Herpetic lesions are a significant problem, and they are difficult to control with therapeutics. Our studies show that the severity of herpetic lesions in a mouse model can be diminished by changing the diet to include increased levels of SCFA, which act to inhibit the involvement of inflammatory T cells. We suggest that changing the diet to include higher levels of SCFA might be a useful approach to reducing the impact of recurrent herpetic lesions in humans.
KEYWORDS: HSV-1, sodium propionate, proinflammatory, anti-inflammatory immune cells
ABSTRACT
This report evaluates a dietary manipulation approach to suppress the severity of ocular infections caused by herpes simplex virus infection. The virus causes chronic damage to the cornea that results from a T-cell-orchestrated inflammatory reaction to the infection. Lesion severity can be limited if cells with regulatory activity predominate over proinflammatory T cells and nonlymphoid inflammatory cells. In this report, we show that this outcome can be achieved by including the short-chain fatty acid (SCFA) salt sodium propionate (SP) in the drinking water. Animals given the SP supplement developed significantly fewer ocular lesions than those receiving no supplement. Corneas and lymphoid organs contained fewer CD4 Th1 and Th17 T cells, neutrophils, and macrophages than those of controls, but a higher frequency of regulatory T cells (Treg) was present. The inclusion of SP in cultures to induce CD4 T cell subsets in vitro reduced the magnitude of Th1 and Th17 responses but expanded Treg induction. Dietary manipulation was an effective approach to limit the severity of viral immuno-inflammatory lesions and may be worth exploring as a means to reduce the impact of herpetic lesions in humans.
IMPORTANCE Herpetic lesions are a significant problem, and they are difficult to control with therapeutics. Our studies show that the severity of herpetic lesions in a mouse model can be diminished by changing the diet to include increased levels of SCFA, which act to inhibit the involvement of inflammatory T cells. We suggest that changing the diet to include higher levels of SCFA might be a useful approach to reducing the impact of recurrent herpetic lesions in humans.
INTRODUCTION
As the coronavirus disease 2019 (COVID-19) pandemic reminds us, virus infections have major consequences, especially if there are no effective vaccines or antiviral therapies to control them. Except for varicella-zoster virus, none of the eight human herpesviruses are controllable by vaccines, and antiviral therapies, when available, are not fully effective. Perhaps the best-known human herpesviruses are herpes simplex virus 1 (HSV-1) and HSV-2, which mostly cause surface lesions on the face or genitalia. These usually manifest as recurrences of productive replication from persistent latent infection in the peripheral nerve ganglia (1). Of particular concern are recurrent lesions in the eye, since they often culminate in severe vision impairment (2, 3). Recurrent lesions in the cornea are in large part immunopathological and are managed with anti-inflammatory drugs, but there is a need for alternative therapies. Investigations, largely from studies in animal models, have defined many of the cellular and mediator events involved in damaging the cornea (4–6). These studies have identified steps that could be counteracted to minimize tissue damage. It is evident that lesion severity is most severe when the inflammatory T cell population is dominated by CD4+ Th1 and Th17 T cells and is ameliorated if T cells with regulatory function (Treg) are expanded or activated (7, 8). Procedures, such as drug therapy, that succeed in adjusting the representation of proinflammatory versus counterinflammatory cell types and their products are useful for diminishing lesion severity (9, 10). Other ways of achieving cellular rebalancing could be to exploit major metabolic differences employed by cells with different immune functions. This approach has largely been explored in the autoimmunity and cancer fields (11–13), but it also represents a logical way of managing lesions caused by infectious agents (14, 15). Our group has reported that differences in glucose metabolism between proinflammatory T cells and those with regulatory function (Treg) can be exploited therapeutically. Thus, therapy with 2-deoxy-glucose (2DG), which diminishes proinflammatory T cells but has no effect on Treg activities, can limit the extent of ocular lesions following HSV infection of mice (16).
Additional approaches that could benefit from metabolic differences among the cell types reacting to infections merit evaluation. A potentially useful strategy is to exploit differences in fatty acid metabolism, since this metabolism can be readily modulated by dietary manipulation. Accordingly, proinflammatory T cells (Th1 and Th17 cells) mainly take in long-chain fatty acids (12, 17); these are metabolized first in the cytoplasm to form acyl coenzyme A (acyl-CoA), which enters the mitochondrial tricarboxylic acid cycle and acts as a source of energy via the oxidative phosphorylation process. On the other hand, anti-inflammatory cell types are more dependent on short-chain fatty acids (SCFA), which can enter the mitochondria by diffusion, and similarly participate in energy generation (18). The SCFA mainly come from bacterial breakdown of dietary complex carbohydrates such as inulin and pectin, a process that becomes more prominent if the diet includes high levels of fiber (19, 20). In the autoimmune-disease field, feeding high-fiber diets, as well as incorporating additional SCFA in the diet, has been shown to limit the extent of lesions. These approaches favor the generation of Treg and counterinflammatory cytokines, thus diminishing lesion severity (12, 13).
The dietary manipulation approach to controlling inflammatory lesions caused by viral infections has so far received minimal attention. In the present report, we address the issue of supplementing the diet with additional SCFA in a test system in which HSV-1 infection causes severe tissue-damaging inflammatory reactions in the cornea of the eye. Our data show that animals which received a standard mouse chow diet supplemented with sodium propionate (SP) developed significantly diminished ocular lesions compared to animals fed the control diet. The lesions in the SP recipients showed reduced angiogenesis, a necessary step in stromal keratitis (SK) pathogenesis (21), and had fewer inflammatory Th1 and Th17 T cells, However, SP recipients showed an increased representation of Treg compared to those fed the control diet. Furthermore, feeding of SP decreased the numbers of nonlymphoid inflammatory cells (neutrophils and macrophages) in lesions. The pattern of results observed in vivo was reflected by in vitro analyses where T cells of various phenotypes were induced from naive T cell precursors in a differentiating medium or in the same medium that contained additional levels of SP. The inclusion of SP significantly enhanced the Treg response and reduced the induction of proinflammatory T cells. Taken together, our results indicate that the severity of virus-induced inflammatory lesions can be diminished by dietary manipulation. Additional approaches to achieve such effects, which we are currently exploring, merit further evaluation.
RESULTS
Effect of dietary supplementation with sodium propionate on the severity of herpetic ocular lesions.
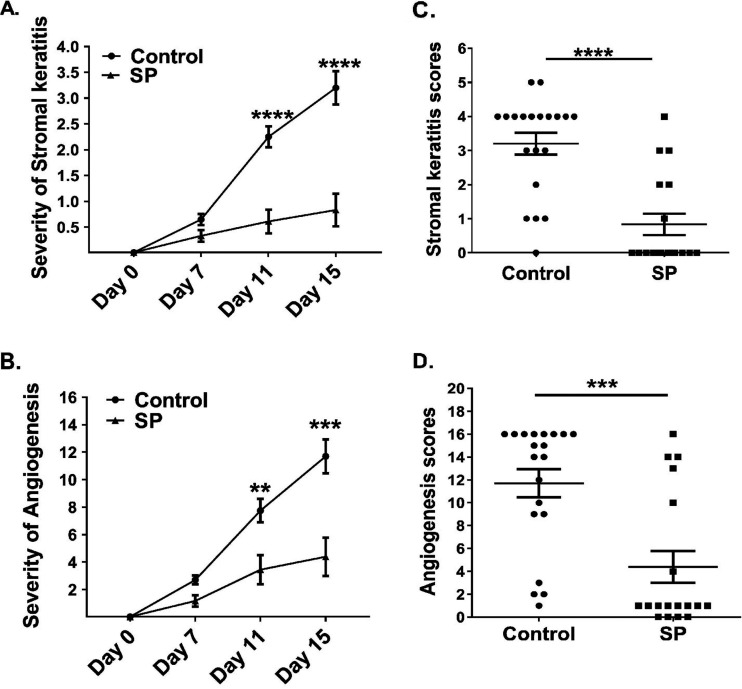
To observe the effect of SP on the pathogenicity of HSV-1 ocular infection, lesions were compared in a group of animals that received SP in the drinking water and a group fed the same diet, but without the SP supplement. Animals were evaluated daily after ocular infection with virus for signs of lesions, which appeared initially as mild angiogenesis and corneal cloudiness and eventually manifested as marked corneal scarring. Lesions, in the form of mild angiogenesis, first became evident around day 4 in the eyes of some mice from the control group but not until day 6 in a minority of the mice that received SP (data not shown). Peak lesion severity in the control group eyes occurred on day 15 in the experiment for which results are shown in Fig. 1; the mean lesion score was 3.2, with 19 of 20 eyes showing detectable lesions (lesion score, ≤1). Moreover, almost 75% of eyes showed a lesion score of 3 or greater. In the SP recipients, lesions at day 15 had a mean score of 0.8, with only 6 of 18 eyes showing detectable lesions (score, ≤1). In this experiment, only 3 of 18 eyes showed notable lesions (score, ≤3) and pathological angiogenesis (Fig. 1A to D). Three additional experiments of the same design involving 12 to 20 eyes per group per experiment showed similar patterns of results. Thus, our results clearly show that supplementing the diet with SP markedly reduced the severity of ocular lesions that resulted from HSV infection.
FIG 1.
Feeding SP reduces the development and severity of SK and angiogenesis after HSV-1 infection. SK and angiogenesis were recorded for each eye of C57BL/6 mice orally fed SP (n, 9 mice) or not (control group; n, 10 mice) after infection with HSV-1. (A, B) The severity of SK (A) and angiogenesis (B) were recorded at days 0, 7, 11, and 15 p.i. (C, D) The severity of SK (C) and angiogenesis (D) was recorded for individual eyes at the 15th day p.i. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
In one experiment, virus levels were compared in ocular swabs taken from SP-fed and control animals at different times after infection. There were no significant differences between the two groups at days 2 and 4, and by day 7, virus was no longer present in either group (data not shown).
Supplementing the diet with SP changes the nature of ocular inflammatory reactions to infection.
Corneas were collected at 15 days postinfection (p.i.) from animals in both the SP supplement-fed and regular-diet groups in order to compare the magnitudes and natures of the cellular responses to HSV-1 infection. Individual samples of the corneas were compared for their cellular compositions.
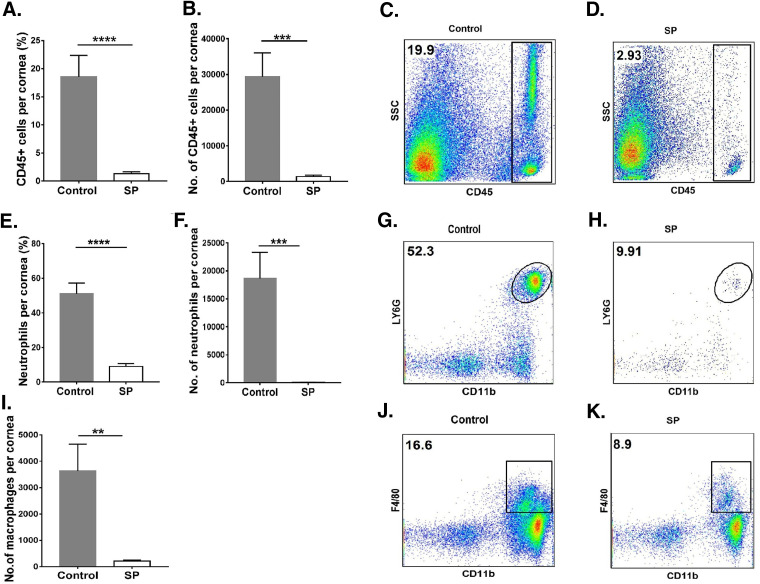
For the control group, the average total-leukocyte level in the cornea was 21.7-fold (P ≤ 0.001) higher than that in the SP-fed group (Fig. 2A to D). Levels of neutrophils were reduced by 210-fold (P ≤ 0.001) in the SP-fed group from those in controls (Fig. 2E to H). The numbers of macrophages were reduced by 17-fold (P ≤ 0.01) in the corneas of the SP-fed group (Fig. 2I to K) from those in control corneas.
FIG 2.
Supplementation of the diet with SP reduces the innate inflammatory response in the cornea. C57BL/6 mice that were orally fed SP or used as controls were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A, B) Total-leukocyte response in corneal tissue, shown as frequencies (A) and numbers (B) of leukocytes. (C, D) Representative FACS plots showing frequencies of total CD45+ cells infiltrating corneal tissue at 15 days p.i. (E, F) Neutrophil accumulation in the cornea, shown as frequencies (E) and numbers (F) of neutrophils. (G, H) FACS plots revealing frequencies of neutrophil (CD45+ CD11b+ Ly6G+) infiltration in corneas. (I) Total numbers of macrophages present in corneas. (J, K) FACS plots showing frequencies of macrophages (CD45+ CD11b+ F4/80+) infiltrating corneas. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
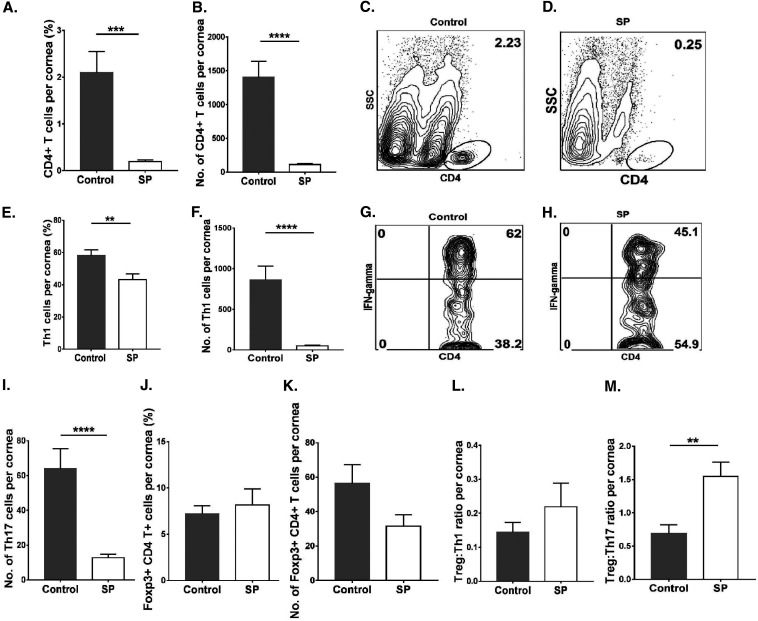
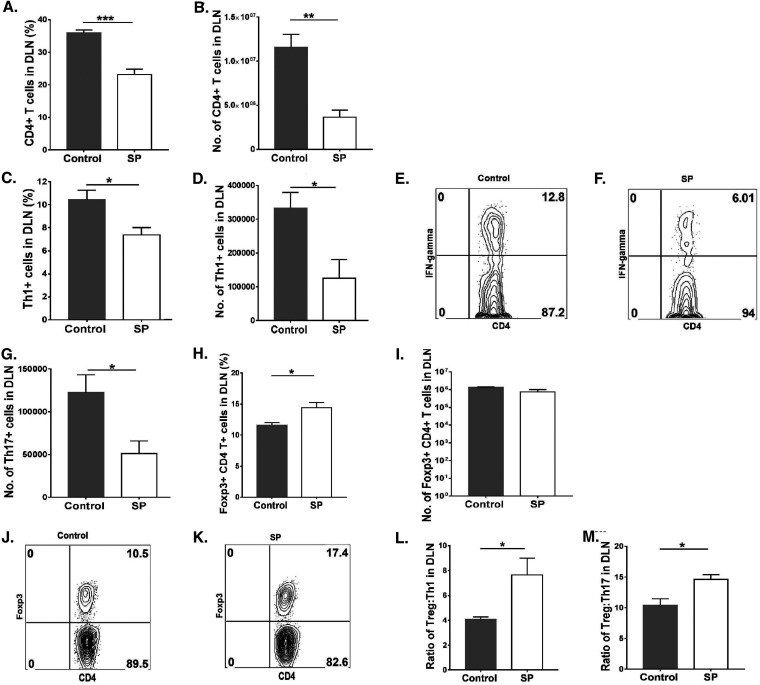
Levels of CD4+ T cells in corneal tissue were 12.3-fold (P ≤ 0.0001) higher in the control group than in the SP-fed group (Fig. 3A to D). The mean numbers of Th1 and Th17 cells infiltrating the corneas of control animals were 16.9-fold (P ≤ 0.0001) and 5.3-fold (P ≤ 0.0001) higher, respectively, than those in the SP-fed group (Fig. 3E to I). The numbers of Treg in the SP- and control-diet-fed mice were also compared, but these were not significantly different (Fig. 3J and K), likely because of a large variation in lesion severity between samples. However, when samples with positive SK responses in both groups were compared, the Treg/Th17 cell and Treg/Th1 cell ratios were 2.3-fold (P ≤ 0.01) and 1.5-fold higher, respectively, in the SP-fed group than in controls (Fig. 3L and M).
FIG 3.
Oral SP feeding resulted in diminished proinflammatory T cell responses in corneas. Orally SP-fed and control C57BL/6 mice were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A, B) Total CD4+ T cell frequencies (A) and numbers (B) in corneal tissue. (C, D) Representative FACS plots showing frequencies of CD4+ T cell infiltration in corneal tissue. (E and F) Th1 cell frequencies (E) and numbers (F) in corneas. (G, H) FACS plots representing frequencies of Th1 (CD4+ IFN-γ+) cell infiltration in corneas. (I) Total numbers of Th17 (CD4+ IL-17A+) cells in corneas. (J, K) Treg (CD4+ Foxp3+) frequencies (J) and numbers (K) in corneas. (L, M) Treg/Th1 cell (L) and Treg/Th17 cell (M) ratios in corneas. For Th1 and Th17 cell enumeration, stimulation was performed for 4 h with PMA-ionomycin. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
We can conclude that at the corneal level, feeding SP to HSV-1-infected mice inhibited inflammatory responses and changed the cellular balance within inflamed tissue.
Dietary supplementation with SP changes the nature of immune cell responses in lymphoid organs after HSV infection.
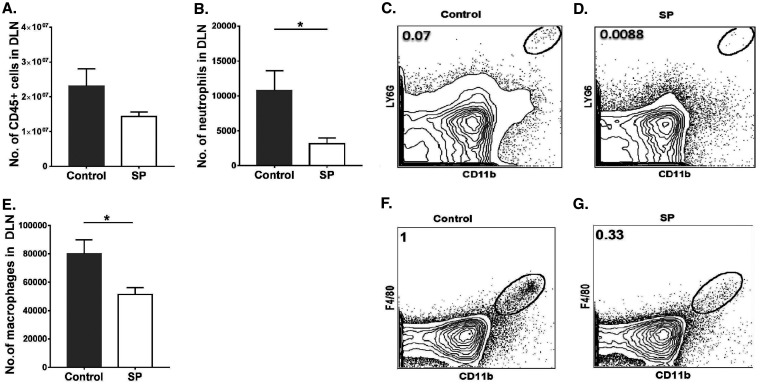
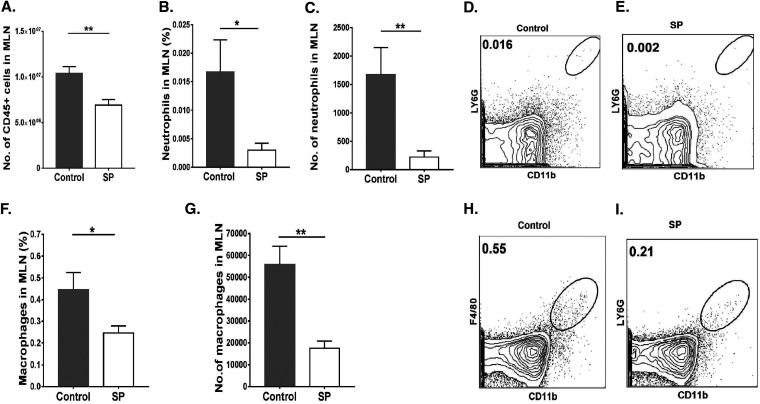
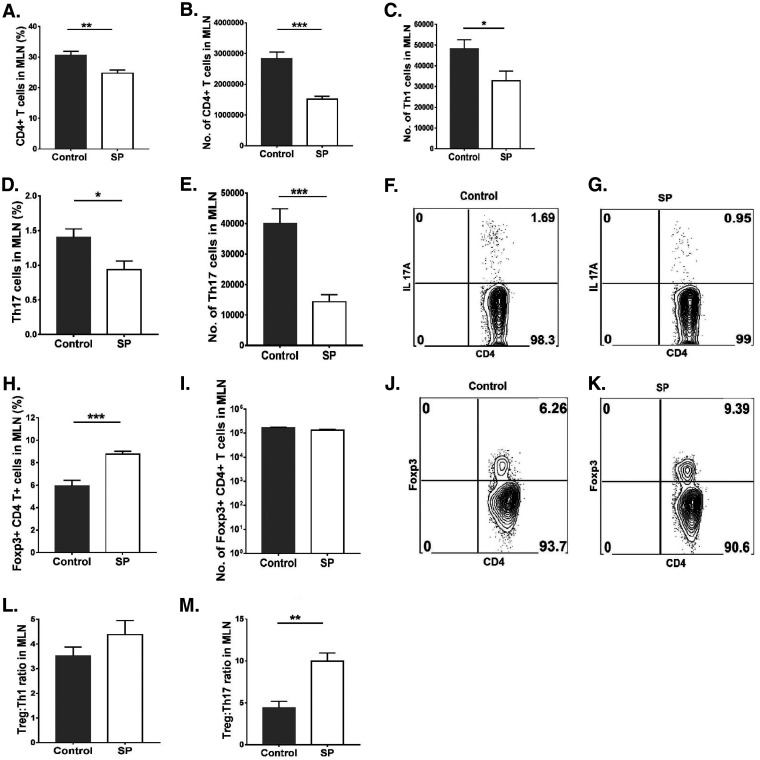
Lymphoid organs were also collected from SP-fed and control mice at 15 days p.i. in order to measure and compare the magnitudes and natures of cellular responses to HSV infection. We focused on lymph nodes draining the eye as well as the mesenteric lymph node (MLN) draining the location of SP administration. In the ocular draining lymph node (DLN), the mean numbers of neutrophils and macrophages were 3.4- and 1.5-fold lower (P ≤ 0.05), respectively, in the SP-fed group than in the control group (Fig. 4A to G). With regard to CD4+ T cells in the DLN, SP recipients showed a 3-fold reduction (P ≤ 0.01) from levels in controls (Fig. 5A and B), and the numbers of Th1 and Th17 T cells were reduced by 2.6-fold (P ≤ 0.05) and 2.4-fold (P ≤ 0.05), respectively (Fig. 5C to G). The numbers of Treg in the corneas of the SP fed group were reduced by 1.7-fold from those in the control group (not significant) (Fig. 5H to K). However, the ratios of total Treg to Th1 cell numbers and total Treg to Th17 cell numbers were significantly (P ≤ 0.05) higher (1.8- and 1.4-fold, respectively) in the SP-fed group than in controls (Fig. 5L and M). Thus, SP feeding reduced the frequency of proinflammatory cells and increased that of anti-inflammatory Treg.
FIG 4.
Mice fed SP presented diminished innate inflammatory responses in ocular DLN tissue samples. Orally SP-fed and control C57BL/6 mice were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A) Total numbers of leukocytes in the DLN. (B) Numbers of neutrophils accumulating in the DLN. (C, D) FACS plots showing the frequencies of neutrophils (CD45+ CD11b+ Ly6G+) in the DLN. (E) Numbers of macrophages in the DLN. (F, G) FACS plots revealing frequencies of macrophages (CD45+ CD11b+ F4/80+) in the DLN. Gating for neutrophils and macrophages in the DLN was carried out as described elsewhere (59, 60). Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
FIG 5.
Feeding SP decreases the level of proinflammatory T cells and enhances the magnitude of anti-inflammatory over proinflammatory cells in the ocular DLN. Orally SP-fed and control C57BL/6 mice were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A, B) Total CD4+ T cell frequencies (A) and numbers (B) in the DLN. (C, D) Th1 cell frequencies (C) and numbers (D) in the DLN. (E, F) FACS plots representing frequencies of Th1 (CD4+ IFN-γ+) cell infiltration in the DLN. (G) Total numbers of Th17 (CD4+ IL-17A+) cells in the DLN. (H, I) Treg (CD4+ Foxp3+) frequencies (H) and numbers (I) in the DLN. (J, K) FACS plots displaying frequencies of Treg (CD4+ Foxp3+) in the DLN. (L, M) Treg/Th1 cell (L) and Treg/Th17 cell (M) ratios in the DLN. For Th1 and Th17 cell enumeration, stimulation was performed for 4 h with PMA-ionomycin. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
A similar analysis of cells in the MLN revealed a mean 1.4-fold reduction in total leukocytes (Fig. 6A) and 7.5-fold and 3-fold reductions in neutrophils and macrophages, respectively, in the SP-fed group from levels in controls (Fig. 6 B to I). SP feeding also resulted in a reduction (P ≤ 0.001) in total CD4+ T cells (1.8-fold) (Fig. 7A and B). With regard to T cell subsets, Th1 and Th17 cells were reduced by 1.4- and 2.7-fold, respectively (P, ≤0.05 and ≤0.001) (Fig. 7C to G). There was also a difference in Treg numbers, with SP feeding increasing the Treg/Th17 cell ratio and, to a lesser extent, the Treg/Th1 cell ratio (not significant) (Fig. 7L and M). The number of Treg in the MLN was also 1.2-fold reduced in the SP-fed group, but not at a significant level (Fig. 7H to K). A similar pattern of cellular changes as a consequence of SP feeding was also observed in the spleen (data not shown).
FIG 6.
Oral feeding of SP reduces neutrophil and macrophage responses in MLN tissue samples. Orally SP-fed and control C57BL/6 mice were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A) Total numbers of leukocytes in the MLN. (B, C) Frequencies (B) and numbers (C) of neutrophils present in the MLN. (D, E) Representative FACS plots showing the frequencies of neutrophils (CD45+ CD11b+ Ly6G+) in the MLN. (F, G) Frequencies (F) and numbers (G) of macrophages in the MLN. (H, I) FACS plots showing frequencies of macrophages (CD45+ CD11b+ F4/80+) in the MLN. Gating for neutrophils and macrophages in the MLN was carried out as described elsewhere (59, 60). Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
FIG 7.
Supplementation with SP decreases proinflammatory T cells and changes the ratio of anti-inflammatory to proinflammatory cells in the MLN. Orally SP-fed and control C57BL/6 mice were infected with HSV-1, and FACS analysis was performed after 15 days p.i. (A, B) Frequencies (A) and numbers (B) of CD4+ T cells in the MLN. (C) Numbers of Th1 cells in the MLN. (D, E) Th17 cell frequencies (D) and numbers (E) in the MLN. (F, G) FACS plots of Th17 cells (CD4+ IL-17A+) in the MLN. (H, I) Treg (CD4+ Foxp3+) frequencies (H) and numbers (I) in the MLN. (J, K) FACS plots displaying frequencies of Treg (CD4+ Foxp3+) in the MLN. (L, M) Treg/Th1 cell (L) and Treg/Th17 cell (M) ratios in the MLN. For enumeration of Th1 and Th17 cells, stimulation was performed for 4 h with PMA-ionomycin. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Thus, a result of SP feeding was to change the representation of different cell types in lymphoid tissues, diminishing inflammatory cells more than Treg.
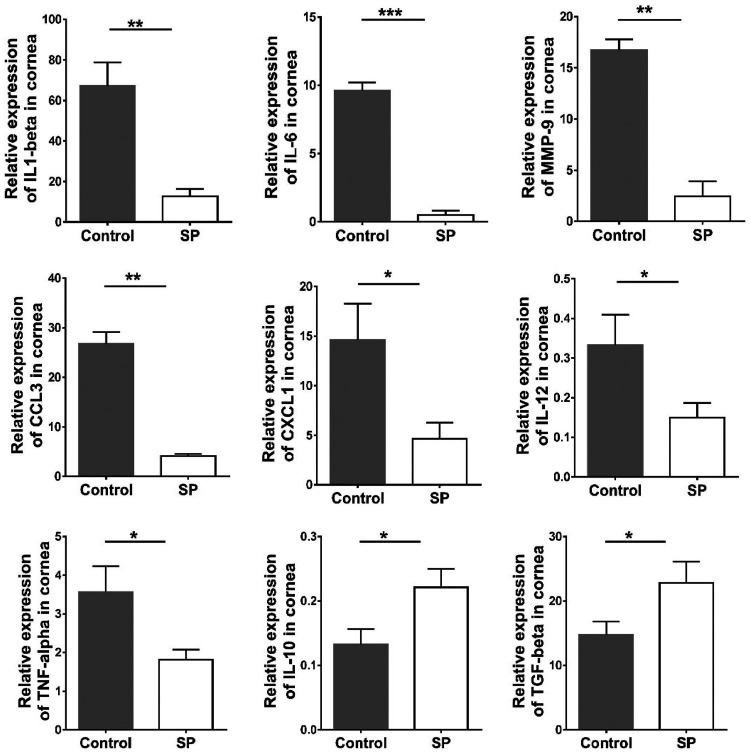
Feeding of SP diminishes proinflammatory cytokines and chemokines after HSV-1 infection.
To gain molecular insight into the effects of SP feeding on the inflammatory response to HSV infection, corneas with average responses from both the SP-fed and control groups were collected at day 15 p.i. and were processed to quantify the expression levels of several mRNAs by real-time PCR (RT-PCR). The focus was on some cytokines and chemokines previously shown to play critical roles in the pathogenesis of stromal keratitis or angiogenesis. In corneal samples from SP-fed mice, there were reductions in the expression levels of interleukin 6 (IL-6) (reduced 19-fold [P ≤ 0.001]), IL-12 (reduced 2.2-fold [P ≤ 0.05]), IL-1β (reduced 4-fold [P ≤ 0.01]), tumor necrosis factor alpha (TNF-α) (reduced 1.9-fold [P ≤ 0.05]), matrix metallopeptidase 9 (MMP-9) (reduced 6-fold [P ≤ 0.01]), CXCL1 (reduced 3-fold [P ≤ 0.05]), and CCL3 (reduced 6.5-fold [P ≤ 0.01]) from those in controls. However, IL-10 and transforming growth factor β (TGF-β) levels were increased in SP-fed samples by 1.7- and 1.5-fold (P ≤ 0.05), respectively. These data indicate that SP feeding reduced the expression of several SK proinflammatory cytokines and chemokines and increased the levels of two cytokines that perform anti-inflammatory functions (Fig. 8).
FIG 8.
Effects of dietary SP supplementation on cytokine and chemokine expression levels in the corneas of HSV-1-infected animals. Orally SP-fed and control C57BL/6 mice infected with HSV-1 were sacrificed on day 15 p.i., and corneas were collected for measurement of the mRNA expression levels of various cytokines (IL-1β, IL-12, IL-6, TNF-α, MMP-9, IL-10, and TGF-β) and chemokines (CXCL1/KC and CCL3) in pooled corneal samples by quantitative RT-PCR. Samples consisted of four corneas per group from control and SP-fed animals. Data represent mean results ± SEM. All data were analyzed by an unpaired Student t test, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Effects of SP on the differentiation in vitro of Th1 cells, Th17 cells, and Treg.
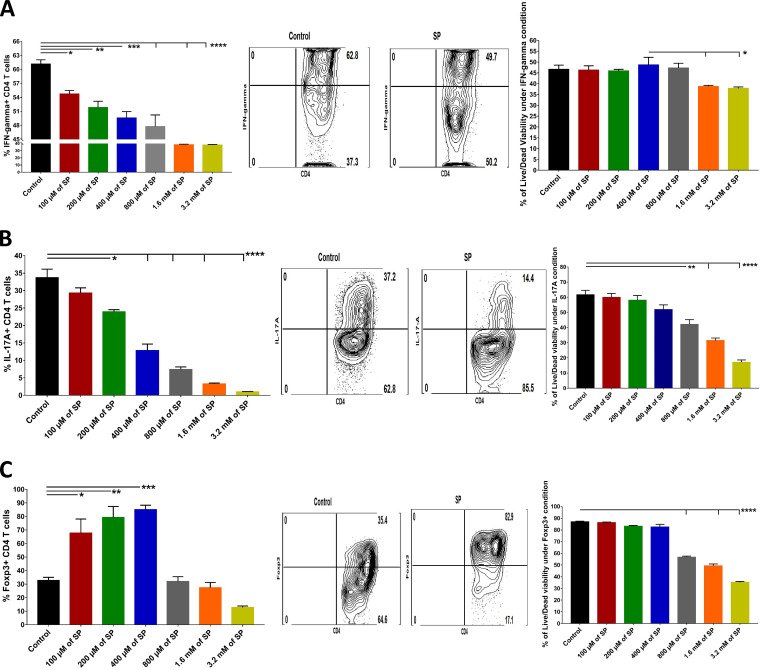
To measure the effects of SP on the induction of CD4 T cell subsets in vitro, naive splenocytes from DO11.10 RAG2−/− mice were cultured under conditions previously shown to induce either Th1 cells, Th17 cells, or Treg with test cultures containing different concentrations of SP. As shown in Fig. 9, the inclusion of SP at concentrations ranging from 100 μM to 3.2 mM inhibited the magnitudes of both Th1 and Th17 T cell responses in a dose-dependent manner, although above 400 μM SP, cell viability was diminished (Fig. 9A and B). With the Treg induction cultures, responses were increased when SP was included from 100 μM to 400 μM but declined at higher concentrations, with cells losing viability (Fig. 9C).
FIG 9.
Effects of addition of SP to induction cultures for Th1 cells, Th17 cells, and Treg. Splenocytes from RAG2–/– mice were cultured ex vivo in the presence of 1 μg/ml of anti-CD3/CD28 Abs as well as rIL-2 (100 U/ml) and TGF-β (0.5 ng/ml) for Treg induction, IL-12 (5 to 10 ng/ml) and anti-IL-4 (10 μg/ml) for Th1 induction, and IL-6 (25 ng/ml) and TGF-β (1 ng/ml) for Th17 induction, along with various concentrations of SP (100 μM to 3.2 mM). After 5 days, cells were harvested and analyzed for the expression of the respective immune phenotype. (A to C) Histogram analysis to measure the effects of SP on the differentiation of Th1 cells (CD4+ IFN-γ+) (A), Th17 cells (CD4+ IL-17A+) (B), and Treg (CD4+ Foxp3+) (C) and on their viability, presented as bar graphs (left) and FACS plots (right). For Th1 and Th17 cell enumeration, stimulation with PMA-ionomycin was performed for 4 h. Data represent mean results ± SEM. All data were analyzed by ANOVA, and significance levels were determined (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
The results of these in vitro assays demonstrate that the addition of SP to induction cultures serves to compromise inflammatory T cell induction but, at an appropriate dose, expands Treg.
DISCUSSION
In this report, we have explored a dietary manipulation approach to controlling the severity of an immuno-inflammatory reaction in the eye to HSV infection. We show that supplementing the diet with SP significantly diminished the severity of SK lesions, raising the prospect that dietary manipulation could represent an approach to diminishing the impact of herpetic lesions in the natural host, humans. Stromal keratitis in humans, as well as in the mouse model used to study the disease, is a lesion which is the consequence of an immunological reaction to the infection (1, 3, 4). A consensus notion is that the immunopathology is orchestrated by T cells but that additional cellular and molecular participants are also involved (7). The T cell orchestrators include CD4, Th1, and Th17 T cells, and perhaps CD8 T cells (22–25), but the tissue damage also involves nonlymphoid cells, particularly neutrophils and macrophages (26, 27). Of interest, the extent of lesion expression can be modulated by cells with regulatory function (Treg), as well as by some cytokines (28, 29). The most studied regulatory cells have been Foxp3+ CD4 T cells, but IL-10-producing T cells and some subtypes of macrophages (M2) may also control the extent of inflammation (29, 30). Hence, an objective of therapy and control is to diminish proinflammatory participants and to expand those components, such as Treg, that inhibit the inflammatory tissue-damaging process. As we show in this report, incorporating the SCFA salt sodium propionate into the diet is an effective way of achieving this objective. Accordingly, mice receiving the SP supplement had diminished SK lesions, and the nature of the ocular inflammatory responses was changed. Such lesions contained fewer neutrophils and macrophages, and the balance of T cell subsets present changed to reflect more representation of Treg relative to Th1 and Th17 cells. Additionally, in comparison to control diet recipients, mice fed the SP supplement had diminished expression of several inflammatory cytokines and chemokines and of some other protein components involved in inflammation.
Stromal keratitis is an important clinical problem in humans and is managed mainly by the use of anti-inflammatory drugs. Since herpetic infections are sustained indefinitely in infected hosts in the form of latency, periodic outbreaks of productive infection, often lesion producing, can be expected to occur. Decreasing the frequency and severity of these episodes, especially in critical locations such as the eye, represents a major objective for those involved in managing herpesvirus infections. Our current results and a previous report demonstrate that this objective can be achieved by making metabolic changes in the infected host (16). Thus, using a mouse model of SK, which, unfortunately, represents a response to primary infection rather than reactivation from latency, as is usually the case with human SK, we show that manipulating metabolism can result in diminished SK lesions. The previous observations had shown that inhibiting glucose utilization with the drug 2DG achieved this effect (16), but there were potential complications if mice were treated with 2DG when replicating virus was still present. In that circumstance, the infection could spread to the central nervous system (CNS) and result in herpes encephalitis (16). The results of the present investigation would seem to have even more promise for practical application. These results clearly show that SK lesions can be markedly diminished merely by supplementing the diet with the SCFA salt sodium propionate. The recipients of such a diet had significantly reduced lesions, likely the consequence of diminished proinflammatory T cell responses, which our group and others had demonstrated act as the usual orchestrators of ocular damage, although other cell types, such as neutrophils and macrophages, may be the actual mediators of the tissue damage (26, 31, 32). Management of the consequences of tissue damage using nutritional modification has so far received minimal investigation with microbial infections, but reports are attesting to the value of this approach. For example, a report showed that the innate neutrophil response of mice to influenza virus infection was diminished upon feeding of SCFA (33), and a second report showed that mice fed a high-fiber diet (which generates SCFA, as described below) resulted in diminished pulmonary inflammatory responses to respiratory syncytial virus infection in mice. This outcome was explained by elevated interferon responses protecting against the virus (15). It is also of interest that feeding additional SCFA can overcome the detrimental effects that high ambient temperatures can have on the outcome of a viral infection, a useful effect in our warming world (34). Those results showed that influenza virus-infected mice were more susceptible at 36°C than at room temperature, but the increased susceptibility was abrogated when the diet was supplemented with SCFA. Additionally, mice infected with influenza virus show changes in their gut microflora and produce less SCFA; as a consequence, they become more susceptible to secondary bacterial infection (35). Interestingly, supplementing the diet with acetate reduced the consequences of secondary superinfection. Thus, evidence is accumulating to attest to the potential value of dietary manipulation in changing the outcome of virus infections.
Far more investigations using dietary manipulation to influence inflammatory reactions have been performed using noninfectious models of inflammatory disease, with the focus on models of autoimmunity (12, 13, 36, 37). A common approach has been to feed animals a diet rich in fiber, or to provide supplements of SCFA salts, which we used in the present study, and to compare the outcome with that for animals fed a control diet. Studies have shown that feeding fiber-rich diets or an SCFA supplement results in diminished inflammatory lesions. How such effects occur has been investigated by several groups; the consensus explanation is that fiber-rich diets favor the expansion of bacterial components of the gut flora, such as Bacteroidetes and Firmicutes species (19, 33). These bacteria can degrade complex carbohydrates such as inulin and pectin into SCFA, which include acetic, butyric, and propionic acids. These products, in turn, are taken up by reacting cells of the immune system and can influence their metabolism, expansion, and function. For example, exposure of developing CD4 Th1 T cells to SCFA affects their amino acid metabolism, although the mechanism by which this occurs needs to be ascertained (38). Developing Th17 cells are inhibited because the nuclear factor erythroid 2-related factor 2/heme oxygenase 1/IL-6 receptor pathway is downgraded (39). Moreover, the presence of SCFA acts to compromise the function of these cells by effects on Rorγt and STAT3 expression (40, 41). Of interest, exposure of Treg to SCFA during their development acts to expand this population of cells and also increases their function via effects on their pivotal transcription factor Foxp3. This is the consequence of enhancement of histone H3 acetylation and inhibition of histone deacetylase (42–44).
In our studies, we also noted reduced numbers of neutrophils and macrophages in the lesions of SP-fed animals. As others have shown, this effect likely occurred by various mechanisms, which include enhancement of neutrophil apoptosis by inducing caspase activity and also downregulation of NF-κB in neutrophils and macrophages, resulting in their reduced responses to chemokines (33, 37, 45, 46). In our studies, too, we detected reduced expression levels of several molecules, including the chemokines CXCL1/KC and CCL3. Additionally, reduced levels of MMP-9, a molecule involved in vascular angiogenesis, an essential step in SK pathogenesis, have been noted (47). Levels of other crucial cytokines, including IL-1β, IL-12, IL-6, and TNF-α, were also reduced in the SP-supplemented group. Moreover, feeding of SCFA enhanced levels of some cytokines, including IL-10 and TGF-β, which are known to have an anti-inflammatory effect (48). Stromal keratitis is an excellent example of a virus-induced lesion that occurs as a consequence of an inflammatory reaction (5). In SK, reports have shown that lesions are minimized in situations where the balance between the T cells participating in the response favors cells with regulatory functions (Treg) (28). Furthermore, certain subsets of Treg, such as those that are able to produce amphiregulin (AMP), may be more instrumental in controlling SK lesion severity than AMP-negative Treg (49). Our studies showed that the consequence of SP supplement feeding was to diminish the numbers of proinflammatory Th1 and Th17 T cells but not to inhibit cells with regulatory function, such as Foxp3+ Treg. In fact, whereas the numbers of Treg were not markedly expanded in vivo, their frequency relative to that of Th1 and Th17 cells was significantly increased. This was evident in corneal tissue with SK lesions as well as in the DLN. A similar pattern of results was also reported in studies on feeding high-fiber diets and propionate or butyrate supplements in autoimmune models of inflammation (12, 13). We could also show, using in vitro T cell induction systems, that the presence of SP in cultures served to reduce inflammatory T cell induction but, in contrast, marginally expanded Treg induction. Accordingly, the expression of inflammatory responses to viruses and some other inflammatory processes can be limited by dietary manipulation. However, it remains to be determined how this approach to lesion management compares with other approaches to the same problem. These include modulating other metabolic events, such as glycolysis, glutamine metabolism, and fatty acid metabolism (50), modulating microRNA (miRNA) responses (51, 52), expanding Treg using some activators (32), and other approaches discussed in a previous review (50). Some of these alternative approaches might be more effective in achieving immune cell rebalancing, but they lack the simplicity of the approach we describe in this report. It is also relevant that uncontrolled inflammatory effects in the lung are a severe, often lethal consequence of COVID-19 infection in some patients (53). This problem is currently treated with steroids or expensive monoclonal antibodies against proinflammatory cytokines such as IL-6 (54, 55). One wonders if the simple and quite inexpensive maneuver of feeding diets rich in fiber or providing nutritional supplements such as SP could be a useful way to diminish the inflammatory consequences of COVID-19 lesions. It is also possible that those persons who suffer from severe and frequent genital herpetic lesions could receive relief if their diets were changed or if they received appropriate supplements. Such issues merit further investigation.
MATERIALS AND METHODS
Mice.
Three- to 4-week-old C57BL/6 mice were purchased from Envigo (USA). DO11.10 RAG2−/− mice were previously purchased from Taconic, and breeding pairs were maintained to continue the strain. All mice were kept in a pathogen-free facility where food, water, bedding, and instruments were autoclaved. All the animals were housed in American Association for Laboratory Animal Science-approved facilities at the University of Tennessee, Knoxville. All investigations followed the guidelines of the Institutional Animal Care and Use Committee and adhered to the guidelines of the Association for Research in Vision and Ophthalmology.
Virus.
HSV-1 strain RE (obtained from Robert Hendricks, University of Pittsburgh) was propagated in Vero cell monolayers (CCL81; American Type Culture Collection, Manassas, VA, USA) as per standard protocols. Briefly, Vero cells were infected at a multiplicity of infection (MOI) of 0.01 with a virus inoculum medium (Dulbecco’s modified Eagle medium [DMEM] without fetal bovine serum [FBS]) containing strain RE for 90 min at 37°C under a 5% CO2 atmosphere in a humidified incubator. After 90 min, the old medium was replaced with fresh medium, and final harvesting was done after 48 h postinoculation. Cell pellets were collected after centrifugation, resuspended in phosphate-buffered saline (PBS), and then kept at −80°C. In order to release the intracellular virus, cells were freeze-thawed three times, and at last, centrifugation was performed at 3,000 rpm for 15 min at 4°C to separate the supernatant and cellular pellet. The supernatant was collected and stored in aliquots at −80°C until use. Viral titration was done in Vero cells, and 1.2 × 108 PFU/ml was calculated after crystal violet staining.
SP administration.
In line with previous studies (56, 57), mice were given sodium propionate (SP) (catalog no. P1880; Sigma) by including it in the drinking water at a 500 mM concentration starting 3 weeks prior to viral infection and continuing until the termination of the study. The control mice received the same food source (regular chow diet) but distilled water for drinking. The drinking sources were changed twice a week for the entire duration of the study.
HSV-1 infection and clinical scoring.
Seven-week-old C57BL/6 mice were given deep anesthesia (a 1.25% solution of 2,2,2-tribromoethanol; catalog no. AC421430500; Acros). The stage of deep anesthesia was confirmed by tail and toe pinch reflex; corneal infections were done by lightly scarifying (15 scratches in checkerboard form on each eye) corneas using a 27-gauge needle; and a 3-μl drop that contained 1 × 104 PFU of HSV-1 RE was administered, followed by eyelid closing and gentle rubbing for 45 s. The day of infection was counted as day 0. These mice were monitored daily for the development of SK lesions. SK lesion severity and angiogenesis in the eyes of mice were examined by slit-lamp biomicroscopy (Kowa Company, Nagoya, Japan) and were recorded at different days (days 7, 11, and 15) postinfection (p.i.). The scoring system was as follows: 0, normal cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing keratitis. The severity of angiogenesis was recorded as described previously (58). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm toward the corneal center. The scores of the four quadrants of the eye were then summed to derive the neovessel index (range, 0 to 16) for each eye at a given time point.
Analysis of virus on eye by swab and plaque assay.
To measure the effect of oral feeding of SP on corneal virus replication, eye swabs were taken on days 2, 4, and 7 p.i., and swabs were kept in 0.5 ml of DMEM and were stored at −80°C for future analysis. To quantify virus, a plaque assay was used as described elsewhere (9). Eye swab samples were titrated and serially diluted on Vero cells on 48-well plates in triplicate. After incubation for 90 min at 37°C, the plates were overlaid with 1% methylcellulose mixed with 10% DMEM and were kept for incubation for 4 days. After 4 days, plates were fixed with 10% formalin for 1 h, followed by staining with crystal violet (0.2% solution in alcohol) for 30 min, and plaques were counted.
Reagents and Abs used.
CD4 (RM4-5), gamma interferon (IFN-γ) (XMG1.2), Foxp3 (FJK-16S), IL-17A (TC11-18H10), F4/80 (BM8), CD11b (M1/70), LY6G (1A8), CD45 (30F11), anti-CD3 (145-2C11), and anti-CD28 (37.51) antibodies (Abs), GolgiPlug (brefeldin A), and a CD16/CD32 (2.4G2) Ab were obtained from either eBioscience or BD Biosciences. Sodium propionate, phorbol myristate acetate (PMA), and ionomycin were from Sigma-Aldrich. The Live/Dead staining kit was obtained from Life Technologies. Recombinant IL-2 (rIL-2) was from PeproTech; IL-12, IL-6, and TGF-β were from R&D Systems, and anti-IL-4 from BioLegend.
Flow cytometric analysis of immune cells.
At day 15 p.i., individual corneas were excised, suspended in medium, and digested with Liberase (Roche Diagnostics, IN) for 45 min at 37°C under a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a 1-ml syringe plunger on a 40-μm cell strainer, and a single-cell suspension was made in 10% RPMI 1640 medium. Single-cell corneal suspensions were divided into two equal parts; one part was processed to detect Treg, and the other was stimulated with PMA (50 ng), ionomycin (500 ng), and brefeldin A (10 mg/ml) for 4 h to enumerate Th1 and Th17 cells. For draining lymph nodes (DLNs), the mesenteric lymph node (MLN), and spleens, single-cell suspensions were made as described above without Liberase treatment, and cells were counted by hemocytometer (catalog no. 02-671-51B; Hausser Scientific). One million cells were either stained directly to detect Treg or stimulated with PMA (50 ng), ionomycin (500 ng), and brefeldin A (10 mg/ml) for 4 h in 96-well U-bottom plates to detect Th1 and Th17 T cells (58).
For flow cytometric staining, all steps were performed at 4°C. Briefly, cells (both stimulated and unstimulated) were reacted with anti-mouse CD16/CD32 (Fc Block; catalog no. 553141; BD Biosciences) for 15 min to eliminate non-antigen-specific binding to the cell surface. After that, cells were reacted with a Live/Dead staining reagent (for 30 min) in PBS, followed by cell surface staining with the respective surface fluorochrome-labeled Abs in fluorescence-activated cell sorter (FACS) buffer (10% FBS in PBS) for 30 min. Subsequently, samples were reacted with fluorochrome-labeled Abs for the detection of intracellular cytokines (IFN-γ and IL-17A) or the Foxp3 transcription factor protein. Finally, the cells were washed three times with FACS buffer and were resuspended in 1% paraformaldehyde. The labeled samples were analyzed to enumerate the cells with different phenotypes using a FACS LSR II system (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR). For Treg determination, unstimulated single-cell suspensions were intracellularly stained for the transcription factor protein Foxp3 by using a Foxp3 intracellular staining kit (catalog no. 00-5523-00; eBioscience) in accordance with the manufacturer’s recommendations. To determine the number of IFN-γ-, IL-17A-producing T cells from stimulated single-cell suspensions, intracellular cytokine staining was performed according to the manufacturer’s recommendations (BD Cytofix/Cytoperm Plus kit; catalog no. 555028; BD Biosciences).
For the study of innate cells such as neutrophils and macrophages, individual corneas were digested with Liberase (Roche Diagnostics, IN), and single-cell suspensions were prepared as described above. Single cell suspensions were also made from the DLN, MLN, and spleen lymphoid tissue. Surface staining was performed for innate cell analysis without stimulation.
During flow cytometric analysis, all doublet cells were gated out, followed by gating of live cells using Live/Dead staining. Cells were identified as Th1 cells if they were CD4+ IFN- γ+, as Th17 cells if they were CD4+ IL-17A+, as Treg if they were CD4+ Foxp3+, as neutrophils if they were CD45+ CD11b+ LY6G+ F4/80−, or as macrophages if they were CD45+ CD11b+ F4/80+ LY6G–.
In vitro Treg, Th1 cell, and Th17 cell differentiation.
To evaluate the effects of SP on the induction of T cell responses in vitro, splenocytes from naive DO11.10 RAG2−/− mice were used as a precursor population as described previously, with minor modifications (16, 58). Briefly, 0.5 × 106 splenocytes after erythrocyte (RBC) lysis and several washes were cultured in 1 ml of 10% RPMI 1640 medium containing rIL-2 (100 U/ml) and TGF-β (0.5 ng/ml) for Treg induction, IL-12 (5 to 10 ng/ml) and anti-IL-4 (10 μg/ml) for Th1 induction, and IL-6 (25 ng/ml) and TGF- β (1 ng/ml) for Th17 induction. All cultures had plate-bound anti-CD3/CD28 Ab (1 μg/ml) and were kept for 5 days at 37°C in a 5% CO2 incubator. Test cultures for the induction of Treg, Th1 cell, and Th17 cell types received different concentrations of SP, which ranged from 100 μM to 3.2 mM. After 5 days, samples were analyzed by flow cytometry to enumerate the numbers of T cells of the different phenotypes that were induced in test and control cultures by using the methodology described in the preceding section.
RT-PCR for quantification of selected RNA levels.
Total RNA was isolated from corneas. At the 15th day p.i., four corneal samples showing average lesions were pooled, and RNA was isolated using a mirVana miRNA isolation kit (Ambion). From corneal samples, cDNA was made by using 500 ng of RNA with the help of an ImProm-II reverse transcription system kit (Promega). A TaqMan gene expression assay for various chemokines and cytokines was obtained from Applied Biosystems, and analysis was carried out by using a 7900 HT Fast real-time PCR (RT-PCR) system (Applied Biosystems). Various predesigned 6-carboxyfluorescein (FAM)-MGM-labeled gene expression probes were purchased with the following identification numbers: for IL-6, Mm00446190_m1; for IL-1β, Mm00434228_m1; for IL-12, Mm01288991_g1; for TNF-α, Mm99999068_m1; for IL-10, Mm99999062_m1; for TGF-β, Mm00436955_m1; for matrix metallopeptidase 9 (MMP-9), Mm00442991_m1; for chemokine (C-X-C motif) ligand 1 (CXCL1), Mm04207460_m1; and for chemokine ligand 3 (CCL3), Mm00441259_g1. Actin β (Mm00607939_s1) was used as an endogenous control for all PCRs.
The expression levels of different molecules were normalized to that of β-actin using a Δ cycle threshold calculation. Relative expression for the control and SP-fed groups was calculated using the 2–ΔΔCT × 1,000 formula.
Statistical analysis.
Statistical significance was determined by either an unpaired Student t test (comparing two groups) or analysis of variance (ANOVA). A P value of <0.05 was regarded as indicating a significant difference between groups (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001). All results are expressed as means ± standard errors of the means (SEM). GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for statistical analysis.
ACKNOWLEDGMENTS
This work was supported by NIH 2020 R21 AI (grant 5R21AI142862-02) and NIH 2020 R01 (grant EY5R01EY005093-35).
We have no financial conflicts of interest.
REFERENCES
- 1.Johnston C, Corey L. 2016. Current concepts for genital herpes simplex virus infection: diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev 29:149–161. doi: 10.1128/CMR.00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalf JF, Kaufman HE. 1976. Herpetic stromal keratitis—evidence for cell-mediated immunopathogenesis. Am J Ophthalmol 82:827–834. doi: 10.1016/0002-9394(76)90057-X. [DOI] [PubMed] [Google Scholar]
- 3.Streilein JW, Dana MR, Ksander BR. 1997. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today 18:443–449. doi: 10.1016/S0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 4.Biswas PS, Rouse BT. 2005. Early events in HSV keratitis—setting the stage for a blinding disease. Microbes Infect 7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Rajasagi NK, Rouse BT. 2019. The role of T cells in herpes stromal keratitis. Front Immunol 10:512. doi: 10.3389/fimmu.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Wang R, Xu C, Zhou H. 2020. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol 11:766. doi: 10.3389/fimmu.2020.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouse BT, Suvas S. 2004. Regulatory cells and infectious agents: détentes cordiale and contraire. J Immunol 173:2211–2215. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 8.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. 2008. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol 82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasagi NK, Bhela S, Varanasi SK, Rouse BT. 2017. Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J Leukoc Biol 102:1159–1171. doi: 10.1189/jlb.3HI1216-511RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varanasi SK, Reddy PBJ, Bhela S, Jaggi U, Gimenez F, Rouse BT. 2017. Azacytidine treatment inhibits the progression of herpes stromal keratitis by enhancing regulatory T cell function. J Virol 91:e02367-16. doi: 10.1128/JVI.02367-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mockler MB, Conroy MJ, Lysaght J. 2014. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front Oncol 4:107. doi: 10.3389/fonc.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thöne J, Demir S, Müller DN, Gold R, Linker RA. 2015. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. 2017. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One 12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 15.Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, dos Santos AÁ, Dias GBM, Vargas JE, Puga R, Mayer FQ, Maito F, Zárate-Bladés CR, Ajami NJ, Sant’Ana MR, Candreva T, Rodrigues HG, Schmiele M, Silva Clerici MTP, Proença-Modena JL, Vieira AT, Mackay CR, Mansur D, Caballero MT, Marzec J, Li J, Wang X, Bell D, Polack FP, Kleeberger SR, Stein RT, Vinolo MAR, de Souza APD. 2019. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun 10:3273. doi: 10.1038/s41467-019-11152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varanasi SK, Donohoe D, Jaggi U, Rouse BT. 2017. Manipulating glucose metabolism during different stages of viral pathogenesis can have either detrimental or beneficial effects. J Immunol 199:1748–1761. doi: 10.4049/jimmunol.1700472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer A, Schliep A, Jörg S, Haghikia A, Gold R, Kleinewietfeld M, Müller DN, Linker RA. 2017. Impact of combined sodium chloride and saturated long-chain fatty acid challenge on the differentiation of T helper cells in neuroinflammation. J Neuroinflammation 14:184. doi: 10.1186/s12974-017-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schönfeld P, Wojtczak L. 2016. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander C, Swanson KS, Fahey GC, Jr, Garleb KA. 2019. Physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv Nutr 10:576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M, Schwarz MA, Lee S, Kumaraguru U, Rouse BT. 2001. Control of stromal keratitis by inhibition of neovascularization. Am J Pathol 159:1021–1029. doi: 10.1016/S0002-9440(10)61777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keadle TL, Morris JL, Pepose JS, Stuart PM. 2002. CD4+ and CD8+ cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microb Pathog 32:255–262. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- 23.Niemialtowski MG, Rouse BT. 1992. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol 149:3035–3039. [PubMed] [Google Scholar]
- 24.Osorio Y, Cai S, Hofman F, Brown D, Ghiasi H. 2004. Involvement of CD8+ T-cells in exacerbation of corneal scarring in mice. Curr Eye Res 29:145–151. doi: 10.1080/02713680490504632. [DOI] [PubMed] [Google Scholar]
- 25.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PBJ, Sehrawat S, Sharma S, Rouse BT. 2011. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol 187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas J, Gangappa S, Kanangat S, Rouse BT. 1997. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol 158:1383–1391. [PubMed] [Google Scholar]
- 27.Jaggi U, Yang M, Matundan HH, Hirose S, Shah PK, Sharifi BG, Ghiasi H. 2020. Increased phagocytosis in the presence of enhanced M2-like macrophage responses correlates with increased primary and latent HSV-1 infection. PLoS Pathog 16:e1008971. doi: 10.1371/journal.ppat.1008971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. 2004. CD4+ CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 29.Sarangi PP, Sehrawat S, Suvas S, Rouse BT. 2008. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. J Immunol 180:6297–6306. doi: 10.4049/jimmunol.180.9.6297. [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Ghiasi H. 2017. Roles of M1 and M2 macrophages in herpes simplex virus 1 infectivity. J Virol 91:e00578-17. doi: 10.1128/JVI.00578-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumpey TM, Chen SH, Oakes JE, Lausch RN. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol 70:898–904. doi: 10.1128/JVI.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy PBJ, Schreiber TH, Rajasagi NK, Suryawanshi A, Mulik S, Veiga-Parga T, Niki T, Hirashima M, Podack ER, Rouse BT. 2012. TNFRSF25 agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions. J Virol 86:10606–10620. doi: 10.1128/JVI.01391-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. 2018. Dietary fiber confers protection against flu by shaping Ly6c—patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama M, Ichinohe T. 2019. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc Natl Acad Sci U S A 116:3118–3125. doi: 10.1073/pnas.1815029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sencio V, Barthelemy A, Tavares LP, Machado MG, Soulard D, Cuinat C, Queiroz-Junior CM, Noordine M-L, Salomé-Desnoulez S, Deryuter L, Foligné B, Wahl C, Frisch B, Vieira AT, Paget C, Milligan G, Ulven T, Wolowczuk I, Faveeuw C, Le Goffic R, Thomas M, Ferreira S, Teixeira MM, Trottein F. 2020. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep 30:2934–2947.e6. doi: 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura YK, Janowitz C, Metea C, Asquith M, Karstens L, Rosenbaum JT, Lin P. 2017. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci Rep 7:11745. doi: 10.1038/s41598-017-12163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira AT, Galvão I, Macia LM, Sernaglia éM, Vinolo MAR, Garcia CC, Tavares LP, Amaral FA, Sousa LP, Martins FS, Mackay CR, Teixeira MM. 2017. Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J Leukoc Biol 101:275–284. doi: 10.1189/jlb.3A1015-453RRR. [DOI] [PubMed] [Google Scholar]
- 38.Maier E, Kurz K, Jenny M, Schennach H, Ueberall F, Fuchs D. 2010. Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food Chem Toxicol 48:1950–1956. doi: 10.1016/j.fct.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Su W, Wan T, Yu J, Zhu W, Tang F, Liu G, Olsen N, Liang D, Zheng SG. 2017. Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway. Biochem Pharmacol 142:111–119. doi: 10.1016/j.bcp.2017.06.136. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Sun M, Wu W, Yang W, Huang X, Xiao Y, Ma C, Xu L, Yao S, Liu Z, Cong Y. 2019. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis 25:1450–1461. doi: 10.1093/ibd/izz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, Lamp B, Nist A, Stiewe T, Shaul Y, Adhikary T, Zaiss MM, Lauth M, Steinhoff U, Visekruna A. 2019. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 44.Bilotta AJ, Cong Y. 2019. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: implication in precision medicine. Precis Clin Med 2:110–119. doi: 10.1093/pcmedi/pbz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filippone A, Lanza M, Campolo M, Casili G, Paterniti I, Cuzzocrea S, Esposito E. 2020. The anti-inflammatory and antioxidant effects of sodium propionate. Int J Mol Sci 21:3026. doi: 10.3390/ijms21083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Di Y, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Zheng M, Kim B, Rouse BT. 2002. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest 110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. 2006. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology 117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varanasi SK, Rajasagi NK, Jaggi U, Rouse BT. 2018. Role of IL-18 induced amphiregulin expression on virus induced ocular lesions. Mucosal Immunol 11:1705–1715. doi: 10.1038/s41385-018-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumbria D, Berber E, Rouse BT. 2019. Factors affecting the tissue damaging consequences of viral infections. Front Microbiol 10:2314. doi: 10.3389/fmicb.2019.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhela S, Mulik S, Reddy PBJ, Richardson RL, Gimenez F, Rajasagi NK, Veiga-Parga T, Osmand AP, Rouse BT. 2014. Critical role of microRNA-155 in herpes simplex encephalitis. J Immunol 192:2734–2743. doi: 10.4049/jimmunol.1302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cokarić Brdovčak M, Zubković A, Jurak I. 2018. Herpes simplex virus 1 deregulation of host microRNAs. Noncoding RNA 4:36. doi: 10.3390/ncrna4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morens DM, Fauci AS. 2020. Emerging pandemic diseases: how we got to COVID-19. Cell 182:1077–1092. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, Yao L, Motaghi S, Couban RJ, Ghadimi M, Bala MM, Gomaa H, Fang F, Xiao Y, Guyatt GH. 2020. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 192:E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morland C, Frøland A-S, Pettersen MN, Storm-Mathisen J, Gundersen V, Rise F, Hassel B. 2018. Propionate enters GABAergic neurons, inhibits GABA transaminase, causes GABA accumulation and lethargy in a model of propionic acidemia. Biochem J 475:749–758. doi: 10.1042/BCJ20170814. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. 2004. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaggi U, Varanasi SK, Bhela S, Rouse BT. 2018. On the role of retinoic acid in virus induced inflammatory response in cornea. Microbes Infect 20:337–345. doi: 10.1016/j.micinf.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Lok LSC, Dennison TW, Mahbubani KM, Saeb-Parsy K, Chilvers ER, Clatworthy MR. 2019. Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc Natl Acad Sci U S A 116:19083–19089. doi: 10.1073/pnas.1905054116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lytle AG, Shen S, McGettigan JP. 2015. Lymph node but not intradermal injection site macrophages are critical for germinal center formation and antibody responses to rabies vaccination. J Virol 89:2842–2848. doi: 10.1128/JVI.03409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]