Abstract
Background
High levels of stromal tumor-infiltrating lymphocytes (sTIL) are associated with increased pathological complete response (pCR) rate and longer survival after neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) patients. Here, we evaluated the value of sTIL in predicting pCR and explored prognosis in TNBC patients treated with neoadjuvant chemotherapy according to body mass index (BMI).
Methods
sTIL were scored centrally on pretreatment biopsies from 2 retrospective series of nonunderweight TNBC patients (n = 445). sTIL and BMI were considered as binary (sTIL: <30.0% vs ≥30.0%; BMI: lean vs overweight and obese) and continuous variables. Associations with pCR (ypT0/isN0) were assessed using logistic regression, and associations with event-free survival and overall survival were assessed using Cox regressions.
Results
236 (53.0%) patients were lean and 209 (47.0%) overweight and obese. pCR was achieved in 181 of 445 (41.7%) patients. Median sTIL was 11.0%, and 99 of 445 (22.2%) tumors had high sTIL. A statistically significant interaction between sTIL and BMI, considered as categorical or continuous variables, for predicting pCR was observed in the multivariable analysis (Pinteraction = .03 and .04, respectively). High sTIL were statistically significantly associated with pCR in lean (odds ratio [OR] = 4.24, 95% confidence interval [CI] = 2.10 to 8.56; P < .001) but not in heavier patients (OR = 1.48, 95% CI = 0.75 to 2.91; P = .26) in the multivariable analysis. High sTIL were further associated with increased event-free survival in lean (hazard ratio [HR] = 0.22, 95% CI = 0.08 to 0.62; P = .004) but not in heavier patients (HR = 0.53, 95% CI = 0.26 to 1.08; P = .08). Similar results were obtained for overall survival.
Conclusion
BMI is modifying the effect of sTIL on pCR and prognosis in TNBC patients treated with neoadjuvant chemotherapy.
Stromal tumor-infiltrating lymphocytes (sTIL) have been extensively studied during the last decade in early breast cancer (1). These are generally quantified using a standardized method on hematoxylin and eosin-stained whole tissue sections (2). sTIL are more prevalent in triple-negative (TNBC) and human epidermal growth factor receptor 2 (HER2)-positive breast cancers as compared to estrogen receptor (ER)-positive breast tumors (3, 4). A pooled analysis of sTIL in TNBC patients treated with adjuvant chemotherapy in the context of clinical trials confirmed the prognostic role of sTIL in these patients and the excellent survival observed for patients with at least 30% sTIL at diagnosis (5). A recent analysis further confirmed the prognostic value of sTIL in systemically untreated patients with early stage TNBC (6). Additionally, a large pooled analysis has demonstrated that higher levels of sTIL predict pathological complete response (pCR) to neoadjuvant chemotherapy in patients from all molecular subtypes (TNBC, HER2-positive, and ER-positive/HER2-negative) but longer survival only in TNBC and HER2-positive breast cancer (4). Of note, in light of the consistent findings in TNBC, sTIL have now been integrated in the new World Health Organization classification of breast tumors, and their evaluation has been recommended by the St Gallen International Consensus Guidelines 2019 (7) for TNBC.
Recent experimental and human data have revealed important differences in the tumor immune microenvironment according to the adiposity of the subject (8–10). Wang et al. (8) demonstrated that the age-related deterioration of the T lymphocytes is exacerbated by obesity and mediated by leptin, which is present at high levels in obese subjects. Consistent results were observed in mice, nonhuman primates, and humans. Specifically, obesity was associated with a decreased proliferative capacity and an increased expression of the exhaustion markers PD-1, LAG3, and TIM3 of the CD8+ cytotoxic T-cell compartment. These observations could potentially explain the improved survival observed in obese, compared with lean, melanoma, non-small cell lung carcinoma and renal cell carcinoma patients treated with checkpoint inhibitors (11–14). Besides the intriguing differences observed for the CD8+ cytotoxic T cells, 2 recent studies highlighted the remodeling that macrophages undergo in the adipose tissue of obese women and obese breast cancer patients (9, 10). Springer et al. (10) recently analyzed macrophages in the mammary tissue from noncancer patients and observed that obesity was associated with an increase in density of M2-biased macrophages. Tiwari et al. (9) analyzed macrophages present in TNBC and observed that obesity was associated with the presence of pro-inflammatory macrophages, which unlike the pro-inflammatory M1 macrophages are protumorigenic. These 2 studies therefore provide evidence regarding the impact of adiposity on the plasticity of macrophages, an effect that might also potentially differ according to the presence or not of cancer.
The proportion of women who are overweight or obese has been increasing over the past decades and is at least 50% in most industrialized countries, reaching even 64% in the United States according to the latest estimates (15). So far, the predictive and prognostic values of sTIL have never been investigated according to the patient’s body mass index (BMI). Here, we investigated whether BMI would modify the effect of sTIL to predict pCR in TNBC patients treated with neoadjuvant chemotherapy and explored the prognostic value of sTIL in this series according to BMI.
Methods
Patients
We considered 2 retrospective, consecutive institutional series of TNBC patients treated with neoadjuvant chemotherapy (UZ Leuven, n = 174; Institut Curie, n = 277). At UZ Leuven, patients were diagnosed between January 1, 2000, and August 31, 2017, and at Institut Curie between January 1, 2002, and June 30, 2012. The type of neoadjuvant chemotherapy was chosen as per local guidelines at the time of diagnosis and was generally based on 1) anthracyclines, 2) anthracyclines and taxanes, or 3) anthracyclines, taxanes, and platinum. In both series, TNBC was defined as ER-negative and progesterone receptor–negative according to American Society of Clinical Oncology and College of American Pathologists guidelines on immunohistochemistry (IHC)-stained slides and HER2-negative by IHC (in cases of score 0 or 1+) or by fluorescent in situ hybridization for cases with an IHC score of 2+ or 3+ (16). pCR was defined as ypT0/is N0. BMI was calculated from the height and weight measurements at the time of patient diagnosis, as the weight in kilograms divided by the square of the height in meters, and was further categorized according to the definition from the World Health Organization: underweight (<18.5 kg/m2), lean (≥18.5 and <25 kg/m2), overweight (≥25 and <30 kg/m2), and obese (≥30 kg/m2). Underweight patients (n = 6) were excluded given their low frequency and potentially adverse prognosis (17). For the main analyses, BMI was binarized as follows: low (lean) vs high BMI (overweight and obese). This study has been approved by the respective ethics committees (UZ Leuven: No. S58586, Institut Curie: No. 1547270).
TIL Assessment
sTIL were scored centrally by an experienced pathologist (GF) on hematoxylin and eosin sections from pretreatment biopsies according to the guidelines of the international TIL working group (2). sTIL were scored on the pretreatment core needle biopsy either using digitalized hematoxylin and eosin slides (Institut Curie) or with standard light microscopy in bright field on hematoxylin and eosin-stained sections (UZ Leuven). The percentage of the mononuclear inflammatory infiltrate present in the peritumoral stroma was recorded for each analyzed field. The average value of 3 to 5 fields with 200× magnification located at the invasive front of the tumor was obtained and used as the actual sTIL score. In addition to considering sTIL as a continuous variable, highly infiltrated tumors were defined as at least 30% sTIL in agreement with recent TNBC literature (5).
Statistical Analysis
Associations between categorical BMI and clinicopathological variables were assessed using the Fisher exact test, association between continuous sTIL and categorical BMI using Kruskal-Wallis, and 2-sample Kolmogorov-Smirnov test. Correlation between continuous BMI and continuous sTIL was assessed using the Spearman rank test. Associations with pCR were assessed using logistic regression. Overdispersion was not considered relevant when the ratio between the residual degrees of freedom and the variance was lower than 2. Associations with event-free survival (EFS) and overall survival (OS) were assessed using stratified log-rank test and Cox proportional hazard regression. EFS was defined as the time from surgery to locoregional, distant recurrence, or death without the evidence of recurrence, and OS was defined as the time from surgery to death for any cause. Median follow-up, as computed using the reverse Kaplan-Meier estimator, was 7.63 years. However, it was curtailed at 5 years to ensure the comparability of the 2-case series in consideration of the declining numerosity of patients followed after that timepoint. Menopausal status (pre vs post), grade (1 + 2 vs 3), and stage (1 + 2 vs 3) at diagnosis were considered as adjustment variables. We accounted for the center effect by stratification or adjustment in models with categorical or continuous variables, respectively. An interaction term was considered between sTIL and BMI. Proportional hazard assumptions were checked by analyzing the Schoenfeld residuals both graphically and through the cox.zph function from the R survival package. Model assumptions, outliers, and leverage effects were checked, using the R stats package, by computing residuals and Cook’s distances. The logistic and Cox regressions were first assessed using sTIL and BMI as binary variables using predefined cutoffs. Then, continuous analyses were performed, and nonlinear effects were explored during the model-building phase resorting to regression splines. For the continuous analyses, odds ratios (OR) and hazard ratios (HR) were computed for a 10.0% increment of sTIL and 1.0% increment in BMI. All P values from regressions were based on Wald test, with the exception of the 1 provided for the joint effects, which is from a likelihood ratio test considering the 2 main terms, sTIL and BMI, as well as their interaction. P values were 2-sided and considered as statistically significant at the conventional level of .05. Consistently, 95% confidence intervals (CIs) were appropriately computed with the functions provided in the stats, survival, and rms packages. All analyses were done using R 3.5.2.
Results
Patient Characteristics
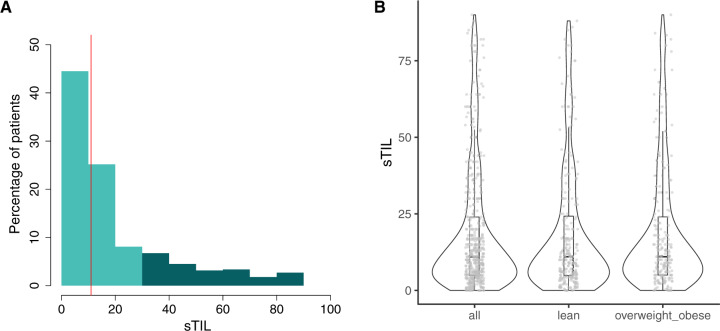
We considered 451 TNBC patients treated with neoadjuvant chemotherapy from 2 retrospective, consecutive institutional series (Supplementary Figures 1 and 2, available online). After excluding underweight patients (n = 6), 236 of 445 (53.0%, 95% CI = 48.4% to 57.7%), 132 of 445 (29.7%, 95% CI = 25.4% to 33.9%), and 77 of 445 (17.3%, 95% CI = 13.8% to 20.8%) patients were lean, overweight, and obese, respectively. Taken together, overweight and obese patients represented 209 of 445 (47.0%, 95% CI = 40.0% to 54.0%) patients. The patient and tumor characteristics are summarized in Table 1. Median age at diagnosis was 49 years, and 183 of 438 (41.8%, 95% CI = 37.2% to 46.4%) were postmenopausal. Median sTIL was 11.0%, and 99 of 445 (22.2%, 95% CI = 18.4% to 26.1%) tumors had high sTIL (defined as ≥30.0%; Figure 2,A). pCR was achieved in 181 of 445 (40.7%, 95% CI = 36.1% to 45.2%) of the patients. For the EFS and OS endpoints, 118 and 90 events were recorded at 5 years, respectively, with 2 deaths without evidence of recurrence. Of these 445 patients, 433 (97.3%) had complete clinicopathologic characteristics for multivariable analyses.
Table 1.
Patient and tumor characteristics according to BMI
| All patients | Lean | Overweight and obese | ||
|---|---|---|---|---|
| Patient and tumor characteristics | (n = 445) | (n = 236) | (n = 209) | P a |
| Age, No. (%) | .002 | |||
| ≤ 40 y | 110 (24.7) | 73 (30.9) | 37 (17.7) | |
| 41-50 y | 128 (28.8) | 70 (29.7) | 58 (27.8) | |
| > 50 y | 207 (46.5) | 93 (39.4) | 114 (54.5) | |
| Age (continuous), y | ||||
| Mean (SD) | 49.4 (11.4) | 47.3 (11.2) | 51.8 (11.2) | <.001 |
| Median (IQR) | 49 (41-57) | 46.5 (39-54) | 52 (43-59) | |
| Range | 25-84 | 25-84 | 27-79 | |
| Menopausal status, No. (%) | ||||
| Pre | 255 (58.2) | 153 (66.2) | 102 (49.3) | <.001 |
| Post | 183 (41.8) | 78 (33.8) | 105 (50.7) | |
| Missing | 7 | 5 | 2 | |
| Tumor size (cT), No. (%) | ||||
| cT1 | 46 (10.3) | 34 (14.4) | 12 (5.7) | <.001 |
| cT2 | 246 (55.3) | 135 (57.2) | 111 (53.1) | |
| cT3 | 108 (24.3) | 55 (23.3) | 53 (25.4) | |
| cT4a-c | 13 (2.9) | 4 (1.7) | 9 (4.3) | |
| cT4d | 32 (7.2) | 8 (3.4) | 24 (11.5) | |
| Nodal status (cN), No. (%) | ||||
| cN0 | 182 (40.9) | 97 (41.1) | 85 (40.7) | .09 |
| cN1 | 193 (43.4) | 110 (46.6) | 83 (39.7) | |
| cN2 | 23 (5.2) | 12 (5.1) | 11 (5.3) | |
| cN3 | 47 (10.6) | 17 (7.2) | 30 (14.4) | |
| Stage, No. (%) | ||||
| Stage I | 19 (4.3) | 15 (6.4) | 4 (1.9) | .008 |
| Stage II | 268 (60.2) | 149 (63.1) | 119 (56.9) | |
| Stage III | 158 (35.5) | 72 (30.5) | 86 (41.1) | |
| Histological grade, No. (%) | ||||
| Grade 1 | 2 (0.5) | 2 (0.9) | 0 (0.0) | .07 |
| Grade 2 | 48 (10.9) | 31 (13.4) | 17 (8.2) | |
| Grade 3 | 390 (88.6) | 199 (85.8) | 191 (91.8) | |
| Missing | 5 | 4 | 1 | |
| sTIL, No. (%) | ||||
| <30.0% | 346 (77.8) | 184 (78.0) | 162 (77.5) | .91 |
| 30.0% | 99 (22.2) | 52 (22.0) | 47 (22.5) | |
| sTil (continuous), No. (%) | ||||
| Mean (SD) | 18.9 (20.5) | 18.7 (20.7) | 19.2 (20.4) | .48 |
| Median (IQR) | 11 (5-24) | 11 (4.8-24.2) | 11 (5-24) | |
| Range | 0-90 | 0-88 | 0-90 | |
| Neoadjuvant treatment, No. (%) | ||||
| Anthra | 57 (12.8) | 32 (13.6) | 25 (12.0) | .73 |
| Anthra-Tax | 296 (66.5) | 159 (67.4) | 137 (65.6) | |
| Anthra-Tax-Carbo | 52 (11.7) | 27 (11.4) | 25 (12.0) | |
| Other | 40 (9) | 18 (7.6) | 22 (10.5) | |
| pCR (ypT0/is N0) No. (%) | ||||
| No | 264 (59.3) | 131 (55.5) | 133 (63.6) | .08 |
| Yes | 181 (40.7) | 105 (44.5) | 76 (36.4) |
P values are from the Fisher exact test and Kruskal-Wallis test when comparing categorical and continuous variables against 2 categories BMI, respectively. BMI = body mass index; IQR = interquartile range.
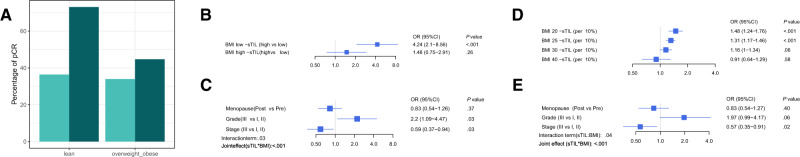
Figure 2.
Association of sTIL with pCR according to BMI. A) pCR rate according to the 2 categories of BMI and sTIL. Low and high sTIL are depicted in light and dark colour, respectively. Multivariable logistic model considering both sTIL and BMI as categorical (B, C) and continuous (D, E) variables. Panels (B) and (D) represent the interaction effect between BMI and sTIL and panels (C, E) the effects of the adjustment variables (menopausal status, grade, stage) of the models. All reported P values are from Wald tests, with the exception of the joint effect that reports a P value from a likelihood ratio test considering the 2 main terms sTIL and BMI as well as their interaction. BMI = body mass index; CI = confidence interval; OR = odds ratio; pCR = pathological complete response; sTIL = stromal tumor-infiltrating lymphocytes.
Association Between BMI and Clinicopathologic Characteristics
BMI was showing association with menopausal status (P < .001), tumor size (P < .001), and stage (P = .008) (Table 1). BMI was not showing association with categorical sTIL. There was also no association when considering binary BMI and continuous sTIL (P = .48 and P = .84 from the Kruskal-Wallis and Kolmorgornov-Smirnoff tests, respectively; Figure 1), or when considering both as continuous variables (Spearman rho = -0.047; P = .32). We further explored the associations considering the 3 main BMI categories (lean, overweight, and obese) and reported these in Supplementary Table 1 (available online) and Supplementary Figure 2 (available online).
Figure 1.
Distribution of sTIL, BMI, and pCR rates. A) Distribution of sTIL. Light and dark colour indicate low (<30.0%) and high (≥30.0%) sTIL, respectively. The vertical line indicates the median. B) Distribution of sTIL for the complete series and according to the 2 categories of BMI. Violin plots indicate the probability density of the data, and box plots represent the median (bold line) and interquartile range (rectangle). Dots report the distribution of the observed values. BMI = body mass index; pCR = pathological complete response; sTIL = stromal tumor-infiltrating lymphocytes.
Association of sTIL With pCR According to BMI
It has previously been demonstrated that increased levels of sTIL are predicting pCR to neoadjuvant chemotherapy in TNBC patients (4). Here, we evaluated whether BMI is modifying this effect. We first considered the predefined cutoffs for both sTIL (<30.0% vs ≥30.0%) (5) and BMI (lean vs overweight and obese). A stable pCR rate was observed in patients with low sTIL tumors across the BMI groups, with 67 of 184 (36.4%, 95% CI = 29.5% to 43.4%) lean and 55 of 162 (34.0%, 95% CI = 26.7% to 41.2%) heavier patients having a pCR (Figure 2,A). However, in patients with high sTIL tumors, we observed a higher pCR rate in lean patients, with 38 of 52 (73.1%, 95% CI = 61.0% to 85.1%) patients having a pCR as opposed to only 21 of 47 (44.7%, 95% CI = 30.5% to 58.9%) in heavier patients. Similar results were seen when exploring the 3 BMI categories (Supplementary Figure 2C, available online), with 16 of 35 (45.7%, 95% CI = 29.2% to 62.2%) overweight and 5 of 12 (41.7%, 95% CI = 13.8% to 69.6%) obese patients achieving a pCR in the high sTIL group (Supplementary Figure 2, available online). On the contrary, a stable pCR rate was observed in the low sTIL group across the BMI categories, with 33.0% (32 of 97, 95% CI = 23.6% to 42.3%) in overweight and 35.4% (23 of 65, 95% CI = 23.8% to 47.0%) in obese patients (Figure 2,A). Regression analyses revealed a statistically significant interaction between sTIL and BMI for predicting pCR in multivariable analysis (Pinteraction = .03) (Figure 2, B and C). sTIL were showing association with pCR in lean but not in heavier patients in multivariable analysis (ORlean = 4.24, 95% CI = 2.10 to 8.56; P < .001; and ORoverweight and obese = 1.48, 95% CI = 0.75 to 2.91; P = .26).
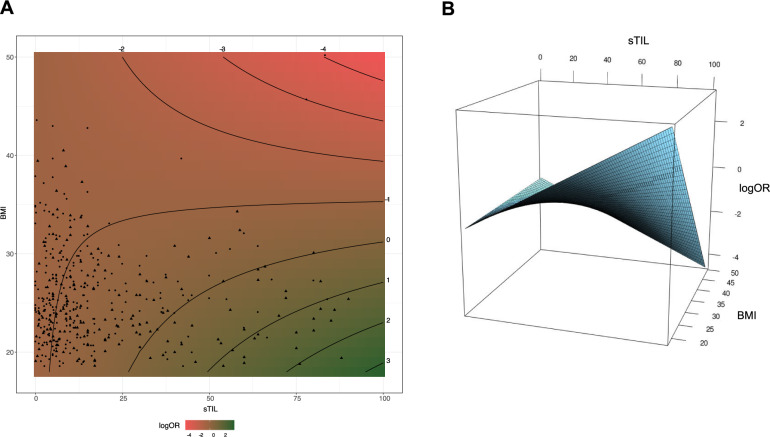
Acknowledging the continuous distribution of both sTIL and BMI, we further carried out an analysis where both were considered as continuous variables. There was no evidence of nonlinear effects. Here, we also observed a statistically significant interaction term between linear sTIL and BMI at the multivariable level (Pinteraction = .04; Figure 2, D and E). We further modeled the OR of having pCR as a function of sTIL and BMI (Figure 3). The plot shows the interaction of BMI with sTIL in predicting pCR. It confirms that for low BMI, higher sTIL levels are associated with higher pCR rates, whereas it is associated with lower pCR rates for higher BMI values.
Figure 3.
Interaction between continuous BMI and sTIL regarding pCR. A) Contour representation of logOR for pCR according to BMI and sTIL. logOR is computed in a logistic model adjusted for menopausal status, grade, and stage. The reference for the logOR is taken at 30.0% sTIL and 20 kg/m2 BMI. The scatter plot refers to the supporting patients with patients achieving pCR as triangles, the others as circles. B) Surface representation of logOR for pCR according to BMI and sTIL. BMI = body mass index; OR = odds ratio; pCR = pathological complete response; sTIL = stromal tumor-infiltrating lymphocytes.
Association of sTIL With Prognosis According to BMI
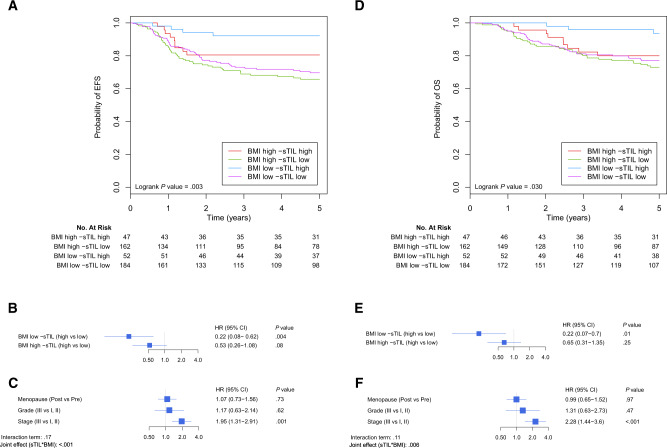
We first explored the prognostic value of sTIL according to BMI for EFS and OS, considering both as binary variables. In lean patients, we observed a clear separation of the curves between high and low sTIL, whereas this difference was less marked in the overweight and obese patients (Figure 4,A). The interaction term, however, did not reach statistical significance at the multivariable level (Pinteraction = .17; Figure 4, B and C). Nevertheless, the confidence intervals of the 2 BMI groups (Figure 4,B) advocate for a trend of an underlying interaction between BMI and sTIL, with higher sTIL being associated with better EFS in lean patients (HR = 0.22, 95% CI = 0.08 to 0.62; P = .004) but not in overweight and obese patients (HR = 0.53, 95% CI = 0.26 to 1.08; P = .08). Similar results were observed for OS (Figure 4, D–F); although the interaction term did not reach statistical significance at the multivariable level (Pinteraction = .12; Figure 4, E and F), higher sTIL were associated with better OS in lean patients (HR = 0.22, 95% CI = 0.07 to 0.70; P = .01) but not in overweight and obese patients (HR = 0.65, 95% CI = 0.31 to 1.35; P = .25). A model including pCR as covariate for the EFS and OS analyses is reported in Supplementary Figure 3 (available online). As expected, it showed a statistically significant protective effect of pCR regarding EFS and OS (HR = 0.18, 95% CI = 0.10 to 0.32; P < .001; and HR = 0.17, 95% CI = 0.09 to 0.34; P < .001 for EFS and OS, respectively) while keeping the same trend considering the interaction between BMI and sTIL regarding the survival. We further evaluated the prognostic value of sTIL according to BMI considering both as continuous variables in Supplementary Figure 4 (available online). The same trend is observed with a protective effect of higher sTIL that decreases with high BMI values (Supplementary Figure 3, B and E, available online). The interaction term did not reach statistical significance (Pinteraction = .27 and Pinteraction = .18 for multivariable analysis of EFS and OS, respectively; Supplementary Figure 4, available online), but the observed increasing hazard ratio of sTIL with higher BMI suggests the existence of a modifying effect of BMI over sTIL. Similarly to Supplementary Figure 4 (available online), we also reported a model including pCR as covariate for the EFS and OS analyses in Supplementary Figure 5 (available online). In this continuous approach, pCR was still showing a statistically significant protective effect considering EFS and OS (HR = 0.19, 95% CI = 0.11 to 0.34; P < .001; and HR = 0.18, 95% CI = 0.09 to 0.35; P < .001 for EFS and OS, respectively), whereas the modifying effect of BMI on sTIL remained visible.
Figure 4.
Association between sTIL and survival according to BMI. A-C) Association between sTIL and EFS according to BMI. D-F) Association of sTIL and OS according to BMI. sTIL and BMI are considered as categorical variables. A, D) Kaplan-Meier curves for the 4 conditions regarding BMI and sTIL as categorical variables. EFS (B, C) and OS (E, F). Cox regressions are adjusted for menopausal status, grade, and stage and stratified for centers. Panels (B, E) represent the interaction between BMI and sTIL, and panels (C, F) the adjustment variables of the models. All reported P values are from Wald tests, except for the joint effect that reports a P value from a likelihood ratio test considering the 2 main terms sTIL and BMI as well as their interaction. BMI = body mass index; CI = confidence interval; EFS = event-free survival; HR = hazard ratio; OS = overall survival; sTIL = stromal tumor-infiltrating lymphocytes.
Discussion
High sTIL levels have been associated with increased pCR rates in TNBC patients treated with a different neoadjuvant chemotherapy regimen (4). Here, we report that BMI is modifying the effect of sTIL in TNBC patients treated with neoadjuvant chemotherapy. Indeed, when defining highly infiltrated tumors as those with at least 30.0% sTIL (5), we observed a pCR rate of 73.1% in lean and 44.7% in overweight and obese patients. These observations were further strengthened by the statistically significant interaction term that was obtained between sTIL and BMI in the regression analyses when considering both sTIL and BMI as categorical variables using predefined cutoffs (Pinteraction = .03). We further repeated the analyses by considering sTIL and BMI as continuous variables to more accurately reflect the degree of tumor immune infiltration and the adiposity of the patient. These analyses confirmed the statistically significant interaction term between sTIL and BMI (Pinteraction = .04). We also provided a continuous surface representation to visualize the odds ratio for achieving pCR according to sTIL level and BMI.
A recent study has further demonstrated that high sTIL levels predict not only pCR in TNBC patients treated with neoadjuvant chemotherapy but also survival in these 632 patients (4). Additionally, a recent pooled analysis of 9 clinical trials comprising 2148 patients has confirmed the strong prognostic role of sTIL in TNBC patients treated with adjuvant chemotherapy (5). In our series, we further observed that BMI would also modify the prognostic value of sTIL. Although the interaction term was not statistically significant, we observed increasing hazard ratios, both for EFS and OS, for sTIL with increasing BMI, consistent with the underlying interaction trend.
The retrospective nature of our study is a limitation that has 3 main implications. First, the neoadjuvant chemotherapy regimen that has been administrated has varied across the years and institutions. Second, BMI was the only measure of adiposity available for these patients. Although practical, BMI might overestimate body fat in physically active individuals and underestimate it in older individuals or in those who have lost muscle mass. Finally, because pCR was the primary endpoint of this study, the duration of the follow-up was limited in a relevant fraction of patients, leading us to curtail the follow-up at 5 years.
Overall, our results could be explained in light of a recent study that suggests the local antitumor immune response is suppressed in obese subjects (8). We might therefore hypothesize that overweight and obese TNBC patients with highly infiltrated tumors that do not respond to neoadjuvant chemotherapy could potentially be good candidates for immunotherapies based on checkpoint inhibition. A retrospective, multicohort analysis performed in patients with metastatic melanoma treated with immunotherapy, targeted therapy, or chemotherapy has found that obesity, as opposed to lean condition, was associated to improved PFS and OS only in those male patients treated with immune therapy or targeted therapy (11). Similarly, a recent retrospective, multicenter analysis carried out mainly in non-small cell lung carcinoma, melanoma, and renal cell carcinoma patients provided similar findings (12). Finally, a meta-analysis of 4 clinical trials investigating atezolizumab reported an association between high BMI and improved survival in patients with non-small cell lung carcinoma (14). However, at this stage, no data are available for TNBC patients.
To conclude, we provide evidence that in TNBC patients treated with neoadjuvant chemotherapy, BMI is modifying the effect of sTIL. Because obesity has reached pandemic proportions, further basic, translational, and clinical research is urgently needed to disentangle the complex interaction between breast cancer, immunity, and increased adiposity.
Funding
This work was supported by the Fondation Cancer Luxemburg (to CD, FC/2018/07).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Role of the authors: GF: Integrity of the data and the accuracy of the data analysis; Study concept and design; Acquisition, analysis, or interpretation of data; Study supervision; Critical revision of the manuscript for important intellectual content. FR: Acquisition, analysis, or interpretation of data; Drafting of the manuscript; Critical revision of the manuscript for important intellectual content; Statistical analysis. ASH: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content; Administrative, technical, or material support. LJ: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content; Administrative, technical, or material support. HW: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. JA: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. KP: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. AS: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. PB: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. IV: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. DDC: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. DM: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. AS: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. ML: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. FR: Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content. EB: Integrity of the data and the accuracy of the data analysis; Study concept and design; Critical revision of the manuscript for important intellectual content; Drafting of the manuscript; Statistical analysis; Study supervision. PN: Integrity of the data and the accuracy of the data analysis; Study concept and design; Critical revision of the manuscript for important intellectual content; Study supervision. CD: Integrity of the data and the accuracy of the data analysis; Study concept and design; Critical revision of the manuscript for important intellectual content; Drafting of the manuscript; Obtained funding; Study supervision.
Prior presentation: Part of this work was presented at the annual San Antonio Breast Cancer Symposium in December 2019 (Desmedt et al. P 01-10-04).
Data Availability
The data that support the findings of this study are available on request from the corresponding author (CD) after approval from the ethics committee. The data are not publicly available since they contain information that could compromise the privacy of the patients.
Supplementary Material
References
- 1. Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13(4):228–241. [DOI] [PubMed] [Google Scholar]
- 2. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. [DOI] [PubMed] [Google Scholar]
- 4. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 5. Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30(12):1941–1949. [DOI] [PubMed] [Google Scholar]
- 7. Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early stage breast cancer. Ann Oncol. 2019;30(10):1541–1557. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiwari P, Blank A, Cui C, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216(6):1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Springer NL, Iyengar NM, Bareja R, et al. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am J Pathol. 2019;189(10):2019–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy WJ, Longo DL.. The surprisingly positive association between obesity and cancer immunotherapy efficacy. JAMA. 2019;321(13):1247. [DOI] [PubMed] [Google Scholar]
- 14. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ.. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;6(4):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon H-G, Han W, Noh D-Y.. Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol. 2009;27(35):5899–5905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (CD) after approval from the ethics committee. The data are not publicly available since they contain information that could compromise the privacy of the patients.