Abstract
Objective
This systematic review aims to evaluate current literature for the prevalence, causes, and effect of low back pain (LBP) in traumatic lower limb amputees, specifically its association with the kinematics and kinetics of the lumbar spine and lower extremities.
Data Sources
Databases (EMBASE, MEDLINE, Scopus, CINAHL, PsycINFO) were searched systematically for eligible studies from inception to January 2018.
Study Selection
The inclusion terms were synonyms of low back pain, lower limb amputation, and trauma, whereas studies involving nontraumatic amputee populations, single cases, or reviews were excluded. 1822 studies were initially identified, of which 44 progressed to full-text reading, and 11 studies were included in the review.
Data Extraction
Two independent reviewers reviewed the included studies, which were evaluated using a quality assessment tool and the Grades of Recommendation, Assessment, Development and Evaluation system for risk of bias, prior to analyzing results and conclusions.
Data Synthesis
There was an LBP prevalence of 52%-64% in traumatic amputees, compared to 48%-77% in the general amputee population (predominantly vascular, tumor, trauma), attributed to a mixture of biomechanical, psychosocial, and personal factors. These factors determined the presence, frequency, and severity of the pain in the amputees, significantly affecting their quality of life. However, little evidence was available on causality.
Conclusion
The high prevalence of LBP in traumatic amputees highlights the necessity to advance research into the underlying mechanics behind LBP, specifically the spinal kinematics and kinetics. This may facilitate improvements in rehabilitation, with the potential to improve quality of life in traumatic amputees.
Keywords: Amputees, Low back pain, Lower limbs, Mechanics, Rehabilitation, Trauma
List of abbreviations: ADIM, abdominal drawing-in maneuver; ADL, activities of daily living; FE, finite element; LBP, low back pain; LLD, limb length discrepancy; PLP, phantom limb pain; ROM, range of motion; TFA, transfemoral amputee; TTA, traumatic transtibial amputee
A traumatic injury is one that is so severe and chaotic that it requires immediate medical treatment1 and can often lead to amputations. Lower limb amputations are of higher prevalence than upper limb2, 3 and of various types, including transfemoral, transtibial, knee disarticulations, and transpelvic.
Lower limb amputation frequently leads to chronic low back pain (LBP) and is considered a secondary disability of amputation.4, 5 People who have experienced an amputation often complain of other pains, such as phantom limb pain (PLP) and residual limb pain. PLP is a pain or sensation relating to a limb or organ that has now been amputated, whereas stump or residual limb pain occurs in the remaining part of the amputated limb.6, 7 There is confusion in the literature about the effect of and difference between PLP and residual limb pain with many articles failing to differentiate between them. However, there is evidence in the research literature that LBP in amputees is more bothersome than any other pain.6
LBP is classified as pain around the lumbar spine (between the thoracic region and the pelvis); it is multidimensional and can be defined by many elements such as pain location, intensity, frequency, and its effect on daily activities.6, 8 Several factors such as physical or biomechanical factors (heavy-lifting work, awkward postures, repetitive straining),6, 9, 10, 11, 12, 13 psychosocial factors (anxiety, depression, job dissatisfaction),12, 13, 14, 15 and personal factors (age, sex, mass, level of amputation, type of prosthesis, amount of daily exercise, smoking)6, 9, 12, 13, 16, 17, 18 have been heavily debated in contributing to LBP among all amputee populations.
Many studies have explored LBP in the general and amputee populations, with the latter presenting a higher LBP prevalence,6, 19 31%-37% in the general population,20, 21 and 47.7%-76.6% for amputees.4, 5, 6, 22, 23, 24, 25, 26, 27, 28 In all these populations, although there appears to be an association between amputation and LBP, the rationale behind this is unclear. This is confounded by the mixed causes, age groups, and clinical presentations of amputees included in past studies. Literature speculates that biomechanics of the lumbar spine influences the frequency and severity of LBP in all populations, but it has been difficult to fully understand why. By analyzing the kinematics and kinetics of amputees during daily activities, which are postulated to be different to that of able-bodied individuals, it will be possible to investigate the potential effect of altered mechanics. A recent systematic review determining the strength of existing research into LBP formulated and rated empirical evidence statements.29 The article highlighted the lack of confidence in the existing research and emphasized the importance of further studies into LBP among amputees, particularly their movement kinematics.29 However, this review was not specific to the role of biomechanics and as such it was unable to draw any conclusions or highlight any evidence in relation to the mechanical aspects of LBP. Conversely, the current review will address this deficit and explore the role of mechanics in the propagation of LBP. Traumatic amputees tend to be younger and fitter than vascular disease or diabetic amputees. Thus, they are more capable of recovering from their injury to a state where they are independently ambulating and achieving high levels of functioning in daily activities.30 Past literature found unilateral traumatic transtibial amputees (TTAs) to have a similar metabolic cost of walking to able-bodied individuals of the same age and mass, and higher gait function (eg, longer stride length and more symmetrical step length) than other TTAs,31 suggesting that traumatic amputees are more capable than the general amputee population. Many achieve high levels of function; thus, the prevalence and characteristics of LBP among amputees may differ in the different amputee populations.
Understanding the propagators and potential mechanisms of LBP specific to traumatic amputees has the potential to inform care and management and the longer-term rehabilitation process. Currently, there appears to be limited information on functional kinematics and kinetics in relation to amputees with and without LBP. This study aimed to systematically review the literature for the prevalence, mechanical etiologies, and consequences of LBP in all presentations of lower extremity traumatic amputees—unilateral, bilateral, transfemoral, transtibial, and knee disarticulation.
Methods
Eligibility criteria
All the following types of traumatic lower limb amputation apart from transpelvic were included: unilateral, bilateral, transfemoral amputees (TFAs), TTAs, and through-knee amputees. For inclusion each study must have assessed LBP and obtained physical measurements of daily tasks via a biomechanical assessment, an objective marker/measure, or an imaging modality. Mixed amputee populations (eg, trauma, vascular, tumor amputation groups) were not accepted as part of this study, nor were single-case studies or review articles. There was no language, publication date, or status limitations imposed on the eligibility.
Search
The search for this review was conducted, from inception to January 9, 2018, over the following databases: EMBASE (since 1947), MEDLINE (since 1946), Scopus, CINAHL, and PsycINFO (since 1806). The search criteria were combinations of synonyms of low back pain, lower limb amputation, and trauma. An exemplar search strategy is given in appendix 1. Potential additional records were found by reviewing references of the included articles, prior to full-text screening.
Study selection
Duplicates were removed from the identified articles and 2 independent reviewers reviewed all the titles and abstracts for inclusion. The full-text articles were independently assessed by 2 reviewers, and tables were completed by both reviewers highlighting articles for exclusion with reasoning. The tables were compared and any discrepancies were discussed to finalize studies for inclusion in the systematic review.
Assessment of risk of bias and quality of evidence
The National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used as a basis for assessing risk of bias and procedural quality of the included articles.32 As with the study selection process, 2 reviewers independently assessed the articles, and a unanimous agreement was reached. The quality of evidence was assessed based on the Grades of Recommendation, Assessment, Development and Evaluation with quality categorized into high, moderate, low, and very low.33
Results
Study selection
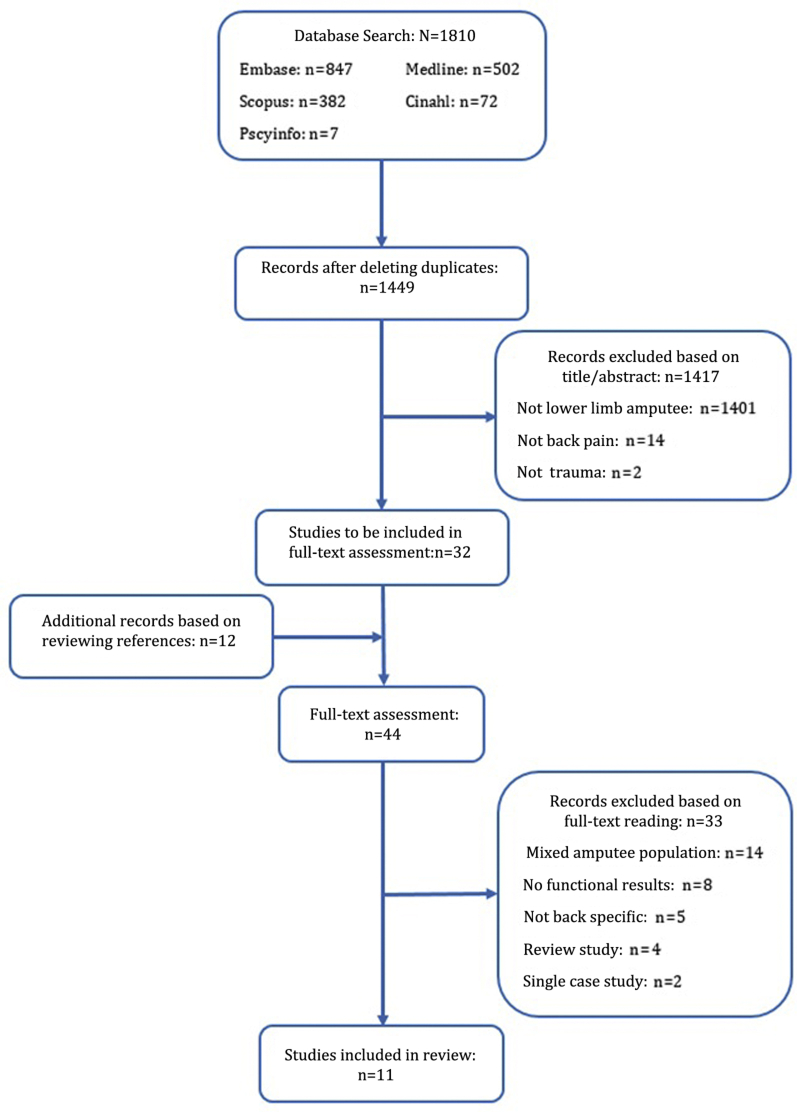
The search yielded 1810 articles. Of these, 361 articles were removed as duplicates; the remaining 1449 records were screened based on their title and abstract, producing a list of 32 full-text articles for inclusion. The references of these articles were reviewed revealing a further 12 articles for inclusion, which resulted in a total of 44 articles progressing to full-text assessment. Upon reading the full texts, 33 articles did not meet the inclusion criteria, resulting in 11 articles being reviewed (fig 1).
Fig 1.
PRISMA flow diagram showing figures for database search, screening, full-text articles, and included studies, with reasons for exclusion at each stage.
Main characteristics of included studies
Table 1 details the main characteristics of the included studies for ease of comparison; the title, aims, participants, and methods used are listed. The studies used questionnaires, biomechanical testing, imaging, or modeling to better understand the prevalence, the mechanics, or the consequences of LBP. The studies using questionnaires were cross-sectional on larger cohorts4, 23, 28 while those with physical testing were on small cohort populations,34, 35, 36, 37, 38, 39 with a mix of demographics and biases. Of note, 94.4% of the all participants were men, and all participants wore their prosthetics during physical tests.
Table 1.
Main characteristics of included studies
| Article | Aims | Participants | Protocol |
|---|---|---|---|
| Ashraf et al28 |
|
Group
|
Design
|
| Devan et al4 |
|
Group
|
Design
|
| Hendershot and Wolf34 |
|
Group
|
Design
|
| Hendershot and Wolf35 |
|
Group
|
Design
|
| Hendershot and Wolf36 |
|
Group
|
Design
|
| Hendershot et al37 |
|
Group
|
Design
|
| Kulkarni et al22 |
|
Group
|
Design
|
| Rahimi et al23 |
|
Group
|
Design
|
| Russell Esposito and Wilken38 |
|
Group
|
Design
|
| Shojaei et al39 |
|
Group
|
Design
|
| Springer and Gill40 |
|
Group
|
Design
|
Abbreviations: HRQoL, health-related quality of life; ML, mediolateral; MRI, magnetic resonance imaging; PA, physical activity; SF-36, Medical Outcomes Study 36-Item Short-Form Health Survey; TKA, through-knee amputee.
Assessment of risk of bias and quality of evidence
Figure 2 examines the quality of the evidence used in the included studies, focusing on the risk of bias. All the studies were cross-sectional and/or cohort studies. None of the studies justified their sample size, implying a potential risk of bias in the choice of sample population. Furthermore, only half of the studies used an able-bodied control group as a comparator.34, 35, 36, 37, 38, 39 When reviewing potential confounders in fig 2, it can be seen that only Devan4 and Kulkarni22 and colleagues took external factors into account.
Fig 2.
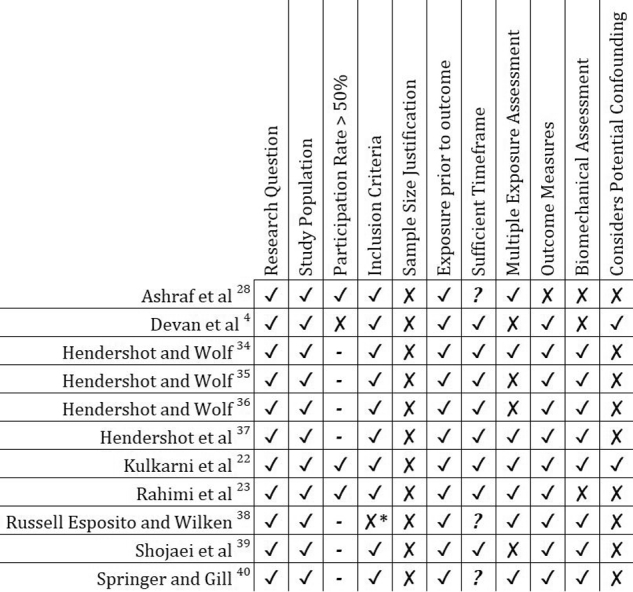
Risk of bias assessment on included studies, based on table 1. ✓ Fulfils criteria. ✗ Does not fulfil criteria. ? Not mentioned in study. – Not applicable. ∗Inclusion or exclusion criteria are not clear (only mention is of unilateral, traumatic amputees).
Despite these shortfalls, it appears reporting of LBP prevalence (52.8-64%)4, 22, 23, 28 is consistent across articles. The participation rates vary across studies, with one recruiting less than 50% of the participants approached4 and many omitting participation rate details,34, 35, 36, 37, 38, 39 again creating a potential bias.
Studies investigating the prevalence of LBP
Many of the included studies4, 22, 23, 28, 38 used questionnaires or interviews to assess the relation between LBP and amputation. In most cases, these questionnaires served as an initial assessment, obtaining subject-specific information, and were followed by technical assessments. From the initial assessment questionnaires, the researchers found an LBP prevalence between 52.8% and 64%4, 22, 23, 28; however, this was reported over different time frames (ranging from 4wk to 6mo). The large population sizes and participation rates exceeding 50% in most of the studies using questionnaires suggest a small possibility of error, indicating moderate to high confidence that these prevalence rates are representative.
Studies investigating the mechanical or anatomical etiologies of LBP
Motion analysis
Five studies22, 34, 36, 37, 38 performed motion analysis on their amputee cohort. A further 235,39 performed additional analysis using data collected in a study by Hendershot and Wolf.34 Table 2 briefly describes the focus of the motion analysis in each of the 5 studies. All the studies did multiple trials of each task.
Table 2.
Details of motion analysis studies
| Article | Gait Analysis Focus | Gait Analysis Specifics | Other Experimental Focuses | Other Experimental Specifics |
|---|---|---|---|---|
| Hendershot and Wolf34 | Joint reaction forces and moments at lower back |
|
___ | ___ |
| Hendershot and Wolf36 | ___ | ___ | Sit-to-stand: General lumbosacral joint kinematics | Measured GRFs at amputees’ feet and bottom |
| Hendershot et al37 | Effect of walking speed on spinal loads |
|
___ | ___ |
| Kulkarni et al22 | Potential causes of LBP |
|
Static Balance Test: With and without vision | Measured GRFs for each limb |
| Russell Esposito and Wilken38 | Pelvic-trunk coordination |
|
___ | ___ |
Abbreviation: GRF, ground reaction force.
From the gait data, Russell Esposito and Wilken38 were able to determine the continuous relative phase between the trunk and pelvis during the gait cycle at each walking speed, to compare coordination, and highlight the effect of LBP on gait. The study showed overall coordination variability was not significantly affected by amputation, LBP, or speed. Amputees were able to maintain pelvis-trunk coordination by modifying their movement in the frontal and sagittal planes, while there were no significant differences in the transverse plane. Similar results were found by Hendershot and Wolf34 regarding the trunk-pelvic motion in the 3 planes.
Hendershot et al37 used the motion data to calculate segment angles and center of mass positions to input into their finite element (FE) model and to determine ground reaction forces for their biomechanical models.34, 36 The models were used to estimate resultant spinal loads, moments, and powers, which increased with increasing speed and increasing level of amputation, although not significantly. Kulkarni et al22 were able to use the force-plate readings and calculate center of pressure displacements, which were greater in the LBP group than in the LBP-free group.
To summarize the self-selected speed findings, the LBP-free group was faster than those with LBP22 and able-bodied participants were faster than the amputees.37 Overall, it was found that the trunk-pelvic range of motion (ROM) was larger in amputees than able-bodied participants. At higher speeds, the trunk motion tends to become larger or faster, and the resultant response of trunk musculature also increases. As walking speed increased, the ROM increased significantly in the frontal and transverse planes in the able-bodied, TFA, and TTA groups. However, in the sagittal plane there was only a significant increase with speed in the TFA group.37 There was a similar finding by Russell Esposito and Wilken38 in that the ROM in the sagittal plane was not as large as in the other 2 planes, but was higher in the TFA-LBP group than in the able-bodied participants.
Hendershot and Wolf34, 35, 36 highlighted the increased use of the trunk and its asymmetrical motion in amputees during simple daily tasks, like walking, standing up, and sitting down. Kulkarni22 reported ground reaction force to be higher on the intact limb in the unilateral participants and also higher in the LBP group. The center of pressure displacements during static standing tasks were greater in the LBP group and also significantly higher when vision was occluded.22 In unilateral participants, Hendershot and Wolf34 characterized the amputated limb as having longer step lengths, shorter stance durations, and longer swing durations.
From the motion analysis data presented, none of the studies were able to understand the effect of these changes noted on spinal loading and thus the relevance with respect to potential mechanisms of LBP is unclear. As a result, the quality of the evidence of the motion analysis studies can be deemed as low.
Modeling
Hendershot34, 35, 36, 37 and Shojaei39 and colleagues used the motion data to calculate forces and moments on the lower back. Using a FE model, Hendershot et al37 found both peak global and local muscle forces around the lower back increased with increasing walking speed. More specifically, from 1.0 to 1.4 m/s, local trunk muscle forces increased by 18% in the control group, 26% in the TTA group, and 36% in TFA group. It is also important to note that with increasing speed, anterior-posterior shear and compression loads also increased more in the amputee groups (∼81% and ∼31%) than in the able-bodied group (∼44% and ∼22%). In the mediolateral shear direction, loads increased by ∼66% in the control and TTA groups, but only by 22% in the TFA group, contrary to the pattern noticed in the other forces. The study concluded that as walking speeds in amputees increased, the more their movement pattern altered, leading to a larger whole-body angular momentum. Usually, legs provide stability against this momentum, but in amputees, the trunk has to increase its contribution, leading to lower back injuries.37
Russell Esposito and Wilken38 supported this in that their amputees demonstrated increased rigidity in some planes of motion in an attempt to stabilize their center of mass. The alterations reported by these 2 studies are also confirmed by Kulkarni et al,22 who state continually adjusting posture to maintain balance results in postural muscle asymmetries leading to LBP. Shojaei et al39 who used an FE model found there was asymmetrical and larger trunk motion in amputees compared to able-bodied participants resulting in increases in local and global trunk muscle forces. This is supported by the findings of Hendershot and Wolf,34 where the peak forces and moments in the lateral, anterior-posterior, and vertical directions of the prosthetic limb were greater in amputees than able-bodied participants, and greater the more proximal the amputation was, leading to substantial increases in spinal loads.
Hendershot and Wolf34, 35, 36 used a 3-dimensional 15-segment biomechanical model to calculate angles, intersegmental forces, moments, and powers at the L5/S1 joint. Joint power is an estimate for the power (ie, rate of work done) and thus represents the flow of energy at the joint; it can be used to describe segmental motion.35, 41 Hendershot and Wolf35 found the total energy generated at the L5/S1 joint during the gait cycles to be 3 times greater among amputees than in able-bodied participants. The moments and powers were not only higher during gait, but also during standing up and sitting down.36 All of these articles showed increased trunk flexion toward the prosthetic limb; individuals alter their motion to maintain stability.37, 38 The models showed the intersegmental forces and moments at the joint to be larger in the amputees compared to able-bodied participants (eg, while walking at 1.4 m/s, the peak compression force is 27.8 N/kg vs 23.5 N/kg),37 which creates a compelling argument towards the origin of the LBP. However, Shojaei39 found the spinal loads to be approximately only 2 times the body weight, and, as such, attributes LBP more to the repetitive loading created during walking.
Generally, the studies are in agreement with each other and there are some findings that strongly direct us into recognizing potential causes of LBP. However, the small cohort sizes express the lack of confidence, indicating only a low level of evidence of the role of intersegmental forces and moments in the propagation of LBP.
Imaging
Two articles used imaging in their research; Kulkarni et al22 used magnetic resonance imaging, whereas Springer and Gill40 used ultrasound imaging. The first imaged the intervertebral discs to investigate and compare how the disc pathology, the facet joint degeneration, and the osteophyte formation changed between participants with and without LBP. The magnetic resonance imaging scans showed little difference in disc pathology and between magnetic resonance degenerative scores of TFA and TTA participants.22 The second article was more specific in their imaging; it compared side-to-side lateral abdominal muscle thicknesses at baseline and during a procedure known as the abdominal drawing-in maneuver (ADIM) using ultrasound. The results showed that the side-to-side symmetry of lateral abdominal muscle thickness at baseline and during ADIM in amputees is comparable to able-bodied participants.40 Both studies found that in the unilateral TFA participants, there was hypertrophy in the psoas muscles on the intact limb side.22, 40 The 2 methods are not comparable and although it is important to note the reasons for imaging and the findings from both the articles, the confidence in the evidence is low.
Studies investigating the consequences of LBP and its effect on daily living
The questionnaires primarily registered the etiology and time of the amputation, along with the presence, frequency, and severity of pain. Ashraf et al28 also collected information on education level, marital and employment status, because these are perceived to alter the psychosocial perception of pain,14, 15, 42 but the information was not used in their analysis.
Rahimi23 and Ashraf28 and colleagues documented frequency of LBP in the last 6 months, whereas Devan et al4 and Russell Esposito and Wilken38 requested frequency in the past 4 weeks. However, Kulkarni et al22 did not provide a timeframe for the occurrence of the LBP. With relation to the location of LBP, only Ashraf28 described where LBP occurred by means of a schematic diagram. To measure severity, Rahimi23 used a subjective severe or not severe; Devan4 and Kulkarni22 used a visual analog pain score. Some studies22, 38 measured limb lengths or limb length discrepancies (LLDs).
The questionnaires show 28%-39% of the participants reported severe interference with activities of daily living (ADLs),4, 22, 28 and of those who reported LBP, 38.7% stated it had been more than 3 years since they experienced a pain-free month.4 One study showed 32.9% of their participants experienced pain in more than 1 location,28 with another stating LBP and PLP were often reported together.23 This compliments Kulkarni’s22 conclusion that those with severe LBP are more likely to report PLP.
Devan,4 Rahimi,23 and Ashraf28 used questionnaires as their main source of information. Rahimi23 used the Medical Outcomes Study 36-Item Short-Form Health Survey, a validated questionnaire for investigating the health-related quality of life in participants. Ashraf28 obtained subjective ratings of their participants’ dependency in a variety of tasks, and Devan4 scored participants using the Physical Activity Scale for Individuals with Physical Disabilities.
The general consensus from the questionnaires is that LBP is a significant contributor to low quality of life,4, 23, 28 but more than 61% of the participants remained independent and were able to continue without any major restrictions to their ADLs.4, 22, 28 Although the findings of these different studies appear consistent, methodological limitations and shortfalls, such as the omission of key confounding factors, limit the validity of the evidence and interpretation of the findings.
Discussion
This review attempted to determine potential causative factors for LBP in traumatic lower limb amputees, with a focus on the biomechanics of the spine and lower limb. Although the included studies have explored the causes of LBP in amputees, most of them have been of low quality, with a number of limitations. A major limitation is the relatively small trial numbers in the physical studies, suggesting the results may not be representative of this population. Furthermore, the lack of sample size justification is perhaps an indication of the size of the available population leading to the researchers using samples of convenience. This is accompanied by a limited number of studies comparing the amputee cohort with an able-bodied control group making it difficult to draw conclusions regarding amputee health. Most studies included appropriate personal and background information on the amputees, but many failed to document known factors associated with a higher risk of LBP. For example, it is known that being overweight influences mobility and is associated with LBP,13, 43 but few studies reported weight or body mass index. Similarly, smoking is a known LBP risk factor,13 but has not been considered. This review has highlighted several areas that require attention in future research which will be discussed below.
Although the findings of this review have identified a clear association between lower limb amputation and LBP, the mechanisms for this association remain unclear. This is similar to the outcomes from studies with mixed amputee populations. The literature found the main contributors to LBP were uneven posture and asymmetric movements of the lumbopelvic region, along with fatigue during functional activities.12, 44 Although this indicates that the biomechanics has a big impact on LBP, the rationale for it has not been identified. The traumatic amputee literature notes the multifaceted nature of LBP amongst amputees.8, 22, 23, 28 Kulkarni et al22 noted that severe LBP participants were more likely to suffer from PLP; others have interpreted this differently, inferring that the presence of PLP was a significant predictor of LBP.42 This stresses the importance of identifying the body regions in which the amputees experience the pain. Only one of the included studies described the locations of the bodily pains.28 In addition, few specified either the frequency, severity, or intensity of pain and failed to use validated pain scales. Kulkarni22 did not mention a timeframe for pain, thus they could have been reporting past, recent, or current pain.
The prevalence of LBP found in the included studies (52-64%)4, 22, 23, 28 was not different from that of the mixed population amputees (47.7%-76.6%).4, 5, 6, 22, 23, 24, 25, 26, 27, 28 The trauma-only amputees had a smaller range of prevalence, and were also toward the lower end of the spectrum, perhaps because they are generally a younger cohort of participants. However, as mentioned above, the uncertainty in these figures is that the researchers did not clearly define LBP limiting generalizability of findings.
Past literature has reported links between the use of prostheses and the prevalence of LBP.27, 45 This suggests that traumatic amputees being younger are therefore more active, and use their prostheses more, so are more prone to LBP in the future, although they may not have experienced it yet. There is a need to understand activity levels, time spent being active on the prostheses, and number of years of prosthetic use with LBP prevalence. There is a theory that the more proximal the amputation, the more pain the participant experiences,6, 25, 43, 46 but we are unable to confirm this in our review of the published traumatic cohort literature. Hendershot’s37 results indicate that the more proximal the amputation, the greater the increase in local muscle force magnitudes, but the small cohort size limits our confidence in the statement and as such further research is required.
Hendershot et al37 and Russell Esposito and Wilken38 found that the spinal loads and trunk-pelvic discoordination increase as walking speed increases, but the cause of these increases requires further investigation. Walking is a highly repetitive task,37 and repeated exposure to these larger alterations can elevate the risk of LBP34, 37; the studies suggest LBP in amputees is due to fatigue. Shojaei et al39 found estimated spinal loads during walking to be twice the body weight. Tissue failure thresholds can be significantly lower for high cycles of loading than for single loading.47 Since the failure threshold for vertebral endplates is approximately 5 kN, which is approximately 6 times the body weight,48 this would suggest that perhaps their LBP is due to loading fatigue.
The gait analyses show that there is a difference in results between the different levels of amputations. Literature states LBP is a result of altered gait and altered movement patterns due to amputation.5, 6, 11, 22, 49, 50, 51 For example, there are theories suggesting TFA participants have increased pelvic tilt to compensate for reduced hip extension,38 which in turn has been linked with LBP.12, 52, 53, 54 Few studies used the gait data to model the body and calculate spinal kinematics. Although they show increases in the intersegmental forces, moments, and powers, the mechanical reasoning is not understood. Furthermore, the studies that investigated the changed mechanics of the amputees did not report LBP prevalence,34, 35, 36, 37, 38, 39 so it is difficult to draw mechanistic conclusions relating the altered gait to LBP. By linking spinal kinematic data and information on LBP presence in various ADLs, it will be possible to come to a better conclusion regarding the effect of altered movement patterns, and which movements most influence LBP.
Kulkarni et al22 found greater center of pressure displacements in those with LBP and in those that had their vision occluded; both relate to limited balance. Balance has been identified as a deficiency in amputees and influences much of the varied motion patterns witnessed in amputees.55, 56, 57, 58, 59, 60, 61 Literature suggests the visual sensory system has a high effect on balance and sway55, 56, 62, 63, 64, 65, 66; because amputees have loss of ankle function, their balance could be compromised.59 Although it is known that the constant perturbations, pelvic tilt, and LBP are connected, the included articles have not explored the connection between balance and LBP.
Imaging is the only means possible to investigate the underlying anatomical structures of the participants, yet only 2 studies employed imaging. In these studies, imaging was focused on specific areas of the lower back. Kulkarni22 looked at the intervertebral disc; literature suggests that lateral bending, perhaps due to LLD or pelvic tilt, can compress the disc and cause it to bulge.67, 68 Springer and Gill40 studied the lateral abdominal muscles during ADIM; ADIM is a basic exercise for lumbar stability, which thickens the transverse abdominis to maintain side-to-side symmetry,40 which has been shown to help minimize LBP.69 In both cases, no differences in muscle anatomy were found between amputees and able-bodied participants. Past literature has shown a correlation between LBP and age-related disc degeneration.70, 71 However, the traumatic amputees in the studies cited are generally young at point of wounding which might explain why there is little difference in disc pathology. Both studies identified hypertrophy in the psoas muscles on the intact side of unilateral amputees. This hypertrophy could lead to an anteriorly rotated pelvis and loss of hip extension with associated implications on spinal posture and loading. Further to this, it would be useful to explore differences in the musculature structure and quality in terms of strength, flexibility, and endurance between able-bodied participants and amputees. Anatomical differences between the different groups would imply that customized rehabilitation techniques must be used on each group to counteract the effect of the altered gait patterns.
The included studies and literature clarify that many factors influence LBP, including psychosocial determinants (eg, job dissatisfaction, anxiety, depression), LLD, mass, age, and level of amputation, all of which are unique to each individual. However, it has not been possible to identify core causative mechanisms, and few studies have proceeded to determine spinal loading in the different amputee types. Mathematical modeling such as FE modeling or inter-segmental modeling is a potential method to understand the mechanical implications on LBP. To date, there is limited research in this area relating to traumatic amputees.
It has been difficult to identify potential causes or factors that contribute to high levels of LBP in traumatic amputees, in part due to poor quality of current literature. Lower limb amputations are common during military or political conflicts, often caused by blast from improvised explosive devices.72, 73 These can be on the battlefield with military personnel, in events such as the Boston marathon bombing in April 2013 where athletes were injured, or in recent episodes of terrorist activities such as the bombings across Sri Lanka in April 2019. The commonality of these events is that often many young and healthy individuals have been hurt. To study this cohort of amputees, military cohorts provide a unique opportunity due to their accessibility and commonality in fitness baseline. Currently, it is particularly difficult to draw conclusions on the traumatic cohorts as most past studies have not differentiated between military trauma from blasts and civilian trauma, such as road traffic accidents. Military amputees are usually fitter and stronger pretrauma than those in the general population who sustain an amputation after trauma.4, 42, 74, 75, 76, 77 Furthermore, the nature of their injury has frequently arisen from blast exposure and as such they may present with other injuries and issues beyond their amputations.78 It is understood that the military cohorts require more complex surgical interventions, frequently more than 1, to stabilize their amputation.73 As a result, rehabilitation post military amputation is more focused and individual than in the general population,73 and amputees have access to higher performing prostheses.79 To fully understand the mechanisms, there is a need for a better methodological study that compares the different amputee groups (TFA/TTA/able-body/LBP/LBP-free/unilateral/bilateral) systemically in terms of personal factors influencing LBP, their anatomical structures, and their biomechanical movements in various conditions.
A potential limitation of this systematic review is that it focuses solely on traumatic amputees and not a mixed population. However, the review found that a number of psycho-social, physiological, and biomechanical factors influence the reporting of LBP in traumatic amputees contributing to 53%-64% prevalence rate. This prevalence rate is lower than that reported in mixed amputee populations, supporting the hypothesis that traumatic amputees are different from other types of amputees, and need to be considered separately.
In summary, LBP in traumatic amputees appears to be multidimensional and our understanding of causative factors is limited. With such a high prevalence of LBP in amputees, there is a necessity to further our understanding so that strategies to manage and prevent LBP, and improve amputee quality of life can be developed.
Acknowledgments
This work was conducted under the auspices of the Royal British Legion Centre for Blast Injury Studies at Imperial College London. We thank the Royal British Legion for financial support.
Footnotes
Disclosures: none
Supplementary Data
References
- 1.University of Florida Health Traumatic injury. https://ufhealth.org/traumatic-injury Available at:
- 2.Ebrahimzadeh M.H., Moradi A., Bozorgnia S., Hallaj-Moghaddam M. Evaluation of disabilities and activities of daily living of war-related bilateral lower extremity amputees. Prosthet Orthot Int. 2016;40:51–57. doi: 10.1177/0309364614547410. [DOI] [PubMed] [Google Scholar]
- 3.Edwards D.S., Phillip R.D., Bosanquet N., Bull A.M., Clasper J.C. What is the magnitude and long-term economic cost of care of the british military Afghanistan amputee cohort? Clin Orthop Relat Res. 2015;473:2848–2855. doi: 10.1007/s11999-015-4250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devan H., Tumilty S., Smith C. Physical activity and lower-back pain in persons with traumatic transfemoral amputation: a national cross-sectional survey. J Rehabil Res Dev. 2012;49:1457–1466. doi: 10.1682/jrrd.2011.09.0155. [DOI] [PubMed] [Google Scholar]
- 5.Ehde D.M., Smith D.G., Czerniecki J.M., Campbell K.M., Malchow D.M., Robinson L.R. Back pain as a secondary disability in persons with lower limb amputations. Arch Phys Med Rehabil. 2001;82:731–734. doi: 10.1053/apmr.2001.21962. [DOI] [PubMed] [Google Scholar]
- 6.Smith D.G., Ehde D.M., Legro M.W., Reiber G.E., del Aguila M., Boone D.A. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;(361):29–38. doi: 10.1097/00003086-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Davis R. Phantom sensation, phantom pain, and stump pain. Arch Phys Med Rehabil. 1993;74:79–91. [PubMed] [Google Scholar]
- 8.Jensen M.P., Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk D.C., Melzack R., editors. Handbook of pain assessment. Guilford Press; New York: 1992. pp. 135–151. [Google Scholar]
- 9.Ebrahimzadeh M.H., Rajabi M.T. Long-term outcomes of patients undergoing war-related amputations of the foot and ankle. J Foot Ankle Surg. 2007;46:429–433. doi: 10.1053/j.jfas.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Gaunaurd I., Gailey R., Hafner B.J., Gomez-Marin O., Kirk-Sanchez N. Postural asymmetries in transfemoral amputees. Prosthet Orthot Int. 2011;35:171–180. doi: 10.1177/0309364611407676. [DOI] [PubMed] [Google Scholar]
- 11.Devan H., Carman A., Hendrick P., Hale L., Ribeiro D.C. Spinal, pelvic, and hip movement asymmetries in people with lower-limb amputation: systematic review. J Rehabil Res Dev. 2015;52:1–19. doi: 10.1682/JRRD.2014.05.0135. [DOI] [PubMed] [Google Scholar]
- 12.Devan H., Hendrick P., Ribeiro D.C., Hale L.A., Carman A. Asymmetrical movements of the lumbopelvic region: is this a potential mechanism for low back pain in people with lower limb amputation? Med Hypotheses. 2014;82:77–85. doi: 10.1016/j.mehy.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 13.da Costa B.R., Vieira E.R. Risk factors for work-related musculoskeletal disorders: A systematic review of recent longitudinal studies. Am J Ind Med. 2010;53:285–323. doi: 10.1002/ajim.20750. [DOI] [PubMed] [Google Scholar]
- 14.Hoogendoorn W.E., van Poppel M.N., Bongers P.M., Koes B.W., Bouter L.M. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine (Phila Pa 1976) 2000;25:2114–2125. doi: 10.1097/00007632-200008150-00017. [DOI] [PubMed] [Google Scholar]
- 15.Pincus T., Burton A.K., Vogel S., Field A.P. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109–E120. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Dionne C.E., Dunn K.M., Croft P.R. Does back pain prevalence really decrease with increasing age? A systematic review. Age Ageing. 2006;35:229–234. doi: 10.1093/ageing/afj055. [DOI] [PubMed] [Google Scholar]
- 17.Deyo R.A., Bass J.E. Lifestyle and low-back pain. The influence of smoking and obesity. Spine (Phila Pa 1976) 1989;14:501–506. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Smith B.H., Elliott A.M., Hannaford P.C., Chambers W.A., Smith W.C. Factors related to the onset and persistence of chronic back pain in the community: results from a general population follow-up study. Spine (Phila Pa 1976) 2004;29:1032–1040. doi: 10.1097/00007632-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 19.Burke M.J., Roman V., Wright V. Bone and joint changes in lower limb amputees. Ann Rheum Dis. 1978;37:252–254. doi: 10.1136/ard.37.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papageorgiou A.C., Croft P.R., Ferry S., Jayson M.I., Silman A.J. Estimating the prevalence of low back pain in the general population. Evidence from the South Manchester Back Pain Survey. Spine (Phila Pa 1976) 1995;20:1889–1894. doi: 10.1097/00007632-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hoy D., Bain C., Williams G. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni J., Gaine W.J., Buckley J.G., Rankine J.J., Adams J. Chronic low back pain in traumatic lower limb amputees. Clin Rehabil. 2005;19:81–86. doi: 10.1191/0269215505cr819oa. [DOI] [PubMed] [Google Scholar]
- 23.Rahimi A., Mousavi B., Soroush M., Masumi M., Montazeri A. Pain and health-related quality of life in war veterans with bilateral lower limb amputations. Trauma Mon. 2012;17:282–286. doi: 10.5812/traumamon.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ephraim P.L., Wegener S.T., MacKenzie E.J., Dillingham T.R., Pezzin L.E. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–1919. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Taghipour H., Moharamzad Y., Mafi A.R. Quality of life among veterans with war-related unilateral lower extremity amputation: a long-term survey in a prosthesis center in Iran. J Orthop Trauma. 2009;23:525–530. doi: 10.1097/BOT.0b013e3181a10241. [DOI] [PubMed] [Google Scholar]
- 26.Smith E., Comiskey C., Ryall N. Prevalence and patterns of back pain and residual limb pain in lower limb amputees at the National Rehabilitation Hospital. Ir J Med Sci. 2008;177:53–57. doi: 10.1007/s11845-007-0111-1. [DOI] [PubMed] [Google Scholar]
- 27.Gailey R., Allen K., Castles J., Kucharik J., Roeder M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev. 2008;45:15–29. doi: 10.1682/jrrd.2006.11.0147. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf A., Shojaee H., Mousavi B. Impact of pain in vertebral column on activities of daily living in the Iranian amputees with bilateral lower limb amputation. Disabil Rehabil. 2012;34:869–872. doi: 10.3109/09638288.2011.623756. [DOI] [PubMed] [Google Scholar]
- 29.Highsmith M.J., Goff L.M., Lewandowski A.L. Low back pain in persons with lower extremity amputation: a systematic review of the literature. Spine J. 2019;19:552–563. doi: 10.1016/j.spinee.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Hammarlund C.S., Carlstrom M., Melchior R., Persson B.M. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: a comparison across three levels of amputation. Prosthet Orthot Int. 2011;35:97–105. doi: 10.1177/0309364610389357. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis H.L., Bennett A.N., Twiste M., Phillip R.D., Etherington J., Baker R. Temporal spatial and metabolic measures of walking in highly functional individuals with lower limb amputations. Arch Phys Med Rehabil. 2017;98:1389–1399. doi: 10.1016/j.apmr.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 32.National Heart, Lung, and Blood Institute Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Available at:
- 33.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendershot B.D., Wolf E.J. Three-dimensional joint reaction forces and moments at the low back during over-ground walking in persons with unilateral lower-extremity amputation. Clin Biomech (Bristol, Avon) 2014;29:235–242. doi: 10.1016/j.clinbiomech.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Hendershot B.D., Wolf E.J. Mediolateral joint powers at the low back among persons with unilateral transfemoral amputation. Arch Phys Med Rehabil. 2015;96:154–157. doi: 10.1016/j.apmr.2014.07.402. [DOI] [PubMed] [Google Scholar]
- 36.Hendershot B.D., Wolf E.J. Persons with unilateral transfemoral amputation have altered lumbosacral kinetics during sitting and standing movements. Gait Posture. 2015;42:204–209. doi: 10.1016/j.gaitpost.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Hendershot B.D., Shojaei I., Acasio J.C., Dearth C.L., Bazrgari B. Walking speed differentially alters spinal loads in persons with traumatic lower limb amputation. J Biomech. 2017;28:28. doi: 10.1016/j.jbiomech.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell Esposito E., Wilken J.M. The relationship between pelvis-trunk coordination and low back pain in individuals with transfemoral amputations. Gait Posture. 2014;40:640–646. doi: 10.1016/j.gaitpost.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Shojaei I., Hendershot B.D., Wolf E.J., Bazrgari B. Persons with unilateral transfemoral amputation experience larger spinal loads during level-ground walking compared to able-bodied individuals. Clin Biomech (Bristol, Avon) 2016;32:157–163. doi: 10.1016/j.clinbiomech.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springer B.A., Gill N.W. Characterization of lateral abdominal muscle thickness in persons with lower extremity amputations. J Orthop Sports Phys Ther. 2007;37:635–643. doi: 10.2519/jospt.2007.2532. [DOI] [PubMed] [Google Scholar]
- 41.Robertson D.G., Winter D.A. Mechanical energy generation, absorption and transfer amongst segments during walking. J Biomech. 1980;13:845–854. doi: 10.1016/0021-9290(80)90172-4. [DOI] [PubMed] [Google Scholar]
- 42.Devan H., Hendrick P., Hale L., Carman A., Dillon M.P., Ribeiro D.C. Exploring factors influencing low back pain in people with nondysvascular lower limb amputation: a national survey. PM R. 2017;9:949–959. doi: 10.1016/j.pmrj.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Ebrahimzadeh M.H., Kachooei A.R., Soroush M.R., Hasankhani E.G., Razi S., Birjandinejad A. Long-term clinical outcomes of war-related hip disarticulation and transpelvic amputation. J Bone Joint Surg Am. 2013;95(1-6):e114. doi: 10.2106/JBJS.L.01160. [DOI] [PubMed] [Google Scholar]
- 44.Devan H., Carman A.B., Hendrick P.A., Ribeiro D.C., Hale L.A. Perceptions of low back pain in people with lower limb amputation: a focus group study. Disabil Rehabil. 2015;37:873–883. doi: 10.3109/09638288.2014.946158. [DOI] [PubMed] [Google Scholar]
- 45.Tranberg R., Zügner R., Kärrholm J. Improvements in hip- and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait Posture. 2011;33:165–168. doi: 10.1016/j.gaitpost.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Friel K., Domholdt E., Smith D.G. Physical and functional measures related to low back pain in individuals with lower-limb amputation: an exploratory pilot study. J Rehabil Res Dev. 2005;42:155–166. doi: 10.1682/jrrd.2004.08.0090. [DOI] [PubMed] [Google Scholar]
- 47.efunda High-cycle fatigue. http://www.efunda.com/formulae/solid_mechanics/fatigue/fatigue_highcycle.cfm Available at:
- 48.Adams M.A., Burton A.K., Dolan P., Bodgduk N. Churchill Livingstone; London: 2007. The biomechanics of back pain. [Google Scholar]
- 49.Skinner H.B., Effeney D.J. Gait analysis in amputees. Am J Phys Med. 1985;64:82–89. [PubMed] [Google Scholar]
- 50.Kelly K.M., Doyle W., Skinner H.B. The relationship between gait parameters and pain in persons with transtibial amputation: a preliminary report. J Rehabil Res Dev. 1998;35:231–237. [PubMed] [Google Scholar]
- 51.Hendershot B.D., Nussbaum M.A. Persons with lower-limb amputation have impaired trunk postural control while maintaining seated balance. Gait Posture. 2013;38:438–442. doi: 10.1016/j.gaitpost.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Devan H., Dillon M.P., Carman A.B. Spinal and pelvic kinematics during gait in people with lower-limb amputation, with and without low back pain: an exploratory study. J Prosth Orthot. 2017;29:121–129. [Google Scholar]
- 53.Fatone S., Stine R., Gottipati P., Dillon M. Pelvic and spinal motion during walking in persons with transfemoral amputation with and without low back pain. Am J Phys Med Rehabil. 2016;95:438–447. doi: 10.1097/PHM.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 54.Rabuffetti M., Recalcati M., Ferrarin M. Trans-femoral amputee gait: socket-pelvis constraints and compensation strategies. Prosthet Orthot Int. 2005;29:183–192. doi: 10.1080/03093640500217182. [DOI] [PubMed] [Google Scholar]
- 55.Ku P.X., Abu Osman N.A., Wan Abas W.A. Balance control in lower extremity amputees during quiet standing: a systematic review. Gait Posture. 2014;39:672–682. doi: 10.1016/j.gaitpost.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Buckley J.G., O'Driscoll D., Bennett S.J. Postural sway and active balance performance in highly active lower-limb amputees. Am J Phys Med Rehabil. 2002;81:13–20. doi: 10.1097/00002060-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Aruin A.S., Nicholas J.J., Latash M.L. Anticipatory postural adjustments during standing in below-the-knee amputees. Clin Biomech (Bristol, Avon) 1997;12:52–59. doi: 10.1016/s0268-0033(96)00053-8. [DOI] [PubMed] [Google Scholar]
- 58.Isakov E., Mizrahi J., Ring H., Susak Z., Hakim N. Standing sway and weight-bearing distribution in people with below-knee amputations. Arch Phys Med Rehabil. 1992;73:174–178. [PubMed] [Google Scholar]
- 59.Barnett C.T., Vanicek N., Polman R.C. Postural responses during volitional and perturbed dynamic balance tasks in new lower limb amputees: a longitudinal study. Gait Posture. 2013;37:319–325. doi: 10.1016/j.gaitpost.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Nederhand M.J., Van Asseldonk E.H., van der Kooij H., Rietman H.S. Dynamic balance control (DBC) in lower leg amputee subjects; contribution of the regulatory activity of the prosthesis side. Clin Biomech (Bristol, Avon) 2012;27:40–45. doi: 10.1016/j.clinbiomech.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Viton J.M., Mouchnino L., Mille M.L. Equilibrium and movement control strategies in trans-tibial amputees. Prosthet Orthot Int. 2000;24:108–116. doi: 10.1080/03093640008726533. [DOI] [PubMed] [Google Scholar]
- 62.Duclos C., Roll R., Kavounoudias A., Mongeau, Roll J.-P., Forget R. Postural changes after sustained neck muscle contraction in persons with a lower leg amputation. J Electromyogr Kinesiol. 2009;19:e214–e222. doi: 10.1016/j.jelekin.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Lenka P., Tiberwala D.N. Effect of stump length on postural steadiness during quiet stance in unilateral trans-tibial amputee. Al Ameen J Med Sci. 2010;3:50. [Google Scholar]
- 64.Nadollek H., Brauer S., Isles R. Outcomes after trans-tibial amputation: the relationship between quiet stance ability, strength of hip abductor muscles and gait. Physiother Res Int. 2002;7:203–214. doi: 10.1002/pri.260. [DOI] [PubMed] [Google Scholar]
- 65.Jarnlo G.B., Thorngren K.G. Standing balance in hip fracture patients. 20 middle-aged patients compared with 20 healthy subjects. Acta Orthop Scand. 1991;62:427–434. doi: 10.3109/17453679108996638. [DOI] [PubMed] [Google Scholar]
- 66.Hermodsson Y., Ekdahl C., Persson B.M., Roxendal G. Standing balance in trans-tibial amputees following vascular disease or trauma: a comparative study with healthy subjects. Prosthet Orthot Int. 1994;18:150–158. doi: 10.3109/03093649409164400. [DOI] [PubMed] [Google Scholar]
- 67.Friberg O. Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine (Phila Pa 1976) 1983;8:643–651. doi: 10.1097/00007632-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Friberg O. Biomechanical significance of the correct length of lower limb prostheses: a clinical and radiological study. Prosthet Orthot Int. 1984;8:124–129. doi: 10.3109/03093648409146072. [DOI] [PubMed] [Google Scholar]
- 69.Hides J., Stanton W., Mendis M.D., Sexton M. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man Ther. 2011;16:573–577. doi: 10.1016/j.math.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Borenstein D.G., O'Mara J.W., Jr., Boden S.D. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am. 2001;83-a:1306–1311. doi: 10.2106/00004623-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Paajanen H., Erkintalo M., Parkkola R., Salminen J., Kormano M. Age-dependent correlation of low-back pain and lumbar disc degeneration. Arch Orthop Trauma Surg. 1997;116:106–107. doi: 10.1007/BF00434112. [DOI] [PubMed] [Google Scholar]
- 72.Clasper J., Ramasamy A. Traumatic amputations. Br J Pain. 2013;7:67–73. doi: 10.1177/2049463713487324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staruch R.M., Jackson P.C., Hodson J. Comparing the surgical timelines of military and civilians traumatic lower limb amputations. Ann Med Surg. 2016;6:81–86. doi: 10.1016/j.amsu.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pohjolainen T., Alaranta H., Wikström J. Primary survival and prosthetic fitting of lower limb amputees. Prosthet Orthot Int. 1989;13:63–69. doi: 10.3109/03093648909078214. [DOI] [PubMed] [Google Scholar]
- 75.Stam H.J., Dommisse A.M., Bussmann H.J. Prevalence of low back pain after transfemoral amputation related to physical activity and other prosthesis-related parameters. Disabil Rehabil. 2004;26:794–797. doi: 10.1080/09638280410001696683. [DOI] [PubMed] [Google Scholar]
- 76.Schoppen T., Boonstra A., Groothoff J.W., de Vries J., Goeken L.N., Eisma W.H. Physical, mental, and social predictors of functional outcome in unilateral lower-limb amputees. Arch Phys Med Rehabil. 2003;84:803–811. doi: 10.1016/s0003-9993(02)04952-3. [DOI] [PubMed] [Google Scholar]
- 77.Christensen J., Ipsen T., Doherty P., Langberg H. Physical and social factors determining quality of life for veterans with lower-limb amputation(s): a systematic review. Disabil Rehabil. 2016;38:2345–2353. doi: 10.3109/09638288.2015.1129446. [DOI] [PubMed] [Google Scholar]
- 78.Bull A.M.J., Clasper J., Mahoney P.F., editors. Blast injury science and engineering: a guide for clinicians and researchers. Springer; Switzerland: 2016. [Google Scholar]
- 79.BBC News Civilian amputees discriminated against, charities claim. https://www.bbc.co.uk/news/av/health-34054433/civilian-amputees-discriminated-against-charities-claim Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.