Abstract
High conservation of extracellular matrix proteins often makes the generation of potent species-specific antibodies challenging. For collagen VII there is a particular preclinical interest in the ability to discriminate between human and murine collagen VII. Deficiency of collagen VII causes dystrophic epidermolysis bullosa (DEB) – a genetic skin blistering disease, which in its most severe forms is highly debilitating. Advances in gene and cell therapy approaches have made curative therapies for genetic diseases a realistic possibility. DEB is one disorder for which substantial progress has been made toward curative therapies and improved management of the disease. However, to increase their efficacy further preclinical studies are needed. The early neonatal lethality of complete collagen VII deficient mice, have led researches to resort to using models maintaining residual collagen VII expression or grafting of DEB model skin on wild-type mice for preclinical therapy studies. These approaches are challenged by collagen VII expression by the murine host. Thus, the ability to selectively visualize human and murine collagen VII would be a substantial advantage. Here, we describe a novel resource toward this end. By immunization with homologous peptides we generated rabbit polyclonal antibodies that recognize either human or murine collagen VII. Testing on additional species, including rat, sheep, dog, and pig, combined sequence alignment and peptide competition binding assays enabled identification of the major antisera recognizing epitopes. The species-specificity was maintained after denaturation and the antibodies allowed us to simultaneously, specifically visualize human and murine collagen VII in situ.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; EB, epidermolysis bullosa; DEB, dystrophic epidermolysis bullosa; JEB, junctional epidermolysis bullosa; NC, non-collagenous domain; pAb, polyclonal antibody; RDEB, recessive dystrophic epidermolysis bullosa
Highlights
-
•
High sequence conservation of murine and human collagen VII makes development of species-specific antibodies challenging.
-
•
Divergence in the immune epitope of a conserved peptide allowed for generation of species-specific collagen VII antibodies.
-
•
The antibodies allow strong, simultaneous visualization of human and murine collagen VII in immunocompetent hosts.
Introduction
The recent clinical advances in gene therapy and discovery of specific, potent gene-editing approaches with limited off-target events have led to a surge in studies aiming to develop curative therapies for orphan genetic diseases. Of these efforts, the group of genetic skin blistering diseases known as epidermolysis bullosa (EB) has enjoyed notable success [1,2]. EB comprises a heterogeneous, in terms of severity and manifestations, group of diseases caused by mutations in >20 genes [3,4]. Many EB variants also present extracutaneous manifestations but the diseases are linked by a de-stabilization of the dermal-epidermal junction (DEJ) as a consequence of loss of abundance or function of the affected proteins [3]. The skin manifestations make topical curative therapies attractive for EB. However, given the extracutaneous involvement for most EB variants, systemic treatment would be desirable. Some curative therapy efforts for laminin-332 deficient junctional EB (JEB) and dystrophic epidermolysis bullosa (DEB), caused by collagen VII deficiency, have already advanced to later clinical trial stages [5]. From the clinical trials on ex vivo gene therapy by transplantation of gene-corrected epidermal grafts for JEB and DEB, it has emerged that the curative effect is less sustained for DEB [6]. This is likely a reflection of key differences in the biology and functions of the two proteins at fault [7], and highlights the need to continue exploring alternative or complementary therapeutic avenues for DEB.
The development of therapies for DEB is challenged by early neonatal lethality of complete collagen VII deficient mice [8]. A collagen VII hypomorphic mouse model that presents most manifestations of severe DEB has been developed [9] and has been widely used by us and others for therapy development and to better understand DEB pathobiology [[10], [11], [12], [13], [14], [15]]. However, for therapies aiming to restore collagen VII abundance the remaining residual expression of wild-type collagen VII can obscure the results. Complementary to genetic mouse models, studies on topical curative therapies, but also other lines of therapies, have frequently used grafting of human skin equivalents or similar onto immunodeficient mice [[16], [17], [18]]. Nevertheless, the evaluation of collagen VII abundance and deposition in the grafts can be influenced by collagen VII expressed by the wild-type host [19]. In this context – and for all other preclinical studies introducing human collagen VII in murine hosts – tools to specifically detect human and murine collagen VII would be valuable.
We and others have shown that the mouse monoclonal collagen VII antibody LH 7: 2 [20,21], which is widely used for diagnostics to detect human collagen VII, recognizes human but not murine collagen VII [12,19]. However, commonly used hosts for human skin and cell grafting such as SCID or athymic nude mice [[16], [17], [18]] are leaky or maintain production of immunoglobulins which distorts staining with mouse monoclonal antibodies. The generation of murine and human specific collagen VII antibodies is challenged by the rather homogenous 84% sequence identity of human and murine collagen VII over the entire polypeptides.
In an effort to generate a potent collagen VII body we previously cloned a 246-amino acid peptide of the central non-collagenous (NC)-1 domain of human collagen VII reported to harbor the epitope of LH 7:2 [22] and raised a rabbit polyclonal antiserum against this peptide [12]. The resulting antiserum was highly potent in western blotting, ELISA and immunofluorescence, unexpectedly – because of the large size of the peptide used for immunization and the homology of human and murine collagen VII – we noted low reactivity to murine collagen VII [12]. Here, we re-raised the antibody in multiple rabbits and observed persistent high human collagen VII and low murine collagen VII reactivity of the generated antibodies. This indicated intrinsic properties of the peptide used for immunization favoring human but not murine collagen VII recognition. Based on this observation and in silico prediction suggesting divergence of the most antigenic sequence between human and murine collagen VII, we raised antibodies against the corresponding murine peptide. The resulting antibodies reacted strongly with murine but not human collagen VII in cell and tissue staining and western blotting. Together these antibodies thus constitute a unique, useful tool to distinguish human and murine collagen VII in situ which we practically showed by injection of human collagen VII into wild-type mouse skin.
Results and discussion
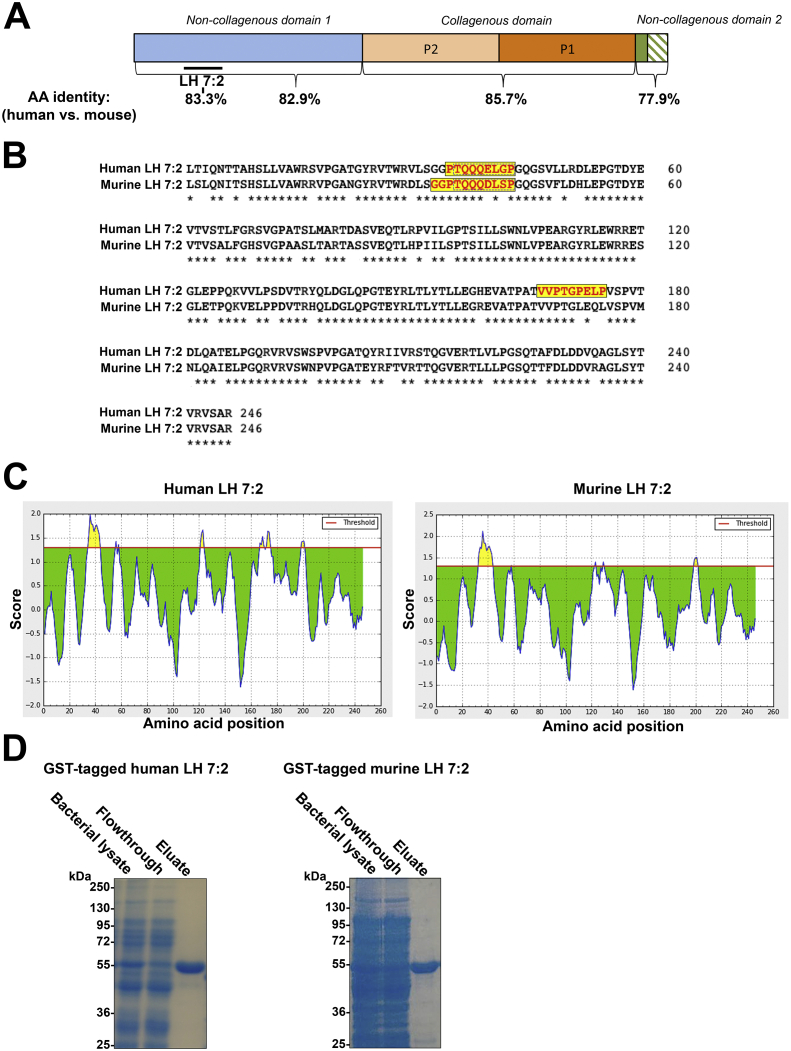
The high amino acid sequence conservation between mouse and human collagen VII makes generation of polyclonal antibodies that show preference for reactivity with one of the species challenging (Fig. 1A). The most sequence-divergent part of human and murine collagen VII is in the C-terminal NC-2 domain, in which an additional stretch of 11 extra amino acids is found in the murine collagen VII alpha 1 chain. However, since the NC-2 domain is cut during maturation of pro-collagen VII to anchoring fibril forming-competent collagen VII [23], even if successfully generated, antibodies directed against the aforementioned amino acid sequence would not detect mature murine collagen VII.
Fig. 1.
Comparison of the murine and human collagen VII alpha 1 chains. A, Schematic illustration of the organization of the collagen VII alpha 1 chain polypeptide with comparison of sequence identity between murine and human. B, Alignment of the human and murine LH 7:2 peptide. Asterisks indicate identical amino acids; the yellow boxes indicate sequences predicted by the BepiPred Linear Epitope prediction algorithm with a specificity of 0.96 (C) to be potential epitopes. Dotted lines within the yellow boxes indicate the prediction results from the BepiPred-2.0 algorithm with a specificity of 0.96. C, Plot of the epitope prediction score from the BepiPred Linear Epitope prediction algorithm with a specificity of 0.96. Peaks (yellow) over cutoff suggest potential epitopes. D, Coomassie brilliant blue-stained gels of purification of GST-tagged human and murine LH 7:2 peptides show that the murine peptide exhibits similar migration as human LH 7:2.
The large N-terminal NC-1 domain of collagen VII has high antigenic properties, as evidenced by many potent monoclonal collagen VII antibodies recognizing epitopes within this domain, as well as antibodies occurring in the autoimmune skin blistering disease epidermolysis bullosa acquisita frequently targeting the NC-1 domain [24]. The collagen VII NC-1 domain is well conserved between mouse and human (Fig. 1B). To generate a potent rabbit polyclonal collagen VII antibody, we previously immunized rabbits with a 246-amino acid peptide reported to contain the epitope of the powerful mouse monoclonal collagen VII antibody LH 7:2 [12,20,22]. During the characterization of the resulting rabbit antiserum we unexpectedly noted minimal reactivity to murine collagen VII but persistent potent recognition of human collagen VII at higher dilutions [12]. To investigate if this response was individual to the rabbit used or was due to intrinsic properties of the peptide used for immunization, we here re-raised the antibody in three additional rabbits (rabbit 1 to 3). The antibodies were highly specific for human collagen VII in western blot and immunofluorescence staining of cultured keratinocytes and skin (Supplemental Fig. 1 and data not shown). Interestingly, when staining wild-type human skin vis-à-vis wild-type murine skin collagen VII we consistently noticed a high reactivity to human collagen VII but low reactivity to murine collagen VII with antisera from all three rabbits (Supplemental Fig. 2). At dilutions >1:2000 antisera from all rabbits strongly stained collagen VII at the DEJ in human skin but negligible in murine skin (Supplemental Fig. 2).
The consistent preference of reactivity to human collagen VII suggested divergence in human and mouse of predominant immunoepitopes in the peptide used for immunization (from hereon called human LH 7:2 (hLH 7:2) and mouse LH 7:2 (mLH 7:2)). The hLH 7:2 peptide and the corresponding murine peptide mLH 7:2 show 83.3% sequence identity (Fig. 1A and B). Antibody epitope prediction of hLH 7:2 and mLH 7:2 using the BepiPred Linear Epitope prediction algorithm with a specificity of 0.96, predicted one predominant immunoepitope for mLH 7:2 which was at the same location as one of the two epitopes above the cutoff for hLH 7:2 (Fig. 1C). In these sequences (11 and 9 amino acids for mouse and human, respectively) two amino acids at the C-termini differed. The BepiPred-2.0 algorithm with a specificity of 0.96 indicated the same epitope but narrowed it to 8 amino acids, consequently 2 of 8 amino acid differed between mouse and human in the sequence. Since the in vitro analyses provided some support for sequence difference in potential dominant immunoepitopes in murine and human LH 7:2 peptides, we cloned, purified and immunized two rabbits with the mLH 7:2 peptide (Fig. 1D).
The resulting antisera, named mLH 7:2 polyclonal antibody (mLH 7:2pAb), was highly specific for collagen VII in western blotting and immunofluorescence staining, as assessed using skin samples from wild-type and collagen VII hypomorphic mice (Supplemental Fig. 3A and B). Notably, the mLH 7:2pAb was potent in western blots and the residual abundance of collagen VII in collagen VII hypomorphic mouse skin [9] was clearly detected.
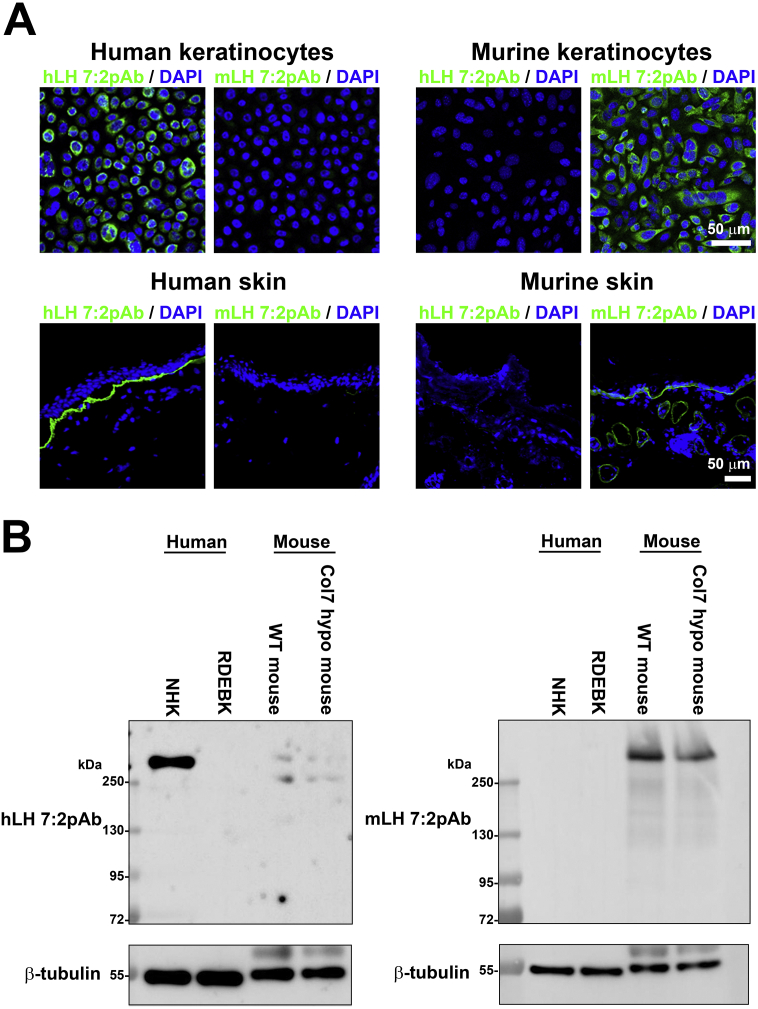
Next, we tested antisera raised against hLH 7:2 (named hLH 7:2pAb) vis-à-vis mLH 7:2pAb at different dilutions on murine and human wild-type keratinocytes. Interestingly, for mLH 7:2pAb at dilutions >1:5000 reactivity to human collagen VII was lost but strong recognition to murine collagen VII retained (Fig. 2A and Supplemental Fig. 4). These findings were replicated on wild-type human and murine skin (Fig. 2A). Based on these observations we thus concluded that hLH 7:2pAb and mLH 7:2pAb recognize human but not murine collagen VII, and vice versa, under native conditions. We were then interested in determining if this specificity was kept under denaturing conditions. It was a possibility that denaturing collagen VII would disrupt the predominant immunoepitope which could impact recognition and species-specificity of the antibodies. Toward this end, we tested the antibodies on western blots of protein lysates from normal human keratinocytes, keratinocytes from donors with complete collagen VII deficient recessive DEB (RDEB), wild-type mouse skin and collagen VII hypomorphic mouse skin (Fig. 2B). Importantly, in this assay at dilutions >1:5000 hLH 7:2pAb detected only human collagen VI and conversely mLH 7:2pAb only murine collagen VII.
Fig. 2.
mLH 7:2pAb recognizes murine but not human collagen VII. A, Human and murine keratinocytes and human and murine skin cryosections all wild-type stained with mLH 7:2pAb and hLH 7:2pAb at 1:20,000 dilutions. Note that at this dilution hLH 7:2pAb recognizes human but not murine collagen VII and mLH 7:2pAb recognizes murine but not human collagen VII. Nuclei counterstained with DAPI, scale bar = 50 μm. B, mLH 7:2pAb and hLH 7:2pAb show species selectivity in western blotting. Western blots of protein lysates from normal human keratinocytes or keratinocytes from donors with complete collagen VII deficient recessive DEB (RDEB), or protein lysates from wild-type or collagen VII hypomorphic mouse skin. The blots were probed with hLH 7:2pAb and mLH 7:2pAb as indicated at a dilution of 1:5000. β-tubulin was used as loading control.
In an effort to find the predominant epitope recognized by the antisera, and to assess the wider applicability of the antisera for investigations in other species that are either commonly used in dermatological research such as pig [25] or in which DEB have been reported to occur such as rat, sheep and dog [[26], [27], [28], [29], [30]] we performed sequence analysis, followed immunostaining of skin from these species. The sequence analysis revealed that the most N-terminal of the two predicted predominant immunoepitopes in the human peptide was almost completely conserved in the ovine, canine and porcine LH: 7.2 (Supplemental Fig. 5A). In contrast, as expected, the corresponding epitope in rat showed most sequence identity with the murine variant (Supplemental Fig. 5A). Next, staining of skin sections revealed strong reactivity of hLH 7:2pAb to ovine, canine and porcine skin but weak to rat skin. Conversely, mLH 7:2pAb stained rat skin but not ovine, canine and porcine skin (Supplemental Fig. 5B). The strong reactivity to ovine skin suggested that the most C-terminal of the two predicted predominant immunoepitope (Supplemental Fig. 5A and B) was not a major determinant of reactivity, as addition of two amino acids in the ovine sequence made this sequence divergent from human.
To experimentally investigate the dependence of the predominant immunoepitopes for recognition of hLH 7:2pAb to collagen VII, we performed peptide competition binding assay to recombinant human collagen VII spotted on nitrocellulose membranes. Diluted hLH 7:2pAb was mixed with 0.1 mg/ml peptides corresponding to the two predicted epitopes in human LH 7:2 (PTQQQELGP and VVPTGPELP, respectively) and then incubated with membranes. Intriguingly, pre-incubation with the PTQQQELGP was able to dramatically reduce hLH 7:2pAb recognition of human collagen VII (Supplemental Fig. 5C). Collectively, these data indicate that the predominant immunoepitope for hLH 7:2pAb is located within or partially contained within the PTQQQELGP sequence (Fig. 1 and Supplemental Fig. 5).
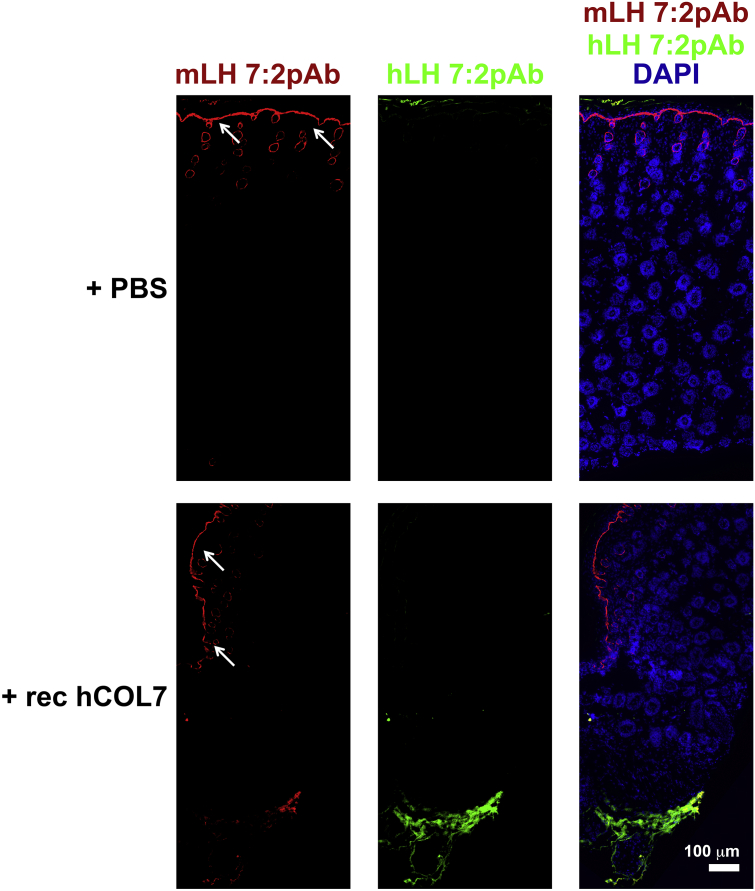
The overarching goal was to develop a tool that would allow simultaneous detection of human and murine collagen VII in situ. To put our antibodies to test we injected wild-type mouse skin with human recombinant collagen VII. Sequential staining of hLH 7:2pAb followed by mLH 7:2pAb could clearly discriminate between the endogenous murine collagen VII and the injected recombinant human collagen VII (Fig. 3).
Fig. 3.
hLH 7:2pAb and mLH 7:2pAb can be used to in situ simultaneously specifically visualize human and murine collagen VII. Skin from newborn wild-type mouse pups received deep intradermal injections of 10 μg human collagen VII or PBS. The skin samples were sectioned and sequential staining of hLH 7:2pAb (green) followed by mLH 7:2pAb (red). Nuclei counterstained with DAPI, scale bar = 100 μm.
To conclude we have generated antibodies allowing specific detection of human and murine collagen VII. To our knowledge one antibody, AP23437PU from Acris raised against the amino acids 97–200 in the NC1 VWFA1 domain, which show 88.6% identity between mouse and human, was previously reported to recognize mouse but not human collagen VII in immunofluorescence staining. Species specificity in other analytical assays was not reported. The antibody has been discontinued. The large size of collagen VII makes positioning of the antibody a potential concern for recognition. Ideally, for best comparative analyses the antibodies of human and murine collagen VII should targeted similarly positioned domains. Because homologous peptides of human and murine collagen VII were used for the generation of the antibodies, there should be limited concerns about differential masking or exposure of epitopes of the two antibodies.
Our antibodies represent a unique and effective tool to discriminate human and murine collagen VII and are made available to any researcher who would wish to use them.
Materials and methods
Cloning
Human COL7A1 cDNA sequence corresponding to the hLH 7:2 peptide (amino acids 333 to 578) was amplified from a pcDNA3.1 vector containing human COL7A1 cDNA and the sequence for mLH 7:2 (amino acids 334 to 579) from Col7a1 from wild-type murine keratinocyte cDNA. The primers used were for human: hLH 7:2_FR CCGGAATTCACCATCCAGAATACCACAG, hLH 7:2_RP CCGCTCGAGACACCCGCACAGTGTAGCT and for mouse: mLH 7:2_FR CCGGAATTCAGCCTCCAGAACATCACAT, mLH 7:2_RPCCGCTCGAGTCATCGAGCAGACACCCGCACC. PCR products were inserted in the PGEX6P1 plasmid using EcoRI and NhoI restriction site and T4 DNA ligase according to the instruction from the manufacturer (Thermo Fisher Scientific). Top10 chemically competent E.coli were transformed with the different constructs and plasmids were then analyzed by sequencing. Plasmids carrying the correct sequences were subsequently then used to transform BL21-DE3 E.coli to allow high level of protein expression.
Peptide purification
BL21-DE3 E.coli bacteria were grown in LB medium and expression of hLH 7:2 and mLH 7:2 epitopes was induced at an optical density of 0.8 using 1 mM of isopropylthiogalactoside for 2 h at 37 °C. Bacteria were then pelleted, washed in PBS and resuspended in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Triton-X-100, 1 mM Pefabloc, 5 mM EDTA and supplemented with complete protease inhibitors (Roche). Lysis was performed by sonication and the lysates were cleared by centrifugation (10,000g, 15 min, 4 °C). GST-fused peptides were purified using Glutathion sepharose beads (GE Healthcare). First 400 μl of clarified lysate was added to 50 μl slurry beads in 1 ml lysis buffer. Tubes were then incubated for 2 h at 4 °C with gentle agitation. After extensive wash, peptides were eluted with a buffer containing 50 mM Tris-HCl pH 8.0 and 10 mM reduced glutathione. Purification efficiency and purity of the proteins were analyzed by SDS-PAGE colored with Coomassie brilliant blue. Finally, Amicon ultra 0.5 ml (Cut-off 10 kDa) was used to concentrate the purified GST-tagged peptides and to exchange buffer to PBS + 0.5 mM EDTA. Proteins were then stored at −80 °C.
Rabbit immunization
Production of polyclonal antibodies targeting mLH 7:2 or hLH 7:2 peptides was done by Eurogentec (Belgium) with immunization of 2 rabbits per peptide following an 87 days protocol. Immunization was performed at 5 times points (Day 0, 14, 28, 56 and 73) with injection of 200 μg peptides per rabbit. Sera from blood was collected by centrifugation at day 87 and stored at −80 °C.
Sequence analysis and epitope prediction
Sequences were retrieved and aligned using Uniprot (https://www.uniprot.org). Epitopes were predicted using IEDB analysis resource (http://tools.immuneepitope.org/bcell/).
Human and mouse samples
Human keratinocytes and skin samples were from people with molecularly confirmed complete collagen VII deficiency (severe generalized RDEB) or healthy control donors. The donors had given written informed consent. Samples from mice were from wild-type mice or collagen VII hypomorphic mice [9]. Breeding and maintenance of collagen VII hypomorphic mice were conducted in accordance by ethical permit G14/93, issued by the regional ethics review board (Regierungspräsidium Freiburg).
Rat, sheep, pig and dog samples
Rat skin was from newborn wild-type Sprague Dawley rats [26]. Cryosections of ovine and canine skin were purchased from Zyagen. Fresh partly de-epithelialized porcine skin was a kind gift from Metzgerei Belledin, Merdingen, Germany.
Western blotting
Proteins from cultured cells were extracted with RIPA buffer and protein from whole skin after crushing of snap-frozen skin and boiling with Laemmli loading buffer containing 4 M urea. Western blotting was performed as previously described [11].
Immunofluorescence
Staining protocol used in experiments presented in Fig. 2 and Supplemental Fig. 4:
1 × 105 keratinocytes were seeded into μ-Slide 8 well chamber slides (ibidi GmbH, Gräfelfing, Germany) and grown to 70–100% confluence. Cells were fixed with 4% formaldehyde solution (SAV Liquid Production GmbH, Flintsbach am Inn, Germany) for 10 min at RT. 8 μm cryosections from samples frozen in OCT (Thermo Fisher Scientific) were fixed in acetone-methanol (1:1) at −20 °C for 10 min. Permeabilization and staining of the cells and cryosections were performed in a single step. Anti-collagen VII antibodies mLH 7:2pAb and hLH 7:2pAb were used at the indicated dilutions, diluted in 0.3% Triton-X, blocking reagent (1:10) and TBS-T. The primary antibodies were applied overnight at 4 °C. After 2 cycles of TBS-T wash, cells were stained with an Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody (Thermo Fisher Scientific) (1:300 in TBS-T) for 1 h at RT. Cells nuclei were stained with DAPI (1:4000 in TBS-T) for 10 min at RT. Cryosections were then mounted in Fluorescence Mounting Medium (Dako). Finally, cells and cryosections were analyzed for collagen VII staining, using an inverted microscope system, which includes the laser scanning confocal microscope Zeiss LSM 700 and the Axio Observer. Z1 (Carl Zeiss).
Staining protocol used in experiments presented in Supplemental Fig. 1, Supplemental Fig. 2 and Supplemental Fig. 3:
Skin cryosections and cells were fixed in acetone, blocked with 3% BSA in PBS and stained with mLH 7:2pAb and hLH 7:2pAb at the indicated dilutions. The slides were after washing in PBS-T, subsequently stained with secondary antibodies, counterstained with DAPI, and mounted in Fluorescence Mounting Medium (Dako). Images were acquired with an Axiocam MRm camera attached to a Zeiss Axio Imager A1 fluorescence microscope (Carl Zeiss), processed using Axiovision 4.9 and ZEN2012 software (Carl Zeiss).
Peptide competition binding assay
Peptides with the sequences PTQQQELGP and VVPTGPELP were produced by Genecust, Boynes, France. The peptides were dissolved to stock concentrations of 1 mg/ml. 100 ng recombinant human collagen VII, expressed and purified as previously described [31], was immobilized on nitrocellulose membranes using a dot blot apparatus (Bio-Rad) and blocked in 5% milk in TBS-T. hLH 7:2pAb at a dilution of 1:1000 in blocking buffer was incubated together with 0.1 mg/ml of the peptides and these solutions were added to the membranes. The membranes where subsequently processed as for western blots.
Injection of recombinant human collagen VII
Human recombinant collagen VII expressed in HEK-293 cells was purified as previously described [31]. 10 μg recombinant collagen VII in PBS was injected deep intradermally in dorsal skin from freshly euthanized wild-type mouse pups. 10 min after the injection the skin was embedded in optimal cutting temperature medium (Sakura) and cryosectioned. Skin cryosections were fixed in 100% ice-cold acetone, blocked with 3% bovine serum albumin and then sequentially stained with hLH 7:2pAb, Alexa-Fluor 488 rabbit anti-goat antibody (Thermo Fisher Scientific), mLH 7:2pAb antisera and Alexa-Fluor 594 rabbit anti-goat antibody (Thermo Fisher Scientific). The sections were rigorously washed in PBS-T between each staining step. Images were acquired and processed as for Supplemental Figs. 1–3.
Author contributions
Conceptualization: OB and AN; Data curation: OB, TK, CG, BL and SH; Formal analysis: OB, UK, and AN; Funding acquisition: UK and AN; Supervision: UK and AN; Writing - original draft: OB and AN; Writing - review & editing: OB, TK, CG, BL, SH, UK and AN.
Declaration of competing interest
The authors state no conflict of interest.
Acknowledgement
The authors thank Univ. Prof. Dr. Eva Rohde and Karin Roider, MSc of the Spinal Cord Injury and Tissue Regeneration Center Salzburg (SCI-TReCS) for providing their Core Facility for Microscopy. This work was supported by DEBRA Austria (to UK) and grants from the German Research Foundation, Deutsche Forschungsgemeinschaft (DFG) (NY-90 2/1, NY-90 3/2, NY 90-5/1, SFB850 project B11 and SFB1160 project B03) to AN and from Debra International (grant Nystrom Bruckner-Tuderman 1) to AN.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mbplus.2019.100017.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hirsch T., Rothoeft T., Teig N., Bauer J.W., Pellegrini G., De Rosa L., Scaglione D., Reichelt J., Klausegger A., Kneisz D., Romano O., Secone Seconetti A., Contin R., Enzo E., Jurman I., Carulli S., Jacobsen F., Luecke T., Lehnhardt M., Fischer M., Kueckelhaus M., Quaglino D., Morgante M., Bicciato S., Bondanza S., De Luca M. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siprashvili Z., Nguyen N.T., Gorell E.S., Loutit K., Khuu P., Furukawa L.K., Lorenz H.P., Leung T.H., Keene D.R., Rieger K.E., Khavari P., Lane A.T., Tang J.Y., Marinkovich M.P. Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA. 2016;316:1808–1817. doi: 10.1001/jama.2016.15588. [DOI] [PubMed] [Google Scholar]
- 3.Has C., Nyström A., Saeidian A.H., Bruckner-Tuderman L., Uitto J. Epidermolysis bullosa: molecular pathology of connective tissue components in the cutaneous basement membrane zone. Matrix Biol. J. Int. Soc. Matrix Biol. 2018;71–72:313–329. doi: 10.1016/j.matbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Vahidnezhad H., Youssefian L., Saeidian A.H., Touati A., Pajouhanfar S., Baghdadi T., Shadmehri A.A., Giunta C., Kraenzlin M., Syx D., Malfait F., Has C., Lwin S.M., Karamzadeh R., Liu L., Guy A., Hamid M., Kariminejad A., Zeinali S., McGrath J.A., Uitto J. Mutations in PLOD3, encoding lysyl hydroxylase 3, cause a complex connective tissue disorder including recessive dystrophic epidermolysis bullosa-like blistering phenotype with abnormal anchoring fibrils and type VII collagen deficiency. Matrix Biol. J. Int. Soc. Matrix Biol. 2019;81:91–106. doi: 10.1016/j.matbio.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Nyström A., Bernasconi R., Bornert O. Therapies for genetic extracellular matrix diseases of the skin. Matrix Biol. J. Int. Soc. Matrix Biol. 2018;71–72:330–347. doi: 10.1016/j.matbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Marinkovich M.P., Tang J.Y. Gene therapy for epidermolysis bullosa. J. Invest. Dermatol. 2019;139:1221–1226. doi: 10.1016/j.jid.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Nyström A., Bruckner-Tuderman L. Gene therapy for epidermolysis bullosa: sticky business. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24:2035–2036. doi: 10.1038/mt.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinonen S., Männikkö M., Klement J.F., Whitaker-Menezes D., Murphy G.F., Uitto J. Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: a model for recessive dystrophic epidermolysis bullosa. J. Cell Sci. 1999;112(Pt 21):3641–3648. doi: 10.1242/jcs.112.21.3641. [DOI] [PubMed] [Google Scholar]
- 9.Fritsch A., Loeckermann S., Kern J.S., Braun A., Bösl M.R., Bley T.A., Schumann H., von Elverfeldt D., Paul D., Erlacher M., Berens von Rautenfeld D., Hausser I., Fässler R., Bruckner-Tuderman L. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J. Clin. Invest. 2008;118:1669–1679. doi: 10.1172/JCI34292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cianfarani F., De Domenico E., Nyström A., Mastroeni S., Abeni D., Baldini E., Ulisse S., Uva P., Bruckner-Tuderman L., Zambruno G., Castiglia D., Odorisio T. Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa. Matrix Biol. J. Int. Soc. Matrix Biol. 2019;81:3–16. doi: 10.1016/j.matbio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Nyström A., Thriene K., Mittapalli V., Kern J.S., Kiritsi D., Dengjel J., Bruckner-Tuderman L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015;7:1211–1228. doi: 10.15252/emmm.201505061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kühl T., Mezger M., Hausser I., Handgretinger R., Bruckner-Tuderman L., Nyström A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:1368–1379. doi: 10.1038/mt.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyström A., Velati D., Mittapalli V.R., Fritsch A., Kern J.S., Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. J. Clin. Invest. 2013;123:3498–3509. doi: 10.1172/JCI68127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyström A., Bornert O., Kühl T., Gretzmeier C., Thriene K., Dengjel J., Pfister-Wartha A., Kiritsi D., Bruckner-Tuderman L. Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E705–E714. doi: 10.1073/pnas.1709111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Oever M., Muldoon D., Mathews W., McElmurry R., Tolar J. miR-29 regulates type VII collagen in recessive dystrophic epidermolysis bullosa. J. Invest. Dermatol. 2016;136:2013–2021. doi: 10.1016/j.jid.2016.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hainzl S., Peking P., Kocher T., Murauer E.M., Larcher F., Del Rio M., Duarte B., Steiner M., Klausegger A., Bauer J.W., Reichelt J., Koller U. COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25:2573–2584. doi: 10.1016/j.ymthe.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turczynski S., Titeux M., Tonasso L., Décha A., Ishida-Yamamoto A., Hovnanian A. Targeted exon skipping restores type VII collagen expression and anchoring fibril formation in an in vivo RDEB model. J. Invest. Dermatol. 2016;136:2387–2395. doi: 10.1016/j.jid.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Bremer J., Bornert O., Nyström A., Gostynski A., Jonkman M.F., Aartsma-Rus A., van den Akker P.C., Pasmooij A.M. Antisense oligonucleotide-mediated exon skipping as a systemic therapeutic approach for recessive dystrophic epidermolysis bullosa. Mol. Ther. Nucleic Acids. 2016;5:e379. doi: 10.1038/mtna.2016.87. [DOI] [PubMed] [Google Scholar]
- 19.Bremer J., Kramer D., Eichhorn D.S., Gostyński A., Diercks G.F.H., Jonkman M.F., van den Akker P.C., Pasmooij A.M.G. Murine type VII collagen distorts outcome in human skin graft mouse model for dystrophic epidermolysis bullosa. Exp. Dermatol. 2018 doi: 10.1111/exd.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heagerty A.H., Kennedy A.R., Leigh I.M., Purkis P., Eady R.A. Identification of an epidermal basement membrane defect in recessive forms of dystrophic epidermolysis bullosa by LH 7:2 monoclonal antibody: use in diagnosis. Br. J. Dermatol. 1986;115:125–131. doi: 10.1111/j.1365-2133.1986.tb05707.x. [DOI] [PubMed] [Google Scholar]
- 21.European Society for Dermatological Research 15th Annual Meeting J. Invest. Dermatol. 1985;84:433–456. doi: 10.1111/1523-1747.ep12265533. [DOI] [Google Scholar]
- 22.Tanaka T., Takahashi K., Furukawa F., Imamura S. The epitope for anti-type VII collagen monoclonal antibody (LH7:2) locates at the central region of the N-terminal non-collagenous domain of type VII collagen. Br. J. Dermatol. 1994;131:472–476. doi: 10.1111/j.1365-2133.1994.tb08546.x. [DOI] [PubMed] [Google Scholar]
- 23.Muir A.M., Massoudi D., Nguyen N., Keene D.R., Lee S.-J., Birk D.E., Davidson J.M., Marinkovich M.P., Greenspan D.S. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biol. J. Int. Soc. Matrix Biol. 2016;56:114–131. doi: 10.1016/j.matbio.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung H.J., Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol. Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd E., Yousef S.A., Pastore M.N., Telaprolu K., Mohammed Y.H., Namjoshi S., Grice J.E., Roberts M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016;8:163–176. doi: 10.2147/CPAA.S64788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyström A., Buttgereit J., Bader M., Shmidt T., Ozcelik C., Hausser I., Bruckner-Tuderman L., Kern J.S. Rat model for dominant dystrophic epidermolysis bullosa: glycine substitution reduces collagen VII stability and shows gene-dosage effect. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niskanen J., Dillard K., Arumilli M., Salmela E., Anttila M., Lohi H., Hytönen M.K. Nonsense variant in COL7A1 causes recessive dystrophic epidermolysis bullosa in central Asian shepherd dogs. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gache Y., Pin D., Gagnoux-Palacios L., Carozzo C., Meneguzzi G. Correction of dog dystrophic epidermolysis bullosa by transplantation of genetically modified epidermal autografts. J. Invest. Dermatol. 2011;131:2069–2078. doi: 10.1038/jid.2011.172. [DOI] [PubMed] [Google Scholar]
- 29.Pérez V., Benavides J., Delgado L., Reyes L.E., García Marín J.F., Ferreras M.C. Dystrophic epidermolysis bullosa in Assaf lambs. J. Comp. Pathol. 2011;145:226–230. doi: 10.1016/j.jcpa.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Bruckner-Tuderman L., Guscetti F., Ehrensperger F. Animal model for dermolytic mechanobullous disease: sheep with recessive dystrophic epidermolysis bullosa lack collagen VII. J. Invest. Dermatol. 1991;96:452–458. doi: 10.1111/1523-1747.ep12470130. [DOI] [PubMed] [Google Scholar]
- 31.Bornert O., Kühl T., Bremer J., van den Akker P.C., Pasmooij A.M., Nyström A. Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24:1302–1311. doi: 10.1038/mt.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material