Abstract
Objective
To synthesize the evidence examining caregiver-mediated mobility interventions in a hospital setting and whether they improve patient, caregiver, or health system outcomes.
Data Sources
We searched MEDLINE, EMBASE, PsycINFO, CINAHL, and Scopus databases from inception to September 7, 2018.
Study Selection
Two reviewers independently selected original research in inpatient settings that reported on an intervention delivered by a caregiver (eg, family, friend, paid worker) and directed to the patient’s mobility. Mobility interventions were categorized based on the level of caregiver engagement using a 3-category framework: inform (provision of education on patient’s condition and management), activate (prompting caregivers to take action in patient care), and collaborate (encouraging interaction with providers or other caregivers).
Data Extraction
One reviewer extracted data, and another checked the data. Quality was assessed using the Cochrane Collaboration’s risk of bias tool and Grading of Recommendations, Assessment, Development and Evaluation approach.
Data Synthesis
Forty studies met the inclusion criteria; most were randomized controlled trials (n=16/40, 40.0%) and investigated older adults (n=18/40, 45.0%) with stroke (n=20/40, 50.0%). Inform (n=2) and activate (n=4) interventions and combined inform-activate (n=5/6, 83.3%) and inform-activate-collaborate (n=6/10, 60.0%) interventions were reported to improve patient mobility. Inform-activate and inform-collaborate interventions were reported to improve caregiver outcomes (eg, burden) (n=13/19, 68.4%). Studies that engaged caregivers in all 3 strategies (inform-activate-collaborate) were reported to improve health system outcomes (eg, hospital readmission) (n=4/6, 66.7%). Most studies were of unclear (n=22/40, 55.0%) or low risk of bias (n=11/40, 27.5%) for most domains.
Conclusions
Engaging caregivers in mobility of hospitalized patients may improve patient mobility as well as caregiver and health system outcomes.
Keywords: Activities of daily living, Caregivers, Hospital, Quality of life, Rehabilitation, Systematic review
List of abbreviations: ADL, activities of daily living; LOS, length of stay; RCT, randomized controlled trial
Many hospitalized patients experience impairments in physical functioning1, 2, 3 and benefit from mobilization; benefits include improved functional outcomes, and reduced bloodstream infection, hospital-acquired pressure ulcers, and risk of deep vein thrombosis.4, 5, 6, 7 Mobility interventions in hospitalized patients improve patient physical and psychological outcomes,4,8 and reduce patients’ hospital length of stay (LOS),8, 9, 10, 11, 12 costs,12 and readmissions.10,11 Early and more frequent mobility interventions may improve motor function by reducing muscle loss and preserving cardiorespiratory function, and thereby result in earlier discharge from the hospital.13 Mobility interventions may increase patients’ self-confidence in their ability to move, which may increase self-efficacy in activities of daily living (ADL) (eg, walking, dressing) and improve patient outcomes.13
Engagement of caregivers (eg, family, friends) in patient mobility may enhance previously reported benefits of patient mobilization.9 Participation of caregivers in patient care improves patient adherence to treatment plans and results in faster recovery.14 Providers (eg, nurses, physicians, physical/occupational therapists, certified nurse assistants) perceive time constraints and limited staffing as barriers to mobilization.15,16 Caregivers may be present more frequently than providers to assist with mobility, which may facilitate increased mobilization episodes compared to when patients are solely dependent on busy providers for mobilization. Caregivers also feel valued when they participate in patient care,17 which contributes to a positive hospital experience for caregivers, patients, and hospital staff.18 Caregiver-mediated mobility interventions may improve patient functional outcomes by potentially increasing mobilization episodes and its associated benefits,4,8 and also reduce caregiver burden.19 Evidence supports that caregiver-mediated interventions improve patient outcomes in various patient populations (eg, pediatric, asthma, stroke).20,21 However, these studies did not specifically target patients’ mobility,19,20 excluded certain populations (eg, pediatric),19,21 targeted caregivers and not patients,19 or included patient care settings outside of the hospital (eg, home).19,21 Little is known about the effect of interventions delivered by caregivers to hospitalized patients (of all ages) that are aimed at improving patients’ mobility or ADL.
The goal of this systematic review was to synthesize the evidence examining caregiver-mediated mobility interventions in hospitalized patients and whether they improve patient, caregiver, or health system outcomes.
Methods
Data sources and searches
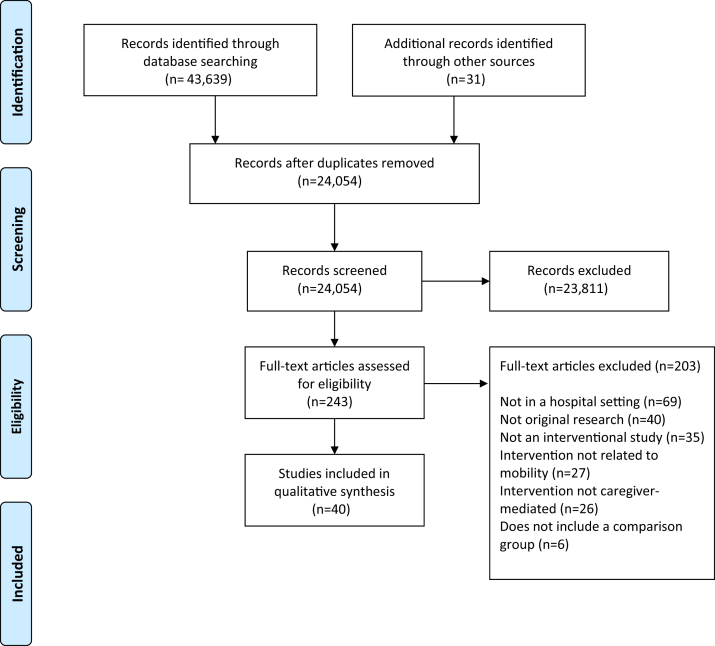
This systematic review is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria (fig 1). A protocol was published a priori on PROSPERO (ID: CRD42017084401). The search strategy was developed with assistance from a medical librarian and experts in caregiver-mediated interventions (H.T.S. and K.M.F) (MEDLINE search strategy provided in supplemental appendix S1, available online only at http://www.archives-pmr.org/). The Medline (OVID), EMBASE (OVID), PsycINFO (ProQUEST), CINAHL (EBSCO), and Scopus (Elsevier) databases were searched from inception to September 7, 2018. The search criteria included subject headings, keywords, and Boolean logic for caregivers, intervention, mobility, and hospital. A caregiver was defined as a family member, friend, or paid helper external to the hospital health care team who regularly looks after the patient (not including volunteers and health or social workers). A hospital was defined as an inpatient institution where patients received treatment.
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart to identify reviewed and included articles.
Study selection
DistillerSR,a a web-based systematic review software, was used to manage and screen references (supplemental fig S1, available online only at http://www.archives-pmr.org/). For the title and abstract screening phase, DistillerAI (a component of DistillerSR) was the second reviewer due to the large number of titles and abstracts (n=24,054). Titles and abstracts were screened by a minimum of 2 human reviewers (K.D.K. and I.Y.) and then screened again by DistillerAI. References included by any reviewer proceeded to the full-text screening stage. Only the human reviewers (K.D.K. and I.Y.) screened references at the full-text stage. References included by both reviewers were included in the final sample.
All search results were uploaded from the databases into DistillerSR, which has features necessary for performing systematic reviews based on the Cochrane guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.22 After a reviewer removed duplicates, a sample of the first 12.4% (n=2989/24,054) results from the search was used to train the DistillerAI software toolkit, a toolkit used in other systematic reviews.23,24 The size of this sample was determined opportunistically based on the recommended true positives and true negatives needed to train the software.25 This sample was independently screened by 2 human reviewers (K.D.K. and I.Y.). Each human reviewer determined relevance to the research question according to the following inclusion criteria: original research (ie, not a review); interventional study design; intervention delivered by the caregiver and directed to a patient; intervention related to mobility; comparator groups; and patient, caregiver, provider, or health system outcomes. With this sample of articles defined as relevant (ie, include) and irrelevant (ie, exclude) to the research question, the DistillerAI software was trained. The DistillerAI software divided the categorized search results into a training set that trained DistillerAI (n=2391/2989, 80.0%), and a test set that compared inclusion and exclusion decisions made by DistillerAI with human-screened decisions (n=598/2989, 20.0%). DistillerAI makes use of traditional machine learning methods (a Naïve Bayes classifier and a Support Vector Machine classifier) to classify references as included or excluded. Hyperparameters to the software were adjusted over a series of 10 training runs until the screening decisions of DistillerAI matched the human screening decisions. With these parameters set, the software was then run on the remaining references as a second reviewer. The remaining titles and abstracts (n=21,065) were screened by at least 1 human reviewer (K.D.K. or I.Y.) and in duplicate by DistillerAI as a second screener for 87.6% (n=21,061) of the references. At the conclusion of title and abstract screening, DistillerAI agreed on 99.1% (n=18,858) of references that were screened in duplicate with I.Y. (n=19,028), and 98.3% (n=1999) of references that were screened in duplicate with K.D.K. (n=2033). There were no limitations on language or time of publication. Where disputes about inclusion occurred, an inclusive approach was used and the article in question was marked for full-text review. This process yielded 243 articles for full-text review.
DistillerAI was only involved in the title and abstract screening stage, and all full-text articles were independently screened by 2 human reviewers (K.D.K. and I.Y.). DistillerAI was not used in the final stage because it was feasible for human reviewers to screen the number of full-text articles (n=243) and because DistillerAI is not yet adequately accurate to replace a human reviewer.26 Additional studies were identified by searching the bibliography of included studies. Disagreements (n=43) between the 2 reviewers were resolved by each reviewer independently rescreening the reference and reaching consensus together based on the inclusion criteria.
Data extraction and quality assessment
One reviewer extracted data independently (I.Y.) and a second reviewer (K.D.K.) checked the data. Information was recorded on study characteristics (eg, study design, location), participant population (eg, infants, older adults), type of caregiver (eg, significant others, adult children), and descriptions of the intervention and control groups. Interventions were classified to be effective in improving patient mobility if the articles reported statistical significance for mobility outcomes. Reviewers then labeled types of caregiver engagement (eg, inform-activate) to be effective if more than 50.0% of the studies in that group suggested statistically significant improvements. The same principle was applied when analyzing effectiveness of the interventions on caregiver and health system outcomes, respectively. Included non-English articles (n=4; 1 Chinese, 1 Korean, 1 Persian, 1 Spanish) were translated using Google Translate (http://translate.google.com), which is an accurate tool for translating non-English articles in systematic reviews.27
Risk of bias was assessed as high, low, or unclear by 2 independent reviewers (K.D.K. and I.Y.) using the Cochrane Collaboration’s risk of bias tool.28 The strength of evidence was assessed using the Grading of Recommendations Assessment Development and Evaluation working group approach,29 which initially ranks randomized controlled trials (RCTs) high and observational studies low, with studies downgraded or upgraded based on methodological quality and quality of evidence. An overall rank (high, moderate, or low) was given based on the Grading of Recommendations Assessment Development and Evaluation working group criteria.29
Data synthesis and analysis
Data were grouped and summarized using STATA.b Meta-analysis was not feasible due to the heterogeneity of reported interventions, participant populations, and outcomes. Interventions were characterized by caregiver engagement based on a caregiver-mediated knowledge translation framework.20 Inform interventions educate caregivers on patients’ disease condition, treatment, or management; activate interventions prompt caregivers to participate in patient care; and collaborate interventions encourage caregivers to engage with providers or other caregivers20 (supplemental table S1, available online only at http://www.archives-pmr.org/). Interventions were further classified into types of caregiver engagement support (see supplemental table S1). For example, an inform intervention can support caregivers by educating them on how they can assist patients in ADL, including walking and transportation. An activate intervention can provide support to caregivers in practical management activities, such as training them to assist patients perform exercises. A collaborate intervention can provide support through safety netting, such as allowing caregivers to contact the study therapist by telerehabilitation if they need additional support, which encourages collaboration between caregivers and providers. Patients were classified as older adults if they were identified as such or if the mean age of participants was more than 65 years. Adult patients were those between 18 and 65 years (inclusive). Outcomes were classified as patient (eg, frequency of ADL, independence in ADL, psychological outcomes like anxiety), caregiver (eg, burden, anxiety, depression), or health system (eg, LOS, hospital readmission) outcomes.
Role of the funding source
The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Results
Study characteristics
A total of 24,054 titles and abstracts were reviewed, of which 40 studies30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 were included in the final synthesis (see fig 1). Studies were published between 1986 and 2017, with a total of 7450 patients (range, 10-1250 patients) (supplemental table S2, available online only at http://www.archives-pmr.org/). Studies included RCTs (n=16/40, 40.0%) and non-RCTs (n=24/40, 60.0%).
Participant characteristics
Half of the studies investigated stroke patients (n=20/40, 50.0%)30,32,37,40, 41, 42, 43, 44, 45, 46, 47,50,52,56, 57, 58, 59, 60,64,66 (see supplemental table S2). The next most common patient populations were patients with hip complications (eg, hip fractures and hip replacement surgery) (n=3/40 studies, 7.5%),33,51,55 heart complications (eg, heart failure, myocardial infarction, coronary surgery) (n=3/40 studies, 7.5%),36,39,62 and cancer (n=2/40 studies, 5.0%).31,69 Patients ranged from premature infants (n=2/40 studies, 5.0%)38,67 to older adults (n=18/40 studies, 45.0%).30,32,33,35,36,39,44,46,48,49,54,56,58,60,62,64,65,68 Patients were mostly men (n=19/35 studies, 54.3%) in studies that reported participant sex (n=35 studies). Caregivers were mostly women in studies that reported caregiver sex (n=15 studies) and mostly significant others (n=15/28 studies, 53.6%) or adult children (n=9/28 studies, 32.1%) in studies that described the caregiver relationship (n=28 studies).
Interventions
The 40 studies investigated a total of 37 unique interventions or programs (supplemental table S3, available online only at http://www.archives-pmr.org/). Two interventions, the family-centered function-focused care34,35 and the London Stroke Carers Training Course,41,46,59 were evaluated in more than 1 study.
The caregiver-mediated mobility interventions engaged caregivers in various aspects of patient care, with most interventions (n=34/40, 85.0%) being multicomponent30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41,44, 45, 46, 47,49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64,66,69 (table 1). Common ways caregivers were involved included being educated on the patient’s condition, being trained in caregiving skills, collaborating with providers (eg, nurses) in goal-setting, or being prepared for patient care on discharge (see supplemental table S3). For example, the family-centered function-focused care program educated caregivers on the patient’s condition, treatment, caregiving techniques, follow-up care, and communicating with postacute providers.34,35 The family-mediated exercise therapy program trained caregivers in delivering individualized exercises and involved them in goal setting.42 Shyu et al64 provided individualized health education to caregivers based on their needs and informed them about referral services post patient discharge.
Table 1.
Type and effectiveness of interventions based on type of caregiver engagement
| Type of Caregiver∗ Engagement (n) | Patient Outcomes† (All) | Patient Outcomes (Mobility) | Caregiver Outcomes | Health System Outcomes |
|---|---|---|---|---|
| Inform (2)65,68 | 2↑65,68 | 2↑65,68 | NR | NR |
| Activate (4)42,43,48,67 | 4↑42,43,48,67 | 4↑42,43,48,67 | 1↑42 | NR |
| Inform-activate (13)31,40,44, 45, 46,52, 53, 54, 55,59,62,66,69 | 9↑31,40,44,46,52,54,62,66,69 |
5↑40,44,52,66,69 1↔46 |
6↑31,44, 45, 46,53,55 1↔59 |
1↑59 3↔44,46,54 |
| Activate-collaborate (1)32 | 1↔32 | 1↔32 | 1↑32 | 1↔32 |
| Inform-collaborate (5)30,39,47,60,64 |
3↑30,39,60 1↔64 |
1↑39 2↔60,64 |
4↑30,47,60,64 | 1↑64 |
| Inform-activate-collaborate (15)33, 34, 35, 36, 37, 38,41,49, 50, 51,56, 57, 58,61,63 |
7↑34, 35, 36,51,56,57,63 4↔33,37,41,50 |
6↑34, 35, 36,51,56,63 4↔33,37,41,50 |
3↑34,35,58 5↔33,38‡,41,50,61 |
4↑34, 35, 36,49 2↔41,50 |
NOTE. ↑ Statistically significant positive effect of intervention. ↓ Statistically significant negative effect of intervention. ↔ No statistically significant effect of intervention.
Abbreviations: n, number of studies reporting each outcome; NR, not reported.
Examples of caregiver engagement: inform: educate caregivers on how they can support patients in ADLs, including walking and transportation; activate: train caregivers on assisting patients perform exercises; collaborate: allow caregivers to contact the study therapist by telerehabilitation if they need additional support.
Includes studies that reported patient mobility.
Study did not analyze outcomes to test for statistical significance.
Most interventions engaged caregivers in multiple strategies: inform-activate-collaborate (n=15/40, 37.5%),33, 34, 35, 36, 37, 38,41,49, 50, 51,56, 57, 58,61,63 inform-activate (n=13/40, 32.5%),31,40,44, 45, 46,52, 53, 54, 55,59,62,66,69 and inform-collaborate (n=5/40, 12.5%)30,39,47,60,64 (see table 1). There was 1 activate-collaborate intervention.32 Activate interventions (n=4/40, 10.0%)42,43,48,67 were the most common single component intervention.42,43,48,67 The remaining 2 interventions were single-component inform interventions.65,68
Outcomes
Studies reported patient (n=31/40, 77.5%), caregiver (n=21/40, 52.5%), and health system (n=12/40, 30.0%) outcomes (table 2). No studies reported provider outcomes. Although all interventions involved mobility, patient mobility outcomes were reported by 26 studies (65.0%).32, 33, 34, 35, 36, 37,39, 40, 41, 42, 43, 44,46,48,50, 51, 52,56,60,63, 64, 65, 66, 67, 68, 69 Most of these studies reported on the patient’s independence in ADL (n=20/26, 76.9%)32, 33, 34, 35,37,40, 41, 42,46,48,50,52,56,60,63, 64, 65, 66,68,69 (supplemental table S4, available online only at http://www.archives-pmr.org/). Other common mobility outcomes were walking performance (n=5/26, 19.2%),32,34,35,39,42 frequency of patients participating in ADL (n=5/26, 19.2%),36,42,46,51,66 and exercise (n=4/26, 15.4%).43,48,68,69 The next most commonly reported patient outcomes were psychological (n=13/31, 41.9% of studies that reported patient outcomes) (eg, quality of life, anxiety, and depression).31, 32, 33,36,39,41,46,50,54,60,64,65,68 Caregiver outcomes were psychological and included caregiver burden (n=12/21, 57.1%), anxiety (n=9/21, 42.9%), depression (n=8/21, 38.1%), and quality of life (n=7/21, 33.3%).30, 31, 32, 33, 34, 35,38,41,42,44, 45, 46, 47,50,53,55,58, 59, 60, 61,64 LOS (8/12, 66.7%) and hospital readmission (7/12, 58.3%) were commonly reported health system outcomes.32,34, 35, 36,41,44,46,49,50,54,59,64
Table 2.
Type and effectiveness of interventions for select subgroups
| Study Population (n) | Patient Outcomes | Caregiver Outcomes | Health System Outcomes |
|---|---|---|---|
| All |
25↑30,31,34, 35, 36,39,40,42, 43, 44,46,48,51,52,54,56,57,60,62,63,65, 66, 67, 68, 69 6↔32,33,37,41,50,64 |
15↑30, 31, 32,34,35,42,44, 45, 46, 47,53,55,58,60,64 6↔33,38‡,41,50,59,61 |
6↑34, 35, 36,49,59,64 6↔32,41,44,46,50,54 |
| Patient population (age) | |||
| Babies/infants/children (4)38,49,67,69 | 2↑67,69 | 1↔38,‡ | 1↑49 |
| Adults (18)31,37,39,40,44, 45, 46, 47,50,53,56,58, 59, 60,62,63,65,68 |
11↑31,39,40,44,46,56,60,62,63,65,68 2↔37,50 |
8↑31,44, 45, 46, 47,53,58,60 2↔50,59 |
1↑59 3↔44,46,50 |
| Older adults∗ (18)30,32, 33, 34, 35, 36,41, 42, 43,48,51,52,54,55,57,61,64,66 |
12↑30,34, 35, 36,42,43,48,51,52,54,57,66 4↔32,33,41,64 |
7↑30,32,34,35,42,55,64 3↔33,41,61 |
4↑34, 35, 36,64 3↔32,41,54 |
| Patient population†(condition) | |||
| Stroke (20)30,32,37,40, 41, 42, 43, 44, 45, 46, 47,50,52,56, 57, 58, 59, 60,64,66 |
11↑30,40,42, 43, 44,46,52,56,57,60,66 5↔32,37,41,50,64 |
10↑30,32,42,44, 45, 46, 47,58,60,64 3↔41,50,59 |
2↑59,64 5↔32,41,44,46,50 |
| Hip complications (3)33,51,55 |
1↑51 1↔33 |
1↑55 1↔33 |
NR |
| Heart complications (3)36,39,62 | 3↑36,39,62 | NR | 1↑36 |
| Cancer (2)31,69 | 2↑31,69 | 1↑31 | NR |
NOTE. ↑ Statistically significant positive effect of intervention. ↓ Statistically significant negative effect of intervention. ↔ No statistically significant effect of intervention.
Abbreviations: n, number of studies reporting each outcome; NR, not reported.
Older adults were defined as patients ≥65 years and not nested within the adults.
Patient populations that were studied in at least 2 studies are reported.
Study did not analyze outcomes to test for statistical significance.
Multicomponent interventions engaging caregivers in inform-activate and inform-activate-collaborate strategies improved mobility and other patient outcomes (eg, quality of life, anxiety, depression) (see table 1). Eighty percent of these interventions improved patient outcomes (n=16/20 that reported patient outcomes). Most of these interventions improved patient mobility: 83.3% of inform-activate (n=5/6 that reported on mobility) and 60.0% of inform-activate-collaborate (n=6/10 that reported on mobility). Improved outcomes included patients’ independence in ADL and increased frequency of performing ADL. Single-component inform (n=2) and activate (n=4) interventions also improved patient mobility. The results were not different between RCTs and non-RCTs, with the exception of inform-activate interventions of RCTs (supplemental table S5, available online only at http://www.archives-pmr.org/).
Multicomponent interventions that engaged caregivers using the inform strategy (ie, inform-activate and inform-collaborate) improved caregiver outcomes (see table 1). Of these studies that reported caregiver outcomes (n=19), 13 reported improved caregiver outcomes (68.4%), such as reduced burden, anxiety, and depression. The results did not differ between RCTs and non-RCTs (see supplemental table S5).
Interventions that engaged caregivers using all 3 strategies (inform-activate-collaborate) improved health system outcomes (see table 1). Among these studies that reported health system outcomes (n=6), 4 reported improved outcomes (66.7%), such as reduced LOS and hospital readmission. No interventions were associated with higher health care costs. The same trend was observed when focusing on non-RCTs, while there was inconclusive evidence supporting inform-activate-collaborate interventions of RCTs (n=1/2, 50.0%) (see supplemental table S5).
Patient populations
Studies commonly investigated adults (n=18/40, 45.0%) and older adults (n=18/40, 45.0%) (see table 2). Patients of all ages who received caregiver-mediated mobility interventions mostly reported improvement in independently performing ADL. Younger patients (ie, babies or infants or children) improved independence in ADL or mobility. Adult patients commonly improved independence in ADL, quality of life, or anxiety (n=11/13, 84.6% of studies in this age group). Older adult patients improved independence in ADL, frequency of ADL, and walking performance (n=12/16, 75.0% studies in this age group).
Patients with stroke, hip complications, heart complications, and cancer commonly reported improved patient and caregiver outcomes with mostly no change in health system outcomes (see table 2). Activate (n=2) and inform-activate (n=4/5) interventions improved mobility in stroke patients, such as independence in ADL and physical functioning (eg, walking performance, balance). Patients with heart complications demonstrated better performance in walking, frequency of ADL, and adherence to treatment. Cancer patients reported improved physical functioning, independence in ADL, and quality of life.
Adverse events
Studies inconsistently reported adverse events, such as cardiovascular events and falls. When reported, the interventions did not increase the occurrence of adverse events and no harms were reported as a consequence of the interventions.34,35,41,51,54
Satisfaction
Participants’ satisfaction with the interventions or patient care were inconsistently reported, and when reported (n=7), different scales were used to quantify satisfaction. The inconsistent reporting of satisfaction may be dependent on study design. More studies reported caregiver satisfaction (n=5/6, 83.3%)30,33,44,46,60 than patient satisfaction (n=3/6, 50.0%).30,31,60 Caregivers reported their satisfaction with patient care, information about patient condition, or self-performance of caregiving. Patients reported their satisfaction with patient care, information they received about their condition, or their relation with nurses. When reported, participants of caregiver-mediated mobility interventions were more satisfied with patient care than those who received standard of care.30,31,33,44,46,60
Risk of bias assessment and strength of evidence
Most of the studies were rated as unclear (n=22/40, 55.0%) or low risk of bias (n=11/40, 27.5%) for most of the domains (supplemental table S6, available online only at http://www.archives-pmr.org/). The domain for blinding of participants and personnel was rated as high risk of bias for all studies because caregiver-mediated mobility interventions do not allow patients and caregivers to be blinded. The risk of bias for blinding of outcome assessors was mostly unclear (n=24/40, 60.0%) or low (n=12/40, 30.0%). Overall, low quality evidence shows that caregiver-mediated mobility interventions improve patient, caregiver, and health system outcomes compared to standard of care (supplemental table S7, available online only at http://www.archives-pmr.org/). The certainty in evidence of RCTs was commonly downgraded due to risk of bias and imprecision.
Discussion
This review identified 40 studies of almost 7500 hospitalized patients where caregiver-mediated mobility interventions that engaged caregivers in multiple strategies improved patient, caregiver, and health system outcomes. Patient and caregiver outcomes were commonly reported, whereas few studies reported health system outcomes. Most caregiver-mediated mobility interventions improved patient mobility, particularly single and multicomponent activate and inform interventions. Caregiver outcomes were improved in multicomponent interventions that engaged caregivers using the inform strategy (eg, inform-activate, inform-collaborate). Health system outcomes were reported to be improved in inform-activate-collaborate interventions.
Single and multicomponent activate and inform caregiver-mediated mobility interventions were reported to improve patient mobility, such as independence in ADL and frequency of performing ADL. A systematic review on caregiver involvement in patient care (classified as a single-component activate intervention) of hospitalized patients also reported improved mobility.70 Inform interventions address caregivers’ needs for knowledge on the patient’s condition, treatment, and illness prognosis, which helps them participate in caring for hospitalized patients.71 However, education-only interventions (which would be classified as single-component inform interventions) generally do not affect outcomes,72,73 suggesting that multicomponent inform interventions may be more effective. In contrast, this review identified single-component inform interventions to improve patient outcomes, although these studies were limited in number and size. If both single and multicomponent inform interventions are effective in improving patient mobility, single component interventions may be preferable due to reduced complexity and need for fewer resources. Single and multicomponent activate and inform caregiver-mediated mobility interventions improve patient mobility, but more evidence is required to identify whether single-component interventions are more or equally effective as multicomponent interventions.
Multicomponent caregiver-mediated mobility interventions that engaged caregivers using the inform strategy (eg, inform-activate or inform-collaborate) were reported to improve caregiver outcomes, including burden, anxiety, and depression. A meta-analysis of caregiver-mediated interventions (classified as inform-activate) in cancer patients also reported improved caregiver outcomes.19 The effectiveness of inform interventions on caregiver outcomes can be explained by caregivers feeling better prepared for caregiving once they have knowledge about the patient’s condition and the expected prognosis.71 When caregivers are prompted to participate in assisting with patient mobility (ie, the activate strategy), they may feel valuable to the patient, thereby improving caregivers’ psychological well-being.74
Multicomponent caregiver-mediated mobility interventions that engaged caregivers using all 3 strategies (inform-activate-collaborate) were reported to improve health system outcomes, including LOS and hospital readmission. The results of this review are similar to that of a family-centered approach for patients undergoing total hip or knee arthroplasty surgery where caregivers were educated on the patient’s condition (inform), assisted patients during the recovery phase (activate), and communicated with providers and other participants (collaborate).75 Similar to the current review, the inform-activate-collaborate intervention also reduced LOS.75 In contrast, an inform-activate intervention that educated parents of premature infants in the neonatal ICU on behavioral interventions reduced LOS.76 Instead of engaging caregivers using the collaborate strategy to avoid intensive staff training,77 the program educated caregivers using audiotapes. However, standardized protocols may not be effective for mobility interventions because they are not tailored to a patient’s unique needs.78 Nurses are vital for engaging caregivers in assisting with the care of hospitalized patients70 by preparing caregivers to be more confident and competent in caregiving.79 Partnership between caregivers and nurses while patients are hospitalized improves the quality and continuity of care for older patients.80 Caregivers delivering mobility interventions may benefit from collaborating with providers (ie, collaborate) rather than following standardized protocols. Equipping caregivers with the necessary knowledge for caregiving at home and providing them information about resources available for further support may lead to better patient outcomes and reduce rehospitalization.81
Including caregivers as additional personnel for delivering inpatient mobilization may increase the associated benefits of mobilization without relying on busy hospital staff. Provider-delivered mobility interventions in hospitals are challenging due to the necessary collaboration between providers with varying degrees of training and patient responsibilities.82 Hoyer et al surveyed nurses and rehabilitation therapists and identified several barriers to delivering inpatient mobilization: increasing workload for nurses, relation between mobilization training and confidence in mobilizing, and patient resistance to mobilization.82 Involving caregivers in patient mobilization may reduce the workload of nurses. Although caregivers may occasionally require support from nurses, providing caregivers support may ultimately require less time. Compared to nurses who are initially unfamiliar with the patient, caregivers may be intimately familiar with the patient and thereby reduce patient resistance to mobilization.14 Classifying interventions using the 3-category framework of caregiver engagement strategies can help identify the most appropriate modes of caregiver engagement based on the patient population (eg, stroke, adults) and desired outcomes (eg, patient-, caregiver-, health system-oriented). Although classification of interventions may be subjective, the potential for bias was mitigated by labeling the intervention based on an objective type of caregiver engagement support (eg, condition and treatment education, lifestyle monitoring). This framework for classifying interventions was described in the evidence-based guidelines for family-centered care in the ICU by Davidson et al, which include caregiver education (inform), teaching caregivers techniques for caregiving (activate), and peer-to-peer support for families (collaborate).83 There is evidence supporting implementation of caregiver-mediated interventions in a hospital setting, and this review identifies that mobility interventions delivered by caregivers may be an effective approach for producing beneficial outcomes of mobilization. Future, higher-quality studies can help determine which type of caregiver engagement strategies is best for different patient populations.
Study strengths and limitations
This review has many strengths, including use of rigorous methodology published a priori in PROSPERO. The search strategy was developed with experts in the field (H.T.S. and K.M.F.). We included all original research publications regardless of language. Diverse patient populations were included, generalizing the findings to hospitalized patients.
Several limitations need to be considered. Even with a rigorous search strategy it is possible that some articles may have been missed, which we attempted to identify by hand-searching bibliographic lists of included articles and searching for follow-up publications of ineligible studies (eg, conference abstracts, protocols). Another limitation is the use of the novel natural language processing tool, DistillerAI, which is yet to be validated. However, all titles and abstracts were screened by at least 1 human reviewer and only the human reviewers were involved in screening full-text articles. Despite the low specificity of DistillerAI, the tool increased the efficiency of screening. A strength of DistillerSR is that it was scored to be the best software for screening in systemic reviews because it met all the mandatory technical criteria for conducting systematic reviews (eg, reference importing, including or excluding references), in addition to reaching the maximum number of criteria that are desirable (eg, project auditing, attaching PDFs).22 The study results are a reflection of controlled environments and interventions that demonstrate efficacy in research settings may not show effectiveness in pragmatic settings.84 Although patients of all ages were included, younger patients (eg, babies, infants, children) were underrepresented. This was unexpected because caregivers, typically parents in these cases, are usually involved in their child’s rehabilitation.85 A possible explanation for this discrepancy is that pediatric rehabilitation usually occurs in outpatient settings,85 whereas we focused on inpatient mobility interventions. The underrepresentation of younger patients may introduce publication bias in our review, limiting our findings to adults and older adults. Another limitation is that caregivers often participate in patient care and their involvement in patient mobilization may be undocumented. This would suggest that our estimates for caregiver-mediated interventions are likely conservative. All studies had a high risk of bias in the domain for blinding of participants and personnel, an inherent limitation in evaluating caregiver-mediated mobility interventions. However, outcome assessors were mostly blinded. Both RCTs and lower quality non-RCTs were included, and when the analyses were restricted to RCTs, the sample size of some interventions became too small to support some of our conclusions. However, the analyses support the aim of summarizing the literature of caregiver-mediated mobility interventions rather than providing conclusive recommendations for care. Outcomes were grouped into 3 broad categories (ie, patient, caregiver, health system), and an intervention was classified to be effective if outcomes were reported to be statistically significant at any time point. For example, a study that reported patients in the intervention group had higher frequency of performing ADL at 1-month postintervention, and another study that reported patients in the intervention group improved on performing ADL independently at 8 weeks postintervention, were both classified as effective interventions. This could lead to inconsistencies in classification of the effectiveness of interventions. However, the purpose of this study was to present overall effectiveness of caregiver-mediated mobility interventions and the outcomes reported are within the scope of this systematic review.
Conclusions
The current evidence suggests that engaging caregivers in patient mobility while patients are in the hospital is beneficial for patients, caregivers, and the health system. Caregiver-mediated interventions appear to be safe, with no increase in reported adverse events. Engaging caregivers to mobilize hospitalized patients may facilitate earlier and more frequent mobilization episodes, which may improve patient mobility, caregiver, and health system outcomes.
Suppliers
-
a.
DistillerSR; Evidence Partners.
-
b.
STATA; StataCorp.
Acknowledgments
We thank H.L. Roberston, MLIS, for her guidance on developing the search strategy for this review and Filipe Lucini, PhD, for reviewing the methods section on machine learning and DistillerAI.
Footnotes
Supported by the Canadian Institutes of Health Research (grant no. TLA-145402 to H.T.S.).
Disclosures: none.
Supplementary Data
References
- 1.Covinsky K.E., Palmer R.M., Fortinsky R.H. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 2.de Saint-Hubert M., Schoevaerdts D., Poulain G., Cornette P., Swine C. Risk factors predicting later functional decline in older hospitalized patients. Acta Clin Belg. 2009;64:187–194. doi: 10.1179/acb.2009.034. [DOI] [PubMed] [Google Scholar]
- 3.Garland A., Dawson N.V., Altmann I. Outcomes up to 5 years after severe, acute respiratory failure. Chest. 2004;126:1897–1904. doi: 10.1378/chest.126.6.1897. [DOI] [PubMed] [Google Scholar]
- 4.Kalisch B.J., Lee S., Dabney B.W. Outcomes of inpatient mobilization: a literature review. J Clin Nurs. 2014;23:1486–1501. doi: 10.1111/jocn.12315. [DOI] [PubMed] [Google Scholar]
- 5.Klein K., Mulkey M., Bena J.F., Albert N.M. Clinical and psychological effects of early mobilization in patients treated in a neurologic ICU: a comparative study. Crit Care Med. 2015;43:865–873. doi: 10.1097/CCM.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 6.Cayley W.E., Jr. Preventing deep vein thrombosis in hospital inpatients. BMJ. 2007;335:147–151. doi: 10.1136/bmj.39247.542477.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zisberg A., Shadmi E., Sinoff G., Gur-Yaish N., Srulovici E., Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273. doi: 10.1111/j.1532-5415.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 8.Pashikanti L., Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26:87–94. doi: 10.1097/NUR.0b013e31824590e6. [DOI] [PubMed] [Google Scholar]
- 9.Peiris C.L., Taylor N.F., Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabil. 2011;92:1490–1500. doi: 10.1016/j.apmr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Brown C.J., Flood K.L. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 11.Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013;144:825–847. doi: 10.1378/chest.12-2930. [DOI] [PubMed] [Google Scholar]
- 12.de Morton N.A., Keating J.L., Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;1:CD005955. doi: 10.1002/14651858.CD005955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cumming T.B., Thrift Amanda G., Collier Janice M. Very early mobilization after stroke fast-tracks return to walking. Stroke. 2011;42:153–158. doi: 10.1161/STROKEAHA.110.594598. [DOI] [PubMed] [Google Scholar]
- 14.Gardner D., Stewart N. Staff involvement with families of patients in critical-care units. Heart Lung. 1978;7:105–110. [Google Scholar]
- 15.Dubb R., Nydahl P., Hermes C. Barriers and strategies for early mobilization of patients in intensive care units. An Am Thorac Soc. 2016;13:724–730. doi: 10.1513/AnnalsATS.201509-586CME. [DOI] [PubMed] [Google Scholar]
- 16.Messer A., Comer L., Forst S. Implementation of a progressive mobilization program in a medical-surgical intensive care unit. Crit Care Nurse. 2015;35:28–42. doi: 10.4037/ccn2015469. [DOI] [PubMed] [Google Scholar]
- 17.Gaglione K.M. Assessing and intervening with families of CCU patients. Nurs Clin North Am. 1984;19:427–432. [PubMed] [Google Scholar]
- 18.Egan J.M. Rehabilitation: the nurse’s responsibility in the intensive care unit. Crit Care Nurs Q. 1979;2:105–112. [Google Scholar]
- 19.Northouse L.L., Katapodi M.C., Song L., Zhang L., Mood D.W. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60:317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiest K.M., McIntosh C.J., Demiantschuk D., Leigh J.P., Stelfox H.T. Translating evidence to patient care through caregivers: a systematic review of caregiver-mediated interventions. BMC Med. 2018;16:105. doi: 10.1186/s12916-018-1097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vloothuis J.D., Mulder M., Veerbeek J.M. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev. 2016;12:CD011058. doi: 10.1002/14651858.CD011058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Mierden S., Tsaioun K., Bleich A., Leenaars C.H. Software tools for literature screening in systematic reviews in biomedical research. Altex. 2019;36:508–517. doi: 10.14573/altex.1902131. [DOI] [PubMed] [Google Scholar]
- 23.Luedke M.W., Blalock D.V., Goldstein K.M. Self-management of epilepsy: a systematic review. Ann Intern Med. 2019;171:117–126. doi: 10.7326/M19-0458. [DOI] [PubMed] [Google Scholar]
- 24.D'Anci K.E., Uhl S., Giradi G., Martin C. Treatments for the prevention and management of suicide: a systematic review. Ann Intern Med. 2019;171:334–342. doi: 10.7326/M19-0869. [DOI] [PubMed] [Google Scholar]
- 25.Evidence Partners. DistillerAI FAQs. https://www.evidencepartners.com/distillerai-faqs/#what-training-set-should-i-use Available at:
- 26.Gartlehner G., Wagner G., Lux L. Assessing the accuracy of machine-assisted abstract screening with DistillerAI: a user study. Syst Rev. 2019;8:277. doi: 10.1186/s13643-019-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson J.L., Kuriyama A., Anton A. The accuracy of Google Translate for abstracting data from non–English-language trials for systematic reviews. Ann Intern Med. 2019;171:677–679. doi: 10.7326/M19-0891. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirrezabal A., Duarte E., Rueda N., Cervantes C., Marco E., Escalada F. Effects of information and training provision in satisfaction of patients and carers in stroke rehabilitation. NeuroRehabilitation. 2013;33:639–647. doi: 10.3233/NRE-130989. [DOI] [PubMed] [Google Scholar]
- 31.Belgacem B., Auclair C., Fedor M.C. A caregiver educational program improves quality of life and burden for cancer patients and their caregivers: a randomised clinical trial. Eur J Oncol Nurs. 2013;17:870–876. doi: 10.1016/j.ejon.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Berg Mvd, Crotty M., Liu E., Killington M., Kwakkel G., Wegen E.V. Early supported discharge by caregiver-mediated exercises and e-health support after stroke: a proof-of-concept trial. Stroke. 2016;47:1885–1892. doi: 10.1161/STROKEAHA.116.013431. [DOI] [PubMed] [Google Scholar]
- 33.Berthelsen C., Kristensson J. The SICAM-trial: evaluating the effect of spouses’ involvement through case management in older patients’ fast-track programmes during and after total hip replacement. J Adv Nurs. 2017;73:112–126. doi: 10.1111/jan.13091. [DOI] [PubMed] [Google Scholar]
- 34.Boltz M., Resnick B., Chippendale T., Galvin J. Testing a family-centered intervention to promote functional and cognitive recovery in hospitalized older adults. J Am Geriatr Soc. 2014;62:2398–2407. doi: 10.1111/jgs.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boltz M., Chippendale T., Resnick B., Galvin J.E. Testing family-centered, function-focused care in hospitalized persons with dementia. Neurodegener Dis Manag. 2015;5:203–215. doi: 10.2217/nmt.15.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deek H., Chang S., Newton P.J. An evaluation of involving family caregivers in the self-care of heart failure patients on hospital readmission: randomised controlled trial (the FAMILY study) Int J Nurs Stud. 2017;75:101–111. doi: 10.1016/j.ijnurstu.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Dignan M.B., Howard G., Toole J.F., Becker C., McLeroy K.R. Evaluation of the north carolina stroke care program. Stroke. 1986;17:382–386. doi: 10.1161/01.str.17.3.382. [DOI] [PubMed] [Google Scholar]
- 38.Dusing S.C., Brown S.E., Drew C.M.V., Thacker L.R., Hendricks-Munõz K.D. Supporting play exploration and early development intervention from NICU to home: a feasibility study. Pediatr Phys Ther. 2015;27:267–274. doi: 10.1097/PEP.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 39.van Elderen-van Kemenade T., Maes S., van den Broek Y. Effects of a health education programme with telephone follow-up during cardiac rehabilitation. Br J Clin Psychol. 1994;33:367–378. doi: 10.1111/j.2044-8260.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 40.El-Senousey M.Y., Aboelsafa A.A., El-Heneedy Y.A., Khalil M.K., Kamel L.I., Abd-El-Aziz A.L. Effect of comprehensive training on ischemic stroke patients. Egypt J Neurol Psychiatry Neurosurg. 2012;49:259–269. [Google Scholar]
- 41.Forster A., Dickerson J., Young J. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet. 2013;382:2069–2076. doi: 10.1016/S0140-6736(13)61603-7. [DOI] [PubMed] [Google Scholar]
- 42.Galvin R., Cusack T., Grady E.O. Family-mediated exercise intervention (FAME): evaluation of a novel form of exercise delivery after stroke. Stroke. 2011;42:681–686. doi: 10.1161/STROKEAHA.110.594689. [DOI] [PubMed] [Google Scholar]
- 43.Harris J.E., Eng J.J., Miller W.C., Dawson A.S. The role of caregiver involvement in upper-limb treatment in individuals with subacute stroke. Phys Ther. 2010;90:1302–1310. doi: 10.2522/ptj.20090349. [DOI] [PubMed] [Google Scholar]
- 44.Hong S., Kim C., Kim E. Effect of a caregiver’s education program on stroke rehabilitation. Ann Rehabil Med. 2017;41:16–24. doi: 10.5535/arm.2017.41.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung B., Kim H. The effect of rehabilitation education program on family caregivers of stroke patients. J Public Health. 2014;2:337–341. [Google Scholar]
- 46.Kalra L., Evans A., Perez I. Training carers of stroke patients: randomised controlled trial. BMJ. 2004;328:1099. doi: 10.1136/bmj.328.7448.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang E., KS H. Effect of a family education program on the family burden of the stroke. J Korean Acad Psych Mental Health Nurs. 2004;13:43–50. [Google Scholar]
- 48.Laitinen-Junkkari P., Meriläinen P., Sinkkonen S. Informal caregivers’ participation in elderly patient care: an interrupted time-series study. Int J Nurs Pract. 2001;7:199–213. doi: 10.1046/j.1440-172x.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- 49.Likitmaskul S., Wongarn R., Kiattisakthavee P. Intensive diabetes education program and multidisciplinary team approach in management of newly diagnosed type 1 diabetes mellitus: a greater patient benefit, experience at Siriraj Hospital. J Med Assoc Thai. 2002;85:S488–S495. [PubMed] [Google Scholar]
- 50.Lindley R.I., Anderson C.S., Billot L. Family-led rehabilitation after stroke in India (ATTEND): a randomised controlled trial. Lancet. 2017;390:588–599. doi: 10.1016/S0140-6736(17)31447-2. [DOI] [PubMed] [Google Scholar]
- 51.Louie S.W., Poon M.-Y., Yu S.-Y., Chan W-l, Au K.-M., Wong K.-M. Effectiveness of a patient/carer empowerment programme for people with hip fractures. Int J Ther Rehabil. 2012;19:673–681. [Google Scholar]
- 52.Maeshima S., Ueyoshi A., Osawa A. Mobility and muscle strength contralateral to hemiplegia from stroke: benefit from self-training with family support. Am J Phys Med Rehabil. 2003;82:456–462. [PubMed] [Google Scholar]
- 53.Martinez RM. del C., Morilla F., del Pino A. Family involvement in the critically ill patient basic care. Enferm Intensiva. 2003;14:96–108. [PubMed] [Google Scholar]
- 54.Martínez-Velilla N., Garrués-Irisarri M., Ibañez-Beroiz B. An exercise program with patient’s involvement and family support can modify the cognitive and affective trajectory of acutely hospitalized older medical patients: a pilot study. Aging Clin Exp Res. 2016;28:483–490. doi: 10.1007/s40520-015-0434-0. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Martin L., Valenza-Demet G., Ariza-Vega P., Valenza C., Castellote-Caballero Y., Jimenez-Moleon J. Effectiveness of an occupational therapy intervention in reducing emotional distress in informal caregivers of hip fracture patients: a randomized controlled trial. Clin Rehabil. 2014;28:772–783. doi: 10.1177/0269215513519343. [DOI] [PubMed] [Google Scholar]
- 56.Mudzi W., Stewart A., Musenge E. Effect of carer education on functional abilities of patients with stroke. Int J Ther Rehabil. 2012;19:380–385. [Google Scholar]
- 57.Nayeri N.D., Mohammadi S., Razi S.P., Kazemnejad A. Investigating the effects of a family-centered care program on stroke patients’ adherence to their therapeutic regimens. Contemp Nurse. 2014;47:88–96. doi: 10.5172/conu.2014.47.1-2.88. [DOI] [PubMed] [Google Scholar]
- 58.Oupra R., Griffiths R., Pryor J., Mott S. Effectiveness of Supportive Educative Learning programme on the level of strain experienced by caregivers of stroke patients in Thailand. Health Soc Care Community. 2010;31:254–262. doi: 10.1111/j.1365-2524.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- 59.Patel A., Knapp M., Evans A., Perez I., Kalra L. Training care givers of stroke patients: economic evaluation. BMJ. 2004;328:1102–1104. doi: 10.1136/bmj.328.7448.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodgers H. Randomized controlled trial of a comprehensive stroke education program for patients and caregivers. Stroke. 1999;30:2585–2591. doi: 10.1161/01.str.30.12.2585. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Gonzalo A., García-Martí C., Ocaña-Colorado A., Baquera-De Micheo M., Morel-Fernández S. Efficiency of an intensive educational program for informal caregivers of hospitalized, dependent patients: cluster randomized trial. BMC Nurs. 2015;14:5. doi: 10.1186/s12912-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanaie N., Mahin R., Mansoureh S. Effect of a family-centered educational program on compliance of patients undergoing coronary bypass graft surgery. Iran J Nurs Midwifery Res. 2013;2:1. [Google Scholar]
- 63.Seel R.T., Douglas J., Dennison A.C., Heaner S., Farris K., Rogers C. Specialized early treatment for persons with disorders of consciousness: program components and outcomes. Arch Phys Med Rehabil. 2013;94:1908–1923. doi: 10.1016/j.apmr.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 64.Shyu Y.L., Kuo L.M., Chen M.C., Chen S.T. A clinical trial of an individualised intervention programme for family caregivers of older stroke victims in Taiwan. J Clin Nurs. 2010;19:1675–1685. doi: 10.1111/j.1365-2702.2009.03124.x. [DOI] [PubMed] [Google Scholar]
- 65.Tang D., Li-Tsang C., Au R. Functional outcomes of burn patients with or without rehabilitation in mainland China. Hong Kong J Occup Ther. 2015;26:15–23. [Google Scholar]
- 66.Torres-Arreola L.D.P., Doubova S.V., Hernandez S.F. Effectiveness of two rehabilitation strategies provided by nurses for stroke patients in Mexico. J Clin Nurs. 2009;18:2993–3002. doi: 10.1111/j.1365-2702.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 67.Ustad T., Evensen K., Campbell S. Early parent-administered physical therapy for preterm infants: a randomized controlled trial. Pediatrics. 2016;138:1–8. doi: 10.1542/peds.2016-0271. [DOI] [PubMed] [Google Scholar]
- 68.Wu F.Y. Influence of rehabilitation education on behavior and lumbar functional exercise in patients following operation for lumbar spinal stenosis. Chin J Clin Rehab. 2005;9:20–22. [Google Scholar]
- 69.Yu L., Mo L., Tang Y., Huang X., Tan J. Effects of nursing intervention models on social adaption capability development in preschool children with malignant tumors: a randomized control trial. Psychooncology. 2014;23:708–712. doi: 10.1002/pon.3572. [DOI] [PubMed] [Google Scholar]
- 70.Mackie B.R., Mitchell M., Marshall P.A. The impact of interventions that promote family involvement in care on adult acute-care wards: an integrative review. Collegian. 2018;25:131–140. [Google Scholar]
- 71.Bellou P., Gerogianni K. The contribution of family in the care of patient in the hospital. Health Sci J. 2007;3 [Google Scholar]
- 72.Wensing M., van der Weijden T., Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract. 1998;48:991–997. [PMC free article] [PubMed] [Google Scholar]
- 73.Oxman A.D., Thomson M.A., Davis D.A., Haynes R.B. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ. 1995;153:1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 74.Davidson J.E., Powers K., Hedayat K.M. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004-2005. Crit Care Med. 2007;35:605–622. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 75.DiGioia A., III, Greenhouse P.K., Levison T.J. Patient and family-centered collaborative care: an orthopaedic model. Clin Orthop Relat Res. 2007;463:13–19. [PubMed] [Google Scholar]
- 76.Melnyk B.M., Feinstein N.F. Reducing hospital expenditures with the COPE (Creating Opportunities for Parent Empowerment) program for parents and premature infants: an analysis of direct healthcare neonatal intensive care unit costs and savings. Nurs Adm Q. 2009;33:32–37. doi: 10.1097/01.NAQ.0000343346.47795.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melnyk B.M., Feinstein N.F., Alpert-Gillis L. Reducing premature infants’ length of stay and improving parents’ mental health outcomes with the Creating Opportunities for Parent Empowerment (COPE) neonatal intensive care unit program: a randomized, controlled trial. Pediatrics. 2006;118:e1414–e1427. doi: 10.1542/peds.2005-2580. [DOI] [PubMed] [Google Scholar]
- 78.Liu B., Moore J.E., Almaawiy U. Outcomes of Mobilisation of Vulnerable Elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing. 2018;47:112–119. doi: 10.1093/ageing/afx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reinhard S.C., Given B., Petlick N.H., Bemis A. Supporting family caregivers in providing care. In: Hughes R., editor. Patient safety and quality: an evidence-based handbook for nurses. Agency for Healthcare Research and Quality; Rockville, MD: 2008. [PubMed] [Google Scholar]
- 80.Hagedoorn E.I., Keers J.C., Jaarsma T., van der Schans C.P., Luttik M.L.A., Paans W. The association of collaboration between family caregivers and nurses in the hospital and their preparedness for caregiving at home. Geriatr Nurs. 2019 doi: 10.1016/j.gerinurse.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Rodakowski J., Rocco P.B., Ortiz M. Caregiver integration during discharge planning for older adults to reduce resource use: a metaanalysis. J Am Geriatr Soc. 2017;65:1748–1755. doi: 10.1111/jgs.14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoyer E.H., Brotman D.J., Chan K.S., Needham D.M. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94:304–312. doi: 10.1097/PHM.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davidson J.E., Aslakson R.A., Long A.C. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45:103–128. doi: 10.1097/CCM.0000000000002169. [DOI] [PubMed] [Google Scholar]
- 84.Godwin M., Ruhland L., Casson I. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siebes R.C., Wijnroks L., Ketelaar M., van Schie P.E., Gorter J.W., Vermeer A. Parent participation in paediatric rehabilitation treatment centres in the Netherlands: a parents’ viewpoint. Child Care Health Dev. 2007;33:196–205. doi: 10.1111/j.1365-2214.2006.00636.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.