Abstract
African swine fever (ASF) is one of the most complex infectious swine diseases and the greatest concern to the pig industry owing to its high mortality and no effective vaccines available to prevent the disease. Since the first outbreak of ASF in pig farms, ASF has been identified in 14 pig farms in four cities/counties in South Korea. The outbreak was resolved in a short period because of the immediate control measures and cooperative efforts. This paper reviews the ASF outbreak and the experience of successfully stopping ASF in pig farms in South Korea through active responses to prevent the spread of ASF. In addition, suitable changes to build a sustainable pig production system and collaborative efforts to overcome the dangerous animal disease, such as ASF, are discussed.
Keywords: African swine fever, disease outbreak, surveillance, South Korea
INTRODUCTION
African swine fever (ASF) was first reported in Kenya in 1921 [1] and is one of the most complex infectious swine diseases causing the greatest concern in the pig industry because of its high mortality [2]. The disease is caused by a large double-stranded DNA virus, a sole member of the Asfarviridae family, which affects domestic pigs and wild boars [3,4].
Since the introduction of ASF to Portugal in 1957, ASF spread to many other European countries, the Caribbean and South America in the 1960s, and Georgia (Caucasus) in 2007 [5]. From there, the disease spread quickly to other neighboring countries, reaching the European Union in 2014 [6]. In 2018, the ASF virus (ASFV) demonstrated its huge capacity for transboundary and trans-continental spread jumping to China, which was several hundreds of kilometers away from previously known infected regions [7], where it spread rapidly with 165 outbreaks detected in 32 provinces. Approximately 1,193,000 pigs were culled as of March 5, 2020 [8]. ASF outbreaks expanded to Mongolia, Vietnam, Cambodia, North Korea, Lao, Myanmar, Philippines, South Korea, Timor-Leste, Indonesia, and India during the last two years [8,9]. In South Korea, after the first report of ASF on September 16, 2019, 14 outbreaks of ASF in pig farms were confirmed up to October 9, 2019 [10]. Furthermore, the first ASF case in a wild boar carcass was detected on October 3, 2019, and as of September 8, 2020, 736 cases of ASFV-infected wild boars were reported [11].
The spread of ASF can be prevented only by early detection and the strict application of classical disease control methods, including surveillance, epidemiological investigation, tracing of pigs, stamping out in infected holdings, biosecurity measures, quarantine, and animal movement control. Owing to the huge/socioeconomic impact of this disease, this review highlights the epidemic prevention policies and control measures during the recent outbreak of ASF in South Korea.
BRIEF DESCRIPTION OF ASF
The ASFV is a large double-stranded DNA virus belonging to the Asfarviridae Family that replicates in the cell cytoplasm of macrophages, which is the main target cell for ASFV replication [12]. The virus genome has approximately 170 to 195 kbp depending on the variation of different multigene families (MGF) and contains between 150 and 167 open reading frames [13]. Viral genotypes have been identified by the partial p72 gene, and 24 genotypes have been reported thus far [14,15]. After its introduction to Georgia in 2007, Genotype II ASFV is currently circulating in Europe and Asia [5]. The etiology and epidemiology for ASFV in Korea have recently been reviewed in detail [16].
Although domestic pigs, wild boar, and soft ticks are susceptible hosts for the ASFV, soft ticks are unlikely to play a role in the current ASF situation in Europe and Asia. ASFV can be transmitted readily between pigs and wild boar through direct contact with infected animals through the oral-nasal route. It can also be transmitted through skin abrasions from sources, including blood, which contains high levels of virus, and other excretions, including saliva, tears, nasal secretions, urine, feces, and secretions from the genital tract. Shedding of the virus from infected animals can start at 2–3 days post-infection through saliva, nasal discharges, and feces [17]. In addition, the ingestion of infected material on contaminated surfaces, feed, or water can lead to infection in pigs. Therefore, considering the physical stability of ASFV, contact with infected carcasses can be an important route of infection between wild boars. Furthermore, interactions between wild boars and domestic pigs can prolong ASFV circulation in both swine populations [18].
ASF can spread to pigs in ASF-free areas through various entry routes, such as trade in live animals and animal products, wild animal migrations, fomites, vehicles, and vector movements [5]. Human activity can propagate ASF over short and long distances. Materials contaminated with the virus, including clothing, boots, vehicles, needles, and hunting tools, act as an indirect transmission source to pigs with human activity. Human actions, such as throwing infected meat at pigs or wild boars, have spread ASF to new areas, particularly long-distance jumps. Because the ASFV is highly resistant to physical and chemical conditions, it remains viable in fresh meat and certain meat products for various periods. The ASFV can be infectious for 15 weeks in chilled meat and for months in cured hams and sausages unless it is inactivated by heat treatment at 60°C for 20 min or 56°C for 70 min [19,20]. Therefore, contaminated pork products fed to pigs have often been sources of ASFV outbreaks and introduction into previously unaffected areas [21,22]. Quarantine inspections have shown that pork items confiscated from travelers contain ASFV, which highlights the risk of the introduction of the ASFV through illegal imports [23].
As there is no effective vaccine or treatment for ASF, preventive measures to mitigate the risk of ASF spread are important for the pig production system. The ASFV is transmitted primarily by direct contact between infected and susceptible pigs. During the introduction of new pigs into the herd, special attention should be given to the management of animal transport, including disinfection of the vehicles, as well as to quarantine procedures with clinical, serological, and virological surveillance [24]. Physical isolation of the herd can be achieved by maintaining adequate distances between farms, fully fencing the herd, and installing a closed entrance to the farm area, which also reduces the risk of transmission from wild boars [25]. The carcasses of domestic pigs and wild boar must also be treated properly.
Wild boars can play an important role in the transmission pathway. They can also be responsible for the transboundary spread of the disease because of their natural dispersal ecology in search of the new territory [26]. As observed in Europe, the transmission of ASF appears to depend largely on the wild boar population density and their interactions with low-biosecurity pig production systems. Good knowledge and management of the wild boar population and good coordination among the Veterinary Services and wildlife authorities are required to prevent and control ASF successfully.
Prevention in countries free of ASF depends on implementing appropriate import policies and biosecurity measures, ensuring that neither infected live pigs nor pork products are introduced into areas free of ASF [27]. This includes cracking down on illegal imports of live pigs and pork products from affected countries, as well as proper disposal of waste food on aircraft, ships, or vehicles coming from affected countries. Several experimental studies have shown that contaminated feed and water can be a source of ASFV infection, despite the difference in infection efficiency [28,29]. Outbreaks in previously ASFV-free areas were attributed to the feeding of susceptible animals with the food waste products from infected pigs. This risk can be resolved by a ban on swill feeding to pigs or the proper heat treatment of swill [21,22]. Furthermore, strict border control is needed to block the introduction of contaminated meat items to naïve areas. Several reports indicated that travelers could bring the ASFV in pork products from the affected countries into other countries [23,30,31]. Various education programs and campaigns have been held to stop people from carrying infectious materials to new areas.
ASF SURVEILLANCE AND DIAGNOSIS
Rapid and reliable detection of the disease is critical for preventing the spread of the disease by implementing strict biosecurity control measures. A diagnosis of ASF cannot be made based on clinical signs and histopathological lesions because of the similarities in the causative agents of classical swine fever, highly pathogenic porcine productive and respiratory syndrome, and Salmonellosis. Only a laboratory diagnosis can identify animals that are or have previously been infected with the ASFV. A differential diagnosis is required, but it is important to report suspected animals to veterinary authorities and undergo a laboratory diagnosis for early detection. An ASF outbreak was confirmed by farmers' notifications on 11 farms and active surveillance on the remaining three farms in South Korea [32]. The outbreak was reported early, which allowed ASF to be controlled successfully within a short period.
Detection of ASF virus and its antibody
Tests for detecting the ASFV are fundamental for the rapid implementation of control measures. These include detecting the viral genome by polymerase chain reaction (PCR) and virus isolation for further analysis of the viral characteristics.
PCR is accepted as the gold standard test for ASF detection because of its high sensitivity, specificity, and high-throughput application to detect the target viral genome in various samples from domestic pigs, wild boars, biological vectors, and even pork products transported illegally. Many PCR tests are available, including conventional and real-time PCR, targeting the VP72-coding region known as a highly conserved gene among all the genotypes of the ASFV [33]. In 2019, 29 suspicious notifications were made, and 14 outbreaks were confirmed by real-time PCR. Three independent regions of the ASFV genome were amplified: the B646L gene encoding p72, the E183L gene encoding p54, and a tandem repeat sequence located between the I73R and I329L genes for genomic analysis. These belonged to the p72 genotype II and intergenic region (IGR) II having an additional tandem repeat sequence between the I73R and I329L. Owing to this genetic stability, it is very difficult to determine the source of introduction and route of spread identified by genetic analysis of the ASFV [34].
Virus isolation from infected animals and the field is essential for the diagnosis and characterization of circulating ASFV, which is based on inoculating the sample material onto susceptible primary cell cultures of a swine origin, from either blood or alveolar monocytes and macrophages. If live ASFV is present in the sample, it will replicate in the cells, showing a cytopathic effect (CPE) and a haemadsorption (HAD) reaction. Cell lysis and CPE usually occur after 48–72 hours of the HAD [35]. The 14 ASFVs were isolated using primary porcine alveolar macrophages (PAMs), which showed a HAD phenomenon within 24 hours.
The presence of ASFV antibodies, which appear one week after infection and last for several years, always indicates a current or past infection because of the absence of vaccines. The recommended ASF serologic tests include the enzyme-linked immunosorbent assay test for antibody screening followed by the indirect fluorescent antibody (IFA) test or indirect immunoperoxidase test (IPT) as confirmation. Passive surveillance is the most effective and efficient method for early detection in ASFV-free areas because of the high lethality and low prevalence of ASF circulating in Europe and Asia [36]. In peracute and acute infections, the animal often dies before antibodies become detectable [37]. All the samples tested during the outbreak in Korea in 2019 were confirmed to be negative in ASF serologic analysis. On the other hand, during outbreaks and in affected countries, active surveillance is necessary to understand the changes in the epidemiological situation and to eradicate the disease. In Spain, antibody detection played a key role in eradicating ASF after 35 years of hard work [38].
Surveillance in South Korea
Disease surveillance is carried out either by testing animals belonging to the suspect case definition (passive surveillance) or by active investigation to detect an infection in a population or a part of a population (active surveillance). Before the occurrence of ASF in Korea, ASF serological surveillance of domestic pigs and wild boars was started in 2009 and from 2014, respectively. Passive surveillance to detect the ASFV for unknown diseased porcine samples from domestic pig farms has been ongoing since 2015. Owing to the elevated risk of ASF introduction to Asian countries, border control has been strengthened in airports and ports with an increase in the number of quarantine detection dogs and X-ray inspections of luggage arriving from affected countries. In quarantine, pork products, including illegal carry-on pork items and food waste from airplanes, have been tested for the ASFV to prevent ASF outbreaks in South Korea. During this quarantine inspection, ASF viral DNA was detected in 51 items as of August 2020, showing that contaminated pork products can introduce the ASFV [23]. All the analyzed contaminated viral fragments belong to genotype II.
On September 16, 2019, the first suspected case of ASF was filed in the passive surveillance system from a farrow-to-finish farm, and it turned out the first ASF outbreak. Thus far, 29 suspected reports from farm animals have been filed, of which 14 were found to be positive. The last outbreak of ASF was on October 9, 2019, in domestic pigs, despite the ongoing cases found in wild boar. After the ASF outbreak, surveillance has been strengthened by increasing the sample size from animals and including vectors and fomites.
ASF CONTROL STRATEGIES AND MEASURES APPLIED IN SOUTH KOREA
Although government authorities have strengthened preventive measures to stop invasions of ASFV from foreign countries and protect pig farms from damage caused by ASF infections, outbreaks can occur. When the first case of ASF was confirmed, immediate responding actions, such as movement control and stamping out, are the most important control measures to stop transmission and spread of the virus into neighboring farms and other regions. Most countries have developed emergency preparedness and contingency plans for immediate action against important contagious animal diseases, such as ASF. In South Korea, there are the ‘Act on the Prevention of Contagious Animal Diseases’ and ‘Enforcement Rule for the Control of Exotic Contagious Animal Diseases’ to provide a legal basis for control strategies against outbreaks of ASF. The Ministry of Agriculture, Food and Rural Affairs (MAFRA) of South Korea also developed ‘the Standard Operation Procedure (SOP) on ASF’ as a contingency plan and a response strategy in August 2018 based on the ‘Enforcement Rule for the Control of Exotic Contagious Animal Diseases’. The first part of the SOP on ASF describes a three-stage warning system according to ASF outbreaks in neighboring countries and South Korea, which shows the action guidelines of each organization, including the central government, local government, and farmers' associations at each alarm stage. When outbreaks of ASF in neighboring countries are reported, the first stage of alarm, called the ‘Yellow (aware) risk stage’, is posted. The report of a suspected case in South Korea results in the second stage is the ‘Orange (warning) risk stage’. As soon as an outbreak of ASF is confirmed in South Korea, MAFRA posts the third stage of alarm called the ‘Red (severe) risk stage.’ The second part of the SOP on ASF defines specific actions to control the outbreak of ASF to provide detailed instructions for carrying out ASF control activities as follows: emergency actions for a suspected case, standstill (lockdown on the movement), destruction and disposal of animals, cleansing and disinfection, epidemiological investigation, operation of control post, designation of slaughterhouses, supply of animal feed, disposal of animal excretions, removal of restrictions for control zones, restocking, response to outbreaks in wild boars and zoo animals, and education of the participants for eradication. Control measures for ASF outbreaks in South Korea have been applied based on this SOP on ASF and sometimes been strengthened, reflecting the specific situations and environmental conditions.
The First outbreak of ASF on the Korean Peninsula
On May 31, 2019, one day after North Korea reported its first confirmed case of ASF with 77 deaths among 99 pigs at a cooperative farm in Usi county near its border with China to the OIE, MAFRA designated 14 cities and counties near the border with North Korea as a ‘Special Monitoring Region.’ In this region, all 624 pig farms were monitored by phone daily, and the farmers were advised to disinfect their farms. Fences were constructed in more than 600 sites to prevent wild boars from approaching pig farms. Blood samples from each of the 624 farms were collected by regional veterinary officers; all were negative for the ASFV. Despite preventive measures and efforts, a suspected ASF case was reported at a farrow-to-finish farm of 2,450 pigs, including 350 sows, in Paju city, Gyeonggi province, on September 16, 2019. The farm was located about 7 km from the border between South Korea and North Korea. As soon as the first outbreak of ASF in South Korea was confirmed, the severe risk stage as the highest alarm was posted. A nationwide standstill order went into effect for 48 h at 06:30 September 17 at all pig farms, slaughterhouses, feed mills, and related transport vehicles. In accordance with the SOP on ASF, the government issued an inter-minister emergency statement and installed posts for movement control and disinfection. The MAFRA strengthened the control measures with disinfection, surveillance, and movement control. A chain of command was established as ‘Headquarters to control ASF’ at central and local governments and ‘Situation control office on ASF’ at central and local veterinary authorities for rapid and effective decision-making and emergency responses to control ASF outbreaks.
In the early stages of an outbreak of ASF, every effort and action must be taken for rapid containment of the virus to the site of the primary infection, eliminating the source of pathogens in the shortest time, and preventing the spread of the virus [39]. As there is no effective vaccine against ASF, stamping out by destruction and safe disposal of all infected and potentially infected pigs is implemented to eliminate the pathogen.
ASF control policies in South Korea
According to the SOP on ASF, three types of zones were established as follows: control zone within a 500 m radius of an infected farm, protection zone within a 3 km radius, and surveillance zone between a 3 km and 10 km radius. The mayor of the city or county with an outbreak should order the culling of pigs in the infected farm and neighboring farms in the control zone. On the other hand, if there are reasons to extend the range of stamping out regarding the situation of the infected region, the commissioner of Animal and Plant Quarantine Agency (APQA) or the governor of the province could recommend an extension to the Minister of MAFRA. With the consultation of the deliberative committee for the control of animal diseases, the Minister could decide to extend the stamping-out range. The decision was made to remove all pigs in the protection zone and nearby high-risk areas, including 27,862 pigs from outbreak farms, 353,101 pigs for preventive culling, and 65,557 pigs for purchase. This action beyond the SOP was taken because it was the first outbreak of ASF in South Korea, and all 14 confirmed farms were located in border areas without any information on ASF progress in North Korea. The spread of the ASFV was blocked successfully, and the disease outbreak was controlled within 23 days. In September and October 2019, since the first outbreak of ASF, the MAFRA announced the special enforced measures to control outbreaks four times. The emergency measures were posted on September 17, and followed by the 1st enforced control measures on September 18, the 2nd enforced control measures on September 24, and the 3rd enforced control measures on October 13. The emergency measures of the first date, September 17, contained the following essential actions: dispatching of the emergency response team, movement control, extension of the stamping-out range, setting the highest stage of alarm, standstill, ban on movement of pigs from Gyeonggi province to other provinces, nationwide disinfection campaign, strict prohibition of swill feeding in pig farms, movement control for epidemiologically related farms for 21 days and related vehicles for 10 days with intensive disinfection, and action to reduce the wild boar populations in the 14 cities and counties in the border areas in cooperation with the Ministry of Environment. In the 1st enforced control measures, the six high-risk cities and counties of Paju, Yeoncheon, Gimpo, Pocheon, Dongducheon, and Cheorwon were designated as ‘Extensive Control Area,’ where the ban on pig movement to other area was implemented, and the shipment of pigs was allowed only to the designated slaughterhouse in the area. Extensive disinfection at farms and livestock-related facilities with the collaboration of control teams and special vehicles of the National Agricultural Cooperative Federation was strongly recommended.
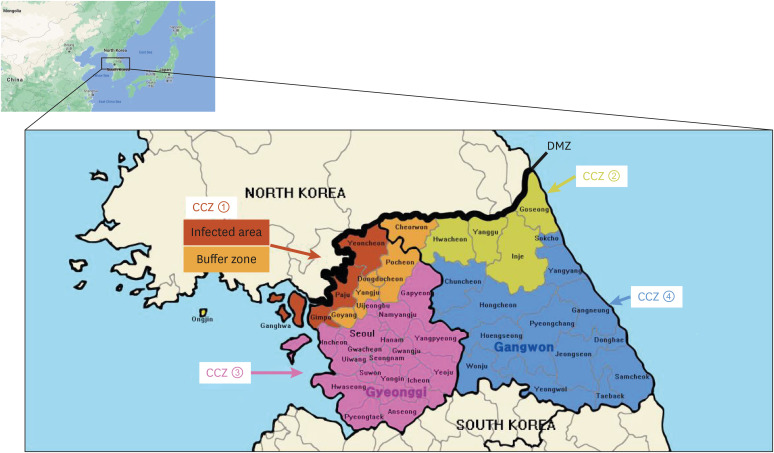
Persons who had contact with pigs, such as veterinarians, insemination technicians, the business people of a feed company, and others, were not allowed to visit pig farms in Gyeonggi and Gangwon provinces except for the treatment of sick animals in farms. The 2nd enforced control measures focused on the separation of infected regions and their vicinity in Gyeonggi and Gangwon provinces into four ‘critical control zones (CCZ)’: Northern zone of Gyeonggi, Northern zone of Gangwon, Southern zone of Gyeonggi, and Southern zone of Gangwon (Fig. 1). Pigs, manure, and related vehicles were allowed to move only in each CCZ to prevent the spread of the ASFV to other areas. The shipment of pigs to the designated slaughterhouse was allowed only after an examination of the pigs by a veterinary officer at a farm immediately before leaving the pigpens. In the Northern CCZ of Gyeonggi, which consisted of 10 cities/counties, the movement of related vehicles was strictly controlled with the system of color-registration and real-time monitoring by GPS. Only the vehicles registered to the pink group could visit pig farms in the Northern CCZ; they were not allowed to move to other regions. Those of the green group could supply feed to the farms in the Northern CCZ but were not allowed to visit pig farms in other regions. All registered vehicles were monitored by GPS with the cooperation of the National Police Agency. Warning messages were sent to drivers moving outside the Northern CCZ, and violations were punishable by up to one year in prison or fines of up to 10 million Korean won (approximately 8,500 USD). The 3rd enforced control measures, which expanded the policy for the control of vehicles' movement taken in the Northern CCZ of Gyeonggi by the 2nd enforced control measures to the Northern CCZ of Gangwon, consisted of four counties, Hwacheon, Yanggu, Inje, and Goseong.
Fig. 1. Four CCZ. The MAFRA of Korea has established four CCZ in Gyeonggi province and Gangwon province to separate infected region and their vicinity. Pigs, manure, and related vehicles were allowed to move only in each CCZ to prevent the spread of African swine fever virus to other areas.
CCZ 1, Northern zone of Gyeonggi province including infected area (red, four cities/counties; Yeoncheon, Paju, Gimpo, and Ganghwa), buffer zone (orange, five cities/counties; Cheorwon, Pocheon, Dongducheon, Yangju, and Goyang,) and Ongjin (yellow); CCZ 2, Northern zone of Gangwon province (green); CCZ 3, Southern zone of Gyeonggi province (pink), CCZ 4; Southern zone of Gangwon province (blue).
CCZ, critical control zone; DMZ, demilitarized zone.
Considering that it was the first outbreak of ASF in the history of South Korea and most of the confirmed farms were located in the Northern part of Gyeonggi, the Korean government decided on very strong preventive measures to block the transmission of the disease to other areas. The so-called ‘vacuuming strategy’ was applied to empty pig farms in four affected cities/counties (Ganghwa, Gimpo, Paju, and Yeoncheon) and two neighboring regions (Pocheon and Cheorwon): stamping-out in 14 infected farms, preventive culling for all pigs within a 3 km radius of the infected farms, and purchasing or slaughtering with compensation by the government for remaining pigs in six target cities/counties. Five cities/counties (Goyang, Yangju, Donducheon, Pocheon, and Cheorwon) adjacent to the affected areas were designated as a ‘buffer zone.’ All pig farms in the buffer zone had been monitoring with a laboratory test for antigen detection of ASF once a week for three weeks. Control posts on the main roads at the edge of the buffer zone were managed, and access of vehicles to all pig farms in the buffer zone was controlled.
Supporting farmers who suffer the economic impact caused by ASF outbreaks is a pillar of control measures [40]. The Korean government compensated the losses of pigs as 100% of the market price and provided further support with a stabilization fund for living, up to 6 months and possibly more reflecting the extent of the damage, up to 3,000 USD per month.
Wild boar control and ASF monitoring
The first case of ASF in a wild boar was detected on October 2, 2019. ASF has been spreading in wild boar populations, as in Europe. As the number of ASFV-positive cases and infected cities/counties continues to increase, 736 positive cases in wild boar have been reported in three cities/counties (Paju, Yeoncheon, and Pocheon) of Gyeonggi province and six (Cheorwon, Hwacheon, Yanggu, Goseong, Inje, and Chuncheon) of Gangwon until September 8, 2020. The control measures to limit the spread of ASF among wild boar in South Korea consist of fencing, population control, and search and safe disposal of carcasses. The confirmed ASF-infected sites are fenced immediately, and a three-layer fencing system is deployed to avoid the southward spread of ASF. The first consists of electrical fences surrounding the area where one or more infected wild boar carcasses have been found, a 1–2 km distance around the confirmed cases. The second layer is a semi-rigid 1.5 m high wire mesh placed at approximately 5–10 km around the electrical fences. A 250 km long fence as a third layer was built crossing Gyeonggi and Gangwon provinces from east to west, 20–30 km south of the second layer [11]. Active carcass search has been performed by the Ministry of Environment, the Forest Service, the Ministry of National Defense, and local governments. Regarding wild boar population control, cage traps are used inside the second-layer fences, and hunting under special permit takes place outside the second-layer fences.
CHANGE AND PREPARATION OF ASF OUTBREAKS FOR THE FUTURE
These waves of ASF outbreaks in Europe and Asia since 2007 have affected the global pig industry. Changes in the pig industry of Asia where huge damage taken by ASF will be inevitable. In many Asian countries, backyard and small-scale pig farms are in the majority, e.g., approximately 65% of the total pig population in Vietnam. This has provoked wide transmission of ASF and economical loss caused by the low biosecurity of these farms [41,42]. Recently there are some reports that animal industries are being transformed and upgraded. Small-scale farmers have gradually withdrawn from the animal industry, and the number of commercial farms is increasing. As many backyard farms traditionally practiced swill feeding, even though the ban on swill feeding was the first step of control against ASF, it is not easy for the government to persuade these farmers to change from swill to commercial feed. The Chinese government prohibited swill feeding nationwide since the first outbreak of ASF in September 2018. They reported that the transmission route of 57 cases (38%) among 150 outbreaks of ASF between September 2018 and December 2019 was swill feeding. In contrast, none of the 15 outbreaks between January and June 2020 involved swill feeding [43].
Biosecurity of pig farms
Enhancing the biosecurity of farms has been emphasized to protect domestic pigs from ASF. Because the ASFV can survive in environmental conditions and act as a transmission source, stricter biosecurity tools should be applied to prevent pigs from being exposed to the ASFV. ASF-infected wild boars are being found continuously in European countries and South Korea. Therefore, pig farms require double fences to prevent access by wild boars, and preventive measures against insects, rodents, and wild animals are needed. Recently, the Korean government started a ‘ban of vehicle entrance close to pig-raising barns’ program that prohibits related vehicles from entering the area of pig houses, such as feeding, transporting pigs, and handling manure. At the first step, this program was applied to the northern areas of Gyeonggi and Gangwon provinces. All pig farms in the areas were investigated and grouped according to the type and program applicability: 1) Group 1 (29 farms, 8%) is a type in which the vehicles do not enter the farm, either immediately or with minor modifications; 2) Group 2 (135 farms, 36%) has vehicle access only to the inner fence that protects the pig houses on the farm and should be installed if there is no inner fence; 3) Group 3 (213 farms, 56%) is a type that requires reconfiguration of the farm to belong to Group 2. Through ongoing referrals and promotions, all Group 3 farms have submitted plans to transition to Group 2 and work towards the transition to Group 2. As of the end of August, 78% of Group 1 (37 farms) and 79% of Group 2 (328 farms) completed the facility implementation. All related vehicles visiting farms are registered and are subjected to real-time monitoring to check the violation of ‘ban of vehicle entrance close to pig raising barns’ using the GPS control system.
Movement control of pigs in the nation
The movement of people and vehicles to outside pig farms is a major route, particularly for long-distance ASF transmission. The second case of ASF, which occurred in China in September 2018, was found at a slaughterhouse located in Zhengzhou City, Henan Province. The pig came from Jamushi City, Heilongjiang Province, and traveled more than 2,000 km through areas with a high pig density [44]. Chinese researchers insist that the long-distance transport of live pigs is unavoidable because the pig-producing field is not distributed uniformly throughout China [45]. Some Asian countries, such as China and Vietnam, have highly complex pig/pork production systems [46]. Other countries also have complex pig value chains that affect the movement of pigs, the feed-supply system, and shipment to slaughterhouses. As a long-term plan, a solution with the modification of the pig value chain should be investigated to avoid the long-distance transportation of pigs, feed, and other materials. When the outbreak of a disease occurs, ‘zoning’ should be applied with a restriction of movement as one of the most important control measures to stop the spread of the disease to other regions. Like ‘zoning’ in the case of an outbreak, ‘functional zoning’ can be considered as a preventive measure that allows the circulation of pigs, feed, other material, manure treatment, and slaughter internally without crossing borders. The solution also includes preventive measures that must be taken at each procedure to reduce the risk. The vehicles for the transportation of pigs and the slaughterhouse can be sources of transmission outside farms. The new transport vehicles must reflect the animal welfare issue and maintain the appropriate conditions during transportation. The management of slaughterhouses should focus on reducing the contamination risk and detecting suspected animals at the site.
Future directions
Animal welfare and preservation of the natural environment may be the major concerns of people in the future. These issues will affect every corner of human life, including activities to control animal diseases. Therefore, new control measures and application tools based on scientific evidence and improving animal welfare and the natural environment should be developed in the near future as a national, regional, and global strategy.
In the 1960s, the attenuated virus was used in a large-scale vaccination program. Out of approximately 550,000 animals that received the vaccine, 128,684 developed post-vaccinal reactions, such as death, pneumonia, locomotor disturbances, skin ulcers, abortions, and disturbance of lactation [47]. It has been almost 60 years since that failure. Still, there are no effective commercial vaccines for ASF. Live attenuated vaccines, which appear most likely, can induce effective immune responses, but there is a concern about their safety, including adverse effects and safety. Recently, several live attenuated viruses showed protective effects without severe side effects on the laboratory scale [48,49,50]. Despite these recent advances, there are many issues to be solved before field applications become possible. Nevertheless, vaccines are a good way to mitigate the threat of ASF.
REGIONAL AND GLOBAL COOPERATION OF ASF SITUATION
As most countries are connected, and there are huge movements of people, animals, and products between countries, diseases can spread quickly and far. In particular, transboundary animal diseases (TADs), such as ASF, foot and mouth disease (FMD), and highly pathogenic avian influenza (HPAI) can affect the infected country and neighboring countries. Hence, collaborative efforts to control the disease should be applied at a regional level. In 2004, the OIE and FAO launched and implemented a joint initiative-the Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs). The initiative combines the strengths of both organizations to achieve commonly agreed objectives and serves as a facilitating mechanism to empower regional alliances in the fight against TADs. Under the GF-TADs umbrella, a Standing Group of Experts on ASF (SGE-ASF) in Europe was established to facilitate coordination and information sharing among infected and at-risk countries. In 2017, the Regional Strategy for the Control of African Swine Fever in Africa was launched jointly by the FAO, African Union-Inter African Bureau for Animal Resources (AU-IBAR), and International Livestock Research Institute (ILRI). Based on the SGE-ASF Europe model, similar initiatives adapted to the regional contexts were launched in the Americas at Ottawa in May 2019 [51].
Learning from the European experience, the SGE-ASF for Asia was launched in April 2019 to build closer cooperation among countries to address ASF in a more collaborative and harmonized manner across Asia. Three meetings of the SGE-ASF for Asia were held in 2019 to discuss recommended actions for the early detection, surveillance, biosecurity, border control, and risk communication. Owing to Covid-19, a virtual conference was held in April 2020 to share the experiences in managing ASF outbreaks. In Asia, ASF was first reported in China in August 2018 and spread to 10 countries in 2019 and India in 2020.
Recently on July 20, 2020, the OIE and FAO launched a joint initiative for the Global Control of ASF in 2020–2025 to support the countries' efforts to protect the economy and food security. Based on a long-standing collaboration between OIE and FAO for the management of animal health-related risks, the joint GF-TADs developed the Global Initiative to encourage national, regional, and global partnerships, to strengthen control measures and minimize the impact of ASF [52]. To achieve the final goal of ‘Global Control of ASF’, three objectives were defined.
1) Improve the capability of countries to control (prevent, respond, and eradicate) ASF using OIE International Standards and best practices based on the latest science.
2) Establish an effective coordination and cooperation framework for the global control of ASF.
3) Facilitate business continuity, ensuring safe production and trade to protect food systems.
The OIE and FAO emphasized that coordinated actions as part of the Global Initiative should take place alongside maintaining transparency regarding the reporting of animal diseases and investing in strong and resilient animal health systems. The Global Initiative aims to strengthen national Veterinary Services' ability to manage risks through the development and implementation of ASF national control programs, with public and private sectors working in partnership. Risk communication with the relevant stakeholder will be essential for managing the risk pathways and high-risk practices.
The document of the Global Initiative mentioned that ASF has never been so widespread, and the scale of the national and regional challenges to control ASF should not be underestimated. Long-term commitment by all involved will be required to tackle this global threat. Control measures should be coordinated at the regional and global levels and embedded into supra-national frameworks that consider the diverse socio-cultural, geographical, political and economic needs, and characteristics of each region.
CONCLUSIONS
In South Korea, after the first outbreak of ASF, 14 cases of ASF in pig farms were confirmed. The spread of the ASFV was tackled successfully in swine farms over 23 days. The outbreak of the disease was controlled in a short period due to early reporting by educated farmers and practitioners, immediate control measures by the government, and cooperative efforts of all stakeholders. The effective control measures against ASF included the following measures: 1) risk-based prevention and surveillance programs, 2) adequate biosecurity in pig production sectors and hunting grounds, 3) pig traceability and movement control, 4) wild boar management, 5) safe culling and disposal of animals and their contaminated products, 6) improved collaboration among the multiple sectors involved, and 7) continued education and awareness-raising programs for all relevant parties. To build a sustainable pig farming industry, it is important to improve identified weaknesses, such as swill feeding, backyard farms, and low biosecurity. Moreover, the collaborative efforts for TAD management, such as ASF, FMD, and HPAI, should be applied at the regional level because they may affect not only the affected countries but also the regions.
Footnotes
Funding: This study was supported by a grant from the Animal and Plant Quarantine Agency (Grant No. B-1543085-2020-22-01), the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Park B.
- Writing - original draft: Kim YJ, Kang HE.
- Writing - review & editing: Kang HE.
References
- 1.Montgomery RE. On a form of swine fever occurring in British East Africa (Kenya Colony) J Comp Pathol Ther. 1921;34:159–191. [Google Scholar]
- 2.VanderWaal K, Deen J. Global trends in infectious diseases of swine. Proc Natl Acad Sci U S A. 2018;115(45):11495–11500. doi: 10.1073/pnas.1806068115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso C, Borca M, Dixon L, Revilla Y, Rodriguez F, Escribano JM Ictv Report Consortium. ICTV virus taxonomy profile: Asfarviridae. J Gen Virol. 2018;99(5):613–614. doi: 10.1099/jgv.0.001049. [DOI] [PubMed] [Google Scholar]
- 4.Probst C, Globig A, Knoll B, Conraths FJ, Depner K. Behaviour of free ranging wild boar towards their dead fellows: potential implications for the transmission of African swine fever. R Soc Open Sci. 2017;4(5):170054. doi: 10.1098/rsos.170054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-Cordón PJ, Montoya M, Reis AL, Dixon LK. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet J. 2018;233:41–48. doi: 10.1016/j.tvjl.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenais E, Depner K, Guberti V, Dietze K, Viltrop A, Ståhl K. Epidemiological considerations on African swine fever in Europe 2014-2018. Porcine Health Manag. 2019;5(1):6. doi: 10.1186/s40813-018-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Li N, Luo Y, Liu Y, Miao F, Chen T, et al. Emergence of African swine Fever in China, 2018. Transbound Emerg Dis. 2018;65(6):1482–1484. doi: 10.1111/tbed.12989. [DOI] [PubMed] [Google Scholar]
- 8.ASF Situation in Asia Update [Internet] Rome: FAO; [Updated 2020]. http://www.fao.org/ag/againfo/programmes/en/empares/ASF/2020/Situation_update_2020_03_05.html. [Google Scholar]
- 9.Situational Updates of ASF in Asia and the Pacific [Internet] Paris: OIE; [Updated 2020]. http://rr-asia.oie.int/en/projects/asf/situational-updates-of-asf/ [Google Scholar]
- 10.Kim HJ, Cho KH, Lee SK, Kim DY, Nah JJ, Kim HJ, et al. Outbreak of African swine fever in South Korea, 2019. Transbound Emerg Dis. 2020;67(2):473–475. doi: 10.1111/tbed.13483. [DOI] [PubMed] [Google Scholar]
- 11.Jo YS, Gortázar C. African swine fever in wild boar, South Korea, 2019. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13532. [DOI] [PubMed] [Google Scholar]
- 12.Galindo I, Alonso C. African swine fever virus: a review. Viruses. 2017;9(5):103. doi: 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon LK, Chapman DA, Netherton CL, Upton C. African swine fever virus replication and genomics. Virus Res. 2013;173(1):3–14. doi: 10.1016/j.virusres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Bastos AD, Penrith ML, Crucière C, Edrich JL, Hutchings G, Roger F, et al. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch Virol. 2003;148(4):693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- 15.Achenbach JE, Gallardo C, Nieto-Pelegrín E, Rivera-Arroyo B, Degefa-Negi T, Arias M, et al. Identification of a new genotype of african swine fever virus in domestic pigs from Ethiopia. Transbound Emerg Dis. 2017;64(5):1393–1404. doi: 10.1111/tbed.12511. [DOI] [PubMed] [Google Scholar]
- 16.Yoo D, Kim H, Lee JY, Yoo HS. African swine fever: etiology, epidemiological status in Korea, and perspective on control. J Vet Sci. 2020;21(2):e38. doi: 10.4142/jvs.2020.21.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinat C, Reis AL, Netherton CL, Goatley L, Pfeiffer DU, Dixon L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet Res (Faisalabad) 2014;45(1):93. doi: 10.1186/s13567-014-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chenais E, Ståhl K, Guberti V, Depner K. Identification of wild boar-habitat epidemiologic cycle in African swine fever epizootic. Emerg Infect Dis. 2018;24(4):810–812. doi: 10.3201/eid2404.172127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EFSA AHAW Panel Scientific opinion on African swine fever. EFSA J. 2014;12:3628. [Google Scholar]
- 20.OIE Technical Disease Card-African Swine Fever. Paris: OIE; 2013. [Google Scholar]
- 21.Kolbasov D, Titov I, Tsybanov S, Gogin A, Malogolovkin A. African swine fever virus, Siberia, Russia, 2017. Emerg Infect Dis. 2018;24(4):796–798. doi: 10.3201/eid2404.171238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costard S, Jones BA, Martínez-López B, Mur L, de la Torre A, Martínez M, et al. Introduction of African swine fever into the European Union through illegal importation of pork and pork products. PLoS One. 2013;8(4):e61104. doi: 10.1371/journal.pone.0061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Lee MJ, Lee SK, Kim DY, Seo SJ, Kang HE, et al. African swine fever virus in pork brought into South Korea by travelers from China, August 2018. Emerg Infect Dis. 2019;25(6):1231–1233. doi: 10.3201/eid2506.181684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. African Swine Fever [Internet] Paris: OIE; [Updated 2019]. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF/pdf. [Google Scholar]
- 25.Food and Agriculture Organization of the United Nations/World Organisation for Animal Health/World Bank. Good Practices for Biosecurity in the Pig Sector-Issues and Options in Developing and Transition Countries. FAO Animal Production and Health Paper No. 169. Rome: FAO; 2010. [Google Scholar]
- 26.Podgórski T, Śmietanka K. Do wild boar movements drive the spread of African swine fever? Transbound Emerg Dis. 2018;65(6):1588–1596. doi: 10.1111/tbed.12910. [DOI] [PubMed] [Google Scholar]
- 27.Sur JH. How far can African swine fever spread? J Vet Sci. 2019;20(4):e41. doi: 10.4142/jvs.2019.20.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niederwerder MC, Stoian AM, Rowland RR, Dritz SS, Petrovan V, Constance LA, et al. Infectious dose of African swine fever virus when consumed naturally in liquid or feed. Emerg Infect Dis. 2019;25(5):891–897. doi: 10.3201/eid2505.181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blázquez E, Pujols J, Segalés J, Rodríguez F, Crenshaw J, Rodríguez C, et al. Commercial feed containing porcine plasma spiked with African swine fever virus is not infective in pigs when administered for 14 consecutive days. PLoS One. 2020;15(7):e0235895. doi: 10.1371/journal.pone.0235895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WH, Lin CY, Chang Ishcol MR, Urbina AN, Assavalapsakul W, Thitithanyanont A, et al. Detection of African swine fever virus in pork products brought to Taiwan by travellers. Emerg Microbes Infect. 2019;8(1):1000–1002. doi: 10.1080/22221751.2019.1636615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito S, Jurado C, Sánchez-Vizcaíno JM, Isoda N. Quantitative risk assessment of African swine fever virus introduction to Japan via pork products brought in air passengers' luggage. Transbound Emerg Dis. 2020;67(2):894–905. doi: 10.1111/tbed.13414. [DOI] [PubMed] [Google Scholar]
- 32.Yoon H, Hong SK, Lee I, Yoo DS, Jung CS, Lee E, et al. Clinical symptoms of African swine fever in domestic pig farms in the Republic of Korea, 2019. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13552. [DOI] [PubMed] [Google Scholar]
- 33.Yu M, Morrissy CJ, Westbury HA. Strong sequence conservation of African swine fever virus p72 protein provides the molecular basis for its antigenic stability. Arch Virol. 1996;141(9):1795–1802. doi: 10.1007/BF01718302. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Cho KH, Ryu JH, Jang MK, Chae HG, Choi JD, et al. Isolation and genetic characterization of African swine fever virus from domestic pig farms in South Korea, 2019. Viruses. 2020;12(11):1237. doi: 10.3390/v12111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltran-Alcrudo D, Arias M, Gallardo C, Kramer S, Penrith ML. African Swine Fever: Detection and Diagnosis – A Manual for Veterinarians. FAO Animal Production and Health Manual No. 19. Rome: FAO; 2017. [Google Scholar]
- 36.Boklund A, Cay B, Depner K, Földi Z, Guberti V, Masiulis M, et al. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018) EFSA J. 2018;16(11):e05494. doi: 10.2903/j.efsa.2018.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pershin A, Shevchenko I, Igolkin A, Zhukov I, Mazloum A, Aronova E, et al. A long-term study of the biological properties of ASF virus isolates originating from various regions of the Russian Federation in 2013–2018. Vet Sci. 2019;6(4):99. doi: 10.3390/vetsci6040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danzetta ML, Marenzoni ML, Iannetti S, Tizzani P, Calistri P, Feliziani F. African swine fever: lessons to learn from past eradication experiences. A systematic review. Front Vet Sci. 2020;7:296. doi: 10.3389/fvets.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penrith ML, Guberti V, Depner K, Lubroth J. Preparation of African Swine Fever Contingency Plans. Rome: FAO; 2019. [Google Scholar]
- 40.How Rapid Actions Can Curb the Spread of ASF: the Experience of Republic of Korea [Internet] Paris: OIE; [Updated 2020]. https://rr-asia.oie.int/en/projects/asf/asf-story-republic-of-korea/ [Google Scholar]
- 41.Tao D, Sun D, Liu Y, Wei S, Yang Z, An T, et al. One year of African swine fever outbreak in China. Acta Trop. 2020;211:105602. doi: 10.1016/j.actatropica.2020.105602. [DOI] [PubMed] [Google Scholar]
- 42.Nga BT, Tran Anh Dao B, Nguyen Thi L, Osaki M, Kawashima K, Song D, et al. Clinical and pathological study of the first outbreak cases of African swine fever in Vietnam, 2019. Front Vet Sci. 2020;7:392. doi: 10.3389/fvets.2020.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Q. ASF Outbreak Management Approaches in China, Virtual Meeting of the Standing Group of Experts on African Swine Fever for Asia, April 2020 [Internet] [Updated 2020]. https://rr-asia.oie.int/wp-content/uploads/2020/04/2-asf-outbreak-management-approaches-in-china.pdf.
- 44.Second Outbreak of ASF Found in China [Internet] [Updated 2020]. https://cahfs.umn.edu/news/second-outbreak-asf-found-china.
- 45.Gao X, Liu T, Liu Y, Xiao J, Wang H. Transmission of African swine fever in China through legal trade of live pigs. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13681. [DOI] [PubMed] [Google Scholar]
- 46.Gong L, Xu R, Wang Z, Deng Q, Wang H, Zhang G. African swine fever recovery in China. Vet Med Sci. 2020;6(4):890–893. doi: 10.1002/vms3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manso-Ribeiro J, Nunes-Petisca JL, Lopez-Frazao F, Sobral M. Vaccination against ASF. Bull Off Int Epizoot. 1963;60:921–937. [Google Scholar]
- 48.Barasona JA, Gallardo C, Cadenas-Fernández E, Jurado C, Rivera B, Rodríguez-Bertos A, et al. First oral vaccination of Eurasian wild boar against African swine fever virus genotype II. Front Vet Sci. 2019;6:137. doi: 10.3389/fvets.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, et al. Development of a highly effective african swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol. 2020;94(7):e02017–e02019. doi: 10.1128/JVI.02017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Zhao D, He X, Liu R, Wang Z, Zhang X, et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci. 2020;63(5):623–634. doi: 10.1007/s11427-020-1657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalpravidh W, Holley C. Strengthening the Cooperation on African Swine Fever Prevention and Control in the Asia-Pacific Region. OIE Regional Commission, 2019 [Internet] [DOI]
- 52.Global Control of African Swine Fever, A GF-TADs Initiative (2020–2025). FAO & OIE [Internet] [Updated 2020]. https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/ASF/ASF_GlobalInitiative_web.pdf.