Abstract
A majority of patients with Alzheimer’s disease (AD) experience some form of sleep disruption, including nocturnal sleep fragmentation, increased daytime napping, decreased slow-wave sleep (SWS, stage N3), and decreased rapid-eye-movement sleep (REM). Clinical studies are investigating whether such sleep disturbances are a consequence of the underlying disease, and whether they also contribute to the clinical and pathological manifestations of AD. Emerging research has provided a direct link between several of these sleep disruptions and AD pathophysiology, suggesting that treating sleep disorders in this population may target basic mechanisms of the disease. Here, we provide a comprehensive review of sleep disturbances associated with the spectrum of AD, ranging from the preclinical stages through dementia. We discuss how sleep interacts with AD pathophysiology and, critically, whether sleep impairments can be targeted to modify the disease course in a subgroup of affected AD patients. Ultimately, larger studies that fully utilize new diagnostic and experimental tools will be required to better define the most relevant sleep disturbance to target in AD, the interventions that best modulate this target symptom, and whether successful early intervention can modify AD risk and prevent dementia.
Keywords: Alzheimer’s disease, sleep, dementia, circadian rhythms, EEG, power spectra
1. Introduction
Alzheimer’s disease (AD) is clinically heterogeneous, with numerous risk factors that affect its clinical and neuropathologic course. Recent advances in neuroimaging and biomarkers, have led to AD being re-conceptualized with pathological processes that begin well before clinical symptoms and which evolve with progressive neuropathological neurodegenerative changes. The clinical symptoms manifest through a spectrum that can include subjective cognitive impairment (SCI), mild cognitive impairment (MCI), and mild-severe AD dementia (Bateman et al., 2012; Jack et al., 2018). Table 1 provides the nomenclature associated with the AD continuum as outlined by Jack and colleagues (2018) (Jack et al., 2018). The long asymptomatic period of AD pathophysiology opens up opportunities for early intervention to prevent clinical symptom evolution and dementia.
Table 1.
The Alzheimer’s disease spectrum. Modified from (Jack et al., 2018).
| Syndromal Categories | Biomarkers | Clinical features |
|---|---|---|
| Preclinical AD | Positive for: A. Aggregated Aβ:
Positive for A/T or A/T/N = Alzheimer’s disease |
Cognitively unimpaired (“asymptomatic”), objective measures within normal range. A subset of individuals may report subjective cognitive decline and/or demonstrate subtle decline on serial cognitive testing. |
| MCI due to AD | Positive for A/T/ or A/T/N | Cognitive performance below expected range for that individual and evidence of decline in cognitive performance from baseline. Amnestic or neurobehavioral disturbances may be prominent. Individuals are able to maintain independence in daily activities. |
| AD dementia | Positive for A/T/N | Progressive cognitive impairment that affects several domains. Individuals are no longer fully independent, requiring assistance with daily life activities. May be subdivided into mild, moderate, and severe stages. |
Recently, there has been growing interest in the sleep disruption associated with AD across this continuum, and as an important component of AD pathophysiology (Mander et al., 2016). It is hypothesized that sleep disturbances might present prior to other clinical symptoms, contribute to the preclinical cascade of neuropathology, and emerge when individuals have objective measurable impairment in cognition (Figure 1).
Figure 1. Hypothesized link between sleep disturbances and the progression of AD.
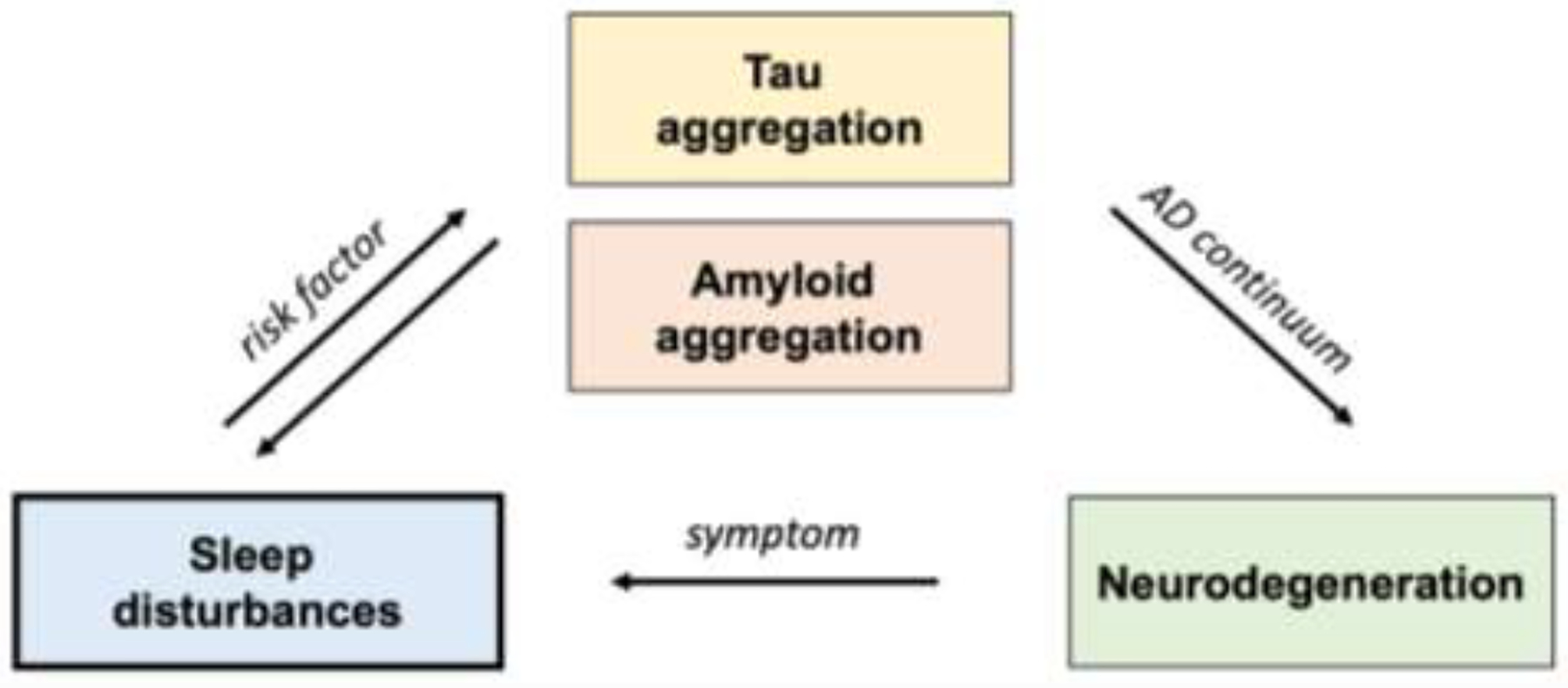
Sleep disturbances may be a risk factor that contributes to the accumulation of tau and amyloid during the earliest stages of the AD continuum and sleep disturbances may also present as a symptom in later disease stages.
Sleep disturbances are common in AD, highly disruptive to quality of life (Petrovsky et al., 2018), increase caregiver burden (Okuda et al., 2019), and are reported to be a leading cause of patients requiring institutional care (Bianchetti, 1995). A recent meta-analysis of 27 observational studies concluded that ~15% of AD cases could be delayed in onset or potentially prevented through effective approaches to address sleep disorders (Bubu et al., 2017). While broad disturbances in sleep have been recognized in AD for decades, more recent efforts have elucidated overlapping mechanisms underlying sleep disturbances and AD pathophysiology, highlighting the potential of treating specific aspects of sleep disruption as a disease modifying therapy (reviewed by (Cedernaes et al., 2017; Mander et al., 2016)).
In this review, we critically evaluate the evidence for the emerging link between sleep and AD pathophysiology. We review subjective and objective methods for sleep assessment in patients along the spectrum of AD, the evidence for sleep disorders as a risk factor for AD, as well as the relationship between sleep and disease pathogenesis. We conclude with a review of potential treatment targets.
The historical and current evaluation of the literature is complicated by the different reference standards for AD diagnosis, from clinically-based diagnosis with lower rates of accuracy, to the new evolving biomarker-based diagnosis and the longstanding historical gold standard of neuropathologically verified disease. Only recently have well-validated and accurate CSF assays and PET biomarkers become available for diagnostic purposes (Jack et al., 2018). Within the biomarker-based criteria there are still challenges to address including studies that have relied exclusively on CSF Aβ for diagnostic reporting. While low levels of CSF Aβ42 are commonly reported for diagnostic purposes, assays and studies vary in the cut-off points used to indicate plaque accumulation (Ju et al., 2013; Molano et al., 2017; Varga et al., 2016). Furthermore, an increase of CSF Aβ42 prior to plaque formation has been proposed (Varga et al., 2016), but requires further validation.
2. Sleep across the Alzheimer’s disease spectrum
Estimates of the prevalence of sleep disorders in AD vary between studies, with one study reporting that 65% of patients with AD or MCI met the diagnostic criteria for a major sleep disorder, including insomnia, sleep disordered breathing, REM sleep behaviour disorder, restless legs syndrome, and excessive daytime sleepiness (Guarnieri et al., 2012). With the increasing availability and utilization of a wide variety of tools including wearables to evaluate sleep, the prevalence of specific sleep disruption across the AD spectrum is expected to be more accurately characterized.
2.1. EEG sleep assessments in Alzheimer’s disease
2.1.1. Sleep staging in Alzheimer’s disease
The gold standard method for evaluating sleep time and architecture is clinical polysomnography (PSG). Table 2 compares the findings from PSG studies in patients with AD and age-matched control participants, listed in chronological order of publication. These generally small sample sized studies show a general trend that AD is associated with shorter sleep duration, longer sleep latency, lower sleep efficiency, less SWS/N3, and less REM. Sleep efficiency and time spent in N3 has also been shown to be reduced with disease progression (Liguori et al., 2014), associated with cognitive impairment (Liguori et al., 2014; Moe, 1995), and exacerbated in APOEε4 carriers (Hita-Yañez, 2012).
Table 2.
EEG studies in patients with Alzheimer’s disease compared to elderly controls
| Reference | Sample | Total Sleep Time | Sleep latency | Sleep efficiency | SWS/N3 | REM |
|---|---|---|---|---|---|---|
| Loewenstein et al., 1982 (Loewenstein, 1982) | AD n = 9; Controls n = 8 | AD = 340.0 min; Controls = 374.0 min (NS) | AD = 25.0 min; Controls 20.0 min (NS) | AD = 77.7%; Controls = 83.8% (NS) | AD = 3.9%; Controls = 10% (*) | AD = 19.9%; Controls 19.7% (NS) |
| Reynold et al., 1985 (Reynolds III, 1985) | AD n = 25; Controls n = 25 | AD = 353.6 min; Controls = 368.7 min (***) | AD = 57.0 min; Controls = 22.2 min (*) | AD = 72.3%; Controls = 84.1% (***) | AD = 1.1%; Controls = 2.5% (NS) | AD = 17.8%; Controls = 20.2% (***) |
| Bliwise et al., 1989 (Bliwise, 1989) | AD n = 28; Controls n = 28 | AD = 363.9 min Controls = 374.8 min (NS) |
Not provided | AD = 68.3% Controls = 78.3% (*) | AD = 2.98%; Controls = 8.50% (**) | AD = 13.6%; Controls = 17.5% (NS) |
| Vitiello et al., 1990 (Vitiello, 1990) | AD n = 44; Controls n = 45 | AD = 398.6 min; Controls = 398.8 (NS) | AD = 13.4 min; Controls 11.0 min (NS) | AD = 81.6%; Controls = 86.0% (NS) | AD = 7.4%; Controls 9.5% (*) |
AD = 18.0%; Controls 19.5% (NS) |
| Moe et al., 1995 (Moe, 1995) | AD n = 78; Controls n = 38 | AD = 346.84 min; Controls = 378.6 min (sig. not provided) | AD = 15.3 min; Controls 11.0 min (NS) | AD = 72.2%; Controls 80.6% (**) | AD = 3.9%; Controls = 6.0% (NS) | AD = 15.0%; Controls=17.7% (NS) |
| Dykierek et al., 1998 (Dykierek, 1998) | AD n = 25; Controls n = 42 | AD = 359.6 min; Controls = 384.9 min (*) | AD = 29.1 min; Controls = 25.3 min (NS) | AD = 74.9%; Controls = 80.0% (*) | AD = 1.2 %; Controls=1.7% (NS) | AD = 15.5%; Controls = 17.2 % (*) |
| Hot et al., 2011 (Hot et al., 2011) | AD n = 14; Controls n = 14 | AD = 386 min; Controls = 371.0 min (NS) | AD = 34 min; Controls = 17 min (NS) | AD = 72%; Controls = 81% (*) | AD = 15.8%; Controls = 14.1% (NS) | AD = 14.5%; Controls = 12.3% (NS) |
| Liguori et al., 2014 (Liguori et al., 2014) | AD n = 48; Controls n=15 | AD = 377.71 min; Controls 382.2 min (NS) | AD = 20.58 min; Controls = 11.67 min (NS) | AD = 71.87%; Controls 89.54% (**) | AD = 8.65%; Controls = 17.87% (**) | AD = 5.03%; Controls = 15.27% (**) |
| Hita-Yanez et al., 2012 (Hita-Yañez, 2012) | MCI n = 25; Controls n = 25 | MCI = 369.8 min; Controls = 397.9 min (NS) | Not provided | Not provided | MCI = 22.0%; Controls = 24.0% (NS) | MCI = 10.1%; Controls = 14.7% (**) |
| Westerberg et al., 2012 (Westerberg et al., 2012) | aMCI n = 8; Controls n = 16 | aMCI = 322.6 min; Controls 362.1 min (NS) | aMCI = 22.3 min; Controls = 17.1 min (NS) | aMCI = 77.2%; Controls = 85.8% (*) | aMCI = 0.2 %; Controls = 2.7% (**) | aMCI = 18.8%; Controls = 23.7% (*) |
| Varga et al., 2016 (Varga et al., 2016) | CSF Biomarker: High Aβ42 n = 18; Low Aβ42 n = 18 | High Aβ42 = 358.4 min; Low Aβ42 = 369.7 min (NS) | Not provided | High Aβ42 = 79.3%; Low Aβ42 = 79.7% (NS) | High Aβ42 = 13.5%; Low Aβ42 = 21.4% (*) | High Aβ42 = 31.1%; Low Aβ42 = 30.8% (NS) |
|
Carnicelli et al., 2019 (Carnicelli et al., 2019) [This study used home-based ambulatory PSG] |
aMCI n = 19; Controls n = 11 | aMCI = 361.9 min; Controls = 414.5 min (NS) | Not provided | Not provided | aMCI = 26.6%; Controls = 23.5 % (NS) | aMCI = 17.0%; Controls = 24.1% (**) |
| Liu et al., 2019 (Liu et al., 2019a) | aMCI n = 32; AD n = 30; Controls n = 30 | aMCI = 355.9; AD = 314.3; Controls n = 400.8 (***) | aMCI = 29.7 min; AD = 32.7 min; Controls = 19.7 min (*) | aMCI = 77.1%; AD = 72.2%; Controls = 82.4% (***) | aMCI = 19.8%; AD n = 15.6%; Controls = 23.5% (***) | aMCI = 18.5%; AD = 16.0%; Controls = 25.3% (***) |
| Liu et al., 2020 (Liu et al., 2020) | aMCI n = 45; Controls n = 22 | Stable MCI = 336.4 min; progressive MCI = 330.8 min; Controls = 372.2 min (***) | Stable MCI = 27.1 min; progressive MCI = 28.6 min; Controls = 20.5 min (*) | Stable MCI = 75.7%; progressive MCI = 76.6%; Controls = 82.5% (**) | Stable MCI = 16.2%; progression MCI = 16.8%; Controls = 18.0% (NS) | Stable MCI = 15.4%; progression MCI = 14.8%; Controls = 17.4% (NS) |
| Liguori et al., 2020 (Liguori et al., 2020a) | SCI n = 54; MCI n = 59; mild AD n = 56; moderate-severe AD n = 48; Controls n = 41 | SCI = 348.1 min; MCI = 349.4 min; mild AD = 318.8 min; moderate-severe AD = 252.9 min; Controls = 367.1 min (***) | SCI = 23.3 min; MCI = 27.7 min; mild AD = 20.5 min; moderate-severe AD = 24.3 min; Controls=13.0 min (NS) | SCI = 77.8%; MCI = 78.5%; mild AD = 74.4%; moderate-severe AD = 67.8%; Controls = 89.2% (***) | SCI = 16.3%; MCI = 16.1%; mild AD = 11.2%; moderate-severe AD = 6.9%; Controls = 17.8% (***) | SCI = 12.6%; MCI = 9.37%; mild AD = 6.41%; moderate-severe AD = 4.6%; Controls = 15.9% (***) |
Table 2: AD = Alzheimer’s disease as defined by the study, Controls = age-matched non-demented control group, MCI = Mild Cognitive Impairment, aMCI = amnestic MCI, SCI = subjective cognitive impairment
p < 0.05,
p < 0.01,
p < 0.001,
NS = not statistically significant.
2.1.2. qEEG in Alzheimer’s disease
Spectral analysis through quantitative EEG (qEEG) may be more sensitive than sleep staging with PSG in identifying the earliest changes in sleep quality associated with AD pathology. Using qEEG to assess power spectra, enables quantification of the distribution of the EEG signal across specific frequencies. With age, EEG power spectra tend to shift, exhibiting lower power in delta (0–4Hz), theta (4–8Hz), and sigma (12–15Hz) frequency bands during sleep (Carrier, 2001; Carrier et al., 2011; Dijk, 1989). This shift toward faster frequency (i.e., reduced power in slower frequency bands) is more pronounced in those with SCI, MCI, and AD compared to healthy elderly controls (Hot et al., 2011; Taillard et al., 2019; Westerberg et al., 2012). These shifts in power spectra may negatively affect memory consolidation (Westerberg et al., 2012) and are associated with tau accumulation (Lucey BP, 2019), and yet would not be detected through traditional sleep staging (Hot et al., 2011).
Characterizing the frequency and coupling of EEG parameters during sleep, such as the alignment of sleep spindles, hippocampal ripples, and slow oscillations is emerging as an important component of sleep in AD (Clemens et al., 2007). Patients with MCI or AD exhibit fewer N2 sleep spindles (Liu et al., 2020; Liu et al., 2019a; Westerberg et al., 2012) and lower sleep spindle power (Taillard et al., 2019) compared to age-matched healthy controls. A recent study of cognitively normal older adults, reported that less coupling between slow oscillations and sleep spindles is associated with tau burden in the medial temporal lobe (Winer et al., 2019), while the number of sleep spindles is negatively associated with CSF tau (Kam et al., 2019).
The importance of evaluating quantitative aspects of sleep in AD has also been highlighted by mouse models of AD, which express a combination of the human transgenes associated with familial AD (e.g., amyloid precursor protein (APP), presenilin 1 (PS1), or tau (MAPT)). Several mouse models of AD exhibit shifts in the power spectra toward faster frequencies, while exhibiting no differences compared to controls in the time spent awake or in NREM or REM sleep stages (Colby-Milley et al., 2015; Ittner et al., 2014; Jyoti et al., 2010; Kent et al., 2019; Kent et al., 2018; Platt et al., 2011). Preliminary data from a mouse model of AD also suggests that coupling of the spindle-band power peak in the frontal cortex and hippocampal ripples is reduced (Zhurakovskaya et al., 2019).
These studies highlight the importance of examining quantitative aspects of EEG when trying to elucidate the relationship between sleep quality and AD.
2.1.3. Multiple sleep latency tests in patients with Alzheimer’s disease
Multiple sleep latency tests, assessing propensity for daytime sleep, typically show higher levels of daytime sleepiness and reduced mean daytime sleep latency (MDSL) in AD compared to controls, and a positive correlation with dementia severity (Bonanni, 2005; Ferman, 2014). Daytime sleepiness is more extreme in dementia with Lewy Bodies (DLB) (Ferman, 2014) and PD (Boddy et al., 2007), suggesting a stronger link with synucleinopathy.
2.2. Actigraphy in Alzheimer’s disease
Given the inherent difficulties of overnight sleep studies in specialized units, actigraphy in the home environment is fast becoming an attractive option as it provides an objective measure of the daily pattern of rest and activity and has been shown to correlate with EEG for estimating sleep time to a high degree (r = 0.91) in patients with dementia (Ancoli-Israel, 1997). As with PSG, actigraphy studies report fragmented sleep-wake rhythms in AD that worsen with disease progression (Hatfield et al., 2004; Liguori et al., 2020b; van Someren; Witting, 1990); however, this has not been a consistent finding across all studies (Basta et al., 2019).
Some studies have used actigraphy to assess the relationship between sleep quality and CSF (Ju et al., 2013; Molano et al., 2017) and PET (Ettore et al., 2019; Lucey BP, 2019; Wilckens et al., 2018) biomarkers in preclinical AD, but the differences in sleep quality between those positive and negative for biomarkers, are small and inconsistent between studies (Ettore et al., 2019; Ju et al., 2013; Lucey BP, 2019).
Benefits of using actigraphy to assess sleep, include its adaptability for use in the home environment, its enabling larger sample sizes than expensive PSG studies, its noninvasive implementation, and its extended use for studies over days, weeks, or even months. However, with present technology it is unable to assess specific sleep stages, power spectra, or circadian rhythmicity. At this point actigraphy lacks sensitivity to identify individuals in the preclinical stage of AD who may present with subtle changes in sleep staging or EEG power shifts, but may be helpful for identifying individuals with severe sleep disturbances.
2.3. Self-reported sleep in Alzheimer’s disease
Sleep assessments using self-report or informant/caregiver assessments, with sleep diaries and standardized sleep questionnaires such as the Pittsburgh Sleep Quality Index (PSQI) (Buysse, 1988) (reviewed by (Ibanez et al., 2018)) have the advantage of being inexpensively conducted and administered to large samples. However, the discrepancies between subjective reporting and objective measures of sleep assessments in AD (Most et al., 2012) need to be considered when evaluating data drawn exclusively from self-report with concerns about the reliability of these measures. However, this approach may be particularly useful in screening for more detailed investigations or identifying those who might benefit from therapies targeting sleep.
In patients with AD dementia, self-reported sleep disturbances are associated with poorer cognitive function (Ancoli-Israel, 1994; Shin et al., 2014) and higher levels of behavioural and psychological symptoms (Kabeshita et al., 2017). The subjective measures of sleep have been less consistent for MCI (Hita-Yanez et al., 2013; J Wams, 2017; Palmer et al., 2018; Yuen et al., 2019) and preclinical AD (Branger et al., 2016; Carvalho et al., 2018; Chen et al., 2018; Fjell et al., 2019; Hwang et al., 2018; Spira et al., 2013; Sprecher et al., 2015; Sprecher, 2017). Self-reported sleep in patients with insomnia has not been associated with CSF biomarker of AD (CSF Aβ42 was higher, not lower, in individuals with insomnia) (Chen et al., 2018), but worse subjective sleep quality has been reported to be associated with CSF biomarkers in those with a family history of AD (Sprecher, 2017). Amyloid deposition measured using PiB-PET has provided more consistent results (Sprecher et al., 2015), with Aβ being associated with self-reported shorter sleep duration (< 6 h) (Spira et al., 2013), poorer sleep quality (Spira et al., 2013), excessive daytime sleepiness (Carvalho et al., 2018), longer sleep latency (Branger et al., 2016), and number of nocturnal awakenings (Branger et al., 2016). These studies showing associations between sleep disturbances and AD biomarkers in cognitively normal individuals, suggest that at least in a subset of individuals sleep disturbances may be an early symptom during the preclinical stage of AD.
2.4. Sleep disordered breathing in Alzheimer’s disease
In addition to changes in sleep quality, there is evidence that sleep disordered breathing is diagnosed in up to 40% of AD patients (Reynolds, 1985). A meta-analysis of 14 studies, with a total of 4 million subjects, concluded that individuals with sleep disordered breathing were 26% more likely to develop cognitive impairment (Leng et al., 2017). Another meta-analysis reported that individuals with AD have a 5x higher rate of presenting with obstructive sleep apnea (Odds Ratio 2.2–16.5) (Emamian et al., 2016). In cognitively normal individuals and individuals with MCI, obstructive sleep apnea is associated with higher sleep fragmentation, reduced SWS, faster changes in Aβ and tau CSF and PET biomarkers, and an increased risk of developing AD (Bubu et al., 2019; Elias et al., 2018; Ju et al., 2016; Lee et al., 2019; Liguori et al., 2019). These findings suggest that sleep disordered breathing is a contributing factor to the sleep disturbances and accumulation of Aβ and tau associated with AD; however, the effect of obstructive sleep apnea on AD biomarkers may depend on whether there is already Aβ accumulation (Sharma et al., 2018), suggesting that sleep fragmentation and/or intermittent hypoxia from apnea may accelerate the neuropathology, but not initiate its development.
3. Sleep disturbance as a risk factor for cognitive decline or dementia
In addition to the evidence that sleep disturbances are associated with AD biomarkers in cognitively normal individuals (e.g., (Branger et al., 2016; Carvalho et al., 2018; Spira et al., 2013)), which suggests that sleep disturbances are an early symptom in the preclinical stage of the AD continuum, longitudinal studies also identify sleep as a risk factor for dementia. Most of these longitudinal studies do not measure AD biomarkers, so it is unknown whether the sleep disturbances occur prior to any pathology, or whether they occur during the preclinical stage of the continuum, when there is underlying neuropathology developing.
A recent meta-analysis of 27 observational studies found that individuals with sleep disturbances have a 1.55 times higher risk of AD, 1.65 higher risk of cognitive impairment, and 3.78 times higher risk of preclinical AD (Bubu et al., 2017). Table 3 summarizes some of the large longitudinal studies that have been conducted in cognitively normal adults to assess sleep as a risk factor for later cognitive decline or dementia. The majority of studies in cognitively normal adults find that sleep disturbances are associated with higher risk for cognitive decline or dementia (Lim et al., 2013a; Luojus et al., 2017; Ohara et al., 2018; Potvin et al., 2012; Suh et al., 2018; Yaffe et al., 2015); however, not all studies find such a relationship (Lysen et al., 2018; Mecca, 2018). Overall, changes in sleep do seem to occur during the preclinical stage of AD and sleep disruption is an important risk factor for AD dementia and could be used as a potential biomarker of underlying AD pathology (Benedict et al., 2015; Hahn et al., 2014; Lim et al., 2013a; Osorio, 2011; Tranah et al., 2011; Yaffe et al., 2014; Yaffe, 2011; Yaffe et al., 2015).
Table 3.
Large longitudinal studies in cognitively normal adults evaluating sleep disturbances as a risk factor for later cognitive decline or dementia
| Reference | Sample | Duration | Methods | Sleep disturbance a risk factor (Yes/No) | Key finding |
|---|---|---|---|---|---|
| Potvin et al., 2012 (Potvin et al., 2012) | n = 1664 | 1 year | Self-report | Yes | Increased incidence of cognitive impairment (MMSE) |
| Keage et al., 2012 (Keage et al., 2012) | n = 2012 | 10 years | Self-report | Yes | Obtaining 6.5 h or less of nighttime sleep and excessive daytime sleepiness were associated with an increased risk of cognitive decline |
| Lim et al., 2013 (Lim et al., 2013a) | n = 737 | 6 years | Actigraphy | Yes | Sleep fragmentation was associated with a 22% increase in annual rate of cognitive decline, and increased risk (1.5-fold) of AD compared to those with the lowest levels of sleep fragmentation |
| Yaffe et al., 2015 (Yaffe et al., 2015) | n=179738 | 8 years | Medical records | Yes | Diagnosis of a sleep disorder was associated with a 27% increased risk of developing dementia |
| Luojus et al., 2017 (Luojus et al., 2017) | n = 2386 | 20 years | Self-report | Yes | Sleep disturbances at baseline had an increased risk of dementia (risk ratio 1.58) |
| Suh et al., 2018 (Suh et al., 2018) | n = 2238 | 4 years | Self-report | Yes | Long sleep latency, long sleep duration and late mid sleep time had increased risk of cognitive decline |
| Ohara et al., 2018 (Ohara et al., 2018) | n = 1517 | 10 years | Self-report | Yes | Short (< 5 h) and long (> 10 h) daily sleep duration and hypnotic use were associated with higher incidence rates of dementia and allcause mortality |
| Lysen et al., 2018 (Lysen et al., 2018) | n = 4835 | 8.5 years | Self-report | No | Poorer subjective sleep quality was not associated with an increased risk of dementia |
| Sindi et al., 2018 (Sindi et al., 2018) | n = 3210 | 3–11 years | Self-report | Yes/No | Late-life sleep disturbances were associated with poorer cognition. Midlife general sleep problems were not associated with late-life MMSE performance |
| Burke et al., 2019 (Burke, 2019) | n = 6782 | 11 years | Self-report | Yes | Sleep disturbance was associated with eventual AD development, at same rate for APOEε4+ and non-carriers |
| Tsai et al., 2020 (Tsai et al., 2020) | n = 3978 diagnosed with obstructive sleep apnea (OSA); n = 15912 healthy controls | Up to 16 years | Medical records | Yes | OSA was associated with increased risk of AD (hazard ratio 2.12). Treated OSA had lower risk of AD. |
Sleep may be a particularly important modifiable risk factor for APOEε4+ individuals (Lim et al., 2013b) as low levels of sleep fragmentation attenuate the increased AD risk of the APOEε4 allele on the rate of cognitive decline and neurofibrillary tangle density (Lim et al., 2013a; Lim et al., 2013b).
There is also a strong link between ever taking benzodiazepines as a sleeping aid and risk of dementia (OR 1.51) (Billioti de Gage et al., 2014). The association between AD risk and benzodiazepine use begins at least five years before diagnosis, suggesting that the sleep disturbances occurr prior to the onset of memory impairment (Billioti de Gage et al., 2014). Similarly, another large retrospective cohort study revealed that those exposed to sedative-hypnotics are at a higher risk of developing AD (HR 1.79) (Lee et al., 2018). The possibility that benzodiazepines or other sedative-hypnotics directly contribute to disease progression remains to be determined.
One limitation of these longitudinal studies is that we cannot ascertain the first onset of sleep disturbances. For example, Ohara and colleagues followed individuals (average 70 years of age at baseline) for 10 years and found that daily sleep duration of less than 5 hours or greater than 10 hours were at a higher risk of dementia (Ohara et al., 2018). We do not know whether these individuals were short or long sleepers throughout their life (perhaps a genetic phenotype that also increases AD risk) or whether there were recent changes in their sleeping habits (suggesting that sleep disturbances may be an early biomarker occurring in the preclinical stage of AD). Given that there is interindividual variability in preferred sleep duration as well as sleep quality, future research assessing sleep as a risk factor for AD should consider incorporating measures of when sleep disturbances first occurred.
4. Beyond a symptom: Sleep disruption contributes to Alzheimer’s disease pathogenesis
Although the precise mechanisms leading to AD are still a major focus of investigation and debate, the accumulation of misfolded Aβ and tau proteins remain the key pathological hallmarks of the disease (Ittner and Götz, 2011), with evidence that sleep disturbances directly contribute to Aβ metabolism, clearance, and deposition (Ju et al., 2014).
4.1. Sleep disruption increases Alzheimer’s disease biomarkers in mouse models
The first evidence that sleep directly affects levels of Aβ and tau metabolites came from mouse models of AD (Di Meco et al., 2014; Holth et al., 2019; Kang et al., 2009; Roh, 2012; Xie et al., 2013; Zhu et al., 2018) where chronic sleep restriction increased cortical Aβ, tau, and memory impairments in the APP/PS1 (Kang et al., 2009), 3xTgAD (Rothman et al., 2013), and P301S (Zhu et al., 2018) transgenic mouse models. In mice, both Aβ and tau increase in interstitial fluid (ISF) with time spent awake while increased wakefulness results in tau pathology spreading to other synaptically connected brain regions (Holth et al., 2019; Kang et al., 2009). By preventing sleep in mice, the daily rhythm of Aβ in ISF is delayed, suggesting that the daily rhythm is driven at least in part by sleep (Holth et al., 2019; Kang et al., 2009), which is of particular interest for human disease given the daily rhythm in CSF Aβ concentrations in humans (Kang et al., 2009).
4.2. Sleep affects Alzheimer’s disease biomarkers in humans
Recently, preliminary research has examined sleep and Aβ dynamics in humans. As seen in Table 4, a number of small studies show how acute sleep deprivation alters Aβ biomarkers in blood plasma (Wei et al., 2017), CSF (Lucey, 2018; Ooms et al., 2014), and PET imaging (Shokri-Kojori et al., 2018), as well as tau biomarkers in blood plasma (Benedict et al., 2020) and CSF (Holth et al., 2019) in cognitively healthy adults; however, there is little agreement between studies in how acute sleep loss affects CSF biomarkers.
Table 4.
Effects of acute sleep loss on biomarkers in cognitively normal adults
| Reference | Sample | Intervention | Marker | Key finding |
|---|---|---|---|---|
| Ooms et al., 2014 (Ooms et al., 2014) | n = 13/ group | 1 night sleep deprivation | CSF | Sleep deprivation prevented the 6% decrease in CSF Aβ42. Morning levels of Aβ40, p-tau, or T-tau did not differ between groups. |
| Wei et al., 2017 (Wei et al., 2017) | n = 20 | 1 night sleep deprivation | Blood | Sleep deprivation was associated with 32.6% higher plasma Aβ40 and 19.3% lower plasma Aβ42/Aβ40 ratio. |
| Ju et al., 2017 (Ju et al., 2017) | n = 17 | Selective disruption of SWS | CSF | SWS disruption was associated with an increase in CSF Aβ40, but there was no effect on CSF Aβ42, tau, or orexin. |
| Olsson et al., 2018;2019 (Olsson et al., 2018, 2019) | n = 13 | Partial sleep deprivation for five nights (max 4h sleep/night) or prolonged sleep deprivation (8 days) | CSF and blood | Sleep deprivation increased CSF orexin. No effect of partial sleep deprivation on CSF or plasma Aβ, tau, NfL, or GFAP or on amount of SWS. |
| Lucey et al., 2018 (Lucey, 2018) | n = 8 | 1 night sleep deprivation | CSF with stable isotope labeling kinetics (SILK) | Overnight CSF Aβ40 and Aβ42 concentrations increased by 30% in sleep-deprived participants |
| Shokri-Kojori et al., 2018 (Shokri-Kojori et al., 2018) | n = 20 | 1 night sleep deprivation | 18F-florbetaben PET | Sleep deprivation was associated with a ~5% increase in Aβ levels clustering around the right hippocampus and thalamus |
| Holth & Fritschi et al., 2019 (Holth et al., 2019) | n = 6 (same cohort as (Lucey, 2018)) | 1 night sleep deprivation | CSF | Sleep deprivation increased CSF tau by 50%, and increased CSF alpha-synuclein. CSF neurofilament light (NfL) and astrocytic glial fibrillary acidic protein (GFAP) were unaffected by sleep deprivation. |
| Barthelemy et al., 2020 (Barthélemy et al., 2020) | n = 8 (same cohort as (Holth et al., 2019; Lucey, 2018)) | 1 night sleep deprivation | CSF | The effects of sleep deprivation varied by tau isoform. CSF nonphosphorylated and phosphorylated T181 and T21, and nonphosphorylated S202, but not phosphorylated S202, increased during an overnight sleep deprivation. |
| Benedict et al., 2020 (Benedict et al., 2020) | n = 15 | 1 night sleep deprivation | Blood | Sleep deprivation was associated with an increase in evening to morning levels of tau, but not Aβ40, Aβ42, or NfL following one night of sleep deprivation. |
In healthy adults, one night of complete or partial sleep deprivation has been associated with higher CSF Aβ42 (Lucey, 2018; Ooms et al., 2014) or no effect (Ju et al., 2017), higher CSF Aβ40 (Ju et al., 2017; Lucey, 2018) or no effect (Ooms et al., 2014), and higher CSF tau (Holth et al., 2019) or no effect (Ju et al., 2017; Olsson et al., 2018, 2019; Ooms et al., 2014). The variable results are likely in part due to the small sample sizes and the role of underlying pathology even in cognitively normal adults and how these might affect CSF biomarker dynamics. Measurement limitations are also a challenge when trying to estimate ISF levels from CSF (Lucey et al., 2015). Furthermore, sleep affects the production/release of the peptides into ISF, as well as clearance, making the interpretation of the CSF levels difficult.
5. Mechanisms underlying the relationship between sleep disturbances and Alzheimer’s disease
There is substantial evidence that sleep disturbances occur throughout the AD spectrum and that sleep may directly affect the accumulation of Aβ and tau. Table 5 summarizes some of the proposed mechanisms driving the relationship between AD and sleep.
Table 5.
Summary of proposed mechanisms underlying the relationship between sleep disturbances and Alzheimer’s disease
| Proposed Mechanism | Link to Alzheimer’s disease |
|---|---|
| Circadian rhythms | Circadian rhythms regulate the timing and consolidation of sleep. In AD, circadian rhythms are delayed, weakened, and misaligned |
| Neurodegeneration | The neurocircuitry important for regulating sleep-wake is vulnerable to early pathological changes in AD, such as tau accumulation and neurodegeneration in the brainstem |
| Neurotransmitters | Neurotransmitters that are important for regulating sleep-wake, such as acetylcholine, orexin, and melatonin, are also affected by AD |
| Neuronal hyperexcitability | More wakefulness/less sleep results in increased activity-regulated amyloid production |
| Glymphatic Clearance | More wakefulness/less sleep results in less clearance of Aβ and tau |
5.1. Circadian rhythms
There is accumulating evidence that circadian rhythmicity is altered in AD (Chauhan et al., 2017). As summarized in Table 6, most studies attempting to assess circadian rhythmicity in patients with AD have used actigraphy to evaluate the timing of physical activity over several days (Harper et al., 2008; Harper, 2001; Hatfield et al., 2004; Satlin, 1995; Tranah et al., 2011; Volicer, 2001). To evaluate circadian rhythmicity of a variable (e.g., physical activity measured by actigraphy) researchers often fit a cosinor regression with a 24h period, as illustrated in Figure 2. When evaluating actigraphy data using the cosinor method, the daily physical activity patterns associated with AD are delayed in phase (i.e., peak later in the day) and have lower amplitudes (Harper, 2001; Hatfield et al., 2004; Satlin, 1995; Volicer, 2001).
Table 6.
Novel interventions currently being investigated to improve sleep in Alzheimer’s disease
| Intervention | Proposed Mechanism |
|---|---|
| Acoustic stimulation | Non-invasive acoustic stimulation to enhance slow wave sleep. |
| Continuous positive airway pressure (CPAP) | A ventilator used as a treatment for obstructive sleep apnea. |
| Gabapentin enacarbil | Anticonvulsant and analgesic treatment used for restless leg syndrome. |
| Nabilone | Cannabinoid-1 and Cannabinoid-2 receptor agonists. These drugs were developed to treat the nausea associated with cancer chemotherapy and weight loss associated with other conditions. There is evidence but no definitive conclusions of sleep-promoting effects of cannabinoids. |
| Nelotanserin | Serotonin receptor (5-HT2A) agonist for the treatment for insomnia. Serotonin is involved in NREM regulation, promoting SWS and sleep consolidation. |
| Piromelatin | Melatonin and serotonin receptor agonists developed as a multimodal sleep aid for the treatment of insomnia. |
| Repetitive transcranial magnetic stimulation | Non-invasive brain stimulation. Short magnetic pulses to stimulate neurons. Proposed as a treatment for insomnia. |
| Suvorexant (MK-4305), Lemborexant | Dual orexin receptor antagonist for the treatment of insomnia. These drugs are intended to block the wake-promoting effects of orexin. |
| Wearable devices: Neuro RX Gamma device and Vielight Neuro Gamma | Non-invasive brain stimulation. Transcranial and intranasal near-infrared LED light to stimulate neurons. Pulsed at the gamma frequency (25–100Hz). |
| Zolpide and zoplicone | GABAa receptor agonist used as a sedative (hypnotic) to treat insomnia. |
Figure 2.

Hypothetical examples of circadian rhythms fit with cosinor regressions. The solid black represents a circadian rhythm from a healthy individual and the dotted line represents a circadian rhythm from an individual with Alzheimer’s disease. The period (peak to peak or trough to trough) is 24h. The amplitude is the difference between the peak (or trough) and the mean value of the cosine wave. The phase/acrophase refers to the timing of a reference point in the cycle (e.g., the peak) relative to time. In this example, the phase of the peak in the healthy individual occurs at ~18h, whereas the phase of the peak in the individual with Alzheimer’s disease occurs later (delayed) at ~21h.
In addition to actigraphy, the Constant Routine (CR) protocol has been developed to study circadian rhythmicity in humans (Duffy and Dijk, 2002). During CR, participants remain awake in constant dim light, a semi-recumbent posture and provided hourly identical snacks and fluids, to try and remove external and behavioural time cues (Duffy and Dijk, 2002). The CR method has only been conducted once in patients with AD, and confirmed that even under the highly controlled conditions, patients with AD exhibit rhythms with dampened amplitude and delayed phase (by ~4 hours) compared to healthy participants (Harper et al., 2005).
Circadian timing is also assessed by measuring clock gene expression. One study found that BMAL mRNA exhibited a delayed phase of ~3 h in saliva of AD patients compared to controls (Weissova et al., 2016). A neuropathology study assessing the relative expression levels of PER1, PER2, and BMAL1 in the bed nucleus of the stria terminalus, cingulate cortex, and pineal gland revealed altered internal phase relationships in AD patients compared to age-matched controls (Cermakian et al., 2011), such that the timing of peak expression was shifted out of normal alignment. Internal misalignment has also been seen in a drosophila model of AD (Chen et al., 2014), suggesting that uncoupling of peripheral circadian oscillators contributes to the circadian changes associated with AD (Chauhan et al., 2017; Kent, 2014); however, internal misalignment was not identified in the APP/PS1 mouse model (Kent et al., 2019).
As with sleep, there is some evidence that changes in circadian rhythms are associated with increased risk of AD. A large study of cognitively normal older adults found that participants exhibiting a peak of daily physical activity occurring after 3:51PM (indicating a phase delay in the circadian rhythm) or a weaker rhythmic pattern (i.e., lower amplitude and robustness) had an increased risk of MCI and dementia (Tranah et al., 2011).
Together, the data suggest that AD is associated with changes in circadian rhythms and that these changes may occur prior to the onset of dementia and underlie part of the relationship between sleep disturbances and AD. Because many sleep disturbances (e.g., sleep fragmentation) overlap between various neurological conditions, changes in circadian rhythms may reflect a more AD-specific biomarker. Circadian rhythm synchrony may be a potential target for interventions aimed at improving sleep, slowing AD progression, and delaying the onset of dementia.
5.2. Neurodegeneration of sleep regulating neural circuitry
Another mechanism underlying the relationship between AD and sleep disturbances may be that Aβ and tau pathology disrupt the function of brain regions important for regulating sleep-wake processes (reviewed by (Weber and Dan, 2016)). Figure 3 illustrates the spread of tau accumulation associated with AD in sleep-wake brain regions, starting with neurons in the locus coeruleus showing the earliest AD-related intraneuronal changes (Braak et al., 2011).
Figure 3.
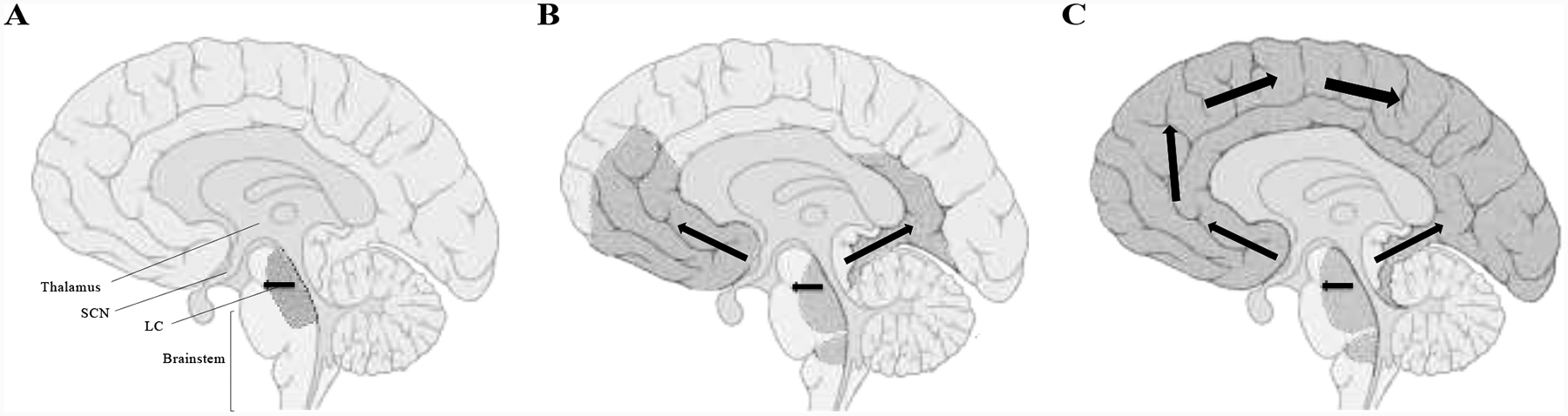
Sagittal brain section illustrating AD-related tau progression throughout the brain with key sleep-wake brain regions identified. The dark grey shading and black arrows indicate the spread of tau tangles from (A) locus coeruleus (LC) and other brainstem nuclei to (B) projections to the cerebral cortex and then (C) widespread cortical pathology. The size of the arrows indicates the progression, with the smallest arrows associated with the onset of tau accumulation in the brainstem and the larger arrows indicating the accumulation in the cortex later in the disease course. Figure 3 is based on the work of (Braak et al., 2011), image adapted from Wikimedia commons (Patrick J. Lynch, medical illustrator / CC BY).
5.2.1. Suprachiasmatic nucleus (SCN)
If disrupted circadian rhythms contribute to sleep disturbances experienced by patients with AD, then AD pathology may be interfering with processes in the SCN of the hypothalamus, which is the master, retinorecipient circadian pacemaker. In fact, there is some evidence for functional disruption of the SCN, defined as a loss of rhythmic clock gene expression, in the earliest preclinical, asymptomatic stages of AD (Wu et al., 2006). Neuropathology has confirmed that patients diagnosed with AD show reduced SCN volume (Swaab, 1985) as well as fewer SCN neurons expressing critical neuropeptides regulating synchrony (e.g., vasopressin and neurotensin) (Harper et al., 2008; Liu, 2000; Stopa, 1999). In AD, the degree of degeneration in SCN (glia/neuron ratio) and neuronal counts has also been shown to correlate with rhythms of physical activity and core body temperature, such that the loss of SCN neurons was associated with lower amplitude and higher fragmentation (Harper et al., 2008). The SCN does accumulate Aβ and tau pathology, but the neurodegeneration in the SCN is the most striking association with AD (Stopa, 1999).
5.2.2. Brainstem
The brainstem structures shown to be critically important for regulating sleep are of particular interest because the brainstem is the first brain region to show intraneuronal changes and accumulation of hyperphosphorylated tau in AD (Braak et al., 2011). For example, the locus coeruleus nucleus (LC) in the pons is important for regulating the sleep-wake cycle (reviewed by (Satoh and Iijima, 2017; Weber and Dan, 2016)). The LC receives dense hypocretin/orexin innervation, which are neuropeptides implicated in the regulation of REM sleep (Bourgin, 2000). As AD progresses there is significant neuronal loss in LC, which is suggested to drive clinical and pathological manifestations of sleep disorders AD (Satoh and Iijima, 2017). A study examining the functional connectivity of the LC and ventral tegmental area (VTA) compared patients with AD and aMCI, to elderly controls using resting-state fMRI at 3T (Serra et al., 2018). There was evidence of VTA disconnection, which was associated with self-reported sleep disorders (Serra et al., 2018).
5.2.3. Thalamus
The intermediate nucleus of the thalamus, which is proposed as the human homologue of the ventrolateral preoptic nucleus, has been shown to play a critical role in the initiation, maintenance, and consolidation of sleep, in part by inhibiting wake-promoting areas, such as the tubermamillary nucleus (Weber and Dan, 2016). Total cell numbers in the intermediate nucleus are not different between AD and age-matched controls (Swaab, 1988); however, sleep fragmentation in AD is associated with fewer galanin-immunoreactive intermediate nucleus neurons (Lim et al., 2014), suggesting that the AD-associated cell loss may be specific to the galaninergic subpopulation. In late-stage AD, galanin-containing fibres innervating the surviving cholinergic neurons are increased up to 200%, likely responding to the cholinergic degeneration of the basal forebrain (Counts, 2003).
5.3. Changes in neurotransmitters important for sleep regulation
The sleep disturbances associated with AD may also result from AD-associated changes in neurotransmitters, such as ACh, orexin, and melatonin.
5.3.1. Acetylcholine
Degeneration of basal forebrain cholinergic nuclei occurs early in AD and is predictive of amyloid burden (Teipel et al., 2014). Cholinesterase inhibitors enhance cholinergic transmission by inhibiting the enzyme acetylcholinesterase (AChEI), and are the most widely prescribed medication for AD (Bartus, 1982). The reduction of ACh likely has a negative effect on sleep-wake state regulation, particularly the regulation of REM sleep (Grace and Horner, 2015). The findings for REM sleep disturbances in AD have been mixed; however, some studies have found that individuals with AD exhibit a longer latency to first episode of REM compared to controls, which may reflect the selective deterioration of the REM-cholinergic system (Bliwise, 1989).
The qEEG data suggesting reduced delta power during sleep associated with AD has been proposed to involve a period of enhanced cholinergic output in the brain as a compensatory response to the neurodegeneration (Kwak, 2006). Indeed, an autopsy series from MCI and AD patients showed increased ACh transferase, the rate limiting enzyme in the synthesis of ACh, in MCI compared to AD (Ikonomovic et al., 2003). Anticholinergic drugs increase EEG delta power (Kikuchi, 1999) and increasing ACh with cholinesterase inhibitors, decreases EEG slow-wave power (Adler, 2001; Balkan, 2003; Brassen, 2003). Interestingly, donepezil, one of the AchEIs is associated with bad dreams, limiting its tolerability for some patients (Singer et al., 2005).
5.3.2. Orexin
Orexin (hypocretin), is a neuropeptide that regulates wakefulness and REM sleep (Sakurai, 2007). In AD, an altered orexinergic system has been proposed to affect the sleep–wake cycle and secondarily influence AD pathology by inducing Aβ accumulation and tau-mediated neurodegeneration (Liguori, 2017). While several studies have found AD and MCI to be associated with higher CSF orexin levels than controls (Gabelle et al., 2017; Liguori et al., 2017; Liguori et al., 2019; Liguori et al., 2014), some studies have found the opposite relationship with AD pathology associated with lower CSF orexin levels (Fronczek et al., 2012), or no difference between individuals with AD and controls (Schmidt et al., 2013; Slats, 2012). Postmortem analysis of the hypothalamus showed 40% less hypocretin-1 immunoreactive neurons in AD compared to healthy controls (Fronczek et al., 2012).
5.3.3. Melatonin
Disrupted melatonin production and rhythms have been identified early in AD (reviewed by (Wu, 2005)). Pineal gland melatonin obtained at autopsy revealed that those with AD had reduced melatonin levels compared to controls (Skene et al., 1990), even in preclinical AD (Wu et al., 2003). In patients with AD, CSF melatonin levels were only 20% of levels in control subjects (Liu et al., 1999), and there is reduced serum melatonin levels as well (Mishima, 1999). One study found that the melatonin receptor type A gene (MTNR1A, variant rs12506228A) is a shared genetic risk factor for intolerance to shift work and AD (Sulkava et al., 2018).
5.4. Activity-regulated amyloid
Given that synaptic activity regulates the release of Aβ (Cirrito et al., 2005; Kamenetz, 2003), it has been proposed that neuronal hyperactivity may contribute to disease progression in AD and that this may be an important pathway by which disrupted sleep drives AD pathogenesis. Kang and colleagues (2009) first reported that there was increased production of Aβ with sleep deprivation in mice, and that promoting sleep with an orexin antagonist reduced Aβ accumulation (Kang et al., 2009). Some, but not all, studies in humans have found similar changes in CSF Aβ in cognitively normal adults following a single night of disrupted sleep or the selective disruption of SWS (Ju et al., 2017; Lucey, 2018; Wei et al., 2017). Measuring Aβ kinetics with SILK (stable isotope labeling kinetics) in serially collected CSF samples, Lucey and colleagues identified 25–30% increases in overnight Aβ38, Aβ40, and Aβ42 production with sleep deprivation (Lucey, 2018). Activity-regulated amyloid is hypothesized to explain why the Default Mode Network (DMN) is selectively vulnerable to the earliest AD pathology.
5.5. Glymphatic clearance
Glymphatic clearance was first described in 2012 as an efficient mechanism facilitated by astrocytic aquaporin 4 water channels that enables convective flow of ISF from para-arterial to para-venous space, removing Aβ and other metabolic waste products from the brain (Iliff et al., 2012) (reviewed by (Boespflug and Iliff, 2018)). Reduced glymphatic clearance is thought to contribute to the decreased clearance (~30% reduction) of Aβ in humans with AD (Mawuenyega, 2010). A recent study found that the AQP4 gene variant rs72878776 was associated with poorer sleep quality and other SNPs moderated the relationship between sleep latency, sleep duration, and Aβ levels (Rainey-Smith et al., 2018).
6. Treating sleep disorders
Treating sleep disorders in patients with AD is becoming a topic of greater importance given its potential role in the pathogenesis and course of the disease. There are several potential mechanisms underlying the relationship between sleep and AD from neurodegeneration of sleep promoting brain regions to changes in neurotransmitters and circadian rhythms, while there is also growing evidence that sleep is not only a symptom of the underlying pathology but that sleep disturbances contribute to disease progression. Given these complexities, there are a number of important potential targets for treatment and prevention in AD that have been explored. The following section will review treatments available for patients along the AD continuum and novel treatments in the pipeline. Detailed reviews have been recently published (Ferini-Strambi et al., 2020; McCleery et al., 2016; Ooms and Ju, 2016).
6.1. Treatments available for patients
AD Therapeutics:
Although the currently available drug therapy for AD is targeting the cognitive symptoms associated with the disease and not sleep, a recent study of patients with AD found that donepezil treatment for 4 months, in combination with memantine, improved self-reported sleep compared to controls who were only treated with memantine (Liu et al., 2019b).
Bright Light and Melatonin:
To treat sleep in AD, several studies have attempted to regulate the circadian system by implementing bright light and melatonin therapy (Riemersma-Van Der Lek, 2008; van Maanen et al., 2016; Xu et al., 2015). Bright light therapy is designed to augment the entraining (synchronizing) effects of daylight to ensure that the body is aligned with the external environment. In practice, bright light is used in the morning and exerts an alerting effect as well as a synchronizing effect on the circadian system (Terman and Terman, 2005; Van Someren et al., 1999). Similarly, melatonin is used to entrain (synchronize) circadian rhythms to the external environment but is prescribed in the evening and offers sedative effects in addition to acting on the circadian system (Arendt et al., 1997).
A meta-analysis of bright light therapy in dementia, found effect sizes ranging from g = 0.30 – 0.38, concluding that bright light >2500 lux can improve sleep in individuals with dementia (van Maanen et al., 2016). Similarly, a meta-analysis of melatonin treatments in dementia, found small effect sizes on sleep efficacy (−0.13 – 3.70%) and duration (3.26 – 45.46 min) (Xu et al., 2015). Of note, one study compared the effects of bright light therapy, melatonin, and the combination, and found that bright light therapy attenuated cognitive decline by 5% and melatonin increased sleep duration by 6% (Riemersma-Van Der Lek, 2008). Although the effects of bright light and melatonin were not additive, the combined treatment was associated with reduced aggression by 9% (Riemersma-Van Der Lek, 2008). A recent pilot study reported that melatonin intake in individuals diagnosed with mild-moderate AD, was associated with shorter sleep onset and higher relative delta power (Cruz-Aguilar et al., 2018).
Hypnotics:
There have been no controlled clinical trials evaluating how benzodiazepines and non-benzodiazepine hypnotics improve sleep in AD, or how these drugs affect Aβ (McCleery et al., 2016); however, a meta-analysis concluded that there is an increased risk of adverse effects in elderly, suggesting that they are not suitable for individuals diagnosed with or at risk of AD (Glass et al., 2005). Anti-depressants with hypnotic action (e.g., mianserin and trazodone) have been proposed to have a better risk/benefit profile for treating sleep disturbances in elderly individuals (Camargos et al., 2014; McCleery et al., 2016; Scoralick et al., 2017). A recent study found that patients diagnosed with AD who had also been treated with trazodone had slower cognitive decline, which is thought to result from the increased SWS associated with trazodone (La, 2019).
Continuous Positive Airway Pressure (CPAP):
Given that OSA is a potentially modifiable risk factor for MCI and dementia, its treatment represents an important consideration. OSA is associated with repeated hypoxic events that induce arousals, resulting in sleep fragmentation and daytime sleepiness as well as lower CSF Aβ40, CSF Aβ42, and SWS. (Ju et al., 2016). CPAP has been shown to improve cognition in AD patients following 6 weeks of treatment (Ancoli-Israel et al., 2008), as well as in MCI after one year of treatment adherence compared to MCI who were nonadherent to the treatment (Richards et al., 2019; Wang et al., 2020). Of note, in one cohort there was 46% of nonadherence (Richards et al., 2019), highlighting the importance of evaluating tolerability, compliance and efficacy in this patient population. Cognitively normal adults diagnosed with OSA, treated for 1–4 months with positive airway pressure showed increased time in N3 sleep stage and increased delta power, but no difference in CSF Aβ40, Aβ42, or tau (Ju et al., 2019). Of note, the STOP-Bang and Berlin questionnaires to identify OSA, are reportedly not effective for screening in patients with AD (Jorge et al., 2019).
6.2. Potential therapeutics in clinical development
Orexin antagonists have been shown to increase sleep and in some cases reduce Aβ accumulation in mouse models of AD (Duncan et al., 2019; Kang et al., 2009; Roh et al., 2014). Lemborexant, a dual competitive antagonist of orexin receptors 1 and 2 is currently being tested in clinical trials as a treatment for insomnia in elderly and patients with dementia (Moline, 2019; Rosenberg et al., 2019). Preliminary data suggests that lemborexant is well-tolerated by elderly individuals (Rosenberg et al., 2019), increases total sleep time, and reduces sleep fragmentation in mild to moderate AD (Moline, 2019). The orexin receptor antagonist, suvorexant, has also been shown to increase total sleep time in patients with mild-moderate AD (Herring et al. 2020) and was approved by the FDA in February 2020 for treating insomnia in AD.
Sodium oxybate, which is a prescription often given to treat narcolepsy, has also been considered as a sleep aid (Lucey, 2018). In a small study of healthy adults, sodium oxybate treatment was found to have no effect on time spent in SWS and had no effect on CSF Aβ (Lucey, 2018).
Novel nonpharmacological interventions to increase SWS have also been investigated, including acoustic stimulation (Papalambros et al., 2017; Papalambros et al., 2019) or a rocking bed (Perrault, 2019), both of which enhance SWS in healthy adults and patients with aMCI (Papalambros et al., 2017; Papalambros et al., 2019; Perrault, 2019), and are associated with improved memory in healthy adults (Papalambros et al., 2017; Perrault, 2019). Similarly, transcranial alternating current stimulation (tACS) has been examined as a way of augmenting endogenous slow-wave oscillations. One study delivered tACS in the 0.5–1.2 Hz frequency range during sleep in young adults and found that tACS during sleep enhanced slow-wave power and coupling with spindles, as well as performance on cognitive tasks (Ketz et al., 2018). Together, these findings suggest that interventions aimed at increasing SWS may hold promise for reducing Aβ levels and improving mnemonic processes; however, safety needs to be ascertained given the electrophysiological abnormalities and risks of recurrent electrical stimulation (e.g., kindling).
Currently, there is a breadth of clinical trials assessing interventions to improve sleep as primary or secondary outcomes in AD and other dementias. Table 6 summarizes the key candidates and proposed mechanisms of action being investigated in the current trials.
7. Conclusion
There is growing evidence suggesting that sleep is a potential therapeutic target and novel outcome measure for AD. Sleep will be an important component of a prevention platform for dementia: personalizing treatment and stratifying risk. Given AD is a heterogenous multifactorial disease, we predict only a subset of AD cases have sleep as a critical risk factor. The goal will be to identify those individuals who would benefit most from interventions targeting sleep as a way to change the trajectory of the underlying pathological cascade.
Consensus is building that SWS is the most important sleep stage to target for preventing AD dementia because not only is a reduction of SWS most often seen in PSG and qEEG studies in human patients and mouse models of AD, but it also seems to be the stage regulating Aβ levels in CSF. However, there is only preliminary and conflicting evidence for acute effects of sleep on Aβ levels in humans. For symptomatic AD patients, sleep and SWS in particular is important to target with interventions in view of the extensive literature demonstrating the important role of sleep for attention, executive control, and declarative and non-declarative memory (Hennevin et al., 2007; McCoy and Strecker, 2011; Oudiette and Paller, 2013; Rasch and Born, 2013; Scullin and Bliwise, 2015), and that specific sleep stages are critical for hippocampal- and non-hippocampal-dependent memory functions and neuroplasticity (Havekes et al., 2016; Kent and Mistlberger, 2017; Kreutzmann et al., 2015; Rasch and Born, 2013). SWS disruption has even been proposed as a critical pathway by which Aβ disrupts hippocampal-dependent memory consolidation (Mander et al., 2015). SWS evaluation will be an important outcome measure when assessing sleep disturbances in patients and those at risk of AD, as well as in the development of novel therapeutics.
While this review focused specifically on the link between sleep and AD pathogenesis, it is important to note that there is a large literature demonstrating how sleep is important for overall health and cognition, as well as how factors such as stress, physical activity, and obesity affect sleep quality. Thus, how sleep disturbances contribute symptomatically to AD as well as the factors that affect sleep quantity and quality are also something to consider but beyond the scope of this review.
A future research priority should be the development of improving techniques to evaluate sleep using EEG in the home environment and to look beyond the traditional sleep staging. Evaluating EEG power spectra shifts, frequency of N2 sleep spindles, and the coupling of EEG features during sleep may provide greater insight of the AD-specific changes in sleep. Overall, this research into the relationship between sleep disturbances and AD advances our theoretical understanding of how broader neuronal network dysfunction may be central to AD pathogenesis.
Supplementary Material
Box 1. Literature search strategy and selection criteria.
References for our review were identified by conducting a systematic PubMed search with the term “Alzheimer’s disease” in combination with “sleep,” “circadian rhythms,” “polysomnography,” “EEG,” “actigraphy,” “dementia,” and “sleep disordered breathing”.
The literature search was performed between February 2018 and March 2020. We also searched references from selected publications. Only articles published in or translated to English were selected in the citations.
We reviewed https://clinicaltrials.gov on March 18, 2020 to identify trials currently in clinical development. Using the key words “sleep” and “Alzheimer’s disease (incl subtypes)”, there were 63 active studies that we reviewed.
Box 2. Summary of sleep disturbances associated with AD.
Patients with AD dementia exhibit lower sleep efficiency and lower amounts of SWS, compared to healthy elderly controls.
PSG, actigraphy, and self-reported methods consistently identify associations between sleep fragmentation and AD pathology.
qEEG identifies an association between AD pathology and power spectra shifts, specifically reduced slow wave activity.
Sleep disordered breathing is associated with AD.
Box 3. Summary of sleep as a risk factor for Alzheimer’s disease.
Large longitudinal studies suggest that sleep disturbances are a risk factor for cognitive decline and dementia.
Adults presenting with poor sleep duration or quality, may represent a subpopulation of individuals that would benefit from interventions targeting sleep as a way to prevent AD.
It is unclear if particular types of sleep disruption can identify individuals who have underlying AD pathology.
Box 4. Summary of the relationship between sleep and levels of Aβ and tau.
There is strong evidence in mouse models of AD that sleep directly affects the accumulation of Aβ and tau.
There is no consensus for how acute sleep loss affects CSF biomarkers in cognitively healthy humans.
Highlights.
Patients with AD dementia exhibit lower sleep efficiency and lower amounts of SWS, compared to healthy elderly controls
Sleep will be an important component of a prevention platform for dementia, as well as a potential therapeutic target and novel outcome measure for clinical trials
There is strong evidence in mouse models of AD that sleep directly affects the accumulation of Aβ and tau, but there is no consensus for how acute sleep loss affects AD biomarkers in cognitively healthy adults
There are a number of potential targets for treatment and prevention in AD that are currently being explored in clinical trials
Acknowledgments
The authors gratefully acknowledge funding from MSFHR (BAK, HBN), CIHR Banting (BAK), and NIH-NINDS K99/R00 NS109909-01 (BAK). The authors would also like to thank Meghan Chen for her input and editing support as well as Alexandre Shadyab for their help with Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler G, & Brassen S, 2001. Short-term rivastigmine treatment reduces EEG slow-wave power in Alzheimer patients. Neuropsychobiology 43, 273–276. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, & Mason W, 1997. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 20, 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Gillin JC, Campbell SS, & Hofstetter CR, 1994. Sleep in non-institutionalized Alzheimer’s disease patients. Aging Clinical and Experimental Research 6, 451–458. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS, 2008. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc 56, 2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S, 1997. Efficacy of melatonin treatment in jet lag, shift work, and blindness. Journal of biological rhythms 12, 604–617. [DOI] [PubMed] [Google Scholar]
- Balkan S, Yaras N, Mihci E, Dora B, AGar A, & Yargicoglu P, 2003. Effect of donepezil on EEG spectral analysis in Alzheimer’s disease. Acta neurologica belgica 103, 164–169. [PubMed] [Google Scholar]
- Barthélemy NR, Liu H, Lu W, Kotzbauer PT, Bateman RJ, Lucey BP, 2020. Sleep deprivation affects tau phosphorylation in human cerebrospinal fluid. Annals of Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, & Lippa AS, 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–414. [DOI] [PubMed] [Google Scholar]
- Basta M, Simos P, Vgontzas A, Koutentaki E, Tziraki S, Zaganas I, Panagiotakis S, Kapetanaki S, Fountoulakis N, Lionis C, 2019. Associations between sleep duration and cognitive impairment in mild cognitive impairment. J Sleep Res, e12864. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC, Dominantly Inherited Alzheimer N, 2012. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Blennow K, Zetterberg H, Cedernaes J, 2020. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, Lind L, Lannfelt L, Schioth HB, 2015. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimer’s & Dementia 11, 1090–1097. [DOI] [PubMed] [Google Scholar]
- Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, & Trabucchi M, 1995. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia and Geriatric Cognitive Disorders 6, 108–112. [DOI] [PubMed] [Google Scholar]
- Billioti de Gage S, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M, Pariente A, Begaud B, 2014. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 349, g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, … & Dement WC, 1989. REM latency in Alzheimer’s disease. Biological Psychiatry 25, 320–328. [DOI] [PubMed] [Google Scholar]
- Boddy F, Rowan EN, Lett D, O’Brien JT, McKeith IG, Burn DJ, 2007. Subjectively reported sleep quality and excessive daytime somnolence in Parkinson’s disease with and without dementia, dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry 22, 529–535. [DOI] [PubMed] [Google Scholar]
- Boespflug EL, Iliff JJ, 2018. The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-beta, and Sleep. Biol Psychiatry 83, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, … & Murri L, 2005. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. Journal of Sleep Research 14, 311–317. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitrón-Reséndiz S, Spier AD, Fabre V, Morte B, Criado JR, … & De Lecea L, 2000. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. Journal of Neuroscience 20, 7760–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K, 2011. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology 70, 960–969. [DOI] [PubMed] [Google Scholar]
- Branger P, Arenaza-Urquijo EM, Tomadesso C, Mezenge F, Andre C, de Flores R, Mutlu J, de La Sayette V, Eustache F, Chetelat G, Rauchs G, 2016. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging 41, 107–114. [DOI] [PubMed] [Google Scholar]
- Brassen S, & Adler G, 2003. Short-term effects of acetylcholinesterase inhibitor treatment on EEG and memory performance in Alzheimer patients: an open, controlled trial. Pharmacopsychiatry 36, 304–308. [DOI] [PubMed] [Google Scholar]
- Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, Anderson WM, 2017. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 40. [DOI] [PubMed] [Google Scholar]
- Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, Hogan MM, Shim AM, Mukhtar F, Sharma N, Mbah AK, Seixas AA, Kam K, Zizi F, Borenstein AR, Mortimer JA, Kip KE, Morgan D, Rosenzweig I, Ayappa I, Rapoport DM, Jean-Louis G, Varga AW, Osorio RS, Alzheimer’s Disease Neuroimaging I, 2019. Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, Hu T, Spadola CE, Burgess A, Li T, & Cadet T, 2019. Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease. Journal of aging and health 31, 322–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ, 1988. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nobrega OT, 2014. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. Am J Geriatr Psychiatry 22, 1565–1574. [DOI] [PubMed] [Google Scholar]
- Carnicelli L, Maestri M, Di Coscio E, Tognoni G, Fabbrini M, Schirru A, Giorgi FS, Siciliano G, Bonuccelli U, Bonanni E, 2019. A longitudinal study of polysomnographic variables in patients with mild cognitive impairment converting to Alzheimer’s disease. J Sleep Res, e12821. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, & Monk TH, 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38, 232–242. [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D, 2011. Sleep slow wave changes during the middle years of life. Eur J Neurosci 33, 758–766. [DOI] [PubMed] [Google Scholar]
- Carvalho DZ, St Louis EK, Knopman DS, Boeve BF, Lowe VJ, Roberts RO, Mielke MM, Przybelski SA, Machulda MM, Petersen RC, Jack CR Jr., Vemuri P, 2018. Association of Excessive Daytime Sleepiness With Longitudinal beta-Amyloid Accumulation in Elderly Persons Without Dementia. JAMA Neurol 75, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C, 2017. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev 31, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Lamont EW, Boudreau P, Boivin DB, 2011. Circadian clock gene expression in brain regions of Alzheimer ‘s disease patients and control subjects. J Biol Rhythms 26, 160–170. [DOI] [PubMed] [Google Scholar]
- Chauhan R, Chen KF, Kent BA, Crowther DC, 2017. Central and peripheral circadian clocks and their role in Alzheimer’s disease. Dis Model Mech 10, 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DW, Wang J, Zhang LL, Wang YJ, Gao CY, 2018. Cerebrospinal Fluid Amyloid-beta Levels are Increased in Patients with Insomnia. J Alzheimers Dis 61, 645–651. [DOI] [PubMed] [Google Scholar]
- Chen KF, Possidente B, Lomas DA, Crowther DC, 2014. The central molecular clock is robust in the face of behavioural arrhythmia in a Drosophila model of Alzheimer’s disease. Dis Model Mech 7, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM, 2005. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J, 2007. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130, 2868–2878. [DOI] [PubMed] [Google Scholar]
- Colby-Milley J, Cavanagh C, Jego S, Breitner JC, Quirion R, Adamantidis A, 2015. Sleep-Wake Cycle Dysfunction in the TgCRND8 Mouse Model of Alzheimer’s Disease: From Early to Advanced Pathological Stages. PLoS One 10, e0130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Perez SE, Ginsberg SD, de Lacalle S, & Mufson EJ, 2003. Galanin in Alzheimer disease. Molecular Interventions 3, 137–155. [DOI] [PubMed] [Google Scholar]
- Cruz-Aguilar MA, Ramirez-Salado I, Guevara MA, Hernandez-Gonzalez M, Benitez-King G, 2018. Melatonin Effects on EEG Activity During Sleep Onset in Mild-to-Moderate Alzheimer’s Disease: A Pilot Study. J Alzheimers Dis Rep 2, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meco A, Joshi YB, Pratico D, 2014. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging 35, 1813–1820. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, & van den Hoofdakker RH, 1989. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiology of Aging 10, 677–682. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, 2002. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms 17, 4–13. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Farlow H, Tirumalaraju C, Yun DH, Wang C, Howard JA, Sanden MN, O’Hara BF, McQuerry KJ, Bachstetter AD, 2019. Effects of the dual orexin receptor antagonist DORA-22 on sleep in 5XFAD mice. Alzheimers Dement (N Y) 5, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykierek P, Stadtmüller G, Schramma P, Bahro M, Van Calker D, Braus DF, … & Berger M, 1998. The value of REM sleep parameters in differentiating Alzheimer’s disease from old-age depression and normal aging. Journal of Psychiatric Research 32, 1–9. [DOI] [PubMed] [Google Scholar]
- Elias A, Cummins T, Tyrrell R, Lamb F, Dore V, Williams R, Rosenfeld JV, Hopwood M, Villemagne VL, Rowe CC, 2018. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-beta and Tau Imaging. J Alzheimers Dis 66, 733–741. [DOI] [PubMed] [Google Scholar]
- Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, Rosenzweig I, Sepehry AA, 2016. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front Aging Neurosci 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettore E, Bakardjian H, Sole M, Levy Nogueira M, Habert MO, Gabelle A, Dubois B, Robert P, David R, 2019. Relationships between objectives sleep parameters and brain amyloid load in subjects at risk to Alzheimer’s disease: the INSIGHT-preAD Study. Sleep. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Galbiati A, Casoni F, Salsone M, 2020. Therapy for Insomnia and Circadian Rhythm Disorder in Alzheimer Disease. Current Treatment Options in Neurology 22, 4. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Dickson DW, Graff-Radford NR, Lin SC, Wszolek Z, … & Parisi JE, 2014. Abnormal daytime sleepiness in dementia with Lewy bodies compared to Alzheimer’s disease using the Multiple Sleep Latency Test. Alzheimer’s Research & Therapy 6, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sørensen Ø, Amlien IK, Bartrés-Faz D, Bros DM, Buchmann N, Demuth I, Drevon CA, Ebmeier KP, Idland A-V, 2019. Self-reported sleep relates to hippocampal atrophy across the adult lifespan–results from the Lifebrain consortium. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, Swaab DF, 2012. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging 33, 1642–1650. [DOI] [PubMed] [Google Scholar]
- Gabelle A, Jaussent I, Hirtz C, Vialaret J, Navucet S, Grasselli C, Robert P, Lehmann S, Dauvilliers Y, 2017. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging 53, 59–66. [DOI] [PubMed] [Google Scholar]
- Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE, 2005. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 331, 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace KP, Horner RL, 2015. Evaluating the Evidence Surrounding Pontine Cholinergic Involvement in REM Sleep Generation. Front Neurol 6, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino FI, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili FM, Passero S, Perri R, Rocchi R, Sucapane P, Tognoni G, Zabberoni S, Sorbi S, 2012. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EA, Wang HX, Andel R, Fratiglioni L, 2014. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry 22, 1262–1271. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A, 2008. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain 131, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, McKee AC, Satlin A, Harlan PC, Goldstein R, & Volicer L, 2001. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Archives of General Psychiatry 58, 353–360. [DOI] [PubMed] [Google Scholar]
- Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A, 2005. Disturbance of Endogenous Circadian Rhythm in Aging and Alzheimer Disease. The American Journal of Geriatric Psychiatry 13, 359–368. [DOI] [PubMed] [Google Scholar]
- Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH, 2004. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 127, 1061–1074. [DOI] [PubMed] [Google Scholar]
- Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, Bruinenberg VM, Poplawski SG, Day JP, Aton SJ, Radwanska K, Meerlo P, Houslay MD, Baillie GS, Abel T, 2016. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Huetz C, Edeline JM, 2007. Neural representations during sleep: from sensory processing to memory traces. Neurobiol Learn Mem 87, 416–440. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Ceesay P, Snyder E, Bliwise D, Budd K, Hutzelmann J, Stevens J, Lines C, Michelson D, 2020. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimer’s & Dementia 16, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hita-Yanez E, Atienza M, Cantero JL, 2013. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep 36, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Gil-Neciga E, & L Cantero J, 2012. Disturbed Sleep Patterns in Elders with Mild Cognitive Impairment: The Role of Memory Decline and ApoE ε 4 Genotype. Current Alzheimer Research 9, 290–297. [DOI] [PubMed] [Google Scholar]
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Lucey BP, 2019. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, Eustache F, 2011. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol Psychol 87, 334–339. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi JW, Hwang SH, Lee YJ, Lee DY, Group KR, 2018. Moderating effect of APOE epsilon4 on the relationship between sleep-wake cycle and brain beta-amyloid. Neurology 90, e1167–e1173. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Silva J, Cauli O, 2018. A survey on sleep questionnaires and diaries. Sleep Med 42, 90–96. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST, 2003. Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer’s neuropathology. J Alzheimers Dis 5, 39–48. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Nagelhus EA, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science Translational Medicine 4, 147ra111–147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner AA, Gladbach A, Bertz J, Suh LS, Ittner LM, 2014. p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun 2, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Götz J, 2011. Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nature Reviews Neuroscience 12, 67. [DOI] [PubMed] [Google Scholar]
- J Wams E, K Wilcock G, G Foster R, & Wulff K, 2017. Sleep-Wake Patterns and Cognition of Older Adults with Amnestic Mild Cognitive Impairment (aMCI): A Comparison with Cognitively Healthy Adults and Moderate Alzheimer’s Disease Patients. Current Alzheimer Research 14, 1030–1041. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge C, Benitez I, Torres G, Dakterzada F, Minguez O, Huerto R, Pujol M, Carnes A, Gaeta AM, Dalmases M, Gibert A, Sanchez de la Torres M, Barbe F, Pinol-Ripoll G, 2019. The STOP-Bang and Berlin questionnaires to identify obstructive sleep apnoea in Alzheimer’s disease patients. Sleep Med 57, 15–20. [DOI] [PubMed] [Google Scholar]
- Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, Crimmins DL, Fagan AM, Holtzman DM, 2016. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 80, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, Lucey BP, Holtzman DM, 2014. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol 10, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM, 2013. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM, 2017. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 140, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Zangrilli MA, Finn MB, Fagan AM, Holtzman DM, 2019. Obstructive sleep apnea treatment, slow wave activity, and amyloid-beta. Ann Neurol 85, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyoti A, Plano A, Riedel G, Platt B, 2010. EEG, activity, and sleep architecture in a transgenic AbetaPPswe/PSEN1A246E Alzheimer’s disease mouse. J Alzheimers Dis 22, 873–887. [DOI] [PubMed] [Google Scholar]
- Kabeshita Y, Adachi H, Matsushita M, Kanemoto H, Sato S, Suzuki Y, Yoshiyama K, Shimomura T, Yoshida T, Shimizu H, Matsumoto T, Mori T, Kashibayashi T, Tanaka H, Hatada Y, Hashimoto M, Nishio Y, Komori K, Tanaka T, Yokoyama K, Tanimukai S, Ikeda M, Takeda M, Mori E, Kudo T, Kazui H, 2017. Sleep disturbances are key symptoms of very early stage Alzheimer disease with behavioral and psychological symptoms: a Japan multi-center cross-sectional study (J-BIRD). Int J Geriatr Psychiatry 32, 222–230. [DOI] [PubMed] [Google Scholar]
- Kam K, Parekh A, Sharma RA, Andrade A, Lewin M, Castillo B, Bubu OM, Chua NJ, Miller MD, Mullins AE, Glodzik L, Mosconi L, Gosselin N, Prathamesh K, Chen Z, Blennow K, Zetterberg H, Bagchi N, Cavedoni B, Rapoport DM, Ayappa I, de Leon MJ, Petkova E, Varga AW, Osorio RS, 2019. Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: novel roles for sleep spindles and tau. Mol Neurodegener 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, … & Malinow R, 2003. APP processing and synaptic function. Neuron 37, 925–937. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM, 2009. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE, 2012. What sleep characteristics predict cognitive decline in the elderly? Sleep Med 13, 886–892. [DOI] [PubMed] [Google Scholar]
- Kent BA, 2014. Synchronizing an aging brain: can entraining circadian clocks by food slow Alzheimer’s disease? Front Aging Neurosci 6, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Michalik M, Marchant EG, Yau KW, Feldman HH, Mistlberger RE, Nygaard HB, 2019. Delayed daily activity and reduced NREM slow wave power in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neurobiology of Aging 78, 74–86. [DOI] [PubMed] [Google Scholar]
- Kent BA, Mistlberger RE, 2017. Sleep and hippocampal neurogenesis: Implications for Alzheimer’s disease. Front Neuroendocrinol 45, 35–52. [DOI] [PubMed] [Google Scholar]
- Kent BA, Strittmatter SM, Nygaard HB, 2018. Sleep and EEG power spectral analysis in three transgenic mouse models of AD: APP/PS1, 3xTgAD, and Tg2576. Journal of Alzhiemer’s Disease 64, 1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. J Neurosci 38, 7314–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Wada Y, Nanbu Y, Nakajima A, Tachibana H, Takeda T, & Hashimoto T, 1999. EEG changes following scopolamine administration in healthy subjects. Neuropsychobiology 39, 219–226. [DOI] [PubMed] [Google Scholar]
- Kreutzmann JC, Havekes R, Abel T, Meerlo P, 2015. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 309, 173–190. [DOI] [PubMed] [Google Scholar]
- Kwak YT, 2006. Quantitative EEG findings in different stages of Alzheimer’s disease. J Clin Neurophysiol 23, 456–461. [DOI] [PubMed] [Google Scholar]
- La AL, Walsh CM, Neylan TC, Vossel KA, Yaffe K, Krystal AD, … & Karageorgiou E, 2019. Long-Term Trazodone Use and Cognition: A Potential Therapeutic Role for Slow-Wave Sleep Enhancers. . Journal of Alzheimer’s Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jung SJ, Choi JW, Shin A, Lee YJ, 2018. Use of sedative-hypnotics and the risk of Alzheimer’s dementia: A retrospective cohort study. PLoS One 13, e0204413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Yang SW, Ju YJ, Ki SK, Chun KH, 2019. Sleep-disordered breathing and Alzheimer’s disease: A nationwide cohort study. Psychiatry Res 273, 624–630. [DOI] [PubMed] [Google Scholar]
- Leng Y, McEvoy CT, Allen IE, Yaffe K, 2017. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol 74, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, 2017. Orexin and Alzheimer’s Disease. Curr Top Behav Neurosci 33, 305–322. [DOI] [PubMed] [Google Scholar]
- Liguori C, Chiaravalloti A, Nuccetelli M, Izzi F, Sancesario G, Cimini A, Bernardini S, Schillaci O, Mercuri NB, Fabio P, 2017. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer’s disease. J Neurol 264, 2215–2223. [DOI] [PubMed] [Google Scholar]
- Liguori C, Mercuri NB, Nuccetelli M, Izzi F, Cordella A, Bernardini S, Placidi F, 2019. Obstructive sleep apnea may induce orexinergic system and cerebral beta-amyloid metabolism dysregulation: is it a further proof for Alzheimer’s disease risk? Sleep Med 56, 171–176. [DOI] [PubMed] [Google Scholar]
- Liguori C, Placidi F, Izzi F, Spanetta M, Mercuri NB, Di Pucchio A, 2020a. Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimer’s Research & Therapy 12, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F, 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 71, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Liguori C, Spanetta M, Izzi F, Franchini F, Nuccetelli M, Sancesario GM, Di Santo S, Bernardini S, Mercuri NB, Placidi F, 2020b. Sleep-Wake Cycle in Alzheimer’s Disease Is Associated with Tau Pathology and Orexin Dysregulation. Journal of Alzheimer’s Disease, 1–8. [DOI] [PubMed] [Google Scholar]
- Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB, 2014. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA, 2013a. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA, 2013b. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 70, 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF, 1999. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-ε4/4 genotype. The Journal of Clinical Endocrinology & Metabolism 84, 323–327. [DOI] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, Hoogendijk WJ, Van Heerikhuize J, Kamphorst W, Unmehopa UA, … & Swaab DF, 2000. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. Journal of Neuropathology & Experimental Neurology 59, 314–322. [DOI] [PubMed] [Google Scholar]
- Liu S, Pan J, Lei Q, He L, Zhong B, Meng Y, Li Z, 2020. Spontaneous K-Complexes may be biomarkers of the progression of amnestic mild cognitive impairment. Sleep Medicine 67, 99–109. [DOI] [PubMed] [Google Scholar]
- Liu S, Pan J, Tang K, Lei Q, He L, Meng Y, Cai X, Li Z, 2019a. Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep and Breathing, 1–15. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang J, Xia M, Liu J, Jiang S, 2019b. Effect of donepezil on Hcy level in serum of Alzheimer’s disease patients and correlation analysis of Hcy and dyssomnia. Exp Ther Med 17, 1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, & Mendelson WB, 1982. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiology of Aging 3, 371–377. [DOI] [PubMed] [Google Scholar]
- Lucey BP, Gonzales C, Das U, Li J, Siemers ER, Slemmon JR, Bateman RJ, Huang Y, Fox GB, Claassen JA, Slats D, Verbeek MM, Tong G, Soares H, Savage MJ, Kennedy M, Forman M, Sjogren M, Margolin R, Chen X, Farlow MR, Dean RA, Waring JF, 2015. An integrated multi-study analysis of intra-subject variability in cerebrospinal fluid amyloid-beta concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimers Res Ther 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, Patterson BW, Baty J, Morris JC, Ovod V and Mawuenyega KG, , 2018. Effect of sleep on overnight CSF amyloid-β kinetics. Annals of Neurology 83, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, M. A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TL, 2019. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease Science Translational Medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luojus MK, Lehto SM, Tolmunen T, Brem AK, Lonnroos E, Kauhanen J, 2017. Self-reported sleep disturbance and incidence of dementia in ageing men. J Epidemiol Community Health 71, 329–335. [DOI] [PubMed] [Google Scholar]
- Lysen TS, Wolters FJ, Luik AI, Ikram MK, Tiemeier H, Ikram MA, 2018. Subjective Sleep Quality is not Associated with Incident Dementia: The Rotterdam Study. J Alzheimers Dis 64, 239–247. [DOI] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP, 2015. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP, 2016. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci 39, 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, … & Bateman RJ, 2010. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330, 1774–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J, Cohen DA, Sharpley AL, 2016. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 11, CD009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE, 2011. The cognitive cost of sleep lost. Neurobiol Learn Mem 96, 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AP, Michalak HR, McDonald JW, Kemp EC, Pugh EA, Becker ML, & Mecca MC, 2018. Sleep Disturbance and the Risk of Cognitive Decline or Clinical Conversion in the ADNI Cohort. Dementia and Geriatric Cognitive Disorders 45, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, & Okawa M, 1999. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep–waking. Biological Psychiatry 45, 417–421. [DOI] [PubMed] [Google Scholar]
- Moe KE, Vitiello MV, Larsen LH, & Prinz PN, 1995. Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. Journal of Sleep Research 4, 15–20. [DOI] [PubMed] [Google Scholar]
- Molano JRV, Roe CM, Ju YS, 2017. The interaction of sleep and amyloid deposition on cognitive performance. J Sleep Res 26, 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moline M, 2019. Response to Treatment with Lemborexant: Subjects with Irregular Sleep-Wake Rhythm Disorder and Alzheimer’s Disease Dementia. (Funder(s): Eisai Inc., Purdue Pharma). AAIC 2019. [Google Scholar]
- Most EI, Aboudan S, Scheltens P, Van Someren EJ, 2012. Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer disease. Am J Geriatr Psychiatry 20, 460–467. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS, 2018. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol 75, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara T, Honda T, Hata J, Yoshida D, Mukai N, Hirakawa Y, Shibata M, Kishimoto H, Kitazono T, Kanba S, Ninomiya T, 2018. Association Between Daily Sleep Duration and Risk of Dementia and Mortality in a Japanese Community. J Am Geriatr Soc 66, 1911–1918. [DOI] [PubMed] [Google Scholar]
- Okuda S, Tetsuka J, Takahashi K, Toda Y, Kubo T, Tokita S, 2019. Association between sleep disturbance in Alzheimer’s disease patients and burden on and health status of their caregivers. J Neurol 266, 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Arlig J, Hedner J, Blennow K, Zetterberg H, 2018. Sleep Deprivation and CSF Biomarkers for Alzheimer Disease. Sleep 41, 1–8. [DOI] [PubMed] [Google Scholar]
- Olsson M, Arlig J, Hedner J, Blennow K, Zetterberg H, 2019. Sleep deprivation and plasma biomarkers for Alzheimer’s disease. Sleep Med 57, 92–93. [DOI] [PubMed] [Google Scholar]
- Ooms S, Ju YE, 2016. Treatment of Sleep Disorders in Dementia. Curr Treat Options Neurol 18, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA, 2014. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 71, 971–977. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Pirraglia E, Agüera-Ortiz LF, During EH, Sacks H, Ayappa I, Walsleben J, Mooney A, Hussain A, Glodzik L, & Frangione B, 2011. Greater risk of Alzheimer’s disease in older adults with insomnia. Journal of the American Geriatrics Society 59, 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Paller KA, 2013. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci 17, 142–149. [DOI] [PubMed] [Google Scholar]
- Palmer K, Mitolo M, Burgio F, Meneghello F, Venneri A, 2018. Sleep Disturbance in Mild Cognitive Impairment and Association With Cognitive Functioning. A Case-Control Study. Front Aging Neurosci 10, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, Paller KA, Zee PC, 2017. Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Front Hum Neurosci 11, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, Paller KA, Zee PC, Malkani RG, 2019. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann Clin Transl Neurol 6, 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault AA, Khani A, Quairiaux C, Kompotis K, Franken P, Muhlethaler M, … & Bayer L, 2019. Whole-Night Continuous Rocking Entrains Spontaneous Neural Oscillations with Benefits for Sleep and Memory. Current Biology. [DOI] [PubMed] [Google Scholar]
- Petrovsky DV, McPhillips MV, Li J, Brody A, Caffee L, Hodgson NA, 2018. Sleep disruption and quality of life in persons with dementia: A state-of-the-art review. Geriatr Nurs 39, 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Welch A, Riedel G, 2011. FDG-PET imaging, EEG and sleep phenotypes as translational biomarkers for research in Alzheimer’s disease. Biochem Soc Trans 39, 874–880. [DOI] [PubMed] [Google Scholar]
- Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, Hudon C, 2012. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 35, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey-Smith SR, Mazzucchelli GN, Villemagne VL, Brown BM, Porter T, Weinborn M, Bucks RS, Milicic L, Sohrabi HR, Taddei K, Ames D, Maruff P, Masters CL, Rowe CC, Salvado O, Martins RN, Laws SM, Group AR, 2018. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl Psychiatry 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J, 2013. About sleep’s role in memory. Physiol Rev 93, 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Restifo K, … Nelson J, 1985. Sleep apnea in Alzheimer’s dementia: Correlation with mental deterioration. The Journal of Clinical Psychiatry 46, 257–261. [PubMed] [Google Scholar]
- Reynolds CF III, Kupfer DJ, Taska LS, Hoch CC, Spiker DG, Sewitch DE, … & Morycz R, 1985. EEG sleep in elderly depressed, demented, and healthy subjects. Biological Psychiatry 20, 431–442. [DOI] [PubMed] [Google Scholar]
- Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, Wang Y, Sawyer A, Weaver T, Lozano A, Carter P, Johnson J, 2019. CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea. J Am Geriatr Soc 67, 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma-Van Der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, & Van Someren EJ, 2008. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299, 2642–2655. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, & Holtzman DM, 2012. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Science Translational Medicine 4, 150ra122–150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, Snider BJ, Li M, Yanagisawa M, de Lecea L, Holtzman DM, 2014. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med 211, 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Filippov G, LoPresti A, Kumar D, Murphy P, Moline M, 2019. Safety of lemborexant in elderly subjects with insomnia: results from a Phase 3 study (Sunrise 1) The American Journal of Geriatric Psychiatry 27, S155–S156. [Google Scholar]
- Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP, 2013. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res 1529, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, 2007. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8, 171–181. [DOI] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Stopa EG, & Harper D, 1995. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiology of Aging 16, 765–771. [DOI] [PubMed] [Google Scholar]
- Satoh A, Iijima KM, 2017. Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: Potential strategies to protect the LC against aging. Brain Res. [DOI] [PubMed] [Google Scholar]
- Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, Kaisers UX, Thiery J, Hegerl U, Schonknecht P, 2013. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. PLoS One 8, e63136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoralick FM, Louzada LL, Quintas JL, Naves JO, Camargos EF, Nobrega OT, 2017. Mirtazapine does not improve sleep disorders in Alzheimer’s disease: results from a double-blind, placebo-controlled pilot study. Psychogeriatrics 17, 89–96. [DOI] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL, 2015. Is cognitive aging associated with levels of REM sleep or slow wave sleep? Sleep 38, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L, D’Amelio M, Di Domenico C, Dipasquale O, Marra C, Mercuri NB, Caltagirone C, Cercignani M, Bozzali M, 2018. In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer’s disease. Neurobiol Aging 72, 72–82. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, Wohlleber M, Miller MD, Andrade A, Lewis C, Tweardy S, Buj M, Yau PL, Sadda R, Mosconi L, Li Y, Butler T, Glodzik L, Fieremans E, Babb JS, Blennow K, Zetterberg H, Lu SE, Badia SG, Romero S, Rosenzweig I, Gosselin N, Jean-Louis G, Rapoport DM, de Leon MJ, Ayappa I, Osorio RS, 2018. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. Am J Respir Crit Care Med 197, 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Han HJ, Shin DJ, Park HM, Lee YB, Park KH, 2014. Sleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer’s disease. J Clin Neurol 10, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND, 2018. beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindi S, Johansson L, Skoog J, Mattsson AD, Sjoberg L, Wang HX, Fratiglioni L, Kulmala J, Soininen H, Solomon A, Johansson B, Skoog I, Kivipelto M, Kareholt I, 2018. Sleep disturbances and later cognitive status: a multi-centre study. Sleep Med 52, 26–33. [DOI] [PubMed] [Google Scholar]
- Singer M, Romero B, Koenig E, Förstl H, Brunner H, 2005. Nightmares in patients with Alzheimer’s disease caused by donepezil. Therapeutic effect depends on the time of intake. Der Nervenarzt 76, 1127–1128, 1129. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pevet P, Ravid D, Swaab DF, 1990. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Research 528, 170–174. [DOI] [PubMed] [Google Scholar]
- Slats D, AHR Claassen J, Jan Lammers G, J Melis R, M Verbeek M, & Overeem S, 2012. Association between hypocretin-1 and amyloid-β42 cerebrospinal fluid levels in Alzheimer’s disease and healthy controls. Current Alzheimer Research 9, 1119–1125. [DOI] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM, 2013. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM, 2015. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36, 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, … & Bendlin BB, 2017. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, & Satlin A, 1999. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. Journal of Neuropathology & Experimental Neurology 58, 29–39. [DOI] [PubMed] [Google Scholar]
- Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH, Lee KH, Han G, Hong JW, Kwak KP, Kim BJ, Kim SG, Kim JL, Kim TH, Ryu SH, Moon SW, Park JH, Seo J, Youn JC, Lee DY, Lee DW, Lee SB, Lee JJ, Jhoo JH, Kim KW, 2018. Sleep and cognitive decline: A prospective nondemented elderly cohort study. Ann Neurol 83, 472–482. [DOI] [PubMed] [Google Scholar]
- Sulkava S, Muggalla P, Sulkava R, Ollila HM, Peuralinna T, Myllykangas L, Kaivola K, Stone DJ, Traynor BJ, Renton AE, Rivera AM, Helisalmi S, Soininen H, Polvikoski T, Hiltunen M, Tienari PJ, Huttunen HJ, Paunio T, 2018. Melatonin receptor type 1A gene linked to Alzheimer’s disease in old age. Sleep 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, & Hofman MA, 1988. Sexual differentiation of the human hypothalamus: ontogeny of the sexually dimorphic nucleus of the preoptic area. Developmental Brain Research 44, 314–318. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, & Partiman TS, 1985. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research 342, 37–44. [DOI] [PubMed] [Google Scholar]
- Taillard J, Sagaspe P, Berthomier C, Brandewinder M, Amieva H, Dartigues JF, Rainfray M, Harston S, Micoulaud-Franchi JA, Philip P, 2019. Non-REM Sleep Characteristics Predict Early Cognitive Impairment in an Aging Population. Front Neurol 10, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S, Heinsen H, Amaro E Jr., Grinberg LT, Krause B, Grothe M, Alzheimer’s Disease Neuroimaging I, 2014. Cholinergic basal forebrain atrophy predicts amyloid burden in Alzheimer’s disease. Neurobiol Aging 35, 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M, Terman JS, 2005. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS spectrums 10, 647–663. [DOI] [PubMed] [Google Scholar]
- Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K, Group SOFR, 2011. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Li HY, Huang CG, Wang RY, Chuang LP, Chen NH, Liu CH, Yang YH, Liu CY, Hsu CM, 2020. Risk of alzheimer’s disease in obstructive sleep apnea patients with or without treatment: Real-world evidence. The Laryngoscope. [DOI] [PubMed] [Google Scholar]
- van Maanen A, Meijer AM, van der Heijden KB, Oort FJ, 2016. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev 29, 52–62. [DOI] [PubMed] [Google Scholar]
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, … & Swaab DF, Circadian rest—activity rhythm disturbances in Alzheimer’s disease. Biological Psychiatry 40, 259–270. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB, 1999. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiology international 16, 505–518. [DOI] [PubMed] [Google Scholar]
- Varga AW, Wohlleber ME, Gimenez S, Romero S, Alonso JF, Ducca EL, Kam K, Lewis C, Tanzi EB, Tweardy S, Kishi A, Parekh A, Fischer E, Gumb T, Alcolea D, Fortea J, Lleo A, Blennow K, Zetterberg H, Mosconi L, Glodzik L, Pirraglia E, Burschtin OE, de Leon MJ, Rapoport DM, Lu SE, Ayappa I, Osorio RS, 2016. Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Abeta42 Levels in Cognitively Normal Elderly. Sleep 39, 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Prinz PN, Williams DE, Frommlet MS, & Ries RK, 1990. Sleep disturbances in patients with mild-stage Alzheimer’s disease. Journal of Gerontology 45, M131–M138. [DOI] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, & Satlin A, 2001. Sundowning and circadian rhythms in Alzheimer’s disease. American Journal of Psychiatry 158, 704–711. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng C, Moelter S, Fuentecilla JL, Kincheloe K, Lozano AJ, Carter P, Gooneratne N, Richards KC, 2020. One Year of Continuous Positive Airway Pressure Adherence Improves Cognition in Older Adults With Mild Apnea and Mild Cognitive Impairment. Nursing Research 69, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Dan Y, 2016. Circuit-based interrogation of sleep control. Nature 538, 51–59. [DOI] [PubMed] [Google Scholar]
- Wei M, Zhao B, Huo K, Deng Y, Shang S, Liu J, Li Y, Ma L, Jiang Y, Dang L, Chen C, Wei S, Zhang J, Yang H, Gao F, Qu Q, 2017. Sleep Deprivation Induced Plasma Amyloid-beta Transport Disturbance in Healthy Young Adults. J Alzheimers Dis 57, 899–906. [DOI] [PubMed] [Google Scholar]
- Weissova K, Bartos A, Sladek M, Novakova M, Sumova A, 2016. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PLoS One 11, e0146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA, 2012. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc 18, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Tudorascu DL, Snitz BE, Price JC, Aizenstein HJ, Lopez OL, Erickson KI, Lopresti BJ, Laymon CM, Minhas D, Mathis CA, Buysse DJ, Klunk WE, Cohen AD, 2018. Sleep moderates the relationship between amyloid beta and memory recall. Neurobiol Aging 71, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, Knight RT, Jagust WJ, Walker MP, 2019. Sleep as a potential biomarker of tau and beta-amyloid burden in the human brain. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, & Swaab DF, 1990. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biological Psychiatry 27, 563–572. [DOI] [PubMed] [Google Scholar]
- Wu YH, & Swaab DF, 2005. The human pineal gland and melatonin in aging and Alzheimer’s disease. Journal of Pineal Research 38, 145–152. [DOI] [PubMed] [Google Scholar]
- Wu YH, Feenstra MG, Zhou JN, Liu RY, Torano JS, Van Kan HJ, Fischer DF, Ravid R, Swaab DF, 2003. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab 88, 5898–5906. [DOI] [PubMed] [Google Scholar]
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF, 2006. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J 20, 1874–1876. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang LL, Dammer EB, Li CB, Xu G, Chen SD, Wang G, 2015. Melatonin for sleep disorders and cognition in dementia: a meta-analysis of randomized controlled trials. Am J Alzheimers Dis Other Demen 30, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, Hoang T, 2014. Connections between sleep and cognition in older adults. The Lancet Neurology 13, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S& Stone KL, 2011. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Nettiksimmons J, Yesavage J, Byers A, 2015. Sleep Quality and Risk of Dementia Among Older Male Veterans. Am J Geriatr Psychiatry 23, 651–654. [DOI] [PubMed] [Google Scholar]
- Yuen K, Rashidi-Ranjbar N, Verhoeff N, Kumar S, Gallagher D, Flint AJ, Herrmann N, Pollock BG, Mulsant BH, Rajji TK, Voineskos AN, Fischer CE, Mah L, Group PA-MS, 2019. Association between Sleep Disturbances and Medial Temporal Lobe Volume in Older Adults with Mild Cognitive Impairment Free of Lifetime History of Depression. J Alzheimers Dis 69, 413–421. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhan G, Fenik P, Brandes M, Bell P, Francois N, Shulman K, Veasey S, 2018. Chronic Sleep Disruption Advances the Temporal Progression of Tauopathy in P301S Mutant Mice. J Neurosci 38, 10255–10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurakovskaya E, Ishchenko I, Gureviciene I, Aliev R, Grohn O, Tanila H, 2019. Impaired hippocampal-cortical coupling but preserved local synchrony during sleep in APP/PS1 mice modeling Alzheimer’s disease. Sci Rep 9, 5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


