Abstract
Objective:
Addressing tobacco use among HIV+ smokers is a priority. Lack of knowledge about how HIV+ smokers respond to tobacco use treatments limits our ability to effectively treat this population of smokers.
Method:
Using data from two clinical trials that provided 12 weeks of varenicline and behavioral counseling, one with smokers with HIV (n=89) and one with smokers without HIV (n=179), we used mixed logistic regression modeling to compare point-prevalence abstinence rates and adherence to the initial target quit date (TQD) and Cox regression for repeated outcomes to evaluate lapse and recovery dynamics between the groups.
Results:
60% of HIV− smokers refrained from smoking at the TQD while only 33% of HIV+ smokers did (OR=0.32, 95% CI: 0.18-0.56, p<0.001). The point-prevalence abstinence rates at week 12 were 31% (HIV−) and 28% (HIV+) (OR=0.7, 95% CI: 0.42-1.16, p=0.16) and the point prevalence abstinence rates at week 24 were 22% (HIV−) and 15% (HIV+) (OR=0.87, 95% CI: 0.49-1.57, p=0.65). While there was no interaction between HIV status and lapse risk (Χ2(3)<1), there was a significant interaction for the recovery model (Χ2(3)=20.4, p<0.001): as the number of events increased, the time to the next recovery became longer among smokers with HIV, compared to smokers without HIV.
Conclusions:
While HIV+ smokers were treated effectively with varenicline, compared to HIV− smokers, they showed significantly lower initial cessation at the TQD and took increasingly longer to recover following lapses.
Public Health Significance:
These results suggest that to ensure that smokers with HIV are optimally treated with varenicline, additional strategies may be needed to increase success with initial quit attempts and to support faster recovery following a lapse.
Keywords: Quit attempts, lapse and recovery, HIV+, varenicline
INTRODUCTION
The widespread use of anti-retroviral therapy (ART) for people with HIV (PWH) has substantially improved life expectancy (Antiretroviral Therapy Cohort, 2008), with certain sub-groups of PWH showing a life expectancy similar to that of the general population (Samji et al., 2013). This medical and public health achievement has led to the critical need to address modifiable health risk behaviors, most notably tobacco use. PWH now lose more life-years to tobacco use than to their HIV infection (Helleberg et al., 2013). Tobacco use among PWH increases the risk for cancer (Altekruse et al., 2018; Reddy et al., 2017), cardiovascular disease (CVD) (Friis-Moller et al., 2003), and respiratory conditions such as COPD and pneumonia (Pacek & Crum, 2015), and is estimated to account for almost one-quarter of all deaths among PWH (Lifson et al., 2010). Unfortunately, a study with a large nationally representative sample reported that 42% of PWH were current smokers (Mdodo et al., 2015). Although the rate of smoking among PWH declined from 2009 to 2014, it was only by ~5% and was still nearly 3 times greater than the rate in the general population (Frazier, Sutton, Brooks, Shouse, & Weiser, 2018).
While interest in quitting smoking is high among PWH (Frazier et al., 2018; Pacek & Cioe, 2015; Pacek & Crum, 2015), particularly when HIV treatment is initiated (D. J. Vidrine et al., 2018) and when tobacco use treatment is integrated with HIV care (Pacek, Rass, & Johnson, 2017), there are insufficient data to indicate that tobacco dependence interventions that are efficacious in the general population are efficacious for PWH (Ledgerwood & Yskes, 2016; Pacek & Cioe, 2015; Pool, Dogar, Lindsay, Weatherburn, & Siddiqi, 2016). For example, while behavioral smoking cessation interventions yield moderate effects vs. medical care alone (Keith, Dong, Shuter, & Himelhoch, 2016), no specific behavioral intervention has been identified as being uniquely effective for treating tobacco use among PWH. With respect to pharmacological treatments for smoking cessation, nicotine patches combined with behavioral counseling among PWH have reported moderate effect sizes (Gritz et al., 2013; Manuel, Lum, Hengl, & Sorensen, 2013; Moadel et al., 2012), but only two studies compared the nicotine patch to no medication (Cropsey et al., 2013; Wewers, Neidig, & Kihm, 2000). Two randomized placebo-controlled trials of varenicline found that, compared to placebo, varenicline significantly increased end-of-treatment (EOT) quit rates, with cessation among varenicline-treated participants of 28-29% (Ashare, Thompson, Serrano, et al., 2019; Mercie et al., 2018). Although the data suggest that existing treatments are better than placebo/no treatment for HIV+ smokers, a better understanding of how a quit attempt typically progresses among these smokers can guide strategies to optimize treatments.
For many smokers, quitting smoking is a dynamic and often cyclical process comprised of periods of abstinence, interrupted by instances of smoking, called lapses (J.R. Hughes, Keely, & Naud, 2004; Shiffman et al., 2006). Between these intermittent lapses, a smoker may be able to regain abstinence for some period of time. However, most quit attempts end in relapse, defined as a return to a regular pattern of smoking (J.R. Hughes et al., 2004; Kenford et al., 1994). As such, much of the research on understanding quit attempt dynamics has focused on behavior early in the quit attempt. Indeed, the ability to maintain early abstinence (e.g., first 1-2 weeks) (Baker et al., 2011; Ferguson, Gitchell, Shiffman, & Sembower, 2009) is associated with greater odds of abstinence at longer-term follow-up. For example, individuals who were able to maintain abstinence for the first week after the target quit date (TQD) were significantly more likely to be abstinent at 6 months post-TQD than those who never achieved initial abstinence (i.e., lapsed on the TQD) (Ashare, Wileyto, Perkins, & Schnoll, 2013). Although adherence to a scheduled TQD is thought to increase the likelihood of success (Fiore, Jaen, & Baker, 2008), recent studies have demonstrated that a more flexible approach, that allows smokers to choose their TQD based on when they feel ready, may be more effective for some smokers (J. R. Hughes, Russ, Arteaga, & Rennard, 2011; Rennard et al., 2012).
While early quitting behavior is clearly important, evaluating patterns beyond the initial lapse has also yielded important predictive information about eventual smoking relapse. The duration between the initial quit attempt (typically the TQD) and relapse, as well as the frequency and quantity of lapses, varies between individuals (Kirchner, Shiffman, & Wileyto, 2012). Moreover, the relationship between previous and future quit attempts is complex. For example, a smoker who recently made a quit attempt and failed (i.e., relapsed) has a greater likelihood of making another quit attempt and has a greater chance of relapsing again (Vangeli, Stapleton, Smit, Borland, & West, 2011; Zhou et al., 2009). However, the number of lapses a smoker has within a given time period may not be predictive of subsequent relapse (Zhou et al., 2009). Rather, faster time to the first lapse and shorter duration between subsequent lapses predict increases relapse rates (Kirchner et al., 2012; P. Wileyto et al., 2004). Although a few studies have evaluated individual characteristics associated with smoking cessation behavior patterns (e.g., nicotine dependence levels, having a live-in partner) (Leyro, Hendricks, & Hall, 2015), no study that we know of has evaluated these dynamics among smokers with HIV. Given the low rates of responsiveness to smoking cessation treatments and high smoking relapse rates among PWH (Mdodo et al., 2015), a more fine-grained analysis of the patterns of abstinence, lapses, and recoveries could inform the development of population-specific interventions targeting relapse prevention (E. P. Wileyto et al., 2005). This study evaluated whether, compared to smokers without HIV, smokers with HIV: (1) have lower point-prevalence abstinence rates after 12 weeks of treatment with varenicline and again at 6-month follow-up, controlling for demographic and smoking-related variables; (2) are less likely to achieve initial abstinence (i.e., quit smoking on the assigned TQD); and (3) will exhibit differential lapse dynamics (i.e., shorter periods between lapses and longer periods between recoveries).
METHODS
Samples
Participants with HIV (HIV+)
Data were from a placebo-controlled varenicline trial for HIV+ smokers (NCT01710137) approved by the IRB. Participants were recruited through the health system of an academic medical center in Philadelphia, advertisements, and through a community-based HIV clinic. To be eligible, individuals had to be age ≥18, have a confirmed HIV diagnosis and be treated with ART with HIV viral loads <1000 copies/ml and CD4+ counts >200 cells/mm3, report daily smoking (at least 1 cigarette per day), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) <2 times upper limit of normal, and creatinine clearance >50 mL/min. Individuals were excluded if they reported a lifetime history of psychosis or a suicide attempt, reported a current or planned pregnancy, reported current use of smoking cessation medications or other tobacco products, and showed unstable or untreated alcohol/substance abuse [Subjects were included if dependence was in the past (greater than 1 year ago) or if the subject was currently receiving treatment (e.g. medication, group therapy, support groups and/or intensive outpatient programs) and was stable for >30 days]. For the current analysis, only those who were randomized to receive varenicline in the trial were included (n=89 out of 179).
Participants without HIV (HIV−)
Data were from a placebo-controlled trial of nicotine replacement therapy and varenicline for smokers in the general population (NCT01314001) conducted at 4 academic medical centers. Randomization was stratified by the nicotine metabolite ratio (NMR), a biomarker that represents individual rate of nicotine clearance. Smokers ages 18-65 who reported smoking ≥10 cigarettes/day for the past 6 months were included. Exclusion criteria included use of non-cigarette tobacco products, e-cigarettes, or current smoking treatment; history of substance abuse treatment, current use of cocaine or methamphetamine, or >25 alcoholic drinks per week; medical contraindications (e.g., pregnancy); history of DSM-IV Axis 1 disorder or suicide risk score >1 on the MINI International Neuropsychiatric Interview (Sheehan et al., 1997), or current major depression; current use of antipsychotics, stimulants, metformin, or medications altering CYP2A6 activity. The detailed study protocol has been described elsewhere (Chenoweth et al., 2014; Schnoll et al., 2014). Only subjects who were randomized to receive varenicline were included (n=267 total). All participants in both trials provided written informed consent.
Procedures
Behavioral Smoking Cessation Counseling
Both studies provided manual-based smoking cessation counseling based on PHS guidelines (Fiore et al., 2008). Both counseling protocols were based on US Public Health Service guidelines and focused on enhancing awareness of the harmful effects of smoking, assisting the person in developing skills to quit and avoid relapse, and instructing the smoker on medication use (Fiore et al., 2008). Each sample received a 1-hour in-person counseling session one week prior to the TQD (for the sample of smokers without HIV this was a group counseling session; for the sample with HIV this was an individual counseling session). Both samples received a 30-minute counseling session on the TQD (this was conducted via phone for the sample without HIV). The sample with HIV received four additional 20-minute in-person counseling sessions (total of 6). The sample without HIV received three additional 20-minute phone counseling sessions (total of 5). Supplementary Table 1 depicts the assessment timeline for each study.
Varenicline Use
Both studies followed FDA guidelines for the varenicline dosing regimen: Day 1-Day 3 (0.5mg once daily); Day 4-Day 7 (0.5mg twice daily); and Day 8-EOT (1.0mg twice daily). Medication adherence was assessed via pill count and adherence was defined as taking at least 80% of prescribed doses.
Measures
Baseline Measures (Both Samples)
Demographic information (e.g., gender, race) were ascertained. Smoking-related data included self-reported smoking behavior (e.g., current smoking rate, number of years smoked), the Fagerström Test for Cigarette Dependence (FTCD) (Fagerstrom, 2012)) and breath carbon monoxide (CO).
HIV-related Health Outcomes (HIV+ Sample Only)
Disease-related characteristics, including current plasma HIV viral load and CD4+ count (within the past 6 months), were collected from medical records. Mode of HIV acquisition and current ART medications and ART adherence were collected through self-report.
Abstinence Outcomes (Both Samples)
Smoking outcomes were derived from the timeline follow-back (TLFB) method (Lerman et al., 2015; Schnoll et al., 2015), which records the number of cigarettes smoked each day throughout the treatment and follow-up periods (TQD through the 6-month follow-up). The assessment time points for each study are shown in Supplementary Table 1. The TLFB has been shown to be a reliable and valid measure of smoking behavior using intervals of 30 days or more (Bernstein, Rosner, & Toll, 2016; Robinson, Sobell, Sobell, & Leo, 2014). The smoking behavior outcomes were: adherence to the TQD (i.e., self-reported abstinence from smoking on the TQD) and 7-day point-prevalence abstinence at Weeks 12 (EOT) and 24 (i.e., no self-reported tobacco use, not even a puff, during the 7 days preceding the assessment and a CO ≤8ppm (Benowitz et al., 2002; J. R. Hughes et al., 2003)).
Lapse and Recovery Variables (Both Samples)
Outcomes are time to lapse (a day that included smoking) and time to recovery after a lapse (first entire day without smoking). As in past work (E. P. Wileyto et al., 2005), time intervals were characterized by the starting day from the timeline (tInitial), and the ending day (tFinal). Time intervals were marked as a transition event if someone who was smoke-free lapsed or someone who had lapsed recovered. When finished, TLFB data were divided into abstinent and smoking intervals (Figure 1A). Intervals were shifted back to the origin, so that time to event was interval length (tFinal - tInitial +1), with a minimum length of 1 day. The sequence of two lapses were used in fitting the lapse model, and the sequence of one recovery and one censoring event were used in fitting the recovery model. Data were censored at Week 12 because there were few transitions between lapse and recovery after Week 12. There were, however subjects who had large numbers of transitions, and we limited data for each subject to the first 12 transition events. Recovery was assessed for all participants, but lapses were only assessed for those who abstained from smoking at least once between the TQD and Week 12.
Figure 1.
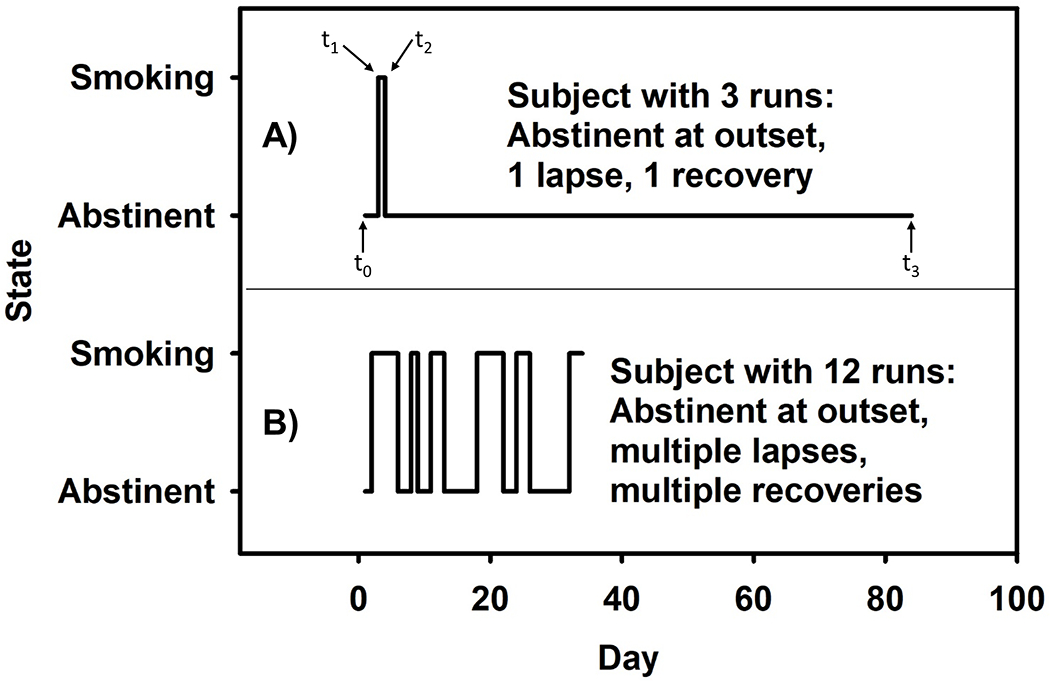
Sample patterns of smoking behavior for two subjects. (A) A subject who had an early lapse event (t1), recovered relatively quickly (t2), and remained abstinent through the end of treatment (t3). (B) A subject who had 5 lapses of varying duration with brief recoveries in between until ultimately relapsing prior to Day 40. This subject was still smoking at EOT.
Data Analysis
Because the sample without HIV was significantly larger than the sample with HIV, propensity score matching was used to create a matched sample of smokers without HIV. Variables used to balance the samples were those that either influenced the outcome or differed between the groups at baseline: sex, age, race, income, cigarettes per day, and FTCD. Subjects were matched to create clusters using a 2:1 ratio (Austin, 2011a; Rassen et al., 2012) with calipers = 0.2 (Austin, 2011b). All analyses were conducted with the matched sample of 89 HIV+ and 178 HIV− smokers.
A mixed logistic regression model was used to evaluate differences in abstinence rates between the HIV+ and HIV− samples with the matching cluster-id included as a random effect. For the lapse/recovery model, we used Cox regression for repeated outcomes, with an indicator for transition event, and standard errors calculated using the cluster-correlated covariance matrix. Those who never stopped smoking were never included in the nonsmoking cohort but were included in the smoking cohort. Transition event (i.e., the number of lapses) was also included as a predictor and the interaction between HIV status and transition event was tested. In a supplemental analysis, we evaluated whether adherence to the TQD predicted abstinence across both samples. Lastly, given differences in exclusion criteria regarding history of or current diagnosis of substance use disorder, we conducted sensitivity analyses excluding subjects with either a current (n=6) or a lifetime (n=12) diagnosis of substance use/dependence. Removing these subjects did not substantially alter the results (data not shown).
RESULTS
Sample Characteristics
Overall, the sample was predominantly male (n=191, 72%) and African American (n=222, 83%); 52% (n=139) had a ≤ high school degree or equivalent, 84% (n=224) reported an annual income of $35k or less, and 24% (n=65) reported being married or living as married. On average, the combined sample was 48.4 years old (SD=10.6), had FTCD scores of 4.7 (SD=1.9) and smoked 13.8 cigarettes/day (SD=5.4). These characteristics for HIV+ and HIV− groups separately are presented in Table 1. By design, the groups did not differ on demographic and smoking-related variables. Varenicline adherence was evaluated as a potential covariate because there was a marginal difference between groups. However, adherence was not related to outcomes nor did it substantially alter the models. Therefore, it was not included in the final models.
Table 1.
Demographic Characteristics and Smoking-related Variables
| Variable | HIV− (n=178) | HIV+ (n=89) | p-value |
|---|---|---|---|
| Demographic variables | |||
|
| |||
| Female sex (n, %)a | 51, 29% | 25, 28% | 0.92 |
| African American race (n, %) a | 149, 84% | 73, 82% | 0.73 |
| Agea | 48.3 (10.9) | 48.7 (10.1) | 0.80 |
| Education HS/GED or less (n, %) | 93, 5% | 46, 52% | 0.93 |
| Income ≤ $35,000 (n, %)a | 149, 84% | 75, 84% | 0.91 |
| Married/Living as Married (n, %) | 43, 24% | 22, 25% | 0.92 |
|
| |||
| Smoking-related variables | |||
|
| |||
| Cigarettes per daya | 13.9 (4.3) | 13.5 (7.1) | 0.55 |
| FTCDa | 4.7 (1.8) | 4.7 (2.2) | 0.99 |
|
| |||
| Treatment Variables | |||
|
| |||
| % Adherent to varenicline (n, %) | 125 (74%) | 52 (63%) | 0.09 |
|
| |||
| Characteristics for HIV sample only | |||
|
| |||
| ART adherence past 2 weeks (%, range) | 99%, 79-100% | ||
| % Plasma HIV viral load <50 copies/ml (n, %) | 75 (84%) | ||
| Plasma CD4 Count (cells/mm3) | 737.2 (329.5) | ||
| HIV acquisition via sex (n, %) | 73, 82% | ||
Note: Unless otherwise noted, values are mean (SD); BMI = Body Mass Index; FTCD = Fagerström Test for Cigarette Dependence.
variable used for propensity score matching;
Abstinence Outcomes
For adherence to the TQD, 60% of HIV− smokers refrained from smoking for that 24-hour period. In contrast, only 33% of HIV+ smokers achieved abstinence on their TQD (OR=0.32, 95% CI: 0.18-0.56, p<0.001). At Week 12, abstinence rates were higher among those who adhered to the TQD both in those with HIV (p=0.004) and those without HIV (p=0.005). At Week 24, adherence to the TQD was associated with abstinence among smokers without HIV (p=0.01), but not among smokers with HIV (p=0.25). At Week 12, the point-prevalence abstinence rates were 31% and 28% for HIV− and HIV+, respectively (OR=0.87, 95% CI: 0.49-1.57, p=0.65). Abstinence rates declined overall at Week 24 and the magnitude of decline was similar between HIV− and HIV+ (HIV− to 21% and HIV+ to 15%; OR=0.63, 95% CI: 0.31-1.29, p=0.21).
Lapse and Recovery Models
Frequency Distribution of Transition Events
Figure 2 displays the distribution of numbers of lapse events. A total of 191 subjects had event time data; 87 of those quit on TQD (met TQD) and their first sequence of days was abstinence. Another 104 kept smoking past TQD, and their first sequence of days was smoking. Among those meeting TQD, there were a total of 264 lapses and 232 recovery events. Among those failing to meet TQD, there were a total of 243 lapses and 274 recoveries. At EOT, 36% of the cohort that met TQD had remained abstinent through treatment, 18% recorded only one lapse, and 46% recorded multiple lapses. Within the cohort that failed to meet TQD, 32% never quit through treatment, 12% recovered once and remained abstinent, and 46% recorded multiple quit events. Thus, a total of 158 subjects (66 HIV+ and 92 HIV−) achieved abstinence for at least one 24-h period between TQD and week 12. Based on this distribution, the number of transition events was collapsed into 4 categories: 1=the first transition; 2=the second transition; 3=transition events 3-8; and 4=transition events 9-12.
Figure 2.
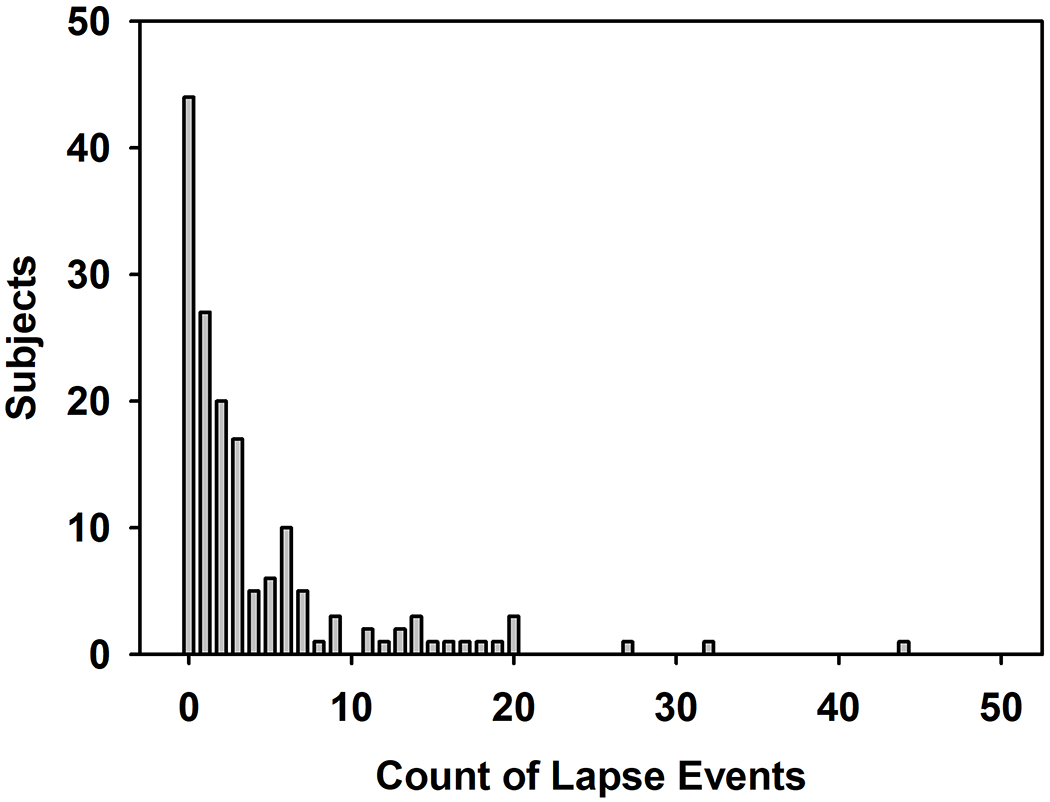
Frequency distribution for the number of lapse events among subjects.
Multivariate Cox Regression Modeling of Time to Lapse and Recovery
The 3-way lapse vs. recovery model by HIV status by transition event interaction was significant suggesting that the time to recovery vs. lapses differed between HIV+ and HIV− smokers (Χ2(3) = 8.5, p = 0.04). Subsequently, we evaluated the 2-way interactions between transition event and HIV status separately for the lapse and recovery models. As shown in Table 3, there was a main effect of transition event such that the time to lapse became shorter with each successive event. However, there was no main effect of HIV status and the overall interaction between HIV status and event sequence was not significant (Χ2(3) = 0.34, p = 0.95). In contrast, for the recovery model, there was a main effect of transition event that was qualified by an interaction with HIV status (Χ2(3) = 20.4, p < 0.001). As shown in Table 3, as the number of events increased, the time to the next recovery became longer among smokers with HIV, compared to smokers without HIV.
Table 3.
Cox regression modeling time to lapse and recovery
| Lapse Modela | Recovery Modelb | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value |
| HIV Status (ref = HIV−) | 0.89 | 0.50, 1.59 | 0.7 | 1.50 | 0.99, 2.28 | 0.06 |
| Transition Event (ref = 1) | ||||||
| Event 2 | 1.86 | 1.24, 2.80 | 0.003 | 2.67 | 1.83, 3.89 | <0.001 |
| Event 3-8 | 2.31 | 1.72, 3.09 | <0.001 | 3.57 | 2.51, 5.07 | <0.001 |
| Event 9-12 | 3.75 | 2.74, 5.13 | <0.001 | 6.18 | 4.27, 8.95 | <0.001 |
| HIV Status X Transition Event (ref = HIV− at Event 1) | ||||||
| HIV x Event 2 | 1.24 | 0.57, 2.68 | 0.58 | 1.00 | 0.59, 1.71 | --- |
| HIV x Event 3-8 | 1.10 | 0.57, 2.10 | 0.78 | 0.60 | 0.37, 0.98 | 0.04 |
| HIV x Event 9-12 | 1.08 | 0.53, 2.20 | 0.83 | 0.44 | 0.26, 0.72 | 0.001 |
Note.
Transition event for a lapse refers to a transition from abstinence to smoking and HR > 1 indicates faster time to relapse;
Transition event for a recovery refers to a transition from smoking to abstinence and HR < 1 indicates longer time to recovery
DISCUSSION
Given the substantial burden of smoking among PWH and the need for more effective treatments (Mdodo et al., 2015), the current study sought to compare patterns of quitting behavior among smokers with and without HIV who were treated with varenicline and behavioral counseling. While our clinical trial of varenicline for HIV+ smokers was consistent with the one other RCT that we know of in demonstrating that varenicline outperformed placebo (Ashare, Thompson, Serrano, et al., 2019; Mercie et al., 2018), the quit rates at Week 12 were still relatively low, compared to the general population. Thus, a better understanding of the dynamic processes that occur during a quit attempt could inform the development of population-specific interventions to optimize treatment (E. P. Wileyto et al., 2005). We compared patterns of abstinence, lapses, and recoveries in a relatively large sample of HIV+ smokers to a matched sample of HIV− smokers and our data suggest that smokers with HIV may exhibit unique patterns of lapse and recover behavior which points to potential implications for treatment development.
Although the differences in abstinence rates were not statistically significant, perhaps due to limited statistical power from the sample sizes, they were meaningfully lower among smokers with HIV compared to those without HIV. A recent US study estimated that a 10% reduction in smoking prevalence in each state could yield a $63 billion reduction in healthcare costs the following year (Lightwood & Glantz, 2016), suggesting that even the observed differences in abstinence rates of 8% at EOT and 7% at 6 months may still be clinically significant. We found a large initial difference between smokers with and without HIV, with more than half of smokers without HIV adhering to the TQD compared to only one-third of PWH. However, this large difference in the ability to achieve early abstinence dissipated by Week 12, as evidenced by the non-significant difference in point-prevalence abstinence rates. In prior studies, maintaining early abstinence was associated with better long-term treatment outcome (Baker et al., 2011; Ferguson et al., 2009). Our data suggest that, across both groups, smokers who were able to quit on the scheduled TQD were more likely to be abstinent at Week 12. However, this relationship was not present at Week 24 among smokers with HIV. This finding may be consistent with emerging evidence that allowing smokers to choose their TQD based on when they feel ready is an effective smoking cessation strategy (J. R. Hughes et al., 2011; Rennard et al., 2012). Thus, future studies should explore whether permitting flexible quit dates is an effective strategy to optimize treatments for tobacco use among smokers with HIV.
As expected, our data showed that each time a smoker had a lapse, the duration to the next lapse decreased. In other words, with each subsequent lapse, the time between lapses becomes shorter, increasing the likelihood of eventual relapse. This pattern was similar for smokers with and without HIV. Thus, cessation strategies that focus on relapse prevention should also be incorporated into interventions for smokers with HIV. Although behavioral strategies have shown limited efficacy, pharmacological approaches have shown greater promise for preventing relapse (Livingstone-Banks et al., 2019). For example, extended treatment with transdermal nicotine (24 weeks) can increase abstinence and reduce the likelihood of lapses compared to 8 weeks of treatment (Schnoll et al., 2010). Extended treatment with varenicline has also been shown to reduce the risk of relapse in the general population (Tonstad et al., 2006) and among smokers with psychiatric comorbidities (Evins et al., 2014). Importantly, the relapse prevention effect of varenicline may be particularly strong among those who had difficulty early in a quit attempt (Hajek, Tonnesen, Arteaga, Russ, & Tonstad, 2009). Thus, the evaluation of these and other behavioral and/or pharmacological strategies, such as episodic use of nicotine replacement to address relapse, is an important direction for future work with smokers with HIV.
In contrast to the lapse model, smokers with HIV had a lower recovery rate compared to smokers without HIV. Specifically, the duration between each successive recovery event increased among smokers with HIV, but not among smokers without HIV. This suggests that each time smokers with HIV recovered from a lapse, they spent more time in a smoking state and had more difficulty achieving abstinence relative to smokers without HIV. Based on this finding, it is possible that intervention strategies that place more emphasis on recovery from lapses will be particularly useful for smokers with HIV. For instance, mindfulness-based approaches have demonstrated higher rates of lapse recovery compared to cognitive behavioral techniques (J. I. Vidrine et al., 2016). Modified pharmacological strategies may also be useful for promoting recovery. There is evidence that continued use of nicotine patches following a lapse improves the likelihood of recovery (Ferguson, Gitchell, & Shiffman, 2012) and that re-treatment with varenicline yields quits rates comparable to those observed in first-time varenicline users (Gonzales et al., 2014). In addition, there is also evidence from randomized controlled trials (Rose & Behm, 2014) as well as large population-based cohort studies (Heckman et al., 2017) suggesting that switching treatments following a lapse may be more beneficial for promoting recovery than repeating the same treatment, indicating the potential use of SMART designs to evaluate smoking cessation treatments in this population. To our knowledge, these strategies have not been tested among smokers with HIV.
Several limitations warrant mention. First, the data used in this study were from participants of two clinical trials that had inclusion and exclusion criteria, which limits the generalizability of the results. In addition, there were some differences between the studies with respect to inclusion criteria. For example, urine drug screens were not completed for the sample with HIV and PWH who reported a current or lifetime diagnosis of substance use or dependence were eligible, whereas this was exclusionary for the sample without HIV. However, results were not substantively affected in sensitivity analyses. Second, although this is the first study to systematically evaluate quitting behavior among smokers with or without HIV treated with varenicline and behavioral counseling, the overall sample was relatively small and, thus, statistical power was limited. Third, since we did not have common measures of potential predictors of lapses and recoveries, we were unable to assess a conceptual model of these quitting dynamics. Kirchner and colleagues showed that components of the abstinence violation effect (internal attribution, abstinence self-efficacy, and guilt) were predictive of lapse behavior among smokers, which may be a useful framework to assess among HIV+ smokers (Kirchner et al., 2012). Fourth, although the assessment timeline and procedures were comparable across studies, it is possible that differences across studies may have contributed to observed differences in lapses and recoveries. For example, while we found only a marginal difference in self-reported varenicline adherence that was unrelated to lapses and recoveries, it is possible that objective indices of adherence would identify larger differences that may be related to quitting behavior. Lastly, we did not have a measure of nicotine metabolism such as the nicotine metabolite ratio for all participants so we could not match the samples in terms of this potentially important variable that may differ by HIV status (Ashare, Thompson, Leone, et al., 2019).
In summary, despite these limitations, the present findings help to increase our understanding of the dynamics of quitting behavior among HIV+ smokers undergoing treatment with varenicline and behavioral counseling and can be used to inform the development and testing of new strategies to increase responsiveness to tobacco use treatments. Future studies with smokes who are HIV+ could be designed to increase initial success with the quit attempt and promote recovery following a smoking lapse. Optimizing smoking cessation treatments for this population in this way may help address the unique burden they face and help to maximize the benefits of advances in medical care.
Supplementary Material
Table 2.
Number of subjects in each transition event for the lapse and recovery models
| Lapse Modela | Recovery Modelb | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Transition Event | HIV− | HIV+ | Total | HIV− | HIV+ | Total |
| Event 1 | 61 | 26 | 87 | 50 | 54 | 104 |
| Event 2 | 31 | 40 | 71 | 41 | 15 | 56 |
| Event 3-8 | 52 | 43 | 95 | 50 | 48 | 98 |
| Event 9-12 | 21 | 19 | 40 | 21 | 20 | 41 |
Note. The sequence of two lapses were used in fitting the lapse model, and the sequence of one recovery and one censoring event were used in fitting the recovery model. Data were censored at Week 12 and were limited to the first 12 transition events for each subject.
Acknowledgments
FUNDING
This research was supported by a grant from the National Institute on Drug Abuse, the National Cancer Institute, the National Institute of General Medical Sciences, and the National Human Genome Research Institute (U01 DA020830) and by grants from the National Institute on Drug Abuse (R01 DA033681 and K24 DA045244) and through core services and support from the Penn Center for AIDS Research (P30 AI045008) and the Penn Mental Health AIDS Research Center (P30 MH097488). This research was undertaken, in part, thanks to funding of a Canada Research Chair in Pharmacogenomics (RFT), a Canadian Institutes of Health Research (Foundation grant FDN-154294) and the Centre for Addiction and Mental Health. Support for this work was also provided by a grant from the National Cancer Institute (R35 CA197461). Pfizer provided medication and placebo free of charge.
DECLARATION OF INTERESTS
Dr. Schnoll received medication and placebo free of charge from Pfizer for clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Palliatech. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator-initiated grant from Novo Nordisk for a drug unrelated to the current study. Dr. Tyndale has consulted for Quinn Emanual and Ethismos.
REFERENCES
- Altekruse SF, Shiels MS, Modur SP, Land SR, Crothers KA, Kitahata MM, … Engels EA (2018). Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS, 32(4), 513–521. doi: 10.1097/QAD.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort, C. (2008). Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet, 372(9635), 293–299. doi: 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Thompson M, Leone F, Metzger D, Gross R, Mounzer K, … Schnoll R (2019). Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS, 33(6), 1083–1088. doi: 10.1097/qad.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Thompson M, Serrano K, Leone F, Metzger D, Frank I, … Schnoll (2019). Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug Alcohol Depend, 200, 26–33. doi: 10.1016/j.drugalcdep.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Perkins KA, & Schnoll RA (2013). The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med, 7(4), 249–254. doi: 10.1097/ADM.0b013e31829363e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC (2011a). An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res, 46(3), 399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC (2011b). Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat, 10(2), 150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, … Fiore MC (2011). New methods for tobacco dependence treatment research. Annals of Behavioral Medicine, 41(2), 192–207. doi: 10.1007/s12160-010-9252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, … Velicer W (2002). Biochemical verification of tobacco use and cessation. Nicotine Tob Research, 4, 149–159. Retrieved from 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Rosner J, & Toll B (2016). Concordance between timeline follow-back and single-question assessment of self-reported smoking in a clinical trial. Subst Abus, 37(3), 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LW Jr., Schnoll RA, George TP, Cinciripini PM, … Tyndale RF (2014). Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev, 23(9), 1773–1782. doi: 10.1158/1055-9965.EPI-14-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, Willig J, … Carpenter MJ (2013). A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non-treatment seeking smokers with HIV. Addict Behav, 38(10), 2541–2546. doi: 10.1016/j.addbeh.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, … Schoenfeld DA (2014). Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA, 311(2), 145–154. doi: 10.1001/jama.2013.285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res, 14(1), 75–78. doi: 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, & Shiffman S (2012). Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction, 107(7), 1349–1353. doi: 10.1111/j.1360-0443.2012.03801.x [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S, & Sembower MA (2009). Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clinical Therapeutics, 31(9), 1957–1965. doi: 10.1016/j.clinthera.2009.08.029 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, & Baker TB, et al. (2008). Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. In. Rockville, MD: U.S. Department of Health and Human Service. Public Health Service. [Google Scholar]
- Frazier EL, Sutton MY, Brooks JT, Shouse RL, & Weiser J (2018). Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009-2014. Prev Med, 111, 231–234. doi: 10.1016/j.ypmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, … Data Collection on Adverse Events of Anti, H. I. V. D. S. G. (2003). Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med, 349(21), 1993–2003. doi: 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng LJ, McRae TD, & Treadow J (2014). Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clin Pharmacol Ther, 96(3), 390–396. doi: 10.1038/clpt.2014.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Danysh HE, Fletcher FE, Tami-Maury I, Fingeret MC, King RM, … Vidrine DJ (2013). Long-term Outcomes of a Cell Phone–Delivered Intervention for Smokers Living With HIV/AIDS. Clinical Infectious Diseases, 57(4), 608–615. doi: 10.1093/cid/cit349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Tonnesen P, Arteaga C, Russ C, & Tonstad S (2009). Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction, 104(9), 1597–1602. doi: 10.1111/j.1360-0443.2009.02646.x [DOI] [PubMed] [Google Scholar]
- Heckman BW, Cummings KM, Kasza KA, Borland R, Burris JL, Fong GT, … Carpenter MJ (2017). Effectiveness of Switching Smoking-Cessation Medications Following Relapse. Am J Prev Med, 53(2), e63–e70. doi: 10.1016/j.amepre.2017.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, … Obel N (2013). Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis, 56(5), 727–734. doi: 10.1093/cid/cis933 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, & Naud S (2004). Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction, 99(1), 29–38. doi: 10.1111/j.1360-0443.2004.00540.x [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, & Swan GE (2003). Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res, 5(1), 13–25. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12745503 [PubMed] [Google Scholar]
- Hughes JR, Russ CI, Arteaga CE, & Rennard SI (2011). Efficacy of a flexible quit date versus an a priori quit date approach to smoking cessation: a cross-study analysis. Addict Behav, 36(12), 1288–1291. doi: 10.1016/j.addbeh.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Keith A, Dong Y, Shuter J, & Himelhoch S (2016). Behavioral Interventions for Tobacco Use in HIV-Infected Smokers: A Meta-Analysis. J Acquir Immune Defic Syndr, 72(5), 527–533. doi: 10.1097/qai.0000000000001007 [DOI] [PubMed] [Google Scholar]
- Kenford S. l., Fiore MC, Jorenby DE, Smith SS, Wetter D, & Baker TB (1994). Predicting smoking cessation: Who will quit with and without the nicotine patch. JAMA: the journal of the American Medical Association, 271(8), 589–594. doi: 10.1001/jama.1994.03510320029025 [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, & Wileyto EP (2012). Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol, 121(1), 187–197. doi: 10.1037/a0024451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, & Yskes R (2016). Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis. Nicotine Tob Res. doi: 10.1093/ntr/ntw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW Jr., Cinciripini P, George TP, Wileyto EP, … Group, P.-P. R. (2015). Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med, 3(2), 131–138. doi: 10.1016/S2213-2600(14)70294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Hendricks PS, & Hall SM (2015). If at first you don’t succeed: characterization of smokers with late smoking abstinence onset. Addict Behav, 45, 34–38. doi: 10.1016/j.addbeh.2015.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR, & Group ISS (2010). Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health, 100(10), 1896–1903. doi: 10.2105/AJPH.2009.188664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood J, & Glantz SA (2016). Smoking Behavior and Healthcare Expenditure in the United States, 1992-2009: Panel Data Estimates. PLoS Med, 13(5), e1002020. doi: 10.1371/journal.pmed.1002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, & Hajek P (2019). Relapse prevention interventions for smoking cessation. Cochrane Database of systematic reviews(2). doi: 10.1002/14651858.CD003999.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel JK, Lum PJ, Hengl NS, & Sorensen JL (2013). Smoking cessation interventions with female smokers living with HIV/AIDS: a randomized pilot study of motivational interviewing. AIDS Care, 25(7), 820–827. doi: 10.1080/09540121.2012.733331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, & Skarbinski J (2015). Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med, 162(5), 335–344. doi: 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- Mercie P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, … Chene G (2018). Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV, 5(3), e126–e135. doi: 10.1016/s2352-3018(18)30002-x [DOI] [PubMed] [Google Scholar]
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, & Shuter J (2012). A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr, 61(2), 208–215. doi: 10.1097/QAI.0b013e3182645679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, & Cioe PA (2015). Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV. Curr HIV/AIDS Rep, 12(4), 413–420. doi: 10.1007/s11904-015-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, & Crum RM (2015). A Review of the Literature Concerning HIV and Cigarette Smoking: Morbidity and Mortality, Associations with Individual- and Social-Level Characteristics, and Smoking Cessation Efforts. Addict Res Theory, 23(1), 10–23. doi: 10.3109/16066359.2014.920013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, & Johnson MW (2017). Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers. AIDS Care, 29(10), 1309–1314. doi: 10.1080/09540121.2017.1330532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool ER, Dogar O, Lindsay RP, Weatherburn P, & Siddiqi K (2016). Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst Rev(6), CD011120. doi: 10.1002/14651858.CD011120.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, & Schneeweiss S (2012). One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf, 21 Suppl 2, 69–80. doi: 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- Reddy KP, Kong CY, Hyle EP, Baggett TP, Huang M, Parker RA, … Walensky RP (2017). Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States. JAMA Intern Med, 177(11), 1613–1621. doi: 10.1001/jamainternmed.2017.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, … Russ C (2012). A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine & Tobacco Research, 14(3), 343–350. doi: 10.1093/ntr/ntr220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav, 28(1), 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Rose JE, & Behm FM (2014). Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry, 171(11), 1199–1205. doi: 10.1176/appi.ajp.2014.13050595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, … Design of Ie, D. E. A. (2013). Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 8(12), e81355. doi: 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, & Tyndale RF (2014). The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl), 231(12), 2515–2523. doi: 10.1007/s00213-013-3421-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, … Hitsman B (2015). Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med, 175(4), 504–511. doi: 10.1001/jamainternmed.2014.8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, & Lerman C (2010). Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med, 152(3), 144–151. doi: 10.1059/0003-4819-152-3-201002020-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, … Dunbar GC (1997). The validity of the Mini International Neuropsychiatric Interview(MINI) according to the SCID-P and its reliability. European Psychiatry, 12(5), 232–241. [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, & Clark DB (2006). Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol, 74(2), 276–285. doi: 10.1037/0022-006X.74.2.276 [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, & Reeves KR (2006). Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA, 296(1), 64–71. doi: 10.1001/jama.296.1.64 [DOI] [PubMed] [Google Scholar]
- Vangeli E, Stapleton J, Smit ES, Borland R, & West R (2011). Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction, 106(12), 2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Frank SG, Savin MJ, Waters AJ, Li Y, Chen S, … Gritz ER (2018). HIV Care Initiation: A Teachable Moment for Smoking Cessation? Nicotine Tob Res, 20(9), 1109–1116. doi: 10.1093/ntr/ntx218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, … Wetter DW (2016). Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. J Consult Clin Psychol, 84(9), 824–838. doi: 10.1037/ccp0000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Neidig JL, & Kihm KE (2000). The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. J Assoc Nurses AIDS Care, 11(6), 37–44. doi: 10.1016/s1055-3290(06)60353-1 [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, … Lerman C (2005). Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research, 7(2), 257–268. doi: 10.1080/14622200500055673 [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, … Lerman C (2004). Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine Tob Res, 6(2), 357–366. doi: 10.1080/1462220042000202463 [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, & West R (2009). Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addict Behav, 34(4), 365–373. doi: 10.1016/j.addbeh.2008.11.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


