Abstract
Background
Insufficient sleep affects circadian hormonal profiles and inflammatory markers, and may modulate attention, executive functioning and decision-making. Medical professionals and specifically resident physicians, who are involved in long-term nightshift schedules during their post-graduate training, are prone to acute and chronic sleep deprivation and disruption, putting them at risk for making medical errors.
Aim
Evaluate the impact of chronic and acute-on-chronic sleep deprivation and disruption among residents on selected physiological and cognitive measures.
Methods
Thirty-three medical and surgical residents were evaluated twice - at baseline and after a 26-hour shift. Eighteen young attending physicians who did not engage in nightshift schedules served as controls and were evaluated once. Measures included morning cortisol and high-sensitivity C-reactive protein (hs-CRP), computerized tests of attention and behavior, the Behavior Rating Inventory of Executive Function, a risk-taking questionnaire and the Pittsburgh Sleep Quality Index.
Results
Residents, but not attendings, reported chronic sleep disruption and deprivation. Residents at baseline exhibited reduced morning cortisol levels and elevated hs-CRP levels, compared to attendings. Residents at baseline had impaired global executive function compared to attendings. A nightshift with acute sleep deprivation further reduced residents’ executive function. Residents at baseline and after a nightshift demonstrated increased impulsivity and slower processing time than attendings. Residents and attendings did not differ in risk-taking tendencies which were assessed in a separate cohort.
Conclusions
In a real-life setting, resident physicians exhibit increased low-grade systemic inflammation (hs-CRP) and impaired HPA-axis function. Their chronic sleep curtailment is associated with greater impulsivity, slower cognitive processing, and impaired executive function. Future research is warranted to understand how improving working schedule by increasing sleep duration may minimize the short-term and potential long-term risks to physicians in training.
Keywords: Resident Training, Executive Function, Shift Work, Sleep and Stress, Cortisol, Burnout, Cognitive Function, Biomarkers
Introduction
Sleep is a pillar of human health1. Insufficient sleep has deleterious effects on health2,3, increasing the risk of cardiovascular disease, premature aging and obesity4. Insufficient sleep is also associated with poor mental and cognitive functioning5,6. Even one night of sleep deprivation, defined as having less than 5 hours of uninterrupted sleep, leads to a detectable decline in cognitive performance7. Sleep disorders have been recently declared a public-health epidemic8, with about 25% of the US adult population suffering from chronic sleep-deprivation9. Sleep deprivation is particularly prevalent among shift-workers, including medical professionals10.
While sleep deprivation and disruption impairs cognitive functions such as attention, executive performance, and risk-taking behaviors, the biological underpinnings of these effects are not fully understood11. It was found that sleep deprivation and disruption affects the circadian hormonal and the immune systems12. For example, short sleep duration and sleep disruption increases C-reactive protein (CRP) levels13,14, and impaired sleep quality changes cortisol secretion patterns15. Relatedly, sleep deprivation increases systemic inflammation, which in turn disrupts the blood-brain barrier16 (for a more extensive review, see ref.17). Furthermore, it has been suggested that hormonal and inflammatory pathways can influence cognitive function18. For example, increased secretion of cortisol impairs some executive functions19 and higher levels of CRP are associated with impaired cognition20.
The first goal of the current study is to provide a comprehensive cognitive and biochemical evaluation of the effects of sleep disruption in the same study. Second, our study aims to assess the differential effects of chronic and acute-on-chronic sleep deprivation on cognitive impairments, and their biological underpinnings. Notably, most research to date has investigated the effect of acute sleep deprivation, i.e. a single night of insufficient sleep. Little information exists regarding the cognitive effects associated with chronic sleep deprivation, i.e. repetitive bouts of insufficient night sleep over prolonged periods of times21. Acute total sleep deprivation cannot be extrapolated to predict the effects of chronic sleep deprivation since they have common but also different neuro-behavioral consequences22.
We chose to focus our investigation on resident physicians. During their postgraduate training, residents are involved in nightshift schedules, which subject them to acute sleep deprivation and disruption, as well as to stress and fatigue23. Since their residency lasts several years, their sleep disruption is also chronic24,25. This provides a unique opportunity to test both chronic and acute-on-chronic sleep deprivation. Furthermore, cognition impairment among physicians is particularly worrisome, as such impairment may cause medical errors that might harm patients26,27. Some studies documented the overall impact of nightshift schedules on residents’ cognitive and performance skills28–35. For example, implementation of schedules with protected sleep period while on call resulted in an increase in overnight sleep duration and improved alertness the morning after the nightshift30. Other studies have focused on the assessment of residents before and after a night with little sleep. For example, the effect of a nightshift on residents’ cognitive performance is comparable to the effect of ingesting alcohol at levels close to the legal limit for driving36. The current study goes beyond previous research on residents’ sleep in (1) testing the hormonal and inflammatory pathways that may connect sleep and cognition, a (2) testing both chronic and acute-on-chronic effects of sleep deprivation and disruption on several cognitive and behavioral measures.
Our study evaluated three groups of physicians: (a) Young attending physicians who do not participate in a nightshift schedule and have no evidence of acute or chronic sleep deprivation; (b) Resident physicians at baseline who participate in a nightshift schedule, and thus suffer by definition from chronic sleep deprivation and disruption. This group has slept well for three consecutive nights and thus did not suffer from acute sleep deprivation; (c) Resident physicians post-call - same residents as in (b) evaluated a second time in the morning after a nightshift. This design allowed us to test the isolated effect of chronic sleep disruption, by comparing residents at baseline to attendings. It further allowed us to examine the additive effect of acute sleep deprivation superimposed on chronic sleep deprivation and disruption by comparing residents to themselves after a nightshift. Since the vast majority of residents suffer from chronic sleep disruption due to shift-work, we did not examine the isolated effect of acute sleep deprivation in a non-sleep deprived population. We expected to find impairments in residents at baseline as compared to attendings, due to chronic sleep deprivation and disruption (even though both groups had slept the night before the assessment). We predicted that acute sleep deprivation would further deteriorate residents’ cognitive and biological impairments and exacerbate the differences between resident and attending physicians.
Methods:
Study population
Fifty-one physicians (16 females) from various medical and surgical specialties (supplemental Table 1) were recruited (age: 32.5±4.9 years, range: 25–46 years), 33 residents and 18 attending physicians (Table 1). To minimize age differences between the groups, we recruited young attendings (age 46 and below), although the residents recruited were still younger on average than attendings. This difference was adjusted for in later analyses. All participants were recruited from the medical staff personnel of the Hadassah Medical Center, Jerusalem, Israel. The experimenter personally called physicians at the hospital and invited them to take part in the study, in return for a breakfast voucher. We screened for stimulant medication use and excluded those physicians who were using stimulants for attention-deficit disorder (n=2). Caffeine consumption was highly prevalent in all participants and was not accounted for in the study. All residents performed between 4 to 8 26-hour nightshifts per month during a residency period lasting 4–6 years (median time in residency – 2 years, interquartile range – 2 years). Thus all residents, by definition, suffered from chronic sleep deprivation and disruption. To ensure acute sleep deprivation in the resident group post-call, only those residents who slept less than 5 hours during a nightshift (lasting 26 hours) were included. Furthermore, baseline residents’ assessment was only performed after residents were off nightshifts for the preceding three nights. All attendings were recruited from specialties that do not involve any nightshifts. Attendings were recruited only if they stated that they have slept more than 5 hours for the preceding three nights. All subjects provided written informed consent, and the study was approved by the Helsinki Committee (IRB) of Hadassah-Hebrew University Medical Center Jerusalem, Israel (protocol # - 0065-16 HMO).
Table 1.
Demographic and Sleep Characteristics in Resident and Attending Physicians
| Baseline Characteristics | Resident Physicians (n=33) | Attending Physicians (n=18) | p value |
|---|---|---|---|
| Age (years) | 30.5±3.7 | 36.3±4.8 | <0.001 |
| Sex (Male/Female) | 21/12 | 14/4 | 0.34 |
| BMI (kg/m2) | 25.1±3.8 | 25.4±2.9 | 0.76 |
| Married (%) | 63.6% | 86.6% | 0.03 |
| Hours slept the night before testing (hours) | 6.1±0.9 | 6.5±0.9 | 0.05 |
| Average sleep per day in past month (hours) | 6.2±0.6 | 6.9±0.7 | 0.001 |
| Self-reported sleep quality (PSQI) | |||
| Sleep quality | 0.8±0.7 | 0.6±0.7 | 0.32 |
| Sleep latency | 0.8±0.9 | 0.4±0.5 | 0.17 |
| Sleep duration | 1±0.7 | 0.5±0.6 | 0.01 |
| Sleep efficiency | 0.1±0.4 | 0±0 | 0.09 |
| Sleep disturbances | 1±0.5 | 0.9±0.4 | 0.67 |
| Sleep medication use | 0.06±0.2 | 0±0 | 0.30 |
| Daytime dysfunction | 1±0.9 | 0.8±0.6 | 0.41 |
| Global PSQI | 5.1±2.5 | 3.4±1.9 | 0.02 |
Data are presented as Mean ± Standard Deviation
Study Design:
All participants completed a demographic questionnaire and the Pittsburgh Sleep Quality Index (PSQI) followed by the MOXO-CPT task, the BRIEF-A questionnaire, and blood draw between 08:00-10:00 AM. Residents completed the assessments on two different occasions, at the same time of the day, after a nightshift and after three nights of normal sleep. To avoid an order effect, residents were randomly assigned to perform the post-call assessment first or second. Risk taking was measured in a separate cohort (30 residents at baseline, 34 residents after nightshift and 28 attendings) (Figure 1, supplementary Table 2).
Figure 1:

Study Design
Sleep Assessment:
The self-rated items of the PSQI assess sleep quality and disturbances over a 1-month time interval. It generates seven component scores37 (range of subscale scores, 0–3), namely, sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbance; use of sleeping medication; and daytime dysfunction. The sum of these seven component scores yields a global score of subjective sleep quality (range, 0–21); higher scores represent poorer subjective sleep quality, and a score above 5 is considered indicative of poor sleep38.
Attention Assessment:
The MOXO-Continuous Performance Test (MOXO-CPT) is an eight-stage standardized computerized test designed to assess attention performance and screen for ADHD related symptoms. The total duration of the test is 18 minutes39,40. Typical CPT tasks require the participant to sustain attention over a continuous stream of stimuli and respond to a pre-specified target41. Performance indices include scores in four domains: attention, timing, impulsivity, and hyperactivity, whereby higher scores (number of correct/incorrect attempts) indicate a higher degree of impairment in the specific domain.
Executive Function Assessment:
The Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) is a 75-item self-report questionnaire exploring everyday behaviors, in which executive functions are implicated. It is designed to capture higher-order cognitive processes required to properly engage in real-world, goal-directed behaviors, and has been validated in different clinical settings42. The BRIEF-A consists of nine subscales (Inhibit, Shift, Emotional Control, Self-Monitor, Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials), two index scales (Behavioral Regulation Index (BRI) and Metacognition Index (MTI)), and a general composite score, the Global Executive Composite (GEC). The BRI is composed of the Inhibit, Shift, Emotional Control, and Self-Monitor scales and measures the ability to appropriately regulate behavioral and emotional responses. The MTI is composed of the Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials scales and measures the ability to actively organize information and solve problems as they are encountered during completion of complex tasks. Higher scores reflect lower executive function, with T scores above 65 considered clinically significant.
Risk-Taking Assessment:
We employed a previously validated questionnaire to examine risk-taking tendency43. We used two parallel sets of questions to increase reliability. The first set consisted of 10 questions in each of which participants had to choose between a lottery and a sure gain. The lottery always offered a 50% chance to gain 100 Shekels (NIS). The sure option offered a sure gain; the size of the gain ranged from 5 NIS in the first question to 95 in the 10th question, in 5 NIS increments. For example, participants had to indicate whether they preferred a 50% chance to gain 100 NIS or a sure gain of 60 NIS. The second set of questions consisted of 11 items with different amounts. The lottery always offered a 50% chance to gain 15 NIS. The sure option offered gains that ranged from 1 NIS in the first question to 11 NIS in the 11th question, in increments of 1 NIS. The number of questions in which participants chose the lottery option was added, yielding a global risk-taking score, ranging from 0 (no risk-taking) to 21 (maximum risk-taking).
Biochemical analyses of inflammatory and hormonal markers:
Venous peripheral blood was drawn from all participants at 08:00-10:00 AM following the cognitive assessments. Serum was sent immediately for analysis at a central laboratory for high-sensitivity C-reactive protein levels (hs-CRP, expressed as mg per dL) and free cortisol levels (expressed as nmol per L).
Statistical analyses:
Continuous variables were compared using paired t-tests between residents at baseline and residents post-call, and unpaired t-tests between residents at baseline and attendings. Dichotomous variables were compared with χ2 tests or Fisher’s exact test. Correlations between variables were assessed by Pearson’s correlation coefficient. hs-CRP and cortisol levels were naturally log-transformed before analysis. To control for age differences between the study groups, we followed up the correlations with linear regression in which the age variable was introduced. Statistical significance was assumed at a p-value < 0.05. All data were analyzed using SPSS version 25 (IBM, NY, USA).
Results
Sleep disruption
In the PSQI, residents reported shorter average sleep duration during the preceding month as compared to attendings (6.2±0.6 vs. 6.9±0.7 hours/night, p=0.001). Residents also reported poorer sleep quality than attendings (PSQI scores: 5.1±2.5 vs. : 3.4±1.9, respectively, p=0.02). Residents slept 3±1.2 (range: 1–5) hours on the nightshift before the study, and of those a mean of 2.4±1.4 hours of continuous uninterrupted sleep (Table 1).
Attention, executive function, and risk-taking
Significant differences emerged when comparing MOXO-CPT scores in residents at baseline to attendings. Residents exhibited increased impulsivity and slower processing speed, whereas attention and hyperactivity did not differ. In a regression analysis where age was also introduced as an independent variable, those differences were not statistically significant. None of the four attention domain scores differed between residents at baseline and residents post-call (Table 2).
Table 2.
Cognitive functioning in Resident and Attending Physicians
| The MOXO-Continuous Performance Test Scores | |||||
|---|---|---|---|---|---|
| Attending Physicians | p value¥ | Resident Physicians Baseline | Resident Physicians post-call | p value* | |
| Attention | 33.3±0.3 | 0.37 | 33.3±0.8 | 33.3±0.6 | 0.89 |
| Timing | 24.7±4.6 | 0.05 | 27.2±3.2 | 26.6±3 | 0.35 |
| Impulsivity | 0.7±0.5 | 0.03 | 1.1±0.76 | 1.2±1.1 | 0.29 |
| Hyperactivity | 0.4±0.4 | 0.09 | 0.8±1.2 | 0.7±06 | 0.42 |
| Behavior Rating Inventory of Executive Function (T scores) | |||||
| Behavioral Regulation | 44.7±7.5 | 0.05 | 49.2±9 | 52.6±9.3 | 0.18 |
| Metacognition | 42.3±5.9 | 0.002 | 48.5±8.8 | 51.3±10.4 | 0.05 |
| Global Executive Composite | 42.7±6.5 | 0.008 | 48.7±8.5 | 53±10.5 | 0.02 |
Data are presented as Mean ± Standard Deviation
Within-Subject comparison before and after night shift
Between-subject comparison of baseline resident physicians’ vs attending physicians’ parameters. MOXO-CPT scores indicate number of correct/incorrect attempts – higher scores indicating poorer performance. Lower T scores on BRIEF-A indicate better executive function.
The BRI and MTI sub-scores of the BRIEF-A, and accordingly the GEC score, were all higher in the residents at baseline as compared to attendings, indicating impaired executive function. Residents post-call had even higher scores than residents at baseline (Table 2, Figure 2). In a regression analysis where age and/or type of specialty (medical/surgical) were introduced, the pattern of differences between the GEC scores of attendings and residents did not change.
Figure 2:
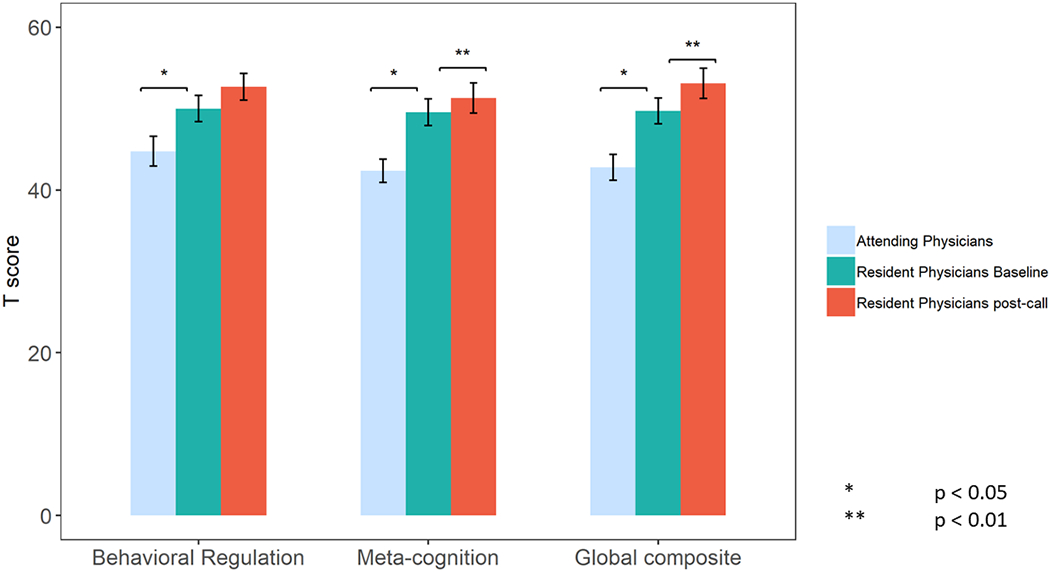
BRIEF-A scores in Attending Physicians and in Resident Physicians both before or after a nightshift
Risk-taking tendencies did not differ between the three groups (Supplemental Table 2).
Biomarkers of inflammation and hormonal function
Average hs-CRP levels in residents at baseline were marginally higher than those of the attendings (0.28±0.24 vs. 0.16±0.14 mg/dL, p=0.067). hs-CRP levels did not significantly differ between residents at baseline and residents post-call (p=0.43). We further conducted a comparison of hs-CRP levels of each group to the previously established threshold of 0.3mg/dL, which has been associated with increased cardiovascular risk and mortality44. hs-CRP levels among residents at baseline were in the vicinity of this risk-associated cut-off value (t(32)=−0.391, p=0.698) while hs-CRP levels among attendings were significantly lower (t(16)=−3.95, p=0.001). Additionally, 16/33 (48.5%) of the residents at baseline had hs-CRP levels of 0.3mg/dL or higher, compared with only 4/17 (23.5%) of the attendings (p=0.129) (Figure 3). In a regression analysis where age was introduced as an independent variable, we obtained the same pattern of results.
Figure 3:
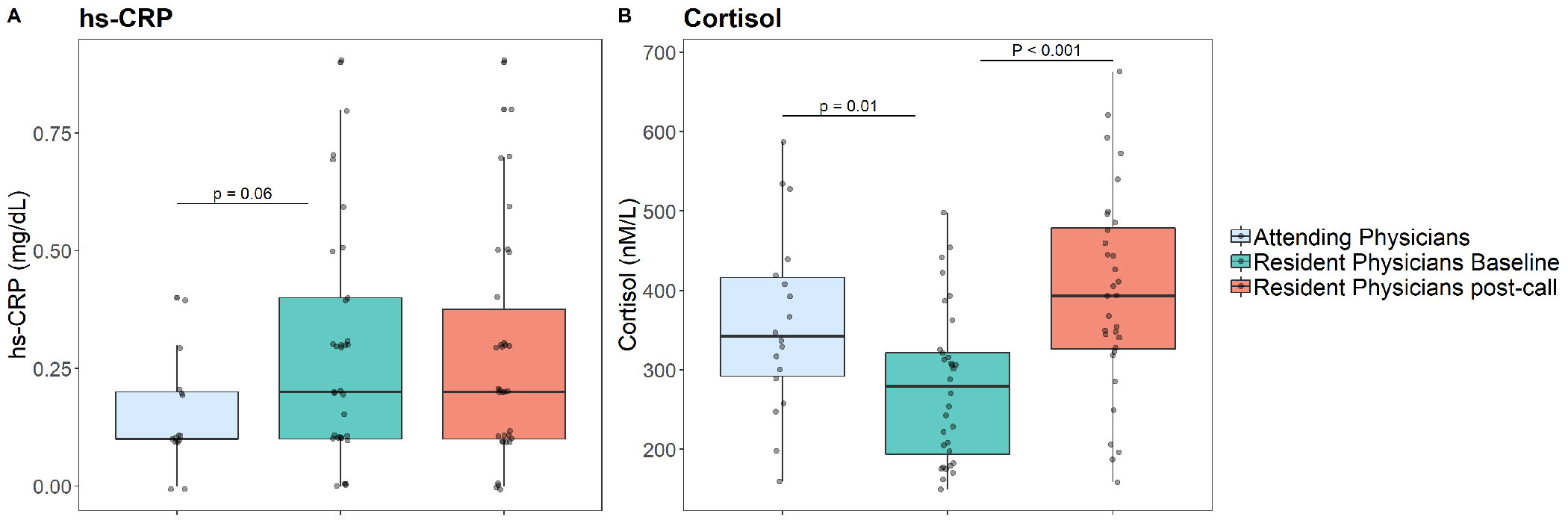
hs-CRP and Cortisol levels in Attending Physicians and in Resident Physicians both before or after a nightshift
Significant differences emerged when comparing morning serum cortisol levels scores in residents at baseline to attendings (275±95 vs. 358±115 nmol/L, p<0.001). Additionally, cortisol levels in residents at baseline were lower than those of attendings post-call (396±127 nmol/L; p<0.001) (Figure 3). In a regression analysis where age was introduced as an independent variable, the same pattern of results emerged.
Associations between sleep disruption, cognition and biomarkers
In a correlation analysis, three out of seven sleep components measured by the PSQI were associated with greater impairment of executive function – lower sleep efficiency, reported sleep disturbances and daytime dysfunction. Furthermore, shorter sleep duration was associated with reduced attention. Finally, daytime dysfunction was associated with higher hs-CRP levels and reported sleep disturbances with increased cortisol. See Table 3 for the full list of correlations of sleep parameters.
Table 3.
Correlations between chronic sleep disturbances and cognitive measures and serum biomarkers
| CRP | CORTISOL | BEHAVIORAL REGULATION | METACOGNITION | GLOBAL EXECUTIVE COMPOSITE | ATTENTION | TIMING | IMPULSIVITY | HYPERACTIVITY | |
|---|---|---|---|---|---|---|---|---|---|
| SLEEP QUALITY | 0.212 | −0.115 | −0.059 | 0.108 | 0.045 | 0.077 | −0.156 | 0.094 | 0.097 |
| SLEEP LATENCY | −0.119 | 0.129 | −0.206 | −0.147 | −0.175 | −0.136 | 0.110 | −0.284 | −0.125 |
| SLEEP DURATION | −0.116 | −0.174 | 0.064 | 0.112 | 0.108 | −0.304* | 0.162 | 0.136 | −0.132 |
| SLEEP EFFICIENCY | 0.137 | 0.027 | 0.378** | 0.209 | 0.310* | −0.046 | −0.079 | 0.030 | 0.068 |
| SLEEP DISTURBANCES | 0.166 | 0.337* | 0.409** | 0.171 | 0.301* | −0.227 | −0.222 | 0.165 | 0.168 |
| SLEEP MEDICATONS | 0.297* | 0.045 | 0.039 | −0.042 | −0.013 | 0.059 | −0.083 | −0.172 | −0.016 |
| DAYTIME DYSFUNCTION | 0.321* | 0.051 | 0.339* | 0.365** | 0.389** | 0.166 | 0.029 | 0.192 | 0.070 |
| GLOBAL PSQI | 0.174 | 0.052 | 0.198 | 0.217 | 0.239 | −0.110 | −0.018 | 0.069 | 0.020 |
Data are presented as r correlation coefficient
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Discussion
The current study explored the effects of chronic and acute-on-chronic sleep deprivation and disruption, associated with residency training, on cognitive performance, systemic inflammation and cortisol levels. Participants were residents before and after a nightshift and young attending physicians. We found that chronic sleep disruption and deprivation, caused by the overall schedules of residency training, was associated with poorer executive functioning and increased impulsivity, and was accompanied by lower morning cortisol levels and a trend towards increased inflammation. Acute partial sleep disruption, following a nightshift, was associated with further executive function impairment in residents and with increased cortisol secretion. Impulsivity and inflammation were not affected beyond the impairment levels associated with chronic sleep deprivation and disruption.
Attention, executive function and risk taking
Cognitive performance and behavior were altered in our cohort of chronically sleep-deprived resident physicians in a dose-response manner. Residents had higher scores than the attendings, indicating a greater executive function impairment. Executive functioning is viewed as the central control system underpinning complex behaviors, such as attention, planning and goal-setting, inhibitory abilities and cognitive flexibility45,46. Sleep deprivation studies have usually reported executive functioning domain-specific associations, such as cognitive control in task-goal switching47, motor response inhibition48, reaction time and inhibitory control49, and divergent thinking50. We tried to overcome these limitations by using the BRIEF-A, a validated questionnaire designed to capture a wide range of higher-order cognitive processes required to properly engage in goal-directed behaviors (although not validated previously on physicians). Our study further expands the current base of knowledge by assessing executive functioning under natural settings and real-life conditions, providing greater ecological validity.
Chronic sleep deprivation was associated with impulsive behavior in residents in our study, which was not further exacerbated by acute sleep deprivation, as measured by the MOXO-CPT computerized tool. Our results are supported by some prior evidence: self-regulation impairments, including loss of the ability to manage cognition and emotion, that are manifest as difficulties in controlling impulsive behavior and maintaining adequate attention are affected by prolonged lack of sleep51,52. Similar to our results, another study found no significant increase in impulsive behavior following acute sleep deprivation53. Impulsivity, as seen here in the context of relatively young, inexperienced physicians, making critical decisions, often under stress, may result in compromised medical care. Indeed, some studies have shown that extended 24-hour shifts increase the rate of medical errors54. At the same time, other studies found that extending physicians’ sleep duration did not improve patient-centered outcomes55–58.
Risk-taking scores were similar across the three groups in our study. Several studies suggested an increase in physicians’ risk-taking behavior after acute sleep deprivation59,60. However, studies investigating risk taking in other settings failed to reach a consistent conclusion61,62.
Biomarkers of inflammation and hormonal function
A trend towards an increased systemic inflammation, as reflected by elevated hs-CRP, was detected in residents compared to attendings. This finding concurs with a long list of studies associating inflammation with total night sleep deprivation, partial night sleep deprivation, or chronic restriction of sleep duration12. From existing literature, it appears that persistent disturbances of sleep are necessary for inflammatory signaling to be translated into an increase in systemic markers of inflammation63. Accordingly, we did not observe increases in hs-CRP levels following a single night of partial sleep deprivation during the nightshift. Our findings are also in line with a large meta-analysis64 where chronic sleep disruption, but not shorter sleep duration, was linked to increased systemic inflammation. Notwithstanding, the elevated hs-CRP levels in the residents group, which were in the higher cardiovascular risk range, deserve attention, and potentially suggest that the working schedules of residents may be fraught with an enhanced risk contributing to both cardiovascular and all-cause mortality44. Physicians are clearly exposed to chronic stress and have been reported at increased risk for burnout and depression 34,35,65–67, all of which are also linked to increased inflammation and cardiovascular disease68. Thus, an improved understanding of the major determinants of the adverse consequences of residents’ schedules is critical to enable personalized tailoring of such training and risk mitigation.
Morning cortisol levels were reduced in the residents group with chronic sleep deprivation (baseline), and rose to normal levels, similar to those of the attendings, following acute sleep deprivation (post call). Since blood samples were drawn between 08:00 and 10:00 AM, coinciding with the physiological peak in blood cortisol, the reduced cortisol concentrations could be related to two major processes: (a) the HPA pathway has reconfigured its responsiveness in the context of chronic stress. The sustained requirements to increase cortisol after partial sleep disruption during a nightshift led to the dampening of the baseline cortisol output. This explanation is supported by recent evidence showing that two nights of 4 hours in bed were associated with a reduction in ACTH response to CRH injection, suggestive of a decreased pituitary sensitivity to CRH in a state of sleep debt69. (b) the normal timing of morning cortisol rise is shifted to a different time of the day. Acute insufficient sleep results in elevation of evening cortisol levels70,71, which has been hypothesized to reflect altered HPA axis recovery from the circadian-driven morning stimulation71–73. As we tested the cortisol levels only once, this study does not permit any inferences as to which of the two possibilities is at play. Still, our findings are important since the homeostatic regulation of cortisol is crucial to a myriad of physiological processes, including innate immunity, which could explain the elevated hs-CRP levels74 in residents at baseline. Moreover, low cortisol has been suggested as a key factor in the development of stress-related disease and burnout15,75, and our findings are in line with the evidence that HPA axis activity decreases with longer duration of chronic stress76. While lower morning cortisol in residents at baseline has not been documented before, prior studies documented dysregulation of the HPA axis in residents after a nightshift77–79.
Strengths and limitations
The strength of our study lies in the comprehensive cognitive and biochemical assessment of the chronic effects of interrupted sleep over time in a real-life environment. Although night shiftwork is associated with shorter sleep duration80, many of the detrimental effects attributable to shiftwork seem to originate from the associated chronic circadian misalignment81. Our study reflects the combined effects of circadian disruption associated with iterative changes in day and night work schedules, as well as the sleep disruption involved in the specific work schedule during clinical residency training.
The observational (rather than experimental) nature of the study encompasses some methodological limitations that should be acknowledged. Apart for disrupting sleep, residency introduces also differences in work load and stress35,82,83. Resident physicians may differ from attendings in age, lifestyle, specialty, income, and professional experience (note that we recruited the youngest, least experienced attendings, and controlled for age in our analyses). While we find that sleep was associated with the different outcomes, it is still possible that other factors also contributed to our observed effects. However, attendings were the closest possible control, as practically all residents in our medical system participate in nightshifts and medical students are less suitable to serve as a control group.
Cognitive functioning was measured in our study through validated, well-established behavioral tools84,85. Sleep was assessed with the PSQI, a widely used questionnaire which has been applied to a large variety of settings, including shift work, with strong reliability and both internal and external validity38. Still, the PSQI is based on subjective reporting, which may have led to underestimation of the true degree of sleep deprivation86. Even if underestimation occurred, it occurred in all groups, and cannot account for the observed differences. Additionally, in contrast to laboratory conditions, we could not monitor all of our cognitive and biological outcome measures throughout the day to assess for circadian changes and for windows of a specific vulnerability.
The current study was cross-sectional, and did not investigate the potential longitudinal effects of the above-mentioned cognitive perturbations. Future research may unravel long-lasting effects of chronic sleep disruption on health and cognitive domains. Future research is also needed to test whether letting residents sleep more (e.g., by limiting the on-call period) may solve some of the problems documented in this study. We note that adding sleep may not be sufficient for improving patient care, due to residents’ overall fatigue and burnout23,87. Such changes in training schedules, while improving sleep, might cause deterioration in education opportunities and interruption in continuity of care56,57 and result in more medical errors58.
Conclusions
In conclusion, our findings suggest that chronic sleep deprivation and disruption caused by postgraduate residency training impose adverse cognitive effects of impaired executive function and increased impulsivity. These are also associated with maladaptive cortisol levels and a trend towards increased low-grade systemic inflammation. Some of the effects of chronic sleep deprivation are further aggravated by the acute sleep restriction of night calls. Further research is needed to examine whether incorporating biological and functional considerations into working schedules, may minimize the short-term and potential long-term risks of physician training.
Supplementary Material
Acknowledgments
Parts of this work were performed as the requirement for MD degree (FM) and Board Certification in Pediatrics (AI) under the supervision of AGH, SCH & IB.
Funding: This work was partly supported by IB internal research grant. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Funding: AGH is supported by The Israel Science Foundation grant 2779/19; DG is supported by the National Institutes of Health grants HL130984 and HL140548; IB is supported by the National Institute for Psychobiology in Israel grant (#1041819). SCH thanks the Recanati Fund of the School of Business Administration at the Hebrew University for funding.
Abbreviation List
- BRIEF-A
Behavior Rating Inventory of Executive Function-Adult Version
- BRI
Behavioral Regulation Index
- GEC
Global Executive Composite
- hs-CRP
high-sensitivity C-Reactive Protein
- MOXO-CPT
The MOXO-Continuous Performance Test
- MTI
Metacognition Index
- PSQI
Pittsburgh Sleep Quality Index
References
- 1.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. January 1 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leger D, Bayon V. Societal costs of insomnia. Sleep medicine reviews. December 2010;14(6):379–389. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. May 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annual review of psychology. January 3 2015;66:143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killgore WD. Effects of sleep deprivation on cognition. Progress in brain research. 2010;185:105–129. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham JEA, Jones SAH, Eskes GA, Rusak B. Acute Sleep Restriction Has Differential Effects on Components of Attention. Frontiers in psychiatry. 2018;9:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. March 15 1999;22(2):171–179. [DOI] [PubMed] [Google Scholar]
- 8.NHLBI. 2017; https://www.nhlbi.nih.gov/health-topics/sleep-deprivation-and-deficiency. [Google Scholar]
- 9.Effect of short sleep duration on daily activities--United States, 2005-2008. MMWR. Morbidity and mortality weekly report March 4 2011;60(8):239–242. [PubMed] [Google Scholar]
- 10.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ (Clinical research ed.). November 1 2016;355:i5210. [DOI] [PubMed] [Google Scholar]
- 11.Owens JA. The ADHD and sleep conundrum redux: moving forward. Sleep medicine reviews. December 2006;10(6):377–379. [DOI] [PubMed] [Google Scholar]
- 12.Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiological reviews. July 1 2019;99(3):1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrie JE, Kivimaki M, Akbaraly TN, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. September 15 2013;178(6):956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HJ, Seeman TE, Kiefe CI, Lauderdale DS, Irwin MR. Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. May 2015;46:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett SM, Lupis SB, Gianferante D, Rohleder N, Wolf JM. Sleep quality but not sleep quantity effects on cortisol responses to acute psychosocial stress. Stress. 2015;18(6):638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. The Journal of neuroscience : the official journal of the Society for Neuroscience. October 29 2014;34(44):14697–14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurtado-Alvarado G, Dominguez-Salazar E, Pavon L, Velazquez-Moctezuma J, Gomez-Gonzalez B. Blood-Brain Barrier Disruption Induced by Chronic Sleep Loss: Low-Grade Inflammation May Be the Link. Journal of immunology research. 2016;2016:4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields GS, Moons WG, Slavich GM. Inflammation, Self-Regulation, and Health: An Immunologic Model of Self-Regulatory Failure. Perspect Psychol Sci. July 2017;12(4):588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields GS, Bonner JC, Moons WG. Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology. August 2015;58:91–103. [DOI] [PubMed] [Google Scholar]
- 20.Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. European archives of psychiatry and clinical neuroscience. September 2015;265(6):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lingenfelser T, Kaschel R, Weber A, Zaiser-Kaschel H, Jakober B, Kuper J. Young hospital doctors after night duty: their task-specific cognitive status and emotional condition. Medical education. November 1994;28(6):566–572. [DOI] [PubMed] [Google Scholar]
- 22.Basner M, Rao H, Goel N, Dinges DF. Sleep deprivation and neurobehavioral dynamics. Current opinion in neurobiology. October 2013;23(5):854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor TS, Teunissen PW, Dornan T, Lingard L. Fatigue in Residency Education: Understanding the Influence of Work Hours Regulations in Europe. Academic medicine : journal of the Association of American Medical Colleges. December 2017;92(12):1733–1739. [DOI] [PubMed] [Google Scholar]
- 24.Mustahsan SM, Ali SM, Khalid F, et al. Sleep deprivation and its consequences on house officers and postgraduate trainees. J Pak Med Assoc. April 2013;63(4):540–543. [PubMed] [Google Scholar]
- 25.Avidan AY. Sleep and fatigue countermeasures for the neurology resident and physician. Continuum (Minneapolis, Minn.). February 2013;19(1 Sleep Disorders):204–222. [DOI] [PubMed] [Google Scholar]
- 26.Mansukhani MP, Kolla BP, Surani S, Varon J, Ramar K. Sleep deprivation in resident physicians, work hour limitations, and related outcomes: a systematic review of the literature. Postgraduate medicine. July 2012;124(4):241–249. [DOI] [PubMed] [Google Scholar]
- 27.Mountain SA, Quon BS, Dodek P, Sharpe R, Ayas NT. The impact of housestaff fatigue on occupational and patient safety. Lung. July-August 2007;185(4):203–209. [DOI] [PubMed] [Google Scholar]
- 28.Veasey S, Rosen R, Barzansky B, Rosen I, Owens J. Sleep Loss and Fatigue in Residency Training. JAMA. 2002/September/04 2002;288(9):1116. [DOI] [PubMed] [Google Scholar]
- 29.Philibert I Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. November 2005;28(11):1392–1402. [DOI] [PubMed] [Google Scholar]
- 30.Volpp KG, Shea JA, Small DS, et al. Effect of a protected sleep period on hours slept during extended overnight in-hospital duty hours among medical interns: a randomized trial. JAMA. December 5 2012;308(21):2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavallo A, Ris MD, Succop P. The night float paradigm to decrease sleep deprivation: good solution or a new problem? Ergonomics. June 10 2003;46(7):653–663. [DOI] [PubMed] [Google Scholar]
- 32.Dawson D, Zee P. Work hours and reducing fatigue-related risk: good research vs good policy. Jama. September 7 2005;294(9):1104–1106. [DOI] [PubMed] [Google Scholar]
- 33.Chang VY, Arora VM, Lev-Ari S, D’Arcy M, Keysar B. Interns overestimate the effectiveness of their hand-off communication. Pediatrics. March 2010;125(3):491–496. [DOI] [PubMed] [Google Scholar]
- 34.Moss M, Good VS, Gozal D, Kleinpell R, Sessler CN. A Critical Care Societies Collaborative Statement: Burnout Syndrome in Critical Care Health-care Professionals. A Call for Action. American journal of respiratory and critical care medicine. July 1 2016;194(1):106–113. [DOI] [PubMed] [Google Scholar]
- 35.Stewart NH, Arora VM. The Impact of Sleep and Circadian Disorders on Physician Burnout. Chest. 2019;156(5):1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. Jama. September 7 2005;294(9):1025–1033. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. May 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 38.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep medicine reviews. February 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 39.Berger I, Cassuto H. The effect of environmental distractors incorporation into a CPT on sustained attention and ADHD diagnosis among adolescents. J Neurosci Methods. January 30 2014;222:62–68. [DOI] [PubMed] [Google Scholar]
- 40.Berger I, Goldzweig G. Objective measures of attention-deficit/hyperactivity disorder: a pilot study. Isr Med Assoc J. September 2010;12(9):531–535. [PubMed] [Google Scholar]
- 41.Shalev L, Ben-Simon A, Mevorach C, Cohen Y, Tsal Y. Conjunctive Continuous Performance Task (CCPT)--a pure measure of sustained attention. Neuropsychologia. July 2011;49(9):2584–2591. [DOI] [PubMed] [Google Scholar]
- 42.Roth RM, Gioia GA. Behavior rating inventory of executive function--adult version Psychological Assessment Resources Lutz, FL; 2005. [Google Scholar]
- 43.Dohmen T, Falk A, Huffman D, Sunde U. Are Risk Aversion and Impatience Related to Cognitive Ability? The American Economic Review. 2010;100(3):1238–1260. [Google Scholar]
- 44.Doran B, Zhu W, Muennig P. Gender differences in cardiovascular mortality by C-reactive protein level in the United States: evidence from the National Health and Nutrition Examination Survey III. Am Heart J. July 2013;166(1):45–51. [DOI] [PubMed] [Google Scholar]
- 45.Kuula L, Pesonen AK, Heinonen K, et al. Naturally occurring circadian rhythm and sleep duration are related to executive functions in early adulthood. J Sleep Res. February 2018;27(1):113–119. [DOI] [PubMed] [Google Scholar]
- 46.Diamond A Executive functions. Annual review of psychology. 2013;64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slama H, Chylinski DO, Deliens G, Leproult R, Schmitz R, Peigneux P. Sleep deprivation triggers cognitive control impairments in task-goal switching. Sleep. December 13 2017. [DOI] [PubMed] [Google Scholar]
- 48.Schaedler T, Santos JS, Vincenzi RA, Pereira SIR, Louzada FM. Executive functioning is preserved in healthy young adults under acute sleep restriction. Sleep Sci. May-Jun 2018;11(3):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bratzke D, Steinborn MB, Rolke B, Ulrich R. Effects of sleep loss and circadian rhythm on executive inhibitory control in the Stroop and Simon tasks. Chronobiol Int. February 2012;29(1):55–61. [DOI] [PubMed] [Google Scholar]
- 50.Vartanian O, Bouak F, Caldwell JL, et al. The effects of a single night of sleep deprivation on fluency and prefrontal cortex function during divergent thinking. Front Hum Neurosci. 2014;8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossa KR, Smith SS, Allan AC, Sullivan KA. The effects of sleep restriction on executive inhibitory control and affect in young adults. J Adolesc Health. August 2014;55(2):287–292. [DOI] [PubMed] [Google Scholar]
- 52.Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-Regulation and Sleep Duration, Sleepiness, and Chronotype in Adolescents. Pediatrics. December 2016;138(6). [DOI] [PubMed] [Google Scholar]
- 53.Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiology & behavior. August 15 2007;91(5):579–587. [DOI] [PubMed] [Google Scholar]
- 54.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. The New England journal of medicine. October 28 2004;351(18):1838–1848. [DOI] [PubMed] [Google Scholar]
- 55.Banfi T, Coletto E, d’Ascanio P, et al. Effects of Sleep Deprivation on Surgeons Dexterity. Frontiers in neurology. 2019;10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desai SV, Feldman L, Brown L, et al. Effect of the 2011 vs 2003 duty hour regulation-compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA internal medicine. April 22 2013;173(8):649–655. [DOI] [PubMed] [Google Scholar]
- 57.Jena AB, Farid M, Blumenthal D, Bhattacharya J. Association of residency work hour reform with long term quality and costs of care of US physicians: observational study. BMJ (Clinical research ed.). July 10 2019;366:l4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landrigan CP, Rahman SA, Sullivan JP, et al. Effect on Patient Safety of a Resident Physician Schedule without 24-Hour Shifts. The New England journal of medicine. June 25 2020;382(26):2514–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capanna MV, Hou R, Garner M, Yuen HM, Hill CM. Risk-taking in junior doctors working night shifts in intensive care. Intensive Care Med. May 2017;43(5):709–710. [DOI] [PubMed] [Google Scholar]
- 60.Aran A, Wasserteil N, Gross I, Mendlovic J, Pollak Y. Medical Decisions of Pediatric Residents Turn Riskier after a 24-Hour Call with No Sleep. Med Decis Making. January 2017;37(1):127–133. [DOI] [PubMed] [Google Scholar]
- 61.Short MA, Weber N. Sleep duration and risk-taking in adolescents: A systematic review and meta-analysis. Sleep medicine reviews. October 2018;41:185–196. [DOI] [PubMed] [Google Scholar]
- 62.Rusnac N, Spitzenstetter F, Tassi P. Chronic sleep loss and risk-taking behavior: Does the origin of sleep loss matter? Behav Sleep Med. June 20 2018:1–11. [DOI] [PubMed] [Google Scholar]
- 63.Irwin MR, Opp MR. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology. January 2017;42(1):129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biological psychiatry. July 1 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dyrbye LN, West CP, Satele D, et al. Burnout among U.S. medical students, residents, and early career physicians relative to the general U.S. population. Academic medicine : journal of the Association of American Medical Colleges. March 2014;89(3):443–451. [DOI] [PubMed] [Google Scholar]
- 66.Michel JB, Sangha DM, Erwin JP 3rd. Burnout Among Cardiologists. Am J Cardiol. March 15 2017;119(6):938–940. [DOI] [PubMed] [Google Scholar]
- 67.Baer TE, Feraco AM, Tuysuzoglu Sagalowsky S, Williams D, Litman HJ, Vinci RJ. Pediatric Resident Burnout and Attitudes Toward Patients. Pediatrics. March 2017;139(3). [DOI] [PubMed] [Google Scholar]
- 68.Wirtz PH, von Kanel R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep. September 20 2017;19(11):111. [DOI] [PubMed] [Google Scholar]
- 69.Guyon A, Morselli LL, Balbo ML, et al. Effects of Insufficient Sleep on Pituitary-Adrenocortical Response to CRH Stimulation in Healthy Men. Sleep. June 1 2017;40(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. August 2014;99(8):2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. October 1997;20(10):865–870. [PubMed] [Google Scholar]
- 72.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. October 23 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 73.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. November 2004;89(11):5762–5771. [DOI] [PubMed] [Google Scholar]
- 74.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41(3):224–233. [DOI] [PubMed] [Google Scholar]
- 75.Suarez EC, Sundy JS. The cortisol:C-reactive protein ratio and negative affect reactivity in depressed adults. Health Psychol. September 2017;36(9):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. November 2005;30(10):1010–1016. [DOI] [PubMed] [Google Scholar]
- 77.Chatterton RT Jr., Dooley SL. Reversal of diurnal cortisol rhythm and suppression of plasma testosterone in obstetric residents on call. Journal of the Society for Gynecologic Investigation. Jan-Feb 1999;6(1):50–54. [DOI] [PubMed] [Google Scholar]
- 78.Coeck C, Jorens PG, Vandevivere J, Mahler C. ACTH and cortisol levels during residency training. The New England journal of medicine. September 5 1991;325(10):738. [DOI] [PubMed] [Google Scholar]
- 79.Knight JM. Physiological and neurobiological aspects of stress and their relevance for residency training. Academic psychiatry : the journal of the American Association of Directors of Psychiatric Residency Training and the Association for Academic Psychiatry. January 1 2013;37(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korsiak J, Tranmer J, Day A, Aronson KJ. Sleep duration as a mediator between an alternating day and night shift work schedule and metabolic syndrome among female hospital employees. Occup Environ Med. February 2018;75(2):132–138. [DOI] [PubMed] [Google Scholar]
- 81.Vetter C, Fischer D, Matera JL, Roenneberg T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. March 30 2015;25(7):907–911. [DOI] [PubMed] [Google Scholar]
- 82.Gaba DM, Howard SK. Fatigue among Clinicians and the Safety of Patients. New England Journal of Medicine. 2002/October/17 2002;347(16):1249–1255. [DOI] [PubMed] [Google Scholar]
- 83.Gerdes J, Kahol K, Smith M, Leyba MJ, Ferrara JJ. Jack Barney award: The effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons. The American Journal of Surgery. 2008/December 2008;196(6):813–820. [DOI] [PubMed] [Google Scholar]
- 84.Roth RM, Lance CE, Isquith PK, Fischer AS, Giancola PR. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function-Adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. August 2013;28(5):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berger I, Slobodin O, Aboud M, Melamed J, Cassuto H. Maturational delay in ADHD: evidence from CPT. Front Hum Neurosci. 2013;7:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. November 2008;19(6):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mendelsohn D, Despot I, Gooderham PA, Singhal A, Redekop GJ, Toyota BD. Impact of work hours and sleep on well-being and burnout for physicians-in-training: the Resident Activity Tracker Evaluation Study. Medical education. March 2019;53(3):306–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


