Abstract
Background
Psoriatic disease (PsD) is a complex systemic disorder with cutaneous and musculoskeletal manifestations. Current evidence on pharmacological interventions, effective across the spectrum of clinical manifestations of early, systemic treatment-naïve PsD, is limited. This review aims to appraise such evidence.
Methods
This systematic review examined seven patient–intervention–comparator–outcome research questions to address the efficacy of the interventions on the following: across the spectrum of clinical manifestations PsD activity; peripheral arthritis; dactylitis; spondylitis; enthesitis; skin; and nails. Early PsD was defined as a disease duration of ≤2 years, except for studies investigating outcomes restricted to the skin. Eligible references were clinical trials or well-designed prospective studies/series reporting on adult humans, untreated, with cutaneous and/or musculoskeletal features of PsD.
Results
Nine references (out of 160 319, publication range 1946–2019) fulfilled the eligibility criteria. No study adopted comprehensive (that is, simultaneous assessment of different PsD manifestations) composite indices as primary outcome measures. Individual studies reported that apremilast and biologics successfully improved outcomes (disease activity index for PsA, minimal disease activity, PsA DAS, psoriasis area and severity index, PsA response criteria) when efficacy analyses were restricted to single manifestations of untreated PsD. Only qualitative synthesis of evidence was possible, owing to the following factors: data heterogeneity (disease classification criteria, outcome measures); unavailable data subsets (focused on early, untreated PsD) at the single study level; and insufficient data on the exposure of participants to previous treatment.
Conclusion
Effective interventions, albeit limited in scope, were found for early, treatment-naïve PsD. No study provided evidence about the management of co-occurring cutaneous and musculoskeletal manifestations in early, treatment-naïve PsD. This review highlights an unmet need in research on early PsD.
Keywords: psoriatic disease, psoriasis, PsA, early stage, systemic treatment naïve, concurrent co-morbidities
Key messages
Evidence on interventions effective across the clinical spectrum of early, untreated psoriatic disease is lacking.
Few agents improved outcomes in early, untreated psoriatic disease, with their efficacy being restricted to single manifestations.
Introduction
Psoriatic disease (PsD) [1, 2] is a complex chronic condition characterized by a range of cutaneous and musculoskeletal (MSK) inflammatory manifestations. Cutaneous lesions vary in morphology (plaques, pustules and nail abnormalities), anatomical location (extensor surfaces of limbs, scalp, skin folds and oro-genital mucosae) and surface area (limited involvement or whole-body erythrodermia). MSK inflammatory manifestations are arthritis [3], enthesitis, dactylitis and spondylitis/sacro-iliitis. Moreover, PsD is associated with ocular involvement (notably, anterior uveitis/iritis) or IBD. Although the definition of PsD is still formally debated [4–6], clinicians (mostly dermatologists and rheumatologists) commonly appreciate the value of recognizing the multifaceted clinical phenotypes of PsD under one umbrella term. Typically, cutaneous and MSK manifestations co-occur, and the management of complex cases would benefit from a multidisciplinary and comprehensive approach [7]. Although full knowledge of PsD pathogenesis remains elusive [8], factors such as genetic susceptibility, environmental triggers/modulators and dysregulated/dysfunctional inflammatory responses are thought to interact in determining the clinical phenotype.
Clinical experience, alongside improved understanding of the multifactorial mechanisms underlying psoriasis [6–8], have led some authors to hypothesize the concept of PsD [1, 2]. Accordingly, PsD is: (a) systemic, because it affects several sites of the human body, mainly the skin and MSK system; (b) heterogeneous, because different clinical phenotypes can stretch across anatomical sites; and (c) both severity and clinical course vary even within the same individual. Although an officially accepted definition of PsD is lacking, in this review the one described above was adopted.
PsD is a relevant health-care matter, whichever the disciplinary perspective taken. For example, the prevalence of cutaneous psoriatic lesions in the general population ranges worldwide from 0.09 to 11.4%, depending on the regions studied [9]. Moreover, the burden of PsD is considerable, through social stigmatization [7], underestimated disease severity and delayed diagnosis by health professionals [10], reduced autonomy and participation in the workforce, and reduced self-fulfilment and impaired quality of life [7, 10].
Despite the abundance of potent pharmacological agents for psoriasis and PsA [11–15], their effects may not perform simultaneously on both the skin and the MSK system. Sometimes, the interventions can even produce domain-restricted clinical effects; for instance, improving peripheral arthritis but not spondylitis. These therapeutic hurdles matter in contexts where the multidisciplinary/holistic approach to patient care aims to address all PsD components at once. Equally important, interventions at an early stage of PsD have potential for exploiting a window of opportunity and thus modifying the course of the condition, although it is not clear whether this concept would apply to all manifestations of PsD.
Furthermore, most trials conducted in the field of PsD have been limited in two ways. Firstly, there has been a focus on patients with severe disease. Secondly, the primary outcome measures have been limited in scope, focusing on either the cutaneous or the MSK manifestations, without taking a broader look at the more comprehensive composite indices that assess the full clinical spectrum of PsD.
This systematic review stems from the hypothesis that published data do not address the simultaneous treatment of the full clinical spectrum of PsD in its early stages.
Objectives
The aim of this systematic review was to assess the available evidence on non-topical pharmacological therapies for early, untreated (DMARD/systemic therapy-naïve) PsD, with a specific focus on: (a) the efficacy of interventions as measured by outcomes that assess the clinical spectrum of PsD; and (b) the safety of such interventions.
Methods
A multidisciplinary panel was gathered composed of dermatologists and rheumatologists, mainly GRAPPA [16] members and from diverse backgrounds (clinicians, academics, methodologists and trainees), supported by expert librarians. Several rounds of discussion took place to produce a formal protocol for a systematic search, modelled on the Cochrane approach [17]. The research questions that were generated followed the patient–intervention–comparator–outcomes standard. Full protocol details are available on PROSPERO [18].
The search targeted clinical trials and prospective cohorts reporting on participants affected by either cutaneous or MSK PsD, and at an early stage. Early stage was defined as a maximum of 2 years of disease duration for MSK publications, although this restriction did not apply to studies assessing outcomes restricted to cutaneous features of PsD. The research questions addressed seven different facets of PsD: (a) disease activity across the clinical spectrum of PsD; (b) peripheral arthritis; (c) dactylitis; (d) axial involvement; (e) entheseal involvement; (f) skin involvement; and (g) nail involvement. Thirty-five individual drugs (Supplementary Data S1, available at Rheumatology Advances in Practice online), in addition to four drug classes (fumaric acid esters, gold compounds, NSAIDs and CSs) were considered interventions of interest. Outcome measures, although formally stated in the research questions, were removed from the final search strategy after one exploratory run of the MEDLINE database in which the number of hits decreased by 66% after applying outcome measures as search terms. This decision aimed to increase the sensitivity of the search strategy, but the restricted focus on disease response (i.e. disease activity) was retained. Consequently, studies adopting patient-reported outcome measures as primary outcomes were not eligible. The duration of interventions described in the original studies did not constitute an exclusion criterion for this review. The systematic search was also set up to evaluate the safety of the interventions described.
One member of the panel (G.D.M.) explored electronic databases for publications in English, French, German and Spanish. Those databases were as follows: The Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8 of 12, August 2019); CINAHL (1981 to August 2019), via the EBSCO interface; EMBASE (both classic and EMBASE, 1947 to August 2019), using the Ovid interface; and MEDLINE (inclusive of Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions, 1946 to August 2019), using the Ovid interface. The relevant search strategies are available in the Supporting Information (Supplementary Data S2-S5, available at Rheumatology Advances in Practice online). Aiming to produce outputs as up to date as possible, the database search activities continued until the time limit of August 2019.
Two other members of the panel (L.C.C. and A.M.) assessed the following online trial registers: the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au); the ISRCTN register (www.isrctn.com); the European Union clinical trials register (www.clinicaltrialsregister.eu); The United States of America National Institutes of Health/National Library of Medicine clinical studies register (www.clinicaltrials.gov); and The World Health Organization international clinical trials platform (www.who.int/trialsearch).
Other resources explored were conference proceedings of the ACR annual meeting; the EULAR annual conference; the American Academy of Dermatology annual conference; the Society for Investigative Dermatology annual meeting; and the European Academy of Dermatology and Venereology annual meeting. The relevant archives between years 2014 and 2019 were explored by two members of the panel (A.B. and S.D.).
The agreed plan for summarizing findings was to create descriptive tables and then attempt a quantitative summary of the evidence. In this case, the five Grading of Recommendations Assessment, Development and Evaluation [19] considerations (that is, study limitations, inconsistency of results, indirectness of the evidence, imprecision and publication bias) would be used to assess the quality of the evidence gathered.
After each round of searching activities, a list of references was generated and stored in an electronic Endnote X9© library. The final list, updated and maintained by the project coordinator (G.D.M.), fed the Web-based systematic review management system Covidence© [20]. The selection process of references of interest was multistep (step 1: screening by title and abstract; and step 2: full-text-assessment) and operated independently by different members of the panel (A.M. and G.D.M., step 1; and G.D.M. and H.M.-O., step 2). Resolution of disagreement related to reference selection consisted of discussion and subsequent consensus between operators. When clarifications were needed, operators tried to contact the original corresponding authors of the specific publications under assessment.
Four clinician panel members (M.F., E.L., D.M.G. and M.W.) performed the data extraction from the final set of selected studies. All these authors assessed the references independently and recorded their evaluations on specifically designed data extraction forms.
Results
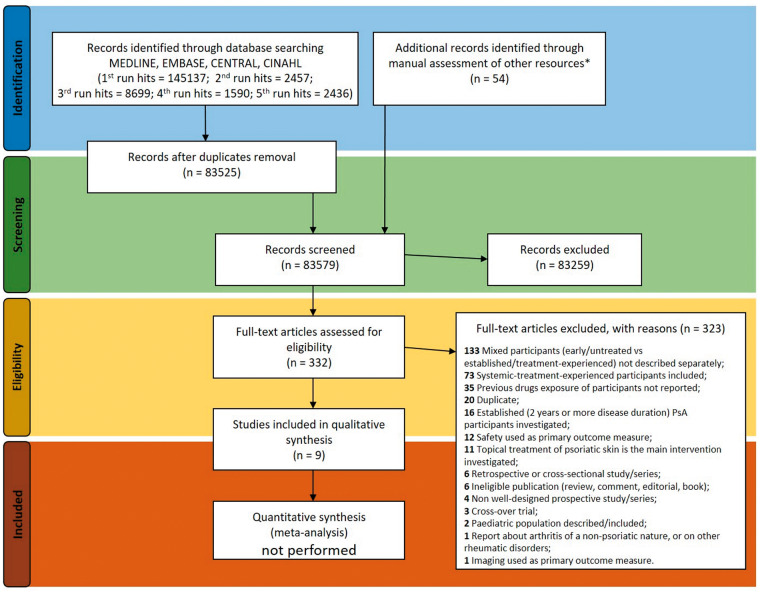
A total of 160 319 references were identified between 19 June 2018 and 17 August 2019, covering all four target languages (Fig. 1). Key information referring to eligibility was often found in the methodology sections and in the summary of baseline characteristics of the single references assessed.
Fig. 1.
Flow diagram of the systematic review activities, in compliance with the PRISMA statement [355]
*Additional resources explored (clinical trials registers and conference proceedings) are described in the Methods section.
Few references (nine in total; Table 1) met the eligibility criteria set by the search protocol. These references were as follows: four full reports published in medical journals [21–24]; one partial report (from proceedings of a conference) about a prospective cohort [25]; one other partial [26] report (from information published on a clinical trials register); and three references that were descriptions of ongoing trials [27–29] with incomplete enrolment to date (at 3 April 2020). Such references were included because the information available upon review suggested that the participants described were treatment naïve.
Table 1.
List of references fulfilling the eligibility criteria set by the search protocol
| Reference (in order of publication, where applicable) | Condition mentioned in inclusion criteria | Condition targeted and primary outcome measure | Intervention | Effect of the intervention | Comments |
|---|---|---|---|---|---|
|
|
|
|
No difference (TJC, SJC, VAS, ESR, CRP, PGA, PhGA) between arms at 6 months |
|
|
|
|
|
85.9% of arm 1 participants achieved PASI75 vs 33.7% of arm 2 (P < 0.001) |
|
|
|
|
|
Mean percentage change in sPGA×BSA greater in arm 1 than in arm 2 (−48.1 vs −10.2%; P <0.0001) | Information available from report suggests that no participant was exposed to systemic/immune-suppressant therapy, for the purpose of treating any condition, before enrolment |
|
|
|
MTX, step up to MTX + bDMARDs if DAPSA remission or MDA not achieved (T2T approach) | 41 participants (53.2%) achieved DAPSA remission or MDA within 7 ± 5 months, using MTX monotherapy (≤25 mg/week) |
|
|
|
|
|
90.7% of arm 1 participants achieved PASI75 vs 22.2% of arm 2 (P < 0.0001) vs 70.4% of arm 3 (P = 0.0137) |
|
|
|
|
|
Not available |
|
|
|
|
|
Not available |
|
|
|
|
|
Not available | Information available from trial register does not exclude that participants might have been exposed to systemic CS or systemic therapy for cutaneous disease before enrolment |
|
|
|
|
|
|
Materials appraised were publicly available documents, such as reports from indexed medical journals (full-text articles), supplementary files and attachments from paper publications or web sites (i.e. clinical trials registers).
ADA: adalimumab; APM: apremilast; bDMARDs: biologic DMARDs; CASPAR: classification criteria [357]; DAPSA: disease activity index for PsA; FAE: fumaric acid esters; GUK: guselkumab; IXK: ixekizumab; MDA: minimal disease activity; MOLL/WRIGHT: classification criteria [356]; MSK: musculoskeletal; NB-UVB: narrow-band ultraviolet B; PASDAS: PsA DAS; PASI75: improvement of the psoriasis area and severity index by 75%; PASI90: improvement of the psoriasis area and severity index (PASI) by 90%; PGA: patient global assessment; PhGA: physician global assessment; PsARC: PsA response criteria; SEC: secukinumab; sPGA: static physician’s global assessment; T2T: treat-to-target; TJC/SJC: tender/swollen joints count; VAS: visual analog scale.
None of the nine studies mentioned above adopted primary outcome measures that assess disease activity across the clinical spectrum of PsD. Moreover, instruments quantifying the disease response restricted to a single feature of PsD were never combined with outcome measures assessing PsD features of a different type as co-primary outcomes. Infrequently, the authors of the original studies had evaluated the effects of their interventions across the clinical spectrum of PsD: only one (out of the nine references that met eligibility criteria) plainly declared that response in cutaneous features would be a secondary outcome assessed alongside the primary MSK measurements. However, it is noteworthy that the majority of references describing ongoing trials did not provide the full trial protocols, making a comprehensive assessment possible only at the time of publication of the final reports.
In general, the interventions, as described in the studies that met the eligibility criteria set by the search protocol, showed efficacy on restricted features of PsD (Table 1). Specifically, the drugs investigated in these studies were fumaric acid esters, LEF, MTX, NSAIDs, SSZ, apremilast and biologic DMARDs (including, but not limited to, adalimumab, guselkumab, ixekizumab and secukinumab). Significant improvements in cutaneous outcome measures (psoriasis area and severity index) were reported for secukinumab, apremilast, guselkumab and ixekizumab. MTX and biologics generated amelioration of MSK features (tender/swollen joint count, disease activity index for PsA or DAPSA, minimal disease activity or MDA, PsA disease activity score or PASDAS, PsA response criteria or PsARC). Representation, in terms of the primary outcomes investigated, of diverse features of PsD (cutaneous and MSK) was fairly balanced. Interestingly, the design of most of these studies was a head-to-head comparison, and the results showed superior efficacy, restricted to cutaneous outcomes, of biologics compared with fumaric acid esters and MTX. Unfortunately, the paucity of data retrieved and the heterogeneity of primary outcome measures adopted prevented an attempt to proceed to quantitative synthesis of the evidence. Such a constraint involved both disease response data and safety data. Consequently, it was deemed appropriate to describe in this report all the nine eligible studies and to forgo a formal quality assessment of these publications. The information available is fully listed and detailed in Table 1.
Excluded references
Of note, in the majority of references deemed not eligible after full-text assessment (30–352, see Supplementary Data S6, available at Rheumatology Advances in Practice online) there were descriptions of different percentages of participants who were, in fact, at early PsD stage or treatment naïve (Table 2). Again, interventions such as MTX, apremilast and biologics were effective in improving MSK symptoms (trials primarily investigating cutaneous outcomes are ongoing). Unfortunately, only aggregate-level reports of the baseline characteristics were available in the methodology sections or in the tables of each original publication. No trial dataset was publicly available to allow selective extraction of information related to the specific subsets of participants who are the focus of this review. Consequently, it was not possible to perform a quantitative synthesis from the excluded references.
Table 2.
Details of references excluded owing to a lack of disaggregate description of participants at baseline
| Characteristic | Number of references (% out of 133 if not specified otherwise) and comments |
|---|---|
| The original study was published in some indexed journal | 117 (87.9), publication year range 1963–2019 |
| The original study was a randomized clinical trial (inclusive of single blinded) | 119 (90.1) |
| Of which statistically powered | 78/119 (65.6) |
| PsD feature required for the enrolment of participants | |
| Cutaneous only | 101 |
| Musculoskeletal only | 15 |
| Musculoskeletal and cutaneous | 9 |
| Cutaneous and musculoskeletal | 3 |
| Cutaneous and metabolic | 2 |
| PsD feature assessed by the primary outcome measure | |
| Cutaneous | 108 (81.9) |
| Musculoskeletal | 24 (18.4) |
| Treatment-naïve participants described | 91 (68.4) |
| Participants at early clinical stage described | 94 (70.7) |
The original reports did mention participants who were contemporaneously treatment naïve and/or at early stage of their condition. References identified that fulfilled these criteria: 133/332 examined by full-text assessment (see flow diagram, Fig. 1).
PsD: psoriatic disease.
Another analysis carried out on the excluded references pertained to the features of PsD selected in the original publications. As shown in Table 2, none of these studies adopted, as a primary outcome measure, composite indices assessing disease activity across the clinical spectrum of PsD. Furthermore, indices evaluating disease response in single PsD features were not combined as co-primary outcomes. However, taking the results from a different perspective, ∼10% of studies among excluded references required the co-occurrence of cutaneous and MSK features of PsD (or vice versa) to allow enrolment. Moreover, 61 original studies (45.9% out of 133 references) did report on several features of PsD among the secondary outcomes at baseline. Furthermore, in 26 of these studies a follow-up of the same secondary outcomes was available at the time of the primary endpoint.
Finally, only a few studies reported that the participants originally enrolled were treatment naïve and at an early stage of PsD. A summary is available in Table 3. The trials mentioned therein are particularly interesting owing to their innovative designs, which were often not limited to a single comparison (i.e. single intervention vs placebo). Again, quantitative synthesis was not possible owing to lack of disaggregate participant descriptions or lack of availability of the original datasets.
Table 3.
A selection of references that formally did not meet the eligibility criteria of this review. A full list of excluded references 30–352 can be found in Supplementary Data S6, available at Rheumatology Advances in Practice online.
| Reference (in order of publication, where applicable) | Condition mentioned in inclusion criteria | Condition targeted and primary outcome measure | Intervention | Effect of the intervention | Comments |
|---|---|---|---|---|---|
|
PsA: peripheral arthritis |
|
|
Higher odds of ACR20 response in arm 1 than in arm 2 (OR 1.91, 95% CI 1.03, 3.55, P = 0.0392) | Participants treated with DMARDs, for the purposes of controlling cutaneous symptoms, before enrolment could meet eligibility criteria |
|
PsA: peripheral arthritis |
|
|
Larger proportion of clinical remission achieved in participants of arm 1 vs arm 2 (75 vs 20%; P < 0.001) |
|
|
PsA: peripheral arthritis |
|
|
Larger proportion of ACR20 achieved in participants of arms 2 and 3 vs arm 1 (28 and 30.7 vs 15.9%; (P = 0.0062 and 0.001, respectively) |
|
|
PsA: peripheral arthritis |
|
|
Larger proportion of DAS remission achieved in participants of arm 1 vs arm 2 (81 vs 42%; P = 0.004) |
|
|
PsA: peripheral arthritis |
|
|
Larger proportion of ACR20 achieved in participants of arm 1 and arm 2 vs arm 3 (P = 0.005 and 0.029, respectively) | Use of DMARDs before enrolment in 12.6% of participants |
|
PsA: peripheral arthritis |
|
|
Not available |
|
The majority of participants originally enrolled in the studies described in this table were treatment naïve and at an early stage of PsD.
ACR20: American College of Rheumatology improvement criteria, improvement by 20%; ACR50 is improvement in the same parameters by 50%; APM: apremilast; BSA: body surface area; csDMARD: conventional synthetic DMARD; bDMARD: biologic DMARD; ETN: etanercept; GOL: golimumab; MDA: minimal disease activity; mNAPSI: modified nail psoriasis and severity index; OR: odds ratio; PASI75: improvement of the psoriasis area and severity index (PASI) by 75%; PASI50 and PASI90 are improvement of PASI by 50% and 90%, respectively; PBO: placebo; PsD: psoriatic disease; SEC: secukinumab; sPGA: static physician’s global assessment; T2T: treat-to-target; TFC: tofacitinib.
Other findings
The assessment of references through full-text reading demonstrated that the search strategy appropriately identified the variety of phenotypes of psoriasis. These ranged from plaque psoriasis to psoriatic nail changes, palmoplantar psoriasis (hyperkeratotic, pustular), genital psoriasis, scalp psoriasis, erythrodermic psoriasis, generalized pustular psoriasis and inverse (skin-fold) psoriasis. The MSK clinical spectrum was also fairly represented, with the notable exception of axial involvement.
A useful by-product of the search strategy adopted was the identification of investigational drugs not originally included in the search terms. Although this development was noticed at an early stage during the selection activities (i.e. screening by title and abstract), it was decided to present a limited report restricted to the references subjected to full-text assessment. Overall, 39 compounds (synthetics, monoclonal antibodies and other molecules) not listed in the search protocol were found (Supplementary Table S1, available at Rheumatology Advances in Practice online).
Discussion
The main finding of this study confirms the hypothesis underpinning the project: few references described interventions in early, untreated PsD; only nine studies met the eligibility criteria, and none demonstrated efficacy across the clinical spectrum of PsD adopting comprehensive outcome measures. This conclusion is also supported by a robust search strategy, designed to maximize sensitivity and capable of reaching beyond the planned search terms. In fact, many identified studies did investigate drugs not originally encompassed in the protocol of this project, including agents such as tildrakizumab and bimekizumab alongside others (Supplementary Table S1, available at Rheumatology Advances in Practice online). It is also worth mentioning that the search strategy designed for this review allowed the retrieval of studies that adopted outcome measures that were not formally encompassed in the research questions [18]. This further expanded the comprehensiveness of the search activities.
Although each study assessed in this review had its own specific eligibility criteria, a few comments are appropriate. Firstly, a substantial number of references were excluded at full-text assessment stage because of the lack of disaggregated data. Namely, it was not possible to extract data pertaining to early, untreated PsD from the samples described in single studies. We would encourage future researchers to share comprehensive datasets for later analyses. Secondly, it was noticed, mainly in rheumatology reports, that MTX-naïve does not equate to treatment-naïve PsD. Trials of such design are enrolling participants who are at an early stage of PsD and yet already exposed to other systemic agents.
Third, the relevant difference in terminology between dermatology researchers and rheumatologists (systemic drugs vs DMARDs) would benefit from clarification. In this review, discerning the eligibility criteria of the studies under assessment required substantial effort, especially in evaluating the exposure to medications before enrolment. Theoretically, any study aiming at enrolling genuine treatment-naïve PsD participants should set eligibility criteria requiring the following: (a) exclusion of patients previously treated for the target condition; (b) exclusion of patients previously treated for PsD features that are not the target condition; and (c) exclusion of patients who were treated with PsD drugs but for different indications (i.e. MTX for neoplasms). Ideally, future clinical trials would incorporate a multidisciplinary approach, assessing the effects of interventions across the heterogeneous clinical domains of PsD. Such a methodological repositioning would allow a deeper appreciation of recent and upcoming therapies. Alternatively, the availability in the public domain of disaggregated trial data would enable later research initiatives to perform separate analyses focused on the clinical spectrum of early, untreated PsD.
Lastly, this review confirmed previous findings [353] about the limited evidence related to the use of systemic CSs in PsD, which are an option for the management of MSK manifestations. Despite concerns related to the potential for triggering cutaneous flares of PsD or producing long-term side effects, clinical trials often allow systemic CSs, especially in rheumatology. This review did not identify any evidence about the short- or long-term effect of CSs in early, untreated PsD. The potential role of CSs, alone or in combination with immune modulators, remains under-investigated.
Limitations
The main limitation of this review is the lack of consensus on the definition of an early stage in PsD. Research from dermatology tends to label psoriasis as chronic when its duration is >6 months, whereas a clear definition of early PsA is lacking in the rheumatology literature [8]. The consensus in the panel relied on a clinical perspective, adopting the time limit of 2 years for MSK features and leaving no limitations for cutaneous features. Ideally, future definitions of early stage might rely only on biomarkers supplemented by clinical phenotype stratification.
Another limitation of this review is that the LILACS repository [354] was not explored. However, it seems unlikely that citations in that database would have been missed by the search terms and strategies adopted for this project. The substantial yield of references, identified from a range of resources explored adopting an extended timeline of publication, decreases the chances of having missed relevant studies.
Conclusions
In conclusion, this review identified the paucity of evidence in early, untreated PsD. No study used composite measures, assessing the full clinical spectrum of PsD, as the primary outcome measure. Furthermore, even separate clinical features of PsD were not adopted as a co-primary endpoints in early, untreated PsD. The co-occurrence of cutaneous and MSK manifestations underscores the clinical importance of producing evidence that addresses efficacy across the PsD spectrum, in order to overcome the present limitations of treatment guidelines. This review succeeded in highlighting an unmet need of the research agenda in cutaneous psoriasis and PsA.
Supplementary Material
Acknowledgements
This paper presents independent research supported by the National Institute for Health Research (NIHR) Leeds and Oxford Biomedical Research Centre (BRC), UK. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The Group for Research into Psoriasis and Psoriatic arthritis (GRAPPA) supported this project. The authors are grateful to Dr John Arnold, NHMRC, C. J. Martin, Research Fellow & Lecturer, School of Health Sciences, University of South Australia, Adelaide, who kindly facilitated the part of the search activities performed in the CINAHL database.
Conceptualization—design of the review strategy, such as research questions and search protocol: all the authors. Funding acquisition: not applicable. Methodology of the systematic search methods: G.D.M., A.O.B., J.E., D.D.G. and L.M. Databases searches, all runs: G.D.M. Conference proceedings and clinical trials repositories search: A.B., L.C.C., S.D. and A.M. Reference selection, step 1 (screening by title and abstract): G.D.M. and A.M. Reference selection, step 2 (full text assessment): G.D.M. and H.M.-O. Data extraction: E.L., D.M.G., F.M. and M.W. Project administration: G.D.M. Manuscript original draft: all authors. Review and editing: all authors. Supervision: H.M.-O. and P.S.H.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement: A.B., S.D., G.D.M. and M.F. have no conflicts to disclose. L.C.C. has received research grants from Abbvie, Celgene, Janssen, Novartis and Pfizer. She has received consulting fees and/or honoraria from Abbvie, Amgen, BMS, Celgene, Galapagos, Gilead, Janssen, Lilly, MSD, Novartis, Pfizer, Prothena, Sun Pharma and UCB. D.D.G. has received consultancy fees and/or grant support from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB. P.S.H. has received consulting fees and/or speaking fees from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly and Company, Janssen, Leo, Novartis, Sun Pharma and UCB. E.L. has received consultancy fees as speaker from Abbvie, Celgene, Novartis and Pfizer. D.M.G. has received grants and/or honoraria from Abbvie, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer and UCB. A.M. has received honoraria from Abbvie, BSM, Celgene, Janssen, MSD, Novartis, Pfizer and UCB. H.M.-O. has received grants and/or honoraria from Abbvie, Celgene, Janssen, Lilly, Novartis, Pfizer and UCB. A.O.B. has received consultancy honoraria from Abbvie, Amgen, BMS, Corrona, Lilly, Novartis, Pfizer and Takeda. She has received grants from Novartis, NIH, Pfizer and Rheumatology Research Foundation. M.W. has received consultancy honoraria from Abbvie, Celgene, Janssen, L’Oreal, UCB, Biogen and Novartis.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References 30–352 were excluded after full text assessment. A list of excluded references can be found in Supplementary Data S6, available at Rheumatology Advances in Practice online.
References
- 1. Scarpa R, Ayala F, Caporaso N, Olivieri I. Psoriasis, psoriatic arthritis, or psoriatic disease? J Rheumatol 2006;33:210–2. [PubMed] [Google Scholar]
- 2. Scarpa R, Altomare G, Marchesoni A et al. Psoriatic disease: concepts and implications. J Eur Acad Dermatol 2010;24:627–30. [DOI] [PubMed] [Google Scholar]
- 3. Helliwell P, Marchesoni A, Peters M, Barker M, Wright V. A re-evaluation of the osteoarticular manifestations of psoriasis. Br J Rheumatol 1991;30:339–45. [DOI] [PubMed] [Google Scholar]
- 4. Ciocon DH, Kimball AB. Psoriasis and psoriatic arthritis: separate or one and the same? Br J Dermatol 2007;157:850–60. [DOI] [PubMed] [Google Scholar]
- 5. Ritchlin C. Psoriatic disease—from skin to bone. Nat Clin Pract Rheumatol 2007;3:698–706. [DOI] [PubMed] [Google Scholar]
- 6. Chimenti MS, Caso F, Alivernini S et al. Amplifying the concept of psoriatic arthritis: the role of autoimmunity in systemic psoriatic disease. Autoimmun Rev 2019;18:565–75. [DOI] [PubMed] [Google Scholar]
- 7. Greb JE, Goldminz AM, Elder JT et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- 8. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019;15:153–66. [DOI] [PubMed] [Google Scholar]
- 9. Michalek IL, John SM. WHO Global Report on Psoriasis. 2016. http://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf; jsessionid=FEF989BAC6EF104C9AD874429D823C10?sequence=1 (11 November 2019, date last accessed).
- 10. van de Kerkhof PC, Reich K, Kavanaugh A et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Eur Acad Dermatol Venereol 2015;29:2002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menter A, Strober BE, Kaplan DH et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019;80:1029–72. [DOI] [PubMed] [Google Scholar]
- 12. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coates LC, Kavanaugh A, Mease PJ et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 14. Megna M, Balato A, Napolitano M et al. Psoriatic disease treatment nowadays: unmet needs among the “jungle of biologic drugs and small molecules”. Clin Rheumatol 2018;37:1739–41. [DOI] [PubMed] [Google Scholar]
- 15. Gossec L, Smolen JS, Ramiro S et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 16. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. 2019. https://www.grappanetwork.org/ (15 January 2020, date last accessed).
- 17. Methodological Expectations of Cochrane Intervention Reviews (MECIR) Manual. 2019. https://community.cochrane.org/mecir-manual (15 January 2020, date last accessed).
- 18. De Marco GB, Coates LC, Dubash S et al. Non topical pharmacological treatment of early, untreated (DMARD-naïve, systemic therapy-näive) psoriatic disease: a systematic review. 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=97366 (15 January 2020, date last accessed).
- 19. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation, 2019. www.covidence.org (15 January 2020, date last accessed).
- 21. Reich K, Augustin M, Thaçi D et al. A 24-week multicentre, randomised, open-label, parallel-group study comparing the efficacy and safety of ixekizumab to fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. Br J Dermatol 2020;182:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scarpa R, Peluso R, Atteno M et al. The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol 2008;27:823–6. [DOI] [PubMed] [Google Scholar]
- 23. Sticherling M, Mrowietz U, Augustin M et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. 2017;177:1024–32. [DOI] [PubMed] [Google Scholar]
- 24. Strober B, Bagel J, Lebwohl M et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol 2017;16:801–8. [PubMed] [Google Scholar]
- 25. Loginova E, Korotaeva T, Gubar E, Gluk-Hova S, Nasonov E. Attainment of remission and minimal disease activity after starting methotrexate subcutaneous therapy. Acta Derm Venereol 2018;98(Suppl 219):10–1. [Google Scholar]
- 26. NCT. A study to compare the efficacy of guselkumab to fumaric acid esters for the treatment of participants with moderate to severe plaque psoriasis. 2016. http://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/701/CN-01559701/frame.html (15 January 2020, date last accessed).
- 27.CTRI. Comparison between methotrexate versus methotrexate plus leflunomide treatment in arthritis associated with psoriasis. 2017. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01885872/full (15 January 2020, date last accessed).
- 28. Euctr GB. Severe psoriatic arthritis—early intervention to control disease: the SPEED trial. 2018. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01909380/full (15 January 2020, date last accessed).
- 29. Iversen L, Eidsmo L, Austad J et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification – rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol 2018;32:1930–9. [DOI] [PubMed] [Google Scholar]
- 353. Gossec L, Smolen JS, Gaujoux-Viala C et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 2012;71:4–12. [DOI] [PubMed] [Google Scholar]
- 354. Latin American and Caribbean Health Sciences Literature (LILACS). 2019. https://lilacs.bvsalud.org/en/ (15 January 2020, date last accessed).
- 355.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Med 2009;6(7). [PMC free article] [PubMed]
- 356. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78. [DOI] [PubMed] [Google Scholar]
- 357. Taylor W, Gladman D, Helliwell P et al. ; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.