Abstract
Background
Recently, there has been an increasing interest in targeting inflammation to reduce major adverse cardiovascular events (MACE) in patients with cardiovascular risk. Statins, PCSK9 inhibitors, and ezetimibe have been shown to reduce MACE owing to reduction in low-density lipoproteins cholesterol (LDL-c). Herein, we investigate whether the intensity of these agents is associated with (i) discernible reduction in inflammation measured by the levels of high-sensitivity C-reactive protein (hsCRP); (ii) reduction in MACE; (iii) if there is an association between the baseline hsCRP and MACE.
Methods and results
Electronic databases were searched for randomized controlled trials (RCTs) that compared statins, ezetimibe, PCSK9 inhibitors with placebos/active controls and reported MACEs and hsCRP (mg/L). Studies were stratified based on baseline hsCRP (<2, 2–3, >3) with subgroup analysis conducted across each stratum. Fourteen RCTs including 133 109 patients randomized into more intensive therapy (MIT) and less intensive therapy were selected. Meta-analysis did not demonstrate any significant differences between use of MIT and hsCRP levels (mean difference, −0.02; CI, −0.06, 0.02; P = 0.31). The MIT significantly reduced the risk of MACE (RR, 0.82; CI, 0.75, 0.91; P < 0.001). The relative risk and absolute risk remained consistent across the strata. However, there was a 0.5% statistically significant absolute risk reduction in all-cause mortality in patients with higher hsCRP (RD, −0.005; CI, −0.009, −0.001; P = 0.01).
Conclusion
Overall, LDL-c lowering therapies reduce relative risk of MACEs particularly in patients with higher baseline hsCRP. However, there appears to be a residual inflammatory risk despite the use of contemporary lipid lowering agents.
Keywords: Inflammation, Randomized control trials, Myocardial infarction, Stroke
Introduction
Low-density lipoprotein cholesterol (LDL-c) has consistently been shown to be associated with coronary artery disease (CAD). Although statins are the best studied agents to demonstrate the beneficial effects of lowering LDL-c towards reducing the major adverse cardiovascular events (MACE), medications with mechanism of action other than HMG-CoA reductase inhibition such as ezetimibe and PCSK9 inhibitors have also shown improved cardiovascular outcomes with reduction of LDL-c.1,2
The current American College of Cardiology/American Heart Association guideline recommends using high-intensity statins in patients with clinical cardiovascular disease.3 This is based on several previous ‘more vs. less statins’ trials which demonstrated that more intensive therapy (MIT) leads to a higher reduction in LDL-c and thus significantly reduced cardiovascular events compared with less intensive therapy (LIT).4,5 However, despite LDL-c lowering, patients appear to be at a residual risk of coronary events. For instance, PCSK-9 inhibitors can drive down the LDL-c to very low levels. Yet such patients appear to be at a residual risk.6,7 Inflammation has long been shown to be associated with atherogenesis and inflammatory markers have been incorporated into the risk prediction algorithms such as the Reynolds risk score.8 The CANTOS trial recently demonstrated inflammation to be a modifiable risk factor in patients with known CAD.9
In the present systematic review and meta-analysis, we examined whether the intensity of contemporary lipid lowering agents (statins, ezetemibe, and PCSK9 inhibitors) are associated with any changes in the inflammatory marker in the form of high-sensitivity C-reactive protein (hsCRP) over time as a surrogate for residual inflammatory risk. We also investigated whether the efficacy of lipid lowering therapy is affected by the baseline inflammatory risk.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10
Data sources and searches
Two authors (S.U.K. and S.T.) devised the search strategy and performed literature search using MEDLINE (via PUBMED), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from inception to March, 2018. Following key search words were used: ‘statins’, ‘PCSK9 inhibitors’, ‘ezetimibe’, ‘high sensitivity C-reactive protein’, ‘hsCRP’, ‘cardiovascular disease’, ‘myocardial infarction’, ‘stroke’, and ‘mortality’. We applied restrictions on humans and randomized controlled trials (RCTs). No restrictions were applied on publication year, language, or text availability. Additional sources included online libraries of www.clinicaltrialresults.com and www.clinicaltrials.gov, and bibliographies of relevant articles. The citations were downloaded to Endnote X7 (Thompson ISI ResearchSoft, Philadelphia, PA, USA) and duplicates were identified and removed. Two authors (S.U.K. and S.T.) independently screened the search results in a two-step process based on predetermined inclusion/exclusion criteria. First citations were evaluated on title and abstract level, followed by full-text screening of the final list of articles. Any disagreements were resolved by discussion or third party review.
Study selection
The priori inclusion criteria were: (i) RCTs comparing statins, PCSK 9 inhibitors, ezetimibe with placebo, or active controls. Consistent with prior reports, the pre-specified intervention groups were MIT vs. LIT which were defined as the drugs vs. placebo/no drug, or higher dose vs. lower dose of drugs or more intensive LDL-C lowering therapy vs. less intensive LDL-C lowering therapy 11,12; (ii) studies had to report baseline hsCRP and effect of the intervention on hsCRP (3) studies had to report at least one clinical event for key outcomes in adult population (≥18 years); and (iii) follow-up ≥3 months and sample size ≥100 patients (to avoid small study effects).
Quality assessment and data extraction
Two authors (M.S.K. and S.T.) independently abstracted data on baseline characteristics of participants in both treatment groups, baseline hsCRP (mg/L), change in hsCRP in each group and difference between the groups, crude point estimates, events, sample size and study duration. We focused on adjusted estimates and data were abstracted from intention-to-treat analysis. Disagreements related to data were resolved by discussion, referring back to the original article or opinion of the third author (S.U.K.). Quality assessment of the trials was assessed using the Cochrane bias risk assessment tool (Supplementary material online, Table S1).
The primary outcome was MACE. The MACE endpoint from each trial was selected to most closely approximate the composite outcome of myocardial infarction (MI) or acute coronary syndrome, cardiovascular mortality, coronary revascularization, and stroke. Secondary endpoints were all-cause mortality, cardiovascular mortality, MI, stroke, coronary revascularization, and change in hsCRP (mg/L). The endpoints were defined as reported in the individual trial.
Data synthesis and analysis
Outcomes were combined using DerSimonian and Laird random effects model. The summary statistics for binomial variables were risk ratio (RR) and risk difference (RD) and continuous variables were calculated as standard mean difference (SMD) with 95% confidence interval (CI). Heterogeneity was assessed using Cochrane Q statistics and was quantified via I2 with values 25–50%, 50–75%, and >75% consistent with low, moderate, and high degree of heterogeneity, respectively. Publication bias was assessed using funnel plot and Egger’s regression test. For all analyses, statistical significance was set as ≤0.05.
To assess whether baseline hsCRP modifies the primary outcome, trials were stratified based on baseline hsCRP (<2, 2–3, >3 mg/L) and a subgroup analysis was conducted across the strata of baseline hsCRP. Comprehensive meta-analysis software version 3.0 (Biostat, Englewood, NJ, USA) was used for all analyses.
Results
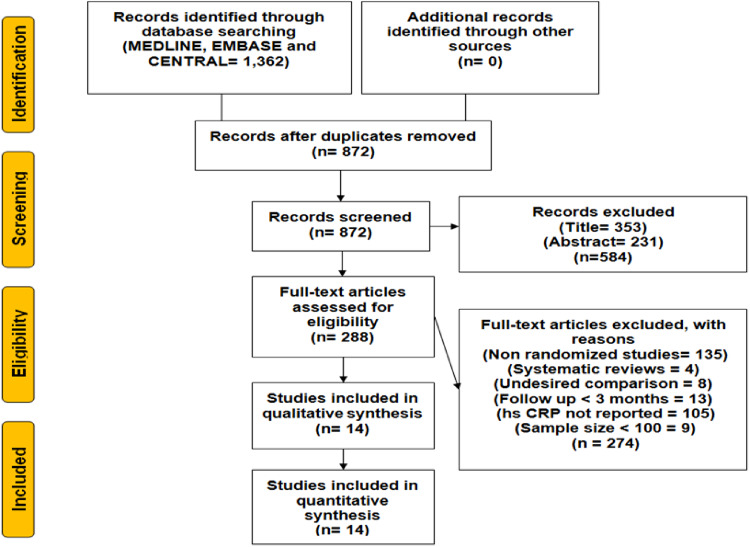
Initial electronic search yielded 1362 records, 490 citations were removed as duplicates and out of remaining 872 articles, 584 citations were excluded at title- and abstract-level screening. Further 288 full-text articles were excluded based on pre-specified inclusion/exclusion criteria. Ultimately, 14 RCTs (133 109 patients) were included in this meta-analysis (Figure 1). Guyton et al. was a three arm study comparing niacin, ezetimibe plus simvastatin, and ezetimibe plus simvastatin plus niacin. Data were extracted from ezetimibe plus simvastatin (intensive therapy) and niacin (control) arms. Overall, statins had nine trials (44 376 patients), ezetimibe had one trial (18144 patients), and four trials assessed PCSK 9 inhibitors (70 589 patients).13–26 The mean age (in years) of study participants was 57 ± 8.5/57 ± 8.3, out of which 61.5%/60.6% were male in the MIT and LIT groups, respectively. The pooled mean baseline hsCRP was 3.1 ± 2.3 mg/L. The mean follow-up duration was 28.3 months (Table 1).
Figure 1.
Flowchart for study selection. Systematic search using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow sheet shows detailed search strategy.
Table 1.
Clinical characteristics of the study participants, number of patients (%), and mean (SD) or median values (IQR) where available
| Studies (year) | Arms | N | Age (years) | Men (%) | CHD (%) | HTN (%) | DM (%) | Baseline hsCRP (mg/L) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| FOURIER (2017)c | Evolocumab | 13 784 | 62.5 ± 9.1 | 75.4 | 80.9 | 80.1 | 36.7 | 1.7 | 26 |
| Placebo | 13 780 | 62.5 ± 8.9 | 75.5 | 81.3 | 80.1 | 36.5 | 1.7 | ||
| INTREPID (2017)a | Pravastatin | 126 | 49.2 ± 8.7 | 88 | NR | NR | NR | 5.5 | 12 |
| Pitavastatin | 126 | 50.1 ± 7.5 | 84 | NR | NR | NR | 4.0 | ||
| SPIRE (2017)a | Bococizumab | 13 720 | 62.9 ± 9.3 | 70.7 | NR | 81.3 | 48.1 | 2.0 | 12 |
| Placebo | 13 718 | 63.0 ± 9.3 | 70.2 | NR | 80.4 | 46.9 | 2.0 | ||
| GLAGOV (2016)e | Evolocumab | 484 | 59.8 ± 9.6 | 72.1 | 100 | 82.2 | 20.2 | 1.6 | 18 |
| Placebo | 484 | 59.8 ± 8.8 | 72.3 | 100 | 83.7 | 21.5 | 1.6 | ||
| HOPE 3 (2016)d | Rosuvastatin | 6361 | 65.8 ± 6.4 | 53.6 | 0.0 | 37.8 | 5.9 | 2.0 | 67 |
| Placebo | 6344 | 65.7 ± 6.3 | 53.9 | 0.0 | 38.0 | 5.6 | 2.0 | ||
| DESCARTES (2014)d | Evolocumab | 599 | 55.9 ± 10.8 | 48.4 | 15.7 | 48.2 | 10.4 | 1.0 | 12 |
| Placebo | 302 | 56.7 ± 10.1 | 46.4 | 13.9 | 49.3 | 13.9 | 1.0 | ||
| ASTRONOMER (2010)d | Rosuvastatin | 134 | 58.0 ± 12.9 | 60.5 | NR | NR | NR | 1.6 | 42 |
| Placebo | 135 | 57.9 ± 14.3 | 53.0 | NR | NR | NR | 1.88 | ||
| AURORA (2009)a | Rosuvastatin | 1389 | 64.1 ± 8.6 | 61.3 | 10.5 | NR | 20.6 | 4.8 | 38 |
| Placebo | 1384 | 64.3 ± 8.7 | 63.0 | 9.8 | NR | 18.0 | 5.2 | ||
| Guyton et al. (2008)b | Ezetimibe + Simvastatin | 272 | 57.5 ± 10.3 | 55.9 | 8.1 | 66.5 | 15.8 | 2.1 | 5.5 |
| Niacin | 272 | 56.4 ± 10.6 | 50.0 | 5.9 | 61.0 | 14.7 | 2.0 | ||
| GISSI-HF (2008)a | Rosuvastatin | 2285 | 68 ± 11 | 76.2 | 31.8 | 55.1 | 27.4 | 2.70 | 47 |
| Placebo | 2289 | 68 ± 11 | 78.6 | 33.8 | 53.5 | 25.0 | 2.17 | ||
| JUPITER (2008)d | Rosuvastatin | 8901 | 66.0 | 61.5 | NR | NR | 0.0 | 4.2 | 60 |
| Placebo | 8901 | 66.0 | 62.1 | NR | NR | 0.0 | 4.3 | ||
| CORONA (2007)d | Rosuvastatin | 2514 | 73 ± 7.1 | 76.0 | 60.0 | 63.0 | 30 | 3.1 | 33 |
| Placebo | 2497 | 73 ± 7.0 | 76.0 | 60.0 | 63.0 | 29 | 3.0 | ||
| Sola et al. (2005)d | Statin | 255 | 55.4 ± 6.4 | 62.0 | 42.0 | 41.0 | 24.0 | 15 | 24 |
| Placebo | 191 | 53.8 ± 5.7 | 63.0 | 46.0 | 36.0 | 27.0 | 14 |
Change in hsCRP was detected aAt 12 weeks, bat 24 weeks, cat 48 weeks, dat 52 weeks, eat 76 weeks.
CHD, coronary heart disease; DM, diabetes mellitus; hsCRP, high-sensitivity C-reactive protein; HTN, hypertension; NA, not available; NR, not reported.
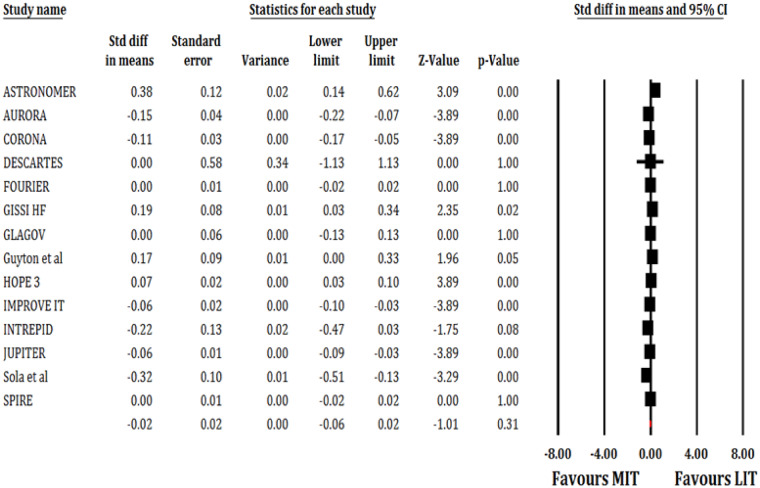
Out of 133 109 patients, 73 609 patients were randomized into MIT arm and 59 500 patients into LIT. The MIT had no significant effect on hsCRP (SMD: −0.02; 95% CI, −0.06, 0.02; P = 0.31; I2 = 86%; Figure 2).
Figure 2.
Forest plot showing effect of MIT on hsCRP level. Squares represent the risk ratio of the individual studies; Horizontal lines represent the 95% confidence intervals (CI) of the risk ratio. The size of the squares reflects the weight that the corresponding study contributes in the meta-analysis. The diamonds represent the pooled risk ratio or the overall effect.
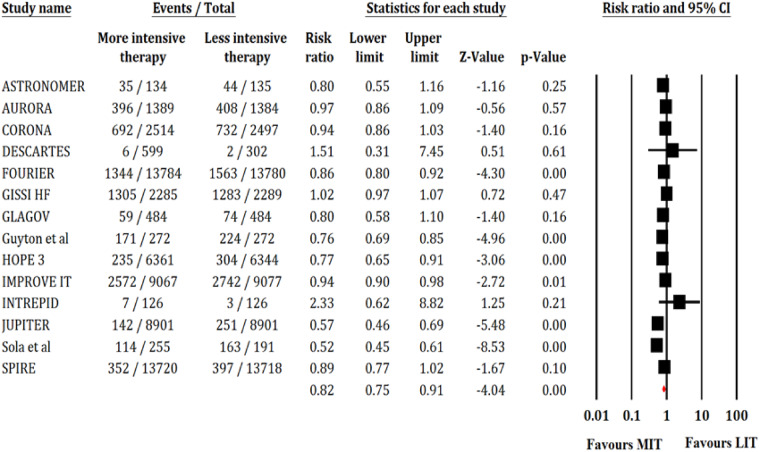
Fourteen trials reported 15 620 events of MACEs out of 119 391 patients. The MIT significantly reduced the risk of MACE compared with LIT (RR, 0.82; 95% CI, 0.75–0.91; P < 0.001; I2 = 88; Figure 3). Fourteen trials reported 4800 events of MI out of 119 391 patients. The MIT significantly reduced the risk of MI (RR, 0.80; 95% CI, 0.72–0.88; P < 0.001; I2 = 49; Supplementary material online, Figure S1). Fourteen trials reported 2088 events of stroke out of 119 391 patients. The MIT was associated with significantly lower risk of stroke compared with LIT (RR, 0.83; 95% CI, 0.72–0.96; P = 0.01; I2 = 50; Supplementary material online, Figure S2). Seven trials reported 6455 patients of coronary revascularization out of 107 394 patients. The MIT significantly reduced the risk of coronary revascularization (RR, 0.81; 95% CI, 0.70–0.93; P < 0.001; I2 = 78; Supplementary material online, Figure S3). Thirteen trials reported 8895 cases of all-cause mortality out of 119 139 patients and 11 trials reported 4673 cases of CV mortality out of 118 149 patients. There were no significant differences between both the arms in terms of all-cause mortality (RR, 0.94; 95% CI, 0.88–1.01; P = 0.09; I2 = 55; Supplementary material online, Figure S4) or CV mortality (RR, 0.99; 95% CI, 0.94–1.04; P = 0.67, I2 = 0; Supplementary material online, Figure S5).
Figure 3.
Forest plot showing effect of MIT on MACE. Other annotations as in Figure 2.
When outcomes were stratified based on baseline hsCRP (mg/L; Table 2), the relative risk reduction, and absolute risk reduction for the MACE remained consistent throughout the baseline hsCRP strata. For MI, there was statistically significant reduction in absolute risk across hsCRP strata. In case of all-cause mortality, there was a significant 0.5% absolute risk reduction noticed in patients with higher baseline hsCRP.
Table 2.
Cardiovascular outcomes based on baseline hsCRP (mg/L), CI, MACE, RR, and RD
| Outcome | Baseline hsCRP (mg/L) | RR (95 % CI) | P-value (I2) | RD (95% CI) | P-value (I2) |
|---|---|---|---|---|---|
| MACE | <2 | 0.73 (0.54–0.99) | 0.04 (91) | −0.126 (−0.254, 0.001) | 0.05 (96) |
| 2–3 | 0.86 (0.73–1.02) | 0.09 (86) | −0.008 (−0.022, 0.006) | 0.25 (87) | |
| >3 | 0.87 (0.76–1.00) | 0.05 (84) | −0.013 (−0.018, −0.008) | <0.001 (5) | |
| P-interaction = 0.55 | P-interaction = 0.18 | ||||
| Myocardial infarction | <2 | 0.72 (0.64–0.81) | <0.001 (0) | −0.015 (−0.026, −0.003) | 0.01 (32) |
| 2–3 | 0.86 (0.73–1.00) | 0.05 (0) | −0.002 (−0.004, 0.000) | 0.05 (0) | |
| >3 | 0.82 (0.70–0.96) | 0.01 (66) | −0.008 (−0.014, −0.002) | 0.01 (45) | |
| P-interaction = 0.15 | P-interaction = 0.02 | ||||
| Stroke | <2 | 0.79 (0.66–0.94) | 0.01 (0) | −0.004 (−0.006, −0.001) | 0.01 (0) |
| 2–3 | 0.83 (0.55–1.26 ) | 0.38 (72) | −0.002 (−0.005, 0.001) | 0.27 (44) | |
| >3 | 0.85 (0.69–1.06) | 0.16 (59) | −0.004 (−0.006, −0.002) | <0.001 (0) | |
| P-interaction = 0.84 | P-interaction = 0.48 | ||||
| Coronary revascularization | <2 | 0.78 (0.72–0.86) | <0.001 (0) | −0.008 (−0.019, 0.004) | 0.18 (71) |
| 2–3 | 0.78 (0.60–1.02) | 0.07 (21) | −0.0005 (−0.0015, 0.0006) | 0.36 (18) | |
| >3 | 0.82 (0.62–1.08) | 0.16 (86) | −0.004 (−0.009, 0.002) | 0.16 (79) | |
| P-interaction = 0.96 | P-interaction =0.96 | ||||
| Cardiovascular mortality | <2 | 1.03 (0.87–1.22) | 0.74 (0) | 0.0004 (−0.0025, 0.0032) | 0.79 (0) |
| 2–3 | 0.97 (0.88–1.06) | 0.49 (0) | −0.0002 (−0.0017, 0.0013) | 0.83 (0) | |
| >3 | 0.99 (0.93–1.06) | 0.85 (0) | −0.0006 (−0.0021, 0.0008) | 0.37 (0) | |
| P-interaction = 0.80 | P-interaction = 0.78 | ||||
| All-cause mortality | <2 | 0.71 (0.35–1.45) | 0.34 (89) | −0.014 (−0.038, 0.010) | 0.25 (90) |
| 2–3 | 1.00 (0.93–1.07) | 0.95 (0) | −0.0002 (−0.0022, 0.0019) | 0.86 (86) | |
| >3 | 0.95 (0.90–1.01) | 0.08 (25) | −0.005 (−0.009, −0.001) | 0.01 (0) | |
| P-interaction = 0.42 | P-interaction = 0.04 | ||||
CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; MACE, major adverse cardiovascular events; RD, risk difference; RR, risk ratio. Note: Statistically significant P-values are mentioned in bold.
Funnel plot and Egger’s regression test did not detect publication bias [P-value (two-tailed) = 0.13; Supplementary material online, Figure S6].
Discussion
In this systematic review and meta-analysis of 14 RCTs of commonly used lipid lowering agents (statins, PCSK9 inhibitors, and ezetemibe) involving 133 109 patients, we found that despite aggressive lipid lowering, the inflammatory risk persists. Importantly, although the relative risk of reduction in MACE is consistent across all strata of hsCRP, the absolute risk reduction seems greatest in patients with highest hsCRP, implying that individuals with highest baseline risk (and hence highest absolute number of events) may benefit the most from aggressive lipid lowering.
A number of preclinical studies have attempted to explore the effects of lipid lowering agents beyond LDL-c lowering. Statins, in particular, have been studied to have pleiotropic role including reducing the vascular and myocardial inflammation. The pleiotropic effects of statins are thought to be mediated by inhibition of protein isoprenylation, which mediated the downstream responses in the mevalonate pathway responsible for formation of intermediates that mediate the signal transduction molecules in the myocardial and vascular inflammation.27,28
For instance, the PRINCE trial demonstrated that pravastatin reduced CRP levels at both 12 and 24 weeks independent to the LDL-c levels at the baseline although the change in LDL was affected by the baseline CRP. Of note, the PRINCE trial was not powered to determine the clinical endpoints.29 However, the JUPITER trial was the first trial that enrolled 17 802 participants with elevated hsCRP and LDL below 130 mg/dL. Compared with placebo, the use of rosuvastatin 20 mg was associated with significant reduction in the levels of hsCRP and a risk ratio of 0.56 (95% CI= 0.46–0.69, P ≤ 0.00001) for combined primary endpoint.24 An analysis of the heart protection study revealed that the beneficial effect of the statins was seen even in patients with hsCRP <1.25 suggesting that even patients with low baseline inflammation are likely to derive the benefit.30 However, although statins appear to reduce systemic inflammation compared with placebos, it appears that more intensity statin therapy does not appear to reduce inflammation as compared with the LIT.
Similarly, PCSK9 inhibitors are being investigated for pleiotropic effects in addition to the aggressive lipid lowering. A recent meta-analysis of seven RCTs enrolling 2564 patients suggested that the PCSK9 inhibitors are associated with no reduction in inflammation as measured by hsCRP. The effect size remained robust independent of type, dosage, or frequency of PCSK9 inhibitor used.31 The PCSK9 trials were conducted with a background of statin therapy and this consideration needs to be taken into account while interpreting the effects of PCSK9 inhibitors on hsCRP.
There are several important limitations of the existing meta-analysis. First, this is a study-level analysis as we did not have access to the individual patient level data. Second, for hsCRP we relied on the baseline data and data available at the longest duration of available follow-up as different studies employed differing follow-up durations for collection of data. Third, we used hsCRP as the surrogate for inflammation and many prior studies such as the historical statin trials have relied on the CRP as the marker for inflammation. This resulted in the exclusion of a large number of statin trials. Fourth, the trials varied widely in terms of the type of statin or PCSK9 inhibitor used and their dosages. Fifth, due to limited number of trials for PCSK9 inhibitors and ezetimibe, a subgroup analysis of separate drugs on hsCRP and MACE could not be performed. Finally, most of these studies had relatively small hsCRP as they did not have a strict inclusion criterion for hsCRP. Similarly, they were not conducted on patients with acute coronary syndrome who would expected to have higher levels of hsCRP given the systemic inflammatory response.
In conclusion, this meta-analysis suggests that despite treatment with conventional lipid lowering agents, a substantial component of inflammatory risk persists. Moreover, patients with higher baseline inflammation may benefit more from aggressive lipid lowering as the baseline risk may be higher. Finally, intensive lipid lowering therapy does not appear to reduce inflammation more than less intensive lipid lowering therapy.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Giugliano RP, Sabatine MS.. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015;65:2638–2651. [DOI] [PubMed] [Google Scholar]
- 2. Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J.. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol 2004;93:1487–1494. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH. et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl 2): S1–S45. [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I. et al. High-dose atorvastatin vs usual dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 6. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD. et al. Risk of incident diabetes with intensive dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 7. Latimer J, Batty JA, Neely RD, Kunadian V.. PCSK9 inhibitors in the prevention of cardiovascular disease. J Thromb Thrombolysis 2016;42:405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ridker PM, Buring JE, Rifai N, Cook NR.. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Everett BM, Thuren T, Macfadyen JG, Chang WH, Ballantyne C. et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M. et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K. et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan SU, Rahman H, Okunrintemi V, Riaz H, Khan MS, Sattur S. et al. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e011581.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J; ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–314. [DOI] [PubMed] [Google Scholar]
- 14. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J. et al. ; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 15. Rogers JK, Jhund PS, Perez AC, Böhm M, Cleland JG, Gullestad L. et al. Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail 2014;2:289–297. [DOI] [PubMed] [Google Scholar]
- 16. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L. et al. ; DESCARTES Investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 17. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA. et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 18. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R. et al. ; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 19. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ. et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 20. Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM.. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol 2008;51:1564–1572. [DOI] [PubMed] [Google Scholar]
- 21. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L. et al. ; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 22. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P. et al. ; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 23. Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA.. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017;4:e284–e294. [DOI] [PubMed] [Google Scholar]
- 24. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ. et al. ; JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 25. Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV.. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol 2006;47:332–337. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F. et al. ; SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017;376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 27. Mathur N, Ramasubbu K, Mann DL.. Spectrum of pleiotropic effects of statins in heart failure. Heart Fail Clin 2008;4:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jain MK, Ridker PM.. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005;4:977–987. [DOI] [PubMed] [Google Scholar]
- 29. Albert MA, Danielson E, Rifai N, Ridker P; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 30.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 31. Sahebkar A, Di Giosia P, Stamerra CA, Grassi D, Pedone C, Ferretti G. et al. Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol 2016;81:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.