Summary:
Many diseases are caused by toxic RNA repeats. Herein, we designed a lead small molecule that binds the structure of the r(CUG) repeat expansion [r(CUG)exp] that causes myotonic dystrophy type 1 (DM1) and Fuch’s endothelial corneal dystrophy (FECD) and rescues disease biology in patient-derived cells and in vivo. Interestingly, the compound’s downstream effects are different in the two diseases, owing to the location of the repeat expansion. In DM1, r(CUG)exp is harbored in the 3’ untranslated region (UTR), and the compound has no effect on the mRNA’s abundance. In FECD, however, r(CUG)exp is located in an intron, and the small molecule facilitates excision of the intron, which is then degraded by the nuclear RNA exosome complex. Thus, structure-specific, RNA-targeting small molecules can act disease-specifically to affect biology, either by disabling its gain-of-function mechanism (DM1) or by stimulating quality control pathways to rid a disease-affected cell of a toxic RNA (FECD).
Graphical Abstract

eTOC BLURB
RNA repeat expansions cause >30 diseases. Angelbello et al. show that a small molecule that binds the repeat expansion r(CUG)exp can improve defects in multiple diseases. When r(CUG)exp is located in an intron, small molecule binding triggers decay of the disease-causing RNA through the nuclear RNA exosome.
Introduction
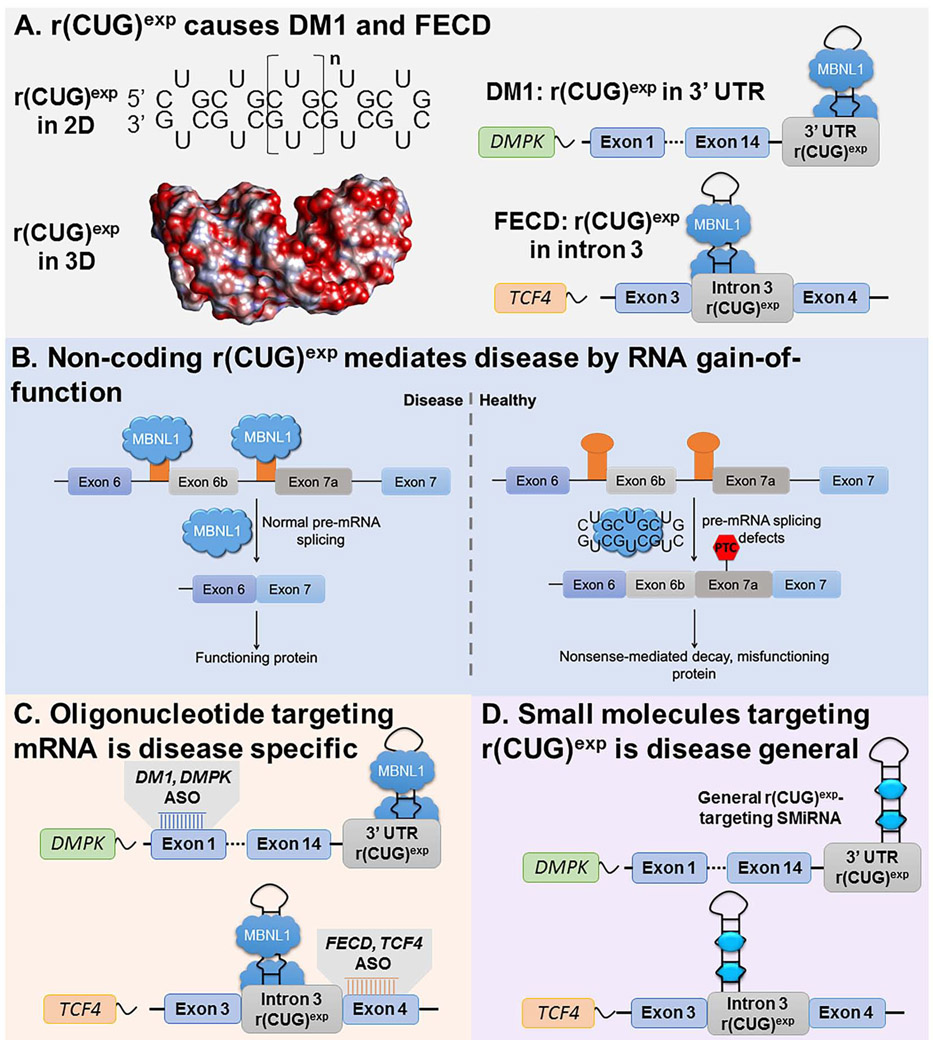
Over 40 diseases are caused by RNA repeat expansions including Huntington’s disease (HD) (Group, 1993), C9orf72-mediated amyotrophic lateral sclerosis/frontotemporal dementia (C9ALS/FTD) (DeJesus-Hernandez et al., 2011), myotonic dystrophy type 1 (DM1) (Brook et al., 1992), and Fuchs endothelial corneal dystrophy (FECD) (Wieben et al., 2012). DM1 is caused by expression of an r(CUG) repeat expansion [r(CUG)exp] harbored in the 3’ untranslated region (UTR) of the dystrophia myotonica protein kinase (DMPK) mRNA (Fig. 1A) (Brook et al., 1992). DM1 is the most common form of adult-onset muscular dystrophy, affecting approximately 1 in 8,000 people. This r(CUG)exp causes disease by a gain-of-function mechanism in which the repeat expansions binds to and sequesters various proteins, particularly the pre-mRNA splicing regulator muscleblind-like 1 protein (MBNL1), in nuclear foci (Taneja et al., 1995). Sequestration of MBNL1 by r(CUG)exp limits the amount of the protein available to regulate pre-mRNA splicing, causing system-wide defects that manifest themselves in DM1 (Fig. 1B) (Jiang et al., 2004; Nakamori et al., 2013).
Fig. 1.
Mechanisms by which r(CUG)exp causes DM1 and FECD and approaches to target the disease-causing RNAs with small molecules. (A) DM1 and FECD are caused by r(CUG)exp found in the 3’ UTR of DMPK mRNA or in intron 3 of TCF4 mRNA, respectively. The repeat expansion forms a structure containing repeating 1×1 UU internal loops that sequesters regulatory proteins such as MBNL1. (B) r(CUG)exp causes disease via RNA gain-of-function in which it sequesters MBNL1 in nuclear foci, resulting in pre-mRNA splicing defects in genes that are regulated by MBNL1; the muscle specific chloride ion channel pre-mRNA (CLCN1) is shown as an example. (C) Using antisense oligonucleotides (ASOs) to target disease-causing RNAs requires customization for each transcript. (D) Developing small molecules that bind r(CUG)exp structure offers a general way to target multiple diseases with the same compound.
Interestingly, it was recently discovered that r(CUG)exp causes another disease, FECD, which manifests itself differently than DM1 as the repeat expansion is harbored in a different gene and in an intron. FECD is a dominantly inherited corneal disease that affects as many as 5% of Caucasian males, resulting in vision impairment (Lorenzetti et al., 1967). The FECD-causing r(CUG)exp resides in intron 3 of the transcription factor 4 (TCF4) pre-mRNA (Wieben et al., 2012). Akin to DM1, r(CUG)exp also sequesters MBNL1 in nuclear foci in FECD, causing pre-mRNA splicing defects (Du et al., 2015; Hale et al., 2019; Mootha et al., 2015). Recent studies have indicated that some TCF4 mRNAs in FECD cells retain intron 3, a phenomenon that also occurs in other genetic disorders caused by intronic repeat expansions (Sznajder et al., 2018).
A common way to rescue the biology of microsatellite diseases is by the binding of antisense oligonucleotides (ASOs) that target unstructured regions in the coding mRNA (Fig. 1C). Although targeting the repeat sequence itself has been investigated (Wheeler et al., 2009), specificity has been limited as tandem repeats are ubiquitous in the genome. Sequence-targeting ASOs also run the risk of parallel knockdown of transcripts from the wild-type allele (Jauvin et al., 2017), which may give rise to loss-of-function phenotypes. Accordingly, an ASO is customized for each disease, complementary to regions outside of the repeating sequence, even if two (or more) diseases are caused by the same repeat expansion.
An alternate approach to the sequence-based recognition of ASOs is the structure-based recognition of small molecules interacting with RNAs (SMIRNAs). That is, an SMIRNA would recognize the structure formed by the repeat expansions, for example the repeating array of 1×1 nucleotide U/U internal loops formed by r(CUG)exp (Rzuczek et al., 2017; Rzuczek et al., 2015). Indeed, many small molecules have been developed to target the structure of r(CUG)exp and improve DM1-associated defects in patient-derived cells and in vivo (Angelbello et al., 2019; Jahromi et al., 2013; Li et al., 2018; Rzuczek et al., 2017; Rzuczek et al., 2015). Using a small molecule that directly recognizes the structure of RNA repeat expansions does not require customization across different diseases involving the same repeat motif, such as DM1 and FECD (Fig. 1D).
Herein, we describe the design of a small molecule that binds r(CUG)exp with nanomolar affinity and is broadly selective. Indeed, the molecule directly engages the target, as studied by the target profiling method Competitive Chemical Cross-Linking and Isolation by Pull-down (C-Chem-CLIP). As a result of this binding, we find that the compound improves r(CUG)exp-mediated defects similarly in both DM1 and FECD. In addition, the small molecule reduces the frequency of intron retention in TCF4 in FECD-affected cells. The excised intron is subsequently degraded by the RNA nuclear exosome complex. Thus, RNA structure-specific ligands can be applied across diseases that are mediated by the same toxic structure. The impact of these compounds on metabolism of the RNA target can vary, depending on where the repeat expansion lies in the host gene, and in some cases may accelerate decay of the toxic element via quality control machinery.
Results
Compound design, in vitro evaluation, and structural analysis.
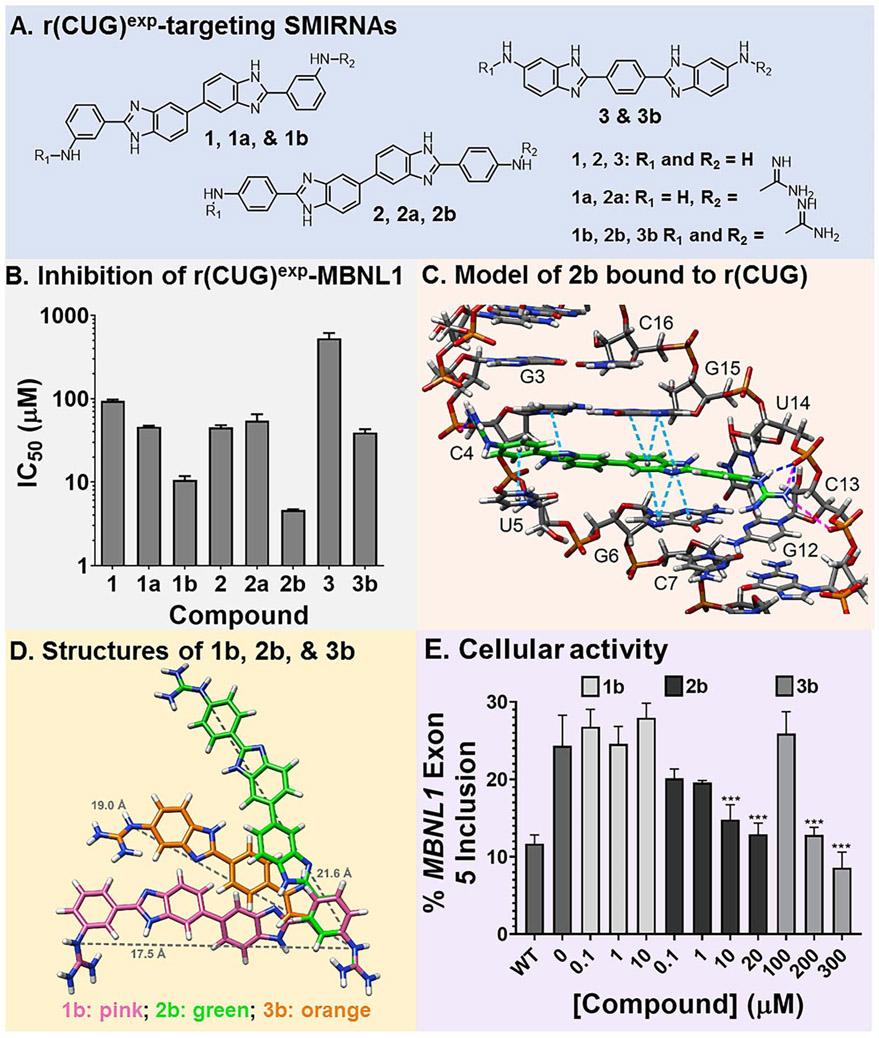
The most avid and bioactive ligands that have been designed to target repeat expansions of r(CUG)exp have been dimers that contain two 5’CUG/3’GUC-binding modules (H) (Rzuczek et al., 2017). A considerable challenge in targeting RNA structures, however, is developing low molecular weight, monomeric compounds that bind with high affinity and specificity. Thus, in this study, monomeric ligands that target r(CUG)exp and affect disease-associated biology were developed. Previously, we reported that aryl diamines separated by a bis-benzimidazole scaffold was a favored sub-structure that targets r(CUG)exp, revealed by chemical similarity searching using H as query molecule (Parkesh et al., 2012; Rzuczek et al., 2015). Compounds that were found to bind r(CUG)exp and inhibit formation of the r(CUG)exp-MBNL1 complex include 1, 2, and 3 (Fig. 2A) (Parkesh et al., 2012; Rzuczek et al., 2015). Further, modeling studies of 1 (H1) suggested that the amino side chains interacted, in part, with the phosphodiester backbone (Parkesh et al., 2012).
Fig. 2.
Design of compounds that bind r(CUG)exp. (A) Structures of small molecules interacting with RNA (SMIRNAs) that target r(CUG)exp. (B) Studying compounds in vitro by using a previously reported TR-FRET assay to identify compounds that inhibit the r(CUG)exp-MBNL1 complex. (C) 3D model of interactions of 2b with the loops formed by r(CUG)exp. Pink lines are ionic interactions, dark blue lines are hydrogen bonding interactions, and light blue lines are stacking interactions. (D) Structures of 1b (pink), 2b (green), and 3b (orange) used to calculate the distance between guanidine substituents (gray line). (E) Quantification of MBNL1 exon 5 inclusion DM1 fibroblasts by 1b, 2b, and 3b (n = 3). **, P < 0.01; ***, P < 0.001, as determined by a one-way ANOVA by comparison to untreated cells. Data are represented as mean ± SD. See also Figures S1 and S2.
One way to enhance activity of RNA-targeting ligands is through guanidinylation, which increases and distributes charge such that interactions are formed with the phosphodiester backbone. Thus, lead compounds 1, 2, and 3 were treated with cyanamide under acidic conditions to obtain singly (1a and 2a) and doubly guanidinylated (1b, 2b, 3b) molecules in one pot reactions (Fig. 2A & Fig. S1). Each compound was evaluated in vitro for inhibition of the r(CUG)12-MBNL1 complex using a previously developed time-resolved fluorescence resonance energy transfer (TR-FRET) assay (Fig. 2B) (Chen et al., 2012). Relative to the parent compounds, each derivative provided a more potent in vitro response, and potency increased with the number of guanidine substituents. In particular, the doubly guanidinylated compounds were at least 10-fold more potent than the parent molecules. The affinity of the most potent compound, 2b (IC50 = 4.6±0.1 μM), which is also the most potent in cellulis (vide infra), was measured by a direct binding assay. The compound bound avidly to a model of r(CUG)exp with a Kd of 40±6 nM, while no measurable binding was observed to a full base-paired RNA lacking 1×1 U/U internal loops, an AT rich DNA hairpin, or other RNA repeat expansions including models of the c9ALS/FTD RNA [r(G4C2)exp] and the HD RNA [r(CAG)exp] (Kd > 5000 nM) (Fig. S1).
To study the features of molecular recognition, we completed dynamic docking to obtain a structural model of 2b bound to an RNA with one copy of a 5’CUG/3’GUC internal loop motif (Fig. 2C and Fig. S1). Indeed, multiple interactions drive molecular recognition of the target. Previous biophysical studies have shown that the 5’CUG/3’GUC loops in the DM1 RNA are dynamic, adopting conformations in which the Us form 0, 1 (most populated), and 2 hydrogen bonds (Parkesh et al., 2011). Upon binding, 2b changes the structure of the RNA such that the loop nucleotides no longer stack on the closing base pairs, replaced by the stacking of 2b. The compound spans the major groove to form inter-strand interactions with the phosphodiester backbone and an array of hydrogen bonds between its bis-benzimidazole imino protons and the nucleobases and the sugar. Collectively, these modeling studies support that specific interactions and shape complementarity drive complex formation between 2b and r(CUG) repeats.
To gain further insights into the shape complementarity and its role in driving complex formation, we overlaid the structures of 1b, 2b, and 3b, which may provide a rationale for 2b’s enhanced potency (Fig. 2D). It is apparent that both the size of 2b and the positioning of its functional groups affords the most complementarity to the structure formed by r(CUG)exp. For example, the shape presented by the 1×1 nucleotide U/U internal loops is best spanned by 2b such that the two ends of the ligand can interact with two phosphates. In contrast, 1b does not have its guanidinium groups in the proper orientations to bind both phosphates simultaneously while compound 3b is too short to accommodate both interactions simultaneously.
Designer ligands improve various DM1 cellular defects.
The doubly guanidinylated compounds (1b, 2b, and 3b), the most potent for inhibition of the r(CUG)exp-MBNl1 complex in vitro, were studied for improving various DM1 defects in patient-derived fibroblasts at concentrations where no toxicity was observed (Fig. S2A). Compounds were first tested for their ability to rescue the MBNL1 exon 5 splicing defect, as MBNL1 protein regulates the splicing of its own pre-mRNA; that is, MBNL1 exon 5 alternative splicing is dysregulated in DM1 fibroblasts (Gates et al., 2011). While 1b failed to improve the splicing pattern, 2b and 3b both rescued splicing dose-dependently, with statistically significant improvement observed at 10 μM and 200 μM, respectively (Fig. 2E and Fig. S2B-D). This dose-dependent rescue of mis-splicing observed upon 2b-treatment was further confirmed by RT-qPCR analysis of MBNL1 exon 5 inclusion (Fig. S2E). 2b also reduced the number of r(CUG)exp- and MBNL1-containing nuclear foci in DM1 fibroblasts, as determined by fluorescence in situ hybridization (FISH) and MBNL1 immunofluorescence (Fig. S2F-G). Notably, 2b did not affect MBNL1 exon 5 inclusion in wild-type fibroblasts (Fig. S2H) nor a NOVA-regulated splicing event in DM1 fibroblasts (Fig. S2I). The observed improvements of DM1-associated molecular defects were not due to transcriptional inhibition of DMPK levels, which were unaffected by 2b treatment as determined by RT-qPCR (Fig. S2J).
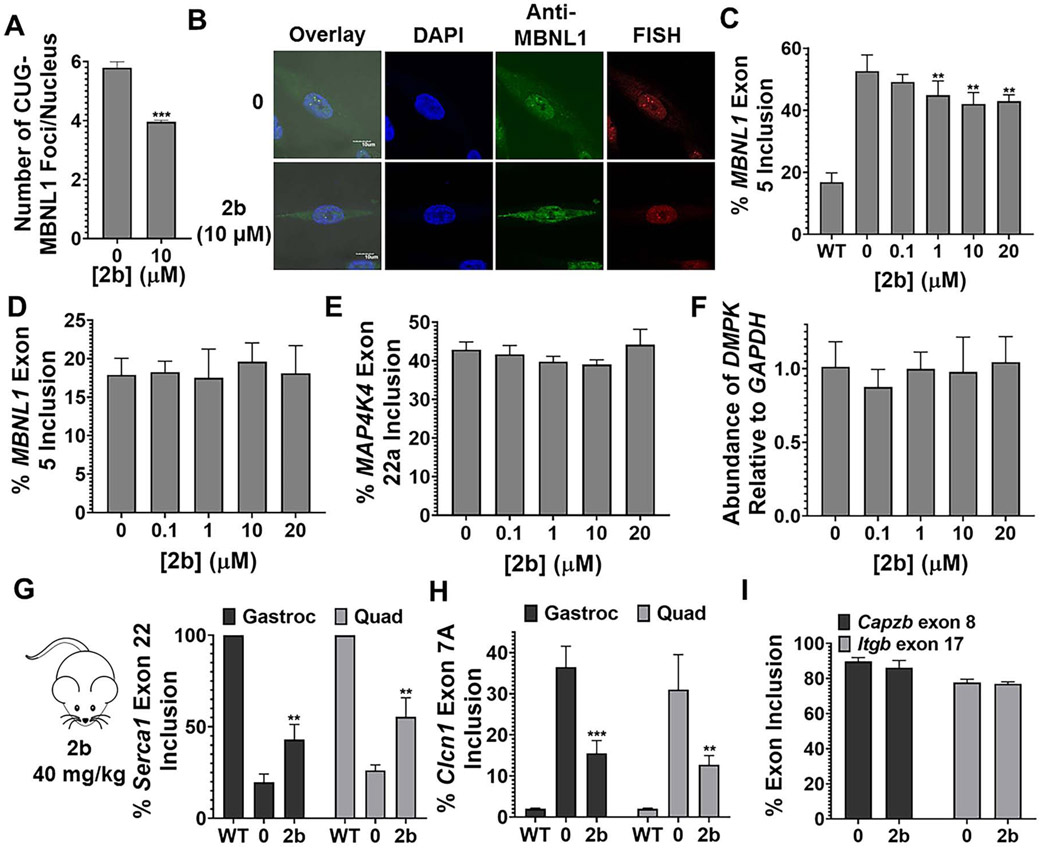
As 2b was clearly the most potent, we studied it further in DM1 patient-derived myotubes, differentiated from fibroblasts (Arandel et al., 2017). In agreement with studies in fibroblasts, 2b reduced the number of nuclear foci/cell at 10 μM dose (Figs. 3A and 3B) and improved the MBNL1 exon 5 splicing defect dose-dependently, significantly upon treatment with 1 μM compound (Fig. 3C and Fig. S3A). As expected from studies in fibroblasts, 2b did not affect MBNL1 exon 5 inclusion in wild-type myotubes (Fig. 3D and Fig. S3B), and no effect was observed on NOVA-regulated MAP4K4 exon 22a splicing (Ule et al., 2005) (Fig. 3E and S3C). Additionally, the levels of DMPK mRNA were not affected by 2b treatment, as measured by RT-qPCR (Fig. 3F), indicating that 2b binds the RNA rather than inhibits transcription. Collectively, these studies in both DM1 patient-derived fibroblasts and myotubes indicate that 2b acts in a manner that is consistent with selectively improving DM1 disease biology.
Fig. 3.
Compound 2b alleviates molecular defects in DM1 myotubes and in a DM1 mouse model. (A) Representative images of r(CUG)exp-MBNL1 foci in DM1 myotubes, imaged by MBNL1 immunostaining and RNA fluorescence in situ hybridization (FISH). (B) Quantification of the number of r(CUG)exp-MBNL1 foci/nucleus in DM1 myotubes (n = 3; 40 nuclei counted/replicate). *** P < 0.001, as determined by a two-tailed Student t-test by comparison to untreated cells. (C) Ability of 2b to improve MBNL1 exon 5 splicing in DM1 myotubes (n = 3). * P < 0.05, ** P < 0.01, *** P < 0.001, as determined by one-way ANOVA by comparison to untreated cells. (D) Evaluation of MBNL1 exon 5 splicing in wild-type myotubes (n = 3). (E) Evaluation of MAP4K4 exon 22a splicing in DM1 myotubes (n = 3). (F) Evaluation of DMPK levels in DM1 myotubes treated with 2b, as determined by RT-qPCR with gene-specific primers (n = 3). (G) Quantification of Serca 1 exon 22 splicing in HSALR mice treated with 2b. (H) Quantification of Clcn1 exon 7a splicing in HSALR mice treated with 2b. “Gastroc” indicates gastrocnemius and “Quad” indicates quadriceps (n = 4 mice/group). **, P < 0.01; *** P < 0.001, as determined by a two-tailed Student t-test relative to vehicle treated (0). (H) Evaluation of Capzb exon 8 and Itgb exon 17 splicing (non-MBNL1 regulated events) in HSALR mice treated with 2b (n = 4 mice/group). Data are represented as mean ± SD. See also Figure S3 and S4.
Designer ligands directly bind to r(CUG)exp in DM1 patient-derived cells.
We next studied if the different potencies of 2b and 3b could be traced to the degree of r(CUG)exp target engagement in cells. To measure target occupancy, we utilized the method Chemical Cross-Linking and Isolation by Pull-down (Chem-CLIP) (Disney, 2019). In Chem-CLIP, a chemical probe is appended with cross-linking (chlorambucil, CA) and purification (biotin) modules (Fig. 4A). The Chem-CLIP probe undergoes a proximity-based cross-linking reaction to conjugate the structure-targeting ligand to the RNAs that it binds in cells. The cross-linked RNA can then be purified using streptavidin beads. Chem-CLIP probe 2H-K4NMeS-CA-Biotin (Fig. S3D) (Rzuczek et al., 2017) binds r(CUG)exp and pulls down mutant, but not wild-type, DMPK mRNA in DM1 fibroblasts, enriching the RNA by 20-fold (100 nM; Fig. 4A).
Fig. 4.
Compounds designed to target r(CUG)exp engage the target in DM1 fibroblasts, as determined by C-Chem-CLIP. (A) Chem-CLIP target engagement in cells using Chem-CLIP probe 2H-K4NMeS-CA-Biotin, which selectively cross-links and enriches r(CUG)exp-containing DMPK transcripts in DM1 fibroblasts. The probe does not enrich DMPK in wild-type fibroblasts. (n = 3 for both DM1 and WT fibroblasts). Data for WT fibroblasts was previously collected in Rzuczek et al. (Rzuczek et al., 2017). **, P < 0.01, as determined by a two-tailed Student t-test. (B) Competitive Chem-CLIP (C-Chem-CLIP) to study target engagement by 2b and 3b. DM1 fibroblasts were co-treated with 100 nM of 2H-K4NMeS-CA-Biotin and varying concentrations of 2b or 3b to calculate the IC50s, or relative target occupancy in cells (n = 3). For 2b the IC50 is 38±9 nM, and for 3b the IC50 is 3400±60 nM. Data are represented as mean ± SD. See also Figure S3.
Thus, this Chem-CLIP probe is suitable to study if ligands bind to r(CUG)exp in cells by using a competition experiment, or C-Chem-CLIP. In C-Chem-CLIP, cells are co-treated with the molecule of interest, in this case 2b and 3b, at varying concentrations in the presence of a constant concentration of the Chem-CLIP probe, in this case 2H-K4NMeS-CA-Biotin (100 nM; Fig. 4B). Occupancy of the query ligand is calculated by its ability to inhibit the pull-down of mutant DMPK mRNA. In these studies, 2b had an IC50 of 38±9 nM, while 3b had an IC50 of 3,400±60 nM, a difference of 100-fold for modest changes in compound structure (Fig. 4B). Since many molecules of MBNL1 and many molecules of compound can each bind r(CUG)exp, target occupancy can account for differences in cellular potency, at least in part. Interestingly, this difference in relative cellular occupancy in C-Chem-CLIP is greater than the relative difference in the ability of the two ligands to inhibit the formation of the r(CUG)exp-MBNL1 complex (Fig. 2B). This suggest that factors other than in vitro potency affect cellular activity and can include uptake and target selectivity that is read out in C-Chem-CLIP, which provides a holistic view of factors that affect RNA target occupancy in cells.
Designer small molecule 2b rescues splicing in vivo.
Lastly, we studied the therapeutic potential of 2b in the HSALR mouse model of DM1 (Mankodi et al., 2000). This transgenic mouse model expresses a human skeletal actin (HSA) transgene containing r(CUG)220 and has several hallmarks of human disease, including pre-mRNA splicing defects (Mankodi et al., 2000). To study if 2b can improve these disease defects in vivo, HSALR and wild-type control (FVB) mice were treated with 40 mg/kg of 2b via intraperitoneal (i.p.) injection every day for 7 days. The compound was well tolerated in both strains. Analysis of RNA isolated from the gastrocnemius and quadriceps muscles of HSALR mice after dosing showed specific improvement of two deregulated splicing events, Serca1 exon 22 and Clcn1 exon 7A (Fig. 3G and Fig. S4). These changes in splicing outcomes were specific to DM1, as two non-MBNL1 regulated splicing events, Capzb exon 8 and Itgb exon 17 (Lin et al., 2006), were not affected (Fig. 3H and Fig. S4). Notably, the splicing of Serca1 exon 22 and Clcn1 exon 7A was unaffected in wild-type mice treated with 2b (Fig. S4). Thus, 2b potently and specifically improved DM1-associated defects in patient-derived cells and in vivo.
Designer small molecule 2b that targets r(CUG)exp improves disease pathways in FECD.
The corneal disease FECD is caused by r(CUG)exp located in intron 3 of TCF4 transcript (Wieben et al., 2012). FECD causes thinning of the cornea that ultimately may require transplantation. In contrast to DM1, it is not a rare disease, but rather a common disease that affects 5% of male Caucasians over the age of 40 (Lorenzetti et al., 1967). The disease is manifest at the molecular levels by similar biochemical events to those observed in DM1, i.e. r(CUG)exp forms foci with MBNL1 and causes pre-mRNA splicing defects (Du et al., 2015; Mootha et al., 2015). Thus, we investigated whether 2b also improves disease defects in FECD patient-derived corneal endothelium cells.
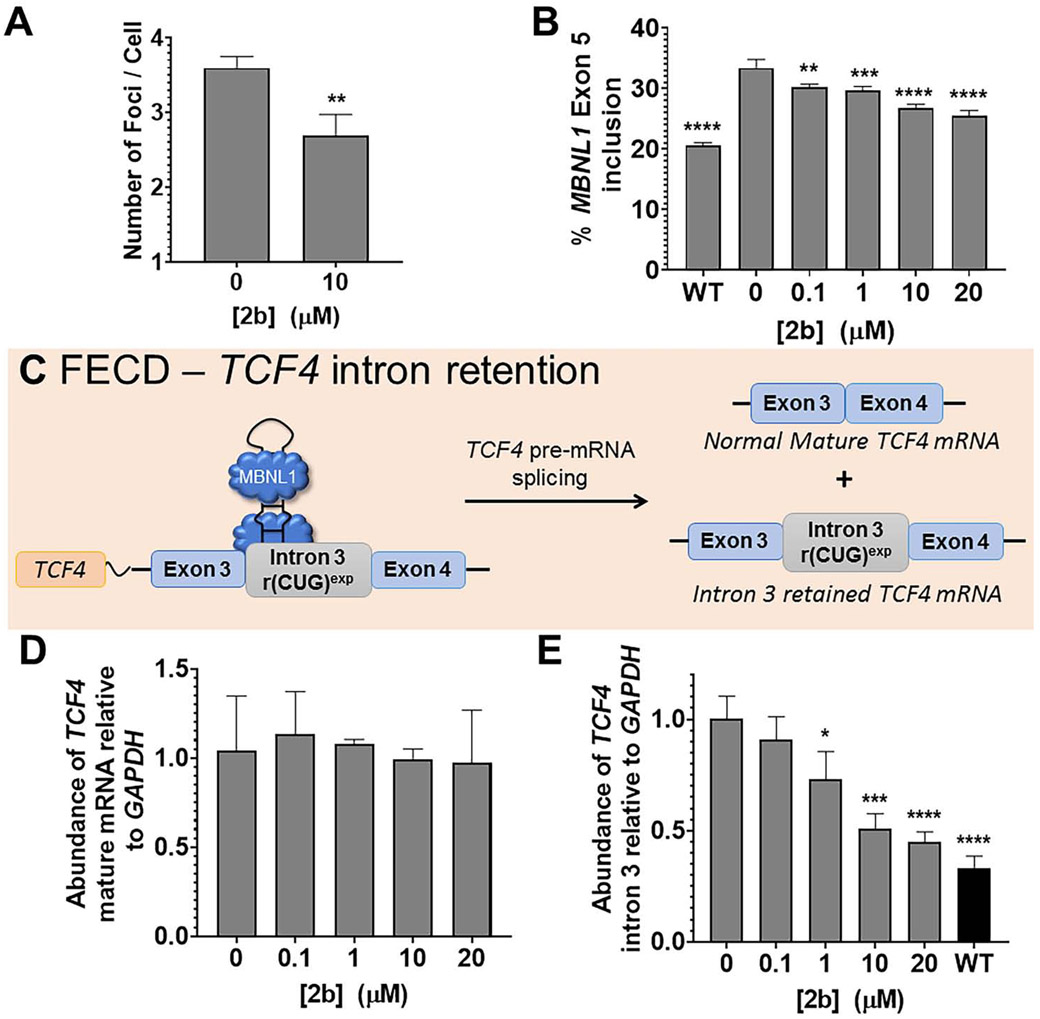
As determined by a cell viability assay, no measurable toxicity was observed up to 20 μM of 2b (Fig. S5A). We then studied whether 2b could reduce r(CUG)exp-MBNL1 foci. As was observed in DM1, 2b (10 μM) reduced the number of foci per FECD cell, from 3.6±0.2 to 2.7±0.3 (P<0.01; Fig. 5A and Fig. S5B-C). As expected, 2b also improved FECD-associated pre-mRNA splicing defects, as a dose-response profile was observed for rescue of the MBNL1 exon 5 splicing (Fig. 5B and Fig. S5D), in accordance with results in DM1 cells (Fig. 2E and 3C). In particular, statistically significant rescue was observed in low μM range in FECD cells (Fig. 5B). The compound had no effect on NOVA-regulated MAP4K4 exon 22a splicing (Fig. S5E), and similar to wild-type fibroblast studies described above, no effect on MBNL1 exon 5 splicing was observed in wild-type corneal endothelium cells (Fig. S5F), demonstrating the functional selectivity of 2b in FECD cells.
Fig. 5.
Compound 2b alleviates molecular defects in FECD cells. (A) Quantification of the number of r(CUG)exp-MBNL1 foci/nucleus (n = 3, 40 nuclei counted/replicate). (B) Quantification of the effect of 2b on MBNL1 exon 5 pre-mRNA splicing (n = 3). Statistical significance was computed by comparison to untreated cells (“0”). (C) Schematic of retention of TCF4 intron 3 in FECD-affected cells. (D) Quantification of the effect of 2b on TCF4 mature mRNA, as determined by RT-qPCR (n = 3). (E) Quantification of the effect of 2b on intron 3-containing TCF4 levels in FECD cells, compared to WT levels, as determined by RT-qPCR (n = 3). Data are represented as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, as determined by a two-tailed Student t-test (panel A) or a one-way ANOVA (panels B, D, and E). See also Figure S5.
Designer small molecule 2b triggers decay of the intronic r(CUG) repeat expansion via the RNA exosome.
Next, we studied the effect of 2b on the levels of the TCF4 transcript and intron 3, as previous studies have shown that intronic RNA repeat expansions, particularly those that are GC-rich, cause intron retention in the mature mRNAs (Fig. 5C) (Sznajder et al., 2018). In myotonic dystrophy type 2 (DM2), intron retention is due, at least in part, to formation of the r(CCUG)exp-MBNL1 complex (Benhamou et al., 2020). Inhibition of this complex via small molecule treatment triggered decay of the retained intron, but the mechanism of its decay was not explored, that is which enzymes were responsible for the intron’s degradation (Benhamou et al., 2020).
To study the levels of TCF4 in FECD cells, we performed RT-qPCR using primers for mature TCF4 mRNA and primers that bind only within intron 3 to detect all r(CUG)exp-containing intron 3 species, i.e., both intron-retained mRNA species and the excised intron. The levels of mature TCF4 mRNA in FECD cells were not affected upon compound treatment (Fig. 5D), however levels of transcripts containing intron 3 were decreased in a dose-dependent manner (Fig. 5E). In particular, as little as 1 μM of 2b reduced levels of the retained intron, and reversion to levels observed in wild-type cells was nearly achieved at 20 μM (Fig. 5E). Importantly, transcripts containing intron 3 were not affected in wild-type cells (Fig. S5).
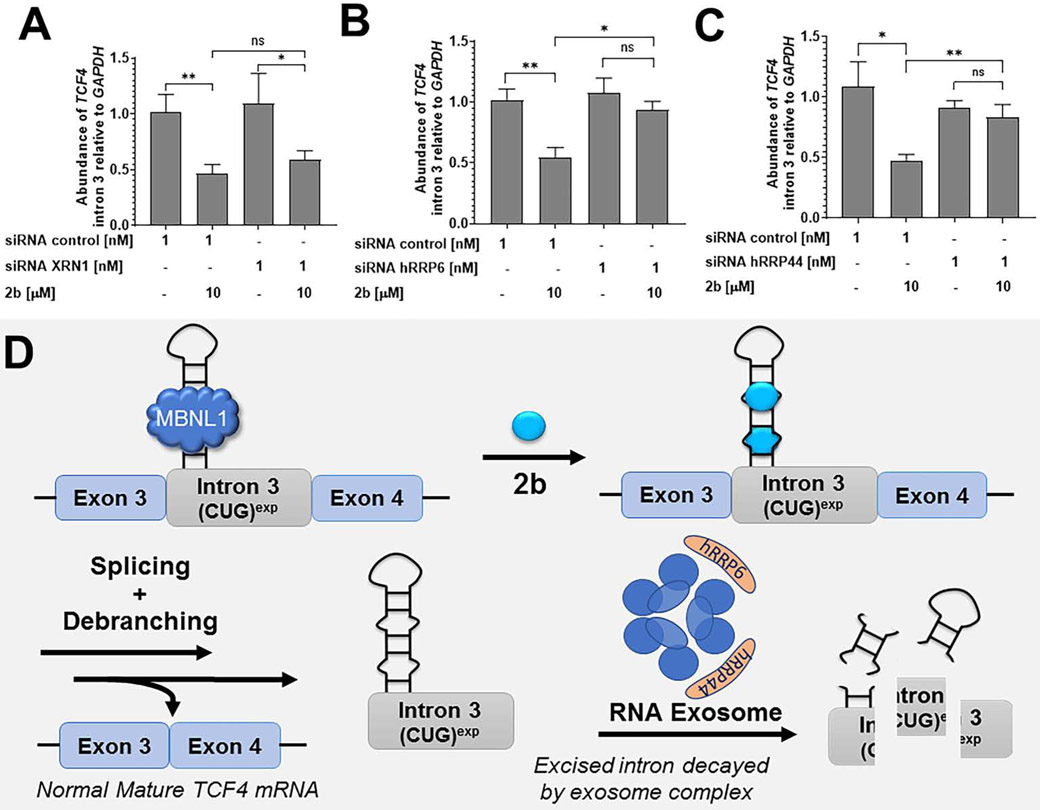
Next, we investigated the mechanism by which 2b decreases levels of intron 3-retaining TCF4 transcripts. Following removal by the spliceosome, introns are processed by debranching enzymes and then degraded by exonucleases. We used an siRNA approach to ablate various exonucleases that may be contributing to the decay of the r(CUG)exp-containing intron. After siRNA knockdown, FECD cells were then treated with 2b; if the exonuclease is contributing to degradation of the intron, 2b would therefore lose its ability to reduce intron 3-containing TCF4 transcripts. We focused our studies on the cytoplasmic 5'-3' exoribonuclease XRN1 and the two catalytic domains of the nuclear RNA exosome (comprised of 11 total subunits) (Garneau et al., 2007), hRRP6 and hRRP44 (Kilchert et al., 2016).
When XRN1 expression was knocked down, no significant change in 2b’s activity to reduce TCF4 intron 3 levels was observed, suggesting that XRN1 is not involved in the degradation of the intron (Fig. 6A and Fig. S6). In contrast, siRNA knock down of hRRP6 and hRRP44 individually both reduced the ability of 2b to decrease the levels of intronic r(CUG)exp. Co-treatment of 2b and a control siRNA did not affect the reduction of intron 3 by 2b (Fig. 6B-C and Fig. S6). Collectively, these results strongly suggest that the native decay of the retained intron triggered by 2b is in part regulated by the nuclear multi-protein intracellular exosome complex (Fig. 6D). Notably, 2b could promote intron decay by facilitating splicing and/or making the intron more accessible to exosomal-mediated degradation by exclusion of MBNL1 or other RNA-binding proteins.
Fig. 6.
Mechanism of r(CUG)exp-containing intron 3 decay in FECD cells. (A) Effect of the siRNA targeting XRN1 in combination with 2b on TCF4 intron 3 levels, as determined by RT-qPCR. Knock-down of XRN1 had no effect on TCF4 intron 3 decay induced by 2b (n = 3). (B) Effect of the siRNA targeting hRRP6 in combination with 2b on TCF4 intron 3 levels, as determined by RT-qPCR (n = 3). Knock-down of hRRP6, a catalytic domain of the exosome complex, decreased TCF4 intron 3 decay generated by 2b. (C) Effect of the siRNA targeting hRRP44 in combination with 2b on TCF4 intron 3 levels, as determined by RT-qPCR (n = 3). Knock-down of hRRP44, a second catalytic domain of the exosome complex, decreased TCF4 intron 3 decay generated by 2b. (D) Proposed decay mechanism, where the exosome complex plays a key role in the decay pathway. Exosome nucleolytic domains are represented in orange including the hRRP6 and hRRP44 subunits. Data are represented as mean ± SD. *P, < 0.05; **P, < 0.01, as determined by a two-tailed Student t-test. See also Figure S6.
Discussion
Implications on the generality of ligands that target RNA structure.
Oligonucleotides, such as, ASOs or siRNAs, are increasingly being used to treat genetic diseases. Their sequence-based recognition is biased for regions in an RNA target that lack structure (Vickers et al., 2000). Because repeating sequences are found throughout the genome, ASOs that target them generally lack selectivity, either through decay by an RNase H-dependent mechanism (Angelbello et al., 2019) or by steric blockade such as with morpholino oligonucleotides (Wheeler et al., 2009). Therefore, ASOs for microsatellite diseases have been developed that recognize sequences unique to each gene, rather than the repeat expansion itself. Thus, a single ASO could not be used for both FECD and DM1, despite sharing a common causative agent, r(CUG)exp.
Small molecule targeting of RNA is still in its relative infancy, and many more studies will be required to truly assess their potential to ameliorate disease pathways and thus serve as therapeutics, not simply chemical probes. Repeat expansions can indeed be targeted in an allele-specific manner by using small molecules that target RNA structure (Angelbello et al., 2019; Rzuczek et al., 2017). This selectivity can be traced at least in part to the differences in structure formed by long toxic repeats, such as r(CUG)exp, and short, non-pathological repeats, which do not generally form stable structures (Angelbello et al., 2019).
Herein, we show that ligands that recognize the toxic structure formed by r(CUG)exp can be applied across diseases to study r(CUG)exp-mediated biology and with potential as treatments for all r(CUG)exp-mediated diseases, provided the compounds could be developed into clinical candidates. There are three known diseases mediated by r(CUG)exp, DM1, FECD, and Huntington’s-like 2 (HDL2) (Margolis and Rudnicki, 2016; Ratovitski et al., 2016). Such an approach would be even more far-reaching for small molecules that bind r(CAG)exp and deactivate disease pathways, as it operates in nearly a dozen different diseases (Paulson, 2018). That is, the overall implications of our studies are that many different diseases caused by the same repeat expansion could be treated with a single ligand and that small molecule mechanisms to alleviate disease biology can be unique to each disease depending on where the repeat expansion is harbored (UTR vs. intron, for example).
Small molecules that target RNA structures can induce targeted degradation.
There is intense interest in targeted degradation as a strategy to ameliorate disease states. Various modalities have been deployed to target biomolecules for degradation, including proteolysis targeting chimeras (PROTACs) for proteins (Coleman and Crews, 2018) and ASOs and ribonuclease targeting chimeras (RIBOTACs) (Costales et al., 2020) for RNAs. All three target biomolecules for destruction by using ligands that possess larger molecular weights and fall outside of Lipinksi guidelines (Lipinski et al., 1997); for PROTACs and RIBOTACs, this is because of their chimeric nature to bind the target and recruit an enzyme.
The mode of action of 2b suggests that intrinsic RNA decay pathways can be harnessed to accelerate the degradation of a disease-causing RNA. Thus, targeted degradation functionality can be accessed by using simple binding ligands with low molecular weights that may be more within the purview of drug-like chemical space. There is a myriad of RNA quality control pathways that can process RNAs in many different manners. It is very likely that specific targeting of RNA structure can facilitate the recognition of RNA by the quality control machinery using very simple ligands such as 2b. Furthermore, the observed selectivity of a small molecule could be even greater than expected if off-target binding occurs in a non-functional site that has no effect on downstream biology (Costales et al., 2017). Thus, although a binding site may not be unique, affecting function and transcript abundance can be selective because only certain transcripts, such as retained repeats in introns, are sensitive to ligand binding.
Structure-binding small molecules can modulate RNA function in various ways, and the mechanistic underpinnings in this study show that one way is through stimulation of exosomal decay. Perhaps, small molecules can stimulate other RNA quality control pathways. Several other diseases are mediated by repeat expansions in retained introns (Sznajder et al., 2018), including DM2 (Benhamou et al., 2020) and c9ALS/FTD (Niblock et al., 2016). As such, it will be interesting to see if the same pathway can be activated with ligands that target these repeat expansions. If so, these targets may be more druggable than they perhaps appear.
STAR Methods:
RESOURCE AVAILABILITY
Lead Contact:
All requests for additional information and reagents should be directed to the Lead Contact, Matthew D. Disney (disney@scripps.edu).
Materials Availability:
This study did not generate new unique reagents.
Data and Code Availability:
This study did not generate any data sets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines:
Compounds were tested in DM1 patient-derived fibroblasts with 500 CUG repeats (GM03987: male, 39 years old at sampling; Coriell Institute) and wild-type fibroblasts (GM07492: male, 17 years old at sampling; Coriell Institute). Compounds were also tested in two cell lines that can be differentiated into myotubes (Arandel et al., 2017): (i) a DM1 (1300 CUG repeats) conditional MyoD-fibroblast cell line (female, 11 years old at sampling) and (ii) a wild-type conditional MyoD-fibroblast cell line (male, 25 years old at sampling) (generous gifts from D. Furling; Centre de Recherche en Myologie (UPMC/Inserm/CNRS), Institut de Myologie, Paris, France). For FECD studies, compounds were tested in cell lines that were telomerase transformed from Fuchs Endothelial Corneal Dystrophy (FECD) endothelial cells obtained from patients at the time of corneal transplant surgery (under Johns Hopkins University IRB approved protocol (NA_00023119). The F35T (disease model) cell line has a mutant allele containing ~4500 r(CUG) repeats and a wild-type allele with 21 r(CUG) repeats in length (female, 62 years old at sampling). The F20T (WT) cell line has two alleles measuring the same length of 15 r(CUG) repeats (male, 54 years old at sampling). Triplet repeats in this region of TCF4 greater than 50 (i.e. 150 bp long) are considered pathogenic for FECD.
Cell culture maintenance:
All cells were maintained at 37 °C with 5% CO2. DM1 fibroblasts and wild-type fibroblasts were maintained in growth medium [1× MEM (Corning), 10% FBS (Sigma), 1% MEM non-essential amino acids (Corning), 1% antibiotic/antimycotic solution (Corning), and 1% Glutagro (Corning)], and cells were treated with compound dissolved in growth medium. Conditional MyoD-fibroblast cell lines (Arandel et al., 2017) were grown in growth medium [1× DMEM (Corning), 10% FBS, 1% antibiotic/antimycotic solution, and 1% glutagro]. For FECD studies, F35T and F20T cells were grown in growth medium [1× OptiMEM-1 (Gibco), 5 ng/ml EGF (Human EGF Cat# J64012), 20 ng/ml NGF (Invitrogen Cat# 13257-019), 20 μg/ml ascorbic acid, 200 mg/l calcium chloride, 0.08% chondroitin sulfate, 50 μg/ml gentamicin, 1% antibiotic/antimycotic solution (Corning), and 8% FBS].
Mouse model of DM1:
All experimental procedures, mouse handling, and husbandry were completed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care. Mice were maintained in standard housing conditions with nesting material and were allowed free access to dry food and water. Mice were housed according to treatment group, except for male FVB mice where vehicle- and 2b-treated mice were housed together.
A mouse model for DM1, HSALR in line 20b (Mankodi et al., 2000), was used to test compound efficacy. HSALR mice express human skeletal actin RNA with 220 r(CUG) repeats in the 3’ UTR. HSALR mice were maintained as homozygotes on an FVB inbred background. Wild-type (FVB/NJ) were purchased from the Jackson Laboratory. For both studies, littermates of the same sex were randomly assigned to experimental groups. For HSALR studies, 4 mice (4 males, 8 weeks old at the beginning of the study) were assigned to each group. On average, HSALR mice weighed 28 g at the beginning of the study. For FVB studies, 3 mice were assigned to each group (2 females, 1 male, 8 weeks old at the beginning of the study). On average, male FVB mice weighed 24 g at the beginning of the study, and female mice weighed 20 g at the beginning of the study.
METHOD DETAILS
TR-FRET assay to measure r(CUG)exp-MBNL1 complex inhibition in vitro:
In vitro activity of compounds was assessed by inhibition of r(CUG)exp-MBNL1 complex formation using a previously reported TR-FRET assay (Chen et al., 2012; Parkesh et al., 2012). Briefly, biotinylated r(CUG)12 (final concentration 80 nM) was folded in 1× Folding Buffer (20 mM HEPES, pH 7.5, 100 mM KCl, and 10 mM NaCl) at 60 °C for 5 min and slowly cooled to room temperature. The buffer was then adjusted to 1× TR-FRET Buffer (1× Folding Buffer supplemented with 2 mM MgCl2, 2 mM CaCl2, 5 mM DTT, 0.1% BSA, 0.05% Tween-20), and the small molecules were added. The solutions were incubated for 1 h at room temperature followed by addition of MBNL1-His6 (60 nM final concentration). The samples were incubated for 15 min, after which 1 μL of antibody solution (1:1 mixture of 8.8 ng/μl Anti-His6-Tb and 800 nM streptavidin XL-665) was added such that the final volume of each sample was 10 μL. The samples were incubated for an additional 15 min at room temperature, followed by measuring the fluorescence of Tb and the FRET between TB and XL-665. The ratio of fluorescence intensity of 545 and 665 nm as compared to the ratios in the absence of small molecule and in the absence of RNA were used to calculate percent inhibition. The resulting curves were fit to equation 1 to determine IC50 values:
| (Eq. 1) |
where y is ratio of fluorescence intensities at 545 nm and 665 nm (F545/F665), x is the concentration of small molecule, B is the maximum observable FRET signal (F545/F665; sample containing RNA and protein but no small molecule added); A is the minimum observable FRET signal (F545/F665; sample containing antibodies but no RNA, protein, or small molecule); and the IC50 is the concentration of small molecule where half of the protein is inhibited from binding r(CUG)12 by small molecule.
Microscale thermophoresis (MST) (Li and Disney, 2018):
MST measurements were performed on a Monolith NT.115 system (NanoTemperTechnologies) with Cy5-labeled AT-rich DNA hairpin (5’-Cy5-CGCGAATTCGCGTTTTCGCGAATTCGCG; IDT), Cy5-labeled r(CUG)12 (5’-Cy5-GCG(CUG)12CGC; Dharmacon), Cy5-labeled r(G4C2)8 (Dharmacon), Cy5-labeled r(CAG)12 (5’-Cy5-GCG(CAG)12CGC; Dharmacon),and Cy5-labeled base pair control (BP) (5’-Cy5-GCG(CUG)6(CAG)6CGC; Dharmacon), which were deprotected according to the manufacturer’s protocol. RNA or DNA (5 nM) was prepared in 1× MST Buffer (8 mM Na2HPO4, 185 mM NaCl, 1mM EDTA) and folded by heating at 60 °C for 5 min and slowly cooling to room temperature. After cooling, Tween-20 was added to a final concentration of 0.05% (v/v). Compound 2b was then added to a final concentration of 5 μM, followed by 1:1 dilutions in 10 μL of 1× MST Buffer. Nucleic acid and compound were then mixed 1:1 (v/v). Samples were incubated for 30 min at room temperature and then loaded into premium capillaries (NanoTemper Technologies). The following parameters were used: 5 – 20 % LED, 80% MST power, Laser-On time = 30 s, Laser-Off time = 5 s. Fluorescence was detected using excitation wavelengths of 605–645 nm and emission wavelengths of 680–685 nm. The resulting data were analyzed by thermophoresis analysis and fitted by quadratic binding equation in MST analysis software (NanoTemper Technologies). The dissociation constant was then determined using Equation 2. The reported Kd values are an average of three independent set of experiments.
| (Eq. 2) |
Computational modeling methods:
To study the binding of 2b to r(CUG)exp, a previously reported methodology was employed (Childs-Disney et al., 2014). Briefly, a model RNA duplex, r(5’-CCGCUGCGG-3’)2, was generated using the nucgen module of AMBER 16 (D.A. Case et al., 2016). Amber99 (Cornell et al., 1995) force field with revised χ (Yildirim et al., 2010) and α/γ torsional parameters taken from the parmbsc0 force field (Pérez et al., 2007) was utilized to describe the RNA. Watson-Crick (WC) base pairing, torsional, and chirality restraints were additionally used to maintain the A-form geometry whenever necessary. Generalized Amber Force Field (GAFF) (Wang et al., 2004) was used to describe 2b. To calculate 2b’s resp charges, Gaussian09 (Frisch et al., 2005) was utilized to first optimize and then calculate the molecular electrostatic potential (MEP). Furthermore, potential energy surface (PES) scans were performed on several torsions of 2b to better describe its structural properties.
The bound states of the 2b-RNA complex were investigated using the dynamic docking methodology, which was successfully employed in our previous studies (Childs-Disney et al., 2014). The details of the binding methodology is as follows: All simulations were conducted using the Amber 16 MD package under the Generalized Born implicit solvent model (Onufriev et al., 2004). First, we placed the compound 40 Å away from the UU internal loop. The distance between the center-of-mass (COM) of the heavy atoms of 2b and the COM of the heavy atoms of the loop closing base pairs was used as the reaction coordinate for conducting the dynamic binding approach. The compound was gradually moved towards the internal loop along the reaction coordinate by decrements of 1 Å until the distance reached 0 Å. Then, compound was moved away from the loop along the reaction coordinate by increments of 1 Å until the distance was 40 Å. While the compound was moving towards the UU internal loop, WC base pairing, torsional, and chirality restraints were imposed on the RNA residues except the loop residues to maintain the A-form geometry. This restraint set was called ‘flex-restraints’ that allowed the loop residues to move naturally while interacting with the compound. While the compound was moved away from the loop region, WC base pairing, torsional, and chirality restraints were imposed on all the RNA residues to transform the RNA structure back to its apo form as observed by NMR and X-ray spectroscopy (Berman et al., 2000; Chang et al., 2008; Chapman et al., 1978; Desaulniers et al., 2005; Geraldes et al., 1982; Hart, 1978; Richarz and Wüthrich, 1978; Rosemeyer et al., 1990; Santos et al., 1983; Sierzputowska-Gracz et al., 1991). We repeated this process 50 times sequentially to produce 50 initial bound states of the 2b-RNA complex. These initial bound states were then utilized to conduct implicit-solvent MD simulations.
Modified ‘flex-restraints’ were imposed on the system in the MD simulations where an extra restraint along the reaction coordinate was added to ensure 2b did not leave the binding site. Each MD simulation was run for 120 ns yielding a total of 6 μs combined MD trajectory, which was used in the cluster analysis. An in-house code was used to calculate the root-mean-square deviation (RMSD) throughout the trajectory and to cluster snapshots having RMSD <= 1.0 Å. MM-PBSA approach was then utilized on clusters having more than 100 snapshots to predict the binding free energies (Miller et al., 2012). Lowest binding free energy structure is displayed in Figures 2B and S1. The structures were visualized and the distance between the guanidines was measured using UCSF Chimera (Pettersen et al., 2004).
Compound treatment:
DM1 and wild-type fibroblasts were treated with compound in growth medium. Conditional MyoD-fibroblasts (~80% confluency) were treated with compound and differentiated in differentiation medium [1× DMEM, 1% antibiotic/antimycotic solution, 0.1 mg/mL iron-transferrin, 0.01 mg/mL insulin (Life Technologies), and 2 μg/mL doxycycline (Sigma)]. For FECD studies, F35T and F20T cells were treated with compound dissolved in growth medium.
siRNA constructs and transfection:
All siRNA constructs were purchased from Dharmacon. The siRNA control is an ON-TARGETplus Non-targeting siRNA #4 (cat: #D-001810-04-05); the siRNA for XRN1 is an ON-TARGETplus Human XRN1 #54464 (cat: #J-013754-11- 0002); the siRNA for hRRP44 is an ON-TARGETplus Human DIS3 (cat: # J-015405-11- 0002), and the siRNA hRRP6 was designed as previously reported (Sense: 5'-G.C.A.A.A.A.U.C.U.G.A.A.A.C.U.U.U.C.C.dT.dT-3’ and Antisense: 5'-G.G.A.A.A.G.U.U.U.C.A.G.A.U.U.U.U.G.C.dT.dT-3') (Kammler et al., 2008). The siRNAs were transfected using Lipofectamine RNAiMAX Reagent (Invitrogen) according to the manufacturer’s protocol.
Toxicity analysis:
DM1 fibroblasts were plated in a 96-well plate and treated with compound as described above. After 48 h, medium was removed, and cells were washed once with 1× PBS followed by the addition of 50 μL of growth medium containing 10% WST-1 reagent (Roche). Cells were incubated for 30 min at 37 °C, and then absorbance was measured at 450 nm (signal) and 690 nm (background) using a Molecular Devices SpectraMax M5 plate reader. The corrected absorbance was used to determine cell viability relative to untreated cells.
FECD (F35T) were plated in a 96-well plate and treated with 2b as described above. After 24 h, cell viability was measured using CellTiter-Glo 2.0 (G9242, Promega Corp) per the manufacturer’s protocol. Briefly, 100 μL of CellTiter-Glo was added to each well, the plate was then incubated for 10 min at room temperature, and luminescence was measured using a Molecular Devices SpectraMax M5 plate reader.
Analysis of pre-mRNA splicing:
Rescue of disease-associated splicing defects by a small molecule was completed as previously described (Rzuczek et al., 2017). Briefly, cells were plated in 12-well plates and treated with compound for 48 h as described above. After 48 h, the cells were lysed, and total RNA was harvested using a Zymo Quick RNA Miniprep Kit per the manufacturer’s recommended protocol. Approximately 200 ng of total RNA was reverse transcribed with 100 units of Superscript III reverse transcriptase (Life Technologies) at 50 °C or qScript cDNA synthesis kit (20 mL total reaction volume, Quanta BioSciences) per the manufacturers’ recommended protocols. Next, 2 μL of the RT reaction was subjected to PCR using GoTaq DNA polymerase (Promega). RT-PCR products were observed after 30 cycles of 95 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min and a final extension at 72 °C for 5 min. Products were separated on a 2% agarose gel run at 100 V for 1 h in 1× TBE buffer, visualized by staining with ethidium bromide, and imaged using a Typhoon 9410 variable mode imager. Gels were quantified using ImageJ.
Evaluation of r(CUG)exp-MBNL1 foci:
RNA-FISH to image nuclear foci was completed as previously described (Rzuczek et al., 2017). Briefly, cells were grown in a MatTek Poly-D-Lysine coated 96-well glass bottom plate and treated as described above. After 48 h, cells were washed with 1× DPBS then fixed with 100 μL of 4% paraformaldehyde in 1× DPBS for 10 min at 37 °C. The cells were washed five times with 1× DPBS at 37 °C for 2 min and then permeabilized by washing twice with 0.1% Triton X-100 in 1× DPBS for 5 min at 37 °C. Cells were then incubated with 100 μL of 30% formamide in 2× SSC for 10 min at room temperature. Then, 1 ng/μL DY547-2'OMe-(CAG)6 was added in 30% formamide in 2× SSC containing 2 μg/μL BSA, 1 μg/μL tRNA, and 2 mM vanadyl complex, and the cells were incubated at 37 °C overnight. The cells were washed with 2× SSC for 30 min at 37 °C, and then immunostaining of MBNL1 was completed as previously described using anti-MBNL1 (EMD Millipore, #MABE70; diluted 1:50) in 2× SSC and incubating at 37 °C for 1 h. Cells were washed once with 0.1% Triton X-100 in 1× DPBS, then incubated with goat anti-mouse IgG-DyLight 488 conjugate (1:200 dilution) in 2× SSC for 1 h at 37 °C. Cells were then washed three times with 0.1% Triton X-100 in 1× DPBS and twice with 1× DPBS. Nuclei were stained using a 1 μg/μL solution of DAPI in 1× DPBS for 5 min at 37 °C. Cells were then washed three times with 1× DPBS and imaged in 1× DPBS using an Olympus FluoView 1000 confocal microscope at 100× magnification. The number of r(CUG)exp-MBNL1 foci were counted in 40 nuclei/replicate (120 total nuclei counted over three independent samples).
RT-qPCR analysis:
Levels of DMPK, TCF4, EXOSC10, and XRN1 mRNAs were measured by RT-qPCR as follows. Cells were plated in 12-well plates and treated as described above. After 48 h, total RNA was extracted, and reverse transcription was performed as described in “Analysis of pre-mRNA splicing”. A 2 μL aliquot of the RT reaction was used for each primer pair for qPCR (see Table S1) with SYBR Green Master Mix (Life Technologies) performed on a QuantStudio 5, 384-well Block Real-Time PCR System (Applied Biosciences). Relative abundance of each transcript was determined by normalizing to GAPDH.
Target occupancy by using C-Chem-CLIP:
Target binding of small molecules was assessed using DM1 patient-derived fibroblasts. Cells were grown as monolayers in 100 mm2 dishes in growth medium. Once cells were ~80% confluent, they were treated with growth medium containing 2b or 3b for 6 h. Then, 2H-K4NMeS-CA-Biotin (100 nM final concentration) was added to the growth medium. After 48 h, the cells were lysed and total RNA was harvested using Trizol reagent (Life Technologies). Approximately 10 μg of total RNA was incubated with streptavidin-agarose beads (100 μL, >15 μg/mL binding capacity; Sigma) for 1 h at room temperature. Then the beads were washed with 1× PBS, and the bound RNA was eluted by adding 100 μL of 95% formamide containing 10 mM EDTA, pH 8.2 for 20 min at 60 °C. The eluted RNA was cleaned up using a Zymo Quick RNA miniprep kit per the manufacturer’s recommend protocol. Approximately 100 ng of RNA was reverse transcribed using a qScript cDNA synthesis kit (10 μL total reaction volume, Quanta BioSciences); 1 μL of the RT reaction was used with each primer set for qPCR with SYBR Green Master Mix (Life Technologies) performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Relative abundance was determined by normalizing to GAPDH.
Evaluation of 2b in HSALR and FVB mice:
Age- and gender-matched HSALR or FVB mice were injected intraperitoneally with either 40 mg/kg of 2b in water for treatment or the same volume of 0.9% NaCl for vehicle-treated mice once per day for 7 days. Mice were weighed daily, and weight did not significantly fluctuate over the course of treatment. Mice were euthanized one day after the last injection, and the quadriceps and gastrocnemius muscle were harvested. RNA was extracted as previous described (Wheeler et al., 2009). Briefly, muscles were homogenized in TRIzol (ThermoFisher) and RNA was extracted per the manufacturer’s recommended protocol. Approximately 1 μg of total RNA was reverse transcribed with 100 units of Superscript III reverse transcriptase (Life Technologies) at 50 °C. Next, 2 μL of the RT reaction was subjected to PCR using GoTaq DNA polymerase (Promega). RT-PCR products were observed after 30 cycles of 95 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min and a final extension at 72 °C for 5 min. Products were separated on a 2% agarose gel run at 100 V for 1 h in 1× TBE buffer, visualized by staining with ethidium bromide, and imaged using a Typhoon 9410 variable mode imager. Gels were quantified using ImageJ.
Synthetic Methods:
Microwave reactions were carried out using a Biotage Initiator+ SP Wave microwave. Compounds were purified by preparative reverse phase HPLC using a Waters 1525 Binary HPLC pump equipped with a Waters 2487 dual absorbance detector system and a Waters Sunfire C18 OBD 5 μm 19 x 150 mm column. Absorbance was monitored at 345 and 220 nm. A gradient of 0-100% MeOH in H2O with 0.1% TFA over 60 min was used for compound purification. Purity was assessed by analytical HPLC using a Waters Symmetry C18 5 μm 4.6 x 150 mm column. Small molecules were analyzed using a gradient of 0-100% methanol in water with 0.1% TFA over 60 min. 1H NMR spectra were collected using a Bruker 400 MHz NMR, while 13C NMR spectra were collected on a Bruker 600 MHz NMR. High resolution mass spectrometry (HR-MS) was completed on an Applied Biosystems MALDI ToF Analyzer 4800 Plus using an α-cyano-4-hydroxycinnamic acid matrix with calibration standards. All compounds evaluated had ≥95% purity.
Synthesis of 1a and 1b.
Compound 1 (10 mg, 16.4 μmol) (Wilson et al., 2010) was suspended in a mixture of water (1.50 mL) and ethanol (300 μL). Then cyanamide (50 mg, 1.2 mmol) and nitric acid (5 drops) were added, and the reaction mixture was heated to 120 °C via a microwave for 6 h. Then, the reaction mixture was diluted in water and purified by preparative reverse phase HPLC as described in the General Methods, affording 2.56 μmoles of 1a (16%, light brown solid) and 467 nmoles of 1b (3%, light brown solid).
1a: 1H NMR (400 MHz, CD3OD): δ 8.10 (m, 2H), 8.02 (d, 1H, J = 1), 7.98 (d, 1H, J = 2), 7.95 (d, 1H, J = 1), 7.93 (d, 1H, J = 2), 7.88 (d, 1H, J = 9), 7.81 (d, 1H, J = 8), 7.72 (m, 2H), 7.50 (m, 1H), 7.41 (m, 3H), 7.07 (m, 1H). HRMS (m/z): [M]+ calcd. for C27H22N8, 459.2040; found, 459.1970.
1b: 1H NMR (400 mHz, CD3OD): δ 8.10 (m, 4H), 7.97 (s, 2H), 7.80 (m, 2H), 7.74 (m, 4H), 7.52 (m, 2H). 13C NMR (600 mHz, CD3OD): δ 158.63, 138.02, 132.64, 130.13, 127.25, 126.64, 125.28, 118.43, 117.05, 116.52, 116.05, 1114.10. HRMS (m/z): [M]+ calcd. for C28H24N10, 501.2258; found, 501.2247.
Synthesis of 2a and 2b.
Compound 2 (10 mg, 16.4 μmol) (Wilson et al., 2010), was suspended in a mixture of water (1.5 mL) and ethanol (300 μL). Then, cyanamide (50 mg, 1.2 mmol) and nitric acid (5 drops) were added, and the reaction mixture was refluxed overnight. Afterwards, the reaction mixture was diluted in water and purified by preparative reverse phase HPLC as described in the General Methods, affording 1.07 μmoles of 2a (7%, light brown solid) and 161 nmoles of 2b (1%, light brown solid).
2a 1H NMR (400 mHz, CD3OD): δ 8.03-8.01 (m, 2H), 7.94-7.79 (m, 10H), 6.86 (d, 2H, J = 9). HRMS (m/z): [M]+ calcd. for C27H22N8, 459.2040; found, 459.1930.
2b 1H NMR (400 mHz, CD3OD): δ 8.25 (d, 4H, J = 9), 8.05 (s, 2H), 7.88 (s, 4H) 7.59 (d, 4H, J = 9). 13C NMR (600 mHz, CD3OD): δ 157.82, 151.77, 140.80, 139.74, 136.91, 135.77, 130.27, 126.43, 125.99, 124.86, 119.09, 117.72, 115.92, 113.89. HRMS (m/z): [M]+ calcd. for C28H24N10, 501.2258; found, 501.2231.
Synthesis of 3b.
2,2′-p-Phenylene-bis(5-aminobenzimidazole) (3) was synthesized as previously described (Liu et al., 2012), and 150 mg (0.44 mmol) was suspended in water (1.5 mL)and ethanol (0.5 mL). Cyanamide (730 mg, 17.6 mmol) was added followed by 6 drops of concentrated nitric acid. The reaction was then placed in a Biotage Initiator+ SP Wave microwave at 130°C for 6 h. Next, the reaction mixture was diluted with water and purified by preparative reverse phase HPLC as described in the “General Methods” section, affording 50 mg of the desired product (27%, yellow solid). 1H NMR (400 mHz, CD3OD): δ 8.36 (s, 4H), 7.79 (d, 2H, J = 9), 7.66 (d, 2H, J = 2), 7.33 (dd, 2H, J = 2, 9). 13C NMR (600 mHz, CD3OD): δ 158.61, 152.86, 138.98, 137.17, 132.73, 131.16, 124.09, 117.05, 113.97. HRMS (m/z): [M]+ calcd. for C22H21N10, 425.1945; found, 425.1849.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data are reported as mean ± SD, unless noted otherwise. The number of replicates for each experiment is indicated in the figure legends and was always at least n = 3. Data were plotted and statistical significance (one-way ANOVA or t-test) was calculated using GraphPad Prism 7 software. Details are provided in the figure legends.
Supplementary Material
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-His6-Tb | Cisbio | CAT# 61HISTLF |
| Anti-MBNL1 | EMD Millipore Corporation | CAT# MABE70 |
| Anti-mouse IgG AlexaFluor488 | Thermo Scientific Pierce | CAT# 7074 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Streptavidin XL-665 | Cisbio | CAT# 610SAXLF |
| MBNL1-His6 protein | Expressed as described in (Chen et al., 2012) | N/A |
| Critical Commercial Assays | ||
| qScript cDNA synthesis | Quanta BioSciences | CAT# 101414-100 |
| Quick-RNA Miniprep Kit | Zymo | CAT# R1055 |
| TRIzol Reagent | Thermo Fisher Scientific | CAT# 15596026 |
| WST-1 Reagent | Roche | CAT# 5015944001 |
| CellTiter-Glo 2.0 | Promega | CAT# G9242 |
| SSIII reverse transcriptase | Life Technologies | CAT# 18080085 |
| GoTaq DNA polymerase | Promega | CAT# M8295 |
| SYBR Green Master Mix | Life Technologies | CAT# 4368708 |
| Experimental Models: Cell Lines | ||
| DM1 Fibroblasts | Coriell Institute | GM03987 |
| Healthy Fibroblasts | Coriell Institute | GM07492 |
| DM1 conditional MyoD fibroblasts | Arandel et al., 2017 | N/A |
| Healthy conditional MyoD fibroblasts | Arandel et al., 2017 | N/A |
| F35T (FECD patient-derived corneal endothelial cells) | Albert S. Jun, M.D., Ph.D.; Wilmer Eye Institute, Johns Hopkins Medical Institutions | N/A |
| F20T (wild-type corneal endothelial cells) | Albert S. Jun, M.D., Ph.D.; Wilmer Eye Institute, Johns Hopkins Medical Institutions | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: HSA LR20b | Mankodi et al., 2000 | N/A |
| Mouse: FVB/NJ | The Jackson Laboratory | 001800 |
| Oligonucleotides | ||
| 5’-Biotin-GCG(CUG)12-CGC | Dharmacon | N/A |
| Control siRNA (ON-TARGET plus) | Dharmacon | CAT# D-001810-04-05 |
| siRNA-XRN1 (ON-TARGET plus) | Dharmacon | CAT# J-013754-11-0002 |
| siRNA-DIS3 (ON-TARGET plus) | Dharmacon | CAT# J-015405-110002 |
| siRNA-hRRP6 | Dharmacon (Kammler et al., 2008) | N/A |
| Primers used for RT-qPCR, see Table S1 | Integrated DNA Technologies | N/A |
| 5’DY547-2’OMe-(CAG)6 | Integrated DNA Technologies | N/A |
| 5’-Cy5-GCG(CUG)12-CGC | Dharmacon | N/A |
| 5’-Cy5-GCG(CAG)12-CGC | Dharmacon | N/A |
| 5’-Cy5-GCG(CUG)6(CAG)6-CGC | Dharmacon | N/A |
| 5’-Cy5-r(G4C2)8 | Dharmacon | N/A |
| 5’-Cy5-CGCGAATTCGCGTTTTCGCGAATTCGCG | Integrated DNA Technologies | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/download.html |
| Olympus Fluoview Software Version 3.0 | Olympus | http://www.olympuscanada.com/cpg_section/cpg_support_downloads.asp?id=1274 |
| GraphPad Prism 7 | GraphPad Prism Software, Inc. | https://www.graphpad.com/scientific-software/prism/ |
| Nucgen module of AMBER 16 | AMBER 2016 | https://ambermd.org/GetAmber.php |
Significance: RNA repeat expansions cause greater than 40 diseases, each with unique pathological mechanisms depending on the location of the RNA repeat within a gene. A common way to target RNA is with oligonucleotide-based modalities, however, they must be customized for each disease by nature of targeting the gene that harbors the repeat, rather than targeting the repeat itself. Herein, we describe the design of a small molecule that binds the structure of a r(CUG) repeat expansion [r(CUG)exp] and reverses molecular defects in two diseases mediated by the same RNA repeat - myotonic dystrophy type 1 (DM1) and Fuchs endothelial corneal dystrophy (FECD). Thus, a single structure-specific ligand has potential therapeutic benefit for multiple diseases. Indeed, the small molecule binds the target with nanomolar affinity and >100-fold specificity vs. many other RNAs and DNA. In DM1, r(CUG)exp is in the 3’ UTR and the compound has no effect on mRNA abundance. In contrast, in FECD, r(CUG)exp is retained in an intron, and the compound facilitates removal of the intron which is then degraded by the nuclear RNA exosome. Therefore, a small molecule binder can stimulate RNA quality control pathways to degrade disease-causing RNAs. This approach can be effective for targeting other disease-causing RNAs, as several other RNA repeat expansions are harbored in retained introns.
HIGHLIGHTS.
Design of a small molecule that selectively binds the structure of r(CUG)exp
The small molecule improves defects in two r(CUG)exp-mediated diseases
Small molecule binding to RNA repeats located in introns trigger native decay
Decay of the repeat-containing intron is mediated by the nuclear RNA exosome
Acknowledgements.
We thank J. Childs-Disney for experimental advice and Prof. Denis Furling [Centre de Recherche en Myologie (UPMC/Inserm/CNRS), Institut de Myologie] for his generous gift of myotube cell lines used in this paper. We also thank the agencies that funded this work including the National Institutes of Health (DP1-NS096898 and R35 NS116846 to M.D.D., F31 NS110269 to A.J.A., and P50-NS048843 to C.A.T.), the Muscular Dystrophy Association (grant 380467 to M.D.D.), the Myotonic US Fellowship Research grant (to R.I.B. and S.C.) and to the National Ataxia Foundation fellowship research grant (to R.I.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests. M.D.D. is a founder of Expansion Therapeutics, and S.G.R. is currently an employee of Expansion Therapeutics. M.D.D. and S.G.R. also have a patent related to this work (US20190152924A1).
Supplemental Information
Supplemental Information includes six figures and one table and can be found with this article online at: [to be filled in]
References:
- Angelbello AJ, Rzuczek SG, Mckee KK, Chen JL, Olafson H, Cameron MD, Moss WN, Wang ET, and Disney MD (2019). Precise small-molecule cleavage of an r(CUG) repeat expansion in a myotonic dystrophy mouse model. Proc. Natl. Acad. Sci. U. S. A 116, 7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandel L, Polay Espinoza M, Matloka M, Bazinet A, De Dea Diniz D, Naouar N, Rau F, Jollet A, Edom-Vovard F, Mamchaoui K, et al. (2017). Immortalized human myotonic dystrophy muscle cell lines to assess therapeutic compounds. Dis. Models Mech 10, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou RI, Angelbello AJ, Wang ET, and Disney MD (2020). A toxic RNA catalyzes the cellular synthesis of Its own inhibitor, shunting it to endogenous decay pathways. Cell Chem. Biol 27, 223–231.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, and Bourne PE (2000). The Protein Data Bank. Nucleic Acids Res. 28, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion J-P, Hudson T, et al. (1992). Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808. [DOI] [PubMed] [Google Scholar]
- Chang Y-C, Herath J, Wang THH, and Chow CS (2008). Synthesis and solution conformation studies of 3-substituted uridine and pseudouridine derivatives. Bioorg. Med. Chem 16, 2676–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman GE, Abercrombie BD, Cary PD, and Bradbury EM (1978). The measurement of small nuclear overhauser effects in the 1H spectra of proteins, and their application to lysozyme. J. Magn. Reson. (1969) 31, 459–469. [Google Scholar]
- Chen CZ, Sobczak K, Hoskins J, Southall N, Marugan JJ, Zheng W, Thornton CA, and Austin CP (2012). Two high-throughput screening assays for aberrant RNA–protein interactions in myotonic dystrophy type 1. Anal. Bioanal. Chem 402, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs-Disney JL, Yildirim I, Park H, Lohman JR, Guan L, Tran T, Sarkar P, Schatz GC, and Disney MD (2014). Structure of the myotonic dystrophy type 2 RNA and designed small molecules that reduce toxicity. ACS Chem. Biol 9, 538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KG, and Crews CM (2018). Proteolysis-targeting chimeras: harnessing the ubiquitin-proteasome system to induce degradation of specific target proteins. Annu. Rev. Cancer Biol 2, 41–58. [Google Scholar]
- Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, and Kollman PA (1995). A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc 117, 5179–5197. [Google Scholar]
- Costales MG, Aikawa H, Li Y, Childs-Disney JL, Abegg D, Hoch DG, Pradeep Velagapudi S, Nakai Y, Khan T, Wang KW, et al. (2020). Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl. Acad. Sci. U. S. A 117, 2406–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costales MG, Haga CL, Velagapudi SP, Childs-Disney JL, Phinney DG, and Disney MD (2017). Small molecule inhibition of microRNA-210 reprograms an oncogenic hypoxic circuit. J. Am. Chem. Soc 139, 3446–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DA, R.M.B., Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Giese TJ, Gohlke H,, Goetz AW, N.H., Izadi S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Lin C, T.L., Luo R, Madej B, Mermelstein D, Merz KM, Monard G, Nguyen H, Nguyen HT, Omelyan I, A.O., Roe DR, Roitberg A, Sagui C, Simmerling CL, Botello-Smith WM, Swails J,, and Walker RC, J.W., Wolf RM, Wu X, Xiao L and Kollman PA (2016). AMBER 2016. University of California, San Francisco. [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaulniers J-P, Chui HMP, and Chow CS (2005). Solution conformations of two naturally occurring RNA nucleosides: 3-Methyluridine and 3-methylpseudouridine. Bioorg. Med. Chem 13, 6777–6781. [DOI] [PubMed] [Google Scholar]
- Disney MD (2019). Targeting RNA with small molecules to capture opportunities at the Intersection of chemistry, biology, and medicine. J. Am. Chem. Soc 141, 6776–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Aleff RA, Soragni E, Kalari K, Nie J, Tang X, Davila J, Kocher J-P, Patel SV, Gottesfeld JM, et al. (2015). RNA toxicity and missplicing in the common eye disease Fuchs endothelial corneal dystrophy. J. Biol. Chem 290, 5979–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, and Petersson G (2005). Gaussian 09; Gaussian, Inc: Wallingford, CT, 2009. [Google Scholar]
- Garneau NL, Wilusz J, and Wilusz CJ (2007). The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol 8, 113–126. [DOI] [PubMed] [Google Scholar]
- Gates DP, Coonrod LA, and Berglund JA (2011). Autoregulated splicing of muscleblind-like 1 (MBNL1) pre-mRNA. J. Biol. Chem 286, 34224–34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes CFGC, Santos H, and Xavier AV (1982). A proton relaxation study of the conformations of some purine mononucleotides in aqueous solution. Can. J. Chem 60, 2976–2983. [Google Scholar]
- The Huntington's Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983. [DOI] [PubMed] [Google Scholar]
- Hale MA, Johnson NE, and Berglund JA (2019). Repeat-associated RNA structure and aberrant splicing. BBA-Gene Regul. Mech 1862, 194405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PA (1978). Conformation of mononucleotides and dinucleoside monophosphates. P[H] and H[H] nuclear Overhauser effects. Biophys. J 24, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi AH, Fu Y, Miller KA, Nguyen L, Luu LM, Baranger AM, and Zimmerman SC (2013). Developing bivalent ligands to target CUG triplet repeats, the causative agent of myotonic dystrophy type 1. J. Med. Chem 56, 9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauvin D, Chrétien J, Pandey SK, Martineau L, Revillod L, Bassez G, Lachon A, McLeod AR, Gourdon G, Wheeler TM, et al. (2017). Targeting DMPK with antisense oligonucleotide improves muscle strength in myotonic dystrophy type 1 mice. Mol. Ther. Nucleic Acids 7, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Mankodi A, Swanson MS, Moxley RT, and Thornton CA (2004). Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Human Mol. Genet 13, 3079–3088. [DOI] [PubMed] [Google Scholar]
- Kammler S, Lykke-Andersen S, and Jensen TH (2008). The RNA exosome component hRrp6 is a target for 5-fluorouracil in human cells. Mol. Cancer Res 6, 990. [DOI] [PubMed] [Google Scholar]
- Kilchert C, Wittmann S, and Vasiljeva L (2016). The regulation and functions of the nuclear RNA exosome complex. Nat. Rev. Mol. Cell Biol 17, 227–239. [DOI] [PubMed] [Google Scholar]
- Li J, Nakamori M, Matsumoto J, Murata A, Dohno C, Kiliszek A, Taylor K, Sobczak K, and Nakatani K (2018). A dimeric 2,9-diamino-1,10-phenanthroline derivative improves alternative splicing in myotonic dystrophy Type 1 cell and mouse models. Chemistry 24, 18115–18122. [DOI] [PubMed] [Google Scholar]
- Li Y, and Disney MD (2018). Precise small molecule degradation of a noncoding RNA identifies cellular binding sites and modulates an oncogenic phenotype. ACS Chem. Biol 13, 3065–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Miller JW, Mankodi A, Kanadia RN, Yuan Y, Moxley RT, Swanson MS, and Thornton CA (2006). Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum. Mol. Genet 15, 2087–2097. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, and Feeney PJ (1997). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev 23, 3–25. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang Q, Xia Q, Dong J, and Xu Q (2012). Synthesis, characterization and properties of polyimides derived from a symmetrical diamine containing bis-benzimidazole rings. Polym. Degrad. Stab 97, 987–994. [Google Scholar]
- Lorenzetti DW, Uotila MH, Parikh N, and Kaufman HE (1967). Central cornea guttata. Incidence in the general population. Am. J. Ophthalmol 64, 1155–1158. [PubMed] [Google Scholar]
- Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, Krym M, and Thornton CA (2000). Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773. [DOI] [PubMed] [Google Scholar]
- Margolis RL, and Rudnicki DD (2016). Pathogenic insights from Huntington's disease-like 2 and other Huntington's disease genocopies. Curr. Opin. Neurol 29, 743–748. [DOI] [PubMed] [Google Scholar]
- Miller BR, McGee TD, Swails JM, Homeyer N, Gohlke H, and Roitberg AE (2012). MMPBSA.py: an efficient program for end-state free energy calculations. JJ. Chem. Theory Comput 8, 3314–3321. [DOI] [PubMed] [Google Scholar]
- Mootha VV, Hussain I, Cunnusamy K, Graham E, Gong X, Neelam S, Xing C, Kittler R, and Petroll WM (2015). TCF4 triplet repeat expansion and nuclear RNA foci in Fuchs' endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci 56, 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamori M, Sobczak K, Puwanant A, Welle S, Eichinger K, Pandya S, Dekdebrun J, Heatwole CR, McDermott MP, Chen T, et al. (2013). Splicing biomarkers of disease severity in myotonic dystrophy. Ann. Neurol 74, 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niblock M, Smith BN, Lee Y-B, Sardone V, Topp S, Troakes C, Al-Sarraj S, Leblond CS, Dion PA, Rouleau GA, et al. (2016). Retention of hexanucleotide repeat-containing intron in C9orf72 mRNA: implications for the pathogenesis of ALS/FTD. Acta Neuropathol. Commun 4, 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onufriev A, Bashford D, and Case DA (2004). Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 55, 383–394. [DOI] [PubMed] [Google Scholar]
- Parkesh R, Childs-Disney JL, Nakamori M, Kumar A, Wang E, Wang T, Hoskins J, Tran T, Housman D, Thornton CA, et al. (2012). Design of a bioactive small molecule that targets the myotonic dystrophy type 1 RNA via an RNA motif–ligand database and chemical similarity searching. J. Am. Chem. Soc 134, 4731–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkesh R, Disney MD, and Fountain M (2011). NMR spectroscopy and molecular dynamics simulation of r(CCGCUGCGG)2 reveal a dynamic UU internal loop found in myotonic dystrophy type 1. Biochemistry 50, 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson H (2018). Repeat expansion diseases. Handb. Clin. Neurol 147, 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez A, Marchán I, Svozil D, Sponer J, Cheatham TE, Laughton CA, and Orozco M (2007). Refinement of the AMBER force field for nucleic acids: improving the description of α/γ conformers. Biophys. J 92, 3817–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Ratovitski T, Chaerkady R, Kammers K, Stewart JC, Zavala A, Pletnikova O, Troncoso JC, Rudnicki DD, Margolis RL, Cole RN, et al. (2016). Quantitative proteomic analysis reveals similarities between Huntington's Disease (HD) and Huntington's Disease-Like 2 (HDL2) human brains. J. Proteome Res 15, 3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarz R, and Wüthrich K (1978). NOE difference spectroscopy: A novel method for observing individual multiplets in proton NMR spectra of biological macromolecules. J. Magn. Reson. (1969) 30, 147–150. [Google Scholar]
- Rosemeyer H, Toth G, Golankiewicz B, Kazimierczuk Z, Bourgeois W, Kretschmer U, Muth HP, and Seela F (1990). Syn-anti conformational analysis of regular and modified nucleosides by 1D 1H NOE difference spectroscopy: a simple graphical method based on conformationally rigid molecules. J. Org. Chem 55, 5784–5790. [Google Scholar]
- Rzuczek SG, Colgan LA, Nakai Y, Cameron MD, Furling D, Yasuda R, and Disney MD (2017). Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nat. Chem. Biol 13, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzuczek SG, Southern MR, and Disney MD (2015). Studying a drug-like, RNA-focused small molecule library identifies compounds that inhibit RNA toxicity in myotonic dystrophy. ACS Chem. Biol 10, 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H, Xavier AV, and Geraldes CFGC (1983). Conformation of purine mononucleotides by H{H} and P{H} nuclear Overhauser effects. Can. J. Chem 61, 1456–1464. [Google Scholar]
- Sierzputowska-Gracz H, Guenther RH, Agris PF, Folkman W, and Golankiewicz B (1991). Structure and conformation of the hypermodified purine nucleoside wyosine and its isomers: A comparison of coupling constants and distance geometry solutions. Magn. Reson. Chem 29, 885–892. [Google Scholar]
- Sznajder ŁJ, Thomas JD, Carrell EM, Reid T, McFarland KN, Cleary JD, Oliveira R, Nutter CA, Bhatt K, Sobczak K, et al. (2018). Intron retention induced by microsatellite expansions as a disease biomarker. Proc. Natl. Acad. Sci. U. S. A 115, 4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja KL, McCurrach M, Schalling M, Housman D, and Singer RH (1995). Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol 128, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule J, Ule A, Spencer J, Williams A, Hu JS, Cline M, Wang H, Clark T, Fraser C, Ruggiu M et al. (2005). Nova regulates brain-specific splicing to shape the synapse. Nat. Genet 37, 844–852. [DOI] [PubMed] [Google Scholar]
- Vickers TA, Wyatt JR, and Freier SM (2000). Effects of RNA secondary structure on cellular antisense activity. Nucleic Acids Res. 28, 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wolf RM, Caldwell JW, Kollman PA, and Case DA (2004). Development and testing of a general amber force field. J. Comput. Chem 25, 1157–1174. [DOI] [PubMed] [Google Scholar]
- Wheeler TM, Sobczak K, Lueck JD, Osborne RJ, Lin X, Dirksen RT, and Thornton CA (2009). Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science 325, 336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben ED, Aleff RA, Tosakulwong N, Butz ML, Highsmith WE, Edwards AO, and Baratz KH (2012). A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS One 7, e49083–e49083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FX, Johnson PD, Vickers R, Storer R, Wynne GM, Roach AG, De Moor O, Dorgan CR, and Davis PJ (2010). Preparation of bibenzo[d]imidazoles and other biheteroaryl compounds as antibacterial agents. Summit Corporation PLC, UK: ., pp. 65. [Google Scholar]
- Yildirim I, Stern HA, Kennedy SD, Tubbs JD, and Turner DH (2010). Reparameterization of RNA χ torsion parameters for the AMBER force field and comparison to NMR spectra for cytidine and uidine. J. Chem. Theory Comput 6, 1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any data sets or code.