Abstract
Folate is increasingly thought to promote gastrointestinal health and regulate the diversity of gut microbiota to alleviate weaning stress in piglets. The present study was conducted to investigate the effects of folate on organ weight, digesta pH, short-chain fatty acids (SCFAs) concentration, and intestinal microbiota in weaned piglets. A total of 28 piglets (6.73 ± 0.62 kg) were allocated to four dietary treatments consisting of a control group, 3, 9, and 18 mg/kg of folate supplementation in a 14-d feeding trial. The results showed that piglets fed with 9 and 18 mg/kg of folate supplementation had greater (P < 0.05) average liver and spleen weight than the control group. Folate supplementation (9 and 18 mg/kg) can significantly increase (P < 0.05) the stomach pH and tend (P < 0.10) to decrease the cecum pH. Folate treatment (9 and 18 mg/kg) had a positive effect on the metabolism of SCFAs in piglets, in particular, compared with the control group, and the content of acetic acid (AA) and valeric acid was markedly increased (P < 0.05) in the cecum and colon, respectively. Moreover, isobutyric acid, butyric acid, and isovaleric acid were tended (P < 0.10) to increase in the colon. Cecum contents samples were used to determine bacterial community diversity by 16S rRNA gene amplicon sequencing. At the genus level, in the cecum, there was a higher (P < 0.05) relative abundance of Lactobacillus reuteri, Lactobacillus salivarius, and Lactobacillus mucosae in the 9 mg/kg folate supplementation group. The functional pathways analysis predicted that folate may modify nutrient metabolism by changing the gut microbiota function of weaned piglets. Furthermore, the data showed that Lactobacillus was positively correlated with AA in the cecum. Overall, these findings suggested that folate treatment could increase the organ weight and the stomach pH of weaned piglets and had beneficial effects on gut health, which might be attributed to the alteration in intestinal microbiota induced by folate and the interaction of the intestinal microbiota with SCFAs.
Keywords: digesta pH, folate, intestinal microbiota, organ weight, short-chain fatty acid, weaned piglets
Introduction
Early weaning stress syndrome in piglets is an important event in the large-scale breeding of pigs, causing huge economic losses to the agriculture enterprises every year. A series of studies have proved that weaning can lead to atrophy of piglet intestinal villi, digestive and absorption disorder, and inflammation, which induce the release of a large number of reactive oxygen species (ROS) free radicals, eventually causing intestinal dysfunction and diseases (Xiong et al., 2015; Zou et al., 2017). Evidences have shown that diet supplementation of appropriate doses of some functional nutrients, such as vitamins, can adjust or improve the immune function and capability of disease resistance and reduce the risk of disease infection or inflammatory responses of weaned piglet (Chen et al., 2019; Li et al., 2019; Wang et al., 2020b).
Folate is considered as a form of the water-soluble vitamin B9 and plays an important role in the growth, health, and maintenance of animal metabolism (Bässler, 1997; Ragaller et al., 2009; Sesay et al., 2017). Folate metabolism also plays an important role in DNA synthesis, chromosomal stability, and integrity via regulated spindle assembly checkpoint signaling (Guo et al., 2017). Mammals, including rats and pigs, cannot synthesize folate, and folate in their bodies is mainly derived from food. Previous studies have shown that folate deficiency can elevate oxidative stress, impair antioxidant enzymatic activities, increase ROS generation, reduce mtDNA biogenesis, and promote mitochondrial oxidative decay (Huang et al., 2001; Hsu et al., 2013). A series of studies from animal models have shown that folate supplementation during critical periods in early development induces different metabolic phenotypes by regulating the promoter DNA methylation status of some specific genes and subsequent mRNA expression abundance. Folate plays a key part in enhancing the immune capacities and resistance against bacterial infection of animals. Folate deficiency was also associated with impaired maturation of dendritic cells (DCs), decreased cytokines (interleukin-6 [IL-6], IL-12, and tumor necrosis factor-alpha [TNF-α]) secretion by DCs stimulated with lipopolysaccharide and inhibit differentiation of CD4(+) T lymphocytes into Th17 cells (Wu et al., 2017). Grieshop et al. (2001) reported that the daily supplementation of folate to pregnant sows can affect the percentage of peripheral lymphocytes and the secondary antibody response level of the piglets at the weaning process. Moreover, Wang et al. (2011) found that supplementation of folate (100 mg/kg) in the diets of lactating sows can increase milk production, improve milk quality, and increase the growth performance of piglets. Supplementation of folate in the diet can resynthesize methionine for protein synthesis during moderate methionine deficiency in piglets (McBreairty et al., 2016).
It is possible and necessary to add a higher dose of folate in the diet of weaned piglets to meet their needs and promote immune function, improve the capability of disease resistance, and relieve inflammation. However, little or no data exist presently quantifying folate supplementation on physicochemical effects in the gastrointestinal tract of weaned piglets. Therefore, the purpose of the present study was to determine the effect of dietary supplementation of folate on organ weights, digesta pH, short-chain fatty acid (SCFAs) concentration, and intestinal microbiota of weaned piglets. This may provide valuable insights into exploring the optimum supplementary dose of folate in weaned piglets.
Materials and Methods
The animal experimental design and procedures used in the present study were approved by the Animal Protocol Review Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Changsha, China).
Animals and treatments
A total of twenty-eight piglets (Duroc × [Landrace × Yorkshire]; 6.73 ± 0.62 kg) weaned at 21 d of age were randomly assigned by body weight (BW) and gender to four treatments, and each treatment included seven replicate pens and one piglet per pen. Each treatment consisted of a basal diet supplemented with different dosages of folic acid. Treatments A, B, C, and D groups were supplemented with 0, 3, 9, and 18 mg/kg of folate, respectively, 0, 10, 30, and 60 times according to the NRC’s (2012) recommendation. The basal diet composition is presented in Table 1 was formulated to meet the nutrient specifications for 7 to 11 kg BW piglets (NRC, 2012). The folate product used in the present study was composed of 80% folate and 20% carrier (Beijing, Blooming Biotechnology Co., Ltd.). The piglets were allowed to consume diets ad libitum and free access to water for 14 d. Previous studies reported that after weaning there are acute phase (first 5 to 7 d) and adaptive phase (second week after weaning), since it takes about 7 d for weaned pigs to learn how to eat out of a feeder and resume an intake that is comparable to that during the preweaning period (Pluske et al., 1997; Le Dividich and Seve, 2000). Therefore, the present study conducted a 14-day animal experimental period.
Table 1.
Ingredient and chemical composition of piglet diets, as-fed basis
| Ingredients | % |
|---|---|
| Corn | 39.7 |
| Extruded corn | 20.0 |
| Soybean meal, 44% crude protein | 9.0 |
| Fish meal, 63% crude protein | 7.0 |
| Spray-dried porcine plasma | 5.0 |
| Whey powder | 9.0 |
| Glucose | 3.0 |
| Soybean oil | 3.8 |
| Limestone | 1.05 |
| Choline chloride | 0.1 |
| Antioxidants premix1 | 0.05 |
| Citric acid | 0.5 |
| NaCl | 0.1 |
| Mineral premix2 | 0.15 |
| l-Lys HCl | 0.45 |
| dl-Met | 0.20 |
| l-Thr | 0.14 |
| l-Trp | 0.02 |
| Vitamin premix3 | 0.74 |
| Total | 100 |
| Metabolizable energy, kcal/kg | 3,393 |
| Crude protein, % | 18.50 |
| Calcium, % | 0.80 |
| Phosphorus, % | 0.38 |
| Folic acid, mg/kg | 0.20 |
| Lys, % | 1.37 |
| Met + Cys, % | 0.75 |
| Thr, % | 0.81 |
| Trp, % | 0.22 |
1Antioxidants premix per kilogram of feed: 50 mg ethoxyquin.
2Mineral premix per kilogram of feed: 150 mg Fe (FeSO4), 100 mg Zn (ZnSO4), 30 mg Mn (MnSO4), 25 mg Cu (CuSO4), 0.5 mg I (KIO3), 0.3 mg Co (CoSO4), and 0.3 mg Se (Na2SeO3).
3Vitamin premix supplied per kilogram of feed: 2,200 IU vitamin A, 220 IU vitamin D3, 0.5 mg vitamin K3, 0.0175 mg vitamin B12, 3.5 mg riboflavin, 30 mg niacin, 10 mg D-pantothenic acid, 0.05 mg biotin, 0.3 mg thiamine, and 7 mg pyridoxine.
Organ weight and digesta pH
On day 14 of the trial, all piglets were sacrificed by captive bolt penetration under general anesthesia via intravenous injection of 4% sodium pentobarbital solution (40 mg/kg BW). Subsequently, piglets were immediately eviscerated from sternum to pubis, and the small intestinal tract and organs (liver, spleen, and kidney) were removed, and, finally, the weight (emptied from digesta and blood) of each organ was recorded. Approximately, 30 mL of digesta samples from the stomach, medial ileum (30 cm), medial cecum (30 cm), and medial colon (30 cm) was collected for the pH analysis using a digital pH meter (Fisher Scientific, Sunnyvale, USA).
Intestinal histology and morphology analysis
The cross sections of the cecum and colon samples preserved in formaldehyde and glutaraldehyde mixing fixative were prepared using standard paraffin embedding techniques. The samples were sectioned at 5 μm thickness and were stained with hematoxylin and eosin. The crypt depth (CD) was measured under a microscope with 40× combined magnification using an image processing and analysis system (version 1; Leica Imaging Systems Ltd, Cambridge, UK). At least, 10 well-oriented intact villi and the associated CD of each section were measured in each piglet.
Determination of SCFAs
The amount of SCFAs in the cecum and colon digesta was measured using gas chromatography (GC) according to the method of Alfa et al. (2018). Briefly, digesta samples were homogenized, and 1 g of them was diluted with ddH2O. After mechanical shaking and centrifuging at 19,152 × g for 15 min, a mixture of supernatant fluid and 25% metaphosphoric acid solution (9:1, v/v) was allowed to stand overnight. Afterward, the solution was centrifuged and filtered through a membrane filter (pore size 0.45 μm). The SCFAs concentrations of samples on a DB-FFAP column (30 m long × 250 μm diameter × 0.25 μm film thickness) were separated by using an Agilent Technologies 7890B GC System with flame ionization detector (FID). Nitrogen was used as the carrier gas with a flow rate of 0.8 mL/min. An FID was used to determine acetate, propionate, butyrate, isobutyrate, valerate, and isovalerate concentrations. The temperatures of the detector and injector temperatures were 280 and 250 °C, respectively.
16S rRNA high-throughput sequencing
The cecum contents from piglets in the control group (without folate supplementation) and the group receiving 9 mg/kg feed of folate (n = 7 per treatment) were collected for microbiota diversity analysis, as described previously (Zou et al., 2019). The total bacterial DNA was extracted from the cecum content (approximately 0.25 g) using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Bacterial community diversity and composition in each cecum sample were determined by 16S ribosomal DNA (rDNA) high-throughput sequencing. The V4 hypervariable region of 16S rRNA gene was PCR amplified using 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primers with barcodes. Paired-end sequencing was performed on a Thermofisher IonS5XL platform (Novogene, Beijing, China).
Bioinformatics analysis of sequencing data
Raw 16S data sequences were obtained, before being screened and assembled using the QIIME (v.1.9.1) software packages. UPARSE (v7.0.1001) was used to analyze these clean reads and determine operational taxonomic units (OTUs). Subsequently, high-quality sequences were aligned against the SILVA (http://www.arb-silva.de/) and clustered into OTUs at a 97% similarity level using the MUSCLE (v3.8.31) algorithm.
Functional metagenomes of all samples were predicted via PICRUSt. OTUs were normalized by copy number, and the gene categories were predicted at level 3 functional difference (Kyoto Encyclopedia of Genes and Genomes [KEGG] Orthology) abundances using the STAMP (V2.1.3) software package.
Statistical analysis
All statistical analyses were performed using SPSS 22.0 statistical package (SPSS Inc., Chicago, IL, USA). Alpha and beta diversities were analyzed using QIIME (v1.9.1). R package software (V2.15.3, R Core Team, Auckland, New Zealand) was used to perform for the statistical analyses of microbial community and create visualized diagrams. Data for microbial metabolic functions were analyzed by Student’s t-test. A value of P < 0.05 was taken to indicate statistical significance. Tendencies were reported when P < 0.1.
Results
Effect of folate supplementation on organ weights and intestinal morphology
The effect of folate supplementation on organ weights is provided in Table 2. The relative weights of the liver, spleen, and kidney were increased by folate supplementation. The liver and spleen of piglets fed 9 and 18 mg/kg folate had higher (P < 0.05) relative weight than the control group. In addition, the relative weight of the kidney in piglets fed diets containing 18 mg/kg folate was significantly increased (P < 0.05) compared with the control group. However, there were no differences in the relative length of the small intestine (m/kg BW) among the four treatments.
Table 2.
Effect of different folate levels on organ index of piglets1
| Supplement folate level, mg/kg | ||||||
|---|---|---|---|---|---|---|
| Items | 0 | 3 | 9 | 18 | SEM | P-value |
| Liver, g/kg BW | 20.71a | 23.42ab | 24.86b | 26.14b | 0.72 | 0.041 |
| Spleen, g/kg BW | 1.59a | 2.03ab | 2.25b | 2.22b | 0.09 | 0.024 |
| Kidney, g/kg BW | 5.32a | 5.78a | 5.94ab | 6.94b | 0.21 | 0.033 |
| Length of the small intestine, m/kg BW | 1.17 | 1.25 | 1.33 | 1.24 | 0.03 | 0.362 |
1 n = 7.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
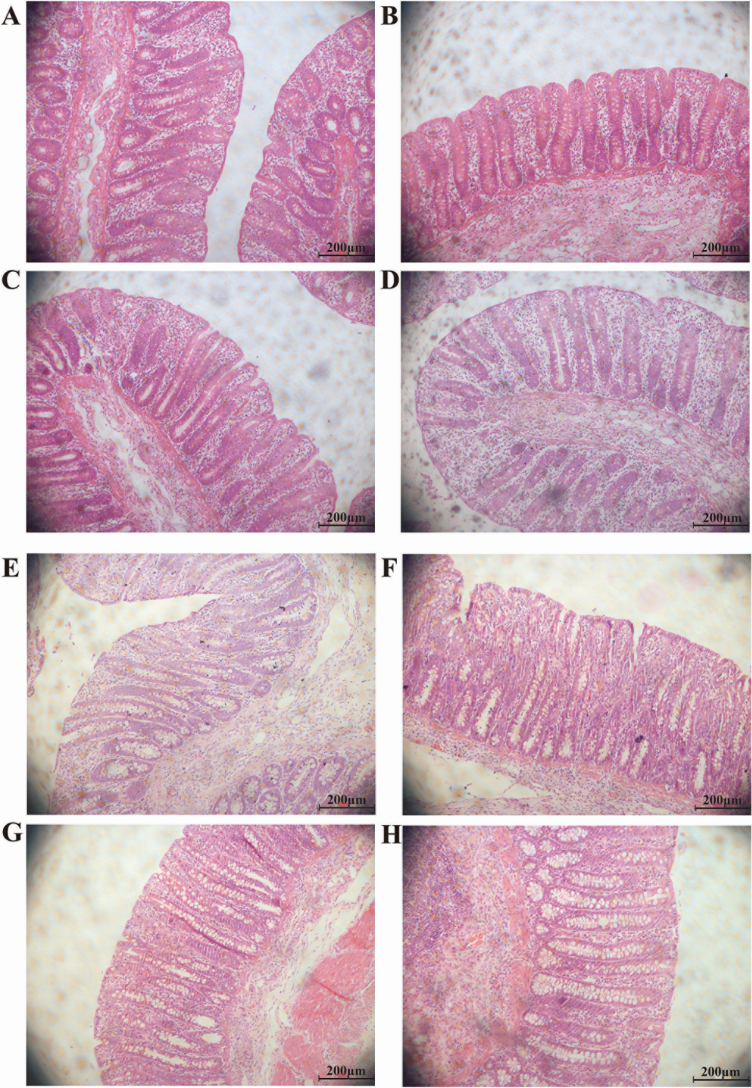
The effect of folate supplementation on the intestinal histology analysis of piglets and on the morphology of cecum and colon is illustrated in Figure 1 and Table 3, respectively. Piglets fed with 9 mg/kg folate had greater (P < 0.05) cecum CD than piglets in the control group. However, there were no differences in colonic CD among the four treatments.
Figure 1.
Intestinal histology analysis of weanling piglets fed different dietary concentrations of folate (magnification 200×). (A) cecum 0 mg/kg folate, (B) cecum 3 mg/kg folate, (C) cecum 9 mg/kg folate, (D) cecum 18 mg/kg folate, (E) colon 0 mg/kg folate, (F) colon 3 mg/kg folate, (G) colon 9 mg/kg folate, and (H) colon 18 mg/kg folate.
Table 3.
Effects of different folate levels on the morphology of the cecum and colon1
| Supplement folate level, mg/kg | ||||||
|---|---|---|---|---|---|---|
| Items | 0 | 3 | 9 | 18 | SEM | P-value |
| Cecum CD, μm | 410.71a | 431.00ab | 478.14b | 454.43ab | 10.12 | 0.094 |
| Colon CD, μm | 339.14 | 312.86 | 341.86 | 329.00 | 6.15 | 0.346 |
1 n = 7.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
Effect of folate supplementation on digesta pH
The effect of folate supplementation on the gastrointestinal digesta pH is presented in Table 4. The stomach pH was higher (P < 0.05) for diets containing folate (3, 9, and 18 mg/kg, respectively) compared with the control group. Additionally, a tendency (P = 0.088) for lower cecum pH in diets containing 9 mg/kg folate was observed. However, there were no differences in the ileum and colon pH compared with the control group.
Table 4.
Effects of different folate levels on the pH value of chyme in different digestive tracts of piglets1
| Supplement folate level, mg/kg | ||||||
|---|---|---|---|---|---|---|
| Items | 0 | 3 | 9 | 18 | SEM | P-value |
| Stomach pH | 3.20a | 5.16b | 4.95b | 5.60b | 0.31 | 0.025 |
| Ileum pH | 6.86 | 7.29 | 7.52 | 7.02 | 0.11 | 0.165 |
| Cecum pH | 6.32a | 6.17a | 5.70b | 5.84ab | 0.10 | 0.088 |
| Colon pH | 6.64 | 6.42 | 6.51 | 6.43 | 0.07 | 0.684 |
1 n = 7.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
Effect of folate supplementation on the SCFAs concentration
Different folate supplementation greatly influences the amount of SCFAs in the cecum and colon of weaned piglets (Table 5). Compared with the control group, diets containing folate (9 and 18 mg/kg) can significantly increase (P < 0.05) the acetic acid (AA) content in the cecum, and the amounts of valeric acid (VA) significantly increased (P < 0.05) in the colon in diets containing folate (18 mg/kg). Furthermore, the amounts of isobutyric acid (IBA), butyric acid (BA), isovaleric acid (IVA), and AA: propionic acid ratio in the colon in diets containing folate (18 mg/kg) have increased trend compared with the control group (P < 0.1). There were no significant differences observed in the total amounts of SCFAs in the cecum and colon of piglets (P > 0.05).
Table 5.
SCFAs concentration (mg/g fresh content) in the cecum and colon of weanling piglets fed different dietary concentrations of folate1
| Supplement folate level, mg/kg | |||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 3 | 9 | 18 | SEM | P-value | |
| Cecum | AA | 3.45a | 4.37ab | 5.32b | 4.82b | 0.24 | 0.03 |
| Propionic acid | 2.32 | 2.61 | 3.00 | 3.10 | 0.18 | 0.38 | |
| IBA | 0.08 | 0.10 | 0.10 | 0.10 | 0.01 | 0.43 | |
| BA | 0.83 | 0.93 | 1.14 | 1.09 | 0.10 | 0.67 | |
| IVA | 0.08 | 0.11 | 0.11 | 0.12 | 0.01 | 0.50 | |
| VA | 0.13 | 0.16 | 0.17 | 0.23 | 0.02 | 0.22 | |
| Acetic: propionic acid ratio | 1.70 | 1.77 | 1.81 | 1.56 | 0.07 | 0.68 | |
| Total SCFAs | 6.89 | 8.28 | 9.84 | 9.46 | 0.49 | 0.12 | |
| Colon | AA | 3.15 | 2.55 | 4.08 | 4.01 | 0.27 | 0.14 |
| Propionic acid | 1.72 | 2.47 | 2.01 | 2.36 | 0.15 | 0.29 | |
| IBA | 0.13b | 0.20ab | 0.23a | 0.24a | 0.02 | 0.06 | |
| BA | 0.74b | 1.22ab | 1.24ab | 1.47b | 0.11 | 0.10 | |
| IVA | 0.22b | 0.32ab | 0.43a | 0.41a | 0.03 | 0.06 | |
| VA | 0.16b | 0.27ab | 0.33ab | 0.43a | 0.04 | 0.04 | |
| Acetic: propionic acid ratio | 1.91a | 1.27b | 2.07a | 1.73ab | 0.11 | 0.07 | |
| Total SCFAs | 6.12b | 7.03ab | 8.33ab | 8.92a | 0.46 | 0.12 | |
1 n = 7.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
Microbial assessment in cecum contents
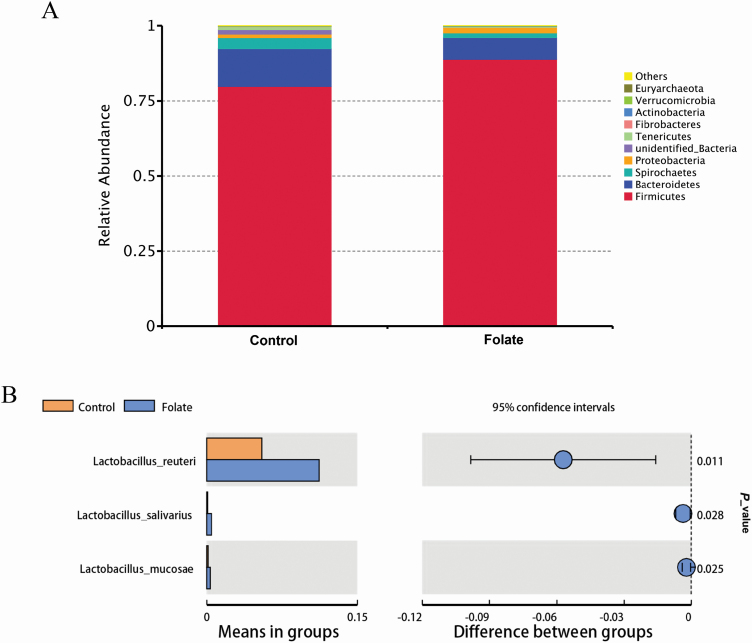
Our results showed that diet supplementation of 9 mg/kg folic acid has a positive effect on intestinal morphology, growth performance, and suppressing diarrhea (data not shown) of weaned piglets. Therefore, we used the cecum content of the 9 mg/kg folate group for 16s rRNA high-throughput sequencing. There were no significant differences between treatments for the indices of diversity, including observed_species, Shannon, Simpson, Chao1, abundance-based coverage estimator (ACE), and phylogenetic diversity (PD)_whole_tree indices, of cecal microbiota (data not shown). In addition, based on an unweighted pair-group method with arithmetic mean analysis, Firmicutes, Bacteroidetes, and Spirochaetes were found to be the dominant bacteria in the cecal microbiota (Figure 2A). At the genus level, there was a higher (P < 0.05) relative abundance of Lactobacillus reuteri, Lactobacillus salivarius, and Lactobacillus mucosae in the cecum when piglets were supplemented with 9 mg/kg folate (Figure 2B).
Figure 2.
Microbial communities in the cecum of weaned piglets between control and folate-supplemented groups after 14-d of feeding. (A) Top 10 cecum bacterial communities at the genus level in weaned piglets and (B) significantly different in weaned piglets. n = 7.
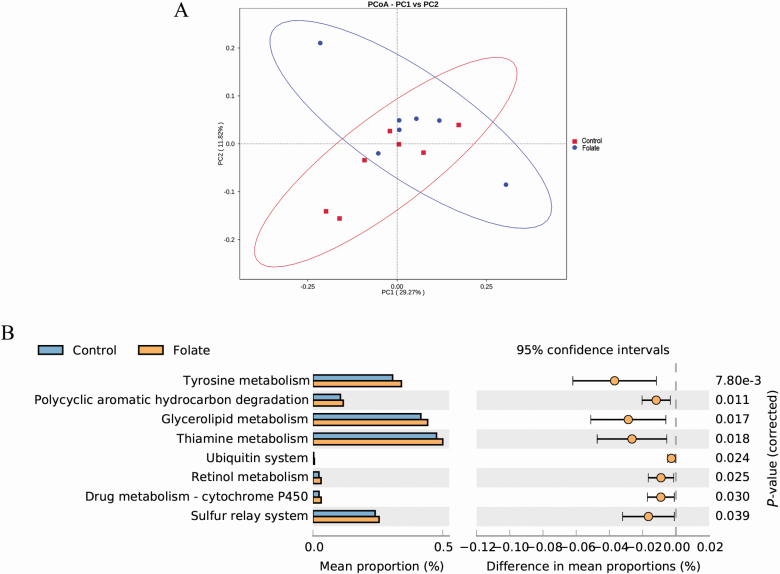
Effect of folate on metabolic function of microbiota
A principal coordinate analysis of functional profiles showed that there was clear clustering between the control group and folate supplementation group based on the KEGG annotation (Figure 3A). Compared with the control group, tyrosine metabolism, polycyclic aromatic hydrocarbon degradation, thiamine metabolism, ubiquitin system, retinol metabolism, drug metabolism-cytochrome P450, and sulfur relay system were significantly increased (P < 0.05) when piglets were supplemented with 9 mg/kg folate (Figure 3B).
Figure 3.
Analysis of functional differences in the cecal microbiota of weaned piglets between control and folate-supplemented groups after 14-d of feeding. (A) Principal components analysis (PCoA) plot of functional profiles among groups and (B) t-test bar plot of significantly different KEGG signal pathways. n = 7.
Discussion
Early weaning of piglets is often accompanied by severe growth inhibition and diarrhea, causing huge economic losses to commercial swine production. In the present study, we provide evidence that folate, when supplemented at the early weaning process, had a positive effect on organ index, digesta pH, SCFAs concentration, and intestinal microbiota of piglets.
The organ index can reflect the strength of organ function to a certain extent. The liver, as an organ with the most vigorous metabolism and the largest digestive gland in the digestive system, has the functions of storing glycogen, secreting bile, and synthesizing secreted proteins. The enhanced dietary folate intake may protect against the accumulation of the mtDNA deletion in the liver of chemotherapeutic drug-treated rats, which indicated that folate is an important nutrient in the maintenance of mitochondrial function (Branda et al., 2002). The kidneys play an important role in excreting metabolites, maintaining fluid balance and acid–base balance in the body. The spleen contains about 40% T cells and 60% B cells, which play a key role in humoral immunity. The increase in the weight of the immune organs is associated with rapid growth and development (Mejicanos et al., 2020). A series of studies have confirmed that vitamins can increase the weight of immune organs, promote the development of immune organs, and then improve the body’s immunity (Partearroyo et al., 2013; Pan et al., 2017; Sun, 2018). But up to now, there is no report on the direct effect of folate on the weight and index of weaned piglet organs. Our results show that the diet supplementation with 9 mg/kg folate can significantly increase the liver, kidney, and spleen weight of weaned piglets, suggesting that folate can improve the metabolism and immune function of weaning-stressed piglets by promoting the development of organs index.
The properties of intestinal folate absorption were documented decades ago. Folates are absorbed primarily in the duodenum and jejunum within the acid microenvironment at the cell surface (Qiu et al., 2006, 2007). The colon is a rich breeding ground for bacteria that synthesize folates and hence are a potential endogenous source of folates (Maynard et al., 2018), whereas the rate of folate net absorption from the colon was estimated to be approximately one-tenth rate of absorption from the small intestine. The mechanism of folates absorption is across the apical brush-border membrane of the proximal small intestine by the proton-coupled folate transporter (Visentin et al., 2014). The animal’s gastrointestinal tract is not only an important organ for the nutrient digestion and absorption but also the largest immune organ (Zou et al., 2017). The proper acidity required to maintain the normal physiological functions of the digestive system of the weaned piglet is an important measure to ensure the health of the piglet. The pH of the gastrointestinal tract of weaned piglets is increased, which is easy to cause the rapid proliferation of Escherichia coli and cause intestinal diseases (Xiong et al., 2019). Our results have shown that diet supplementation with 9 mg/kg folate can decrease the pH value of the cecum (P = 0.088). However, it is worth noting that folate treatment caused a significant increase (P < 0.05) in the stomach pH value of weaned piglets. The possible reason was that piglets have increased feed intake and did not have strict fasting before slaughter, resulting in slow emptying of a gastric chime. The cecum pH value of weaned piglets may be related to the promotion of the growth of cecal Lactobacillus by folate. Furthermore, the lactic acid produced by the metabolism of Lactobacilli can reduce the pH of the cecal content (Li et al., 2020).
It is well known that SCFAs play an important role in maintaining the morphology and function of intestinal epithelial cells, gut microbiota balance, and intestinal immune response (Liu et al., 2018). It is well known that the main way for animals to obtain SCFAs is through the fermentation of hindgut microbiota. On the other hand, the released SCFAs could be metabolized by the intestinal microorganisms as a carbon source and converted into other SCFAs. For example, AA could be converted into BA, propionic acid could be converted into AA, and BA could be converted into propionic acid and AA (Barcenilla et al., 2000; Duncan Sylvia et al., 2004). SCFAs can provide 60% to 70% of the energy of colonic epithelium. Generally, the intestinal tract uses BA first, followed by propionic acid and AA. Shibata et al. (2017) reported that SCFAs can inhibit the colonization and growth of some pathogens (Salmonella and E.coli) by reducing the pH value of the large intestine. Hung and Suzuki (2018) found that SCFAs, such as acetate, propionate, and butyrate, transported by monocarboxylate transporter (MCT)-1 suppress TNF-α-induced inflammatory signaling in Caco-2 cells and mouse colons. In addition, SCFAs act as specific G protein-coupled receptor signaling molecules and are involved in the regulation of host energy (glucose and lipid) metabolism (den Besten et al., 2013). The findings in the present study indicated that folate treatment could generate more SCFAs (AA, IBA, BA, IVA, and VA) and promote the intestinal health of the weaned piglets, which are closely correlated with the decreased cecal pH of weaned piglets in the folate treatment. It is worth noting that the dietary supplementation of 3 mg/kg folate caused a decrease in the concentration of AA in the colon (P > 0.05), due to which AA may be converted into BA by intestinal microbiota.
The previous studies show that ruminal total volatile fatty acids concentration, cellulolytic bacteria abundance, and enzyme activity increased with folate supplementation in steers (Wang et al., 2016, 2020a). Zhu et al. (2019) reported that vitamin B12 can be used as a growth substrate for beneficial bacteria to promote the growth of Lactobacillus so as to competitively inhibit the proliferation of E. coli, thereby improving the structure of the piglet’s intestinal microflora and promoting the intestinal microecological balance. Zhang et al. (2020) found that the intestinal dysbacteriosis was significantly correlated with folate deficiency in a folate-deficient rat model, and supplementation of folic acid bacteria can restore the destruction of the intestinal microbiota and restore serum folate and homocysteine to normal levels. In the present study, we employed 16S rRNA sequencing to investigate the effects of folate on the richness and diversity of cecal microbiota and found that folate had positive influences on Lactobacillus of the cecum segment. Most of the Lactobacillus that produce folate belong to the Bifidobacterium, Streptococcus, Lactobacillus, and Lactococcus genus. Among them, the relative production of folic acid of Bifidobacterium and Streptococcus is lower than that of Lactobacillus and Lactococcus genus. Therefore, our results show that the addition of folate to the diet can alleviate the decrease in the number of intestinal lactobacilli in piglets caused by waned stress.
Conclusions
The results of the present study indicated that diet supplementation with folate can improve the weight of the liver, kidney, and spleen in piglets at the weaning process. Piglets supplemented with 9 mg/kg folate can increase cecum CD and decrease the cecum pH compared with the control group. Further investigation indicated that there were close interactions between cecum pH value and SCFAs. In addition, diet supplementation with 9 mg/kg folate had greater proportions of beneficial bacteria (Lactobacillus) in piglet cecum. These findings will provide valuable guidance for the regulation of organ index, digesta pH, intestinal microbiota, and SCAFs concentration through folate to improve weaned piglet health and productivity.
Acknowledgments
This work was supported by the Research Foundation of Education Bureau of Hunan Province, China (18B476) and Hunan Provincial Natural Science Foundation of China (2018JJ3094).
Glossary
Abbreviations
- AA
acetic acid
- BA
butyric acid
- BW
body weight
- DCs
dendritic cells
- IBA
isobutyric acid
- IL
interleukin
- IVA
isovaleric acid
- OUT
operational taxonomic unit
- PCoA
principal co-ordinates analysis
- ROS
reactive oxygen species
- SCFA
short-chain fatty acids
- TNF-α
tumor necrosis factor-α
- VA
valeric acid
Author Contributions
L.W., J.L., Y.H., and Y.Y. designed the study. L.W. and Y.H. carried out the animal trials and sample analysis. W.L., L.Z., J.L., H.Y., and Y.Y. wrote and revised the manuscript.
Conflict of interest statement
The authors have no real or perceived conflicts of interest.
Literature Cited
- Alfa, M. J., D. Strang, P. S. Tappia, M. Graham, G. Van Domselaar, J. D. Forbes, V. Laminman, N. Olson, P. DeGagne, D. Bray, . et al. 2018. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 37:797–807. doi: 10.1016/j.clnu.2017.03.025 [DOI] [PubMed] [Google Scholar]
- Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. . 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654–1661. doi: 10.1128/aem.66.4.1654-1661.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bässler, K. H 1997. Enzymatic effects of folic acid and vitamin B12. Int. J. Vitam. Nutr. Res. 67:385–388. PMID: 9350482. [PubMed] [Google Scholar]
- den Besten, G., K. van Eunen, A. K. Groen, K. Venema, D. J. Reijngoud, and B. M. Bakker. . 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54:2325–2340. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda, R. F., E. M. Brooks, Z. Chen, S. J. Naud, and J. A. Nicklas. . 2002. Dietary modulation of mitochondrial DNA deletions and copy number after chemotherapy in rats. Mutat Res 501(1–2):29–36. doi: 10.1016/s0027-5107(02)00014-3 [DOI] [PubMed] [Google Scholar]
- Chen, C., Z. Wang, J. Li, Y. Li, P. Huang, X. Ding, J. Yin, S. He, H. Yang, and Y. Yin. . 2019. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets1. J. Anim. Sci. 97:1212–1221. doi: 10.1093/jas/skz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan Sylvia H., H. Grietje, E. Lobley Gerald, A. Calder, C. Stewart, H. Flint. 2004. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 91(6):915–23. doi: 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- Grieshop, C. R., T. S. Stahly, and R. C. Ewan. . 2001. Effect of gestational folic acid supplementation of sows on offspring muscle development and postnatal growth response. ISU Swine Research Report (ASLR646). Iowa State: Iowa State University Digital Repository; p. 35–37. https://lib.dr.iastate.edu/swinereports_2000/13 [Google Scholar]
- Guo, X., J. Ni, Y. Zhu, T. Zhou, X. Ma, J. Xue, and X. Wang. . 2017. Folate deficiency induces mitotic aberrations and chromosomal instability by compromising the spindle assembly checkpoint in cultured human colon cells. Mutagenesis 32:547–560. doi: 10.1093/mutage/gex030 [DOI] [PubMed] [Google Scholar]
- Hsu, H. C., J. F. Chiou, Y. H. Wang, C. H. Chen, S. Y. Mau, C. T. Ho, P. J. Chang, T. Z. Liu, and C. H. Chen. . 2013. Folate deficiency triggers an oxidative-nitrosative stress-mediated apoptotic cell death and impedes insulin biosynthesis in RINm5F pancreatic islet β-cells: relevant to the pathogenesis of diabetes. PLoS One. 8:e77931. doi: 10.1371/journal.pone.0077931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R. F., Y. C. Hsu, H. L. Lin, and F. L. Yang. . 2001. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 131:33–38. doi: 10.1093/jn/131.1.33 [DOI] [PubMed] [Google Scholar]
- Hung, T. V., and T. Suzuki. . 2018. Short-chain fatty acids suppress inflammatory reactions in Caco-2 cells and mouse colons. J. Agric. Food Chem. 66:108–117. doi: 10.1021/acs.jafc.7b04233 [DOI] [PubMed] [Google Scholar]
- Le Dividich, J., and B. Seve.. 2000. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domest. Anim. Endocrin. 19:63–74. doi: 10.1016/S0739-7240(00)00067-9 [DOI] [PubMed] [Google Scholar]
- Li, M., Y. Wang, H. Cui, Y. Li, Y. Sun, and H. J. Qiu. . 2020. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front. Vet. Sci. 7:49. doi: 10.3389/fvets.2020.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., L. Yin, L. Wang, J. Li, P. Huang, H. Yang, and Y. Yin. . 2019. Effects of vitamin B6 on growth, diarrhea rate, intestinal morphology, function, and inflammatory factors expression in a high-protein diet fed to weaned piglets1. J. Anim. Sci. 97:4865–4874. doi: 10.1093/jas/skz338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B., W. Wang, X. Zhu, X. Sun, J. Xiao, D. Li, Y. Cui, C. Wang, and Y. Shi. . 2018. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 9:2344. doi: 10.3389/fmicb.2018.02344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard, C., I. Cummins, J. Green, and D. Weinkove. . 2018. A bacterial route for folic acid supplementation. BMC Biol. 16:67. doi: 10.1186/s12915-018-0534-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBreairty, L. E., J. L. Robinson, S. V. Harding, E. W. Randell, J. A. Brunton, and R. F. Bertolo. . 2016. Betaine is as effective as folate at re-synthesizing methionine for protein synthesis during moderate methionine deficiency in piglets. Eur. J. Nutr. 55:2423–2430. doi: 10.1007/s00394-015-1049-0 [DOI] [PubMed] [Google Scholar]
- Mejicanos, G. A., G. Gonzalez-Ortiz, and C. M. Nyachoti. . 2020. Effect of dietary supplementation of xylanase in a wheat-based diet containing canola meal on growth performance, nutrient digestibility, organ weight, and short-chain fatty acid concentration in digesta when fed to weaned pigs. J. Anim. Sci. 98(3). doi: 10.1093/jas/skaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) 2012. Nutrient requirements of swine 11th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- Pan, J. H., L. Feng, W. D. Jiang, P. Wu, S. Y. Kuang, L. Tang, Y. A. Zhang, X. Q. Zhou, and Y. Liu. . 2017. Vitamin E deficiency depressed fish growth, disease resistance, and the immunity and structural integrity of immune organs in grass carp (Ctenopharyngodon idella): referring to NF-κB, TOR and Nrf2 signaling. Fish Shellfish Immunol. 60:219–236. doi: 10.1016/j.fsi.2016.11.044 [DOI] [PubMed] [Google Scholar]
- Partearroyo, T., N. Úbeda, A. Montero, M. Achón, and G. Varela-Moreiras. . 2013. Vitamin B(12) and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoprolipheration in aged rats. Nutrients 5:4836–4848. doi: 10.3390/nu5124836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluske, J. R., D. J. Hampson, and I. H. Williams.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Qiu, A., M. Jansen, A. Sakaris, S. H. Min, S. Chattopadhyay, E. Tsai, C. Sandoval, R. Zhao, M. H. Akabas, and I. D. Goldman. . 2006. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127:917–928. doi: 10.1016/j.cell.2006.09.041 [DOI] [PubMed] [Google Scholar]
- Qiu, A., S. H. Min, M. Jansen, U. Malhotra, E. Tsai, D. C. Cabelof, L. H. Matherly, R. Zhao, M. H. Akabas, and I. D. Goldman. . 2007. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am. J. Physiol. Cell Physiol. 293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007 [DOI] [PubMed] [Google Scholar]
- Ragaller, V., L. Hüther, and P. Lebzien. . 2009. Folic acid in ruminant nutrition: a review. Br. J. Nutr. 101:153–164. doi: 10.1017/S0007114508051556 [DOI] [PubMed] [Google Scholar]
- Sesay, D. F., H. M. Habte-Tsion, Q. Zhou, M. Ren, J. Xie, B. Liu, R. Chen, and L. Pan. . 2017. The effect of dietary folic acid on biochemical parameters and gene expression of three heat shock proteins (HSPs) of blunt snout bream (Megalobrama amblycephala) fingerling under acute high temperature stress. Fish Physiol. Biochem. 43:923–940. doi: 10.1007/s10695-016-0311-6 [DOI] [PubMed] [Google Scholar]
- Shibata, N., J. Kunisawa, and H. Kiyono. . 2017. Dietary and microbial metabolites in the regulation of host immunity. Front. Microbiol. 8:2171. doi: 10.3389/fmicb.2017.02171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J 2018. Dietary vitamin D, vitamin D receptor, and microbiome. Curr. Opin. Clin. Nutr. Metab. Care 21:471–474. doi: 10.1097/MCO.0000000000000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin, M., N. Diop-Bove, R. Zhao, and I. D. Goldman. . 2014. The intestinal absorption of folates. Annu. Rev. Physiol. 76:251–274. doi: 10.1146/annurev-physiol-020911-153251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., J. Li, Y. Wang, L. Wang, Y. Yin, L. Yin, H. Yang, and Y. Yin. . 2020b. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J. Anim. Sci. 98(2). doi: 10.1093/jas/skaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Q. Liu, G. Guo, W. Huo, L. Ma, Y. Zhang, C. Pei, S. Zhang, and H. Wang. . 2016. Effects of dietary supplementation of rumen-protected folic acid on rumen fermentation, degradability and excretion of urinary purine derivatives in growing steers. Arch. Anim. Nutr. 70:441–454. doi: 10.1080/1745039X.2016.1233677 [DOI] [PubMed] [Google Scholar]
- Wang, C., C. Liu, G. W. Zhang, H. S. Du, Z. Z. Wu, Q. Liu, G. Guo, W. J. Huo, J. Zhang, C. X. Pei, . et al. 2020a. Effects of rumen-protected folic acid and betaine supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Br. J. Nutr. 123:1109–1116. doi: 10.1017/S0007114520000331 [DOI] [PubMed] [Google Scholar]
- Wang, S. P., Y. L. Yin, Y. Qian, L. L. Li, F. N. Li, B. E. Tan, X. S. Tang, and R. L. Huang. . 2011. Effects of folic acid on the performance of suckling piglets and sows during lactation. J. Sci. Food Agric. 91:2371–2377. doi: 10.1002/jsfa.4469 [DOI] [PubMed] [Google Scholar]
- Wu, C. H., T. C. Huang, and B. F. Lin. . 2017. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J. Nutr. Biochem. 41:65–72. doi: 10.1016/j.jnutbio.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Xiong, X., B. Tan, M. Song, P. Ji, K. Kim, Y. Yin, and Y. Liu. . 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., H. Yang, B. Tan, C. Yang, M. Wu, G. Liu, S. W. Kim, T. Li, L. Li, J. Wang, . et al. 2015. Differential expression of proteins involved in energy production along the crypt-villus axis in early-weaning pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 309:G229–G237. doi: 10.1152/ajpgi.00095.2015 [DOI] [PubMed] [Google Scholar]
- Zhang, J., D. Cai, M. Yang, Y. Hao, Y. Zhu, Z. Chen, T. Aziz, A. Sarwar, and Z. Yang. . 2020. Screening of folate-producing lactic acid bacteria and modulatory effects of folate-biofortified yogurt on gut dysbacteriosis of folate-deficient rats. Food Funct. 11:6308–6318. doi: 10.1039/d0fo00480d [DOI] [PubMed] [Google Scholar]
- Zhu, X., S. Xiang, X. Feng, H. Wang, S. Tian, Y. Xu, L. Shi, L. Yang, M. Li, Y. Shen, . et al. 2019. Impact of cyanocobalamin and methylcobalamin on inflammatory bowel disease and the intestinal microbiota composition. J. Agric. Food Chem. 67(3):916–926. doi: 10.1021/acs.jafc.8b05730 [DOI] [PubMed] [Google Scholar]
- Zou, L., X. Xiong, H. Liu, J. Zhou, Y. Liu, and Y. Yin. . 2019. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J. Sci. Food Agric. 99:1643–1650. doi: 10.1002/jsfa.9348 [DOI] [PubMed] [Google Scholar]
- Zou, L., X. Xiong, X. Wang, H. Yang, J. Li, P. Huang, and Y. Yin. . 2017. Current advances in renewal mechanisms of intestinal epithelial cells along the crypt-villus axis. Sci. Sin. Vitae 47:190–200. doi: 10.1360/N052016-00240 [DOI] [Google Scholar]