Abstract
Objective
In this study, we evaluated the effect of neonatal ketamine exposure on anxiety-like and exploratory behaviours in adult the Balb/c and C57BL/6 strains of mice which anxiety responses are different.
Methods
Ketamine was administered at two different doses single dose (10, 20 mg/kg, 0.1 ml/10 g body weight, intraperitoneally) and repeated doses (10, 20 mg/kg every 240 minutes; thrice times) on the 7th postnatal day to male Balb/c and C57BL/6 mice. In adulthood, open-field (OF) and elevated plus maze (EPM) apparatuses were used to evaluate exploratory and anxiety-like behaviour.
Results
In the C57BL/6 mice, the 20 mg/kg single dose decreased open-arm time and total-arm entries in EPM and increased time of central latency and decreased distance travelled in OF. Both the 10 and 20 mg/kg repetitive doses increased time of central latency and decreased time spent in the centre, frequency of rearing and centre crossing in OF and decreased open-arm time, total-arm entries, number of open-arm entries in EPM. The 20 mg/kg repetitive dose decreased number of head dipping behaviours in EPM. In the Balb/c mice, both the single and repetitive 10−20 mg/kg doses had no significant effect on anxiety-like and exploratory behaviours.
Conclusion
There were no significant differences in anxiety-like and exploratory behaviour in different strains by the single 10 mg/kg dose. However, in the C57BL/6 mice, both the single and repetitive 20 mg/kg doses and the 10 mg/kg repetitive dose increased anxiety-like behaviour and decreased exploratory behaviour in EPM and OF. In conclusion, hereditary factors may be effective on the effect of neonatal ketamine treatment on anxiety-like and exploratory behaviour.
Keywords: Anxiety, Exploratory behavior, Ketamine, BALB C mice, C57BL mice.
INTRODUCTION
A non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine has been used as a dissociative anaesthetic agent in paediatric and adult patients since 1960s. Ketamine, a non-competitive antagonist of the phencyclidine binding sites of the NMDA receptors of the ionotropic receptors of glutamate, the excitatory neurotransmitter in the central nervous system, also has an effect on other neurotransmitter systems. Currently, ketamine shows potential as a new therapeutic target of major depressive disorder [1,2]. Many studies have approved ketamine’s efficacy on anxiety behaviour in humans as well as in animals [3 -5]. However, studies on the effect of ketamine in anxiety-like behaviour showed conflicting results. Ketamine has shown attenuation of anxiety-like behaviour but also anxiogenic effects in humans and animals.
Few studies have shown that ketamine exposure in the neonatal period may have long-lasting behavioural effects [6]. Additionally, the mechanism of neonatal ketamine exposure on anxiety-related response in animal models still remains largely unknown. NMDAR blockade is known to cause behavioural changes in adulthood when it is performed in critical brain development [7,8]. However, the molecular mechanisms underlying response neonatal ketamine exposure are likely to be more complex than blockade of NMDAR. It is known that the relationship between physical and social environmental factors and gene expression in the central nervous system affects the physiological and behavioural structure of the brain [9]. Although hereditary factors are known to have effects on behaviour, the effects of neonatal ketamine exposure on adult behaviour related to hereditary factors have not been previously studied in the literature.
The administration of non-competitive NMDA receptor antagonists, such as ketamine and MK-801 to rodents during a critical period of development leads to neurotoxicity or neurodegeneration in brain. In previous studies shown that the administration of ketamine during critical developmental periods result in a dose-related and exposure-time dependent increase in neurotoxicity [10 -12]. Hayashi et al. [13] demonstrated that a single dose of ketamine not increase in neuronal degeneration while repeated doses of ketamine at 90 minutes intervals on postnatal day 7 (PD7) in rats increased degenerate neurons in multiple brain regions. Furthermore, ketamine neurotoxicity was observed only after of repeated injections given during a period of about 9 hours while MK-801 neurotoxicity reported after a single exposure [12]. Also, we investigated the effects of single and repetitive neonatal ketamine administrations on anxiety-like behaviour in two different strains of adult mice which anxiety responses are different (lowly C57BL/6 and highly anxious Balb/c). In this study, we chose C57BL/6 and highly anxious Balb/c mice because these strains have been shown to differ in anxiety-like behaviour response to neuropharmacological agents [14].
METHODS
The experimental protocols were approved by the Ethics Committee of Cukurova University, Faculty of Medicine, Medical Sciences Experimental Research and Application Center (Date: March 1, 2013 and Decision number: 6/4). The procedures in the study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals.
Animals
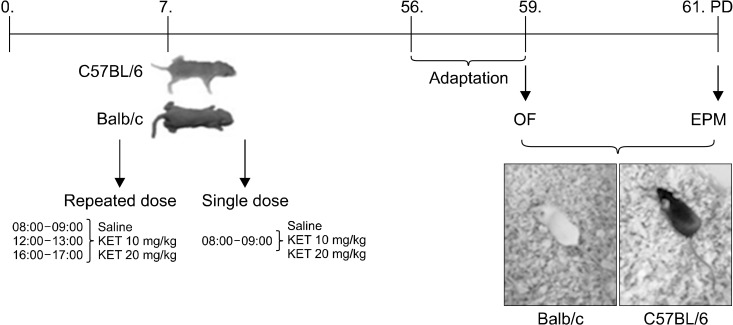
Adult (8−10-week-old) male Balb/c and C57BL/6 mice were used for the behavioural tests. The mice were bred and housed at the Physiology Laboratory of Cukurova University Faculty of Medicine. Food and water were given without restriction. The ambient temperature was kept at 21 ± 2°C, and behavioural tests were performed at this temperature. The animals were housed in a room with a 12-hour light/dark cycle (05:00−17:00 light, 17:00−05:00 dark). The day of birth was counted as the postnatal day 0. The behavioural tests were performed between 9:00 and 12:00. Male mice were used in this study because of the oestrus cycle in female mice and changes in anxiety-like behaviours during this cycle Animals were handled and allowed to adapt to the room they were kept in. Three days before the behavioural tests, the mice were gently handled (each mouse 1 min/day) at the same time using both hands covered with fine latex gloves, and they were placed in cages and transported to the testing room (5 min/day) (Fig. 1).
Fig. 1.
Timeline of the experimental procedures. OF, open field test; EPM, elevated plus maze; KET, ketamine; PD, post-natal day.
Application of Single Dose Ketamine
The male mice pups were injected on PD7 intraperitoneally with ketamine hydrochloride (100 mg/ml, Ketasol; Richter Pharma AG, Wels, Austria) at doses of 10 and 20 mg/kg and a volume of 0.1 ml/10 g body weight, once a day (8:00−9:00). The same volume of saline (0.9% NaCl) was injected to the mice in the control group.
Application of Repeated Dose Ketamine
The male mice pups were injected on PD7 intraperitoneally with ketamine hydrochloride (100 mg/ml, Ketasol; Richter Pharma AG,) one every 240 minutes (three times 8:00−9:00, 12:00−13:00, 16:00−17:00) the repeated doses of 10 or 20 mg/kg and a volume of 0.1 ml/10 g body weight. The same volume of saline (0.9% NaCl) was injected to the control group mice.
The mother was placed in another cage next to the home cage, and then, saline or ketamine was gently injected to each pup at the same time using both hands covered with fine latex gloves (injection time for each mouse: 1 minute). After the injections on the pups, their mothers were immediately returned to the home cage. All pups from the same litter received the same treatment and randomly assigned to groups of saline or ketamine on PD21.
Apparatus
Open-field
We performed the open-field (OF) test as previously described [15,16]. In the OF experiments, exploratory locomotor activities were measured using the following criteria: the distance travelled in the centre and peripheral fields, time spent in the centre versus the peripheral field, the frequency of centre crossing and latency of entering the centre. The frequency of rearing (vertical activity) was evaluated as well. For the OF test, the mice were placed onto any corner of the apparatus. Their behaviours were recorded for five minutes using a video camera. The Ethovision software (Ethovision XT version 4.1; Noldus, Leesburg, VA, USA) was used to calculate the total distance travelled and time spent in the centre. The latency of entering the centre and the frequency of rearing were also manually recorded onto the computer. The OF apparatus was illuminated by 165 lx.
Elevated plus maze
We performed the elevated plus maze (EPM) test as previously described [15]. The animal was placed at the centre of the plus-maze facing the enclosed arm and observed for 5 minutes. In the EPM, exploratory locomotor activities and anxiety-like behaviours were evaluated. The EPM was illuminated by 165 lx (the open arms). The maze apparatus was cleaned after each trial. The following parameters were registered: number of entries in the open arm, total entries (open and closed arms), time of permanence in the open and closed arms. The Ethovision software was used to calculate the parameters. Mice naturally prefer the safe closed arm and abstain from the anxiety-inducing open arm. The fear of height is hereditary in mice and novelty-induced, anxiety-like behaviours were evaluated by the EPM.
Statistical Analysis
The data are expressed as mean ± standard error. Tukey’s HSD test was used in pairwise comparisons following two-way ANOVA in groups with normal distribution and homogeneous variance. Mann−Whitney Utest was used in pairwise comparisons following Kruskal−Wallis test in non-normally distributed groups and non- homogeneous variance.
RESULTS
Open-field
Single dose ketamine application
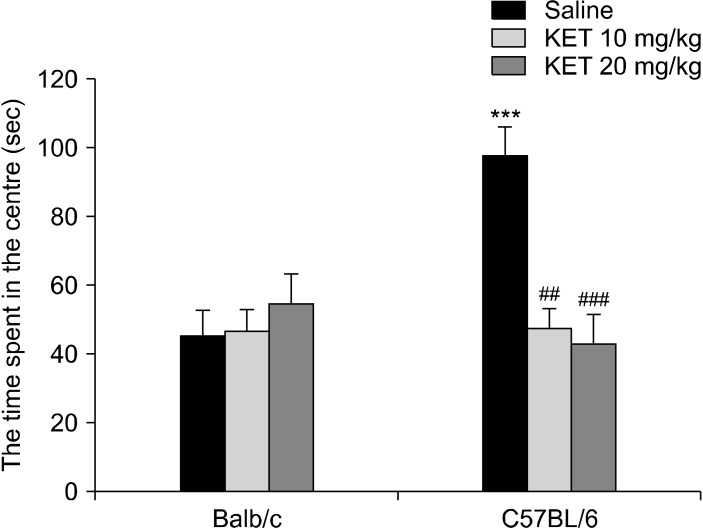
There was a significant difference in the time of central latency (H[5, n = 60] = 11.11, p < 0.05) and rearing (H[5, n = 60] = 40.27, p < 0.01) in the OF. The C57BL/6 control mice had a shorter latency of entering the centre than the Balb/c control mice (p < 0.05) (Table 1). In the C57BL/6 mice, the treatment with 20 mg/kg dose of ketamine caused an increase in the time of central latency in comparison to the control mice (p < 0.05). In the C57BL/6 mice, there was more rearing in comparison to the Balb/c mice (p < 0.05). In the OF test, regarding the times spent in the centre, a two-way ANOVA showed a significant strain effect (F[1,54] = 4.57, p < 0.05), a drug effect (F[2,54] = 6.06, p < 0.05) or a strain X drug interaction effect was confirmed (F[2,54] = 9.69, p < 0.001) (Fig. 2). The C57BL/6 control mice had increased times spent in the centre in comparison to the Balb/c control mice (p < 0.001). In the C57BL/6 mice, with treatment by 10 mg/kg and 20 mg/kg ketamine, there were decreased levels of time spent in the centre in comparison to the control mice (respectively p < 0.01, p < 0.001).
Table 1.
Behaviours in the open field of adult mice subjected to a single dose of neonatal ketamine exposure
| The open filed | Balb/c | C57BL/6 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Saline (n = 11) | KET10 (n = 11) | KET20 (n = 12) | Saline (n = 9) | KET10 (n = 7) | KET20 (n = 10) | ||
| The time of centre latency | 29.3 ± 5.9 | 19.5 ± 5.7 | 18.6 ± 3.0 | 8.7 ± 1.4* | 17.5 ± 7.3 | 46.1 ± 15.9# | |
| Frequency of rearing | 29.3 ± 2.2 | 24.8 ± 1.7 | 25.3 ± 2.6 | 46.8 ± 3.6* | 57.4 ± 3.5 | 46.4 ± 2.7 | |
| Frequency of centre crossing | 24.3 ± 2.4 | 26.7 ± 3.3 | 31.0 ± 3.8 | 23.3 ± 1.6 | 27.5 ± 3.2 | 19.3 ± 2.8 | |
Values are presented as mean ± standard error.
KET10, ketamine 10 mg/kg; KET20, ketamine 20 mg/kg.
*p < 0.05 in comparison to the Balb/c control (saline) group, # p < 0.05 in comparison to the C57BL/6 control (saline) group.
Fig. 2.
The time spent in the centre of the open field in the adult mice exposed to a single dose of ketamine in their neonatal periods. The data are expressed as mean ± standard error. KET, ketamine. ***p < 0.001 in comparison to the Balb/c control (saline) group, ## p < 0.01, ### p < 0.001 in comparison to the C57BL/6 control (saline) group. The statistical analysis included a two-way ANOVA followed by Tukey’s HSD test.
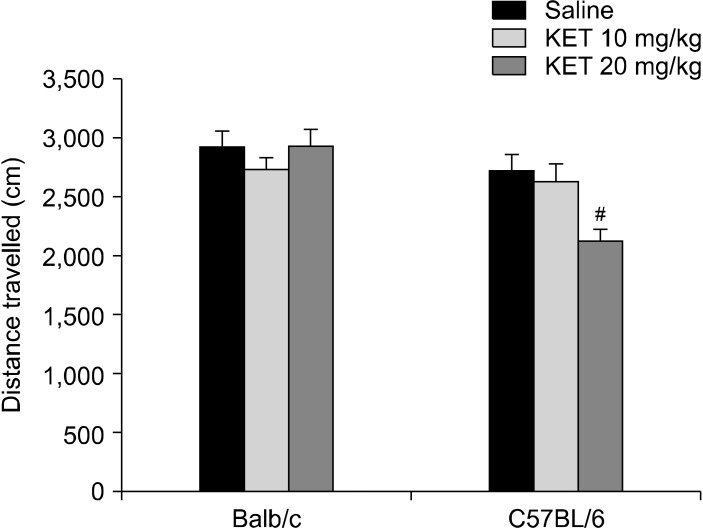
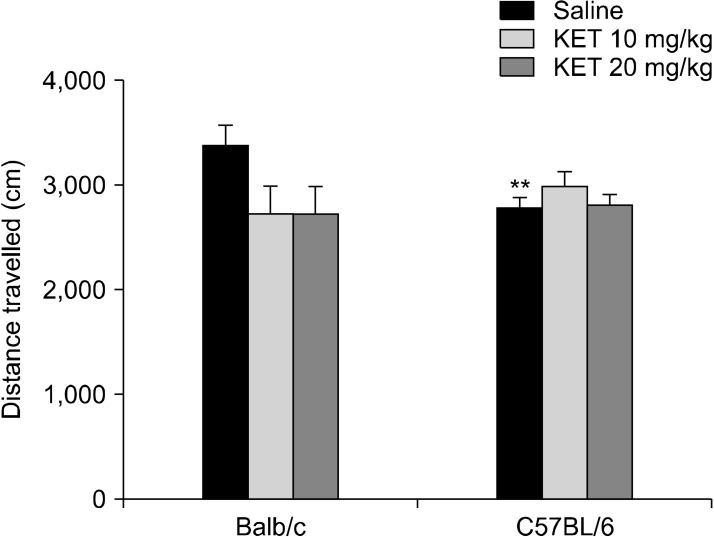
There was a significant difference in the distance travelled based on the strain X drug interaction (F[2,54] = 3.80, p < 0.05). In the C57BL/6 mice, the 20 mg/kg dose of ketamine caused a decrease in the distance travelled in comparison to the control mice (p < 0.05) (Fig. 3). The frequency of centre crossing had no significant drug effect (F[2,54] = 0.52, p > 0.05), strain effect (F[1,54] = 2.38, p > 0.05) or strain X drug interaction effect (F[2,54] = 2.43, p < 0.05) (Table 1).
Fig. 3.
Distance travelled in the open-field test (cm) in the adult mice subjected to a single neonatal dose of ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. # p < 0.05 in comparison to the C57BL/6 control (saline) group. Statistical analysis included a two-way ANOVA followed by Tukey’s HSD test.
Repeated dose ketamine application
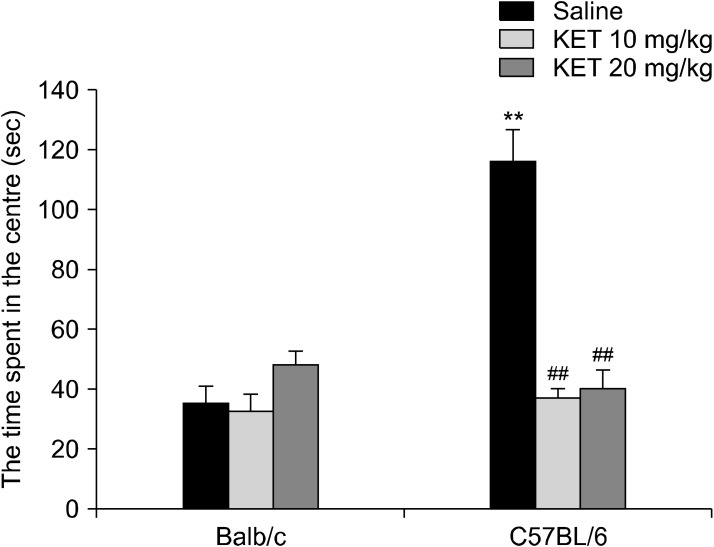
There was a significant difference in the time of centre latency (H[5, n = 63] = 31.96, p < 0.001) in the OF. C57BL/6 control mice were decreased in the time of centre latency compared to Balb/c control mice (p < 0.001). In C57BL/6 mice with treatment 10 and 20 mg/kg ketamine were increase in the time of centre latency (respectively p < 0.001, p < 0.01). For frequency of rearing, significant strain effect (F[1,57] = 56.28, p < 0.001), drug effect (F[2,57] = 21.95, p < 0.001) and strain X drug interaction (F[2,57] = 7.26, p < 0.01) were confirmed by using two-way ANOVA. The C57BL/6 control mice had increased frequency of rearing in comparison to the Balb/c control mice (p < 0.001). In the C57BL/6 mice, there was a decrease in frequency of rearing by ketamine application at the 10 mg/kg and 20 mg/kg doses in comparison to the C57BL/6 control mice (p < 0.001) (Table 2). In the OF test, with a two-way ANOVA test, the time spent in the centre showed a significant strain effect (F[1,57] = 20.87, p < 0.001), drug effect (F[2,57] = 21.94, p < 0.001) and a strain X drug interaction effect (F[2,57] = 28.87, p < 0.001). The C57BL/6 control mice had increased times spent in the centre in comparison to the Balb/c control mice (p < 0.001). In the C57BL/6 mice, with treatment by 10 mg/kg and 20 mg/kg ketamine, there were decreased levels of time spent in the centre in comparison to the control mice (p < 0.001) (Fig. 4). The distance travelled had no significant drug effect (F[2,57] = 0.84, p > 0.05), strain effect (F[1,57] = 1.26, p > 0.05) or strain X drug interaction effect (F[2,57] = 2.68, p > 0.05) (Fig. 5). In the OF, the groups differed significantly in terms of their frequencies of centre crossing (H[5, n = 63] = 14.62, p < 0.05). The frequency of centre crossing was increased in the C57BL/6 control mice in comparison to the Balb/c control mice (p < 0.05). In the C57BL/6 mice, the two different doses of ketamine application both caused a decrease in the frequency of centre crossing on the apparatus (p < 0.01) (Table 2).
Table 2.
The behaviours in the open field for adult mice subjected to repetitive doses of neonatal ketamine exposure
| The open filed | Balb/c | C57BL/6 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Saline (n = 13) | KET10 (n = 11) | KET20 (n = 9) | Saline (n = 10) | KET10 (n = 10) | KET20 (n = 10) | ||
| The time of centre latency | 48.9 ± 12.1 | 37.5 ± 15.7 | 30.4 ± 5.5 | 3.4 ± 0.9*** | 43.5 ± 6.1### | 21.2 ± 6.5## | |
| Frequency of rearing | 20.7 ± 2.0 | 15.7 ± 2.6 | 14.2 ± 3.1 | 45.5 ± 2.8*** | 29.6 ± 2.2### | 20.5 ± 1.6### | |
| Frequency of centre crossing | 25.0 ± 2.9 | 23.9 ± 5.3 | 29.5 ± 3.1 | 37.1 ± 2.5* | 22.4 ± 1.3## | 22.8 ± 2.7## | |
Values are presented as mean ± standard error.
KET10, ketamine 10 mg/kg; KET20, ketamine 20 mg/kg.
*p < 0.05, ***p < 0.001 compared to Balb/c control (saline) group, ## p < 0.01, ### p < 0.001 compared to C57BL/6 control (saline) group.
Fig. 4.
The time spent in the centre of the open field in the adult mice subjected to repetitive doses neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. **p < 0.01 in comparison to the Balb/c control (saline) group, ## p < 0.01 in comparison to the C57BL/6 control (saline) group. Statistical analysis included a two-way ANOVA followed by Tukey’s HSD test.
Fig. 5.
The distance travelled in the open-field test (cm) in the adult mice subjected to the repetitive doses of neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. **p < 0.01 in comparison to the Balb/c control (saline) group. Mann−Whitney Utest.
Elevated Plus Maze
Single dose ketamine application
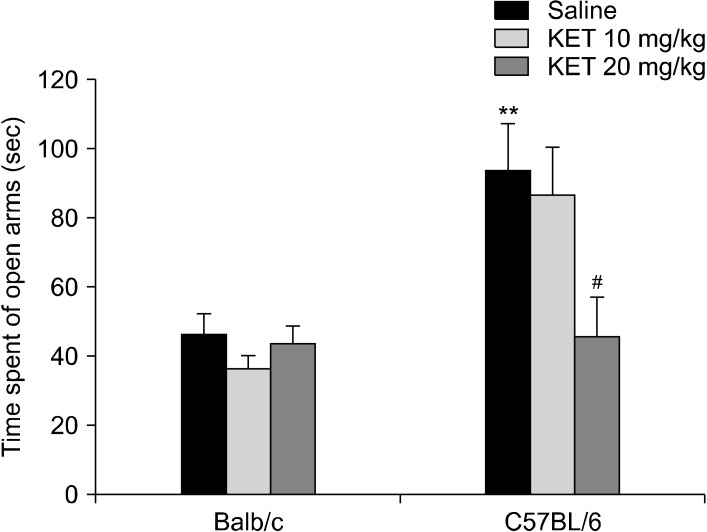
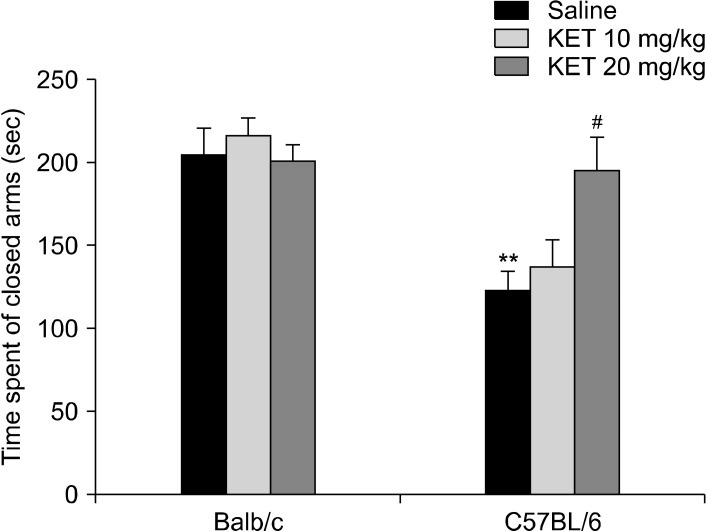
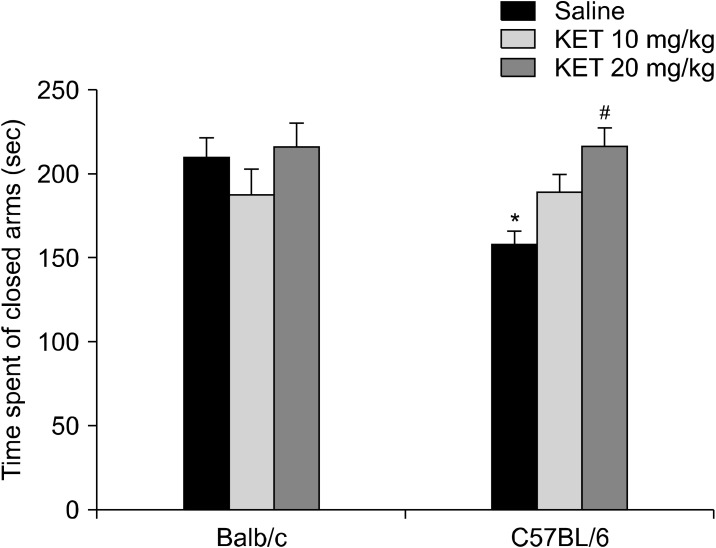
In the elevated plus maze, the groups differed significantly in terms of their of open-arm time (H[5, n = 60] = 22.87, p < 0.001) (Fig. 6), number of open-arm entries (H[5, n = 60] = 25.39, p < 0.001), total arm entries (H[5, n = 60] = 27.19, p < 0.001) and number of head dipping (H[5, n = 60] = 32.05, p < 0.001) (Table 3). The C57BL/6 control mice had increased open-arm time, number of open-arm entries, total arm entries and number of head dipping in comparison to the Balb/c control mice (p < 0.01). In the C57BL/6 mice, treatment with 20 mg/kg ketamine decreased the open-arm time, the number of open-arm and total arm entries and the number of head dipping occasions (p < 0.05) in comparison to the control mice. In the EPM, the of closed-arm time values were examined by a two-way ANOVA test, and significant effects were confirmed based on strain (F[1,54] = 22.25, p < 0.001) and strain X drug interaction (F[2,54] = 4.91, p < 0.05) (Fig. 7). The C57BL/6 control mice had decreased in the of closed-arm time in comparison to the Balb/c control mice (p < 0.01). In the C57BL/6 mice, the 20 mg/kg dose of ketamine caused an increase in closed-arm time in comparison to the C57BL/6 control mice (p < 0.05). For the latency to enter the open arm of the EPM a strain effect (F[1,54] = 0.003, p > 0.05), a strain X drug interaction effect (F[2,54] = 1.53, p > 0.05) or a drug effect (F[2,54] = 0.49, p > 0.05) were not confirmed.
Fig. 6.
The time spent in the open arms of the elevated plus maze (sec) in the adult mice subjected to a single dose of neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. **p < 0.01 in comparison to the Balb/c control (saline) group, # p < 0.05 in comparison to the C57BL/6 control (saline) group. Mann−Whitney Utest.
Table 3.
The behaviours in the elevated plus maze of adult mice subjected to a single dose ketamine neonatal exposure
| The elevated plus maze | Balb/c | C57BL/6 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Saline (n = 11) | KET10 (n = 11) | KET20 (n = 12) | Saline (n = 9) | KET10 (n = 7) | KET20 (n = 10) | ||
| Number entries of in the open arm | 3.2 ± 0.5 | 2.6 ± 0.3 | 2.5 ± 0.2 | 7.0 ± 0.8** | 6.5 ± 0.7 | 4.1 ± 0.9# | |
| Number of total arm entries | 9.0 ± 0.9 | 7.8 ± 0.7 | 7.5 ± 0.6 | 14.3 ± 1.0** | 15.0 ± 0.8 | 9.5 ± 1.5# | |
| Enter latency to open arm(s) | 41.4 ± 20.8 | 23.3 ± 3.8 | 21.8 ± 6.2 | 17.1 ± 8.8 | 18.4 ± 13.2 | 53.3 ± 29.7 | |
| The number of head dipping | 1.7 ± 0.5 | 1.2 ± 0.4 | 0.6 ± 0.3 | 18.5 ± 3.0** | 18.4 ± 3.9 | 9.4 ± 2.6# | |
Values are presented as mean ± standard error.
KET10, ketamine 10 mg/kg; KET20, ketamine 20 mg/kg.
**p < 0.01 in comparison to the Balb/c control (saline) group, # p < 0.05, in comparison to the C57BL/6 control (saline) group.
Fig. 7.
The time spent in the closed arms (sec) in the adult mice subjected to a single dose of neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. **p < 0.01 in comparison to the Balb/c control (saline) group, # p < 0.05 in comparison to the C57BL/6 control (saline) group. Statistical analysis included a two-way ANOVA followed by Tukey’s HSD test.
Repeated dose ketamine application
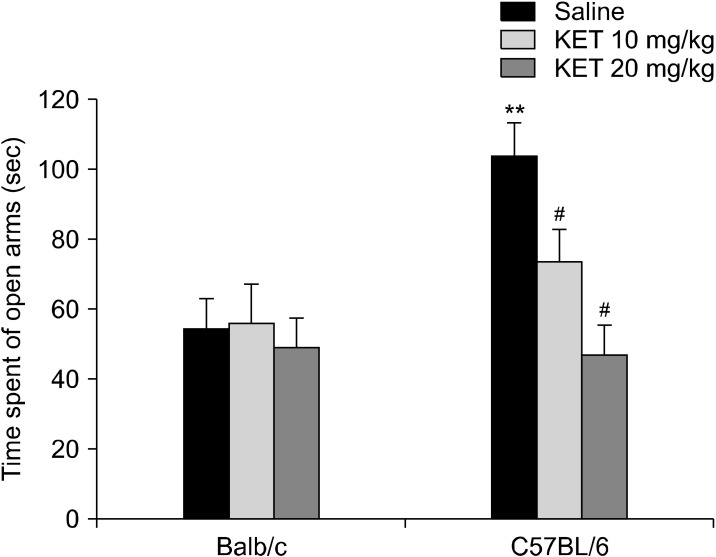
A significant difference was observed for the time spent in the open arms (H[5, n = 63] = 17.54, p < 0.01) (Fig. 8), the number of open-arm entries (H[5, n = 63] = 28.66, p < 0.001) and total arm entries (H[5, n = 63] = 38.46, p < 0.001) (Table 4). The Balb/c control mice spent less time in the open-arms in comparison to the C57BL/6 control mice (p < 0.01). In C57BL/6 mice, treatment with 10 mg/kg and 20 mg/kg ketamine decreased the open-arm time (p < 0.05). The C57BL/6 control mice had increased numbers of open-arm entries and total arm entries in comparison to the Balb/c control mice (p < 0.001). In the C57BL/6 mice, treatment with 10 and 20 mg/kg ketamine decreased the number of open-arm entries and total arm entries in comparison to the C57BL/6 control mice (p < 0.01). In the EPM, a two-way ANOVA test showed that the time spent in the closed-arms was affected significantly by the drug (F[2,57] = 4.07, p < 0.05) and strain X drug interaction (F[2,57] = 3.44, p < 0.05), whereas a strain effect was not confirmed (F[1,57] = 3.09, p > 0.05) (Fig. 9). The C57BL/6 control mice spent less time in the closed-arms in comparison to the Balb/c control mice (p < 0.05). In the C57BL/6 mice, treatment with 20 mg/kg ketamine increased the closed-arms in comparison to the C57BL/6 control mice (p < 0.05). Significant strain effect (F[1,57] = 34.35, p < 0.001), drug effect (F[2,57] = 8.23, p < 0.01) and strain X drug interaction effect (F[2,57] = 3.79, p < 0.05) were confirmed for the number of head dipping occasions (Table 4). the C57BL/6 control mice had increased numbers of head dipping occasions in comparison to the Balb/c control mice (p < 0.001). the C57BL/6 mice with treatment of 20 mg/kg had decreased numbers of head dipping occasions in comparison to the C57BL/6 control mice (p < 0.001). A significant difference was not observed for the latency to enter the open arm of the EPM (H[5, n = 63] = 8.8, p > 0.05).
Fig. 8.
The time spent in the open arms (sec) of the elevated plus maze in the adult mice subjected to repetitive doses of neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. **p < 0.01 in comparison to the Balb/c control (saline) group, # p < 0.05 in comparison to the C57BL/6 control (saline) group. Mann−Whitney Utest.
Table 4.
The behaviours in the elevated plus maze for adult mice subjected to repetitive doses of neonatal ketamine exposure
| The elevated plus maze | Balb/c | C57BL/6 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Saline (n = 13) | KET10 (n = 11) | KET20 (n = 9) | Saline (n = 10) | KET10 (n = 10) | KET20 (n = 10) | ||
| Number entries of in the open arm | 4.6 ± 0.5 | 5.5 ± 0.8 | 4.5 ± 0.4 | 18.3 ± 2.9*** | 7.2 ± 0.8 | 5.9 ± 0.7## | |
| Number of total arm entries | 11.5 ± 0.9 | 12.0 ± 1.2 | 10.4 ± 0.6 | 33.3 ± 3.9*** | 16.9 ± 0.8 | 17.6 ± 1.2## | |
| Enter latency to open arm(s) | 11.6 ± 3.2 | 17.8 ± 5.3 | 16.3 ± 4.9 | 6.6 ± 1.9 | 14.1 ± 2.5 | 38.9 ± 13.0 | |
| The number of head dipping | 3.3 ± 0.8 | 3.7 ± 0.8 | 2.2 ± 0.4 | 10.6 ± 0.9*** | 9.1 ± 1.4 | 4.0 ± 1.1### | |
Values are presented as mean ± standard error of the mean.
KET10, ketamine 10 mg/kg; KET20, ketamine 20 mg/kg.
***p < 0.001 in comparison to the Balb/c control (saline) group, ## p < 0.01, ### p < 0.001 in comparison to the C57BL/6 control (saline) group.
Fig. 9.
Time spent in the closed arms of the elevated plus maze in the adult mice subjected to repetitive doses of neonatal ketamine exposure. The data are expressed as mean ± standard error. KET, ketamine. *p < 0.05 in comparison to the Balb/c control (saline) group, # p < 0.05 in comparison to the C57BL/6 control (saline) group. Statistical analysis included a two-way ANOVA followed by Tukey’s HSD test.
DISCUSSION
There were no significant differences in the anxiety-like behaviours and locomotor activity in two different strains of mice with the single 10 mg/kg dose of ketamine. However, in the C57BL/6 mice, both the single and repetitive 20 mg/kg doses of ketamine and the 10 mg/kg repetitive dose of ketamine increased anxiety-like behaviour in the EPM test and OF test.
Although, in the C57BL/6 mice, both the single and repetitive 20 mg/kg doses of ketamine increased anxiety-like behaviour, surprisingly, the Balb/c mice did not experience an effect on anxiety-like behaviour. In previous studies, long-term behavioural changes in rodents treated with ketamine during their early development period have been evaluated to a limited degree [6,17]. In a recent study, Sampaio et al. [17] reported that neonatal ketamine exposure did not alter locomotor activity and anxiety-related parameters in the OF test by a single neonatal ketamine (20 mg/kg) exposure on PD7 in rats. Their study used rats and only the open-field test, whereas our study used both the EPM and OF tests to investigate anxiety-related behaviours. Moreover, Walf and Frye [18] proposed using the EPM test in rats due to both its reliability and validity in detecting anxiety-related behaviours. In studies, generally, the effects of neonatal exposure to ketamine on cognitive functions and locomotor activity were evaluated of the adulthood period, but not anxiety-like behaviour. Furthermore, current research results suggest that ketamine administration during the early development period induced apoptosis in the brain. For example, Scallet et al. [11] found that, in the medial of the amygdala and in the dorsolateral thalamus, ketamine causes apoptosis in rates that received injections by both single and repetitive doses of 20 mg/kg. However, they did not report such an effect at the dose of 10 mg/kg injection. Soriano et al. [19] observed apoptosis in the frontal cortex and hippocampus on PD7 in rats that were administered repetitive ketamine injections at the dose of 20 mg/kg. Zou et al. [10] found the potential neurotoxic effects of ketamine that was administered by doses of 10, or 20 mg/kg using single or multiple injections at 2-hours intervals on PD7 in rats. Moreover, cognitive deficits were found on PDs 5 and 6 in Rhesus monkeys exposed to ketamine anaesthesia for 24 hours [20]. Current research results suggest that ketamine-induced apoptosis has a dose-, sex-, time-, and strain-dependent profile [21,22]. Perhaps, the increase in the anxiety-like behaviours in our study may have been due to apoptosis in the amygdala or other regions.
In our previous study shown that different doses of ketamine application has also different effects in different strains of adult mice [16]. In this study, we investigated the effect of acute ketamine in adult mice. We found that a subanesthetic dose of ketamine decreased exploratory locomotion in C57BL/6 adult mice and increased exploratory locomotion in Balb/c adult mice. In our another study, NMDA receptor hypofunction was induced 7−10 days postnatally using MK-801 in Balb/c and C57BL/6 mice [15]. In this study, MK-801 decreased anxiety-like behaviors in BALB/c mice while increased anxiety-like behaviors in C57BL/6 mice. However, in the present study, ketamine increased only anxiety-like behaviour in C57BL/6 pups but not Balb/c mice. A non-competitive NMDA receptor antagonist, MK-801 binds more selectively to NMDA receptors with compared to ketamine as known. It is reported that ketamine and MK-801 have different physico-chemical properties (e.g., ketamine is smaller and more strongly cationic than MK-801) [23]. Moreover, ketamine can act through NMDA receptors as well as the monoaminergic systems, cholinergic systems and opiate receptors [24]. This pharmacological profile of ketamine makes it difficult to explain ketamine-induced behaviors only by NMDA receptor blockade. Taken together, it suggest that ketamine effects on anxiety-like related behaviors may depend on several factors, as the duration of ketamine administration, doses and tested strain, sex.
It is known that ketamine is a racemic mixture of equal amounts of (S)- and (R)- ketamine enantiomers. In studies, subanesthetic administration of pure S- and R-ketamine in healthy individuals reveals regional changes in brain metabolism, each associated with different behavioural responses [25]. It is reported that (S)-ketamine binds with higher affinity to the phencyclidine binding site of the NMDA receptor in human brain than (R)-ketamine [26]. In the literature, it not information about (S)- and (R)- ketamine affinity to the phencyclidine binding site of the NMDA receptor in these two strains. However, in these two mouse strains were shown both anatomical and biological differences in the brain [27,28]. Previous study shown that the hippocampal volume of C57BL/6 pups were significantly larger than of Balb/c in the PD7 [28]. C57BL/6 and Balb/c mice were shown to have not only different anxiety-like behavior and stress response but also levels of monoamines, their metabolites and related proteins [29 -31]. Yochum et al. [30], found that in the Balb/c mice were decreased serotonergic activity and increased dopaminergic activity compared to C57BL/6 mice in the hippocampus on both PD3 and PD10. Zolotarev et al. [32], reported that NMDA, 5HT2A and nicotinic Ach receptor density was higher in adult C57BL/6 mice than in adult Balb/c mice in the prefrontal cortex and hippocampus. In another study have observed that ketamine application alters NMDAR subunit gene expression in developing rats brain [33]. Ketamine application increased NMDAR subunit GR1N1, GRIN2A, and GRIN2C mRNA expression in the frontal cortex. It has been suggested that ketamine administration -induced neurotoxicity is caused by GRIN1 up-regulation. Taken together, the high NMDA receptor level in C57BL/6 mice may have increased susceptibility to neurotoxicity induced by ketamine administration.
In our study, PD7 was selected as the injection time. In the literature, it is reported that PD7 in rodents is important for brain development [34]. In particular, it is considered to be a period of qualitative and quantitative changes on the neurotransmitter systems [35 -37]. During development in rats, particularly on PDs 7−14, the brain is highly sensitive to the toxic effects of NMDA receptor modulation. It is known that there is a relationship between NMDA receptor blockade and apoptosis in the early period of brain development [12,38]. Additionally, in the long-term, the effects on behavioural changes by ketamine may involve multi-system changes, including the glutamatergic and serotonergic systems [39]. It is reported that the developmental processes of neurotransmitter systems is different among such strains [30]. The developmental differences of neurochemical systems may explain the behavioural differences in two strains that were shown by ketamine treatment [24,30]. Perhaps, the effects of ketamine in the Balb/c mice were not observed to be significant because the development processes of the neurotransmitter systems were not the same between the strains [30,33].
In conclusion, single and repetitive ketamine administrations in the neonatal period may affect adult-term anxiety-like and exploratory behaviours. These effects of ketamine may be affected by hereditary factors. It should not be ignored that ketamine may affect anxiety-like behaviour in adulthood when it is used in paediatric patients. Moreover, further comprehensive research is needed to resolve confusion regarding the doses, developmental stages and durations of ketamine administration in the neonatal period that are safe for paediatric patients.
Acknowledgments
Funding for this study was provided by the Scientific Research Office of Cukurova University (I.U BAP, Project numbers: TF2013BAP22).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Kübra Akillioglu. Experimental conduct: Kübra Akillioglu and Mustafa Karadepe. Data analysis: Kübra Akillioglu and Mustafa Karadepe. Writing−manuscript: Kübra Akillioglu. All authors read and approved the manuscript.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 4.Silvestre JS, Nadal R, Pallarés M, Ferré N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- 5.Pitychoutis PM, Thelen C, Sens J, Mauch J, Pandit R. Sex differences in the neurochemical and behavioral antidepressant-like effects of ketamine in mice. Biol Psychiatry. 2016;79(9 Suppl):154s. [Google Scholar]
- 6.Huang L, Hayes S, Yang G. Long-lasting behavioral effects in neonatal mice with multiple exposures to ketamine-xylazine anesthesia. Neurotoxicol Teratol. 2017;60:75–81. doi: 10.1016/j.ntt.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawabe K. Effects of chronic forced-swim stress on behavioral properties in rats with neonatal repeated MK-801 treatment. Pharmacol Biochem Behav. 2017;159:48–54. doi: 10.1016/j.pbb.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Li JT, Zhao YY, Wang HL, Wang XD, Su YA, Si TM. Long-term effects of neonatal exposure to MK-801 on recognition memory and excitatory-inhibitory balance in rat hippocampus. Neuroscience. 2015;308:134–143. doi: 10.1016/j.neuroscience.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Min JA, Lee HJ, Lee SH, Park YM, Kang SG, Park YG, et al. RORA polymorphism interacts with childhood maltreatment in determining anxiety sensitivity by sex: a preliminary study in healthy young adults. Clin Psychopharmacol Neurosci. 2017;15:402–406. doi: 10.9758/cpn.2017.15.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, et al. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr Anaesth. 2002;12:770–774. doi: 10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- 14.Fowler SC, Zarcone TJ, Vorontsova E. Haloperiodol-induced microcatalepsy differs in CD-1, BALB/c, and C57BL/6 mice. Exp Clin Psychopharmacol. 2001;9:277–284. doi: 10.1037//1064-1297.9.3.277. [DOI] [PubMed] [Google Scholar]
- 15.Akillioglu K, Binokay S, Kocahan S. The effect of neonatal N-methyl-D-aspartate receptor blockade on exploratory and anxiety-like behaviors in adult BALB/c and C57BL/6 mice. Behav Brain Res. 2012;233:157–161. doi: 10.1016/j.bbr.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Akillioglu K, Melik EB, Melik E, Boga A. Effect of ketamine on exploratory behaviour in BALB/C and C57BL/6 mice. Pharmacol Biochem Behav. 2012;100:513–517. doi: 10.1016/j.pbb.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio TB, de Oliveira LF, Constantino LC, Costa AP, Poluceno GG, Martins WC, et al. Long-term neurobehavioral consequences of a single ketamine neonatal exposure in rats: effects on cellular viability and glutamate transport in frontal cortex and hippocampus. Neurotox Res. 2018;34:649–659. doi: 10.1007/s12640-018-9927-x. [DOI] [PubMed] [Google Scholar]
- 18.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL, et al. Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology. 2010;112:1155–1163. doi: 10.1097/ALN.0b013e3181d3e0c2. [DOI] [PubMed] [Google Scholar]
- 20.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damazio Pacheco F, Canever L, Antunes Mastella G, Gomes Wessler P, Kunz Godoi A, Hubbe I, et al. Effects of ketamine on prepubertal Wistar rats: implications on behavioral parameters for Childhood-Onset Schizophrenia. Int J Dev Neurosci. 2019;79:49–53. doi: 10.1016/j.ijdevneu.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res. 2016;312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindefors N, Barati S, O'Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res. 1997;759:205–212. doi: 10.1016/s0006-8993(97)00255-2. [DOI] [PubMed] [Google Scholar]
- 25.Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 26.Oye I, Hustveit O, Maurset A, Moberg ER, Paulsen O, Skoglund LA. The chiral forms of ketamine as probes for NMDA receptor functions in humans. In: Kameyama T, Nabeshima T, Domino EF, editors. NMDA receptor related agents. BPP Books; Kameyama T, Nabeshima T, Domino EF, editors. NMDA receptor related agents. Biochemistry, pharmacology and behavior. Ann Arbor (MI): 1991. pp. 381–390. [Google Scholar]
- 27.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 28.Flood ZC, Engel DL, Simon CC, Negherbon KR, Murphy LJ, Tamavimok W, et al. Brain growth trajectories in mouse strains with central and peripheral serotonin differences: relevance to autism models. Neuroscience. 2012;210:286–295. doi: 10.1016/j.neuroscience.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Narkevich VB, Kudrin VS, Klodt PM, Pokrovskiĭ AA, Kozlovskaia MM, Maĭskiĭ AI, et al. [Effects of heptapeptide selank on the content of monoamines and their metabolites in the brain of BALB/C and C57Bl/6 mice: a comparative study] Eksp Klin Farmakol. 2008;71:8–12. [PubMed] [Google Scholar]
- 30.Yochum CL, Medvecky CM, Cheh MA, Bhattacharya P, Wagner GC. Differential development of central dopaminergic and serotonergic systems in BALB/c and C57BL/6J mice. Brain Res. 2010;1349:97–104. doi: 10.1016/j.brainres.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Anderzhanova EA, Bächli H, Buneeva OA, Narkevich VB, Medvedev AE, Thoeringer CK, et al. Strain differences in profiles of dopaminergic neurotransmission in the prefrontal cortex of the BALB/C vs. C57Bl/6 mice: consequences of stress and afobazole. Eur J Pharmacol. 2013;708:95–104. doi: 10.1016/j.ejphar.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Zolotarev YA, Kovalev GI, Kost NV, Voevodina ME, Sokolov OY, Dadayan AK, et al. Anxiolytic activity of the neuroprotective peptide HLDF-6 and its effects on brain neurotransmitter systems in BALB/c and C57BL/6 mice. J Psychopharmacol. 2016;30:922–935. doi: 10.1177/0269881116660705. [DOI] [PubMed] [Google Scholar]
- 33.Shi Q, Guo L, Patterson TA, Dial S, Li Q, Sadovova N, et al. Gene expression profiling in the developing rat brain exposed to ketamine. Neuroscience. 2010;166:852–863. doi: 10.1016/j.neuroscience.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph LM, Sengelaub DR. Critical period for estrogen-dependent motoneuron dendrite growth is coincident with ERa expression in target musculature. Dev Neurobiol. 2013;73:72–84. doi: 10.1002/dneu.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110:926–941. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 38.Cheung HM, Yew DTW. Effects of perinatal exposure to ketamine on the developing brain. Front Neurosci. 2019;13:138. doi: 10.3389/fnins.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]