Abstract
Polybrominated diphenyl ethers (PBDEs), a major class of flame retardants incorporated into numerous consumer products, leach out into dust resulting in widespread exposure. There is evidence from in vitro and in vivo animal studies that PBDEs affect ovarian granulosa cell function and follicular development, yet human studies of their association with female infertility are inconclusive. Here, we tested the hypothesis that exposure to the PBDEs in follicular fluid is associated with dysregulation of gene expression in the mural and cumulus granulosa cells collected from women undergoing in vitro fertilization by intracytoplasmic sperm injection. The median concentration of the ∑ 10PBDEs detected in the follicular fluid samples (n = 37) was 15.04 pg/g wet weight. RNA microarray analyses revealed that many genes were differentially expressed in mural and cumulus granulosa cells. Highest vs lowest quartile exposure to the Σ 10PBDEs or to 2 predominant PBDE congeners, BDE-47 or BDE-153, was associated with significant effects on gene expression in both cell types. Mural granulosa cells were generally more sensitive to PBDE exposure compared to cumulus cells. Overall, gene expression changes associated with BDE-47 exposure were similar to those for ∑ 10PBDEs but distinct from those associated with BDE-153 exposure. Interestingly, exposure to BDE-47 and ∑ 10PBDEs activated the expression of genes in pathways that are important in innate immunity and inflammation. To the best of our knowledge, this is the first demonstration that exposure to these environmental chemicals is associated with the dysregulation of pathways that play an essential role in ovulation.
Keywords: polybrominated diphenyl ethers, ovary, granulosa cell, gene expression
Flame-retardant chemicals are produced in high quantities, are widespread in the environment, can bioaccumulate, and are detected in a wide variety of biological samples (1). These chemicals are incorporated into numerous products, such as plastics, textiles, and foams; they can contribute up to 30% of the weight of the components in electronic equipment found in offices and homes, synthetic building materials, furniture, and motor vehicles (1). Brominated flame retardants, including the polybrominated diphenyl ethers (PBDEs), represent a major class of flame retardants (1). This diverse family of chemicals consists of 209 members that share a common core structure of 2 aromatic rings with varying numbers of bromine atoms. With time, these flame retardants leach out into domestic environments, resulting in repetitive exposure through inhalation and ingestion (2, 3). Because PBDEs are lipophilic and persistent (4), they biomagnify through the food chain (2) and bioaccumulate in human milk, serum, adipose tissues (5), and fetal tissues (6). PBDEs are still detected in substantial amounts in house dust, in foods such as fish, and in body fluids even though they have been regulated or banned in North America (2, 7-10).
There is increasing evidence that PBDEs are endocrine disruptors, affecting androgenic, estrogenic, and thyroid hormone activities (11). PBDEs may also affect immune function (12, 13). PBDEs have been detected in the serum of women of reproductive age (14, 15) and in follicular fluid (15-17); thus, they may represent a risk to reproductive health (18, 19). Previously, we reported that in vitro exposure to a PBDE mixture reflecting the profile detected in human follicular fluid significantly affected steroidogenesis and induced oxidative stress in KGN cells, a human granulosa cell line (20). In the ovary, granulosa cells line the fluid-filled antral follicles surrounding the oocyte; effects on granulosa cell function are likely to have an impact on the oocyte and, thus, on female fertility. The dietary exposure of adult female rats to a PBDE mixture formulated to be representative of North American house dust prior to mating did not affect pregnancy rate, maternal health, litter size, or fetal weights (21). However, this mixture did affect folliculogenesis in the ovary, resulting in an approximately 50% increase in the numbers of preantral and antral follicles; this effect was accompanied by changes in gene expression, a decrease in circulating 17-hydroxypregnenolone, and an increase in testosterone (22). Interestingly, in the female offspring of these dams, there was an increase in abnormal ovarian follicles, early vaginal opening, and onset of puberty, in addition to effects on ovarian gene expression (23). Together, these in vitro and in vivo animal studies suggest that exposure to an environmentally relevant PBDE mixture may affect granulosa cell functions and follicular development.
Epidemiological studies have reported that high levels of PBDEs in serum or follicular fluid are associated with a longer time to conceive or a failure in embryo implantation after in vitro fertilization (IVF) (15, 24). Further, preconception serum PBDE concentrations have been associated with increased pregnancy loss (measured as conversion to a negative hCG pregnancy test or the onset of menstruation) (25). Together, these studies provide data to suggest that exposure to PBDEs may have adverse effects on female fertility. However, in another study (15), an increased probability of implantation and clinical pregnancy in Caucasian women who underwent IVF was associated with an interquartile increase in serum BDE-153 concentrations, whereas an increase in serum BDE-47 concentrations was associated with a decrease in the probability of clinical pregnancy in women of other races. Clearly, the specific PBDE congeners to which women are exposed and differences among the populations studied are both important determinants of outcome.
Changes in the gene expression profile of granulosa cells, the somatic cells that surround the oocyte (cumulus cells) or line the follicle wall (mural cells), may provide insight into the mechanisms underlying the success or failure of IVF (26). Indeed, it has been suggested that changes in cumulus cell gene expression may be a marker for pregnancy outcome after IVF (27, 28). Oocytes and granulosa cells are both directly exposed to the persistent environmental endocrine-disrupting chemicals found in ovarian follicular fluid, yet studies associating exposure to these chemicals with the outcome of IVF do not prove causality and are often inconsistent (29).
We hypothesize that exposure to the PBDEs in follicular fluid will result in changes in the gene expression profile of follicular cells and suggest that these changes will provide insight into the impact of these chemicals on granulosa cell functions. To test this hypothesis, we investigated the association between follicular fluid PBDE concentrations and changes in mural and cumulus granulosa cell gene expression in women undergoing IVF by intracytoplasmic sperm injection (ICSI).
Materials and Methods
Sample collection
Thirty-seven women undergoing IVF with ICSI were recruited at the OVO Clinic and the McGill Reproductive Centre located in Montreal, Quebec, Canada, between January 2012 and February 2014. The criteria for recruiting these women were ages between 18 and 40 years, failure to achieve natural pregnancy for a period of 12 months, normal uterus and fallopian tubes, normal hormone levels on day 3 of the menstrual cycle, normal ovarian reserve, and couple registration for an IVF with ICSI procedure. Ethics approval for the study was obtained from the McGill University Health Centre (No. 11-121-SDR) and Health Canada (No. 2012-0012) Research Ethics Boards. Informed written consent was provided by each study participant.
Mature ovarian follicles were obtained 36 hours after hCG administration and ad hoc ovarian stimulation. Mural granulosa cells and follicular fluid were collected once the cumulus-oocyte complexes were isolated. Cumulus cells were collected after oocyte denudation, the first step required during the preparation of oocytes for the ICSI procedure, by an embryologist. Follicular fluid samples were centrifuged for 5 minutes at 900g and then stored at –20 °C for future PBDE analysis. Mural granulosa cells were purified using a Red Blood Cell Lysis Buffer (BioLegend) and a density gradient cell separation medium of Ficoll and sodium diatrizoate (Histopaque 1077; Sigma-Aldrich), following the manufacturers’ instructions. Mural and cumulus cells were both stored in RNAprotect Cell Reagent (Qiagen) at –80 °C for later gene expression analyses.
Measurements of polybrominated diphenyl ether levels in follicular fluid samples
Thawed follicular fluid samples were weighed in Erlenmeyer flasks and fortified with 13C PBDE–labeled congeners (Cambridge Isotope Laboratories; Wellington Laboratories) as surrogate standards to correct for loss during sample preparation. Both ethanol and water (~ 25 mL) saturated with ammonium sulphate and 75-mL of hexane were added to the samples and homogenized using a Polytron. Following phase separation, the hexane layer was removed, a second aliquot of hexane was added, and the samples were rehomogenized. This hexane layer was removed as before and combined with the first. These raw extracts were rinsed with deionized water (15 mL), and after the water phase was discarded, the hexane factions were dried over a bed of precleaned sodium sulfate and collected in a round-bottom flask. Each sample was evaporated to dryness using rotary evaporation, and gravimetric lipid determination was performed. The remaining extract was passed through a column packed with 2 g of activated silica gel prerinsed with 10-mL hexane, and PBDEs were eluted in 4-mL hexane. The extracts were then diluted in hexane to 100 mL and mixed gently with repeated exposure to 10-mL concentrated sulfuric acid and rinsed sequentially with deionized water, 1% potassium hydroxide, and finally with deionized water. The samples were passed through a bed of anhydrous sodium sulfate and reduced in volume to 1 mL using rotary evaporation. Further cleanup of the PBDE fraction was achieved using 2 columns in vertical series: i) 2-g acidified silica gel and ii) 1.5 g of 100% activated Florisil (Sigma-Aldrich) eluted with 40-mL hexane to collect PBDE 209. The Florisil column alone was eluted further with 60-mL dichloromethane, to isolate the remaining PBDEs and this fraction was then evaporated to dryness and reconstituted in 1-mL hexane. A final cleanup step was performed using a 0.4-g 18% Carbopack C:Celite column in which the analytes were eluted with 2-mL hexane, 1-mL (1:1) hexane: dichloromethane and 25-mL toluene, evaporated to dryness, reconstituted with performance standards in 10-µL toluene and placed into vials for gas chromatographic–high-resolution mass spectrometric analysis.
PBDE analyses were performed using a Micromass AutoSpec Ultima high-resolution mass spectrometer (Waters Corp) coupled to an Agilent 6890 gas chromatograph, as previously described (21).
Two reagent blank samples and a low-level certified serum reference material (NIST SRM 1957) were included with each set of samples analyzed in the present study. An aliquot of a serum sample containing measurable and low PBDE concentrations, which was repeatedly tested in our laboratory, was also included with each set of samples as an internal quality control. Each quality control sample was fortified with stable isotope analogues of the analytes, as surrogate standards, prior to extraction, to allow for recovery correction using isotope dilution, as for the samples of unknown concentration. PBDE concentrations are expressed as picogram per gram (pg/g) wet weight of follicular fluid. When values were negative, they were replaced by the limit of detection for the congener divided by the square root of 2.
Transcriptome analyses
Samples were grouped by cell type (mural or cumulus) and then by PBDE exposure to evaluate differences in gene expression between granulosa cell types and the association of these gene expression profiles with exposure to PBDEs. Gene expression in each cell type was analyzed in the context of exposure to the combination of 10 PBDEs detected in more than 80% of the follicular fluid samples (Σ 10PBDE) and of the 2 predominant BDEs measured in the follicular fluid samples, BDE-47 and BDE-153. Classification was based on patients with the highest and lowest quartiles of considered PBDE concentrations in follicular fluid within the sample set of 37 individuals; sample sizes and median PBDE concentrations in each group are provided in Supplementary Data Figure S1 (30). Gene expression fold changes were determined based on the ratio of the highest to lowest exposure groups. Gene lists were generated for each group; genes were filtered to those for which the absolute fold change of expression varied by 1.5-fold or greater. Moderated t tests were used to identify significant changes in gene expression, in relation to PBDE exposure.
Microarrays of mural and cumulus granulosa cells were performed on samples from the same individual when possible. Total RNA was extracted using an RNeasy Plus Mini kit (Qiagen), as per the manufacturer’s instructions. Total RNA was quantified and checked for quality using an Agilent 2100 Bioanalyzer (Agilent Technologies). The RNA integrity number value for all but 3 samples was greater than 7.0; these 3 samples were excluded from further analyses. Complementary DNA (cDNA) was labeled with Cy3 from 25 ng of total RNA using the One-Color Low Input Quick Amp kit (Agilent Technologies). Labeled cDNA was hybridized to SurePrint G3 Human Gene Expression v3 8x60K Microarrays (Agilent Technologies). Labeling and hybridization of cumulus and mural granulosa cells was conducted in 2 batches. Thirty-two arrays were used for analysis of gene expression in the lowest- and highest-PBDE exposure groups (corresponding to biological replicates of 4-7 per group, Supplementary Data Fig. S1) (30). Arrays were scanned with an Agilent SureScan G2600D Microarray scanner (Agilent Technologies). Probe intensities were converted to numerical values that were analyzed using GeneSpring v.14.9-GX-PA (Agilent Technologies). In all cases, samples were normalized to the 75th percentile using a scale algorithm determined from the median of all samples, and log2 transformed. Baseline values were not subtracted from normalized probe values; only probes detected at levels above baseline were retained for analysis.
For comparisons between cell types, 2 gene-level experiments were set up in GeneSpring v.14.9-GX-PA to identify probes that were detected in each cell type. Genes that were mutually exclusive from the 2 lists were classified as cell-type specific. To identify genes that were differentially regulated following exposure to PBDEs, individual experiments for each cell type and PBDE congener grouping were imported into GeneSpring and normalized, as described earlier. From these arrays, gene-level experiments were generated from detected probes. All raw and normalized microarray data have been uploaded to GEO (Gene Expression Omnibus; Accession Nos. GSE110916, GSE110917, GSE110918, GSE110919, GSE110921, GSE110922, and GSE110924).
RNA for select genes was analyzed by quantitative polymerase chain reaction (qPCR) to validate the microarray data. Total RNA was extracted from mural granulosa cells using the RNeasy Plus Mini Kit (Qiagen). SuperScript IV VILO Master Mix (Thermo Fisher Scientific) was used to synthesize cDNA from 0.1 µg and 0.5 µg of total RNA extracted from mural granulosa cells. cDNA was amplified with primers from the RT2 qPCR Primer Assay (Qiagen) (Supplementary Data Table S1), and RT2 SYBRgreen Master Mix containing ROX passive reference (Qiagen). Samples were cycled through the following conditions: 10 minutes at 95 °C, and 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C with a StepOnePlus Real-Time PCR System (Thermo Fisher). Each sample was amplified in quadruplicate, and all samples were amplified on a single 96-well plate for a specific primer in each run. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a housekeeping gene. Relative expression was determined using the 2–ΔΔCT method (31) with the reference sample arbitrarily set to 1 because all samples with the same primer were amplified on the same plate. Samples flagged for high variation or CT values greater than 35 were excluded from analysis.
Pathway and upstream regulator analyses
Genes with significantly greater than 1.5-fold differential regulation were exported for analysis with Ingenuity Pathway Analysis software (Qiagen Bioinformatics). Using a contingency table to calculate a P value with a Fisher exact test, this software examines the imported gene set for enrichment against curated pathways. To predict the directionality (upregulation or downregulation) of an enriched pathway or status of upstream regulators (activated or inhibited), a z score was calculated. Based on known associations between different interacting molecules, associations within the data set that were in accordance with the literature were given a value of 1, and those in the opposite direction were given a value of –1. These values were added and divided by the square root of the number of effector molecules examined. A z score above 2 indicated upregulation/activation, whereas one below –2 indicated downregulation/inhibition. Values between but not including –2 and 2 were not used for predictions.
Results
Participant characteristics
The characteristics of the study participants are presented in Table 1. Only women undergoing ICSI were recruited for this study. The median age of study participants was 36 years (range, 25-41 years); the median BMI was 25.59. Relatively few of the participants were smokers (5%); 76% of the women had a university education and 59% identified themselves as Caucasian. The cycle outcomes were no pregnancy in 68% of the women, a biochemical pregnancy in 3%, and a clinical pregnancy in 30%. Of the ICSI cycles completed, 19% resulted in a live term birth.
Table 1.
Study population characteristics (N = 37)a
| % | No. | Median (min-max) | |
|---|---|---|---|
| Age, y | 36 (25-41) | ||
| Height, m | 1.65 (1.47-1.76) | ||
| Weight, kg | 68.8 (45.36-136.36) | ||
| BMI, kg/m2 | 25.59 (18.83-44.52) | ||
| Smoker | |||
| Yes | 5 | 2 | |
| No | 35 | 13 | |
| Never | 38 | 14 | |
| Second-hand smoke exposure | |||
| Yes | 35 | 13 | |
| No | 54 | 20 | |
| Education | |||
| Secondary | 14 | 5 | |
| University | 49 | 18 | |
| MSc/PhD/MD | 27 | 10 | |
| Other | 5 | 2 | |
| Ethnicity | |||
| Caucasian | 59 | 22 | |
| Hispanic | 8 | 3 | |
| African American | 5 | 2 | |
| Asian | 8 | 3 | |
| Other | 5 | 2 | |
| Day 3 embryo count | |||
| 0 | 14 | 5 | |
| 1-4 | 24 | 9 | |
| 5-8 | 35 | 13 | |
| 9-12 | 19 | 7 | |
| 13-16 | 8 | 3 | |
| Cycle outcome | |||
| No pregnancy | 68 | 25 | |
| Biochemical pregnancy | 3 | 1 | |
| Clinical pregnancy | 30 | 11 | |
| Pregnancy outcome | |||
| No pregnancy | 70 | 26 | |
| Early fetal loss | 5 | 2 | |
| Term birth | 19 | 7 |
Abbreviations: BMI, body mass index; MD, doctor of medicine degree; min-max, minimum to maximum; MSc, master of science degree; PhD, doctor of philosophy degree.
a Self-reported patient demographics are based on a standardized survey and medical chart review. Percentages do not add up to 100% in all cases because respondents left some questions blank or the data were not available from the patient’s chart.
Follicular fluid polybrominated diphenyl ether levels
The concentrations of 23 PBDEs from follicular fluid samples were measured by high-resolution mass spectrometry. BDE-17, BDE-37, BDE-75, BDE-71, BDE-77, BDE-119, BDE-126, BDE-160, BDE-138, BDE-181, BDE-190, BDE-205, and BDE-209 were below the level of detection in the majority of samples. Ten PBDEs (BDE-15, BDE-28, BDE-47, BDE-66, BDE-100, BDE-99, BDE-85, BDE-154, BDE-153, and BDE-183) were detected in more than 80% of the samples. The quantifications of these 10 PBDE congeners are listed in Table 2. The median ∑ 10PBDE concentration in the follicular fluid samples was 15.04 pg/g wet weight (minimum 2.95; maximum 104.70). The most abundant PBDE congeners were tetra-BDE-47, penta-BDE-99, penta-BDE-100, and hexa-BDE-153. Correlations and respective P values among specific PBDE congeners are provided in Supplementary Data Table S2 (30). Major components of the penta-BDE commercial formulation (BDE-47, BDE-99, and BDE-100) were moderately to highly correlated with each other (0.37-0.92). BDE-154 and BDE-153, found both in the penta-BDE and octa-BDE formulations, showed very different results: Whereas BDE-154 had low to high correlations with the penta-BDEs (0.21-0.96), BDE-153 showed no significant relationship.
Table 2.
Follicular fluid polybrominated diphenyl ether congener concentrations in the study population
| LOD | Detect freq., % | GMa | 95% CI | Min | 25th percentile | Median | 75th percentile | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Di-BDE-15 | 0.02 | 84 | 0.09 | 0.10-0.18 | < LOD | 0.04 | 0.13 | 0.21 | 0.50 |
| Tri-BDE-28 | 0.03 | 97 | 0.38 | 0.42-0.86 | < LOD | 0.21 | 0.41 | 0.90 | 2.77 |
| Tetra-BDE-47 | 0.02 | 100 | 8.84 | 8.59-19.38 | 1.89 | 4.52 | 7.84 | 18.66 | 76.30 |
| Tetra-BDE-66 | 0.03 | 84 | 0.05 | 0.04-0.12 | < LOD | < LOD | 0.05 | 0.08 | 0.59 |
| Penta-BDE-100 | 0.01 | 100 | 1.14 | 1.15-2.45 | 0.11 | 0.57 | 1.14 | 2.43 | 9.28 |
| Penta-BDE-99 | 0.03 | 92 | 0.99 | 0.90-5.61 | < LOD | 0.40 | 1.44 | 2.48 | 41.04 |
| Penta-BDE-85 | 0.04 | 86 | 0.11 | 0.11-0.46 | < LOD | 0.05 | 0.10 | 0.22 | 2.71 |
| Hexa-BDE-154 | 0.08 | 89 | 0.15 | 0.13-0.58 | < LOD | < LOD | 0.16 | 0.34 | 3.70 |
| Hexa-BDE-153 | 0.16 | 97 | 3.11 | 3.02-9.92 | < LOD | 1.58 | 3.44 | 6.53 | 58.07 |
| Hepta-BDE-183 | 0.52 | 81 | 0.25 | 0.28-0.45 | < LOD | < LOD | < LOD | < LOD | 0.99 |
| Σ 10 PBDE | – | – | 18.36 | 18.47-36.07 | 2.95 | 9.10 | 15.04 | 36.25 | 104.70 |
PBDEs are expressed as pg/g wet weight of follicular fluid.
Abbreviations: freq., frequency; GM, geometric mean; LOD, limit of detection; Max, maximum; Min, minimum; PBDE, polybrominated diphenyl ether.
a GM was calculated based on inputing values below the LOD at LOD/sqrt (2).
Differences in gene expression between mural and cumulus granulosa cells
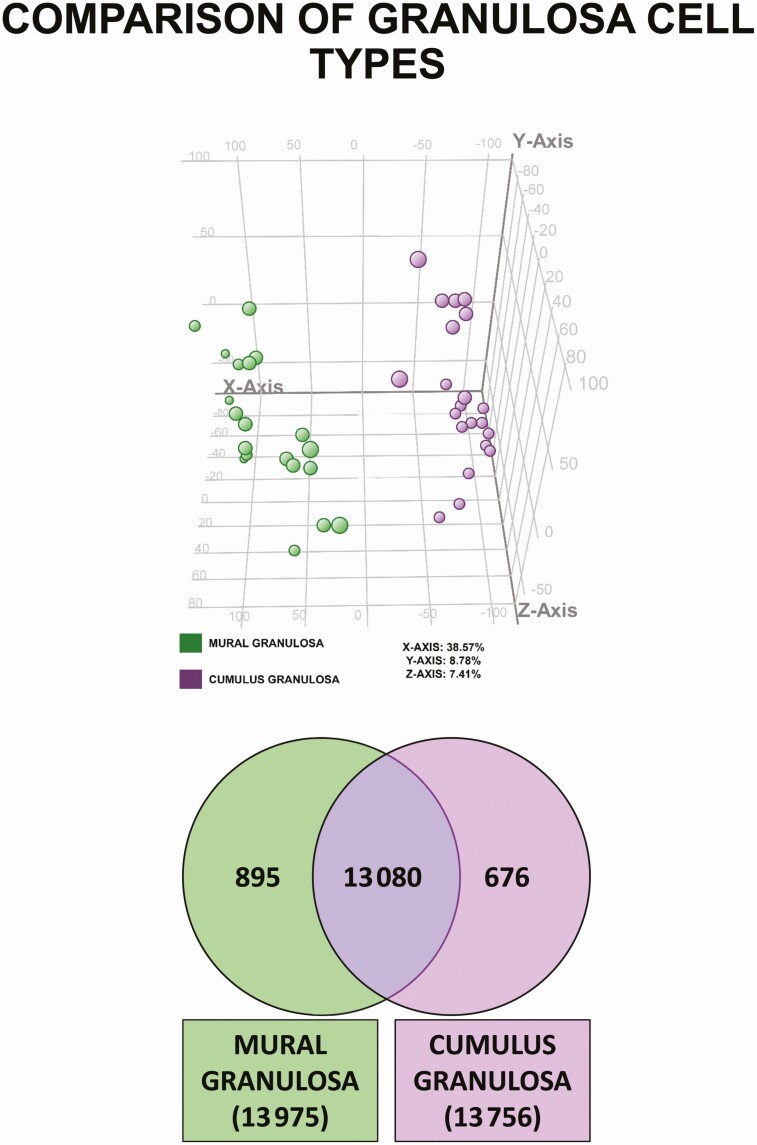
Principal component analysis was used to survey the global gene expression of mural and cumulus granulosa cells (Fig. 1). The algorithm separates samples in 3 dimensions, but most of the differences among samples are represented by separation on the x axis, which comprises 38.7% of the variation among samples. Based on the distribution of these samples within the dimensions of the graph and the groupings that are formed, the x axis is separated in samples by granulosa cell type.
Figure 1.
Differential gene expression in mural and cumulus granulosa cells. A, Principal component analysis plot of all entities in either mural or cumulus granulosa cells. B, Identification of genes that are expressed in common and differentially between both cell types. A full list of genes is provided in the Supplementary Data 1 Excel file (30).
Recognizing that there is differential gene expression between the 2 cell types, a Venn diagram was generated to identify genes that are uniquely expressed by each cell type. This strategy identified 13 080 unique Entrez Gene GeneIDs expressed in common between both cell types as well as genes expressed exclusively in mural (895 genes) or in cumulus (676) granulosa cells (see Fig. 1; gene lists are provided in the Supplementary Data 1 Excel file) (30). The top 10 uniquely expressed genes for each cell type and those with the largest fold change between both cell types are shown in Tables 3 to 5, respectively; complete gene lists are provided in the Supplementary Data 1 Excel file (30). A comparison of the top 10 uniquely expressed genes in mural and cumulus cells (Tables 3 and 4) revealed that these genes have distinct functions; for example, a number of the genes that are highly expressed in mural cells play roles in host defense and chemotaxis, whereas some of those in cumulus cells are important in transmembrane signaling and in cell proliferation and survival. Among the top genes that are differentially regulated in cumulus and mural cells (Table 5) are 2 for chemokines (CCL4L2 and CCL3) that induce the chemotaxis of cells, and 4 for calcium binding proteins (S100A12, S100P, S100A9, and S100A8) that play a prominent role in regulation of inflammatory processes and may act as alarmins or danger-associated molecular pattern molecules to stimulate an innate immune response. Others (S1PR4, SELL) may play a role in mediating the migration or adherence of lymphocytes.
Table 3.
Top 10 uniquely expressed mural granulosa cell genes
| Gene name | Gene symbol | Normalized, log |
|---|---|---|
| Defensin, α 3, neutrophil-specific | DEFA3 | 12.783955 |
| ATP synthase, H+ transporting, mitochondrial Fo complex, subunit G2 | ATP5L2 | 12.557222 |
| Complement component 5a receptor 1 | C5AR1 | 11.83879 |
| Hydroxycarboxylic acid receptor 3 | HCAR3 | 11.787745 |
| Chemokine (C-C motif) ligand 3-like 3 | CCL3L3 | 11.646143 |
| Aquaporin 9 | AQP9 | 11.604592 |
| Lymphotoxin β (TNF superfamily, member 3) | LTB | 11.566189 |
| Fc fragment of IgG, low-affinity IIc, receptor for (CD32) (gene/pseudogene) | FCGR2C | 11.552032 |
| Lysosomal protein transmembrane 5 | LAPTM5 | 11.465715 |
| Prokineticin 2 | PROK2 | 11.382727 |
Abbreviations: ATP, adenosine 5′-triphosphate; IgG, immunoglobulin G; TNF, tumor necrosis factor.
Table 5.
Top 10 absolute differentially expressed genes (cumulus vs mural)
| Gene name | Gene symbol | Fold change | P |
|---|---|---|---|
| Chemokine (C-C motif) ligand 4-like 2 | CCL4L2 | 137.3465 | 7.28E-20 |
| Chemokine (C-C motif) ligand 3 | CCL3 | 135.7144 | 6.31E-19 |
| S100 calcium binding protein A12 | S100A12 | 115.1581 | 3.86E-19 |
| S100 calcium binding protein P | S100P | 99.47706 | 9.50E-21 |
| Interleukin 1, β | IL1B | 85.96865 | 5.46E-19 |
| S100 calcium binding protein A9 | S100A9 | 78.41742 | 5.10E-20 |
| Sphingosine-1-phosphate receptor 4 | S1PR4 | 76.09713 | 5.87E-22 |
| CD52 molecule | CD52 | 69.04562 | 2.73E-22 |
| Selectin L | SELL | 64.49516 | 4.26E-22 |
| S100 calcium binding protein A8 | S100A8 | 63.96976 | 1.83E-20 |
Table 4.
Top 10 uniquely expressed cumulus granulosa cell genes
| Gene name | Gene symbol | Normalized, log |
|---|---|---|
| Guanine nucleotide binding protein (G protein), α inhibiting activity polypeptide 2 | GNAI2 | 14.44725 |
| Tenascin C | TNC | 11.25721 |
| KIAA0100 | KIAA0100 | 10.51303 |
| Acidic repeat containing | ACRC | 10.47444 |
| Proline-rich protein BstNI subfamily 2 | PRB2 | 10.34489 |
| Autophagy/beclin-1 regulator 1 | AMBRA1 | 10.07974 |
| Forkhead box G1 | FOXG1 | 10.03152 |
| Phosphatidylinositol-4-phosphate 3-kinase, catalytic subunit type 2 α | PIK3C2A | 9.734154 |
| Secretogranin II | SCG2 | 9.08257 |
| Pregnancy upregulated nonubiquitous CaM kinase | PNCK | 9.014592 |
Exposure to follicular polybrominated diphenyl ethers is associated with differential gene expression responses in cumulus and mural granulosa cells
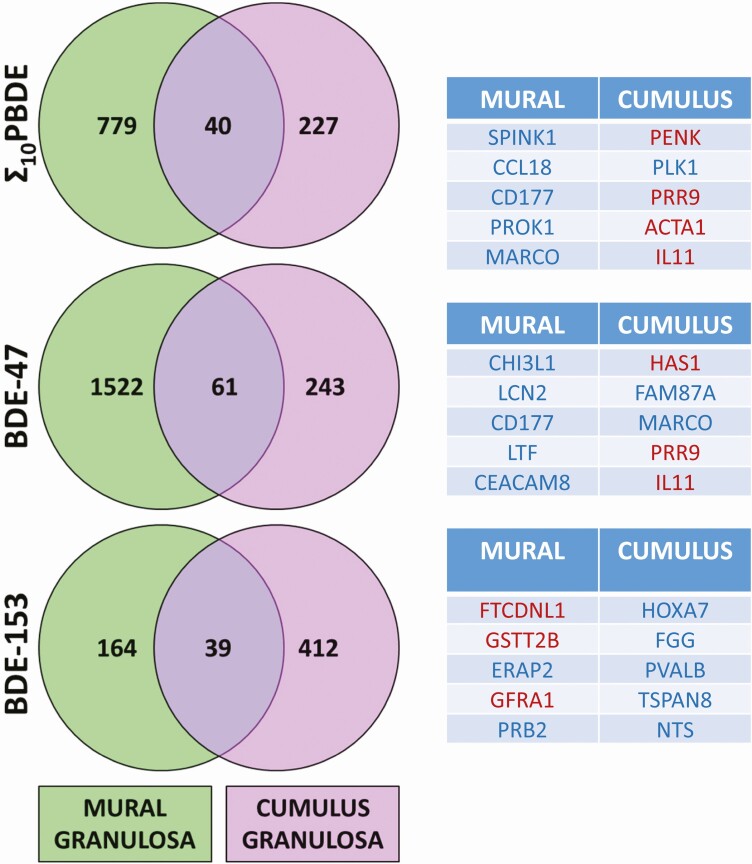
Data were grouped into high vs low Σ 10PBDEs, BDE-47, or BDE-153, categories to determine whether significant changes in gene expression in mural and cumulus granulosa cells were associated with PBDE exposure (Fig. 2). The overlap of each Venn diagram represents genes that are differentially regulated in both cell types in high-exposure individuals. Thus, the expression of 40, 61, and 39 genes is significantly altered between these 2 cell types in association with Σ 10PBDEs, BDE-47, or BDE-153, respectively. Many more genes are differentially regulated in a cell-type–specific manner. In mural cells, 779, 1522, and 164 genes are differentially regulated across the Σ 10PBDE, BDE-47, and BDE-153 exposure groups, respectively; in cumulus cells, 227, 243, and 412 genes are differentially regulated across the same groups. A comparison of the findings in the microarray experiment for mural granulosa cell samples from the high and low BDE-47 groups with reverse transcriptase–qPCR values is presented in Supplementary Data Fig. S2 (30).
Figure 2.
Association of polybrominated diphenyl ether exposure with granulosa cell gene expression. Each Venn diagram shows the number of genes that are significantly differentially regulated by 1.5-fold or more, determined by a moderated t test. This analysis was conducted both for mural and cumulus granulosa cell types. The overlap of each Venn diagram represents genes that are differentially regulated in both cell types in the highest- vs the lowest-exposure individuals. A full list of genes is provided in the Supplementary Data 1 Excel file. The tables report the top 5 genes that are differentially regulated (upregulated [blue] or downregulated [red]) for each cell type.
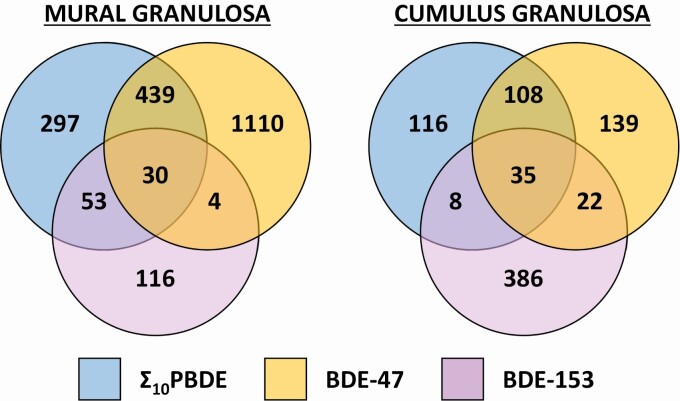
Gene expression varied by PBDE congener exposure. Although some genes are commonly regulated across the Σ 10PBDEs and BDE-47 groups (CD177, PRR9, IL11), the majority are not. Furthermore, BDE-153 exposure is associated with a very different gene expression profile compared to the former 2 groups. This difference is highlighted in Fig. 3, in which differential gene expression is compared across treatments within a cell type. A list of the genes in each part of the Venn diagram is provided in the Supplemental Data 1 Excel files (30).
Figure 3.
Polybrominated diphenyl ether (PBDE) congener-dependent differences in gene expression between mural and cumulus granulosa cells. Genes that are differentially regulated by greater than 1.5-fold and deemed significant by moderated t test are represented for a PBDE congener. This was performed both for mural and granulosa cell types. A full list of genes is provided in the Supplementary Data 1 Excel file (30).
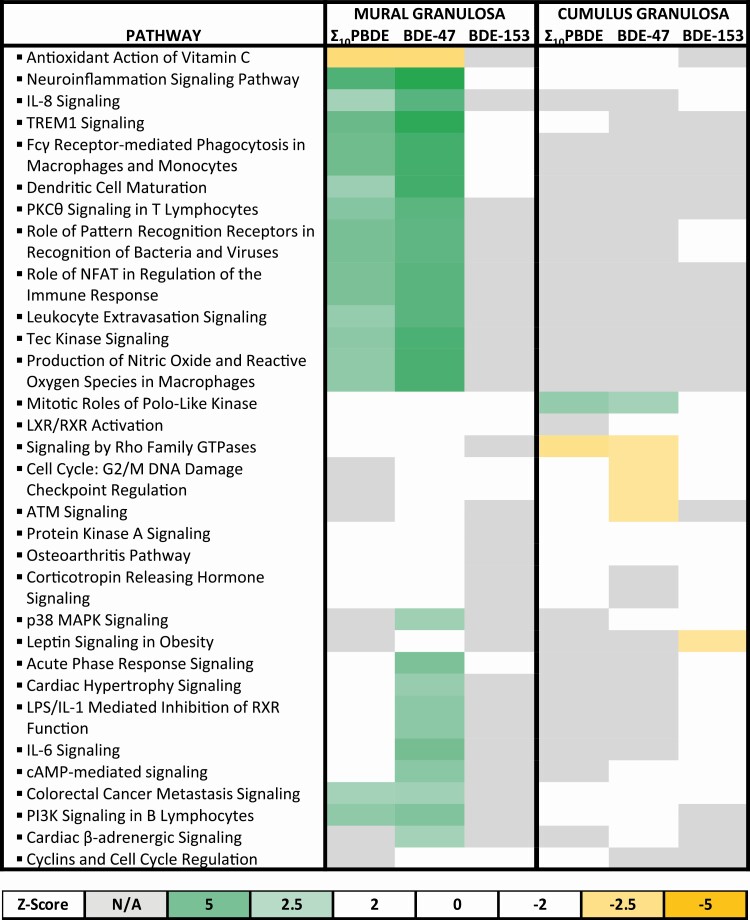
Signal transduction pathways associated with exposure to polybrominated diphenyl ethers
Because gene expression is dependent both on the cell type (ie, mural vs cumulus granulosa cells) and the PBDE congeners to which they are highly exposed, we next queried how these changes in gene expression may affect physiological functions. Signal transduction pathways or cellular processes potentially altered by the changes in gene expression associated with cell type and exposure levels of Σ 10PBDEs, BDE-47, or BDE-153 are presented in Fig. 4. The similarities and differences among the 6 PBDE exposure groups were compared by selecting the top 10 pathways with the lowest P values observed within each data set. The directionality of the regulation of many of these pathways could be predicted by the directionality of the expression of genes listed in the underlying gene set, that is, the signaling pathways potentially upregulated or downregulated, as indicated by the green or yellow boxes, respectively, in Fig. 4. The regulation of signaling pathways in mural granulosa cells was generally more sensitive to PBDE exposure in comparison to cumulus granulosa cells. In cumulus granulosa cells, the only pathway predicted to be activated in association with exposure to Σ 10PBDEs or BDE-47 is mitotic roles of polo-like kinases; pathways related to signaling by ρ family GTPases and cell-cycle checkpoints in response to DNA damage are predicted to be inhibited. One of the most striking differences among the different data sets is the activation of effectors involved in immune signaling that is associated specifically in mural granulosa cells with high exposure to BDE-47 or Σ 10PBDEs. Many of these pathways are not significantly enriched, and none are predicted to be activated in mural granulosa cells in the BDE-153 group. Several genes are shared among the 11 pathways related to immune function that are clustered in association with high exposure to Σ 10PBDEs or to BDE-47 in mural granulosa cells. Genes found in at least 9 of these pathways include GRB2, MAPK1, PIK3CG, FGFR1, MAPK3, PIK3CD, PIK3R5, and PLCG2 for BDE-47, and MAPK1, KL, PIK3CD, and PLCG2 for Σ 10PBDEs.
Figure 4.
Pathway enrichment in mural and cumulus granulosa cells exposed to polybrominated diphenyl ether (PBDE) congeners. Following the identification of significantly regulated genes, Ingenuity Pathway Analysis software was used to identify gene enrichment in the data set. This provided a list of several pathways. In some cases, pathways were predicted to be activated (green) or inhibited (yellow), depending on relative fold changes provided. The calculated z score is a statistical measure of the match between the expected relationship direction and the observed gene expression. Values greater than 2 or less than –2 are considered significant. When there are not enough data in the data set to make a prediction, the box is undefined (gray), whereas when there is no correlation between the observed data set and the expected relationship, the calculated z score is zero (white).
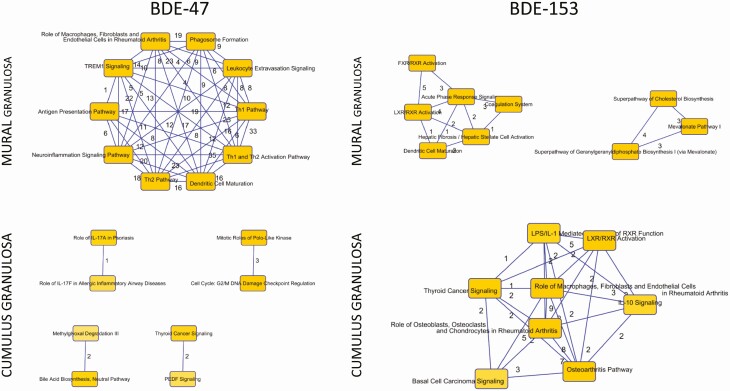
The top 10 pathways associated with exposure to BDE-47 or BDE-153 were plotted to identify clusters that were functionally related by common mediators. Fig. 5 shows the connectivity among pathways and the numbers of genes they have in common. These are also presented in a table in the Supplemental Data 2 Excel file (30) with a full listing of the pathway names, the entities within each pathway from the uploaded data set, and the P value and z score for each pathway. In mural granulosa cells with high exposure to BDE-47, the top upstream regulators are enriched for functions involving the inflammatory response; in cumulus cells, cell-cycle progression and checkpoint regulation are affected. Both in mural and cumulus cells, BDE-153 exposure is associated with pathways involved in lipid metabolism, molecular transport, and small-molecule biochemistry.
Figure 5.
Identification of common identities among pathways in mural and cumulus granulosa cells exposed to polybrominated diphenyl ether (PBDE) congeners BDE-47 or BDE-153. Based on pathways that were enriched within the data set, the top 10 pathways were selected and queried for common effector genes. Here the numbers represent the number of genes in common between the relevant pathways. Full details are provided in the Supplementary Data 2 Excel file (30).
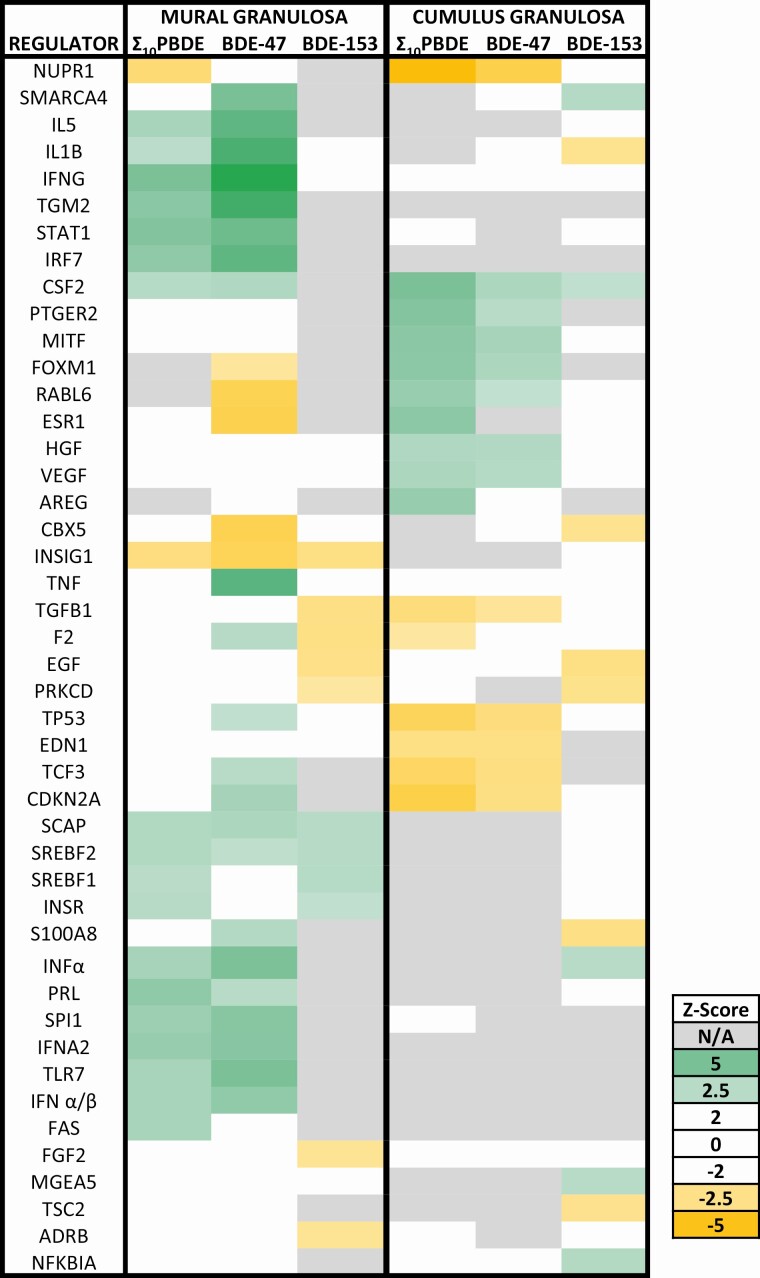
Regulators of gene expression following polybrominated diphenyl ether exposure
Based on differential gene expression in the data sets, upstream activators are predicted to be either activated or inhibited in association with exposure to high levels of PDBEs (Fig. 6). High exposure to Σ 10PBDEs is associated with inhibition of nuclear protein 1 (NUPR1), a transcriptional regulator, both in mural and cumulus granulosa cells; upregulation of this transcriptional regulator helps cells respond to stress by participating in the regulation of cell cycle, apoptosis, autophagy, and DNA repair responses (32). In cumulus cells, Σ 10PBDEs exposure is also associated with inhibition of other stress response transcription factors, including tumor protein 53 (TP53) and cyclin-dependent kinase inhibitor 2A (CDKN2A), which work together to arrest cell cycle progression. Interestingly, estrogen receptor 1 (ESR1) activation is predicted to be inhibited in mural cells in association with BDE-47 exposure but activated in cumulus cells in the high Σ 10PBDE–exposed group. In mural cells, exposure to Σ 10PBDEs and to BDE-47 is associated with the activation of interleukin 5 (IL5) and interleukin 1β (IL1B). IL1B, a potent proinflammatory cytokine, is involved in the induction of interferon γ (IFNG), another important immunoregulator. IFNG and several other interferons (INFα, IFNA2, IFNα/β) are predicted to be strongly activated. Interestingly, both the ESR1 and insulin-induced gene 1 are predicted to be strongly inhibited in association with BDE-47 exposure in mural cells. Thus, the mediators of PBDE action that are predicted to be activated or inhibited are dependent both on the PBDE congener and the granulosa cell type. A number of these upstream regulators play a role in the immune response or in mediating hormone action.
Figure 6.
Based on differential gene expression, predictions were made as to the status of upstream regulators of gene expression. Those in green are predicted to be activated, whereas those in yellow are predicted to be inhibited. The calculated z score is a statistical measure of the match between the expected relationship direction and the observed gene expression. Values greater than 2 or less than –2 are considered significant. When there are not enough data in the data set to make a prediction, the box is undefined (gray), whereas when there is no correlation between the observed data set and the expected relationship, the calculated z score is zero (white).
Discussion
Polybrominated diphenyl ethers are present in follicular fluid
The median Σ 10PBDEs in the follicular fluid samples collected from 37 women living in Montreal between 2012 and 2014 is 15.04 pg/g wet weight (see Table 2); PBDE detection frequencies vary from 81% (BDE-183) to 100% (BDE-47 and BDE-100). The most abundant PBDE congeners are BDE-47, BDE-99, BDE-100, and BDE-153. Previously, Johnson et al (16) reported that 7 PBDEs (BDE-28, BDE-47, BDE-99, BDE-100, BDE-154, BDE-153, and BDE-183) were detected at frequencies that varied from 3% to –70% in follicular fluid samples collected in Boston. The mean Σ 7PBDEs in follicular fluid in their study was 58 pg/g wet weight; the predominant PBDEs detected were BDE-47 (26 pg/g wet weight), BDE-99 (14 pg/g wet weight), and BDE-153 (7 pg/g wet weight); comparison of the PBDE levels in follicular fluid and serum revealed that follicular fluid levels were generally similar to or lower than those in serum, although the ratios were highly variable. The same 7 PBDEs (mean Σ 7PBDEs: 50 ± 24 ng/g lipid weight) were detected in follicular fluid samples collected in China (17). However, the detection frequency of BDE-47 was only 28%; the predominant BDEs were BDE-100 and BDE-99, with detection frequencies of 65% and 90%, respectively. It is difficult to directly compare these studies because there are differences in the ways in which the PBDE levels were reported in follicular fluid and in the detection frequencies of the various PBDEs; differences in detection frequencies may be a function of the use of different flame-retardant formulations in these countries and the timing of regulatory actions among countries. Nevertheless, PBDEs are clearly present in follicular fluid samples collected from North America and China.
Mural and cumulus granulosa cells have distinct gene expression profiles
Analyses of the global gene expression profiles of cumulus and mural granulosa cells reveals that many genes are expressed uniquely in 1 cell type whereas others are differentially expressed in the 2 cell types (see Fig. 1). These findings are consistent with previous reports (33-35) and the fact that there are important functional differences between cumulus and mural granulosa cells. Cumulus granulosa cells are in direct contact with the oocyte and play an important role in enabling its growth and developmental competence. Here, the top uniquely expressed gene in cumulus cells is guanine nucleotide-binding protein G(i) subunit α-2; this gene is implicated in cell division (36). Others, such as autophagy/beclin-1 regulator 1, may play a role in regulating cell survival (37). In agreement with a previous study (35), 2 other genes, tenascin C and forkhead box G1, are highly expressed in cumulus cells and have roles in cell proliferation and differentiation.
Mural granulosa cells support follicle growth and tissue remodeling, including angiogenesis (38), and play a role in the immune response (35). Here, we observe a general enrichment of genes involved in immune responses in the mural granulosa cell population, including components of the complement cascade (Supplementary Data 2 Excel file) (30). A previous study identified most components of the complement system in human follicular fluid and reported finding transcripts for many of these factors in mural granulosa cells (39). Using single-cell RNA-sequencing analyses, Fan and colleagues reported that human mural granulosa cells express the complement regulatory proteins, CD55 and CD59 (40). The inflammatory response and innate immunity signaling pathways play an essential role in ovulation and are upregulated in the ovarian follicle (41). Furthermore, there is evidence that granulosa cells themselves function as innate immune cells during ovulation (42, 43). It has been suggested that an inappropriate inflammatory response may be associated with infertility (27, 42).
Polybrominated diphenyl ether (PBDE) exposure is associated with BDE-congener and granulosa cell-type specific effects on the transcriptome
Several studies have reported that PBDE exposure, in vitro or in an animal model, was associated with an upregulation of key inflammatory mediators, such as the interleukins and tumor necrosis factor-α (44, 45). In contrast, other studies have reported suppression of the release of inflammatory mediators and an impaired innate immune response after PBDE exposure (46, 47). This is the first demonstration that exposure to Σ 10PBDEs, BDE-47, or BDE-153 is associated with distinct BDE-congener–specific effects on gene expression in mural and cumulus granulosa cells (see Fig. 2). However, further confirmation of these findings, with qPCR and protein analyses, is warranted.
Overall, the gene expression changes associated with BDE-47 exposure are similar to those for Σ 10PBDEs, but distinct from those associated with BDE-153 exposure. CD177 is among the top 5 upregulated genes associated with exposure to Σ 10PBDEs and BDE-47 in mural granulosa cells. CD177 is involved in the innate immune system and neutrophil functions. Interestingly, macrophage receptor with collagenous structure (MARCO) is upregulated in association with exposure to Σ 10PBDEs and BDE-47 in both cumulus and mural granulosa cells. MARCO is a pattern-recognition molecule and may play a role in macrophage function. Interleukin-11 (IL11) and proline-rich protein 9 (PRR9) are both among the top 5 genes downregulated in cumulus cells in association with exposure to Σ 10PBDEs and BDE-47. IL11 is a cytokine associated with the innate immunity; there is little known about the function of PRR9.
Analyses of the signal transduction pathways associated with exposure to Σ 10PBDEs, BDE-47, or BDE-153 reinforce the strong differences in response to PBDEs between mural and cumulus granulosa cells (see Figs. 4 and 5). Strikingly, 11 pathways related to immune function are predicted to be activated only in mural cells in association with exposure to Σ 10PBDEs or BDE-47; mitogen-activated protein kinase 1 (MAPK1) and MAPK3 are among the genes found in at least 9 of these 11 pathways. The MAPKs play a central role in cell signaling, including in the activation of toll-like receptors and the interleukin signaling pathways (48, 49). Three phosphatidylinositol-3 kinases, PIK3CD, PIK3CG, and PIK3R5, are also found in most of these pathways; these PI3Ks play multiple roles in the immune response, in B- and T-cell signaling, in cytokine production, and in natural killer cell activation (50) Finally, phospholipase C γ 2 (PLCG2) is involved in the IL-2 pathway, B- and T-cell receptor signaling, and the adaptive immune system (51). The distinct “nodes” associated with exposure to BDE-47 or BDE-153 in mural or cumulus granulosa cells are graphically depicted in Fig. 5.
Interleukins (IL1B and IL5) and interferons (IFNG, INFα, IFNA2, IFNα/β) are the predicted top upstream regulators of differential gene expression in mural granulosa cells associated with BDE-47 and Σ 10PBDEs exposure (see Fig. 6). These signaling molecules play significant roles as modulators of the function of both the innate and adaptive immune system (52). Importantly, human granulosa cells activate the innate immune system and trigger a “sterile” inflammatory reaction in the absence of any pathogen (42). In “professional” immune cells, such as macrophages, the innate immune system activates an inflammatory response by assembling inflammasomes, cytosolic multiprotein oligomers (53). These inflammasomes can activate caspase 1 and trigger the proteolytic cleavage of proinflammatory cytokines such as IL1B. Previously, IL1B has been shown to be a potent suppressor of the follicle-stimulating hormone–induced differentiation of ovarian granulosa cells (54). The inhibition of inflammasome activation has been reported to decrease the inflammatory neurotoxicity and kidney damage induced in mice by treatment with BDE-47 (55, 56).
Together, our data suggest that the PBDE congeners found in follicular fluid have a marked impact on the gene expression profile of mural granulosa cells. Specifically, exposure to BDE-47 and Σ 10PBDEs was associated with activation of the expression of genes involved in innate immunity and the inflammatory response. To the best of our knowledge this is the first demonstration that exposure to environmental chemicals may dysregulate the innate immune response in ovarian granulosa cells. Further research is needed to determine whether other environmental chemicals found in follicular fluid act via a similar mechanism to alter follicular development and fertility.
Conclusions
Our findings demonstrate that multiple PBDEs were detected ubiquitously in follicular fluid samples collected from women during an IVF procedure. Furthermore, we provide evidence that exposure to PBDEs is associated with the differential modulation of gene expression in ovarian mural and cumulus granulosa cells. Importantly, many of these changes in gene expression may affect the inflammatory response that is involved in oocyte maturation and ovulation and influence the production of mature oocytes and hence fertility.
Acknowledgments
The authors acknowledge Patricia Monnier, Jacques Kadoch, Nicola Dean, Lalita Garufi, Lydia Goff, Brïte Pauchet, and Marie-Eve Ruest for their valuable assistance with patient recruitment and sample collection, and Yaned Gaitan for her technical assistance.
Financial Support: This work was supported by the Canadian Institutes of Health Research (CIHR) Institute for Human Development, Child and Youth Health (Team grant RHF100625 to C.G. and B.F.H.). P.L.C.L. is the recipient of a Fonds de recherche du Québec – Santé fellowship. T.C.N. is the recipient of studentships from the Réseau Québécois en Reproduction NSERC-CREATE and the CIHR REDIH Program. B.R. and B.F.H. are James McGill Professors.
Author Contributions: C.G., B.F.H., P.L.C.L., T.C.N., P.M., and B.R. were responsible for the experimental design, data analyses, and manuscript preparation. W.Y.S. was responsible for the granulosa cell collections. P.L.C.L., T.C.N., and A.R.S. conducted most of the experiments. D.F.K.R. performed analytical data analysis for accuracy. All authors approved the final version of the manuscript.
Glossary
Abbreviations
- cDNA
complementary DNA
- ESR1
estrogen receptor 1
- ICSI
intracytoplasmic sperm injection
- IFNG
interferon γ
- IL1B
interleukin 1β
- IVF
in vitro fertilization
- MAPK
mitogen-activated protein kinase
- MARCO
macrophage receptor with collagenous structure
- PBDEs
polybrominated diphenyl ethers
- PRR9
proline rich protein 9
- qPCR
quantitative polymerase chain reaction
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Gene expression data are available at GEO (Gene Expression Omnibus) accession numbers GSE110916, GSE110917, GSE110918, GSE110919, GSE110921, GSE110922, and GSE110924. All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Segev O, Kushmaro A, Brenner A. Environmental impact of flame retardants (persistence and biodegradability). Int J Environ Res Public Health. 2009;6(2):478-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, He C, Han W, et al. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: a review. Environ Res. 2020;187:109531. [DOI] [PubMed] [Google Scholar]
- 3. Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—a review on occurrence and human exposure. Environ Pollut. 2012;169:217-229. [DOI] [PubMed] [Google Scholar]
- 4. Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112(1):9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945-956. [DOI] [PubMed] [Google Scholar]
- 6. Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from Greater Montreal, Quebec: a longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117(4):605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbasi G, Saini A, Goosey E, Diamond ML. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci Total Environ. 2016;545-546:299-307. [DOI] [PubMed] [Google Scholar]
- 9. Klinčić D, Dvoršćak M, Jagić K, Mendaš G, Herceg Romanić S. Levels and distribution of polybrominated diphenyl ethers in humans and environmental compartments: a comprehensive review of the last five years of research. Environ Sci Pollut Res Int. 2020;27(6):5744-5758. [DOI] [PubMed] [Google Scholar]
- 10. Sjödin A, Jones RS, Wong L-Y, Caudill SP, Calafat AM. Polybrominated diphenyl ethers and biphenyl in serum: time trend study from the National Health and Nutrition Examination Survey for years 2005/06 through 2013/14. Environ Sci Technol. 2019;53(10):6018-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darnerud PO. Brominated flame retardants as possible endocrine disrupters. Int J Androl. 2008;31(2):152-160. [DOI] [PubMed] [Google Scholar]
- 12. Mynster Kronborg T, Frohnert Hansen J, Nielsen CH, et al. Effects of the commercial flame retardant mixture DE-71 on cytokine production by human immune cells. PLoS One. 2016;11(4):e0154621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaylord A, Trasande L, Kannan K, et al. Persistent organic pollutant exposure and celiac disease: a pilot study. Environ Res. 2020;186:109439. [DOI] [PubMed] [Google Scholar]
- 14. Drage DS, Heffernan AL, Cunningham TK, et al. Serum measures of hexabromocyclododecane (HBCDD) and polybrominated diphenyl ethers (PBDEs) in reproductive-aged women in the United Kingdom. Environ Res. 2019;177:108631. [DOI] [PubMed] [Google Scholar]
- 15. Ingle ME, Mínguez-Alarcón L, Carignan CC, et al. ; EARTH Study Team . Exploring reproductive associations of serum polybrominated diphenyl ether and hydroxylated brominated diphenyl ether concentrations among women undergoing in vitro fertilization. Hum Reprod. 2020;35(5):1199-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson PI, Altshul L, Cramer DW, Missmer SA, Hauser R, Meeker JD. Serum and follicular fluid concentrations of polybrominated diphenyl ethers and in-vitro fertilization outcome. Environ Int. 2012;45:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Yan M, Nie H, Wang W, Wang J. Persistent halogenated organic pollutants in follicular fluid of women undergoing in vitro fertilization from China: occurrence, congener profiles, and possible sources. Environ Pollut. 2019;244:1-8. [DOI] [PubMed] [Google Scholar]
- 18. Linares V, Bellés M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89(3):335-356. [DOI] [PubMed] [Google Scholar]
- 19. Lyche JL, Rosseland C, Berge G, Polder A. Human health risk associated with brominated flame-retardants (BFRs). Environ Int. 2015;74:170-180. [DOI] [PubMed] [Google Scholar]
- 20. Lefevre PL, Wade M, Goodyer C, Hales BF, Robaire B. A Mixture reflecting polybrominated diphenyl ether (PBDE) profiles detected in human follicular fluid significantly affects steroidogenesis and induces oxidative stress in a female human granulosa cell line. Endocrinology. 2016;157(7):2698-2711. [DOI] [PubMed] [Google Scholar]
- 21. Berger RG, Lefèvre PL, Ernest SR, et al. Exposure to an environmentally relevant mixture of brominated flame retardants affects fetal development in Sprague-Dawley rats. Toxicology. 2014;320:56-66. [DOI] [PubMed] [Google Scholar]
- 22. Lefèvre PL, Berger RG, Ernest SR, et al. Exposure of female rats to an environmentally relevant mixture of brominated flame retardants targets the ovary, affecting folliculogenesis and steroidogenesis. Biol Reprod. 2016;94(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allais A, Albert O, Lefèvre PLC, Wade MG, Hales BF, Robaire B. In utero and lactational exposure to flame retardants disrupts rat ovarian follicular development and advances puberty. Toxicol Sci. 2020;175(2):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harley KG, Marks AR, Chevrier J, Bradman A, Sjödin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118(5):699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi G, Wang YB, Sundaram R, et al. Polybrominated diphenyl ethers and incident pregnancy loss: the LIFE Study. Environ Res. 2019;168:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fortin CS, Leader A, Mahutte N, et al. Gene expression analysis of follicular cells revealed inflammation as a potential IVF failure cause. J Assist Reprod Genet. 2019;36(6):1195-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96(1):47-52.e2. [DOI] [PubMed] [Google Scholar]
- 28. Borup R, Thuesen LL, Andersen CY, et al. Competence classification of cumulus and granulosa cell transcriptome in embryos matched by morphology and female age. PLoS One. 2016;11(4):e0153562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Björvang RD, Gennings C, Lin PI, et al. Persistent organic pollutants, pre-pregnancy use of combined oral contraceptives, age, and time-to-pregnancy in the SELMA cohort. Environ Health. 2020;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hales B. Data from: Polybrominated diphenyl ethers in human follicular fluid dysregulate mural and cumulus granulosa cell gene expression. Dryad Digital Repository 2020. Deposited September 24, 2020. doi: 10.5061/dryad.nk98sf7rp [DOI] [PMC free article] [PubMed]
- 31. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101-1108. [DOI] [PubMed] [Google Scholar]
- 32. Gironella M, Malicet C, Cano C, et al. p8/nupr1 regulates DNA-repair activity after double-strand gamma irradiation-induced DNA damage. J Cell Physiol. 2009;221(3):594-602. [DOI] [PubMed] [Google Scholar]
- 33. Burnik Papler T, Vrtacnik Bokal E, Maver A, Kopitar AN, Lovrečić L. Transcriptomic analysis and meta-analysis of human granulosa and cumulus cells. PLoS One. 2015;10(8):e0136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grøndahl ML, Andersen CY, Bogstad J, Borgbo T, Boujida VH, Borup R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol Hum Reprod. 2012;18(12):572-584. [DOI] [PubMed] [Google Scholar]
- 35. Kõks S, Velthut A, Sarapik A, et al. The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. Mol Hum Reprod. 2010;16(4):229-240. [DOI] [PubMed] [Google Scholar]
- 36. Cho H, Kehrl JH. Localization of Gi alpha proteins in the centrosomes and at the midbody: implication for their role in cell division. J Cell Biol. 2007;178(2):245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He C, Wei Y, Sun K, et al. Beclin 2 functions in autophagy, degradation of G protein–coupled receptors, and metabolism. Cell. 2013;154(5):1085-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grøndahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2013;100(4):994-1001. [DOI] [PubMed] [Google Scholar]
- 39. Yoo SW, Bolbot T, Koulova A, et al. Complement factors are secreted in human follicular fluid by granulosa cells and are possible oocyte maturation factors. J Obstet Gynaecol Res. 2013;39(2):522-527. [DOI] [PubMed] [Google Scholar]
- 40. Fan X, Bialecka M, Moustakas I, et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat Commun. 2019;10(1):3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab. 2008;19(6):191-196. [DOI] [PubMed] [Google Scholar]
- 42. Poulsen LC, Bøtkjær JA, Østrup O, et al. Two waves of transcriptomic changes in periovulatory human granulosa cells. Hum Reprod. 2020;35(5):1230-1245. [DOI] [PubMed] [Google Scholar]
- 43. Poulsen LC, Englund ALM, Wissing MLM, Yding Andersen C, Borup R, Grøndahl ML. Human granulosa cells function as innate immune cells executing an inflammatory reaction during ovulation: a microarray analysis. Mol Cell Endocrinol. 2019;486:34-46. [DOI] [PubMed] [Google Scholar]
- 44. Jing L, Sun Y, Wang Y, et al. Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats. Chemosphere. 2019;223:675-685. [DOI] [PubMed] [Google Scholar]
- 45. Park HR, Kamau PW, Loch-Caruso R. Involvement of reactive oxygen species in brominated diphenyl ether-47-induced inflammatory cytokine release from human extravillous trophoblasts in vitro. Toxicol Appl Pharmacol. 2014;274(2):283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hennigar SR, Myers JL, Tagliaferro AR. Exposure of alveolar macrophages to polybrominated diphenyl ethers suppresses the release of pro-inflammatory products in vitro. Exp Biol Med (Maywood). 2012;237(4):429-434. [DOI] [PubMed] [Google Scholar]
- 47. Longo V, Longo A, Di Sano C, et al. In vitro exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) impairs innate inflammatory response. Chemosphere. 2019;219:845-854. [DOI] [PubMed] [Google Scholar]
- 48. Peroval MY, Boyd AC, Young JR, Smith AL. A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PLoS One. 2013;8(2):e51243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kitanaka N, Nakano R, Sugiura K, et al. Interleukin-1β promotes interleulin-6 expression via ERK1/2 signaling pathway in canine dermal fibroblasts. PLoS One. 2019;14(7):e0220262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-γ2 (PLCγ2) for Dectin-1–induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol. 2009;39(5):1369-1378. [DOI] [PubMed] [Google Scholar]
- 52. Guarda G, Braun M, Staehli F, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213-223. [DOI] [PubMed] [Google Scholar]
- 53. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10(2):417-426. [DOI] [PubMed] [Google Scholar]
- 54. Gottschall PE, Katsuura G, Arimura A. Interleukin-1 beta is more potent than interleukin-1 alpha in suppressing follicle-stimulating hormone-induced differentiation of ovarian granulosa cells. Biochem Biophys Res Commun. 1989;163(2):764-770. [DOI] [PubMed] [Google Scholar]
- 55. Shan Q, Zheng GH, Han XR, et al. Troxerutin protects kidney tissue against BDE-47–induced inflammatory damage through CXCR4-TXNIP/NLRP3 signaling. Oxid Med Cell Longev. 2018;2018:9865495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhuang J, Wen X, Zhang YQ, et al. TDP-43 upregulation mediated by the NLRP3 inflammasome induces cognitive impairment in 2 2′,4,4′-tetrabromodiphenyl ether (BDE-47)-treated mice. Brain Behav Immun. 2017;65:99-110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gene expression data are available at GEO (Gene Expression Omnibus) accession numbers GSE110916, GSE110917, GSE110918, GSE110919, GSE110921, GSE110922, and GSE110924. All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”