ABSTRACT
Zika virus (ZIKV) is an emerging arthropod-borne flavivirus that, upon infection, results in teratogenic effects and neurological disorders. ZIKV infections pose serious global public health concerns, prompting scientists to increase research on antivirals and vaccines against the virus. These efforts are still ongoing as the pathogenesis and immune evasion mechanisms of ZIKV have not yet been fully elaborated. Currently, no specific vaccines or drugs have been approved for ZIKV; however, some are undergoing clinical trials. Notably, several strategies have been used to develop antivirals, including drugs that target viral and host proteins. Additionally, drug repurposing is preferred since it is less costly and takes less time than other strategies because the drugs used have already been approved for human use. Likewise, different platforms have been evaluated for the design of vaccines, including DNA, mRNA, peptide, protein, viral vectors, virus-like particles (VLPSs), inactivated-virus, and live-attenuated virus vaccines. These vaccines have been shown to induce specific humoral and cellular immune responses and reduce viremia and viral RNA both in vitro and in vivo. Importantly, most of these vaccines have entered clinical trials. Understanding the viral disease mechanism will provide better strategies for developing therapeutic agents against ZIKV. This review provides a comprehensive summary of the viral pathogenesis of ZIKV and current advancements in the development of vaccines and drugs against this virus.
KEYWORDS: Antivirals, pathogenesis, sfRNAs, therapeutics, vaccines, zika virus
Graphical abstract
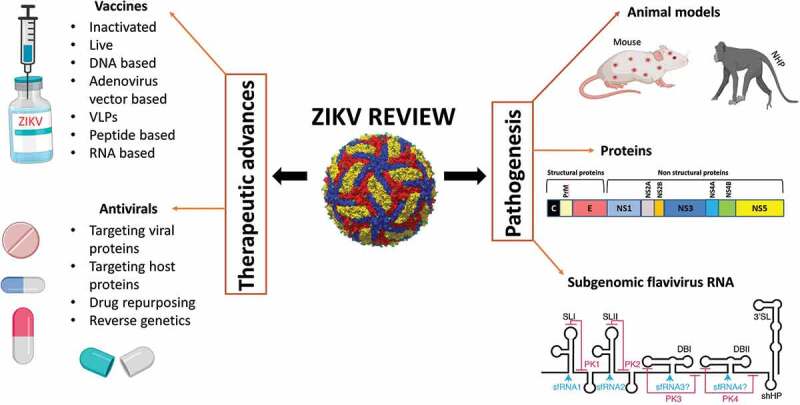
Introduction
Zika virus (ZIKV) is a mosquito-borne, single-stranded positive-sense, enveloped RNA virus that belongs to the Flaviviridae family [1]. ZIKV is closely related to other flaviviruses, such as Dengue virus (DENV), West Nile virus (WNV), Yellow Fever (YFV), and Japanese Encephalitis virus (JEV). Since its first isolation in Uganda (1947), sporadic global spread of ZIKV has been observed over the last 7 decades. ZIKV was first isolated from a sentinel rhesus monkey [2], with the first human isolation reported in Nigeria in 1952 [3]. Despite the virus’s long history and sporadic infection cases, it was not until 2016 that the WHO declared the virus to be a global public health emergency due to an expansive and major outbreak in Brazil [4]. Phylogenetic analysis of ZIKV shows that the virus is spread in 3 lineages: West African, East African, and Asian [5]. Currently, no specific vaccines or drugs are approved for ZIKV; however, some are undergoing clinical trials. Of note, the pandemic currently seems to be declining despite the absence of therapeutic measures.
ZIKV is primarily transmitted by Aedes mosquitoes; however, other forms of transmission exist, including vertical [6], sexual [7], blood, and laboratory acquired [8]. There is also the possibility of acquiring ZIKV through body fluids, such as stool [9], semen [10], breast milk [11], urine [12] and saliva [13]. Contrary to other mosquito-borne flaviviruses, ZIKV is a unique flavivirus because it can persist for months in immune-privileged sites, such as the eyes and testes [14,15]. The wide range of tropism of ZIKV is summarized in Figure 1, which also explains the unique characters of this virus. Approximately 80% of ZIKV infections are asymptomatic, with the 20% of symptomatic infections resulting in ZIKV-associated infections, such as microcephaly in children [16] and Guillan Barre` syndrome in adults. Other congenital ZIKV-related diseases in children include cerebral malformations, ophthalmological and hearing defects, and arthrogryposis [17]. Vector-borne transmission of ZIKV occurs in two mosquito-driven cycles: (i) a sylvatic cycle, in which the virus cycles between non-human primates (NHPs) and arboreal mosquitoes; (ii) an urban cycle, in which the virus cycles between humans and urban mosquitoes [18]. ZIKV is mostly maintained in the sylvatic cycle [19–21]. However, in this type of transmission, humans are usually incidental hosts. Currently, direct human to human transmission has become common in the urban infectious cycle as the virus can be spread perinatally [6], sexually [7], and through breastfeeding [11] or blood transfusion [8], as mentioned above, making ZIKV a human pathogen. Despite zoonotic transmission being primarily maintained by monkeys and mosquitoes, ZIKV antibodies have also been detected in other animal species, including water buffalo, elephants, goats, hippos, impala, kongoni, lions, sheep, rodents, wildebeest, and zebras [22,23].
Figure 1.
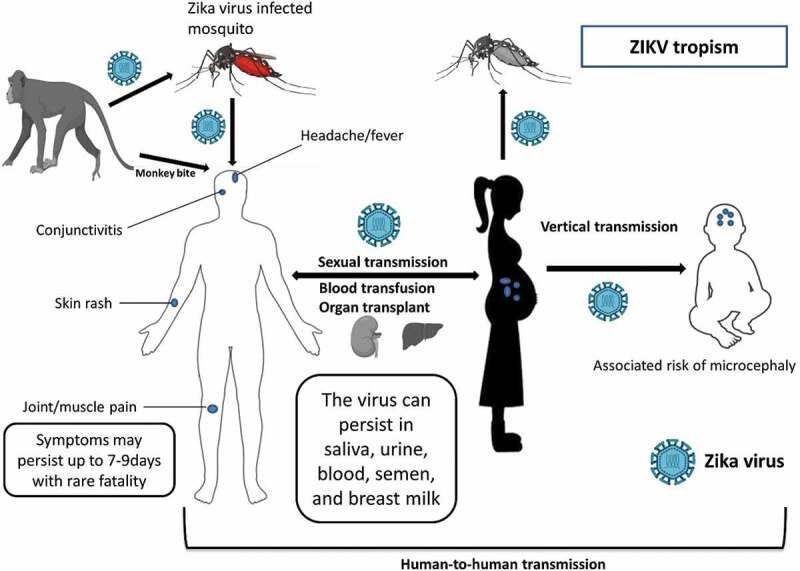
The biology of ZIKV, showing the transmission modes, tropism, and disease symptoms associated with ZIKV
ZIKV is a spherical, enveloped virus with icosahedral symmetry. The diameter of the mature virus is approximately 50 nm, and the diameter of the immature virus is closer to 60 nm [24–27]. ZIKV has a positive-sense single-stranded RNA genome approximately 10.7 kilobases in length. Located at the 5ʹ and 3ʹ ends of the genome are two flanking untranslated regions (UTRs), which in the ZIKV MR766 strain are 106 and 428 nucleotides long, respectively [28]. The ZIKV genome lacks a 3ʹ poly (A) tract and ends with CUOH, similar to other flaviviruses. The genome contains a single open reading frame (ORF) that encodes a polyprotein composed of approximately 3400 amino acids [28], which after being processed by host and viral proteases, yields three structural proteins (C, PrM, E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5). In this review, we examine various studies to discuss the pathogenesis of ZIKV and the current development of therapeutics for treating infections of this virus.
ZIKV pathogenesis
Little is known about the pathogenesis of ZIKV since it involves complex interactions between viral and host factors. The molecular mechanisms of virus–host interactions have been studied both in vitro and in vivo to provide more insight into the pathogenesis of ZIKV. The fact that ZIKV causes teratogenic effects conveys an urgent need for the rapid development of antiviral therapies [29]. WNV and Powassan virus (POWV) have also been shown to result in significant fetal injury in mice [30]. ZIKV infection begins with a single mosquito bite of a ZIKV-infected person. Viral replication begins in the epithelial cells of the mosquito midgut and proceeds to the salivary glands. The mosquito can spread the virus after a 10-day incubation period, when its saliva becomes infected [31]. The incubation period in humans is 3–12 days [32], and symptoms appear after 6–11 days. ZIKV is cleared within 24 days in 99% of patients [33].
Pathogenesis in humans
Following a mosquito bite from a ZIKV-infected mosquito, ZIKV infects and replicates in dendritic cells, spreading through the blood to other parts of the human body. In most cases, the virus is self-limiting; however, infections in pregnant women result in teratogenic effects [17]. In pregnant women, viral infection extends for a longer period since the virus replicates in the fetal brain for months with increasing effects during the early months of pregnancy [34].
ZIKV first binds to cellular receptors that are specific to different flaviviruses. ZIKV receptor members include DC-SIGN (dendritic cell-specific intracellular adhesion molecule 3-grabbing nonintegrin) and phosphatidylserine receptor proteins: TYRO 3, AXL, TIM, and TAM [35]. These receptors facilitate ZIKV entry into macrophages, monocytes, neural progenitor cells (NPCs), and fetal cells. These receptors play important roles in ZIKV infection, such as adhesion, migration, replication and evasion of the immune system, cytokine release, and antigen signaling pathways [35]. Experimentally, knocking out receptors does not result in total protection from viral infection because different flaviviruses use different receptors for entry [36].
AXL appears to play a major role in viral pathogenesis because it makes human skin fibroblasts permissive to ZIKV infection and replication [35]. AXL is highly expressed in the developing human cortex throughout neurogenesis and is overexpressed in glial cells. Surprisingly, studies by Wells et al. have shown that AXL is not required for ZIKV infection, even if it is highly expressed in NPCs: genetic ablation of AXL does not protect human NPCs or cerebral organoids from ZIKV infection [36] and an anti-AXL antibody does not reduce ZIKV infection in NPCs [37]. Hastings et al. also demonstrated that TAM receptors are not essential for ZIKV infection [38]. Generally, these studies suggest the existence of redundant entry receptors for ZIKV infection. TIM-1 (T cell immunoglobulin mucin domain 1) is a predominant ZIKV entry factor expressed in human placental cells [6]. In skin fibroblasts, ZIKV induces the expression of pattern recognition receptors (PRRs), such as toll-like receptors (TLR3), RIG-1, and melanoma differentiation associated gene 5 (MDA5), enhancing the antiviral response against ZIKV infection [35].
Pathogenesis in animal models
Mouse models
Animal models have been used to study ZIKV infection in pregnant women and fetuses to optimize the development of vaccines and antiviral therapies [39]. Neonatal mouse models have been used to study the pathogenesis of ZIKV because they are more vulnerable to ZIKV infection than adult mice. Immunocompetent adult mice also demonstrate extensive ZIKV tropism in the brain, blood, spinal cord, spleen, kidney, and eye. Male Ifnar-deficient mice show high levels of ZIKV in their testes, providing more insights into the persistence of ZIKV in immunocompromised tissues [40]. Rag-1 mice also exhibit pathogenesis of ZIKV in the epididymis and testicular cells [15].
The A129 mouse model, which is deficient in type-I IFN receptor, has also been used to study ZIKV pathogenesis. Different routes of viral administration have been used, including intraperitoneal and intravaginal. Three-week old mice infected with ZIKV (FSS13025) exhibited neurological disease and a high fatality rate from 6 days postinfection. However, older mice (11 week old) displayed 50% mortality in the 5th week and no mortality in the 11th week [41]. A129 mice infected with the MR766 (African) strain also exhibited weight loss and high mortality 6 days postinfection. Viral titers were highest in the brain and spleen [42], and a similar viral tropism was observed in an Asian lineage strain, except that it did not cause severe disease [43].
AG129 mice, with deficiencies in both type I and II IFN receptors, were injected intraperitoneally with ZIKV (FSS13025) and displayed neurologic disease with a high mortality rate 6 days postinfection [41]. However, intravaginal administration of the virus had a delayed lethality that manifested 22 days postinfection [44]. The H/PF/2013 (Asian) strain displayed lethality at 8 days postinfection, with the highest virus titers quantified on day 2 [45]. AG129 mice injected intraperitoneally with the MR766 (African) strain were characterized by a hunched back and hind-limb paralysis and succumbed 18 days postinfection [46]. In the same study, SCID mice were used in comparison with AG129 mice injected with the same strain. Surprisingly, lethality was delayed in SCID compared to AG129 mice. Mice succumbed 40 days postinfection on average [46].
The pathogenesis of ZIKV in the central nervous system (CNS) was also studied in the Swiss mouse model. One-day-old mice injected with ZIKV (SPH 2015) displayed paralysis and neurological disease characterized by inflammation within the cerebral cortex [47]. BALB/c immunosuppressed mice injected with ZIKV PRVABC59 (Asian) displayed widespread viremia and inflammation of various tissues, especially orchitis, which may result in male infertility; however, treatment with Type 1 interferon (IFN 1) greatly reduced ZIKV infection [48]. Seven-week old C57BL/6 mice challenged with the ZIKV H/PF/2013 (Asian) and Senegal 1984 (African) strains also displayed widespread virus dissemination to immunosuppressed tissues, such as the testis and epididymis. Treatment with IFN 1 greatly improved the clinical outcomes of the mice [15].
Six-week-old TKO mice were used to study ZIKV dissemination to the CNS using ZIKV FSS13025 (2010 Cambodian isolate). Mice were more susceptible to ZIKV infection since they lacked 3 interferon regulatory factors (IRF3, IRF5, and IRF7), and ZIKV infection resulted in the death of neural progenitor cells [49]. Pregnant female mice have also been used to demonstrate infection by ZIKV in different trophoblasts and fetal endothelial cells of the placenta, resulting in congenital malformations [34].
Non-human primate (NHP) models
Rhesus macaques have been used to study ZIKV pathogenesis in the brain using a French Polynesian ZIKV strain [50], a finding reported across New World and Old World macaque species [51]. ZIKV RNA accumulates in the brain, cerebrospinal fluid, urine, and saliva for at least 3 weeks. This study showed that ZIKV infection elicited host immune responses that include ZIKV-specific T-cells and nAb responses. Prolonged detection of viral RNA in urine and saliva, even after viral clearance in the blood, showed that ZIKV persisted in certain tissues [50]. Pigtail macaques infected with a Cambodian ZIKV isolate (FSS13025) were shown to develop fetal brain lesions during their pregnancy [52]. This was the first reported case of a fetal brain injury in a NHP after maternal infection with ZIKV infection. The pigtail macaque model provides a novel model for testing vaccines and other therapeutics against ZIKV [52]. Another study involving rhesus and cynomolgus macaques infected with 2 ZIKV isolates of Thai and Puerto Rican origin showed that ZIKV also persisted for more than 3 weeks in the saliva and semen, even after no traces of virus remained in the blood. This study demonstrated that ZIKV infection elicited rapid innate and adaptive immune responses in macaques, protecting them from reinfection [53].
NHPs have provided an ideal model for studying infections during pregnancies since they mimic humans in many ways, including placenta and brain development. These models have also shown susceptibility to many other flaviviruses, making them excellent models for ZIKV studies.
ZIKV protein functions and role in viral pathogenesis
The ZIKV genome is translated into 3 structural proteins (C, PrM and E) and 7 nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5), which are summarized in Figure 2. The structural proteins are essential for genomic replication and host immunity [54].
Figure 2.
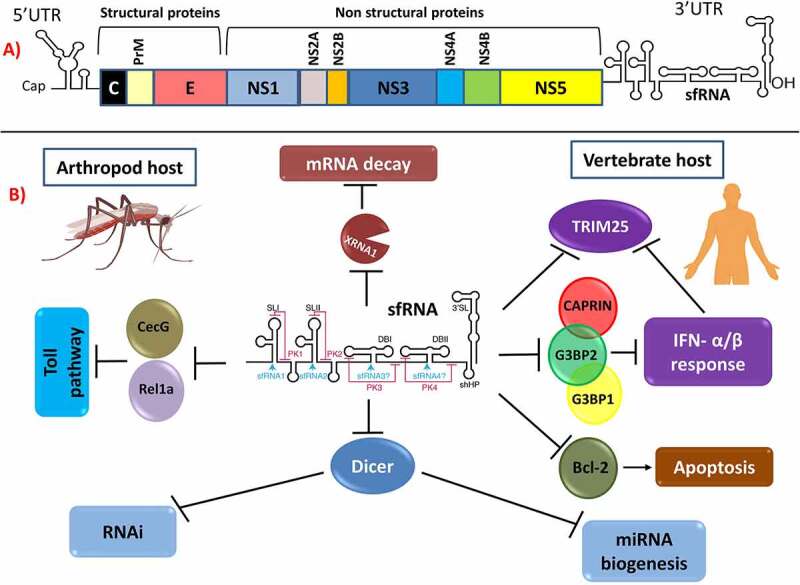
Subgenomic flaviviral RNA location and its roles in pathogenesis. (a) ZIKV genome scheme showing the location of sfRNA at the 3ʹUTR. (b) sfRNA pathogenic functions in arthropod and vector hosts. (a) and (b) are reproduced with permission and minor modifications from Slonchak and Khromykn [78]
Glycoprotein E is a major target for antibodies as it is responsible for facilitating viral entry into the host. ZIKV is capable of mediating the antibody response by the changing amino acids that surround the Asn154 glycosylation site in the virus E protein [55], which implicates glycosylation as an important player not only in the structure and tropism of the virus but also in its pathogenesis and evasion of the immune system.
The primary roles of PrM and capsid in ZIKV pathogenesis are still not well defined. However, the PrM protein has a conserved region that is responsible for viral maturation, egression and secretion [56]. Therefore, inhibiting the function of PrM will interfere with viral infectivity and pathogenicity. The capsid protein has been shown to play an important role in viral assembly since it is the primary structural protein that interacts with the viral genome within the viral particle [57]. Generally, flavivirus capsids have been shown to localize in the nucleoli in addition to their association with the phospholipid membranes of the ER and lipid droplets. Localization of the capsid in the nucleoli may indicate that it plays a role in pathogenesis apart from viral packaging [57].
NS are important for entry, translation, replication and pathogenesis of the virus. However, little information is known about the functional significance of ZIKV NS proteins or their role in ZIKV-induced pathogenesis. NS proteins play a major role in evading host immune responses specifically by interfering with the interferon (IFN) I response [58]. NS1 has been described as a pathogenicity factor and the most enigmatic protein of flaviviruses since it plays a major role in replication, as well as in immune evasion. NS1 is secreted from infected cells in the form of hexamers (sNS1), which are the hallmark of flavivirus NS1 [59]. Notably, sNS1 has been used as a ZIKV diagnostic marker and incorporated into vaccine development [59].
ZIKV NS1 and NS4B inhibit IFN I expression, leading to autophagic degradation of NS2B and NS3 through the autophagy pathway, inhibiting viral replication [58]. NS1 and NS4B suppress IFN I signaling by targeting TBK1, consequently inhibiting the production of interferons. NS2B3 (NS2B-NS3) has been shown to inhibit the JAK-STAT pathway by enhancing degradation of Jak-1. It is therefore clear that the co-operation among NS1, NS2B3 and NS4B proteins generally helps to enhance viral replication by evading IFN 1 responses.
The NS5 protein is the largest (approximately 900 amino acids) and most conserved of the flavivirus proteins. The NS5 protein contains a methyltransferase for RNA capping and a polymerase for viral RNA synthesis [60]. NS5 is also an IFN antagonist that degrades STAT2, which in turn, limits type I IFN signaling and leads to increased viral replication. STAT2 is a signaling molecule required in the IFN I pathway. The mechanism of STAT2 degradation in ZIKV by the NS5 protein is distinct from that in DENV. Expression of ZIKV NS5 alone results in STAT 2 degradation and does not require maturation of the N terminus of NS5 and does not involve UBR4 [61]. The interaction is also host-specific since NS5 is unable to degrade murine STAT2, leading to susceptibility to ZIKV infection in immunocompetent mice. ZIKV has been shown to bind and degrade STAT2 through proteasomal degradation. Antagonism of STAT1 and STAT2 phosphorylation results in ZIKV disease [62].
ZIKV NS2A has been shown to play a central role in recruiting viral RNA, the structural protein prM/E, and the viral NS2B/NS3 protease to the virion assembly site and in engineering virion morphogenesis [63]. A single mutation interfering with these interactions did not significantly affect viral RNA replication but selectively abolished virion assembly, demonstrating the specific role of these interactions in viral morphogenesis [64].
The flavivirus NS4A and NS4B proteins have also been shown to inhibit JAK/STAT and RLR signaling through multiple mechanisms [65]. In ZIKV, overexpression of NS4A and NS4B in fetal neuronal stem cells (fNSCs) reduces neurosphere formation and inhibits differentiation [66]. The effects of these two proteins were further linked to increased mediated Akt-mTOR signaling and were ZIKV-specific because DENV failed to show a similar effect [66].
sfRNA functions and role in viral pathogenesis
Subgenomic flavivirus RNA (sfRNA) is a highly structured 0.3–0.7 kb long noncoding RNA and is said to be the most abundant viral RNA species in infected cells. sfRNA is produced in all arthropod-borne flaviviruses during viral infection and represents the 3ʹ-terminal highly conserved region of the 3ʹ UTR (untranslated region) [67]. sfRNAs have many isoforms with different 5ʹ ends, including sfRNA-1, sfRNA-2, sfRNA-3, sfRNA4, and other new isoforms yet to be described.
ZIKV-infected cells produce sfRNAs in their 3ʹ-untranslated regions that accumulate during infection and resist degradation by host 5ʹ-3ʹ exonucleases in infected cells, such as XRN1. ZIKV-infected cells have been suggested to play a role in the replication cycle of the virus and in evasion of the immune system [68,69].
Flavivirus sfRNAs contain stem-loop (SL) and dumbbell (DBL) structures made up of nucleotides that form pseudoknots (PK). During ZIKV infection, two XRN1 resistant RNA are produced, xrRNA1 as a result of XRNA stalling at SL1 and xrRNA2 as a result of XRNA stalling at SL2 [68,70]. These two RNAs form three-way junctions of coaxial stacking of helices P1 and P2, while P3 is located at the acute angle of P1. Three-way junctions are highly structured elements of nucleic acids, such as rRNA, and have a unique topology in ZIKV. These two different resistant RNAs can occur as the result of cellular mechanisms [70].
sfRNAs have several functions in flavivirus infections, as summarized in Figure 2. First, these RNAs are directly linked to cytopathic effects (CPEs). In a cell culture experiment of the pathogenicity of WNV and DENV, genomes harboring mutations that interfered with the formation of full-length sfRNA produced no visible plaques, and replication was inefficient in both insect and mammalian cell lines [67]. Funk et al., demonstrated the role of sfRNA in pathogenesis both in vitro (Vero cells) and in vivo (mice). All mutant viruses that did not produce sfRNA1 were highly attenuated in mice and can be used as potential vaccine candidates [71].
The second function of sfRNAs is suppression of IFN responses in vertebrates. ZIKV sfRNA functions both as a RIG-1 and MDA-5 agonist and has stronger activity than DENV serotype 2, which only affects RIG-1 [68]. In WNV, sfRNAs directly antagonize IFN- stimulated gene (ISG) products, such as protein kinase R and RNase L, which bind RNAs [69]. DENV sfRNA antagonizes proteins that modulate viral infection, which include G3BP1, G3BP2, and CAPRIN1, colocalizing with them. Most flaviviruses produce and use this type of RNA in their mechanisms of interacting with the host. sfRNA was first characterized in Murray Valley Viral infections [72] and was later found in Japanese Encephalitis Virus (JEV) [73] and WNV [74]. sfRNAs help flaviviruses evade the innate immune system [69,75,76]. Mutations in sfRNA, such as deletions, lead to significant effects on the viral replication and life cycle of DENV and WNV in cells that exhibit IFN I responses [69,77]. These mutations may also be the cause of the emergence of new pathogenic viral strains as a result of viral evolution [78].
Third, sfRNAs have been shown to induce apoptosis in cells through the Bcl-2 mediated PI3k/Akt signaling pathway [79]. However, overexpression alone did not induce apoptosis, indicating that its action requires flavivirus replication.
Fourth, several studies have shown that sfRNA also plays an important role in the flaviviral life cycle and dissemination in infected insects [80–82]. sfRNAs also determine the infection and transmission rates of WNV and DENV in mosquitoes [80,83]. Their fifth function entails the ability of sfRNAs to suppress RNAi and miRNA pathways [82].
The sixth function includes the generation of sfRNA by XRN1, which represses the activity of the exoribonuclease that plays a major role in cellular mRNA decay, dysregulating host mRNA stability. During flavivirus infections, changes in the half-life of mRNA have a significant effect on the expression of normal short-lived mRNAs that encode cytokines compared to long-lived transcripts. Flaviviruses can therefore escape cellular-mediated immunity by taking advantage of this dysregulation [84].
Lastly, sfRNAs play a role in the replication of flaviviruses through downregulation of RNA synthesis and translation [85]. Transfection of JEV infected cells with a -sfRNA to counter effect of +sfRNA elevated the antigenome levels implicating that sfRNAs inhibits antigenome synthesis.
Development of ZIKV vaccines
Following the emergence of ZIKV, rapid and promising vaccine development has been ongoing by incorporating the lessons learned from the design of other flavivirus vaccines. Despite the development of several ZIKV vaccines, a few challenges have arisen considering the possibility of crossreactivity and adverse effects in immunocompromised individuals. Therefore, development of a safe and efficacious ZIKV vaccine is of great importance. Currently, multiple vaccine platforms are being incorporated to create new vaccines, such as DNA, mRNA, peptide, protein, viral vectors, virus-like particles (VLPs), inactivated-virus, and live-attenuated virus. These platforms and the current vaccines are briefly discussed below and summarized in Table 1.
Table 1.
Current Advances in ZIKV Vaccine Development [152]
| Platform | Candidate Vaccine | Immunogen | Adjuvant Type | Registry ID | Sponsor Name | Phase | Study start date | Completion Date anticipated | Age | Sample size | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA-based | GLS-5700 | prME | None | NCT02809443 | GeneOne Life Science/Inovio Pharmaceuticals | 1 | 7/1/16 | 12/1/17 | Adult | 40 | [96] |
| GLS-5700 | prME | None | NCT02887482 | GeneOne Life Science/Inovio Pharmaceuticals | 1 | 8/1/16 | 6/1/18 | Adult | 160 | [96,153] | |
| VRC-ZKADNA085-00-VP | prME | None | NCT02840487 | NIAID | 1 | 8/2/16 | 12/28/19 | Adult | 80 | [95] | |
| VRC-ZKADNA090-00-VP | prME | None | NCT02996461 | NIAID | 1 | 12/16/16 | 12/28/19 | Adult | 45 | [95] | |
| VRC-ZKADNA090-00-VP | prME | None | NCT03110770 | NIAID | 2 | 3/29/17 | 1/1/20 | Child/Adult | 2338 | [94,95] | |
| IWTO* | ZIKV PIV | whole virus | Aluminum salts | NCT02963909 | NIAID | 1 | 11/1/16 | 10/30/18 | Adult | 75 | [89] |
| ZIKV PIV | whole virus | Aluminum salts | NCT02952833 | NIAID | 1 | 10/14/16 | 12/5/18 | Adult | 91 | [89] | |
| ZIKV PIV | whole virus | Aluminum salts | NCT02937233 | BIDMC | 1 | 12/8/16 | 6/4/18 | Adult | 36 | [89] | |
| ZIKV PIV | whole virus | Aluminum salts | NCT03008122 | NIAID | 1 | 2/24/17 | 1/15/20 | Adult | 90 | [87,89] | |
| PIZV or TAK-426 | whole virus | Aluminum salts | NCT03343626 | Takeda | 1 | 11/13/17 | 2/28/21 | Adult | 240 | [87,89] | |
| VLA1601 | whole virus | Aluminum salts | NCT03425149 | Valneva Austria GmbH | 1 | 2/24/18 | 11/16/18 | Adult | 67 | [87,89] | |
| BBV121 | whole virus | Aluminum salts | CTRI/2017/05/008539 | Bharat Biotech | 1 | 6/1/17 | Not listed | Adult | 48 | [90] | |
| Recombinant viral vector | rZIKV/D4Δ30-713 | prME in DENV backbone | None | NCT03611946 | NIAID | 1 | 7/6/18 | 9/30/19 | Adult | 28 | - |
| MV-Zika | prME | None | NCT02996890 | Themis Bioscience | 1 | 4/4/17 | 4/17/18 | Adult | 48 | [154] | |
| Peptide | AGS-v | Mosquito salivary proteins | Not specified | NCT03055000 | NIH | 1 | 2/15/17 | 12/28/18 | Adult | 49 | - |
| mRNA | mRNA-1325 | prME | None | NCT03014089 | Moderna Therapeutics | 2 | 12/1/16 | 2/1/19 | Adult | 90 | [103,155] |
IWOT* Inactivated whole target organism
Inactivated virus and live-attenuated virus vaccines
Traditionally, the development of vaccines has involved the use of inactivated and attenuated organisms. A similar approach has been used to design vaccines for several flaviviruses, including DENV, TBEV, JEV, and YFV.
For ZIKV, Shan et al developed a vaccine using an infectious cDNA clone with 10 nucleotide deletions within the 3`untranslated region of the viral genome. The vaccine was immunogenic and protected both immunodeficient AG129 and CD-1 mice, causing higher T-cell responses than the wild-type virus. Mechanistically, the attenuated vaccine also elicited increased sensitivity to type-I IFN and downregulated RNA synthesis. The attenuated 10-del ZIKV did not infect mosquitoes after oral feeding with spiked-blood meals, representing an additional safety feature of the vaccine [86]. Sumathy et al. designed an alum-absorbed inactivated virus vaccine using the Ugandan ZIKV strain MR766. The inactivated vaccine was shown to protect AG129 and BALB/c mice from viral infection. Two doses of the vaccine-elicited high titers of neutralizing antibodies in BALB/c mice. Importantly, vaccine antisera were able to protect passively immunized mice against virus challenge [87].
Xie et al. used a reverse genetics approach to develop two chimeric viruses (CHV) by swapping the prM-E (premembrane and envelope) structural genes between DENV-2 and ZIKV, incorporating a full-length cDNA clone. Chimeric ZIKV with DENV-2 prM-E (Chimeric Virus 1) and Chimeric DENV-2 with ZIKV prM-E genes (Chimeric Virus 2) were highly attenuated in AG129 mice and were immunogenic as indicated by their decreased viremia and weight loss. These chimeric viruses also protected mice against DENV-2 and ZIKV challenge. Notably, this study demonstrated that the PrM-E genes are major determinants of DENV and ZIKV thermostability and that the hydrogen-bond interaction between Q350 and T351 in the CD loop of the ZIKV E protein is not required for virion thermostability. Additionally, the conformation of the extended CD loop is important for viral assembly or release. Potentially, these viruses can be developed into effective vaccines against ZIKV [88]. Currently, 5 of 7 inactivated virus vaccines have completed phase 1 clinical trials. ZPIV is a purified, formalin-inactivated ZIKV vaccine candidate that has been completed and has been demonstrated to be well-tolerated and immunogenic in a phase 1 human clinical trial [89]. In all the vaccines, formalin was used to inactivate the virus [87,89,90].
DNA-based vaccines
DNA-based vaccines offer several advantages compared to other vaccine platforms. DNA-based vaccines are easy to manufacture, transport, and store. Importantly, DNA-based vaccines have been shown to produce both antibody and T-cell immune responses, exhibiting an added advantage over other protein based-vaccines [91]. However, DNA-based vaccines have a major disadvantage with respect to administration, requiring special equipment for electroporation to facilitate DNA entry into the cell [92].
Larroca et al. designed a vaccine expressing full-length prM-E genes using a Brazilian strain, BeH815744. The vaccine elicited high Env-specific neutralizing antibodies and good T cell immune responses. A single-dose immunization provided complete protection in BALB/c, SJL and C57BL/6 mice [93]. Dowd et al. developed a DNA vaccine candidate, VRC ZKADNAO85-00-VP, using a vector (VRC 5288) incorporating a full-length prM-E gene from the ZIKV strain H/PF/2013 with a JEV stem and transmembrane regions to facilitate protein expression. This vaccine is currently undergoing phase 1 and 2 clinical trials [94]. Gaudinski et al. compared two vectors, VRC5288 and VRC5283, which differ in their final 98 amino acids of the envelope protein. VRC5283, which encodes the wild-type ZIKV sequence, showed higher immunogenicity and was more efficient thanVRC5288. Nonetheless, both vaccines were tolerable in healthy individuals. Currently, VRC5283 is in Phase 2 investigation undergoing clinical trials to optimize its safety, immunogenicity, and dose delivery regimens [95].
Tebas et al. evaluated the safety and immunogenicity of a DNA vaccine, GLS-5700, which is currently under development. The vaccine is composed of a prM-E sequence from an infectious ZIKV clone and was tested in 40 participants with no adverse effects. Inoculated individuals developed ZIKV specific antibodies, and this trial successfully demonstrated the safety and efficacy of the vaccine [96]. Development of a synthetic DNA vaccines is appropriate for emerging infectious diseases such as ZIKV since they can be manipulated for the rapid design of novel antigens. This platform can be used for the rapid development of vaccines, using relevant antigens expressed in the emergent pathogen [97].
Adenovirus vector-based vaccines
Recombinant adenoviral vectors have been widely used in the development of vaccines, offering several advantages, including safety, ease of manufacturing, and broad and strong immune responses. Human and chimpanzee adenoviruses have been extensively explored in the design of vaccine vectors.
Abbink et al. designed a rhesus adenoviral vector (RhAd52) expressing prM-Env using a ZIKV Brazilian strain and tested its efficacy by intramuscular immunization in monkeys. A single dose of immunization-induced neutralizing antibodies protected monkeys against ZIKV [98]. Similar work with chimpanzee adenovirus vectors (ChAd) has demonstrated protective immune responses in the A129 mouse model [99].
Kim et al. designed a recombinant E1/E3-deleted adenoviral vector (pAd.ZIKV-Efl) from the ZIKV strain BeH815744 that expressed a codon-optimized Env-antigen. The extracellular region of the ZIKV envelope was linked to the T4 fibrin trimerization domain, facilitating protein expression. Additionally, the vector was designed with a polyhistidine tag and a Tobacco Etch Virus (TEV) to increase protein folding and facilitate purification. The vaccine was shown to protect C57BL/6 mice from lethal challenge with the ZIKV DAKAR41542 strain [100]. Notably, in these vaccine studies, the ZIKV E subunit protein production yield was very low, a finding that has also been highlighted by Larroca et al. [93]. The low yield of the E protein was probably due to the absence of preM, which is important for protein stability. Therefore, preM is an indispensable factor in the development of ZIKV E protein-based vaccines.
Virus-like particles
Virus-like particles (VLPs) have emerged as a powerful platform for the development of vaccines due to their ability to produce broad and strong immune responses. VLPs are self-assembling platforms that resemble viruses but are noninfectious and nonreplicating. Boigard et al. used VLPs to develop a ZIKV immunogenic vaccine by coexpressing the C-prM-Env with the NS2B/NS3 protease to test their efficacy. The ZIKV VLP immunogen was tested in BALB/c mice and elicited high antibody titers against ZIKV FSS13025 and MR-766 strains; however, VLP immunization did not enhance DENV infection when ADE tests were conducted [101].
Peptide-based vaccines
Immune-informatics approaches are becoming an interesting platform for the development of vaccines as they can allow the manipulation of conformational or linear epitopes to optimize epitope-based vaccines. The availability of information in the genome sequence has simplified the prediction of T-cell epitopes in developing ZIKV epitope-based vaccines, which elicit immune responses. Dikhit et al. used this in silico approach to predict 9 promiscuous epitopes in 5 proteins by combining human leukocyte antigen-binding specificity and population coverage [102]. These epitopes consisted of capsid (MVLAILAFL), Env (RLKGVSYSL and RLITANPVI), NS2A (AILAALTPL), NS4B (LLVAHYMYL and LVAHYMYLI), and NS5 (SLINGVVRL, ALNTFTNLV and YLSTQVRYL). Altogether, these epitopes elicited immune responses from human CD8 + T cells and bound to at least one HLA molecule from the majority of the population. Moreover, further research needs to be performed both in vitro and in vivo to understand the immunological relevance of these epitopes in the development of vaccines against ZIKV [102].
RNA-based vaccines
RNA provides a good platform for developing ZIKV vaccines since it can be easily modified and incorporated into vaccines to improve their immunogenicity and eliminate side effects. RNA vaccines have an added advantage over DNA vaccines in that they have no risk of possible integration into the human genome. Specifically, mRNA has proven to be a highly effective platform in the design of ZIKV vaccines.
Pardi et al. developed a novel anti-ZIKV vaccine in which the prM-E proteins were encoded by mRNA. The mRNA was encapsulated using lipid-nanoparticles (mRNA-LNPs), which subsequently enhanced protein expression. The vaccine-elicited antibodies that protected C57BL/6 mice from a lethal ZIKV H/PF/2013 challenge [103].
Richner et al. also used this approach to develop a full-length prM-E RNA sequence of ZIKV into LNPs to increase shuttling between cells. The immunodominant fusion loop in domain II (DII-FL) was mutated, crippling the reactivity of the antibodies targeting this region and resulting in no ADE in response to DENV infection. The vaccine protected immunodeficient AG129 and BABL/c mice, as well as immunocompetent C57BL/6 mice, with the production of high and durable neutralizing antibodies [104].
Chahal et al. also employed RNA nanotechnology in designing an RNA vaccine candidate expressing prM-E as an open reading frame using the Asian ZIKV isolate Z1106033. Immunization through intramuscular injection elicited ZIKV E protein-specific IgG responses and protected C57BL/6 mice. Additionally, immunization also resulted in good CD8 + T cell responses against the peptide (IGVSNRDFV) derived from the envelope protein [105].
ZIKV drug development
Currently, there are no specific drugs to treat or prevent ZIKV infections, and treatment involves the administration of fluids and plenty of rest by infected individuals. Painkillers (e.g., Paracetamol) are used to alleviate headaches, fever, and myalgia. Four strategies have been employed in the development of ZIKV antiviral drugs, including targeting viral proteins (Table 2), targeting host proteins (Table 3), repurposing of clinically approved drugs, and using reverse genetic systems, such as infectious cDNA clones and replicons.
Table 2.
ZIKV Drugs Targeting Viral Proteins
| Targets | Inhibitors | IC 50 µM | ZIKV Strains | Cell lines tested | Biochemical Assays | Animal tests | Pregnancy Category | Reference |
|---|---|---|---|---|---|---|---|---|
| Envelope | EGCG | 21.4 | ZIKVBR, MR 766 | Vero E6 | Cell viability | Wistar (SPF) rats | - | [115,156] |
| Crotoxin | 0.23, 0.32 µg/µl | - | Vero E6, C6/36 | WB | BALB/c | - | [114] | |
| Pinocembrin | 17.4 | PRAVBC59 | JEG-3 | WB,QRT-PCR,IFA,TOA | - | Phase 1 Trials | [117] | |
| Psiloxylon mauritianum | 19.5 µg/ml | MR766, ZIKV PF-13 | Vero, A549 | TOA, IFA, RT-qPCR, FISH, Flow cytometry, COMET | - | - | [157] | |
| Porphyrins (Co-protoporphyrin IX (CoPPIX) and Sn-protoporphyrin IX (SnPPIX) |
2.49, 5.78 | MR766, ZIKVBR | BHK-21, Vero, HeLa, | TOA, IFA, TEM, WB, ELISA | Rats and humans | - | [116] | |
| Suramin | 47 | BeH823339 | - | DSC, NMR | C57BL/6 | - | [118,119] | |
| Ivermectin | ~5.00 | MEX_1_7/2015 | Huh-7, HeLa, NPC, JEG3, HAEC | Flow cytometry, IFA | C57BL/6 J | - | [120,121] | |
| NS5 polymerase | BCX4430 | 3.8 ± 2.5, 4.7 ± 0.6, 4.7 ± 2.2 | PRVABC59 | Vero76, Huh-7, RD | Immunohistochemistry(IHC), qRT-PCR | AG129 mice, Rhesus macaques | Phase 1 trials | [158] |
| 7DMA | 1.3 | MR766 | Vero, BHK, C6/36 | TOA, IFA, RT-PCR | AG129 mice | - | [46] | |
| Emetine | 0.0298 | MR 766, PRAVBC59, FSS13025 | SNB-19, HEK 293, Vero E6, hNSC | Western blot(WB), IF | AG129 and SJL mice | - | [109] | |
| Cephaeline | 0.0189 | MR 766, PRAVBC59, FSS13025 |
SNB-19, HEK 293, Vero E6, hNSC | WB, IF, NS5 polymerase inhibition | AG129 and SJL mice | - | [109] | |
| Ribavirin | Unknown | MR766, PRVABC59, and PC-740 | A549, hNPCs, HDFs and Vero | MTT assay, qRT-PCR, western-blot | 129 SV | - | [110,135] | |
| Sofosbuvir | unknown | ZIKVBR, MR766 | VeroE6 | Cell viability, Caspase assay | Swiss Albino mice | B | [131] | |
| Favipiravir | - | PRVVABC59, P6-740 | Vero. hNPCs | qRT-PCR, WB, IFA | 129 SV | - | [110,135] | |
| NS5 Methyltransferase | SAM analog sinefungin, S-adenosyl-L-homocysteine (SAH | 1.18, 0.43 | H/PF/2013 | unknown | Radioactive methyltransferase assay | ND | - | [159] |
| F3043-0013, F0922-0796, F1609-0442, and F1750-0048 | 4.8 ± 2.4, 12.5 ± 7.4, 17.5 ± 8.4 and 17.6 ± 3.1 | - | Vero | Plaque Reduction Assay | ND | - | [132] | |
| NS2B/NS3 | Lopinavir-ritonavir | 4.78g/ml | PRVABC59 | Vero, Huh-7, in silico | NS2B-NS3 protease activity | - | B | [123] |
| Novobiocin | 42.63, 62.24 | PRVVABC59 | Vero, Huh-7, in silico | NS2B-NS3 protease activity | BALB/c mice | C | [123] | |
| Aprotinin | 0.361 | Z1106033 | in silico | X-ray crystallography | - | B | [125] | |
| Bromocriptine | 13.04 | PRVABC59 | Vero | NS2B-NS3 protease assay | - | B | [126] | |
| NSC135618 | 1.0 | PRVABC59 | A549, hNPCs, HPEC, | IFA, qRT-PCR,WB, mass spectrometry | - | - | [160] | |
| Peptide Z2 | 1.75 | SZ01/2016, GZ01/2016 | C6/36, Vero, Huh-7 | IFA, flow cytometry, RT-qPCR, | C57BL/6 mice | - | [161] | |
| Erythrosin B | 0.62 ± 0.12 | PRVABC59 | HPECs, A549, HNPC | IFA, qRT-PCR, protein thermal shift assay, WB | - | B | [124] | |
| Temoporfin, Niclosamide, Nitazoxanide | 0.024, 0.48, 1.48 | PRVABC59 | A549, HPECs, hNPCs | IFA, WB, qRT-PCR, SPR | - | - | [107] | |
| NSC157058, NSC86314, NSC716903 |
0.82 ± 0.09, 1.12 ± 0.11, 3.01 | FSS13025 | hfNPCs | Fluorescent peptide, IF, RT-PCR | - | - | [162] | |
| Myricitin, Quercetin, Luteolin, Isorhamnetin and Apigerin | 1.3 ± 0.1, 2.4 ± 0.2, 2.7 ± 0.3, 15.5 ± 0.7, 56.3 ± 0.9 and 3.5 ± 0.2 | ZIKV 8375 | - | NMR | - | - | [122] |
Table 3.
ZIKV Drugs targeting host proteins
| Targets | Inhibitors | IC 50 µM | ZIKV Strains | Tested Cell Lines | Animal tests | Biochemical Assays | Pregnancy Category | Reference |
|---|---|---|---|---|---|---|---|---|
| IMP dehydrogenase inhibition | Merimepodib | 0.6 | FSS13025, MR766 | Vero E6 | - | qRT-PCR. | - | [163] |
| Pyrimidine synthesis inhibitor | Cyclosporine A | unknown | MEX_1_7/2015, ZIKV DAK_41525 | Huh-7, hNSC | - | IF, Flow cytometry | C | [120] |
| Purine biosynthesis inhibitor | 6-methylmercaptopurine riboside (6MMPr) | 24.5, 20.3 | ZIKV PE243 | Vero, SH-SY5Y | - | qRT-PCR. TOA, Flow cytometry, ifa | - | [141] |
| Purine biosynthesis inhibitor | Methotrexate | 0.28 | PRVABC59, H/PAN/2016/BEI-259,634 | Vero, hNPCs | - | IFA | - | [164] |
| Nucleoside metabolic inhibitor | Palonosetron | 16.3 | ZIKVBR: (KX197192.1) | Huh-7 | - | IFA | B | [165] |
| Cholesterol metabolism inhibitor | Mevastatin | 3.42 5.05 |
MR766 PRVABC59 |
Vero 76 | ND | WB, RT-qPCR | - | [166] |
| Cholesterol metabolism inhibitor | Imipramine | - | PF-25,013-18 | HFF1, Vero | C57BL/6 N | IFA, WB, RT-QPCR, Flow cytometry | C | [167,168] |
| Cholesterol metabolism inhibitor | Benzamil | - | PRVABC59 | Vero, RPE (Retinal Pigment Epithelial) | - | qRT-PCR, IFA | B | [169] |
| Cholesterol metabolism inhibitor | Gsw4869 | - | PRVABC59 | Human fetal astrocytes, NPCs | - | TEM, WB, IFA, IHC, qRT-PCR | - | [143] |
| Cholesterol metabolism inhibitor | 7-KC | 4.064 | ZIKV SPH, ZIKV Ibh 365 | Vero, Human neurons | - | Autophagy TOA, QRT-PCR, | ||
| SREBP pathway inhibitor | Nordihydroguaiaretic acid (NDGA) | 9.1 | ZIKV PA259459 | Vero | ND | ELISA,WB, qPCR | Not approved | [145] |
| SREBP pathway inhibitor | Tetra-O-methyl nordihydroguaiaretic acid (M4N) | 5.7 | ZIKV PA259459 | Vero | ND | ELISA, WB, qPCR | - | [145] |
| caspase 3 activity inhibitor | PHA-690,509 | 1.72 | MR766, FSS13025, PRVABC59 | SNB-19, hNPCs | - | WB, IHC, TOA, caspase-3 assay, | D | [146] |
| caspase 3 activity inhibitor | Bithioniol | PRVABC59 | Vero, RAW264.7 | - | - | [147] | ||
| caspase 3 activity inhibitor | Phloretin | 22.85, 9.31 | MR766, PRVABC59 | Vero | - | IFA, WB | - | |
| Caspase inhibitor | Emricasan | 0.17, 0.13, 0.19 | MR766, PRVABC59, FSS13025 | SNB-19, hNPCs | 3D brain organoids | WB, IHC,TOA, caspase-3 assay, | - | [146] |
| Protein Metabolism inhibitor | Celgosvir | 50 | MR766, PRVABC59 | Vero 76 | ND | WB, RT-qPCR | - | [166] |
| Protein Metabolism inhibitor | Fatostatin | ≤1.2 | PA259459 | Vero | - | WB, IFA, ELISA, RT-QPCR | - | [145] |
| Protein Metabolism inhibitor | Metformin | PA259459 | Vero, BHK-21 | ND | Lipidomics, IFA, RT-QPCR | - | [170] | |
| Protein metabolism inhibitor | Cyclosporine A | 1 | MEX_1_7/2015, ZIKV DAK_41525 | Huh-7, hNSC | C57BL/6 | IFA, Flow cytometry | C | [120] |
| Endocytosis and Endosomal Fusion inhibitors(EEF) | 25-hydroxycholesterol (25HC | 0.188 | ZIKV SZ01 | Vero | A129 mice, Rhesus macaque | Plaque assay, IFA, | - | [134] |
| EEF | Arbidol | 12.09 ± 0.77, 10.57 ± 0.74 | MR66, ZIKV Paraiba-01 | Vero, PS, HBCA, UKF-NB-4 | 129 SV | IFA | - | [135,171] |
| EEF | K22 | 2.1–2.5 | PR-2015; PRVABC59 | Vero Huh-7 | - | IFA, TEM, TOA | - | [136] |
| EEF | Tenovin 1 | 0.7 | Mex2-81, FSS13025, MR766 | Jeg3, BHK-21, Vero, Human U2OS | - | RT-QPCR, TOA,IFA | - | [137] |
| EEF | Peptide EV37 | - | PRVABC59 | Huh-7, Huh 7.5.1, BHK-21, HEK293T | - | TOA, QRT-PCR, WB, Flow cytometry | - | [138] |
| EEF | Chlorpromazine | - | BeH819015, PE243/2015 | BHK-21. Huh-7, Vero E6 | C57BL/6 | IFA | - | [172,173] |
| EEF | Amodiaquine | - | ZIKV (strain PLCal_ZV) | Vero, Huh-7 | SCID-beige mice | QRT-PCR, Flow cytometry | - | [139] |
| EEF | Chloroquine | 9.82, 14.20, 12.36 | MR766, ZIKVBR | Vero, hBMECs, hNPC | A129, SJL mice | qRT-PCR,Flow cytometry, IF | - | [108] |
| Apoptosis and vATPase inhibitor | Obatoclax, Saliphenylhalamide (SaliPhe) | 0.04 0.01, 0.05 ± 0.02 | ZIKV FB-GWUH-2016, MR766, H/PF/2013 | RPE | 3D brain organoids | TDA, IFA, qRT-PCR, | - | [174] |
| Inhibits mTOR, STAT3 and NF-κB signaling pathways | Niclosamide | 0.28 | MR766, PRVABC59, FSS13025 | SNB-19, hNPCs | Chick embryos | WB, IF, TOA,caspase-3 assay, | B | [146,175,176] |
| Innate immunity and Epigenetics | GSK-126 | - | H/PF/2013 | Vero,MRC-5 | C57BL/6 J | RT-QCR, QPCR, WB, ChIP, IFA, IHC | - | [150] |
| TNF-α neutralizing antibody | Infliximab | - | - | Swiss mice | - | B | [177] | |
| NMDA receptor blocker | Memantine | HS-2015-BA-01 | hNPCs | SV129 | IHC | B | [178] | |
| Unknown | Azithromycin | SPH2015, PRVABC59, FSS13025 | HPSCs, U87 glial cell | ICR mice | - | B | [106] | |
| Unknown | Ebelsen | PRVABC59 | AG129 | IFA, IHC, WB, qRT-PCR, Histology | - | [179] |
ND – Not Done DSC- Differential Scanning Calorimetry CPE -Cytopathic Effects PRNT-Plaque Reduction Neutralization Test SPR-Surface Plasmon Resonance NMR-Nuclear Magnetic Resonance
Drug(s) repurposing has rapidly emerged as a strategy for developing ZIKV drug(s) from clinically approved drug(s). Drug repurposing is faster and most cost-effective than other platforms of drug design. These drugs include the antibiotic azithromycin [106], the antiparasitic drug nitazoxanide [107], the antimalarial drug chloroquine [108], the antiprotozoal drug emetine [109], the antiviral drug ribavirin and favipiravir [110], and many other FDA-approved drugs. High-throughput screening has also been extensively performed for both repurposed drugs and compound screening.
Importantly, the reverse genetic system has also been incorporated into the design of antivirals by using infectious cDNA clones, ZIKV replicons, and VLPs. The use of reporter genes, such as luciferase and EGFP, in designing recombinant ZIKV has provided a novel platform in determining the mechanisms of drug inhibition. The flavivirus replicon has been proven to be safe when working with the virus since it is not infectious. VLPs have also been used in drug and vaccine development for several flaviviruses, such as WNV, JEV, YFV, DENV, and ZIKV [111].
ZIKV infections are usually characterized by mild illness and uneventful recovery; therefore, when designing anti-ZIKV strategies, the primary target population should be highly considered, namely, immunocompromised individuals, pregnant women and their fetuses [112]. Currently, five pregnancy categories for anti-ZIKV drugs have been defined: Category A, B, C, D and X, all of which have considerations for use in pregnancy [113]. For Category A, adequate and well-controlled studies have failed to demonstrate a risk to fetuses in the first trimester or later of pregnancy. In Category B, animal reproduction studies have failed to demonstrate a risk to fetuses, but there are no adequate or well-controlled studies in pregnant women. For Category C, animal reproduction studies have shown an adverse effect on fetuses, and while there are no adequate or well-controlled studies in humans, their potential benefits may warrant use of these drugs in pregnant women, despite the potential risks. In Category D, there is positive evidence of a human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but the potential benefits of these drugs may warrant their use in pregnant women, despite these potential risks. For Category X, studies in animals or humans have demonstrated fetal abnormalities, and/or there is positive evidence of a human fetal risk based on adverse reaction data from investigational or marketing experience. Therefore, the risks involved in the use of these drugs in pregnant women clearly outweigh their potential benefits. In this section, we summarize drug development using host and viral proteins as targets for drug design.
Antivirals targeting viral proteins
Flavivirus host proteins (E, NS1, NS2B-NS3, NS3 and NS5) have been used as targets for developing therapeutics against ZIKV. Different compounds act directly by binding to the E protein and impairing E-mediated membrane fusion. Most of these compounds have been effective in vitro; however, only a few of them have reached clinical trials. These compounds include Crotoxin, a venom from Crotalus durissus terrificus, and epigallocatechin gallate (EGCG), a polyphenol that inhibits ZIKV entry into host cells [114,115]. Co-protoporphyrin IX (CoPPIX) and Sn-protoporphyrin IX (SnPPIX) have also been shown to exhibit anti-ZIKV activity by interfering with viral morphology and entry into host cells [116]. Pinocembrin (natural flavonoid) evidenced efficacy against ZIKV and is now in Phase 1 trials [117]. Suramin and Ivermectin have also shown anti-ZIKV activity both in vitro and in vivo [118–121].
The ZIKV NS2B-NS3 protease and NS3-helicase play a major role in viral replication. Consequently, several compounds have been developed to target these two proteins. Natural flavonoids, including myricetin, quercetin, luteolin, isorhamnetin and apigerin, have been shown to noncompetitively inhibit ZIKV protease [122]. Lopinavir-ritonavir and novobiocin have also been shown to exhibit anti-ZIKV effects [123]. Novobiocin was shown to protect mice against a lethal ZIKV challenge, reducing viremia and histopathological damage [123]. Erythrosin B, a category B drug, was found to noncompetitively inhibit DENV2 and ZIKV NS2B-NS3 proteases [124] and was also shown to reduce viral titers in YFV, JEV, and WNV, with low cytotoxicity and a micromolar potency [124]. In a screening of 2816 approved drugs, 3 potent drugs, temoporfin, niclosamide, and nitazoxanide, were identified as inhibitors of ZIKV protease [107]. Moreover, temoporfin was shown to inhibit ZIKV replication in human placental cells and protected mice from succumbing to ZIKV infection [107]. Aprotinin, used to reduce bleeding during complex surgery [125], and bromocriptine [126] were found to be potent inhibitors of ZIKV NS2B-NS3. In both, molecular models were developed to predict binding with the NS2B-NS3 protease.
NS5 is an RNA-dependent RNA polymerase that plays an important role in viral genome replication. Several inhibitors have been developed using NS5 as a therapeutic target. BCX4430, an adenosine analogue, has broad-spectrum activity against a wide range of RNA viruses with potent in vivo activity against YFV, WNV, Tick-borne Encephalitis virus (TBEV), Marburg and Ebola viruses [127–129]. The compound is now in clinical trials and is a promising antiviral agent against ZIKV infection [130]. Other compounds tested in mice (AG129) include 7-deaza-2ʹ-C-methyladenosine, a potent inhibitor of ZIKV replication, which was shown to delay ZIKV pathogenesis in a robust mouse model (AG129) [46]. Emetine and cephaeline also inhibited ZIKV polymerase both in vitro and in AG129 mice [109]. Favipiravir and ribavirin also inhibited ZIKV NS5 in both Asian and African strains [110]. Finally, sofosbuvir protected ZIKV-infected mice from mortality by decreasing viral RNA levels in different tissues, preventing acute neuromotor and long-term memory sequelae [131]. Flavivirus NS5 methyltranferases are also central players in viral replication and have also been used as targets for drug design. Methltransferase inhibitors include S-adenosyl-L-methionine (SAM) and/or S-adenosyl-L-homocysteine (SAH) analogues. Other NS5 inhibitory compounds include F3043-0013, F0922-0796, F1609-0442, and F1750-0048 [132].
Antivirals targeting host proteins
Antivirals acting on host proteins interfere with different parts of the ZIKV life cycle, impairing viral replication. The first step in the ZIKV life cycle is binding of the virus to receptors (DC-SIGN, TYRO 3, AXL, TIM, and TAM) [35], followed by internalization by endocytosis to reach endosomes [37]. Different compounds have been developed using this target. Endocytosis and endosomal fusion (EEF) inhibitors include 25-hydroxycholesterol (25HC), a natural product of lipid metabolism shown to reduce viremia in mice and monkeys. Moreover, 25HC protected infected fetal mice from microcephaly [134]. Chloroquine, an antimalarial and anti-inflammatory drug, reduced ZIKV titers in ZIKV-infected cells and protected mouse neurospheres from morphological damage [108]. Arbidol, also known as umifenovir, an approved drug both in Russia and China, was shown to reduce viral titers in ZIKV with a micromolar effect [135]. Additionally, the drug also reduced viral multiplication in WNV and tick-borne encephalitis virus (TBEV) with a strong cell-dependent effect [135]. K22, a small compound inhibitor with potential activity against a broad range of coronaviruses, was also shown to inhibit ZIKV. K22 efficiently interfered with the replication of other flaviviruses, including JEV, WNV and, to a certain extent, Usutu virus (USUV), Wesselsbron virus (WESSV), hepacivirus (HCV), and bovine viral diarrhea virus (BVDV) [136]. Another EEF inhibitor is Tenovin-1, which inhibits ZIKV multiplication in primary fibroblasts [137]. A venom peptide, Ev37, from the scorpion Euscorpiops validusin inhibited ZIKV, DENV-2, hepatitis C virus (HCV) and herpes simplex virus type 1 (HSV-1) infections in a dose-dependent manner. The drug showed low cytotoxic effects in vitro; however, it had no effect on Sendai virus (SeV) or adenovirus (AdV) [138]. Finally, Amodiaquine, an antimalarial drug, was shown to exhibit antiviral activity in both ZIKV and DENV at a micromolar concentrations in vitro [139].
Different drugs have been developed by targeting lipid metabolism, the endoplasmic reticulum (ER), and nucleoside biosynthesis, impairing ZIKV replication. Pyrimidine synthesis inhibitors include cyclosporine A, which inhibits ZIKV in different cell lines [120]. Cyclosporin A was also shown to be effective against the WNV NS5 protein [140]. Purine synthesis inhibitors include 6MMPr, which decreases ZIKV infectious titers by more than 99% in a dose and time-dependent manner [141]. Methotrexate (MTX) decreases ZIKV titers in a dose-dependent manner in Vero and hNSCs cells by antagonizing dihydrofolate reductase (DHFR) [142]. Cholesterol metabolism inhibitors include GW4869, a neutral sphingomyelinase-2 (nSMase2) inhibitor that effectively inhibits ZIKV propagation in human astrocytes and decreases extracellular vesicle (EV) levels [143]. 7-Ketocholesterol (7-KC) inhibited ZIKV replication specifically in viral budding, release from the host, and viral integrity [144]. Nordihydroguaiaretic acid (NDGA) and its methylated derivative tetra-O-methyl nordihydroguaiaretic acid (M4N) were shown to inhibit ZIKV and WNV infections by interfering with the sterol regulatory element-binding protein (SREBP) pathway [145]. In the same line, fatostatin was also shown to inhibit the SREBP pathway in ZIKV [145]. Caspase-3 activity inhibitors include PHA-690509, which reduces ZIKV multiplication in vitro [146]. Bithiniol, a broad spectrum compound, also inhibited ZIKV caspase activity, hence inhibiting ZIKV pathogenicity [147]. Additionally, metformin was shown to inhibit ZIKV replication by inactivating adenosine monophosphate-activated protein kinase (AMPK), a regulator of lipid metabolism [148]. Other inhibitors include compounds that inhibit the NMDA receptor, such as memantine, a blocker of the N-methyl-D-aspartate receptor (NMDAR) that was shown to inhibit neuronal damage as a result of ZIKV infection [149]. Finally, the innate immune response has been used as drug target in the case of GSK-126, which is currently in clinical trials [150].
Currently, a few drugs, including Pinocembrin and BCX4430, also known as Galidesivir, have completed phase 1 clinical trials performed by Biocryst Pharmaceuticals. Galidesivir was administered intravenously in 24 healthy volunteers. In trials, this drug was shown to be safe and tolerable. Intramuscular administration and animal models have also been used to show that this drug has survival benefits against several pathogens, including Ebola [129], Marburg, YFV, WNV [127] and ZIKV [129]. Additionally, Galidesivir has shown broad-spectrum activity in vitro against more than 20 RNA viruses in nine different families, including filoviruses, togaviruses, bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, and flaviviruses [151]. Nevertheless, this drug has been shown to confer resistance as a result of an E460D substitution in the NS5 protein of TBEV [128], which represents a major challenge in the development of viral replication inhibitors since the viruses are rapidly evolving.
In summary, many ZIKV antiviral drugs have been developed despite the fact that most of them never reach clinical trials. As an RNA virus, ZIKV is prone to rapid evolution as a result of mutations in both structural and nonstructural proteins, possibly leading to drug resistance. Therefore, additional research is needed to understand this hypothesis. Drug combinations should be considered for effective treatment of ZIKV.
Conclusions and future perspectives
Since the reemergence of ZIKV, several studies have been performed to understand the pathogenesis of the virus, including the use of mice and other animal models, such as macaques. However, questions remain to be answered concerning the different mechanisms through which the virus escapes the immune system and causes disease in mammalian hosts. Having many routes of transmission and a wide range of tropism, ZIKV has become a crucial human pathogen in need of quick therapeutic measures and vaccines that convey long-term immunity. Understanding ZIKV interactions with the host will give more insights into the development of these therapeutic measures. Despite the rapid development of ZIKV vaccines, more research is needed to complete clinical trials for ZIKV therapeutics. Importantly, more studies should be performed on the development of vaccines that are safe, especially for pregnant women, unborn fetuses, infants, and elderly individuals. The role of sfRNAs is still not well-defined in ZIKV pathogenesis and disease; therefore, future studies in this field will be of novel importance.
Funding Statement
This work was supported by the Ministry of Science and Technology of China [2018ZX10101004, 2013FY113500]; the Science and Technology Bureau of Wuhan, China [2018-201261638501]; the Chinese Academy of Sciences [153211KYSB20160001, ZDRW ZS-2016-4]; the Wuhan Institute of Virology, China [WIV-135-PY2], and Sino-Africa Joint Research Center, Chinese Academy of Sciences [SAJC201605].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Nyaruaba R, Mwaliko C, Mwau M, et al. Arboviruses in the East African Community partner states: a review of medically important mosquito-borne Arboviruses. Pathog Glob Health. 2019;113:209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dick GWA, Kitchen SF, Haddow AJ, et al. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46. DOI: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- [3].MacNamara FN. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. [DOI] [PubMed] [Google Scholar]
- [4].Lupton K. Zika virus disease: a public health emergency of international concern. Br J Nurs. 2016;25:198–202. [DOI] [PubMed] [Google Scholar]
- [5].Lanciotti RS, Lambert AJ, Holodniy M, et al. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg Infect Dis. 2016;22:933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tabata T, Petitt M, Puerta-Guardo H, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Musso D, Roche C, Robin E, et al. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Musso D, Nhan T-X, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19. DOI: 10.2807/1560-7917.ES2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- [9].Li C, Deng Y-Q, Zu S, et al. Zika virus shedding in the stool and infection through the anorectal mucosa in mice. Emerg Microbes Infect. 2018;7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Atkinson B, Hearn P, Afrough B, et al. Detection of Zika Virus in Semen. Emerg Infect Dis. 2016;22:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Regla-Nava JA, Viramontes KM, Vozdolska T, et al. Detection of Zika virus in mouse mammary gland and breast milk. PLoS Negl Trop Dis. 2019;13:e0007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gourinat A-C, O’Connor O, Calvez E, et al. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Musso D, Roche C, Nhan T-X, et al. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55. [DOI] [PubMed] [Google Scholar]
- [14].Jampol LM, Goldstein DA. Zika Virus . Infection and the Eye. JAMA Ophthalmol. 2016;134:535–536. [DOI] [PubMed] [Google Scholar]
- [15].Govero J, Esakky P, Scheaffer SM, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mlakar J, Korva M, Tul N, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. [DOI] [PubMed] [Google Scholar]
- [17].Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika Virus and Birth Defects — reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. [DOI] [PubMed] [Google Scholar]
- [18].Song B-H, Yun S-I, Woolley M, et al. Zika virus: history, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017;308. DOI: 10.1016/j.jneuroim.2017.03.001 [DOI] [PubMed] [Google Scholar]
- [19].Boorman JPT, Porterfield JS. A simple technique for infection of mosquitoes with viruses transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50:238–242. [DOI] [PubMed] [Google Scholar]
- [20].Haddow AJ, Williams MC, Woodall JP, et al. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ. 1964;31: 57–69. pmid:14230895; PubMed Central PMCID: PMC2555143. [PMC free article] [PubMed] [Google Scholar]
- [21].Kirya BG, Okia NO. A yellow fever epizootic in Zika Forest, Uganda, during 1972: part 2: monkey serology. Trans R Soc Trop Med Hyg. 1977;71:300–303. [DOI] [PubMed] [Google Scholar]
- [22].Darwish MA, Hoogstraal H, Roberts TJ, et al. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77:442–445. [DOI] [PubMed] [Google Scholar]
- [23].Henderson BE, Hewitt LE, Lule M. Serology of wild mammals. Virus Res Inst Annu Rep. 1968;409:48–51. [Google Scholar]
- [24].Sirohi D, Chen Z, Sun L, et al. The 3.8 Åresolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hasan SS, Sevvana M, Kuhn RJ, et al. Structural biology of Zika virus and other flaviviruses. Nat Struct Mol Biol. 2018;25:13–20. [DOI] [PubMed] [Google Scholar]
- [26].Kostyuchenko VA, Lim EXY, Zhang S, et al. Structure of the thermally stable Zika virus. Nature. 2016;533:425. [DOI] [PubMed] [Google Scholar]
- [27].Prasad VM, Miller AS, Klose T, et al. Structure of the immature Zika virus at 9 Å resolution. Nat Struct Mol Biol. 2017;24:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuno G, Chang G-J-J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687–696. [DOI] [PubMed] [Google Scholar]
- [29].Adibi J, Marques E, Cartus A, et al. Teratogenic effects of the Zika virus and the role of the placenta. Lancet. 2016;387. DOI: 10.1016/S0140-6736(16)00650-4 [DOI] [PubMed] [Google Scholar]
- [30].Platt D, Smith A, Arora N, et al. Zika virus–related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med. 2018;10:eaao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Agumadu VC, Zika Virus: RK. A Review of Literature. Cureus. 2018;10:e3025–e3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burke RM, Pandya P, Nastouli E, et al. Zika virus infection during pregnancy: what, where, and why? Br J Gen Pract. 2016;66:122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lessler J, Ott C, Carcelen A, et al. Times to key events in Zika virus infection and implications for blood donation: A systematic review. Bull World Health Organ. 2016;94:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miner JJ, Cao B, Govero J, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wells MF, Salick MR, Wiskow O, et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell. 2016;19:703–708. [DOI] [PubMed] [Google Scholar]
- [37].Meertens L, Labeau A, Dejarnac O, et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 2017;18:324–333. [DOI] [PubMed] [Google Scholar]
- [38].Hastings A, Yockey LJ, Jagger B, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558–568. DOI: 10.1016/j.celrep.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol. 2017;91:JVI.00009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma W, Li S, Ma S, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2017;168:542. [DOI] [PubMed] [Google Scholar]
- [41].Rossi SL, Tesh RB, Azar SR, et al. Characterization of a novel murine model to study zika virus. Am J Trop Med Hyg. 2016;94:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dowall SD, Graham VA, Rayner E, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dowall S, Graham V, Rayner E, et al. Lineage-dependent differences in the disease progression of Zika virus infection in type-I interferon receptor knockout (A129) mice. PLoS Negl Trop Dis. 2017;11:e0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tang W, Young M, Mamidi A, et al. Model of Zika virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Aliota M, Caine L, Walker E, et al. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zmurko J, Marques RE, Schols D, et al. The viral polymerase inhibitor 7-Deaza-2ʹ-C-Methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis. 2016;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fernandes N, Nogueira J, Réssio R, et al. Experimental Zika virus infection induces spinal cord injury and encephalitis in newborn Swiss mice. Exp Toxicol Pathol. 2016;69. DOI: 10.1016/j.etp.2016.11.004. [DOI] [PubMed] [Google Scholar]
- [48].Chan JFW, Zhang AJ, Chan CCS, et al. Zika virus infection in dexamethasone-immunosuppressed mice demonstrating disseminated infection with multi-organ involvement including orchitis effectively treated by recombinant type I interferons. EBioMedicine. 2016;14:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li H, Saucedo-Cuevas L, Regla-Nava AJ, et al. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016;19: DOI: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berry N, Ferguson D, Ham C, et al. High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci Rep. 2019;9:14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Osuna CE, Lim S-Y, Deleage C, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lindenbach B, Thiel HJ, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia, PA: Lippincott-Raven; 2007. p. 1101–1152. [Google Scholar]
- [55].Sirohi D, Chen Z, Sun L, et al. The 3. 8 Å resolution cryo-EM structure of Zika virus. Science. 2016;5316(1–7). 80: DOI: 10.1126/science.aaf5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li L, Lok S-M, Yu I-M, et al. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–1834. [DOI] [PubMed] [Google Scholar]
- [57].Sotcheff S, Routh A. Understanding flavivirus capsid protein functions: the tip of the iceberg. Pathog (Basel, Switzerland). 2020;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wu Y, Liu Q, Zhou J, et al. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017;3:17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rastogi M, Sharma N, Singh SK. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol J. 2016;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Best S. The many faces of the flavivirus NS5 protein in antagonism of Type I interferon signaling. J Virol. 2016;91:JVI.01970–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Grant A, Ponia SS, Tripathi S, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe. 2016;19:882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Culshaw A, Mongkolsapaya J, Screaton G. The immunology of Zika Virus. F1000Research 2018;7:203. DOI: 10.12688/f1000research.12271.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang X, Xie X, Xia H, et al. Zika Virus NS2A-Mediated Virion Assembly. MBio. 2019;10:e02375–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Avila G, Nogales A, Park J-G, et al. A natural polymorphism in Zika virus NS2A protein responsible of virulence in mice. Sci Rep. 2019;9:19968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Muñoz-Jordán J, Sánchez G, Laurent-Rolle M, et al. Inhibition of interferon signaling by Dengue virus. Proc Natl Acad Sci U S A. 2003;100:14333–14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liang Q, Luo Z, Zeng J, et al. Zika Virus NS4A and NS4B proteins deregulate akt-mtor signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell. 2016;19. DOI: 10.1016/j.stem.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pijlman GP, Funk A, Kondratieva N, et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe. 2008;4:579–591. [DOI] [PubMed] [Google Scholar]
- [68].Donald CL, Brennan B, Cumberworth SL, et al. Full Genome Sequence and sfRNA Interferon Antagonist Activity of Zika Virus from Recife, Brazil. PLoS Negl Trop Dis. 2016;10. DOI: 10.1371/journal.pntd.0005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schuessler A, Funk A, Lazear H, et al. West nile virus noncoding subgenomic RNA contributes to viral evasion of the Type I interferon-MEDIATED antiviral response. J Virol. 2012;86:5708–5718. DOI: 10.1128/JVI.00207-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Akiyama BM, Laurence HM, Massey AR, et al. Zika virus produces noncoding RNAs using a multi-pseudoknot structure that confounds a cellular exonuclease. Science. 2017;354:1148–1152. DOI: 10.1126/science.aah3963.Zika [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Funk A, Truong K, Nagasaki T, et al. RNA structures required for production of subgenomic flavivirus RNA. J Virol. 2010;84:11407–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Urosevic N, van Maanen M, Mansfield J, et al. Molecular characterization of virus-specific RNA produced in the brains of flavivirus-susceptible and -resistant mice after challenge with Murray Valley encephalitis virus. J Gen Virol. 1997;78(Pt 1):23–29. [DOI] [PubMed] [Google Scholar]
- [73].Lin K-C, Chang H-L, Chang R-Y. Accumulation of a 3ʹ-terminal genome fragment in Japanese encephalitis virus-infected mammalian and mosquito cells. J Virol. 2004;78:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pijlman G, Funk A, Kondratieva N, et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe. 2009;4:579–591. [DOI] [PubMed] [Google Scholar]
- [75].Chang R-Y, Hsu T-W, Chen Y-L, et al. Japanese encephalitis virus non-coding RNA inhibits activation of interferon by blocking nuclear translocation of interferon regulatory factor 3. Vet Microbiol. 2013;166:11–21. [DOI] [PubMed] [Google Scholar]
- [76].Manokaran G, Finol E, Wang C, et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon Expression for Epidemiological Fitness. Science. 2015;350:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bidet K, Dadlani D, Garcia-Blanco MA, et al. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mrnas and are targeted by a dengue virus non-coding RNA. PLOS Pathog. 2014;10:e1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Slonchak A, Khromykh AA. Subgenomic flaviviral RNAs: what do we know after the first decade of research. Antiviral Res. 2018;159:13–25. [DOI] [PubMed] [Google Scholar]
- [79].Liu Y, Liu H, Zou J, et al. Dengue virus subgenomic RNA induces apoptosis through the Bcl-2-mediated PI3k/Akt signaling pathway. Virology. 2014;448:15–25. [DOI] [PubMed] [Google Scholar]
- [80].Pompon J, Manuel M, Ng GK, et al. Dengue subgenomic flaviviral RNA disrupts immunity in mosquito salivary glands to increase virus transmission. PLoS Pathog. 2017;13. DOI: 10.1371/journal.ppat.1006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Moon SL, Dodd BJT, Brackney DE, et al. Flavivirus sfRNA suppresses antiviral RNA interference in cultured cells and mosquitoes and directly interacts with the RNAi machinery. Virology. 2015;485:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schnettler E, Sterken MG, Leung JY, et al. Noncoding Flavivirus RNA Displays RNA Interference Suppressor Activity in Insect and Mammalian Cells. J Virol. 2012;86:13486–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Göertz GP, Fros JJ, Miesen P, et al. Noncoding subgenomic flavivirus RNA is processed by the mosquito RNA interference machinery and determines West Nile virus transmission by culex pipiens mosquitoes. J Virol. 2016;90:10145–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Moon SL, Dodd BJT, Brackney DE, et al. Flavivirus sfRNA suppresses antiviral RNA interference in cultured cells and mosquitoes and directly interacts with the RNAi machinery. Virology. 2012;485:2029–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fan YH, Nadar M, Chen CC, et al. Small noncoding RNA modulates Japanese encephalitis virus replication and translation in trans. Virol J. 2011;8:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shan C, Muruato AE, Nunes BTD, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017;23:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sumathy K, Kulkarni B, Gondu RK, et al. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep. 2017;7:46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xie X, Yang Y, Muruato AE, et al. Understanding Zika Virus Stability and Developing a Chimeric Vaccine through Functional Analysis. MBio. 2017;8:e02134–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Modjarrad K, Lin L, George SL, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 2018;391:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Baldwin WR, Livengood JA, Giebler HA, et al. Purified inactivated Zika vaccine candidates afford protection against lethal challenge in mice. Sci Rep. 2018;8:16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Moreno S, Timón M. DNA vaccination: an immunological perspective. Inmunologia. 2004;23:41–55. [Google Scholar]
- [92].Das Neves Almeida R, Racine T, Magalhães KG, et al. Zika Virus vaccines: challenges and perspectives. Vaccines (Basel). 2018;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Larocca RA, Abbink P, Peron JPS, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474. DOI: 10.1038/nature18952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dowd KA, Ko S-Y, Morabito KM, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354:237LP– 240. DOI: 10.1126/science.aai9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gaudinski MR, Houser KV, Morabito KM, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 2018;391:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tebas P, Roberts CC, Muthumani K, et al. Safety and Immunogenicity of an Anti–Zika Virus DNA Vaccine — preliminary Report. N Engl J Med. 2017. DOI: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Maslow JN. Vaccine development for emerging virulent infectious diseases. Vaccine. 2017;35:5437–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Abbink P, Larocca RA, De La Barrera RA, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].López-Camacho C, De Lorenzo G, Slon-Campos JL, et al. Immunogenicity and Efficacy of Zika Virus Envelope Domain III in DNA, Protein, and ChAdOx1 Adenoviral-Vectored Vaccines. Vaccines (Basel). 2020;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim E, Erdos G, Huang S, et al. Preventative vaccines for Zika virus outbreak: preliminary evaluation. EBioMedicine. 2016;13:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Boigard H, Alimova A, Martin GR, et al. Zika virus-like particle (VLP) based vaccine. PLoS Negl Trop Dis. 2017;11:e0005608–e0005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dikhit MR, Ansari MY, Vijaymahantesh K, et al. Computational prediction and analysis of potential antigenic CTL epitopes in Zika virus: A first step towards vaccine development. Infect Genet Evol. 2016;45:187–197. [DOI] [PubMed] [Google Scholar]
- [103].Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Richner JM, Himansu S, Dowd KA, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114–1125.e10. DOI: 10.1016/j.cell.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chahal JS, Fang T, Woodham AW, et al. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 2017;7:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Retallack H, Di Lullo E, Arias C, et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci. 2016;113:201618029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li Z, Brecher M, Deng Y-Q, et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017;27. DOI: 10.1038/cr.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Delvecchio R, Higa LM, Pezzuto P, et al. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yang S, Xu M, Lee EM, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kim J-A, Seong R-K, Kumar M, et al. Favipiravir and ribavirin inhibit replication of Asian and African strains of Zika Virus in different cell models. Viruses. 2018;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dai S, Zhang Y, Xu B, et al. Establishment of baculovirus-expressed VLPs induced syncytial formation assay for flavivirus antiviral screening. Viruses. 2018;10:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Saiz J-C, Vázquez-Calvo Á, Blázquez AB, et al. Zika virus: the latest newcomer. Front Microbiol. 2016;7:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Drugs.com . Prescription drug information, interactions & side effects. n.d.
- [114].Russo R, Junior N, Cintra A, et al. Expression, purification and virucidal activity of two recombinant isoforms of phospholipase A2 from Crotalus durissus terrificus venom. Arch Virol. 2019;164. DOI: 10.1007/s00705-019-04172-6. [DOI] [PubMed] [Google Scholar]
- [115].Carneiro BM, Batista MN, Braga ACS, et al. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215–218. [DOI] [PubMed] [Google Scholar]
- [116].Cruz-Oliveira C, Carvalho C, Neris R. Co-protoporphyrin IX and Sn- protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Sci Rep. 2018;8. DOI: 10.1038/s41598-018-27855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Le LJ, Loe M, Lee R, et al. Antiviral activity of pinocembrin against Zika virus replication. Antiviral Res. 2019;167. DOI: 10.1016/j.antiviral.2019.04.003. [DOI] [PubMed] [Google Scholar]
- [118].Aparecida Coronado M, Josef Eberle R, Bleffert N, et al. Zika virus NS2B/NS3 proteinase: A new target for an old drug - Suramin a lead compound for NS2B/NS3 proteinase inhibition-. Antiviral Res. 2018;160. DOI: 10.1016/j.antiviral.2018.10.019. [DOI] [PubMed] [Google Scholar]
- [119].Ahe D, Huehnchen P, Balkaya M, et al. Suramin-induced neurotoxicity: preclinical models and neuroprotective strategies. Molecules. 2018;23:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Barrows NJ, Campos RK, Powell ST, et al. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Davis JA, Paylor R, McDonald MP, et al. Behavioral effects of ivermectin in mice. Lab Anim Sci. 1999;49:288–296. [PubMed] [Google Scholar]
- [122].Roy A, Lim L, Srivastava S, et al. Solution conformations of Zika NS2B-NS3pro and its inhibition by natural products from edible plants. PLoS One. 2017;12. DOI: 10.1371/journal.pone.0180632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yuan S, Chan JF-W, den-Haan H, et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antiviral Res. 2017;145:33–43. [DOI] [PubMed] [Google Scholar]
- [124].Li Z, Sakamuru S, Huang R, et al. Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antiviral Res. 2017;150: DOI: 10.1016/j.antiviral.2017.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chen X, Yang K, Wu C, et al. Mechanisms of activation and inhibition of Zika virus NS2B-NS3 protease. Cell Res. 2016;26:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chan JF-W, Chik KK-H, Yuan S, et al. Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antiviral Res. 2017;141:29–37. [DOI] [PubMed] [Google Scholar]
- [127].Eyer L, Zouharová D, Širmarová J, et al. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antiviral Res. 2017;142:63–67. [DOI] [PubMed] [Google Scholar]
- [128].Eyer L, Nougairède A, Uhlířová M, et al. An E460D substitution in the NS5 protein of tick-borne encephalitis virus confers resistance to the inhibitor Galidesivir (BCX4430) and also attenuates the virus for mice. J Virol. 2019. DOI: 10.1101/563544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Taylor R, Kotian P, Warren T, et al. BCX4430 – A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. 2016;9. DOI: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Julander J, Siddharthan V, Evans J, et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res. 2016;137. DOI: 10.1016/j.antiviral.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ferreira AC, Zaverucha-do-Valle C, Reis PA, et al. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci Rep. 2017;7:9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Stephen P, Baz M, Boivin G, et al. Structural Insight into NS5 of Zika Virus Leading to the Discovery of MTase Inhibitors. J Am Chem Soc. 2016;138. DOI: 10.1021/jacs.6b10399. [DOI] [PubMed] [Google Scholar]
- [133].Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Li C, Deng Y-Q, Wang S, et al. 25-hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity. 2017;46:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Oestereich L, Rieger T, Neumann M, et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (Favipiravir) in a mouse model for crimean-congo hemorrhagic fever. PLoS Negl Trop Dis. 2014;8:e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].García-Nicolás O, V’kovski P, Vielle N, et al. The small-compound inhibitor K22 displays broad antiviral activity against different members of the family flaviviridae and offers potential as a panviral inhibitor. Antimicrob Agents Chemother. 2018;62. DOI: 10.1128/AAC.01206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Rausch K, Hackett BA, Weinbren NL, et al. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep. 2017;18:804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Li F, Lang Y, Ji Z, et al. A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. J Biol Chem. 2018;294:jbc.RA118.005015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Han Y, Mesplède T, Xu H, et al. The antimalarial drug amodiaquine possesses anti-ZIKA virus activities. J Med Virol. 2018;90. DOI: 10.1002/jmv.25031. [DOI] [PubMed] [Google Scholar]
- [140].Qing M, Yang F, Zhang B, et al. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother. 2009;53:3226LP– 3235. DOI: 10.1128/AAC.00189-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Carvalho O, Félix D, de Mendonça L, et al. The thiopurine nucleoside analogue 6-methylmercaptopurine riboside (6MMPr) effectively blocks Zika virus replication. Int J Antimicrob Agents. 2017;50. DOI: 10.1016/j.ijantimicag.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [142].Beck S, Zhu Z, De Oliveira M, et al. Mechanism of action of methotrexate against Zika virus. Viruses. 2019;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Huang Y, Li Y, Zhang H, et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018;4. DOI: 10.1038/s41421-018-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Willard KA, Elling CL, Stice SL, et al. The oxysterol 7-ketocholesterol reduces zika virus titers in vero cells and human neurons. Viruses. 2019;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Merino-Ramos T, Jiménez de Oya N, Saiz J-C, et al. Antiviral activity of nordihydroguaiaretic acid and its derivative Tetra-O-Methyl nordihydroguaiaretic acid against West Nile virus and Zika virus Antimicrob Agents Chemother. 2017;61:e00376–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Xu M, Lee EM, Wen Z, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Leonardi W, Zilbermintz L, Cheng L, et al. Bithionol blocks pathogenicity of bacterial toxins, ricin, and Zika virus OPEN. Sci Rep. 2016;6. DOI: 10.1038/srep34475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Oya N, Blázquez A-B, Casas J, et al. Direct Activation of Adenosine Monophosphate-Activated Protein Kinase (AMPK) by PF-06409577 inhibits flavivirus infection through modification of host-cell lipid metabolism. Antimicrob Agents Chemother. 2018;62(AAC.00360–18). DOI: 10.1128/AAC.00360-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Costa VV, Del Sarto JL, Rocha RF, et al. N-Methyl-D-Aspartate (NMDA) receptor blockade prevents neuronal death induced by Zika virus infection. MBio. 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Arbuckle JH, Gardina PJ, Gordon DN, et al. Inhibitors of the Histone Methyltransferases EZH2/1 Induce a Potent Antiviral State and Suppress Infection by Diverse Viral Pathogens. MBio. 2017;8:e01141–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].BioCryst Completes Phase 1 Clinical Trial of Galidesivir - Drugs.com MedNews. n.d.
- [152].WHO . WHO vaccine pipeline tracker. WHO 2016.
- [153].Muthumani K, Griffin BD, Agarwal S, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. Npj Vaccines. 2016;1:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hörner C, Bodmer B, Fiedler A, et al. Vaccine candidate mediates protection against Zika virus in an allogeneic mouse pregnancy model. J Virol. 2018;93. DOI: 10.1128/JVI.01485-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Richner JM, Jagger BW, Shan C, et al. Vaccine mediated protection against Zika virus induced congenital disease contributed to the design of safety and other experiments with ZIKV-NS1-LAV in mouse models. Cell. 2017;170:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Isbrucker RA, Edwards JA, Wolz E, et al. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 3: teratogenicity and reproductive toxicity studies in rats. Food Chem Toxicol. 2006;44:651–661. [DOI] [PubMed] [Google Scholar]
- [157].Clain E, Haddad J, Koishi SL, et al. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an endemic medicinal plant from Reunion Island, inhibits the early stages of Dengue and Zika Virus infection. Int J Mol Sci. 2019;20:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Julander JG, Siddharthan V, Evans J, et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res. 2017;137:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Coutard B, Barral K, Lichière J, et al. Zika virus Methyltransferase: structure and functions for drug design perspectives. J Virol. 2017;91:e02202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Brecher M, Li Z, Liu B, et al. A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 2017;13:e1006411. DOI: 10.1371/journal.ppat.1006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Yu Y, Deng YQ, Zou P, et al. A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat Commun. 2017;8. DOI: 10.1038/ncomms15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Shiryaev S, Farhy C, Pinto A, et al. Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists. Antiviral Res. 2017;143. DOI: 10.1016/j.antiviral.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Tong X, Smith J, Bukreyeva N, et al. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antiviral Res. 2017;149. DOI: 10.1016/j.antiviral.2017.11.004 [DOI] [PubMed] [Google Scholar]
- [164].Beck S, Zhu Z, Oliveira MF, et al. Mechanism of action of methotrexate against Zika virus. Viruses. 2019;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Pascoalino BS, Courtemanche G, Cordeiro MT, et al. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA-approved library. F1000Research 2016;5:2523. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Adcock RS, Chu Y-K, Golden JE, et al. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res. 2017;138:47–56. [DOI] [PubMed] [Google Scholar]
- [167].Costa-Nunes JP, Cline BH, Araújo-Correia M, et al. animal models of depression and drug delivery with food as an effective dosing method: evidences from studies with celecoxib and dicholine succinate. Biomed Res Int. 2015;2015:596126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wichit S, Hamel R, Bernard E, et al. Imipramine inhibits Chikungunya virus replication in human skin fibroblasts through interference with intracellular cholesterol trafficking. Sci Rep. 2017;7. DOI: 10.1038/s41598-017-03316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Singh PK, Khatri I, Jha A, et al. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci Rep. 2018;8: DOI: 10.1038/s41598-018-29329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cheng F, da Silva ASR, Huang AI-C, et al. Suppression of Zika Virus Infection and Replication in Endothelial Cells and Astrocytes by PKA Inhibitor PKI 14-22. J Virol. 2018;92:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Haviernik J, Štefánik M, Fojtíková M, et al. Arbidol (Umifenovir): A Broad-spectrum Antiviral Drug that Inhibits Medically Important Arthropod-borne Flaviviruses. Viruses. 2018. DOI: 10.3390/v10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Varghese FS, Rausalu K, Hakanen M, et al. Obatoclax inhibits alphavirus membrane fusion by neutralizing the acidic environment of endocytic compartments. Antimicrob Agents Chemother. 2017;61:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Petruska J, Frank D, Freeman G, et al. Toxicity and carcinogenicity studies of chlorpromazine hydrochloride and p -Cresidine in the p53 heterozygous mouse model. Toxicol Pathol. 2002;30:696–704. [DOI] [PubMed] [Google Scholar]
- [174].Kuivanen S, Bespalov MM, Nandania J, et al. Obatoclax, saliphenylhalamide and gemcitabine inhibit Zika virus infection in vitro and differentially affect cellular signaling, transcription and metabolism. Antiviral Res. 2017;139:117–128. [DOI] [PubMed] [Google Scholar]
- [175].Cairns D, Boorgu DSSK, Levin M, et al. Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells. Biol Open. 2018;7:bio031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kao JC, HuangFu WC, Tsai TT, et al. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Negl Trop Dis. 2018;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Freitas L, Leal D, Neris R, et al. da Silva Frost P. Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci Transl Med. 2018;10. DOI: 10.1126/scitranslmed.aar2749. [DOI] [PubMed] [Google Scholar]
- [178].Costa V, Del Sarto J, Rocha R, et al. N -Methyl-d-Aspartate (NMDA) receptor blockade prevents neuronal death induced by Zika virus infection. MBio. 2017;8:e00350–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Simanjuntak Y, Liang -J-J, Chen S-Y, et al. Ebselen alleviates testicular pathology in mice with Zika virus infection and prevents its sexual transmission. PLOS Pathog. 2018;14:e1006854. [DOI] [PMC free article] [PubMed] [Google Scholar]


