Supplemental Digital Content is Available in the Text.
Acute pain facial expressions can be detected/scored in human fetuses. We propose a seven-item scale to differentiate pain facial expressions from rest/acoustic stimuli ones.
Keywords: Pain, Fetal, Ultrasound
Abstract
Introduction:
The question of whether the human fetus experiences pain has received substantial attention in recent times. With the advent of high-definition 4-dimensional ultrasound (4D-US), it is possible to record fetal body and facial expressions.
Objective:
To determine whether human fetuses demonstrate discriminative acute behavioral responses to nociceptive input.
Methods:
This cross-sectional study included 5 fetuses with diaphragmatic hernia with indication of intrauterine surgery (fetoscopic endoluminal tracheal occlusion) and 8 healthy fetuses, who were scanned with 4D-US in 1 of 3 conditions: (1) acute pain group: Fetuses undergoing intrauterine surgery were assessed in the preoperative period during the anesthetic injection into the thigh; (2) control group at rest: Facial expressions at rest were recorded during scheduled ultrasound examinations; and (3) control group acoustic startle: Fetal facial expressions were recorded during acoustic stimulus (500–4000 Hz; 60–115 dB).
Results:
Raters blinded to the fetuses’ groups scored 65 pictures of fetal facial expressions based on the presence of 12 items (facial movements). Analyses of redundancy and usefulness excluded 5 items for being of low discrimination capacity (P>0.2). The final version of the pain assessment tool consisted of a total of 7 items: brow lowering/eyes squeezed shut/deepening of the nasolabial furrow/open lips/horizontal mouth stretch/vertical mouth stretch/neck deflection. Odd ratios for a facial expression to be detected in acute pain compared with control conditions ranged from 11 (neck deflection) to 1,400 (horizontal mouth stretch). Using the seven-item final tool, we showed that 5 is the cutoff value discriminating pain from nonpainful startle and rest.
Conclusions:
This study inaugurates the possibility to study pain responses during the intrauterine life, which may have implications for the postoperative management of pain after intrauterine surgical interventions
1. Introduction
The question of whether the human fetus experiences pain has received substantial attention in recent times.2,9 With the advent of high-definition 4-dimensional ultrasound (4D-US) machines, it has become possible, using high-quality films, to record fetal body and facial expressions.20 A recent report describing 2 important methodological advances has addressed this challenge by introducing5: (1) the possibility of applying a scale based on facial expression originally developed for acute behavioral responses to nociceptive input assessment in neonates (ie, after blood draw) to fetuses and of applying it (2) right after (time anchor) an acute pinprick stimulus (external stimulus) caused by an anesthetic injection administered before an intrauterine surgical procedure. Since normal fetuses may display several physiological facial expressions that are unrelated to nociception,32 an ideal scoring system should have items that discriminate acute pain (AP) from rest/nonpainful stimulation states.
In this study, we had 2 aims. The first was to adapt items on the facial scoring system used for neonates to those with clear discriminatory weight when comparing acute behavioral responses with nociceptive input response against rest or acoustic startle in fetuses. The second was to explore a potential cutoff value that would allow for the categorization of facial expressions as related to acute behavioral responses to nociceptive input when compared with both control conditions. This is the first attempt to assess specific AP-related facial codes in human fetuses, and it has the potential for theoretical and practical implications such as intrauterine postsurgical pain management and follow-up of potential pain induced by fetal malformations such as gastroschisis and bowel atresia.
2. Methods
The study was approved by our institution's Ethics Review Board (2.649.528). All patients gave consent to participate in the study and to record the behavioral reactions of the fetuses. Ultrasound scanning was performed in the third trimester of pregnancy by a researcher (with no role in the anesthetic fetal procedure) using a 4D-US machine (Voluson E8 by GE Healthcare, Zipf, Austria). We assessed 5 fetuses with diaphragmatic hernia with no concomitant neurological malformations or diseases and 8 healthy fetuses in 1 of 3 conditions:
(1) Acute pain group: Fetuses with diaphragmatic hernia with indication of intrauterine surgery (fetoscopic endoluminal tracheal occlusion) were assessed in the preoperative period during the anesthetic injection into the thigh, according to the model previously described assessing acute behavioral responses to nociceptive input.5 A second ultrasound machine was operated by a fetal medicine specialist exclusively to record the facial expressions of the fetus for 45 seconds before and 45 seconds after the anesthetic injection to the thigh, guided by the main ultrasound machine.
(2) Control group at rest (Co-Re): The facial expressions of third-trimester fetuses at rest were recorded during scheduled ultrasound examinations. Scans were performed, after a 5-minute period of rest for the mother, in a quiet and dark room for 45 seconds;
(3) Control group acoustic startle (Co-AS): Acoustic stimulation was used to improve the efficiency of fetal heart rate testing to assess fetal wellbeing. Fetal facial expressions were recorded for 45 seconds before and after the acoustic stimulus. The stimulator similar to a bike horn (Kobo; Kobo horn, São Paulo, Brazil) was applied to the maternal abdomen next to the fetal cephalic pole for 4 seconds. We used stimulation in the form of 3 pulses for 4 seconds, producing a 500- to 4000-Hz sound at 60–115 dB, which provides the intrauterine acoustic perception of a slightly laud acute sound, being safe and reliable.19,25
The fetuses without congenital disease were confirmed to be in good health by neonatal examination after birth. Recordings from the initial rest 45 seconds from the AP and Co-AS groups were made to ascertain that the facial US film was in a good recording window and to ascertain the moment of AP/acoustic startle initiation, but were not analyzed in the study as part of the “rest” condition. Rest condition films were scored exclusively from the Co-Re group, so that all groups had one 45-second recordings used for the study. After performing the ultrasound examinations on the 3 groups, films from all the fetuses were anonymized. Films were then reviewed by 2 ultrasonographists, who selected screenshots from each film. To provide the highest number of facial expressions possible, a screenshot was taken each time the ultrasonographist judged that facial expressions were clearly visible, similar to the procedure of choosing the most representative screenshots of a given organ during US recordings as part of everyday practice of fetal medicine specialists. As expected, several facial movements were allowed to occur in each screenshot. Each group of screenshots was then reviewed by 2 blinded researchers who independently scored the facial pictures of each fetus using the Neonatal Facial Coding System (10 items), plus 2 additional ones: yawning and neck deflection, for a total of 12 items: brow lowering, eyes squeezed shut, deepening of the nasolabial furrow, open lips, horizontal mouth stretch, vertical mouth stretch, lip purse, taut tongue, tongue protrusion, chin quiver, neck deflection, and yawning. These 2 last supplementary items were included because of clinical maternal-fetal medicine research observations of fetal reactions after painful stimulus and rest. In all cases, a maximal of 5 screenshots were enough to comprise all facial expressions in each condition,5 so that each recording (condition) provided 5 screenshots. Each item was given 1 point if present, zero if absent on a given screenshot. Importantly, each of the 12 items could only be scored only once for each film. This means that opening the mouth for 1 second or for 3 seconds would provide a score of only 1 for this specific item in both cases. Also, in the (very rare) event of an item occurring more than once, for instance, tongue protrusion occurring on 2 separate occasions during the same 45-second recording, in this case, the final score for this specific item would also be 1. Instances where the final score for each fetus differed between the 2 researchers were resolved by a face-to-face meeting between them and a third specialist until consensus was reached. The product of the screenshot assessment was a single score (between zero and 12) for each fetus. This score was assessed for redundancy of items, where items that always appeared or were always absent in blocks were merged. Items that were never scored were suppressed. This gave rise to a reduced scale that only contained the more discriminant items. The reduced scale score of each fetus was analyzed by considering the condition of the fetus in the recording: AP, rest, or acoustic startle. This allowed researchers to determine a cutoff score that was exclusively related to pain and provided a potential “pain cutoff” for the new reduced scoring system.
2.1. Statistics analyses
Our statistical analysis initially evaluated distributions, frequencies, and percentages for each of the considered items (facial expressions). We assumed that the observed items were independent variables, and differences between the AP and Co-Re/Co-AS groups were compared with nonparametric tests.3 Specifically, we applied the Barnard test (given the independence of rows and columns in 2 × 2 contingency tables and the small sample size). In our case, rows corresponded to the observation of a specific item while columns corresponded to groups. All analyses were performed with the statistical language, R,37 with the additional “Barnard” package. We assessed redundancy (a measure of co-occurrence between 2 expression items) by merging items that always appeared (or were always absent) in blocks. The key idea of assessing the redundancy of expression items is to find closely related ones.29,40 Results were also expressed in terms of odds ratio, which was calculated using the additional “fmsb” R package. Anonymized data can be made available to qualified investigators on request to the corresponding author.
3. Results
All 13 fetuses were in the third trimester of gestation (31.1 ± 2.8 weeks), and mothers were 28.7 ± 5.5 years old. A total of 13 films (n = 5: AP; n = 4: Co-Re; n = 4: Co-AS) providing a total of 65 pictures of fetal facial expressions were produced (Fig. 1). First, we reduced the items on the facial scoring system scale by determining which items were redundant: those that were concomitantly present or absent in both the AP and control conditions (ie, those with no discriminative capacities). Data from both control situations were merged and contingency tables of 2 × 2 correlations were created. We assessed the concordance between pairs of items6 to investigate whether they agree sufficiently to allow for the removal of redundant items. The results showed that 2 items were not scored on the screenshots from the 4D-US: “taut tongue” and “chin quiver.” Furthermore, “lips pursing,” “tongue protrusion,” and “yawning” had very high P-values (>0.20), as they occurred in similar frequencies in the AP and control groups (Co-Re/Co-AS) (Fig. 2). These items were excluded from the scale in subsequent analyses, and the final version of the pain assessment tool consisted of a total of 7 items with lower redundancy (Fig. 3): (1) “brow lowering,” (2) “eyes squeezed shut,” (3) “deepening of the nasolabial furrow,” (4) “open lips,” (5) “horizontal mouth stretch,” (6) “vertical mouth stretch,” and the newly added item, (7) “neck deflection” (Figs. 4A and B). Once the scale was reduced and the redundant items were excluded, we proceeded with the odds ratio calculation for each set of screenshots (ie, for each condition: AP and Co-Re/Co-AS) for each fetus. Figure 5 depicts the odds ratio of each of the 7 selected facial expressions (items) to be present in the AP scores and in the other 2 control ones. Odds ratio ranged from 11 (neck deflection) to 1,400 (horizontal mouth stretch). Figure 6 indicates that no control fetus scored higher than 4, while in the AP group, no score was less than 5, which suggested that this might be used as an initial cutoff value for the 7-item scale.
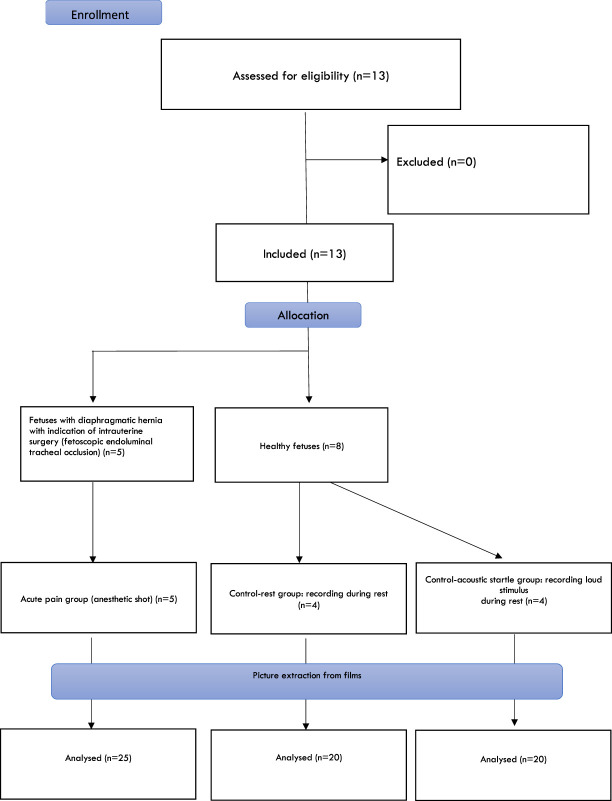
Figure 1.
Enrollment and allocation. A total of 13 fetuses were assessed (n = 5: acute pain; n = 4: control group at rest; n = 4: control group acoustic startle) providing a total of 65 pictures of fetal facial expressions.
Figure 2.
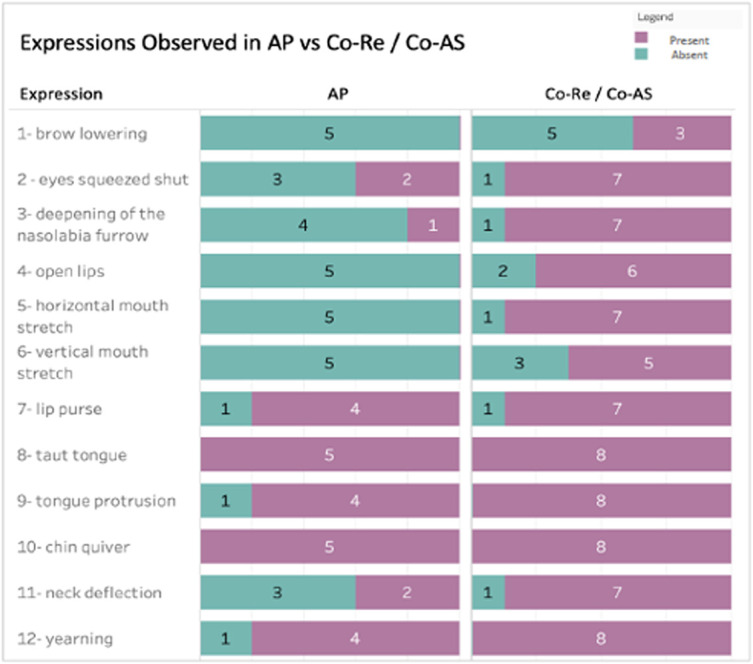
Expressions observed in acute pain (AP) vs control groups (Co-Re—control group at rest and Co-AS—control group acoustic startle). It shows how the considered facial expressions were distributed between AP and Co-Re/Co-AS groups. In the first column, one can observe the facial expressions in fetuses under AP: The green color indicates the number of fetuses that facial expression was present, while the purple color indicates the number of fetuses that the facial expression was absent. The colors in the second column indicate the same, but for the control groups merged together (Co-Re + Co-AS). It can be observed that the “8-taut tongue” and “10-chin quiver” expressions were not detected in any of the tested fetuses. It is also clear that the AP and Co-Re/Co-AS fetuses presented different results regarding the facial expressions “3—deepening of the nasolabial furrow,” “4—open lips,” “5—horizontal mouth stretch,” and “6—vertical mouth stretch.”
Figure 3.
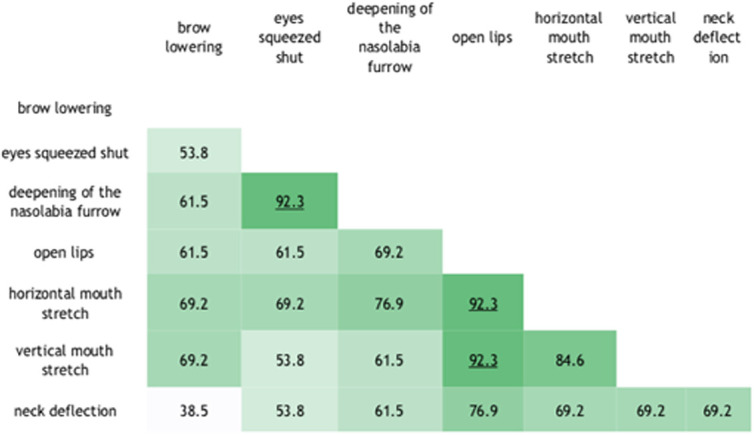
Concordance between pairs of items, given in percentages. It shows the concordance between the different items (facial expressions); concordance values above 90% are indicated in dark green. Higher concordances were found between deepening of the nasolabial furrow and eyes squeezes shut; open lips and horizontal mouth stretch; and open lips and vertical mouth stretch.
Figure 4.
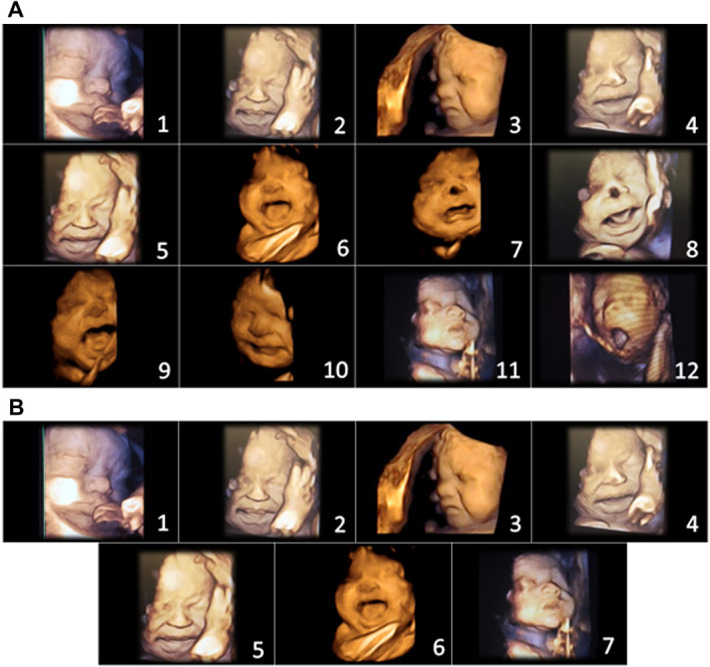
(A) Initial items from neonatal facial coding system and 2 supplementary items. 1. Brow lowering. 2. Eyes squeezed shut. 3. Deepening of the nasolabial furrow. 4. Open lips. 5. Horizontal mouth stretch. 6. Vertical mouth stretch. 7. Lip purse. 8. Taut tongue. 9. Tongue protrusion. 10. Chin quiver. 11. Neck deflection. 12. Yawning. (B) Final items from the Fetal-5 Scale. 1. Brow lowering. 2. Eyes squeezed shut. 3. Deepening of the nasolabial furrow. 4. Open lips. 5. Horizontal mouth stretch. 6. Vertical mouth stretch. 7. Neck deflection.
Figure 5.
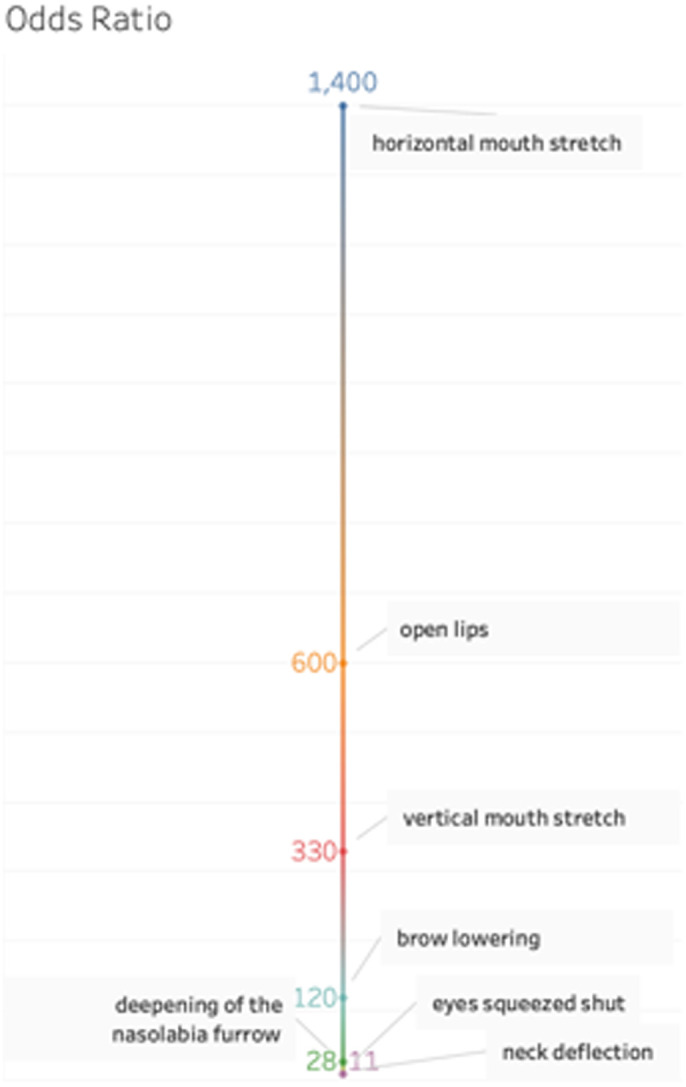
Odds ratio associated with each facial expression from the Fetal-5 Scale. This shows the odds ratio of observing the facial expression for a fetus from the acute pain group and the odds of observing the expression when the fetus is from both control groups. It should be noted that the items “horizontal mouth stretch,” “open lips,” “vertical mouth stretch,” and “brow lowering” were very relevant in the acute pain group.
Figure 6.
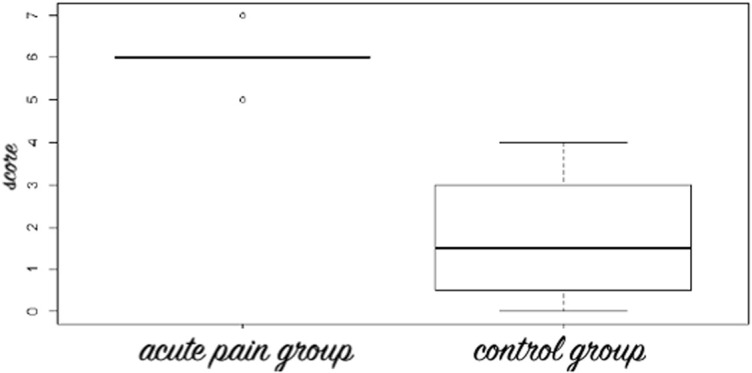
Box plot of mean scores of the acute pain and control (Co-Re—control group at rest and Co-AS—control group acoustic startle) groups for the Fetal-7 Scoring system where zero stands for the absence of the items related to facial expressions and 7 means for the presence of them. Note that all fetuses in the acute pain group scored at or above 5.
4. Discussion
In this report, we have described a facial expression-based score that was able to differentiate acute mechanical nociceptive responses from nonpainful startle reactions and from rest in human fetuses. We reduced the classical Neonatal Facial Coding System to remove facial responses that are either undetectable in static images or that are unable to aid in discriminating pain from control facial expressions. Although no single item was specific for nociceptive response, the quantitative grouping of items provided a higher score in the nociceptive compared with the non-nociceptive stimulations.
We also added a supplementary item to the final scale, neck deflection, which increased the utility of the seven-item tool for AP detection. This new scale has 7 one-point items (one for each specific facial movement), ranging from zero to 7, and those that are present are added together to give an overall score. In all instances tested, scores equal to or higher than 5 were related to acute nociceptive-related facial responses. This new tool used a temporal anchor, which in our case was an acute pinprick reaction provided by an anesthetic injection administered intramuscularly before a surgical procedure. This temporal anchor allows for the detection of a clear temporal relationship between the stimulus and the facial response. It is widely acknowledged that pain cannot be inferred from the stimulus attributes. So, it must pointed out that what was measured in the AP group is not pain per se, but, instead, the acute facial expression responses after a clear noxious stimulus. The current IASP pain definition31 states that pain is “an unpleasant sensory and emotional experience … resembling that associated with actual tissue damage,” and that (note): “verbal description is only one of several behaviors to express pain, inability to communicate does not negate the possibility that a human … experiences pain.” Thus, according to this definition, our data indicate AP group participants exhibited an acute nociceptive-related facial response, that may have been experienced as pain, something that remains to be determined.
The use of 2 control conditions, instead of the simple observation of pain gestalts, allowed the deletion of items that either did not occur frequently, or that could not be clearly detected in screenshots, such as “taut tongue” and “chin quivering.” This study has cleared the way for a formal test–retest assessment and has the potential to be useful in a clinical setting to monitor anesthesia or to assess AP situations related to postoperative periods. The possibility of measuring acute nociceptive responses in fetuses also opens some very important discussions, one being the ethical and neuroscientific issues related to the determination of when a human fetus begins to feel pain. Here, we have studied third trimester fetuses, when the basic circuitry responsible for nociceptive experiences is believed to be fully operant. Indeed, the first synapses are formed in the spinal cord by the fifth gestational week,26 while thalamic projections from the thalamus to the developing cortex commences after 12 to 15 gestational weeks.21,38 It is only by 23 to 24 weeks' gestation that major corticocortical, thalamocortical, basal forebrain bundles form synapses within the cortical plate.16 This period is also the moment when free nerve endings and their respective projection enter the spinal cord and become fully developed.11 At the 25th gestational week, brain blood flow, noradrenalin discharge,15 and behavioral modifications triggered by a noxious stimulus are promptly detected.7,8,14,35 However, several local environmental factors may influence the perception and experience of nociceptive stimuli in the developing fetus when compared with preterm newborns of similar age. These factors include the loss of the warmness and the buoyancy provided by the uterus and its amniotic fluid, as well as the spinal and brain plastic changes brought about by leaving a laying-down position to a new gravity-influenced posture where a profusion of somatosensory and arthresthesic imputs is the rule.9 All these factors and the beginning of after-birth neuronal and behavioral development create an environment where pain will emerge from nociception,36 awareness, and past experiences. After-birth motor and cognitive responses are generated along with the perception of feelings created by the integration of processed external input with internal information (embodied cognition),1,12 allowing the brain to continuously model the external world based on perception and active inference (action/motor/cognitive states).13 Given this developmental perspective, conscience and experience are necessary for fetal pain to emerge from nociception. One could argue that fetal (and likely neonatal) nociceptive experiences would be qualitatively dissimilar from those experienced by a 4-year-old child, or an aphasic patient suffering pain and also displaying a corresponding facial expression because the lack of self-awareness. On the other hand, using active inference as a model, one could propose that if the fetus is considered as a Markov blanket, then, after receiving a nociceptive stimulus, and after acting (enacting) as if in pain, the fetus is likely modeling a new version of the world where AP is then possible, and starts to act (through facial expression) “as if” in pain,4,27,34 and would then start to model his personal experiences based on this event.33
This study deals exclusively with acute nociceptive conditions. It is largely known that chronic pain has little in common with acute nociceptive inputs18; experimental animal and human pain studies have demonstrated that facial expression and behavior may differ between these 2 situations.30 Also, it has also been shown that chronic pain can also be deduced from facial-expression derived scales in nonverbal human adults.23 Repetitive painful procedures in preterm newborns are associated with adult-life health-related issues, something that could potentially happen after intrauterine stress caused by surgery10 and after-surgery settings.17,39 It will be necessary to test whether the current scale could be used to explore and monitor recurring AP conditions that could potentially occur in certain fetal diseases, such as gastroschisis (which occurs when a fetus has not developed an abdominal wall and the floating viscera is in direct contact with the amniotic fluid and their body), or in instances of intrauterine interventions such as thoracoamniotic shunts (when thoracic drainages are necessary because of recurrent pleural effusion compressing vital organs).24,28
Another limitation of this study is that the AP group was recorded during the intramuscular injection of anesthetics. A recent neonatal procedural pain platform estimated that intramuscular injections are among the most severe procedures a preterm can undergo as measured by the behavioral reactivity scores triggered by the process.22 We do not know, so far, if our approach is sensitive enough to detect lighter sensory stimuli, which remains to be formally tested. Also, because of the small sample size of this report, one cannot attest that the scores observed in the acute nociceptive stimulus group were specific for nociception and would not similarly appear after other non-nociceptive stimuli of similar salience. Further studies with larger samples will shed light into this current limitation.
In summary, we have reported that, in fetal humans undergoing perianesthetic puncture, changes in facial expression can be detected, quantified, and scored using a similar scale to the one routinely used in newborns undergoing blood sampling or anesthetic injections. Moreover, there were group items (facial expressions) that were quantitatively different between groups, with a possible cutoff value discriminating the types of stimuli. The validation of these preliminary findings in larger samples may provide a useful tool to be used in clinical practice.
Disclosures
The authors have no conflicts of interest to declare.
Supplemental video content
Illustrative videos from the three groups. Legends indicate the group and the moment of stimulation in the case of acute pain and acoustic startle groups. a. Acute pain triggered by the anesthetic shot: http://links.lww.com/PR9/A91, b. control group at rest (Co-Re): http://links.lww.com/PR9/A92, and c. control group acoustic startle (Co-AS): http://links.lww.com/PR9/A93.
Acknowledgments
The authors thank Ms. Ester Minorescu for secretariat help and support. No conflict of interest related to the research or manuscript.
Author contributions: L.S. Bernardes: conceptualization, data curation, formal analysis, funding acquisition, project administration, and writing—original draft writing; M.A. Carvalho: data curation; S.B. Harnik: methodology; M.J. Teixeira: review and editing; J. Ottolia: data curation and visualization; D. Castro: methodology; A. Velloso: conceptualization, software, and methodology; R. Francisco: review and editing; C. Listik: writing—review and editing; R. Galhardoni: project administration; V. Aparecida da Silva: investigation and methodology; L.I. Moreira: investigation and methodology, A. M. Fernandes: project administration resources; and A. G.d. Amorim Filho: methodology; D. Ciampi de Andrade: conceptualization, data curation, formal analysis, funding acquisition, project administration, writing—original draft writing, and supervision.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Lisandra S. Bernardes, Email: lisbernardes@yahoo.com.
Mariana A. Carvalho, Email: marixoxim@gmail.com.
Simone B. Harnik, Email: siharnik@gmail.com.
Manoel J. Teixeira, Email: manoeljacobsen@gmail.com.
Juliana Ottolia, Email: julianaottolia@gmail.com.
Daniella Castro, Email: daniella@kunumi.com.
Adriano Velloso, Email: adrianov@dcc.ufmg.br.
Rossana Francisco, Email: rossana.francisco@hc.fm.usp.br.
Clarice Listik, Email: clarice.listik@gmail.com.
Ricardo Galhardoni, Email: rgalhardoni@gmail.com.
Valquiria Aparecida da Silva, Email: valquiria.ase@gmail.com.
Larissa I. Moreira, Email: larissaiulle@gmail.com.
Antonio G. de Amorim Filho, Email: amorim.doc@gmail.com.
Ana M. Fernandes, Email: anamerciaf@hotmail.com.
References
- [1].Allen M, Friston KJ. From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 2018;195:2459–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apkarian AV. Definitions of nociception, pain, and chronic pain with implications regarding science and society. Neurosci Lett 2019;702:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barnard GA. A new test for 2 × 2 tables. Nature 1945:156:3177. [Google Scholar]
- [4].Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci 2015;16:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernardes LS, Ottolia JF, Cecchini M, Filho AGA, Teixeira MJ, Francisco RPV, de Andrade DC; Grupo de Estudo da Dor Fetal (Fetal Pain Study Group). On the feasibility of accessing acute pain-related facial expressions in the human fetus and its potential implications: a case report. Pain Rep 2018;3:e673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- [7].Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. PAIN 1993;52:287–99. [DOI] [PubMed] [Google Scholar]
- [8].de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 2006;82:257–66. [DOI] [PubMed] [Google Scholar]
- [9].Derbyshire SW. Can fetuses feel pain? BMJ 2006;332:909–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fisk NM, Gitau R, Teixeira JM, Giannakoulopoulos X, Cameron AD, Glover VA. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology 2001;95:828–35. [DOI] [PubMed] [Google Scholar]
- [11].Fitzgerald M. Prenatal growth of fine-diameter primary afferents into the rat spinal cord: a transganglionic tracer study. J Comp Neurol 1987;261:98–104. [DOI] [PubMed] [Google Scholar]
- [12].Friston K, Kiebel S. Predictive coding under the free-energy principle. Philos Trans R Soc Lond B Biol Sci 2009;364:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Friston K, Mattout J, Kilner J. Action understanding and active inference. Biol Cybern 2011;104:137–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet 1994;344:77–81. [DOI] [PubMed] [Google Scholar]
- [15].Giannakoulopoulos X, Teixeira J, Fisk N, Glover V. Human fetal and maternal noradrenaline responses to invasive procedures. Pediatr Res 1999;45(4 pt 1):494–9. [DOI] [PubMed] [Google Scholar]
- [16].Glover V, Fisk NM. Fetal pain: implications for research and practice. Br J Obstet Gynaecol 1999;106:881–6. [DOI] [PubMed] [Google Scholar]
- [17].Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med J 2013;4:e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013;136(pt 9):2751–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jardri R, Pins D, Houfflin-Debarge V, Chaffiotte C, Rocourt N, Pruvo JP, Steinling M, Delion P, Thomas P. Fetal cortical activation to sound at 33 weeks of gestation: a functional MRI study. Neuroimage 2008;42:10–18. [DOI] [PubMed] [Google Scholar]
- [20].Kim TH, Lee JJ, Chung SH, Lee HH, Lee KH, Choi KY, Lee SH. Efficacy of assessment in fetal behaviour by four dimensional ultrasonography. J Obstet Gynaecol 2010;30:439–43. [DOI] [PubMed] [Google Scholar]
- [21].Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 2002;267:1–6. [DOI] [PubMed] [Google Scholar]
- [22].Laudiano-Dray MP, Pillai Riddell R, Jones L, Iyer R, Whitehead K, Fitzgerald M, Fabrizi L, Meek J. Quantification of neonatal procedural pain severity: a platform for estimating total pain burden in individual infants. PAIN 2020;161:1270–7. [DOI] [PubMed] [Google Scholar]
- [23].Lautenbacher S, Kunz M. Facial pain expression in dementia: a review of the experimental and clinical evidence. Curr Alzheimer Res 2017;14:501–5. [DOI] [PubMed] [Google Scholar]
- [24].Mahieu-Caputo D, Muller F, Jouvet P, Thalabard JC, Jouannic JM, Nihoul-Fekété C, Dumez Y, Dommergues M. Amniotic fluid beta-endorphin: a prognostic marker for gastroschisis? J Pediatr Surg 2002;37:1602–6. [DOI] [PubMed] [Google Scholar]
- [25].Nomura RM, Kwon C, Miyadahira S, Zugaib M. Computerized cardiotocography analysis of fetal heart response to acoustic stimulation [in Portuguese]. Rev Bras Ginecol Obstet 2009;31:547–51. [PubMed] [Google Scholar]
- [26].Okado N. Onset of synapse formation in the human spinal cord. J Comp Neurol 1981;201:211–19. [DOI] [PubMed] [Google Scholar]
- [27].Ondobaka S, Kilner J, Friston K. The role of interoceptive inference in theory of mind. Brain Cogn 2017;112:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peranteau WH, Adzick NS, Boelig MM, Flake AW, Hedrick HL, Howell LJ, Moldenhauer JS, Khalek N, Martinez-Poyer J, Johnson MP. Thoracoamniotic shunts for the management of fetal lung lesions and pleural effusions: a single-institution review and predictors of survival in 75 cases. J Pediatr Surg 2015;50:301–5. [DOI] [PubMed] [Google Scholar]
- [29].Qu Y, Fang Y, Yan F. Feature selection algorithm based on association rules. J Phys Conf Ser 2019;1168:052012. [Google Scholar]
- [30].Rahavard BB, Candido KD, Knezevic NN. Different pain responses to chronic and acute pain in various ethnic/racial groups. Pain Manag 2017;7:427–53. [DOI] [PubMed] [Google Scholar]
- [31].Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. PAIN 2020;161:1976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Reissland N, Francis B, Mason J, Lincoln K. Do facial expressions develop before birth? PLoS One 2011;6:e24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sekulic S, Gebauer-Bukurov K, Cvijanovic M, Kopitovic A, Ilic D, Petrovic D, Capo I, Pericin-Starcevic I, Christ O, Topalidou A. Appearance of fetal pain could be associated with maturation of the mesodiencephalic structures. J Pain Res 2016;9:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013;17:565–73. [DOI] [PubMed] [Google Scholar]
- [35].Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci 2006;26:3662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slater R, Worley A, Fabrizi L, Roberts S, Meek J, Boyd S, Fitzgerald M. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain 2010;14:321–6. [DOI] [PubMed] [Google Scholar]
- [37].Team RC. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2018. [Google Scholar]
- [38].Ulfig N, Neudorfer F, Bohl J. Transient structures of the human fetal brain: subplate, thalamic reticular complex, ganglionic eminence. Histol Histopathol 2000;15:771–90. [DOI] [PubMed] [Google Scholar]
- [39].Walker SM. Long-term effects of neonatal pain. Semin Fetal Neonatal Med 2019;24:101005. [DOI] [PubMed] [Google Scholar]
- [40].Xie J, Wu J, Qian Q. Feature selection algorithm based on association rules mining method. 2009 Eighth IEEE/ACIS International Conference on Computer and Information Science, Shanghai, China, 2009. pp. 357–362.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Illustrative videos from the three groups. Legends indicate the group and the moment of stimulation in the case of acute pain and acoustic startle groups. a. Acute pain triggered by the anesthetic shot: http://links.lww.com/PR9/A91, b. control group at rest (Co-Re): http://links.lww.com/PR9/A92, and c. control group acoustic startle (Co-AS): http://links.lww.com/PR9/A93.