Abstract
Plasmacytoid dendritic cells (pDCs) are a unique subpopulation of immune cells, distinct from classical dendritic cells. pDCs are generated in the bone marrow, and following development, they typically home to secondary lymphoid tissues. Nevertheless, while peripheral tissues are generally devoid of pDCs during steady state, few tissues, including the lung, kidney, vagina, and in particular ocular tissues harbor resident pDCs. pDCs were originally appreciated for their potential to produce large quantities of type I interferons in viral immunity. Subsequent studies have now unraveled their pivotal role in mediating immune responses, in particular in the induction of tolerance. In this review, we summarize our current knowledge on pDCs in ocular tissues in both mice and humans, in particular in the cornea, limbus, conjunctiva, choroid, retina, and lacrimal gland. Further, we will review our current understanding on the significance of pDCs in ameliorating inflammatory responses during herpes simplex virus keratitis, sterile inflammations, and corneal transplantation. Moreover, we describe their novel and pivotal neuroprotective role, their key function in preserving corneal angiogenic privilege, as well as their potential application, as a cell-based therapy for ocular diseases.
Keywords: plasmacytoid dendritic cells, tolerance, transplantation, viral keratitis, angiogenesis, neuroprotection
1. Introduction on Plasmacytoid Dendritic Cells
1.1. Identification of Plasmacytoid Dendritic Cells
The discovery of plasmacytoid dendritic cells (pDCs) began with observations in human tissues. In 1958, the pathologists Lennert and Remmele noted a previously unappreciated cell type in human lymph nodes in cases of non-specific lymphoid hyperplasia. These cells appeared medium sized, were referred to as a “lymphoblast”, and were noted to be present in clusters (Lennert et al. 1958). Considering that these cell clusters were later observed in T cell-associated (paracortical) areas of lymph nodes, and that electron microscopy studies indicated an abundant rough endoplasmic reticulum resembling plasma cells (Muller-Hermelink et al. 1973), Lennert et al. later referred to them as “T-associated plasma cells” (Lennert et al. 1975). The advent of immunostaining techniques later revealed that these cells expressed the T-helper (Th) marker CD4 (clones Leu-3a and OKT4), but lacked common T cell and B cell lineage markers; thus, they were described as “plasmacytoid T cells” (Feller et al. 1983, Muller-Hermelink et al. 1983, Papadimitriou et al. 1983, Vollenweider et al. 1983, Harris et al. 1987). Yet, more extensive immunophenotyping revealed that these cells also expressed the myelomonocytic markers Ki-M6 and Ki-M7 (Horny et al. 1987), and thus in 1988 Facchetti et al. proposed renaming them to “plasmacytoid monocytes” (Facchetti et al. 1988). In 1997, Grouard et al. showed that freshly isolated plasmacytoid T cells/monocytes were morphologically nearly identical to CD4+ CD11cneg Linneg immature cells, which differentiate into dendritic cells (DCs) in human peripheral blood, and upon cultures with interleukin (IL)-3 and CD40 ligand, they effectively promoted proliferation of naïve CD4+ CD45RA+ Th cells (Grouard et al. 1997). Few months later, Olweus et al. confirmed the phenotype of plasmacytoid T cells/monocytes and their capacity to induce naïve T cell proliferation following ex vivo stimulation (Olweus et al. 1997). In 1999, Rissoan et al. then designated these cells as “type 2 DC precursors (pDC2s)”, as their ex vivo cultures with naïve CD4+ T cells demonstrated that they favored production of a Th2 cytokine profile in naïve T cells, in contrast to monocytic precursors of myeloid (conventional or classical) DCs (cDCs), which promoted a Th1 response (Rissoan et al. 1999). However, since further studies indicated that both cDCs and pDC2s were able to interact with both Th1 and Th2 cells (Boonstra et al. 2003), the term “plasmacytoid dendritic cell” (pDC) was more commonly used.
Considering the importance of interferons (IFNs) in viral infections, in entirely independent line of studies, Trinchieri et al. showed that an unknown type of lymphocytes isolated from peripheral blood that did not belong to B or T cells, had a strong capacity to secrete IFNs (Trinchieri et al. 1978). In fact, the majority of IFNs in the blood were secreted by a rare subpopulation of immune cells that was initially termed natural “IFN producing cells” (IPCs) (Ronnblom et al. 1983). IPCs were distinct from cDCs, monocytes, natural killer (NK) cells, T cells, and B cells (Abb et al. 1983, Ronnblom et al. 1983, Perussia et al. 1985, Fitzgerald-Bocarsly et al. 1988, Chehimi et al. 1989, Feldman et al. 1990, Ferbas et al. 1994, Svensson et al. 1996). Further, several studies showed that IPCs co-purified with cells with a dendritic morphology, expressed major histocompatibility complex (MHC)-II, and morphologically resembled DCs based on their large size, and veiled and ruffled morphology (Fitzgerald-Bocarsly et al. 1988, Ferbas et al. 1994). Moreover, Chehimi et al. demonstrated that IPCs and cDCs were distinct populations, by showing that cDCs, but not IPCs were potent inducers of strong mixed lymphocyte reactions (Chehimi et al. 1989). Svensson et al. showed that IPCs could promote T cell proliferation, suggesting that IPCs resembled immature but not mature cDCs (Svensson et al. 1996). Thus, by the mid-1990s cumulative evidence suggested that IPCs might belong to the DC family. In the late 1990s, Siegal et al., and Cella et al., independently demonstrated that IPCs in fact hold the same identity as the independently identified pDCs (Cella et al. 1999, Siegal et al. 1999).
Following the discovery of pDCs in humans, investigators aimed to unravel their murine counterparts. In 2001, a few years after unifying the identity of pDCs and IPCs in humans, Nakano et al., Asselin-Paturel et al., and Bjorck independently recognized a subpopulation of DCs in murine lymph nodes and spleens that displayed a plasmacytoid morphology (Asselin-Paturel et al. 2001, Bjorck 2001, Nakano et al. 2001). They demonstrated that these cells have the capacity to stimulate naïve T cells and produce IFN-α when stimulated in vitro and in vivo, proposing that they are equivalent to human pDCs (Asselin-Paturel et al. 2001, Bjorck 2001, Nakano et al. 2001). Fig. 1 demonstrates scanning electron micrograph (Fig. 1A) and transmission electron micrograph (Fig. 1B) of pDCs isolated from human peripheral blood as well as pDCs in murine spleen during steady state (Fig. 1C). Upon verification of these observations by additional groups (Brawand et al. 2002, Martin et al. 2002, O’Keeffe et al. 2003), later studies identified pDCs in monkeys (Coates et al. 2003), pigs (Summerfield et al. 2003), rats (Hubert et al. 2004), and sheep (Pascale et al. 2008), suggesting that pDCs may be preserved during evolution.
Figure 1. Illustration of plasmacytoid dendritic cells.
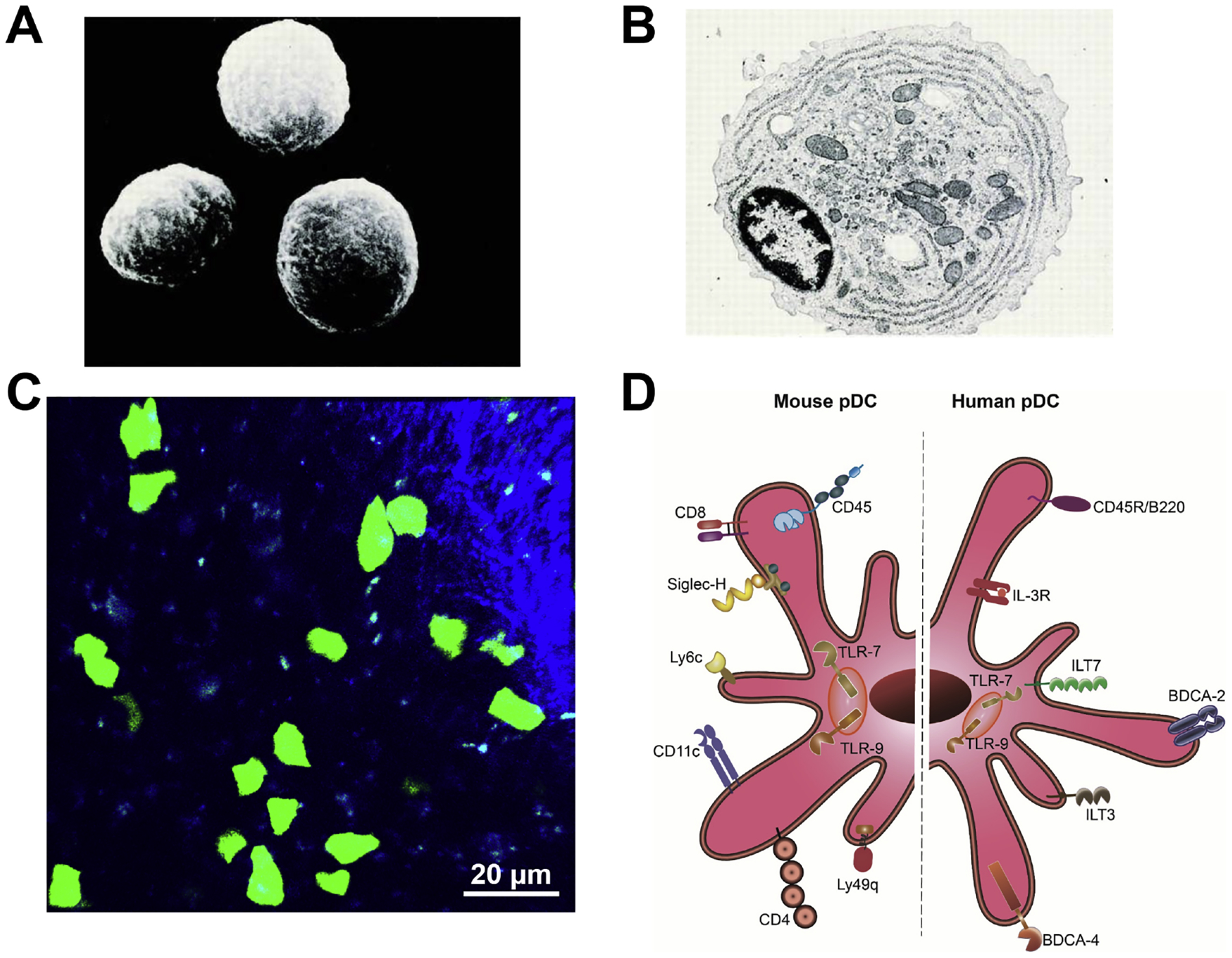
(A, B) Scanning electron micrograph (A) and transmission electron micrograph (B) of human pDCs isolated from peripheral blood. Magnification: ×3,500 in (A) and ×8,000 in (B); ©1997 Grouard et al. Originally published in J Exp Med. https://doi.org/10.1084/jem.185.6.1101. (C) Representative image of splenic pDCs in a DPE-GFP×RAG1−/− mouse with GFP-tagged pDCs. Scale bar: 20 μm. (D) Schematic representation of pDC markers in mice and humans. In humans, pDCs express the specific markers BDCA-2 and ILT-7, and share expression of BDCA-4, IL-3Rα, and ILT-3 with other immune cells. In mice, pDCs express PDCA-1, Siglec-H, CD11c, CD45R/B220, Ly6C, and Ly49Q. Both human and murine pDCs express intracellular receptors TLR-7 and TLR-9.
1.2. Phenotypic Markers of Plasmacytoid Dendritic Cells
1.2.1. Plasmacytoid Dendritic Cell Markers in Human
The original discovery of human pDCs showed that pDCs do not express CD3 (T cell marker), CD20, CD22 (both expressed by B cells and plasma cells), but do express CD4, CD68, and IL-3Rα (CD123)(Lennert et al. 1975, Horny et al. 1987, Facchetti et al. 1988, Grouard et al. 1997). Later, it was shown that pDCs specifically express blood dendritic cell antigen (BDCA)-2 (CD303) (Dzionek et al. 2000), Ig-superfamily receptor or immunoglobulin-like transcript (ILT)-7 (Rissoan et al. 2002, Cao et al. 2006), and share expression of BDCA-4 (CD304; neuropilin-1) with other cells (Dzionek et al. 2000). Further, in contrast to mice, human pDCs do not express CD11c (Facchetti et al. 1988, Grouard et al. 1997, Olweus et al. 1997).
BDCA-2 is a type II C-type lectin, which can take up antigens and inhibit secretion of IFN-α/β and tumor necrosis factor (TNF)-α (Dzionek et al. 2001, Cao et al. 2006). Although it is deemed pDC-specific in humans, expression of BDCA-2 is down-regulated in pDCs, when cultured with IL-3 (Dzionek et al. 2000). ILT-7, similar to BDCA-2, is considered a human pDC-specific cell surface receptor, which can regulate secretion of IFN-α and TNF-α in stimulated pDCs (Cao et al. 2006, Cho et al. 2008). BDCA-4, a type I transmembrane receptor, is a member of the class 3 semaphorin subfamily (Kolodkin et al. 1997), a co-receptor for vascular endothelial growth factor (VEGF)-A, and is expressed by human pDCs, as well as by some other murine and human cell populations such as immune cells (Tordjman et al. 2002, Bruder et al. 2004, Delgado et al. 2005, de Paulis et al. 2006, Ghez et al. 2006, Bles et al. 2007, Lepelletier et al. 2007, Battaglia et al. 2008, Fantin et al. 2010, Carrer et al. 2012, Mendes-da-Cruz et al. 2014, Miyauchi et al. 2018), vascular endothelial cells (Herzog et al. 2001) and in multiple cancers (Jubb et al. 2012, Li et al. 2016, Zhu et al. 2018, Ma et al. 2019, Yang et al. 2019). While this molecule has broad implications in axonal guiadance and angionegensis (Bagri et al. 2002, Plein et al. 2014), in pDCs, it may be implicated in IFN-α secretion (Grage-Griebenow et al. 2007) and similar to cDCs, may contribute to pDC-T cell interactions (Chaudhary et al. 2014).
Thus, in humans, pDCs can be indentified by their expression of CD4, IL-3Rα, CD45R/B220, BDCA-2, BDCA-4, ILT-3, and IL-7, and lack of expression of T cell and B cell lineage markers, CD3 and CD19, as well as the myeloid marker, CD11b, and cDC marker, CD11c (Table 1). Among these markers, BDCA-2 and ILT-7 are considered specific pDC markers. Accurate detection of human pDCs requires a core panel consisting of CD45, BDCA-2, IL-3Rα, ILT-7, CD11c and CD11b (Barchet et al. 2005, Rogers et al. 2013, Swiecki et al. 2015).
Table 1.
pDCs Markers in Humans and Mice
| Marker | Human | Mouse |
|---|---|---|
| CD1a (Langerhans cell marker) | − (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) |
N/A |
| CD3 (T cell marker; T cell co-receptor) | − (Muller-Hermelink et al. 1983) (Harris et al. 1987) (Horny et al. 1987) (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) |
− (Asselin-Paturel et al. 2003) |
| CD4 (T cell co-receptor) | + (Muller-Hermelink et al. 1983) (Harris et al. 1987) (Horny et al. 1987) (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) |
+ (Nakano et al. 2001) (Martin et al. 2002) (Omatsu et al. 2005) |
| CD8 (T cell co-receptor) | − (Muller-Hermelink et al. 1983) (Harris et al. 1987) (Horny et al. 1987) (Facchetti et al. 1988) (Grouard et al. 1997) (Bendriss-Vermare et al. 2001) |
+/− (Asselin-Paturel et al. 2001) (Nakano et al. 2001) (Martin et al. 2002) (Asselin-Paturel et al. 2003) low (Castellaneta et al. 2004) (Omatsu et al. 2005) |
| CD11b (αM integrin; usually non-covalently associates with β2 integrin [CD18]) | − (Harris et al. 1987) (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) |
− (Nakano et al. 2001) (Martin et al. 2002) (Asselin-Paturel et al. 2003) (Castellaneta et al. 2004) |
| CD11c (αx integrin; usually forms a heterodimer with β2 integrin [CD18]) | − (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) |
+/++ (Nakano et al. 2001) (Asselin-Paturel et al. 2003) (Castellaneta et al. 2004) (Omatsu et al. 2005) (Contractor et al. 2007) and NK cells: (Blasius et al. 2007) |
| CD14 (LPS receptor; mainly expressed by monocytes and macrophages) | − (Facchetti et al. 1988) (Grouard et al. 1997) (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) |
|
| CD16 (low affinity IgG receptor, mainly expressed by NK cells, activated monocytes, and macrophages) | − (Grouard et al. 1997) (Olweus et al. 1997) |
|
| CD19 (member of the Ig superfamily, expressed on all stages of B cell development from pro-B cells to mature B cells) | − (Grouard et al. 1997) (Olweus et al. 1997) |
− (Nakano et al. 2001) (Martin et al. 2002) (Asselin-Paturel et al. 2003) (Contractor et al. 2007) |
| CD45R/B220 (an isoform of CD45, expressed at all developmental stages of B cells, from pro-B cells through mature B cells) | + (Nakano et al. 2001) (Asselin-Paturel et al. 2003) (Castellaneta et al. 2004) (Omatsu et al. 2005) (Contractor et al. 2007) And NK cells: (Blasius et al. 2007) NK cell progenitors: (Rolink et al. 1996) |
|
| CD123 (IL3Ra) | ++ (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) also present on blood monocytes: (Buelens et al. 2002) cDCs: (Masten et al. 2006) cDC precursors: (Breton et al. 2016), (See et al. 2017) |
− (As IL-3Ra) (Martin et al. 2002) − (Asselin-Paturel et al. 2001) Low (Bjorck 2001) Low: (O’Keeffe et al. 2002) |
| CD56 (A single transmembrane glycoprotein member of the Ig superfamily, mainly expressed by NK and NKT cells) | − (Grouard et al. 1997) (Olweus et al. 1997) (Bendriss-Vermare et al. 2001) |
|
| CD303 (BDCA-2; a type II transmembrane glycoprotein member of the C-type lectin superfamily) | +* (Dzionek et al. 2000) |
N/A |
| CD304 (BDCA-4; a type I transmembrane protein implicated in a variety of biologic functions; VEGF165/semaphorin-3A receptor) | (Dzionek et al. 2000) | N/A |
| ILT3 (a type I membrane protein expressed by DCs and monocytes) | + And monocytes, macrophages, and cDCs: (Cella et al. 1997) (Cao et al. 2006) |
|
| ILT7 (a member of leukocyte immunoglobulin-like receptor family) | +* (Cao et al. 2006) |
|
| Gr-1 (Ly6C/Ly6G) | + (Nakano et al. 2001) low (Asselin-Paturel et al. 2003) low (Castellaneta et al. 2004) (Contractor et al. 2007) |
|
| Ly6C (a member of the Ly6 family of GPI linked protein, expressed by various murine immune cells) | N/A | + (Asselin-Paturel et al. 2003) (Omatsu et al. 2005) |
| Ly6G (a member of the Ly6 family of GPI linked protein, expressed on the majority of myeloid cells and granulocytes) | − (Asselin-Paturel et al. 2003) |
|
| Ly49Q (a type II C-type lectin membrane-associated polypeptide) | + (Omatsu et al. 2005) |
|
| PDCA-1 (Bst-2; a type II transmembrane glycoprotein, an IFN-induced response factor) | + Also tumor cells: (Walter-Yohrling et al. 2003) |
+ (Asselin-Paturel et al. 2003) (Blasius et al. 2006) (Contractor et al. 2007) Also other cells: (Blasius et al. 2006) |
| Siglec-H (a member of CD33-related Siglec family) | N/A | + |
| CD45 (a single chain type I membrane glycoprotein; expressed by all immune cells) | + (Facchetti et al. 1988) |
+ |
−: negative; +: present; ++:high levels
considered pDC specific
N/A: Not applicable
ILT: immunoglobulin-like transcript
BDCA: blood dendritic cell antigen
PDCA-1: plasmacytoid dendritic cell antigen-1
Bst-2: Bone marrow stromal antigen-2
1.2.2. Plasmacytoid Dendritic Cell Markers in Mice
In mice, detecting pDCs is more sophisticated as compared to humans. Murine pDCs were originally identified as CD11bneg CD4+ CD11clow/int CD45R/B220+ Gr-1+ Ly6C+cells (Asselin-Paturel et al. 2001, Bjorck 2001, Nakano et al. 2001, Brawand et al. 2002, Martin et al. 2002, O’Keeffe et al. 2003). However, to date, no single marker is considered sufficiently specific to uniquely distinguish pDCs during steady state and inflammation in mice. The first purported pDC marker, the 120G8 antibody, recognizes splenic and bone marrow pDCs during steady state, and allows depletion pDCs both functionally and physically (Asselin-Paturel et al. 2003). A year later, plasmacytoid dendritic cell antigen (PDCA)-1 was introduced as another antibody. This antibody also recognizes and depletes pDCs (Krug et al. 2004). Later, Blasius et al. generated an antibody, which recognizes pDCs and reacts with bone marrow stromal cell antigen 2 (BST-2; also, known as CD317) (Blasius et al. 2006). They showed that both 120G8 and PDCA-1 antibodies in fact recognize BST-2 as well (Blasius et al. 2006), suggesting that all three antibodies (120G8, PDCA-1 and BST-2) share a similar target. Although in mice, BST-2 is predominantly expressed on pDCs during steady state in multiple lymphoid organs, it is also expressed by other cell lines, such as embryonal carcinoma cell line P19, and is upregulated on multiple immune and non-immune cells during inflammation (Blasius et al. 2006, Holmgren et al. 2015). Further studies showed that expression of PDCA-1 is not necessarily restricted to pDCs even during steady state, as rare subpopulations of B cells and plasma cells may share expression of PDCA-1 with pDCs (Vinay et al. 2010, Vinay et al. 2012). Current studies suggest that PDCA-1, as a type II transmembrane glycoprotein, may tether viral membranes to host cell membranes and thus, prevent the release of multiple enveloped viruses from infected cells (Martin-Serrano et al. 2011, Hotter et al. 2013). Furthermore, PDCA-1 may regulate IFN production by pDCs (Swiecki et al. 2012) and can amplify NF-κB signaling (Cocka et al. 2012).
Another potentially specific pDC marker initially designated as 440c (Blasius et al. 2004), recognizes a member of the Sialic acid-binding immunoglobulin-type lectin (Siglec) family of I-type lectins, called Siglec-H, suggesting that Siglec-H is selectively expressed by murine pDCs (Blasius et al. 2006). Later studies indicated that pDCs share expression of Siglec-H with other immune cells, including marginal zone macrophages in the spleen, medullary cord macrophages in lymph nodes, and central nervous system microglia (Zhang et al. 2006, Konishi et al. 2017). Current data suggests that Siglec-H is an endocytic receptor, which mediates antigen uptake by pDCs and may regulate IFN and cytokine production in these cells, as well as interaction of pDCs with T cells (Blasius et al. 2004, Blasius et al. 2006, Zhang et al. 2006, Takagi et al. 2011).
Gr-1 was among the first array of markers by which murine pDCs were originally identified (Asselin-Paturel et al. 2001, Nakano et al. 2001). Prior to the discovery of murine pDCs, it was acknowledged that the Gr-1 antibody strongly reacts with granulocyte marker Ly6G, but also recognizes Ly6C as another member of Ly6 complex (Fleming et al. 1993). Later, it was shown that murine pDCs do not express Ly6G (Asselin-Paturel et al. 2003), but do express Ly6C, and thus, the antibody against Gr-1 (that recognizes Ly6C), can also detect pDCs. However, pDCs have a lower affinity to binding this antibody compared to neutrophils, and are thus, stained with less intensity with Gr-1 (Shortman et al. 2002). Moreover, although Ly6C is highly expressed on pDCs (Asselin-Paturel et al. 2003), due to its well-known common expression by subsets of multiple immune and non-immune cells (Jutila et al. 1988), its solo application for differentiating pDCs is discouraged (Colonna et al. 2004).
Another important murine pDC marker is Ly49Q, a type II C-type lectin polypeptide (Rahim et al. 2014). Gr-1+ cells expressing Ly49Q, were shown to also express CD11c and CD45R/B220, corroborating their identity as pDCs (Toyama-Sorimachi et al. 2005). However, in addition to pDCs, stimulated macrophages and cDCs may also express Ly49Q following treatment with IFN-α or IFN-γ (Toyama-Sorimachi et al. 2005). Further, in a few murine strains, all myeloid cells express low levels of this molecule (Toyama-Sorimachi et al. 2005). In addition to the potential role in pDC maturation (the process of up-regulation of MHC-II and antigen presentation to prime adaptive immune cells) (Toma-Hirano et al. 2009), through interaction with MHC-I, Ly49Q may regulate cytokine production, including IFN-α, in pDCs (Tai et al. 2008).
In summary, murine pDCs express PDCA-1, Siglec-H, CD11clow/int, CD45R/B220, Ly49Q, Ly6C, and Gr-1 (Table 1). Thus, to accurately identify murine pDCs, assessment of multiple markers, such as a core panel of PDCA-1, CD45R/B220, Siglec-H, CD11b, CD11c, Ly6C, Ly49Q, CD3, and CD19 is recommended (Barchet et al. 2005, Jegalian et al. 2009, Rogers et al. 2013, Swiecki et al. 2015).
1.2.3. Plasmacytoid Dendritic Cells Repertoire of Toll-like Receptors
As members of the innate immune system, pDCs are equipped with a specific repertoire of pattern recognition receptors. Both in humans and mice, pDCs are known to particularly express endosomal/lysosomal receptors toll-like receptor (TLR)-7 and TLR-9, which enables them to detect single stranded RNA and double stranded DNA, respectively (Kadowaki et al. 2001, Krug et al. 2001, Hornung et al. 2002, Edwards et al. 2003). Although traditionally pDCs were thought to express only TLR-7 and TLR-9, some reports suggests that pDCs may also express TLRs-1, −6, and −10 (Kadowaki et al. 2001, Krug et al. 2001, Hornung et al. 2002, Edwards et al. 2003, Hasan et al. 2005, Raieli et al. 2019), and may also up-regulate TLRs-2 and −4 (Hernandez et al. 2011, Zheng et al. 2012). Fig. 1D summarizes the markers and receptors expressed by pDCs in mice and humans.
1.3. Development of Plasmacytoid Dendritic Cells
The development of pDCs is defined by their remarkable plasticity. pDCs are continuously generated in the bone marrow and after terminal differentiation, they egress into the bloodstream (Sawai et al. 2013, Chistiakov et al. 2014). However, a minor subset of pre-pDCs may enter the bloodstream and differentiate into pDCs or alternatively into cDCs, depending on the tissue environment (Schlitzer et al. 2012). In contrast to many other immune cells that originate from either myeloid or lymphoid progenitors, at least in mice, pDCs can arise from both common lymphoid and myeloid progenitors (Wu et al. 2001, Karsunky et al. 2003, Karsunky et al. 2005, Rodrigues et al. 2018, Dress et al. 2019). In fact, both lineages have been shown to lead to a common peripheral pDC phenotype with the characteristic set of pDC surface markers as described above, although myeloid-derived pDCs may show a higher cytokine secretory and T cell-stimulating capacities (Yang et al. 2005).
1.3.1. Plasmacytoid Dendritic Cell Development in Mice
In mice, the evidence of the development of pDCs from both lineages is strongly supported by multiple immunophenotyping studies on the developing progenitors in the bone marrow, as well as recent single cell sequencing approaches on the characterizing pre-pDCs and pDCs (Rodrigues et al. 2018, Dress et al. 2019). Our current knowledge suggests that from the myeloid pathway, pDCs are derived either directly from myeloid macrophage/DC progenitors (MDPs), or via the MDP to common DC progenitor (CDP) pathway (Karsunky et al. 2005). In mice, CDPs are lineage marker-negative (Linneg), indicating they are not expressing mature cell lineage markers (Karsunky et al. 2005). In addition, CDPs are fms-like tyrosine kinase (FLT)3+ (also known as CD135), receptor tyrosine kinase KIT low or intermediate (c-Kitlow/int), and macrophage colony stimulating factor receptor (M-CSFR)+. Exposure to FLT3-ligand (FLT3-L), and to a lesser degree M-CSF, induces CDPs toward pDCs (Karsunky et al. 2005).
pDC developmental paradigms are encoded by the cumulative effects of several key transcription factors. Among the key transcription factors in pDC development, are transcription factor 4 (TCF-4), also known as basic helix-loop-helix transcription factor or E protein (E2–2) and its protein co-factor MTG16, as well as B-cell lymphoma/leukemia 11A (BCL-11A), and interferon-regulatory factor 8 (IRF-8) (Cisse et al. 2008, Ghosh et al. 2010, Ippolito et al. 2014, Sichien et al. 2016, Grajkowska et al. 2017). As with most developmental paradigms, there are counteracting players involved. The DNA-binding protein inhibitor ID-2 acts by binding to and inhibiting E2–2. Therefore, if ID-2 is expressed by CDPs, pDC development is suppressed, as E2–2 is blocked from activating its downstream targets (Ghosh et al. 2014). When E2–2 is expressed by CDPs, it activates the interferon regulatory factors (IRF)-4 and IRF-8, two closely related transcription factors that have a central role in pDC development (Tamura et al. 2005). Studies have demonstrated that pDCs are diminished in both IRF-4 and IRF-8 knockout animals and that thus, both IRF-4 and IRF-8 are important in pDC development (Tamura et al. 2005). In fact, the expression of E2–2 is not only required for the initial development of pDCs from progenitors in the bone marrow, but also for the continued maintenance of the pDC phenotype in the periphery (Ghosh et al. 2010).
PU.1 is another key transcription factor in pDC development that regulates the expression of the Flt3 gene and as such, PU.1 deletion blocks pDC development (Carotta et al. 2010). Phosphoinositide 3-kinase, a mammalian target of rapamycin (mTOR), signals downstream of FLT3 (Sathaliyawala et al. 2010), and inhibition of mTOR reduces pDC numbers, whereas deletion of the Pten gene, a negative regulator of mTOR signaling, increases pDC differentiation (Sathaliyawala et al. 2010). Spi-B has also been highlighted as a master regulator of the pDC fate and is required for pDC development (Sasaki et al. 2012). Yet, another transcription factor, X-box binding protein-1 (XBP-1), has been implicated for both differentiation and survival of pDCs. Knockout of XBP-1 results in decreased numbers of pDCs, and stimulation of XBP-1−/− pDCs resulted in decreased survival (Iwakoshi et al. 2007). Other transcription factors involved in pDC development include DNA-binding protein Ikaros, hypoxia-inducible factor 1α, growth factor independent 1, and nuclear polyadenylated RNA-binding protein 2 (Rathinam et al. 2005, Allman et al. 2006, Balzarolo et al. 2012, Backer et al. 2017). Several studies have shown that knockout mice lacking these transcription factors show reduced, if not eliminated pDCs in the examined tissues (Rathinam et al. 2005, Allman et al. 2006, Balzarolo et al. 2012, Backer et al. 2017).
Lymphoid progenitors (LPs) can also commit to the pDC lineage. pDC precursors of lymphoid origin include the lymphoid primed progenitors (LMPP), which give rise to MDPs, and the common lymphoid progenitor (CLP) (Vogt et al. 2009, Sathe et al. 2013). Although pDCs may originate from CLPs, they may develop independent of key enzymes required for B and T cell development. In this regard, it has been demonstrated that in RAG1−/− mice, which lack mature B and T cells (Mombaerts et al. 1992), and in mice with disruptive Ig H chain mu membrane exon that lack B cells in the peripheral blood (Kitamura et al. 1992), the density and phenotype of pDCs is comparable to wild-type mice (Nikolic et al. 2002). In contrast, the lymphoid cytokine IL-7 seems to play a role in pDC development as shown in IL-7 deficient mice, which contain few pDCs in the adult (Vogt et al. 2009). As in the myeloid pathway, lymphoid progenitors expressing FLT3, when exposed to FLT-3L, develop into pDC (Lyman et al. 1993, Maraskovsky et al. 1996).
1.3.2. Plasmacytoid Dendritic Cell Development in Humans
In humans, a new paradigm regarding pDC development has recently emerged, with noted differences from the murine model (Collin et al. 2018). Given the invasive nature of bone marrow biopsies, it is difficult to obtain and analyze human bone marrow of humans. The blood is easily attainable, and studies from those hematopoietic precursors have more recently been probed (Mohamedali et al. 2015). In the classical model of hematopoiesis, it was thought that progenitor cells divide and give rise to cells of alternative fates (e.g., CDP giving rise to pDCs and cDCs) with equal probability. However, this notion has been challenged based on available empirical data. The revised model suggests that priming occurs early on during hematopoiesis, which determines the ultimate fate of the cell, although the cell will transiently pass through phenotypes that were previously considered progenitors (Collin et al. 2018). In fact, a study by Lee et al. assessing clonal assays of human CD34+ progenitor cells showed no human MDP or CDP progenitors (Lee et al. 2017). This concept has also been supported by single-cell analysis of cord blood (Karamitros et al. 2018). Given the lack of empirical evidence for specific progenitors, it has been proposed that there is no need to distinguish between myeloid and lymphoid lineages, as both result from an initial lymphoid-primed multipotent progenitor (Collin et al. 2018). This revised model can make sense of the lymphoid and myeloid features of pDCs (such as shared cell markers with both lineages), which the previous classical model fell short of.
Aside from the revised model of hematopoiesis in humans, the developmental paradigms among mice and men are quite similar, sharing many of the same core transcription factors. E2–2 is a major transcription factor in pDC development along with Spi-B (Schotte et al. 2004), IRF-4, IRF-8 (Sontag et al. 2017), and ZEB2, Zinc Finger E-Box Binding Homeobox 2, working in conjunction with MTG16 to repress ID-2 expression (Villani et al. 2017), which mirrors murine pDC development. In humans, a loss of E2–2 or heterozygous mutation causes Pitt–Hopkins syndrome in which mature type I IFN-secreting pDC are significantly reduced (Cisse et al. 2008). Further, the cytokine thrombopoietin, along with FLT3-L, can synergistically promote human pDC development (Cisse et al. 2008, Nagasawa et al. 2008, Ghosh et al. 2010). Overall, it has been difficult to directly examine the ontogeny of human pDCs, as it is not feasible to perform fate-mapping experiments in humans as researchers have done in the murine systems. Fig. 2 summarizes development of pDCs from bone marrow stems cells and highlights the key transcription factors involved in their development.
Figure 2. Schematic diagram on development of plasmacytoid dendritic cells.
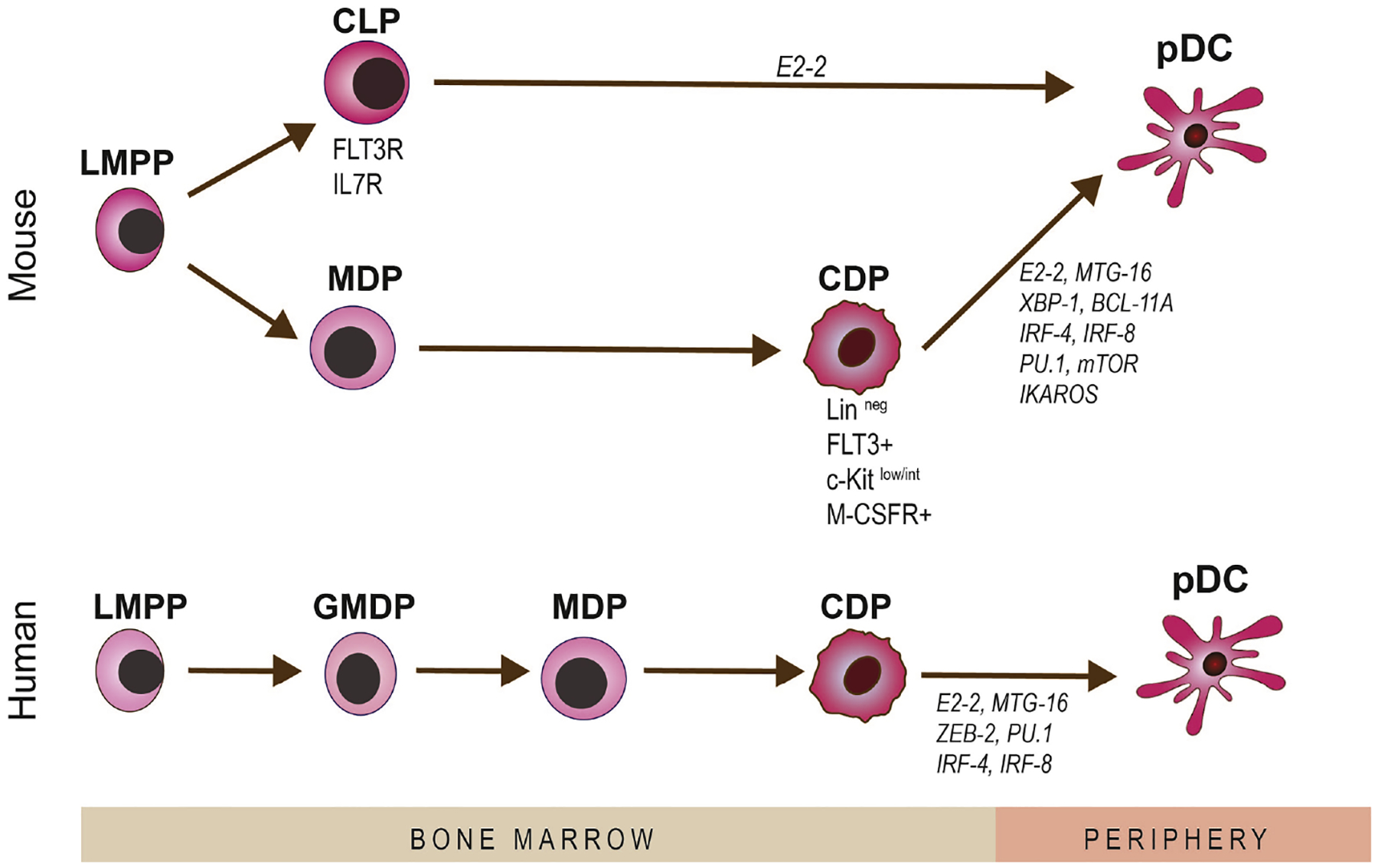
As bone marrow-derived cells, pDCs can origin from both myeloid and lymphoid precursors, in mice. The cellular precursors of pDCs and transaction factors involved in development of pDCs are shown.
1.4. Tissue Distribution of Plasmacytoid Dendritic Cells
pDCs are generally considered as rare, but potent immune cells, comprising close to only 1% of immune cells in the bone marrow. Studies in mice and humans have widely confirmed that after generation in the bone marrow, pDCs enter the blood steam, where they constitute less than 1% of immune cells (Asselin-Paturel et al. 2003, Chowdhury et al. 2010, Murray et al. 2019). While circulating in the blood stream, during steady state they typically home to secondary lymphoid organs, including the spleen, lymph nodes, tonsils, thymus, and Peyer’s patches of the gut (Grouard et al. 1997, Bendriss-Vermare et al. 2001, Nakano et al. 2001, Jameson et al. 2002, Asselin-Paturel et al. 2003, Castellaneta et al. 2004, Omatsu et al. 2005, Contractor et al. 2007, Boor et al. 2019). Based on the current understanding, pDCs are typically absent in peripheral tissues during steady state, with few exceptions. However, they are recruited from the blood to sites of inflammation during microbial infections, tumors, and autoimmune conditions (Nestle et al. 2005, Santoro et al. 2005, Smit et al. 2006, Sozzani et al. 2010).
Nevertheless, few peripheral tissues do host pDCs during steady state, albeit in low densities. Among these peripheral tissues, lungs were among the first tissues in which resident pDCs were noted during steady state (Donnenberg et al. 2003, de Heer et al. 2004). Attempting to assess the role of antigen presenting cells (APCs) in the prevention of immune responses to allergens, de Heer et al. showed that pDCs reside in the interalveolar interstitium, at almost twice-higher densities as resident cDCs (de Heer et al. 2004). Later studies confirmed the presence of resident pDCs in murine and human lungs; however, at lower densities compared with cDCs (Donnenberg et al. 2003, Lommatzsch et al. 2007, Venet et al. 2010, Ten Berge et al. 2012).
The kidney is another peripheral tissue in which resident pDCs have been reported during steady state by Coates et al., who showed that murine kidneys host CD11c+ CD45R/B220+ CD8αneg cells, presumably of pDC phenotype (Coates et al. 2004). Resident pDCs were reported in the tubulo–interstitium, and rarely within the glomeruli of normal human kidneys, although their density was less as compared to cDCs (Woltman et al. 2007). Assessing the role of pDCs in vaginal herpes simplex virus (HSV)-2 infection, Lund et al., showed that the murine vagina also hosts resident pDCs during steady state in a density comparable to cDCs (Lund et al. 2006). In line with their findings, Agrawal et al. later showed the presence of pDCs the cervical mucosa of healthy individuals (Agrawal et al. 2009).
Moreover, based on currently available RNA sequencing performed on tissue samples from 95 human individuals, E2–2 is expressed in the brain, heart, fat tissue, adrenal gland, ovary, and testis, in addition to lymphoid tissues and the lung, suggesting the potential presence of pDCs in these tissues (Fagerberg et al. 2014). Table 2 summarizes the non-ocular tissues, including secondary lymphoid organs and peripheral tissues in which pDCs have been reported during steady state.
Table 2.
Distribution of pDCs in Tissues During Steady State
N/A: Not applicable
N/E: Not evaluated
In patients with hepatic tumors, in sample distant from tumor (at least 1 cm)
Of note, although detailed morphologic assessment of resident pDCs in peripheral tissues needs further investigation, current evidence suggests that the morphology of pDCs in peripheral tissues may differ from circulating pDCs in the blood stream or lymphoid organs. As depicted in Fig. 1A–C, while electron microscopy of freshly isolated pDCs from blood circulation as well as histologic staining and MPM of pDCs in lymphoid organs (Fig. 1C) indicate that they appear as spherical cells without dendritic projections (Grouard et al. 1997, Jegalian et al. 2009), histologic staining of pDCs in the peripheral tissues, such as resident pDCs in kidney and lung, suggest that they exhibit more elongated cell bodies with few stub-like projections (Masten et al. 2006, Woltman et al. 2007). Thus, pDCs may show a distinct morphology in the blood stream and lymphoid organs compared with peripheral tissues.
2. Resident Immune Cells in Ocular Tissues
The notion of ocular immune privilege has been described decades ago (Medawar 1948); however, this concept has undergone extensive modification and revision since its initial conception (Forrester 2009, Hori et al. 2010). While many pillars have been proposed that contribute to ocular immune privilege, from a lack or limited expression of MHC-II on resident APCs in ocular tissues (Streilein et al. 1979, Wang et al. 1987, Baudouin et al. 1988), to lack of lymphatics and blood vessels (Medawar 1948), and an immunosuppressive microenvironment in the ocular tissues (Streilein et al. 1992, Taylor et al. 1994, Stuart et al. 2005, Hori 2008, Taylor 2009), these pillars have failed to explain immune privilege in its entirety (Paunicka et al. 2015, Hamrah et al. 2016, Hori et al. 2019). Work from several groups in the field has now demonstrated that there are in fact resident immune cells present in essentially all ocular tissues (Steptoe et al. 1995, Butler et al. 1996, Gomes et al. 1997, Hamrah et al. 2002, Hamrah et al. 2003, Hamrah et al. 2003, Hamrah et al. 2003, Forrester et al. 2005, Xu et al. 2007). Herein, we briefly review our current understanding on resident immune cell populations in ocular tissues and discuss what is known with respect to the function of these cells, in order to provide a framework as to how pDCs complement known functions of other immune cells in reinstating homeostasis in ocular tissues. Fig. 3A illustrates the presence and distribution of resident immune cells in ocular tissues.
Figure 3. Schematic illustration of distribution of various resident immune cells in the ocular tissues.
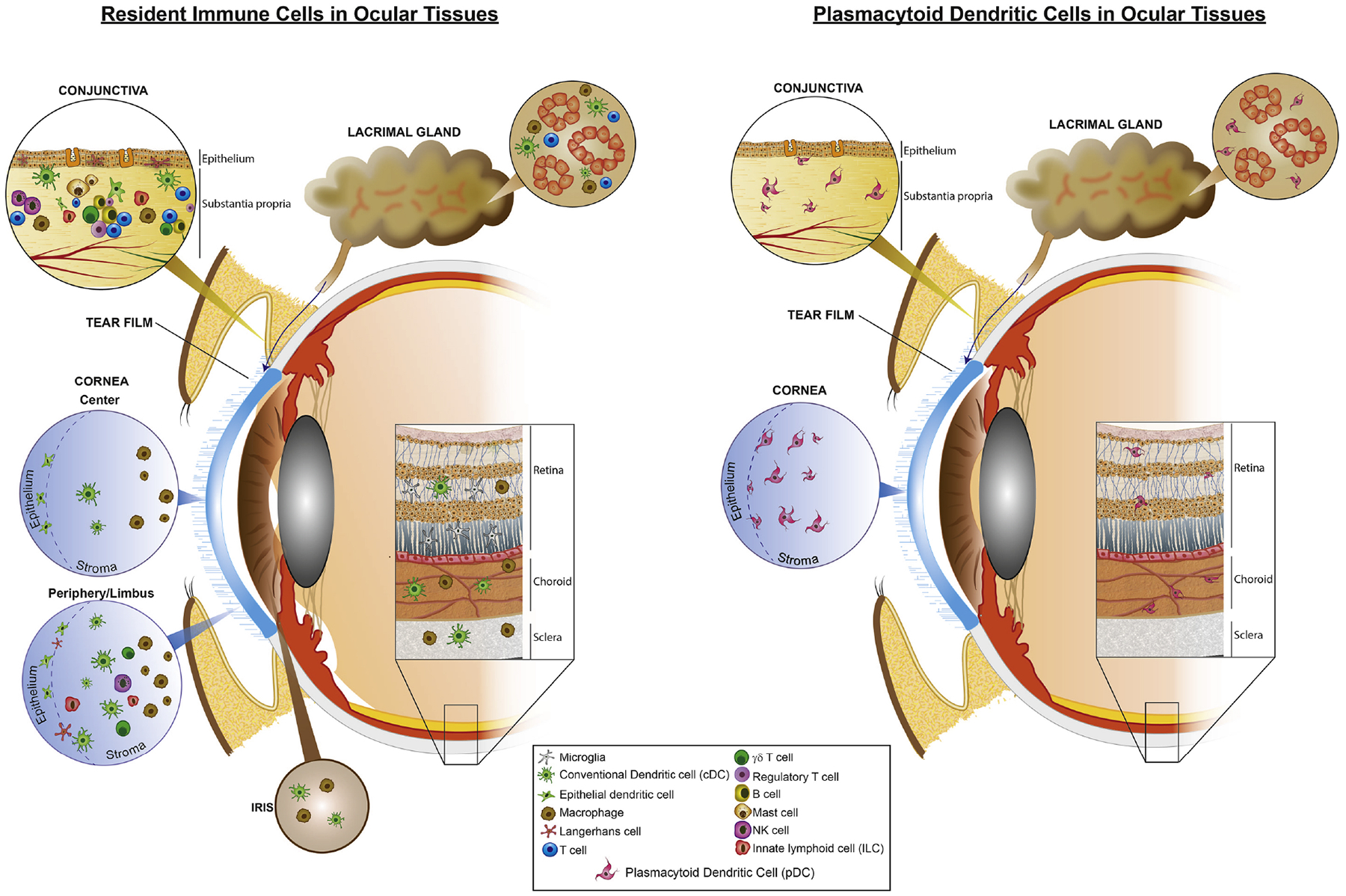
(A) Resident immune cells are located in different parts of the ocular system. In the conjunctiva, cDCs, macrophages, B cell, and T cells are detected; in cornea, cDCs and macrophages comprise the main resident immune cells; in choroid, cDCs and macrophages and in retina microglia, perivascular macrophages, and cDCs are considered the main resident immune cells; in the lacrimal gland, cDCs, macrophages, and B cells are predominant resident immune cells. (B) To date, resident pDCs are reported in the central and peripheral cornea, limbus, bulbar conjunctiva, choroid, retina, and lacrimal gland
2.1. Cornea
Despite the traditional view that cornea is a collagenous tissue devoid of resident immune cells, it has now been demonstrated that the normal cornea is in fact home to different populations of resident immune cells, in particular innate immune cells, including APCs (Rodrigues et al. 1981, Vantrappen et al. 1985, Brissette-Storkus et al. 2002, Hamrah et al. 2002, Hamrah et al. 2003, Hamrah et al. 2003). Interestingly, resident corneal immune cells are not distributed evenly. While the majority of immune cells follow a decrease in density from the peripheral towards the central cornea, their localization throughout different corneal layers varies among subpopulations (Hamrah et al. 2002, Hamrah et al. 2003, Hamrah et al. 2003).
Subpopulations of cDCs are among the first populations of resident APCs that were detected in the cornea (Rodrigues et al. 1981, Chandler et al. 1985, Seto et al. 1987, Hamrah et al. 2002, Yamagami et al. 2005). While initial reports, studying MHC-II, suggested that cDCs, including LCs, are confined to limbus and peripheral cornea, it was later shown that MHC-IIneg cDCs are also located in the central corneal epithelium and stroma, with a decreasing density from periphery to center (Hamrah et al. 2002, Liu et al. 2002, Hamrah et al. 2003, Hamrah et al. 2003). Nevertheless, upon inflammatory stimuli, corneal cDCs increase and mature throughout the cornea, expressing increased levels of MHC-II and co-stimulatory molecules (Hamrah et al. 2003). In addition to cDCs, macrophages are shown to reside in the posterior corneal stroma during steady state in the peripheral and central cornea (Brissette-Storkus et al. 2002, Hamrah et al. 2003, Hamrah et al. 2003). Nakamura et al. demonstrated that 2 weeks following adoptive transfer of bone marrow-derived cells from eGFP-expressing mice to irradiated, syngeneic mice, eGFP+ cells appear in the corneal limbus and periphery, confirming the bone marrow origin of resident corneal immune cells (Nakamura et al. 2005). Further studies, taking advantage of transgenic CD11cDTR-eGFP mice that express enhanced green fluorescent protein (eGFP) under the control of CD11c promoter, confirmed the presence of eGFP+ cDCs in the cornea during steady state (Knickelbein et al. 2009). Similar to cDCs, studies on transgenic CX3CR1eGFP mice in which macrophages express eGFP, confirmed their presence in the cornea during steady state (Chinnery et al. 2007).
More recently, intravital multi-photon microscopy studies on corneas of CD11cDTR-eGFP and MHC-IIeGFP mice have provided in vivo insights into their behavior (Seyed-Razavi et al. 2019). These studies have shown that during acute corneal inflammation, cDCs respond to stimuli by exhibiting less volume and more sphericity, as well as increasing their motility, highlighting the alterations in their morphology and kinetics following inflammation (Seyed-Razavi et al. 2019). In fact, resident corneal APCs play important roles in mediating immune responses to corneal insults, such as during infectious keratitis, dry eye disease (DED), and corneal transplantation (Liu et al. 2002, Buela et al. 2015, Hu et al. 2015, Hua et al. 2016, Ramke et al. 2016, Choi et al. 2017, Maruoka et al. 2018). Following corneal transplantation, corneal cDCs have been demonstrated to migrate to the mandibular draining lymph nodes (dLNs) and elicit adaptive immune responses (Liu et al. 2002). Further, cDCs are also vital for corneal wound healing in diabetic mice (Gao et al. 2016) and in corneal nerve survival in DED (Choi et al. 2017). In addition to antigen presentation and wound healing (Li et al. 2013, Bellner et al. 2015), corneal macrophages contribute to angiogenesis and lymphangiogenesis under pathologic conditions (Cursiefen et al. 2004, Maruyama et al. 2005, Xu et al. 2007, Maruyama et al. 2012, Kiesewetter et al. 2019).
Notably, resident APCs, including both cDCs and macrophages are located closely to the corneal nerves (Cruzat et al. 2011, Leppin et al. 2014, Seyed-Razavi et al. 2014, Paunicka et al. 2015, Gao et al. 2016, Hamrah et al. 2016, Hori et al. 2019). However, they dissociate after corneal injury, implying that neuro-immune crosstalk has a potential role in corneal health and disease (Seyed-Razavi et al. 2014). Employing multi-photon microscopy in double-transgenic CD11ceYFP×Thy1YFP mice, we have recently assessed the significance of corneal nerves on alterations in morphology and kinetics of CD11c+ cDCs in DED. We observed that in mice with DED, cDCs are less frequently associated with nerves, and that association with nerves diminishes alterations in cDC morphology and kinetics observed in DED (Jamali et al. 2020).
In addition to cDCs and macrophages, subpopulations of T cells, such as γδ T cells (Li et al. 2007), type 2 innate lymphoid cell (ILCs) (Liu et al. 2017), and NK cells (Liu et al. 2012) have been reported in the limbus during steady state, and are involved in maintaining corneal immune privilege (Skelsey et al. 2001) and wound healing (Li et al. 2007, Li et al. 2011, Liu et al. 2012, Liu et al. 2017). Although the various functions of resident immune cells have been assessed in various pathological conditions and diseases, their contribution to the maintenance of homeostasis has not been demonstrated. For instance, it is not clear if/how resident immune cells may contribute to angiogenic privilege of the cornea, or how they may interact with corneal nerves to mediate corneal nerve health and function during steady state. Further, although role of various immune cells during viral keratitis has been studied, it is not yet known how much they contribute to production of type I IFNs, an important cytokine involved in antiviral immunity.
2.2. Conjunctiva
In contrast to the avascular cornea, the conjunctiva contains a variety of resident immune cells during steady state. In fact, the conjunctiva is unique among the ocular tissues as it considered as part of mucosal immune system, which is designated as conjunctiva-associated lymphoid tissue. Immune cells in the conjunctiva are either scattered in the tissue or clustered in structured aggregates resembling follicles (Fix et al. 1989, Hingorani et al. 1997). The majority of the resident immune cells in the conjunctiva reside in the substantia propria, however, to a lesser extent, they are also found in the epithelium (Fix et al. 1989, Gomes et al. 1997, Hingorani et al. 1997).
Among innate immune cells, mast cells, NK cells, cDCs, LCs, macrophages, and rarely γδ T cells and ILCs are reported in the normal conjunctiva (Allansmith et al. 1978, Sacks et al. 1986, Sacks et al. 1986, Soukiasian et al. 1992, Baddeley et al. 1995, Gomes et al. 1997, Hingorani et al. 1997, Knop et al. 2000, Ohbayashi et al. 2007, Yoon et al. 2018). While during steady state, cDCs appear to be the most common professional APCs in the conjunctiva, LCs have also been reported in the conjunctival epithelium and sub-epithelial layers (Rodrigues et al. 1981, Ohbayashi et al. 2007), with regional differences in distribution of both (Sacks et al. 1986). Macrophages tend to majorly populate in the substantia propria and can be rarely found among the epithelial cells, however, they are not detected among ductal cells (Gomes et al. 1997). Macrophages are increased in the course of experimental allergic conjunctivitis and contribute to antigen uptake and presentation (Fukushima et al. 2010, Ishida et al. 2010). Noteworthy, the density of APCs increases in the conjunctiva during aging (Bian et al. 2019). γδ T cells constitute a minor subpopulation of T cells in the conjunctival epithelium and substantia propria during the steady state (Soukiasian et al. 1992) and are shown to play a pivotal role in promoting clinical severity and eosinophilic infiltration in the conjunctiva in murine model of allergic conjunctivitis (Reyes et al. 2011).
In addition to innate immune cells, adaptive immune cells are present in the conjunctiva. Plasma cells are detected in the conjunctiva, in particular in the substantia propria or superficial layers of conjunctival epithelium during steady state (Allansmith et al. 1978, Bhan et al. 1982, Franklin et al. 1984, Vantrappen et al. 1985, Sacks et al. 1986, Hingorani et al. 1997, Knop et al. 2000, Siebelmann et al. 2013). Similar to resident innate immune cells, B and T cells are more frequently found in the conjunctival substantia propria rather than epithelium (Gomes et al. 1997). T cell population outnumber B cells in the conjunctiva (Sacks et al. 1986, Gomes et al. 1997, Hingorani et al. 1997). It has also been reported that the conjunctiva is endowed with a significant regulatory T cell population (Nesburn et al. 2007), likely contributing to the immune privileged status of the ocular surface.
It has been suggested that development of immune cells in the conjunctiva is age-dependent; resident immune cells peak in adolescence, they tend to decline through adulthood (Siebelmann et al. 2013). Nevertheless, similar to the cornea, the turnover of the immune cells in the conjunctiva needs further investigation.
2.3. Choroid, Iris, and Ciliary Body
Populations of APCs have also been described throughout the entirety of the uveal tract. In the iris and ciliary body, the described resident immune cell populations are predominantly macrophages, based on their expression of F4/80. Subpopulations could also be delineated by the expression of either Mac-1 or MHC-II (Williamson et al. 1989). Further, less frequently, resident cDCs have also been identified within these tissues. Immunohistochemistry studies has revealed distinct localization of resident macrophages and cDCs; while cDCs are present within the epithelial layers and the stroma, macrophages reside in the substantia propria (McMenamin et al. 1992).
Interestingly, the turnover rate of iris-resident macrophages and cDCs differ substantially. Bone marrow transplantation into irradiated mice indicated that cDCs have a turnover rate with a half-life of 3 days, whereas macrophages had a turnover rate with a half-life of 10–12 days (Steptoe et al. 1996). Further, presence of CD34+ progenitor immune cells in the iris has been shown, which may suggest potential in situ renewal of at least some subtype of immune cells in this tissue during steady or following inflammatory stimuli (Vrapciu et al. 2014). Iris-resident cDCs have been shown to induce T cell proliferation in vitro, whereas macrophages failed to do so (Steptoe et al. 1995). Later studies revealed that while resident iris macrophages could not stimulate proliferation in unprimed T cells, they were capable of promoting the proliferation of primed, antigen-specific T cells (Steptoe et al. 2009). This distinction between resident cDCs and macrophages can be explained in that upon antigen uptake, resident cDCs migrate to the dLNs to induce T cell responses, whereas the resident macrophages may potentiate those responses within the tissue.
In the choroid, resident cDCs appear to have an immature phenotype, as indicated by little to no expression of the co-stimulatory molecules CD80 and CD86. The lack of these molecules suggests that these cDCs are not capable of antigen presentation, but rather, points toward a role in antigen capture (McMenamin 1999). A close association between these resident immune cells and retinal pigment epithelial cells has also been described (Forrester et al. 1994). Choroidal cDCs are poor antigen presenters unless activated in vitro. Interestingly, choroidal macrophages are poor antigen presenters, even after activation, but improve the antigen presentation by cDCs when co-cultured (Forrester et al. 2005). More recently, choroidal resident myeloid cells have been investigated ex vivo by time-lapse confocal microscopy. These experiments utilized young and aged CX3CR1eGFP/+ mice to address if immune-vascular associations are altered during aging. These studies, highlighting close interaction of resident immune cells and choroidal vasculature and show that the density of myeloid cells increases with age (Kumar et al. 2014).
In the uveal track, resident immune cells, including cDCs and macrophages play an important role in mediating immune responses, in part through acting as local APCs in conditions such as autoimmune uveitis (Butler et al. 1996, McMenamin et al. 1997, Jiang et al. 1999), and macular degeneration (Luhmann et al. 2009, Cherepanoff et al. 2010, Bretz et al. 2018). Although our understanding of the role of resident immune cells in the uveal track in disease states has significantly increased, their role in maintaining immune and vasculature homeostasis during steady state remains mainly elusive.
2.4. Retina
The retina, being an extension of the central nervous system, is host to microglia. During steady state, microglia are typically detected in three layers of the retina: (1) nerve fiber layer/ganglion cell layer, (2) inner plexiform layer, and (3) outer plexiform layer (Hume et al. 1983, Diaz-Araya et al. 1995, Provis et al. 1995, McMenamin et al. 2019). However, some reports suggest that in adult retinas, they are absent from the nerve fiber layer/ganglion cell layer. Microglia are widely implicated in mediating immune responses, neurodevelopment, neuronal survival, and synaptic pruning throughout the central nervous system (Silverman et al. 2018). Microglia are tissue-resident long-lived cells; however, during injury it has been shown that bone marrow-derived cells may infiltrate into the retina and differentiate into microglia-like cells (Xu et al. 2007, Kaneko et al. 2008).
In addition to microglia, the retina hosts small fractions of other resident immune cells, such as macrophages and cDCs. Retinal perivascular macrophages are located in close association to epiretinal vessels, extending their processes around the vessels or bridging adjacent vessels (Cuff et al. 1996). These cells constitute a distinct population from microglia, since unlike microglia, they lack expression of Iba-1, although they share expression of F4/80 and CD11b with microglia (Mendes-Jorge et al. 2009). In fact, in a series of experiments, O’Koren et al. demonstrated that despite similarities, it is possible to distinguish microglia from macrophages within the retina via extensive phenotyping by flow cytometry (O’Koren et al. 2016). Interestingly, they demonstrated that microglia, at least within the retina, maintain a stable phenotype even during neuroinflammation in the light-induced retinal degeneration model (O’Koren et al. 2016). More recently, it has been demonstrated microglia residing in inner plexiform and outer plexiform layers harbor distinct properties, since only survival of the microglia in the inner plexiform layer is dependent on ganglion cells’ secretion of IL-34 (O’Koren et al. 2019). Further, solely microglia in the inner plexiform layer contribute to feedback regulation of cone-bipolar cell axons and thus, visual information (O’Koren et al. 2019).
During retinal injury, both retinal microglia and infiltrating macrophages contribute to removal of debris. Additionally, infiltrating macrophages re-enter the circulation, a possible indication of their antigen-presenting potential (Joly et al. 2009). Further, microglia have been shown to interact with retinal pigmented epithelium (RPE) cells. When injected into the subretinal space of naïve mice, microglia caused alterations in RPE cells including increased expression of pro-inflammatory and pro-angiogenic molecules, with a concurrent increase in the extent of choroidal neovascularization (Ma et al. 2009). It has since been demonstrated that modulating interferon-β signaling can provide benefit in choroidal neovascularization, as mice benefit from systemic interferon-β therapy in a laser burn model (Luckoff et al. 2016).
In addition to microglia and peri-vascular macrophages, resident cDCs have also been reported in the retina (Gregerson et al. 2003, Xu et al. 2007). In a study, using CD11cDTR-GFP mice, it has been shown that GFP+ cDCs were observed in the retina, expressing CD11b and intermediate levels of CD45, further confirming the presence of resident cDCs in the retina (Lehmann et al. 2010). Resident retinal cDCs selectively up-regulate MHC-II expression following retinal injury, suggesting their role in antigen presentation and identification in conditions such as autoimmune uveoretinitis (Xu et al. 2007, Lehmann et al. 2010). Nevertheless, considering the recent discovery of resident cDCs, their functions during steady state and disease needs to be further elucidated.
2.5. Lacrimal Gland
The lacrimal gland forms part of the lacrimal functional unit, key for maintenance and homeostasis of the tear film at the ocular surface. As such, resident immune cell populations within the lacrimal gland will briefly be discussed. A variety of immune cells are present in the lacrimal gland, including macrophages, cDCs, and unique subpopulations of lymphocytes (Pappo et al. 1988, Wieczorek et al. 1988, Gomes et al. 1997, Saitoh-Inagawa et al. 2000). In the lacrimal gland, APCs are the predominant immune cell type followed by lymphocytes.
These immune cell populations appear to be involved in the homeostatic function of the lacrimal gland. For instance, following exposure to environmental antigens or ocular immunization with toxins, such as cholera toxin, the density of antibody-secreting cells in the lacrimal gland increases, leading to an increase in tear antibody levels against the antigens (Allansmith et al. 1987, Saitoh-Inagawa et al. 2000). Also, if the parasympathetic innervation to the lacrimal gland is severed, mRNA levels of mediators such as NFκB, MHC-II, macrophage metalloelastase, and CD53 are increased at early and late time points (Nguyen et al. 2006). Similarly, inflammatory cytokines, such as CCL2, CCL4, IL-6, MHC-II, are increased the lacrimal glands of rabbits which were housed in warmer temperatures and/or lower humidity (Mircheff et al. 2011). Additionally, ex vivo studies have demonstrated that inflammatory cytokines such as IL-1β and IL-6 directly impact secretory function and decrease chloride flux in response to treatment with carbachol, a muscarinic agonist (Selvam et al. 2013). Thus, within the lacrimal gland there are complex interactions between the parasympathetic nerves, resident immune cells, and even environmental cues, likely relayed by corneal afferents (Stern et al. 2004). Perturbation of any of these components can result in lacrimal gland dysfunction. Additional work is warranted in this area to unravel the complexities of such interactions and to uncover contributions of various immune cells.
2.6. Perspective
Previous studies have elucidated the presence of a variety of immune cells in ocular tissues during steady state and their role in different pathological settings. This has led to the potential for therapeutic targeting of immune pathways in ocular diseases. However, our understanding of the homeostatic function of immune cells in ocular tissues is lacking. An additional area that requires further investigation are whether these immune cell populations are replenished by in situ proliferation, local progenitors, or bone marrow-derived immune cells recruited from blood stream. While it appears as though there is a distinction in functions of cDCs and macrophages in these settings, studies have indicated close associations between resident immune cells and nerves as well as resident immune cells and vasculature. Understanding the neuro-immune and immune-vascular crosstalks in these tissues remain to be elucidated.
3. Distribution of Plasmacytoid Dendritic Cells in Ocular Tissues
Considering that innate immune cells are found in ocular tissues during steady state, more recent studies have assessed if murine or human ocular tissues also host resident pDCs. Notably, considering the difficulty of accessing healthy human ocular tissues, most of our knowledge on tissue-resident pDCs is derived from murine studies. Under this section, we briefly review our current understanding on presence of resident pDCs in ocular tissues and the alterations in their density or phenotype in various ocular diseases. Fig. 3B summarizes our current knowledge on distribution of resident pDCs in the ocular tissues.
3.1. Cornea
Investigations into the potential existence of pDCs in ocular tissues date back to 2005, when Sosnova et al. first showed that a subpopulation of double-positive CD45R/B220+ Gr-1+ cells among CD45+ bone marrow-derived cells in the cornea (Sosnova et al. 2005). Although this study first brought up the potential presence of pDCs in normal murine corneas, considering that the expression of CD45R/B220 and Gr-1 is not restricted to pDCs, these data remained inconclusive. Extensive studies on the identification of pDCs in ocular tissues awaited until 2010, when it was demonstrated that during steady state, pDCs, initially specified as CD45+ PDCA-1+ CD45R/B220+ cells, reside in the anterior stroma in both the central and peripheral murine cornea, with their particular localization immediately below to the corneal basal epithelium (Zheng et al. 2010). These studies further showed that corneal pDCs express TLR-7 and TLR-9 (Zheng et al. 2010), consistent with prior studies in other tissues (Kadowaki et al. 2001, Krug et al. 2001, Hornung et al. 2002, Edwards et al. 2003). In addition to CD45R/B220, co-expression of CD11c was shown on CD45+ PDCA-1+ cells. Interestingly, as illustrated in Fig. 4A, both populations of CD45+ PDCA-1neg CD11chigh cDCs (Fig. 4A, arrow heads) and CD45+ PDCA-1+ CD11clow presumable pDCs (Fig. 4A, arrows) are detectable in the murine limbus during the steady state. Nevertheless, clear characterization of pDCs via confocal microscopy seems technically impractical since it requires co-staining with multiple markers such as PDCA-1, Siglec-H, CD11c, CD45R/B220, and Ly6C. Thus, unequivocal confirmation of the presence of pDCs in the murine cornea during steady state awaited thorough flow cytometric evaluations, which demonstrated that the majority of CD45+ PDCA-1+ CD45R/B220+ cells in the cornea co-express CD11c (low), Ly6C, Gr-1, and Ly49Q, but are negative for CD11b, F4/80, Ly6G, CD3, and CD19 (manuscript under review). Interestingly, pDCs appear in the cornea during the embryonic stage of life, suggesting their early homing to ocular tissues during development (Abou-Slaybi et al. 2019). Surprisingly, despite their rarity in the peripheral blood and secondary lymphoid organs, pDCs constitute approximately 0.4% of total corneal cells and 15–25% of corneal immune cells (CD45+ cells) in corneal single cell suspensions during the steady state. In addition studies on corneal inflammation have shown that both sterile and infectious inflammatory stimuli, including thermal cautery, stromal suture placement, or HSV-1 keratitis, result in increased corneal pDC density in both the peripheral and central cornea (Zheng et al. 2010, Blanco et al. 2017) (manuscript currently under review).
Figure 4. Presence of resident plasmacytoid dendritic cells in ocular tissues.
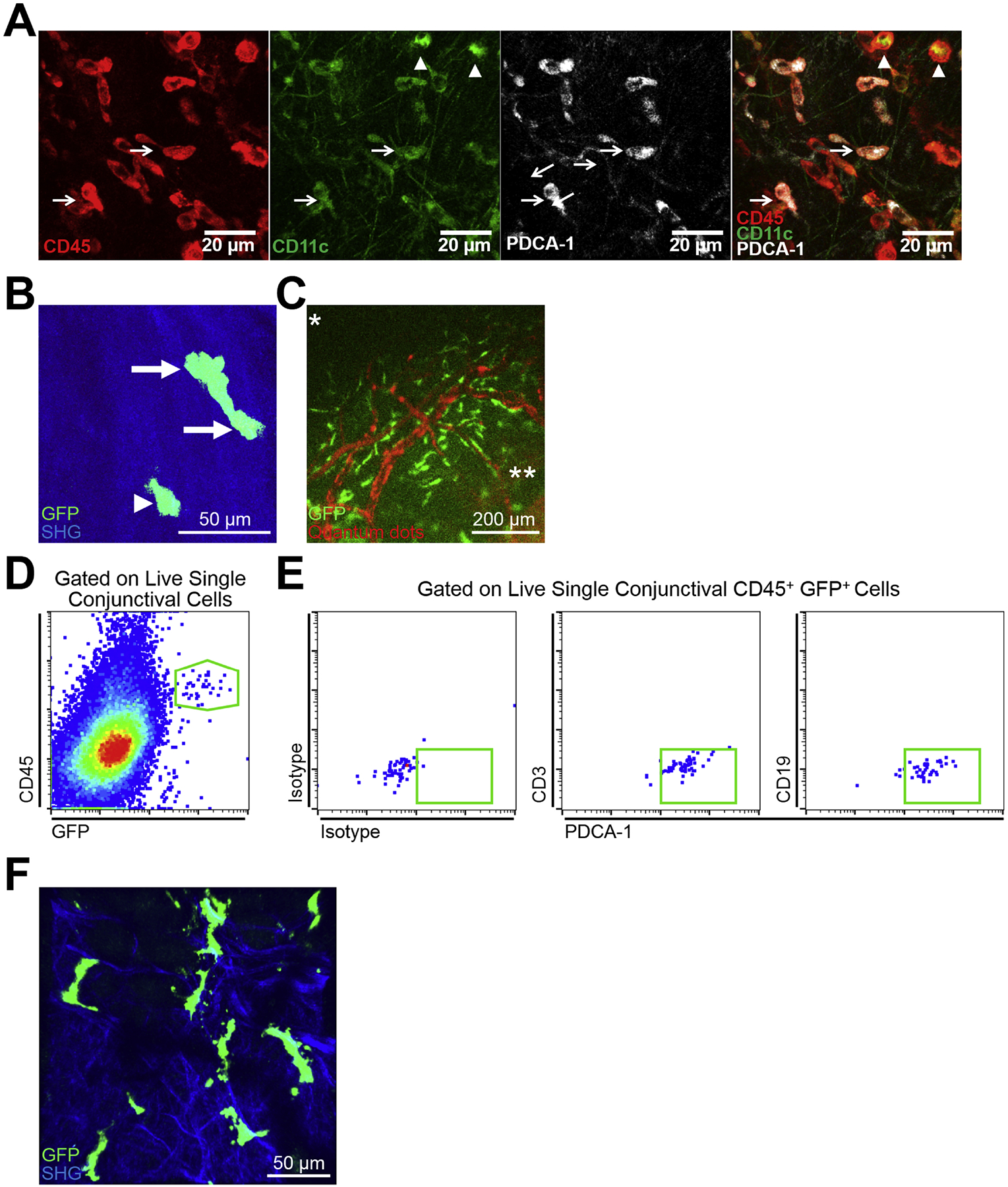
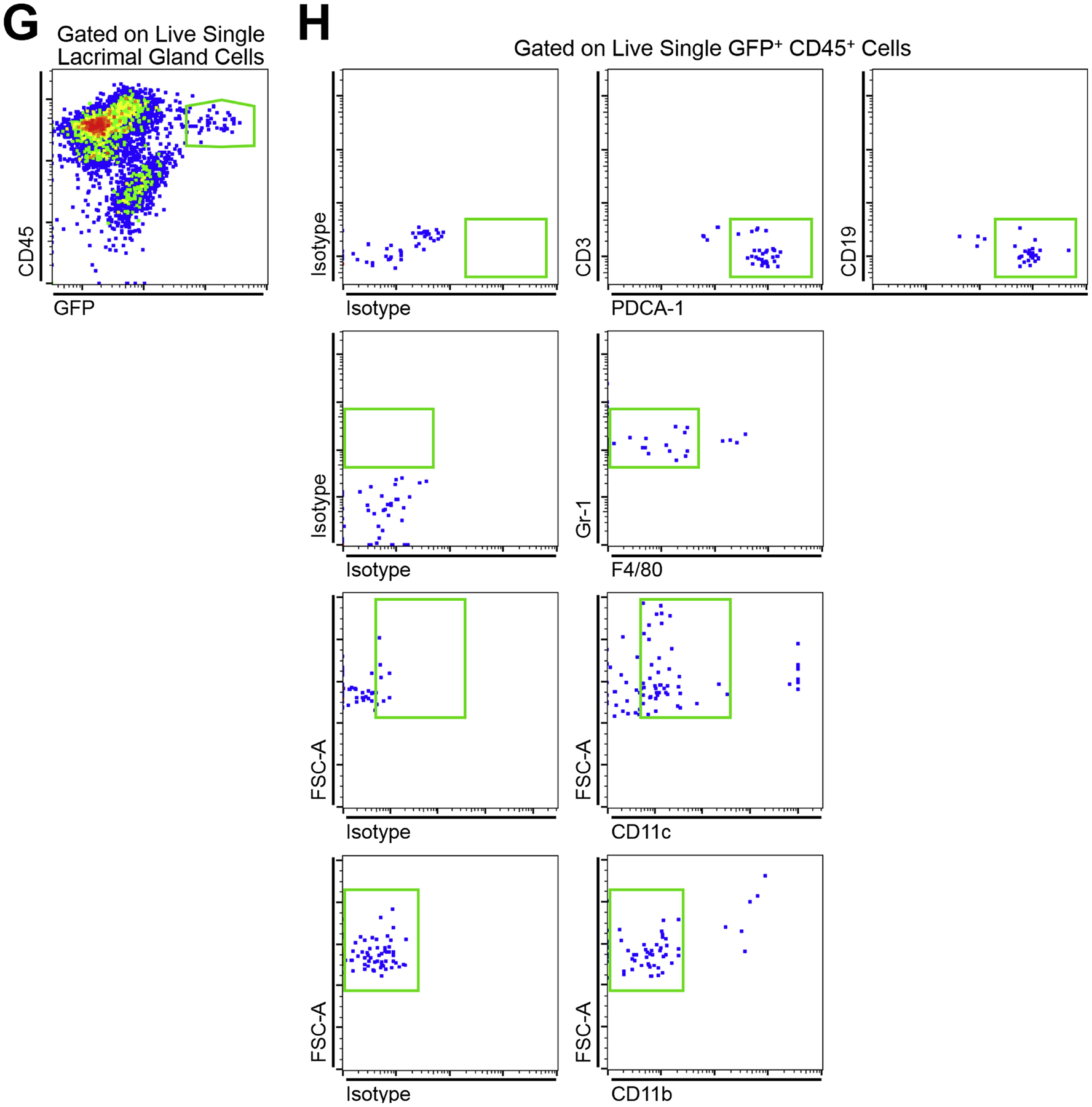
(A) Representative confocal micrographs of the limbus of a wild-type C57BL/6 mouse indicting CD45+ PDCA-1neg CD11chigh cDCs (arrow heads) as well as CD45+ PDCA-1+ CD11clow pDCs (arrows) during steady state. Scale bar: 20 μm. (B) Representative reconstructed multiphoton micrograph of cornea of a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged plasmacytoid dendritic cells (green), highlighting typical morphology of resident corneal plasmacytoid dendritic cells with knob-like extensions (arrows) as well as less common morphology of corneal plasmacytoid dendritic cells with a round cell body without long stellates (arrow head). Second harmonic generation (SHG; blue) delineates corneal stroma. Scale bar: 50 μm. (C) Representative fluorescent microscopy image of the limbus in a transgenic DPE-GFP×RAG1−/− mouse receiving intravenous injection of quantum dots (red), reveals strategic localization of plasmacytoid dendritic cells (green) in close proximity to vessels in the limbus. Scale bar: 200 μm. (D) Representative flow cytometric dot plot on pooled conjunctiva of DPE-GFP×RAG1−/− mice during steady state indicating presence of CD45+ GFP+ cells among conjunctival single cells following gating out debris, dead cells, and debris (not shown). (E) Representative flow cytometric dot plots gated on live single CD45+ GFP+ cells of pooled conjunctiva of DPE-GFP×RAG1−/− mice during steady state depicting the identity of the CD45+ GFP+ cells as mainly plasmacytoid dendritic cells based on expression of PDCA-1 and lack of expression of CD3 and CD19. (F) Representative reconstructed multiphoton micrograph of the lacrimal gland of a transgenic DPE-GFP×RAG1−/− mouse, illustrating presence of resident lacrimal gland plasmacytoid dendritic cells. Second harmonic generation (SHG; blue) delineates lacrimal gland stroma. Scale bar: 50 μm. (G) Representative flow cytometric dot plot on lacrimal gland of a DPE-GFP×RAG1−/− mouse during steady state validating the presence of CD45+ GFP+ cells among lacrimal gland single cells following gating out debris, dead cells, and debris (not shown). (H) Representative flow cytometric dot plots gated on live single CD45+ GFP+ cells of lacrimal gland of a DPE-GFP×RAG1−/− mouse during steady state. Flow plots demonstrate that the majority of the CD45+ GFP+ cells are plasmacytoid dendritic cells based on expression of PDCA-1, moderate to low levels of CD11c, Gr-1 as well as lack of expression of CD3, CD19, CD11b, and F4/80; nevertheless, a minor population lack expression of PDCA-1 or express F4/80, CD11b, and/or high levels of CD11c.
The advent of intravital multiphoton microscopy has enabled in vivo imaging of immune cells of interest with high resolution over time in living animals, in particular in the cornea (Sumen et al. 2004, Seyed-Razavi et al. 2019). In this regard, DPE-GFP×RAG1−/− transgenic mice provide a potent source for studying pDCs in vivo, since in these mice, which lack RAG1, GFP is expressed under the control of CD4, leaving pDCs as the solely GFP-tagged cells in the tested organs (Iparraguirre et al. 2008). Taking advantage of this technology and availability of the transgenic mice, precise localization, morphology, and kinetics of pDCs in the cornea have recently been studied (Blanco et al. 2017). The findings of the study confirmed the presence of corneal pDCs during steady state without the need for application of immunofluorescence staining, confirming their higher density in the peripheral cornea. pDCs generally appear with a central cell body and few stub-like extensions (Fig. 4B, arrows), however, they do not possess thin dendrites as observed in cDCs. In addition, a minor population of pDCs in the cornea harbored a round cell body without cytoplasmic extensions (Fig. 4B, arrow head). In contrast, cDCs tend to have a round cell body with multiple fine dendrites, while macrophages generally exhibit shorter stellates compared to pDCs. Further, the study showed that morphology and migratory properties of pDCs are altered during inflammation, regardless of the etiology. In fact, while pDCs are sessile during steady state, during inflammation, their migratory behavior is significantly altered, as shown by their higher mean speed and longer displacement in the cornea (Blanco et al. 2017) (manuscript under review). These findings suggest that corneal pDCs sense and respond to inflammatory stimuli.
Of note, in the limbus, the distribution of pDCs is uniquely organized. In DPE-GFP×RAG1−/− mice, GFP+ pDCs engulf the limbal vessels (Fig. 4C and Supplementary Video 1), and in rare occasion are found in the lumen of the vessels, patrolling the intravascular space (Jamali et al. 2020). In the limbus, the majority (approximately 85%) of the resident pDCs accompany limbal vessels, with higher frequency around larger vessels (Jamali et al. 2020). Considering the critical localization of pDCs, it might be postulated that they may promptly participate in dampening immune responses, by traveling to dLNs in order to prevent unnecessary immune responses. Further, as reviewed in the relevant sections below, they may contribute to vascular integrity and corneal angiogenic privilege.
Following identification of pDCs in murine corneas during steady state, the presence of resident pDCs in human corneas has also been confirmed (manuscript under review). Performing flow cytometry on single cell suspensions of eyebank corneas from healthy individuals, it was shown that similar to mice, approximately 1–2% of corneal single cell population express CD45, among which about 15–20% co-express BDCA-2 and BDCA-4, suggesting that the normal human cornea is also endowed with resident pDCs (manuscript under review). In a recent study on human cadaveric corneas and limbal explant cultures, Luznik et al. suggested the presence of pDCs as judged by expression of BDCA-2, CD123, and lack of expression of CD11c on a fraction of CD45+ immune cells (Luznik et al. 2019).. Nevertheless, further evidence is needed to support the findings of this study due to the technical shortcoming of the study, such as lack of presentation of a viability marker, fluorescence minus one controls, as well as discrepancies in presented gating strategies and the density of pDCs in the peripheral cornea.
3.2. Conjunctiva
In the conjunctiva, in an initial study in 2007, investigators reported that PDCA-1+ CD11c+ cells, as presumable pDCs, are very rarely detected in the conjunctiva during steady state. However, following allergen stimulation (without subsequent challenge), pDCs are well noticed in the subepithelial layer of the conjunctiva throughout the substantia propria of bulbar, forniceal, and tarsal conjunctiva (Ohbayashi et al. 2007). Further, as early as 24 hours (h) following allergen challenge, pDCs are significantly increased compared with stimulated but not challenged mice in the conjunctiva, in particular in the forniceal conjunctiva (Ohbayashi et al. 2007). Interestingly, pDCs reach higher densities compared cDCs (Ohbayashi et al. 2007). Similarly, Stern et al. demonstrated that while during steady state PDCA-1+ CD11clow pDCs constitute a minor fraction of immune cells on the ocular surface (combining corneal and conjunctival tissue), their density remarkably increases as early as 1 day following induction of DED by subcutaneous administration of scopolamine and environmental desiccating stress (Stern et al. 2012). Despite these initial findings that demonstrated resident PDCA-1+ CD11c+/low cells in the conjunctiva during steady state and their increase following allergic or desiccating stimuli, conclusive evidence could not be drawn regarding presence of resident pDCs in the conjunctiva as only two pDC markers that can also be found on other cells, were used in these studies.
Recently, following the observation of CD45+ PDCA-1+ CD11clow cells in the bulbar conjunctiva and limbus of naïve mice by confocal microscopy, the presence of PDCA-1+ Ly6C+ CD11bneg F4/80neg pDCs among CD45+ cells in the bulbar conjunctiva was confirmed during steady state by flow cytometry. In fact, these cells constitute approximately 15% of total immune cells in this tissue. Further, immunophenotyping of these cells using fluorescence minus one controls revealed that as expected for pDCs, CD45+ PDCA-1+ Ly6C+ CD11bneg F4/80neg cells are also CD11c+ and Ly49Q+, but lack expression of CD3, CD19, and Ly6G (Jamali et al. 2020). As depicted in Fig. 4C, the presence of resident pDCs in the conjunctiva has also been observed in DPE-GFP×RAG1−/− mice. Importantly, the GFP+ cells in the conjunctiva of these mice express CD45 (Fig. 4D) as well as PDCA-1 (Fig. 4E), but lack CD3 and CD19 (Fig. 4E), confirming their identity as pDCs. Notably, although the majority of GFP+ cells in the conjunctiva of these transgenic mice aligned with pDC identity, considering that CD4 might be expressed by other APCs, such as minor subtypes of macrophages or cDCs (Vremec et al. 2000, Bialecki et al. 2011, Abtin et al. 2014, Bain et al. 2018), together with the presence of a minor subpopulation of GFP+ cells that lacked expression of PDCA-1, it may be postulated that a minor population of GFP+ cells in the conjunctiva of these transgenic mice may represent subtypes of cDCs or macrophages. In this regard, although expression of other immune cell markers such as myeloid cell marker, CD11b, as well as monocyte/macrophage markers, F4/80 and CD68, was not assessed on conjunctival GFP+ cells to further support the identity of these cells as pDCs in the conjunctiva of DPE-GFP×RAG1−/− mice, in the cornea they mainly appeared negative for CD68 (manuscript under review). Further, during steady state, conjunctival pDCs express moderate levels of MHC-II, higher levels of co-inhibitory molecules PD-L1 and B7-H3, and minor to negligible levels of ICAM-1, CD40, and CD86, suggesting their potential tolerogenic functions (Jamali et al. 2020). Similar to these findings on the phenotype of resident conjunctival pDCs, murine resident lung pDCs express negligible levels of the co-stimulatory molecule CD40, and low levels of CD80 and CD86, yet considerable levels of PD-L1 (de Heer et al. 2004). Further, in human lung specimens, pDCs also express negligible levels of co-stimulatory molecules CD40 and CD80, and low levels of CD86 and ICAM-1 (Demedts et al. 2005). Similarly, pDCs detected in the kidney during steady state do not express CD40, and only express minor levels of CD80 and CD86 (Coates et al. 2004).
In humans, the presence of pDCs in the normal conjunctiva has not yet been explored. However, pDCs have been detected in peri- and intra-granuloma infiltrates in conjunctival biopsies of children with primary chronic blepharitis leading to granulomatous conjunctivitis (BAïZ et al. 2012). In summary, current evidence indicates that pDCs reside in the conjunctiva during steady state, at least in mice. However, considering our limited knowledge on their life cycle, it is interesting to study the longevity of these tissue-resident pDCs and to assess how these cells keep their niche in the conjunctiva during steady state.
3.3. Choroid and Retina
Currently, there are few studies on the presence of tissue resident pDCs in murine or human choroid and retina. In a recent preliminary report, Baban et al. assessed the presence of CD11c+ CD45R/B220+ cells in human eyebank retinas by immunohistochemistry (Baban et al. 2015). They observed that presumable pDCs reside in the normal human retina and that pDC density is decreased in the retinas from diabetic patients (Baban et al. 2015). More recently, murine retinas and choroid have been shown to host pDCs during steady state (Gupta et al. 2017). Using flow cytometry of collagen-digested tissues, CD45+ PDCA-1+ CD45R/B220+ pDCs were shown to constitute less than 5–10% of CD45+ cells in the retina and choroid during steady state. The pDC identity of these cells has been further confirmed as they expressed CD11c and Gr-1, and are negative for CD3 and CD19 (Gupta et al. 2017). Using transgenic DPE-GFP×RAG1−/− mice with GFP-tagged pDCs, the presence of GFP+ cells in the choroid/retinal tissues has been demonstrated during steady state, in close proximity to the vasculature. Further, GFP+ cells in the aforementioned tissues expressed TLR-7 and TLR-9, the main intracellular receptors of pDCs (Gupta et al. 2017) (manuscript under preparation). Similar to the other ocular tissues, our knowledge is currently limited on how pDCs renew in the choroid and retina. Therefore, further studies are necessary to unravel the life cycle of pDCs in these tissues and to demonstrate how these cells regenerate following cell death.
3.4. Lacrimal Gland
As the main source of the tear aqueous layer, the lacrimal gland plays a key role in ocular surface homeostasis. Considering the putative role of pDCs in Sjögren’s syndrome and DED, as well as prior research indicating the presence of immune cells, and more specifically APCs in the lacrimal gland, the presence of pDCs in the lacrimal gland has been assessed. Using multiple pDC markers, it has been shown that during steady state, approximately 3–4% of CD45+ cells in the murine lacrimal gland are CD11bneg F4/80neg CD3neg CD19neg PDCA-1+ Gr-1+ CD11clow, suggestive of the presence of pDCs in the lacrimal gland (manuscript under preparation). GFP+ cells were also detected in the lacrimal gland of transgenic DPE-GFP×RAG1−/− mice (Fig. 4F), with the majority expressing CD45 (Fig. 4G), PDCA-1, moderate to low levels of CD11c, and Gr-1 (Fig. 4H), but as expected, were negative for CD11b, CD3 and CD19 (Fig. 4H), confirming the majority of them align with a pDC identity. Of note, a minor population of GFP+ cells did not express PDCA-1 and were positive for CD11b and/or high expressed levels of CD11c, in the lacrimal gland, suggesting that a minority of the GFP+ cells may represent other immune cells, such as cDCs or macrophages (manuscript under preparation). The GFP+ cells in the lacrimal gland of these transgenic mice express high levels of E2–2, TLR-7, and TLR-9, further suggesting their identity as pDCs (manuscript under preparation). Taken together, these observations suggest that similar to the cornea, conjunctiva, choroid, and retina, pDCs may also reside in the lacrimal gland during steady state in mice, although in sparse numbers. Fig. 3B summarizes our current knowledge on distribution of resident pDCs in the ocular tissues.
4. Plasmacytoid Dendritic Cell Functions
4.1. General Immune Functions of Plasmacytoid Dendritic Cells
As members of innate immunity, pDCs contribute to a wide range of functions. Despite their diverse role, they exert their functions through two main approaches: (1) secretion of soluble molecules, and (2) interaction with other immune cells. Although pDCs were originally appreciated for their production of type I IFNs, they secret multiple immunomodulatory and pro-inflammatory cytokines and chemokines (chemotactic cytokines), including type I IFNs (IFN-α, IFN-β, IFN-ω, and IFN-τ), type II IFN (IFN-γ), type III IFNs (including IFN-λ1 [IL-29], IFN-λ2 [IL-28a], and IFN-λ3 [IL-28b]), TNF-α, IL-4, IL-6, IL-8, IL-10, IL-12, CCL3, CCL4, and CXCL10 (Coccia et al. 2004, Cox et al. 2005, Kamogawa-Schifter et al. 2005, Omatsu et al. 2005, Ito et al. 2006, Decalf et al. 2007, Smolen et al. 2014, Doyle et al. 2019). Through secretion of these cytokines and chemokines, pDCs communicate with other immune cells and surrounding cells in tissues, in order to direct pro-inflammatory or anti-inflammatory responses.
In addition to employing their secretary machinery, pDCs regulate immune response by directly interacting with other cells of the immune system. Freshly isolated splenic pDCs, as immature APCs, display a poor capacity in inducing naïve T cell proliferation. However, following stimulation, they up-regulate expression of T cell co-stimulatory molecules, such CD40, CD80, CD86, adhesion molecule CD54, and the maturation marker MHC-II, and can promote T cell proliferation (Grouard et al. 1997, Nakano et al. 2001), albeit, with a lower efficiency compared to cDCs (Abe et al. 2005, Tokita et al. 2008). In addition to priming effector T cells, pDCs may mediate the generation of regulatory T cells (Tregs), which can suppress allospecific responses (Gilliet et al. 2002, Moseman et al. 2004, Ito et al. 2007).
Through these mechanisms pDCs bridge innate and adaptive immunity. Thus, it is not surprising that pDCs play a key role in the development or progress of miscellaneous conditions. In the sections below, we describe how pDCs are implicated in the pathogenesis/immune response to pathogens, autoimmune diseases, as well as tumors, and organ transplantation in non-ocular and ocular tissues.
4.2. Role of Plasmacytoid Dendritic Cells in Infectious Diseases
4.2.1. Viral Infections
4.2.1.1. Non-ocular Viral Infections
In 1957, Isaacs and Lindenmann found that supernatants of virally infected cells produce proteins that interfere with viral replication, called interferons (Isaacs et al. 1957). About four decades later, investigators discovered that pDCs are the main producers of type I IFNs among immune cells upon viral exposure or following exposure to unmethylated CpG-DNA sequences typically found in viruses and bacteria (Cella et al. 1999, Siegal et al. 1999, Kadowaki et al. 2001). Over several years, multiple additional studies revealed that pDCs are involved in anti-viral immunity against multiple viruses (Swiecki et al. 2010, Swiecki et al. 2015). Following viral encounter, pDCs are redistributed from the circulation to the lymph nodes or peripheral tissues to the site of infection, where they secrete type I IFNs (Donaghy et al. 2001, Penna et al. 2001, Barron et al. 2003, Yoneyama et al. 2005, Gerlini et al. 2006, Lund et al. 2006, Smit et al. 2006, GeurtsvanKessel et al. 2008, Brown et al. 2009, Donaghy et al. 2009, Gao et al. 2009, Kim et al. 2009, Lukens et al. 2009, Wolf et al. 2009, Huch et al. 2010, Lehmann et al. 2010, Davidson et al. 2011, Dunmire et al. 2015).
Although pDCs produce type I IFNs during viral infections, particularly in the early time points after viral exposure (Krug et al. 2004, Smit et al. 2006, Swiecki et al. 2010, Swiecki et al. 2013), their contribution in eliciting immune responses and promoting viral clearance is not always imperative and dependent on the secretion of type I IFNs and pro-inflammatory cytokines and chemokines (Krug et al. 2002, Penna et al. 2002, Jego et al. 2003, Swiecki et al. 2013). In fact, the contribution of pDCs to viral immune responses goes beyond secretion of type I IFNs, as they regulate different subpopulations of immune cells, including T cells, B cells, cDCs, and NK cells (Penna et al. 2002, Jego et al. 2003, Krug et al. 2004, Yoneyama et al. 2005, Tsuchida et al. 2012, Swiecki et al. 2013, Lynch et al. 2018). For instance, pDC depletion in local vaginal HSV-2 infection does not affect IFN-α levels, viral load, or mortality, but absence of pDCs in systemic HSV-1 and HSV-2 infections leads to decreased IFN-α levels, as well as decreased NK cell activation and reduced production of IFN-γ by virus-specific CD8+ T cells, without affecting their proliferative capacity or accumulation in the site of inflammation (Swiecki et al. 2013). In subcutaneous HSV-1 infection, pDCs poorly induce virus-specific T cell responses; however, in their absence, cDCs lose their capacity to prime CD4+ or CD8+ T cells (Yoneyama et al. 2005). In summary, during systemic HSV-1 and HSV-2 infections, pDCs act in concert with other immune cells including cDCs, NK cells, and T cells to direct the immune response. However, they are not indispensable in local infections.
In influenza virus infections, depletion of pDCs does not affect viral clearance and generation of virus-specific CD8+ cytotoxic or memory T cells (GeurtsvanKessel et al. 2008, Wolf et al. 2009), and has controversial effects on production of anti-viral neutralizing antibodies (GeurtsvanKessel et al. 2008, Wolf et al. 2009). Further, although pDC depletion may not affect viral clearance (GeurtsvanKessel et al. 2008, Wolf et al. 2009), in the absence of pDCs, infiltration of T cells to the lungs is delayed (Wolf et al. 2009), which can be explained by expression of both macrophage inflammatory protein (MIP)-1α and MIP-1β chemoattractant proteins necessary for recruitment of effector Th1 and CD8+ T cells (Bonecchi et al. 1998, Loetscher et al. 1998, Penna et al. 2002, Castellino et al. 2006). pDCs may even destroy viral-specific CD8+ T cells through FasL-Fas signaling, when encountering lethal, but not sublethal doses of influenza virus, contributing to the mortality due to lethal infections in mice (Langlois et al. 2010).
Furthermore, it has been shown that in respiratoty synsytial virus (RSV) infections, pDC depletion leads to increased airway hyperreactivity, accumulation of inflammatory cells, and prevention of viral clearance (Smit et al. 2006). Noteworthy, the increased immunopathologic severity of the RSV infection following pDC depletion, is not only due to decreased production of IFN-α and delayed viral clearance, but also due to lack of modulation of T cell responses in the absence of pDCs, since pDC depletion enhances production of both Th1 and Th2 cytokines in the lungs and dLNs (Smit et al. 2006). In line with these findings, it has been demonstrated that pDC depletion augments the severity of airway inflammation induced by the pneumonia virus of mice (PVM) in neonatal mice and is accompanied by decreased neuropilin-1+ Tregs, as pDCs express semaphorin 4A and promote the generation of neuropilin-1+ Tregs (Lynch et al. 2018). In contrast, adoptive transfer of pDCs decreases airway inflammation through inhibition of Th1 and Th2 responses, favoring Treg generation (Tsuchida et al. 2012).
In murine cytomegalovirus (CMV) infection, depletion of pDCs leads to reduced levels of type I IFN and increased levels of IL-12p70, with reduced NK cytotoxic activity, and higher viral loads, particularly in early time points; however, at later time points, pDCs seem to limit secretion of IFN-γ by NK cells (Krug et al. 2004, Swiecki et al. 2010). Further, although following pDC depletion expression of MHC-II by cDCs is considerably confined, the ability of cDCs to prime CD8+ T cells is not affected (Swiecki et al. 2010). Similarly, in vesicular stomatitis virus (VSV), pDC depletion abolishes early IFN-α increase. However, dissimilar to CMV, pDCs promote accumulation and survival of antigen-specific T cells in VSV (Swiecki et al. 2010). In line with these findings, it has been shown that pDCs can induce cDC maturation and promote CD8+ T cell clonal expansion by cDCs (Yoneyama et al. 2005).
In summary, in viral infections in various non-ocular tissues, pDCs play a vital role in viral encounters through production of type I IFNs and through modulating innate and adaptive immune responses, based on the offending agent and its route of entry. Considering the key role of pDCs in viral infections, below we review our understanding on their contribution to HSV-1 keratitis.
4.2.1.1. Ocular Viral Infections
As described above, classically, pDCs were acknowledged for their pivotal role in viral challenges (Cella et al. 1999, Siegal et al. 1999, Coccia et al. 2004, Krug et al. 2004, Lund et al. 2006, Smit et al. 2006, Swiecki et al. 2010). Thus, viral infections were among the first conditions in which the role of pDCs were investigated in ocular diseases. In this regard, in an early report, Kittan et al. assessed the distribution and function of pDCs in acute retinal necrosis caused by HSV or VZV in humans. They observed that in individuals with acute retinal necrosis, pDCs seem to display a lower frequency in the blood stream compared to controls. However, they observed low numbers of pDCs in the vitreous of one (out of two) of the examined patients, suggesting potential redistribution of pDCs to the site of inflammation. Further, although pDCs isolated from the peripheral blood of the individuals with acute retinal necrosis expressed higher levels of co-stimulatory molecules, their capacity to produce IFN-α was limited compared to controls (Kittan et al. 2007).
Considering constant exposure of the ocular surface to the environment and thus pathogens, shortly following the identification of resident corneal pDCs, their significance was investigated in viral infections of the cornea. In this regard, due to the importance of HSV-1 keratitis as the leading cause of infectious blindness in developed countries (Liesegang 2001), studies on the role of pDCs in corneal infections mostly focused on HSV-1 keratitis (Hu et al. 2013, Sendra et al. 2015, Sendra et al. 2016).
Dissimilar to other tissues, primary infection of HSV-1 in humans rarely accompanies clinical symptoms and signs in the cornea (Darougar et al. 1985, Liesegang et al. 1989); Typically, during primary ocular or mucosal infection, HSV-1 invades the sensory dendrites and is transferred to neuronal cell bodies in trigeminal ganglion (TG), where it remains in a dormant state. However, following the resolution of the primary infection, the dormant virus in TG can be re-activated by various stressors, traveling back to the cornea via sensory dendrites in a retrograde fashion, leading to recurrent epithelial keratitis manifested as corneal inflammation, neovascularization, scarring, perforation and in severe cases blindness (Liesegang 1999, Giménez et al. 2013, Rowe et al. 2013). Although the HSV-1 virus entry to TG is thought to follow a non-corneal HSV-1 infection, it may also arise from “front door” transmission through the cornea (Kaye et al. 1992, Kovacs et al. 2009, Shah et al. 2010).
To directly assess the contribution of pDCs in immune responses in HSV-1 keratitis, our group has examined how density of pDCs is altered during the course of acute HSV-1 keratitis. We observed that as early as 1 day following HSV-1 inoculation, pDCs are increased in both peripheral and central corneas compared with sham-inoculated controls and their increase tends to progress till day 6 post inoculation (Hu et al. 2013) (manuscript under review). This observation was further validated by flow cytometry, showing considerable increase in the density of CD45+ CD45R/B220+ PDCA-1+ pDCs, which co-expressed CD11c, Ly49Q, Ly6C, and Gr-1, but are negative for CD11b, F4/80, Ly6G, CD3, and CD19 on day 3 following HSV-1 inoculation (manuscript under review). To evaluate how depletion of pDCs alters immune response to HSV-1 keratitis, transgenic BDCA-2-DTR mice which express simian DTR under the control of pDC specific BDCA-2 promoter (Swiecki et al. 2010) have been used. It has been shown that pDC depletion prior to HSV-1 inoculation is accompanied by deterioration of clinical severity of HSV-1 keratitis, enhanced infiltration of immune cells to the cornea, increased viral load in the cornea, and viral transmission to dLNs and TG (Hu et al. 2013, Sendra et al. 2017) (manuscript under review; Fig. 5). Further, it has been shown that pDC depletion is accompanied by reduced IFN-α levels in the cornea, and blocking TLR-9 in pDC-sufficient corneas prevents HSV-1 induced IFN-α response, suggesting an important role of pDCs signaling through TLR-9 in IFN-α responses in acute HSV-1 keratitis (manuscript under review). Nevertheless, the study does not clarify whether the observed deterioration of clinical severity of HSV-1 keratitis or enhanced tissue damage in pDC-depleted corneas is linked to reduced IFN-α secretion or potentially other properties of pDCs.
Figure 5. Schematic illustration on the role of plasmacytoid dendritic cells during herpes simplex virus-1 keratitis.
During HSV-1 keratitis, local depletion of pDCs in the cornea is accompanied by increased infiltration of cellular members of innate and adaptive immunity, including cDCs, macrophages, and ex-Tregs, enhanced viral load, and reduced IFN-α level. In the draining lymph nodes, corneal pDC depletion leads to re-programming of Tregs to effector ex-Tregs, enhanced density of Th1 cells and decreased Tregs.
In contrast to the cornea, the periorbital skin, including eye lids, similar to skin covering other body sites, clinically manifests signs of primary HSV-1 infection (Darougar et al. 1985). Noteworthy, the skin is known to be devoid of resident pDCs during steady state, and thus, it might be postulated that the presence of tissue-resident pDCs in the cornea during steady state may, at least in part, explain how the cornea is preserved from signs of primary HSV-1 infection in humans. Nevertheless, this hypothesis needs to be further tested by evaluating immune response to skin tissue, with prior adoptive transfer of pDCs or alternatively, in murine corneas depleted from pDCs and inoculated with low dose of HSV-1, which is usually infective for skin.
The role of pDCs in mediating adaptive immune responses in HSV-1 keratitis has also been studied. It has been shown that following corneal HSV-1 inoculation, the density of pDCs increases remarkably in the dLNs, with a major shift towards mature (MHC-II+) pDCs. Further, although the distribution of pDCs in subcapsular, paracortical, and cortical areas of the dLN is remained unchanged following corneal HSV-1 inoculation, their motility and displacement is enhanced in the dLNs (Sendra et al. 2016). Further, it has been reported that depletion of corneal pDCs in BDCA-2-DTR mice prior to HSV-1 inoculation is accompanied by alterations in the dLN cytokine milieu, leading to decreased density of Tregs (Sendra et al. 2017) (manuscript under preparation). Recently, it has been shown that during HSV-1 keratitis, Tregs may become unstable and can be reprogrammed to effector T cells. Such ex-Tregs harbor pathogenic properties and can propagate the severity of keratitis (Bhela et al. 2017). Recent studies highlight that pDCs not only favor generation of Tregs during HSV-1 keratitis, but they also prevent reprogramming of Tregs to pathogenic effector ex-Tregs. In this regard, it is shown that, local corneal depletion of pDCs is accompanied by enhanced density of ex-Tregs in the dLN as well as increased recruitment of ex-Tregs to the cornea in vivo. Further, in vitro experiments indicated that co-culture of pDCs with Tregs prolongs expression of Foxp3 in Tregs and diminishes their reprogramming to effector T cells (manuscript under review).
Our current knowledge on the role of pDCs in mediating innate and adaptive immune responses in HSV-1 keratitis is depicted in Fig. 5. Despite these findings, our knowledge is limited on how pDCs mediate several aspects of immune responses in HSV-1 keratitis. For instance, although it has been reported that following inflammation, a higher frequency of corneal pDCs express the proliferation marker Ki-67 (Schwarzenbacher et al. 2017), currently the contribution of extravasating pDCs from the blood versus the potential in situ proliferating resident pDCs and their respective functions following HSV-1 keratitis remains to be elucidated. Further, molecular mechanisms through which pDCs may prevent viral entry and transmission to TG are not studied. In addition, future experiments may reveal if pDCs may interact with other cells/structures in the cornea, such as epithelial cells, stromal cells, and corneal nerves to mediate the immune responses following exposure to HSV-1 and to re-establish homeostasis following resolution of the keratitis. Thus, further studies are needed to assess if the findings on the important role of pDCs in controlling HSV-1 keratitis can be generalized to other viral causes of keratitis. In this regard, studying the significance of pDCs in mediating immune response and clearing the virus in viral conjunctivitis and more importantly sight-threatening viral infections such as varicella zoster virus (VZV) keratitis or CMV retinitis, warrants further investigation.
4.2.2. Bacterial Infections
In addition to their pivotal role in viral infections, pDCs contribute to immune responses during bacterial infections. Early evidence on their potential role in bacterial infections was provided by Svensson et al., who showed that upon stimulation with Staphylococcus (S.) aureus, pDCs increase and produce type I IFNs (Svensson et al. 1996). Later, it was shown that exposure to S. aureus and other bacteria, such as Neisseria meningitides and Haemophilus influenza may trigger secretion of cytokines, such as IL-6 and TNF-α by pDCs (Michea et al. 2013). Similarly, exposure to gram positive bacteria can enhance cytokine production by pDC and their capability to promote CD4+ T cell expansion and proliferation (Raieli et al. 2019).
During Listeria (L.) monocytogenes infection, pDCs tend to accumulate in the lymph nodes and spleen and up-regulate expression of the co-stimulatory molecule CD86 and maturation marker MHC-II (Tam et al. 2006). pDC depletion leads to reduced levels of pro-inflammatory serum cytokines, including IFN-γ, IL-6, and IL-12p40, reduced pathogen load, and improved survival (Takagi et al. 2011). Further, it has been shown that although bone marrow pDCs encountered with L. monocytogenes produce IFN-α and IFN-β in a MyD88-dependent fashion in vitro, their contribution to the production of type I IFNs in L. monocytogenes in vivo is minimal (Stockinger et al. 2009). In line with these findings, depleting pDCs with anti-PDCA-1 antibody during L. monocytogenes infection does not affect type I IFNs levels (Solodova et al. 2011). Thus, in bacterial infections, pDCs may not serve as the main source of type I IFNs, but may play a pivotal role in mediating both innate and adaptive immune responses through other yet unknown mechanisms.
Protective effects of pDCs are also evident in Citrobacter (C.) rodentium bacterial colitis, where pDCs are increased in the spleen and infiltrate the colon. Systemic pDC depletion in C. rodentium results in overall poor health, necessitating euthanasia (Rahman et al. 2019). Although pDC depletion does not affect the density of infiltrating immune cells, CD3+ CD4+ Th cells, and Tregs, it is accompanied by increased pro-inflammatory serum cytokines, increased vascular permeability and higher bacterial burden, suggesting the importance of pDCs in conserving the architecture of mucosal barrier (Rahman et al. 2019).
The molecular mechanisms, through which pDCs sense and respond to bacteria, are still controversial. While a study suggested that production of IFN-α following exposure to S. aureus is not mediated through TLR-2 and may require TLR-7/TLR-9 activation (Parcina et al. 2008), other evidence suggests that secretion of IFN-α can be abolished via blocking TLR-2 (Raieli et al. 2019). Additionally, the secretion of other pro-inflammatory cytokines and up-regulation of co-stimulatory molecules by pDCs may be mediated through TLR-1 (Raieli et al. 2019).
In summary, bacterial infection or exposure to bacteria in vitro alters pDCs properties and enables them to alter the inflammatory milieu and to prime naïve T cells to differentiate into other T cell populations. However, unique features of the immune responses mediated by pDCs to various bacteria and modulating different T cell and B cell responses by pDCs in both mice and human remains to be elucidated. In ocular tissues, Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus species, and Pseudomonas aeruginosa are among the main causes of serious bacterial infections of the conjunctiva and cornea. Considering the role of pDCs in mediating immune response in various bacterial infections, it is worthwhile to study if and how pDCs may control potentially blinding bacterial infections.
4.2.3. Parasitic Infections
To date, few studies have studied the role of pDCs in parasitic infections, such as toxoplasmosis and malaria infections. Toxoplasmosis, caused by the obligate intracellular protozoan Toxoplasma gondii, is the most common cause of infective retinitis in immunocompetent patients. Following systemic T. gondii infection, through signaling of TLR-11, pDCs in the lymph nodes proliferate and acquire a mature state by up-regulating MHC-II and co-stimulatory molecules, suggesting an early role for pDCs in T cell activation (Pepper et al. 2008). Further, Koblansky et al. showed that while pDCs express both TLR-11 and TLR-12, the role of TLR-12 is more prominent in the induction of immune responses during toxoplasmosis, as exposure to profilin-like protein from T. gondii enhances production of IL-12p40 in pDCs. However, this effect is abrogated in pDCs isolated from TLR-12−/−, but not TLR-11−/− mice (Koblansky et al. 2013). Further, in TLR-11−/− mice, depletion of pDCs succumb the relative resistance to toxoplasmosis, enhancing the mortality of mice upon T. gondii challenge (Koblansky et al. 2013). The effect of T. gondii on pDCs resembles exposure of these cells to IL-10, since both result in the abolishment of cytokine production, by regulating downstream effects of TLR-9 signaling, such as IRF7 and STAT3, thereby blocking IFN-α production (Pierog et al. 2018).
In summary, pDCs mediate various innate and adaptive immune responses to offending pathogens ranging from viruses to parasites. While in viral exposures pDCs mainly employ TLR-7 and TLR-9 for responding to the pathogen, their machinery for sensing other microorganisms is less known and may include other TLRs.
4.3. Role of Plasmacytoid Dendritic Cells in Autoimmunity and Sterile Inflammation
4.3.1. Sjögren’s Syndrome
Sjögren’s syndrome (SS) is a systemic autoimmune disease, which although primarily affects salivary and lacrimal glands, can also affect other organs (Zoukhri 2006). Initial evidence on the potential implication of pDCs in the pathogenesis of SS originated from genome-wide gene expression profiling of minor salivary gland in individuals with SS. The study demonstrated that in individuals with SS, IFN-inducible genes are up-regulated, suggesting that pDCs, as the main producers of type I IFNs, may participate in pathogenesis of SS (Gottenberg et al. 2006). Similarly, in monocytes of individuals with SS, a cluster of IFN-inducible genes are up-regulated, which together with higher expression of CD40 on pDCs in these individuals, suggests that pDCs may play a role in the pathogenesis of SS (Wildenberg et al. 2008). Further, the density of circulating pDCs is decreased in individuals with SS (Vogelsang et al. 2010) and while pDCs were not found in salivary glands of healthy individuals, they accumulated in the main or minor salivary glands in individuals with SS, signifying they are redistributed from circulation to affected tissues in SS (Gottenberg et al. 2006, Vogelsang et al. 2010).
Behavior of pDCs is altered in the course of SS. In fact, miRNome analysis of circulating pDCs has shown that pDCs isolated from individuals with SS exhibit distinctive expression of miRNAs involved in regulation of apoptosis, autophagy, and survival, compared with pDCs isolated from healthy controls (Hillen et al. 2019). Additionally, transcriptome of peripheral pDCs from individuals with SS patients are similar to pDCs stimulated by TLR-7, as both exhibit low ribosomal proteins expression (RPL11, RPL27 and RPS11) compared with pDCs isolated from healthy volunteers. Further, it has been shown that TLR-stimulated pDCs from individuals with SS produce remarkably higher levels of IFN-α and IFN-b compared with pDCs from healthy individuals, altogether, indicating that pDCs are activated during the course of the SS (Hillen et al. 2019). Further, pDCs are suggested to have an indirect role in B cell recruitment to minor salivary glands, and thus to contribute to the development of SS, as IFN-α secretion by pDCs promotes release of CXCL13 by macrophages, which in turn leads to the recruitment of B cells (Zhao et al. 2016). Studies also assessed alterations in the functions of pDCs during SS. In vitro studies found that pDCs can phagocytose autoantigens, which exist in apoptotic bodies of epithelial cells of human submandibular gland in individuals with SS. Exposure to these apoptotic particles led to the production of inflammatory cytokines such as IFN-α, IL-6, IL-8 and TNF-α by pDCs through TLR-7 and TLR-9. Additionally, it is known that sex hormones are altered in SS, with decreased estrogen and dihydrotestosterone levels. These sex hormones had no effect on TLR-7 and TLR-9 expression by pDCs, and did not alter their pro-inflammatory cytokine production in vitro; however, they were protective of the epithelial cells, reducing their apoptosis, and thus, limiting exposure of pDCs to autoantigens (Ainola et al. 2018)
In summary, pDCs accumulate in the salivary glands in the course of SS and exhibit characteristics of activation. However, it is not clear if they are in fact the initiators of the disease or if they contribute to the progress of the disease through secretion of type I IFNs and cytokines. Of note, lacrimal gland dysfunction is one of the hallmarks of SS, and it remains unknown how pDCs are altered in the lacrimal gland in the course of the disease and how they may contribute to the disease pathophysiology. Thus, further studies are necessary to evaluate the role of pDCs in the lacrimal gland in SS.
4.3.2. Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by loss of tolerance to self-antigens, with a wide range of clinical manifestations (Dorner et al. 2019). Ocular involvement is common in individuals with SLE, affecting about one third of patients. In addition to its most common associate, Sjögren’s syndrome (please see relevant section above), ocular manifestation of SLE range from involvement of ocular adnexa to vision-threatening retinal vasculitis to optic neuropathy (Palejwala et al. 2012, Shoughy et al. 2016, Silpa-archa et al. 2016). In SLE, several autoantibodies against self-molecules in the nucleus, cytoplasm, and cell surface, in addition to soluble molecules, such as IgG and coagulation factors, participate in the pathogenesis of the disease (Dorner et al. 2019). Due to the presence of a variety of autoantibodies, SLE can virtually manifest in any tissue. Neutrophil extracellular traps containing nucleic acid antigens, as well as apoptotic cells, may expose DNA and nuclear proteins that interact with autoantibodies to form immune complexes. pDCs harbor Fc receptors on their surface and are thus capable of engulfing immune complexes. Following internalization of immune complexes, fusion with endosomes allows the engagement of TLR-7 and TLR-9, mediating pathogenic production IFN-α, along with other cytokines such as TNF-α (Tian et al. 2007, Sakata et al. 2018, Smith et al. 2019). Secreted type I IFNs subsequently activate and sustain autoantibody generating B cells (Jego et al. 2005, Banchereau et al. 2006).
Early depletion of pDCs in SLE ameliorates the disease progression in mice, by limiting aberrant B cells and subsequent autoantibody generation (Rowland et al. 2014). Conversely, pDC repopulation results in reduced splenic weight, decreased autoantibodies, B cells and CD4+ T cells. Thus, temporary pDC depletion results in favorable outcomes, suggesting a pathogenic role of pDCs, at least at the onset of SLE. In Tcf4+/−Tlr7.tg mice, which develop a SLE-like disease, absence of pDCs leads to reduced splenomegaly, serum anti-RNA IgG levels, normalization of the density of peripheral CD11c+ MHC-IIneg cells, and increased survival. Therefore, lack of pDCs ameliorates the immune activation observed in the Tlr7.Tg SLE-like mice (Sisirak et al. 2014). Moreover, pDCs from mice with late SLE are unable to produce IFN-α upon TLR stimulation (Liao et al. 2015). Together, these results indicate that pDCs lose the ability to produce IFN-α in the course of SLE, and therefore, may not be necessary for disease progression.
In humans, circulating pDCs are increased in individuals with SLE as compared to healthy individuals (Jin et al. 2008), and have an increased ability to stimulate and expand T cells. Conversely, pDCs from healthy individuals are not capable of stimulating T cells, but are instead capable of inducing Tregs (Jin et al. 2010). Furthermore, pDCs during steady state can induce both Bregs (CD24+ CD38hi) that co-express IL-10 and plasmablasts through secretion of IFN-α and CD40 signaling. In healthy conditions, Bregs provide a negative feedback and reduce IFN-α production by pDCs via secretion of IL-10. However, during SLE, pDCs fail to induce the differentiation of Bregs and instead solely promote antibody-producing plasmablasts(Menon et al. 2016). Targeting the pDC specific receptor BDCA-2 with a humanized monoclonal antibody (24F4A) results in blocking pDC-mediated IFN-α expression in the serum of SLE patients (Gardet et al. 2019). Furthermore, injections of 24F4A in monkeys inhibit pDC activation by SLE-associated immune complexes (Pellerin et al. 2015). Application of BIIB059, another humanized monoclonal antibody against BDCA-2, which is currently under investigation in a phase II clinical study (NCT02847598), reduces skin damage and increases internalization of BDCA-2 in pDCs, which correlates with reduced levels of circulating IFN-α (Furie et al. 2019). Since IFN-α has been shown to be one of the pathogenic mediators during SLE, current clinical trials with anti-IFN-α antibodies are ongoing and are in phase III clinical trials (NCT01438489) (Furie et al. 2017).
In summary, although secretion of type I IFNs is necessary for immune response to pathogens, secretion of high levels of IFN-α by murine pDCs at the disease onset activates and sustains B cells to produce autoantibodies; however, the role of pDCs in disease progression is to be elucidated.
4.3.3. Rheumatoid Arthritis
Although pDCs seem to serve as culprit in SLE, this may not be the case in the majority of other autoimmune disease in which the role of pDCs has been investigated. Therefore, in order to provide a more balanced view of potential protective and destructive roles of pDCs in autoimmune conditions, we briefly review our current understanding of the role of pDCs in rheumatoid arthritis, a condition in which pDCs may play a protective role. Rheumatoid arthritis is a chronic disease that primarily affects joints, but can include vasculitis or other systemic comorbidities. It has been established that between 50% and 70% of individuals with rheumatoid arthritis share autoantibodies against citrullinated peptide and against IgG (rheumatoid factor) (Nell-Duxneuner et al. 2010, Barra et al. 2011). In the synovial milieu, a Th1/Th2 imbalance towards Th1 cells has been described in RA patients, and this imbalance is driven by pDCs, cDCs and B cells (McInnes et al. 2007). Despite the fact that pDCs can exert a Th1 response by producing IFN-α, the involvement of IFN-α production by pDCs during rheumatoid arthritis remains controversial (Nehmar et al. 2017). A recent study employed different strategies to deplete pDCs to explore the contribution of type I IFNs and pDCs in a mouse model of rheumatoid arthritis. To induce rheumatoid arthritis in this study, sera of K/BxN mice, which contain pathogenic antibodies against the ubiquitous protein glucose-6-phosphate isomerase, necessary to develop the majority of rheumatoid arthritis features (Monach et al. 2007), were injected into mice. Following induction of rheumatoid arthritis, Ikaros−/− mice, which lack peripheral pDCs (Allman et al. 2006), displayed lasting bone erosion and paw swelling, accompanied by an increased influx of immune cells, mainly neutrophils in the peri-articular tissues (Nehmar et al. 2017). Similarly, depletion of pDCs via different methods leads to increased paw swelling and serum levels of IL-6, but does not significantly alter histological findings (Nehmar et al. 2017). In another model of rheumatoid arthritis, depletion of pDCs exacerbates collagen-specific proliferation of T cells, autoreactive B cells, and disease pathology, evident by extensive synovial hyperplasia, cartilage degradation, and pannus invasion (Jongbloed et al. 2009).
In individuals with rheumatoid arthritis, the density of blood pDCs is decreased and pDCs possess an immature phenotype, defined by decreased expression of CD40L, CD80, CD83, CD86, and adhesion molecule, L-selectin (CD62L) (Jongbloed et al. 2006, Cooles et al. 2018, Nehmar et al. 2018). Further, in individuals with rheumatoid arthritis, pDCs are able to favor generation of IL-10-secreting Treg cells from allogeneic naïve CD4+ CD25neg T cells cell (Kavousanaki et al. 2010). Peripheral pDCs show increased CCR7 (a key chemokine receptor for migration of DCs to lymph nodes), which is inversely correlated with pDC frequency (Cravens et al. 2007, Seth et al. 2011). In addition, the CCR7 ligand, CCL19/CCL21, is increased in synovial joints in rheumatoid arthritis, supporting migration of pDCs to the joints in the early course of the disease (Pickens et al. 2011). Taken together, the presence of pDCs seems to limit the immune response and reduce inflammation-induced tissue damage in rheumatoid arthritis, suggesting a beneficial role for pDCs in this condition (Jongbloed et al. 2009, Kavousanaki et al. 2010, Nehmar et al. 2017).
In summary, the role of pDCs in pathogenesis of autoimmune diseases depends on the nature of the disease. During early SLE, when IFN-α signature is prominent, pDCs may participate in the pathogenesis and progression of the disease. However, in rheumatoid arthritis, pDCs tend to promote tolerance, re-establish homeostasis, and thus, favor clinical outcome of the disease. In ocular diseases, it would be interesting to assess if pDCs contribute to pathogenesis of autoimmune conditions, such as uveitis. Of note, considering the important role of IL-23/IL-17 signaling pathway in induction of autoimmune uveitis (Zhong et al. 2020) and counter-regulatory role of Tregs in this process (Grégoire et al. 2016, Zhuang et al. 2017), it may be postulated that pDCs may contribute to the pathogenesis of autoimmune uveitis by mediating T cell priming and preferably inducing particular T cell responses. Findings of such studies may pave the way for novel therapies targeting immune responses and thus ameliorating clinical severity and sequela of the disease.
4.3.4. Sterile Inflammation
Homeostatic properties of pDCs are not confined to induction of tolerance to antigens as described in the following section. In fact, pDCs drive anti-inflammatory responses in various conditions. For example, in an acute immune-mediated liver injury model, pDC depletion is accompanied by severe liver injury, judged by increased serum aminotransferase levels, increased serum IFN-γ and IL-6 levels, as well as to decreased infiltration of Tregs to the liver (Koda et al. 2019). Along the same lines, adoptive transfer of pDCs results in decreased serum aminotransferase, IL-6, and MCP-1 levels, reduced generation of IFN-γ+ Th1 and Th17 effector T cells, enhanced generation of generation of Tregs through IL-35 and favors mice survival (Koda et al. 2019).
In the ocular tissues, sterile inflammation occurs in response to contact lens wear, allergens, self-antigens, and mechanical, thermal and chemical traumas and burns. Among ocular tissues, the cornea is unique to study immune responses due to its accessibility and simplicity of clinical examination, as well as its immune and angiogenic privilege. To study immune responses in the cornea, sterile models of inflammation, including thermal cautery, alkali burn, and corneal suture placement have been widely used (Pfister et al. 1978, Williamson et al. 1987, Ormerod et al. 1989, Sano et al. 1995, Streilein et al. 1996, Hamrah et al. 2002, Cursiefen et al. 2004, Giacomini et al. 2014). Recently, it has been shown that depletion of corneal pDCs in BDCA-2-DTR mice prior to suture placement is accompanied by enhanced clinical opacity of the cornea as compared to controls, as well as augmented influx of inflammatory immune (CD45+) cells in general, including neutrophils and macrophages (Sendra et al. 2014, Sendra et al. 2017). pDCs may also regulate adaptive immune response in the dLNs, as their depletion prior to corneal suture placement results in increased the density of CD8+ T cells and B cells (Sendra et al. 2014). This model also demonstrates that the role of pDCs in mediating innate and adaptive immune response is not dependent on IFN-α, but rather might be mediated through other pathways, as neutralization of IFN-α using subconjunctival administration of anti-IFN-α antibody does not alter clinical severity of inflammation, density of recruited immune cells, or density of subpopulations of CD4+ T cells in the dLN (Sendra et al. 2017) (manuscript under preparation).
Experimental DED is another instance in which the role of pDCs in sterile ocular surface inflammation is studied. In this regard, Stern et al. evaluated IFN-α production in the tears of feeble mice, which carry a mutation in Slc15a4, abrogating IFN-α and cytokine production relatively specially in pDCs (Blasius et al. 2010, Stern et al. 2013). They demonstrated that compared with wild-type mice, feeble mice show significantly lower amount of IFN-α in their tears, suggesting that pDCs are the major source of IFN-α during desiccating stress-induced experimental DED (Stern et al. 2013). Fig. 6 summarizes our current understating on significance of pDCs in sterile corneal inflammation.
Figure 6. Schematic illustration on the role of plasmacytoid dendritic cells during sterile corneal inflammation.
During sterile corneal inflammation induced by suture placement, local depletion of pDCs leads to increased accumulation of immune cells in particular cDCs and macrophages.
In summary, pDCs may contribute to amelioration of inflammation in sterile inflammations. Thus, it is worthwhile to assess the role of pDCs in other sterile inflammatory conditions such as chemical and mechanical traumas and evaluate if they may be involved in homeostasis and supporting stems cells and regeneration of corneal epithelium following traumas. Further, although capacity of pDCs in secreting type I IFNs is well known, it is interesting to study if contribution of pDCs to inflammatory processes such as DED goes beyond secretion of type I IFNs and if so, how such functions of pDCs are regulated.
4.4. Role of Plasmacytoid Dendritic Cells in Tolerance
In addition to directing immune responses to pathogens and their involvement in pathogenesis of autoimmune diseases, pDCs are pivotal in inducing tolerance and suppressing inflammatory responses. In fact, pDCs provide a tolerogenic microenvironment, maintaining homeostasis, through various mechanisms, ranging from secretion of tolerogenic cytokines and growth factor, the development of Tregs in the thymus and peripheral tissues, to mediating T cell activities (de Heer et al. 2004, Martin-Gayo et al. 2010).
In general, freshly isolated pDCs from human blood, unlike cDCs, express ICOS-L upon in vitro stimulation, through which they may prevent expansion of naïve CD4+ T cells and augment generation of IL-10 producing Tregs (Ito et al. 2007). Further, upon stimulation with TLR-9 agonists, human peripheral blood pDCs shift cytokine production pattern of naïve CD4+ T cells towards enhanced production of TGF-β and IL-10, and decrease secretion of IL-2. They also favor generation of Tregs, which can suppress autologous and allogeneic T cell proliferation in an Ag-nonspecific manner in vitro (Moseman et al. 2004). Dissimilar to cDCs, when stimulated pDCs are cultured with naïve CD8+ T cells, they prime CD8+ Tregs with poor secondary proliferative capacity and cytotoxic activity against allogeneic cells. These Tregs prevent allospecific proliferation of naïve CD8+ T cells through secretion of IL-10 (Gilliet et al. 2002). Compared with cDCs, even stimulated pDCs exhibit lower capacity in promoting proliferation of allogeneic T cell. Further, pDCs also induce remarkable apoptosis in allogeneic CD4+ T cells via Tregs (Tokita et al. 2008). Notably, it is shown that pDCs isolated from different tissues, for instance liver or spleen may display different tolerogenic capacities (Tokita et al. 2008). In the sections below, we briefly review the roles of pDCs in inducing tolerance to organ transplants, tumors, and oral antigens, as three main areas in which immune responses are currently under comprehensive investigations and where they may apply to respective ocular diseases.
4.4.1. Transplantation
4.4.1.1. Transplantation in Non-ocular Tissues
Recent studies have unraveled tolerogenic effects of pDCs in multiple organ transplants, such as heart, kidney, and hematopoietic stem cells, by highlighting their role in the generation or promotion of the functions of Tregs, as well as mediating T cell anergy (Abe et al. 2005, Ochando et al. 2006, Li et al. 2010, Rajasekar et al. 2010, Oh et al. 2019). For instance, in a rat model of heart transplantation, it has been shown that accumulation of pDCs in the graft is necessary for induction of tolerance and graft survival (Li et al. 2010). Dissecting the cellular players in mediating immune responses to grafts, Ochando et al. demonstrated that, in a murine model of cardiac transplant, pDCs capture alloantigens in the graft and egress to the dLN via blood circulation, where they induce generation of CD4+ CD25+ Foxp3+ Tregs. Furthermore, depletion of pDCs accelerates graft rejection in tolerized mice, while intravenous adoptive transfer of pDCs isolated from tolerized mice enhances Treg generation and favors graft survival (Ochando et al. 2006). Similarly, it has been shown that pDCs preferentially promote tolerogenic a function of CD8+ Tregs on suppressing CD4+ T cell proliferation, via a contact-dependent effect on CD8+ Tregs (Li et al. 2010). In addition to favoring the generation of Tregs, pDCs may promote T cell anergy. In this regard, it has been shown that ex vivo CpG-ODN stimulated pDCs isolated from the bone marrow, are less efficient in inducing allogeneic naïve T cell proliferation compared to cDCs, which can be in part attributed to their expression of the co-inhibitory molecule PD-L1 (B7-H1) (Abe et al. 2005). Furthermore, intravenous adoptive transfer of pDCs induces non-specific hypo-responsiveness to challenge with donor or third-party irradiated splenocytes ex vivo, including reduced T cell proliferative, as well as IL-2 and IFN-γ secretory capacities (Abe et al. 2005). Interestingly, the addition of IL-2 to T cells, isolated from mice receiving pDCs, could not retrieve T cell proliferative capacity, indicating that pDC-induced hypo-responsiveness is not reversible (Abe et al. 2005). Moreover, pre-operative adoptive transfer of in vitro-propagated donor or third party pDCs significantly improves survival of fully MHC-mismatched heart transplants (Abe et al. 2005). Confirming these findings, it has been shown that adoptive transfer of mobilized donor pDCs prior to surgery can substantially promote heart allograft survival compared with subtypes of cDCs (Bjorck et al. 2005).
In kidney transplants, naïve pDCs isolated form syngeneic or accepted allogeneic transplants can generate Tregs from CD4+ CD25neg T cells in vitro. Further, adoptive transfer of Tregs, generated ex vivo by co-culturing pDCs and CD4+ CD25neg T cells, enhances graft survival (Oh et al. 2019). In contrast, pDCs are also implicated in immune responses to viral pathogens occurring following kidney transplantation. It has been shown that pDCs treated with conditioned media isolated from CMV-infected human kidney proximal tubular epithelial cells exhibit phagocytic activity and can increase CD4+ and CD8+ T cell proliferation and cytokine production and thus, may contribute to kidney transplant rejection (Ruben et al. 2018). Tolerogenic effects of pDCs in transplantation are not confined to solid tissues. In fact, it has been shown that in patients undergoing HLA-matched hematopoietic stem cell transplant due to hematologic malignancies, higher graft pDC count is associated with increased risk of relapse and poor overall survival, persumably due to attenuation of graft-versus-leukemia effect (Rajasekar et al. 2010).
In summary, pDCs mediate the induction of tolerance to grafts and promote reestablishment of homesotasis in the transplanted tissue via generation of Tregs, induction of anergy, and production of anti-inflammatory cytokines, as well as by controling opportunistic infections. Consideirng that corneal trasnplntation is the most common solid tissue transplant with a high rate of rejection in high-risk individuals, it is thus necessesary to study the impact of pDCs in corneal transplntation and to assess if tolerogenic properties of pDCs can be utilized to improve transplantation outcomes. In the follwing section, we summarize our current knowledge on the significance of pDCs in corneal transplantation.
4.4.1.2. Corneal Transplantation
The role of resident professional APCs, such as cDCs in eliciting immune responses to corneal allografts is well documented (Hori et al. 2019). Immature APCs take up corneal alloantigens following transplantation and undergo maturation via upregulation of MHC-II and co-stimulatory molecules. They process the antigens and transfer them to the dLNs, where they prime naïve T cells to effector CD4+ IFN-γ+ Th1 cells. The effector Th1 cells then infiltrate the cornea, inducing immune rejection of the graft (Qazi et al. 2013, Amouzegar et al. 2016, Hori et al. 2019).
The role of pDCs in corneal transplantation is less studied compared to other solid organ transplants. In a recent study, Tahvildari et al. have shown that depletion of pDCs in recipient mice enhances graft opacity and infiltration of both innate and adaptive immune cells, in particular, CD68+ macrophages and CD3+ CD4+ T cells (Tahvildari et al. 2017) (manuscript under preparation). Moreover, using ELISPOT assay, it has been reported that in pDC-depleted recipients, IFN-γ+ T cells are increased in both direct and indirect allosensitization compared to controls. They further showed that pDC depletion prior to corneal transplantation is accompanied by increased frequencies of CD4+ IFN-γ+ Th1 cells and CD4+ IL-17+ Th17 cells, as well as decreased expression of CD25 among Tregs in the dLNs, leading to acceleration of immune rejection in pDC-depleted mice (Tahvildari et al. 2017) (manuscript under preparation). Fig. 7 illustrates the role of pDCs in corneal transplantation.
Figure 7. Schematic illustration on the role of plasmacytoid dendritic cells in corneal allograft.
During allogeneic corneal transplantation, local depletion of pDCs prior to the procedure, enhances recruitment of innate immune cells including cDCs, macrophages, and CD4+ Th cells to the cornea and leads to enhanced generation of effector Th1 and Th17 and reduced density of Tregs in the draining lymph nodes.
Although current evidence suggests a critical role for pDCs in induction of tolerance to allogeneic transplants via suppressing the generation of effector T cells in the dLNs, several questions remain unanswered. For instance, it is not clear if pDCs mediate Treg induction in corneal transplantation or if they may affect several aspects of Treg biology, such as their survival, T suppressor activity, or fate. Furthermore, the molecular mechanism and signaling pathways through which pDCs exert their allosuppressive effects on subpopulations of T cells is not yet clear. Further studies may also unravel if tolerogenic properties of pDCs can be employed to induce specific tolerance of anergy in high-risk corneal transplantations and thus promote graft survival.
4.4.2. Allergic Diseases
4.4.2.1. Non-ocular Allergic Diseases
In this section, we will review our current understanding on the role of pDCs in the induction of tolerance to allergens, mainly to oral and airway allergens, and subsequently discuss potential contribution of pDCs to ocular allergies. Oral tolerance is the phenomenon by which tolerance is induced through the oral administration of antigens. This phenomenon has important implications for conditions such as allergy and asthma, and may, perhaps, be utilized in the future to promote tolerance in the context of autoimmunity (Weiner et al. 2011). While the role of Tregs in this context has received much attention, there are several studies that underscore the importance of pDCs in oral tolerance. Studies on mucosal-associated DCs revealed that CD8α+ DCs are promoters of Treg suppressive abilities. Further phenotypic characterization of these CD8α+ DCs indicated that both pDCs and cDCs are present in this population (Bilsborough et al. 2003, Fleeton et al. 2004). A series of in vitro proliferation assays indicated that CD8α+ pDCs are less capable of supporting T cell proliferation, and in fact, favor T cell suppression, even after maturation by exposure to CpG oligonucleotides (Bilsborough et al. 2003).
Additional reports have demonstrated that pDCs have an indispensable role in the induction of oral tolerance, as depletion of pDCs prevented tolerance. These reports investigated the contribution of liver-derived DCs to oral tolerance, and noted that the majority (60–80%) of liver-derived CD11c+ CD11bneg NK1.1neg cells are pDCs (Goubier et al. 2008). As indicated by adoptive transfer and pDC depletion experiments, pDCs are able to limit the response of CD4+ and CD8+ T cells in response to oral challenge with 2,4-dinitro-1-fluorobenzene or ovalbumin. This effect is mediated by the ability of pDCs to induce anergy or deletion of CD4+ and CD8+ T cells in an antigen-specific manner (Goubier et al. 2008). The role of pDCs in tolerance induction is further confirmed by investigating the contribution of oral mucosa associated DCs. It is found that oral DC subsets, both cDCs and pDCs, are capable of promoting tolerance and are prone to polarizing naïve T cells towards Th1 or Treg phenotypes, in contrast to their splenic counterparts (Mascarell et al. 2008). Additional studies have led to the proposal of a two-step model for the induction of oral tolerance. In this first step, antigen-specific T cells are deleted or rendered anergic by pDCs, while simultaneously, pDCs promote the suppressive functions of Tregs. In the second stage, residual antigen-specific T cells are suppressed by Tregs upon antigen re-exposure, resulting in tolerance (Dubois et al. 2009).
Oral administration of probiotic Lactobacillus gasseri OLL2809, which induces oral tolerance, is accompanied by enhancing the ratio of pDCs as compared with cDCs in the lamina propria in the small intestine (Aoki-Yoshida et al. 2016). Moreover, it has been demonstrated that mesenteric LN-derived pDCs are potent inducers of CD4+ Foxp3+ Tregs, and neither inhibition of indoleamine 2,3-dioxygenase (IDO) nor blockade of B7 family members of co-stimulatory molecules can prevent generation of antigen-specific Tregs by pDCs in vitro (Uto et al. 2018). Rather, autocrine secretion of TGF-β mediates generation of such antigen-specific Tregs (Uto et al. 2018). In line with these in vitro findings, pDC-depleted mice fail to generate sufficient Tregs in the mesenteric LNs and fail to demonstrate protective tolerance to the antigen following feeding with OVA (Uto et al. 2018).
Findings from pre-clinical models have begun to be confirmed in humans (Hoffmann et al. 2006, Palomares et al. 2012). One such study investigated a variety of clinical parameters and white blood cell counts in a group of patients allergic to wheat. After the initial challenge with flour, there was reduced clinical response to the allergen upon subsequent challenge. This was associated with reduced numbers of circulating pDCs and Tregs, as well as reduced expression of MHC on DCs. This suggests that pDCs and Tregs are recruited to the site of challenge and promote a tolerogenic response (Hoffmann et al. 2006). Another report utilized the tonsils as a source of pDCs and tonsillar Tregs and peripheral blood as a source of naïve T cells from atopic and non-atopic individuals. Co-culture experiments revealed that pDCs are capable of inducing Foxp3+ Tregs. Additionally, it was found that pDCs are decreased in atopic individuals compared to non-atopic individuals (Palomares et al. 2012).
Thus, in the context of oral tolerance, pDCs clearly have a central role. The studies described above also highlight that in each of the studied areas, pDCs have similar roles for the elimination of antigen-specific T cells and promoting the suppressive abilities of Tregs. These effects have proven to be relevant in animal models of allergy and atopy, and there are correlations between pDCs and Tregs in non-atopic humans, which is lost in atopic individuals. Altogether these findings suggest that pDCs represent a promising therapeutic target or, perhaps, cell-based therapy for the treatment of allergy and atopy. There is additional potential for utilizing pDC-driven oral tolerance to treat autoimmune diseases, however, this area requires further investigation.
Similar to oral tolerance, it is shown that pDCs promote tolerance to common allergens. In this regard, it has been shown that compared with control mice subjected to repeated exposure to ovalbumin aerosols, initial intratracheal administration of ovalbumin suppresses the airway inflammation, suggesting induction classic immunologic tolerance. However, only in mice undergoing depletion of resident pDCs in the lung, challenging mice with ovalbumin aerosols following initial intratracheal immunization, leads to eosinophilic infiltration around vessels and bronchi, goblet cell hyperplasia, and detection of ovalbumin-specific IgE in the serum, characteristic of asthma (de Heer et al. 2004). pDCs capture intratracheally administrated ovalbumin and migrate to dLNs, where among ovalbumin+ cells, pDCs tend to be more frequently ovalbumin+. In stark contrast with cDCs, pDCs are not capable to induce ovalbumin-specific T cell proliferation and fail to secret higher amounts of pro-inflammatory cytokines following co-culture with T cells. In fact, pDCs induce differentiation of Tregs with suppressing effects on antigen-specific T cell proliferation. Adoptive transfer of pDCs pulsed with ovalbumin prior to sensitizing mice with intraperitoneal injection of alum- ovalbumin and subsequent challenge with ovalbumin aerosols significantly inhibits inflammation in the airways and T cell cytokine production. Thus, pDCs are vital for inducing tolerance to allergens and preserving tolerance to inert antigens (de Heer et al. 2004).
4.4.2.2. Ocular Allergic Diseases
In ocular allergies, in an early study summarized above, it was shown that pDCs, which were rarely observed in the conjunctiva during steady state, tended to significantly increase in this tissue following allergen challenge, reaching higher numbers than cDCs (Ohbayashi et al. 2007). However, the significance of these cells in the pathogenesis of allergic conjunctivitis warrants further investigation. In light of the essential role of pDCs in maintaining and inducing tolerance to oral antigens, as well as the pivotal role of pDCs in preventing allergic reactions in the respiratory system, as reviewed above, it might be postulated that pDCs promote tolerance to allergens on the ocular surface and thus diminish disease severity in individuals with allergic conjunctivitis.
Further studies on humans and murine models of allergic conjunctivitis may evaluate if the phenotype of pDCs and their tolerogenic properties are altered in allergic conjunctivitis and if so how such alterations can be reverted to promote tolerance and decrease severity of the disease. Further, it is intriguing to study if pDCs can be employed to induce tolerance to allergens after induction of immune response due to prior exposure to allergens and thus can be used to desensitize individuals to specific allergens and thus treat allergic conjunctivitis.
4.4.3. Tumors
Within the tumor microenvironment, pDCs have been found to infiltrate primary and metastatic tumors, as well as peri-tumoral tissues in multiple malignancies, including breast, ovarian, head and neck, gastric, liver, lung cancers, malignant melanomas, and lymphomas (Facchetti et al. 1989, Hartmann et al. 2003, Vermi et al. 2003, Kutzner et al. 2009, Conrad et al. 2012, Faget et al. 2012, Sawant et al. 2012, Aspord et al. 2013, Huang et al. 2014, Pedroza-Gonzalez et al. 2015, Sorrentino et al. 2015). Considering the well-known anti-tumor effects of IFN-α, studies aimed to evaluate if pDCs promote anti-tumoral immune responses through their secretion of IFN-α. However, tumor-associated pDCs fail to effectively produce type I IFNs (Zou et al. 2001, Hartmann et al. 2003, Labidi-Galy et al. 2011, Sisirak et al. 2012, Le Mercier et al. 2013, Dey et al. 2015, Terra et al. 2018), at least in part, due to secretion of immunomodulatory molecules by tumor cells, such as IL-10 and TGF-β (Bekeredjian-Ding et al. 2009, Sisirak et al. 2013, Bruchhage et al. 2018).
Although tumor-associated pDCs have a limited IFN-a production capacity, they do promote tolerance by suppressing T cell proliferation, cytotoxic activity, and IFN-γ secretion in vitro (Wei et al. 2005). Further, based on depletion studies, pDCs promote IL-10 secretion by CD4+ T cells (Dey et al. 2015) and favor the accumulation of myeloid-derived suppressor cells in tumors (Sawant et al. 2012). In addition, pDC depletion leads to decreased density of Tregs in the tumor and metastases, as well as attenuated suppressive capacity of existing Tregs, suggesting a central role of pDCs in promoting Tregs (Sawant et al. 2012, Dey et al. 2015). Furthermore, pDCs isolated from tumor-draining LNs are shown to express IDO, and can activate suppressor activity of resting Tregs on CD4+ and CD8+ T cells in an IDO-dependent manner (Sharma et al. 2007). In addition to promoting Tregs, pDCs express PD-L1, which via interacting with PD-1 on T cells, limits proliferation and cytotoxic activity of both CD4+ and CD8+ T cells, as well as the cytolytic activity of NK cells (Ray et al. 2015). Expression of Granzyme B by pDCs can also regulate T cells, as secreted Granzyme B can inhibit CD4+ T cell expansion (Jahrsdorfer et al. 2010). Similarly, tumor-associated pDCs express ICOS ligand (ICOS-L), which is necessary for survival and proliferation of ICOS+ Tregs (Conrad et al. 2012, Faget et al. 2012). Further, via ICOS/ICOS-L signaling, stimulation of pDCs with tumor lysate induces CD4+ Foxp3neg IL-10+ Tregs from naïve CD4+ T cells, which exhibit potent suppressive effects on T cell expansion (Pedroza-Gonzalez et al. 2015).
Although pDCs generally provide a tolerogenic microenvironment for tumors, upon stimulation, they are able alter these tolerogenic properties. For instance, stimulation of naïve pDCs through TLR-7 or TLR-9 enhances immune responses to tumors by multiple mechanisms: (1) enhancing direct cytotoxic activity of pDCs via enhancing their expression of Granzyme B and TRAIL (Drobits et al. 2012, Kalb et al. 2012, Wu et al. 2017); (2) stimulating expression of TRAIL, CD69, and IFN-γ by NK cells (Lelaidier et al. 2015), (3) cross priming and activating CD8+ T cells and Th17 T cells (Lou et al. 2007, Liu et al. 2008, Guery et al. 2014), and (4) augmenting infiltration of NK cells and CD8+ T cells in tumors (Liu et al. 2008, Le Mercier et al. 2013, Guery et al. 2014, Wu et al. 2017). Thus, stimulation of pDCs may alter intrinsic pDC functions and deserve further studies as a therapeutic strategy.
In summary, pDCs generally favor immune irresponsiveness to tumors via directly inhibiting T cells and NK cells or through promotion of Tregs. Nevertheless, pDCs may also affect other aspects of tumor biology, including direct effects on tumor cell proliferation, secretary functions, migration, invasion, metastasis as well as metabolism and angiogenesis in tumors, all of which warrant further studies. In ocular tissues, it is important to assess if pDCs play a similar tolerogenic role through directing suppressive immune responses for ocular neoplasia, such as in choroidal or conjunctival melanomas.
4.5. Plasmacytoid Dendritic Cell Function in Graft-Versus-Host Disease
Whereas transplant rejection occurs due to a host-mediated immune response, graft-versus-host disease (GVHD) reflects the opposite scenario, in which donor T cells primed by either donor or host APCs induce an immune response against the host. GVHD affects many organs. In particular, it may involve ocular tissues. Among ocular manifestations, DED is the most common presentation of ocular GVHD following HSCT (Munir et al. 2017). Our knowledge on the significance of pDCs in pathogenesis of ocular involvement of GVHD is limited and therefore, warrants detailed studies. However, we herein discuss our understanding on the role of pDCs in this disease.
In one of the early studies addressing the role of pDCs in GVHD, peripheral blood samples from individuals that had undergone hematopoietic stem cell transplantation (HSCT) were acquired and their immune cell subsets were profiled (Clark et al. 2003). This study found that pDC density in the peripheral blood of individuals with chronic GVHD are higher compared to control individuals who had undergone HSCT but did not develop GVHD (Clark et al. 2003). However, this difference is not due to an increase in pDCs in the GVHD group, rather it is due to decreased pDCs in control HSCT group without GVHD, since in the GVHD group, density of pDC in peripheral blood were comparable to healthy volunteers (Clark et al. 2003). In a murine model of GVHD, investigators irradiated MHC-II-deficient mice and reconstituted the APC populations (either pDCs, cDCs, or B cells), followed by T cells, showing that pDCs are capable of priming alloreactive T cells to induce GVHD (Koyama et al. 2009). A caveat to this study however, is that this is an artificial model, and it is unclear if pDCs would have the same effect following reconstitution with all APC populations. An additional study revealed that depletion of host-derived cDCs, pDCs, or B cells was insufficient to prevent the onset of GVHD (Li et al. 2012).
While it may be tempting to speculate that pDCs may thus be pathogenic, the possibility that pDC may promote tolerance cannot be excluded. In fact, there is a growing body of direct evidence that pDCs are protective in GVHD. One such study utilized a murine model of GVHD, where irradiated mice were reconstituted with STAT1−/− bone marrow, resulting in expanded pDC and Treg populations and GVHD resistance. Additionally, depletion of pDCs after reconstitution with STAT1−/− bone marrow reversed this effect (Capitini et al. 2014). Further, CCR9+ pDCs, which constitute the majority of pDCs in the dLNs, have potent tolerogenic capabilities, as they can effectively induce Tregs, which in turn inhibit CD4+ T cell proliferation considerably (Hadeiba et al. 2008). In addition, adoptive transfer of allogeneic CCR9+ pDCs is protective in GVHD, as it leads to decreased priming of naïve donor T cells towards IFN-γ+ Th1 and Th17 effector T cells in the dLNs and spleen, as well as to an enhanced density of CD25+ Foxp3+ Tregs in the dLN, with beneficial effects on animal survival (Hadeiba et al. 2008). In a large clinical study, multivariable analysis indicated that after adjustment for several factors, recipients of higher number of bone marrow pDCs showed improved 3-year survival, fewer deaths due to GVHD as well as rejection (Waller et al. 2014). Interestingly, the study did not observe a similar protective role for higher number of pDCs in grafts in individuals receiving granulocyte colony stimulating factor-mobilized peripheral blood HSCT. In follow-up studies, in a murine model of lethal GVHD, it was then confirmed that transplantation of bone marrow pDCs considerably improved survival, potentially due to secretion of IL-12, a capacity which was limited in pDCs isolated from the spleen following treatment with granulocyte colony stimulating factor (Hassan et al. 2017). In addition to potential differences in the IL-12 secretion capacity, other differences between bone marrow and mobilized peripheral blood pDCs may underlie this observation. For instance, bone marrow pDCs may contain higher numbers of less mature precursor pDCs with distinct antigen presentation capacity or chemokine receptor repertoire. In this regard, it has been shown that mobilized peripheral blood pDCs expressed higher levels of CCR7, lymph node homing receptor for pDCs, but lower levels of L-selectin (CD62L), CXCR3, and CCR9, which may facilitate homing of pDCs to inflamed tissues (Hosoba et al. 2014). Further studies have shown that an increase in pDC density in donor bone marrow has a protective effect by limiting GVHD (Hassan et al. 2019). In this regard, it has been shown that expansion of the pDCs in vivo by treating donor mice with FLT3-L, prior to reconstitution of irradiated recipient mice, leads to improved engraftment and protection from GVHD (Hassan et al. 2019). As such, increasing pDC numbers prior to bone marrow reconstitution may improve patient outcomes through their tolerogenic nature and capacity to induce Tregs. As expansion of pDCs in the prior study was done in vivo, it is interesting to study if ex vivo treatment of donor bone marrow cells with FLT3-L may yield a similar effect, or alternatively, supplementing the treatment regimen in recipients of HSCT with FLT3-L may promote engraftment.
In summary, pDCs are considered multifaceted cells of the innate immune system with diverse immune functions (Swiecki et al. 2015). While they were originally appreciated for their potent capacity in producing type I IFNs, our current knowledge suggests they are crucial implementer of tolerance and ameliorate inflammation.
4.6. Role of Plasmacytoid Dendritic Cells in Neuroprotection
The cornea is the most densely innervated tissue in the body, with approximately 300–600 times higher nerve density compared to the skin (Rozsa et al. 1982). Corneal nerves are in majority sensory, and arise from the ophthalmic branch of trigeminal nerves. Anatomically, they can be can be observed as the subbasal nerve plexus, the most densely innervated region of the cornea, which runs parallel to the superficial corneal surface between the Bowman’s layer and the basal epithelium and the stromal plexus, which consists of thicker nerve fiber bundles in the corneal stroma (Millodot 1984, Marfurt et al. 1993, Muller et al. 1996, Muller et al. 1997, Al-Aqaba et al. 2010, Marfurt et al. 2010, Belmonte et al. 2017, Cruzat et al. 2017). In addition to their vital role in initialing the corneal blink reflex and stimulating tear production, recent studies suggest that they play a crucial role in the development of multiple ocular surface diseases, including DED and neurotrophic keratopathy (Bonini et al. 2003, Dastjerdi et al. 2009, Hamrah et al. 2010, Hamrah et al. 2013, Hamrah et al. 2016, Mo et al. 2017, Neelam et al. 2018, Al-Aqaba et al. 2019, McKay et al. 2019). Constant exposure of the cornea to the external environment in the form of chemical irritants and pathogens, poses hazards to this intricate innervation. Further, similar to other peripheral nerves, corneal nerves need constant trophic support, such as members of neurotrophins family including NGF, BDNF, NT-3, NT-4/5 as well non-neurotrophin growth factors such as GDNF, neurturin, artemin, persephin, PEDF, NPFF and neuropoetic cytokines for their maintenance, proper function, or regeneration following injury (Daniele et al. 1992, Lambiase et al. 1998, Bonini et al. 2000, Kerschensteiner et al. 2003, Reichard et al. 2014, Dai et al. 2015, He et al. 2015, Razavi et al. 2015, Zhou et al. 2015). However, it is known that inflammatory conditions, as well as common surgical interventions such as cataract surgery, keratorefractive surgeries or keratoplasties, can result in at least partial corneal denervation (Wilson et al. 2001, Savini et al. 2004, Hamrah et al. 2010, Cruzat et al. 2011, Kurbanyan et al. 2012, Hamrah et al. 2013). Despite these insults, corneal nerves have a marked capacity for regeneration (Muller et al. 2015). Peripheral nerves are dependent on cues and survival signals from the tissues, which they innervate. Within the cornea, it remains to be determined which cell type(s) are responsible for this signaling. However, considering the well-studied roles for immune cells in wound healing and tissue repair/remodeling, exploring a potential contribution of immune cells in providing trophic support for corneal nerve is warranted. Early evidence on the potential communication of corneal nerves and pDCs stems from confocal microscopy of whole-mounted cornea labeled for βIII-tubulin (pan-neuronal marker), CD45, and PDCA-1 for visualizing corneal nerves (βIII-tubulin+) and pDCs (CD45+ PDCA-1+ cells). As demonstrated in Supplementary Video 2, confocal micrographs reveal that corneal pDCs are present within the anterior stroma, in close association with the corneal nerves, suggesting that pDCs may contribute to neuro-immune crosstalk (Zheng et al. 2010) (manuscript under review).
Recently, the significance of resident corneal pDCs in homeostasis of corneal nerves has been investigated. It has been reported that depletion of corneal pDCs in naïve BDCA-2-DTR mice leads to an abrupt and robust degeneration of the corneal nerves, evident as early as one day following pDC depletion, and continues to progress while pDCs remain depleted, as determined by confocal microscopy on corneal whole-mounts stained with βIII-tubulin. Further, depletion of corneal pDCs is accompanied by reduced corneal sensitivity. These observations suggest that pDCs are indeed crucial for the maintenance and health of corneal nerves in vivo (Jamali et al. 2015). Analysis of TG neurons following corneal pDC depletion, has indicated an upregulation of several neurodegenerative markers, including tau and calpain-1, suggesting that depletion of pDCs and the ensuing nerve loss is at least in part mediated by tau oligomer neurotoxicity and subsequent calpain activation (Kenyon et al. 2019). More interestingly, pDCs can induce nerve regeneration as their repopulation, following initial depletion, is accompanied by nerve regeneration (Jamali et al. 2015). Direct neurotrophic properties of pDCs are shown in co-culture of TG neurons with freshly isolated splenic pDCs, which exhibited longer neurite outgrowth and expression of higher levels of neuroregenerative markers compared with TG neuron monocultures (Jamali et al. 2015). Further, pDCs exert their in vitro neurotrophic molecules through secretion of nerve growth factor (NGF; manuscript under preparation). Fig. 8 demonstrates the neuroprotective role of corneal pDCs.
Figure 8. Schematic illustration on the role of plasmacytoid dendritic cells in homeostasis of corneal nerves.
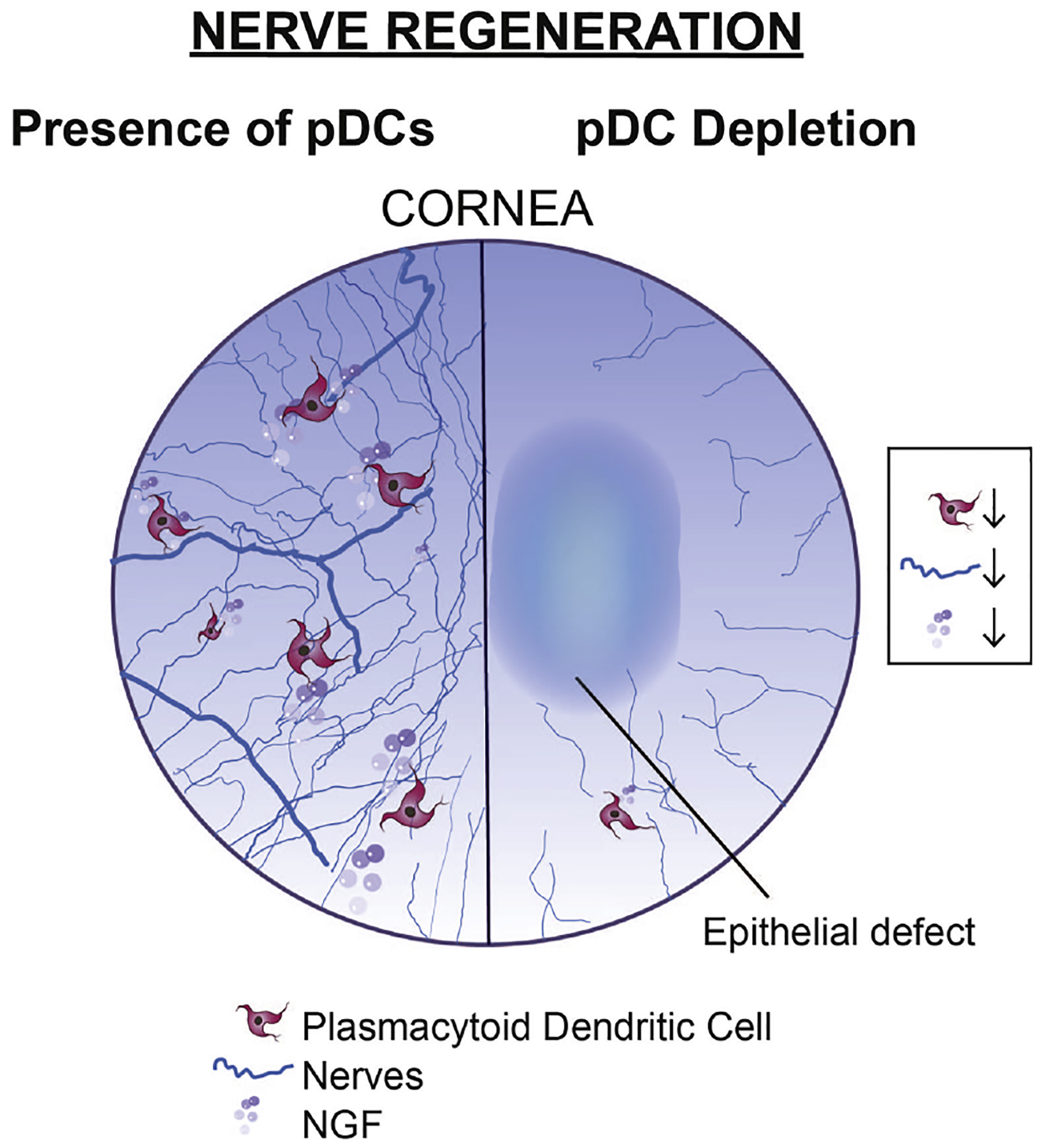
Local depletion of pDCs in the cornea during steady state is accompanied by decreased levels of neurotrophic molecule NGF in the cornea, leading to corneal nerve degeneration, and compromise of epithelial integrity.
Interestingly, the role of corneal cDCs in diabetes, as a neurotrophic corneal condition, has recently been investigated. It is shown that in diabetic mice, cornea encompasses less nerves and also hosts fewer cDCs. Further, it was reported that depletion of cDCs in CD11c-DTR-GFP mice, delays corneal nerve regeneration following nerve injury, which can be rescued by exogenous administration of CNTF, suggesting that cDCs may facilitate corneal nerve regeneration after wounding through secretion of neurotrophic molecule CNTF (Gao et al. 2016). Nevertheless, in contrast to pDCs, depletion of cDCs has not been reported to lead to corneal nerve degeneration during steady state.
Despite the reported neurotrophic properties of corneal pDCs, as a novel function of pDCs, our understating is limited as to whether resident pDCs in other ocular tissues or other body sites may also display neurotrophic properties. Further, it would be important to assess how pDCs are attracted towards the subbasal nerves in the cornea and how production of NGF in pDCs is regulated in this tissue. Also, considering the widely acknowledged trophic effects of the corneal nerves for epithelium, studies on role of pDCs on epithelial integrity may be important, as persistent epithelial defects, which may in turn facilitate access of pathogens, predisposes the cornea to infectious keratitis.
4.7. Role of Plasmacytoid Dendritic Cells in Vasculature Integrity and Angiogenic Privilege
Significant role of infiltrating innate immune cells, in particular macrophages and cDCs, in promoting neovascularization has been appreciated for decades (Ribatti et al. 2009, Bruno et al. 2014). As mentioned above and illustrated in Fig. 4B, in our initial observations on the presence of pDCs in the conjunctiva and limbus, we reported that pDCs in a transgenic DPE-GFP×RAG1−/− mice with GFP-tagged pDCs (Iparraguirre et al. 2008) accompany limbal blood vessels at a high density compared to their densities in the conjunctiva and cornea (Jamali et al. 2020). Intravital multiphoton microscopy of GFP-tagged pDCs in the limbal region of the cornea demonstrates that during steady state (Supplementary Video 1) (Jamali et al. 2020) and in suture-induced neovascularized corneas, pDCs engulf limbal vessels (Supplementary Video 3). More detailed assessment of the pDCs shows that these cells are not statically residing by limbal vessels, rather they actively interact with the vasculature, for instance by extending their stellates around the newly-formed vessels (Supplementary Video 3; magnified region of interest in Supplementary Video 4). In addition to pDCs accompanying vessels, a fraction of pDCs also dynamically patrol intravascular spaces (Supplementary Video 5). Thus, collectively, our observations further suggest that pDCs may play a role regulating vasculature, in particular during dynamic process of inflammatory neovascularization, leading us to explore the hypothesis that pDCs, as another innate immune cell population, may also play a role angiogenesis.
In order to explore the potential role of pDCs in regulating neovascularization, we depleted pDCs locally in the cornea in transgenic BDCA-2-DTR mice. We reported that depletion of pDCs during steady state is accompanied by breakdown of angiogenic privilege (Jamali et al. 2016). Similarly, local depletion of pDCs, enhanced corneal neovascularization following suture placement (Jamali et al. 2016). Upon dissecting the molecular mechanisms through which pDCs may contribute to corneal angiogenic privilege and prevent corneal neovascularization induced by suture placement, it has been reported that pDCs secret a wide range of anti-angiogenic (angiostatic) molecules, including endostatin, thrombospondin (TSP)-1, platelet factor (PF)-4/CXCL4, and tissue inhibitor of metalloproteinase (TIMP)-3, and can thus inhibit endothelial cell proliferation in vitro (Jamali et al. 2016, Harris et al. 2018, Harris et al. 2019). Fig. 9 illustrates the significant role of pDCs in corneal angiogenic privilege.
Figure 9. Schematic illustration on the role of plasmacytoid dendritic cells in corneal angiogenic privilege.
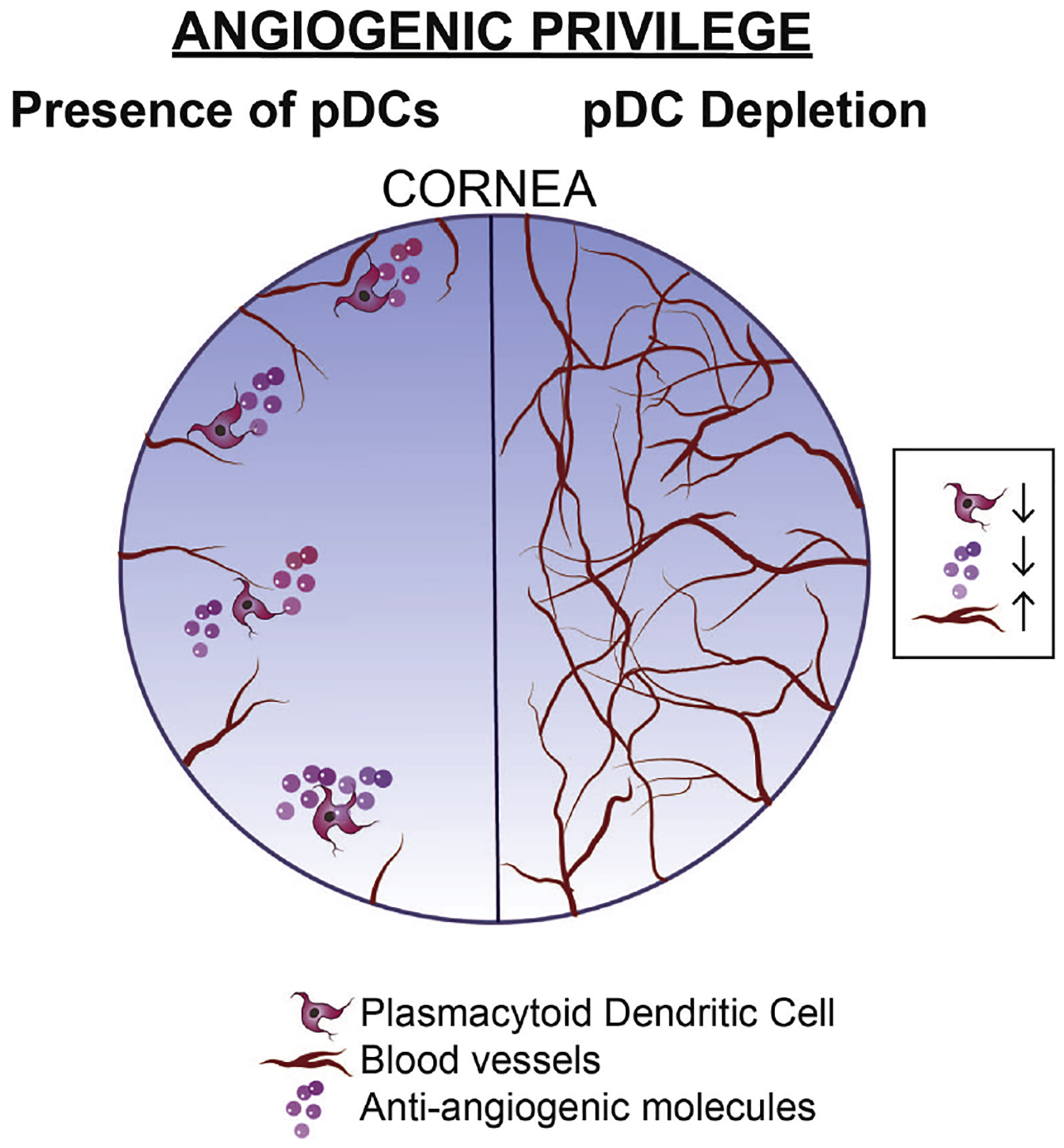
While during steady state cornea enjoys angiogenic privilege, local depletion of pDCs in the cornea is accompanied by decreased levels of anti-angiogenic molecules, leading to break down of corneal angiogenic privilege.
4.8. Therapeutic Potential of Plasmacytoid Dendritic Cells
Considering the tolerogenic, anti-inflammatory, anti-angiogenic, and neuroprotective properties of pDCs, it is worthwhile to assess their potential in treating various ocular conditions in which inflammation, neovascularization, or nerve degeneration play key roles in their pathogenesis. These conditions may range from neovascular ocular diseases, including age-related macular degeneration, retinopathy of prematurity, diabetic retinopathy, corneal traumas, to inflammatory diseases such as microbial keratitis, uveitis, and endophthalmitis, and neurodegenerative conditions such as neurotrophic keratopathy. Potential therapeutic use of pDCs can be examined via different approaches, including systemic or local adoptive transfer of syngeneic naïve or ex vivo-stimulated pDCs, or administration of their rich culture supernatant. In this regard, the potential efficacy of local adoptive transfer of naïve pDCs isolated from murine spleen in enhancing corneal nerve regeneration following nerve damage has been assessed, as has been their efficacy in preventing corneal neovascularization, and treating acute HSV-1 keratitis (Jamali et al. 2017, Sendra et al. 2017, Jamali et al. 2019).
In this regard, a technique for transferring small number of pDCs locally to the cornea has been recently proposed (manuscript under review). In this method, following isolating pDCs from spleen or bone marrow of mice, the central cornea epithelium is mechanically removed via an Algerbrush and pDCs are locally transferred to the cornea using a fibrin sealant. As depicted in Fig. 10, transferred pDCs can be detected in the cornea following the procedure, indicating feasibility of the procedure in transferring pDCs. To assess the significance of local adoptive transfer of pDCs in enhancing nerve regeneration, paracentral/central corneal nerves were severed by using a trephine and following debridement of central cornea, pDCs were adoptively transferred locally to the cornea using fibrin sealant. Local adoptive transfer of pDCs enhances NGF levels and corneal nerve regeneration compared with application of fibrin sealant without cells or adoptive transfer of CD11b+ myeloid cells (mainly containing cDCs and macrophages) control groups (Jamali et al. 2017). Similarly, adoptive transfer of splenic pDCs diminishes corneal neovascularization induced by suture placement compared with application of fibrin sealant without cells or adoptive transfer of CD11b+ myeloid cells (Jamali et al. 2019). Moreover, pre-loading the cornea with pDCs locally 24 h prior to inoculation of HSV-1, is accompanied by less corneal opacity and viral load by enhancing the levels of anti-viral IFN-α and anti-inflammatory TGF-β (Sendra et al. 2017). Collectively, these observations suggest that local adoptive transfer of pDCs can suppress sterile and infectious corneal diseases.
Figure 10. Representative confocal micrograph of whole-mounted cornea showing successful local adoptive transfer of plasmacytoid dendritic cells.
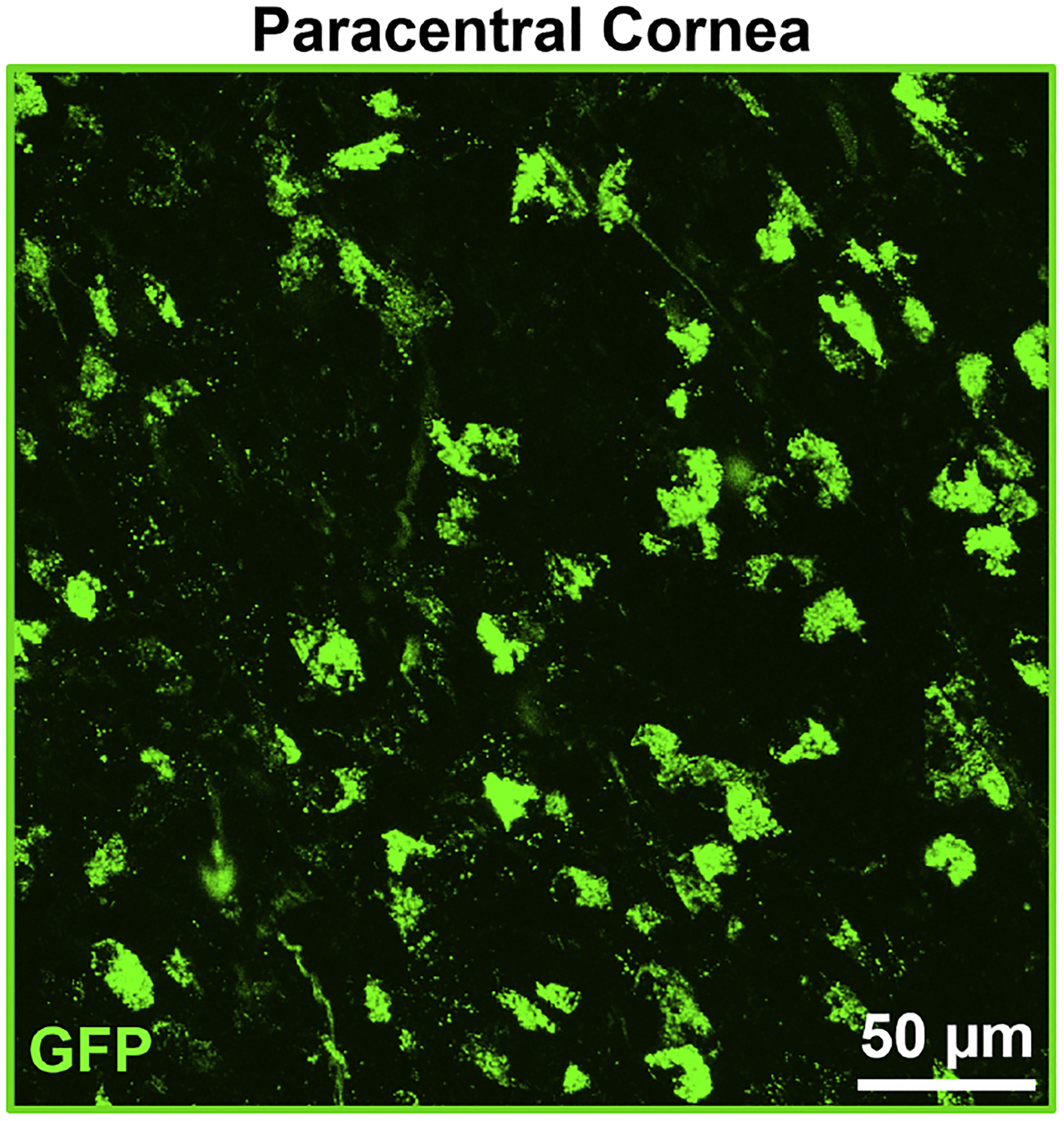
The figure illustrates a representative image of the paracentral cornea, 48 h following adoptive transfer of 10,000 GFP+ pDCs isolated from the spleen of DPE-GFP×RAG1−/− mouse. Scale bar: 50 μm.
Although trials in therapeutic application of pDCs to prevent corneal neovascularization and enhance corneal nerve regeneration in mice are promising, several concerns need to be considered in future studies. First, in these studies, the corneal epithelium was mechanically removed to facilitate migration of pDCs into the corneal stroma. Considering that mice do not have a Bowman’s layer, feasibility of locally transferring pDCs to the cornea might be more challenging for humans with thicker corneal epithelium and the presence of a protective Bowman’s layer as barriers. Similarly, feasibility of systemic or local adoptive transfer of pDCs to choroid and retina needs to be elucidated. More importantly, potential long-term side effects of systemic and local adoptive transfer of pDCs need to be carefully examined. While pDCs may show initial protective effects, considering their marked plasticity, it is not yet clear if adoptively transferred pDCs may alter their properties in the inflammatory microenvironment they are introduced to. This concern warrants further attention in the light of studies which show bone marrow pDCs may give rise to cDCs with pro-inflammatory properties during inflammation (Zuniga et al. 2004, Liou et al. 2008). Nevertheless, using blood or splenic pDCs that are shown to have less plastic capacity (Zuniga et al. 2004) may dampen this shortcoming. On the flip side, considering the tolerogenic property of pDCs, adoptively transferred pDCs may interfere with immune surveillance against tumors, facilitating development of primary tumors in ocular tissues or elsewhere, progression of cancers in remission, or development of secondary tumors.
5. Future Directions
5.1. Life Cycle of Plasmacytoid Dendritic Cells
Life cycle and longevity of pDCs is an aspect of their biology, which deserves detailed exploration, in particular in peripheral tissues during steady state. While our current knowledge suggests that pDCs leave the bone marrow following terminal development, they may alter their phenotype and may convert to cDCs under certain conditions in vitro and in vivo (Grouard et al. 1997, O’Keeffe et al. 2003, Zuniga et al. 2004, Liou et al. 2008). It is worthwhile to dissect the molecular signaling that derives such conversion and also it is interesting to evaluate if other immune cells, such as their closet counterparts, cDCs, may convert to pDCs during the steady state or following inflammatory stimuli such as viral challenges. Based on our current understandings, pDCs in secondary lymphoid tissues, such as in the spleen, have been shown to be long-lived, with low proliferative capacity judged by lower expression of proliferative marker, Ki-67 as well as BrdU incorporation, compared with cDCs (O’Keeffe et al. 2002, Liu et al. 2007). Further, it has been shown that upon irradiating one of the parabiont pairs, pDCs in the secondary lymphoid tissues are replenished from the other parabiont partner, suggesting contribution of blood-derived pDCs or pDC precursors in repopulating the pool of pDCs in the secondary lymphoid organs (Liu et al. 2007). Nevertheless, it needs to be elucidated if pDCs in peripheral tissues such as lung, kidney, and ocular tissues also follow a similar pattern. Further, our knowledge is limited about the longevity of the resident pDCs in the ocular tissues and if they repopulate these tissues from blood pool, in situ proliferation, or potential precursors residing in these tissues.
5.2. Molecular Regulation of Plasmacytoid Dendritic Cell Function
Considering that production of type I IFNs has been the major focus of the studies on pDCs for several decades, it is not surprising that our knowledge on regulation of pDC functions has been predisposed to unravel how pDCs receive danger signal to produce type I IFNs in various viral and bacterial infections, how their IFN production machinery is assembled, and how IFN secretion is mediated. Considering the clinical importance of various viral, bacterial, and parasitic infections of the conjunctiva, cornea, and retina and feasibility of clinical and pathological examinations on these tissues, future studies can use these ocular tissues to assess the molecular mechanisms through which pDCs are activated upon exposure to pathogens. Nevertheless, considering the recently explored versatile immune and non-immune functions of pDCs such as promoting Tregs, neuroprotection, and anti-angiogenic properties, it is of particular interest to evaluate how these functions are regulated and if such regulation is dependent or independent of pathways that mediate IFN production in pDCs.
One approach to explore potential molecules that may regulate pDCs relies on identifying cell surface and intracellular receptors that are expressed by pDCs. In this regard, one set of candidates are currently known pDC markers which are coupled with intracellular signaling molecules, such as Ly6C and Siglec-H in mice and BDCA-2 and ILT-7 in humans. Another set of candidates include receptors, which are expressed by other immune cells, in particular by innate immune cells more close to pDCs, such as their classical counterpart, cDCs and macrophages. In this regard, purinergic receptors might be of particular interest. To date, four receptors have been reported which bind to ATP and its derivatives with varying specificities and affinities and all have been widely studied on other immune cells. ATP is typically found at negligible concentrations within the extracellular space, however, upon cellular injury, ATP or its related catabolites leaks out into the extracellular milieu (Van Belle et al. 1987, Pedata et al. 2001). This serves as a potent danger signal that attracts and activates immune cells. Similar to immune cells, purinergic signaling within the central nervous system leads to activation and migration of microglia and promotes the release of neurotrophic factors from microglia and astrocytes. Interestingly, it is shown that freshly isolated peripheral blood pDCs express adenosine receptor A1 and upon ex vivo stimulation, these cells downregulate adenosine receptor A1 and instead express adenosine receptor A2a (Schnurr et al. 2004). Further, it has been shown that adenosine receptor A1 and A2a may differentially regulate pDCs, since stimulation of adenosine receptor A1 in freshly stimulated pDCs serves as a potent pDC chemoattractant, while signaling through adenosine receptor A2a in stimulated pDCs is coupled with reduced production of pro-inflammatory molecules IFN-α, IL-6, and IL-12 (Schnurr et al. 2004). Additionally, it has recently been shown that pDCs express members of ATP-gated P2X receptor cation channel family, namely, P2rx4 and P2rx7 and extracellular ATP signaling through P2rx7 may induce apoptosis in pDCs, another instance which suggests purinergic reporters may regulate pDC behavior (Furuta et al. 2017).
Another interesting set of receptors that may play a role in regulating pDC functions are receptors for neurotrophic molecules. Currently, it has been shown that pDCs express p75NTR, which can regulate functions of pDCs in asthma (Bandola et al. 2017). Interestingly, it has been shown that NGF can mediate several functions of pDCs through signaling through p75NTR. For instance, it was shown that NGF can increase allergen-specific T cell proliferation and cytokine secretion in patients with asthma, delay the onset of autoimmune diabetes and intensified graft-versus-host disease murine models (Bandola et al. 2017). In this regard, it is interesting to evaluate if other functions of pDCs in mediating immune responses to infectious diseases, promoting of Tregs and induction of tolerance to oral antigens and alloantigens, neurotrophic and anti-angiogenic properties of pDCs can be regulated by NGF or other neurotrophic molecules via signaling through p75NTR or other neurotrophic factor receptors.
5.3. Cellular Regulation of Plasmacytoid Dendritic Cell Function
Considering the complexity of in vivo interactions of cells in tissues, understanding the cellular players that regulate diverse and sometimes opposing functions of pDCs is as crucial as dissecting the signaling pathways that can regulate pDCs. Potentially cells that may alter pDC properties might be tissue-specific and vary among different tissues. For instance, while in the cornea subbasal nerves, epithelium, stromal keratocytes, and corneal immune cells may serve as potential cellular candidates that affect pDC behavior, in the limbus, choroid, and retina, vascular endothelial cells may mainly regulate pDCs since pDCs reside in close proximity to vasculature in these tissues.
Another important avenue is the exploration of potential crosstalk between pDCs and cellular members of the vascular system, such as vascular endothelial cells, pericytes, and vascular smooth muscle cells. As demonstrated above, pDCs in the limbus stably engulf vasculature and patrol intravascular spaces; it might be postulated that expression of certain chemokines by vasculature potently attracts pDCs and leads to special pDC-vessel arrangement observed in the limbus, choroid, and retina. It would be interesting to assess if such interactions are involved in particular pDC functions, for instance for entering blood/lymphatic system to deliver antigen to dLNs.
In summary, pDCs are the most recently identified immune cells in ocular tissues, which in addition to mediating immune response to pathogens, may contribute to several aspects of ocular tissue homeostasis including preserving ocular immune privilege, nerve maintenance and function, as well as regulating the ocular vasculature. Future studies are paramount to evaluate their biology and their role in various ocular conditions ranging from infectious or non-infectious ocular diseases such as conjunctivitis, keratitis, and uveitis to vascular diseases such as corneal neovascularization, diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration.
Supplementary Material
Supplementary Video 1. Multiphoton microscopy of normal limbus of transgenic DPE-GFP×RAG1−/− mouse. Representative 3D reconstructed multiphoton microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) during steady state. The video strategic localization of pDCs in intimate proximity to limbal vessels.
Supplementary Video 2. Confocal microcopy of naïve whole-mounted cornea indicating close proximity of plasmacytoid dendritic cells and corneal nerves. Representative 3D reconstructed confocal micrograph of a corneal whole-mount, stained for pan-immune cell marker, CD45 (red), pDC marker, PDCA-1 (green), and nerve marker (βIII-tubulin), showing close proximity of resident corneal pDCs and subbasal nerve plexus during steady state.
Supplementary Video 3. Multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video demonstrates interaction of pDCs with limbal vessels and alterations in the morphology of pDCs following suture placement. Further the video highlights that pDCs engulf the vessels and actively extend their dendrites over the vessels.
Supplementary Video 4. Magnified multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Magnified representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video highlights pDCs extending their dendrites over the vessels.
Supplementary Video 5. Multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video highlights patrolling movements of pDCs in limbal vessels and their morphologic alterations following induction of neovascularization by suture placement.
Highlights.
The cornea, limbus, conjunctiva, choroid, retina, and lacrimal glands are endowed with resident plasmacytoid dendritic cells.
Corneal plasmacytoid dendritic cells secret type I interferons during herpes simplex virus-1 keratitis and limit viral propagation, dissemination to the corneal stroma, draining lymph nodes, and trigeminal ganglion. They also prevent re-programing of Tregs to effector ex-Tregs.
Corneal plasmacytoid dendritic cells promote graft survival by inhibiting effector Th1 cells in the draining lymph nodes after corneal transplantation.
Corneal plasmacytoid dendritic cells are pivotal for corneal nerve maintenance and function through secretion of neurotrophic molecules.
Resident plasmacytoid dendritic cells, which accompany limbal vessels, produce anti-angiogenic molecules and contribute to corneal angiogenic privilege.
Financial Support:
NIH R01-EY022695 (PH), NIH R01-EY026963 (PH), NIH R01-EY029602 (PH), NIH R21-EY025393 (PH), Eversight Eye and Vision Research Grant (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Eye Bank Association of America (AJ), Research to Prevent Blindness Challenge Grant, Tufts Medical Center Institutional Support
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abb J, et al. (1983). “Phenotype of human alpha-interferon producing leucocytes identified by monoclonal antibodies.” Clin Exp Immunol 52(1): 179–184. [PMC free article] [PubMed] [Google Scholar]
- Abe M, et al. (2005). “Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival.” Am J Transplant 5(8): 1808–1819. [DOI] [PubMed] [Google Scholar]
- Abou-Slaybi A, et al. (2019). “Analysis of leukocyte populations and nerves in developing murine corneas.” The Journal of Immunology 202(1 Supplement): 117.115–117.115. [Google Scholar]
- Abtin A, et al. (2014). “Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection.” Nat Immunol 15(1): 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal T, et al. (2009). “Recruitment of myeloid and plasmacytoid dendritic cells in cervical mucosa during Chlamydia trachomatis infection.” Clin Microbiol Infect 15(1): 50–59. [DOI] [PubMed] [Google Scholar]
- Ainola M, et al. (2018). “Activation of plasmacytoid dendritic cells by apoptotic particles - mechanism for the loss of immunological tolerance in Sjogren’s syndrome.” Clin Exp Immunol 191(3): 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aqaba MA, et al. (2019). “Corneal nerves in health and disease.” Prog Retin Eye Res 73: 100762. [DOI] [PubMed] [Google Scholar]
- Al-Aqaba MA, et al. (2010). “Architecture and distribution of human corneal nerves.” Br J Ophthalmol 94(6): 784–789. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, et al. (1978). “Number of inflammatory cells in the normal conjunctiva.” Am J Ophthalmol 86(2): 250–259. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, et al. (1987). “The immune response of the lacrimal gland to antigenic exposure.” Curr Eye Res 6(7): 921–927. [DOI] [PubMed] [Google Scholar]
- Allman D, et al. (2006). “Ikaros is required for plasmacytoid dendritic cell differentiation.” Blood 108(13): 4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouzegar A, et al. (2016). “Alloimmunity and Tolerance in Corneal Transplantation.” J Immunol 196(10): 3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki-Yoshida A, et al. (2016). “Enhancement of Oral Tolerance Induction in DO11.10 Mice by Lactobacillus gasseri OLL2809 via Increase of Effector Regulatory T Cells.” PLoS One 11(7): e0158643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C, et al. (2013). “Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL.” Cancer Immunol Res 1(6): 402–415. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, et al. (2001). “Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology.” Nat Immunol 2(12): 1144–1150. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, et al. (2003). “Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody.” J Immunol 171(12): 6466–6477. [DOI] [PubMed] [Google Scholar]
- Baban B, et al. (2015). “Dendritic cells-mediated polarization of retinal macrophages in human diabetic retina.” Investigative Ophthalmology & Visual Science 56(7): 4291–4291. [Google Scholar]
- Backer V, et al. (2017). “Knockdown of myeloid cell hypoxia-inducible factor-1alpha ameliorates the acute pathology in DSS-induced colitis.” PLoS One 12(12): e0190074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley SM, et al. (1995). “Mast cell distribution and neutral protease expression in acute and chronic allergic conjunctivitis.” Clin Exp Allergy 25(1): 41–50. [DOI] [PubMed] [Google Scholar]
- Bagri A, et al. (2002). “Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance.” Adv Exp Med Biol 515: 13–31. [PubMed] [Google Scholar]
- Baharom F, et al. (2017). “Human Lung Dendritic Cells: Spatial Distribution and Phenotypic Identification in Endobronchial Biopsies Using Immunohistochemistry and Flow Cytometry.” J Vis Exp(119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, et al. (2018). “Origin, Differentiation, and Function of Intestinal Macrophages.” Front Immunol 9: 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAïZ H, et al. (2012). “Granulomatous conjunctivitis associated with chronic blepharitis in children.” 90. [Google Scholar]
- Balzarolo M, et al. (2012). “The transcriptional regulator NAB2 reveals a two-step induction of TRAIL in activated plasmacytoid DCs.” Eur J Immunol 42(11): 3019–3027. [DOI] [PubMed] [Google Scholar]
- Banchereau J, et al. (2006). “Type I interferon in systemic lupus erythematosus and other autoimmune diseases.” Immunity 25(3): 383–392. [DOI] [PubMed] [Google Scholar]
- Bandola J, et al. (2017). “Neurotrophin Receptor p75NTR Regulates Immune Function of Plasmacytoid Dendritic Cells.” Front Immunol 8: 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchet W, et al. (2005). “Plasmacytoid dendritic cells--virus experts of innate immunity.” Semin Immunol 17(4): 253–261. [DOI] [PubMed] [Google Scholar]
- Barra L, et al. (2011). “Lack of seroconversion of rheumatoid factor and anti-cyclic citrullinated peptide in patients with early inflammatory arthritis: a systematic literature review.” Rheumatology (Oxford) 50(2): 311–316. [DOI] [PubMed] [Google Scholar]
- Barron MA, et al. (2003). “Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals.” J Infect Dis 187(1): 26–37. [DOI] [PubMed] [Google Scholar]
- Battaglia A, et al. (2008). “Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer.” Immunology 123(1): 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, et al. (1988). “HLA DR and DQ distribution in normal human ocular structures.” Curr Eye Res 7(9): 903–911. [DOI] [PubMed] [Google Scholar]
- Bekeredjian-Ding I, et al. (2009). “Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha.” Immunology 128(3): 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellner L, et al. (2015). “Heme oxygenase-2 deletion impairs macrophage function: implication in wound healing.” Faseb j 29(1): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, et al. (2017). “TFOS DEWS II pain and sensation report.” Ocul Surf 15(3): 404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendriss-Vermare N, et al. (2001). “Human thymus contains IFN-alpha-producing CD11c(−), myeloid CD11c(+), and mature interdigitating dendritic cells.” J Clin Invest 107(7): 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan AK, et al. (1982). “T-cell subsets and Langerhans cells in normal and diseased conjunctiva.” Am J Ophthalmol 94(2): 205–212. [DOI] [PubMed] [Google Scholar]
- Bhela S, et al. (2017). “The Plasticity and Stability of Regulatory T Cells during Viral-Induced Inflammatory Lesions.” J Immunol 199(4): 1342–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki E, et al. (2011). “Spleen-resident CD4+ and CD4− CD8α- dendritic cell subsets differ in their ability to prime invariant natural killer T lymphocytes.” PLoS One 6(10): e26919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian F, et al. (2019). “Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells.” Mucosal Immunol 12(4): 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsborough J, et al. (2003). “Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentation and support function of T cells with regulatory properties.” Immunology 109: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck P (2001). “Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice.” Blood 98(13): 3520–3526. [DOI] [PubMed] [Google Scholar]
- Bjorck P, et al. (2005). “Promotion of long-term heart allograft survival by combination of mobilized donor plasmacytoid dendritic cells and anti-CD154 monoclonal antibody.” J Heart Lung Transplant 24(8): 1118–1120. [DOI] [PubMed] [Google Scholar]
- Blanco T, et al. (2017). “Plasmacytoid Dendritic Cells in the Mouse Cornea: a Multiphoton Intravital Microscopy Study.” Investigative Ophthalmology & Visual Science 58(8): 980–980. [Google Scholar]
- Blasius A, et al. (2004). “A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha.” Blood 103(11): 4201–4206. [DOI] [PubMed] [Google Scholar]
- Blasius AL, et al. (2010). “Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells.” Proc Natl Acad Sci U S A 107(46): 19973–19978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, et al. (2007). “Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells.” J Exp Med 204(11): 2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, et al. (2006). “Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12.” Blood 107(6): 2474–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, et al. (2006). “Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation.” J Immunol 177(5): 3260–3265. [DOI] [PubMed] [Google Scholar]
- Bles N, et al. (2007). “Gene expression profiling defines ATP as a key regulator of human dendritic cell functions.” J Immunol 179(6): 3550–3558. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, et al. (1998). “Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s.” J Exp Med 187(1): 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S, et al. (2000). “Topical treatment with nerve growth factor for neurotrophic keratitis.” Ophthalmology 107(7): 1347–1351; discussion 1351–1342. [DOI] [PubMed] [Google Scholar]
- Bonini S, et al. (2003). “Neurotrophic keratitis.” Eye (Lond) 17(8): 989–995. [DOI] [PubMed] [Google Scholar]
- Boonstra A, et al. (2003). “Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation.” J Exp Med 197(1): 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor PPC, et al. (2019). “Characterization of Antigen-Presenting Cell Subsets in Human Liver-Draining Lymph Nodes.” Front Immunol 10: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand P, et al. (2002). “Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs.” J Immunol 169(12): 6711–6719. [DOI] [PubMed] [Google Scholar]
- Breton G, et al. (2016). “Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs.” J Exp Med 213(13): 2861–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz CA, et al. (2018). “Erythropoietin Signaling Increases Choroidal Macrophages and Cytokine Expression, and Exacerbates Choroidal Neovascularization.” Sci Rep 8(1): 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette-Storkus CS, et al. (2002). “Identification of a novel macrophage population in the normal mouse corneal stroma.” Invest Ophthalmol Vis Sci 43(7): 2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Brown KN, et al. (2009). “Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection.” PLoS Pathog 5(5): e1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchhage KL, et al. (2018). “IL-10 in the microenvironment of HNSCC inhibits the CpG ODN induced IFN-alpha secretion of pDCs.” Oncol Lett 15(3): 3985–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder D, et al. (2004). “Neuropilin-1: a surface marker of regulatory T cells.” Eur J Immunol 34(3): 623–630. [DOI] [PubMed] [Google Scholar]
- Bruno A, et al. (2014). “Orchestration of angiogenesis by immune cells.” Front Oncol 4: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buela KA, et al. (2015). “Cornea-infiltrating and lymph node dendritic cells contribute to CD4+ T cell expansion after herpes simplex virus-1 ocular infection.” J Immunol 194(1): 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelens C, et al. (2002). “Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties.” Blood 99(3): 993–998. [DOI] [PubMed] [Google Scholar]
- Butler TL, et al. (1996). “Resident and infiltrating immune cells in the uveal tract in the early and late stages of experimental autoimmune uveoretinitis.” Invest Ophthalmol Vis Sci 37(11): 2195–2210. [PubMed] [Google Scholar]
- Cao W, et al. (2006). “Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production.” J Exp Med 203(6): 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitini CM, et al. (2014). “Absence of STAT1 in donor-derived plasmacytoid dendritic cells results in increased STAT3 and attenuates murine GVHD.” Blood 124(12): 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotta S, et al. (2010). “The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner.” Immunity 32(5): 628–641. [DOI] [PubMed] [Google Scholar]
- Carrer A, et al. (2012). “Neuropilin-1 identifies a subset of bone marrow Gr1- monocytes that can induce tumor vessel normalization and inhibit tumor growth.” Cancer Res 72(24): 6371–6381. [DOI] [PubMed] [Google Scholar]
- Castellaneta A, et al. (2004). “Identification and characterization of intestinal Peyer’s patch interferon-alpha producing (plasmacytoid) dendritic cells.” Hum Immunol 65(2): 104–113. [DOI] [PubMed] [Google Scholar]
- Castellino F, et al. (2006). “Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction.” Nature 440(7086): 890–895. [DOI] [PubMed] [Google Scholar]
- Cella M, et al. (1997). “A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing.” J Exp Med 185(10): 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, et al. (1999). “Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon.” Nat Med 5(8): 919–923. [DOI] [PubMed] [Google Scholar]
- Chandler JW, et al. (1985). “Presence of Langerhans cells in the central corneas of normal human infants.” Invest Ophthalmol Vis Sci 26(1): 113–116. [PubMed] [Google Scholar]
- Chaudhary B, et al. (2014). “Neuropilin 1: function and therapeutic potential in cancer.” Cancer Immunol Immunother 63(2): 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J, et al. (1989). “Dendritic cells and IFN-alpha-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood.” Immunology 68(4): 486–490. [PMC free article] [PubMed] [Google Scholar]
- Cherepanoff S, et al. (2010). “Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration.” Br J Ophthalmol 94(7): 918–925. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, et al. (2007). “The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium.” Invest Ophthalmol Vis Sci 48(4): 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, et al. (2014). “Plasmacytoid dendritic cells: development, functions, and role in atherosclerotic inflammation.” Front Physiol 5: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, et al. (2008). “SAGE library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type I IFN production.” Int Immunol 20(1): 155–164. [DOI] [PubMed] [Google Scholar]
- Choi EY, et al. (2017). “Langerhans cells prevent subbasal nerve damage and upregulate neurotrophic factors in dry eye disease.” PLoS One 12(4): e0176153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, et al. (2010). “Enumeration and phenotypic assessment of human plasmacytoid and myeloid dendritic cells in whole blood.” Cytometry A 77(4): 328–337. [DOI] [PubMed] [Google Scholar]
- Cisse B, et al. (2008). “Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development.” Cell 135(1): 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, et al. (2003). “Origin and subset distribution of peripheral blood dendritic cells in patients with chronic graft-versus-host disease.” Transplantation 75(2): 221–225. [DOI] [PubMed] [Google Scholar]
- Coates PT, et al. (2003). “Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand.” Blood 102(7): 2513–2521. [DOI] [PubMed] [Google Scholar]
- Coates PT, et al. (2004). “In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival.” Transplantation 77(7): 1080–1089. [DOI] [PubMed] [Google Scholar]
- Coccia EM, et al. (2004). “Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells.” Eur J Immunol 34(3): 796–805. [DOI] [PubMed] [Google Scholar]
- Cocka LJ, et al. (2012). “Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities.” PLoS Pathog 8(9): e1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, et al. (2018). “Human dendritic cell subsets: an update.” Immunology 154(1): 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, et al. (2004). “Plasmacytoid dendritic cells in immunity.” Nat Immunol 5(12): 1219–1226. [DOI] [PubMed] [Google Scholar]
- Conrad C, et al. (2012). “Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells.” Cancer Res 72(20): 5240–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor N, et al. (2007). “Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype.” J Immunol 179(5): 2690–2694. [DOI] [PubMed] [Google Scholar]
- Cooles FAH, et al. (2018). “Phenotypic and Transcriptomic Analysis of Peripheral Blood Plasmacytoid and Conventional Dendritic Cells in Early Drug Naive Rheumatoid Arthritis.” Front Immunol 9: 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K, et al. (2005). “Plasmacytoid dendritic cells (PDC) are the major DC subset innately producing cytokines in human lymph nodes.” J Leukoc Biol 78(5): 1142–1152. [DOI] [PubMed] [Google Scholar]
- Cravens PD, et al. (2007). “Human peripheral blood dendritic cells and monocyte subsets display similar chemokine receptor expression profiles with differential migratory responses.” Scand J Immunol 65(6): 514–524. [DOI] [PubMed] [Google Scholar]
- Cruzat A, et al. (2017). “In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease.” Ocul Surf 15(1): 15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, et al. (2011). “Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis.” Invest Ophthalmol Vis Sci 52(8): 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, et al. (1996). “The ordered array of perivascular macrophages is disrupted by IL-1-induced inflammation in the rabbit retina.” Glia 17(4): 307–316. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, et al. (2004). “VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment.” J Clin Invest 113(7): 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, et al. (2015). “Neuropeptide FF Promotes Recovery of Corneal Nerve Injury Associated With Hyperglycemia.” Invest Ophthalmol Vis Sci 56(13): 7754–7765. [DOI] [PubMed] [Google Scholar]
- Daniele S, et al. (1992). “Treatment of persistent epithelial defects in neurotrophic keratitis with epidermal growth factor: a preliminary open study.” Graefes Arch Clin Exp Ophthalmol 230(4): 314–317. [DOI] [PubMed] [Google Scholar]
- Darougar S, et al. (1985). “Epidemiological and clinical features of primary herpes simplex virus ocular infection.” Br J Ophthalmol 69(1): 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi MH, et al. (2009). “Corneal nerve alterations in dry eye-associated ocular surface disease.” Int Ophthalmol Clin 49(1): 11–20. [DOI] [PubMed] [Google Scholar]
- Davidson S, et al. (2011). “Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway.” J Immunol 186(10): 5938–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer HJ, et al. (2004). “Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen.” J Exp Med 200(1): 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paulis A, et al. (2006). “Expression and functions of the vascular endothelial growth factors and their receptors in human basophils.” J Immunol 177(10): 7322–7331. [DOI] [PubMed] [Google Scholar]
- Decalf J, et al. (2007). “Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients.” J Exp Med 204(10): 2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, et al. (2005). “Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo.” J Leukoc Biol 78(6): 1327–1338. [DOI] [PubMed] [Google Scholar]
- Demedts IK, et al. (2005). “Identification and characterization of human pulmonary dendritic cells.” Am J Respir Cell Mol Biol 32(3): 177–184. [DOI] [PubMed] [Google Scholar]
- Dey M, et al. (2015). “Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma.” J Immunol 195(1): 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Araya CM, et al. (1995). “Development of microglial topography in human retina.” J Comp Neurol 363(1): 53–68. [DOI] [PubMed] [Google Scholar]
- Donaghy H, et al. (2009). “Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection.” J Virol 83(4): 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy H, et al. (2001). “Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load.” Blood 98(8): 2574–2576. [DOI] [PubMed] [Google Scholar]
- Donnenberg VS, et al. (2003). “Identification, rare-event detection and analysis of dendritic cell subsets in broncho-alveolar lavage fluid and peripheral blood by flow cytometry.” Front Biosci 8: s1175–1180. [DOI] [PubMed] [Google Scholar]
- Dorner T, et al. (2019). “Novel paradigms in systemic lupus erythematosus.” Lancet 393(10188): 2344–2358. [DOI] [PubMed] [Google Scholar]
- Doyle EH, et al. (2019). “Individual liver plasmacytoid dendritic cells are capable of producing IFNalpha and multiple additional cytokines during chronic HCV infection.” PLoS Pathog 15(7): e1007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dress RJ, et al. (2019). “Plasmacytoid dendritic cells develop from Ly6D(+) lymphoid progenitors distinct from the myeloid lineage.” Nat Immunol 20(7): 852–864. [DOI] [PubMed] [Google Scholar]
- Drobits B, et al. (2012). “Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells.” J Clin Invest 122(2): 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, et al. (2009). “Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance.” Gastroenterology 137(3): 1019–1028. [DOI] [PubMed] [Google Scholar]
- Dunmire SK, et al. (2015). “The Incubation Period of Primary Epstein-Barr Virus Infection: Viral Dynamics and Immunologic Events.” PLoS Pathog 11(12): e1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionek A, et al. (2000). “BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood.” J Immunol 165(11): 6037–6046. [DOI] [PubMed] [Google Scholar]
- Dzionek A, et al. (2001). “BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction.” J Exp Med 194(12): 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, et al. (2003). “Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines.” Eur J Immunol 33(4): 827–833. [DOI] [PubMed] [Google Scholar]
- Facchetti F, et al. (1988). “Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin.” Am J Pathol 133(1): 15–21. [PMC free article] [PubMed] [Google Scholar]
- Facchetti F, et al. (1989). “Plasmacytoid monocytes (so-called plasmacytoid T cells) in Hodgkin’s disease.” J Pathol 158(1): 57–65. [DOI] [PubMed] [Google Scholar]
- Fagerberg L, et al. (2014). “Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics.” Mol Cell Proteomics 13(2): 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget J, et al. (2012). “ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells.” Cancer Res 72(23): 6130–6141. [DOI] [PubMed] [Google Scholar]
- Fantin A, et al. (2010). “Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction.” Blood 116(5): 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, et al. (1990). “Sequential enrichment and immunocytochemical visualization of human interferon-alpha-producing cells.” J Interferon Res 10(4): 435–446. [DOI] [PubMed] [Google Scholar]
- Feller AC, et al. (1983). “Immunohistology and aetiology of histiocytic necrotizing lymphadenitis. Report of three instructive cases.” Histopathology 7(6): 825–839. [DOI] [PubMed] [Google Scholar]
- Ferbas JJ, et al. (1994). “CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection.” J Immunol 152(9): 4649–4662. [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, et al. (1988). “Human mononuclear cells which produce interferon-alpha during NK(HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors.” J Leukoc Biol 43(4): 323–334. [DOI] [PubMed] [Google Scholar]
- Fix AS, et al. (1989). “Conjunctiva-associated lymphoid tissue (CALT) in normal and Bordetella avium-infected turkeys.” Vet Pathol 26(3): 222–230. [DOI] [PubMed] [Google Scholar]
- Fleeton M, et al. (2004). “Involvement of dendritic cell subsets in the induction of oral tolerance and immunity.” Ann N Y Acad Sci 1029: 60–65. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, et al. (1993). “Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family.” J Immunol 151(5): 2399–2408. [PubMed] [Google Scholar]
- Forrester JV (2009). “Privilege revisited: an evaluation of the eye’s defence mechanisms.” Eye (Lond) 23(4): 756–766. [DOI] [PubMed] [Google Scholar]
- Forrester JV, et al. (2005). “Choroidal dendritic cells require activation to present antigen and resident choroidal macrophages potentiate this response.” Br J Ophthalmol 89(3): 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester JV, et al. (1994). “Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye: implications for induction of autoimmune uveoretinitis.” Invest Ophthalmol Vis Sci 35: 64–77. [PubMed] [Google Scholar]
- Franklin RM, et al. (1984). “Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system.” Invest Ophthalmol Vis Sci 25(2): 181–187. [PubMed] [Google Scholar]
- Fukushima A, et al. (2010). “Participation of CD11b and F4/80 molecules in the conjunctival eosinophilia of experimental allergic conjunctivitis.” Int Arch Allergy Immunol 151(2): 129–136. [DOI] [PubMed] [Google Scholar]
- Furie R, et al. (2017). “Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus.” Arthritis Rheumatol 69(2): 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie R, et al. (2019). “Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus.” J Clin Invest 129(3): 1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, et al. (2017). “E-NPP3 controls plasmacytoid dendritic cell numbers in the small intestine.” PLoS One 12(2): e0172509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, et al. (2016). “Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea.” Sci Rep 6: 36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, et al. (2016). “Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea.” J Clin Invest 126(5): 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, et al. (2009). “Dynamic accumulation of plasmacytoid dendritic cells in lymph nodes is regulated by interferon-beta.” Blood 114(13): 2623–2631. [DOI] [PubMed] [Google Scholar]
- Gardet A, et al. (2019). “Effect of in vivo Hydroxychloroquine and ex vivo Anti-BDCA2 mAb Treatment on pDC IFNalpha Production From Patients Affected With Cutaneous Lupus Erythematosus.” Front Immunol 10: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlini G, et al. (2006). “Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions.” J Invest Dermatol 126(2): 507–509. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, et al. (2008). “Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells.” J Exp Med 205(7): 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez D, et al. (2006). “Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry.” J Virol 80(14): 6844–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, et al. (2014). “ETO family protein Mtg16 regulates the balance of dendritic cell subsets by repressing Id2.” J Exp Med 211(8): 1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh HS, et al. (2010). “Continuous expression of the transcription factor e2–2 maintains the cell fate of mature plasmacytoid dendritic cells.” Immunity 33(6): 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini C, et al. (2014). “Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: an overview of two common animal models of corneal neovascularization.” Exp Eye Res 121: 1–4. [DOI] [PubMed] [Google Scholar]
- Gilliet M, et al. (2002). “Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells.” J Exp Med 195(6): 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez F, et al. (2013). “Pathogenesis of herpes stromal keratitis--a focus on corneal neovascularization.” Prog Retin Eye Res 33: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes JA, et al. (1997). “Phenotypic analysis of resident lymphoid cells in the conjunctiva and adnexal tissues of rat.” Exp Eye Res 64(6): 991–997. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, et al. (2006). “Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome.” Proc Natl Acad Sci U S A 103(8): 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubier A, et al. (2008). “Plasmacytoid dendritic cells mediate oral tolerance.” Immunity 29(3): 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grage-Griebenow E, et al. (2007). “Anti-BDCA-4 (neuropilin-1) antibody can suppress virus-induced IFN-alpha production of plasmacytoid dendritic cells.” Immunol Cell Biol 85(5): 383–390. [DOI] [PubMed] [Google Scholar]
- Grajkowska LT, et al. (2017). “Isoform-Specific Expression and Feedback Regulation of E Protein TCF4 Control Dendritic Cell Lineage Specification.” Immunity 46(1): 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson DS, et al. (2003). “CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40.” Invest Ophthalmol Vis Sci 44(7): 3083–3093. [DOI] [PubMed] [Google Scholar]
- Grégoire S, et al. (2016). “Treatment of Uveitis by In Situ Administration of Ex Vivo-Activated Polyclonal Regulatory T Cells.” J Immunol 196(5): 2109–2118. [DOI] [PubMed] [Google Scholar]
- Grouard G, et al. (1997). “The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand.” J Exp Med 185(6): 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery L, et al. (2014). “Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression.” Cancer Res 74(22): 6430–6440. [DOI] [PubMed] [Google Scholar]
- Gupta A, et al. (2017). “Novel Characterization of Resident Plasmacytoid Dendritic Cells in the Retina and Choroid.” Investigative Ophthalmology & Visual Science 58(8): 5377–5377. [Google Scholar]
- Hadeiba H, et al. (2008). “CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease.” Nat Immunol 9(11): 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, et al. (2013). “Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study.” Ophthalmology 120(1): 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, et al. (2013). “Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study.” Ophthalmology 120(1): 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, et al. (2010). “Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study.” Ophthalmology 117(10): 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, et al. (2003). “Corneal immunity is mediated by heterogeneous population of antigen-presenting cells.” J Leukoc Biol 74(2): 172–178. [DOI] [PubMed] [Google Scholar]
- Hamrah P, et al. (2003). “Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation.” Arch Ophthalmol 121(8): 1132–1140. [DOI] [PubMed] [Google Scholar]
- Hamrah P, et al. (2003). “The corneal stroma is endowed with a significant number of resident dendritic cells.” Invest Ophthalmol Vis Sci 44(2): 581–589. [DOI] [PubMed] [Google Scholar]
- Hamrah P, et al. (2016). “Translational Immunoimaging and Neuroimaging Demonstrate Corneal Neuroimmune Crosstalk.” Cornea 35 Suppl 1: S20–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, et al. (2002). “Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells.” Invest Ophthalmol Vis Sci 43(3): 639–646. [PubMed] [Google Scholar]
- Harris DL, et al. (2018). “Plasmacytoid Dendritic Cells and Their Role in Vascular Endothelial Cell Proliferation and Differentiation In Vitro.” Investigative Ophthalmology & Visual Science 59(9): 3340–3340.30025076 [Google Scholar]
- Harris DL, et al. (2019). “Plasmacytoid Dendritic Cells Inhibit Vascular Endothelial Cell Proliferation and Differentiation through the Angiostatic Molecule Platelet Factor-4.” Investigative Ophthalmology & Visual Science 60(9): 951–951. [Google Scholar]
- Harris NL, et al. (1987). ““Plasmacytoid T cells” in Castleman’s disease. Immunohistologic phenotype.” Am J Surg Pathol 11(2): 109–113. [DOI] [PubMed] [Google Scholar]
- Hartmann E, et al. (2003). “Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer.” Cancer Res 63(19): 6478–6487. [PubMed] [Google Scholar]
- Hasan U, et al. (2005). “Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88.” J Immunol 174(5): 2942–2950. [DOI] [PubMed] [Google Scholar]
- Hassan M, et al. (2017). “Reduced GvHD in Recipients of BM Derived Versus G-CSF Mobilized Plasmacytoid Dendritic Cells: Role of Inducible IL-12.” Blood 130(Supplement 1): 4431–4431. [Google Scholar]
- Hassan M, et al. (2019). “Flt3L Treatment of Bone Marrow Donors Increases Graft Plasmacytoid Dendritic Cell Content and Improves Allogeneic Transplantation Outcomes.” Biol Blood Marrow Transplant 25(6): 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, et al. (2015). “The PEDF Neuroprotective Domain Plus DHA Induces Corneal Nerve Regeneration After Experimental Surgery.” Invest Ophthalmol Vis Sci 56(6): 3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JC, et al. (2011). “Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens.” AIDS Res Hum Retroviruses 27(10): 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog Y, et al. (2001). “Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins.” Mech Dev 109(1): 115–119. [DOI] [PubMed] [Google Scholar]
- Hillen MR, et al. (2019). “Dysregulated miRNome of plasmacytoid dendritic cells from patients with Sjogren’s syndrome is associated with processes at the centre of their function.” Rheumatology (Oxford). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen MR, et al. (2019). “Plasmacytoid DCs From Patients With Sjogren’s Syndrome Are Transcriptionally Primed for Enhanced Pro-inflammatory Cytokine Production.” Front Immunol 10: 2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani M, et al. (1997). “Characterisation of the normal conjunctival leukocyte population.” Exp Eye Res 64(6): 905–912. [DOI] [PubMed] [Google Scholar]
- Hoffmann HJ, et al. (2006). “Response of respiratory flour allergics in an ingested flour challenge may involve plasmacytoid dendritic cells, CD25+ and CD152+ T cells.” Int Arch Allergy Immunol 140(3): 252–260. [DOI] [PubMed] [Google Scholar]
- Holmgren AM, et al. (2015). “Bst2/Tetherin Is Induced in Neurons by Type I Interferon and Viral Infection but Is Dispensable for Protection against Neurotropic Viral Challenge.” J Virol 89(21): 11011–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori J (2008). “Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation.” J Ocul Biol Dis Infor 1(2–4): 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori J, et al. (2010). “Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye.” Ocul Immunol Inflamm 18(5): 325–333. [DOI] [PubMed] [Google Scholar]
- Hori J, et al. (2019). “Immune privilege in corneal transplantation.” Prog Retin Eye Res 72: 100758. [DOI] [PubMed] [Google Scholar]
- Hornung V, et al. (2002). “Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides.” J Immunol 168(9): 4531–4537. [DOI] [PubMed] [Google Scholar]
- Horny HP, et al. (1987). “Immunocytology of plasmacytoid T cells: marker analysis indicates a unique phenotype of this enigmatic cell.” Hum Pathol 18(1): 28–32. [DOI] [PubMed] [Google Scholar]
- Hosoba S, et al. (2014). “Chemokine and lymph node homing receptor expression on pDC vary by graft source.” Oncoimmunology 3(10): e958957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotter D, et al. (2013). “Emerging role of the host restriction factor tetherin in viral immune sensing.” J Mol Biol 425(24): 4956–4964. [DOI] [PubMed] [Google Scholar]
- Hu K, et al. (2013). “The role of corneal Plasmacytoid Dendritic Cells in acute herpes simplex virus infection.” Investigative Ophthalmology & Visual Science 54(15): 2158–2158. [Google Scholar]
- Hu K, et al. (2015). “A Dual Role for Corneal Dendritic Cells in Herpes Simplex Keratitis: Local Suppression of Corneal Damage and Promotion of Systemic Viral Dissemination.” PLoS One 10(9): e0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, et al. (2016). “Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis.” Invest Ophthalmol Vis Sci 57(3): 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XM, et al. (2014). “Role of plasmacytoid dendritic cells and inducible costimulator-positive regulatory T cells in the immunosuppression microenvironment of gastric cancer.” Cancer Sci 105(2): 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert FX, et al. (2004). “Rat plasmacytoid dendritic cells are an abundant subset of MHC class II+ CD4+CD11b-OX62- and type I IFN-producing cells that exhibit selective expression of Toll-like receptors 7 and 9 and strong responsiveness to CpG.” J Immunol 172(12): 7485–7494. [DOI] [PubMed] [Google Scholar]
- Huch JH, et al. (2010). “Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection.” J Virol 84(8): 4060–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA, et al. (1983). “Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers.” J Cell Biol 97(1): 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iparraguirre A, et al. (2008). “Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide.” J Leukoc Biol 83(3): 610–620. [DOI] [PubMed] [Google Scholar]
- Ippolito GC, et al. (2014). “Dendritic cell fate is determined by BCL11A.” Proc Natl Acad Sci U S A 111(11): E998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A, et al. (1957). “Virus interference. I. The interferon.” Proc R Soc Lond B Biol Sci 147(927): 258–267. [DOI] [PubMed] [Google Scholar]
- Ishida W, et al. (2010). “Conjunctival macrophages act as antigen-presenting cells in the conjunctiva during the development of experimental allergic conjunctivitis.” Mol Vis 16: 1280–1285. [PMC free article] [PubMed] [Google Scholar]
- Ito T, et al. (2006). “Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells.” Blood 107(6): 2423–2431. [DOI] [PubMed] [Google Scholar]
- Ito T, et al. (2007). “Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand.” J Exp Med 204(1): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakoshi NN, et al. (2007). “The transcription factor XBP-1 is essential for the development and survival of dendritic cells.” J Exp Med 204(10): 2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdorfer B, et al. (2010). “Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion.” Blood 115(6): 1156–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali A, et al. (2020). “Resident Plasmacytoid Dendritic Cells Patrol Vessels in the Naive Limbus and Conjunctiva.” Ocul Surf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali A, et al. (2019). “Local Adoptive Transfer of Plasmacytoid Dendritic Cells as a Novel Therapeutic Approach for Corneal Neovascularization.” Investigative Ophthalmology & Visual Science 60(9): 897–897. [Google Scholar]
- Jamali A, et al. (2015). “Plasmacytoid Dendritic Cells Demonstrate Vital Neuro-protective Properties in the Cornea and Induce Corneal Nerve Regeneration.” Investigative Ophthalmology & Visual Science 56(7): 4355–4355. [Google Scholar]
- Jamali A, et al. (2016). “Plasmacytoid Dendritic Cells Maintain Corneal Heme-Angiogenic Privilege Through Secretion of Anti-Angiogenic Molecules.” Investigative Ophthalmology & Visual Science 57(12): 1430–1430. [Google Scholar]
- Jamali A, et al. (2017). “Local Adoptive Transfer of Plasmacytoid Dendritic Cells as a Novel Therapeutic Approach for Corneal Nerve Regeneration.” Investigative Ophthalmology & Visual Science 58(8): 993–993. [Google Scholar]
- Jamali A, et al. (2020). “Intravital Multiphoton Microscopy of the Ocular Surface: Alterations in Conventional Dendritic Cell Morphology and Kinetics in Dry Eye Disease.” Frontiers in Immunology 11(742). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B, et al. (2002). “Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques.” J Virol 76(4): 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegalian AG, et al. (2009). “Plasmacytoid dendritic cells: physiologic roles and pathologic states.” Adv Anat Pathol 16(6): 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, et al. (2003). “Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6.” Immunity 19(2): 225–234. [DOI] [PubMed] [Google Scholar]
- Jego G, et al. (2005). “Dendritic cells control B cell growth and differentiation.” Curr Dir Autoimmun 8: 124–139. [DOI] [PubMed] [Google Scholar]
- Jiang HR, et al. (1999). “Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice.” Invest Ophthalmol Vis Sci 40(13): 3177–3185. [PubMed] [Google Scholar]
- Jin O, et al. (2010). “Abnormalities in circulating plasmacytoid dendritic cells in patients with systemic lupus erythematosus.” Arthritis Res Ther 12(4): R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin O, et al. (2008). “Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression.” Lupus 17(7): 654–662. [DOI] [PubMed] [Google Scholar]
- Joly S, et al. (2009). “Cooperative phagocytes: resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions.” Am J Pathol 174(6): 2310–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed SL, et al. (2009). “Plasmacytoid dendritic cells regulate breach of self-tolerance in autoimmune arthritis.” J Immunol 182(2): 963–968. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, et al. (2006). “Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis.” Arthritis Res Ther 8(1): R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb AM, et al. (2012). “Neuropilin-1 expression in cancer and development.” J Pathol 226(1): 50–60. [DOI] [PubMed] [Google Scholar]
- Jutila MA, et al. (1988). “Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma.” Eur J Immunol 18(11): 1819–1826. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, et al. (2001). “Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN.” J Immunol 166(4): 2291–2295. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, et al. (2001). “Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens.” J Exp Med 194(6): 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb ML, et al. (2012). “TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity.” J Immunol 188(4): 1583–1591. [DOI] [PubMed] [Google Scholar]
- Kamogawa-Schifter Y, et al. (2005). “Ly49Q defines 2 pDC subsets in mice.” Blood 105(7): 2787–2792. [DOI] [PubMed] [Google Scholar]
- Kaneko H, et al. (2008). “Characteristics of bone marrow-derived microglia in the normal and injured retina.” Invest Ophthalmol Vis Sci 49(9): 4162–4168. [DOI] [PubMed] [Google Scholar]
- Karamitros D, et al. (2018). “Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells.” Nat Immunol 19(1): 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, et al. (2003). “Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo.” J Exp Med 198(2): 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, et al. (2005). “Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors.” Exp Hematol 33(2): 173–181. [DOI] [PubMed] [Google Scholar]
- Kavousanaki M, et al. (2010). “Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy.” Arthritis Rheum 62(1): 53–63. [DOI] [PubMed] [Google Scholar]
- Kaye SB, et al. (1992). “Non-traumatic acquisition of herpes simplex virus infection through the eye.” Br J Ophthalmol 76(7): 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon B, et al. (2019). “Corneal Plasmacytoid Dendritic Cell Depletion Results in Increased Expression of Neurodegenerative Markers in the Trigeminal Ganglion.” Investigative Ophthalmology & Visual Science 60(9): 900–900. [Google Scholar]
- Kerschensteiner M, et al. (2003). “Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases.” Ann Neurol 53(3): 292–304. [DOI] [PubMed] [Google Scholar]
- Kiesewetter A, et al. (2019). “Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis.” Sci Rep 9(1): 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, et al. (2009). “Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses.” PLoS One 4(1): e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D, et al. (1992). “Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion.” Nature 356(6365): 154–156. [DOI] [PubMed] [Google Scholar]
- Kittan NA, et al. (2007). “Impaired plasmacytoid dendritic cell innate immune responses in patients with herpes virus-associated acute retinal necrosis.” J Immunol 179(6): 4219–4230. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, et al. (2009). “Stratification of Antigen-presenting Cells within the Normal Cornea.” Ophthalmol Eye Dis 1: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop N, et al. (2000). “Conjunctiva-associated lymphoid tissue in the human eye.” Invest Ophthalmol Vis Sci 41(6): 1270–1279. [PubMed] [Google Scholar]
- Koblansky AA, et al. (2013). “Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii.” Immunity 38(1): 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda Y, et al. (2019). “Plasmacytoid dendritic cells protect against immune-mediated acute liver injury via IL-35.” J Clin Invest 129(8): 3201–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, et al. (1997). “Neuropilin is a semaphorin III receptor.” Cell 90(4): 753–762. [DOI] [PubMed] [Google Scholar]
- Konishi H, et al. (2017). “Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes.” Glia 65(12): 1927–1943. [DOI] [PubMed] [Google Scholar]
- Kovacs SK, et al. (2009). “Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice.” Curr Eye Res 34(10): 896–904. [DOI] [PubMed] [Google Scholar]
- Koyama M, et al. (2009). “Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells.” Blood 113(9): 2088–2095. [DOI] [PubMed] [Google Scholar]
- Krug A, et al. (2004). “TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function.” Immunity 21(1): 107–119. [DOI] [PubMed] [Google Scholar]
- Krug A, et al. (2001). “Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12.” Eur J Immunol 31(10): 3026–3037. [DOI] [PubMed] [Google Scholar]
- Krug A, et al. (2002). “IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation.” J Immunol 169(11): 6079–6083. [DOI] [PubMed] [Google Scholar]
- Kumar A, et al. (2014). “Vascular associations and dynamic process motility in perivascular myeloid cells of the mouse choroid: implications for function and senescent change.” Invest Ophthalmol Vis Sci 55(3): 1787–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurbanyan K, et al. (2012). “Corneal nerve alterations in acute Acanthamoeba and fungal keratitis: an in vivo confocal microscopy study.” Eye (Lond) 26(1): 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzner H, et al. (2009). “CD123-positive plasmacytoid dendritic cells in primary cutaneous marginal zone B-cell lymphoma: diagnostic and pathogenetic implications.” Am J Surg Pathol 33(9): 1307–1313. [DOI] [PubMed] [Google Scholar]
- Labidi-Galy SI, et al. (2011). “Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer.” Cancer Res 71(16): 5423–5434. [DOI] [PubMed] [Google Scholar]
- Lambiase A, et al. (1998). “Topical treatment with nerve growth factor for corneal neurotrophic ulcers.” N Engl J Med 338(17): 1174–1180. [DOI] [PubMed] [Google Scholar]
- Langlois RA, et al. (2010). “Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells.” J Immunol 184(8): 4440–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mercier I, et al. (2013). “Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment.” Cancer Res 73(15): 4629–4640. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. (2017). “Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors.” Nat Immunol 18(8): 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, et al. (2010). “Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients.” PLoS One 5(6): e11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, et al. (2010). “Dendritic cells are early responders to retinal injury.” Neurobiol Dis 40(1): 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelaidier M, et al. (2015). “TRAIL-mediated killing of acute lymphoblastic leukemia by plasmacytoid dendritic cell-activated natural killer cells.” Oncotarget 6(30): 29440–29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennert K, et al. (1975). “Letter: T-associated plasma-cells.” Lancet 1(7914): 1031–1032. [DOI] [PubMed] [Google Scholar]
- Lennert K, et al. (1958). “[Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes].” Acta Haematol 19(2): 99–113. [DOI] [PubMed] [Google Scholar]
- Lepelletier Y, et al. (2007). “Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions.” Proc Natl Acad Sci U S A 104(13): 5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppin K, et al. (2014). “Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea.” Invest Ophthalmol Vis Sci 55(6): 3603–3615. [DOI] [PubMed] [Google Scholar]
- Li H, et al. (2012). “Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction.” J Immunol 188(8): 3804–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. (2016). “Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors.” J Exp Clin Cancer Res 35: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. (2013). “Macrophage depletion impairs corneal wound healing after autologous transplantation in mice.” PLoS One 8(4): e61799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, et al. (2010). “Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance.” J Immunol 185(2): 823–833. [DOI] [PubMed] [Google Scholar]
- Li Z, et al. (2011). “CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing.” Faseb j 25(8): 2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. (2007). “gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion.” Am J Pathol 171(3): 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, et al. (2015). “Cutting Edge: Plasmacytoid Dendritic Cells in Late-Stage Lupus Mice Defective in Producing IFN-alpha.” J Immunol 195(10): 4578–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ (1999). “Classification of herpes simplex virus keratitis and anterior uveitis.” Cornea 18(2): 127–143. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ (2001). “Herpes simplex virus epidemiology and ocular importance.” Cornea 20(1): 1–13. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ, et al. (1989). “Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982.” Arch Ophthalmol 107(8): 1155–1159. [DOI] [PubMed] [Google Scholar]
- Liou LY, et al. (2008). “In vivo conversion of BM plasmacytoid DC into CD11b+ conventional DC during virus infection.” Eur J Immunol 38(12): 3388–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. (2008). “Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice.” J Clin Invest 118(3): 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. (2017). “Local Group 2 Innate Lymphoid Cells Promote Corneal Regeneration after Epithelial Abrasion.” Am J Pathol 187(6): 1313–1326. [DOI] [PubMed] [Google Scholar]
- Liu K, et al. (2007). “Origin of dendritic cells in peripheral lymphoid organs of mice.” Nat Immunol 8(6): 578–583. [DOI] [PubMed] [Google Scholar]
- Liu Q, et al. (2012). “NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing.” Am J Pathol 181(2): 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. (2002). “Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts.” J Exp Med 195(2): 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, et al. (1998). “CCR5 is characteristic of Th1 lymphocytes.” Nature 391(6665): 344–345. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, et al. (2007). “Airway dendritic cell phenotypes in inflammatory diseases of the human lung.” Eur Respir J 30(5): 878–886. [DOI] [PubMed] [Google Scholar]
- Lou Y, et al. (2007). “Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses.” J Immunol 178(3): 1534–1541. [DOI] [PubMed] [Google Scholar]
- Luckoff A, et al. (2016). “Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization.” EMBO Mol Med 8(6): 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann UF, et al. (2009). “The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages.” Invest Ophthalmol Vis Sci 50(12): 5934–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens MV, et al. (2009). “Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node.” J Virol 83(14): 7235–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, et al. (2006). “Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ.” J Immunol 177(11): 7510–7514. [DOI] [PubMed] [Google Scholar]
- Luznik Z, et al. (2019). “Identification and characterization of dendritic cell subtypes in preserved and cultured cadaveric human corneolimbal tissue on amniotic membrane.” Acta Ophthalmol 97(2): e184–e193. [DOI] [PubMed] [Google Scholar]
- Lyman SD, et al. (1993). “Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells.” Cell 75(6): 1157–1167. [DOI] [PubMed] [Google Scholar]
- Lynch JP, et al. (2018). “Plasmacytoid dendritic cells protect from viral bronchiolitis and asthma through semaphorin 4a-mediated T reg expansion.” J Exp Med 215(2): 537–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, et al. (2019). “The miR-141/neuropilin-1 axis is associated with the clinicopathology and contributes to the growth and metastasis of pancreatic cancer.” Cancer Cell Int 19: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, et al. (2009). “Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD.” PLoS One 4(11): e7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E, et al. (1996). “Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified.” J Exp Med 184(5): 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, et al. (2010). “Anatomy of the human corneal innervation.” Exp Eye Res 90(4): 478–492. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, et al. (1993). “Immunohistochemical localization of tyrosine hydroxylase in corneal nerves.” J Comp Neurol 336(4): 517–531. [DOI] [PubMed] [Google Scholar]
- Martin-Gayo E, et al. (2010). “Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development.” Blood 115(26): 5366–5375. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, et al. (2011). “Host factors involved in retroviral budding and release.” Nat Rev Microbiol 9(7): 519–531. [DOI] [PubMed] [Google Scholar]
- Martin P, et al. (2002). “Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential.” Blood 100(2): 383–390. [DOI] [PubMed] [Google Scholar]
- Maruoka S, et al. (2018). “Activation of Dendritic Cells in Dry Eye Mouse Model.” Invest Ophthalmol Vis Sci 59(8): 3269–3277. [DOI] [PubMed] [Google Scholar]
- Maruyama K, et al. (2005). “Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages.” J Clin Invest 115(9): 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, et al. (2012). “The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages.” Invest Ophthalmol Vis Sci 53(6): 3145–3153. [DOI] [PubMed] [Google Scholar]
- Mascarell L, et al. (2008). “Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells.” J Allergy Clin Immunol 122(3): 603–609 e605. [DOI] [PubMed] [Google Scholar]
- Masten BJ, et al. (2006). “Characterization of myeloid and plasmacytoid dendritic cells in human lung.” J Immunol 177(11): 7784–7793. [DOI] [PubMed] [Google Scholar]
- McInnes IB, et al. (2007). “Cytokines in the pathogenesis of rheumatoid arthritis.” Nat Rev Immunol 7(6): 429–442. [DOI] [PubMed] [Google Scholar]
- McKay TB, et al. (2019). “Corneal pain and experimental model development.” Prog Retin Eye Res 71: 88–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG (1999). “Dendritic cells and macrophages in the uveal tract of the normal mouse eye.” Br J Ophthalmol 89: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, et al. (1997). “Resident and infiltrating cells in the rat iris during the early stages of experimental melanin protein-induced uveitis (EMIU).” Ocul Immunol Inflamm 5(4): 223–233. [DOI] [PubMed] [Google Scholar]
- McMenamin PG, et al. (1992). “Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye: distribution, phenotype and relation to retinal microglia.” Immunology 77: 385–393. [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, et al. (2019). “Immune cells in the retina and choroid: Two different tissue environments that require different defenses and surveillance.” Prog Retin Eye Res 70: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB (1948). “Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye.” Br J Exp Pathol 29(1): 58–69. [PMC free article] [PubMed] [Google Scholar]
- Mendes-da-Cruz DA, et al. (2014). “Semaphorin 3F and neuropilin-2 control the migration of human T-cell precursors.” PLoS One 9(7): e103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Jorge L, et al. (2009). “Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier.” Invest Ophthalmol Vis Sci 50(12): 5997–6005. [DOI] [PubMed] [Google Scholar]
- Menon M, et al. (2016). “A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus.” Immunity 44(3): 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michea P, et al. (2013). “Epithelial control of the human pDC response to extracellular bacteria.” Eur J Immunol 43(5): 1264–1273. [DOI] [PubMed] [Google Scholar]
- Millodot M (1984). “A review of research on the sensitivity of the cornea.” Ophthalmic Physiol Opt 4(4): 305–318. [PubMed] [Google Scholar]
- Mircheff AK, et al. (2011). “Systematic variations in immune response-related gene transcript abundance suggest new questions about environmental influences on lacrimal gland immunoregulation.” Curr Eye Res 36(4): 285–294. [DOI] [PubMed] [Google Scholar]
- Miyauchi JT, et al. (2018). “Deletion of Neuropilin 1 from Microglia or Bone Marrow-Derived Macrophages Slows Glioma Progression.” Cancer Res 78(3): 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J, et al. (2017). “Effect of Corneal Nerve Ablation on Immune Tolerance Induced by Corneal Allografts, Oral Immunization, or Anterior Chamber Injection of Antigens.” Invest Ophthalmol Vis Sci 58(1): 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamedali AM, et al. (2015). “High concordance of genomic and cytogenetic aberrations between peripheral blood and bone marrow in myelodysplastic syndrome (MDS).” Leukemia 29(9): 1928–1938. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, et al. (1992). “RAG-1-deficient mice have no mature B and T lymphocytes.” Cell 68(5): 869–877. [DOI] [PubMed] [Google Scholar]
- Monach PA, et al. (2007). “Circulating C3 is necessary and sufficient for induction of autoantibody-mediated arthritis in a mouse model.” Arthritis Rheum 56(9): 2968–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseman EA, et al. (2004). “Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells.” J Immunol 173(7): 4433–4442. [DOI] [PubMed] [Google Scholar]
- Muller-Hermelink HK, et al. (1973). “[Pseudofollicular nests of plasmacells (of a special type?) in paracortical pulp of human lymph nodes (author’ s transl)].” Virchows Arch B Cell Pathol 14(1): 47–56. [PubMed] [Google Scholar]
- Muller-Hermelink HK, et al. (1983). “Malignant lymphoma of plasmacytoid T-cells. Morphologic and immunologic studies characterizing a special type of T-cell.” Am J Surg Pathol 7(8): 849–862. [PubMed] [Google Scholar]
- Muller LJ, et al. (1996). “Ultrastructural organization of human corneal nerves.” Invest Ophthalmol Vis Sci 37(4): 476–488. [PubMed] [Google Scholar]
- Muller LJ, et al. (1997). “Architecture of human corneal nerves.” Invest Ophthalmol Vis Sci 38(5): 985–994. [PubMed] [Google Scholar]
- Muller RT, et al. (2015). “Degeneration and Regeneration of Subbasal Corneal Nerves after Infectious Keratitis: A Longitudinal In Vivo Confocal Microscopy Study.” Ophthalmology 122(11): 2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, et al. (2017). “A Review of Ocular Graft-Versus-Host Disease.” Optom Vis Sci 94(5): 545–555. [DOI] [PubMed] [Google Scholar]
- Murray L, et al. (2019). “CLEC4C gene expression can be used to quantify circulating plasmacytoid dendritic cells.” J Immunol Methods 464: 126–130. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, et al. (2008). “Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2–2 and the Ets factor Spi-B.” Eur J Immunol 38(9): 2389–2400. [DOI] [PubMed] [Google Scholar]
- Nakamura T, et al. (2005). “Characterization and distribution of bone marrow-derived cells in mouse cornea.” Invest Ophthalmol Vis Sci 46(2): 497–503. [DOI] [PubMed] [Google Scholar]
- Nakano H, et al. (2001). “CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells.” J Exp Med 194(8): 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam S, et al. (2018). “Induction of Contrasuppressor Cells and Loss of Immune Privilege Produced by Corneal Nerve Ablation.” Invest Ophthalmol Vis Sci 59(11): 4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehmar R, et al. (2017). “Therapeutic Modulation of Plasmacytoid Dendritic Cells in Experimental Arthritis.” Arthritis Rheumatol 69(11): 2124–2135. [DOI] [PubMed] [Google Scholar]
- Nehmar R, et al. (2018). “Therapeutic Perspectives for Interferons and Plasmacytoid Dendritic Cells in Rheumatoid Arthritis.” Trends Mol Med 24(4): 338–347. [DOI] [PubMed] [Google Scholar]
- Nell-Duxneuner V, et al. (2010). “Autoantibody profiling in patients with very early rheumatoid arthritis: a follow-up study.” Ann Rheum Dis 69(1): 169–174. [DOI] [PubMed] [Google Scholar]
- Nesburn AB, et al. (2007). “Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection.” J Virol 81(14): 7647–7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, et al. (2005). “Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production.” J Exp Med 202(1): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DH, et al. (2006). “Loss of parasympathetic innervation leads to sustained expression of pro-inflammatory genes in the rat lacrimal gland.” Auton Neurosci 124(1–2): 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic T, et al. (2002). “A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics.” Eur J Immunol 32(3): 686–692. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M, et al. (2002). “Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus.” J Exp Med 196(10): 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe M, et al. (2003). “Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors.” Blood 101(4): 1453–1459. [DOI] [PubMed] [Google Scholar]
- O’Koren EG, et al. (2016). “Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina.” Sci Rep 6: 20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, et al. (2019). “Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration.” Immunity 50(3): 723–737.e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochando JC, et al. (2006). “Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts.” Nat Immunol 7(6): 652–662. [DOI] [PubMed] [Google Scholar]
- Oh NA, et al. (2019). “Plasmacytoid dendritic cell driven induction of Tregs is strain-specific and correlates with spontaneous acceptance of kidney allografts.” Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi M, et al. (2007). “Dynamic changes in conjunctival dendritic cell numbers, anatomical position and phenotype during experimental allergic conjunctivitis.” Exp Mol Pathol 83(2): 216–223. [DOI] [PubMed] [Google Scholar]
- Olweus J, et al. (1997). “Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin.” Proc Natl Acad Sci U S A 94(23): 12551–12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, et al. (2005). “Development of murine plasmacytoid dendritic cells defined by increased expression of an inhibitory NK receptor, Ly49Q.” J Immunol 174(11): 6657–6662. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, et al. (1989). “Standard models of corneal injury using alkali-immersed filter discs.” Invest Ophthalmol Vis Sci 30(10): 2148–2153. [PubMed] [Google Scholar]
- Palejwala NV, et al. (2012). “Ocular manifestations of systemic lupus erythematosus: a review of the literature.” Autoimmune Dis 2012: 290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomares O, et al. (2012). “Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance.” J Allergy Clin Immunol 129(2): 510–520, 520 e511–519. [DOI] [PubMed] [Google Scholar]
- Papadimitriou CS, et al. (1983). “Comparative immunostaining of T-associated plasma cells and other lymph-node cells in paraffin sections.” Virchows Arch B Cell Pathol Incl Mol Pathol 43(1): 31–36. [DOI] [PubMed] [Google Scholar]
- Pappo J, et al. (1988). “Phenotype of Mononuclear Leukocytes Resident in Rat Major Salivary and Lacrimal Glands.” Immunology 64: 295–300. [PMC free article] [PubMed] [Google Scholar]
- Parcina M, et al. (2008). “Staphylococcus aureus-induced plasmacytoid dendritic cell activation is based on an IgG-mediated memory response.” J Immunol 181(6): 3823–3833. [DOI] [PubMed] [Google Scholar]
- Pascale F, et al. (2008). “Plasmacytoid dendritic cells migrate in afferent skin lymph.” J Immunol 180(9): 5963–5972. [DOI] [PubMed] [Google Scholar]
- Paunicka KJ, et al. (2015). “Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye.” Am J Transplant 15(6): 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, et al. (2001). “Adenosine extracellular brain concentrations and role of A2A receptors in ischemia.” Ann N Y Acad Sci 939: 74–84. [DOI] [PubMed] [Google Scholar]
- Pedroza-Gonzalez A, et al. (2015). “Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors.” Oncoimmunology 4(6): e1008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin A, et al. (2015). “Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms.” EMBO Mol Med 7(4): 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, et al. (2001). “Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells.” J Immunol 167(4): 1862–1866. [DOI] [PubMed] [Google Scholar]
- Penna G, et al. (2002). “Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells.” J Immunol 169(12): 6673–6676. [DOI] [PubMed] [Google Scholar]
- Pepper M, et al. (2008). “Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines.” J Immunol 180(9): 6229–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B, et al. (1985). “A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses.” Nat Immun Cell Growth Regul 4(3): 120–137. [PubMed] [Google Scholar]
- Pfister RR, et al. (1978). “Topical ascorbate decreases the incidence of corneal ulceration after experimental alkali burns.” Invest Ophthalmol Vis Sci 17(10): 1019–1024. [PubMed] [Google Scholar]
- Pickens SR, et al. (2011). “Characterization of CCL19 and CCL21 in rheumatoid arthritis.” Arthritis Rheum 63(4): 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierog PL, et al. (2018). “Toxoplasma gondii Inactivates Human Plasmacytoid Dendritic Cells by Functional Mimicry of IL-10.” J Immunol 200(1): 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plein A, et al. (2014). “Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability.” Microcirculation 21(4): 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, et al. (1995). “Human retinal microglia: expression of immune markers and relationship to the glia limitans.” Glia 14(4): 243–256. [DOI] [PubMed] [Google Scholar]
- Qazi Y, et al. (2013). “Corneal Allograft Rejection: Immunopathogenesis to Therapeutics.” J Clin Cell Immunol 2013(Suppl 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim MM, et al. (2014). “Ly49 receptors: innate and adaptive immune paradigms.” Front Immunol 5: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, et al. (2019). “Plasmacytoid Dendritic Cells Provide Protection Against Bacterial-Induced Colitis.” Front Immunol 10: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raieli S, et al. (2019). “TLR1/2 orchestrate human plasmacytoid predendritic cell response to gram+ bacteria.” PLoS Biol 17(4): e3000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar R, et al. (2010). “Dendritic cell count in the graft predicts relapse in patients with hematologic malignancies undergoing an HLA-matched related allogeneic peripheral blood stem cell transplant.” Biol Blood Marrow Transplant 16(6): 854–860. [DOI] [PubMed] [Google Scholar]
- Ramke M, et al. (2016). “Resident corneal c-fms(+) macrophages and dendritic cells mediate early cellular infiltration in adenovirus keratitis.” Exp Eye Res 147: 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, et al. (2005). “The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function.” Immunity 22(6): 717–728. [DOI] [PubMed] [Google Scholar]
- Ray A, et al. (2015). “Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells.” Leukemia 29(6): 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi S, et al. (2015). “Neurotrophic factors and their effects in the treatment of multiple sclerosis.” Adv Biomed Res 4: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard M, et al. (2014). “In vivo visualisation of murine corneal nerve fibre regeneration in response to ciliary neurotrophic factor.” Exp Eye Res 120: 20–27. [DOI] [PubMed] [Google Scholar]
- Reyes NJ, et al. (2011). “gammadelta T cells are required for maximal expression of allergic conjunctivitis.” Invest Ophthalmol Vis Sci 52(5): 2211–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, et al. (2009). “Immune cells and angiogenesis.” J Cell Mol Med 13(9a): 2822–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan MC, et al. (2002). “Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells.” Blood 100(9): 3295–3303. [DOI] [PubMed] [Google Scholar]
- Rissoan MC, et al. (1999). “Reciprocal control of T helper cell and dendritic cell differentiation.” Science 283(5405): 1183–1186. [DOI] [PubMed] [Google Scholar]
- Rodrigues MM, et al. (1981). “Langerhans cells in the normal conjunctiva and peripheral cornea of selected species.” Invest Ophthalmol Vis Sci 21(5): 759–765. [PubMed] [Google Scholar]
- Rodrigues PF, et al. (2018). “Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells.” Nat Immunol 19(7): 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, et al. (2013). “Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance?” Am J Transplant 13(5): 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A, et al. (1996). “A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors.” J Exp Med 183(1): 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom L, et al. (1983). “Properties of human natural interferon-producing cells stimulated by tumor cell lines.” Eur J Immunol 13(6): 471–476. [DOI] [PubMed] [Google Scholar]
- Rowe AM, et al. (2013). “Herpes keratitis.” Prog Retin Eye Res 32: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland SL, et al. (2014). “Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model.” J Exp Med 211(10): 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozsa AJ, et al. (1982). “Density and organization of free nerve endings in the corneal epithelium of the rabbit.” Pain 14(2): 105–120. [DOI] [PubMed] [Google Scholar]
- Ruben JM, et al. (2018). “Human plasmacytoid dendritic cells acquire phagocytic capacity by TLR9 ligation in the presence of soluble factors produced by renal epithelial cells.” Kidney Int 93(2): 355–364. [DOI] [PubMed] [Google Scholar]
- Sacks E, et al. (1986). “A comparison of conjunctival and nonocular dendritic cells utilizing new monoclonal antibodies.” Ophthalmology 93(8): 1089–1097. [DOI] [PubMed] [Google Scholar]
- Sacks EH, et al. (1986). “Lymphocytic subpopulations in the normal human conjunctiva. A monoclonal antibody study.” Ophthalmology 93(10): 1276–1283. [DOI] [PubMed] [Google Scholar]
- Saitoh-Inagawa W, et al. (2000). “Unique characteristics of lacrimal glands as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+ alphabeta T cells.” Invest Ophthalmol Vis Sci 41(1): 138–144. [PubMed] [Google Scholar]
- Sakata K, et al. (2018). “Up-Regulation of TLR7-Mediated IFN-α Production by Plasmacytoid Dendritic Cells in Patients With Systemic Lupus Erythematosus.” Front Immunol 9: 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, et al. (1995). “Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction.” Invest Ophthalmol Vis Sci 36(11): 2176–2185. [PubMed] [Google Scholar]
- Santoro A, et al. (2005). “Recruitment of dendritic cells in oral lichen planus.” J Pathol 205(4): 426–434. [DOI] [PubMed] [Google Scholar]
- Sasaki I, et al. (2012). “Spi-B is critical for plasmacytoid dendritic cell function and development.” Blood 120(24): 4733–4743. [DOI] [PubMed] [Google Scholar]
- Sathaliyawala T, et al. (2010). “Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling.” Immunity 33(4): 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe P, et al. (2013). “Convergent differentiation: myeloid and lymphoid pathways to murine plasmacytoid dendritic cells.” Blood 121(1): 11–19. [DOI] [PubMed] [Google Scholar]
- Savini G, et al. (2004). “Ocular surface changes in laser in situ keratomileusis-induced neurotrophic epitheliopathy.” J Refract Surg 20(6): 803–809. [DOI] [PubMed] [Google Scholar]
- Sawai CM, et al. (2013). “Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells.” J Exp Med 210(11): 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant A, et al. (2012). “Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells.” J Immunol 189(9): 4258–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, et al. (2012). “Tissue-specific differentiation of a circulating CCR9- pDC-like common dendritic cell precursor.” Blood 119(25): 6063–6071. [DOI] [PubMed] [Google Scholar]
- Schnurr M, et al. (2004). “Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells.” Blood 103(4): 1391–1397. [DOI] [PubMed] [Google Scholar]
- Schotte R, et al. (2004). “The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development.” J Exp Med 200(11): 1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbacher L, et al. (2017). “Turnover of Resident Plasmacytoid Dendritic Cells in the Cornea.” Investigative Ophthalmology & Visual Science 58(8): 992–992. [Google Scholar]
- See P, et al. (2017). “Mapping the human DC lineage through the integration of high-dimensional techniques.” Science 356(6342). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam S, et al. (2013). “Diverse mediators modulate the chloride ion fluxes that drive lacrimal fluid production.” Invest Ophthalmol Vis Sci 54(4): 2927–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendra V, et al. (2015). “Plasmacytoid Dendritic Cells Mediate Adaptive Immunity in Acute Herpes Simplex Virus Keratitis.” Investigative Ophthalmology & Visual Science 56(7): 1856–1856. [Google Scholar]
- Sendra V, et al. (2016). “Plasmacytoid Dendritic Cells Mediate T cell Responses by Direct Interaction in Lymph Nodes during Herpes Simplex Virus-1 Keratitis.” Investigative Ophthalmology & Visual Science 57(12): 331–331. [Google Scholar]
- Sendra VG, et al. (2014). “Role of plasmacytoid dendritic cell in the immune regulation in sutured inflamed cornea.” Investigative Ophthalmology & Visual Science 55(13): 1694–1694. [Google Scholar]
- Sendra VG, et al. (2017). “Plasmacytoid Dendritic Cells Modulate Corneal Inflammation Through Transforming Growth Factor (TGF)-β1.” Investigative Ophthalmology & Visual Science 58(8): 3618–3618. [Google Scholar]
- Seth S, et al. (2011). “CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions.” J Immunol 186(6): 3364–3372. [DOI] [PubMed] [Google Scholar]
- Seto SK, et al. (1987). “HLA-DR+/T6- Langerhans cells of the human cornea.” Invest Ophthalmol Vis Sci 28(10): 1719–1722. [PubMed] [Google Scholar]
- Seyed-Razavi Y, et al. (2014). “A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea.” Invest Ophthalmol Vis Sci 55(3): 1313–1320. [DOI] [PubMed] [Google Scholar]
- Seyed-Razavi Y, et al. (2019). “Kinetics of corneal leukocytes by intravital multiphoton microscopy.” Faseb j 33(2): 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, et al. (2010). “HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection.” Mol Vis 16: 2476–2486. [PMC free article] [PubMed] [Google Scholar]
- Sharma MD, et al. (2007). “Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase.” J Clin Invest 117(9): 2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, et al. (2002). “Mouse and human dendritic cell subtypes.” Nat Rev Immunol 2(3): 151–161. [DOI] [PubMed] [Google Scholar]
- Shoughy SS, et al. (2016). “Ocular findings in systemic lupus erythematosus.” Saudi J Ophthalmol 30(2): 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichien D, et al. (2016). “IRF8 Transcription Factor Controls Survival and Function of Terminally Differentiated Conventional and Plasmacytoid Dendritic Cells, Respectively.” Immunity 45(3): 626–640. [DOI] [PubMed] [Google Scholar]
- Siebelmann S, et al. (2013). “Development, alteration and real time dynamics of conjunctiva-associated lymphoid tissue.” PLoS One 8(12): e82355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, et al. (1999). “The nature of the principal type 1 interferon-producing cells in human blood.” Science 284(5421): 1835–1837. [DOI] [PubMed] [Google Scholar]
- Silpa-archa S, et al. (2016). “Ocular manifestations in systemic lupus erythematosus.” Br J Ophthalmol 100(1): 135–141. [DOI] [PubMed] [Google Scholar]
- Silverman SM, et al. (2018). “Microglia in the Retina: Roles in Development, Maturity, and Disease.” Annu Rev Vis Sci 4: 45–77. [DOI] [PubMed] [Google Scholar]
- Sisirak V, et al. (2012). “Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression.” Cancer Res 72(20): 5188–5197. [DOI] [PubMed] [Google Scholar]
- Sisirak V, et al. (2014). “Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus.” J Exp Med 211(10): 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisirak V, et al. (2013). “Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells.” Int J Cancer 133(3): 771–778. [DOI] [PubMed] [Google Scholar]
- Skelsey ME, et al. (2001). “Gamma delta T cells are needed for ocular immune privilege and corneal graft survival.” J Immunol 166(7): 4327–4333. [DOI] [PubMed] [Google Scholar]
- Smit JJ, et al. (2006). “Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus.” J Exp Med 203(5): 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, et al. (2019). “Control of TLR7-mediated type I IFN signaling in pDCs through CXCR4 engagement-A new target for lupus treatment.” Sci Adv 5(7): eaav9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen KK, et al. (2014). “Single-cell analysis of innate cytokine responses to pattern recognition receptor stimulation in children across four continents.” J Immunol 193(6): 3003–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodova E, et al. (2011). “Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage.” PLoS One 6(4): e18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag S, et al. (2017). “Modelling IRF8 Deficient Human Hematopoiesis and Dendritic Cell Development with Engineered iPS Cells.” Stem Cells 35(4): 898–908. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, et al. (2015). “Human lung cancer-derived immunosuppressive plasmacytoid dendritic cells release IL-1alpha in an AIM2 inflammasome-dependent manner.” Am J Pathol 185(11): 3115–3124. [DOI] [PubMed] [Google Scholar]
- Sosnova M, et al. (2005). “CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers.” Stem Cells 23(4): 507–515. [DOI] [PubMed] [Google Scholar]
- Soukiasian SH, et al. (1992). “The T cell receptor in normal and inflamed human conjunctiva.” Invest Ophthalmol Vis Sci 33(2): 453–459. [PubMed] [Google Scholar]
- Sozzani S, et al. (2010). “Trafficking properties of plasmacytoid dendritic cells in health and disease.” Trends Immunol 31(7): 270–277. [DOI] [PubMed] [Google Scholar]
- Steptoe RJ, et al. (1995). “Functional studies of major histocompatibility class II-positive dendritic cells and resident tissue macrophages isolated from the rat iris.” Immunology 85: 630–637. [PMC free article] [PubMed] [Google Scholar]
- Steptoe RJ, et al. (1995). “Functional studies of major histocompatibility class II-positive dendritic cells and resident tissue macrophages isolated from the rat iris.” Immunology 85(4): 630–637. [PMC free article] [PubMed] [Google Scholar]
- Steptoe RJ, et al. (1996). “Origin and steady-state turnover of major histocompatibility complex Class II-positive dendritic cells and resident-tissue macrophages in the iris of the rat eye.” J Neuroimmunol 68: 67–76. [DOI] [PubMed] [Google Scholar]
- Steptoe RJ, et al. (2009). “Resident tissue macrophages within the normal rat iris lack immunosuppressive activity and are effective antigen-presenting cells.” Ocular Immunology and Inflammation 8(3): 177–187. [PubMed] [Google Scholar]
- Stern M, et al. (2013). “Plasmacytoid Dendritic Cells are the Primary Source of IFN-α During the Immunopathogenesis of Desiccating Stress-Induced Dry Eye Disease.” Investigative Ophthalmology & Visual Science 54(15): 4323–4323. [Google Scholar]
- Stern ME, et al. (2004). “The role of the lacrimal functional unit in the pathophysiology of dry eye.” Exp Eye Res 78(3): 409–416. [DOI] [PubMed] [Google Scholar]
- Stern ME, et al. (2012). “Plasmacytoid Dendritic Cells are Modulated During the Immunopathogenesis of Desiccating Stress-Induced Experimental Dry Eye.” Investigative Ophthalmology & Visual Science 53(14): 2327–2327. [Google Scholar]
- Stockinger S, et al. (2009). “Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes.” PLoS Pathog 5(3): e1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, et al. (1996). “Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas.” Invest Ophthalmol Vis Sci 37(2): 413–424. [PubMed] [Google Scholar]
- Streilein JW, et al. (1979). “Corneal allografts fail to express Ia antigens.” Nature 282(5736): 326–327. [DOI] [PubMed] [Google Scholar]
- Streilein JW, et al. (1992). “Eye-derived cytokines and the immunosuppressive intraocular microenvironment: a review.” Curr Eye Res 11 Suppl: 41–47. [DOI] [PubMed] [Google Scholar]
- Stuart PM, et al. (2005). “The role of Fas ligand as an effector molecule in corneal graft rejection.” Eur J Immunol 35(9): 2591–2597. [DOI] [PubMed] [Google Scholar]
- Sumen C, et al. (2004). “Intravital microscopy: visualizing immunity in context.” Immunity 21(3): 315–329. [DOI] [PubMed] [Google Scholar]
- Summerfield A, et al. (2003). “Porcine peripheral blood dendritic cells and natural interferon-producing cells.” Immunology 110(4): 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson H, et al. (1996). “Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus.” J Interferon Cytokine Res 16(1): 7–16. [DOI] [PubMed] [Google Scholar]
- Svensson H, et al. (1996). “The cell surface phenotype of human natural interferon-alpha producing cells as determined by flow cytometry.” Scand J Immunol 44(2): 164–172. [DOI] [PubMed] [Google Scholar]
- Swiecki M, et al. (2015). “The multifaceted biology of plasmacytoid dendritic cells.” Nat Rev Immunol 15(8): 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, et al. (2010). “Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual.” Immunity 33(6): 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, et al. (2013). “Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections.” PLoS Pathog 9(10): e1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, et al. (2012). “Cutting edge: paradoxical roles of BST2/tetherin in promoting type I IFN response and viral infection.” J Immunol 188(6): 2488–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildari M, et al. (2017). “The Role of Corneal Plasmacytoid Dendritic Cells in Immune Sensitization after Corneal Transplantation.” Investigative Ophthalmology & Visual Science 58(8): 2068–2068. [Google Scholar]
- Tai LH, et al. (2008). “Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC.” J Exp Med 205(13): 3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, et al. (2011). “Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo.” Immunity 35(6): 958–971. [DOI] [PubMed] [Google Scholar]
- Tam MA, et al. (2006). “Differential expansion, activation and effector functions of conventional and plasmacytoid dendritic cells in mouse tissues transiently infected with Listeria monocytogenes.” Cell Microbiol 8(7): 1172–1187. [DOI] [PubMed] [Google Scholar]
- Tamura T, et al. (2005). “IFN regulatory factor-4 and −8 govern dendritic cell subset development and their functional diversity.” J Immunol 174(5): 2573–2581. [DOI] [PubMed] [Google Scholar]
- Tanis W, et al. (2004). “Human hepatic lymph nodes contain normal numbers of mature myeloid dendritic cells but few plasmacytoid dendritic cells.” Clin Immunol 110(1): 81–88. [DOI] [PubMed] [Google Scholar]
- Taylor AW (2009). “Ocular immune privilege.” Eye (Lond) 23(10): 1885–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, et al. (1994). “Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor.” J Immunol 153(3): 1080–1086. [PubMed] [Google Scholar]
- Ten Berge B, et al. (2012). “Evidence for local dendritic cell activation in pulmonary sarcoidosis.” Respir Res 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra M, et al. (2018). “Tumor-Derived TGFbeta Alters the Ability of Plasmacytoid Dendritic Cells to Respond to Innate Immune Signaling.” Cancer Res 78(11): 3014–3026. [DOI] [PubMed] [Google Scholar]
- Tian J, et al. (2007). “Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE.” Nat Immunol 8(5): 487–496. [DOI] [PubMed] [Google Scholar]
- Tokita D, et al. (2008). “Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells.” J Hepatol 49(6): 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma-Hirano M, et al. (2009). “Ly49Q ligand expressed by activated B cells induces plasmacytoid DC maturation.” Eur J Immunol 39(5): 1344–1352. [DOI] [PubMed] [Google Scholar]
- Tordjman R, et al. (2002). “A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response.” Nat Immunol 3(5): 477–482. [DOI] [PubMed] [Google Scholar]
- Toyama-Sorimachi N, et al. (2005). “Inhibitory NK receptor Ly49Q is expressed on subsets of dendritic cells in a cellular maturation- and cytokine stimulation-dependent manner.” J Immunol 174(8): 4621–4629. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, et al. (1978). “Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation.” J Exp Med 147(5): 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, et al. (2012). “Effect of respiratory syncytial virus infection on plasmacytoid dendritic cell regulation of allergic airway inflammation.” Int Arch Allergy Immunol 157(1): 21–30. [DOI] [PubMed] [Google Scholar]
- Uto T, et al. (2018). “Critical role of plasmacytoid dendritic cells in induction of oral tolerance.” J Allergy Clin Immunol 141(6): 2156–2167.e2159. [DOI] [PubMed] [Google Scholar]
- Van Belle H, et al. (1987). “Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia.” Am J Physiol 252(5 Pt 2): H886–893. [DOI] [PubMed] [Google Scholar]
- Vantrappen L, et al. (1985). “Lymphocytes and Langerhans cells in the normal human cornea.” Invest Ophthalmol Vis Sci 26(2): 220–225. [PubMed] [Google Scholar]
- Velasquez-Lopera MM, et al. (2008). “Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes.” Clin Exp Immunol 154(1): 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F, et al. (2010). “Plasmacytoid dendritic cells control lung inflammation and monocyte recruitment in indirect acute lung injury in mice.” Am J Pathol 176(2): 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermi W, et al. (2003). “Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas.” J Pathol 200(2): 255–268. [DOI] [PubMed] [Google Scholar]
- Villani AC, et al. (2017). “Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors.” Science 356(6335). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, et al. (2010). “PDCA expression by B lymphocytes reveals important functional attributes.” J Immunol 184(2): 807–815. [DOI] [PubMed] [Google Scholar]
- Vinay DS, et al. (2012). “Exposure of a distinct PDCA-1+ (CD317) B cell population to agonistic anti-4–1BB (CD137) inhibits T and B cell responses both in vitro and in vivo.” PLoS One 7(11): e50272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsang P, et al. (2010). “Levels of plasmacytoid dendritic cells and type-2 myeloid dendritic cells are reduced in peripheral blood of patients with primary Sjogren’s syndrome.” Ann Rheum Dis 69(6): 1235–1238. [DOI] [PubMed] [Google Scholar]
- Vogt TK, et al. (2009). “Novel function for interleukin-7 in dendritic cell development.” Blood 113(17): 3961–3968. [DOI] [PubMed] [Google Scholar]
- Vollenweider R, et al. (1983). “Plasmacytoid T-cell clusters in non-specific lymphadenitis.” Virchows Arch B Cell Pathol Incl Mol Pathol 44(1): 1–14. [DOI] [PubMed] [Google Scholar]
- Vrapciu AD, et al. (2014). “Stem potentialities of the human iris - An in situ immunohistochemical study.” Acta Histochem 116(8): 1509–1513. [DOI] [PubMed] [Google Scholar]
- Vremec D, et al. (2000). “CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen.” J Immunol 164(6): 2978–2986. [DOI] [PubMed] [Google Scholar]
- Waller EK, et al. (2014). “Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201.” J Clin Oncol 32(22): 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter-Yohrling J, et al. (2003). “Identification of genes expressed in malignant cells that promote invasion.” Cancer Res 63(24): 8939–8947. [PubMed] [Google Scholar]
- Wang HM, et al. (1987). “The distribution and ontogeny of MHC antigens in murine ocular tissue.” Invest Ophthalmol Vis Sci 28(8): 1383–1389. [PubMed] [Google Scholar]
- Wei S, et al. (2005). “Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma.” Cancer Res 65(12): 5020–5026. [DOI] [PubMed] [Google Scholar]
- Weiner HL, et al. (2011). “Oral tolerance.” Immunol Rev 241(1): 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek R, et al. (1988). “The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions.” Ophthalmology 95(1): 100–109. [DOI] [PubMed] [Google Scholar]
- Wildenberg ME, et al. (2008). “Systemic increase in type I interferon activity in Sjogren’s syndrome: a putative role for plasmacytoid dendritic cells.” Eur J Immunol 38(7): 2024–2033. [DOI] [PubMed] [Google Scholar]
- Williamson J, et al. (1989). “Immunoregulatory Properties of Bone Marrow-Derived Cells in the Iris and Ciliary Body.” Immunology 67: 96–102. [PMC free article] [PubMed] [Google Scholar]
- Williamson JS, et al. (1987). “Immunobiology of Langerhans cells on the ocular surface. I. Langerhans cells within the central cornea interfere with induction of anterior chamber associated immune deviation.” Invest Ophthalmol Vis Sci 28(9): 1527–1532. [PubMed] [Google Scholar]
- Wilson SE, et al. (2001). “Laser in situ keratomileusis-induced neurotrophic epitheliopathy.” Am J Ophthalmol 132(3): 405–406. [DOI] [PubMed] [Google Scholar]
- Wolf AI, et al. (2009). “Plasmacytoid dendritic cells are dispensable during primary influenza virus infection.” J Immunol 182(2): 871–879. [DOI] [PubMed] [Google Scholar]
- Woltman AM, et al. (2007). “Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions.” Kidney Int 71(10): 1001–1008. [DOI] [PubMed] [Google Scholar]
- Wu J, et al. (2017). “TLR-activated plasmacytoid dendritic cells inhibit breast cancer cell growth in vitro and in vivo.” Oncotarget 8(7): 11708–11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. (2001). “Development of thymic and splenic dendritic cell populations from different hemopoietic precursors.” Blood 98(12): 3376–3382. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. (2007). “Turnover of resident retinal microglia in the normal adult mouse.” Glia 55(11): 1189–1198. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. (2007). “LYVE-1–Positive Macrophages Are Present in Normal Murine Eyes.” Investigative Opthalmology & Visual Science 48(5). [DOI] [PubMed] [Google Scholar]
- Xu H, et al. (2007). “Identification of novel dendritic cell populations in normal mouse retina.” Invest Ophthalmol Vis Sci 48(4): 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami S, et al. (2005). “Distinct populations of dendritic cells in the normal human donor corneal epithelium.” Invest Ophthalmol Vis Sci 46(12): 4489–4494. [DOI] [PubMed] [Google Scholar]
- Yang GX, et al. (2005). “Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors.” J Immunol 175(11): 7281–7287. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. (2019). “Neuropilin-1 is associated with the prognosis of cervical cancer in Henan Chinese population.” Onco Targets Ther 12: 2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama H, et al. (2005). “Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs.” J Exp Med 202(3): 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, et al. (2018). “Distribution of Interleukin-22-secreting Immune Cells in Conjunctival Associated Lymphoid Tissue.” Korean J Ophthalmol 32(2): 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. (2006). “Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors.” Blood 107(9): 3600–3608. [DOI] [PubMed] [Google Scholar]
- Zhao J, et al. (2016). “Association of plasmacytoid dendritic cells with B cell infiltration in minor salivary glands in patients with Sjogren’s syndrome.” Mod Rheumatol 26(5): 716–724. [DOI] [PubMed] [Google Scholar]
- Zheng D, et al. (2012). “Lipopolysaccharide-pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease.” Kidney Int 81(9): 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, et al. (2010). “Identification of Novel Subsets of Plasmacytoid and Conventional Dendritic Cells in the Cornea.” Investigative Ophthalmology & Visual Science 51(13): 1544–1544. [Google Scholar]
- Zhong Z, et al. (2020). “Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis.” Prog Retin Eye Res: 100866. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. (2015). “Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing.” Stem Cells 33(5): 1566–1576. [DOI] [PubMed] [Google Scholar]
- Zhu H, et al. (2018). “Neuropilin-1 regulated by miR-320 contributes to the growth and metastasis of cholangiocarcinoma cells.” Liver Int 38(1): 125–135. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, et al. (2017). “Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis.” Sci Rep 7: 40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, et al. (2001). “Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells.” Nat Med 7(12): 1339–1346. [DOI] [PubMed] [Google Scholar]
- Zoukhri D (2006). “Effect of inflammation on lacrimal gland function.” Exp Eye Res 82(5): 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga EI, et al. (2004). “Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection.” Nat Immunol 5(12): 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1. Multiphoton microscopy of normal limbus of transgenic DPE-GFP×RAG1−/− mouse. Representative 3D reconstructed multiphoton microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) during steady state. The video strategic localization of pDCs in intimate proximity to limbal vessels.
Supplementary Video 2. Confocal microcopy of naïve whole-mounted cornea indicating close proximity of plasmacytoid dendritic cells and corneal nerves. Representative 3D reconstructed confocal micrograph of a corneal whole-mount, stained for pan-immune cell marker, CD45 (red), pDC marker, PDCA-1 (green), and nerve marker (βIII-tubulin), showing close proximity of resident corneal pDCs and subbasal nerve plexus during steady state.
Supplementary Video 3. Multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video demonstrates interaction of pDCs with limbal vessels and alterations in the morphology of pDCs following suture placement. Further the video highlights that pDCs engulf the vessels and actively extend their dendrites over the vessels.
Supplementary Video 4. Magnified multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Magnified representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video highlights pDCs extending their dendrites over the vessels.
Supplementary Video 5. Multiphoton microscopy of limbus of transgenic DPE-GFP×RAG1−/− mouse on day 7 following corneal suture placement. Representative intravital microscopy of the limbus in a transgenic DPE-GFP×RAG1−/− mouse with specifically GFP-tagged pDCs (green) receiving intravenous injection of quantum dots (red), on day 7 following corneal suture placement. The video highlights patrolling movements of pDCs in limbal vessels and their morphologic alterations following induction of neovascularization by suture placement.


