int J Sport Nutr Exerc Metab. 2003;13(4):466–78. [DOI] [PubMed] [Google Scholar]
- 18. McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2018;81:25–34. [DOI] [PubMed] [Google Scholar]
- 19. Phinney SD Ketogenic diets and physical performance. Nutr Metab. 2004;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98(1):160–7. [DOI] [PubMed] [Google Scholar]
- 21. Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, Wallis GA. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Am J Clin Nutr. 2017;105(4):864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mika A, Macaluso F, Barone R, Di Felice V, Sledzinski T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front Physiol. 2019;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace B SK, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. Proceedings of the ACM International Health Informatics Symposium (IHI). Miami, FL; 2012:819–24. [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SMet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 26. Greene DA, Varley BJ, Hartwig TB, Chapman P, Rigney M. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and Olympic weightlifting athletes. J Strength Cond Res. 2018;32(12):3373–82. [DOI] [PubMed] [Google Scholar]
- 27. Heatherly AJ, Killen LG, Smith AF, Waldman HS, Seltmann CL, Hollingsworth A, O'Neal EK. Effects of ad libitum low-carbohydrate high-fat dieting in middle-age male runners. Med Sci Sports Exerc. 2018;50(3):570–9. [DOI] [PubMed] [Google Scholar]
- 28. Prins PJ, Noakes TD, Welton GL, Haley SJ, Esbenshade NJ, Atwell AD, Scott KE, Abraham J, Raabe AS, Buxton JDet al. . High rates of fat oxidation induced by a low-carbohydrate, high-fat diet, do not impair 5-km running performance in competitive recreational athletes. J Sports Sci Med. 2019;18(4):738–50. [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw DM, Merien F, Braakhuis A, Maunder ED, Dulson DK. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sports Exerc. 2019;51(10):2135–46. [DOI] [PubMed] [Google Scholar]
- 30. Sjodin A, Hellstrom F, Sehlstedt E, Svensson M, Buren J. Effects of a ketogenic diet on muscle fatigue in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. 2020;12(4):955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinn C, Wood M, Williden M, Chatterton S, Maunder E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr. 2017;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson JM, Lowery RP, Roberts MD, Sharp MH, Joy JM, Shields KA, Partl J, Volek JS, D'Agostino D. The effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training males. J Strength Cond Res. 2017. doi: 10.1519/JSC.0000000000001935. [DOI] [PubMed] [Google Scholar]
- 33. Dostal T, Plews DJ, Hofmann P, Laursen PB, Cipryan L. Effects of a 12-week very-low carbohydrate high-fat diet on maximal aerobic capacity, high-intensity intermittent exercise, and cardiac autonomic regulation: non-randomized parallel-group study. Front Physiol. 2019;10:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vargas-Molina S, Petro JL, Romance R, Kreider RB, Schoenfeld BJ, Bonilla DA, Benitez-Porres J. Effects of a ketogenic diet on body composition and strength in trained women. J Int Soc Sports Nutr. 2020;17(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kephart WC, Pledge CD, Roberson PA, Mumford PW, Romero MA, Mobley CB, Martin JS, Young KC, Lowery RP, Wilson JMet al. . The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in CrossFit trainees: a pilot study. Sports (Basel). 2018;6(1). doi: 10.3390/sports6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LaFountain RA, Miller VJ, Barnhart EC, Hyde PN, Crabtree CD, McSwiney FT, Beeler MK, Buga A, Sapper TN, Short JAet al. . Extended ketogenic diet and physical training intervention in military personnel. Mil Med. 2019;184(9-10):e538–47. [DOI] [PubMed] [Google Scholar]
- 37. Burke LM, Sharma AP, Heikura IA, Forbes SF, Holloway M, McKay AKA, Bone JL, Leckey JJ, Welvaert M, Ross ML. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One. 2020;15(6):e0234027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsafnat G, Glasziou P, Choong MK, Dunn A, Galgani F, Coiera E. Systematic review automation technologies. Syst Rev. 2014;3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherrier M, Li H.. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;110(3):562–73. [DOI] [PubMed] [Google Scholar]
- 40. Burke LM Ketogenic low CHO, high fat diet: the future of elite endurance sport?. J Physiol. 2020. epub ahead of print. doi: 10.1113/JP278928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egan B, D'Agostino DP. Fueling performance: ketones enter the mix. Cell Metab. 2016;24(3):373–5. [DOI] [PubMed] [Google Scholar]
- 42. Pinckaers PJ, Churchward-Venne TA, Bailey D, van Loon LJ. Ketone bodies and exercise performance: the next magic bullet or merely hype?. Sports Med. 2017;47(3):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arkinstall MJ, Tunstall RJ, Cameron-Smith D, Hawley JA. Regulation of metabolic genes in human skeletal muscle by short-term exercise and diet manipulation. Am J Physiol Endocrinol Metab. 2004;287(1):E25–31. [DOI] [PubMed] [Google Scholar]
- 44. Boyle KE, Canham JP, Consitt LA, Zheng D, Koves TR, Gavin TP, Holbert D, Neufer PD, Ilkayeva O, Muoio DMet al. . A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J Clin Endocrinol Metab. 2011;96(3):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cameron-Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A, Hawley JA, Hargreaves M. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr. 2003;77(2):313–8. [DOI] [PubMed] [Google Scholar]
- 46. Mulya A, Haus JM, Solomon TP, Kelly KR, Malin SK, Rocco M, Barkoukis H, Kirwan JP. Exercise training-induced improvement in skeletal muscle PGC-1alpha-mediated fat metabolism is independent of dietary glycemic index. Obesity. 2017;25(4):721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol. 2002;541(Pt 1):261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism. 2005;54(8):1048–55. [DOI] [PubMed] [Google Scholar]
- 49. Margolis LM, Wilson MA, Whitney CC, Carrigan CT, Murphy NE, Hatch AM, Montain SJ, Pasiakos SM. Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metabolism. 2019;97:1–8. [DOI] [PubMed] [Google Scholar]
- 50. Edwards LM, Holloway CJ, Murray AJ, Knight NS, Carter EE, Kemp GJ, Thompson CH, Tyler DJ, Neubauer S, Robbins PAet al. . Endurance exercise training blunts the deleterious effect of high-fat feeding on whole body efficiency. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galbo H, Holst JJ, Christensen NJ. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979;107(1):19–32. [DOI] [PubMed] [Google Scholar]
- 52. Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1271–8. [DOI] [PubMed] [Google Scholar]
- 54. Greiwe JS, Hickner RC, Hansen PA, Racette SB, Chen MM, Holloszy JO. Effects of endurance exercise training on muscle glycogen accumulation in humans. J Appl Physiol. 1999;87(1):222–6. [DOI] [PubMed] [Google Scholar]
- 55. van Loon LJ, Jeukendrup AE, Saris WH, Wagenmakers AJ. Effect of training status on fuel selection during submaximal exercise with glucose ingestion. J Appl Physiol. 1999;87(4):1413–20. [DOI] [PubMed] [Google Scholar]
- 56. San-Millan I, Brooks GA.. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med. 2018;48(2):467–79. [DOI] [PubMed] [Google Scholar]
- 57. Chang CK, Borer K, Lin PJ. Low-carbohydrate-high-fat diet: can it help exercise performance?. J Hum Kinet. 2017;56:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bartlett JD, Hawley JA, Morton JP. Carbohydrate availability and exercise training adaptation: too much of a good thing?. Eur J Sport Sci. 2015;15(1):3–12. [DOI] [PubMed] [Google Scholar]
- 59. Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, Burke LM. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. 2006;290(2):E380–8. [DOI] [PubMed] [Google Scholar]
- 60. Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T, Maughan RJ. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab. 2018;28(5):451–63. [DOI] [PubMed] [Google Scholar]
- 61. Devries MC. Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol. 2016;101(2):243–9. [DOI] [PubMed] [Google Scholar]
- 62. Durkalec-Michalski K, Nowaczyk PM, Siedzik K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J Int Soc Sports Nutr. 2019;16(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McSwiney FT, Doyle L. Low-carbohydrate ketogenic diets in male endurance athletes demonstrate different micronutrient contents and changes in corpuscular haemoglobin over 12 weeks. Sports (Basel). 2019;7(9). doi: 10.3390/sports7090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McKay AKA, Peeling P, Pyne DB, Welvaert M, Tee N, Leckey JJ, Sharma AP, Ross MLR, Garvican-Lewis LA, Swinkels DWet al. . Chronic adherence to a ketogenic diet modifies iron metabolism in elite athletes. Med Sci Sports Exerc. 2019;51(3):548–55. [DOI] [PubMed] [Google Scholar]
- 65. Hennigar SR, McClung JP, Pasiakos SM. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017;31(9):3719–28. [DOI] [PubMed] [Google Scholar]
- 66. McClung JP, Karl JP, Cable SJ, Williams KW, Nindl BC, Young AJ, Lieberman HR. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124–31. [DOI] [PubMed] [Google Scholar]
- 67. McClung JP, Karl JP, Cable SJ, Williams KW, Young AJ, Lieberman HR. Longitudinal decrements in iron status during military training in female soldiers. Br J Nutr. 2009;102(4):605–9. [DOI] [PubMed] [Google Scholar]
- 68. Vargas S, Romance R, Petro JL, Bonilla DA, Galancho I, Espinar S, Kreider RB, Benitez-Porres J. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J Int Soc Sports Nutr. 2018;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, Rood JC, Carbone JW, Combs GF Jr, Young AJ. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. 2013;27(9):3837–47. [DOI] [PubMed] [Google Scholar]
- 70. Murphy NE, Carrigan CT, Philip Karl J, Pasiakos SM, Margolis LM. Threshold of energy deficit and lower-body performance declines in military personnel: a meta-regression. Sports Med. 2018;48(9):2169–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.