Abstract
We report the case of a 28-year-old man, diagnosed with a non-secreting, non-metastatic suprasellar germinoma treated with chemoradiation who developed, four months after completion of radiation therapy, multiple discrete demyelinating lesions mimicking multiple sclerosis (MS). The patient had no previous diagnosis of MS and the neuroimaging studies performed both at the time of diagnosis and after chemotherapy, pre-irradiation, showed no evidence of white matter lesions. He remained asymptomatic, with no focal neurological deficits. Biochemical analysis of the CSF was positive for the intrathecal synthesis of IgG with oligoclonal bands. Follow-up MRI six months later showed a spontaneous decrease in lesion size and resolution of associated inflammatory signs, with lesions remaining stable in number. We discuss the potential origin of these white matter lesions, which may correspond to MS-like late-delayed demyelination secondary to chemoradiation therapy, in a previously predisposed patient.
Keywords: Chemorradiation-induced demyelination, Multiple sclerosis-like demyelination, Chemorradiation-induced neurotoxicity, Neuro-oncology, MRI
Highlights
-
•
Chemoradiation therapy can induce multiple sclerosis-like demyelinating lesions
-
•
Neurotoxicity is a well-known side effect of chemo and radiation therapy
-
•
Radiation-induced demyelination is dose-dependent and can be seen 4 to 6 months following radiotherapy
-
•
Chemoradiation induced demyelination and MS share a common pathophysiology
1. Introduction
Chemotherapy and radiation therapy-induced neurotoxicity is a well-known secondary effect in cancer patients with both treatments having a negative impact upon neural precursor cells, mainly of oligodendrocyte lineage affecting axonal myelination [1,2].
Recent research has shown that chemotherapy depletes oligodendrocyte lineage cells in humans and leads to a persistent try-glial dysregulation via microglial activation and induction of a chronic inflammatory state that disrupts the gliogenic microenvironement and glial homeostasis [1]. This mechanism resembles other neurological diseases featuring myelin dysfunction such as MS [3] and Alzheimer's disease [4]. Activated microglia blocks the proliferation and dysregulates the differentiation of oligodendrocyte precursor cells (OPCs) leading to dysmyelination. Moreover, the activation of reactive astrocytes, promotes oligodendrocyte death increasing neurotoxicity [1].
A similar process takes place after radiation exposure with cranial irradiation inducing chronic microglial inflammation and leading to decreased hippocampal neurogenesis [7,8].
Radiotherapy can lead to necrosis of white matter tracts, axonal degeneration and vascular injury [9]. Demyelination, one of the hallmarks of this radiation-induced neurotoxicity, is presumed to result from the enhanced radiosensitivity of OPCs [2,10]. Moreover, radiation-induced damage to the microvasculature, prompting to hemorrhagic and ischemic events, local necrosis and blood-brain-barrier disruption (with resulting vasogenic edema), facilitates CNS influx of inflammatory cells, further contributing to a pro-inflammatory state and persistent demyelination [9].
A diffusion tensor MR imaging study has shown that early demyelination is dose-dependent, affecting regions exposed to high radiation doses, up to three months after radiotherapy. However, this process is continuous and progressive diffuse demyelination, not limited to high-dose volumes, can be seen 4 to 6 months following radiotherapy [11]. This case report concerns a patient with no prior clinical or radiological signs of MS who, 4 months after being treated with chemoradiation for a suprasellar germinoma, developed demyelinating lesions diagnostic of MS, according to MAGNIMS criteria [12].
To the best of our knowledge MS-like demyelinating plaques have not been previously described as a direct consequence of chemotherapy and/or radiotherapy in non-MS patients.
2. Case report
The patient is a previously healthy 28 year-old-man who presented with progressive fatigue, polyuria, polydipsia and anejaculation. His neurological and neuroophthalmological exams were unremarkable and his family history was non-contributory. Laboratory investigation disclosed hypopituitarism including diabetes insipida, hypogonadothrophic hypopituitarism and central hypothyroidism.
Magnetic resonance imaging (MRI) of the brain, sella turcica and neuroaxis (Fig. 1) revealed a mass lesion in the pituitary infundibulum and pituitary stalk, showing moderate enhancement after gadolinium administration. The brain parenchyma was unremarkable and there were no signs of subependymal or leptomeningeal enhancement to suggest cerebrospinal fluid (CSF) seeding.
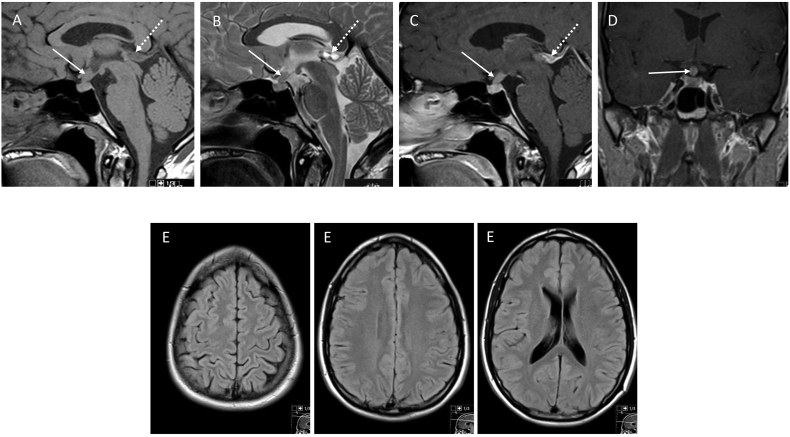
Fig. 1.
MRI of the brain at diagnosis: Sagittal T1W (A), T2W (B) and CE T1W (C) and coronal CE T1W (D) images demonstrate an enhancing mass lesion in the infundibulum and pituitary stalk protruding into the suprasellar cistern (arrows). An incidental peripheral enhancing epiphyseal cyst is also noted (dashed arrows). Axial FLAIR images (E) throughout the brain at this stage were unremarkable with no evidence of demyelinating WM lesions.
Lumbar puncture disclosed normal opening pressure and crystalline CSF. Cytologic analysis was negative for neoplastic cells and biochemical analysis showed the presence of intrathecal synthesis of IgG with oligoclonal bands (IgG 3.78 mg/dl, Freedman pattern 2). Bacteriologic and virologic CSF testing were also negative. Seric and CSF levels of α-fetoprotein and β-HCG were normal.
Surgical biopsy of the pituitary stalk mass, performed under neuronavigation revealed a germinoma.
With a diagnosis of a non-secreting, non-metastatic supra-sellar germinoma the patient was treated according to the SIOP (International Society of Paediatric Oncology) protocol with a 3 multidrug chemotherapy regimen including carboplatin, etoposide and ifosfamide followed by radiation therapy.
MRI performed 10 days after completing the chemotherapy regimen showed a complete macroscopic response and no signs of complication (Supplementary Fig. 1).
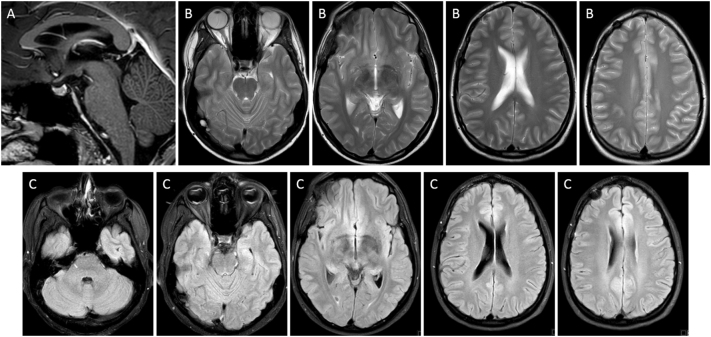
Supplementary Fig. 1.
MRI of the brain after 4 cycles of chemotherapy (cisplatin, etoposide and ifosfamide) and before radiation therapy: Sagittal T1W (A), Axial T2W (B) and FLAIR (C) images throughout the brain show complete macroscopic response of the pituitary stalk lesion and no signs of treatment complication namely, no evidence of white matter lesions.
The patient then received whole-ventricular irradiation (24 Gy given in 15 fractions of 1.6 Gy/cycle/day) using Volumetric Modulated Arc Therapy (VMAT) with concomitant memantine.
According to Common Terminology Criteria for Adverse Events (CTCAE), toxicity included grade 1 hepatotoxicity, grade 3 neutropenia and grade 4 thrombocytopenia during CT and grade 2 headache and vomiting during RT.
Four months after completing the treatment protocol, MRI of the brain and spine (Fig. 2) showed complete tumor response and was remarkable for the interval appearance of multiple discrete white matter lesions affecting the posterior fossa and supratentorium, distributed throughout the deep and periventricular white matter with a typical orientation perpendicular to the body of the lateral ventricles and involving the calloso-septal interface (“Dawson's fingers”). Some of these lesions showed a subtle halo of restricted diffusion and perilesional edema suggesting inflammatory activity. No lesions were found in the spinal cord or optic nerves.
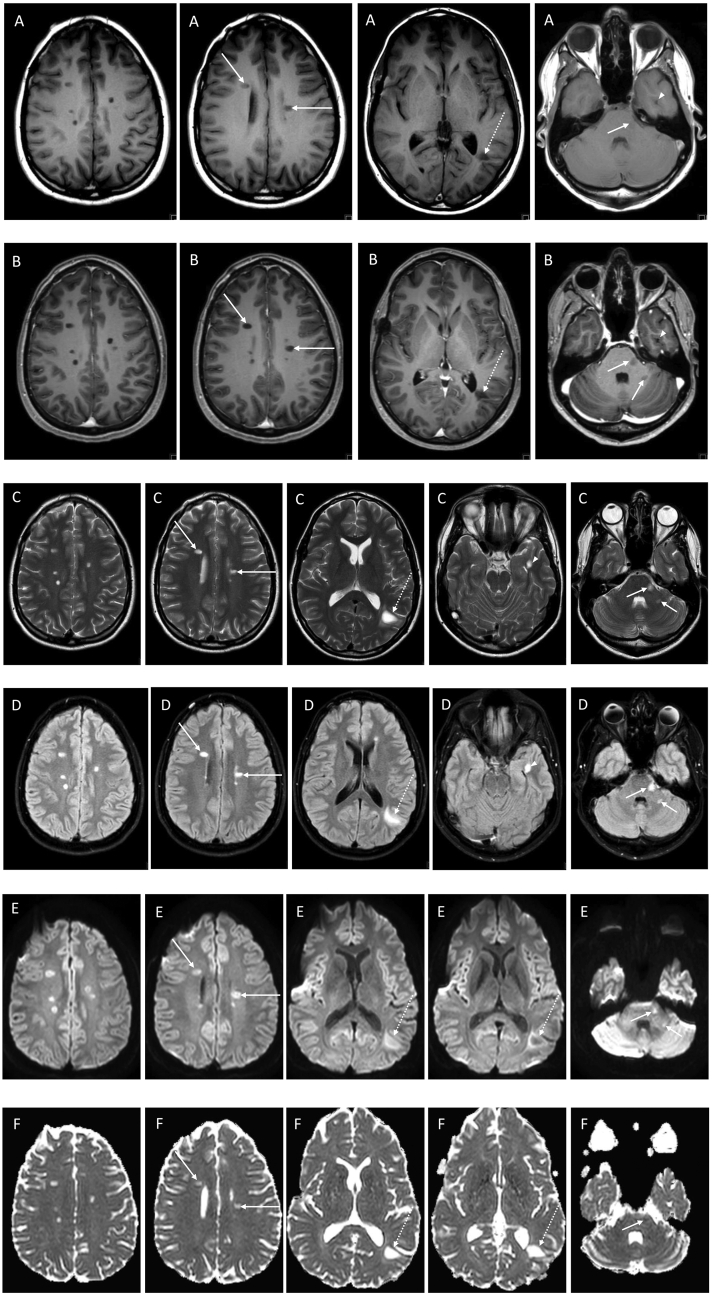
Fig. 2.
MRI of the brain 3 months after completion of the CRT protocol: Pre- (A) and post‑gadolinium (B) axial T1W, axial T2W (C), axial FLAIR (D) and DWI images, b1000 (E) and ADC maps (F) demonstrate the interval appearance of multiple discrete deep and periventricular white matter lesions hypointense on T1 and hyperintense on T2W images, with no contrast enhancement on post‑gadolinium T1W images, most with facilitated diffusion and a few with a thin rim of restricted diffusion. Most lesions are located in the deep white matter of the centrum semi-ovale, some affecting the pericallosal region oriented perpendicular to the body of the lateral ventricles (arrows), with the largest lesion in the peri-atrial white matter on the left side (dashed arrows). This lesion shows a peripheral digitiform T2W/FLAIR hyperintense rim consistent with peripheral edema with no significant mass effect upon the ventricular trigone or adjacent sulci. Also noted are 2 lesions in the posterior fossa, one in the left lateral aspect of the pons and the other in the posterior aspect of the middle cerebellar peduncle (short arrows) and a lesion in the left temporal lobe adjacent to the lateral margin of the temporal horn (arrowhead).
A second lumbar puncture continued to show oligoclonal bands and intrathecal synthesis of IgG in the CSF (IgG 2.01 mg/dl, Freedman pattern 2) with no additional biocytochemical changes. Panel of infection, autoimmunity, including autoimmune encephalitis and anti-neuronal antibodies (Ab), were negative. Visual evoked potentials (VEP) showed normal amplitude and median latencies of the main peak (P100) with no asymmetries.
Clinical evaluation did not reveal focal neurological deficits. The patient complained of mild memory impairment recalling words, difficulty concentrating which prevented him from resuming his professional life and, although he was a sportsman before, he had no thrive for sports. No active treatment was deemed appropriate and the patient remained under surveillance.
Subsequent MRI, performed 10 months after treatment (Supplementary Fig. 2), showed a slight decrease in the size of the largest demyelinating lesion located in the peri-atrial white matter and resolution of the associated vasogenic edema. It also showed interval disappearance of the faint peripheral contrast enhancement and restricted diffusion of the lesions. No new demyelinating lesions and no evidence of tumor recurrence were seen.
On the last follow up visit, one year after treatment, the patient remained asymptomatic with no focal neurologic deficits, specifically denying memory and concentration difficulties. He resumed his full-time job and his normal social habits.
3. Discussion
This case is remarkable for the appearance of a neuroimaging picture compatible with MS, 4 months after chemoradiotherapy (CRT) for a suprasellar germinoma, in a previously healthy young adult with no family history of MS and no previous white matter lesions, showing intrathecal synthesis of IgG and oligoclonal bands in the CSF.
There are 2 potential explanations for this occurrence: a toxic effect from CRT leading to an unusual demyelinating pattern simulating MS or the coincidental development of a clinically silent MS in a previously predisposed patient. The following discussion will address the existing evidence for both these hypotheses.
-
1.
CRT-induced toxicity simulating MS
Neurotoxicity is a well-known side effect of both chemo and radiation therapy and share a common denominator: depletion of oligodendrocyte precursor cells and disruption of oligodendrocyte lineage dynamics leading to axonal demyelination, triggered by microglial activation and inflammation [1].
Several chemotherapy agents, in particular antimetabolites and alkylating drugs, have been shown to induce an acute and most often reversible leukoencephalopathy via microglial activation and inflammation [3,5,6]. Carboplatin and ifosfamide are both neurotoxic alkylating agents. The former most often associated with neurovascular dysregulation leading to posterior reversible leukoencephalopathy [13,14] and, the latter, responsible for a toxic leukeoencephalopathy syndrome seen in 10–20% of patients which is not usually associated with structural white matter changes on conventional MRI studies [15,16].
Neuroimaging findings of radiation-induced leukoencephalopathy comprise discrete or diffuse and confluent white matter lesions, solid contrast enhancing lesions, and necrotic lesions with thick, irregular, contrast-enhancing borders eliciting vasogenic brain edema which, in a chronic stage, may evolve to cystic porencephaly and brain atrophy [[17], [18], [19]]. Advanced diffusion tensor MR imaging techniques, have been shown to depict early microstructural white matter changes, related to increased vascular permeability and neuroinflammation across all radiation doses, even below 10 Gy [20].
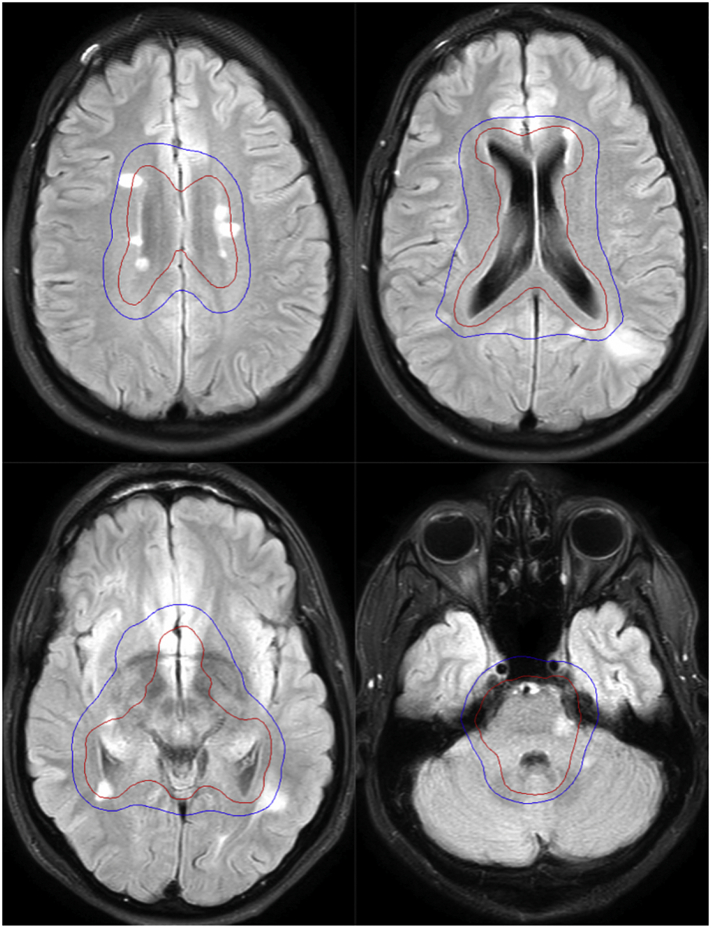
In our case the demyelinating lesions disclosed on the MRI scan 4 months after RT, strongly suggest an acute/subacute inflammatory demyelinating process with multiple discrete lesions showing restricted diffusion and vasogenic edema. Interestingly, the superimposition of the irradiated volumes on the post-RT axial FLAIR MR images (Fig. 3), demonstrates that almost all demyelinating lesions lay within the 20 Gy isodose curve. Therefore, although the distribution and pattern of the white matter lesions resemble that of MS, it is conceivable that they may have resulted from the superimposed neurotoxic effect of radiation therapy upon an already susceptible ground of microglial inflammation induced by the previous chemotherapy.
-
2.
Coincidental clinically silent MS in a predisposed patient
Fig. 3.
Dose distribution after registration of CT planning upon axial FLAIR images of the follow-up MRI scan obtained 3 months after treatment. The 24 Gy isodose curve (red) shows the volume irradiated with the prescribed dose. The 20 Gy isodose curve (blue) represents the volume that received at least 20 Gy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The morphology and distribution of the demyelinating lesions disclosed on the post-radiation MRI scan have a typical pattern for MS according to the MAGNIMS criteria [12]. These neuroimaging findings, in an otherwise asymptomatic patient, strongly favor a radiologically isolated syndrome (RIS), a clinically silent form of MS. Multiple factors (clinical, laboratory and radiologic) have been associated with the likelihood to progress from subclinical to clinical MS including the presence of OCBs, younger age, male gender, positive family history and abnormal visual evoked potentials [21]. On MRI, the presence of gadolinium-enhancing and spinal cord lesions are predictors of conversion to a full-blown MS [12,21].
Interestingly, our patient had oligoclonal bands in the CSF prior to the development of the WM lesions. While not exclusive for MS, OCBs have a reported positive predictive value (PPV) ranging between 61 and 94% depending on the reference population and on the integration with other CSF findings [22]. However, OCBs are present in 6% of cancer patients and have been reported in at least 6 cases of germinoma [[23], [24], [25]].
Although the development of this RIS could have been coincidental, the temporal relationship with the CRT is hard to be neglected. Eventhough the effects of brain irradiation in MS patients remain elusive, it seems intuitive that MS patients carry a higher risk of chemoradiation-induced neurotoxicity as both processes target oligodendrocytes. In addition, it is likely that RT-induced BBB disruption, facilitates the influx of autoreactive T-lymphocytes in MS-predisposed patients.
Previous studies have reported an increased susceptibility of MS patients to radiation-induced demyelination, in some cases, precipitating disease reactivation in patients with long-lasting quiescent disease [[26], [27], [28], [29], [30], [31]]. The largest review study found in the literature is a retrospective evaluation of 15 MS patients, treated with external beam radiation therapy between 1976 and 2014 [32].This study supported the impression that MS patients are at higher risk for neurotoxicity compared to non-MS patients. Moreover, 3 patients who had probable MS, evolved to full-blown MS after irradiation. It is conceivable that since the use of more conformal radiotherapy techniques IMRT, VMAT and radiosurgery, sparing healthy brain tissue, these results may not be reproduced. Large retrospective studies will be required to clarify this issue.
In our case, while irradiating the whole ventricular system, the periventricular WM included in the low-dose bath encompasses most of the lesions, making it quite likely that radiation therapy was the trigger for the development of the white matter lesions following prior chemo-sensitization in a potentially predisposed patient (with CSF OCBs). The weight of each independent factor is hard to determine. In fact, we favor this hypothesis as the most likely explanation for the appearance of the demyelinating lesions.
To our knowledge such a pattern of demyelination has not yet been described in association with radiation nor with the chemotherapy agents used in this multidrug regimen (etoposide, carboplatin and ifosfamide).
Since the clinical and imaging features and the temporal evolution of the demyelinating lesions of our patient did not suggest other demyelinating diseases such as acute disseminated encephalomyelitis (ADEM) or neuromyelitis optica (NMO) we did not search for aquaporin 4 (AQP4) or myelin oligodendrocyte glycoprotein (MOG) antibodies. In fact, an MRI of the neuro-axis excluded spinal cord lesions and the visual evoked potentials were normal. However, since MOG antibody-associated inflammatory demyelinating diseases represent an oligodendropathy [33,34], it would be interesting to find whether or not these antibodies were present in our patient.
In a short follow-up period of one year, the patient did not develop neurological symptoms and there has been no progression of the neuroimaging findings. He will remain in close surveillance to ascertain whether or not he will evolve to a full-blown MS.
The following are the supplementary data related to this article.
Follow-up MRI of the brain 6 months after CRT: Post-gadolinium axial T1W (A) and axial FLAIR (B) images demonstrate a slight decrease in the size of the largest peri-trigonal lesion with resolution of the peripheral edema and restricted diffusion (dashed arrows). The remainder demyelinating lesions remain similar in size and number and no new lesions appeared in the interval.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statement
Alexandra Borges: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Daniela Garcez: Conceptualization, Data curation, Writing - review & editing. Cátia Pedro: Data curation, Writing - review & editing. João Passos: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
None.
References
- 1.Gibson E.M., Nagaraja S., Ocampo A., Tam L.T., Wood L.S., Pallegar P.N. Methotrexate chemotherapy induces persistent triglial dysregulation that underlies chemotherapy-related cognitive impairment. Cell. 2019;176(1–2):43–55. doi: 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helson L. Radiation-induced demyelination and remyelination in the central nervous system: a literature review. Anticancer Res. 2018;38(9):4999–5002. doi: 10.21873/anticanres.12818. [DOI] [PubMed] [Google Scholar]
- 3.Zrzavy T., Hametner S., Wimmer I., Butovsky O., Weiner H.L., Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140:1900–1913. doi: 10.1093/brain/awx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagher N.N., Najafi A.R., Kayala K.M., Elmore M.R., White T.E., Medeiros R., West B.L., Green K.N. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seigers R., Schagen S.B., Coppens C.M., van der Most P.J., van Dam F.S., Koolhaas J.M., Buwalda B. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav. Brain Res. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Acharya M.M., Martirosian V., Chmielewski N.N., Hanna N., Tran K.K., Liao A.C., Christie L.A., Parihar V.K., Limoli C.L. Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 2015;75:676–686. doi: 10.1158/0008-5472.CAN-14-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monje M.L., Mizumatsu S., Fike J.R., Palmer T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 8.Monje M.L., Vogel H., Masek M., Ligon K.L., Fisher P.G., Palmer T.D. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 9.Karunamuni R.A., White N.S., McDonald C.R. Multi-component diffusion characterization of radiation-induced white matter damage. Med. Phys. 2017;44:1747–1754. doi: 10.1002/mp.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene-Schloesser D., Robbins M.E., Peiffer A.M., Shaw E.G., Wheeler K.T., Chan M.D. Radiation-induced brain injury: a review. Front. Oncol. 2012 Jul 19;2:73. doi: 10.3389/fonc.2012.00073. (eCollection 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagesh V., Tsien C.I., Chenevert T.L., Ross B.D., Lawrence T.S., Junick L., Cao Y. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int. J. Radiat. Oncol. Biol. Phys. 2008 Mar 15;70(4):1002–1010. doi: 10.1016/j.ijrobp.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi M., Rocca M.A., Ciccarelli O., De Stefano N., Evangelou N., Kappos L., Rovira A., Sastre-Garriga J., Tintorè M., Frederiksen J.L., Gasperini C., Palace J., Reich D.S., Banwell B., Montalban X., Barkhof F., MAGNIMS Study Group MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016 Mar;15(3):292–303. doi: 10.1016/S1474-4422(15)00393-2. (Epub 2016 Jan 26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan S.A., MacEneaney P., O’Reilly S.P. Reversible posterior leukoencephalopathy induced by carboplatin and etoposide. Med. Oncol. 2012;29:1287–1291. doi: 10.1007/s12032-011-9898-8. [DOI] [PubMed] [Google Scholar]
- 14.Raut T.P., Mathur T., Ambulkar I. Severe reversible form of chemotherapy induced acute disseminated leuko-encephalopathy: a case report with review of literature. Ann. Clin. Lab. Res. 2017;5:1. [Google Scholar]
- 15.Özütemiz C., Roshan S.K., Kroll N.J., Benson J.C., Rykken J.B., Oswood M.C., Zhang L., McKinney A.M. Acute toxic leukoencephalopathy: etiologies, imaging findings, and outcomes in 101 patients. AJNR. February 2019;40(2):267–275. doi: 10.3174/ajnr.A5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich J., Marienhagen J., Schalke B., Bogdahn U., Schlachetzki F. Vascular neurotoxicity following chemotherapy with cisplatin, ifosfamide, and etoposide. Ann. Pharmacother. 2004 Feb;38(2):242–246. doi: 10.1345/aph.1D106. (Epub 2003 Dec 15) [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.X., King A.D., Zhou H., Leung S.F., Abrigo J., Chan Y.L., Hu C.W., Yeung D.K., Ahuja A.T. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010 Jan;254(1):210–218. doi: 10.1148/radiol.09090428. (Epub 2009. Dec 17) [DOI] [PubMed] [Google Scholar]
- 18.Burns T.C., Awad A.J., Li M.D., Grant G.A. Radiation-induced brain injury: low-hanging fruit for neuroregeneration. Neurosurg. Focus. 2016 May;40(5) doi: 10.3171/2016. [DOI] [PubMed] [Google Scholar]
- 19.Bompaire F., Lahutte M., Buffat S., Soussain C., Ardisson A.E., Terziev R. New insights in radiation-induced leukoencephalopathy: a prospective cross-sectional study. Support Care Cancer. 2018;26:4217–4226. doi: 10.1007/s00520-018-4296-9. [DOI] [PubMed] [Google Scholar]
- 20.Connor M., Karunamuni R., McDonald C., White N., Pettersson N., Moiseenko V., Hattangadi-Gluth J. Dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2016;121(2):209–216. doi: 10.1016/j.radonc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siva A. Asymptomatic MS. Clin. Neurol. Neurosurg. 2013 Dec;115(Suppl. 1):S1–S5. doi: 10.1016/j.clineuro.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Deisenhammer F., Zetterberg H., Fitzner B., Zettl U.K. The cerebrospinal fluid in multiple sclerosis. Front. Immunol. 2019;10:726. doi: 10.3389/fimmu.2019.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen O., Biran I., Steiner I. Cerebrospinal fluid oligoclonal IgG bands in patients with spinal arteriovenous malformation and structural central nervous system lesions. Arch. Neurol. 2000 Apr;57(4):553–557. doi: 10.1001/archneur.57.4.553. [DOI] [PubMed] [Google Scholar]
- 24.Pierzchlewicz K., Bilska M., Jurkiewicz E., Chmielewski D., Moszczyńska E., Daszkiewicz P., Ciołkowski M., Grajkowska W., Kotulska K. Germinoma mimicking brain inflammation: a case report. Child Neurol. Open. 2019;6 doi: 10.1177/2329048X19848181. 2329048X19848181. Published online 2019 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kageyama Hiroshi, Suzuki Takamoto, Ohara Yukou. Intramedullary spinal cord germinoma clinically mimicking multiple sclerosis: a case report. Surg. Neurol. Int. 2019;10:201. doi: 10.25259/SNI_466_2019. 10.25259/SNI_466_2019 Published online 2019 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy C.B., Hashimoto S.A., Graeb D., Thiessen B.A. Clinical exacerbation of multiple sclerosis following radiotherapy. Arch. Neurol. 2003;60:273–275. doi: 10.1001/archneur.60.2.273. [DOI] [PubMed] [Google Scholar]
- 27.Lampert P., Tom M.I., Rider W.D. Disseminated demyelination of the brain following Co60 (gamma) radiation. Arch. Pathol. 1959;68:322–330. [PubMed] [Google Scholar]
- 28.Tourtellotte W.W., Potvin A.R., Baumhefner R.W. Multiple sclerosis de novo CNS IgG synthesis: effect of CNS irradiation. Arch. Neurol. 1980;37:620–624. doi: 10.1001/archneur.1980.00500590044005. [DOI] [PubMed] [Google Scholar]
- 29.Shulga A.I. The radiotherapy of patients with disseminated multiple sclerosis [in Russian] Med. Radiol. 1967;12:45–47. [PubMed] [Google Scholar]
- 30.McMeekin R.R., Hardman J.M., Kempe L.G. Multiple sclerosis after x-radiation: activation by treatment of metastatic glomus tumor. Arch. Otolaryngol. 1969;90:617–621. doi: 10.1001/archotol.1969.00770030619017. [DOI] [PubMed] [Google Scholar]
- 31.Peterson K., Rosenblum M.K., Powers J.M. Effect of brain irradiation on demyelinating lesions. Neurology. 1993;43:2105–2112. doi: 10.1212/wnl.43.10.2105. [DOI] [PubMed] [Google Scholar]
- 32.Miller R.C., Lachance D.H., Lucchinetti C.F., Keegan B.M., Gavrilova R.H., Brown P.D., Weinshenker B.G., Rodriguez M. 66(4) 2006 Nov 15. Multiple Sclerosis, Brain Radiotherapy, and Risk of Neurotoxicity: The Mayo Clinic Experience; pp. 1178–1186. (Epub 2006 Sep 11) [DOI] [PubMed] [Google Scholar]
- 33.Zamvil S., Slavin A.J. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e62. doi: 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reindl M., Rostasy K. MOG antibody-associated diseases. Neurol. Neuroimmunol. Neuroinflamm. 2015;2(1):e60. doi: 10.1212/NXI.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Follow-up MRI of the brain 6 months after CRT: Post-gadolinium axial T1W (A) and axial FLAIR (B) images demonstrate a slight decrease in the size of the largest peri-trigonal lesion with resolution of the peripheral edema and restricted diffusion (dashed arrows). The remainder demyelinating lesions remain similar in size and number and no new lesions appeared in the interval.