Abstract
Mammalian sperm cells must respond to cues originating from along the female reproductive tract and from the layers of the egg in order to complete their fertilization journey. Dynamic regulation of ion signalling is, therefore, essential for sperm cells to adapt to their constantly changing environment. Over the past 15 years, direct electrophysiological recordings together with genetically modified mouse models and human genetics have confirmed the importance of ion channels, including the principal Ca2+-selective plasma membrane ion channel CatSper, for sperm activity. Sperm ion channels and membrane receptors are attractive targets for both the development of contraceptives and infertility treatment drugs. Furthermore, in this era of assisted reproductive technologies, understanding the signalling processes implicated in defective sperm function, particularly those arising from genetic abnormalities, is of the utmost importance not only for the development of infertility treatments but also to assess the overall health of a patient and his children. Future studies to improve reproductive health care and overall health care as a function of the ability to reproduce should include identification and analyses of gene variants that underlie human infertility and research into fertility-related molecules.
Regulation of ion balance is essential for sperm motility and fertility. In particular, ions pass through channels at least 1,000 times faster than through transporters1. Thus, ion channels enable sperm to rapidly respond to guidance cues in the female reproductive tract. Calcium (Ca2+) influx through Ca2+-permeable ion channels can affect cell signalling by altering local electrostatic fields and protein conformations2. The speed and effectiveness of Ca2+ signalling is a consequence of a more than ~10,000-fold gradient maintained across the cell plasma membrane; the intracellular Ca2+ concentration (10–100 nM free) is, therefore, low when compared with the extracellular concentration (~1–2 mM)2,3.
Ca2+ signalling is a conserved mechanism to modulate cell motility by increasing flagellar asymmetry. Ca2+ influx is required for altering flagellar waveform in Chlamydomonas4, the steering and turning of sperm from sea urchins5,6 and fish7, and sperm hyperactivation in mammals8. The axoneme of motile cilia and flagella have Ca2+-binding sites that regulate flagellar curvature by modulating the motor protein dynein ATPase9-12. Although various molecular mechanisms can chelate, compartmentalize or extrude Ca2+ upon its entry into the cytosol2, specialized Ca2+-selective ion channels are the only Ca2+ entry sites in the sperm flagella13,14.
In mammals, flagellar Ca2+ entry is facilitated by the cation channel of sperm (CatSper), the sperm-specific Ca2+ channel complex13,15-18 (FIG. 1). CatSper-dependent Ca2+ entry induces hyperactivated motility during sperm capacitation16,17. Hyperactivated motility is the swimming pattern observed in most sperm retrieved from the oviductal ampulla at the time of fertilization and is characterized by a deep and asymmetrical flagellar bend8,19,20. Sperm hyperactivation helps to free sperm cells from the oviductal epithelium21,22, to facilitate their upstream progression23, and to penetrate the zona pellucida to fuse with the egg15. Specific signals, such as progesterone and other secretion factors, which are present within the oviduct around ovulation, stimulate hyperactivation of bovine and human sperm24,25. Studies have suggested that human CatSper functions as a polymodal sensor that translates physical and chemical cues in the reproductive tract into a Ca2+ response23,26. Thus, elucidating the molecular regulatory mechanisms by which CatSper is regulated is fundamentally important to understanding sperm hyperactivation and mammalian fertilization.
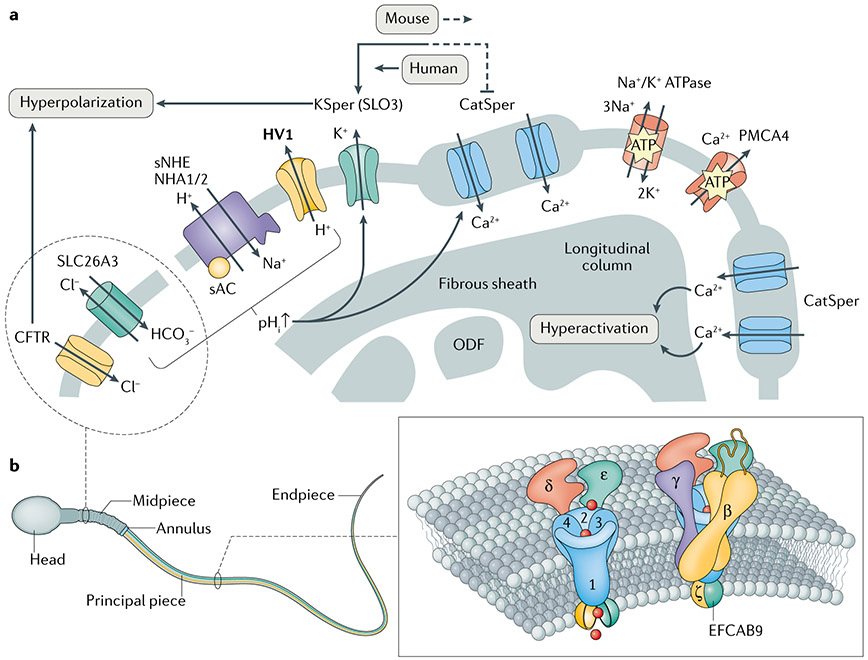
Fig. 1 ∣. Spermatozoan ion channels and membrane receptors.
a ∣ Partial cross-sectional view of the mammalian flagellar membrane that harbours ion channels and receptors. Intracellular alkalinization is induced by sNHE and HV1 in mouse and human sperm, respectively. HCO3− transporters such as SLC26A3, which interacts with the Cl−-permeable CFTR, can also carry HCO3− into sperm cells from the female reproductive tract to trigger activation of soluble adenylyl cyclase (sAC). Consequently, in mouse sperm, the pH-sensitive channels KSper and CatSper are activated and result in membrane hyperpolarization and Ca2+ influx, respectively. The hyperpolarization contributed from both KSper and CFTR during capacitation can inhibit further CatSper activation. By contrast, human KSper exhibits less pH sensitivity and is robustly activated by Ca2+ via CatSper. CatSper molecules are compartmentalized into four linear nanodomains along the principal piece of the tail; each nanodomain is apparently composed of two CatSper rows (two nanodomains are depicted in blue). Na+/K+ ATPase α4 and PMCA4 function as transporters and sustain cytoplasmic ion homeostasis in sperm. As Na+/K+ ATPase α4 contributes to membrane potential maintenance, both transporters are associated with Ca2+ regulation, directly or indirectly. Unlike the other proteins that are localized in the principal piece, CFTR and SLC26A3 localize within the sperm head and/or midpiece (dashed line). HV1 expression is species-specific (in bold type). b ∣ A CatSper channel complex linear nanodomain. CatSper is comprised of at least ten subunits including the pore-forming CATSPER1–4, the non-pore forming CATSPERβ, CATSPERγ, CATSPERδ and CATSPERε, and cytosolic CATSPERζ and EF-hand calcium-binding domain-containing protein 9 (EFCAB9). Note that large extracellular domains of the non-pore-forming transmembrane subunits are predicted to surround the channel pore. ODF, outer dense fibre.
Within the past two decades, the development of genetically modified mouse models and the application of direct electrophysiological recordings have improved our understanding of the molecular basis of mammalian sperm sensory signalling27. Species-specific variations in sperm signalling such as differences in overall receptor expression, molecular composition, and/or regulatory mode of primary ion channels have also been revealed28. Furthermore, application of state-of-the-art techniques such as super-resolution imaging29-32, imaging flow cytometry33-35 and cryo-electron tomography36-38 to sperm cells has begun to more directly inform the molecular and structural bases of sperm motility. Thus, the signal transduction pathways that lead to the mechanical transitions in the axoneme to regulate sperm motility have, at least partially, been revealed.
In this Review, we explore the literature regarding ion channel signalling in the context of regulating mammalian sperm motility and male fertility, focusing on sperm ion channels and membrane transporters for which genetic and/or electrophysiological evidence is available to support their roles in male fertility in mice and humans. We summarize important findings, current controversies and challenges, and provide insights into molecular mechanisms, future perspectives and clinical developments in the field.
Sperm intracellular alkalinization
Mammalian sperm remain quiescent during maturation and storage in the acidic luminal environment of the epididymis (pH 6.6–6.8)39-41. Variations in pH along the female reproductive tract is one of the physiological cues that stimulates sperm capacitation, including hyperactivated motility and the acrosome reaction42. In humans, the pH of luminal fluid in the female reproductive tract increases gradually from the vagina (pH ~4.4) towards the cervix (pH 6.5–7.5); the pH of cervical mucus is in the range 5.4–8.5 and the pH of the uterus is >7 (REFS43,44). The luminal pH is normally highest in the fallopian tubes (73–7.7 in humans and ~7.9 in pigs and rabbits)43. Thus, spermatozoa encounter a drastic extracellular pH change during their journey through the female reproductive tract. Upon ejaculation, human spermatozoa are mixed with seminal plasma (pH 7.2–8.4), which alkalinizes the acidic vaginal environment45,46. The optimal pH for sperm motility is in the range 7.0–8.5 in bulls47 and humans48, which is consistent with the pH of the oviductal fluid. Intracellular alkalinization of sperm cells during capacitation can be caused by HCO3− uptake from the fluid in the female reproductive tract through HCO3− transporters and extrusion of H+ through proton carriers (FIG. 1 a).
Proton carriers
Carrier-mediated mechanisms constitute the major route for proton transport across the plasma membrane in sperm39. Membrane transporters such as sodium–hydrogen exchangers (NHEs) and the voltage-gated proton-selective ion channel HV1 are the best-studied examples.
Sodium–hydrogen exchangers.
NHEs, encoded by the Slc9 gene family, transport Na+ into cells and H+ out of cells49, thereby regulating intracellular pH (FIG. 1a). NHE1 (REF.50), NHE5 (REF.51) and NHE8 (REF.52) are expressed in multiple tissue types in mammals, including the testis. However, two NHEs — sNHE (encoded by Slc9c1)53,54 and NHA1 (encoded by Slc9b1)54 — are expressed specifically in sperm flagellum. Gene knockout studies in mice have demonstrated the importance of pH regulation by NHEs in sperm physiology (TABLE 1). In these studies, loss of sNHE53 or NHA1 and NHA2 (encoded by Slc9b2)55 together caused male infertility and knockout of NHE8 (encoded by Slc9a8) in male germ cells resulted in defects in acrosome formation and male infertility56. However, the extent of the contribution made by each NHE in controlling intracellular pH in mouse sperm is not clear. For example, impaired sperm motility in sNHE-deficient sperm was only partially rescued by artificial alkalinization but was completely rescued by administration of cyclic AMP (cAMP) analogues53. The intracellular cAMP levels and protein expression of soluble adenylyl cyclase (sAC) were attenuated in the absence of sNHE, NHA1 and NHA2 (REFS55,57), suggesting a functional redundancy in NHEs for sAC expression and/or their relationship with cAMP signalling pathways to facilitate sperm motility regulation. In sea urchins, sNHE can control intracellular pH homeostasis more rapidly than typical transporters by responding to hyperpolarization and cAMP58. As no other NHE has been reported in the sea urchin genome, sNHE might act as a solo voltage-dependent NHE. Whether other, as-yet-uncharacterized, NHEs are involved in pH regulation in mammalian sperm is unknown.
Table 1 ∣.
Genetic studies of sperm ion channels and membrane transporters implicated in male fertility
| Gene | Tissue expression |
Protein localization in sperm |
Phenotypes of mutation | Species | Refs |
|---|---|---|---|---|---|
| Ion channels | |||||
| Kcnu1 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: impaired In vivo fertility: infertile |
Knockout mouse | 70,84 |
| Lrrc52 | Testis | ND | Spermatogenesis: ND Hyperactivated motility: ND In vitro fertilization: impaired In vivo fertility: subfertile |
Knockout mouse | 86 |
| Catsper1 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: impaired In vivo fertility: infertile |
Knockout mouse | 15,16 |
| CATSPER1 | Testis | Principal piece | Spermatogenesis: impaired Motilitya: reduced In vitro fertilization: ND In vivo fertility: infertile |
Humanb | 100b |
| Catsper2 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: ND In vivo fertility: infertile |
Knockout mouse | 17 |
| CATSPER2 | Testis | Principal piece | Spermatogenesis: impaired Motilitya: reduced In vitro fertilization: ND In vivo fertility: infertile |
Humanc,d | 102c; 103c; 104d;111c;216c |
| Catsper3 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: ND In vivo fertility: infertile |
Knockout mouse | 18,95 |
| Catsper4 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: ND In vivo fertility: infertile |
Knockout mouse | 18,95 |
| CATSPERE | Testis | Principal piecee | Spermatogenesis: normal Motilitya: normal In vitro fertilization: impaired In vivo fertility: infertile |
Humanf | 29e;106f |
| Catsperz | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: impaired In vivo fertility: subfertile |
Knockout mouse | 29 |
| Cacna1e | Ubiquitous | Head | Spermatogenesis: ND Hyperactivated motility: normal In vitro fertilization: impaired In vivo fertility: mildly subfertile |
Knockout mouse | 160,161 |
| CFTR | Multiple | Head/midpiece | Spermatogenesis: impaired Hyperactivated motility: ND In vitro fertilization: impaired In vivo fertility: impaired |
Human, knockout mouse | 178,180-183,188,251 |
| Pkdrej | Testis | Heade | Spermatogenesis: normal Hyperactivated motility: normal but slow In vitro fertilization: ND In vivo fertility: fertile |
Knockout mouse | 171,172e; 252 |
| Trpv4 | Ubiquitous | Head and tail | Spermatogenesis: normal Hyperactivated motility: normal but delayed In vitro fertilization: ND In vivo fertility: fertile |
Knockout mouse | 147,151,152,253 |
| Membrane transporters | |||||
| Atp1a4 | Testis | Principal piece | Spermatogenesis: normal Hyperactivated motility: reduced In vitro fertilization: impaired In vivo fertility: infertile |
Knockout mouse | 82 |
| Slc9a8 | Ubiquitous | Head (acrosome) | Spermatogenesis: impaired Motilitya: reduced In vitro fertilization: ND In vivo fertility: infertile |
Knockout mouse | 56 |
| Slc9b1 | Testis | Principal piece | Spermatogenesis: normal Motilitya: reduced In vitro fertilization: ND In vivo fertility: subfertileg |
Knockout mouse | 55 |
| Slc9b2 | Ubiquitous | Principal piece | Spermatogenesis: normal Motilitya: reduced In vitro fertilization: ND In vivo fertility: subfertileg |
Knockout mouse | 55 |
| Slc9c1 | Testis | Principal piece | Spermatogenesis: normal Motilitya: reduced In vitro fertilization: impaired In vivo fertility: infertile |
Knockout mouse | 53 |
| P2rx2 | Ubiquitous | Midpiece | Spermatogenesis: normal Hyperactivated motility: normal In vitro fertilization: normal In vivo fertility: mildly subfertile |
Knockout mouse | 154 |
| Cnnm4 | Ubiquitous | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: impaired In vivo fertility: subfertile |
Knockout mouse | 195 |
| Atp2b4 | Ubiquitous | Principal piece | Spermatogenesis: normal Hyperactivated motility: impaired In vitro fertilization: normal In vivo fertility: infertile |
Knockout mouse | 191-193 |
| SLC26A3 | Multiple | Head/midpiece | Spermatogenesis: ND Hyperactivated motility: ND In vitro fertilization: ND In vivo fertility: subfertile |
Human, knockout mouse | 182-185,254 |
ND, Not determined.
Motility was tested in the standard bath solution without capacitation components.
Two separate insertion mutations in exon 1 (c.539–540insT and c.948–949insATGGC).
Deaf infertility syndrome with a deletion encompassing CATSPEP2 and STRC together.
Copy number variation in the region of 43894500 to 43950000 in 15q 15.3 encompassing a heterozygous deletion of CATSPEP2.
Protein localization information only.
Homozygous in-frame 6-bp deletion in exon 18 (c.2393_2398delCTATGG).
Slc9bl/Slc9b2-double-knockout mice are completely infertile.
Proton channels.
The HV1 proton channel has been suggested to control human sperm intracellular pH59. HV1 is homologous with the voltage sensor domain (VSD) of voltage-gated channels and functions as a dimeric channel complex, but does not contain a separate pore domain60,61. Molecular dynamic simulations of HV1 homology models suggest that HV1 probably contains an internal water wire within the central crevice of the VSD for selective proton transfer62. Full-length HV1 and N-terminal cleaved HV1Sper have been detected in human sperm63. Both channels exhibit voltage-dependent activation, which requires a pH difference across the membrane (ΔpH), and are inhibited by zinc59,63. The voltage dependence of heterologously expressed HV1Sper is also affected by simultaneous changes in intracellular and extracellular pH. Electrophysiological recordings of human sperm have not been able to distinguish HV1Sper from HV1 current63; thus, uncovering the molecular mechanisms by which HV1 and HV1Sper regulate the pH of human sperm requires further studies. Such studies would rely on human genetic evidence because HV1 is absent in mouse sperm59. Interestingly, a 2018 study found that the HV1 channels are arranged in bilateral lines along one side of the flagellar membrane32, which suggests that the combined effect of H+ efflux through many HV1 channels alters the local intraflagellar pH and, in doing so, can regulate pH-sensitive molecules.
Intracellular alkalinization has been predicted to precede membrane potential hyperpolarization and Ca2+ signalling in mouse and human spermatozoa after ejaculation13,64-67. Thus, understanding the molecular mechanism of sperm pH regulation and sensing is crucial. HV1 and sNHE are both confined to the principal piece of the flagella53,59. Interestingly, the ion channels CatSper and KSper (sperm-specific K+ channel), which are both pH-sensitive, are also found in the principal piece (FIG. 1a), suggesting compartmentalized pH regulation and sensing machineries.
Membrane potential hyperpolarization
Electrochemical gradients across cellular plasma membranes are generally maintained by Na+/K+ ATPase68. Resting membrane potential is primarily set by K+ channels and is typically about −70 mV in somatic cells, including nerve fibres, in the absence of excitation69. By comparison, it is less polarized in mammalian spermatozoa (for example, about −40 mV in mouse and human sperm)64,65,70. During capacitation, the sperm membrane hyperpolarizes — K+ efflux through activated KSper is primarily responsible for this change in membrane potential64 (FIG. 1a). In turn, hyperpolarization regulates various membrane proteins, including the voltage-gated proton channel HV1, ion exchangers and Ca2+ channels. Abnormal depolarization of membrane potential might be associated with human male subfertility71-74; thus, understanding the molecular interactions and regulatory mechanisms of Na+/K+ exchange and KSper is critical to understanding sperm physiology. Genetic evidence regarding which mutations in genes encoding Na+/K+ ATPases and KSper are associated with fertility defects in men remains to be collected.
Na+/K+ transporters
Na+/K+ ATPase transporters contribute to the regulation of membrane potential owing to unequal exchange of cytoplasmic Na+ for extracellular K+ (REF.68). The transporter consists of a group of isozymes that contain α-subunits and β-subunits. The catalytic α-subunit of Na+/K+ ATPase facilitates ion permeation of the plasma membrane68. The α1 subunit is expressed in a variety of tissue and cell types in humans and rats, including sperm75,76; however, the α4 subunit is specific to male germ cells76. When rat or human α4 subunit is expressed in transgenic mice, it is particularly abundantly expressed in the sperm flagellum, which is consistent with its expression in wild-type rat and human sperm77,78. Treatment of rat sperm with a low concentration of ouabain (which selectively inhibits α4) increased intracellular Na+, depolarized the membrane potential, increased intracellular calcium and decreased intracellular pH79. Because α4 does not directly transport H+, it has been proposed that α4 provides electrochemical energy that facilitates pH regulation by NHEs77,80. In particular, sNHE has a putative voltage sensor53 and so could potentially be regulated by an α4-established membrane potential. Measuring intracellular pH under various transmembrane sodium gradients will help to test this possibility.
The potential contribution of the α1 subunit to sperm function remains to be clarified, as α1 subunit knockout is embryonically lethal in mice81. By contrast, disruption of Atp1α4, which encodes the α4 subunit, in mice causes complete sterility82 (TABLE 1), demonstrating that α4-mediated ion transport is critical for sperm function. Furthermore, Na+/K+ ATPase α4-deficient sperm from the knockout mice are severely bent at the junction of the midpiece and the principal piece, exhibit increased intracellular Na+ levels and have depolarized membrane potentials, consistent with the phenotypes observed in mouse sperm with altered osmoregulation83. Contrastingly, transgenic mouse sperm that express functional rat Atp1α4 exhibit increased membrane hyperpolarization and also demonstrate increased total sperm motility and hyperactivated motility77; these effects starkly contrast those observed in the Atp1α4-disrupted mice that are completely sterile82, demonstrating the importance of functional ATP1A4 in sperm motility and fertility. Expression of human ATP1A4 in transgenic mice results in similar motility phenotypes but not in a significant change in sperm membrane potential78. The expression of rat or human α4 does not affect the acrosome reaction in transgenic mouse sperm77,78. These studies demonstrate that α4 activity primarily maintains sperm intracellular Na+ levels and contributes to setting membrane potential. As a result, α4 affects several vital parameters, such as intracellular pH and Ca2+, which are essential for sperm motility and hyperactivation.
The KSper channel
KSper was first recorded in mouse sperm in 2007 (REF64). Upon intracellular alkalinization, KSper is activated and further hyperpolarizes the spermatozoan cellular membrane (FIG. 1a). Genetic disruption of Kcnu1 demonstrated that SLO3, the pore-forming α-subunit, mediates KSper in mouse sperm70,84 (TABLE 1). Despite their normal morphology and motility, mouse spermatozoa lacking SLO3 are infertile owing to impaired hyperpolarization during capacitation. However, a residual K+ current has been observed in Kcnu1-knockout sperm at very positive potentials84, which suggests that another voltage-gated K+ current might exist in mouse sperm. However, a subsequent study clarified that all voltage-gated outward current is abolished in sperm of Kcnu1/Catsper1-double-knockout mice14, demonstrating that the residual K+ current observed in Kcnu1-knockout sperm is caused by K+ efflux through CatSper and provides evidence that KSper and CatSper are the sole mediators of voltage-dependent K+ and Ca2+ currents, respectively, in uncapacitated mouse epididymal sperm in response to alkalinization. Heterologous expression of leucine-rich repeat-containing protein 52 (LRRC52), an auxiliary subunit of SLO3, modulates SLO3 gating by shifting its voltage and pH dependence to more negative values, close to those of native KSper currents85,86. Consistently, upon deletion of Lrrc52, the activity of KSper at rest is attenuated and its activation requires increasingly positive voltages and higher pH, resulting in a more depolarized membrane potential86. Abrogation of LRRC52 results in male subfertility and reduced litter sizes86 (TABLE 1), supporting the association of this abnormal depolarization of membrane potential with male subfertility in humans71-74.
Native human KSper (hKSper), unlike mouse KSper, is sensitive to both intracellular alkalinization65,87 and calcium65,88. It exhibits Ca2+ sensitivity in the same manner as another SLO family member SLO1 (also known as BK channel and encoded by Kcnma1), and pH sensitivity in the same manner as SLO3. These unusual characteristics — whereby hKSper displays hybrid characteristics of the mouse counterpart, SLO3, which is pH-sensitive, and SLO1, which is Ca2+-sensitive — have resulted in controversy regarding the exact molecular composition of hKSper. However, studies have shown that heterologous expression of human SLO3 in Xenopus and 293T cells does give rise to currents that exhibit the properties of native hKSper in human sperm65,89,90 — that is, activation by Ca2+ and alkalinization, inhibition by progesterone, and a single-channel conductance of 70 pS (REF.65). Moreover, SLO3 and LRRC52 were identified in human sperm by mass spectrometry65, further supporting the view that SLO3 and LRRC52 comprise hKSper. A 2017 study showed that a single-nucleotide polymorphism of human SLO3 (C382R) can endow the channel with enhanced pH and Ca2+ sensitivities89, suggesting that species-specific SLO3 variants could have acquired different Ca2+ sensitivity. KSper sensitivity to intracellular pH and Ca2+ might define its role in mouse and human sperm: in mouse sperm, the KSper-dependent, capacitation-associated membrane hyperpolarization is an upstream signalling event that increases the force driving Ca2+ influx through CatSper91; in humans, KSper probably functions downstream of CatSper because activation of hKSper requires an increase in cytosolic Ca2+ (REF92); KSper-induced hyperpolarization would further affect CatSper. An improved understanding of how sperm membrane potential is regulated during capacitation will provide insights into species-specific fertilization processes.
Ca2+ influx and signal transduction
The intracellular Ca2+ increase in sperm, which is required for hyperactivated motility and the acrosome reaction, originates primarily from influx of extracellular Ca2+; in these specialized cells, the organelles that are typically used for intracellular Ca2+ storage and release, such as the endoplasmic reticulum, are less developed than in somatic cells. Mouse spermatogenic cells possess T-type Cav channels93, but electrophysiological recordings have demonstrated that Cav currents gradually decrease during spermiogenesis and become undetectable in epididymal sperm94, suggesting that Cav currents are only required during spermatogenesis and not in mature sperm cells. So far, CatSper is the only Ca2+ channel in which genetic mutations have been reported to cause male infertility. Thus, the flagellar-specific Ca2+ channel CatSper is a predominant pathway by which Ca2+ enters mammalian sperm.
Molecular organization of CatSper
CatSper was first identified in 2001 as a gene (Catsper1) encoding a putative sperm Ca2+ channel with a single repeat of six transmembrane domains in human and mouse testis15. The topology of CATSPER1 is unlike that of conventional voltage-gated Ca2+ channels, which are typically composed of four repeats of six transmembrane domains. Subsequently, Catsper2 (REF17), Catsper3 and Catsper4 (REFS18,95) were identified. CatSper currents are absent in mouse sperm in which one subunit (Catsper1, Catsper2, Catsper3 or Catsper4) has been knocked out18, indicating that the CatSper pore is a heterotetramer composed of CATSPER1–4 and that one of each is required for channel function. In addition, CatSper channels comprise at least six additional subunits that do not form the pore (FIG. 1b): four transmembrane proteins that are predicted to contain large extracellular domains (ECDs), denoted CATSPERβ96, CATSPERγ97, CATSPERδ98 and CATSPERε29; and two small cytoplasmic proteins, CATSPERζ29 and EF-hand calcium-binding domain-containing protein 9 (EFCAB9)31. CatSper is the most complex ion channel known31,98. Knocking out any one of the four genes that encode CATSPER1–4 in mice renders males infertile15,18,95,99 (TABLE 1) and CATSPER loss-of-function mutations have also been identified in infertile men100-104. Sperm cells in which Catsper1, Catsper2, Catsper3 or Catsper4 has been knocked out fail to develop hyperactivated motility16-18,99, which is consistent with the inability of Catsper1-null sperm to fertilize oocytes with an intact zona pellucida15. Direct whole-cell patch clamp studies of spermatozoa have demonstrated that CatSper is Ca2+-selective channel activated by intracellular alkalinization13,67.
Deletion of Catsperd, which encodes one of the nonpore-forming transmembrane subunits, CATSPERδ, also abrogates CatSper current and hyperactivated motility, resulting in infertility in male mice98 (TABLE 1). This phenotype arises from the loss of not only CATSPERδ but also each transmembrane domain-containing CATSPER protein in sperm cells of Catsperd-null males, and is similar to the all-or-nothing expression pattern of the CatSper pore-forming subunits29-31,98. By contrast, knocking out Catsperz and/or Efcab9 does not completely eliminate the formation of functional CatSper channels and correspondingly results in male subfertility29,31 (TABLE 1). Consistently, reduced CATSPERζ protein expression has been reported in men with asthenozoospermia105, suggesting that these two non-transmembrane subunits, CATSPERζ and EFCAB9, function as true auxiliary subunits that could modulate CatSper expression levels and/or channel kinetics. Interestingly, Catsperz orthologues have been found only in mammals, implying that its regulatory function might be specific to mammalian CatSper channels29. Direct analyses of the functions of the other non-pore-forming transmembrane subunits, CATSPERβ, CATSPERγ and CATSPERε await their investigation using genetic disruption in mice and/or the discovery of human genetic abnormalities related to these proteins.
Spatial organization of CatSper
Flagellar Ca2+ signalling nanodomains.
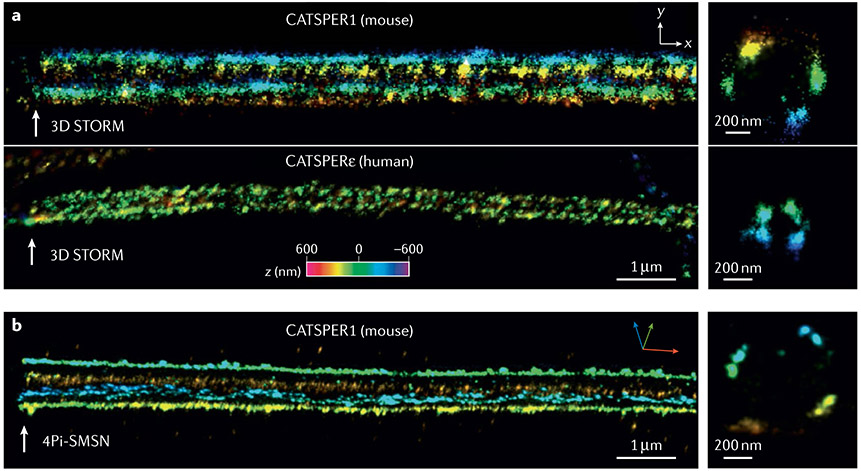
Super-resolution microscopy has been used to demonstrate that the macromolecular CatSper channel forms four linear (also called quadrilateral) Ca2+ signalling nanodomains along the sperm tails in both mice and humans29-31 (FIGS 1,2) and organizes a network of intracellular signalling molecules such as calmodulin-dependent protein kinase II (CaMKII) and calcineurin30. Compartmentalized domains enable specific and fast-triggering downstream events and are common cellular adaptations for effective Ca2+ signalling in many biological systems2. A 2019 study in mice demonstrated that each of the four CatSper nanodomains is further resolved into two row structures31 (FIG. 2b). CATSPERζ and/or EFCAB9 deficiency disrupts not only the linearity of the CatSper nanodomains but also the two-row organization, renders the proximal sperm flagella rigid and alters sperm motility29,31. As a result, the presence and integrity of these nanodomains serves as an indicator of fertilizing capability. These studies also indicate that CATSPERζ and EFCAB9 regulate the compartmentalization of Ca2+ signalling in mammalian sperm, and might, therefore, modulate the mechanism by which CatSper facilitates Ca2+ influx.
Fig. 2 ∣. Quadrilinear CatSper Ca2+ signalling nanodomains in mammalian sperm.
a ∣ 3D stochastic optical reconstruction microscopy (STORM) images. Distributions of CATSPER1 (mouse, upper) and CATSPERε (human, lower) suggest conservation of the CatSper nanodomains in mammalian sperm. b ∣ 3D 4Pi single-molecule switching nanoscopy (SMSN) images of CATSPER1 in mouse sperm. Note that each nanodomain is further resolved into two row structures. The colour encodes the relative distance from the focal plane along the z axis (color scale bar in x–y projection). Adapted from (REF.29), CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
CatSper assembly and trafficking to the flagellar membrane.
Despite the absolute requirement of the CatSper channel for male fertility and the importance of CatSper nanodomain formation for sperm Ca2+ signalling, knowledge of how CatSper is assembled and organized into nanodomains is limited. This lack is largely due to an inability to heterologously reconstitute CatSper under laboratory conditions. Although the timing of translation of CatSper subunits is not known, mouse CATSPERδ has been suggested to assist the assembly of the CatSper channel in the endoplasmic reticulum or the Golgi, as CATSPER1 is degraded in its absence98. CATSPERδ and CATSPERε might dimerize, as they have a region of high homology at their C terminus29. In 2018, a 6-bp in-frame deletion within CATSPERE in an infertile man was reported; the sperm cells of this patient did not respond to progesterone to elicit CatSper-mediated Ca2+ increase and in vitro fertilization (IVF) failed106,107. This alteration occurred within the region of CATSPERε that exhibits high homology with CATSPERδ, suggesting that CATSPERε might also have a role in the assembly of the large CatSper complex together with CATSPERδ. Studies in mice have suggested that only a correctly assembled CatSper channel, probably with all of its transmembrane subunits in place, is trafficked to the flagellar membrane98.
Questions remain regarding how CatSper traffics to the flagellar membrane and whether intraflagellar transport (IFT) machinery and adaptor proteins exist that specifically interact with CatSper or other sperm ion channels and transporters is unknown. Studies using genetically altered mouse models have shown that IFT is essential for mammalian spermiogenesis but that epididymal spermatozoa are devoid of IFT proteins108. For example, IFT25 and IFT27, which are dispensable for ciliogenesis in somatic cells, are required for flagellar formation as they participate in assembling and transporting structural components of the fibrous sheath that is unique to spermatozoa109,110. Identification of the CatSper interactome from spermatids, not mature sperm, and application of super-resolution microscopy could provide valuable information on the trafficking of flagellar membrane proteins and quadrilateral compartmentalization (FIGS 1,2) during spermiogenesis.
Human versus mouse CatSper.
Interestingly, two studies in infertile patients who lacked CatSper current demonstrated that CatSper assembly in humans is strikingly different from CatSper assembly in mice103,104. Infertile men who had lost one genomic copy of CATSPER2 (owing to a copy number variation)104 or both copies owing to contiguous deletion of a genomic region encompassing CATSPER2 and STRC (15q15.1–15q15.3, deletion of which leads to the rare deafness–infertility syndrome)103,111,112 had otherwise normal semen parameters despite substantially reduced or absent expression of CATSPER2, respectively, in their spermatozoa. The protein expression levels of the other three CatSper pore-forming subunits in these unique human CatSper-deficient sperm cells were only marginally changed, or were not changed at all103,104, in contrast to the changes observed in mouse CatSper-knockout sperm. Moreover, the uniquely quadrilateral sub-flagellar compartmentalization was observed for CATSPER3 and CATSPER4 in the principal piece of human CATSPER2-deficient spermatozoa103. These results suggest that human CATSPER1, CATSPER3 and CATSPER4 proteins can still traffic to the flagella, but they cannot form functional channels without CATSPER2. Thus, the all-or-nothing expression pattern of the CatSper complex apparently does not exist in human sperm cells. Formation of trimeric complexes lacking CATSPER2 and their proper trafficking to the flagella is unlikely to happen because a tetramer of an ion channel pore is often assembled as a dimer of dimers. These findings illustrate the species-specific differences in the assembly and trafficking patterns of CatSper.
A more thorough understanding of the organization of CatSper into Ca2+ signalling nanodomains requires further investigation. For example, probing the absence or presence and the sub-flagellar localizations of other CatSper auxiliary subunits in these human CATSPER2-deficient sperm will provide more insight into their roles in channel assembly and trafficking and domain organization. Additional unidentified channel subunits that specifically function in trafficking might also exist. This possibility was implied by a recent CatSper proteome study of mouse sperm cells, which showed several candidate CatSper-associated proteins, including one with a conserved domain involved in membrane trafficking31. The likely existence of additional unknown CatSper subunits highlights the molecular and regulatory complexity of this important channel.
Modulation of CatSper activity
Molecular mechanisms of alkaline activation and Ca2+ sensitivity of CatSper.
Ca2+ entry through CatSper requires channel activation by intracellular alkalinization13,31,66,67. The pH sensitivity of CatSper was initially attributed to a conserved histidine-rich region in the N terminus of CatSper1 among mammals13,15, but, subsequently, the molecular basis by which CatSper can sense pH and Ca2+ has been elucidated. The mechanism relies upon EFCAB9, a testis-specific protein that has co-evolved with other core members of the CatSper complex31. EFCAB9 was identified in a comparative proteomics screen for proteins differentially expressed in Catsper1-null sperm when compared with wild-type sperm31. EFCAB9 is the only CatSper subunit that contains known Ca2+- and calmodulin-binding motifs. Three EF-hand motifs were identified in EFCAB9 and it was shown to bind CATSPERζ in a pH-dependent and Ca2+-dependent manner31. An increased Ca2+ concentration facilitated EFCAB9–CATSPERζ complex formation, whereas alkalinization impeded the interaction31. That EFCAB9 and CATSPERζ work as one functional unit is supported by mouse models in which either Efcab9 or Catsperz or both has been knocked out. These three mouse models exhibit identical phenotypes and demonstrate that the expression of these proteins is interdependent: if Efcab9 has been knocked out, CATSPERζ is absent, and vice versa31. Although EFCAB9-deficient sperm exhibit reduced CatSper expression, when intracellular pH is low, wild-type and Efcab9-null sperm show a similarly dense CatSper current (ICatSper)31, indicating that EFCAB9–CATSPERζ normally limits CatSper-mediated Ca2+ entry. When intracellular pH rises, CatSper is activated in a Ca2+-dependent manner, but in the absence of EFCAB9, the channel is less sensitive to intracellular Ca2+ changes and less responsive to alkalinization31. Thus, a working model was generated: before capacitation, the pH-sensing and Ca2+-binding EFCAB9–CATSPERζ complex stabilizes the closed CatSper pore; upon alkalinization and Ca2+ entry through CatSper, EFCAB9–CATSPERζ undergoes structural rearrangements releasing its inhibition of the channel. Further opening of the channel is followed by Ca2+ entry, which would be bound by EFCAB9, stabilizing its prolonged open state31.
Steroid hormones in human CatSper regulation.
Both mouse and human CatSper channels are activated by intracellular alkalinization; however, activation of CatSper by physiological ligands has only been reported in humans66,67 and rhesus macaques113, suggesting that CatSper is regulated by species-specific mechanisms. For example, progesterone, a steroid hormone secreted by cumulus cells, can robustly evoke human66,67,102, but not mouse67, CatSper currents. Similarly, prostaglandin E1 (PGE1), which is abundant in the seminal plasma and also secreted by cumulus cells, was found to activate human, but not mouse, CatSper67. α/β hydrolase domain-containing protein 2 (ABHD2) has been identified as the non-genomic progesterone receptor in human sperm114. ABHD2 abolishes endocannabinoid 2-arachidonoylglycerol (2-AG) inhibition of CatSper as a progesterone-dependent lipid hydrolase, enabling CatSper activation114. Other steroid hormones, such as pregnenolone sulfate and testosterone, also modulate CatSper-mediated Ca2+ influx into human sperm; they probably bind to the same sites as progesterone115. Whether testosterone and other steroids function as human CatSper agonists or antagonists remains controversial115-117, and the mechanisms by which these ligands bind to and modulate CatSper activity remain largely unknown.
Additional ligands and chemicals have been reported to activate human CatSper directly or indirectly115,118-120. These include structurally diverse endocrine-disrupting chemicals (EDCs) such as p,p′-dichlorodiphenyldichloroethylene, 4-methylbenzylidene camphor and triclosan119-121. Thus, the presence of any of these EDCs in the female reproductive tract could interfere with human sperm function by modulating CatSper activity.
The non-pore-forming CatSper transmembrane subunits have been suggested to bind factors that alter CatSper gating as they have large ECDs29,96-98 (FIG. 1b). They could potentially bind ligands and function as sensory transducers in the polymodal Ca2+ signalling exhibited by human sperm. Disrupting one of the genes, CatSperd, in mice results in an identical phenotype to that seen in mice with knockout of the pore-forming CatSper subunits at the organismal and cellular levels98, illustrating that CATSPERδ is essential to form a functional CatSper. In the future, biochemical and structural characterization of isolated ECDs from the non-pore-forming CatSper transmembrane subunits, in addition to characterization of the cytoplasmic CatSper subunits themselves, is a sensible approach to improving our understanding of the molecular mechanisms of CatSper.
Signalling pathways in capacitation
Sperm capacitation involves a cascade of signalling pathways (FIG. 1a). Studies have indicated that different pathways directly or indirectly regulate CatSper, the central signalling hub in network with multiple signalling proteins.
Signalling crosstalk and CatSper
During capacitation, the rise in intracellular pH activates not only CatSper but also KSper. Activation of KSper hyperpolarizes the membrane to further drive Ca2+ influx through CatSper in mouse sperm64,70,84. HCO3−, Ca2+ and bovine serum albumin (BSA) have long been recognized as indispensable for sperm capacitation and fertilization in vitro122, engaging signalling pathways that regulate CatSper-mediated calcium signalling.
HCO3− uptake and cAMP-dependent PKA activation.
At the molecular level, capacitation is initiated when spermatozoa are exposed to a high concentration of HCO3− in the luminal fluid of the female reproductive tract, which also has a higher Ca2+ concentration than the luminal fluid of the epididymis39. HCO3− enters the sperm through HCO3− transporters42 and activates a unique sAC123, resulting in increased cAMP levels. The cAMP-dependent PKA pathway is important in the regulation of sperm motility: sAC-deficient male mice are infertile124, as are mice deficient in the catalytic subunit of PKA, Cα2 (REF.125). HCO3− also stimulates Ca2+ entry into sperm by raising intracellular pH (FIG. 1a). However, the role of cAMP in the regulation of Ca2+ influx is less clear. Membrane-permeable analogues of cyclic nucleotides stimulate Ca2+ entry in mouse15,96,126,127 and human sperm26. However, a series of studies have also shown that an increase in intracellular cAMP (stimulated by HCO3−, 3-isobutyl-1-methylxanthine, uncaging of cAMP, or adenosine) fails to stimulate Ca2+ influx in mouse128 and human sperm66. Furthermore, membrane-permeable analogues of cyclic nucleotides, but not physiological concentrations of cAMP or cGMP, activate human CatSper when applied extracellularly, but not intracellularly26,129. However, these data are inconsistent with the findings of studies in mouse sperm, in which cAMP directly applied intracellularly through a pipette activated CatSper130, but cyclic nucleotide analogues applied extracellularly to the bath solution had no effect13. Thus, cAMP and PKA regulation of CatSper-mediated Ca2+ influx is probably species-specific and needs to be further clarified.
Calcium influx induced by loss of cholesterol.
Together with high levels of HCO3− and Ca2+ concentration, serum albumin is a key component in mammalian sperm capacitation in vivo as well as in vitro131,132. Although BSA is known to induce Ca2+ influx in sperm in vitro, the molecular mechanism by which this occurs has not been fully elucidated. However, BSA-induced Ca2+ influx is obliterated in Catsper1-null spermatozoa133. As cholesterol release from the sperm plasma membrane by BSA is associated with activation of cAMP–PKA pathways during sperm capacitation in both mice134 and humans135, lipid signalling by cholesterol efflux might participate in regulating CatSper-mediated Ca2+ signalling via the cAMP–PKA pathway. In this respect, caveolin 1 — a protein associated with cholesterol-rich lipid rafts — has been found in the CatSper Ca2+ signalling nanodomains in mouse sperm. The role of the CatSper channel as a domain organizer is notable, as caveolin 1 localization is dysregulated in the absence of the CatSper channel but CatSper nanodomains remain intact in the absence of caveolin 1 (REF.30).
Downstream signalling that leads to motility regulation at the axoneme.
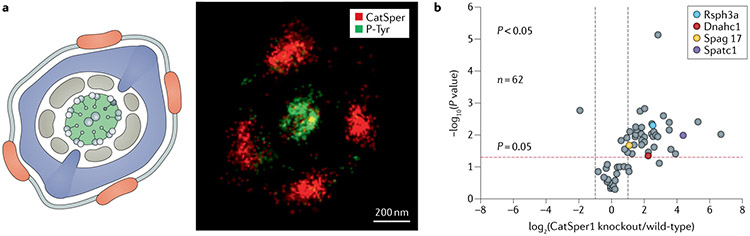
Under capacitating conditions, cAMP-stimulated PKA activity leads to sperm motility changes within a minute136-138. By contrast, hyperactivated motility and a capacitation-associated increase in tyrosine phosphorylation (P-Tyr) occur much later in time139. The cAMP–PKA pathway is thought to control downstream P-Tyr because permeable cAMP analogues are able to induce P-Tyr, even in the absence of HCO3− and calcium ions140 and administration of PKA inhibitors completely blocks P-Tyr development141. Interestingly, earlier onset and increased P-Tyr was observed in Catsper1-null and Catsperd-null spermatozoa following incubation under capacitation conditions30, suggesting that PKA activity and P-Tyr are suppressed by a CatSper-mediated Ca2+ signalling pathway. Super-resolution imaging has revealed a striking spatial confinement of P-Tyr to the axoneme in capacitated wild-type sperm (FIG. 3a); P-Tyr spreads and fills the extra-axonemal region in the absence of CatSper30. As P-Tyr requires PKA activation, the signal transduction to suppress P-Tyr in the periaxoneme has been suggested to involve active protein phosphatase 1 (PP1) and/or protein phosphatase 2A (PP2A) to limit PKA activity and protein tyrosine phosphatase30. In the same study, multiple tyrosine-phosphorylated proteins were identified from capacitated mouse sperm30, including axonemal proteins and a testis-specific tyrosine kinase, FER/FERT (FIG. 3b). Subsequently, a 2016 study revealed that the capacitation-associated P-Tyr increases are mostly eliminated in sperm from kinase-inactivating mutant (D743R) FerDR/DR males142, demonstrating that FER/FERT is the master tyrosine kinase responsible for capacitation-associated P-Tyr. However, FerDR/DR males are fertile143, although sperm from these mice do display reduced fertilizing ability in vitro142. Thus, P-Tyr is not essential for mouse fertilization in vivo but might have a functional role, such as timing motility regulation and/or determining the lifespan of sperm in the female oviduct.
Fig. 3 ∣. Capacitation-associated protein tyrosine phosphorylation.
a ∣ Tyrosine phosphorylation (P-Tyr) exhibits subflagellar localization. A cross-sectional view of the principal piece (left) shows CATSPER (red), fibrous sheath (blue), outer dense fibres (brown), and 9+2 axoneme (line structures in the centre). A two-colour 3D stochastic optical reconstruction microscopy (STORM) cross-sectional image of a capacitated wild-type spermatozoon (right) shows CATSPER1 (red) and P-Tyr (green). Note that P-Tyr is localized in the centre of the cross-section, corresponding to the axoneme. b ∣ P-Tyr identified by quantitative whole-sperm proteome analysis from capacitated wild-type and Catsper1-null mice. Each protein is represented as a dot in a volcano plot of statistical significance (y-axis) against the average protein fold-change (x-axis) of Catsper1-null compared with wild-type spermatozoa. Four axonemal proteins (more than twofold change and P < 0.05) are marked in colour.
A Ca2+ signal can be directly translated into mechanical changes in the axoneme: transient treatment with the Ca2+ ionophore A23187 can bypass the P-Tyr development in mouse sperm and the CatSper signalling network to induce hyperactivated motility in vitro144,145. Nevertheless, CatSper-mediated Ca2+ signal transduction, originating from the linear CatSper nanodomains, is required for flagellar Ca2+ regulation in vivo. Disruption of the nanodomain compartmentalization29,31, or loss of calcineurin30,146 or EFCAB9 (REF.31), both of which are Ca2+-binding proteins from the nanodomains, leads to changes in the flagellar envelope and fertility defects in mice. Discovery of more Ca2+-dependent molecules associated with the CatSper nanodomains and/or the axoneme will further elucidate the downstream Ca2+ signalling affecting dynamic motility regulation.
Other channels in sperm
In addition to CatSper and KSper, HIV1 and sNHE, a number of other channels are involved in regulating sperm function.
DSper and TRPV4
The presence of a channel that facilitates influx of Na+, as is observed in neuronal excitation, has been hypothesized in sperm and named ‘DSper’28. The DSper current was recorded in human sperm as a non-CatSper, non-selective cation conductance with outward rectification and pronounced temperature sensitivity147. In this study, DSper was potentiated during capacitation and was not diminished by either Mg2+ or NNC55-0396 administration, which block and inhibit CatSper, respectively. DSper is reversibly activated by warm temperatures (22–37°C; T1/2 34°C in uncapacitated human sperm, T1/2 31 °C in capacitated human sperm). Pharmacological screening of human sperm has suggested that TRPV4 mediates this temperature-dependent DSper current147. Capacitated rabbit and human sperm cells have been shown to move towards higher temperatures in vitro148, and subsequent study showed that capacitated human sperm swim up a temperature gradient by modulating hyperactivated motility149, However, in humans, sperm thermotaxis remains controversial because temperature differences within the female reproductive tract have not been reported and sperm capacitation is not linked to ovulation150. Regardless, direct recording of human spermatozoa by whole-sperm patch clamp147 and mouse genetics studies151 suggest that TRPV4 could be a molecular basis of temperature sensing at least in mouse and human sperm. However, there is a discrepancy regarding the protein distribution of TRPV4 in human sperm: data from two separate studies that used antibodies raised against the same epitope are conflicting. In one study, TRPV4 was reported in the post-acrosomal and neck regions, but not in the flagella152, whereas in the other study, TRPV4 was found in both the acrosome and the flagella147. This discrepancy highlights the limitation of immunolabelling in determining protein distribution in sperm cells without stringent controls. In mouse sperm, TRPV4 was detected along the tail and in the head and the signal was absent in Trpv4-knockout sperm151, validating this specific distribution in mice. Mouse genetics support the involvement of TRPV4 in sperm thermotaxis151. Unlike wild-type sperm, Trpv4-knockout sperm failed to respond to increasing temperature and exhibited delayed hyperactivated motility, despite still being capable of fertilization151. Specific DSper current via TRPV4 might have been undetectable in the previous mouse sperm recordings13, as in the absence of external Ca2+, the Na+ moving through CatSper might have been masking the Na+ movement through DSper. Isolating the DSper current and confirming the existence of DSper-dependent thermotactic behaviour in mouse or human CatSper-deficient sperm will clarify whether and how TRPV4 contributes to the molecular basis of mammalian sperm thermotaxis.
P2X2
Extracellular ATP has been reported to raise the intracellular concentration of Ca2+ and to stimulate the acrosome reaction in sperm153. Mouse spermatozoa have an ATP-gated current (IATP), which is the only ion current detected from the midpiece of mammalian spermatozoa by patch-clamp recordings154. IATP is an intrinsically inwardly rectifying, cation-non-selective and divalent-permeable current, and is mediated by the homomeric P2X2 purinergic receptor. Consistent with the properties of P2X2 current in Xenopus oocytes155,156, IATP is also activated by Zn2+ and by an acidic extracellular pH in mouse sperm154. In light of these observations, P2X2 might function during sperm maturation in the epididymis where Zn2+ is abundant and the pH is more acidic than in the oviduct. Neither oviductal fluid nor cumulus cells from ovulated mice evoked sperm IATP, suggesting that the female reproductive tract is not the primary site of sperm P2X2 function in mice154. The genetic disruption of P2rx2 abolishes IATP in mouse sperm but does not affect sperm progressive motility, hyperactivation or the acrosome reaction154. However, frequent mating renders P2rx2-null male mice less fertile, suggesting that ATP-activated Ca2+ influx confers an advantage under high sexual demands154.
Subtle sperm fertility phenotypes in the ion-channelknockout mice discussed in this Review could be manifested more prominently in natural mating settings, in which sperm competition presumably exerts a stronger force than it does in a more controlled environment. In vitro, ATP supplementation rescues the immobility observed in metabolite starvation-induced sperm from both wild-type and P2rx2-null males154. Presumably, IATP can deliver Ca2+ to the mitochondria to drive ATP production. The function of IATP in human sperm is not clear, as ATP-gated current has not been found in human sperm26. The source of extracellular ATP for P2X2 activation also remains uncertain.
Cav2.3
The voltage-gated calcium channel Cav2.3, encoded by Cacnale, mediates R-type Ca2+ currents in neurons157. Cav2.3 was thought to have a function in sperm physiology based on its immunological detection in mouse sperm158,159. However, male mice lacking Cav2.3 are only mildly subfertile160 (TABLE 1). Non-capacitated Cav2.3-lacking sperm swim more linearly and exhibit a lower rising rate of Ca2+ transients induced by BSA in their heads than wild-type sperm160. This subtle but interesting difference prompted the question as to whether Cav2.3 functions in the acrosome reaction, and this was investigated in a study to determine whether membrane lipids can stimulate the acrosome reaction via modulation of Cav2.3 during capacitation161. SNX-482, a Cav2.3-specific blocker, reduced acrosome reactions induced by cholera toxin B or GM1 in sperm incubated under capacitating conditions, and Cacnale-null sperm exhibited significant reductions in the rates of the acrosome reaction and successful IVF161. Intriguingly, Ca2+ transients in the sperm head were associated with lipid modulation of the activity and localization of Cav2.3 (REF.161). The calcium transients occurred only at the apical acrosome of wild-type sperm but in the equatorial segment of Cacna1e-null sperm with faster kinetics. These Cav2.3 data were not recorded using the current gold-standard method of patch clamping mouse sperm, implying that the resulting readout could be indirect14,162. Careful modification of the recording conditions required for recording from mouse sperm incubated under capacitated conditions and/or acrosome-reacted sperm might enable further biophysical characterization of the contribution of Cav2.3 to the acrosome reaction. Using CATSPER-null sperm for Cav2.3 patch clamp measurements will also help visualize the current mediated by Cav2.3 by eliminating the major contribution of Ca2+ influx mediated by CatSper.
PKD1, PKDREJ and PKD2
PKD1 and PKD2 encode polycystin 1 (PC1) and polycystin 2 (PC2 or TRPP2), respectively. PC1 is a putative transmembrane receptor, whereas PC2 can independently form a TRP-like ion channel163. Together PC1 and PC2 can form a heterotetrameric receptor channel complex and co-localize in renal cilia164-166. Mutations in either PKD1 or PKD2 can cause autosomal dominant polycystic kidney disease (ADPKD), which results in the formation and expansion of collecting tubule-derived renal cysts164,167. Men with ADPKD also exhibit an increased rate of infertility and necrospermia168-170. Additionally, immotile sperm that lack the two central axoneme microtubules (9+0) have been identified in infertile men with PKD170.
A PKD1 homologue, Pkdrej, has also been implicated in fertility and has been studied in some detail in mice171. The PKD1 homologue PKDREJ (polycystic kidney disease and receptor for egg jelly) was found to be expressed specifically in the testicular tissues of humans172 and mice173 and localizes to the plasma membrane of the mouse sperm head173. However, no direct evidence supports a role for PKDREJ in acrosomal exocytosis. Mice that are homozygous for a disrupted Pkdrej allele (Pkdrejtm/tm) are still fertile (TABLE 1) but less fertile than wild-type mice when compared using sequential mating trials or artificial insemination assays171. Pkdrejtm/tm sperm navigate the female reproductive tract in vivo with reduced efficiency compared with wild-type sperm, as shown by fewer sperm reaching the cumulus matrix surrounding the egg over the same amount of time, and are slower to develop the ability to undergo a zona pellucida-induced acrosome reaction under capacitating conditions. However, as a comparable proportion of Pkdrejtm/tm sperm and wild-type sperm developed hyperactivated motility over the same time course in vitro, PKDREJ has been suggested to be a chronoregulator, not a master regulator, of capacitation171.
CFTR and functionally related transporters
Cystic fibrosis transmembrane regulator (CFTR) is a Cl−-permeable and HCO3−-permeable anion channel. HCO3− conductance through CFTR is low compared with that of Cl−174,175. Mutations in the gene encoding CFTR are the cause of cystic fibrosis, an autosomal recessive, monogenetic disease that results in severe phenotypes including progressive lung disease. Another cystic fibrosis phenotype is male infertility, which affects 97–98% of men with cystic fibrosis owing to congenital bilateral absence of the vas deferens (CBAVD)176. However, mutations in CFTR have also been found in 8.9% of otherwise healthy men with reduced sperm counts and/or with poor sperm quality; for example, sperm that exhibited reduced motility or abnormal morphology, or both177.
Xu and colleagues characterized the role of CFTR in sperm using a heterozygous (Cftr+/−) cystic fibrosis mouse model178 — a heterozygous mouse was used because the homozygotes rarely survive past weaning179. When compared with wild-type sperm, fewer Cftr+/− sperm could achieve capacitation; they exhibited decreased membrane hyperpolarization and cAMP production in response to HCO3−, decreased motility and reduced fertility in vitro and in vivo178 In accordance with these data, CFTR inhibitor-172, a CFTR channel blocker, inhibits the acrosome reaction, HCO3−-dependent increases in intracellular pH and membrane hyperpolarization, and inhibits an HCO3−-dependent increase in cAMP concentration in mouse sperm178,180. Likewise, another CFTR inhibitor, diphenylamine-2-carboxylic acid (DPC), also blocks capacitation-associated hyperpolarization (and seems to inhibit capacitation in general) as well as Cl− influx181. By contrast, genistein, which activates CFTR, induced hyperpolarization under noncapacitating conditions in mouse sperm and resulted in Cl− influx: intracellular Cl− was measured using N-(ethoxycarbonylmethyl)-6-meth oxyquinolinium bromide (MQAE), a fluorescent Cl− probe181. External Cl− was required for the genistein-induced hyperpolarization181. This external Cl− dependence of capacitation was also observed in guinea pig sperm182, suggesting a role of CFTR in sperm capacitation in guinea pig. In the same study, the Cl−/HCO3− exchanger known as solute carrier family 26, number 3 (SLC26A3) was suggested to work together with CFTR by pumping out Cl− that had entered through CFTR182. In support of this interaction, mouse SLC26A3 and CFTR were co-immunoprecipitated, together with other members of solute carriers (SLC26A6 and SLC9A3R1)183. This CFTR-SLC interaction model was supported by a study showing that pharmacological inhibition or blocking SLC26A3 with an antibody could inhibit the acrosome reaction and hyperactivated motility182. Furthermore inhibition of CFTR and SLC26A3 was found to inhibit db-cAMP-induced Cl− influx, capacitation-associated hyperpolarization and pH change, and HCO3− was found to induce hyperpolarization183.
Human mutations in SLC26A3 can result in congenital chloride diarrhoea (CLD), and men with CLD often also have subfertility and oligoasthenoteratozoospermia184. Accordingly, Slc26A3 knockout in mice also results in CLD and subfertility185. As both CFTR and SLC26A3 are expressed in the epithelial cells of the male reproductive tract as well as in the sperm cells themselves184,186, their respective roles in sperm versus other fertility-related processes can be difficult to determine. In the future, assessing SLC26A3 protein expression and function in heterozygous Cftr+/− mice could inform its role in CFTR regulation and sperm capacitation.
Direct whole-cell patch recordings from mouse testicular sperm demonstrated a Cl− component to the membrane current that is ATP-dependent and is stimulated by cAMP, cGMP and genistein, and inhibited by CFTR inhibitor-172 and DPC187. However, Cl− current could still be recorded from CFTR loss-of-function (ΔF508) mouse testicular sperm, although in ΔF508 mice this current is less sensitive to cAMP and CFTR inhibitor-172 (REF.187). In the same study, Cl− current was also recorded from mouse wild-type epididymal sperm, but the effects of the CFTR-modulating compounds observed were not as substantial as seen in the testicular sperm. Thus, further work is required to clarify the extent to which CFTR conducts Cl− current in mature sperm cells.
The precise localization of CFTR within sperm also requires clarification. Immunocytochemistry studies have shown that CFTR is localized in the equatorial segment of human178,180, mouse178 and guinea pig sperm cells182 but have also demonstrated its presence in the midpiece of human and mouse sperm181,183. CFTR has also been found simultaneously in both the equatorial segment of mouse sperm heads and the sperm midpiece188. Likewise, immunocytochemistry has demonstrated that SLC26A3 localizes within the heads of guinea pig sperm182 but was shown to localize to the midpiece of mouse sperm in a separate study183. This discrepancy highlights the importance of genetic studies including the generation of knockout mice, for example, in sperm studies, as sperm cells have been repeatedly shown to be prone to non-specific antibody binding.
To date, the mouse and human sperm ion channels and membrane transporters that are clearly implicated in male fertility have been largely localized in the flagella (FIG. 1a; TABLE 1). Identifying and investigating channels located in other compartments of the sperm cells, such as acrosomal channels, requires further studies using current state-of-the-art techniques that provide specificity, sensitivity and high resolution in time and space, as well as evidence from genetic studies in both mice and humans.
Sperm Ca2+ homeostasis
To support dynamic cellular Ca2+ signalling and to prevent Ca2+ intoxication, Ca2+ must be efficiently cleared from the cytosol after its entry through Ca2+-permeable channels.
PMCA4
Plasma membrane Ca2+-ATPases (PMCAs) are highly conserved Ca2+ extrusion pumps that maintain low basal levels of intracellular Ca2+ (REF.189). Two isoforms, PMCA1 and PMCA4 (encoded by Atp2b1 and Atp2b4, respectively), are expressed abundantly in testis and highly conserved across species190. Genetic knockout of PMCA4 in mice results in a >90% reduction in total PMCA expression191, indicating that PMCA4 is the primary PMCA in sperm cells. PMCA4-deficient male mice produce sperm cells with normal morphology but are totally infertile owing to impaired sperm motility and defective hyperactivated motility191-193 (TABLE 1). Surprisingly, PMCA4-deficient mouse sperm are able to bind to zona pellucida and fertilize eggs in vitro192, suggesting that infertility in vivo is probably due to inefficient sperm navigation in the female reproductive tract. Interestingly, PMCA4 is primarily localized in the sperm principal piece but, unlike CatSper, does not exhibit a distinct pattern of distribution on the flagellar surface30. Ultrastructural analysis has revealed that mitochondria from PMCA4-deficient sperm are more condensed than wild-type sperm when incubated under capacitating conditions191; this characteristic is indicative of Ca2+ overload. Whether PMCA4 participates in regulating CatSper-relevant Ca2+ signalling and how sperm mitochondria are involved in Ca2+ signalling during capacitation remains to be clarified.
Mg2+ transporter
A Mg2+ transporter, CNNM4 (also known as ancient conserved domain-containing protein 4, ACDP4) is highly expressed in mature ameloblasts and intestinal epithelia194. Interestingly, CNNM4-deficient male mice are almost infertile195. Cnnm4-null spermatozoa exhibit rapid motility decreases and fail to develop hyperactivated motility195. Mg2+ levels are significantly increased in CNNM4-deficient mouse sperm, whereas Ca2+ levels are not affected and removal of Mg2+ from the medium can rescue Cnnm4-null sperm motility195, suggesting that abnormally high levels of Mg2+ are detrimental to sperm function. In addition, Cnnm4-null sperm exhibit excessive P-Tyr with impaired Ca2+ influx195, similar to the phenotype of CatSper-deficient sperm30. These results suggest that CNNM4 might be involved in sperm Ca2+ homeostasis and/or could be functionally linked to CatSper. Additional manipulation to a Cnnm2 allele, which encodes another CNNM family Mg2+ transporter, to make CNNM2 non-functional rendered the resulting Cnnm2+/−/Cnnm4−/− male mice completely infertile. The Cnnm2+/−/Cnnm4−/− sperm exhibited a more severe reduction in motility and a loss of BSA-induced Ca2+ response, compared with Cnnm4−/− sperm, suggesting that CNNM2 works together with CNNM4 to regulate intracellular Mg2+ homeostasis and male fertility196. Whether the Mg2+ efflux activities of CNNMs are associated with Ca+ homeostasis and how they can potentially regulate sperm Ca2+ signalling await further investigation.
Clinical implications
The manipulation of ion channels in order to affect fertility could be leveraged for clinical application; for example, to produce male contraceptives or fertility treatments. Additionally, male fertility can reflect overall health as the dysregulated expression of ubiquitous proteins implicated in non-reproductive diseases might also result in altered sperm function.
Novel non-hormonal male contraceptives
Most contraceptive strategies have been developed for use by women, but interest in generating a novel male contraceptive remains. One strategy is the use of hormones to inhibit endogenous testosterone production and, as a result, to block spermatogenesis197. To bypass potential adverse effects associated with hormone use, non-hormonal contraceptives are more desirable.
Ion channels in sperm are attractive targets for the development of contraceptives and, conversely, for drugs that could be used to treat infertility. Like G-protein-coupled receptors, ion channels are good drug targets as they are implicated in a variety of pathophysiologies and present druggable sites at cell surfaces198,199. In fact, ~15% of current drug targets are ion channels199,200.
The sperm-specific nature of the CatSper ion channel complex means that targeting it should result in few unintended off-target effects. Some compounds have been identified that inhibit CatSper, but they are non-specific and also inhibit KSper with comparable potency and so are probably not specific enough to be used as contraceptives201. RU1968, a ligand of steroidal sigma receptors, has been shown to suppress progesteronestimulated Ca2+ signalling and prostaglandinstimulated Ca2+ signalling in human sperm202. RU1968 has also been shown to inhibit human CatSper with ~15-fold higher potency than human KSper, and not to inhibit mouse KSper at all201, demonstrating the specificity for CatSper inhibition. Hopefully RU1968 and other CatSper inhibitors can be used as a template for the design of drugs that could be used in contraception.
Many sperm ion channels have yet to be thoroughly explored as therapeutic targets at least partially (as has been asserted for K+ channel pharmacology) owing to difficulties in establishing robust, high-capacity functional assays that could be used to interrogate large chemical libraries for potential drugs with activity against not only the targets themselves but also related targets to determine the specificity of screening hits199. This difficulty has been somewhat overcome in sperm with the development of a high-throughput, automated screening platform to assess the effect of small molecules on human sperm motility and ability to undergo the acrosome reaction203. The drug library (ReFRAME) used in the study comprises drugs that have either been approved for use or have undergone preclinical profiling204; thus, a hit in this type of screen could accelerate the search for a marketable drug for use as a male contraceptive or to modify fertility.
Fertility as a proxy for overall health in men
The relationship between subfertility and overall health in men is becoming increasingly apparent205,206. For example, as ~10% of the genome is implicated in fertility, subfertility and/or infertility, phenotypes can indicate abnormalities in other biological processes, such as those resulting in fibrosis205.
In addition to genetic fertility associations, subfertility and infertility are also associated with developmental, lifestyle, oncological and cardiovascular disorders205. Lifestyle factors associated with infertility in men include obesity207, tobacco abuse208 and stress208. For example, a large meta-analysis of 21 studies including 13,077 men showed a J-shaped association between the risk of abnormal sperm concentration and BMI, in which increased BMI was negatively correlated with semen sperm concentration207. It is well known that cancer treatment can impair male fertility209, and studies have now also supported a link between male infertility and the risk of developing testicular cancer210,211, suggesting that male infertility might serve as a marker of oncological risk. Thus, assessing fertility and sperm functionality could offer unique insights into overall male health: each sperm cell is designed to function outside of the male body as a single cell and is, therefore, particularly amenable to in vitro analysis212. However, although sperm analysis can be a convenient and non-invasive tool, male reproductive capability is generally not evaluated until late in a man’s life, usually after failure to reproduce206,213. Thus, it is recommended that men who exhibit health issues such as cystic fibrosis or altered fertility should consider genetic counselling to better understand other health problems to which they might be predisposed and the risk associated with passing their genes onto their children.
Specific notable mutations.
Mutations in CFTR have been identified not only in men who exhibit cystic fibrosis but also in those with CBAVD and/or sperm of reduced quality176,177. Although cystic fibrosis is a recessive disease, mutagenesis of one CFTR copy can result in altered sperm parameters in mice178 and could be associated with CBAVD in men214. Abnormal mucociliary clearance is associated with cystic fibrosis, although this primarily results from the abnormal biophysical properties of the airway mucus and not ciliopathy215. However, a relationship is seen between fertility and ciliopathies216.
Mutations in the genes encoding PKD1 or PKD2, which both localize to primary cilia, can result in ADPKD, which is associated with increased rates of infertility164,167-170. As the motility of cilia and flagella are both conferred by an axoneme, it is tempting to speculate that similar axonemal defects could result in similar phenotypes.
Because the CATSPER complex is a sperm-specific ion channel, mutations in CATSPER genes are less likely to cause more widespread health problems than alterations in other ion channels such as CFTR. For example, a CATSPERE in-frame 6-bp deletion results in normal sperm motility in humans but failure to fertilize in IVF owing to defective hyperactivation and lack of Ca2+ response to progesterone106,107. In such cases, intracyto-plasmic sperm injection can be used to achieve fertilization and can result in clinical pregnancy. Although mutations in CATSPER are most likely to affect fertility, they can be associated with other health problems, such as deafness. Deafness–infertility syndrome is a very rare syndrome characterized by both deafness and male infertility and is associated with homozygous deletions of STRC (which is expressed in the inner ear) and CATSPER2 on chromosome 15q15 (REFS111,112).
These examples illustrate the importance of diagnosis of the underlying cause of male infertility. Mutations giving rise to channelopathies that can affect male fertility can also have more wide-ranging effects on other body systems that men might wish to be aware of before beginning to consider assisted reproductive technology (ART).
Current controversies
A large body of evidence exists regarding the role of ion channels in sperm, but limitations of the experimental approaches used in the field must be taken into account and whether the findings from in vitro and/or animal studies are physiologically relevant and not species-specific must be considered before these data can be extrapolated to humans.
Interpreting data from indirect approaches
Determining protein locations in sperm cells solely based on antibody detection has generated controversies in the field and needs to be considered critically. Studies have produced inconsistent data, including the specific localizations of some ion channels and receptors, including CFTR, SLC26A3 and TRPV4, in sperm cells.
The identification of CatSper as a primary Ca2+ channel in sperm illustrates the importance of genetic and/or other direct evidence, such as direct electrophysiological recording from sperm cells. Before the discovery of CatSper, the N-type and R-type voltage-gated Ca2+ channels, Cav2.2 and Cav2.3, were thought to be the Ca2+ entry channels in sperm, as they had been immunologically detected in mouse sperm159,217. T-type voltage-gated Ca2+ currents were also suggested to contribute to the Ca2+ influx in spermatozoa, as the corresponding currents were recorded in mouse testicular sperm93-218-219. However, whole-cell patch clamping of mouse epididymal sperm cells, combined with genetic analysis, clarified that CatSper is the primary facilitator of Ca2+ influx in mature sperm and is specifically localized in the principal piece of the sperm tail13,18. Furthermore, T-type currents were shown to be diminished in spermatids and were not detected in mature sperm13,94, highlighting the importance of a direct approach. Immunological methods such as immunostaining and immunoblotting have also been used to demonstrate the expression of many neurotransmitter receptors in mammalian sperm, including receptors for norepinephrine, aspartate, serotonin, acetylcholine, GABA and glycine220,221. However, no neurotransmitter-mediated currents were detected by whole-sperm cell electrophysiological recordings when functional expression of the corresponding receptors was tested154, suggesting either a non-functional presence of these receptors or non-specific detection.
Studies that solely relied on pharmacological interrogation also illustrate the unreliable nature of indirect approaches. Sperm cells are particularly prone to non-specific antibody binding and are particularly amenable to chemical inhibitors and/or activators, probably owing to their small dimensions and relative lack of cytoplasm (that is, their low copy number of the channels or receptors and large lipid surface-to-volume ratio), which demonstrates the risk of detecting artefacts in sperm cells when genetic or other more direct evidence (for example, electrophysiology data) is lacking.
Compounding implications from in vitro studies
Various capacitation-associated changes have been described, including activation of cAMP/PKA141, increases in protein P-Tyr139, a rise in intracellular pH222 and Ca2+ (REFS223-226), membrane hyperpolarization227,228, and modulation of the lipid content of the sperm plasma membrane132. In particular, a substantial body of work has documented the involvement of P-Tyr in regulating sperm motility and fertility during sperm capacitation229. As a result, P-Tyr has been used as a hallmark of sperm capacitation for decades. However, mounting evidence now indicates that P-Tyr is not actually required for hyperactivated motility144 or fertility142 in mice. This new interpretation has arisen because sperm capacitation is typically studied using in vitro analyses that reflect an average value for entire sperm populations at a given time. Inducing capacitation in mouse or human sperm in vitro using buffer containing HCO3− and BS A results in a heterogeneous population of sperm, in which as few as ~15% of mouse sperm are hyperactivated230; 2–14% of human sperm are acrosome-reacted231. Similar to the fact that in vitro experiments do not necessarily reflect the in vivo processes, contact with the zona pellucida was believed to induce the acrosome reaction, especially after an observation that the mouse acrosome reaction can be induced by zona pellucida sperm-binding protein 3 (ZP3; one of four glycoproteins that make up the zona pellucida) in vitro232. Subsequently, mouse spermatozoa were found to begin undergoing acrosome reaction in the isthmus region of the fallopian tube before arriving at the ampulla233,234 and reacted spermatozoa were able to penetrate the zona in vivo235; the small number of mouse spermatozoa that arrive at the lumen of the ampulla and cumulus oophorus are all fully capacitated and acrosome-reacted in vivo236. Even so, determining the exact site of the acrosome reaction in other mammalian species awaits further technological development and the physiological functions of the acrosome reaction and P-Tyr are currently being re-evaluated.
Currently available tools based on flow cytometry combined with sorting have been used to detect and/or separate the in vitro capacitated heterogeneous sperm populations based on various capacitation parameters: P-Tyr35,237, acrosome reaction status238,239 and membrane hyperpolarization74,240. However, the dynamics of how these parameters change in each individual sperm and the extent to which these parameters reflect the fertilizing ability of each sperm has not been established. The direct linking of molecular information with motility and fertilizing capacity awaits a new approach to be developed.
Critical appreciation of species-specific regulation
Conclusions from studies using different animal models should be carefully interpreted for two reasons. First, it is becoming apparent that some controversies in the field are partly due to a lack of understanding of marked species-specific regulation. Studies that exemplify this are those that have investigated the different molecular mechanisms of CatSper and KSper activation: CatSper is universally activated by intracellular alkalinization as demonstrated by sperm cells from sea urchin241, mouse13, rat28, human67, cow242 and horse243; however, only human and primate CatSper currents are robustly potentiated by hormones such as progesterone66,67,113. Similarly, human KSper is more sensitive to Ca2+ than to alkalinization compared with mouse KSper. These discrepancies illustrate the divergence of molecular mechanisms that regulate CatSper and KSper across different species. In the future, these phenomena should be kept in mind, as other sperm ion channels and membrane receptors might have also diverged in a species-specific manner.
Second, the mouse model is limited in its ability to reflect the sperm biology of other species such as humans and domestic animals, at least in part because epididymal, not ejaculated, mouse sperm are usually used in in vitro experiments28,244. Ejaculated sperm cells are mixed with secretory factors from male glands and ultimately with other molecules and ions in the female reproductive tract. Conclusions drawn from in vitro experiments using mouse sperm must consider the absence of these factors. Future studies should ensure that in vitro experiments are combined with genetic modifications and/or that in vitro systems are supplemented with the appropriate secretory factors from the male and female reproductive tracts in order to better inform our understanding of species-specific regulation of ion channels and membrane receptors including CatSper.
Future of the field
In order to better understand the regulation and role of ion channels in sperm, future studies must seek more evidence from in vivo studies and genetics evidence.
Seeking more in vivo context
Sperm analysis is currently performed using functionally heterogeneous sperm populations; these populations are typically capacitated in vitro and include degenerating cells30. Researchers must consider that the population mean of these analyses of the changes undergone by the sperm is often presented and cannot be used to faithfully recapitulate the time-dependent and space-dependent changes that sperm undergo in the oviduct245,246, nor the physiological and signalling state of the small number of spermatozoa that reach the vicinity of, and fertilize, the eggs247,248. Consistent with this notion, a greater number of sperm are required for IVF than the number of sperm present at the site of fertilization in vivo249. Studies that better emulate in vivo conditions and/or the development of new systems that can directly link the molecular changes of individual cells to motility and fertilizing ability are research priorities in the future. Examples of such studies include motility-correlative molecular imaging of sperm cells30, probing acrosome reaction states of sperm from genetically modified mice encoding fluorescence proteins in the acrosome along the female reproductive tracts233,236,250, and electrophysiological recordings taken from capacitated human sperm147. Such studies, in which an effort is made to understand sperm function in a more physiologically relevant manner, are anticipated in the future.
Identifying gene variants in human fertility
Decades of mouse genetics studies have resulted in an extensive list of genes encoding spermatozoan ion channels and transporters implicated in male infertility (TABLE 1). However, only a few of these genes, for example CatSper-encoding genes or CFTR, have been implicated in human infertility, which is surprising considering advances in tools such as whole-exome analysis, but illustrates the practical difficulties of studying the inheritance of infertility traits in humans. Damaging mutations in CatSper might have occurred and, therefore, have been detected, with increased frequency owing to the large number of genes required to form this multi-subunit channel complex. Targeted genomic analysis of large cohorts with a specific functional characteristic (for example, infertile men with normal semen parameters but with deficits in Ca2+ signalling or abnormal membrane potential) and analyses of large families that exhibit Mendelian inheritance of these infertility traits might accelerate the discovery of human variants in these genes. As our understanding has grown that clinically assessing male reproductive health could provide unique insights into the general health of a patient and his children, identification of human infertility genetic variants could serve as a guideline for counselling general health, as well as candidate drug targets for screening for contraception, and could also provide insights into drug design and mechanisms of action.
In the current era of ARTs, in which natural barriers to egg fertilization are removed, defining the genetic defects underlying infertility is of the utmost importance, as genetic mutations that lead to male infertility are able to be passed to the next generation. Understanding the long-term effect of using ARTs needs to be prioritized in research.
Conclusions
Technical advances have substantially improved our understanding of the role of ion channels and membrane receptors in sperm function during fertilization. Current knowledge of the ion channels and membrane transporters found in sperm have been greatly shaped by gene knockout studies in mice and genetic evidence from humans. In particular, our understanding of the CatSper channel — its molecular and spatial organization, the regulatory mechanism by which it is regulated, and the CatSper-based signalling pathways that are required to trigger hyperactivated motility — are now fairly well understood. However, the field is not without controversies and debates, in particular regarding the way in which in vitro data can be applied in vivo, and how data collected from one species can be applied to others; these areas need to be addressed. Many features of ion channels are unique to sperm as the sperm-specific channel isoforms have unique properties that are not found in other cell types. Thus, understanding the ion channel mechanisms in sperm cells will advance our knowledge of causes of male infertility and should inspire improvements in assisted reproduction and the development of new contraceptives, and improve diagnoses of infertility.
Key points.
Mammalian sperm cells undergo intracellular alkalinization during their fertilization journey as they encounter a drastic extracellular pH change in the female reproductive tract.
During capacitation,the sperm membrane potential hyperpolarizes, primarily via KSper activation and K+ efflux.
Increases in intracellular Ca2+ are required for inducing hyperactivated motility and acrosome reaction, two key physiological events essential for fertilization.
CatSper,the multi-subunit Ca2+ channel, is the predominant Ca2+ entry pathway in sperm cells and organizes into linear Ca2+ signalling nanodomains along the flagella.
CatSper-mediated Ca2+ signalling is integrated into other sperm capacitation signalling pathways including phosphorylation cascades.
Improved understanding of spermatozoan ion channels and transporters will help elucidate the delicate and dynamic regulation of Ca2+ homeostasis in sperm motility and fertility.
Acknowledgements
The authors thank Jae Yeon Hwang for valuable discussion and critical reading of the draft manuscript. This work was supported by start-up funds from Yale University School of Medicine, Grantham Foundation, and NIH (R01 HD 096745) to J.-J.C.
Glossary
- CatSper
Sperm-specific calcium channel.
- NHEs
Sodium–hydrogen exchangers.
- HV1
Proton channel.
- KSper
Native sperm-specific potassium current/channel.
- Na+/K+ ATPase
Sodium–potassium adenosine triphosphatase; also known as the sodium–potassium pump.
- SLO3
The mediator of KSper, which is also used as the name of the protein or channel expressed heterologously.
- Quadrilateral compartmentalization
The four linear Ca2+ signalling nanodomains.
- DSper
Depolarizing channel of sperm.
- TRPV4
Transient receptor potential cation channel subfamily V member 4.
- P2X2
P2X purinoceptor 2.
- Cav2.3
R type, voltage-dependent, calcium channel, α 1 E subunit.
- PKD
Polycystin, transient receptor potential cation channel, autosomal dominant polycystic kidney disease protein.
- PKDREJ
Polycystin family receptor for egg jelly.
- CFTR
Cystic fibrosis transmembrane conductance regulator.
- PMCA4
Plasma membrane calcium ATPase 4.
- CNNM4
Cyclin and CBS domain divalent metal cation transport mediator 4.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Urology thanks P. Lishko, M. Yeste, Y. Okamura, P. Visconti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hille B Ion channels of excitable membranes (2001). [Google Scholar]
- 2.Clapham DE Calcium signaling. Cell 131, 1047–1058 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Bagur R & Hajnóczky G Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 66, 780–788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessen M, Fay RB & Witman GB Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol 86, 446–455 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhmer M et al. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 24, 2741–2752 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood CD, Nishigaki T, Furuta T, Baba SA & Darszon A Real-time analysis of the role of Ca(2+) in flagellar movement and motility in single sea urchin sperm. J. Cell Biol 169, 725–731 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagimachi R et al. Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol. Reprod 96, 780–799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suarez SS, Varosi SM & Dai X Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl Acad. Sci. USA 90, 4660–4664 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EF Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell 13, 3303–3313 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno K et al. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca(2+)-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol. Cell 101, 91–103 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Bannai H, Yoshimura M, Takahashi K & Shingyoji C Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J. Cell Sci 113, 831–839 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Tash JS et al. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J. Cell Biol 106, 1625–1633 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirichok Y, Navarro B & Clapham DE Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Zeng XH, Navarro B, Xia XM, Clapham DE & Lingle CJ Simultaneous knockout of Slo3 an d CatSper1 abolishes all alkalization- and voltageactivated current in mouse spermatozoa. J. Gen. Physiol 142, 305–313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren D et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson AE et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl Acad. Sci. USA 100, 14864–14868 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quill TA et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl Acad. Sci. USA 100, 14869–14874 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi H et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl Acad. Sci. USA 104, 1219–1223 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagimachi R The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil 23, 193–196 (1970). [DOI] [PubMed] [Google Scholar]
- 20.Suarez SS & Ho HC Hyperactivated motility in sperm. Reprod. Domest. Anim 38, 119–124 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Pacey AA, Davies N, Warren MA, Barratt CL & Cooke ID Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum. Reprod 10, 2603–2609 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Ho K, Wolff CA & Suarez SS CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev 21, 345–350 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Miki K & Clapham DE Rheotaxis guides mammalian sperm. Curr. Biol 23, 443–452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coy P, Garcia-Vazquez FA, Visconti PE & Aviles M Roles of the oviduct in mammalian fertilization. Reproduction 144, 649–660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter RH Components of oviduct physiology in eutherian mammals. Biol. Rev. Camb. Philos. Soc 87, 244–255 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Brenker C et al. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 31, 1654–1665 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lishko PV et al. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol 453–475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MR, Mansell SA, Meyers SA & Lishko PV Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium 58, 105–113 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Chung JJ et al. CatSperζ regulates the structural continuity of sperm Ca(2+) signaling domains and is required for normal fertility. eLife 6, e23082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung JJ et al. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157, 808–822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JY et al. Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 177, 1480–1494.e19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MR et al. Asymmetrically positioned flagellar control units regulate human sperm rotation. Cell Rep. 24, 2606–2613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerns K, Zigo M, Drobnis EZ, Sutovsky M & Sutovsky P Zinc ion flux during mammalian sperm capacitation. Nat. Commun 9, 2061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matamoros-Volante A & Trevino CL Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci 133, jcs238816 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Matamoros-Volante A et al. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: the case of capacitation-induced tyrosine phosphorylation. Mol. Hum. Reprod 24, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J & Nicastro D Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, eaar1968 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabeo D, Croft JT & Hoog JL Axonemal doublet microtubules can split into two complete singlets in human sperm flagellum tips. FEBS Lett. 593, 892–902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zabeo D et al. A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep 8, 2727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernardino RL, Carrageta DF, Sousa M, Alves MG & Oliveira PF pH and male fertility: making sense on pH homeodynamics throughout the male reproductive tract. Cell Mol. Life Sci 76, 3783–3800 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine N & Marsh DJ Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J. Physiol 213, 557–570 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wales RG, Wallace JC & White IG Composition of bull epididymal and testicular fluid. J. Reprod. Fertil 12, 139–144 (1966). [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wang DK & Chen LM The physiology of bicarbonate transporters in mammalian reproduction. Biol. Reprod 86, 99 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Ng KYB, Mingels R, Morgan H, Macklon N & Cheong Y In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum. Reprod. Update 24, 15–34 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Breckenridge MA, Pederson DP & Pommerenke WT A pH study of human cervical secretions. Fertil. Steril 1, 427–434 (1950). [DOI] [PubMed] [Google Scholar]
- 45.Fox CA, Meldrum SJ & Watson BW Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil 33, 69–75 (1973). [DOI] [PubMed] [Google Scholar]
- 46.Owen DH & Katz DF A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl 26, 459–469 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Tampion D & Gibbons RA Effect of pH on the swimming rate of bull spermatozoa. J. Reprod. Fertil 5, 249–258 (1963). [DOI] [PubMed] [Google Scholar]
- 48.Moghissi KS, Dabich D, Levine J & Neuhaus OW Mechanism of sperm migration. Fertil. Steril 15,15–23 (1964). [DOI] [PubMed] [Google Scholar]
- 49.Orlowski J & Grinstein S Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflug. Arch 447, 549–565 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Garcia MA & Meizel S Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol. Reprod. Dev 52, 189–195 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Klanke CA et al. Molecular cloning and physical and genetic mapping of a novel human Na+/H+ exchanger (NHE5/SLC9A5) to chromosome 16q22.1. Genomics 25, 615–622 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Goyal S, Vanden Heuvel G & Aronson PS Renal expression of novel Na+/H+ exchanger isoform NHE8. Am. J. Physiol. Ren. Physiol 284, F467–F473 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Wang D, King SM, Quill TA, Doolittle LK & Garbers DL A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol 5, 1117–1122 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Liu T et al. A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Front. Biosci 2, 566–581 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Chen SR et al. Sodium-hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis. 7, e2152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberheide K, Puchkov D & Jentsch TJ Loss of the Na(+)/H(+) exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem 292, 10845–10854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl Acad. Sci. USA 104, 9325–9330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Windler F et al. The solute carrier SLC9C1 is a Na(+)/H(+)-exchanger gated by an S4-type voltagesensor and cyclic-nucleotide binding. Nat. Commun 9, 2809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lishko PV, Botchkina IL, Fedorenko A & Kirichok Y Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Lee SY, Letts JA & Mackinnon R Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl Acad. Sci. USA 105, 7692–7695 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tombola F, Ulbrich MH & Isacoff EY The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsey IS et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol 17, 869–875 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger TK et al. Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J. Physiol 595, 1533–1546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navarro B, Kirichok Y & Clapham DE KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl Acad. Sci. USA 104, 7688–7692 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brenker C et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 3, e01438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strunker T et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471,382–386 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Lishko PV, Botchkina IL & Kirichok Y Progesterone activates the principal Ca2+ channel of human sperm. Nature 471,387–391 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Clausen MV, Hilbers F & Poulsen H The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol 8, 371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huxley AF & Stampfli R Direct determination of membrane resting potential and action potential in single myelinated nerve fibers. J. Physiol 112, 476–495 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santi CM et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041–1046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calzada L & Tellez J Defective function of membrane potential (psi) on sperm of infertile men. Arch. Androl 38, 151–155 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Brown SG et al. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod 31, 1147–1157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baro Graf C et al. Membrane potential assessment by fluorimetry as a predictor tool of human sperm fertilizing capacity. Front. Cell Dev. Biol 7, 383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina LCP et al. Membrane potential determined by flow cytometry predicts fertilizing ability of human sperm. Front. Cell Dev. Biol 7, 387 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez G, Nguyen ANT, Timmerberg B, Tash JS & Blanco G The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod 12, 565–576 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Wagoner K, Sanchez G, Nguyen AN, Enders GC & Blanco G Different expression and activity of the α1 and α4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction 130, 627–641 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Jimenez T et al. Increased expression of the Na, K-ATPase alpha4 isoform enhances sperm motility in transgenic mice. Biol. Reprod 84, 153–161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDermott J, Sanchez G, Nangia AK & Blanco G Role of human Na,K-ATPase alpha 4 in sperm function, derived from studies in transgenic mice. Mol. Reprod. Dev 82, 167–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jimenez T, Sanchez G, Wertheimer E & Blanco G Activity of the Na,K-ATPase α4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction 139, 835–845 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Blanco G, Melton RJ, Sanchez G & Mercer RW Functional characterization of a testes-specific α-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry 38, 13661–13669 (1999). [DOI] [PubMed] [Google Scholar]
- 81.James P F. et al. Identification of a specific role for the Na,K-ATPase α2 isoform as a regulator of calcium in the heart. Mol. Cell 3, 555–563 (1999). [DOI] [PubMed] [Google Scholar]
- 82.Jimenez T, McDermott JP, Sanchez G & Blanco G Na,K-ATPase α4 isoform is essential for sperm fertility. Proc. Natl Acad. Sci. USA 108, 644–649 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper TG et al. Mouse models of infertility due to swollen spermatozoa. Mol. Cell Endocrinol 216, 55–63 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Zeng XH, Yang C, Kim ST, Lingle CJ & Xia XM Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl Acad. Sci. USA 108, 5879–5884 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C, Zeng XH, Zhou Y, Xia XM & Lingle CJ LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proc. Natl Acad. Sci. USA 108, 19419–19424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng XH, Yang C, Xia XM, Liu M & Lingle CJ SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc. Natl Acad. Sci. USA 112, 2599–2604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mansell SA, Publicover SJ, Barratt CL & Wilson SM Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol. Hum. Reprod 20, 392–408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mannowetz N, Naidoo NM, Choo SA, Smith JF & Lishko PV Slo1 is the principal potassium channel of human spermatozoa. eLife 2, e01009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geng Y et al. A genetic variant of the sperm-specific SLO3 K(+) channel has altered pH and Ca(2+) sensitivities. J. Biol. Chem 292, 8978–8987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wijerathne TD, Kim J, Yang D & Lee KP Intracellular calcium-dependent regulation of the sperm-specific calcium-activated potassium channel, hSlo3, by the BKCa activator LDD175. Korean J. Physiol. Pharmacol 21,241–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chavez JC et al. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem 289, 32266–32275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown SG, Publicover SJ, Barratt CLR & Martins da Silva SJ Human sperm ion channel (dys) function: implications for fertilization. Hum. Reprod. Update 25, 758–776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lievano A et al. T-type Ca2+ channels and α1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 388, 150–154 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Xia J & Ren D Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol. Reprod 80, 1092–1098 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin J et al. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod 77, 37–44 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Xia J, Cho KH, Clapham DE & Ren D CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem 282, 18945–18952 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Wang H, Liu J, Cho KH & Ren D A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol. Reprod 81, 539–544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L & Clapham DE A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun 2, 153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlson AE et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem 280, 32238–32244 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Avenarius MR et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet 84, 505–510 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hildebrand MS et al. Genetic male infertility and mutation of CATSPER ion channels. Eur. J. Hum. Genet 18, 1178–1184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith JF et al. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl Acad. Sci. USA 110, 6823–6828 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schiffer C et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling. EMBO J. 39, e102363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo T et al. A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum. Reprod 34, 414–423 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Sinha A, Singh V, Singh S & Yadav S Proteomic analyses reveal lower expression of TEX40 and ATP6V0A2 proteins related to calcium ion entry and acrosomal acidification in asthenozoospermic males. Life Sci. 218, 81–88 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Brown SG et al. Homozygous in-frame deletion in CATSPERE in a man producing spermatozoa with loss of CatSper function and compromised fertilizing capacity. Hum. Reprod 33, 1812–1816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams HL et al. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum. Reprod 30, 2737–2746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.San Agustin JT, Pazour GJ & Witman GB Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol. Biol. Cell 26, 4358–4372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y et al. Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev. Biol 432, 125–139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu H et al. IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol. Reprod 96, 993–1006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y et al. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J. Med. Genet 44, 233–240 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avidan N et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet 11,497–502 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Sumigama S et al. Progesterone accelerates the completion of sperm capacitation and activates CatSper channel in spermatozoa from the rhesus macaque. Biol. Reprod 93, 130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller MR et al. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555–559 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mannowetz N, Miller MR & Lishko PV Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl Acad. Sci. USA 114, 5743–5748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brenker C et al. Action of steroids and plant triterpenoids on CatSper Ca(2+) channels in human sperm. Proc. Natl Acad. Sci. USA 115, E344–E346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mannowetz N, Mundt N & Lishko PV Reply to Brenker et al.: The plant triterpenoid pristimerin inhibits calcium influx into human spermatozoa via CatSper. Proc. Natl Acad. Sci. USA 115, E347–E348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Diao R et al. CCR6 is required for ligand-induced CatSper activation in human sperm. Oncotarget 8, 91445–91458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schiffer C et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 15, 758–765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tavares RS et al. p,p′-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Hum. Reprod 28, 3167–3177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou QX et al. Diethylstilbestrol activates CatSper and disturbs progesterone actions in human spermatozoa. Hum. Reprod 32, 290–298 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Bailey JL Factors regulating sperm capacitation. Syst. Biol. Reprod. Med 56, 334–348 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Jaiswal BS & Conti M Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl Acad. Sci. USA 100, 10676–10681 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie F et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol 296, 353–362 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Nolan MA et al. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc. Natl Acad. Sci. USA 101, 13483–13488 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia J, Reigada D, Mitchell CH & Ren D CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol. Reprod 77, 551–559 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Kobori H, Miyazaki S & Kuwabara Y Characterization of intracellular Ca(2+) increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol. Reprod 63, 113–120 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Carlson AE, Hille B & Babcock DF External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol 312, 183–192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang T et al. The Ca(2+) channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J. Biol. Chem 295, 13181–13193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orta G et al. CatSper channels are regulated by protein kinase A. J. Biol. Chem 293, 16830–16841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gadella BM & Harrison RA The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 127, 2407–2420 (2000). [DOI] [PubMed] [Google Scholar]
- 132.Visconti PE et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev. Biol 214, 429–443 (1999). [DOI] [PubMed] [Google Scholar]
- 133.Xia J & Ren D The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod. Biol. Endocrinol 7, 119 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Visconti PE et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: β-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem 274, 3235–3242 (1999). [DOI] [PubMed] [Google Scholar]
- 135.Osheroff JE et al. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod 5, 1017–1026 (1999). [DOI] [PubMed] [Google Scholar]
- 136.Harrison RA Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol. Reprod. Dev 67, 337–352 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Battistone MA et al. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod 19, 570–580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wennemuth G et al. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development 130, 1317–1326 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Visconti PE et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein-tyrosine phosphorylation. Development 121, 1129–1137 (1995). [DOI] [PubMed] [Google Scholar]
- 140.Salicioni AM et al. Signalling pathways involved in sperm capacitation. Soc. Reprod. Fertil Suppl. 65, 245–259 (2007). [PubMed] [Google Scholar]
- 141.Visconti PE et al. Capacitation of mouse spermatozoa. II. Protein-tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121, 1139–1150 (1995). [DOI] [PubMed] [Google Scholar]
- 142.Alvau A et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 143, 2325–2333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Craig AWB, Zirngibl R, Williams K, Cole LA & Greer PA Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell. Biol 21, 603–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tateno H et al. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl Acad. Sci. USA 110, 18543–18548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Navarrete FA et al. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci. Rep 6, 33589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyata H et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 350, 442–445 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Mundt N, Spehr M & Lishko PV TRPV4 is the temperature-sensitive ion channel of human sperm. eLife 7, e35853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bahat A et al. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med 9, 149 (2003). [DOI] [PubMed] [Google Scholar]
- 149.Boryshpolets S, Pérez-Cerezales S & Eisenbach M Behavioral mechanism of human sperm in thermotaxis: a role for hyperactivation. Hum. Reprod 30, 884–892 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Aitken RJ & Nixon B Sperm capacitation: a distant landscape glimpsed but unexplored. Mol. Hum. Reprod 19, 785–793 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Hamano K, Kawanishi T, Mizuno A, Suzuki M & Takagi Y Involvement of transient receptor potential vanilloid (TRPV) 4 in mouse sperm thermotaxis. J. Reprod. Dev 62, 415–422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar A et al. TRPV4 is endogenously expressed in vertebrate spermatozoa and regulates intracellular calcium in human sperm. Biochem. Biophys. Res. Commun 473, 781–788 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Bjorkgren I & Lishko P V. Purinergic signaling in testes revealed. J. Gen. Physiol 148, 207–211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Navarro B, Miki K & Clapham DE ATP-activated P2X2 current in mouse spermatozoa. Proc. Natl Acad. Sci. USA 108, 14342–14347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.King BF, Wildman SS, Ziganshina LE, Pintor J & Burnstock G Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br. J. Pharmacol 121, 1445–1453 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wildman SS, King BF & Burnstock G Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol 123, 1214–1220 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Catterall WA, Goldin AL & Waxman SG International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev 57, 397–409 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Westenbroek RE & Babcock DF Discrete regional distributions suggest diverse functional roles of calcium channel α1 subunits in sperm. Dev. Biol 207, 457–469 (1999). [DOI] [PubMed] [Google Scholar]
- 159.Wennemuth G, Westenbroek RE, Xu T, Hille B & Babcock DF CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem 275, 21210–21217 (2000). [DOI] [PubMed] [Google Scholar]
- 160.Sakata Y et al. Ca(v)2.3 (α1E) Ca2+ channel participates in the control of sperm function. FEBS Lett. 516, 229–233 (2002). [DOI] [PubMed] [Google Scholar]
- 161.Cohen R et al. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Dev. Cell 28, 310–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kirichok Y & Lishko PV Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod 17, 478–499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen PS et al. The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763–773.e11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cordido A, Besada-Cerecedo L & Garcia-Gonzalez MA The genetic and cellular basis of autosomal dominant polycystic kidney disease–a primer for clinicians. Front. Pediatr 5, 279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoder BK, Hou X & Guay-Woodford LM The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol 13, 2508–2516 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Su Q et al. Structure of the human PKD1-PKD2 complex. Science 361, eaat9819 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Kierszenbaum AL Polycystins: what polycystic kidney disease tells us about sperm. Mol. Reprod. Dev 67, 385–388 (2004). [DOI] [PubMed] [Google Scholar]
- 168.Vora N, Perrone R & Bianchi DW Reproductive issues for adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis 51, 307–318 (2008). [DOI] [PubMed] [Google Scholar]
- 169.Li Vecchi M, Cianfrone P, Damiano R & Fuiano G Infertility in adults with polycystic kidney disease. Nephrol. Dial. Transpl 18, 190–191 (2003). [DOI] [PubMed] [Google Scholar]
- 170.Okada H et al. Assisted reproduction for infertile patients with 9+0 immotile spermatozoa associated with autosomal dominant polycystic kidney disease. Hum. Reprod 14, 110–113 (1999). [DOI] [PubMed] [Google Scholar]
- 171.Sutton KA, Jungnickel MK & Florman HM A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl Acad. Sci. USA 105, 8661–8666 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hughes J, Ward CJ, Aspinwall R, Butler R & Harris PC Identification of a human homologue of the sea urchin receptor for egg jelly: a polycystic kidney disease-like protein. Hum. Mol. Genet 8, 543–549 (1999). [DOI] [PubMed] [Google Scholar]
- 173.Butscheid Y et al. Polycystic kidney disease and receptor for egg jelly is a plasma membrane protein of mouse sperm head. Mol. Reprod. Dev 73, 350–360 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Linsdell P et al. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J. Gen. Physiol 110, 355–364 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Illek B, Yankaskas JR & Machen TE cAMP and genistein stimulate HCO3- conductance through CFTR in human airway epithelia. Am. J. Physiol 272, L752–L761 (1997). [DOI] [PubMed] [Google Scholar]
- 176.Chen H, Ruan YC, Xu WM, Chen J & Chan HC Regulation of male fertility by CFTR and implications in male infertility. Hum. Reprod. Update 18, 703–713 (2012). [DOI] [PubMed] [Google Scholar]
- 177.van der Ven K, Messer L, van der Ven H, Jeyendran RS & Ober C Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum. Reprod 11,513–517 (1996). [DOI] [PubMed] [Google Scholar]
- 178.Xu WM et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl Acad. Sci. USA 104, 9816–9821 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Snouwaert JN et al. An animal model for cystic fibrosis made by gene targeting. Science 257, 1083–1088 (1992). [DOI] [PubMed] [Google Scholar]
- 180.Li CY et al. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum. Reprod 25, 317–327 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Hernandez-Gonzalez EO et al. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J. Biol. Chem 282, 24397–24406 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Chen WY et al. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol. Reprod 80, 115–123 (2009). [DOI] [PubMed] [Google Scholar]
- 183.Chavez JC et al. Participation of the Cl−/HCO3− exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol. Reprod 86, 1–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoglund P et al. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil. Steril. 85, 232–235 (2006). [DOI] [PubMed] [Google Scholar]
- 185.Schweinfest CW et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem 281, 37962–37971 (2006). [DOI] [PubMed] [Google Scholar]
- 186.Wang YY et al. Loss of SLC9A3 decreases CFTR protein and causes obstructed azoospermia in mice. PLoS Genet. 13, e1006715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Figueiras-Fierro D et al. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J. Cell Physiol 228, 590–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rode B et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum. Mol. Genet 21, 1287–1298 (2012). [DOI] [PubMed] [Google Scholar]
- 189.Strehler EE & Zacharias DA Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev 81, 21–50 (2001). [DOI] [PubMed] [Google Scholar]
- 190.Keeton TP, Burk SE & Shull GE Alternative splicing of exons encoding the calmodulin-binding domains and C termini of plasma membrane Ca(2+)-ATPase isoforms 1, 2, 3, and 4. J. Biol. Chem 268, 2740–2748 (1993). [PubMed] [Google Scholar]
- 191.Okunade GW et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem 279, 33742–33750 (2004). [DOI] [PubMed] [Google Scholar]
- 192.Schuh K et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem 279, 28220–28226 (2004). [DOI] [PubMed] [Google Scholar]
- 193.Prasad V, Okunade GW, Miller ML & Shull GE Phenotypes of SERCA and PMCA knockout mice. Biochem. Biophys. Res. Commun 322, 1192–1203 (2004). [DOI] [PubMed] [Google Scholar]
- 194.Yamazaki D et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 9, e1003983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamazaki D et al. The Mg2+ transporter CNNM4 regulates sperm Ca2+ homeostasis and is essential for reproduction. J. Cell Sci 129, 1940–1949 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Yamazaki D, Funato Y, Miyata H, Ikawa M & Miki H Complementary role of CNNM2 in sperm motility and Ca(2+) influx during capacitation. Biochem. Biophys. Res. Commun 474, 441–446 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Long JE, Lee MS & Blithe DL Male contraceptive development: update on novel hormonal and nonhormonal methods. Clin. Chem 65, 153–160 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB & Gloriam DE Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. DrugDiscov 16, 829–842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Garcia ML & Kaczorowski GJ Ion channels find a pathway for therapeutic success. Proc. Natl Acad. Sci. USA 113, 5472–5474 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McManus OB HTS assays for developing the molecular pharmacology of ion channels. Curr. Opin. Pharmacol 15, 91–96 (2014). [DOI] [PubMed] [Google Scholar]
- 201.Rennhack A et al. A novel cross-species inhibitor to study the function of CatSper Ca(2+) channels in sperm. Br. J. Pharmacol 175, 3144–3161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schaefer M, Habenicht UF, Brautigam M & Gudermann T Steroidal sigma receptor ligands affect signaling pathways in human spermatozoa. Biol. Reprod 63, 57–63 (2000). [DOI] [PubMed] [Google Scholar]
- 203.Gruber FS, Johnston ZC, Barratt CL & Andrews PD A phenotypic screening platform utilising human spermatozoa identifies compounds with contraceptive activity. eLife 9, e51739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Janes J et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl Acad. Sci. USA 115, 10750–10755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Choy JT & Eisenberg ML Male infertility as a window to health. Fertil. Steril 110, 810–814 (2018). [DOI] [PubMed] [Google Scholar]
- 206.De Jonge C & Barratt CLR The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology 7, 762–768 (2019). [DOI] [PubMed] [Google Scholar]
- 207.Sermondade N et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 19, 221–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li Y, Lin H, Li Y & Cao J Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil. Steril 95, 116–123 (2011). [DOI] [PubMed] [Google Scholar]
- 209.Trottmann M et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur. Urol 52, 355–367 (2007). [DOI] [PubMed] [Google Scholar]
- 210.Jacobsen R et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ 321, 789–792 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Walsh TJ, Croughan MS, Schembri M, Chan JM & Turek PJ Increased risk of testicular germ cell cancer among infertile men. Arch. Intern. Med 169, 351–356 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Breuss MW et al. Autism risk in offspring can be assessed through quantification of male sperm mosaicism. Nat. Med 26, 143–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leung AK, Henry MA & Mehta A Gaps in male infertility health services research. Transl. Androl. Urol 7, S303–S309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu J, Chen Z, Ni Y & Li Z CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum. Reprod 27, 25–35 (2012). [DOI] [PubMed] [Google Scholar]
- 215.Tilley AE, Walters MS, Shaykhiev R & Crystal RG Cilia dysfunction in lung disease. Annu. Rev. Physiol 77, 379–406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Inaba K & Mizuno K Sperm dysfunction and ciliopathy. Reprod. Med. Biol 15, 77–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Serrano CJ, Treviño CL, Felix R & Darszon A Voltage-dependent Ca(2+) channel subunit expression and immunolocalization in mouse spermatogenic cells and sperm. FEBS Lett. 462, 171–176 (1999). [DOI] [PubMed] [Google Scholar]
- 218.Santi CM, Darszon A & Hernandez-Cruz A A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am. J. Physiol 271, C1583–C1593 (1996). [DOI] [PubMed] [Google Scholar]
- 219.Arnoult C, Cardullo RA, Lemos JR & Florman HM Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc. Natl Acad. Sci. USA 93, 13004–13009 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Meizel S The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol. Rev. Camb. Philos. Soc 79, 713–732 (2004). [DOI] [PubMed] [Google Scholar]
- 221.Kurata S, Hiradate Y, Umezu K, Hara K & Tanemura K Capacitation of mouse sperm is modulated by gamma-aminobutyric acid (GABA) concentration. J. Reprod. Dev 65, 327–334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zeng Y, Oberdorf JA & Florman HM pH regulation in mouse sperm: identification of Na(+)-, Cl(−)-, and HCO3(−)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol 173, 510–520 (1996). [DOI] [PubMed] [Google Scholar]
- 223.Singh JP, Babcock DF & Lardy HA Increased calcium-ion influx is a component of capacitation of spermatozoa. Biochem. J 172, 549–556 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ruknudin A & Silver IA Ca2+ uptake during capacitation of mouse spermatozoa and the effect of an anion transport inhibitor on Ca2+ uptake. Mol. Reprod. Dev 26, 63–68 (1990). [DOI] [PubMed] [Google Scholar]
- 225.Zhou R, Shi B, Chou KC, Oswalt MD & Haug A Changes in intracellular calcium of porcine sperm during in vitro incubation with seminal plasma and a capacitating medium. Biochem. Biophys. Res. Commun 172, 47–53 (1990). [DOI] [PubMed] [Google Scholar]
- 226.Baldi E et al. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl 12, 323–330 (1991). [PubMed] [Google Scholar]
- 227.Zeng Y, Clark EN & Florman HM Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol 171, 554–563 (1995). [DOI] [PubMed] [Google Scholar]
- 228.Demarco IA et al. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J. Biol. Chem 278, 7001–7009 (2003). [DOI] [PubMed] [Google Scholar]
- 229.Naz RK & Rajesh PB Role of tyrosine phosphorylation in sperm capacitation / acrosome reaction. Reprod. Biol. Endocrinol 2, 75 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Neill JM & Olds-Clarke P A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res. 18, 121–140 (1987). [DOI] [PubMed] [Google Scholar]
- 231.Cohen-Dayag A, Tur-Kaspa I, Dor J, Mashiach S & Eisenbach M Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. Proc. Natl Acad. Sci. USA 92, 11039–11043 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bleil JD & Wassarman PM Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol 95, 317–324 (1983). [DOI] [PubMed] [Google Scholar]
- 233.Hino T et al. The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol. Reprod 95, 50 (2016). [DOI] [PubMed] [Google Scholar]
- 234.La Spina FA et al. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol 411, 172–182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jin M et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl Acad. Sci. USA 108, 4892–4896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Muro Y et al. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol. Reprod 94, 80 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Sidhu KS et al. A flow cytometric assay for global estimation of tyrosine phosphorylation associated with capacitation of spermatozoa from two marsupial species, the tammar wallaby (Macropus eugenii) and the brushtail possum (Trichosurus vulpecula). Reproduction 127, 95–103 (2004). [DOI] [PubMed] [Google Scholar]
- 238.Zoppino FC, Halón ND, Bustos MA, Pavarotti MA & Mayorga LS Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril 97, 1309–1315 (2012). [DOI] [PubMed] [Google Scholar]
- 239.Uhler ML, Leung A, Chan SY, Schmid I & Wang C Assessment of human sperm acrosome reaction by flow cytometry: validation and evaluation of the method by fluorescence-activated cell sorting. Fertil. Steril 60, 1076–1081 (1993). [DOI] [PubMed] [Google Scholar]
- 240.Escoffier J et al. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod 92, 121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seifert R et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 34, 379–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Marquez B & Suarez SS Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol. Reprod 76, 660–665 (2007). [DOI] [PubMed] [Google Scholar]
- 243.Loux SC et al. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol. Reprod 89, 123 (2013). [DOI] [PubMed] [Google Scholar]
- 244.Roldan ERS Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology 150, 388–395 (2020). [DOI] [PubMed] [Google Scholar]
- 245.Chang H & Suarez SS Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol. Reprod 86, 141–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Demott RP & Suarez SS Hyperactivated sperm progress in the mouse oviduct. Biol. Reprod 46, 779–785 (1992). [DOI] [PubMed] [Google Scholar]
- 247.Suarez SS Sperm transport and motility in the mouse oviduct: observations in situ. Biol. Reprod 36, 203–210 (1987). [DOI] [PubMed] [Google Scholar]
- 248.Williams M et al. Sperm numbers and distribution within the human fallopian tube around ovulation. Hum. Reprod 8, 2019–2026 (1993). [DOI] [PubMed] [Google Scholar]
- 249.Suarez SS Interactions of spermatozoa with the female reproductive tract: inspiration for assisted reproduction. Reprod. Fertil. Dev 19, 103–110 (2007). [DOI] [PubMed] [Google Scholar]
- 250.Ishikawa Y, Usui T, Yamashita M, Kanemori Y & Baba T Surfing and swimming of ejaculated sperm in the mouse oviduct. Biol. Reprod 94, 89 (2016). [DOI] [PubMed] [Google Scholar]
- 251.Xu WM et al. Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS ONE 6, e19120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Miyata H et al. Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proc. Natl Acad. Sci. USA 113, 7704–7710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Suzuki M, Mizuno A, Kodaira K & Imai M Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem 278, 22664–22668 (2003). [DOI] [PubMed] [Google Scholar]
- 254.Wedenoja S et al. A missense mutation in SLC26A3 is associated with human male subfertility and impaired activation of CFTR. Sci. Rep 7, 14208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]