Abstract
Brush border microvilli enable functions that are critical for epithelial homeostasis, including solute uptake and host defense. However, the mechanisms that regulate the assembly and morphology of these protrusions are poorly understood. The parallel actin bundles that support microvilli have their pointed-end rootlets anchored in a filamentous meshwork referred to as the “terminal web.” Although classic electron microscopy studies revealed complex ultrastructure, the composition and function of the terminal web remain unclear. Here we identify nonmuscle myosin-2C (NM2C) as a component of the terminal web. NM2C is found in a dense, isotropic layer of puncta across the subapical domain, which transects the rootlets of microvillar actin bundles. Puncta are separated by ∼210 nm, the expected size of filaments formed by NM2C. In intestinal organoid cultures, the terminal web NM2C network is highly dynamic and exhibits continuous remodeling. Using pharmacological and genetic perturbations in cultured intestinal epithelial cells, we found that NM2C controls the length of growing microvilli by regulating actin turnover in a manner that requires a fully active motor domain. Our findings answer a decades-old question on the function of terminal web myosin and hold broad implications for understanding apical morphogenesis in diverse epithelial systems.
INTRODUCTION
Hollow organs such as the intestinal track, kidney tubules, and brain ventricles are lined with solute-transporting epithelial cells, which build apical specializations to enhance their functional capacity. In the small intestine, enterocytes present thousands of microvilli on their apical surface, which are tightly packed into a highly ordered array known as the brush border (Crawley et al., 2014). Microvilli serve two general functions—they markedly increase the membrane surface area for nutrient absorption and also provide the first line of defense against harmful compounds and microbes found in the luminal compartment (Vallance et al., 2002; Helander and Fändriks, 2014). Owing to the constant regeneration of the mammalian gut epithelium, continuous differentiation of enterocytes and assembly of the brush border are critical for maintaining homeostasis throughout an organism’s lifetime. Despite microvilli occupying a critical physiological interface, mechanisms that regulate apical morphogenesis remain unclear.
A single microvillus is supported by a core of 20–30 actin filaments, bundled together in parallel by actin bundlers villin, espin, and fimbrin (Bretscher and Weber, 1979, 1980; Mooseker et al., 1980; Bartles et al., 1998), with the barbed ends oriented at the distal tips and the pointed ends anchored in a dense, filamentous network known as the terminal web (Leblond et al., 1960; Mooseker and Tilney, 1975; Hull and Staehelin, 1979). While the terminal web was first described several decades ago in ultrastructural studies, little is known about how it contributes to the apical domain structure, microvillar organization, or brush border function. Interestingly, knockout (KO) mouse models lacking major brush border structural components, such as PACSIN-2, plastin-1, or multiple actin-bundling proteins (villin, espin, and plastin-1), exhibit significant perturbations to the terminal web (Grimm-Gunter et al., 2009; Revenu et al., 2012; Postema et al., 2019). All of these models exhibit a common phenotype, where the terminal web thins and microvilli become disorganized or shortened, which further suggests that terminal web architecture and brush border morphology are intimately linked.
Previous biochemical and ultrastructural studies of the terminal web identified several components of this filamentous network, including intermediate filaments and spectrins (Glenney et al., 1982; Glenney and Glenney, 1983; Mooseker et al., 1984; Howe et al., 1985). Early deep-etch electron microscopy (EM) studies also identified “8 nm filaments,” which appeared to cross-link microvillar actin bundle rootlets (Hirokawa and Heuser, 1981). However, these structures did not label with myosin subfragment 1 (S1), suggesting that they were not composed of actin. Additional immunogold labeling EM data led to speculation that 8-nm filaments contained myosin (Mooseker and Tilney, 1975; Hirokawa et al., 1982). This was further supported by immunofluorescence labeling studies that made use of a pan-myosin antibody raised against myosin-2 epitopes (Drenckhahn and Gröschel-Stewart, 1980). However, the identity of the myosin and the organization and function of 8-nm filaments in brush border structure and function remained unclear for decades.
All class 2 myosins are constitutive dimers of heavy chains, each composed of an N-terminal motor domain, followed by a tandem pair of IQ motifs that are constitutively bound by regulatory and essential light chains (RLC and ELC, respectively), a long coiled-coil, and a nonhelical tail piece at the C-terminus (Heissler and Manstein, 2013). A fully functional myosin-2 molecule consists of two heavy chains (HC), each bound by one RLC and one ELC. Class 2 myosins are regulated by reversible phosphorylation at sites on the RLC, the C-terminal tailpiece, or both, which controls activation and assembly of contractile units, respectively (Jana et al., 2009; Dulyaninova and Bresnick, 2013; Betapudi, 2014). Driven by electrostatic interactions between their coiled-coil tails, skeletal, cardiac, and nonmuscle myosin-2 (NM2) variants self-assemble into bipolar filaments that serve as fundamental force-generating units (McLachlan and Karn, 1982; Atkinson and Stewart, 1992).
Nonmuscle class 2 myosins (NM2A encoded by MYH9, NM2B encoded by MYH10, and NM2C encoded by MYH14) are ubiquitously expressed and have been implicated in diverse contractile activities including cytokinesis, cell motility, and the control of cell morphology (Betapudi, 2014). At higher levels of biological complexity, these motors have been implicated in shaping and bending of tissues, collective cell migration, and regulating the paracellular permeability of epithelial sheets (Chantler et al., 2010; Betapudi, 2014). NM2 paralogues have distinct kinetic properties, which allow these motors to perform specific and distinct functions within the cell (Heissler and Manstein, 2011; Billington et al., 2013). In the context of in vitro sliding filament assays, tail-less NM2C heavy meromyosin (HMM) fragments move actin filaments at ∼0.05 µm/s, slower than both NM2B (∼0.08 µm/s) and NM2A (∼0.29 µm/s) (Kim et al., 2005). Bipolar filaments assembled by NM2C (∼293 nm) tend to be shorter than those assembled by NM2B (∼323 nm) or NM2A (∼301 nm). Filaments composed of NM2C also contain fewer molecules than those assembled by NM2A and NM2B, resulting in thinner structures (∼8 nm vs. ∼11 nm wide) and presumably a lower potential for generating force (Billington et al., 2013).
A previous proteomic study by our laboratory revealed that brush border fractions isolated from mouse small intestine contain all three NM2 paralogues, with NM2C exhibiting high-level abundance (McConnell et al., 2011). Although NM2C remains the most poorly understood paralogue with regard to biophysical properties and physiological function, previous work established that this isoform exhibits specific expression in pituitary gland, glial cells, as well as inner ear sensory, intestinal, and kidney epithelia (Golomb et al., 2004). Mutations in MYH14 have been linked to hearing loss, peripheral neuropathies, and developmental defects in the lower gastrointestinal tract (Donaudy et al., 2004; Fu et al., 2016; Kim et al., 2016, 2017; Zhu et al., 2017; Almutawa et al., 2019; Han et al., 2019; Lerat et al., 2019; Song et al., 2020). The parallel perturbation of both inner ear and intestinal epithelial systems by mutations in MYH14 is intriguing, as actin bundled–supported stereocilia found on the apical surface of hair cells are closely related to microvilli found on solute-transporting epithelia and may share mechanisms of assembly and maintenance (Prost et al., 2007; Mathur and Yang, 2015). Previous studies of a NM2C-EGFP–expressing mouse revealed that this motor is highly enriched at the junctional margins of sensory and intestinal epithelial cells, where cell–cell contacts are formed (Ebrahim et al., 2014). At these sites, NM2 assembles into circumferential sarcomere-like structures, characterized by a linear array of puncta that are uniformly separated by ∼300–400 nm, a distance comparable to the expected length of contractile filaments formed by NM2 isoforms (Billington et al., 2013; Ebrahim et al., 2014). Treatment of NM2C-EGFP mouse epithelial tissues with myosin-2 inhibitor blebbistatin (which prevents force generation) increased the interpuncta distance, indicating that under normal conditions the circumferential NM2 band is under tension (Ebrahim et al., 2014). Careful inspection of images in this previous study also revealed that the NM2C signal is not confined to the circumferential/junctional array, as dimmer puncta also appear to span the cell medially, throughout the entire subapical region.
In this paper, we report that NM2C localizes at the pointed ends of microvillar actin bundles, where it forms a highly dynamic array of puncta that are confined to the plane of the subapical terminal web. Although these puncta demonstrate approximately twofold lower levels of NM2C enrichment relative to those found in a circumferential array, based on their similar spacing and dynamics, we propose that they represent NM2C contractile filaments. Using pharmacological and genetic perturbations in cultured intestinal epithelial cells, we found that the activity of this motor controls the length of growing microvilli by regulating actin disassembly at the pointed ends. These findings address a decades-old question on the function of the terminal web myosin-2 and hold important implications for understanding brush border assembly and apical morphogenesis in epithelial systems.
RESULTS
NM2C is enriched in the enterocyte brush border terminal web in vivo
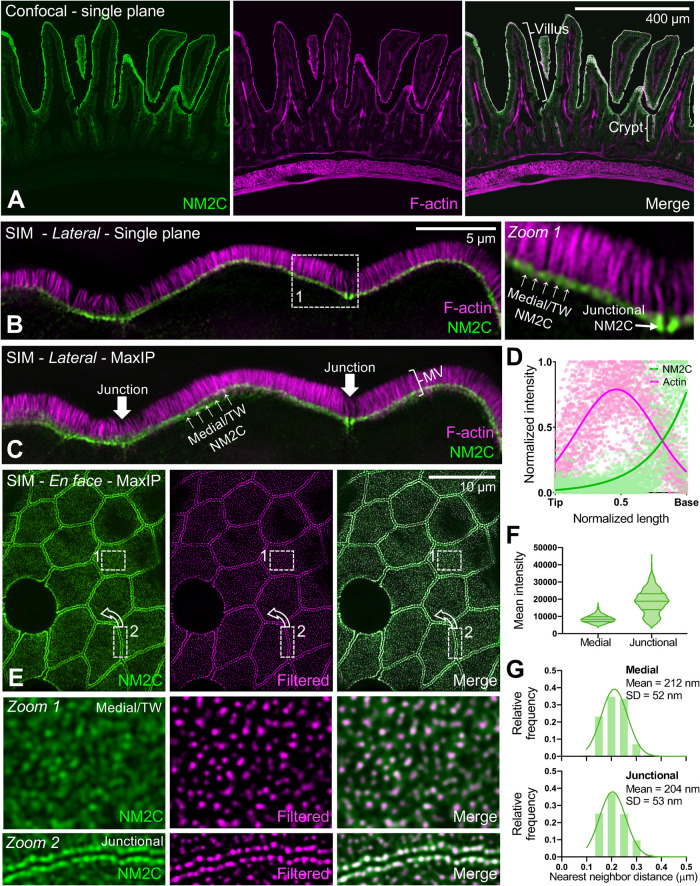
Although all three NM2 isoforms are detectable in the brush border proteome (McConnell et al., 2011), our analysis of recently generated single-cell RNAseq (scRNAseq) data from the intestinal tract (Banerjee et al., 2020; Southard-Smith et al., 2020) revealed that NM2C is preferentially expressed in mature villus enterocytes relative to NM2A or NM2B, the latter of which was barely detectable (Supplemental Figure 1). We therefore focused subsequent studies on characterizing the function of NM2C. To investigate the subcellular distribution of this motor in intestinal tissues in more detail, we took advantage of mice that express an enhanced green fluorescent protein (EGFP)-tagged form of NM2C from the endogenous locus (Ebrahim et al., 2014). In this model, EGFP is fused in frame to the C-terminus of the NM2C heavy chain. Consistent with scRNAseq analysis, confocal imaging of frozen intestinal tissue sections revealed the strong apical signal of NM2C along the full length of villi (Figure 1A). Lower levels of NM2C were also observed on the apical surface of immature epithelial cells in the stem cell–containing crypt compartments (Figure 1A, merge). To gain additional resolution at the subcellular scale, tissue sections from NM2C-EGFP mice were subject to superresolution structured illumination microscopy (SIM). SIM images of enterocytes were acquired with the apical-basolateral axis either parallel or perpendicular to the focal plane (lateral or en face, respectively). Lateral views revealed that the apical NM2C-EGFP signal observed in lower-magnification images was confined to a band of signal that overlapped specifically with the rootlets of microvilli, near the pointed ends of core actin bundles (Figure 1, B and C). This band of signal spanned the entire apical surface at the level of the terminal web, and its intensity increased significantly where it connected to the circumferential/junctional array at the margins of the cell (Figure 1C, white arrows). This was further confirmed with quantitative line scan analysis of NM2C-EGFP fluorescence intensities along individual microvilli visualized in SIM images (Figure 1D). En face images of NM2C-EGFP tissue samples revealed that the terminal web NM2C signal was strikingly punctate; the differential enrichment of NM2C in medial versus junctional puncta was also apparent in these images (Figure 1E). Intensity analysis revealed that medial puncta in the terminal web were approximately half the intensity of junctional puncta (Figure 1F). Moreover, nearest neighbor distance calculations showed that medial and junctional puncta are minimally separated by approximately the same distance, ∼210 nm (Figure 1G). Thus, in addition to its localization in the circumferential band at the margins of enterocytes, NM2C is also enriched throughout the terminal web, where it is well-positioned to interact directly with the rootlets of microvillar core actin bundles.
FIGURE 1:
NM2C localizes to the enterocyte terminal web. (A) Single-plane confocal microscopy of small intestinal tissue from a mouse endogenously expressing EGFP-NM2C (green), costained with phalliodin-568 to visualize F-actin (magenta). Representative villus and crypt regions are highlighted by brackets in merge panel. (B) Single-plane SIM image of small intestinal tissue from a mouse endogenously expressing EGFP-NM2C (green), co-stained with phalloidin-568 to visualize F-actin (magenta). Zoom 1 shows region bound by the dashed white box; medial and junctional populations are highlighted. (C) Maximum intensity projection (MaxIP) of lateral view shown in B. (D) Normalized intensity plots of NM2C and F-signals taken from line scans drawn parallel to the microvillar axis (n = 45) reveal enrichment of NM2C at the base of microvilli in the terminal web. (E) En face SIM MaxIP images of small intestinal tissue from a mouse endogenously expressing EGFP-NM2C (green); medial/terminal web and junctional populations are highlighted in zooms 1 and 2, respectively. SIM image (left) is shown in parallel with a radiality map that was calculated using NanoF-SRRF (middle), which accentuated intensity peaks from individual NM2C puncta and allowed for more precise localization of their position. Merge image (right) shows composite of the original SIM image with the SRRF-filtered image. (F) Mean intensity of medial/terminal web vs. junctional NM2C puncta from SIM images. (G) Histograms of nearest neighbor distances generated by localizing medial (top) vs. junctional (bottom) NM2C puncta in SRRF-filtered SIM images. For F and G, n = 2480 medial puncta and n = 1019 junctional puncta. Scale is indicated on individual image panels.
Terminal web and junctional NM2C puncta exhibit continuous remodeling
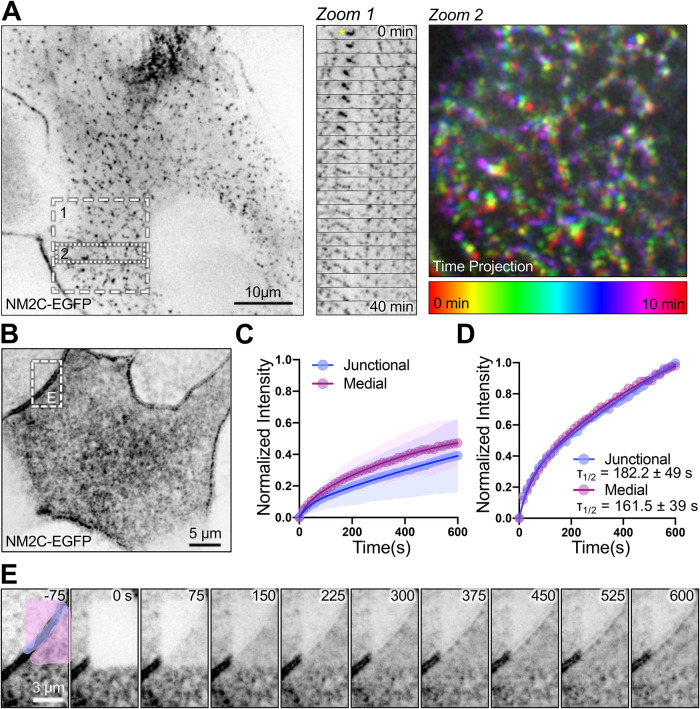
To enable live imaging studies of NM2C dynamics in the terminal web, we isolated crypts from NM2C-EGFP mice, which were then expanded into 2-dimensional (2D) organoid monolayers by plating on a thin layer of Matrigel. Confocal imaging of 2D organoids revealed a pattern of apical NM2C-EGFP distribution similar to that observed in fixed native tissue sections, with prominent junctional bands and a layer of medial puncta at the level of the terminal web (Figure 2A; Supplemental Movie 1). Time-lapse imaging showed that both networks are highly dynamic and continuously remodeling, with puncta across the surface translocating, fusing, and splitting on a timescale of minutes (Figure 2A, zooms 1 and 2; Supplemental Movie 1). We also performed photokinetic studies to examine the turnover rates of NM2C-EGFP puncta in the medial versus junctional populations (Figure 2, B–E). Fluorescence recovery after photobleaching (FRAP) analysis revealed that, despite the twofold difference in puncta intensity (Figure 1F), the recovery rates for these two populations were remarkably similar (Figure 2D). Thus, medial NM2C-EGFP puncta exhibit spacing and turnover kinetics that are similar to junctional puncta. Given that the circumferential/junctional belt of NM2 is an established contractile array (Ebrahim et al., 2014), these data suggest that terminal web NM2C might also hold the potential to exert mechanical force.
FIGURE 2:
Medial and junctional population NM2C puncta exhibit similar dynamics. (A) Live spinning-disk confocal imaging of an organoid monolayer (i.e., 2D) derived from EGFP-NM2C–expressing mouse small intestinal tissues; signal is inverted to facilitate visualization of dim structures. Zoom 1 shows a montage sampled at 2-min intervals that reveals extensive remodeling of medial NM2C puncta over 40 min; puncta marked with a yellow asterisk at t = 0 min exhibit striking expansion during the time lapse. Zoom 2 shows a color-coded time projection that reveals large-scale motion of the medial NM2C network over 10 min. (B) FRAP analysis was performed on EGFP-NM2C puncta in 2D organoid monolayers to determine turnover rates in the junctional vs. medial populations. (C) FRAP recovery curves for junctional and medial NM2C populations normalized to prebleach intensity show that both populations exhibit large immobile fractions (∼50%). (D) Kinetic analysis of data sets normalized to peak postbleach intensities indicates that junctional and medial NM2C signals recover at comparable rates; τ1/2 for both populations are shown on the plot. (E) Image montage of FRAP time-lapse data shown in B; junctional population is highlighted in purple, whereas medial NM2C is shown in pink.
Movie S1.
Related to Figure 2. Live imaging of the apical surface of a 2D intestinal organoid monolayer derived from a NM2C-EGFP expressing mouse. Spinning disk confocal images were acquired every 60 seconds for 40 minutes; playback is 15 frames per second; field width is 53 μm.
Activation of NM2 leads to shortening of microvilli
To further investigate the function of NM2C in the terminal web, we sought an appropriate cell culture model that recapitulates the localization that we observed in native tissue. Based on previous studies from our laboratory and others (Baas et al., 2004; Grega-Larson et al., 2015), Ls174T-W4 (W4) cells mimic partially differentiated intestinal epithelial cells and thus provide a model for studying actively growing microvilli (Postema et al., 2018, 2019). This cell line is derived from human colonic epithelium and can be induced via doxycycline treatment to acquire apical-basolateral polarity and assemble a brush border (Baas et al., 2004; ten Klooster et al., 2009). In this model, microvilli extend from the cell surface parallel to the focal plane, which facilitates length measurements and assessment of protein localization along the protrusion axis. Of critical importance, W4 cells do not grow in monolayers and polarize as single cells. This unique advantage allowed us to specifically interrogate the function of the terminal web population of NM2C in the absence of the circumferential actin-myosin belt that normally assembles when cell–cell junctions are formed. SIM imaging of anti-NM2C–stained W4 cells revealed striking localization at the base of microvilli, in a band that resembled the terminal web observed in native tissue samples (Supplemental Figure 2A). Importantly, an NM2C construct with a C-terminal HaloTag also demonstrated similar terminal web enrichment in W4 cells (Supplemental Figure 2B). Thus, both native and overexpressed NM2C localization in W4 cells are comparable to that observed in native intestinal tissue.
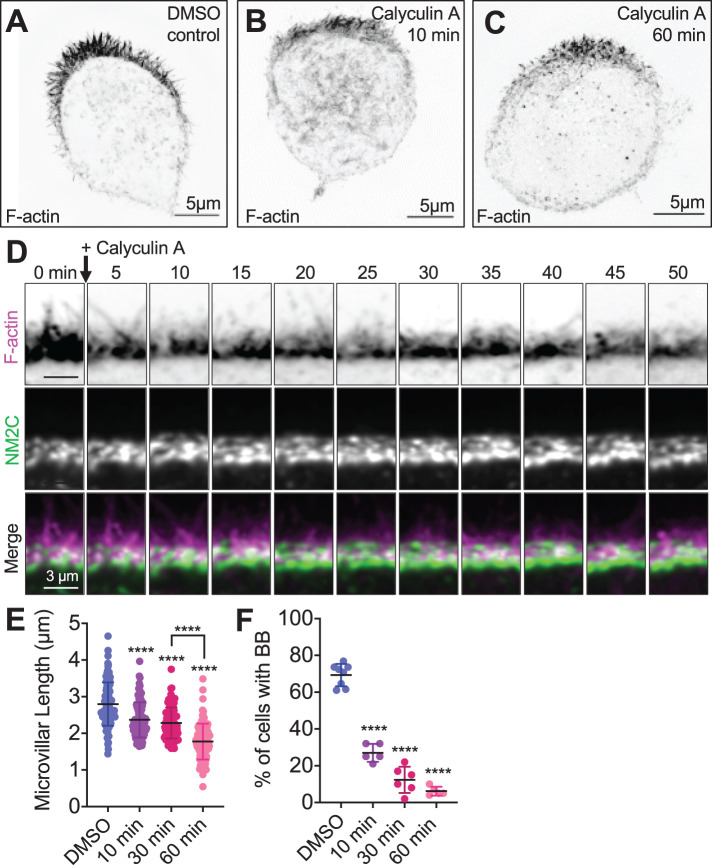
To probe NM2 function in the W4 cell terminal web, we first treated polarized W4 cells with calyculin A, a threonine/serine phosphatase inhibitor that is commonly used to elevate levels of phosphorylated myosin RLC and, thus, increase active motor units (Suganuma et al., 1990; Suzuki and Itoh, 1993; Watanabe et al., 2007; Dulyaninova and Bresnick, 2013). While calyculin A does not specifically target NM2C activity, antibody staining experiments in W4 cells (Supplemental Figure 2) indicate that this isoform specifically enriches in the terminal web relative to the other major isoform, NM2A; this is also consistent with previous proteomic analysis (McConnell et al., 2011) and scRNAseq data presented in this paper (Supplemental Figure 1), which allude to NM2C as the dominant terminal web isoform. Strikingly, treatment with 4 nM calyculin A resulted in marked shortening of microvilli over the course of 60 min (2.8 ± 0.6 µm control vs. 1.8 ± 0.5 µm calyculin A; Figure 3, A–E; Supplemental Movie 2). In calyculin A–treated W4 cells expressing Halo-NM2C, microvillar shortening was temporally paralleled by increased enrichment of NM2C in the terminal web (Figure 3D; Supplemental Movie 2). In some cases, W4 cells appeared to lose nearly all surface microvilli following calyculin A treatment (Figure 3C). Indeed, the fraction of W4 cells that exhibited F-actin–enriched brush borders also decreased significantly over the 60 min course of these experiments (Figure 3F). These drug treatment studies suggest that increased NM2 activity is linked to microvillar shortening over time, and at longer time points, complete loss of the apical brush border.
FIGURE 3:
Activation of NM2 shortens epithelial microvilli. SIM MaxIP images of representative phalloidin-stained (F-actin) Ls174T-W4 cells fixed after (A) 60 min exposure to dimethyl sulfoxide (DMSO) vehicle control, (B) 10 min exposure to 4 nM calyculin A, or (C) 60 min exposure to 4 nM calyculin A; signals are inverted to facilitate visualization of dim structures. (D) Image montage from spinning-disk confocal time-lapse data shows the impact of calyculin A treatment on apical microvilli in a Ls174T-W4 cell–expressing F-actin probe EGFP-UtrCH (magenta) with Halo-NM2C (green) labeled with JF585. (E) Quantification of microvillar length in Ls174T-W4 cells fixed after 10, 30, and 60 min in 4 nM calyculin A; each data point is a single microvillus, at least 10 microvilli per cell, minimum of 10 cells per condition. (F) Quantification of the percentage of brush border–positive cells as a function of time in calyculin A. Each data point is the percentage of cells with a brush border in a stitched 5 × 5 40× image. Each data point is the percentage of cells with a brush border in a stitched 5 × 5 40× image. Data in E and F were tested using ordinary one-way ANOVA with Dunnett’s multiple comparisons; ****, p < 0.000 vs. DMSO-negative controls unless overwise noted.
Movie S2.
Related to Figure 3. Calyculin A shortens Ls174T-W4 cell microvilli. Spinning disk confocal imaging of an induced Ls174T-W4 cell expressing EGFP-UtrCH (magenta) and Halo-NM2C labeled with JF585 (green). Images were acquired every 15 seconds for 60 minutes; playback is 15 frames per second; field width is 23 μm.
Inhibition of NM2 leads to microvillar elongation
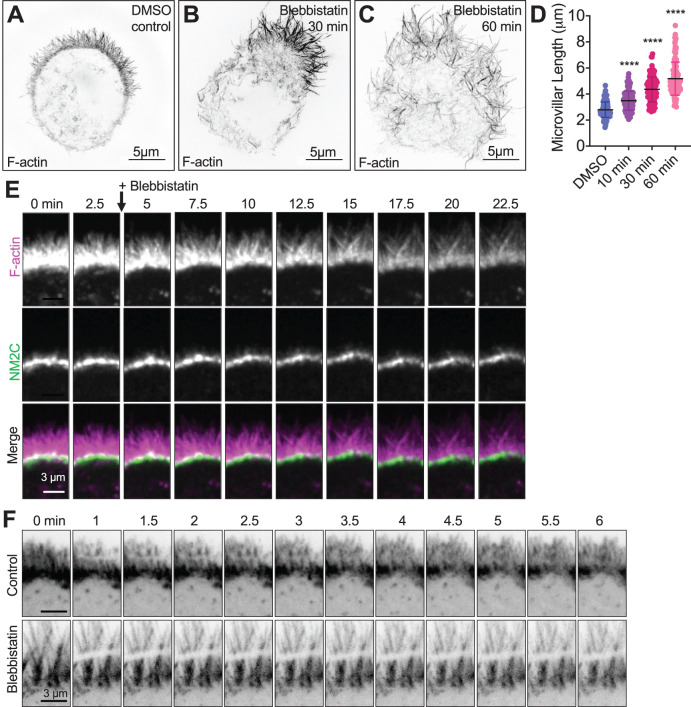
To investigate the impact of NM2 loss-of-function on the brush border actin cytoskeleton, we treated W4 cells with the well characterized NM2 inhibitor, blebbistatin, which binds to and locks the NM2 motor domain in a non–force producing state (Kovács et al., 2004; Limouze et al., 2004). After treating polarized W4 cells with 20 µM blebbistatin, we noted that microvilli became significantly less dynamic (Supplemental Movie 3) and subsequently exhibited a marked elongation, in some cases doubling their length (2.8 ± 0.6 µm control vs. 5.2 ± 1.3 µm blebbistatin treated) over the 60 min experimental time course (Figure 4, A–E). In contrast to the accumulation of NM2C observed in response to treatment with NM2 activators, inhibition with blebbistatin promoted a relatively static population of NM2C in the terminal web (Figure 4F; Supplemental Movie 3). Moreover, although W4 cell microvilli normally extend from a single clearly defined apical “cap,” we noted that blebbistatin induced the dispersion of protrusions across the cell surface (Figure 4C). Thus, inhibition of NM2 leads to unregulated microvillar growth and disorganization of the brush border on the epithelial cell surface.
FIGURE 4:
Inhibition of NM2 elongates microvilli and limits actin turnover. SIM MaxIP images of representative phalloidin-stained (F-actin) Ls174T-W4 cells fixed after (A) 60 min exposure to 20 μM DMSO vehicle control, (B) 10 min exposure to 20 μM blebbistatin, or (C) 60 min exposure to 20 μM blebbistatin; signals are inverted to facilitate visualization of dim structures. (D) Quantification of microvillar length from Ls174T-W4 cells fixed after 10, 30, and 60 min in 20 µM blebbistatin; each data point is a single microvillus, minimum of 10 cells per condition, at least 10 microvilli per cell. Ordinary one-way ANOVA with Dunnett’s multiple comparisons test; ****, p < 0.0001 vs. DMSO control. (E) Image montage of the brush border of a Ls174T-W4 cell expressing EGFP-UtrCH (magenta) and Halo-NM2C labeled with JF585 (green), imaged for 22.5 min after the addition of blebbistatin using spinning-disk confocal microscopy; 2.5 min interval between frames. (F) Image montage of FRAP analysis of Ls174T-W4 cells expressing mNeon-Green β-actin in the absence (top row) or presence (bottom row) of 20 µM blebbistatin for 15 min before imaging.
Movie S3.
Related to Figure 4. Blebbistatin elongates Ls174T-W4 cell microvilli. Spinning disk confocal imaging of an induced Ls174T-W4 cell expressing EGFP-UtrCH (magenta) and Halo-NM2C labeled with JF585 (green). Images were acquired every 30 seconds for 30 minutes; playback is 5 frames per second; field width is 29 μm.
Blebbistatin reverses the impact of calyculin A on microvillar length
Blebbistatin is a well-established and specific inhibitor of NM2, whereas calyculin A has broader effects on a group of threonine/serine phosphatases (Suganuma et al., 1990; Suzuki and Itoh, 1993; Kovács et al., 2004; Limouze et al., 2004). To determine whether the microvillar shortening effects we observed with calyculin A could be countered with blebbistatin treatment, polarized W4 cells were subjected to serial drug treatments (Supplemental Movie 4). Cells were first treated with 4 nM calyculin A for 50 min until microvillar length was significantly diminished. At the 50 min mark, calyculin A was chased with 20 µM blebbistatin. These time-lapse data revealed that specific inhibition of NM2 with blebbistatin was sufficient to drive rapid elongation of the shortened microvilli that result from calyculin A treatment (Supplemental Movie 4). On the basis of these data, we conclude that the impact of calyculin A on microvillar length is most likely mediated by an increase in terminal web NM2 activity. In combination with the calyculin A experiments described above, these data strongly suggest that under normal conditions, terminal web-localized NM2C plays a role in limiting the length of microvilli and promoting their confinement in the apical domain.
Movie S4.
Blebbistatin rescues microvilli shortened by Calyculin A. Spinning disk confocal imaging of an induced Ls174T-W4 cell expressing EGFP-UtrCH. Images were acquired every 30 seconds for 78 minutes; playback is 20 frames per second.
NM2 promotes actin turnover in brush border microvilli
How does terminal web NM2C limit the length of microvilli under normal conditions? Time-lapse analysis of W4 cells treated with 20 µM blebbistatin revealed that microvilli, which normally exhibit striking dynamics on the apical surface (e.g., elongation, shortening, and waving or pivoting around the base), immediately become static and subsequently begin to elongate (Supplemental Movie 3). A previous study from our group established that the parallel actin bundles that support microvilli exhibit robust treadmilling (i.e., retrograde flow), where actin monomer incorporation at tip-oriented barbed ends is balanced by subunit removal from the pointed ends that emerge from the base of these structures (Meenderink et al., 2019). Early in differentiation, this treadmilling activity is coupled to directed motion of microvilli across the apical surface, which in turn promotes the adherent packing and organization of these protrusions (Meenderink et al., 2019). In treadmilling actin structures, steady-state length can be increased by raising the incorporation rate at the barbed ends, lowering the disassembly rate at the pointed ends, or some combination of the two (Bonder et al., 1983; Brenner and Korn, 1983; Wang, 1985). To examine the possibility that NM2C mechanical activity promotes the treadmilling of microvillar cores by driving the disassembly of actin bundles in the terminal web (where the pointed ends reside), we performed FRAP on W4 cells transfected with mNeonGreen-β-actin, in the absence and presence of 20 µM blebbistatin. In control W4 cells, a rectangular region of interest (ROI) was bleached across the brush border and recovered rapidly, within <4 min, presumably due to treadmilling of individual actin cores (Figure 4F; Supplemental Movie 5). However, in W4 cells treated with 20 µM blebbistatin, bleached ROIs remained almost completely static and showed little to no recovery, even after up to 10 min (Figure 4F; Supplemental Movie 6). On the basis of these findings, we conclude that under normal conditions, NM2C promotes the disassembly of the pointed ends of core actin bundles within the terminal web. In the W4 model, without this activity (e.g., in response to blebbistatin treatment), treadmilling stalls and core bundles elongate as a result.
Movie S5.
Related to Figure 4. Microvillar core actin bundles are rapidly turned over. Spinning disk confocal imaging of a FRAP control experiment on induced Ls174T-W4 cell expressing mNeonGreen β-actin. ROI is bleached using a 405-laser line at 30% power with 100 μs dwell time. 5 frames acquired pre-bleach at 60 second intervals;22 frames acquired post-bleach at 30 second intervals for 10 minutes. Playback is 3 frames per second.
Movie S6.
Related to Figure 4. Inhibition of NM2 limits actin turnover. Spinning disk confocal imaging of a FRAP experiment on induced Ls174T-W4 cell expressing mNeonGreen-β-actin, treated with blebbistatin for 15 minutes prior to imaging. ROI is bleached using a 405-laser line at 30% power with 100 μs dwell time. 5 frames acquired pre-bleach at 60 second intervals; 22 frames acquired post-bleach at 30 second intervals for 10 minutes. Playback is 3 frames per second.
NM2C motor domain activity is required for limiting microvillar length
All myosin motor domains contain highly conserved residues that participate directly in actin and ATP binding, as well as ATP hydrolysis and force production (Chantler et al., 2010; Heissler and Manstein, 2013). Previous studies established that mutations to these residues disrupt myosin activity in predictable ways (Shimada et al., 1997; Kuhlman and Bagshaw, 1998; Sasaki et al., 1998, 2003; Kambara et al., 1999). To determine which facets of motor activity are needed for limiting microvillar length in W4 cells, we generated variants of NM2C with mutations predicted to lock the motor domain in weak or strong binding states (E497A or N252A, respectively; Sasaki et al., 1998; Shimada et al., 1997). We also targeted R784, which was identified as a critical residue in the NM2C crystal structure (Chinthalapudi et al., 2017). R784 rests at the interface of the converter, N-terminal subdomain, and lever arm in the NM2C motor domain, and mutation of this residue leads to failure of converter rotation and impaired nucleotide binding, hydrolysis, and release (Chinthalapudi et al., 2017). This general structure/function approach has been employed by our laboratory and others in the past to examine the requirement for myosin motor activity in different biological contexts (Belyantseva et al., 2005; Sakai et al., 2011; Weck et al., 2016). Typically, we would employ a knockdown (KD)/rescue strategy to first eliminate endogenous NM2C expression and then reexpress NM2C mutant variants to assess their function. However, NM2C KD was not well tolerated by W4 cells (unpublished data) as stable NM2C KD cells lines using lentiviral transduction of short hairpin RNAs were multinucleated and lacked a clearly defined apical brush border, which obfuscated analyss of microvillar morphology (unpublished data). As an alternate approach for assaying the impact of NM2C motor domain perturbations, we turned to an over-expression approach. Indeed, Halo-NM2C overexpression significantly shortened W4 cell microvilli (3.6 µm control vs. 2.6 µm Halo-NM2C; Figure 5, A, B, and G). As a point of comparison, we also overexpressed EGFP-NM2A, which exerted an even more potent length reduction effect (3.6 µm control vs. 2.3 µm in NM2A; Figure 5, C and G). Strikingly, all three NM2C variants with function-disrupting mutations in the motor domain were unable to significantly reduce microvillar length when overexpressed in W4 cells (Figure 5, D–G). From these data, we conclude that normal motor domain catalytic and mechanical activities are needed for NM2C to exert a length-limiting effect on microvilli.
FIGURE 5:
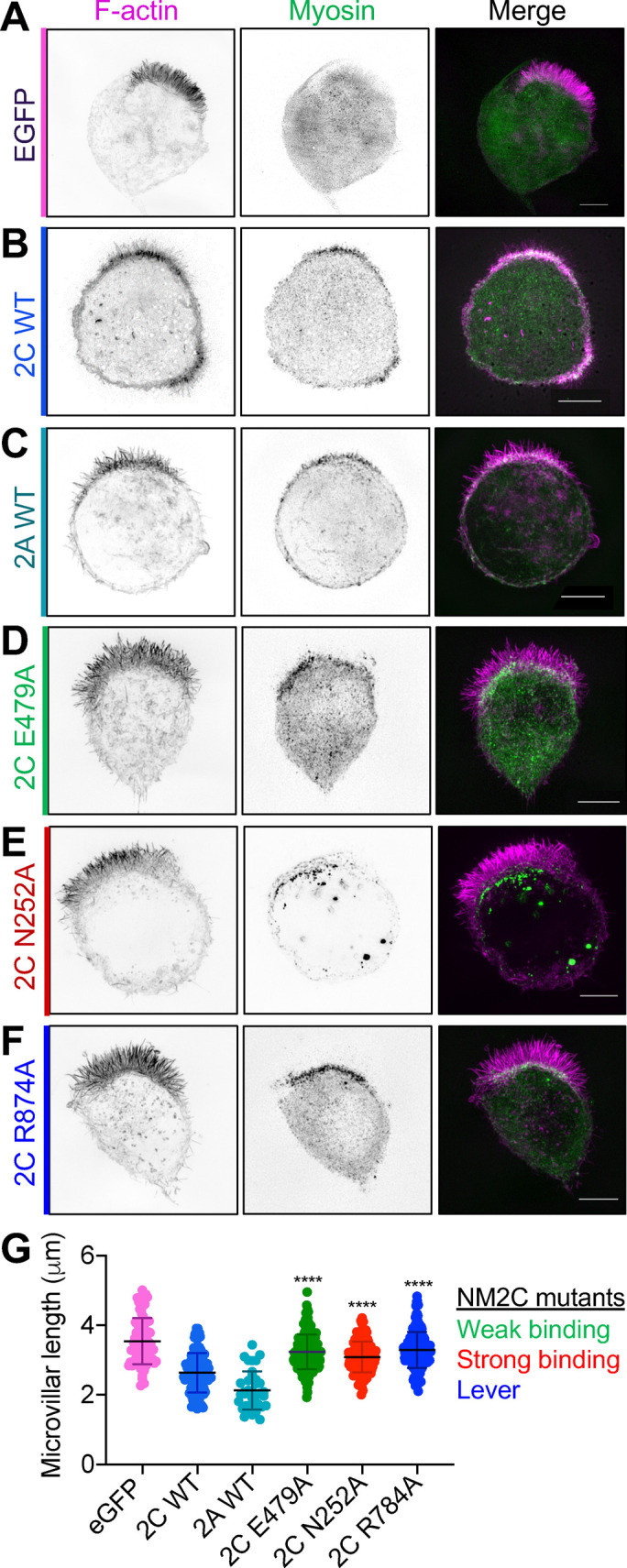
A functional NM2C motor domain is required for microvillar length regulation. SIM MaxIP images of representative Ls174T-W4 cell overexpressing (A) EGFP as a negative control, (B) WT Halo-NM2C, (C) WT EGFP-NM2A, (D) Halo-NM2C-E479A, (E) Halo-NM2C-N252A, or (F) Halo-NM2C-R874A. All Halo-NM2 constructs are labeled with JF585 (green), and cells are also costained with phalloidin to visualize F-actin (magenta). All scale bars = 5 μm. (G) Microvillar length quantification for each overexpression condition; measurements were made from 10 cells per construct, 8–10 microvilli measured per cell. Each data point is a single microvillus. Testing was performed using an ordinary one-way ANOVA with Dunnett’s multiple comparisons test; ****, p < 0.0001 vs. WT NM2C.
DISCUSSION
NM2C activity limits the length of dynamic epithelial microvilli
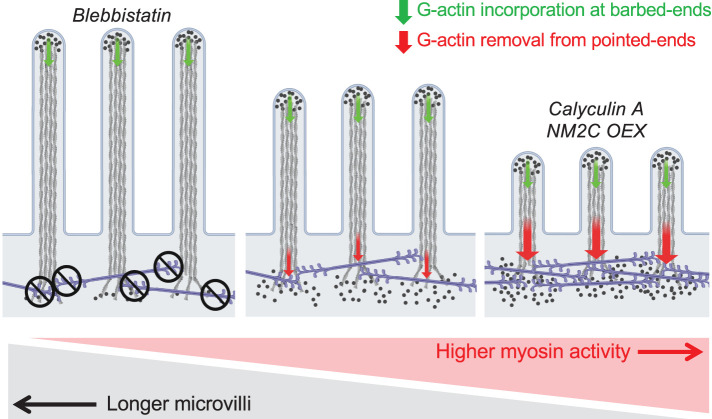
In this work, we establish that the poorly studied NM2C isoform, which is highly expressed in transporting and sensory epithelia, not only localizes to the well-characterized junctional network, but is also enriched in a second network that fully spans the cell at the level of the subapical terminal web. Our superresolution imaging studies show that terminal web NM2C is ideally positioned to interact directly with the rootlets of parallel actin bundles that support apical microvilli. Using a combination of chemical and genetic perturbations to increase or decrease the activity of this motor, we observed striking shortening or elongation of microvilli, respectively (Figure 6). Interestingly, previous studies from our laboratory revealed a similar effect of NM2 inhibition (with blebbistatin) on the nascent microvilli that exhibit robust translocation across the apical surface of LLC-PK1-CL4 kidney epithelial cells early in differentiation (Meenderink et al., 2019). In that context, inhibition of NM2 did not impair forward movement of microvilli, which was found to be driven by actin incorporation into core bundle filaments, although it did lead to the elongation of motile protrusions from their trailing ends (Meenderink et al., 2019). Thus, in two entirely different cellular contexts, blebbistatin exerts similar elongating effects on microvillar morphology. We note here that the time courses of the blebbistatin effects in these two cell culture models are quite different, and the slower onset in the W4 system is most likely explained by higher expression levels of efflux pumps in intestinal epithelial cells (e.g., MDR1; Lonsdale et al., 2013; Lizio et al., 2015). Nevertheless, together these findings lead us to conclude that NM2C activity limits the length of microvilli, most likely by promoting disassembly of core bundle actin filaments near or at their pointed ends, which are embedded in the subapical terminal web. Because microvillar length control mechanisms are still poorly defined, these findings represent an important first step toward understanding how the dimensions of these structures are controlled.
FIGURE 6:
Model of contractility-dependent actin turnover in brush border microvilli. Cartoon summarizing the phenotyping observations linking NM2 inhibition, activation, and overexpression to perturbations to microvillus length. NM2C might also play a role in physically sequestering microvilli on the apical surface, a function that is not captured in this graphic summary.
Organization of NM2C in the terminal web
The presence of a filamentforming myosin in the terminal web was first suggested decades ago in high-resolution transmission electron microscopy (TEM) images of microvillar rootlets (Mooseker and Tilney, 1975). These iconic images revealed that the space between core bundle rootlets is spanned by small filaments with dimensions comparable to those of thick filaments found in muscle. On the basis of these early observations, Mooseker and Tilney (1975) postulated that adjacent microvilli were physically linked by a filament-forming myosin, in a manner analogous to the organization of muscle sarcomeres. Indeed, the ability of NM2 to generate force at functionally significant levels is linked to its capacity to form bipolar filaments reminiscent of the thick filaments found in muscle sarcomeres (Huxley, 1957, 1969; Huxley and Simmons, 1971; Vicente-Manzanares et al., 2009; Stam et al., 2015). Based on in vitro studies with purified motor, NM2 filaments are composed of relatively small numbers (∼10s) of molecules, which self-associate via their long coiled-coil tail domains, leaving their N-terminal motor domains grouped at either end; the resulting structures extend 290–320 nm end-to-end (Billington et al., 2013). In this configuration, NM2 motor groups are capable of generating opposing forces of equal magnitude on actin structures bound by opposite ends of the bipolar filament, such as the rootlets of microvillar core bundles.
Our superresolution imaging studies of native intestinal tissues revealed striking networks of NM2C puncta in the circumferential/junctional band (Ebrahim et al., 2014), as well as the medial terminal web (Figure 1). Given that NM2C in the mouse model employed here is tagged on its C-terminus with EGFP, these puncta likely mark the center positions of bipolar filaments. Nearest neighbor measurements indicate that adjacent puncta in both junctional and medial populations are separated by comparable mean distances (204 vs. 212 nm, respectively; Figure 1), suggesting that these networks may be organized in similar ways. This is further supported by our FRAP studies of NM2C-EGFP dynamics in organoid monolayers, which showed that the turnover kinetics for these two populations are comparable. These similarities in spatial organization and turnover kinetics suggest that the junctional and medial populations of NM2C may be functionally analogous. Because the junctional band of NM2C is an established contractile array (Keller et al., 1985; Turner et al., 1997; Ebrahim et al., 2014), these points might additionally argue that medial/terminal web NM2C is also capable of exerting force on the rootlets of microvillar actin core bundles. We also note here that the interpuncta NM2C spacing measured in our samples (∼210 nm) is shorter than the mean length of NM2C filaments measured in vitro (∼290 nm) but comparable to the length of the NM2C filament bare zones (Billington et al., 2013). The superresolution measurements of puncta spacing reported here are also smaller than previous measurements on the NM2C junctional network in intestinal or stomach epithelial tissues (∼400–500 nm; Ebrahim et al., 2014). The large range of spacing measurements from distinct biological contexts suggests a high degree of plasticity in NM2 contractile network organization, which probably reflects specific mechanical needs in these different environments.
Contractility-dependent actin turnover as a conserved function for NM2
Our experiments with NM2 activation (calyculin A) and inhibition (blebbistatin) demonstrate that tuning the level of myosin contractility in polarized epithelial cells has a profound impact on microvillar length, with higher activity leading to shorter protrusions. Although calyculin A and blebbistatin both exert effects on all NM2 isoforms (Suzuki and Itoh, 1993; Limouze et al., 2004), previous proteomic characterizations of brush borders isolated from mouse small intestine indicate that NM2C is by far the most abundant isoform in this system (∼3-fold greater than NM2A, ∼12-fold greater than NM2B; McConnell et al., 2011); this is further confirmed by the scRNAseq data we present here (Supplemental Figure 1). Moreover, our localization studies performed in W4 cells demonstrate that NM2C specifically enriches in the terminal web, whereas NM2A exhibits a nonpolarized distribution over the entire cell cortex (Supplemental Figure 2, A vs. C). Taken together with our overexpression studies that establish NM2C is capable of shortening microvilli when present at high levels (Figure 5, B and G), we conclude that the effects of drug treatments reported here primarily reflect an impact on NM2C activity in the terminal web.
The idea that NM2 activity can promote actin network turnover is broadly consistent with findings from other diverse systems. Indeed, previous studies on mechanisms of neuronal growth cone motility showed that the rate of actin turnover (i.e., the treadmilling/retrograde flow rate) at the leading edge decreases by ∼50% when cells are treated with blebbistatin (Medeiros et al., 2006). In this system, blebbistatin exposure also resulted in a striking elongation of filopodial actin bundles from their basal ends, which are normally embedded in a meshwork of lamellipodial actin filaments. NM2 contractility has also been implicated in the disassembly of actin filaments at the apical junctional complex in response to Ca2+ depletion (Ivanov et al., 2004) and in the recycling of filaments that is required for normal contractile ring constriction during cytokinesis (Guha et al., 2005; Murthy and Wadsworth, 2005). Thus, the NM2C-dependent microvillar length control mechanism that we identify here represents a new facet of a broadly conserved class of function for filament-forming NM2 isoforms.
Potential mechanisms for NM2-driven contractility-dependent actin turnover
How does NM2C activity promote the shortening of microvillar actin bundles? Previous work established that growing and nascent microvilli are supported by highly dynamic core bundles of parallel actin filaments, which collectively exhibit robust treadmilling (Tyska and Mooseker, 2002; Meenderink et al., 2019). In treadmilling actin networks, new subunits incorporate at filament barbed ends, whereas disassembly dominates at the pointed ends. If assembly and disassembly rates are matched, the steady-state length of the structure can remain constant. To shorten a treadmilling bundle, the assembly rate must decrease, or the disassembly rate must increase (or some combination of the two). On the basis of its localization, we propose that NM2C activity shortens microvilli by accelerating, either directly or indirectly, core actin bundle disassembly in the terminal web; under normal conditions this activity would contribute to actin bundle treadmilling and turnover. This is consistent with our observations showing that the robust turnover dynamics of W4 microvillar actin bundles is attenuated following blebbistatin treatment (Figure 4F).
NM2C might act directly on microvillar actin filament pointed ends to drive their disassembly in the terminal web. Previous in vitro studies with purified actin and myosin-2 established that motor activity alone is capable of promoting filament disassembly (Haviv et al., 2008). Interestingly, NM2C does exhibit kinetic properties (a moderate duty ratio and potentially strain-sensitive catalytic cycle) that would allow it to strain actin filaments (Billington et al., 2013). NM2C could also function indirectly by promoting the activity of other factors capable of disassembling filaments. This would be supported by recent studies on Aplysia growth cones that revealed that NM2 contractility can enhance the localization and severing activity of the severing protein cofilin (Zhang et al., 2019). The proposed mechanisms by which NM2 might promote cofilin activity are based on its competition with cofilin binding, which creates distinct stiff and compliant zones (cofilin undecorated vs. decorated, respectively) along the filament, which are mechanically susceptible to severing at the boundaries of these regions (McCullough et al., 2008; De La Cruz et al., 2015; Schramm et al., 2017). Although there are currently no data to support a role for cofilin in controlling the length of epithelial microvilli, this could be one area of focus for future studies.
Additional roles for NM2C in polarized epithelia
In addition to promoting the turnover of actin filaments that constitute the microvillar core bundles, NM2C might also participate in other roles critical for normal epithelial cell polarization and function. For example, we noticed that microvilli undergo a striking dispersion across the cell surface in response to blebbistatin treatment (Figure 4, A–C; Supplemental Movie 4). Thus, by binding directly to core actin bundles, NM2 might play a role in apicobasal polarity reinforcement and constraint of the size of the apical domain (Ivanov et al., 2005). NM2C could also contribute to the organelle exclusion properties of the terminal web. The high density of cytoskeletal (actin and intermediate) filaments in the terminal web creates a zone of organelle exclusion that prevents endomembrane compartments from making direct contact with the apical surface. If NM2C forms filaments that span the gaps between microvillar rootlets, these structures might participate in regulating the movement of vesicles through this region. Interestingly, the presence of a terminal web meshwork is a unique feature of the intestinal epithelium; other brush border–presenting cells, such as those lining the kidney proximal tubule, lack a terminal web and zone of organelle exclusion (Mooseker et al., 1985; Coudrier et al., 1988). Importantly, these organs also lack strong NM2C localization, suggesting that NM2C may play a specific role in the compartmentalization of subcellular space in the intestinal epithelium (Golomb et al., 2004).
Deeper insight into how NM2C contributes to epithelial physiology will come from careful analysis of in vivo loss-of-function models. Although a mouse model null for MYH14 expression was previously described, the authors reported no overt physiological phenotypes (Ma et al., 2010). Other studies with the same KO mouse model revealed that in the absence of NM2C, NM2A and NM2B exhibit elevated levels in the intestinal epithelium as assessed by immunofluorescence signal (Ebrahim et al., 2014). Thus, an apparent lack of overt phenotypes at the whole animal level may be due to functional compensation by NM2A and NM2B. Phenotypic characterization of the NM2C KO mouse represents an exciting direction for future studies and might also provide a unique opportunity to investigate how the epithelium responds to higher levels of NM2 contractility, as would be expected based on the accelerated kinetic properties of NM2B and NM2A relative to NM2C (Heissler and Manstein, 2011; Billington et al., 2013).
MATERIALS AND METHODS
Frozen tissue preparation
All animal studies were conducted humanely according to National Institutes of Health and American Veterinary Medical Association guidelines by a protocol approved by the Institutional Animal Care and Use Committee, Vanderbilt University. Segments of small intestine were removed and flushed with cold phosphate-buffered saline (PBS) supplemented with 1.2 mM CaCl2 and 1 mM MgCl2 and then fixed with 2% paraformaldehyde (PFA)/PBS for 15 min. After initial fixation, the intestinal tube was cut along its length and fixed for an additional 2 h in 2% PFA/PBS at room temperature with gentle agitation. Samples were washed with PBS three times and subdissected before being floated lumen-side down in 30% sucrose with 1% sodium azide/PBS at 4°C overnight. Samples were then streaked through OCT embedding medium before being oriented in a block filled with fresh OCT and snap-frozen in dry ice-cooled acetone. Samples were then sectioned (10 µm thick for confocal imaging or 2 µm thick for SIM imaging) and mounted on slides for phalloidin staining.
Cell culture
Ls174T-W4 (W4) cells (female Hs colon epithelial cells) were cultured in DMEM with high glucose and 2 mM l-glutamine supplemented with 10% tetracycline-free fetal bovine serum (FBS), blasticidin (10 µg/ml), G418 (1 mg/ml), and phleomycin (20 µg/ml); cells were grown at 37°C and 5% CO2 as our group and others have previously described. This cell line was generous gift from Hans Clevers (Utrecht University, Netherlands).
Cloning and constructs
pEGFP-NM2C was obtained from Addgene (plasmid #10843). mNEON-green-β-actin was purchased from Allele Biotechnology. The pHalo-C1-NM2C (Halo-NM2C) construct was generated via PCR using pEGFP-NM2C as a template.The NM2C open reading frame PCR product was TOPO cloned into the pCR8/GW/TOPO vector (K250020; Invitrogen) and then shuttled into the pHalo-C1 backbone, adapted for Gateway cloning using the Gateway conversion kit (11828029; Invitrogen). To generate pHalo-NM2C mutant variants, E479A, R257A, N252A, and R784A were introduced into pHalo-NM2C using the QuikChange site-directed mutagenesis kit (200524; Agilent).
Transfections
All transfections were performed using Lipofectamine2000 (#11668019; Invitrogen) according to the manufacturer’s instructions, and W4 cells were allowed to recover overnight. Following recovery, W4 cells were seeded onto plates or coverslips, incubated overnight in the absence or presence of 1 μg/ml doxycycline to induce polarity, and then prepared for immunofluorescence or live-cell imaging.
Drug treatments
For fix and stain experiments, W4 cells were split onto glass coverslips and incubated for 12 h in the presence of 1 μg/ml doxycycline to induce apicobasal polarity and brush border formation. Induced W4 cells were then incubated with either 20 µM blebbistatin (B592500; Toronto Research Chemicals), 4 nM calyculin A (PHZ1044; Invitrogen), or 1 mM 4-hydroxyacetophenone (278564; Sigma-Aldrich) for 10, 30, or 60 min. After drug incubation, cells were fixed using methods described below. For live-cell imaging experiments, W4 cells were transfected with the appropriate construct and then split onto 35 mm glass bottom dishes (Invitro Scientific; product number, D35-20-1.5-N). Cells were imaged 24–72 h after transfection. Once on the microscope, blebbistatin, calyculin A, or 4-hydroxyacteophenone was added after ∼3–5 min of baseline imaging; cells were then imaged for an additional 20–60 min, depending on the drug treatment.
Cell and tissue staining
For SIM or laser scanning confocal imaging, W4 cells were plated onto glass coverslips and allowed to adhere overnight in the presence of 1 μg/ml doxycycline. For NM2C staining, cells were then washed with warm PBS and fixed with warm 4% PFA for 15 min at 37°C. Cells were subsequently washed three times with PBS and then permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. Cells were then blocked for 1 h at room temperature in 5% bovine serum albumin (BSA) in PBS. Finally, cells were incubated with primary antibody (Proteintech anti-NM2C, #20716-1-AP) diluted in 5% BSA/PBS, at 37°C for 1.5 h, followed by three washes with fresh PBS. Cells were then incubated for 1 h with the goat anti-rabbit Alexa Fluor 488F(ab’)2 Fragment (1:1000. A11070; Invitrogen) and Alexa Fluor 568 Phallodin (A12380; Invitrogen) at 37°C. Coverslips were washed three times with PBS and mounted on glass slides in ProLong Gold Antifade Mounting Media (P36930; Invitrogen). Frozen tissues sections were washed in PBS three times and stained with Alexa Fluor 568 Phallodin (A12380; Invitrogen) for 2 h at 37°C. Samples were then washed with PBS three times and mounted in ProLong Gold Antifade Mounting Media (P36930; Invitrogen). For transfection with Halo-tagged constructs, cells were incubated for 30 min with Janelia Fluor HaloTag ligand (gift from Luke Lavis, Howard Hughes Medical Institute Janelia Farms) of the appropriate color, diluted to 100 nM after fixation with 4% PFA. Alexa Fluor 488-phalloidin (1:200; A12379; Invitrogen) was diluted in PBS and incubated for 1 h at room temperature. Coverslips were washed three times with PBS and then mounted on glass slides in ProLong Gold (P36930; Invitrogen).
Light microscopy and image processing
Superresolution imaging was performed using a Nikon N-SIM Structured Illumination Microscope equipped with an Andor DU-897 EM-CCD, four excitation lasers (405, 488, 561, and 647 nm), and a 100×/1.49 NA total internal reflection fluorescence (TIRF) objective. SIM images were reconstructed using Nikon Elements. For live-cell spinning-disk confocal microscopy, W4 cells were transfected with the appropriate marker construct in a six-well plate. Cells were then split onto 35 mm glass bottom dishes (Invitro Scientific; product number, D35-20-1.5-N) and incubated in the presence or absence of 1 µg/ml doxycycline for a minimum of 12 h and up to 36 h. Cells transfected with Halo-tagged constructs were incubated with appropriately colored Janelia Fluor HaloTag ligand diluted to 100 nM in the standard cell culture media described above. Cells were incubated for 30 min with HaloTag ligand-media, the media was removed, and cells were washed with 37°C PBS. PBS was replaced with standard cell culture media, and the cells were allowed to equilibrate to new media for 15 min at 37°C. Live-cell imaging was performed on a Nikon Ti2 inverted light microscope equipped with a Yokogawa CSU-X1 spinning-disk head, Andor DU-897 EMCCD camera, or a Photometrics Prime 95B sCMOS camera, 488 and 561 nm excitation lasers, a 405 nm photo-stimulation laser directed by a Bruker miniscanner to enable targeted photoactivation, photoconversion, and photobleaching, and a 100×/1.49 NA TIRF objective. Images were acquired every 15–60 s for 20–60 min. For photokinetic studies of actin dynamics, baseline images were obtained for several frames before bleaching and then for an additional 3–10 min of recovery at 10 s intervals. Bleaching was performed on a line ROI (0.1 μm in width and 3–15 μm in length) using a 405 nm laser at 30% power with a 10 μs dwell time. During imaging, cells were maintained with humidity at 37°C with 5% CO2 using a stage-top incubation system. Image acquisition was controlled with Nikon Elements software. Three-dimensional (3D) time series images were oversampled in the z-dimension with z-steps ranging from 0.09 to 0.18 μm to allow for deconvolution (Nikon Elements Automatic or Richardson–Lucy algorithms). Images were contrast enhanced, cropped, and aligned using ImageJ software (National Institutes of Health [NIH]) or Nikon Elements. Two-dimensional images were generally viewed as a maximum intensity projection (MaxIP); 3D depth coding was performed using ImageJ software (NIH).
Image analysis, quantification, and statistics
All image analysis was performed using FIJI or Nikon Elements software, and all quantitative data are from at least three independent experiments. Radiality maps from en face SIM images showing terminal web NM2C-EGFP puncta were generated using NanoF-Super-Resolution Radial Fluctuations (SRRF) in FIJI (Gustafsson et al., 2016) with a ring radius of 2, radiality magnification of 1, and six ring axes. To perform line-scan analysis, a line was drawn along the axis of microvilli that were in plane with a distinct tip and base visible. The intensity of the NM2C signal was recorded and normalized with the lowest intensity set to 0 and the maximum set to 1. The microvillar length axis from individual scans was also normalized with the base set to 0 and the tip set to 1. Normalized line scans were then plotted together and fitted to a single Gaussian using nonlinear regression analysis (Prism v.7; GraphPad). For quantification of the percentage of cells with brush border, cells were scored as “brush border positive” if they displayed polarized F-actin accumulation as visualized using a 40× objective on a Nikon A1R laser-scanning confocal microscope as we have previously described. Microvillar length measurements were performed on projected SIM images by tracing individual microvillar actin bundles using FIJI. For analyses in which individual microvilli were measured, at least 10 microvillar actin bundles were scored per cell and at least 10 cells measured per experiment. Percent brush border and microvillar length data were analyzed with a D’Agostino and Pearson omnibus normality test to determine normal distribution. Normally distributed data were statistically analyzed to determine significance using the ordinary one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons to compare means between data sets. Statistical analyses performed are stated in the figure legends. All graphs were generated, and statistical analyses performed using Prism (v.7; GraphPad).
Supplementary Material
Acknowledgments
We thank all members of the Tyska laboratory, the Vanderbilt Microtubule and Motors Club, and the Vanderbilt Epithelial Biology Center for advice and support. This work was supported in part by National Institutes of Health (NIH) grants R01-DK111949 and R01-DK095811 (M.J.T.). Microscopy was performed in part through the Vanderbilt Cell Imaging Shared Resource, supported by the Vanderbilt Digestive Disease Research Center funded by NIH grant P30DK058404 (PI: Peek). C.R.C. was supported in part by Vanderbilt Molecular Biophysics Training Program T32-GM008320 (PI: Chazin).
Abbreviations used:
- EGFP
enhanced green fluorescent protein
- ELC
essential light chain
- EM
electron microscopy
- FRAP
fluorescence recovery after photobleaching
- HC
heavy chain
- KD
knockdown
- KO
knockout
- NM2
nonmuscle myosin-2
- NM2A
nonmuscle myosin-2A
- NM2B
nonmuscle myosin-2B
- NM2C
nonmuscle myosin-2C
- RLC
regulatory light chain
- scRNAseq
single cell RNA sequencing
- SIM
structured illumination microscopy
- SRRF
super-resolution radial fluctuations
- TEM
transmission electron microscopy
- W4
Ls174T-W4 cell.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E20-09-0582) on October 7, 2020.
REFERENCES
- Almutawa W, Smith C, Sabouny R, Smit RB, Zhao T, Wong R, Lee-Glover L, Desrochers-Goyette J, Ilamathi HS, Care4Rare Canada Consortium, et al. (2019). The R941L mutation in MYH14 disrupts mitochondrial fission and associates with peripheral neuropathy. EBioMedicine , 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson SJ, Stewart M (1992). Molecular interactions in myosin assembly. Role of the 28-residue charge repeat in the rod. J Mol Biol , 7–13. [DOI] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, Van Der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC (2004). Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD Annette. Cell , 5. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Herring CA, Chen B, Kim H, Simmons AJ, Southard-Smith AN, Allaman MM, White JR, Macedonia MC, Mckinley ET, et al. (2020). Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology, DOI: 10.1053/j.gastro.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles JR, Zheng L, Li A, Wierda A, Chen B (1998). Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol , 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB (2005). Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol , 148–156. [DOI] [PubMed] [Google Scholar]
- Betapudi V (2014). Life without double-headed non-muscle myosin II motor proteins. Front Chem , 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington N, Wang A, Mao J, Adelstein RS, Sellers JR (2013). Characterization of three full-length human nonmuscle myosin II paralogs. J Biol Chem , 33398–33410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder EM, Fishkind DJ, Mooseker MS (1983). Direct measurement of critical concentrations and assembly rate constants at the two ends of an actin filament. Cell , 491–501. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Korn ED (1983). On the mechanism of actin monomer-polymer subunit exchange at steady state. J Biol Chem , 5013–5020. [PubMed] [Google Scholar]
- Bretscher A, Weber K (1979). Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci USA , 2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Weber K (1980). Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol , 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Wylie SR, Wheeler-Jones CP, McGonnell IM (2010). Conventional myosins— unconventional functions. Biophys Rev , 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinthalapudi K, Heissler SM, Preller M, Sellers JR, Manstein DJ (2017). Mechanistic insights into the active site and allosteric communication pathways in human nonmuscle myosin-2C. eLife , e32742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E, Kerjaschki D, Louvard D (1988). Cytoskeleton organization and submembranous interactions in intestinal and renal bursh borders. Kidney Int , 309–320. [DOI] [PubMed] [Google Scholar]
- Crawley SW, Mooseker MS, Tyska MJ (2014). Shaping the intestinal brush border. J Cell Biol , 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz EM, Martiel JL, Blanchoin L (2015). Mechanical heterogeneity favors fragmentation of strained actin filaments. Biophys J , 2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaudy F, Snoeckx R, Pfister M, Zenner HP, Blin N, Di Stazio M, Ferrara A, Lanzara C, Ficarella R, Declau FD, et al. (2004). Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4). Am J Hum Genet , 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D, Gröschel-Stewart U (1980). Localization of myosin, actin, and tropomyosin in rat intestinal epithelium: immunohistochemical studies at the light and electron microscope levels. J Cell Biol , 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulyaninova NG, Bresnick AR (2013). The heavy chain has its day: regulation of myosin-II assembly. Bioarchitecture , 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Fujita T, Millis BA, Kozin E, Ma X, Kawamoto S, Baird MA, Davidson M, Yonemura S, Hisa Y, et al. (2014). NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol , 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zhang L, Jin Y, Sun X, Zhang A, Wen Z, Zhou Y, Xia M, Gao J (2016). Loss of Myh14 increases susceptibility to noise-induced hearing loss in CBA/CaJ mice. Neural Plast , 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney JR, Glenney P (1983). Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell , 503–512. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Glenney P, Osborn M, Weber K (1982). An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell , 843–854. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS (2004). Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem , 2800–2808. [DOI] [PubMed] [Google Scholar]
- Grega-Larson NE, Crawley SW, Erwin AL, Tyska MJ (2015). Cordon bleu promotes the assembly of brush border microvilli. Mol Biol Cell , 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm-Gunter E-MS, Revenu C, Ramos S, Hurbain I, Smyth N, Ferrary E, Louvard D, Robine S, Rivero F (2009). Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol Biol Cell , 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Zhou M, Wang Y (2005). Cortical actin turnover during cytokinesis requires myosin II. Curr Biol , 732–736. [DOI] [PubMed] [Google Scholar]
- Gustafsson N, Culley S, Ashdown G, Owen DM, Pereira PM, Henriques R (2016). Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat Commun , 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JJ, Nguyen PD, Oh DY, Han JH, Kim AR, Kim MY, Park HR, Tran LH, Dung NH, Koo JW, et al. (2019). Elucidation of the unique mutation spectrum of severe hearing loss in a Vietnamese pediatric population. Sci Rep , 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A (2008). A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol , 325–330. [DOI] [PubMed] [Google Scholar]
- Heissler SM, Manstein DJ (2011). Comparative kinetic and functional characterization of the motor domains of human nonmuscle myosin-2C isoforms. J Biol Chem , 21191–21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler SM, Manstein DJ (2013). Nonmuscle myosin-2: mix and match. Cell Mol Life Sci , 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander HF, Fändriks L (2014). Surface area of the digestive tract– revisited. Scand J Gastroenterol , 681–689. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Heuser JE (1981). Quick-freeze, deep-etch visualization of the cytoskeleton beneath surface differentiations of intestinal epithelial cells. J Cell Biol , 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Tilney LG, Fujiwara K, Heuser JE (1982). Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J Cell Biol , 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Sacramone LM, Mooseker MS, Morrow JS (1985). Mechanisms of cytoskeletal regulation: modulation of membrane affinity in avian brush border and erythrocyte spectrins. J Cell Biol , 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull BE, Staehelin LA (1979). The terminal web. A reevaluation of its structure and function. J Cell Biol , 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H (1957). The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol , 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H (1969). The mechanism of muscular contraction. Science , 1356–1366. [PubMed] [Google Scholar]
- Huxley H, Simmons R (1971). Proposed mechanism of force generation in striated muscle. Nature , 533–538. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA (2005). Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell , 2636–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Parkos CA, Nusrat A (2004). Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell , 2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SS, Kim KY, Mao J, Kawamoto S, Sellers JR, Adelstein RS (2009). An alternatively spliced isoform of non-muscle myosin II-C is not regulated by myosin light chain phosphorylation. J Biol Chem , 11563–11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara T, Rhodes TE, Ikebe R, Yamada M, White HD, Ikebe M (1999). Functional significance of the conserved residues in the flexible hinge region of the myosin motor domain. J Biol Chem , 16400–16406. [DOI] [PubMed] [Google Scholar]
- Keller TCS, Conzelman KA, Chasan R, Mooseker MS (1985). Role of myosin in terminal web contraction in isolated intestinal epithelial brush borders. J Cell Biol , 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Kim AR, Han JH, Lee C, Oh DY, Choi BY (2017). Discovery of MYH14 as an important and unique deafness gene causing prelingually severe autosomal dominant nonsyndromic hearing loss. J Gene Med , 1–6. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kovács M, Kawamoto S, Sellers JR, Adelstein RS (2005). Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem , 22769–22775. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee S, Park HJ, Kang TH, Sagong B, Baek JI, Oh SK, Choi JY, Lee KY, Kim UK (2016). Genetic association of MYH genes with hereditary hearing loss in Korea. Gene , 177–182. [DOI] [PubMed] [Google Scholar]
- Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Seller JR (2004). Mechanism of blebbistatin inhibition of myosin II. J Biol Chem , 35557–35563. [DOI] [PubMed] [Google Scholar]
- Kuhlman PA, Bagshaw CR (1998). ATPase kinetics of the Dictyostelium discoideum myosin II motor domain. J Muscle Res Cell Motil , 491–504. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Puchtler H, Clermont Y (1960). Structures corresponding to terminal bars and terminal web in many types of cells. Nature , 784–788. [DOI] [PubMed] [Google Scholar]
- Lerat J, Magdelaine C, Roux AF, Darnaud L, Beauvais-Dzugan H, Naud S, Richard L, Derouault P, Ghorab K, Magy L, et al. (2019). Hearing loss in inherited peripheral neuropathies: molecular diagnosis by NGS in a French series. Mol Genet Genomic Med , 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limouze J, Straight AF, Mitchison T, Sellers JR (2004). Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil , 337–341. [DOI] [PubMed] [Google Scholar]
- Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, Abugessaisa I, Fukufa S, Hori F, Ishikawa-Kato S, et al. (2015). Gateways to the FANTOM5 promoter level mammalian expressionatlas. Genome Biol , 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, et al. (2013). The Genotype-Tissue Expression (GTEx) project. Nat Genet , 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS (2010). Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis. Mol Biol Cell , 4953–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Yang J (2015). Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta , 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell RE, Benesh AE, Mao S, Tabb DL, Tyska MJ (2011). Proteomic analysis of the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol , 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough RB, Blanchoin L, Martiel J-L, De La Cruz EM (2008). Cofilin increases the bending flexibility of actin filaments: implications for severing and cell mechanics. J Mol Biol , 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan AD, Karn J (1982). Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature , 226–231. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P (2006). Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol , 216–226. [DOI] [PubMed] [Google Scholar]
- Meenderink LM, Gaeta IM, Postema MM, Cencer CS, Chinowsky CR, Krystofiak ES, Millis BA, Tyska MJ (2019). Actin dynamics drive microvillar motility and clustering during brush border assembly. Dev Cell , 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Bonder EM, Conzelman KA, Fishkind DJ, Howe CL, Keller TC (1984). Brush border cytoskeleton and integration of cellular functions. J Cell Biol , 104S–112S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Bonder EM, Conzelman KA, Fishkind DJ, Howe CL, Keller TC (1985). The cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol , 209–241.3916317 [Google Scholar]
- Mooseker MS, Graves TA, Wharton KA, Falco N, Howe CL (1980). Regulation of microvillus structure : calcium-dependent solation and cross-linking of actin filaments in the microviili of intestinal epithelial cells. J Cell Biol , 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Tilney LG (1975). Organization of an actin filament-membrane complex: filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol , 725–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K, Wadsworth P (2005). Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol , 724–731. [DOI] [PubMed] [Google Scholar]
- Postema MM, Grega-Larson NE, Meenderink LM, Tyska MJ (2019). PACSIN2-dependent apical endocytosis regulates the morphology of epithelial microvilli. Mol Biol Cell , 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postema MM, Grega-Larson NE, Neininger AC, Tyska MJ (2018). IRTKS (BAIAP2L1) elongates epithelial microvilli using EPS8-dependent and independent mechanisms. Curr Biol , 2876–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost J, Barbetta C, Joanny JF (2007). Dynamical control of the shape and size of stereocilia and microvilli. Biophys J , 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, Loew D, Delacour D, Gilet J, Bro-Laroche E, Rivero F, et al. (2012). A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol Biol Cell , 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Umeki N, Ikebe R, Ikebe M (2011). Cargo binding activates myosin VIIA motor function in cells. Proc Natl Acad Sci USA , 7028–7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Ohkura R, Sutoh K (2003). Dictyostelium myosin II mutations that uncouple the converter swing and ATP hydrolysis cycle. Biochemistry , 90–95. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Shimada T, Sutoh K (1998). Mutational analysis of the switch II loop of Dictyostelium myosin II. J Biol Chem , 20334–20340. [DOI] [PubMed] [Google Scholar]
- Schramm AC, Hocky GM, Voth GA, Blanchoin L, Martiel J-L, De La Cruz EM (2017). Actin filament strain promotes severing and cofilin dissociation. Biophys J , 2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Sasaki N, Ohkura R, Sutoh K (1997). Alanine scanning mutagenesis of the switch I region in the ATPase site of Dictyostelium discoideum myosin II. Biochemistry , 14037–14043. [DOI] [PubMed] [Google Scholar]
- Song MH, Jung J, Rim JH, Choi HJ, Lee HJ, Noh B, Lee JS, Gee HY, Choi JY (2020). Genetic inheritance of late-onset, down-sloping hearing loss and its implications for auditory rehabilitation. Ear Hear , 114–124. [DOI] [PubMed] [Google Scholar]
- Southard-Smith AN, Simmons AJ, Chen B, Jones AL, Solano MAR, Vega PN, Scurrah CR, Zhao Y, Brenan MJ, Xuan J, et al. (2020). Dual indexed library design enables compatibility of in-drop single-cell RNA-sequencing with exAMP chemistry sequencing platforms. BMC Genomics , 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam S, Alberts J, Gardel ML, Munro E (2015). Isoforms confer characteristic force generation and mechanosensation by myosin II filaments. Biophys J , 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M, Fujiki H, Furuya-Suguri H, Yoshizawa S, Yasumoto S, Kato Y, Fusetani N, Sugimura T (1990). Calyculin A, an inhibitor of protein phosphatases, a potent tumor promoter on CD-I mouse skin. Cancer Res , 3521–3525. [PubMed] [Google Scholar]
- Suzuki A, Itoh T (1993). Effects of calyculin A on tension and myosin phosphorylation in skinned smooth muscle of the rabbit mesenteric artery. Br J Pharmacol , 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Colland F, de Koning J, Maurice MM, Hornbeck P, Clevers H (2009). Mst4 and ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell , 551–562. [DOI] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL (1997). Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol , C1378–C1385. [DOI] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS (2002). MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J , 1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance BA, Chan C, Robertson ML, Finlay BB (2002). Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Can J Gastroenterol , 771–778. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol , 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL (1985). Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol , 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hosoya H, Yonemura S (2007). Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol Biol Cell , 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck ML, Crawley SW, Stone CR, Tyska MJ (2016). Myosin-7b promotes distal tip localization of the intermicrovillar adhesion complex. Curr Biol , 2717–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Ajeti V, Tsai N, Fereydooni A, Burns W, Murrell M, De La Cruz EM, Forscher P (2019). Regulation of axon growth by myosin II– dependent mechanocatalysis of cofilin activity. J Cell Biol , 2329–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Peng L, Chen G, Jiang W, Shen Z, Du C, Zang R, Su Y, Xie H, Li H, et al. (2017). Mutations of MYH14 are associated to anorectal malformations with recto-perineal fistulas in a small subset of Chinese population. Clin Genet , 503–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.