Abstract
Disproportionate cervical cancer burden falls on women in low-income countries; and there are new efforts to scale up prevention worldwide, including via “screen and treat” for detection and removal of abnormal cervical lesions. This study examines Malawian women’s experiences with “screen and treat;” this is an under-explored topic in the literature, which has focused largely on knowledge about and attitudes toward screening, but not on experiences with screening. We interviewed 47 women who have been screened at least once for cervical cancer. The interview guide and analysis approach was informed by the Multi-Level Health Outcomes Framework. Women were recruited at facilities that offer “screen and treat,” and asked about their experiences with screening. The average age of respondents was 40 years, and approximately half were HIV-negative. Although women were knowledgeable about the benefits of screening, they articulated many barriers including being turned away because of stock-outs of equipment, far distances to services, discomfort with male providers, and poor communication with providers. Alongside the many health education campaigns to increase awareness and demand for “screen and treat” services, the global public health community must also address implementation barriers in the resource-constrained health systems where burden is greatest. Particular attention should be paid to quality and person-centeredness of “screen and treat” services, to optimize uptake and engagement in care.
Keywords: Cervical cancer, Screening, Global health
BACKGROUND
Cervical cancer remains a major cause of mortality and morbidity in low-income countries, despite substantial reductions in burden among wealthier nations [6, 1]. This global inequity persists due to low rates of primary and secondary prevention in resource-constrained settings. The World Health Organization (WHO) has announced a “global call to action” for cervical cancer elimination worldwide, with specific time-bound targets for member states to achieve increased coverage of vaccination, screening, and appropriate treatment and care for women with cervical disease [46]. Although human papillomavirus (HPV) vaccine is an extremely effective public health tool toward eliminating cervical cancer, it likely will not achieve high population coverage for many years [16]. Secondary prevention therefore remains a priority, particularly in low-resource, high-burden contexts. In Malawi, which is estimated to have the greatest cervical cancer burden in the world [6, 1], fewer than one-third of adult women have ever been screened, and only approximately 40% of screen-positive cases are treated [40].
Malawi offers cervical cancer screening via a “screen and treat” approach, as recommended by the WHO for low-resource settings: trained providers examine the cervix using visual inspection with acetic acid (VIA), which identifies abnormal cells that can be removed immediately using either cryotherapy or thermocoagulation, with complicated cases referred to cancer specialists [45]. The recommended screening schedule per Malawi clinical guidelines is once every 3 years for HIV-negative women and once every 1–2 years for HIV-positive women [31, 30, 25]. Although there is ample evidence that “screen and treat” is safe, effective and acceptable [8, 14, 28, 32, 33, 42, 36], very few studies have investigated women’s experiences with this model of care.
This study explores experiences in a “screen and treat” program, using qualitative data collected during interviews with women in Malawi. The design was informed by the Multi-Level Health Outcomes Framework (MHOF) [3] which uses a socio-ecological perspective to study determinants of health behaviors (Figure 1). The MHOF posits that health behaviors – in this study, participation in a screen and treat program – are affected by factors at the individual, provider and system levels, as well as more distal influences. The MHOF has been used to inform a range of cancer prevention and control studies, from formative research to intervention design [3, 4, 2]. Although the MHOF acknowledges that a single study like this one can only focus on a subset of possible determinants of health outcomes, it situates these within the broader context of potentially relevant factors such as health systems, norms and social factors, and policies. This study focuses on constructs in the Provider and Clinic/Health System domains of the MHOF.
Figure 1:
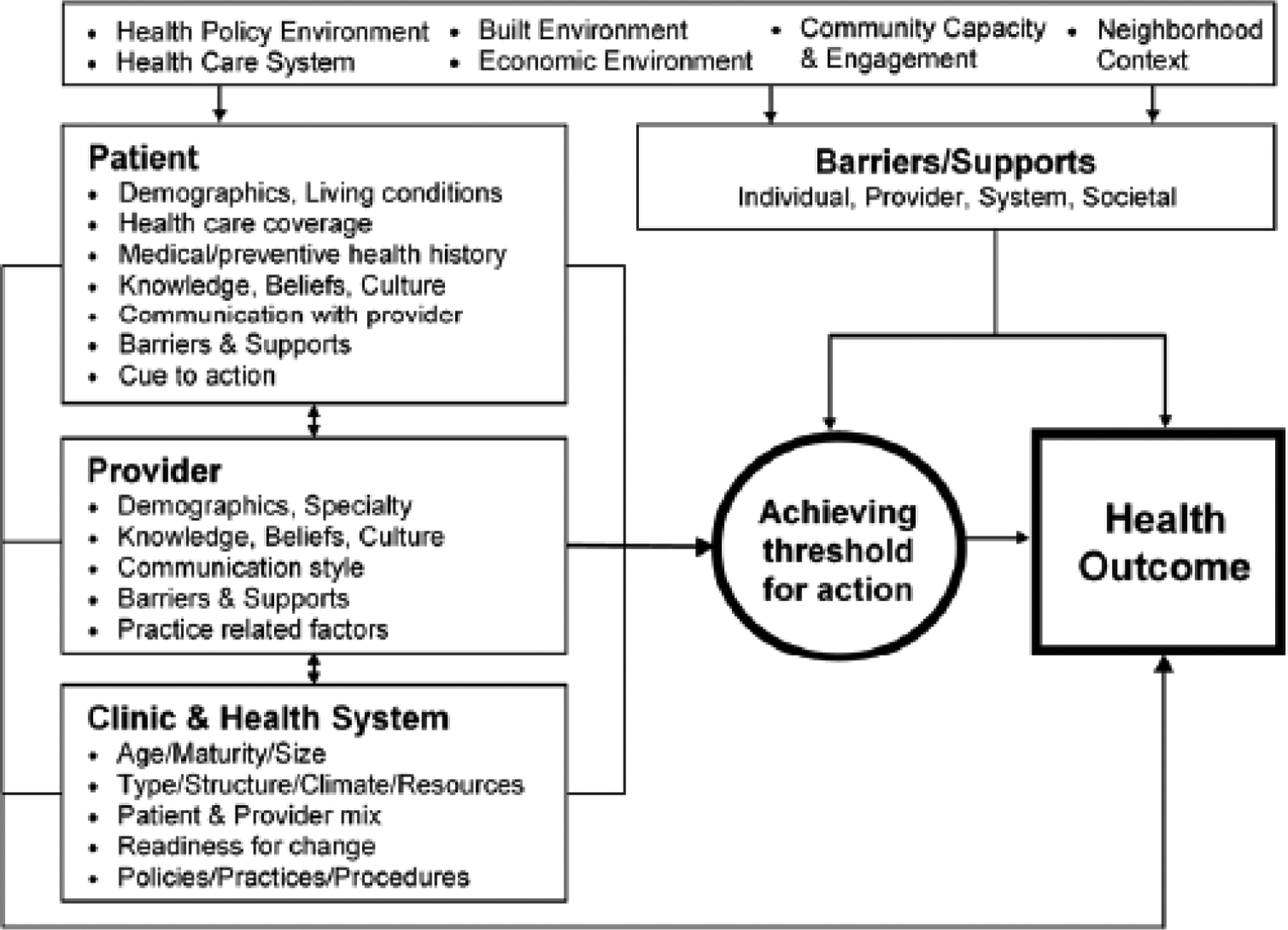
The Multi-Level Health Outcomes Framework
METHODS
Site and participant selection
We invited female adult clients (age 18 and older) at two district hospitals and one mission hospital in central Malawi to participate in an interview. The three health facilities all offer “screen and treat” using VIA and thermocoagulation, and have been doing so since at least 2015. These services are offered free-of-charge for all women at the district hospitals; at mission hospitals, “screen and treat” is free of charge for women on HIV treatment, and for-fee for all other women (approximately US$ 1.25 for screening, and US$ 2.75 for treatment).
In order to capture both immediate and longer-range opinions, two types of clients were recruited: those who had engaged in screening on the day of data collection, and women from the general patient population who had been screened for cervical cancer at least one time previously. Clients were recruited using convenience sampling, from the waiting area of either the outpatient department or the antiretroviral therapy (ART) clinic at each participating facility. Women in the queue were approached, and if they were eligible, told they could come for the interview after seeing the clinician that day. We aimed to conduct 20 interviews at each facility and included women who had never been screened for cervical cancer, although this paper presents results only from women who had ever been screened (that day or previously).
Data collection
The semi-structured interview guide included questions about experiences with cervical cancer screening (see Appendix). All interviews were conducted in Chichewa (the local language) by female Malawian researchers who are highly experienced with qualitative interviews about health services, and were trained specifically for this study about “screen and treat.” All participants were given an opportunity to ask questions before providing informed consent prior to the interview. Data were collected anonymously, and interviews were audio recorded with permission of the participant. Interviews were conducted in a private space at the health facility. Interviews were on average 26 minutes long.
Data analysis
The audio recorded interviews were transcribed and translated to English by highly experienced research staff. The transcripts were coded using broad themes about the screening experience; two authors (CM and PK) piloted the codebook on a subset of interviews, refined it through three rounds of iterative coding, and then each worked independently to code a portion of the client interviews using NVivo software (QSR International, v11). Analysis focused on identifying thematic similarities and differences, and comparing specifically by respondent age and self-reported HIV status.
Ethical review
This study was approved by the Institutional Review Board at the University of California Los Angeles and the Malawi National Health Sciences Research Committee.
RESULTS
Description of the study sample
We conducted interviews with 47 women, who were on average 40 years old (Table 1). Some women who were approached for the study did not participate (nine were not interested in participating, seven had time constraints, two felt ill, and one did not have spousal permission). Approximately half reported being HIV-negative, and nearly all HIV-positive women (86%) reported being on ART.
Table 1.
Respondent characteristics (n=47)
| Age: average (median), range | 39.9 (41), 18–62 |
| HIV status: Negative | 24 (51.1%) |
| Positive on ART | 19 (40.4%) |
| Positive not on ART | 3 (6.4%) |
| HIV status unknown/unreported | 1 (2.1%) |
| Number of times screened†, average (median), range J | 11 (1), 1–4 |
Does not include 2 women who reported being screened annually
Just over one-third of respondents were screened for the first time on the day of data collection (n=17, 36.2%). Among those who had been screened previously (n=30), 16 had been screened once before (median year of screen: 2017), five had been screened once previously plus were screened at the facility on the day of data collection, one had been screened twice before (most recently in 2017), four had been screened twice previously plus were screened at the facility on the day of data collection, two had been screened three times previously plus were screened at the facility on the day of data collection, and two women, both HIV-positive and on ART, reported annual screening (Figure 2).
Figure 2:
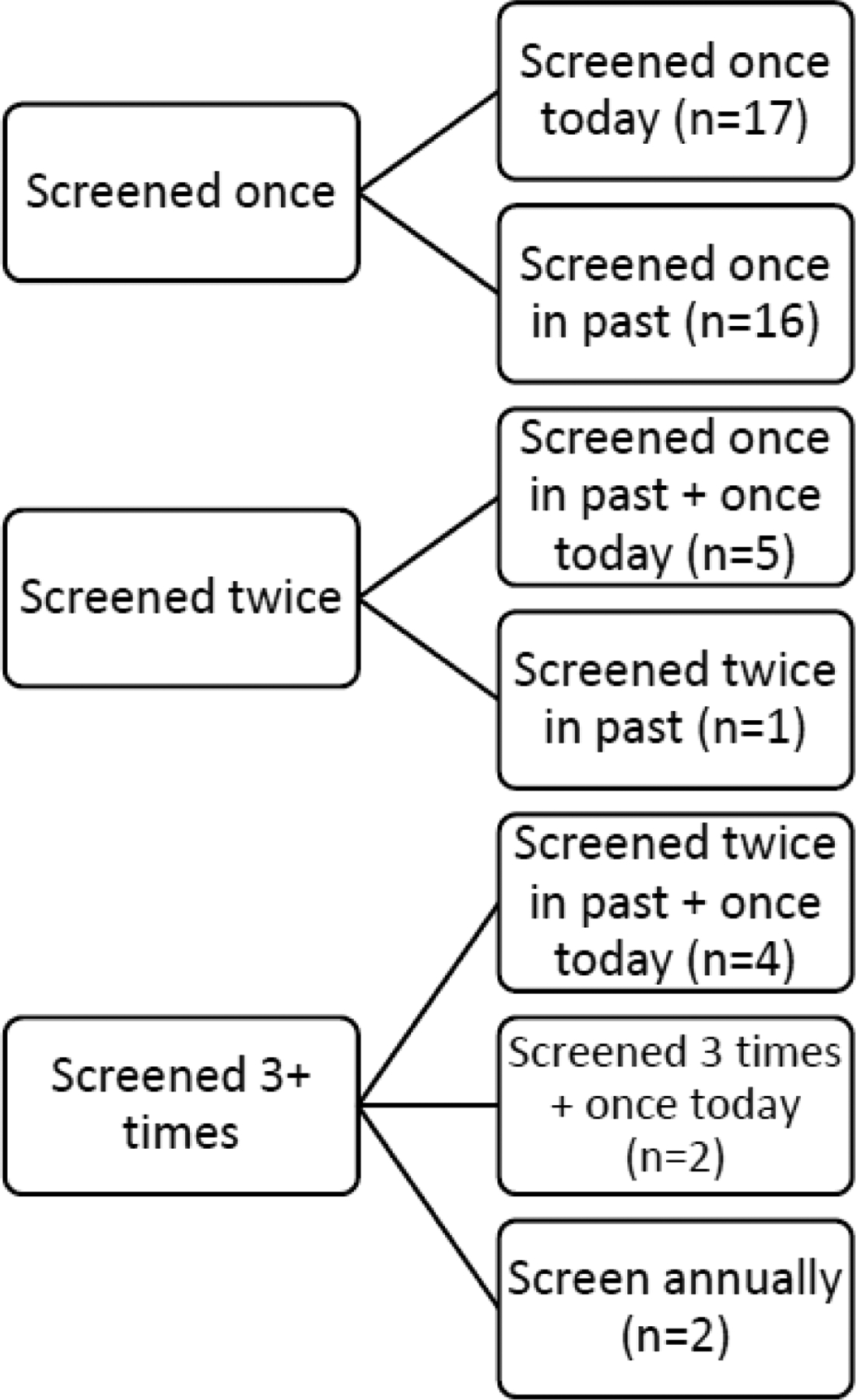
Screening history for women included in the study
Knowledge of “screen and treat”
All respondents were asked to describe “screen and treat.” Several women (n=8) indicated they knew the general concept but could not give details because they were distracted during screening (“I didn’t see how it was happening, I was just protecting my body” [45, HIV-positive on ART]) or could not remember what was involved in screening. The majority of women explained the screening procedure in some detail, although a few women reported misconceptions about the role of vinegar (“When they screen the cervix and find small lesions, they use vinegar to burn them so that they shouldn’t spread” [21, HIV-positive on ART]). But many women were able to articulate the process succinctly and correctly:
“They use vinegar to see what level the cancer is, and if the rashes have just started then they treat it before it spreads.” (39, HIV-positive on ART)
“They use vinegar. When it changes colors, that means you have the signs of cervical cancer, but when there isn’t any reaction, that means you are okay.” (42, HIV-negative)
“Screen and treat” program counseling and education
Women find counseling to be helpful and informative
Women were asked about the content of cervical cancer counseling or education they have received. Most women were able to recall this information, although five women – all HIV-negative – said they had not received any information (two of these women had been screened within the last week and the others had been screened three years ago or more). All respondents who reported having received counseling/education information said that it was helpful (“They taught us things which were helpful and useful, and which we can apply in our daily lives to prevent the cancer” [42, HIV-negative]). Several women, mostly first-time screeners, said that this taught them new information and corrected some of their misconceptions (“I had thought that maybe cancer begins after your immune system is weak like how HIV works, but now I have understood the difference” [32, HIV-negative]). Many mentioned that the pictorial tools used during health education were informative.
Counseling focuses on providing information about cervical cancer and screening
The most common counseling/health education topic was about the importance of universal screening regardless of symptoms or risk factors (“It is hard to recognize cervical cancer because there are no signs unless you have been screened” [33, HIV-negative]), and especially how early detection can prevent advanced disease (“At an early stage, they can help you and you can be cured. At a late stage, they just give you painkillers but there isn’t any help they can give you in terms of cancer” [54, HIV-positive on ART]). Several women spoke of learning about logistics of the screening process during counseling/health education.
“The information I received was that when you get in the screening room, you get undressed and the procedure is painless. They showed us the instruments used for cervical screening… It was encouraging and if there was anyone who was afraid the fear was gone I’m sure” (37, HIV-positive on ART).
Women of all ages, HIV status and screening history spoke of learning about cervical cancer risk factors. The most common message was about sexual transmission, and specifically about the viral origin.
“You could have one husband but maybe they sleep around and they could carry the cancer to you” (53, HIV-negative)
“Today I have also learned that cancer is a virus, something I did not know. And that men are the carriers of the virus… All I knew was that AIDS was caused by a virus but not cervical cancer” (35, HIV-negative)
It was common for women to mention having been counseled on symptoms of cervical cancer, including vaginal discharge or bleeding, itching, warts, and pain.
“They said sometimes you have pus discharge from the vagina or a discharge in form of smelly water. Those could be signs that cervical cancer has begun” (22, HIV-negative)
Women report limited opportunities to ask questions
Respondents were asked if they had any opportunity to ask questions during counseling. It was more common for women to report they were not given such an opportunity (n=28 [60%], versus 19 women [40%] who said they were given the opportunity), although most such respondents were not bothered by this (“They just explained it and I just accepted it” [53, HIV-negative]). This was reported by women across all three sites. For some women, the extensive pre-screening education session obviated their need to ask questions (“Since they had already explained a lot before testing us, after that they just told us the next date to come again and we didn’t think of asking much” [30, HIV-positive on ART]). One respondent wished she had asked about whether the procedure would be painful, and another who received treatment spoke about being too embarrassed about being examined by a male doctor to ask any questions. Among women who said they were given a chance to ask questions, most said they did not ask anything. Two women said they had asked about the screening schedule and follow-up, and one woman asked “what if you buy strong soap and use it every day, would that stop the virus from spreading? And they said no, the virus cannot be controlled by soap” (52, HIV-positive on ART).
Screening process
Screening was not painful even though women had heard it would be
Women reported that screening was painless, despite commonly having heard rumors that it is painful or dangerous. Only one woman mentioned that she found screening, namely the speculum, to be painful.
“People were threatening that when they screen, they laser you and remove your uterus; and they were trying to discourage us. But I was determined that ‘whatever happens will happen, but I need to know my status’.” (22, HIV-negative)
“People said they insert the metallic instruments on the inside and I thought I would die from that but it is not like that.” (40, HIV-positive)
Respondents disagreed about acceptability of the exam being performed by a male clinician
Several women mentioned concerns about being examined by men. This was due to embarrassment (“for a male doctor to undress you and see your private parts is so embarrassing” [45, HIV-positive on ART]). Women, especially older women, said they did not want to be screened by male providers:
“When we come here for [routine visits] we are assisted by male doctors, and to talk to them about cervical cancer it is a bit difficult. One time I heard a lady explaining about cervical cancer and that made me feel free.” (52, HIV-positive on ART)
“Female doctors should be the ones who do screening… The labor ward is different because you are in pain, you don’t have a choice. But here you are not in pain, so female doctors should be the ones doing the screening.” (54, HIV-positive on ART)
Others, particularly younger women in the sample, were dismissive of this concern:
“For someone who has given birth like I have, if a male doctor wants to check me, can I refuse saying I am shy? I can just go ahead because they are looking to help me… Why would one be shy? When we go for childbirth sometimes we are helped by males and it is fine. They are sympathetic.” (35, HIV-positive on ART)
“Cervical cancer screening does not require a male or a female doctor -- anyone with the expertise can do it. When you are shy and say ‘I cannot get undressed before a male doctor,’ then you will not be helped if the person who can help you better is a male doctor.” (41, HIV-positive on ART)
Women planned to be screened again in the future
When asked about their intention to be screened again in the future, all women said that they would be rescreened. Common reasons were wanting to know one’s status, and the possibility that one can be affected later (“I have a man in the house, and they said that we get the virus from the man… So, I should come every year to be checked” [32, HIV-negative]).
Most women clearly understood the importance of screening often, even after a normal screen result:
“You may say you are fine but the disease may have spread in your body. One cannot just relax and say they are fine [after screening], the problem could come. That is why they said one should keep coming [to be screened], so that they know.” (34, HIV-positive on ART)
“They told me that I do not have cervical cancer but that I have to come back next year because I am HIV positive. When you are HIV positive your immune system is weak, making me prone to having cervical cancer, so I need to come back each year to get screened.” (41, HIV-positive on ART)
Outcomes of screening
Nearly all women (n=42, 89%) said they had a normal VIA outcome. Among these women, most HIV-negative women were instructed to return in 3 years unless they experienced problems sooner (n=12, mostly first-time or recent screeners), two were told to return within the next year, one in two years, and one in five years (and two did not recall the follow-up period they had been told). All HIV-positive women reported they had been told to follow up in 1 year (n=14). Ten women (both HIV-positive and -negative) did not recall the recommended follow-up period.
Only three women reported on an abnormal screen result (two HIV-negative and one HIV-positive); all were first-time screeners when the abnormal result was found. Only one of these women successfully received treatment on the same day as screening. The others reported delays, both related to needing their husband’s approval.
“They told me that they will treat me using their machines, and they asked me whether they should treat me right away or whether I needed time to think. I told them to treat me right away and that I did not require any time to think, so they have treated me.” (24, HIV-negative)
“They said that I should not sleep with a man for four weeks, and I said that I need to tell my husband first. They said, that is my right; and I told them I will be back.” (43, HIV-positive on ART)
“On Friday I was told that I have cancer signs and they need to treat. I went back home and people started saying that I will never have any more children, and that people who are treated for lesions never give birth again… My husband got scared and yesterday he said that our marriage is over due to this problem because he wants children. [The doctor] said that is a lie, you can bear children because we do not tamper with the uterus, we only treat the cervix; and after you heal you are able to have children. But I don’t think it will be of any use, I think my husband will think that I just want to get back together with him.” (23, HIV-negative)
Some women report challenges communicating with providers
Many women said they were comfortable talking with providers about cervical cancer (n=11) – primarily because providers were seen as experts who can offer help (“Because they know about the disease, and they can tell us how to prevent the disease and how to cure the disease” [21, HIV-positive on ART]). However, some women reported communication challenges with providers, including harsh treatment.
“It is hard [to talk with the health workers] because the people who work here are difficult to talk to. They are angry and they shout at you so we avoid them.” (47, HIV status known)
“Some people come to the hospital without washing their private part. Instead of nicely telling them to wash up, you shout at them, and the person fears to come get screened.” (32, HIV-positive on ART)
Challenges accessing the “screen and treat” program
Equipment stockouts are common
Women commonly spoke about facing challenges when trying to access “screen and treat” services. The most frequent issue was around stock-outs of supplies (n=9); this was mentioned at two of the three sites:
“Most days when we come here, we find that there are no instruments for screening, and that is discouraging.” (32, HIV-negative)
“It has been seven years since the last time I was screened. Each time I came here they told me that there were no screening instruments. I noticed that I have a lot of vaginal discharge, and yesterday they were announcing about the discharge that comes due to warts [HPV], so I decided to come and get screened because I keep on having warts constantly.” (35, HIV-negative)
“When I came to get screened, I learned that the screening equipment was not sufficient. The nurses said, ‘we are only screening 10 women because we have only sterilized 10 instruments so all other women have to come back next Wednesday.’ I came back the following Wednesday, and they told me they are not screening. I came back later and they said, ‘we are only screening 5 people, the rest come back next week.’ We started feeling discouraged.” (56, HIV-negative)
“When we came yesterday, we were sent back… They said, ‘we do not have enough equipment, come back tomorrow.’ But when some are sent home, they will never come back.” (53, HIV-negative)
Unpredictable service availability is a challenge, especially for women who travel far or wait long
Women commonly mentioned abrupt cancellation and rescheduling of screening services. This was particularly difficult for women who had to travel long distances to receive these services.
“To get help is not easy. When you have gotten help, that means you were strong by coming here. I come from a far distance and spend MK2000 [US$ 2.70] to and from. Today they told me that I cannot be screened and I should come another day. If you should come and be sent back three times, people get discouraged since each time we go we don’t get help.” (31, HIV-negative)
“We come and they tell us ‘there are too many of you,’ and then they just screen nine women and tell the rest to come back the following day. But by then one would have come from afar and used up their transport money. This could discourage one to not get screened.” (50, HIV-negative)
DISCUSSION
Our study found similar challenges reported elsewhere about implementation of “screen and treat” programs, including supply/equipment stock-outs, lack of available providers, and hesitations about male providers [15, 10, 24]. However, relatively few women in our study were eligible for, and received, same-day lesion removal so we were limited in our ability to analyze experiences for these women.
We found that some women experienced harsh communication from providers. This has been reported in other women’s health care settings including maternity wards and family planning clinics [43, 22, 5]. Such challenges may be due to over-burdened and -stressed providers, lack of training on person-centered care, or insufficient supervision and mentorship. We urgently need interventions to address this across the women’s health care spectrum, including for cervical cancer screening.
Our findings suggest that a multi-level intervention approach within the health system may be most beneficial to increase uptake of cervical cancer screening. Interventions will need to address supply chain challenges around stock-outs of equipment, increase the number of providers trained for “screen and treat” (especially female providers), ensure that enough trained providers are available on days when services are offered and have sufficient space to deliver the service, and improve provider-patient communication, which may include addressing issues with space and privacy where counseling can be effectively delivered. A recent systematic review found scarce high-quality evidence on “screen and treat” implementation strategies that took a health systems approach [21]; the most common strategy was to integrate VIA with other services such as HIV care, or other maternal and child health care services [44, 12, 19, 35]; but there are limited data on the costs of such models and their potential spillover effects, including whether other services are negatively affected. And as our results indicate, a broader intervention approach – reaching from interpersonal relationships to health system strengthening – may be needed to maximize uptake and impact of such programs.
In this study, education and counseling activities at health facilities that precede screening were perceived as very instructive and helpful. Respondents commonly mentioned ways in which these messages had corrected a misconception about cervical cancer (susceptibility, causes) or about screening (benefits, process), and the need for and frequency of follow-up. However, more than half of women said they had not been given an opportunity to ask questions. Although this was largely not perceived as a challenge, it suggests an area for strengthening screening programs and increasing the patient-centeredness of care [29]. Lessons from other areas, including within reproductive and maternal health, may be applicable for further research relating to patient-centered quality of cervical cancer care [23, 38]. Patient-centered care is respectful and responsive to each individual woman’s needs, preferences and values. Examples of approaches from reproductive health include: providing tailored counseling; ensuring privacy and confidentiality; fostering providers’ skills around supportive care provision; and strengthening women’s decision-making, communication with partners, and sense of autonomy around service use [13]. Such intervention approaches are likely to be applicable to cervical cancer prevention as well. Communication with partners and autonomy in care decision-making are particularly salient issues in Malawi, where male partner “permission” for health service utilization is a common barrier [26, 7]. After thermocoagulation, women need to abstain from sex for four weeks, which requires support from spouses and sexual partners. Further work is needed on how to engage male partners in “screen and treat” programs.
Some limitations of this research should be noted. First, data were collected at only three sites, all of which are hospitals, so these results may not generalize to other contexts; however, these were selected from among the very few facilities offering “screen and treat” services with thermocoagulation in Malawi, so likely are a fair representation of the current situation. Second, although all interviews were conducted by highly experienced Malawian qualitative researchers, there is a risk of reporting bias (recall bias or social desirability bias). Recall bias may have particularly affected respondents who had been screened a long time ago for cervical cancer. Third, very few women in our sample received same-day lesion removal, so we were limited in our ability to analyze experiences with treatment. Future research should focus on treatment experiences in the context of “screen and treat.” Lastly, because investments for “screen and treat” in Malawi have focused on providing services at HIV treatment sites, and this informed the sampling frame for the study, HIV-positive women are over-represented in our sample.
CONCLUSIONS
In the context of growing global attention around cervical cancer – including articulating its importance toward meeting the Sustainable Development Goals [37], and acknowledging its relevance in the global fight against HIV/AIDS [20] – we must both increase demand for services, and ensure that services are being implemented with attention to quality of care, and in the most effective, equitable and efficient manner. Rigorous research is needed about implementation strategies for “screen and treat,” and ways to address both the demand- and supply-side challenges in delivering these services [21]. Women’s experiences are core to understanding current challenges and identifying areas for improvement.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the excellent work done by Allena Makwenda and Esnart Sanudi, who conducted these interviews; and the support for data collection from Partners in Hope. We thank all the women who generously shared their time and opinions for this research.
Funding: This research was supported by the NIH/National Center for Advancing Translational Sciences (grant number KL2TR001882, PI Wong, UCLA Clinical and Translational Science Institute, Institutional Development Core), and funding from the Jonsson Comprehensive Cancer Center at UCLA.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: The authors declare that they have no conflicts of interest or competing interests.
Availability of data and material: Data can be made available to interested parties upon reasonable request to the corresponding author.
Code availability: Not applicable
Ethics approval: This study was approved by the Institutional Review Board at the University of California Los Angeles, and the Malawi National Health Sciences Research Committee; and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Consent to publish: Not applicable
REFERENCES
- 1.Arbyn Marc, Weiderpass Elisabete, Bruni Laia, Silvia de Sanjosé Mona Saraiya, Ferlay Jacques, and Bray Freddie. 2019. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. The lancet global health. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastani Roshan, Gallardo Nilsa V, and Maxwell Annette E. 2001. Barriers to colorectal cancer screening among ethnically diverse high-and average-risk individuals. Journal of Psychosocial Oncology 19 (3–4):65–84. [Google Scholar]
- 3.Bastani Roshan, Glenn Beth A., Taylor Vicky M., Chen Moon S., Nguyen Tung T., Stewart Susan L., and Maxwell Annette E.. 2010. Integrating theory into community interventions to reduce liver cancer disparities: The Health Behavior Framework. Preventive medicine 50 (1):63–67. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastani Roshan, Annette E Maxwell Clarence Bradford, Prabhu Das Irene, and Yan Kang X. 1999. Tailored risk notification for women with a family history of breast cancer. Preventive medicine 29 (5):355–364. [DOI] [PubMed] [Google Scholar]
- 5.Bohren Meghan A, Hedieh Mehrtash, Bukola Fawole, Thae Maung Maung, Mamadou Dioulde Balde, Ernest Maya, Soe Soe Thwin, Aderoba Adeniyi K, Vogel Joshua P, and Theresa Azonima Irinyenikan. 2019. How women are treated during facility-based childbirth in four countries: a cross-sectional study with labour observations and community-based surveys. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L, Torre Lindsey A, and Ahmedin Jemal. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (6):394–424. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, Amy G, Gloria Hamela, Ann Gottert, Gretchen S Stuart, and Gift Kamanga. 2015. Reasons for intrauterine device use, discontinuation and non-use in Malawi: a qualitative study of women and their partners. African journal of reproductive health 19 (4):50–57. [PubMed] [Google Scholar]
- 8.Campbell Christine, Kafwafwa Savel, Brown Hilary, Walker Graeme, Madetsa Belito, Deeny Miriam, Kabota Beatrice, Morton David, Reynier Ter Haar, and Liz Grant. 2016. Use of thermo-coagulation as an alternative treatment modality in a ‘screen-and-treat’programme of cervical screening in rural Malawi. International Journal of Cancer 139 (4):908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfell Karen. 2019. Towards the global elimination of cervical cancer. Papillomavirus Research:100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chidyaonga-Maseko Fresier, Maureen Leah Chirwa, and Adamson Sinjani Muula. 2015. Underutilization of cervical cancer prevention services in low and middle income countries: A review of contributing factors. Pan African Medical Journal 21 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Sanjose Silvia, and Holme Francesca. 2019. What is needed now for successful scale-up of screening? Papillomavirus Research 7:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeGregorio Geneva, Manga Simon, Kiyang Edith, Manjuh Florence, Bradford Leslie, Cholli Preetam, Wamai Richard, Ogembo Rebecca, Sando Zacharie, and Liu Yuxin. 2017. Implementing a Fee-for-Service Cervical Cancer Screening and Treatment Program in Cameroon: Challenges and Opportunities. The oncologist 22 (7):850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond-Smith Nadia, Warnock Ruby, and Sudhinaraset May. 2018. Interventions to improve the person-centered quality of family planning services: a narrative review. Reproductive health 15 (1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolman L, Sauvaget C, Muwonge R, and Sankaranarayanan R. 2014. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: A systematic review. BJOG: An International Journal of Obstetrics & Gynaecology 121 (8):929–942. doi: 10.1111/1471-0528.12655. [DOI] [PubMed] [Google Scholar]
- 15.Fort, Victoria K, Mary Sue Makin, Siegler Aaron J, Ault Kevin, and Rochat Roger. 2011. Barriers to cervical cancer screening in Mulanje, Malawi: A qualitative study. Patient preference and adherence 5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall, Michaela T, Simms Kate T, Jie-Bin Lew, Smith Megan A, Brotherton Julia ML, Saville Marion, Frazer Ian H, and Canfell Karen. 2019. The projected timeframe until cervical cancer elimination in Australia: a modelling study. The Lancet Public Health 4 (1):e19–e27. [DOI] [PubMed] [Google Scholar]
- 17.Hami, Melanie Y, Ehlers Valerie J, and Van der Wal Dirk M. 2014. Women’s perceived susceptibility to and utilisation of cervical cancer screening services in Malawi. Health SA Gesondheid 19 (1). [Google Scholar]
- 18.Huchko Megan J. 2018. Cervical Cancer Prevention in East Africa: Moving from Evidence to Implementation. In Global Perspectives on Women’s Sexual and Reproductive Health Across the Lifecourse, 367–390. Springer. [Google Scholar]
- 19.Huchko, Megan J, Bukusi Elizabeth A, and Cohen Craig R. 2011. Building capacity for cervical cancer screening in outpatient HIV clinics in the Nyanza province of western Kenya. International Journal of Gynecology & Obstetrics 114 (2):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huchko, Megan J, Maloba May, Nakalembe Miriam, and Cohen Craig R. 2015. The time has come to make cervical cancer prevention an essential part of comprehensive sexual and reproductive health services for HIV-positive women in low-income countries. Journal of the International AIDS Society 18:20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, Lauren G, Allison Armstrong, Joyce Caroline M, Teitelman Anne M, and Buttenheim Alison M. 2018. Implementation strategies to improve cervical cancer prevention in sub-Saharan Africa: A systematic review. Implementation Science 13 (1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawale Paul, Mindry Deborah, Stramotas Stephanie, Chilikoh Peter, Phoya Ann, Henry Katherine, Elashoff David, Jansen Perry, and Hoffman Risa. 2014. Factors associated with desire for children among HIV-infected women and men: a quantitative and qualitative analysis from Malawi and implications for the delivery of safer conception counseling. AIDS care 26 (6):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson Elysia, Vail Daniel, Godfrey M Mbaruku Angela Kimweri, Freedman Lynn P, and Kruk Margaret E. 2015. Moving toward patient-centered care in Africa: A discrete choice experiment of preferences for delivery care among 3,003 Tanzanian women. PloS one 10 (8):e0135621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim Jennifer NW, and Ojo AA. 2017. Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: A systematic review. European journal of cancer care 26 (1):e12444. [DOI] [PubMed] [Google Scholar]
- 25.Malawi Ministry of Health and Population. 2019. National Service Delivery Guidelines for Cervical Cancer Prevention and Control. Lilongwe Malawi. [Google Scholar]
- 26.Manda-Taylor Lucinda, Mwale Daniel, Phiri Tamara, Walsh Aisling, Matthews Anne, Brugha Ruairi, Mwapasa Victor, and Byrne Elaine. 2017. Changing times? Gender roles and relationships in maternal, newborn and child health in Malawi. BMC Pregnancy and Childbirth 17 (1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maree, Johanna E, and Kampinda-Banda Mary. 2018. Knowledge and practices of cervical cancer and its prevention among Malawian women. Journal of Cancer Education:1–7. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy CM, Ramphul M, Madden M, and Hickey K. 2016. The use and success of cold coagulation for the treatment of high grade squamous cervical intra-epithelial neoplasia: a retrospective review. European Journal of Obstetrics & Gynecology and Reproductive Biology 203:225–228. [DOI] [PubMed] [Google Scholar]
- 29.Medicine, Institute of. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 30.Ministry of Health and Population. 2018. Clinical Management of HIV in Children and Adults. Lilongwe Malawi: Ministry of Health and Population. [Google Scholar]
- 31.Ministry of Health Malawi. 2014. National Reproductive Health Service Delivery Guidelines, ed. Support for Service Delivery Integration-Services (SSDI-Services). [Google Scholar]
- 32.Nessa Ashrafun, Naud Paolo, Pulikottil Okkuru Esmy Smita Joshi, Rema Prabhakaran, Wesley Ramani, Kamal Mohammed, Sauvaget Catherine, Muwonge Richard, and Sankaranarayanan Rengaswamy. 2017. Efficacy, Safety, and Acceptability of Thermal Coagulation to Treat Cervical Intraepithelial Neoplasia: Pooled Data From Bangladesh, Brazil and India. Journal of Clinical Gynecology and Obstetrics 6 (3–4):58–64. [Google Scholar]
- 33.Oga, Emmanuel A, Brown Jessica P, Brown Clayton, Dareng Eileen, Adekanmbi Victor, Odutola Michael, Olaniyan Olayinka, Offiong Richard, Obende Kayode, and Adewole Ayodele Stephen. 2016. Recurrence of cervical intraepithelial lesions after thermo-coagulation in HIV-positive and HIV-negative Nigerian women. BMC Women’s Health 16 (1):25. doi:PMC4864941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouedraogo Yacouba, Furlane Gahan, Fruhauf Timothee, Badolo Ousmane, Bonkoungou Moumouni, Pleah Tsigue, Lankoande Jean, Bicaba Isabelle, and Bazant Eva S. 2018. Expanding the single-visit approach for cervical cancer prevention: successes and lessons from Burkina Faso. Global Health: Science and Practice 6 (2):288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramogola-Masire Doreen, Ronny de Klerk Barati Monare, Ratshaa Bakgaki, Friedman Harvey M, and Zetola Nicola M. 2012. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. Journal of acquired immune deficiency syndromes (1999) 59 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randall, Thomas C, Catherine Sauvaget, Richard Muwonge, Trimble Edward L, and Jeronimo Jose. 2018. Worthy of further consideration: An updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Preventive medicine 118:81–91. [DOI] [PubMed] [Google Scholar]
- 37.Singhrao Ruby, Huchko Megan, and Yamey Gavin. 2013. Reproductive and maternal health in the post-2015 era: cervical cancer must be a priority. PLoS medicine 10 (8):e1001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudhinaraset May, Afulani Patience, Nadia Diamond-Smith Sanghita Bhattacharyya, Donnay France, and Montagu Dominic. 2017. Advancing a conceptual model to improve maternal health quality: The Person-Centered Care Framework for Reproductive Health Equity. Gates open research 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapera O, Dreyer G, Kadzatsa W, Nyakabau AM, Stray-Pedersen B, and Hendricks SJH. 2019. Health system constraints affecting treatment and care among women with cervical cancer in Harare, Zimbabwe. BMC Health Services Research 19 (1):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Government of Malawi Ministry of Health. 2017. National Cervical Cancer Control Strategy 2016–2020, ed. UNFPA Malawi. Lilongwe, Malawi. [Google Scholar]
- 41.Tsu Vivien Davis, Denise Njama-Meya Jeanette Lim, Murray Marjorie, and Silvia de Sanjose. 2018. Opportunities and challenges for introducing HPV testing for cervical cancer screening in sub-Saharan Africa. Preventive medicine 114:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viviano Manuela, Kenfack Bruno, Catarino Rosa, Tincho Eveline, Temogne Liliane, Benski Anne-Caroline, Tebeu Pierre-Marie, Ulrike Meyer-Hamme Pierre Vassilakos, and Petignat Patrick. 2017. Feasibility of thermocoagulation in a screen-and-treat approach for the treatment of cervical precancerous lesions in sub-Saharan Africa. BMC Women’s Health 17 (1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren, Charlotte E, Rebecca Njue, Charity Ndwiga, and Timothy Abuya. 2017. Manifestations and drivers of mistreatment of women during childbirth in Kenya: implications for measurement and developing interventions. BMC Pregnancy and Childbirth 17 (1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Were E, Nyaberi Z, and Buziba N. 2010. Integrating cervical cancer and genital tract infection screening into mother, child health and family planning clinics in Eldoret, Kenya. African health sciences 10 (1). [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. 2013. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Geneva, Switzerland. [PubMed] [Google Scholar]
- 46.World Health Organization. 2018. Cervical Cancer: An NCD We Can Overcome, ed. Tedros Adhanom Ghebreyesus. Intercontinental Hotel Geneva. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


