Abstract
Background:
Chronic kidney disease (CKD) is associated with reduced insulin sensitivity, through mechanisms that are not well understood. Low vitamin K intake and incomplete carboxylation of the vitamin K-dependent protein osteocalcin may promote insulin resistance. We assessed relationships of osteocalcin concentration, carboxylation and fragmentation with CKD and glucose homeostasis in a cross-sectional study.
Methods:
We included 87 participants without diabetes: 50 (27 female) with moderate to severe CKD (estimated GFR <60 mL/min/1.73m2 not treated with dialysis) and 37 (17 female) healthy controls. Total osteocalcin was measured by immunoassay, and osteocalcin carboxylation and fragmentation status by LC-ESI based mass spectrometric immunoassay. Endpoints included glucose tolerance (based on 2-hour oral glucose tolerance test), insulin sensitivity (hyperinsulinemic-euglycemic clamp), and pancreatic beta-cell function (intravenous glucose tolerance test).
Results:
The total plasma osteocalcin concentration was higher in the CKD group [mean (SD) 102.9 (147.5)] vs. controls [53.6 (51.1) ng/mL, p=0.03], and more osteocalcin was circulating as fragments. The extent of osteocalcin carbocylation did not differ between individuals with and without CKD. Osteocalcin concentration, carboxylation, and fragmentation were not associated with any measure of glucose homeostasis in multivariable-adjusted analyses.
Conclusions:
In CKD, circulating osteocalcin concentrations are elevated, in part due to larger proportions of fragmented forms. However, osteocalcin carboxylation status is not significantly different between individuals with and without CKD. Our data also do not provide support for the hypothesis that differences in osteocalcin carboxylation may explain reduced insulin sensitivity in individuals with CKD.
Keywords: Vitamin K, insulin sensitivity, insulin resistance, glucose tolerance
Introduction
Vitamin K is an essential cofactor in the posttranslational carboxylation of glutamate (Glu) residues to γ-carboxy-glutamate (Gla) in vitamin K-dependent proteins (VKDPs).[1] Hepatic VKDPs play key roles in blood coagulation, while extrahepatic VKDPs are involved in a variety of biological processes.[1] These include the mineralization of bone and teeth through the protein osteocalcin, and the prevention of ectopic mineral deposition through matrix gla-protein.[2] Overt vitamin K deficiency, clinically defined by prolonged prothrombin time, is rare in adults. However, vitamin K has become of interest with the discovery that the carboxylation of extrahepatic VKDPs such as osteocalcin (OC) is incomplete in most individuals in the United States,[3, 4] suggesting that functional vitamin K deficiency may exist at commonly consumed levels of dietary vitamin K.
People with chronic kidney disease (CKD) may be at particular risk of functional vitamin K deficiency. Dietary vitamin K intake tends to be lower in CKD patients,[5] possibly as a result of the specific dietary regimen recommended to this population. In a rat model, CKD is associated with changes in vitamin K metabolism, independent of vitamin K intake, including a reduced expression in the rate-limiting vitamin K recycling enzyme, Vitamin K epOxide Reductase Complex (VKORC).[6] In CKD, clearance of circulating VKDPs and their fragments may also be reduced, which would lead to accumulation of post-translationally modified forms of VKDPs with potentially varied biologic activities. Patients with CKD are at increased risk of vascular calcifications and dysfunctional mineral and bone metabolism, conditions that may benefit from adequate carboxylation of extrahepatic VKDPs such as OC.[7]
Higher vitamin K intake has been linked to greater glucose tolerance, higher insulin sensitivity, and a reduced risk of type 2 diabetes [8–11], and vitamin K supplementation improves insulin sensitivity and/or glucose tolerance in some,[12–15] but not all studies.[16] The plasma concentration of the proportion of OC that is carboxylated [expressed as either %cOC or as the inverse, the % undercarboxylated (uc) OC], increases with higher vitamin K intake, is used as a biomarker of vitamin K status [17] and is associated with better insulin sensitivity.[18] This relationship may be confounded, however, by the fact that the total plasma OC concentration, and with that likely that of cOC, tends to be lower in individuals with type 2 diabetes (reviewed in [19]).
An important gap in our understating of osteocalcin metabolism in CKD is that existing studies have focused largely on CKD patients undergoing dialysis [20–24], making it impossible to disentangle the impact of CKD vs. dialysis on osteocalcin-related endpoints. The literature is also limited in that commonly used immunoassays to measure cOC or ucOC do not provide complete information on how many of the three glutamate residues within circulating OC molecules are carboxylated. Current research has also neglected the fact that OC circulates partly as truncated fragments,[4] which may differ in their biological function from the full-length molecule. Given the interest in improving our understanding of the role of vitamin K and osteocalcin in CKD-associated metabolic disease, cardiovascular disease, and disturbances in bone and mineral metabolism, it is essential to establish the basic physiology of osteocalcin, and specific changes that may occur in CKD.
We conducted a secondary analysis based on the StUdy of Glucose And Insulin in Renal Disease (SUGAR) [25] to assess to which degree OC concentration, carboxylation, and fragmentation are altered in CKD, and whether OC concentration, carboxylation and fragmentation are associated with glucose homeostasis in this population. SUGAR was a cross-sectional observational study in patients with moderate to severe CKD and controls matched for age, sex, and race [25]. A key finding of SUGAR was that patients with CKD were less insulin sensitive than controls, even after adjustment for key factors determining insulin sensitivity, including physical activity, age, sex, race, fat mass, and fat-free mass [25]. We used a state-of-the art mass spectrometry assay [4] to characterize the carboxylation and fragmentation status of circulating OC in individuals with and without pre-dialysis CKD. We hypothesized that CKD is associated with undercarboxylation of OC and accumulation of OC fragments. Further, we hypothesized that OC carboxylation status, i.e., the degree to which circulating OC is carboxylated, is associated with insulin sensitivity, such that the lower OC carboxylation status among CKD patients may partly explain their lower insulin sensitivity.
Methods
Study design and subjects
This is a secondary analysis based on SUGAR, a cross-sectional observational study comparing glucose homeostasis in nondiabetic patients with moderate to severe chronic kidney disease vs. healthy controls. Details of the study design have been previously published.[25] In short, patients with CKD, defined as eGFR <60 ml/min/1.73m2 were recruited from the patient population of an academic medical center, and from registries based on previous studies in this population. Healthy controls without CKD, defined as eGFR ≥60 ml/min/1.73m2 and urine albumin-to-creatinine ratios <30 mg/g, were recruited, with the goal of matching the CKD population for age, sex, and race. Exclusion criteria for both groups included age<18 years old, diabetes mellitus, maintenance dialysis or fistula in place, history of kidney transplantation, use of medications known to affect insulin sensitivity, fasting serum glucose ≥ 126 mg/dL, and hemoglobin <10 g/dL.
The sample size of SUGAR was determined to provide sufficient power to detect differences in insulin sensitivity between participants with vs. without CKD, and no power calculation was conducted for the secondary analyses reported here. Of 98 SUGAR participants, 6 were excluded from the present analysis because we were unable to obtain reliable measurements on OC carboxylation and fragmentation from their plasma, and another 5 were excluded due to warfarin use, given that warfarin is a vitamin K antagonist. Thus, the present analysis was based on 87 participants, 50 with CKD and 37 healthy controls.
Clinical procedures
All participants were admitted clinic after an overnight fast. Blood was collected in tubes containing EDTA, and plasma was separated within 60 minutes and frozen at −70°C. Insulin sensitivity was assessed using a hyperinsulinemic-euglycemic clamp, pancreatic β-cell function using a short frequently sampled intravenous glucose tolerance test (FS-IVGTT), and oral glucose tolerance using a 2-hour 75-g oral glucose tolerance test (OGTT).[25] Body composition was measured using a whole body dual-energy x-ray absorptiometry (DEXA) scan on a GE Lunar, Prodigy, or iDXA scanner (GE Healthcare, Waukesha WI). Demographics and medical history were reported by participants, and medication use was ascertained by the inventory method. The Human Activity Profile maximum activity score was used to quantify physical activity.[26] Food intake was recorded using a documented 3-day dietary record, and analyzed using Nutrition Data System for Research software (University of Minnesota, Minneapolis, MN).
Laboratory analyses and calculation of glucose homeostasis variables
Total OC concentration was measured using a sandwich immunoassay from Mesoscale Discovery Diagnostics, LLC (Gaithersburg, MD). The immunoassay utilizes eletrochemiluminescence technology, with an intra-assay coefficient of 6.9% reported by the manufacturer. Measurement of osteocalcin carboxylation and fragmentation in CKD patient and control plasma samples was conducted blindly and thus with cases and controls run in random order by a mass spectrometric immunoassay (MSIA) that employed liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS). The analysis has been previously described,[4] with the exception that approximately one-third of the samples in this study were analyzed on a more advanced LC-ESI-MS instrument due to the fact that the original instrument was damaged by a power surge part way through analysis. Both instruments were Bruker quadrupole-time-of-flight (Q-TOF) instruments for which the ion sources were identical. Briefly, anti-human osteocalcin (clone 2H9F11F8 from AbD Serotec/BioRad, Hercules, CA) was immobilized to MSIA pipette tips (ThermoFisher Scientific, Waltham, MA) then exposed to individual human plasma samples. Following a series of previously described rinses [4] and elution with 12 μL of 0.4% trifluoroacetic acid, samples were injected and analyzed by LC-ESI-MS on a Bruker MicrOTOF-Q quadrupole-time-of-flight (Q-TOF) instrument as previously described [4] or injected onto a Dionex Ultimate 3000 HPLC equipped with a 1:100 flow splitter connected to a Bruker maXis 4G Q-TOF mass spectrometer. LC methods on both instruments were similar, but in the latter case a slightly different trap-and-elute form of LC-MS was carried out in which 10 μL of sample was loaded via a loading pump at 10 μl/min in 80% water containing 0.1% formic acid (Solvent A) / 20% acetonitrile (Solvent B) onto an Optimize Technologies peptide captrap configured for uni-directional flow on a 10-port diverter valve. The trap was then rinsed at this solvent composition with the HPLC loading pump at 10 μl/min for 3 minutes. The flow over the captrap was then switched to the micro pump running at 3 μL/min and a composition of 20/80 A/B, directed to the MS inlet. This flow rate and solvent composition was held until 8.1 min at which point the switching valve was reset and the run completed. Both mass spectrometers were operated in positive ion, TOF-only mode, and set to acquire spectra in the m/z range of 300 to 3000. ESI settings for the Agilent G1385A capillary microflow nebulizer ion source were as follows: End Plate Offset −500 V, Capillary −4500 V, Nebulizer nitrogen 2 Bar, Dry Gas nitrogen 3.0 L/min at 225°C. All other post-source ion optics settings were configured to be as close as possible between the two instruments. Data were acquired in profile mode at a digitizer sampling rate of 2 GHz (MicrOTOF-Q) or 4 GHz (maXis). Spectra rate control was by summation at 1 Hz. Data from the two instruments were processed identically: Approximately 30 seconds of recorded spectra were averaged across the chromatographic peak apex of osteocalcin. The electrospray ionization charge-state envelope was deconvoluted with Bruker DataAnalysis v4.2 software to a mass range of 1000 Da on either side of any identified peak. Deconvoluted spectra were baseline subtracted, and for each osteocalcin proteoform the most intense isotope peak height (i.e., height of the X+3 isotope peak) was calculated, tabulated and exported to a spreadsheet for further analysis. (Peak heights were used for quantification as opposed to peak areas due to full isotopic resolution and occasional obfuscation of the lightest or heaviest isotope.) Thus the final raw data collected from each sample consisted of a single averaged mass spectrum. The relative abundances of the various OC proteoforms were calculated from this mass spectrum making the data from each individual sample self-normalized. This obviated the need for standard curves as well as any remaining concern about possible bias between the two different instruments used to collect the data. The gamma-carboxylation score was calculated as the expected value of the number of gamma carboxylations, i.e., as the number of carboxylations times the fraction that had that many carboxylations, summed over the number of possible carboxylations.
Laboratory analyses and the calculation of variables of glucose homeostasis have been described previously.[25] The glucose disposal rate was calculated as the glucose infusion rate during the last 30 minutes of the clamp, adjusted for the drift in plasma glucose concentration. Insulin sensitivity (SI) was calculated as (glucose disposal rate × concentration of infused glucose)/(insulin concentration at steady state - fasting insulin concentration). As the primary measure of insulin secretion, acute insulin response to glucose (AIRg) was calculated as incremental insulin area under the curve 2–10 minutes after the IVGTT dextrose infusion. A standard 75-gram oral glucose tolerance test (OGTT) was performed approximately one week after the IVGTT and clamp, and glucose area under the curve (AUC) was calculated as a measure of glucose tolerance.
Nutritional data analyses and calculations
Three-day dietary records were analyzed using the Nutrition Data System for Research1 (NDSR) developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis. Average vitamin K1 intake was calculated by taking the mean of total vitamin K1 intake consumed over the 3 days covered by the dietary record. Intake data were transformed into μg/1,000 kcal to adjust for potential over- vs. underreporting of food intake. Due to the lack of complete data on food vitamin K2 content in NDSR and inconsistencies of available data in existing nutrient databases, no data on vitamin K2 or total vitamin K intake were obtained.
Statistical analyses
We described participant characteristics by CKD status using mean and standard deviation for continuous variables (or by median and interquartile range where the variable was highly skewed), or number and percent for categorical variables. We explored bivariate relationships via scatterplots and using Pearson’s correlation coefficients, and tested differences in dietary outcomes and OC concentration, carboxylation, and fragmentation by CKD status using the Student’s t test, assuming unequal variances. In the primary analysis, we fitted linear regression models to test associations of OC concentration, carboxylation, and fragmentation with glucose tolerance, insulin sensitivity, and pancreatic beta-cell function. The resulting estimates and corresponding 95% confidence intervals (calculated using robust Huber-White estimates of standard error) are presented both unadjusted and adjusted for a small number of potential confounding factors: age, sex, race (white/black/other), fat mass, and CKD status (yes/no). To account for a small (<5%) number of participants who were missing data on body composition, we used multiple imputation using chained equations in all regression analyses, and imputations were combined using Rubin’s rules.[27] Finally, we performed a principal components analysis of the percentage of circulating OC in different fragments to assess the proportion of explained variability. All analyses were performed using R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org), and a nominal p-value of < 0.05 was taken as evidence of statistical significance for all analyses.
Results
Participants with CKD and healthy controls were largely similar with regard to most baseline characteristics, including gender and racial distribution, smoking status, and habitual physical activity (Table 1). Participants with CKD tended to be slightly older and heavier, and more individuals had a history of cardiovascular disease and antihypertensive medication use. Dietary vitamin K1 intakes were lower in participants with CKD compared to controls [56 (46) vs. 106 (124) μg/1,000 kcal (mean SD); p=0.03]. As reported in detail in the parent study,[25] the main difference between patients with CKD and controls in measures of glucose homeostasis was lower insulin sensitivity, as measured by hyperinsulinemic euglycemic clamp.
Table 1.
Characteristics of study participants
| Controls (N = 37) | CKD (N = 50) | |
|---|---|---|
| Age, years | 60.7 (12.6) | 62.6 (14.1) |
| BMI, kg/m2 | 27.7 (6.5) | 30.5 (6.2) |
| Women | N=17 (46%) | N=27 (54%) |
| Race | ||
| White | N=32 (86%) | N=35 (70%) |
| Black | N=4 (11%) | N=10 (20%) |
| Asian/Pacific Islander | N=1 (3%) | N=5 (10%) |
| History of cardiovascular disease | N=2 (5%) | N=14 (28%) |
| Current smoking | N=3 (8%) | N=7 (14%) |
| Median physical activity, HAP score | 83.0 (76.0–90.0) | 78.0 (73.2–82.0) |
| Use of antihypertensive medication | N=12 (32%) | N=44 (88%) |
| Systolic blood pressure, mmHg | 123.2 (13.4) | 132.9 (15.5) |
| Median serum creatinine, mg/dL | 0.9 (0.8–0.9) | 1.7 (1.5–2.1) |
| Median serum cystatin C, mg/dL | 0.9 (0.7–1.0) | 1.6 (1.4–2.1) |
| eGFR, mL/min/1.73 m2 | 89.2 (16.8) | 36.9 (12.9) |
| Median urine AER, mg/24hr | 5.8 (3.6–8.7) | 91.7 (14.6–294.3) |
| Fasting glucose (mg/dL) | 99.8 (10.3) | 102.1 (8.7) |
| Fasting insulin (uU/mL) | 7.3 (5.4) | 10.2 (5.3) |
| HOMA-IR | 1.7 (1.2) | 2.5 (1.6) |
| Insulin sensitivity (mg/min per uU/mL) | 5.1 (2.1) | 4.1 (2.1) |
| Acute insulin response to glucose (uU × min/mL) | 467.3 (481.7) | 472.2 (354.1) |
| Glucose are-under-the-curve (mg/mL × min) | 190.4 (36.2) | 193.8 (31.6) |
| Aspartate transaminase, IU/L | 25.0 (21.0–27.0) | 22.0 (18.2–25.8) |
| Alanine transaminase, IU/L | 19.0 (17.0–22.0) | 19.0 (15.0–24.0) |
Means (standard deviation) for continuous variables, and N (%) for categorical variables, except as noted. AER: albumin excretion rate; BMI: body mass index; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HAP: human activity profile, HOMA-IR: homeostasis model assessment index of insulin resistance.
The mean circulating concentration of total OC was almost twice as high in participants with CKD, resulting in higher absolute concentrations of almost all subfractions (by both gamma-carboxylation and fragmentation status) in participants with CKD (Table 2). The distribution of OC molecules with 0, 1, 2, and 3 gamma-carboxyl groups were similar between participants with and without CKD, also reflected in a similar gamma-carboxylation score (Table 2, Figure 1). In contrast, the distribution of circulating OC fragments differed significantly between individuals with CKD and controls. Specifically, CKD patients had lower relative proportions of the fully intact Y[1–49]V fragment, the Y[1–48]P fragment, and the L[2–49]V fragment, but a higher percentage of the more truncated fragments pE[4–49V], pE[4–48]P, and W[5–49]V (Figure 1) (“pE” indicates pyroglutamic acid which formed spontaneously from N-terminal glutamine). Principal component analyses based on the distribution of the different circulating OC fragments showed a statistically significant difference between participants with CKD and controls for the first principal component, which had the greatest factor loadings for the intact Y[1–49]V fragment as well as the truncated pE[4–49V], pE[4–48]P, and W[5–49]V fragments (Figure 2).
Table 2.
Osteocalcin concentration, carboxylation, and fragmentation by chronic kidney disease status
| Osteocalcin characteristic | Controls (N = 37) | CKD (N = 50) | p-value (t-tests) |
|---|---|---|---|
| Total osteocalcin concentration (ng/mL) | 53.6 (51.1) | 102.9 (147.5) | 0.03 |
| ɣ-carboxylation status (%) | |||
| 0 ɣ-carboxyl groups | 13.2 (8.3) | 15.2 (10.2) | 0.30 |
| ≤1 ɣ-carboxyl groups | 18.0 (10.6) | 20.9 (14.7) | 0.28 |
| ≤2 ɣ-carboxyl groups | 30.0 (13.9) | 34.4 (18.3) | 0.20 |
| 3 ɣ-carboxyl groups | 70.0 | 65.6 | |
| ɣ-carboxylation score | 2.4 (0.3) | 2.3 (0.4) | 0.24 |
| Absolute concentration by carboxylation status (ng/mL) | |||
| Fully uncarboxylated osteocalcin | 6.6 (6.9) | 17.9 (32.3) | 0.02 |
| ≤1 ɣ-carboxyl groups | 9.3 (9.7) | 24.9 (45.9) | 0.03 |
| ≤2 ɣ-carboxyl groups | 15.4 (14.5) | 41.5 (75.8) | 0.02 |
| 3 ɣ-carboxyl groups | 37.5 | 67.5 | |
| Fragments (%) | |||
| Y[1–49]V (Intact) | 36.7 (7.1) | 31.6 (9.2) | 0.005 |
| Y[3–49]V | 20.5 (3.5) | 21.1 (5.8) | 0.52 |
| Y[1–48]P | 6.7 (1.9) | 4.2 (2.7) | < 0.0001 |
| L[2–49]V | 4.8 (1.6) | 3.5 (2.5) | 0.003 |
| pE[4–49]V | 18.1 (4.5) | 22.6 (7.2) | 0.0006 |
| Y[3–48]P | 4.8 (2.1) | 4.3 (2.4) | 0.38 |
| pE[4–48]P | 3.8 (2.7) | 6.2 (2.8) | 0.0001 |
| W[5–49]V | 4.7 (2.9) | 6.8 (3.3) | 0.002 |
| Absolute concentration of fragments (ng/mL) | |||
| Y[1–49]V (Intact) | 19.1 (17.8) | 29.8 (40.8) | 0.11 |
| Y[3–49]V | 10.3 (8.6) | 24.5 (42.9) | 0.03 |
| Y[1–48]P | 3.8 (4.2) | 4.3 (7.9) | 0.71 |
| L[2–49]V | 2.7 (2.8) | 3.9 (7.1) | 0.27 |
| pE[4–49]V | 10.1 (10.7) | 22.7 (29.4) | 0.008 |
| Y[3–48]P | 2.7 (2.8) | 4.3 (8.0) | 0.19 |
| pE[4–48]P | 2.4 (3.2) | 6.2 (10.0) | 0.02 |
| W[5–49]V | 2.6 (3.5) | 7.2 (11.1) | 0.009 |
Means (standard deviations). CKD: chronic kidney disease.
Figure 1.
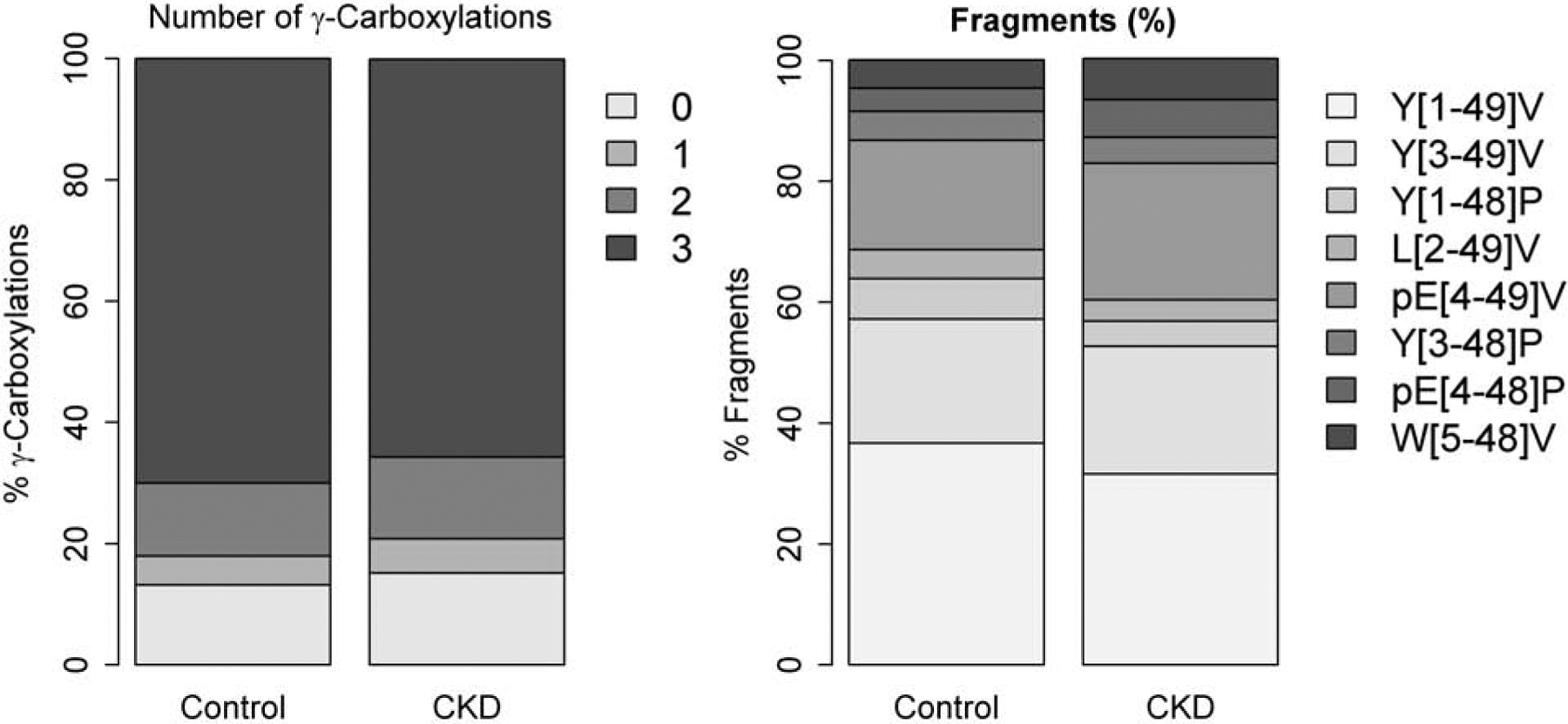
Mean number of ɣ-carboxylations and fragments in circulating osteocalcin in participants with chronic kidney disease (CKD) (n=50) and controls (n=37).
Figure 2.
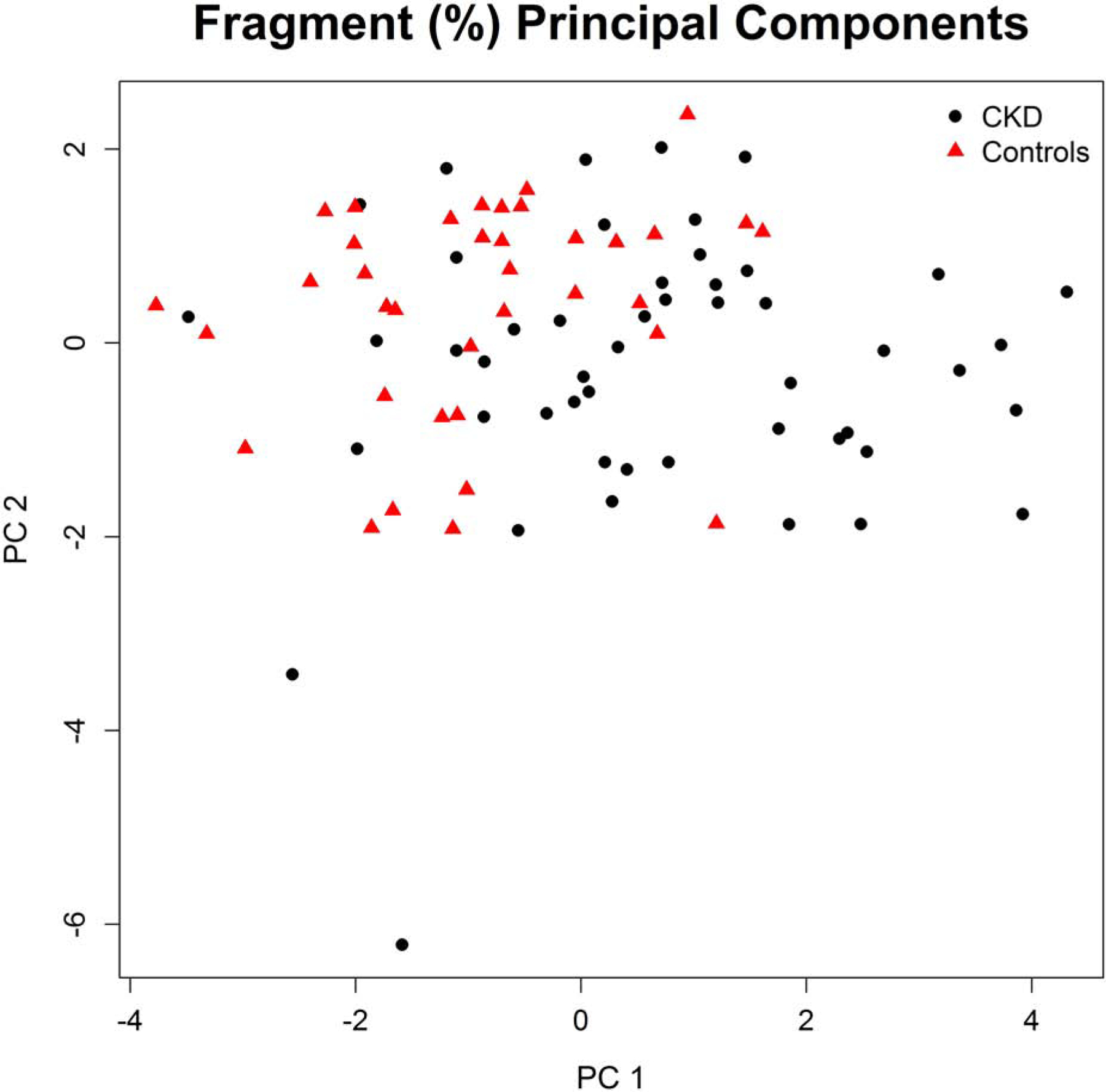
Visualization of principal component analysis of the proportions of different circulating osteocalcin fragments. The first principal component (PC1) has the greatest factor loadings for the following fragments: Y[1–49]V (Intact), pE[4–49]V, pE[4–48]P, and W[5–49]V. The second principal component (PC2) has the greatest factor loadings for the following fragments: Y[3–49]V, Y[1–48]P, L[2–49]V, and Y[3–48]P. Together, PC1 and PC2 explain 39% and 22% of the total variability in fragment proportions, respectively, or cumulatively explain 62% of the total variability in the proportions of different circulating osteocalcin fragments. The value of PC1 differed significantly (p < 0.0001) between participants with chronic kidney disease (CKD) and controls.
Comprehensive analyses of the relationship between the total OC concentration, the OC gamma-carboxylation status, and the OC fragmentation status and glucose tolerance did not suggest any association (Table 3, Figure 3). Specifically, total OC concentration was not associated with AUC glucose over the 2-hour OGTT in the unadjusted model 1 or the multivariable model 2 that was adjusted for age, sex, race, fat mass, and CKD (Table 3). Similarly, no association was seen between the percentage of intact OC fragments or the absolute circulating concentration of any of the eight individual OC fragments and AUC glucose (Table 3, some data for individual fragments not shown). We also did not detect an association of OC carboxylation score or the percentage of fully uncarboxylated OC, the percentage of OC with <=1 carboxyl groups, or the percentage of OC with <=2 carboxyl groups with AUC glucose (Table 3, some data not shown).
Table 3.
Associations of osteocalcin concentration, carboxylation, and fragmentation with glucose tolerance, insulin sensitivity, and pancreatic beta-cell function.
| Glucose tolerance (AUC glucose in 2-h OGTT, mg/dL × min) | Insulin sensitivity [clamp, (mg/min)/(uU/mL)] | Pancreatic beta-cell function [AIRg, (uU*min/mL)] | |
|---|---|---|---|
| Osteocalcin concentration, ng/mL (per doubling) | |||
| Model 1 | 33 (−229; 294), p=0.81 | 0.03 (−0.15; 0.21), p=0.73 | −9.1 (−31.1; 12.0), p=0.42 |
| Model 2 | 35 (−228; 297), p=0.80 | 0.05 (−0.13; 0.22); p=0.60 | 0.0 (−30.1; 30.2), p=0.99 |
| Osteocalcin gamma-carboxylation score (per additional mean number of carboxyl-groups) | |||
| Model 1 | −1,295 (−3,147; 558), p=0.17 | 0.21 (−0.82; 1.23), p=0.69 | 69.7 (−158.3; 297.7), p=0.55 |
| Model 2 | −1,126 (−2,942; 690), p=0.22 | −0.21 (−1.40; 0.99), p=0.74 | −14.3 (−192.5; 164.1), p=0.88 |
| Fully uncarboxylated osteocalcin, % of total (per 10 percentage point increase) | |||
| Model 1 | 530 (−248; 1,309), p=0.18 | −0.12 (−0.51; 0.27), p=0.54 | −3.0 (−104.6, 98.6), p=0.95 |
| Model 2 | 472 (−291; 1,235), p=0.23 | 0.02 (−0.43; 0.47), p=0.92 | 20.9 (−63.6; 105.4), p=0.63 |
| Fully uncarboxylated osteocalcin, ng/mL (per doubling) | |||
| Model 1 | 153 (−19; 325), p=0.08 | 0.01 (−0.14; 0.16); p=0.89 | −27.1 (−49.0; −5.2), p=0.02 |
| Model 2 | 99 (−109; 308), p=0.35 | 0.04 (−0.15; 0.23), p=0.68 | −1.3 (−25.8; 23.3), p=0.92 |
| Intact osteocalcin fragments, % of total (per 10 percentage point increase) | |||
| Model 1 | −94 (−959; 770), p=0.83 | 0.30 (−0.11; 0.72), p=0.15 | 74.5 (−49.7; 198.8), p=0.24 |
| Model 2 | 50 (−870; 970), p=0.92 | 0.10 (−0.42; 0.62); p=0.71 | 11.1 (−85.2; 107.5), p=0.82 |
| Intact osteocalcin fragments, ng/mL (per doubling) | |||
| Model 1 | 35 (−274; 344), p=0.82 | 0.08 (−0.13; 0.30), p=0.46 | −2.9 (−29.3; 23.5), p=0.83 |
| Model 2 | 52 (−261; 365), p=0.75 | 0.07 (−0.13; 0.28), p=0.48 | 2.5 (−33.2; 38.2), p=0.89 |
Data shown are beta-coefficients (95% confidence intervals). Abbreviations: AUC: area-under-the-curve, AIRg: acute insulin response to glucose in an intravenous glucose tolerance test. Model 1: unadjusted. Model 2: adjusted for age, sex, race (white/black/other), fat mass, and CKD (yes/no).
Figure 3.
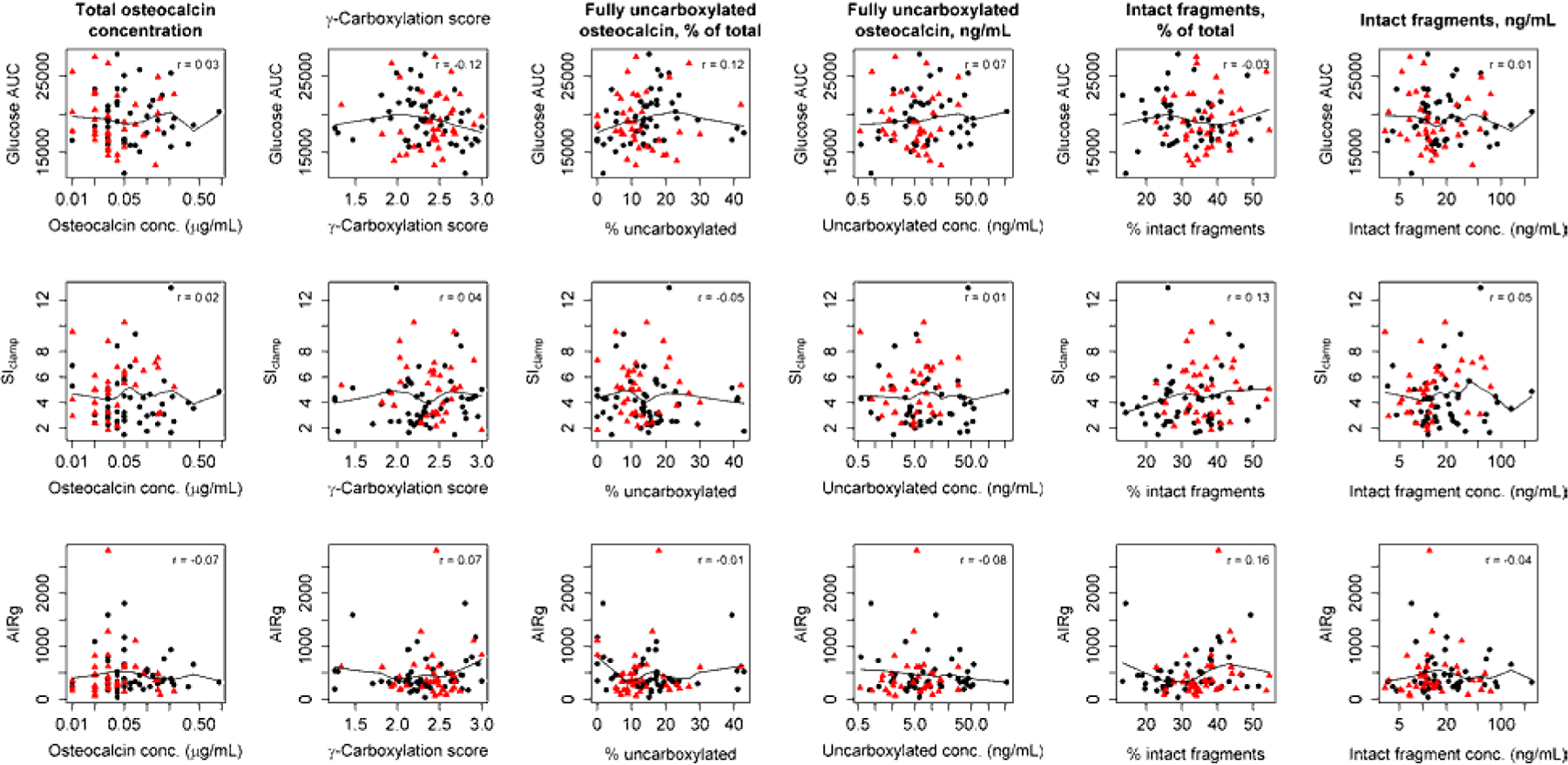
Relationship between total osteocalcin concentration, the osteocalcin carboxylation score, the percentage of fully uncarboxylated osteocalcin, the concentration of fully uncarboxylated osteocalcin, the percentage of intact osteocalcin fragments, and the concentration of intact fragments and glucose tolerance, as measured by the area-under-the-curve (AUC) glucose in a 2-hour oral glucose tolerance test (first row); insulin sensitivity (Si), as measured by hyperinsulinemic-euglycemic clamp (second row); and pancreatic beta-cell function, as measured by the acute insulin response to glucose (AIRg) during a frequently sampled intravenous glucose tolerance test (third row). Black dots: chronic kidney disease. Red triangles: controls.
The absolute concentration of fully uncarboxylated OC was significantly associated with pancreatic beta-cell insulin secretion, as measured by the acute insulin response to glucose (AIRg), in the unadjusted model 1 (Table 3). This association was strongly attenuated and no longer statistically significant after adjustment for age, sex, race (white/black/other), fat mass, and CKD status. OC concentration and fragmentation status were not associated with AIRg. We also did not detect any association between any OC-related measure based on absolute concentration, carboxylation, or fragmentation and insulin sensitivity (Table 3 & Figure 3).
Discussion
Comparing people with versus without CKD, we observed notable differences in the absolute concentration of circulating OC and a shift in the distribution of circulating OC fragments, with a lower percentage of intact OC and a higher percentage of most truncated OC fragments. We also confirmed previous observations of lower dietary vitamin K1 intakes among participants with CKD compared to healthy controls. In spite of these lower vitamin K intakes, however, the degree to which OC was carboxylated did not differ between individuals with CKD and controls. Also in contrast to our hypothesis, we did not detect any association of OC concentration, carboxylation, or fragmentation with insulin sensitivity, insulin secretion, or glucose tolerance after multivariable adjustment. Our data therefore do not provide support for the hypothesis that differences in OC concentration, carboxylation, or fragmentation mediate the relationship between CKD and reduced insulin sensitivity.
Our results are consistent with previous reports that dietary vitamin K1 intake tends to be lower in individuals with CKD compared to healthy controls.[5] Using our gold-standard mass spectrometry-based measure of OC carboxylation, however, we could not confirm reports of reduced OC carboxylation that had been seen in a rat model of CKD.[6] Prior reports indicated a higher percentage of ucOC relative to fully carboxylated OC, as well as a higher absolute concentration of ucOC in CKD patients undergoing hemodialysis.[20–24] Our data show that these findings do not extend to earlier-stage CKD patients not undergoing hemodialysis, suggesting that increased relative and absolute ucOC concentrations may occur only in end-stage CKD or may be a result of hemodialysis rather than CKD. Our data also show that absolute total OC concentrations are elevated in CKD, with an elevated fraction of truncated fragments, suggesting impaired degradation of OC, which is known to occur in liver and kidneys.[28] Our data therefore raise the possibility that earlier findings of higher absolute concentrations of ucOC in patients with CKD may be at least partly due to higher total concentrations of OC, including fragments.
Our finding of increased overall OC concentrations and an increased proportion of truncated fragments in CKD is novel, as previous studies had focused predominantly on the carboxylation status of circulating OC in this patient population. The increased concentration of OC fragments, in particular, is intriguing, as it is reminiscent of the observed accumulation of truncated parathyroid hormone (PTH) fragments in CKD.[29] This is noteworthy in that, as described for PTH,[29] the fragmented forms of OC may differ functionally. It is also important to consider that the affinity of the fragmented OC molecules to the different antibodies used in laboratory assays may differ from that of intact OC. More research is therefore needed to clarify whether OC function or measurement are affected by the increased proportion of truncated fragments in CKD.
We previously demonstrated that insulin sensitivity is reduced in individuals with CKD.[25] Given that OC carboxylation was neither associated with CKD nor insulin sensitivity, our analyses provide no support for the hypothesis that differences in OC carboxylation status may mediate the relationship between CKD and reduced insulin sensitivity. Our data do suggest a potential very modest association between the absolute amount of uncarboxylated OC and both glucose tolerance and pancreatic beta-cell function. However, the strength of these associations was weak in unadjusted analyses, and strongly attenuated by adjustment for classical determinants of metabolic health, making it unlikely that a true cause-effect relationship underlies these observed associations. In mouse models, uncarboxylated OC improves both pancreatic insulin secretion and insulin sensitivity,[19] which suggests that lower vitamin K intake should improve glucose tolerance. However, it is now clear that experimental evidence from mice on the role of OC in glucose homeostasis does not translate to humans.[30] In observational studies in humans, higher vitamin K intake has been linked to greater glucose tolerance, higher insulin sensitivity, and a reduced risk of type 2 diabetes [8–11], and vitamin K supplementation improves insulin sensitivity and/or glucose tolerance in some,[12–15] but not all studies.[16] Because higher vitamin K intake leads to greater carboxylation of OC,[4, 31, 32] these data would suggest that greater OC carboxylation should be associated with better glucose tolerance and insulin sensitivity. This consideration is consistent with data demonstrating an inverse association between the absolute concentration of carboxylated OC and the homeostasis model assessment (HOMA) insulin resistance index,[18] but not supported by data showing no association between ucOC, cOC, and incident type 2 diabetes.[33] Overall, the human data on the role of dietary vitamin K and OC carboxylation in the regulation of glucose homeostasis is inconsistent. In our study, using gold-standard measures of OC carboxylation, insulin sensitivity, and glucose tolerance, we did not detect even a trend towards an association independent of potential confounding factors. To our knowledge, we are also the first to report that the total concentration and fragmentation status of OC is not associated with glucose tolerance, insulin sensitivity, or pancreatic beta-cell function. Thus, our data suggest that OC metabolism is not a modulator of glucose homeostasis in humans.
Our study had some limitations, including its relatively small sample size that may have limited our power to detect associations. It is also important to emphasize that this was an exploratory secondary analyses without a clear hierarchy of endpoints that is subject to potential false positive findings due to an inflated overall Type I error rate. Assessment of dietary vitamin K intakes should be considered estimates rather than measurements, given that it was based on a subjective assessment method (3-day diet records) that is prone to misreporting and biases, and potentially consisted of too few days to reliably assess the intake of this micronutrient.[34] The lack of reliable data on dietary vitamin K2 intakes is another potential limitation, making it impossible to assess the relationship of total vitamin K intake to osteocalcin carboxylation, CKD, and glucose homeostasis. Strengths of our analyses include the very comprehensive assessment of OC carboxylation and fragmentation, and the use of gold-standard measures of glucose tolerance, insulin sensitivity, and pancreatic beta-cell function. Our study population was well defined as individuals with nondiabetic early stage (pre-hemodialysis) CKD and controls. These analyses therefore fill a noteable gap in the literature due to the fact that most previous studies in this area included only CKD patients undergoing hemodialysis.
In conclusion, the major difference in circulating OC in people with CKD compared to healthy controls is accumulation of fragments (truncated from the N-terminus), with a resulting higher concentration of total but not intact OC. In contrast, CKD was not associated with differential carboxylation of OC. In addition, we observed no significant independent association of OC parameters with factors involved in glucose homeostasis.
Practical application.
Osteocalcin carboxylation, a measure of vitamin K status, is not different in people with CKD compared to healthy controls. Individuals with CKD have higher total circulating OC concentrations, which is largely due to an increased circulating concentration of OC fragments.
Acknowledgments
We are indebted to the participants who graciously volunteered to be a part of this study, and to the staff of the University of Washington Translational Research Unit. We thank Cassianne Robinson-Cohen for her guidance on study procedures, Nicole Robinson and Connor Henry for their contributions to data collection, Tamara Chin and Alexandra Kozedub for their work on the euglycemic clamps, and John Ruzinski, Denise Rock, and Charles Ellis for their work in the laboratory.
Support and financial disclosure
This work was supported by R01DK087726, R01DK088762, and R01DK099199 (to Ian H. de Boer) and an unrestricted fund from the Northwest Kidney Centers, and by the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-70. None of the funders had any involvement in designing the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. None of the authors has any conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Card DJ, Gorska R, Cutler J, et al. Vitamin K metabolism: current knowledge and future research. Mol Nutr Food Res. 2014;58(8):1590–1600 [DOI] [PubMed] [Google Scholar]
- 2.Willems BA, Vermeer C, Reutelingsperger CP, et al. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. 2014;58(8):1620–1635 [DOI] [PubMed] [Google Scholar]
- 3.Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr. 2012;3(2):149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehder DS, Gundberg CM, Booth SL, et al. Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry. Mol Cell Proteomics. 2015;14(6):1546–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung CL, Sahni S, Cheung BM, et al. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34(2):235–240 [DOI] [PubMed] [Google Scholar]
- 6.McCabe KM, Booth SL, Fu X, et al. Vitamin K Metabolism in a Rat Model of Chronic Kidney Disease. Am J Nephrol. 2017;45(1):4–13 [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira RB, Stinghen AEM, Massy ZA. Vitamin K role in mineral and bone disorder of chronic kidney disease. Clin Chim Acta. 2020;502:66–72 [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto N, Nishiike T, Iguchi H, et al. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr Metab. 1999;12(1):37–41 [PubMed] [Google Scholar]
- 9.Yoshida M, Booth SL, Meigs JB, et al. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. 2008;88(1):210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beulens JW, van der AD, Grobbee DE, et al. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010;33(8):1699–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarrola-Jurado N, Salas-Salvado J, Martinez-Gonzalez MA, et al. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am J Clin Nutr. 2012;96(5):1113–1118 [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto N, Nishiike T, Iguchi H, et al. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19(4):259–263 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31(11):2092–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HJ, Yu J, Choi H, et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011;34(9):e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasekhi H, Karandish M, Jalali MT, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891–895 [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Binkley N, Vella A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am J Clin Nutr. 2010;92(6):1528–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea MK, Booth SL. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients. 2016;8(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea MK, Gundberg CM, Meigs JB, et al. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90(5):1230–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Brennan-Speranza TC, Levinger I, et al. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients. 2018;10(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cranenburg EC, Schurgers LJ, Uiterwijk HH, et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012;82(5):605–610 [DOI] [PubMed] [Google Scholar]
- 21.Elliott MJ, Booth SL, Hopman WM, et al. Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can J Kidney Health Dis. 2014;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilkey RM, Morton AR, Boffa MB, et al. Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis. 2007;49(3):432–439 [DOI] [PubMed] [Google Scholar]
- 23.Westenfeld R, Krueger T, Schlieper G, et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;59(2):186–195 [DOI] [PubMed] [Google Scholar]
- 24.Nagata Y, Inaba M, Imanishi Y, et al. Increased undercarboxylated osteocalcin/intact osteocalcin ratio in patients undergoing hemodialysis. Osteoporos Int. 2015;26(3):1053–1061 [DOI] [PubMed] [Google Scholar]
- 25.de Boer IH, Zelnick L, Afkarian M, et al. Impaired Glucose and Insulin Homeostasis in Moderate-Severe CKD. J Am Soc Nephrol. 2016;27(9):2861–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson M, de Morton N. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21(2):151–162 [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley & Sons, New York, 1987. [Google Scholar]
- 28.Farrugia W, Melick RA. Metabolism of osteocalcin. Calcif Tissue Int. 1986;39(4):234–238 [DOI] [PubMed] [Google Scholar]
- 29.Evenepoel P, Bover J, Urena Torres P. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016;90(6):1184–1190 [DOI] [PubMed] [Google Scholar]
- 30.Booth SL, Centi AJ, Gundberg C. Bone as an endocrine organ relevant to diabetes. Curr Diab Rep. 2014;14(12):556. [DOI] [PubMed] [Google Scholar]
- 31.van Summeren MJ, Braam LA, Lilien MR, et al. The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br J Nutr. 2009;102(8):1171–1178 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura E, Aoki M, Watanabe F, et al. Low-dose menaquinone-4 improves gamma-carboxylation of osteocalcin in young males: a non-placebo-controlled dose-response study. Nutr J. 2014;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwakenberg SR, Gundberg CM, Spijkerman AM, et al. Osteocalcin Is Not Associated with the Risk of Type 2 Diabetes: Findings from the EPIC-NL Study. PLoS One. 2015;10(9):e0138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Presse N, Payette H, Shatenstein B, et al. A minimum of six days of diet recording is needed to assess usual vitamin K intake among older adults. J Nutr. 2011;141(2):341–346 [DOI] [PubMed] [Google Scholar]


