An important component of plant resistance towards xylem vascular wilt pathogens is the induction of vertical and horizontal structural barriers, restricting the pathogen to infected vessels, thus avoiding plant wilting.
Keywords: Gels, inducible defenses, lignin, physico-chemical barriers, plant-pathogen interactions, structural defenses, suberin, tyloses, vascular pathogens, wilt
Abstract
Xylem vascular wilt pathogens cause devastating diseases in plants. Proliferation of these pathogens in the xylem causes massive disruption of water and mineral transport, resulting in severe wilting and death of the infected plants. Upon reaching the xylem vascular tissue, these pathogens multiply profusely, spreading vertically within the xylem sap, and horizontally between vessels and to the surrounding tissues. Plant resistance to these pathogens is very complex. One of the most effective defense responses in resistant plants is the formation of physico-chemical barriers in the xylem tissue. Vertical spread within the vessel lumen is restricted by structural barriers, namely, tyloses and gels. Horizontal spread to the apoplast and surrounding healthy vessels and tissues is prevented by vascular coating of the colonized vessels with lignin and suberin. Both vertical and horizontal barriers compartmentalize the pathogen at the infection site and contribute to their elimination. Induction of these defenses are tightly coordinated, both temporally and spatially, to avoid detrimental consequences such as cavitation and embolism. We discuss current knowledge on mechanisms underlying plant-inducible structural barriers against major xylem-colonizing pathogens. This knowledge may be applied to engineer metabolic pathways of vascular coating compounds in specific cells, to produce plants resistant towards xylem colonizers.
Introduction
The plant immune system has been shaped by hundreds of millions of years of interactions with microbial pathogens that are constantly trying to evade or overcome host plant defense reactions. Pathogens that colonize the xylem - a vital component for transporting water and minerals from the roots to the aerial parts - are particularly pernicious, causing major destruction in numerous host plants worldwide (Yadeta and Thomma, 2013). Despite the xylem being nutritionally poor in comparison with other plant tissues, a select group of pathogens comprising a few genera of bacteria, fungi and oomycetes, have evolved to target this plant tissue as a niche for colonization. Proliferation of these wilt pathogens causes massive disruption in the xylem vessels through the production of toxins, exopolysaccharides, hyphal mass, conidia and other pathogen propagules that cause destruction as well as physical occlusion of xylem elements, resulting in severe wilting and plant death (Zaini et al., 2018).
Invasion strategies used by vascular wilt pathogens
Vascular plant pathogens utilize diverse strategies to get access into the vessels. Vascular wilt pathogenic fungi (Fusarium oxysporum, Verticillium albo-atrum, Verticiullium dahliae, Ceratocystis fimbriata, Ophiostoma novo-ulmi), oomycetes (Pythium spp) or the soil-borne bacterium Ralstonia solanacearum invade plant roots and advance inter- or intracellularly through the root cortex to reach the xylem, where they proliferate and spread systemically to aerial plant parts (Bae et al., 2015). In order to get access into the vasculature, these pathogens generally target root extremities and the junction between primary and lateral roots where the epidermal barrier may be compromised, and the endodermis as well as Casparian strip are either not fully differentiated or reoriented by outgrowth of lateral roots (Vasse et al., 1995; Álvarez et al., 2010).
In contrast to these root invaders, many vascular bacteria, such as Xylella fastidiosa, are directly inoculated into the xylem by insect vectors that feed on the plant host (Wang et al., 2017). Furthermore, a few species of vascular bacteria reach the xylem tissues via natural plant openings in the aerial parts of the plant, such as leaf hydathodes (Xanthomonas oryzae pv. Oryzae;Pradhan et al., 2012), flower nectarthodes (Erwinia amylovora;Bubán et al., 2003) or stem lenticels (Pseudomonas syringae pv. actinidiae;Renzi et al., 2012). These bacteria multiply in intercellular spaces before eventually colonizing the xylem vessels. Moreover, there are vascular wilt pathogens which are transmitted through graft-infected rootstock/scion, as has been reported for the fungus Ceratocystis fagacearum on oak trees (Blaedow and Juzwik, 2010), or the bacterium X. fastidiosa on pecan (Sanderlin and Melanson, 2006). Another method of infection of vascular wilt pathogens occurs through the use of infected mother plants as a source for clonal propagation. This affects many plant species cultivated in nurseries, such as strawberry plants infected with Fusarium oxysporum f. sp. fragariae (Pastrana et al., 2019), olive plants infected with V. dahliae (Morello et al., 2016), and it is also common in grapevine mother plants that can harbor various vascular wilt fungi (Gramaje et al., 2018).
Immunity against vascular wilt pathogens
Plants are protected against pathogens by a well-orchestrated immune system (Jones and Dangl, 2006). Pattern-triggered immunity (PTI) is initiated upon recognition of conserved microbial features at the plasma membrane by pattern-recognition receptors (PRRs) in the plant. Effector-triggered immunity (ETI) is activated by recognition of pathogen-secreted effectors via intracellular nucleotide-binding leucine-rich repeat proteins (NLRs). ETI is commonly known as a stronger immune response, while PTI offers durable and broad-spectrum resistance (Katagiri and Tsuda, 2010). Yet, these two layers of immunity operate synergistically, converging on several downstream responses including the oxidative burst, activation of kinase signaling cascades, expression of defense-related genes and accumulation of physico-chemical barriers (Thomma et al., 2011; Ngou et al., 2020, Preprint; Yuan et al., 2020, Preprint).
Although PTI and ETI function in the interactions of plants with vascular pathogens, there are very few cases where these processes have been analyzed specifically in the xylem and surrounding tissues of infected plants. Several recent studies have shown that PTI can be triggered in plants by treatment or over-expression of conserved molecular patterns present in wilt pathogens. For example, an extracellular polysaccharide from the wilt bacterium R. solanacearum has been shown to act as a PTI elicitor in resistant tomato, leading to induced expression of defense response genes (Milling et al., 2011; Prakasha et al., 2017). Furthermore, in Nicotiana benthamiana and tomato, PTI is triggered upon perception of the COLD SHOCK PROTEIN 22 (csp22) peptide from R. solanacearum by the PRR COLD SHOCK PROTEIN RECEPTOR (CORE; Wang et al., 2016). In accordance, treatment of tomato with csp22 conferred increased resistance to R. solanacearum in tomato plants. Additionally, transgenic Arabidopsis thaliana plants expressing the tomato csp22 receptor (SlCORE) gained the ability to respond to csp22 and were more resistant to R. solanacearum infection (Wei et al., 2018). Similarly, V. dahliae, possesses two cellulose-degrading glycoside hydrolase family 12 (GH12) proteins, namely VdEG1 and VdEG3, which in N. benthamiana are recognized by the PRR complexes LRR-RLP (Leucine Rich Repeat-Receptor-Like Protein)/SOBIR1/BAK1 (SUPPRESSOR OF BAK1-INTERACTING RECEPTOR-LIKE KINASE 1-1/BRASSINOSTEROID-INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE) and LRR-RLKs (Receptor-Like Kinase)/BAK1, respectively, thereby triggering PTI responses (Gui et al., 2017).
Concomitantly, some bacterial wilt pathogens have evolved modifications in the sequences of their conserved patterns, so that they are no longer recognizable by cognate PRRs. This occurs in X. fastidiosa, which contains a lipopolysaccharide featuring a masking motif that evades recognition by grapevine (Rapicavoli et al., 2018), or flagellin from R. solanacearum, with a polymorphic sequence that avoids perception by many host plants (Wei et al., 2020).
An interesting emerging concept is that roots may be relatively insensitive to most conserved molecular patterns, which may prevent mounting defense responses against commensal organisms in an environment such as the soil, full of microbes. Underlying this phenomenon, it has been recently shown that root PTI is only activated upon local tissue damage (Zhou et al., 2020). This work convincingly shows how localized cell death upregulates PRR expression in the neighboring cells, leading to restricted responsiveness to conserved molecular patterns of root invaders (Zhou et al., 2020).
ETI responses against vascular pathogens have also been documented in the literature. For instance, in the interaction between tomato and the vascular wilt fungus F. oxysporum f. sp. lycopersici, several R protein-effector pairs have been identified. The NLR I2 perceives the Avr2 effector; I, a receptor-like protein with an extracellular LRR domain (LRR-RLP) perceives Avr1; and I3, a S-receptor-like kinase (SRLK) perceives Avr3 (Houterman et al., 2009; Catanzariti et al., 2015, 2017). Interestingly, the I2 gene is primarily expressed in the xylem vascular tissue, probably partly explaining why the fungus reaches the vasculature before being contained in an incompatible interaction (van der Does et al., 2019; Mes et al., 2000). Similarly, effector AvrFom2 from F. oxysporum f. sp. melonis is perceived by the NLR Fom-2 in melon (Joobeur et al., 2004). On the other hand, the effector Ave1 from V. albo-atrum induces resistance in several plant species, mediated by the receptor-like protein Ve1 (de Jonge et al., 2012; Song et al., 2018).
With respect to bacterial vascular wilt pathogens, the RRS1-R/RPS4 (Resistance to Ralstonia solanacearum 1-Recessive/ Resistant to Pseudomonas syringae 4) NLR pair in Arabidopsis mediates immunity against R. solanacearum carrying the PopP2 effector (Deslandes et al., 2002; Le Roux et al., 2015; Sarris et al., 2015). The rice LRR-RLK protein Xa21 confers broad-spectrum immunity to X. oryzae pv. oryzae, mediated through recognition of the small protein Ax21 (Park and Ronald, 2012). In addition, the rice NLR Xo1 recognizes the transcription activator-like effector Tal2H from X. oryzae pvs. oryzicola and oryzae (Triplett et al., 2016). Similarly, the NLR Bs4 in tomato perceives the avirulence protein AvrBs4 from Xanthomonas campestris pv. vesicatoria (Schornack et al., 2004). Furthermore, the Arabidopsis NLR ZAR1 (HOPZ-ACTIVATED RESISTANCE 1) recognizes the X. campestris effector AvrAC/XopAC by forming a complex with PBL2 (PROBABLE SERINE/THREONINE-PROTEIN KINASE 2), which acts as a decoy, and RKS1 (RESISTANCE-RELATED kinase 1), a pseudokinase (Wang et al., 2015).
Besides individual examples of R genes, quantitative traits have been shown to underscore immunity against xylem colonizers in many different hosts. Stable resistance towards R. solanacearum in tomato is controlled by two major quantitative trait loci (QTLs), namely Bwr-6 and Bwr-12, located in chromosomes six and twelve, respectively, and three minor QTLs, Bwr-3, Bwr-4 and Bwr-8 (Thoquet et al., 1996; Mangin et al., 1999; Wang et al., 2000, 2013; Carmeille et al., 2006). Some of these loci have been shown to be strain and/or environment-specific, and involve an extremely complex genetic basis of resistance. Similarly, QTLs for Fusarium wilt resistance have been mapped in several hosts such as chickpea (Sabbavarapu et al., 2013), cotton (Ulloa et al., 2013; Wang et al., 2018), and watermelon (Lambel et al., 2014). QTLs termed RESISTANCE TO FUSARIUM (RFO1-RFO7) have been identified in Arabidopsis that provide broad spectrum immunity to multiple formae speciales of F. oxysporum (Chen et al., 2014). RFO1 was found to encode a wall-associated kinase-like kinase 22 (WAKL22) and RFO2 encodes a receptor-like protein (Diener and Ausubel, 2005). Likewise, resistance to Verticillium correlates with mapping of QTLs in several hosts such as strawberry (Antanaviciute et al., 2015) and cotton (Palanga et al., 2017). A major QTL conferring resistance to X. fastidiosa was identified in a Vitis arizonica linkage map and named as ‘Pierce’s disease resistance 1’ (PdR1), which has been introgressed into commercial cultivars (Krivanek et al., 2006). However, the majority of available QTL mapping data for wilt resistance traits lack resolution. This may be partly due to the high sensitivity of wilt development to temperature variation, and also due to the fact that wilt resistance comprises a complex array of multilayered mechanisms involving the coordinated action of different plant tissues or cell types (Bani et al., 2018). Importantly, this extremely complex polygenic and quantitative resistance underscores the structural physico-chemical defense mechanisms induced by vascular pathogens in resistant plants.
Inducible structural barriers to restrict the spread of vascular wilt pathogens
Inducible structural barriers formed at and around the vasculature upon colonization constitute one of the most important defense components against wilt diseases. If a pathogen manages to reach the xylem, this transport system becomes an excellent channel of inoculum dissemination throughout the plant. As a consequence, plants have evolved effective structural defense mechanisms to prevent vessel colonization or movement between vessels once vascular colonization has occurred (Beckman and Roberts, 1995) Timely formation of these physico-chemical vascular barriers early upon pathogen perception can lead to confinement of the vascular pathogen at the infected vessel, avoiding the spread of wilt diseases (Robb et al., 2007; Zaini et al., 2018; Planas-Marquès et al., 2019).
Some of these structural reinforcements induced by pathogens were already reported in classic botanical studies in the 19th century (Zimmermann, 1979). Moreover, during the 1980s and 1990s, they were intensely studied from anatomical and biochemical points of view, as they were recognized as important components of defense reactions (Newcombe and Robb, 1988; Nicholson, 1992; Niemann, 1994). In trees, the sequential response to confine fungal pathogen progression or tissue damage at the site of infection or injury was initially explained by the CODIT model (compartmentalization of decay in trees; Shigo and Marx, 1977). The model described four “walls” or barriers pre-formed or formed in response to wounding, that restrict pathogen colonization. In particular, wall 1 defined vessel plugging structures formed as a response to vascular wilt pathogen invasion.
However, most of the research in the field of plant-pathogen interactions started refocusing during the 1990s when Arabidopsis gained momentum as a model species. This led to the identification of striking similarities between plant and animal immune systems, the type III secretion system in bacteria being discovered, and avr-R gene pairs being identified as a corollary of Flor’s gene-for-gene model (Nishimura and Dangl, 2010). We now think it is time to revisit the role of inducible structural defenses in plant-pathogen interactions. These defenses are extremely important to block progression of the devastating vascular pathogens, and we are far from understanding how these types of defense mechanisms are controlled.
In this review, we summarize how inducible vascular structures compartmentalize the vascular wilt pathogens leading to resistance. For this, we classify inducible structural barriers using a bi-dimensional perspective. We define a vertical and a horizontal component of resistance as those structures that restrict the vertical and horizontal movement of the pathogen, respectively, within the xylem vascular tissue (Fig. 1, Table 1). Furthermore, we review current knowledge on mechanisms underlying plant-inducible structural defenses against major xylem-colonizing pathogens, highlighting their correlation with biochemical changes and genetic interactions. Finally, we discuss future perspectives in the study of inducible vascular structural defenses, taking into account recent technological advances and how this may be translated into increasing resistance to vascular wilt pathogens in the field. Such physico-chemical defense responses are key traits desired for effective management of vascular wilt pathogens (Ferreira et al., 2017).
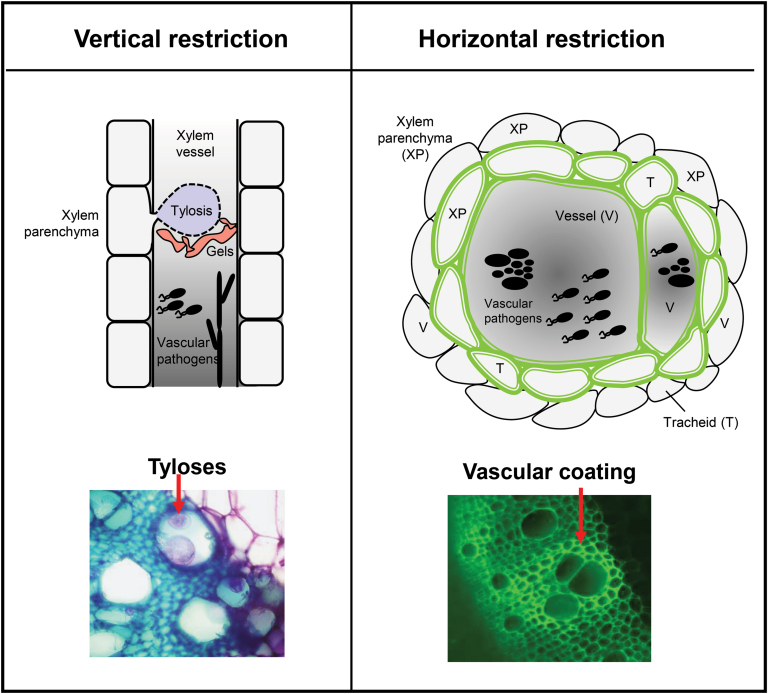
Fig. 1.
The two dimensions of plant physico-chemical barriers induced against xylem vascular wilt pathogens. To counter invasion by xylem vascular wilt pathogens, resistant plants induce two-dimensional physico-chemical defenses that restrict vertical and horizontal movement of the pathogen. Vertical spread within the vessel lumen is mainly restricted by tyloses and gels (left). In contrast, horizontal spread of the pathogen to surrounding healthy vessels is prevented by reinforcement of the walls of colonized vessels (V) and the surrounding xylem parenchyma (XP) and tracheids (T), through vascular coating with mainly lignin and suberin (shown as a green color in the diagram). Synchronized formation of vertical and horizontal barriers early after pathogen invasion results in compartmentalization of the pathogen inoculum at the site of infection, thereby preventing wilt, and constitute a major component of resistance. To visualize tyloses and vascular coating (panels below), tomato root cross-sections were obtained after R. solanacearum soil soak inoculation, and fixed in 70% ethanol. For tyloses, cross-sections were stained with 0.1% toluidine blue and observed using a Leica DM6B-Z microscope under bright field conditions, and images were recorded through a MC190-HD-0518131623 camera. To visualize phenolic vascular coating, the cross-sections were illuminated by UV using a Leica DM6B-Z microscope, and the auto-florescence emitted from phenolic deposits was observed using a HC PL FLUOTAR objective. Images were captured using a Leica-DFC9000GT-VSC07341 camera. In the left panel, the arrow points towards R. solanacearum-induced formation of tyloses inside vessel lumen, which appear pink to violet color upon staining with 0.1% toluidine blue. In the right panel, the arrow points towards R. solanacearum-induced auto-fluorescence emitted from phenolics, deposited in the walls of vessels and the surrounding tracheids and parenchyma cells. Scale bar=120 µm.
Table 1.
List of plant pathosystems in which (I) vertical or (II) horizontal restriction of pathogen movement inside the plant has been shown.
| I. | Vertical restriction | ||
|---|---|---|---|
| Structure | Host | Pathogen | Reference |
| Tylose formation | Banana | Fusarium oxysporum f.sp. cubense | (Vander Molen et al., 1987) |
| Butternut | Ophiognomonia clavigignenti-juglandacearum | (Rioux et al., 2018) | |
| Cotton | Fusarium oxysporum f. sp. vasinfectum | (Shi et al., 1991) | |
| Cucurbits | Fusarium oxysporum f. sp. melonis | (Seo and Kim, 2017) | |
| Elm | Ophiostoma novo-ulmi | (Plichta et al., 2016) | |
| Grapevine | Xylella fastidiosa | (Sun et al., 2013) | |
| Phaeomoniella chlamydospora | (Pouzoulet et al., 2013) | ||
| Potato | Rasltonia solanacearum | (Ferreira et al., 2017) | |
| Tomato | Rasltonia solanacearum | (Grimault et al., 1994) | |
| Verticillium albo-atrum | (Hutson and Smith, 1980) | ||
| Verticillium dahliae | (Tjamos and Smith, 1975) | ||
| Fusarium oxysporum f. sp. lycopersici | (Hutson and Smith, 1980) | ||
| Gel deposition | Banana | Fusarium oxysporum f.sp. cubense | (Vander Molen et al., 1987) |
| Butternut | Ophiognomonia clavigignenti-juglandacearum | (Rioux et al., 2018) | |
| Carnation | Fusarium oxysporum f.sp. dianthi | (Baayen and Elgersma, 1985) | |
| Elm | Ophiostoma novo-ulmi | (Plichta et al., 2016) | |
| Grapevine | Xylella fastidiosa | (Sun et al., 2013) | |
| Pea | Fusarium oxysporum f. sp. pisi | (Bishop and Cooper, 1983) | |
| Plane tree | Ceratocystis fimbriata f. sp platani | (Clérivet et al., 2000) | |
| Tomato | Rasltonia solanacearum | (Grimault et al., 1994; Kim et al., 2016) | |
| Verticillium albo-atrum | (Hutson and Smith, 1980) | ||
| Fusarium oxysporum f. sp. lycopersici | (Hutson and Smith, 1980) | ||
| II. | Horizontal restriction | ||
| Structure | Host | Pathogen | Reference |
| Lignin deposition | Banana | Fusarium oxysporum f. sp. cubense | (De Ascensao and Dubery, 2000) |
| Cotton | Verticillium dahliae | (Xu et al., 2011; Bu et al., 2014) | |
| Dutch elm | Ophiostoma novo-ulmi | (Martin et al., 2007) | |
| Flax | Fusarium oxysporum f. sp. lini | (Galindo-González and Deyholos, 2016) | |
| Oilseed rape | Verticillium longisporum | (Eynck et al., 2009) | |
| Olive | Xylella fastidiosa | (Sabella et al., 2018) | |
| Pepper | Verticillium dahliae | (Novo et al., 2017) | |
| Potato | Rasltonia solanacearum | (Ferreira et al., 2017) | |
| Tomato | Verticillium dahliae | (Street et al., 1986; Hu et al., 2019) | |
| Rasltonia solanacearum | (Ishihara et al., 2012; Ferreira et al., 2017) | ||
| Suberin deposition | Alfalfa | Verticillium albo-atrum | (Newcombe and Robb, 1988) |
| Butternut | Ophiognomonia clavigignenti-juglandacearum | (Rioux et al., 2018) | |
| Dutch elm | Ophiostoma novo-ulmi | (Martín et al., 2008) | |
| Grapevine | Phaeomoniella chlamydospora | (Pouzoulet et al., 2013) | |
| Tomato | Verticillium albo-atrum | (Street et al., 1986; Robb et al., 1991) |
Vertical restriction of vascular colonization
Once they reach the xylem, different lumen occlusions constitute vertical barriers to the anti-gravitational movement of vascular pathogens. Vascular occlusion is an effective means of slowing down vertical progression of the pathogen, or even confining it to the infection site, preventing systemic infection. The most prominent of these occlusions are tyloses and gels, which we will review in this section, focusing on the aspects related to plant defense. As an important defense strategy by vascular pathogens, mechanisms to evade or subvert vertical occlusions and secure colonization have evolved on the pathogen side, as part of the evolutionary arms race. Inhibition of vertical occlusions has been reported for various wilt fungi, although the mechanisms for this virulence mechanism are not fully understood (Beckman and Roberts, 1995; Pouzoulet et al., 2017).
Formation of tyloses
Tyloses (singular tylosis) are balloon-like overgrowths of the protoplast of adjacent living parenchyma cells that protrude into xylem vessels through its pits (Fig. 1; Bonsen and Kucera, 1990). Tyloses are considered inducible defense structures against xylem intruders because their formation can prevent spreading of the pathogen and also protect healthy parts of the plant by blocking the infected vessels (Leśniewska et al., 2017). However, formation of tyloses is not only linked to pathogen attack, as they can be induced by several other environmental stimuli, such as pruning, wounding, flooding and frost (Davison and Tay, 1985; Cochard and Tyree, 1990; Sun et al., 2007; De Micco et al., 2016). The process of tylosis formation is tightly controlled and has certain commonalities with regular cell enlargement (Bishop and Cooper, 1984). Hormones such as auxin, ethylene and jasmonate seem to have a prominent role in the formation of these structures (Vander Molen et al., 1987; Leśniewska et al., 2017).
The formation of tyloses in response to pathogen attack has been extensively reported (Table 1; Cooper and Williams, 2004; Sun et al., 2013; Ferreira et al., 2017; Rioux et al., 2018). Restricted formation of tyloses has been observed and specifically induced in the infected vessels of resistant tomato and potato varieties, effectively restricting R. solanacearum to the infected vascular bundles (Grimault et al., 1994; Ferreira et al., 2017). Such specific induction was not observed in susceptible tomato cultivars infected with R. solanacearum, where the formation of tyloses appeared delayed and less focused, with numerous non-colonized vessels occluded by tyloses, and pathogen growth unrestricted (Grimault et al., 1994). Tylosis formation in resistant tomato cultivars has also been observed upon inoculation with the pathogenic fungus F. oxysporum f. sp. lycopersici and V. albo-atrum (Hutson and Smith, 1980). Similarly, in a banana cultivar resistant to race 1 of F. oxysporum f. sp. cubense, tylosis initially appeared as early as within two days post-inoculation in the lumen of xylem vessels of the root (Vander Molen et al., 1987).
In addition to acting as structural barriers, tyloses can act as storage organs of antimicrobial compounds. Although they are predominantly composed of pectic substances (Rioux et al., 1998; Eynck et al., 2009), fungicidal compounds such as elemental sulfur have been detected in tyloses of tomato lines resistant to V. albo-atrum, which was thought to inhibit spore germination (Williams et al., 2002). In addition, a fully developed tylosis also develops a lignified and/or suberized cell wall, which may protect the exposed cell from the xylem pathogen (Pouzoulet et al., 2013).
However, the formation of tyloses is not always linked to resistance. For instance, a tomato variety susceptible to V. albo-atrum formed a few miniature tyloses in response to infection that the fungal hyphae were able to easily surpass within the vessel (Tjamos and Smith, 1975). More important than the quantity or frequency of tyloses formed, the successful restriction of a vascular pathogen depends on synchronization and specificity of tylose production to pathogen-colonized vessels (Bishop and Cooper, 1984). Sun et al., (2013) reported tyloses as the predominant type of occlusion that formed in grapevines with differing resistance to X. fastidiosa. Excessive tylosis formation in response to X. fastidiosa infection in the susceptible grapevine cultivar led to heavy blockage of vessels and development of wilting symptoms, and did not significantly affect pathogen spread. In contrast, in resistant grapevines, tylosis development was specific and mainly limited to a few internodes close to the point of inoculation, impacting less of the vessels and indicating that timing and localization are key (Sun et al., 2013). Interestingly, the anatomy of xylem vessels also plays a role in augmenting pathogen compartmentalization by tyloses. Cultivars of grapevine having larger vessel size are known to be susceptible to vascular wilt pathogens such as X. fastidiosa, Eutypa lata, Phaeoacremonium aleophilum, Phaeomoniella chlamydospora, Diplodia seriata, and Neofusicoccum parvum (Pouzoulet et al., 2017, 2019, 2020; Deyett et al., 2019). Likewise, Dutch elm cultivars having larger vessel diameters are susceptible to Ophiostoma novo-ulmi (Pouzoulet et al., 2014; Venturas et al., 2014). Across the grapevine genotypes, it has been observed that the extent of P. chlamydospora compartmentalization is a function of the diameter of the host xylem vessels. Genotypes with increased number of xylem vessels above 100 µm in diameter resulted in increased infection of host tissue (Pouzoulet et al., 2020). Though numerous tyloses are formed in large vessels of susceptible grapevine cultivars in response to the wilt pathogen P. chlamydospora, the compartmentalization process is not as efficient as in narrow diameter vessels, due to the presence of large escape routes (Pouzoulet et al., 2017).
Deposition of gels
Deposition of electrodense material corresponding to gels or gums in the lumen of the xylem vessels is another feature commonly observed during xylem invasion by vascular pathogens, and acts as one of the multiple factors that contribute to induced structural defense (Vander Molen et al., 1977). Besides its role in immunity, these occluding structures also form in response to other stimuli such as wounding or aging (Ratnayake et al., 2013).
Gels are commonly secreted by xylem parenchyma cells and they are transported across pit membranes into vessel elements (Fig. 1; Bishop and Cooper, 1984). Tyloses have also been observed to secrete gels into the lumen of vessels, thereby multiplying the clogging effect (Bonsen and Kucera, 1990; Rioux et al., 1998). Gels appear fibrillar, forming thin networks of varying electron density that ultimately fill and clog the vessel lumen (Sun et al., 2008). Gels initially appear as translucent fibres arising from several places along lateral walls of vessels, and later form a continuous layer with wavy edges toward the vessel lumen. Subsequently, gels turn yellow and are interspersed with small particles, coinciding with vessel occlusions (Sun et al., 2008). Although the main component of gels are pectic substances such as partially esterified pectic polysaccharides (Rioux et al., 1998; Clérivet et al., 2000), they may also accumulate antimicrobial compounds such as elemental sulfur and phytoalexins (Cooper and Williams, 2004; Sun et al., 2008). Furthermore, these gels are strengthened by deposition of lignin and other phenolic compounds, which make these plugs strong physical barriers (Kpemoua et al., 1996; Rioux et al., 1998).
Formation of vascular gels is considered an important part of resistance towards several wilt diseases (Table 1). For example, F. oxysporum f. sp. dianthi colonization is restricted due to the formation of gels in the vascular lumen of carnation plants (Baayen and Elgersma, 1985). Moreover, in most resistant cultivars these gels are often observed together with tyloses (Vander Molen et al., 1987; Grimault et al., 1994). In banana plants resistant to F. oxysporum f. sp cubense, formation of vascular occlusions including both gels and tyloses have been observed (Vander Molen et al., 1977). Gel formation in the xylem lumen is also a trait of tomato cultivars resistant towards the vascular bacterium R. solanacearum (Grimault et al., 1994). In other cases, such as pea plants resistant to F. oxysporum f. sp. pisi, vascular gels, but not tyloses, are observed after infection (Bishop and Cooper, 1984).
Horizontal restriction of vascular colonization
The mechanisms mentioned above are part of host responses that restrict vertical movement of vascular pathogens to healthy regions of the host. In addition, resistant plants can often develop a protective vascular coating upon invasion by vascular pathogens, posing a horizontal barrier to further colonization of adjacent healthy tissues. Vascular coating involves physico-chemical structural modifications in the cell walls of xylem tissues that result in confinement of the pathogen to the infected vessels (Figs 1, 2A).
Fig. 2.
Lignin and suberin have a major role in vascular coating induced by xylem vascular wilt pathogens. A. Schematic structure of reinforced cell walls of xylem vessels and parenchyma cells in resistant plants upon infection with xylem vascular wilt pathogens. B. The phenypropanoid pathway provides precursors for both lignin and suberin biosynthesis. Phenylalanine, derived from the shikimate pathway, undergoes several enzymatic reactions as part of the phenylproanoid pathway. The resulting precursors yield the monolignols p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, which are the building blocks of lignin. In parallel, the phenypropanoid metabolites feruloyl-CoA, caffeoyl-CoA, and p-coumaroyl-CoA bifurcate into the suberin pathway. In the suberin pathway, these metabolites can be conjugated to aromatic amine compounds such as tyramine by the action of THT, or can be linked to aliphatic compounds by the action of FHT, to yield suberin monomers. Lignin and suberin monomers are then transported to the cell wall, where they are subsequently polymerized into the reinforcing matrices that constitute vascular coating structures. Abbreviations: PAL: phenylalanine ammonia–lyase; C4H: cinnamate–4–hydroxylase; C3H: coumarate 3-hydroxylase; 4CL: 4–coumarate–CoA ligase; HCT: hydroxycinnamoyl–CoA shikimate/quinate hydroxycinnamoyl transferase; COMT: caffeic acid 3-O-methyltransferase; CCOMT: caffeoyl CoA 3-O-methyltransferase; CCR: cinnamoyl CoA reductase; CAD: cinnamoyl alcohol dehydrogenase; PRX: peroxidase; CYP86A1: fatty acid cytochrome P450 oxidases; FAR: fatty acyl-CoA reductase; TyDC: tyrosine decarboxylase; FHT: feruloyl transferase; THT: tyramine hydroxycinnamoyl transferase.
Xylem pits are the primary routes of vessel-to-vessel and vessel-to-parenchyma cell water transport. Pits are covered by a pit membrane, which is impermeable to particulate matter like bacteria and other pathogens (Choat et al., 2008). For a pathogen to achieve successful horizontal transfer into vessels, it has to either form openings in vessel walls or degrade pit membranes, thereby reaching the adjacent parenchyma cells and vessels (Nakaho et al., 2000). To avoid the breach by pathogens, resistant plants with altered composition and structure of homogalacturonans (HGs) and xyloglucans (XyGs) in pit membranes have evolved; however, these compounds are potential targets of the pathogen’s cell wall degrading enzymes. Grapevine genotypes resistant to X. fastidiosa lacked fucosylated XyGs and weakly methylesterified HGs (ME-HGs), and contained a small amount of heavily ME-HGs. In contrast, pit membranes of susceptible genotypes all had substantial amounts of fucosylated XyGs and weakly ME-HGs, but lacked heavily ME-HGs (Sun et al., 2011).
In addition, reinforcement occurs at vessel walls, parenchyma cells and pit membranes, to confine the spread of vascular pathogens. Ultra-microscopic studies showed that the pit membranes, as well as vessels walls and parenchyma cells, form a conspicuously thick coating in the form of an electron dense amorphous layer, as part of the defense response against vascular pathogens (Street et al., 1986; Benhamou, 1995; Daayf et al., 1997; Nakaho et al., 2000; Araujo et al., 2014). Such reinforcement acts to limit the horizontal movement of the pathogen from the protoxylem or the primary xylem to the surrounding cells (Street et al., 1986; Benhamou, 1995; Daayf et al., 1997; Nakaho et al., 2000; Araujo et al., 2014). Besides, its deposition acts as a shield against pathogen-derived metabolites such as toxins and enzymes, and makes water and nutrients inaccessible for pathogens, thereby impeding their growth (Araujo et al., 2014).
Importantly, the timing of synthesis of the vascular coating plays a crucial role in immunity. Even if vascular coating can be observed in response to vascular pathogens in susceptible plants, these structures form at late time points, compared with their induction in resistant plants (Shi et al., 1991; Daayf et al., 1997). The specific composition of vascular deposits varies depending on the particular host-pathogen interaction. However, phenolics are the most important compounds, as they act as building blocks of the secondary cell wall, and they also have direct antimicrobial activity (Eynck et al., 2009). Among the phenolic polymers constituting vascular coating structures, the principal players are lignin and suberin, described in further detail below. We also outline the role of callose, a non-phenolic compound that plays an important role in the formation of horizontal vascular barriers in certain interactions.
Deposition of lignin
Lignin is a complex phenolic polymer that constitutes a major component of secondary cell walls in vascular plants. Lignin imparts strength to secondary cell walls, being deposited in spaces between cellulose, hemicellulose and pectin (Kang et al., 2019; Fig. 2A). The building blocks of the lignin polymer are monolignols, synthesized from phenylalanine via the phenylpropanoid pathway, where numerous enzymes are involved (Fig. 2B). Monolignols are then transported from the cytosol into the apoplast, where they are polymerized to lignin units by the oxidative activities of laccases and peroxidases.
Lignin is a particularly important element in cell walls of xylem tissue cells, not only because of its structural function, but also because it facilitates water retention in vascular bundles due to its hydrophobic nature. In addition, lignin is resistant to biodegradation and acts as a potent structural barrier against pathogens. Although lignin is an integral part of pre-existing structural barriers in plants, its deposition can also be induced in the xylem upon pathogen attack to form additional reinforcements that further restrict pathogen colonization. This inducible deposition of lignin as a vascular coating can be an important component of resistance towards certain pathogens (Table 1).
Restriction of pathogen colonization by lignin has been elegantly shown using a non-vascular pathogen, the leaf-infecting bacterium Pseudomonas syringae pv tomato (Pto) on Arabidopsis thaliana (Lee et al., 2019). It was shown that infection with either virulent or avirulent strains of the pathogen induced localized lignification at the site of pathogen attack. However, lignin deposition was more conspicuous upon avirulent Pto recognition, leading to confinement of the pathogen, and restricting hypersensitive response cell death to the infection site. In contrast, virulent strains could overcome induced lignification, as the deposition in this case was milder, leading to unrestricted disease progression.
Interestingly, Casparian strip membrane domain proteins (CASPs), were shown to be involved in pathogen-induced lignin deposition (Lee et al., 2019). The Casparian strip acts as a diffusion barrier that controls the uptake of water and molecules from the soil into the water-conducting tissues, and prevents the entry of pathogens and other harmful substances. Both the Casparian strip and the above-mentioned pathogen-induced lignification mechanism involve lignin-containing structures that function as anti-pathogenic physical barriers, serving parallel functions. In addition to lignin, suberin (discussed in detail later) is a chief component of the Casparian strip (Doblas et al., 2017). Recent findings show that the Casparian strip possesses a barrier surveillance pathway comprised of the receptor-like cytoplasmic kinase SCHENGEN1 and the LRR-RLK SCHENGEN3, hence bearing a striking resemblance to signaling pathways for perception of pathogen-associated molecular patterns (Alassimone et al., 2016; Fujita et al., 2020). These receptors in the Casparian strip domain interact with peptides (CASPARIAN STRIP INTEGRITY FACTORS, CIF1/2) expressed in the stele, leading to Casparian strip formation. This spatial separation of receptor and ligand constitutes a surveillance system where interaction stops after effective sealing by the strip, but any breach in the barrier leads to re-interaction and further strengthening (Doblas et al., 2017).
Lignin deposits seem to also play a crucial role in spatial growth restriction of vascular pathogens. There are several examples showing a pronounced transcriptional upregulation of genes involved in lignin biosynthesis in resistant plants following infection with various vascular pathogens (Table 1). This occurs, for example, in cotton after V. dahliae infection, flax after infection with F. oxysporum, tomato after infection with R. solanacearum, or olive tree after X. fastidiosa infection (Xu et al., 2011; Ishihara et al., 2012; Galindo-González and Deyholos, 2016; Sabella et al., 2018). Furthermore, there are several examples showing that resistance/tolerance to vascular pathogens is accompanied by an increase in lignin content and enhanced cell wall lignification upon infection. This includes examples of different pathosystems such as V. dahliae—pepper, O. novo-ulmi—Ulmus minor, V. longisporum—rapeseed, F. oxysporum—banana or tomato, and R. solanacearum—potato (Street et al., 1986; De Ascensao and Dubery, 2000; Martín et al., 2005; Martin et al., 2007; Eynck et al., 2009; Ferreira et al., 2017; Novo et al., 2017).
Nevertheless, the mechanisms that orchestrate timely and effective induction of lignin deposition in resistant plants upon vascular colonization remain vastly unknown. Some progress has been made using cotton plants infected with V. dahliae. In this pathosystem, two proteins potentially regulating V. dahliae-induced vascular lignin deposition have been identified. GhUMC1, a copper-binding protein, is involved in resistance mediated by lignin deposition and jasmonate signaling (Zhu et al., 2018). GhUMC1 knock-down plants are more susceptible to the pathogen, and lignin vascular coating is drastically reduced. On the other hand, the proline-rich protein GhHyPRP1 acts as a negative regulator of defense against V. dahlia, and was shown to induce lignin deposition (Zhu et al., 2018). In accordance, GhHyPRP1 knock-down plants displayed more lignin deposition upon infection and were more resistant to the pathogen. Interestingly, PevD1, a V. dahliae secreted protein, has been shown to activate defenses in cotton, triggering the expression of phenylpropanoid genes and lignin accumulation (Bu et al., 2014). This may indicate that PevD1 acts as an avirulence effector in cotton triggering a defense reaction that includes lignin deposition in the vasculature, which may lead to pathogen confinement into infected vessels. It remains to be determined whether the differential lignin deposition phenotypes observed in resistant cultivars is a direct consequence of pathogen effector recognition via its secreted effectors, and if so, to what degree the mechanisms are conserved between different pathosystems. Moreover, the molecular players involved also need to be identified.
Deposition of suberin
Suberin is a heteropolymer that deposits as a poly-lamellar structure between the plasma membrane and the cell wall, forming a hydrophobic protective barrier (Fig. 2A). Suberin is deposited in specialized tissues such as root and tuber epidermis, root endodermis, and seed coats. In addition, suberin is formed in response to several stresses such as wounding, salt injury and pathogen attack (Dixon and Paiva, 1995; Bernards, 2002). Besides providing strength to the cell wall, suberin prevents water loss and pathogen entry by sealing off the layer of suberized cells.
Suberin consists of a polyphenolic and a polyaliphatic domain. The polyphenolic domain is predominantly formed by esters and amides of ferulic acid, as well as other hydroxycinnamic acids, such as caffeic acid and p-coumaric acid (Negrel et al., 1995; Lashbrooke et al., 2016; Woolfson et al., 2011; Woolfson, 2018). The aliphatic domain consists of a glycerol-based fatty acid-derived polyester comprised primarily of ω-hydroxyacids, α, ω-dicarboxylic acids, fatty alcohols, and small amounts of hydroxycinnamic acids (mainly alkyl ferulates; Beisson et al., 2012). Ferulic esters composed of ferulic acid esterified to fatty acids are considered as one of the monomers of suberin polymer (Negrel et al., 1995).
Significant progress elucidating suberin biosynthesis has been achieved in the last two decades using molecular genetics approaches, especially in the model species A. thaliana and in potato tuber periderm (Ranathunge et al., 2011). Suberin shares the phenypropanoid pathway with lignin (Fig. 2B). This pathway provides the precursors for its polyphenolic domain, which are used by downstream suberin-specific enzymes such as tyramine N-feruloyltransferase (THT) and feruloyl trasferase (FHT; Fig. 2B; Serra et al., 2010; Woolfson, 2018; Woolfson et al., 2011). Similarly, several genes involved in the aliphatic metabolism of suberin have been described, such as fatty acid cytochrome P450 oxidases (CYP86A1), fatty acyl-CoA reductase (FARs), β-ketoacyl-CoA synthases (KCS2/Daisy and KCS20), glycerol-3-phosphate acyltransferase5 (GPAT5), as well as ATP-BINDING CASSETTE G (ABCG) genes involved in the delivery of suberin monomers to the site of suberization (ABCG2, ABCG6, and ABCG20; Beisson et al., 2007; Höfer et al., 2008; Franke et al., 2009; Vishwanath et al., 2013; Yadav et al., 2014). In addition, a few upstream regulators of the suberin biosynthetic pathway have been identified in A. thaliana, such as the MYB transcription factors MYB41, MYB107 and SUBERMAN (MYB39; Kosma et al., 2014; Gou et al., 2017; Cohen et al., 2020).
Suberin reinforcement in the xylem vascular tissue has been long recognized as a potent barrier to colonization by pathogens (Robb et al., 1991). There are numerous studies reporting that suberin deposition in the xylem tissue upon infection contributes to resistance (Table 1). For example, vascular deposits have been observed after infection with V. albo-atrum in resistant tomato and alfalfa (Street et al., 1986; Newcombe and Robb, 1988; Robb et al., 1991). Similarly, induction of suberin deposition is an important line of defense in Ulmus minor against O. novo-ulmi (Martín et al., 2008). Interestingly, exogenous application of phenolic compounds further increased resistance of trees to this pathogen through formation of suberin-like compounds in xylem tissues (Martín et al., 2008). Another example of xylem tissue suberization induced by a vascular pathogen is provided by the histological characterization of grapevine infection by the wilt fungus Phaeomoniella chlamydospora (Pouzoulet et al., 2013, 2017). It was shown that deposition of suberin in paravascular parenchyma cells is an effective barrier against horizontal P. chlamydospora colonization from one vessel to the adjacent vessel. Suberin was also shown to form deposits in tyloses induced by infection with this pathogen (Pouzoulet et al., 2013). Interestingly, inhibition of tylose formation in grapevine by P. chlamydospora led to vascular coating of surrounding parenchyma cells with phenolic compounds, including suberin (Pouzoulet et al., 2017). This indicates that sequential/superimposed defense mechanisms are in place to restrict pathogen progression to the infection site, once it has reached the vasculature.
Although suberin deposition seems to be an important component of defense responses against vascular pathogens, very little is known about its regulation. Similar to lignin, the effectiveness of vascular suberization as a structural barrier against horizontal colonization by vascular pathogens largely depends on the spatio-temporal control of its deposition, i.e. formation of suberin deposits early after pathogen detection at the site of vascular invasion. The phytohormones abscisic acid (ABA) and ethylene have both been shown to regulate suberization (Soliday et al., 1978; Cottle and Kolattukudy, 1982; Barberon et al., 2016). Since these two hormones are involved in defense against various vascular pathogens, a possibility exists that pathogen-induced vascular suberization correlates with an increase of hormone concentrations during immune responses. However, the mechanistic links between these hormonal pathways and suberin biosynthesis during pathogen-triggered suberin vascular coating remain to be established.
Deposition of callose
Callose is a linear amorphous cell wall polysaccharide formed by hundreds of glucose units linked by β-1,3 glucosidic bonds (Stone, 2009). This homopolysaccharide is synthesized from uridine diphosphate glucose by callose synthases (also known as CalS or GSL for glucan synthase-like), large multisubunit complexes at the plasma membrane (Ellinger and Voigt, 2014). Callose is not a particularly abundant polymer in the cell wall, but has very relevant regulatory roles in development, plasmodesmata function, as well as in immunity (Schneider et al., 2016). Pathogen-induced callose deposition has been shown to be localized to callosic papillae, providing structural defense against various pathogens (Schneider et al., 2016). In addition, callose can constitute a matrix for accumulation of antimicrobial compounds, thereby providing targeted delivery of chemical defenses at the sites of pathogen attack (Luna et al., 2011).
In the vasculature, callose has also been shown to act as a structural barrier against fungal wilt pathogens, restricting their horizontal vessel-to-vessel movement. Tomato plants resistant to F. oxysporum f. sp. lycopersici form callose deposits in paravascular parenchyma cells and at pit membranes in response to infection by this pathogen (Beckman et al., 1982; Mueller and Beckman, 1988). In addition, application of the microbe-associated molecular pattern chitosan, a derivative of chitin, restricts colonization of F. oxysporum f.sp. lycopersici by inducing a vascular coating composed of callose and phenolic compounds (Benhamou et al., 1994). Furthermore, cotton roots infected with V. dahliae showed reinforcement with callose deposits (Daayf et al., 1997). In contrast, infection by the bacterial wilt pathogen R. solanacearum caused deposition of callose in both tolerant and susceptible potato plants, indicating that in this interaction callose may not be as important for resistance towards the pathogen (Ferreira et al., 2017). Additional research is needed to clarify the precise role and regulation of callose as a structural defense mechanism induced upon perception of vascular wilt pathogens.
Concluding remarks and future prospects
During the last few decades, much evidence has accumulated showing the importance of physico-chemical barriers as a crucial component of resistance towards xylem vascular pathogens. From all the research until now in this field, it becomes clear that the localization and timing of formation of these vascular structures is key for their effectiveness as barriers for pathogen confinement. Resistant plants are able to form vertical and horizontal barriers quickly upon pathogen invasion of the vasculature, confining them to infected vessels and avoiding spread to the rest of the plant. In susceptible plants, formation of the same vascular structures is observed, but not targeted to infected vessels, and later in time, once the pathogen has spread throughout the plant they have no effect on disease progression. However, the mechanisms regulating the spatial and temporal formation of vascular structures leading to effective pathogen confinement, and their precise composition, are old questions that remain unanswered. In fact, it remains unclear as to how vascular wilt pathogens are perceived at the vasculature, and how this perception is transduced into timely and restricted formation of structural defenses. Since effective mechanisms of resistance are very much sought after in breeding programs, in the coming years it will be important to make an effort to advance knowledge in this area, even though inducible structural defenses are governed by complex polygenic traits.
Major technological advances in the last few years have placed plant molecular biologists in a privileged position to make significant advances. Of particularly relevance is the CRISPR-Cas9 technology, which has proven extremely efficient for Solanaceous crops such as tomato, pepper and eggplant, which are severely affected by wilt diseases caused by xylem vascular pathogens (Hu et al., 2019; Li et al., 2019; Wang et al., 2019). Importantly, an array of technologies have emerged that allow the study of specific processes in a cell, or in a tissue-specific manner. These techniques will become instrumental in the study of plant-pathogen interactions, which constitute a localized phenomenon by its very nature. This is particularly the case for colonization of vascular cells by vascular pathogens, since the ability to confine the invading agent is a key feature of resistant plants. With the advent of single-cell technologies it will be possible to attain astonishing resolution when investigating the processes occurring at infected cells and surrounding areas. For instance, RNA sequencing of laser-dissected areas or single cells allows profiling of the transcriptomic landscape after infection at relevant sites. In turn, this will allow the identification of marker genes associated with the formation of structural defenses and the subsequent generation of transgenic marker lines, to be able to track relevant cells/tissues at early time points after infection for their analysis. In addition, extremely sensitive analytical techniques have been developed in recent years that allow the identification and quantification of proteins, small molecules and metabolites, and the interactions between them. This includes Raman spectroscopy or MALDI (matrix-assisted laser desorption ionization) spectrometry imaging, both of which can be extremely useful in zonal responses such as structural resistance. All this knowledge could lead in the future to the engineering of metabolic pathways of vascular coating compounds in specific cells, to produce resistant plants against xylem colonizers.
With this article we hope to contribute towards raising awareness of the importance of attaining a better understanding of the structural physico-chemical barriers as a crucial component of resistance towards xylem vascular pathogens. Disease management through host resistance is the most efficient and eco-friendly approach to control pathogens. However, a lot has to be learnt about the complex genetic interactions which govern induced structural resistance in various hosts, to be able to deploy this trait in future cultivars and fight vascular pathogens, agents of the most devastating plant diseases in the field.
Acknowledgements
The authors would like to thank all members of the Bacterial plant diseases and cell death lab for helpful comments. We apologize to all authors whose work has been omitted because of space limitations. Research in the lab is funded by the Spanish Ministry of Economy and Competitiveness with grants 2016-78002-R (AGL) and RyC 2014–16158 (NSC), by the Ministry of Science and Innovation / Spanish State Research Agency PID2019-108595RB-I00 / AEI / 10.13039/501100011033, and through the “Severo Ochoa Programme for Centres of Excellence in R&D” (SEV-2015-0533). AK is the recipient of a Netaji Subhas—Indian Council of Agricultural Research (ICAR) International Fellowship. This work was also supported by the CERCA Programme / Generalitat de Catalunya.
Author contribution
AK, MP-M, MC, MV, and NSC conceptualized and wrote the article, and obtained funds.
References
- Alassimone J, Fujita S, Doblas VG, et al. 2016. Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nature Plants 2, 16113. [DOI] [PubMed] [Google Scholar]
- Álvarez B, Biosca EG, López MM. 2010. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. In: Méndez-Vilas A, ed. Technology and education topics in applied microbiology and microbial biotechnology. Current Research, Technology and Education Topics in Applied Microbiology. Badajoz: Formatex, 267–279. [Google Scholar]
- Antanaviciute L, Šurbanovski N, Harrison N, McLeary KJ, Simpson DW, Wilson F, Sargent DJ, Harrison RJ. 2015. Mapping QTL associated with Verticillium dahliae resistance in the cultivated strawberry (Fragaria × ananassa). Horticulture Research 2, 15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo L, Bispo WM, Cacique IS, Moreira WR, Rodrigues FÁ. 2014. Resistance in mango against infection by Ceratocystis fimbriata. Phytopathology 104, 820–833. [DOI] [PubMed] [Google Scholar]
- Baayen RP, Elgersma DM. 1985. Colonization and histopathology of susceptible and resistant carnation cultivars infected with Fusarium oxysporum f. sp. dianthi. Netherlands Journal of Plant Pathology 91, 119–135. [Google Scholar]
- Bae C, Han SW, Song YR, Kim BY, Lee HJ, Lee JM, Yeam I, Heu S, Oh CS. 2015. Infection processes of xylem-colonizing pathogenic bacteria: possible explanations for the scarcity of qualitative disease resistance genes against them in crops. Theoretical and Applied Genetics 128, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Bani M, Pérez-De-Luque A, Rubiales D, Rispail N. 2018. Physical and chemical barriers in root tissues contribute to quantitative resistance to Fusarium oxysporum f. sp. pisi in pea. Frontiers in Plant Science 9, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andeersen TG, Humbel BM, Nawrath C, Takano J, Salt DE. 2016. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447–459. [DOI] [PubMed] [Google Scholar]
- Beckman CH, Mueller WC, Tessier BJ, Harrison NA. 1982. Recognition and callose deposition in response to vascular infection in Fusarium wilt-resistant or susceptible tomato plants. Physiological Plant Pathology 20, 1–10. [Google Scholar]
- Beckman CH, Roberts EM. 1995. On the nature and genetic basis for resistance and tolerance to fungal wilt diseases of plants. Advances in Botanical Research 21, 35–77. [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. 2007. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. The Plant cell 19, 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson F, Li-Beisson Y, Pollard M. 2012. Solving the puzzles of cutin and suberin polymer biosynthesis. Current Opinion in Plant Biology 15, 329–337. [DOI] [PubMed] [Google Scholar]
- Benhamou N, Lafontaine P, Nicole M. 1994. Induction of systemic resistance to Fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology 84, 1432–1444. [Google Scholar]
- Benhamou N 1995. Ultrastructural and cytochemical aspects of the response of eggplant parenchyma cells in direct contact with Verticillium-infected xylem vessels. Physiological and Molecular Plant Pathology 46, 321–338. [Google Scholar]
- Bernards MA 2002. Demystifying suberin. Canadian Journal of Botany 80, 227–240. [Google Scholar]
- Bernards MA 2018. Differential induction of polar and non-polar metabolism during wound-induced suberization in potato (Solanum tuberosum L.) tubers. The Plant Journal 93, 931–942. [DOI] [PubMed] [Google Scholar]
- Bishop CD, Cooper RM. 1983. An ultrastructural study of root invasion in three vascular wilt diseases. Physiological Plant Pathology 22, 15–27. [Google Scholar]
- Bishop CD, Cooper RM. 1984. Ultrastructure of vascular colonization by fungal wilt pathogens. II. Invasion of resistant cultivars. Physiological Plant Pathology 24, 277–289. [Google Scholar]
- Blaedow RA, Juzwik J. 2010. Spatial and temporal distribution of Ceratocystis fagacearum in roots and root grafts of oak wilt affected red oaks. Arboriculture and Urban Forestry 36, 28–34. [Google Scholar]
- Bonsen KJM, Kucera LJ. 1990. Vessel occlusions in plants: morphological, functional and evolutionary aspects. IAWA Bulletin 11, 393–399. [Google Scholar]
- Bu B, Qiu D, Zeng H, Guo L, Yuan J, Yang X. 2014. A fungal protein elicitor PevD1 induces Verticillium wilt resistance in cotton. Plant Cell Reports 33, 461–470. [DOI] [PubMed] [Google Scholar]
- Bubán T, Orosz-Kovács Z, Farkas Á. 2003. The nectary as the primary site of infection by Erwinia amylovora (Burr.) Winslow et al.: A mini review. Plant Systematics and Evolution 238, 183–194. [Google Scholar]
- Carmeille A, Caranta C, Dintinger J, Prior P, Luisetti J, Besse P. 2006. Identification of QTLs for Ralstonia solanacearum race 3-phylotype II resistance in tomato. Theoretical and Applied Genetics 113, 110–121. [DOI] [PubMed] [Google Scholar]
- Catanzariti AM, Do HT, Bru P, de Sain M, Thatcher LF, Rep M, Jones DA. 2017. The tomato I gene for Fusarium wilt resistance encodes an atypical leucine-rich repeat receptor-like protein whose function is nevertheless dependent on SOBIR1 and SERK3/BAK1. The Plant Journal 89, 1195–1209. [DOI] [PubMed] [Google Scholar]
- Catanzariti AM, Lim GT, Jones DA. 2015. The tomato I-3 gene: a novel gene for resistance to Fusarium wilt disease. New Phytologist 207, 106–118. [DOI] [PubMed] [Google Scholar]
- Chen YC, Wong CL, Muzzi F, Vlaardingerbroek I, Kidd BN, Schenk PM. 2014. Root defense analysis against Fusarium oxysporum reveals new regulators to confer resistance. Scientific Reports 4, 5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Cobb AR, Jansen S. 2008. Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytologist 177, 608–625. [DOI] [PubMed] [Google Scholar]
- Clérivet A, Déon V, Alami I, Lopez F, Geiger JP, Nicole M. 2000. Tyloses and gels associated with cellulose accumulation in vessels are responses of plane tree seedlings (Platanus x acerifolia) to the vascular fungus Ceratocystis fimbriata f. sp platani. Trees 15, 25–31. [Google Scholar]
- Cochard H, Tyree MT. 1990. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiology 6, 393–407. [DOI] [PubMed] [Google Scholar]
- Cohen H, Fedyuk V, Wang C, Wu S, Aharoni A. 2020. SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. The Plant Journal 102, 431–447. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Williams JS. 2004. Elemental sulphur as an induced antifungal substance in plant defence. Journal of Experimental Botany 55, 1947–1953. [DOI] [PubMed] [Google Scholar]
- Cottle W, Kolattukudy PE. 1982. Abscisic acid stimulation of suberization: induction of enzymes and deposition of polymeric components and associated waxes in tissue cultures of potato tuber. Plant Physiology 70, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daayf F, Nicole M, Boher B, Pando A, Geiger JP. 1997. Early vascular defense reactions of cotton roots infected with a defoliating mutant strain of Verticillium dahliae. European Journal of Plant Pathology 103, 125–136. [Google Scholar]
- Davison EM, Tay FCS. 1985. The effect of waterlogging on seedlings of Eucalyptus marginata. New Phytologist 101, 743–753. [Google Scholar]
- De Ascensao AR, Dubery IA. 2000. Panama disease: cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. sp. cubense race four. Phytopathology 90, 1173–1180. [DOI] [PubMed] [Google Scholar]
- de Jonge R, van Esse HP, Maruthachalam K, et al. 2012. Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proceedings of the National Academy of Sciences, USA 109, 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Micco V, Balzano A, Wheeler EA, Baas P. 2016. Tyloses and gums: a review of structure, function and occurrence of vessel occlusions. IAWA Journal 37, 186–205. [Google Scholar]
- Deslandes L, Olivier J, Theulieres F, Hirsch J, Feng DX, Bittner-Eddy P, Beynon J, Marco Y. 2002. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proceedings of the National Academy of Sciences, USA 99, 2404–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyett E, Pouzoulet J, Yang JI, Ashworth VE, Castro C, Roper MC, Rolshausen PE. 2019. Assessment of Pierce’s disease susceptibility in Vitis vinifera cultivars with different pedigrees. Plant Pathology 68, 1079–1087. [Google Scholar]
- Diener AC, Ausubel FM. 2005. RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171, 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. The Plant Cell 7, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas VG, Smakowska-Luzan E, Fujita S, Alassimone J, Barberon M, Madalinski M, Belkhadir Y, Geldner N. 2017. Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 355, 280–284. [DOI] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. 2014. Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Annals of Botany 114, 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eynck C, Koopmann B, Karlovsky P, von Tiedemann A. 2009. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 99, 802–811. [DOI] [PubMed] [Google Scholar]
- Ferreira V, Pianzzola MJ, Vilaró FL, Galván GA, Tondo ML, Rodriguez MV, Orellano EG, Valls M, Siri MI. 2017. Interspecific potato breeding lines display differential colonization patterns and induced defense responses after Ralstonia solanacearum infection. Frontiers in Plant Science 8, 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R, Höfer R, Briesen I, Emsermann M, Efremova N, Yephremov A, Schreiber L. 2009. The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. The Plant Journal 57, 80–95. [DOI] [PubMed] [Google Scholar]
- Fujita S, De Bellis D, Edel KH, et al. 2020. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. The EMBO Journal 39, e103894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-González L, Deyholos MK. 2016. RNA-seq transcriptome response of flax (Linum usitatissimum L.) to the pathogenic fungus Fusarium oxysporum f. sp. lini. Frontiers in Plant Science 7, 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou M, Hou G, Yang H, Zhang X, Cai Y, Kai G, Liu CJ. 2017. The MYB107 transcription factor positively regulates suberin biosynthesis. Plant Physiology 173, 1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaje D, Úrbez-Torres JR, Sosnowski MR. 2018. Managing grapevine trunk diseases with respect to etiology and epidemiology: current strategies and future prospects. Plant Disease 102, 12–39. [DOI] [PubMed] [Google Scholar]
- Grimault V, Gélie B, Lemattre M, Prior P, Schmit J. 1994. Comparative histology of resistant and susceptible tomato cultivars infected by Pseudomonas solanacearum. Physiological and Molecular Plant Pathology 44, 105–123. [Google Scholar]
- Gui YJ, Chen JY, Zhang DD, et al. 2017. Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environmental Microbiology 19, 1914–1932. [DOI] [PubMed] [Google Scholar]
- Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R. 2008. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. Journal of Experimental Botany 59, 2347–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houterman PM, Ma L, van Ooijen G, de Vroomen MJ, Cornelissen BJ, Takken FL, Rep M. 2009. The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. The Plant Journal 58, 970–978. [DOI] [PubMed] [Google Scholar]
- Hu N, Xian Z, Li N, Liu Y, Huang W, Yan F, Su D, Chen J, Li Z. 2019. Rapid and user-friendly open-source CRISPR/Cas9 system for single- or multi-site editing of tomato genome. Horticulture Research 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson RA, Smith IM. 1980. Phytoalexins and tyloses in tomato cultivars infected with Fusarium oxysporum f.sp. lycopersici or Verticillium albo-atrum. Physiological Plant Pathology 17, 245–257. [Google Scholar]
- Ishihara T, Mitsuhara I, Hideki T, Kazuhiro N. 2012. Transcriptome analysis of quantitative resistance- specific response upon Ralstonia solanacearum infection in tomato. PLoS Pathogens 7, e46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Joobeur T, King JJ, Nolin SJ, Thomas CE, Dean RA. 2004. The Fusarium wilt resistance locus Fom-2 of melon contains a single resistance gene with complex features. The Plant Journal 39, 283–297. [DOI] [PubMed] [Google Scholar]
- Kang X, Kirui A, Dickwella Widanage MC, Mentink-Vigier F, Cosgrove DJ, Wang T. 2019. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nature Communications 10, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Tsuda K. 2010. Understanding the plant immune system. Molecular Plant-Microbe Interactions 23, 1531–1536. [DOI] [PubMed] [Google Scholar]
- Kim SG, Hur OS, Ro NY, Ko HC, Rhee JH, Sung JS, Ryu KY, Lee SY, Baek HJ. 2016. Evaluation of resistance to Ralstonia solanacearum in tomato genetic resources at seedling stage. The Plant Pathology Journal 32, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosma DK, Murmu J, Razeq FM, Santos P, Bourgault R, Molina I, Rowland O. 2014. AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. The Plant Journal 80, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kpemoua K, Boher B, Nicole M, Calatayud P, Geiger J. 1996. Cytochemistry of defense responses in cassava infected by Xanthomonas campestris pv. manihotis. Canadian Journal of Microbiology 42, 1131–1143. [Google Scholar]
- Krivanek AF, Riaz S, Walker MA. 2006. Identification and molecular mapping of PdR1 a primary resistance gene to Pierce’s disease in Vitis. Theoretical and Applied Genetics 112, 1125–1131. [DOI] [PubMed] [Google Scholar]
- Lambel S, Lanini B, Vivoda E, Fauve J, Patrick Wechter W, Harris-Shultz KR, Massey L, Levi A. 2014. A major QTL associated with Fusarium oxysporum race 1 resistance identified in genetic populations derived from closely related watermelon lines using selective genotyping and genotyping-by-sequencing for SNP discovery. Theoretical and Applied Genetics 127, 2105–2115. [DOI] [PubMed] [Google Scholar]
- Lashbrooke J, Cohen H, Levy-Samocha D, et al. 2016. MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. The Plant Cell 28, 2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux C, Huet G, Jauneau A, et al. 2015. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 161, 1074–1088. [DOI] [PubMed] [Google Scholar]
- Lee MH, Jeon HS, Kim SH, Chung JH, Roppolo D, Lee HJ, Cho HJ, Tobimatsu Y, Ralph J, Park OK. 2019. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. The EMBO Journal 38, e101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leśniewska J, Öhman D, Krzesłowska M, Kushwah S, Barciszewska-Pacak M, Kleczkowski LA, Sundberg B, Moritz T, Mellerowicz EJ. 2017. Defense responses in aspen with altered pectin methylesterase activity reveal the hormonal inducers of tyloses. Plant Physiology 173, 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu C, Zhao R, Wang L, Chen L, Yu W, Zhang S, Sheng J, Shen L. 2019. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biology 19, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. 2011. Callose deposition: a multifaceted plant defense response. Molecular Plant-Microbe Interactions 24, 183–193. [DOI] [PubMed] [Google Scholar]
- Mangin B, Thoquet P, Olivier J, Grimsley NH. 1999. Temporal and multiple quantitative trait loci analyses of resistance to bacterial wilt in tomato permit the resolution of linked loci. Genetics 151, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín JA, Solla A, Coimbra MA, Gil L. 2005. Metabolic distinction of Ulmus minor xylem tissues after inoculation with Ophiostoma novo-ulmi. Phytochemistry 66, 2458–2467. [DOI] [PubMed] [Google Scholar]
- Martín JA, Solla A, Domingues MR, Coimbra MA, Gil L. 2008. Exogenous phenol increase resistance of Ulmus minor to Dutch elm disease through formation of suberin-like compounds on xylem tissues. Environmental and Experimental Botany 64, 97–104. [Google Scholar]
- Martin JA, Solla A, Woodward S, Gil L. 2007. Detection of differential changes in lignin composition of elm xylem tissues inoculated with Ophiostoma novo-ulmi using fourier transform-infrared spectroscopy. Forest Pathology 37, 187–191. [Google Scholar]
- Mes JJ, van Doorn AA, Wijbrandi J, Simons G, Cornelissen BJ, Haring MA. 2000. Expression of the Fusarium resistance gene I-2 colocalizes with the site of fungal containment. The Plant Journal 23, 183–193. [DOI] [PubMed] [Google Scholar]
- Milling A, Babujee L, Allen C. 2011. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One 6, e15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello P, Díez CM, Codes M, Rallo L, Barranco D, Trapero A, Moral J. 2016. Sanitation of olive plants infected by Verticillium dahliae using heat treatments. Plant Pathology 65, 412–421. [Google Scholar]
- Mueller W, Beckman C. 1988. Correlated light and electron microscope studies of callose deposits in vascular paerenchyma cells of tomato plants inoculated with Fusarium oxysporum f.sp. lycopersici. Physiological and Molecular Plant Pathology 33, 201–208. [Google Scholar]
- Nakaho K, Hibino H, Miyagawa H. 2000. Possible mechanisms limiting movement of Ralstonia solanacearum in resistant tomato tissues. Journal of Phytopathology 148, 181–190. [Google Scholar]
- Negrel J, Lotfy S, Javelle F. 1995. Modulation of the activity of two hydroxycinnamoyl transferases in wound-healing potato tuber discs in response to pectinase or abscisic acid. Journal of Plant Physiology 146, 318–322. [Google Scholar]
- Newcombe G, Robb J. 1988. The function and relative importance of the vascular coating response in highly resistant, moderately resistant and susceptible alfalfa infected by Verticillium albo-atrum. Physiological and Molecular Plant Pathology 33, 47–58. [Google Scholar]
- Ngou BPM, Ahn H, Ding P, Jones JDG. 2020. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. BioRxiv 10.1101/2020.04.10.0341731-29. [Preprint]. [DOI] [PubMed] [Google Scholar]
- Nicholson RL 1992. Phenolic compounds and their role in disease resistance. Annual Reviews in Phytopathology 30, 369–389. [Google Scholar]
- Niemann AH 1994. A crucial role of phenolic metabolism in resistance of carnations to wilt diseases. Acta Horticulturae 381, 565–571. [Google Scholar]
- Nishimura MT, Dangl JL. 2010. Arabidopsis and the plant immune system. The Plant Journal 61, 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo M, Silvar C, Merino F, Martínez-Cortés T, Lu F, Ralph J, Pomar F. 2017. Deciphering the role of the phenylpropanoid metabolism in the tolerance of Capsicum annuum L. to Verticillium dahliae Kleb. Plant Science 258, 12–20. [DOI] [PubMed] [Google Scholar]
- Palanga KK, Jamshed M, Rashid MHO, et al. 2017. Quantitative Trait Locus mapping for Verticillium wilt resistance in an upland cotton recombinant inbred line using SNP-based high density genetic map. Frontiers in Plant Science 8, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Ronald PC. 2012. Cleavage and nuclear localization of the rice XA21 immune receptor. Nature Communications 3, 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana AM, Watson DC, Gordon TR. 2019. Transmission of Fusarium oxysporum f. sp. fragariae through stolons in strawberry plants. Plant Disease 103, 1249–1251. [DOI] [PubMed] [Google Scholar]
- Planas-Marquès M, Kressin JP, Kashyap A, Panthee DR, Louws FJ, Coll NS, Valls M. 2019. Four bottlenecks restrict colonization and invasion by the pathogen Ralstonia solanacearum in resistant tomato. Journal of Experimental Botany 71, 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta R, Urban J, Gebauer R, Dvořák M, Ďurkovič J. 2016. Long-term impact of Ophiostoma novo-ulmi on leaf traits and transpiration of branches in the Dutch elm hybrid ‘Dodoens’. Tree Physiology 36, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzoulet J, Jacques A, Besson X, Dayde J, Mailhac N. 2013. Histopathological study of response of Vitis vinifera ‘Cabernet Sauvignon’ to bark and wood injury with and without inoculation by Phaeomoniella chlamydospora. Phytopathologia Mediterranea 52, 313–323. [Google Scholar]
- Pouzoulet J, Pivovaroff AL, Santiago LS, Rolshausen PE. 2014. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Frontiers in Plant Science 5, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzoulet J, Rolshausen P, Charbois R, Chen J, Guillaumie S, Ollat N, Gambetta GA, Delmas CEL. 2020. Behind the curtain of the compartmentalization process: exploring how xylem vessel diameter impacts vascular pathogen resistance. Plant, Cell & Environment, doi: 10.1111/pce.13848. [DOI] [PubMed] [Google Scholar]
- Pouzoulet J, Scudiero E, Schiavon M, Rolshausen PE. 2017. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Frontiers in Plant Science 8, 1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouzoulet J, Scudiero E, Schiavon M, Santiago LS, Rolshausen PE. 2019. Modeling of xylem vessel occlusion in grapevine. Tree Physiology 39, 1438–1445. [DOI] [PubMed] [Google Scholar]
- Pradhan BB, Ranjan M, Chatterjee S. 2012. XadM, a novel adhesin of Xanthomonas oryzae pv. oryzae, exhibits similarity to Rhs family proteins and is required for optimum attachment, biofilm formation, and virulence. Molecular Plant-Microbe Interactions 25, 1157–1170. [DOI] [PubMed] [Google Scholar]
- Prakasha A, Darren Grice I, Vinay Kumar KS, Sadashiva MP, Shankar HN, Umesha S. 2017. Extracellular polysaccharide from Ralstonia solanacearum; a strong inducer of eggplant defense against bacterial wilt. Biological Control 110, 107–116. [Google Scholar]
- Ranathunge K, Schreiber L, Franke R. 2011. Suberin research in the genomics era–new interest for an old polymer. Plant Science 180, 399–413. [DOI] [PubMed] [Google Scholar]
- Rapicavoli JN, Blanco-Ulate B, Muszyński A, Figueroa-Balderas R, Morales-Cruz A, Azadi P, Dobruchowska JM, Castro C, Cantu D, Roper MC. 2018. Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nature Communications 9, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake K, Joyce DC, Webb RI. 2013. Cu2+ inhibition of gel secretion in the xylem and its potential implications for water uptake of cut Acacia holosericea stems. Physiologia Plantarum 148, 538–548. [DOI] [PubMed] [Google Scholar]
- Renzi M, Copini P, Taddei AR, Rossetti A, Gallipoli L, Mazzaglia A, Balestra GM. 2012. Bacterial canker on kiwifruit in Italy: anatomical changes in the wood and in the primary infection sites. Phytopathology 102, 827–840. [DOI] [PubMed] [Google Scholar]
- Rioux D, Blais M, Nadeau-Thibodeau N, Lagacé M, DesRochers P, Klimaszewska K, Bernier L. 2018. First extensive microscopic study of butternut defense mechanisms following inoculation with the canker pathogen Ophiognomonia clavigignenti-juglandacearum reveals compartmentalization of tissue damage. Phytopathology 108, 1237–1252. [DOI] [PubMed] [Google Scholar]
- Rioux D, Nicole M, Simard M, Ouellette GB. 1998. Immunocytochemical evidence that secretion of pectin occurs during gel (Gum) and tylosis formation in trees. Phytopathology 88, 494–505. [DOI] [PubMed] [Google Scholar]
- Robb J, Lee B, Nazar RN. 2007. Gene suppression in a tolerant tomato-vascular pathogen interaction. Planta 226, 299–309. [DOI] [PubMed] [Google Scholar]
- Robb J, Lee SW, Mohan R, Kolattukudy PE. 1991. Chemical characterization of stress-induced vascular coating in tomato. Plant Physiology 97, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbavarapu MM, Sharma M, Chamarthi SK, et al. 2013. Molecular mapping of QTLs for resistance to Fusarium wilt (race 1) and Ascochyta blight in chickpea (Cicer arietinum L.). Euphytica 193, 121–133. [Google Scholar]
- Sabella E, Luvisi A, Aprile A, Negro C, Vergine M, Nicolì F, Miceli A, De Bellis L. 2018. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees ‘Leccino’. Journal of Plant Physiology 220, 60–68. [DOI] [PubMed] [Google Scholar]
- Sanderlin RS, Melanson RA. 2006. Transmission of Xylella fastidiosa through pecan rootstock. HortScience 41, 1455–1456. [Google Scholar]
- Sarris PF, Duxbury Z, Huh SU, et al. 2015. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Schneider R, Hanak T, Persson S, Voigt CA. 2016. Cellulose and callose synthesis and organization in focus, what’s new? Current Opinion in Plant Biology 34, 9–16. [DOI] [PubMed] [Google Scholar]
- Schornack S, Ballvora A, Gürlebeck D, Peart J, Baulcombe D, Ganal M, Baker B, Bonas U, Lahaye T. 2004. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. The Plant Journal: for cell and molecular biology 37, 46–60. [DOI] [PubMed] [Google Scholar]
- Seo Y, Kim YH. 2017. Pathological interrelations of soil-borne diseases in Cucurbits caused by Fusarium species and Meloidogyne incognita. The Plant Pathology Journal 33, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra O, Hohn C, Franke R, Prat S, Molinas M, Figueras M. 2010. A feruloyl transferase involved in the biosynthesis of suberin and suberin-associated wax is required for maturation and sealing properties of potato periderm. The Plant journal 62, 277–290. [DOI] [PubMed] [Google Scholar]
- Shi J, Mueller WC, Beckman CH. 1991. Ultrastructural responses of vessel contact cells in cotton plants resistant or susceptible to infection by Fusarium oxysporum f. sp. vasinfectum. Physiological and Molecular Plant Pathology 38, 211–222. [Google Scholar]
- Shigo AL, Marx HG. 1977. Compartmentalization of decay in trees. U. S. Department of Agriculture. Agriculture Information Bulletin. 405, 1–73. [Google Scholar]
- Soliday CL, Dean BB, Kolattukudy PE. 1978. Suberization: inhibition by washing and stimulation by abscisic acid in potato disks and tissue culture. Plant Physiology 61, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu L, Wang Y, Valkenburg DJ, Zhang X, Zhu L, Thomma BPHJ. 2018. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnology Journal 16, 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA 2009. Chemistry of β-glucans. In: Bacic A, Fincher G, Stone B, eds. Chemistry, biochemistry and biology of 1–3 Beta glucans and related polysaccharides. San Diego, USA: Academic press, 5–46. [Google Scholar]
- Street PFS, Robb J, Ellis BE. 1986. Secretion of vascular coating components by xylem parenchyma cells of tomatoes infected with Verticillium albo-atrum. Protoplasma 132, 1–11. [Google Scholar]
- Sun Q, Greve LC, Labavitch JM. 2011. Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiology 155, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA. 2008. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): tyloses in summer and gels in winter. American Journal of Botany 95, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Reid MS, Matthews MA. 2007. Ethylene and not embolism is required for wound-induced tylose development in stems of grapevines. Plant Physiology 145, 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Sun Y, Walker MA, Labavitch JM. 2013. Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiology 161, 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nürnberger T, Joosten MHAJ. 2011. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 23, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoquet P, Olivier J, Sperisen C, Rogowsky P, Laterrot H, Grimsley N. 1996. Quantitative trait loci determining resistance to bacterial wilt in tomato ‘Hawaii7996’. Molecular Plant-Microbe Interactions 9, 826–836. [Google Scholar]
- Tjamos EC, Smith IM. 1975. The expression of resistance to Verticillium albo-atrum in monogenically resistant tomato varieties. Physiological Plant Pathology 6, 215–225. [Google Scholar]
- Triplett LR, Cohen SP, Heffelfinger C, Schmidt CL, Huerta AI, Tekete C, Verdier V, Bogdanove AJ, Leach JE. 2016. A resistance locus in the American heirloom rice variety Carolina Gold Select is triggered by TAL effectors with diverse predicted targets and is effective against African strains of Xanthomonas oryzae pv. oryzicola. The Plant Journal 87, 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa M, Hutmacher RB, Roberts PA, Wright SD, Nichols RL, Michael Davis R. 2013. Inheritance and QTL mapping of Fusarium wilt race 4 resistance in cotton. Theoretical and Applied Genetics 126, 1405–1418. [DOI] [PubMed] [Google Scholar]
- van der Does HC, Constantin ME, Houterman PM, Takken FLW, Cornelissen BJC, Haring MA, van den Burg HA, Rep M. 2019. Fusarium oxysporum colonizes the stem of resistant tomato plants, the extent varying with the R-gene present. European Journal of Plant Pathology 154, 55–65. [Google Scholar]
- Vander Molen GE, Beckman CH, Rodehorst E. 1987. The ultrastructure of tylose formation in resistant banana following inoculation with Fusarium oxysporum f.sp. cubense. Physiological and Molecular Plant Pathology 31, 185–200. [Google Scholar]
- Vander Molen GE, Beckman CH, Rodehorst E. 1977. Vascular gelation: a general response phenomenon following infection. Physiological Plant Pathology 11, 95–100. [Google Scholar]
- Vasse J, Frey P, Trigalet A. 1995. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Molecular Plant-Microbe Interactions 8, 241–251. [Google Scholar]
- Venturas M, López R, Martín JA, Gascó A, Gil L. 2014. Heritability of Ulmus minor resistance to Dutch elm disease and its relationship to vessel size, but not to xylem vulnerability to drought. Plant Pathology 63, 500–509. [Google Scholar]
- Vishwanath SJ, Kosma DK, Pulsifer IP, Scandola S, Pascal S, Joubès J, Dittrich-Domergue F, Lessire R, Rowland O, Domergue F. 2013. Suberin-associated fatty alcohols in Arabidopsis: distributions in roots and contributions to seed coat barrier properties. Plant Physiology 163, 1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulloa M, Duong T, Roberts PA. 2018. Quantitative Trait Loci mapping of multiple independent loci for resistance to Fusarium oxysporum f. sp. vasinfectum Races 1 and 4 in an interspecific cotton population. Phytopathology 108, 759–767. [DOI] [PubMed] [Google Scholar]
- Wang G, Roux B, Feng F, et al. 2015. The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host & Microbe 18, 285–295. [DOI] [PubMed] [Google Scholar]
- Wang JF, Ho FI, Truong HTH, Huang SM, Balatero CH, Dittapongpitch V, Hidayati N. 2013. Identification of major QTLs associated with stable resistance of tomato cultivar ‘Hawaii 7996’ to Ralstonia solanacearum. Euphytica 190, 241–252. [Google Scholar]
- Wang JF, Olivier J, Thoquet P, Mangin B, Sauviac L, Grimsley NH. 2000. Resistance of tomato line Hawaii7996 to Ralstonia solanacearum Pss4 in Taiwan is controlled mainly by a major strain-specific locus. Molecular Plant-Microbe Interactions 13, 6–13. [DOI] [PubMed] [Google Scholar]
- Wang L, Albert M, Einig E, Fürst U, Krust D, Felix G. 2016. The pattern-recognition receptor CORE of Solanaceae detects bacterial cold-shock protein. Nature Plants 2, 16185. [DOI] [PubMed] [Google Scholar]
- Wang P, Lee Y, Igo MM, Roper MC. 2017. Tolerance to oxidative stress is required for maximal xylem colonization by the xylem-limited bacterial phytopathogen, Xylella fastidiosa. Molecular Plant Pathology 18, 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang H, Zhu H. 2019. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Horticulture Research 6, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Balaceanu A, Rufian JS, Segonzac C, Zhao A, Morcillo RJL, Macho AP. 2020. An immune receptor complex evolved in soybean to perceive a polymorphic bacterial flagellin. Nature Communications 11, 3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Caceres-Moreno C, Jimenez-Gongora T, Wang K, Sang Y, Lozano-Duran R, Macho AP. 2018. The Ralstonia solanacearum csp22 peptide, but not flagellin-derived peptides, is perceived by plants from the Solanaceae family. Plant Biotechnology Journal 16, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Hall SA, Hawkesford MJ, Beale MH, Cooper RM. 2002. Elemental sulfur and thiol accumulation in tomato and defense against a fungal vascular pathogen. Plant Physiology 128, 150–159. [PMC free article] [PubMed] [Google Scholar]
- Woolfson KN, Haggitt ML, Zhang Y, et al. 2011. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-seq-dependent transcriptional analysis and histochemistry. Journal of Experimental Botany 62, 5607–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfson KN 2018. Suberin biosynthesis and deposition in the wound-healing potato (Solanum tuberosum L.) tuber model. PhD thesis, The University of Western Ontario, London, Canada; https://ir.lib.uwo.ca/cgi/viewcontent.cgi?article=8075&context=etd. Accessed July 2020. [Google Scholar]
- Xu L, Zhu L, Tu L, Liu L, Yuan D, Jin L, Long L, Zhang X. 2011. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. Journal of Experimental Botany 62, 5607–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Molina I, Ranathunge K, Castillo IQ, Rothstein SJ, Reed JW. 2014. ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. The Plant Cell 26, 3569–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadeta KA, J Thomma BP. 2013. The xylem as battleground for plant hosts and vascular wilt pathogens. Frontiers in Plant Science 4, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Jiang Z, Bi G, Nomura K, Liu M, He SY, Zhou J-M, Xin X-F. 2020. Pattern-recognition receptors are required for NLR-mediated plant immunity. BioRxiv 10.1101/2020.04.10.031294. [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaini PA, Nascimento R, Gouran H, Cantu D, Chakraborty S, Phu M, Goulart LR, Dandekar AM. 2018. Molecular profiling of Pierce’s disease outlines the response circuitry of Vitis vinifera to Xylella fastidiosa infection. Frontiers in Plant Science 9, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Emonet A, Dénervaud Tendon V, Marhavy P, Wu D, Lahaye T, Geldner N. 2020. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 180, 440–453.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gao E, Shaban M, Wang Y, Wang H, Nie X, Zhu L. 2018. GhUMC1, a blue copper-binding protein, regulates lignin synthesis and cotton immune response. Biochemical and Biophysical Research Communications 504, 75–81. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH 1979. The discovery of tylose formation by a Viennese lady in 1845. IAWA BULLETIN, 2–3, 51-56. [Google Scholar]