Abstract
Self-reported experiences of discrimination are associated with a number of negative health outcomes. However, the neurobiological correlates of discrimination remain elusive. Recent neuroimaging work suggests that the amygdala is sensitive to forms of social adversity and the insula is involved in assessments of trust. We hypothesized that functional connectivity (FC) of these brain regions may be associated with discrimination in older Black adults. One-hundred and twenty-four nondemented older Black adults participating in the Minority Aging Research Study or the Clinical Core study of the Rush Alzheimer’s Disease Center completed a measure of self-reported experiences of discrimination and a 3T MRI brain scan including structural T1 and resting-state fMRI EPIBOLD sequences. The right and left amygdala and insula regions were anatomically delineated as ROIs according to the Harvard-Oxford Brain Atlas and whole-brain voxelwise FC analyses were conducted using default parameters in the CONN toolbox. In regression analyses controlling for demographics and global cognition, self-reported experiences of discrimination were associated with (1) greater FC between the left insula and the bilateral intracalcarine cortex, (2) weaker FC between the left insula and the left dorsolateral prefrontal cortex, and (3) weaker FC between the right insula and the left supplementary motor area. Amygdala analyses yielded no significant findings. Greater self-reported experiences of discrimination are associated with differential insula functional connectivity in older adults. More specifically, results suggest that discrimination is associated with differential connectivity of a key region (the insula) involved in trust perception.
Keywords: discrimination, resting-state fMRI, functional connectivity, insula
INTRODUCTION
The experience of discrimination, whether it be based on skin color, recent immigration status, religious affiliation, gender identity, sexual preference, or disability, can have a devastating impact upon a person’s sense of autonomy and wellbeing. The negative impact of discrimination on health has primarily focused on young and middle-aged adults (Versey and Curtin, 2016; Shepherd et al., 2017; Pascoe and Smart Richman, 2009; Basanez et al., 2013; Cheng and Mallickrodt, 2015; Chou et al., 2008; Clark, 2014; Pascoe and Smart Richman, 2009; Stock et al., 2017), but an emerging literature has begun to investigate the adverse health outcomes of discrimination and unfair treatment among older adults. Older Black adults are a particularly interesting population to interrogate the negative consequences of discrimination based on the historical context of their experiences in the United States. It is well-documented that Black adults born and raised in the early 1900’s lived through oppression and social disadvantage throughout much of their lives (National Research Council, 2004; Angel and Angel, 2006; Smiley and Fakunle, 2016; Noonan et al., 2016). Self-reported experiences of discrimination, a measure of perceptions of everyday mistreatment, have been used to understand whether and how stressful social conditions may impact health in an aging population. From this important body of literature, discrimination has become an important social determinant of health among Black adults and robustly associated with a number of poor mental and physical health outcomes, including depressive and other psychological symptoms, cardiovascular risk factors, and mortality (e.g., Lewis et al., 2010, Nadimpalli et al., 2015; Sutin et al., 2015; Barnes et al., 2008). Reports of discrimination (reviewed in Lewis et al., 2015) have even been linked to markers of premature aging including telomere length (Chae et al., 2014), oxidative stress (Szanton et al., 2012), and allostatic load (Brody et al., 2014).
Recent work has demonstrated that perceptions of discrimination are also associated with cognitive function (Barnes et al., 2012; Zahodne et al. 2017). Older Black adults who report more experiences of discrimination have lower episodic memory (Barnes et al., 2012) and faster rates of episodic memory decline (Zahodne et al., 2017). Although the underlying mechanisms linking discrimination to cognition are unknown, inflammatory mechanisms have been proposed by some investigators (Lewis et al., 2010; Friedman et al., 2009) and one recent report demonstrated that C-reactive protein may partially mediate the negative association between discrimination and episodic memory at baseline but not over time (Zahodne et al., 2019). However, whether self-reported experiences of discrimination may directly have an impact on the function and structure of the aging brain has not been the subject of investigation in older adults despite studies investigating other negative interpersonal experiences as they relate to brain aging in animal and human studies.
Multiple negative interpersonal experiences over time appear to have a cumulative impact upon the functional architecture of the brain. In work that perhaps may be most relevant to the study of discrimination, previous studies have demonstrated that chronic negative social experiences are associated with long-lasting changes in the functional architecture of resting state brain networks (Soares et al., 2013; Dias-Ferreira et al., 2009). For example, a growing body of evidence demonstrates that adverse social stressors, such as experiences of poverty (Barch et al., 2016) and trauma (Fortenbaugh et al., 2017), are associated with changes in the functional connectivity of the amygdala, a brain region historically known as an emotion processing center (Janak and Tye, 2015) for negative affect. Similarly, perceptions of trustworthiness of others appear to involve the functional connectivity of the insula (Castle et al., 2012; Belfi et al., 2015; Tashjian et al., 2019; Todorov et al., 2008), a brain region that is involved in the development of “gut” reactions to complex multisensory social situations (Jones, Ward, and Critchley, 2010; Bermudez-Rattoni, 2014). Given that self-reported experiences of discrimination have been frequently associated with both perceived social stress (Spence et al., 2016; Yang and Chen, 2018) and biological markers of stress (Ong et al., 2017; Cuevas et al, 2019), and are associated with greater mistrust (Glover et al., 2017; Hammond, 2010; Yang and Chen, 2018), it is plausible that experiences of discrimination could affect changes in the functional connectivity of the amygdala and insula in particular given their implication in social constructs closely linked to discrimination. However, to our knowledge, no study has investigated the link between self-reported experiences of discrimination and functional connectivity of these two regions in older adults.
We hypothesized that functional connectivity of the amygdala and insula would be associated with self-reported experiences of discrimination in a group of non-demented older Black adults after adjusting for the effects of age, education, sex, and cognitive function. Given the lack of previous neuroimaging work on discrimination in older adults, we have chosen to focus on these two brain regions specifically given their well-established links to social constructs associated with discrimination. We investigated this hypothesis in a merged data set of over a hundred non-demented older Black participants from three ongoing longitudinal cohort studies of aging at the Rush Alzheimer’s Disease Center that are harmonized in data collection: the Minority Aging Research Study (MARS), the Rush African American Clinical Core, and the Rush Memory and Aging Project (MAP). Blacks were the focus of the present study given greater rates of endorsed self-reported experiences of discrimination attributable to historical institutionalized racism and social injustice (Barnes et al., 2004). To our knowledge this is the first neuroimaging study of discrimination in older adults, and the largest neuroimaging study of discrimination in any age group.
METHODS
Participants
Participants were enrolled in one of three longitudinal cohort studies administered by the Rush Alzheimer’s Disease Center (RADC). These included the Minority Aging Research Study (MARS; Barnes et al., 2012), the Clinical Core study, and the Memory and Aging Project (MAP; Bennett et al., 2018). These longitudinal-epidemiologic cohort studies of aging are based in the Chicago area, harmonized in data collection with the same team of evaluators and data management approaches, and allow for cross-utilization of protocols and data pooling at the item level. Participants in all three studies signed an informed consent and the studies were approved by an Institutional Review Board of Rush University Medical Center. Inclusion criteria are that persons are at least 65 years of age, are nondemented at enrollment, and submit to annual assessments. In addition, the Memory and Aging Project also required organ donation (brain, select muscle and nerve) at the time of death as an inclusion criterion. The only exclusion criterion for all three studies was no known dementia or prescribed medication for dementia. On August 31, 2017, all Black older adult participants who had completed a 3T brain MRI scan and a self-reported experiences of discrimination scale were identified, yielding 150 possible participant datasets for use in the present study. Of these, 24 datasets were determined to have failed internal MRI quality assurance metrics, leaving 126 usable participant MRI datasets. Of these 126 participants, one participant was deemed to have probable Alzheimer’s dementia according to previously published criteria (Barnes et al., 2018; Bennett et al., 2012), leaving 125. When considering the cohort studies that participants came from, only 1 participant came from MAP, 79 came from MARS, and 45 came from the Clinical Core. Since only 1 came from MAP and this person was noticeably younger than the rest of the sample, we removed this participant, leaving 124 non-demented older Black participant datasets for the current analysis. No statistical differences were observed on any variable of interest between participants from MARS or the Clinical Core (Supplementary Table 1). On average, MRI scans were collected within 3 months of completing the behavioral measures (mean number of days=92.07, standard deviation of days=49.40, range of days=7–192).
Self-Reported Experiences of Discrimination
Self-reported experiences of discrimination were assessed using a 9-item scale that asked participants to indicate how frequently they experience mistreatment in everyday life without reference to age, race, or any other social status characteristic (Williams et al., 1997). Examples of individual items include “You are treated with less respect than other people”, “You are treated with less courtesy than other people”, and “People act as if they are better than you are.” Frequency for each item is rated on a four-point scale (“often”, “sometimes”, “rarely”, and “never”), and an item is scored 1 when “often” or “sometimes” is endorsed (otherwise it is scored 0). The range for this measure is 0 to 9. This scale has been used in prior studies of older Black adults and was found to have good internal consistency and validity (Barnes et al., 2004; Lewis et al., 2010).
Cognition
Global cognitive ability was determined using a well-established battery of 18 cognitive tests measuring a broad array of cognitive abilities (Wilson et al., 2015; Bennett et al., 2018; Wilson et al., 2003). The battery included 7 measures of episodic memory (Word List Memory, Word List Recall and Word List Recognition from the procedures established by the CERAD; immediate and delayed recall of Logical Memory Story A; and immediate and delayed recall of the East Boston Story), 2 measures of semantic memory (Verbal Fluency and Boston Naming), 2 measures of visuospatial ability (Judgment of Line Orientation and Standard Progressive Matrices), 4 measures of perceptual speed (oral version of the Symbol Digit Modalities Test, Number Comparison, Stroop Color Naming, Stroop Word Reading [Interference]), and 3 measures of working memory (Digit Span subtests forward and backward of the Wechsler Memory Scale-Revised and Digit Ordering). As previously described (Wilson et al., 2015), scores on the tests were transformed into individual z-scores according to the mean and standard deviation of the baseline cognitive evaluation of the parent study sample, and averaging the z-scores across all tests produced a global cognitive ability composite that was utilized in the present study. The cognitive measures utilized by the Rush Alzheimer’s Disease Center have been found to be sensitive to cognition, cognitive impairment, and cognitive decline in older Black adults (Lamar et al., 2019; Turner et al., 2017; Turner et al., 2015; Arvanitakis et al., 2018; Lim et al., 2018).
Neuroimaging
Brain Magnetic Resonance Imaging (MRI) scans were conducted on all participants with a Phillips 3 Tesla MRI scanner at the same scanner site. A 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence was used to collect high resolution T1-weighted anatomical data. Parameters of the MPRAGE scans were as follows: echo time (TE) = 3.7 ms, repetition time (TR) = 8.1 s, preparation time = 961.9 ms, flip-angle = 8°, field-of-view (FOV) = 24.0 cm × 22.8 cm, 181 sagittal slices, slice thickness = 1 mm, no gap, 240 × 228 acquisition matrix, parallel imaging acceleration factor of 2 along the phase encoding direction, for a total imaging time of 5 minutes and 57.5 seconds. Resting state MRI data was acquired using a 2D gradient echo echo-planar imaging (EPI) sequence with the following parameters: TR = 3000 ms; TE = 30 ms; flip angle = 80°; 45 axial slices; 3.3 mm slice thickness; acquisition/reconstruction matrix 64 × 59; FOV = 21.2 cm × 19.9 cm; 160 time-points/volumes; scan time = 8 min 17.9 sec. Participants were instructed to keep their eyes closed.
Preprocessing and quality assurance of functional and structural MRI data was performed according to the default pipeline implemented in the CONN Toolbox version 18.a (https://web.conn-toolbox.org/home; Whitfield-Gabrieli and Nieto-Castanon, 2012). Briefly, this included functional scan realignment, slice-timing correction, coregistration to MPRAGE, spatial normalization and smoothing according to a full-width half-maximum (FWHM) isotropic Gaussian kernel filter of 8 mm. Anatomical images were segmented according to grey matter, white matter, and cerebrospinal fluid maps, and functional and structural images were normalized to Montreal Neurological Institute (MNI) space (MNI152). These steps were conducted using Statistical Parametric Mapping version 12 (SPM12) software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). As a general quality assurance procedure in our group prior to any preprocessing, functional scans were checked according to an initial head movement exclusion of 1.9 mm translation in any axis and less than 1.9° angular rotation in any axis over any 10 second interval. In preprocessing, functional scans were then subjected to artifact and motion outlier identification using the Artifact Detection Toolbox (ART; https://www.nitrc.org/projects/artifact_detect/) according to conservative settings (95th percentile of normative sample). These settings identified time points as outliers if the global mean signal intensity exceeded 3 standard deviations or if movement from a preceding image exceeded a 0.5 mm deviation. These time points were included as regressors along with principal components delineated from anatomical noise regions (10 components for white matter, 5 components for cerebrospinal fluid) and realignment parameters during a denoising step. Finally, a band-pass filter was applied to the functional data with a frequency window of 0.008–0.09 Hz.
Statistical Analysis
Anatomically-derived regions of interest (ROIs) were delineated according to the Harvard-Oxford Brain Atlas per the CONN Toolbox protocol (Whitfield-Gabrieli and Nieto-Castanon, 2012). In the present study, these included the left and right amygdala and left and right insula (4 ROIs total, separately considered). A mean signal time course for each ROI was calculated, and analyses were conducted by examining the correlations between the ROI signal time course and the time series of every other voxel in the brain. The voxels showing significant functional connectivity to the ROIs were identified as those whose correlation differed significantly from 0 based on Fisher’s z-transformation of the correlation. In order to interrogate clusters of voxels functionally connected to each ROI, two thresholds were sequentially applied. First, on individual voxels (p-value<0.007), next on individual clusters (corrected for multiple comparisons according to a False Discover Rate (FDR) p-value<0.05, two-tailed). A two-tailed test was selected as we a priori believed the experience of self-reported experiences of discrimination could involve differences in functional connectivity of the regions of interest in either direction (strengthening or weakening). Next, results were then correlated with self-reported experiences of discrimination, while controlling for age, education, sex, and global cognition. We controlled for age and sex as these are common demographic factors that are controlled in neuroimaging studies. We controlled education and global cognition as these may impact a person’s ability to self-report experiences of discrimination. Functional connectivity analyses were conducted using the CONN Toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012).
RESULTS
Descriptive Data
The sample consisted of 124 older Black adults. The mean education of the sample was beyond the high school years and the sample was predominantly female. As would be expected in a nondemented group of older adults, the mean MMSE score was within the average range and the global cognition score was near 0. Self-reported experiences of discrimination were endorsed at a range of 0 to 7, with a mean of 1.33 (s.d.=1.85).
Functional Connectivity
Amygdala
Functional connectivity analyses to the left and right amygdala as defined by the Harvard-Oxford Brain Atlas did not yield any significant associations with self-reported experiences of discrimination adjusting for age, education, sex, and global cognition.
Insula
Self-reported experiences of discrimination were significantly associated with functional connectivity between the left insula ROI and two clusters in models adjusting for age, education, sex, and global cognitive function. Greater self-reported experiences of discrimination were associated with greater functional connectivity between the left insula and a cluster primarily within the medial intracalcarine cortex (cluster size=1469 voxels, t-value=4.41, FDR cluster p-value<0.001). Greater self-reported experiences of discrimination were associated with weaker functional connectivity between the left insula and a cluster primarily within the right dorsolateral prefrontal cortex (cluster size=471 voxels, t-value=−4.91, FDR cluster p-value=0.016). Self-reported experiences of discrimination were significantly associated with functional connectivity of the right insula and one cluster. Greater self-reported experiences of discrimination were associated with weaker functional connectivity between the right insula and a cluster primarily in the left supplementary motor cortex region (cluster size=778 voxels, t-value=−5.26, FDR cluster p-value=0.002).
DISCUSSION
The current study examined the association between self-reported experiences of discrimination and the functional connectivity of the amygdala and insula with other brain regions in a group of 124 non-demented older Black adults participating in one of three community-based cohort studies of aging. We found that self-reported experiences of discrimination were associated with variations in insula functional connectivity to particular brain regions after adjusting for the effects of age, education, sex, and global cognitive ability. Specifically, greater self-reported experiences of discrimination were associated with stronger functional connectivity between the left insula and bilateral intracalcarine cortex, weaker functional connectivity between the left insula and right dorsolateral prefrontal cortex, and weaker functional connectivity between the right insula and left supplementary motor cortex. Contrary to our hypothesis, functional connectivity of the amygdala was not associated with self-reported experiences of discrimination in statistical models. Findings suggest that the experience of discrimination may correspond with differential brain associations indicative of increased or decreased connectivity to a key region involved in trust perception.
Little is known about the neurobiological underpinnings of accumulated self-reported experiences of discrimination in older adults. To our knowledge, only one neuroimaging study directly measured brain activity during an experience that was designed to mimic discrimination, and this was done in a small sample of young adults. In this study, eighteen Black individuals with a mean age of 21.4 years old underwent event-related functional MRI brain imaging while experimentally undergoing a social exclusion experience (Masten et al., 2011). In persons who attributed the social exclusion to discrimination, authors observed correlated neural activity in the rostral anterior cingulate, lateral temporal cortex, brainstem, dorsal anterior cingulate, and motor cortices. Activity in these regions was interpreted as neurobiological mechanisms for greater emotional regulation and corresponding “reduced social pain” among the young participants.
A key brain region for human emotional regulation is the insula, which has been implicated in a number of higher order cognitive, emotional, and perceptual functions (Jones, Ward, and Critchley, 2010). Once chiefly believed to be involved in gustatory perception, a meta-analysis found that the insula represented an anatomical overlap of multiple functional brain systems devoted to multisensory perception, socioemotional processing, and cognitive processing, and therefore argued this region as key for maintaining the integrated subjective experience of an individual’s world (Kurth et al., 2010). One function ascribed to the insula is development of a “gut” reaction to a perceived situation or stimuli (Jones, Ward, and Critchley, 2010), and developing recognition memory for this complex integrated association (Bermudez-Rattoni, 2014). This response is similar to a conditioned taste aversion to a disliked food (e.g., Gallo, Roldan, and Bures, 1992), but extends far beyond the gustatory realm.
A function recently ascribed to the insula is assessment of trustworthiness. In a functional neuroimaging study (Castle et al., 2012), both younger and older participants rated faces for how trustworthy (or untrustworthy) they were. Activity of the insula was associated with assessments of untrustworthiness, and younger participants had greater insula activity than older participants to faces that were deemed untrustworthy. The study investigators interpreted the diminished insula activity to untrustworthy faces in older adults as a potential neurobiological mechanism for greater susceptibility to scam and fraud in advanced age. It stands to reason that this brain region, which is involved in the development of “gut” reactions to complex multisensory social situations and assessment of trustworthiness of persons, would be functionally linked to the experience of social discrimination, a far-reaching and complex socioemotional, perceptual, and cognitive experience.
We observed self-reported experiences of discrimination were associated with stronger functional connectivity between the left insula and the bilateral intracalcarine cortex. Given the intracalcarine cortex’s well-established role in visual perception (Grill-Spector and Malach, 2004), this stronger connectivity might be interpreted as a greater necessary integration between visual perceptual systems and the insula due to a proactive need to process and assess social situations for potential discrimination and trust from a “gut” perspective. Self-reported experiences of discrimination were also associated with weaker functional connectivity between the left insula and right dorsolateral prefrontal cortex. Since the dorsolateral prefrontal cortex is associated with higher order control of cognitive and other systems (Miller and Cohen, 2001), this finding could reflect an un-reigning or release of the insula in its integrative “gut” discernment, trust assessment role. Finally, self-reported experiences of discrimination were associated with weaker functional connectivity between the right insula and left supplementary motor cortex. The interpretation of this association is less apparent. The functions of the supplementary motor area appear to be heterogeneous and less well known, though there is general agreement that they involve the linking of cognition with motor action (Nachev, Kennard, and Husain, 2008). It is possible that older Black adults who have experienced more discrimination have had to learn to separate “gut” feelings from physical reactions as a socially normative response, though this interpretation is admittedly speculative. More research is needed to understand the links between these brain regions and the insula in the context of aging.
The amygdala has recently been implicated as sensitive to self-reported experiences of discrimination in a sample of 90 middle-aged adults participating in an HIV study (Clark et al., 2018). Given the amygdala’s role as an emotional processing center, it is reasonable to expect functional connectivity variations to this region may be associated with self-reported experiences of discrimination. Contrary to this recent study, we did not observe any associated functional connectivity variations to the amygdala. Multiple reasons could account for the difference in findings between our study and this previous study. Our participants were on average much older, and this could suggest that the amygdala may be more sensitive to self-reported experiences of discrimination in younger samples. The Clark et al. study also utilized a spherical seed-region of interest approach to delineate functional connectivity to the amygdala, while we utilized an anatomical region of interest approach defined by the Harvard-Oxford Brain Atlas. Differences in location of regions of interest, even those commonly described as being within the same anatomical brain region, have been known to yield quantitatively different results (Marrelec and Fransson, 2011). It is notable that the Clark et al. study did not consider the insula as a region of interest for self-reported experiences of discrimination, despite the insula and amygdala sharing strong reciprocal interconnections (e.g., Gallo, Roldan, and Bures, 1992).
Limitations of the present study need to be acknowledged. First, the present study is cross-sectional and therefore causal inferences cannot be made on the association between insula functional connectivity variations and self-reported experiences of discrimination. A possible alternative interpretation of these results is that participants who show this pattern of connectivity between brain regions may be more inclined to report a higher rate of discrimination. However, it is also important to note that Black adults are able to accurately interpret ambiguous social interactions such as social partners’ implicit bias (e.g., Dovidio et al., 2002; McConnell & Liebold, 2001; Sekaquaptewa et al., 2003). Second, we do not have real-world documentation of discriminatory behavior perpetrated against participants. As such, this is a study of self-reported experiences of discrimination and not actual experienced discrimination. Third and related to this point, no data was available on how traumatic was the experienced discrimination. It is reasonable to assume that more traumatic experiences may be associated with more variation in insula functional connectivity, though this could not be tested. Fourth, the amount of self-reported experiences of discrimination was generally low in our sample. This is consistent with previous work (Barnes et al., 2008) and may reflect the emphasis of the scale on minor versus major experiences of discrimination, or the cohort experience of older Black adults who were more socially expected to manage discrimination than younger Black adults after the civil rights movement (Brown and Barnes-Nacoste, 1993). Fifth, our study was constrained by our focus on the amygdala and insula. It is possible other brain regions may be involved in perceived discrimination that we have not considered in this study. Sixth, there appears to be distinctions between the functions and functional connectivity characteristics of the anterior and posterior insula. Our current methodology did not allow for the exploration of whether our findings are driven by different parts of the insula, though this may be a direction of future study. Finally, we do not know whether the discrimination endorsed by participants is due to race specifically, though it should be noted that a recent review found the health impact of discrimination on Black adults is the same regardless of whether or not it is based on race (Lewis et al., 2015).
Despite these limitations, the present study has a number of strengths. These include (1) the use of a large, well-characterized sample of older Black adults, (2) the ability to account for multiple factors that could impact brain functional connectivity findings such as age, education, sex, and global cognition, and (3) robust neuroimaging statistical constraints to control for multiple comparisons and minimize the chance of spurious findings. Our findings suggest that self-reported experiences of discrimination may possibly have an impact on brain functional architecture in old age specifically involving the insula. Longitudinal studies are needed to clarify the causal direction of this link. The implications of this work are far-reaching and suggest that self-reported experiences of discrimination may be linked with functional neurobiology. As such, this may be viewed as a compelling example of a social determinant of health interacting with brain functional architecture. To our knowledge, this is the first neuroimaging study to demonstrate self-reported experiences of discrimination are associated with differential functional connectivity of brain regions in older adults, and the largest neuroimaging study of self-reported experiences of discrimination in any age group.
Supplementary Material
Figure 1.
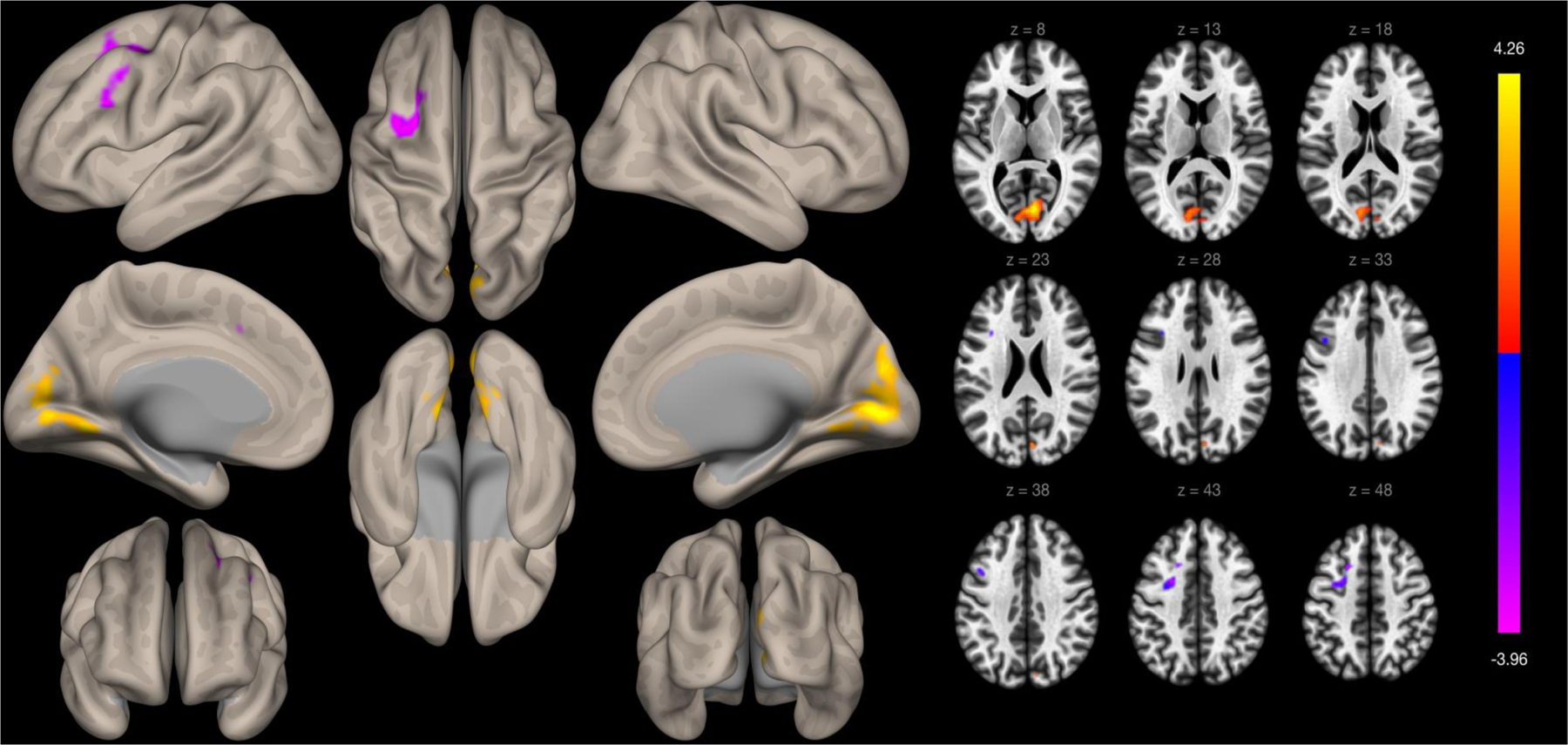
Whole-brain voxelwise functional connectivity to the left insula defined by the Harvard-Oxford Brain Atlas. Clusters are presented on cortical surface area maps on the left side of the figure and in axial slices on the right side of the figure.
Figure 2.
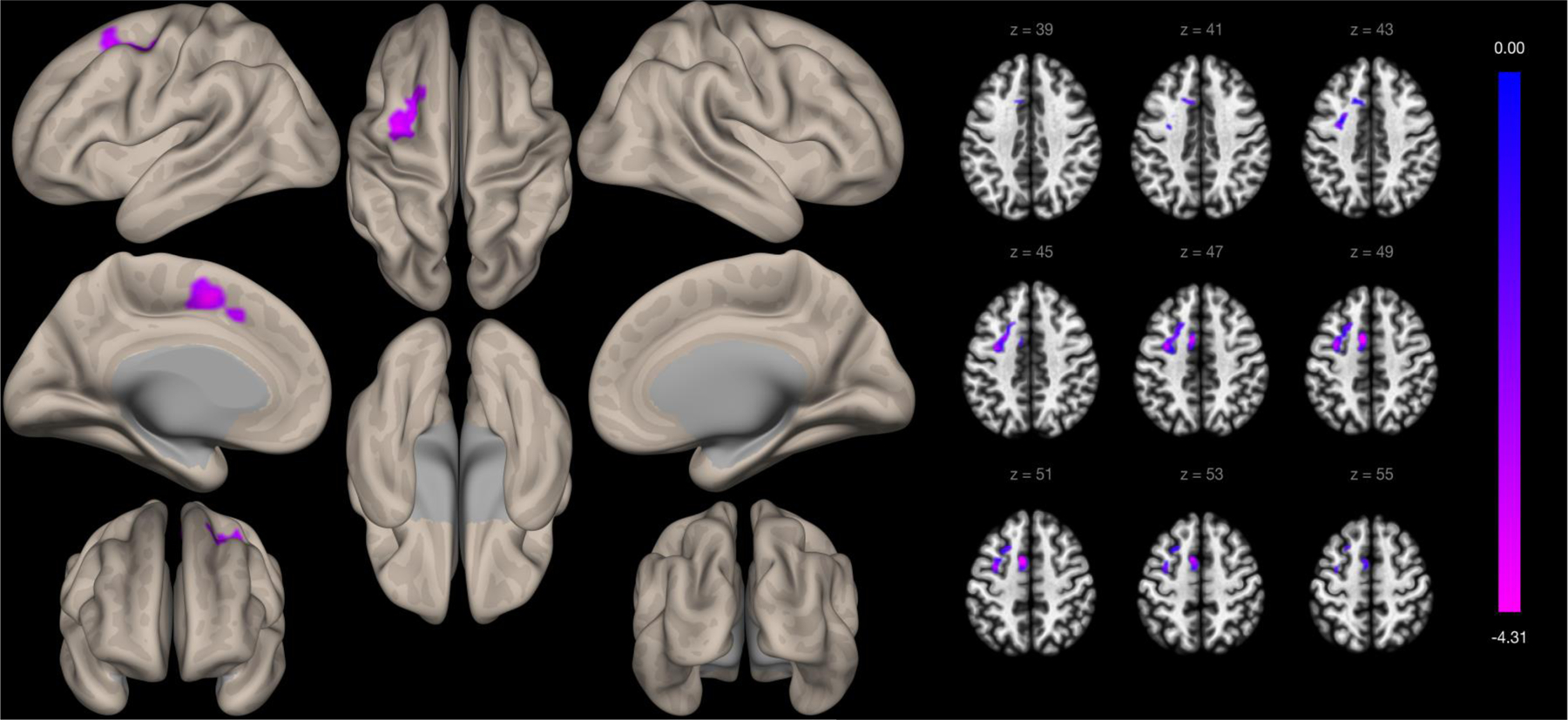
Whole-brain voxelwise functional connectivity to the right insula defined by the Harvard-Oxford Brain Atlas. Clusters are presented on cortical surface area maps on the left side of the figure and in axial slices on the right side of the figure.
Table 1.
Demographic, cognitive, and self-reported experiences of discrimination descriptive data.
| Sample (n = 124) | |
|---|---|
| Age (years) | |
| Mean (SD) | 74.95 (6.28) |
| Range | 62.29 – 90.10 |
| Education (years) | |
| Mean (SD) | 15.36 (3.43) |
| Range | 9 – 30 |
| Sex (% Female) | 85.6% (n = 107) |
| MMSE | |
| Mean (SD) | 28.23 (1.64) |
| Range | 24–30 |
| Global Cognition Z-score | |
| Mean (SD) | 0.12 (0.47) |
| Range | −1.29 – 1.15 |
| Self-Reported Experiences of Discrimination | |
| Mean (SD) | 1.33 (1.85) |
| Range | 0 – 7 |
Table 2.
Significant clusters identified in functional connectivity analyses.
| Region of Interest | Cluster # | Location (x, y, z) | Total # voxels in cluster | Brain regions | # voxels in specific region | t-value | Size p-value FDR | Size p-value FWE |
|---|---|---|---|---|---|---|---|---|
| Left Insula | 1 | 6, −78, 6 | 1469 | R Intracalcarine cortex | 360 | 4.41 | 0.000006 | 0.000007 |
| R Lingual gyrus | 217 | |||||||
| L Lingual gyrus | 199 | |||||||
| L Intracalcarine cortex | 178 | |||||||
| R Cuneal cortex | 120 | |||||||
| R Supracalcarine cortex | 53 | |||||||
| L Cerebellum 4 and 5 | 34 | |||||||
| L Cuneal cortex | 33 | |||||||
| L Cerebellum 6 | 15 | |||||||
| L Supracalcarine cortex | 9 | |||||||
| R Occipital Pole | 6 | |||||||
| L Occipital Fusiform Gyrus | 3 | |||||||
| Precuneus Cortex | 2 | |||||||
| Vermis 4 & 5 | 2 | |||||||
| L temporal occipital fusiform cortex | 1 | |||||||
| R Cerebellum 4 5 | 1 | |||||||
| 2 | −20, 16, 46 | 471 | L Middle frontal gyrus | 195 | −4.91 | 0.016304 | 0.034069 | |
| L Superior frontal gyrus | 63 | |||||||
| L Precentral gyrus | 31 | |||||||
| L Interior frontal gyrus, pars opercularis | 7 | |||||||
| L Paracingulate | 1 | |||||||
| Right Insula | 1 | −8, 4, 50 | 778 | L Supplementary motor cortex | 175 | −5.26 | 0.001694 | 0.001591 |
| L Middle frontal gyrus | 129 | |||||||
| L Precentral gyrus | 116 | |||||||
| L Superior frontal gyrus | 104 | |||||||
| L Paracingulate gyrus | 35 |
Acknowledgements:
This work was supported by the National Institute on Aging at the National Institutes of Health grants RF1AG022018 and R01AG056405 to L.L.B, P30AG010161 and R01AG017917 to D.A.B., and R01AG055430 to S.D.H. The authors gratefully thank the Minority Aging Research Study, Rush Memory and Aging Project, and Clinical Core staff and participants.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Angel JL, & Angel RJ (2006). Minority group status and healthful aging: social structure still matters. Am J Public Health, 96, 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, et al. (2018). Body Mass Index and Decline in Cognitive Function in Older Black and White Persons. J Gerontol A Biol Sci Med Sci, 73 (2), 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barch D, Pagliacco D, Belden A, Harms MP, Gaffrey M, Sylvester CM, Tillman R, & Luby J (2016). Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty school-age depression. Am J Psychiatry, 173, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes LL, de Leon CFM, Lewis TT, Bienias JL, Wilson RS, Evans DA (2008). Perceived Discrimination and Mortality in a Population-Based Study of Older Adults. Am J Public Health, 98, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, & Wilson RS (2012). Perceived discrimination and cognition in older African Americans. J Int Neuropsychol Soc, 18, 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Bennett DA, & Evans DA (2004). Racial differences in perceived discrimination in a community population of older Blacks and Whites. J Aging Health, 16, 315–337. [DOI] [PubMed] [Google Scholar]
- 7.Barnes LL, Shah R, Aggarwal NT, Bennett DA, Schneider JA (2012). The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res, 9(6), 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basanez T, Unger JB, Soto D, Crano W, & Baezconde-Garbanati L (2013). Perceived discrimination as a risk factor for depressive symptoms and substance use among Hispanic adolescents in Los Angeles. Ethn Heal, 18, 244–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfi AM, Koscik TR, & Tranel D (2015). Damage to the insula is associated with abnormal interpersonal trust. Neuropsychologia, 71, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, & Schneider JA (2012). Religious Orders Study and Rush Memory and Aging Project. Curr Alzheimer Res, 9(6), 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermundez-Rattoni F (2014). The forgotten insular cortex: Its role on recognition memory formation. Neurobiology of Learning and Memory, 109, 207–216. [DOI] [PubMed] [Google Scholar]
- 12.Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SR (2014). Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev, 85, 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RE, & Barnes-Nacoste RW (1993). Group consciousness and political behavior. In Jackson JS, Chatters LM, & Taylor RJ (Eds.), Aging in Black America (pp. 217–232). Newbury Park, CA: Sage. [Google Scholar]
- 14.Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, & Taylor SE (2012). Neural and behavioral age differences in perceptions of trust. PNAS, 109, 20848–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, et al. (2014). Discrimination, racial bias, and telomere length in African-American men. Am J Prev Med, 46, 103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng HL, & Mallinckrodt B (2015). Racial/ethnic discrimination, posttraumatic stress symptoms, and alcohol problems in a longitudinal study of Hispanic/Latino college students. J Couns Psychol, 62, 38–49. [DOI] [PubMed] [Google Scholar]
- 17.Chou K (2012). Perceived discrimination and depression among new migrants to Hong Kong: the moderating role of social support and neighborhood collective efficacy. J Affect Disord, 138(1–2), 63–70. [DOI] [PubMed] [Google Scholar]
- 18.Clark TT (2014). Perceived discrimination, depressive symptoms, and substance use in young adulthood. Addict Behav, 39, 1021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark US, Miller ER, & Hegde RR (2018). Experiences of discrimination are associated with greater resting amygdala activity and functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging, 3, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coley SL, Mendes de Leon CF, Ward EC, Barnes LL, Skarupski KA, & Jacobs EA (2017). Perceived discrimination and health-related quality-of-life: gender differences among older African Americans. Qual Life Res, 26, 3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocker J, & Major B (1989). Social stigma and self-esteem: The self-protective properties of stigma. Psychological Review, 96, 608–630. [Google Scholar]
- 22.Cuevas A, Wang K, Williams D, Mattei J, Tucker KL, & Falcon L The association between perceived discrimination and allostatic load in the Boston Puerto Rican Health Study. Psychosomatic Medicine, 81, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, et al. (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325, 621–625. [DOI] [PubMed] [Google Scholar]
- 24.Dovidio JF, Kawakami K, Gaertner SL. Implicit and explicit prejudice and interracial interaction. J Pers Soc Psychol. 2002;82(1):62–68. [DOI] [PubMed] [Google Scholar]
- 25.Fortenbaugh FC, Corbo V, Poole V, McGlinchy R, Milberg W, Salat D, DeGutis J, & Esterman M (2017). Interpersonal early-life trauma alters amygdala connectivity and sustained attention performance. Brain and Behavior, 7, e00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman EM, Williams DR, Singer BH, Ryff CD (2009). Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain Behav Immun, 23, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo M, Roldan G, & Bures J (1992). Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behavioral Brain Research, 52, 91–97. [DOI] [PubMed] [Google Scholar]
- 28.Glover LM, Sims M, & Winters K (2017). Perceived discrimination and reported trust and satisfaction with providers in African Americans: The Jackson Heart Study. Ethn Dis, 27, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill-Spector K, & Malach R (2004). The human visual cortex. Ann Rev Neurosci, 27, 649–677. [DOI] [PubMed] [Google Scholar]
- 30.Hammond WP (2010). Psychosocial correlates of medical mistrust among African American men. Am J Community Psychol, 45, 87–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janak PH, & Tye KM (2015). From circuits to behavior in the amygdala. Nature, 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CL, Ward J, & Critchley HD (2010). The neuropsychological impact of insular cortex lesions. J Neurol Neurosurg Psychiatry, 81, 611–618. [DOI] [PubMed] [Google Scholar]
- 33.Kurth F, Zilles K, Fox PT, Laird AR, & Eickhoff SB (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct, 214, 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamar M, et al. (2019). Relationship of Early-Life Residence and Educational Experience to Level and Change in Cognitive Functioning: Results of the Minority Aging Research Study. J Gerontol B Psychol Sci Soc Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis TT, Aiello AE, Leurgans S, Kelly J, & Barnes LL (2010). Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun, 24, 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol, 11, 407–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim ASP, et al. (2018). Seasonal Plasticity of Cognition and Related Biological Measures in Adults With and Without Alzheimer Disease: Analysis of Multiple Cohorts. PLoS Med, 15 (9), e1002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrelec G, & Fransson P (2011). Assessing the influence of different ROI selection strategies on functional connectivity analyses of fMRI data acquired during steady-state conditions. PlosOne, 6(4), e14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masten CL, Telzer EH, & Eisenberger NI (2011). An fMRI investigation of attributing negative social treatment to racial discrimination. J Cog Neurosci, 23, 1042–1051. [DOI] [PubMed] [Google Scholar]
- 40.McConnell AR, & Leibold JM (2001). Relations among the Implicit Association Test, discriminatory behavior, and explicit measures of racial attitudes. Journal of Experimental Social Psychology, 37(5), 435–442. [Google Scholar]
- 41.Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- 42.Nachev P, Kennard C, & Husain M (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9, 856–869. [DOI] [PubMed] [Google Scholar]
- 43.Nadimpalli SB, James BD, Yu L, Cothran F, & Barnes LL (2015). The association between discrimination and depressive symptoms among older African Americans: the role of psychological and social factors. Exp Aging Res, 41, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council. (2004). Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Anderson NB, Bulatao RA, and Cohen B, Editors. Panel on Race, Ethnicity, and Health in Later Life. Committee on Population, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 45.Noonan AS, Velasco-Mondragon HE, & Wagner FA (2016). Improving the health of African Americans in the USA: an overdue opportunity for social justice. Public Health Rev, 37, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong AD, Williams DR, Nwizu U, Gruenwald TL (2017). Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultur Divers Ethnic Minor Psychol, 23, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135(4), 531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekaquaptewa D, Espinoza P, Thompson M, Vargas P, von Hippel W (2003). Stereotypic explanatory bias: Implicit stereotyping as a predictor of discrimination. Journal of Experimental Social Psychology, 39, 75–82 [Google Scholar]
- 49.Shepherd CCJ, Li J, Cooper MN, Hopkins KD, & Farrant BM (2017). The impact of racial discrimination on the health of Australian Indigenous children aged 5–10 years: analysis of national longitudinal data. Int J Equity Health, 16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smiley C, & Fakunle D (2016). From “brute” to “thug:” the demonization and criminalization of unarmed Black male victims in America. J Human Behav Soc Environ, 26, 350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spence ND, Wells S, Graham K, & George J (2016). Racial discrimination, cultural resilience, and stress. Can J Psychiatry, 61, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares JM, Sampaio A, Ferreira LM, Santos NC, & Marques P (2013). Stress impact on brain networks. PLoS ONE, 8(6), e66500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanley Damian A., Peter Sokol-Hessner, Fareri Dominic S., Perino Michael T., Delgado Mauricio R., Banaji Mahzarin R., and Phelps Elizabeth A.. 2012. Race and reputation: Perceived racial group trustworthiness influences the neural correlates of trust decisions. Philosophical Transactions of the Royal Society B: Biological Sciences 367(1589): 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock ML, Peterson LM, Molloy BK, & Lambert SF (2017). Past racial discrimination exacerbates the effects of racial exclusion on negative affect, perceived control, and alcohol-risk cognitions among Black young adults. J Behav Med, 40(3), 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutin AR, Stephan Y, Carretta H, & Terracciano A (2015). Perceived discrimination and physical, cognitive, and emotional health in older adulthood. Am J Geriatr Psychiatry, 23(2), 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szanton SL, Rifkind JM, Mohanty JG, Miller ER 3rd, Thorpe RJ, et al. (2012). Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med, 19, 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tashjian SM, Guassi Moreira JF, Galvan A (2019). Multivoxel pattern analysis reveals a neural phenotype for trust bias. J Cogn Neurosci, 19, 1–16. [DOI] [PubMed] [Google Scholar]
- 58.Todorov A, Baron SG, & Oosterhof NN (2008). Evaluating face trustworthiness: a model based approach. SCAN, 3, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner A, et al. (2015). Depressive Symptoms and Cognitive Decline in Older African Americans: Two Scales and Their Factors. Am J Psychiatry, 23 (6), 568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Versey HS, & Curtin N (2016). The differential impact of discrimination on Black and White women. Soc Sci Res, 57, 99–115. [DOI] [PubMed] [Google Scholar]
- 61.Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- 62.Williams DR, Yu Y, Jackson JS, & Anderson NB (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. [DOI] [PubMed] [Google Scholar]
- 63.Wilson RS, Barnes LL, Bennett DA (2003). Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology, 25, 634–642. [DOI] [PubMed] [Google Scholar]
- 64.Wilson RS, Boyle PA, Yu L, Segawa E, Sytsma J, Bennett DA. (2015) Conscientiousness, dementia related pathology, and trajectories of cognitive aging. Psychol Aging, 30, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang T-C, & Chen D (2018). A multi-group path analysis of the relationship between perceived racial discrimination and self-rated stress: How does it vary across racial/ethnic groups? Ethn Health, 23, 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zahodne LB, Sol K, & Kraal Z (2017). Psychosocial pathways to racial/ethnic inequalities in late-life memory trajectories. J Gerontol B Psychol Sci Soc Sci, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zahodne LB, Kraal AZ, Sharifan N, Zaheed AB, & Sol K (2019). Inflammatory mechanisms underlying the effects of everyday discrimination on age-related memory decline. Brain Behav Immun, 75, 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


