Abstract
Traumatic brain injury (TBI) is a major cause of death and disability in children in both developed and developing nations. Children and adolescents suffer from TBI at a higher rate than the general population, and specific developmental issues require a unique context since findings from adult research do not necessarily directly translate to children. Findings in pediatric cohorts tend to lag behind those in adult samples. This may be due, in part, both to the smaller number of investigators engaged in research with this population and may also be related to changes in safety laws and clinical practice that have altered length of hospital stays, treatment, and access to this population. The ENIGMA (Enhancing NeuroImaging Genetics through Meta-Analysis) Pediatric Moderate-Severe TBI (msTBI) group aims to advance research in this area through global collaborative meta-analysis of neuroimaging data. In this paper, we discuss important challenges in pediatric TBI research and opportunities that we believe the ENIGMA Pediatric msTBI group can provide to address them. With the paucity of research studies examining neuroimaging biomarkers in pediatric patients with TBI and the challenges of recruiting large numbers of participants, collaborating to improve statistical power and to address technical challenges like lesions will significantly advance the field. We conclude with recommendations for future research in this field of study.
Introduction
Traumatic brain injury (TBI) is a significant, global public health issue that affects more than 3 million children worldwide annually [Dewan et al., 2016]. The annual economic burden of pediatric TBI in the United States alone may exceed $75 million [Brener et al., 2004]. TBI in children remains a particular concern given evidence for increased vulnerability to injury in the immature brain as well as unresolved questions related to injury that occurs within a dynamic developmental context. A growing body of literature has documented neural changes in pediatric patients with TBI, including widespread gray and white matter (GM and WM) loss and altered WM organization as long-term consequences of moderate/severe TBI (msTBI), along with alterations in brain function and connectivity [Dennis et al., 2018b; Keightley et al., 2014; Krogsrud et al., 2016] (see Figure 1). For an in-depth review of the current state of the field in neuroimaging of pediatric msTBI, see recent reviews [Dennis et al., 2018b; Rao et al., 2016; Roberts et al., 2016]. The corpus callosum appears to be particularly vulnerable to injury [Bigler et al., 2013; Dennis et al., 2015a; Ewing-Cobbs et al., 2012; Levin et al., 2000; Lindsey et al., 2019] since, during an injury, transverse forces may strain the falx cerebri, and stress the underlying corpus callosum [Hernandez et al., 2019]. The rapid synaptogenesis in childhood and myelination that continues throughout adolescence may increase the vulnerability of the brain when TBI occurs during development. Because msTBI can permanently alter normal developmental trajectories, understanding how early brain insult affects and interacts with brain development in the short- and long-term is essential for predicting outcome after TBI [Babikian and Asarnow, 2009; Dennis et al., 2014; Ewing-Cobbs et al., 2008]. While children and adults share some similarities that impact injury and recovery from msTBI, several important differences exist, and the literature regarding predictors of outcome after pediatric TBI is limited. Limited or insensitive measurement tools, misconception of the benefits of neuroplasticity in children, and the complex interplay of factors that can influence recovery in the developing brain contribute to this (for a review, see [Anderson et al., 2012]). As detailed in the introductory paper of this Special Issue [Wilde et al., 2019a], we have recently begun a collaborative effort to address some of the gaps in our knowledge of recovery after TBI via the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) Brain Injury working group. Briefly, ENIGMA is a framework for global collaborative analysis of neuroimaging studies for which raw data sharing is not necessary, lowering the bar for participation [Thompson et al., 2019]. This manuscript is focused on pediatric msTBI, with the aims of briefly describing the limitations and challenges reflected in the current msTBI literature including heterogeneity in injury specifics, sample characteristics, and study parameters, and providing clinical and research recommendations for future studies. We will also describe how the ENIGMA Pediatric msTBI group is uniquely poised to address these issues.
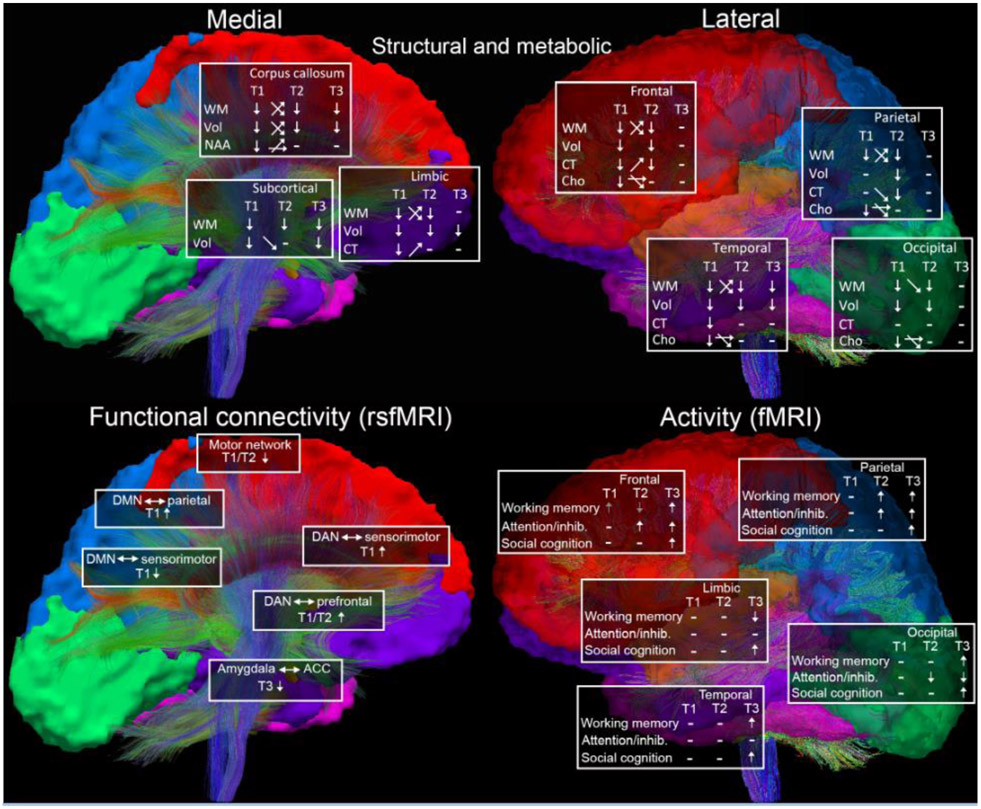
Figure 1.
Overview of reported neuroimaging differences in pediatric msTBI for structural, functional, and metabolic modalities (top panel, WM=white matter/DTI, Vol=volume, CT=cortical thickness, NAA=N-acetylaspartate, Cho=choline) and functional modalities (resting state fMRI left bottom, fMRI right bottom), divided by general brain region. T1=post-acute (2-6 months post-injury), T2=chronic (6 months-2 years post-injury), T3=long-term (>2 years post-injury). Arrows at timepoints indicate higher or lower relative to control group, arrows in between time points reflect changes in TBI group over time (increase, decrease, or no change). Mixed changes across the TBI group, or mixed results reported across studies, are reflected in crossed arrows. Dashes indicate no reported differences. For fMRI, gray arrows indicate that effects did not account for group differences in task performance. For rsfMRI, the seed is left of the arrow (alterations in the motor network were within network connections). DMN=default mode network, DAN=dorsal attention network, ACC=anterior cingulate cortex. Reported task-fMRI differences are divided by task - working memory, attention/inhibition, and social cognition. Adapted from [Dennis et al., 2018b].
The primary aims of the ENIGMA Pediatric msTBI group are:
Establish a collaborative network of researchers focused on TBI in pediatric patients, including those with an interest in neuroimaging, clinical, neuropsychological (cognitive, emotional, and behavioral) and other outcome, and fluid biomarker data
Leverage our combined existing and future datasets to determine factors associated with outcome, better understand the interaction between injury and development, and identify predictors of poor outcome that may suggest additional treatment windows or targets
Evaluate existing tools and develop and test new pipelines for processing neuroimaging data that include consideration of lesions, pediatric-based tissue changes and templates, and allow for longitudinal modeling of injury/recovery
Support the advancement of junior investigators and trainees through opportunities to lead and participate in analyses and to learn and develop new analysis techniques
Provide an opportunity for researchers planning new studies to discuss options for improving data comparability going forward, including sharing protocols and recommending measures and identifying collaborators with similar interests
Provide a forum for researchers to promote reproducibility across samples and techniques, discuss challenges and potential solutions, and debate unresolved scientific questions
Challenges and Opportunities
Numerous challenges exist for pediatric msTBI neuroimaging research that centralize on the complex relation between neuropathology and outcome, and involve the influence of demographic characteristics, interaction with developmental stage, heterogeneity of pathology, variability among methodological approaches, and moderating environmental factors in the post-injury phase. The dynamic nature of brain development requires large sample sizes to accurately model—and control for—multiple injury-related variables. The multifaceted quest for a better understanding of msTBI warrants a multimodal approach and datasets that enable construction of sophisticated models that account for the complexity of pediatric msTBI. The ENIGMA consortium provides a compelling opportunity to address these challenges by combining data across multiple cohorts using a meta-analytic approach and applying big data techniques such as machine learning to identify clinically meaningful patient subtypes. ENIGMA has created a set of validated MRI processing procedures that can be employed by collaborators and are freely available on the ENIGMA website. While including data with variations in protocol has some disadvantages, such an approach can lead to robust, generalizable effects [Dennis et al., 2018c]. Small sample sizes (mostly between 5-50 in TBI group, see tables in [Dennis et al., 2018b]), cross-sectional study designs, and basic image analyses of the published literature in msTBI have limited research to date. The ENIGMA approach is not well-suited to address some questions (e.g., responses to specific treatments) but many other questions regarding risk factors, relations to outcome and moderating factors that influence outcomes can benefit greatly from increased sample size. In the sections that follow, we describe individual differences between children and between injuries in response to TBI and potential mechanisms (suggested in smaller-scale studies with divergent findings) that could be addressed more definitively with harmonized data combined across cohorts.
Demographic variables - Age:
Brain injuries that occur during critical neurodevelopmental periods can alter regions undergoing accelerated neuronal maturation [Anderson et al., 2011; Anderson and Yeates, 2010], leading to cognitive, behavioral, emotional, and psychosocial dysfunction [Beauchamp and Anderson, 2010; Rosema et al., 2012]. Interpretation of structural and functional imaging data acquired from children after msTBI is informed by a developmental context that involves maturational changes in structure, function, and connectivity. Inter-individual variability in imaging findings, including sex differences (see section below), environmental effects, and genetic variation, also affect typical age-related trajectories. Brain plasticity—which is central to and beneficial in typical development—may be a source of vulnerability when it follows pediatric msTBI [Anderson et al., 2011; Ismail et al., 2017], as injury can derail predetermined developmental processes, deplete natural resources, and prevent typical recovery trajectories [Hebb, 1949; Pascual-Leone et al., 2005].
Increases in GM density follow a regionally-specific chronobiologic sequence characterized by early maturation of primary sensorimotor cortices and later development of higher order association cortex, including prefrontal and parietal cortical regions involved in executive function [Giedd et al., 1999; Gogtay et al., 2004] (Figure 2) as well as developmental changes in subcortical structures with connections to these regions. Due to the structure of the skull and mechanics of closed head injuries, the prefrontal cortex is particularly vulnerable to structural damage [Bigler, 2007]. Prefrontal GM volume peaks during adolescence and is followed by an apparent reduction due to continuing WM development [Gogtay et al., 2004; Natu et al., 2019]. Consequently, the loss of prefrontal GM volume after msTBI typically observed in adults is superimposed on age-related maturational changes in children and adolescents. Although WM volume typically increases with age through at least age 25 to 30 years [Schmithorst and Yuan, 2010], the trajectory may be affected by msTBI, depending on the severity of injury [Genc et al., 2017]. Reduced volumetric growth of the corpus callosum after msTBI in children is a prime example of how developmental context may modify the long-term effects of brain injury [Levin et al., 2000]. Training can lead to improvements in brain structure, however, demonstrating how experience can positively alter developmental trajectories [Keller and Just, 2009].
Figure 2.
Gray matter maturation between 5-20 years of age. Right lateral and top views of the dynamic sequence of GM maturation over the cortical surface. The sidebar shows a color representation in units of GM volume. A, precentral gyrus and primary motor cortex; B, superior frontal gyrus, posterior end near central sulcus; C, inferior frontal gyrus, posterior end; D, inferior frontal sulcus, anterior end in the ventrolateral prefrontal cortex; E, inferior frontal sulcus in the dorsolateral prefrontal cortex; F, anterior limit of superior frontal sulcus; G, frontal pole; H, primary sensory cortex in postcentral gyrus; I, supramarginal gyrus (area 40); J, angular gyrus (area 39); K, occipital pole; L–N, anterior, middle, and posterior portions of STG; O–Q, anterior, middle, and posterior points along the inferior temporal gyrus anterior end. Reprinted from [Gogtay et al., 2004].
With functional neuroimaging, maturation affects the strength of connections in neural networks that underpin executive functions, including working memory, inhibition, and shifting response set [Bunge et al., 2002; Rubia et al., 2006]. The magnitude and regional specialization of task-related activation also depend on maturational status, evolving from a relatively diffuse pattern in young children to distributed activation of key regions (such as lateral prefrontal cortex and parietal cortex) in adolescents [Rubia et al., 2006]. Following msTBI in children, changes in the anatomic distribution of activation may reflect potentially altered brain development and reorganization of systems in brain tissue where maturation is underway, but likely incomplete. For example, the neuroanatomic distribution of activation during a social cognition task was altered in adolescents studied at least one year post-msTBI compared to healthy controls, with patients recruiting both areas associated and not typically associated with social cognition [Newsome et al., 2010]. Similar post-injury effects on the development of the default mode network (DMN) may affect resting fluctuations in activation causing aberrant functional connectivity [Stephens et al., 2018]. Differentiating maturational changes from recovery processes and their interactions is difficult, and their synergies contribute to inter-individual variability in imaging data following msTBI.
The ENIGMA Pediatric msTBI group will investigate the complex impact of age at injury on neuroimaging measures and outcomes. While younger children have historically been considered to have greater plasticity, children who are older at the time of injury—with more mature brain structure, function, and connectivity—may have more neural and cognitive reserves to draw upon compared to those injured at a young age. Few studies have specifically examined the impact of age at injury, with conflicting results [Dennis et al., 2017c; Ewing-Cobbs et al., 2016]. Most studies have included patients between 8-18 years of age, a period of considerable brain change [Dennis and Thompson, 2013; Gogtay et al., 2004; Sowell et al., 2002] (Figure 2). A sensitive or critical growth period - or more likely, periods - of greater vulnerability for brain injury and more negative outcomes could exist. With the increased sample size of the ENIGMA Pediatric msTBI group, we will be able to examine the impact of age in several ways, both linear and non-linear relationships and discrete periods across development such as early childhood, late childhood (8-12 years) vs. early adolescence (12-15 years) [Zielinski et al., 2010].
Demographic variables - Sex:
Another open question is how dynamic neurobiological processes are influenced by sex, especially when studying a population that spans puberty. For an in-depth review of sex differences in pediatric TBI, see [Arambula et al., 2019]. Throughout development, many factors may be modified by sex, including biological, cognitive, socio-emotional, and psychological factors. Sex differences are a major topic across TBI neuroimaging and developmental neuroimaging in general [Etchell et al., 2018; Herting and Sowell, 2017; Ingalhalikar et al., 2014; Jahanshad and Thompson, 2017] and will be a central focus for the ENIGMA Pediatric msTBI group. Premorbid sex-related considerations include molecular differences in synapse protein expression, neurotransmitter sensitivity, recruitment of second-messenger cascades involved in sex-differentiation, synaptic density differences by brain region, and the role of sex and growth hormones (which may themselves be altered due to trauma to the hypothalamus and pituitary gland) [Arambula et al., 2019]. Also important are physical factors (e.g., neck strength and body mass), cognitive factors (e.g., intelligence, education, adaptive abilities), and socio-emotional factors (e.g., risk-taking behaviors, socioeconomic status, and psychiatric history) [Arambula et al., 2019].
Boys are approximately twice as likely to sustain a TBI than girls [Coronado et al., 2011] with the highest incidence in boys 0-4 years of age in the United States. Effects of sex on outcome are conflicting, with some evidence indicating longer recoveries and worse prognoses in females, while others show that females have reduced mortality and better cognitive function after injury [Arambula et al., 2019]. Research on sex-specific effects in msTBI is principally focused on animal models, adult patients, or retrospective observational cohort studies. Some studies of sex differences in TBI using animal models have shown significant differences, with greater survival rate in females acutely [Roof and Hall, 2000] and greater preservation of neuronal integrity weeks after injury [Semple et al., 2017], while others have not reported differences [Russell et al., 2011]. These may suggest protective effects of hormones like estrogen and progesterone [Cutler et al., 2007; Djebaili et al., 2005; Roof and Hall, 2000]. Preclinical studies have also shown that progesterone may have a neuroprotective effect [Arambula et al., 2019], although large clinical trials have failed to demonstrate a benefit from administering progesterone to adults with msTBI [Goldstein et al., 2017; Wright et al., 2015]. The role of hormones in outcomes after pediatric TBI is unknown but the incidence of pituitary dysfunction after injury supports further investigation [Acerini and Tasker, 2007; Bondanelli et al., 2004; Rose and Auble, 2012]. Gonadal hormonal changes surrounding puberty remain understudied in relation to outcomes. Observational studies using hospital records are also conflicting, with some reporting greater injury severity in males while others report longer hospital stays for females [Morrison et al., 2004; Slewa-Younan et al., 2004]. In children, particularly, variability in age at puberty and the spectrum of gender must also be considered. Options are limited with legacy data, but when possible, we will examine the impact of pubertal status and encourage future data collection among our group to include more in-depth characterization of pubertal status and hormone measurements. In the ENIGMA Pediatric msTBI group, we will investigate sex as a potential moderating factor as well as whether potential sex differences vary across childhood and adolescence.
Pre-injury variables:
Another question that we intend to examine is how different comparison groups impact findings [Boedhoe et al., 2019]. Most of the published literature has included an uninjured, healthy control (HC) group (e.g., [Dennis et al., 2015b; Wilde et al., 2005]). However, HC groups do not always account for premorbid (psychiatric or neuropsychological) characteristics that may predispose children to injury (e.g., impulsivity or hyperactivity), post-injury effects of medication (e.g., analgesics), long-term medical care, time away from school, or psychological effects of exposure to trauma [Daviss et al., 2000; Delaney-Black et al., 2002; Hajek et al., 2010; Holbrook et al., 2005; Ponsford et al., 2012]. Thus, children with mild (uncomplicated) TBI, orthopedic injury (OI), or other extracranial traumatic injury have been used to account for these pre- and post-injury characteristics [Dennis et al., 2013; Wilde et al., 2019b; Wu et al., 2010; Yeates et al., 2013]. Despite similarities in premorbid characteristics and exposure to traumatic stress among patients with TBI and OI, using children with OI as a comparison group has several limitations. Premorbid social and behavioral difficulties may not be similar [Loder et al., 1995], and children with TBI may experience trauma differently from children with OI [Basson et al., 1991]. The literature provides an incomplete understanding of how functional outcomes are influenced by analgesic medications frequently prescribed for pain in patients with more severe extracranial injury [Borsook, 2012]. Less understood is the possibility of occult brain injury with OI, which could obscure differences between groups in outcome measures, including estimates of brain alterations and neuropsychological impairments. For example, brain structure of children with mild TBI and OI may differ from healthy children who are unexposed to traumatic injury [Wilde et al., 2019b]. While these recent findings support similarities between children with mild TBI and OI, we still lack a complete understanding of the underlying neurobiological mechanisms accounting for these similarities and work is greatly needed in this area. Even in the absence of an orthopedic or extracranial injury, systemic trauma that is associated with a subsequent inflammatory response may alter blood brain barrier function and permeability, causing neuroinflammatory responses that might also result in structural and functional brain changes [McDonald et al., 2016; Nicholson et al., 2018; Sheeler, 2016; Yang et al., 2016]. With limited sample sizes, collecting both HC and OI controls has been beyond the capabilities of most studies to date. The ENIGMA Pediatric msTBI group will be able to examine this, comparing TBI to all control participants or just HC or OI.
Pre-injury psychiatric or developmental disorders are highly prevalent (30-50%) in children with msTBI and comorbidity is common [Brown et al., 1981; Max et al., 2012]. Pre-injury attention-deficit hyperactivity disorder (ADHD) is over-represented in populations with TBI, complicating research given that pre-injury symptoms of ADHD and post-injury sequelae present very similarly. Oppositional Defiant Disorder and anxiety disorders are also common before and after injury [Gerring et al., 1998]. Up to 60% of children sustaining an msTBI may develop novel psychiatric disorders [Brown et al., 1981; Max et al., 2012], which are heterogeneous and additional comorbidity is typical. These novel psychiatric disorders have demonstrable lesion correlates and show distinct neuropsychological profiles [Max et al., 2015] including when compared to developmental or pre-injury disorders [Ornstein et al., 2014]. Heterogeneity in novel psychiatric disorders combined with relatively small sample sizes complicate efforts to understand their neurobiological substrates. Until now, this problem has been dealt with by examining children with the novel psychiatric disorder of interest [Max et al., 2011]. Other studies examine symptom counts or behavioral checklist scores of selected behavioral or psychological traits such as oppositional defiant behavior, PTSD, or attention deficits [Max et al., 1998]. However, examining symptom load may have less clinical validity in the absence of diagnostic categories. As discussed in the introductory paper [Wilde et al., 2019a], working groups within ENIGMA are focused on these comorbid disorders, including ADHD [Boedhoe et al., 2019; Hoogman et al., 2019], anxiety [Groenewold et al., 2018], and PTSD [Dennis et al., 2019], facilitating well-powered cross-disorder analyses. We will work with these other ENIGMA groups to compare the neural signatures of psychiatric disorders after injury to those in the absence of injury to see whether they share common traits. We will also encourage groups collecting new data to include assessments of psychological and behavioral symptoms, such as the CBCL, K-SADS/KSADS-PL-DSM-5, and measures of quality of life, including those identified in the NINDS Common Data Elements for pediatric TBI.
Injury variables:
The mechanism of childhood injury is important to consider. During childhood, the primary mechanism of TBI varies considerably by age and can present in multiple ways, creating an important distinction between pediatric and adult TBI [Giza et al., 2007; Keenan and Bratton, 2006]. Falls, motor-vehicle accidents, and sports-related injuries are the primary cause of injury in children under age 18 [Coronado et al., 2015; Taylor et al., 2017]. Each of these injury types induces unique biomechanical forces to the brain [Bayly et al., 2012]. Additionally, very young children are more likely to sustain TBI as a result of intentional injury or abuse, and may therefore experience repeated and more severe forms of injury [Sills et al., 2005]. Variations in underlying injuries are closely related to the causal mechanisms, though heterogeneous clinical presentations suggest that similar variability exists in the underlying pathological features of the damaged, developing brain. Inter-individual differences in body size can impact outcome, as well [Bigler et al., 2013; Rosenbaum and Lipton, 2012]. Relative to adults, children tend to be at greater risk of diffuse brain damage and chronic neurocognitive deficits resulting from injury, because brain tissue, skull, and head and neck musculature are not fully developed [Margulies and Thibault, 2000], rendering them more vulnerable, in general, to the biomechanical forces of injury [Bigler, 2007; Huisman, 2015].
Recovery following pediatric brain injury is highly dynamic, so it is important to assess children within a circumscribed window post-injury [King et al., 2019]. Within the first week of injury, primary injuries (e.g., bleeding and edema) can impact neuroimaging metrics. For example, bleeds can impact diffusion MRI metrics and complicate interpretation if the timing of the assessment varies between patients and/or across studies.
Anatomic lesions are characteristic of msTBI and may include focal or diffuse axonal injury as well as contusions. As a consequence of gliotic or encephalomalacic changes over time, these lesions can cause structural deformations in the brain that can complicate image registration, normalization, and quantification both in the acute and chronic stages of recovery (Figure 3). Attempts to model morphometric differences may not be robust in the presence of TBI, and some morphometric approaches lack validation in TBI [Goh et al., 2014; Kim et al., 2008]. Lesion variability and heterogeneity are hallmarks of TBI research [Bigler et al., 2013] so pooling datasets across centers may be necessary to provide sufficient power to perform subgroup analyses according to lesion characteristics. Furthermore, type of injury (axonal injury, focal lesions, and diffuse microlesions), severity (mild, moderate, severe), and secondary issues associated with systemic function including comorbid injuries or complications (especially those that affect pulmonary and cardiovascular function, see [Crawford et al., 2019] and post-traumatic seizure: [Bennett et al., 2017; Liesemer et al., 2011]) likely impact the relationship between lesion findings and functional outcomes. Single-site studies often lack the statistical power to consider multiple moderating factors, which could increase our understanding of the extent to which brain metrics are preserved or affected by the type and severity of injury. Importantly, advances in lesion-symptom mapping methods and the development of novel analysis pipelines are beginning to allow investigators to use volumetric measurements of lesion load to relate lesion topography to clinical and behavioral outcome measures in individuals with TBI.
Figure 3.
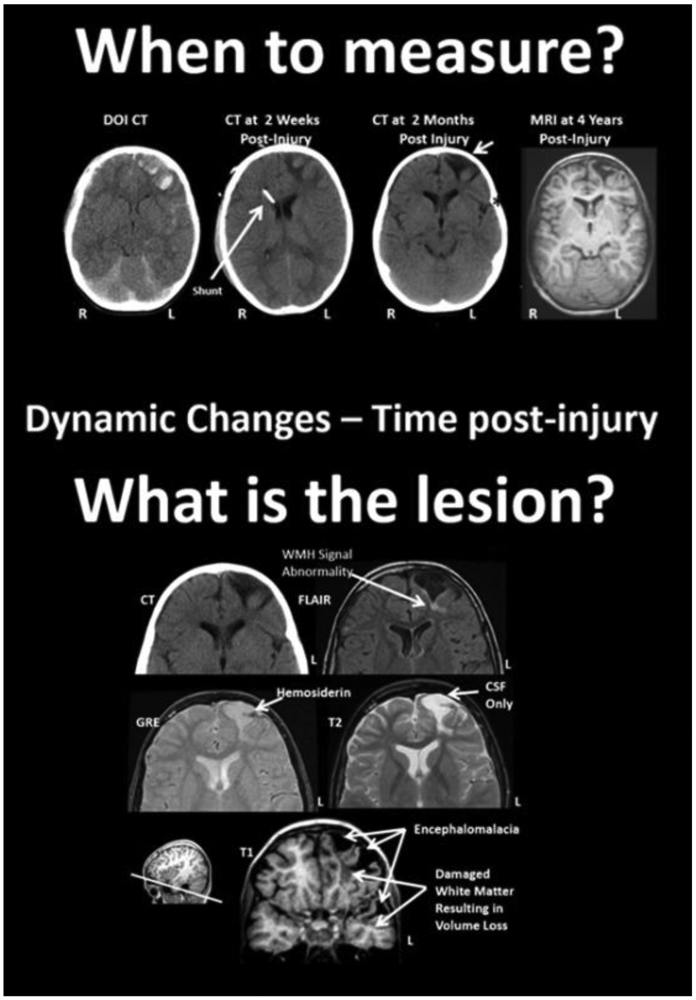
Difficulties in assessing lesions - dynamic changes post-injury and multi-modal assessment. Top: Parenchymal changes over time from the DOI (day of injury) scan to 4 years post-injury. The DOI through the scan at 2 months are all from CT and the far right image is a T1-weighted MRI, at 4 years post-injury. The slice level of these images are all at the level of the anterior horn of the lateral ventricle. Note that where the most prominent acute hemorrhages and frontal contusions occur on the DOI scan that this also becomes the center-point for tissue dissolution (encephalomalacia) over time. Shortly after the DOI scan was obtained the child received an intraventricular shunt to aid in intracranial pressure reduction and monitoring. By 2 months post-injury the focal encephalomalacia is very prominent (white arrow) and there is also prominence of the Sylvian fissure (black asterisk) along with ventricular enlargement, indicative of some generalized cerebral volume loss. R, right; L, left. Bottom: Different MRI sequences yield different facets of the brain injury and residual pathology. FLAIR, fluid attenuated inversion recovery; MRI sequence GRE, gradient recalled echo MRI sequence. Reprinted from [Bigler, 2016].
Post-injury variables:
Questions regarding post-injury moderating factors (other than specific treatments) that influence outcomes can greatly benefit from the ENIGMA approach. For example, complex interrelationships exist between outcomes from pediatric TBI and family factors [Babikian et al., 2015] such as family functioning [Yeates et al., 2010], parent marital status [Raj et al., 2018], family stressors and resources [Yeates et al., 2004], and the family’s response and adjustment to the injury [Wade et al., 2006]. While some of these factors may have been present prior to the injury, the injury itself could contribute additional stress (e.g., financial, emotional) that further affects the child’s environment following the injury. While pre-injury and injury variables may not be modifiable, some post-injury factors may be targeted to improve outcome. Additional environmental factors that could contribute to post-injury outcome include access to appropriate resources and academic support and access to rehabilitation [Semrud-Clikeman, 2010]. Our working group will examine whether measures of family functioning impact outcome and encourage more in-depth characterization of the post-injury environment in future studies.
Neuropsychological assessment:
Including age-appropriate neuropsychological assessment is imperative, but consistency in measurement can be a challenge to implement in developing populations as measures that are appropriate for older adolescents may not be suitable for younger children, both due to differences in the constructs as they are applied (or are applicable) over the age spectrum, but also in item content. In addition, although similar cognitive constructs can be measured, specific test batteries often differ. Additionally, practice effects in longitudinal assessments can undermine the validity of assessments and findings derived from repeated measures. In recognition of this problem, the National Institutes of Health (NIH) introduced a standardized means to collect normative data with a computer-based cognitive assessment battery (the NIH Cognitive Toolbox), which has been adapted for children 3-15 years of age [Bauer and Zelazo, 2013]. Cognitive development is inextricably linked to brain and physiological maturation. Therefore, pediatric TBI neuroimaging outcome studies must ensure use of repeatable, age-appropriate, and standardized neurocognitive measures to monitor whether significant delays and impairments have emerged with increasing time since injury [Li and Liu, 2013; Rabinowitz and Levin, 2014]. The full extent of functional deficits may not become apparent until years after the injury [Ryan et al., 2015]. As respective deficits become more protracted, the slower, atypical development can be exacerbated, increasing the functional gap in maturation in comparison to typically developing children [Anderson et al., 2005; Babikian and Asarnow, 2009; Wells et al., 2009]. Cultural and language differences must also be considered, as measures in which the normative sample is largely white and English-speaking may not generalize to other groups. The Common Cognitive Endpoints subgroup was recently formed within the ENIGMA Brain Injury group with the goal of testing and validating different strategies for combining data across disparate neuropsychological scales including traditional approaches (e.g., Z-scoring), item response theory, and machine learning approaches (e.g., random forest models). This is particularly critical and challenging for pediatrics as the emergence and saliency of different cognitive domains (in addition to the ability to measure them) may shift across childhood and adolescence. As discussed above, collaboration with ENIGMA groups focused on common comorbidities of TBI (i.e. ADHD) will be critical in teasing apart the myriad factors that can influence neurocognition.
Processing tools:
The vast majority of neuroimaging processing tools have been developed and tested on data from healthy adult participants. While these tools are likely appropriate for older adolescents, they may not perform adequately in younger children. Therefore, standard software tools such as FreeSurfer [Dale et al., 1999; Fischl et al., 1999] and FSL [Jenkinson et al., 2012; Smith et al., 2004] are not necessarily suitable without modification or adaptation as these tools model the brains of children and adolescents using adult brain templates that are simply scaled to size. This may result in tissue segmentation and spatial normalization errors [Schoemaker et al., 2016]. Age-appropriate methods and templates can improve results for brain extraction, normalization, and segmentation [Alexander et al., 2017; Fonov et al., 2011; Lorenzen et al., 2006; Sanchez et al., 2012]. With very young children, specialized image acquisition may be necessary, as the contrast between gray and white matter is lower due to incomplete myelination.
Regulations:
In the US, general regulations for pediatric research require a balance between the necessity to protect the child from harm and the opportunity to participate in and benefit from research (US DHHS, 2018). Although ethical guidelines for pediatric research vary to some extent across countries, the core Belmont principles of respect for persons, beneficence, and justice remain universal [Department of Health, Education, and Welfare and National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 2014]. International collaborations offer many benefits [Hicks, 2015]; however, variation in international ethical policies across different socioeconomic, medical, and cultural contexts can pose additional challenges [Palk et al., 2019]. Parental consent is required for minors, but parents or legal guardians may not be immediately available in emergency situations; emergency waivers of consent may be considered with additional ethical considerations [Adelson, 2010]. In longitudinal and prospective cohort studies evaluating outcomes in pediatric TBI, re-consenting participants who have reached adulthood while the study is still in progress can prove challenging. Children who participate in acute msTBI studies may not remember doing so and may experience anxiety or confusion if they are re-contacted as adults [Resnik, 2009]. While non-accidental trauma (NAT) is not a focus of the ENIGMA Pediatric msTBI group, mandated reporting and medicolegal significance in cases of NAT is an additional challenge when working with patients under 18 years old. As ENIGMA is a globally-based effort, we intend to consider ways in which responsible data sharing can be expanded but operate within the policies and requirements of different countries.
Recommendations for Harmonized Data Collection and Analysis
In an attempt to facilitate data sharing and ensure greater consistency in data collection, the National Institute of Neurological Disease and Stroke (NINDS)/International Common Data Elements initiative detailed recommended elements (including variables, definitions, and case report forms) for pediatric studies, including demographic (sex, race/ethnicity, education/parental education, and socioeconomic status), clinical and injury variables, as well as recommended outcome measures in a host of outcome domains (cognitive, psychiatric, physical, family, and functional outcome), biomarkers, and neuroimaging. Canada has also recently published recommendations for common data elements (CDE) for studies in children with TBI, though these are focused on mild TBI. Additionally, recent recommendations have been published regarding common variables that can be collected from electronic health records to facilitate studies and clinical trials in pediatric TBI from the US National Institute of Child Health and Human Development Pediatric Trials Network. The following sections include recommendations from the ENIGMA Pediatric msTBI group, incorporating CDEs mentioned above with additional input from researcher experience.
Non-imaging biomarkers:
Blood-based biomarkers collected at the time of recruitment or neuroimaging can provide complementary information in pediatric msTBI neuroimaging studies and may explain some of the interindividual variability in injury response. Very few genetic and biomarker studies have been conducted in pediatric msTBI due to the sample size required; the ENIGMA consortium provides the opportunity to coordinate collection methods and CDEs to enable multifactorial understanding of the injured pediatric brain. Recent work, for example, by the ENIGMA Epigenetics group, was able to detect two genome-wide significant loci where the level of methylation was associated with hippocampal volume [Jia et al., 2018], but imaging and epigenetic data from over 3,000 individuals were needed to perform a sufficiently powered study.
Products of different pathological processes initiated by brain injury and repair enter the circulation through the disrupted blood-brain barrier or the glymphatic system. Cell-specific macromolecules released following CNS injury include neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), and myelin-basic protein (MBP) that are indicators of damage to neurons, astrocytes, and oligodendrocytes, respectively. Other neuronal markers can reflect damage to specific parts of the neuron (e.g., NF-L, tau). Inflammatory responses can be assessed by detection of cytokines such as Interleukin 6 (IL-6), and cell death by cytochrome c [Au and Clark, 2017]. Other emerging biomarkers such as novel protein biomarkers, miRNA, and metabolites [Banoei et al., 2018] may also provide information about these injury processes.
Blood collection procedures may be anxiety-inducing for both children and parents and may be best coordinated with clinical blood draws. Many biomarkers are elevated for a short period of time after injury; however, depending on the research question it may be appropriate to consider TBI biomarkers released by pathways active at later time points (subacute and chronic), including neurodegeneration markers such as total Tau protein, phosphorylated Tau, amyloid beta peptides, and inflammatory markers [Wang et al., 2018]. Consideration of the best complementary biomarker analysis for the research question is paramount as age-related restrictions on research blood volumes limits the number of exploratory tests that can be performed on any one sample.
Variability in the timing, collection method, biofluid type, and storage of biomarkers can make combining data from different studies difficult. When possible, blood collection should be coordinated with existing biobanks to ensure standardization of procedures and linking of data. A major consideration for pediatric TBI biomarkers is that most will have a variable pattern of release dependent on developmental stage (i.e., neonatal, toddler, school-age, or adolescent). For this reason, normal reference values need to be obtained from well-powered age-matched control samples [Shores and Everett, 2018].
Behavioral preparation for scanning:
Although children generally tolerate MRI scanning well, some children may experience anxiety, claustrophobia, fatigue, or restlessness which can lead to excessive motion and distraction from tasks. Preparation before scanning is essential, especially for the injured child [Bookheimer, 2000; Byars et al., 2002; Gaillard et al., 2001; Rivkin, 2000; Wilke et al., 2003]. We recommend a multi-step procedure to ensure the child’s comfort in the scanner environment, ideally including the use of a mock scanner, exposure to MRI sounds through audio samples, description of study goals, incentives to encourage cooperation, breaks (if necessary) between series of sequences, and distractions to alleviate boredom or anxiety (e.g., watching a movie, when appropriate to the study protocol). Behavioral intervention methods can improve compliance and reduce attrition of pediatric participants [de Bie et al., 2010; Caeyenberghs et al., 2009; Gabrielsen et al., 2018] as well as reduce the need for sedation or general anesthesia [Edwards and Arthurs, 2011; Wilder et al., 2009]. Additionally, efforts are underway to optimize rapid and simultaneous multi-modal acquisition sequences.
MRS:
Magnetic resonance spectroscopy (MRS) is a non-invasive imaging technique that has been used to measure neural metabolites in pediatric TBI across all injury severities and at various time points post-injury. The most commonly reported metabolite change following pediatric TBI is reduced N-acetyl-aspartate (NAA), which is considered to reflect neuronal loss or dysfunction [Ashwal et al., 2000; Babikian et al., 2006; Babikian et al., 2010; Babikian et al., 2018; Dennis et al., 2018a; Holshouser et al., 2005; Holshouser et al., 2019; Hunter et al., 2005; Walz et al., 2008]. In general, MRS is highly sensitive to metabolic alterations following TBI, and these changes appear to correlate with cognitive and behavioral outcomes; however, the extent of metabolite change differs depending on injury severity, the region studied, the specific scan acquisition technique used, and time of assessment post-injury. The ENIGMA Brain Injury MRS subgroup has recently come together to define and encourage the use of standardized data acquisition and analysis protocols that can be used across all clinical MR platforms. For greater detail about the use of MRS in TBI we refer the reader to a companion paper in this special issue [Bartnik-Olson et al., 2019].
Diffusion MRI:
While ENIGMA dMRI protocols are relatively simple and appropriate for a range of acquisitions, for future data collection, we encourage the use of advanced dMRI acquisition and processing techniques to detect white matter alterations and inform what the biological underpinnings may be [Imms et al., 2019]. Specifically, future dMRI research should employ high angular resolution diffusion imaging (HARDI) with multiple diffusion weightings and an accelerated acquisition speed (e.g., multiband/compressive sensing) to minimize the impacts of motion. Acquisitions with phase encoding in opposite directions can provide additional correction for susceptibility-induced distortions beyond the current ENIGMA EPI correction steps. Moreover, researchers may benefit from employing robust estimation approaches for dMRI metrics (e.g., Slicewise OutLIer Detection (SOLID); [Sairanen et al., 2018]) and apply models that can resolve crossing fiber orientations (e.g., constrained spherical deconvolution, or the tensor distribution function model, [Nir et al., 2017; Zavaliangos-Petropulu et al., 2018]). Researchers should extract specific measures of white matter, such as fiber density and cross-section [Raffelt et al., 2017], to overcome the limitations of the commonly used DTI metric fractional anisotropy (FA). Deviations in FA have been widely demonstrated in patients with TBI as compared to control groups (for meta-analyses, see [Hunter et al., 2019; Wallace et al., 2018]). Increased WM pathology (observed as FA abnormalities) predicts greater behavioral deficits in TBI patients [Caeyenberghs et al., 2010; Caeyenberghs et al., 2011]. Yet, despite this large body of dMRI work in TBI patients, few attempts have been made to investigate these findings in more detail and pinpoint how FA is affected, or how it leads to or is associated with behavioral deficits. Tractography approaches that solely exploit orientational information (e.g., FA) are not able to capture the capacity of WM pathways to facilitate communication between brain areas. FA can be modulated by changes in myelination, axon density/diameter, inflammation, and the layout of the axons within the voxel [Jones et al., 2013]. When possible, researchers are encouraged to move beyond basic diffusion metrics averaged within regions of interest and instead use analytic techniques that calculate metrics specific to differently-oriented fiber bundles (fixel-based analysis framework [Raffelt et al., 2017]), though implementing these across sites will take further development. Finally, alternative myelin-sensitive sequences, such as quantitative magnetization transfer imaging and multi-component relaxometry (myelin water fraction mapping) [Dalamagkas et al., 2019; Lee et al., 2019; Tang et al., 2019] can complement the information obtained by dMRI acquisitions.
Task fMRI:
Task related blood oxygen-level-dependent (BOLD) fMRI is the most commonly used functional neuroimaging method in both adult and pediatric msTBI. With a few exceptions, [Khetani et al., 2019; Kramer et al., 2009], the frequent finding is that children and adolescents with msTBI demonstrate increased activation throughout the brain, often interpreted as compensatory recruitment patterns [Olsen et al., 2019; Turner et al., 2011]. The ENIGMA consortium is currently engaged in efforts to develop fMRI and rsfMRI processing and analytical protocols [Adhikari et al., 2018; Veer et al., 2019].
The BOLD signal is heavily influenced by task performance, effort, lesion characteristics (especially hemorrhagic lesions), changes in neurovascular coupling, and participant movement. Tasks should be designed to avoid floor and ceiling effects, and designs in which task load can be manipulated in a parametric fashion are especially powerful (e.g. working memory tasks; [Olsen et al., 2019]). Task performance should be matched or adjusted for when comparing task fMRI activations between groups [Price et al., 2006] to ensure that patients are similarly engaged in the task. This can be particularly challenging when studying children, as cognitive abilities vary substantially with age, in addition to injury effects. We recommend linking BOLD alterations to cognitive and clinical measures performed outside the scanner to determine functional and clinical relevance. Because of the heterogeneity of TBI pathology, task fMRI analyses may benefit from using a whole-brain approach when studying brain function. Accordingly, and especially when combining data across sites, a domain-general task that provides widespread activations is recommended (e.g. working memory or executive function)[Dennis et al., 2018b; Newsome et al., 2007; Scheibel, 2017; Westfall et al., 2015]. One potential approach to combining data across different tasks and sites is to focus on modeling the BOLD signal based on common task-general dimensions, such as task load or difficulty. Core brain regions linked to working memory and cognitive control, such as the dorsolateral prefrontal cortex, medial frontal cortex, and the insula, consistently show increased BOLD activation as an effect of increased task demands, regardless of task type [Hillary et al., 2006; Olsen et al., 2013; Olsen et al., 2015; Power and Petersen, 2013]. Investigating how the BOLD signal in domain-general core brain regions responds to relative variations in task load, and how these responses are linked to external cognitive and clinical measures, may be a good approach to learning more about the functional consequences of msTBI.
Connectomics:
Given the widespread effects of TBI, especially in more severe injuries, typical approaches that treat brain regions as being independent of each other may not capture the full effects of the injury. A graph theoretical approach allows researchers to examine network topology based on a connectivity matrix for all region pairs, quantitatively characterizing both global and local network organization. Graph theoretic approaches using dMRI tractography (structural connectivity) have been examined in pediatric TBI populations [Caeyenberghs et al., 2012; Dennis et al., 2017a; Dennis et al., 2017b; Königs et al., 2017; Verhelst et al., 2018; Watson et al., 2019; Yuan et al., 2017]. While the network metrics differ across studies— likely due to methodological differences— widespread alterations in network topology are seen in TBI patients compared to orthopedic injury or healthy control subjects. In addition to simple group differences there are associations with injury severity and cognitive outcomes [Dennis et al., 2017a; Dennis et al., 2017b; Königs et al., 2017; Watson et al., 2019; Yuan et al., 2017]. These connectomic methods have the potential to elucidate diagnosis, prognosis, and ultimately therapeutic approaches to brain injury, based on conceptualizing the brain network itself as the substrate of pathology.
Several limitations hamper the meta-analysis of network topology across studies: brain region definition (e.g., different brain parcellations), edge weight definition (e.g., tractography streamline count vs. mean FA [Königs et al., 2017]), and network construction differences [Dell’Italia et al., 2018; Hallquist and Hillary, 2019]. Finally, no two injuries are identical, and some subjects may be “missing” brain regions that are present in others [Bigler et al., 2013]. Some of these problems can be mitigated by the ENIGMA approach: combining data from children with variable age at injury and injury location would facilitate modeling such effects on outcomes. Significant efforts are also being dedicated to specialized harmonization approaches for diffusion MRI, recognizing that protocols differ in their ability to detect neural pathways, depending on their spatial and angular resolution. Several harmonization methods are being tested in ENIGMA to mitigate this [Moyer et al., 2019; Nir et al., 2019; Zhu et al., 2019]. Furthermore, data from large-scale studies of typically-developing children and adolescents (e.g., Adolescent Brain Cognitive Development, Human Connectome Project in Development) allow for the creation of robust normative reference values, providing important context for alterations shown in TBI patients (see Supplementary Table 1).
GM metrics:
GM metrics - cortical thickness, gyrification, surface area, and signal intensity covariance - can also be harnessed to recover large-scale network-level topology using clinical MRI sequences [Bigler, 2016; Hasan et al., 2018; Hutchinson et al., 2018; Wilde et al., 2015]. All cortical thickness methods aim to quantify the distance between tissues along the cortical mantle. The ENIGMA cortical protocols (including critical quality control steps) rely on FreeSurfer to generate average measures of thickness and surface area averaged within regions of interest. Although the relationship between surface area, gyrification, and cortical thickness is mathematically straightforward, each process appears to be under distinct mechanisms of genetic control [Chen et al., 2015; Panizzon et al., 2009]. Intensity covariance approaches quantify interrelationships of signal density across all voxels of the brain, giving rise to conserved structural coherence maps that recapitulate functional brain networks, and have been shown to outperform other metrics including thickness in modeling age and sex effects in brain development [Gennatas et al., 2017; Zielinski et al., 2010].
Despite the well-characterized cellular, morphological, and gross anatomical changes that accompany TBI [Bigler, 2013; Sta Maria et al., 2019], there is a striking lack of research related to interregional correlations in brain structure metrics such as thickness, surface area, and gyrification, and such reports in pediatric TBI are exceedingly sparse. Use of these metrics to describe structural network-based changes post-injury represents an intriguing approach to characterizing distributed pathologic changes related to mechanism (blunt vs. shear), lesion location (cortical or subcortical area), lesion type (hemorrhage, contusion, microbleed), and lesion extent, and more clearly characterize the effect of network-based changes on outcomes after TBI.
Linking distributed patterns of injury after TBI will enable diagnostic approaches centered on brain networks as pathological substrates, and provide a path forward for both documenting dynamic changes after injury as well as devising therapeutic strategies to optimize recovery of impacted brain networks. Substantial hurdles remain, such as the impact of localized edema and blurring of the gray-white junction on cortical segmentation, the impact of acute blood or subsequent encephalomalacia on network-based structural techniques, and the characterization of gray-white tissue compartments in younger children with changing tissue contrast related to maturity. However, the ENIGMA framework is poised to undertake such a multifaceted effort. Large samples of heterogeneous patient populations are necessary to tease out common influences of network-level changes post-TBI, enabling new tools aimed at optimizing network recovery, and rescuing networks in decline.
Multimodal imaging:
As detailed above, there are a number of imaging sequences that can provide important information in assessing TBI-related alterations, but none can give a complete picture of disruption alone. To date, the vast majority of studies published have focused on a single modality, but future studies would benefit from simultaneous utilization and integration of the rich datasets which are often collected. For example, dMRI can reveal WM disruption, but has limited capacity to distinguish demyelination from inflammation. Combining dMRI with MRS can provide additional evidence that WM disruption is due in part to neuronal loss [Dennis et al., 2018a]. DMRI combined with rsfMRI could provide a more complete picture of disruption and may reveal compensatory increases in functional connectivity related to disrupted structural connectivity. With large sample sizes and advancing big data techniques such as Symmetric Multivariate Linear Reduction (SyMILR), it will be possible to reduce high-dimensional multi-modal data to components that may reveal patterns that bridge modalities [Avants et al., 2014; Kandel et al., 2015].
Conclusions
Numerous hurdles exist in the field of pediatric msTBI, many of which pertain to heterogeneity in risk factors, injury variables, and ongoing patterns of development during childhood and adolescence, a period of dynamic maturation. With larger sample sizes and multi-site collaboration, ENIGMA Pediatric msTBI will address some of these issues, and big data approaches and machine learning should aid identification of patient subgroups. While a multi-cohort approach is not appropriate for all questions, it has the promise to address some critical questions in the field and to generate hypotheses for future studies. Researchers and clinicians interested in joining this effort are encouraged to contact the authors of this paper.
Supplementary Material
Acknowledgments:
We wish to acknowledge multiple funding sources, including the following: K99 NS096116 to Dr. Dennis, NHMRC Career Development Fellowship and an ACURF Program grant by the Australian Catholic University (ACU) to Dr. Caeyenberghs, NRF SARChI Chair of Clinical Neurosciences to Dr. Figaji, NICHD R43HD09703901 to Dr. Levin, R01 HD088438 to Dr. Max, and U54 EB020403, R01 MH116147, R56 AG058854, P41 EB015922, R01 MH111671 to Dr. Thompson. Dr. Yeates is supported by the Ronald and Irene Ward Chair in Pediatric Brain Injury, funded by the Alberta Children's Hospital Foundation. The authors wish to acknowledge the leadership of Dr. Paul Thompson (ENIGMA PI) as well as the leadership of Drs. Frank Hillary, Alexander Olsen, Inga Koerte, David Baron, Alexander Lin, Brenda Bartnik-Olson, Carrie Esopenko, and Neda Jahanshad, as well as ENIGMA support personnel and all working group members and contributors. We alos wish to acknowledge the constructive feedback and suggestions of anonymous reviewers. Finally, we wish to acknowledge the participation of the children and family members and the efforts of our many colleagues that make this work possible.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest:
Dr. Max and Dr. Giza report medico-legal consultation approximately equally for plaintiffs and defendants at approximately 10%. Dr. Thompson received partial research support from Biogen, Inc. for research unrelated to this manuscript.
References
- Acerini CL, Tasker RC (2007): Traumatic brain injury induced hypothalamic-pituitary dysfunction: a paediatric perspective. Pituitary 10:373–380. [DOI] [PubMed] [Google Scholar]
- Adelson P (2010): Clinical trials for pediatric TBI. Pediatric traumatic brain injury: New frontiers in clinical and translational research:54–67. [Google Scholar]
- Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, Kugel H, Engelen J, Dietsche B, Krug A, Kircher T, Fieremans E, Veraart J, Novikov DS, Boedhoe PSW, van der Werf YD, van den Heuvel OA, Ipser J, Uhlmann A, Stein DJ, Dickie E, Voineskos AN, Malhotra AK, Pizzagalli F, Calhoun VD, Waller L, Veer IM, Walter H, Buchanan RW, Glahn DC, Hong LE, Thompson PM, Kochunov P (2018): A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain Imaging Behav. 10.1007/s11682-018-9941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B, Murray AL, Loh WY, Matthews LG, Adamson C, Beare R, Chen J, Kelly CE, Rees S, Warfield SK, Anderson PJ, Doyle LW, Spittle AJ, Cheong JLY, Seal ML, Thompson DK (2017): A new neonatal cortical and subcortical brain atlas: the Melbourne Children’s Regional Infant Brain (M-CRIB) atlas. Neuroimage 147:841–851. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J (2005): Functional plasticity or vulnerability after early brain injury? Pediatrics 116:1374–1382. [DOI] [PubMed] [Google Scholar]
- Anderson V, Godfrey C, Rosenfeld JV, Catroppa C (2012): Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics 129:e254–61. [DOI] [PubMed] [Google Scholar]
- Anderson V, Yeates KO (2010): Pediatric Traumatic Brain Injury: New Frontiers in Clinical and Translational Research. Cambridge University Press. [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A (2011): Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 134:2197–2221. [DOI] [PubMed] [Google Scholar]
- Arambula SE, Reinl EL, El Demerdash N, McCarthy MM, Robertson CL (2019): Sex differences in pediatric traumatic brain injury. Experimental Neurology. http://dx.doi.Org/10.1016/j.expneurol.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwal S, Holshouser BA, Shu SK, Simmons PL, Perkin RM, Tomasi LG, Knierim DS, Sheridan C, Craig K, Andrews GH, Hinshaw DB (2000): Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol 23:114–125. [DOI] [PubMed] [Google Scholar]
- Au AK, Clark RSB (2017): Paediatric traumatic brain injury: prognostic insights and outlooks. Curr Opin Neurol 30:565–572. [DOI] [PubMed] [Google Scholar]
- Avants BB, Libon DJ, Rascovsky K, Boller A, McMillan CT, Massimo L, Coslett HB, Chatterjee A, Gross RG, Grossman M (2014): Sparse canonical correlation analysis relates network-level atrophy to multivariate cognitive measures in a neurodegenerative population. Neuroimage 84:698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Alger JR, Ellis-Blied MU, Giza CC, Dennis E, Olsen A, Mink R, Babbitt C, Johnson J, Thompson PM, Asarnow RF (2018): Whole Brain Magnetic Resonance Spectroscopic Determinants of Functional Outcomes in Pediatric Moderate/Severe Traumatic Brain Injury. J Neurotrauma 35:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Asarnow R (2009): Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology 23:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Marion SD, Copeland S, Alger JR, O’Neill J, Cazalis F, Mink R, Giza CC, Vu JA, Hilleary SM (2010): Metabolic levels in the corpus callosum and their structural and behavioral correlates after moderate to severe pediatric TBI. J Neurotrauma 27:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA (2006): MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging 24:801–811. [DOI] [PubMed] [Google Scholar]
- Babikian T, Merkley T, Savage RC, Giza CC, Levin H (2015): Chronic Aspects of Pediatric Traumatic Brain Injury: Review of the Literature. J Neurotrauma 32:1849–1860. [DOI] [PubMed] [Google Scholar]
- Banoei MM, Casault C, Metwaly SM, Winston BW (2018): Metabolomics and Biomarker Discovery in Traumatic Brain Injury. J Neurotrauma 35:1831–1848. [DOI] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D, Alia-Klein N, Potter AS, Paulus MP, Prouty D, Zucker RA, Sher KJ (2018): Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson B, Alger J, Babikian T, Harris AD, Holshouser B, Kirov II, Maudsley AA, Thompson PM, Dennis EL, Tate DF, Wilde EA, Lin A (2019): The Clinical Utility of Magnetic Resonance Spectroscopy in Traumatic Brain Injury: Recommendations from the ENIGMA MRS Working Group. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson MD, Guinn JE, McElligott J, Vitale R, Brown W, Fielding LP (1991): Behavioral disturbances in children after trauma. J Trauma 31:1363–1368. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Zelazo PD (2013): IX. NIH Toolbox Cognition Battery (CB): summary, conclusions, and implications for cognitive development. Monogr Soc Res Child Dev 78:133–146. [DOI] [PubMed] [Google Scholar]
- Bayly PV, Clayton EH, Genin GM (2012): Quantitative imaging methods for the development and validation of brain biomechanics models. Annu Rev Biomed Eng 14:369–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Anderson V (2010): SOCIAL: an integrative framework for the development of social skills. Psychol Bull 136:39–64. [DOI] [PubMed] [Google Scholar]
- Bennett KS, DeWitt PE, Harlaar N, Bennett TD (2017): Seizures in Children With Severe Traumatic Brain Injury. Pediatr Crit Care Med 18:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie HMA, Boersma M, Wattjes MP, Adriaanse S, Vermeulen RJ, Oostrom KJ, Huisman J, Veltman DJ, Delemarre-Van de Waal HA (2010): Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr 169:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2007): Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 21:515–531. [DOI] [PubMed] [Google Scholar]
- Bigler ED (2013): Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci 7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2016): Systems Biology, Neuroimaging, Neuropsychology, Neuroconnectivity and Traumatic Brain Injury. Front Syst Neurosci 10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, Taylor HG, Rubin KH, Vannatta K, Gerhardt CA, Stancin T, Owen Yeates K (2013): Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology 27:438–451. [DOI] [PubMed] [Google Scholar]
- Boedhoe PSW, van Rooij D, Hoogman M, Twisk JWR, Schmaal L, Abe Y, Alonso P, Ameis SH, Anikin A, Anticevic A, Aherson P, Arango C, Arnold PD, Assogna F, Auzias G, Banaschewski T, Baranov A, Batistuzzo MC, Baumeister S, Baur-Streubel R, Behrmann M, Bellgrove MA, Benedetti F, Beucke JC, Biederman J, Bollettini I, Bose A, Bralten J, Bramati IE, Brandeis D, Brem S, Brennan BP, Busatto GF, Calderoni S, Calvo A, Calvo R, Castellanos FX, Cercignani M, Chaim-Avancini TM, Chantiluke KC, Cheng Y, Cho KIK, Christakou A, Coghill D, Conzelmann A, Cubillo AI, Dale AM, Dallaspezia S, Daly E, Denys D, Deruelle C, Di Martino A, Dinstein I, Doyle AE, Durston S, Earl EA, Ecker C, Ehrlich S, Ely BA, Epstein JN, Ethofer T, Fair DA, Fallgatter AJ, Faraone SV, Fedor J, Feng X, Feusner JD, Fitzgerald J, Fitzgerald KD, Fouche J-P, Freitag CM, Fridgeirsson EA, Frodl T, Gabel MC, Gallagher L, Gogberashvili T, Gori I, Gruner P, Gürsel DA, Haar S, Haavik J, Hall GB, Harrison NA, Hartman CA, Heslenfeld DJ, Hirano Y, Hoekstra PJ, Hoexter MQ, Hohmann S, Høvik MF, Hu H, Huyser C, Jahanshad N, Jalbrzikowski M, James A, Janssen J, Jaspers-Fayer F, Jernigan TL, Kapilushniy D, Kardatzki B, Karkashadze G, Kathmann N, Kaufmann C, Kelly C, Khadka S, King JA, Koch K, Kohls G, Kohls K, Kuno M, Kuntsi J, Kvale G, Kwon JS, Lázaro L, Lera-Miguel S, Lesch K-P, Hoekstra L, Liu Y, Lochner C, Louza MR, Luna B, Lundervold AJ, Malpas CB, Marques P, Marsh R, Martínez-Zalacaín I, Mataix-Cols D, Mattos P, McCarthy H, McGrath J, Mehta MA, Menchón JM, Mennes M, Martinho MM, Moreira PS, Morer A, Morgado P, Muratori F, Murphy CM, Murphy DGM, Nakagawa A, Nakamae T, Nakao T, Namazova-Baranova L, Narayanaswamy JC, Nicolau R, Nigg JT, Novotny SE, Nurmi EL, Weiss EO, O’Gorman Tuura RL, O’Hearn K, O’Neill J, Oosterlaan J, Oranje B, Paloyelis Y, Parellada M, Pauli P, Perriello C, Piacentini J, Piras F, Piras F, Plessen KJ, Puig O, Antoni Ramos-Quiroga J, Janardhan Reddy YC, Reif A, Reneman L, Retico A, Rosa PGP, Rubia K, Rus OG, Sakai Y, Schrantee A, Schwarz L, Schweren LJS, Seitz J, Shaw P, Shook D, Silk TJ, Blair Simpson H, Skokauskas N, Vila JCS, Solovieva A, Soreni N, Soriano-Mas C, Spalletta G, Stern ER, Stevens MC, Evelyn Stewart S, Sudre G, Szeszko PR, Tamm L, Taylor MJ, Tolin DF, Tosetti M, Tovar-Moll F, Tsuchiyagaito A, van Erp TGM, van Wingen GA, Vance A, Venkatasubramanian G, Vilarroya O, Vives-Gilabert Y, von Polier GG, Walitza S, Wallace GL, Wang Z, Wolfers T, Yoncheva YN, Yun J-Y, Zanetti MV, Zhou F, Ziegler GC, Zierhut KC, Zwiers MP, the ENIGMA-ADHD working group, the ENIGMA-ASD working group, the ENIGMA-OCD working group, Thompson PM, Stein DJ, Buitelaar J, Franke B, van den Heuvel OA (2019): Subcortical brain volume, regional cortical thickness and cortical surface area across attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and obsessive-compulsive disorder (OCD). bioRxiv. https://www.biorxiv.org/content/10.1101/673012v1. [Google Scholar]
- Bondanelli M, De Marinis L, Ambrosio MR, Monesi M, Valle D, Zatelli MC, Fusco A, Bianchi A, Farneti M, Degli ECI (2004): Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma 21:685–696. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY (2000): Methodological issues in pediatric neuroimaging. Ment Retard Dev Disabil Res Rev 6:161–165. [DOI] [PubMed] [Google Scholar]
- Borsook D (2012): Neurological diseases and pain. Brain 135:320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener I, Harman JS, Kelleher KJ, Yeates KO (2004): Medical costs of mild to moderate traumatic brain injury in children. J Head Trauma Rehabil 19:405–412. [DOI] [PubMed] [Google Scholar]
- Brown G, Chadwick O, Shaffer D, Rutter M, Traub M (1981): A prospective study of children with head injuries: III. Psychiatric sequelae. Psychol Med 11:63–78. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE (2002): Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E (2002): Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol 17:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, De Decker C, Heitger M, Drijkoningen D, Vander Linden C, Sunaert S, Swinnen SP (2012): Brain connectivity and postural control in young traumatic brain injury patients: a diffusion MRI based network analysis. Neuroimage: clinical 1:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Coxon J, Leunissen I, Drijkoningen D, Geurts M, Gooijers J, Michiels K, Sunaert S, Swinnen SP (2011): Bimanual Coordination and Corpus Callosum Microstructure in Young Adults with Traumatic Brain Injury: A Diffusion Tensor Imaging Study. J Neurotrauma 28:897–913. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Geurts M, Taymans T, Vander Linden C, Smits-Engelsman BCM, Sunaert S, Swinnen SP (2010): Brain-behavior relationships in young traumatic brain injury patients: fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia 48:1472–1482. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Wenderoth N, Smits-Engelsman BCM, Sunaert S, Swinnen SP (2009): Neural correlates of motor dysfunction in children with traumatic brain injury: exploration of compensatory recruitment patterns. Brain 132:684–694. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Imaging P, Neurocognition and Genetics Study, Peng Q, Schork AJ, Lo M-T, Fan C-C, Wang Y, Desikan RS, Bettella F, Hagler DJ, Westlye LT, Kremen WS, Jernigan TL, Le Hellard S, Steen VM, Espeseth T, Huentelman M, Håberg AK, Agartz I, Djurovic S, Andreassen OA, Schork N, Dale AM, Alzheimer’s Disease Neuroimaging Initiative (2015): Large-scale genomics unveil polygenic architecture of human cortical surface area. Nature Communications. 10.1038/ncomms8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD, Centers for Disease, Control, Prevention (2011): Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ 60:1–32. [PubMed] [Google Scholar]
- Coronado VG, Haileyesus T, Cheng TA, Bell JM, Haarbauer-Krupa J, Lionbarger MR, Flores-Herrera J, McGuire LC, Gilchrist J (2015): Trends in Sports- and Recreation-Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001-2012. J Head Trauma Rehabil 30:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AM, Yang S, Hu P, Li Y, Lozanova P, Scalea TM, Stein DM (2019): Concomitant chest trauma and traumatic brain injury, biomarkers correlate with worse outcomes. J Trauma Acute Care Surg 87:S146–S151. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG (2007): Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma 24:1475–1486. [DOI] [PubMed] [Google Scholar]
- Dalamagkas K, Tsintou M, Rathi Y, O’Donnell LJ, Pasternak O, Gong X, Zhu A, Savadjiev P, Papadimitriou GM, Kubicki M, Yeterian EH, Makris N (2019): Individual variations of the human corticospinal tract and its hand-related motor fibers using diffusion MRI tractography. Brain Imaging Behav. http://dx.doi.Org/10.1007/s11682-018-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno ltMI (1999): Cortical Surface-Based Analysis. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Daviss WB, Mooney D, Racusin R, Ford JD, Fleischer A, McHugo GJ (2000): Predicting posttraumatic stress after hospitalization for pediatric injury. J Am Acad Child Adolesc Psychiatry 39:576–583. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ondersma SJ, Nordstrom-Klee B, Templin T, Ager J, Janisse J, Sokol RJ (2002): Violence Exposure, Trauma, and IQ and/or Reading Deficits Among Urban Children. Archives of Pediatrics & Adolescent Medicine. http://dx.doi.Org/10.1001/archpedi.156.3.280. [DOI] [PubMed] [Google Scholar]
- Dell’Italia J, Johnson MA, Vespa PM, Monti MM (2018): Network Analysis in Disorders of Consciousness: Four Problems and One Proposed Solution (Exponential Random Graph Models). Front Neurol 9:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Ellis MU, Marion SD, Jin Y, Moran L, Olsen A, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, Asarnow RF (2015a): Callosal Function in Pediatric Traumatic Brain Injury Linked to Disrupted White Matter Integrity. Journal of Neuroscience 35:10202–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Babikian T, Alger J, Rashid F, Villalon-Reina JE, Jin Y, Olsen A, Mink R, Babbitt C, Johnson J, Others (2018a): Magnetic resonance spectroscopy of fiber tracts in children with traumatic brain injury: A combined MRS--Diffusion MRI study. Hum Brain Mapp 39:3759–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Babikian T, Giza CC, Thompson PM, Asarnow RF (2018b): Neuroimaging of the Injured Pediatric Brain: Methods and New Lessons. Neuroscientist 24:652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Disner SG, Fani N, Salminen LE, Logue M, Clarke EK, Haswell CC, Averill CL, Baugh LA, Bomyea J, Bruce SE, Cha J, Choi K, Davenport ND, Densmore M, du Plessis S, Forster GL, Frijling JL, Gonenc A, Gruber S, Grupe DW, Guenette JP, Hayes J, Hofmann D, Ipser J, Jovanovic T, Kelly S, Kennis M, Kinzel P, Koch SBJ, Koerte I, Koopowitz S, Korgaonkar M, Krystal J, Lebois LAM, Li G, Magnotta VA, Manthey A, May GJ, Menefee DS, Nawijn L, Nelson SM, Neufeld RWJ, Nitschke JB, O’Doherty D, Peverill M, Ressler KJ, Roos A, Sheridan MA, Sierk A, Simmons A, Simons RM, Simons JS, Stevens J, Suarez-Jimenez B, Sullivan DR, Théberge J, Tran JK, van den Heuvel L, van der Werff SJA, van Rooij SJH, van Zuiden M, Velez C, Verfaellie M, Vermeiren RRJM, Wade BSC, Wager T, Walter H, Winternitz S, Wolff J, York G, Zhu Y, Zhu X, Abdallah CG, Bryant R, Daniels JK, Davidson RJ, Fercho KA, Franz C, Geuze E, Gordon EM, Kaufman ML, Kremen WS, Lagopoulos J, Lanius RA, Lyons MJ, McCauley SR, McGlinchey R, McLaughlin KA, Milberg W, Neria Y, Olff M, Seedat S, Shenton M, Sponheim SR, Stein DJ, Stein MB, Straube T, Tate DF, van der Wee NJA, Veltman DJ, Wang L, Wilde EA, Thompson PM, Kochunov P, Jahanshad N, Morey RA (2019): Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: results from the PGC-ENIGMA PTSD consortium. Mol Psychiatry. http://dx.doi.Org/10.1038/S41380-019-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jin Y, Villalon-Reina J, Zhan L, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC (2015b): White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. Neuroimage: Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Rashid F, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Asarnow RF, Thompson PM (2017a): Altered network topology in pediatric traumatic brain injury. In: . 13th International Conference on Medical Information Processing and Analysis. International Society for Optics and Photonics. Vol. 10572, p 105720P. [Google Scholar]
- Dennis EL, Rashid F, Jahanshad N, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Asarnow RF, Thompson PM (2017b): A NETWORK APPROACH TO EXAMINING INJURY SEVERITY IN PEDIATRIC TBI. Proc IEEE Int Symp Biomed Imaging 2017:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM (2013): Mapping connectivity in the developing brain. Int J Dev Neurosci 31:525–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Wilde EA, Newsome MR, Scheibel RS, Troyanskaya M, Velez C, Wade BSC, Drennon AM, York GE, Bigler ED, Abildskov TJ, Taylor BA, Jaramillo CA, Eapen B, Belanger H, Gupta V, Morey R, Haswell C, Levin HS, Hinds SR 2nd, Walker WC, Thompson PM, Tate DF (2018c): ENIGMA MILITARY BRAIN INJURY: A COORDINATED META-ANALYSIS OF DIFFUSION MRI FROM MULTIPLE COHORTS. Proc IEEE Int Symp Biomed Imaging 2018:1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis L.; Rashid E, Ellis F;., U.; Babikian M, Villalon-Reina T;., E.; Jin J, Olsen Y;., Mink A;., Babbitt R;., Johnson C;., Giza J;., C.; Thompson C, M.; Asarnow P, F R (2017c): Diverging White Matter Trajectories in Children after Traumatic Brain Injury: The RAPBI Study. Neurology 88:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Bigler ED, Abildskov T, Agostino A, Taylor HG, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Yeates KO (2013): Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Dev Cogn Neurosci 5:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Simic N, Sinopoli KJ, Wilkinson A, Yeates KO, Taylor HG, Bigler ED, Fletcher JM (2014): Functional plasticity in childhood brain disorders: when, what, how, and whom to assess. Neuropsychol Rev 24:389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health, Education, and Welfare, National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (2014): The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. J Am Coll Dent 81:4–13. [PubMed] [Google Scholar]
- Dewan MC, Mummareddy N, Wellons JC 3rd, Bonfield CM (2016): Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg 91:497–509.e1. [DOI] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts S, Blanken LME, Bookheimer SY, Braden BB, Byrge L, Castellanos FX, Dapretto M, Delorme R, Fair DA, Fishman I, Fitzgerald J, Gallagher L, Keehn RJJ, Kennedy DP, Lainhart JE, Luna B, Mostofsky SH, Müller R-A, Nebel MB, Nigg JT, O’Hearn K, Solomon M, Toro R, Vaidya CJ, Wenderoth N, White T, Craddock RC, Lord C, Leventhal B, Milham MP (2017): Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data 4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, Deen B, Delmonte S, Dinstein I, Ertl-Wagner B, Fair DA, Gallagher L, Kennedy DP, Keown CL, Keysers C, Lainhart JE, Lord C, Luna B, Menon V, Minshew NJ, Monk CS, Mueller S, Müller R-A, Nebel MB, Nigg JT, O’Hearn K, Pelphrey KA, Peltier SJ, Rudie JD, Sunaert S, Thioux M, Tyszka JM, Uddin LQ, Verhoeven JS, Wenderoth N, Wiggins JL, Mostofsky SH, Milham MP (2014): The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG (2005): The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma 22:106–118. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Arthurs OJ (2011): Paediatric MRI under sedation: is it necessary? What is the evidence for the alternatives? Pediatr Radiol 41:1353. [DOI] [PubMed] [Google Scholar]
- Etchell A, Adhikari A, Weinberg LS, Choo AL, Garnett EO, Chow HM, Chang S-E (2018): A systematic literature review of sex differences in childhood language and brain development. Neuropsychologia 114:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM (2008): Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Mendez D, Treble A, Payne C, Bachevalier J (2012): Social communication in young children with traumatic brain injury: relations with corpus callosum morphometry. Int J Dev Neurosci 30:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Johnson CP, Juranek J, DeMaster D, Prasad M, Duque G, Kramer L, Cox CS, Swank PR (2016): Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: Impact of age at injury and time since injury on pathway integrity. Hum Brain Mapp 37:3929–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Rose EJ, Darcey VL, Belcher AM, VanMeter JW (2016): Neurodevelopmental Precursors and Consequences of Substance Use during Adolescence: Promises and Pitfalls of Longitudinal Neuroimaging Strategies. Front Hum Neurosci 10:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR (2011): Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. https://www.sciencedirect.com/science/article/pii/S1053811910010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen TP, Anderson JS, Stephenson KG, Beck J, King JB, Kellems R, Top DN Jr, Russell NCC, Anderberg E, Lundwall RA, Hansen B, South M (2018): Functional MRI connectivity of children with autism and low verbal and cognitive performance. Mol Autism 9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B (2001): Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage 13:239–249. [DOI] [PubMed] [Google Scholar]
- Genc S, Anderson V, Ryan NP, Malpas CB, Catroppa C, Beauchamp MH, Silk TJ (2017): Recovery of White Matter following Pediatric Traumatic Brain Injury Depends on Injury Severity. J Neurotrauma 34:798–806. [DOI] [PubMed] [Google Scholar]
- Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, Hakonarson H, Gur RE, Gur RC (2017): Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood. J Neurosci 37:5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerring JP, Brady KD, Chen A, Vasa R, Grados M, Bandeen-Roche KJ, Nick Bryan R, Denckla MB (1998): Premorbid Prevalence of ADHD and Development of Secondary ADHD After Closed Head Injury. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1097/00004583-199806000-00015. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Giza CC, Mink RB, Madikians A (2007): Pediatric traumatic brain injury: not just little adults. Curr Opin Crit Care 13:143–152. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapaport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh SYM, Irimia A, Torgerson CM, Van Horn JD (2014): Neuroinformatics challenges to the structural, connectomic, functional and electrophysiological multimodal imaging of human traumatic brain injury. Front Neuroinform 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein FC, Caveney AF, Hertzberg VS, Silbergleit R, Yeatts SD, Palesch YY, Levin HS, Wright DW (2017): Very Early Administration of Progesterone Does Not Improve Neuropsychological Outcomes in Subjects with Moderate to Severe Traumatic Brain Injury. J Neurotrauma 34:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold N, Bas-Hoogendam JM, Amod AR, van Velzen L, Aghajani M, Filippi C, Gold A, Ching CRK, Roelofs K, Furmark T, Others (2018): F27. subcortical volumes in social anxiety disorder: preliminary results from enigma-anxiety. Biol Psychiatry 83:S247–S248. [Google Scholar]
- Hajek CA, Yeates KO, Gerry Taylor H, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M (2010): Relationships among post-concussive symptoms and symptoms of PTSD in children following mild traumatic brain injury. Brain Inj 24:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hillary FG (2019): Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small-world. Netw Neurosci 3:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Keser Z, Schulz PE, Wilde EA (2018): Multimodal Advanced Imaging for Concussion. Neuroimaging Clin N Am 28:31–42. [DOI] [PubMed] [Google Scholar]
- Hebb DO (1949): The organization of behavior ; a neuropsycholocigal theory. A Wiley Book in Clinical Psychology:62–78. [Google Scholar]
- Hernandez F, Giordano C, Goubran M, Parivash S, Grant G, Zeineh M, Camarillo D (2019): Lateral impacts correlate with falx cerebri displacement and corpus callosum trauma in sports-related concussions. Biomech Model Mechanobiol. 10.1007/s10237-018-01106-0. [DOI] [PubMed] [Google Scholar]
- Herting MM, Sowell ER (2017): Puberty and structural brain development in humans. Front Neuroendocrinol 44:122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R (2015): Ethical and regulatory considerations in the design of traumatic brain injury clinical studies. Traumatic Brain Injury, Part II. 10.1016/b978-0-444-63521-1.00046-7. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J (2006): Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp 27:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Hoyt DB, Coimbra R, Potenza B, Sise M, Anderson JP (2005): Long-term posttraumatic stress disorder persists after major trauma in adolescents: new data on risk factors and functional outcome. J Trauma 58:764–9; discussion 769–71. [DOI] [PubMed] [Google Scholar]
- Holshouser B, Pivonka-Jones J, Nichols JG, Oyoyo U, Tong K, Ghosh N, Ashwal S (2019): Longitudinal Metabolite Changes after Traumatic Brain Injury: A Prospective Pediatric Magnetic Resonance Spectroscopic Imaging Study. J Neurotrauma 36:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, Ashwal S (2005): Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR Am J Neuroradiol 26:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, Jahanshad N, Sudre G, Wolfers T, Earl EA, Soliva Vila JC, Vives-Gilabert Y, Khadka S, Novotny SE, Hartman CA, Heslenfeld DJ, Schweren LJS, Ambrosino S, Oranje B, de Zeeuw P, Chaim-Avancini TM, Rosa PGP, Zanetti MV, Malpas CB, Kohls G, von Polier GG, Seitz J, Biederman J, Doyle AE, Dale AM, van Erp TGM, Epstein JN, Jernigan TL, Baur-Streubel R, Ziegler GC, Zierhut KC, Schrantee A, Høvik MF, Lundervold AJ, Kelly C, McCarthy H, Skokauskas N, O’Gorman Tuura RL, Calvo A, Lera-Miguel S, Nicolau R, Chantiluke KC, Christakou A, Vance A, Cercignani M, Gabel MC, Asherson P, Baumeister S, Brandeis D, Hohmann S, Bramati IE, Tovar-Moll F, Fallgatter AJ, Kardatzki B, Schwarz L, Anikin A, Baranov A, Gogberashvili T, Kapilushniy D, Solovieva A, El Marroun H, White T, Karkashadze G, Namazova-Baranova L, Ethofer T, Mattos P, Banaschewski T, Coghill D, Plessen KJ, Kuntsi J, Mehta MA, Paloyelis Y, Harrison NA, Bellgrove MA, Silk TJ, Cubillo Al, Rubia K, Lazaro L, Brem S, Walitza S, Frodl T, Zentis M, Castellanos FX, Yoncheva YN, Haavik J, Reneman L, Conzelmann A, Lesch K-P, Pauli P, Reif A, Tamm L, Konrad K, Oberwelland Weiss E, Busatto GF, Louza MR, Durston S, Hoekstra PJ, Oosterlaan J, Stevens MC, Ramos-Quiroga JA, Vilarroya O, Fair DA, Nigg JT, Thompson PM, Buitelaar JK, Faraone SV, Shaw P, Tiemeier H, Bralten J, Franke B (2019): Brain Imaging of the Cortex in ADHD: A Coordinated Analysis of Large-Scale Clinical and Population-Based Samples. Am J Psychiatry 176:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Styner MA, Gao W, Yap P-T, Wang L, Baluyot K, Yacoub E, Chen G, Potts T, Salzwedel A, Li G, Gilmore JH, Piven J, Smith JK, Shen D, Ugurbil K, Zhu H, Lin W, Elison JT (2019): The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. Neuroimage 185:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman TAGM (2015): Accidental Head Trauma in Children. In: Rossi A, editor. Pediatric Neuroradiology. Berlin, Heidelberg: Springer Berlin Heidelberg. pp 1–35. [Google Scholar]
- Hunter JV, Thornton RJ, Wang ZJ, Levin HS, Roberson G, Brooks WM, Swank PR (2005): Late proton MR spectroscopy in children after traumatic brain injury: correlation with cognitive outcomes. AJNR Am J Neuroradiol 26:482–488. [PMC free article] [PubMed] [Google Scholar]
- Hunter LE, Lubin N, Glassman NR, Xue X, Spira M, Lipton ML (2019): Comparing Region of Interest versus Voxel-Wise Diffusion Tensor Imaging Analytic Methods in Mild and Moderate Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J Neurotrauma 36:1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EB, Schwerin SC, Avram AV, Juliano SL, Pierpaoli C (2018): Diffusion MRI and the detection of alterations following traumatic brain injury. J Neurosci Res 96:612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imms P, Clemente A, Cook M, D’Souza W, Wilson PH, Jones DK, Caeyenberghs K (2019): The structural connectome in traumatic brain injury: A meta-analysis of graph metrics. Neurosci Biobehav Rev 99:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014): Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail FY, Fatemi A, Johnston MV (2017): Cerebral plasticity: Windows of opportunity in the developing brain. Eur J Paediatr Neurol 21:23–48. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Thompson PM (2017): Multimodal neuroimaging of male and female brain structure in health and disease across the life span. J Neurosci Res 95:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012): FSL. Neuroimage. http://dx.doi.Org/10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jia T, Chu C, Liu Y, van Dongen J, Armstrong NJ, Bastin ME, Carrillo-Roa T, den Braber A, Harris M, Jansen R, Liu J, Luciano M, Ori APS, Santiañez RR, Ruggeri B, Sarkisyan D, Shin J, Sungeun K, Gutiérrez DT, Ent DV, Ames D, Artiges E, Bakalkin G, Banaschewski T, Bokde ALW, Brodaty H, Bromberg U, Brouwer R, Büchel C, Quinlan EB, Cahn W, de Zubicaray GI, Ekström TJ, Flor H, Fröhner JH, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, Jahanshad N, Jiang J, Kwok JB, Martin NG, Martinot J-L, Mather KA, McMahon KL, McRae AF, Nees F, Orfanos DP, Paus T, Poustka L, Samann PG, Schofield PR, Smolka MN, Strike LT, Teeuw J, Thalamuthu A, Trollor J, Walter H, Wardlaw JM, Wen W, Whelan R, Apostolova LG, Binder EB, Boomsma DI, Calhoun V, Crespo-Facorro B, Deary IJ, Pol HH, Ophoff RA, Pausova Z, Sachdev PS, Saykin A, Wright MJ, Thompson PM, Schumann G, Desrivières S (2018): Epigenome-wide meta-analysis of blood DNA methylation and its association with subcortical volumes: findings from the ENIGMA Epigenetics Working Group. bioRxiv. https://www.biorxiv.org/content/10.1101/460444v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: the do’s and don'ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Kandel BM, Wang DJJ, Gee JC, Avants BB (2015): Eigenanatomy: sparse dimensionality reduction for multimodal medical image analysis. Methods 73:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Tolvanen M, Scheinin NM, Uusitupa H-M, Korja R, Ekholm E, Tuulari JJ, Pajulo M, Huotilainen M, Paunio T, Karlsson H, FinnBrain Birth Cohort Study Group (2018): Cohort Profile: The FinnBrain Birth Cohort Study (FinnBrain). Int J Epidemiol 47:15–16j. [DOI] [PubMed] [Google Scholar]
- Keenan HT, Bratton SL (2006): Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci 28:256–263. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Sinopoli KJ, Davis KD, Mikulis DJ, Wennberg R, Tartaglia MC, Chen J-K, Tator CH (2014): Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front Hum Neurosci 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA (2009): Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron 64:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani A, Rohr CS, Sojoudi A, Bray S, Barlow KM (2019): Alteration in Cerebral Activation during a Working Memory Task after Pediatric Mild Traumatic Brain Injury: A Prospective Controlled Cohort Study. J Neurotrauma. http://dx.doi.Org/10.1089/neu.2018.6117. [DOI] [PubMed] [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, Detre JA, Gee JC (2008): Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage 39:1014–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DJ, Ellis KR, Seri S, Wood AG (2019): A systematic review of cross-sectional differences and longitudinal changes to the morphometry of the brain following paediatric traumatic brain injury. Neuroimage Clin 23:101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs M, van Heurn LWE, Bakx R, Jeroen Vermeulen R, Carel Goslings J, Bwee Tien Poll-The, van der Wees M, Catsman-Berrevoets CE, Oosterlaan J, Pouwels PJW (2017): The structural connectome of children with traumatic brain injury. Human Brain Mapping. 10.1002/hbm.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ME, Chiu C-YP, Shear PK, Wade SL (2009): Neural correlates of verbal associative memory and mnemonic strategy use following childhood traumatic brain injury. J Pediatr Rehabil Med 2:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsrud SK, Fjell AM, Tamnes CK, Grydeland H, Mork L, Due-Tønnessen P, Bjørnerud A, Sampaio-Baptista C, Andersson J, Johansen-Berg H, Walhovd KB (2016): Changes in white matter microstructure in the developing brain--A longitudinal diffusion tensor imaging study of children from 4 to 11years of age. Neuroimage 124:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen GY, Schober M, Fabio A, Wisniewski SR, Grant MJC, Shafi N, Bennett TD, Hirtz D, Bell MJ (2016): Structure, Process, and Culture Differences of Pediatric Trauma Centers Participating in an International Comparative Effectiveness Study of Children with Severe Traumatic Brain Injury. Neurocrit Care 24:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, Bloomberg JJ, Seidler RD (2019): Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 10.1001/jamaneurol.2018.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, Fletcher JM (2000): Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 54:647–653. [DOI] [PubMed] [Google Scholar]
- Liesemer K, Bratton SL, Zebrack CM, Brockmeyer D, Statler KD (2011): Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma 28:755–762. [DOI] [PubMed] [Google Scholar]
- Li L, Liu J (2013): The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Dev Med Child Neurol 55:37–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey HM, Lalani SJ, Mietchen J, Gale SD, Wilde EA, Faber J, MacLeod MC, Hunter JV, Chu ZD, Aitken ME, Ewing-Cobbs L, Levin HS (2019): Acute pediatric traumatic brain injury severity predicts long-term verbal memory performance through suppression by white matter integrity on diffusion tensor imaging. Brain Imaging Behav. 10.1007/s11682-019-00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder RT, Warschausky S, Schwartz EM, Hensinger RN, Greenfield ML (1995): The psychosocial characteristics of children with fractures. J Pediatr Orthop 15:41–46. [DOI] [PubMed] [Google Scholar]
- Lorenzen P, Prastawa M, Davis B, Gerig G, Bullitt E, Joshi S (2006): Multi-modal image set registration and atlas formation. Med Image Anal 10:440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies SS, Thibault KL (2000): Infant skull and suture properties: measurements and implications for mechanisms of pediatric brain injury. J Biomech Eng 122:364–371. [DOI] [PubMed] [Google Scholar]
- Max JE, Castillo CS, Bokura H, Robin DA, Lindgren SD, Smith WL Jr, Sato Y, Mattheis PJ (1998): Oppositional defiant disorder symptomatology after traumatic brain injury: a prospective study. J Nerv Ment Dis 186:325–332. [DOI] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, Saunders A, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT (2011): Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci 23:29–39. [DOI] [PubMed] [Google Scholar]
- Max JE, Wilde EA, Bigler ED, Hanten G, Dennis M, Schachar RJ, Saunders AE, Ewing-Cobbs L, Chapman SB, Thompson WK, Yang TT, Levin HS (2015): Personality Change Due to Traumatic Brain Injury in Children and Adolescents: Neurocognitive Correlates. J Neuropsychiatry Clin Neurosci 27:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Wilde EA, Bigler ED, MacLeod M, Vasquez AC, Schmidt AT, Chapman SB, Hotz G, Yang TT, Levin HS (2012): Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsychiatry Clin Neurosci 24:427–436. [DOI] [PubMed] [Google Scholar]
- McDonald SJ, Sun M, Agoston DV, Shultz SR (2016): The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J Neuroinflammation 13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Squeglia LM, Jacobus J, Silk JS (2018): Adolescent Brain Development: Implications for Understanding Risk and Resilience Processes Through Neuroimaging Research. J Res Adolesc 28:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison WE, Arbelaez JJ, Fackler JC, De Maio A, Paidas CN (2004): Gender and age effects on outcome after pediatric traumatic brain injury. Pediatr Crit Care Med 5:145–151. [DOI] [PubMed] [Google Scholar]
- Moyer D, Ver Steeg G, Tax CMW, Thompson PM (2019): Scanner Invariant Representations for Diffusion MRI Harmonization. arXiv [q-bio.QM]. arXiv. http://arxiv.org/abs/1904.05375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, Zhen Z, Cox S, Weiner KS, Weiskopf N, Others (2019): Apparent thinning of human visual cortex during childhood is associated with myelination. Proceedings of the National Academy of Sciences 116:20750–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Hanten G, Chu Z, Steinberg JL, Hunter JV, Lu H, Vasquez AC, Li X, Lin X, Cook L, Levin HS (2010): Brain activation while thinking about the self from another person’s perspective after traumatic brain injury in adolescents. Neuropsychology 24:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Hunter JV, Wang ZJ, Chu Z, Li X, Levin HS (2007): Brain activation during working memory after traumatic brain injury in children. Neurocase 13:16–24. [DOI] [PubMed] [Google Scholar]
- Nicholson SE, Watts LT, Burmeister DM, Merrill D, Scroggins S, Zou Y, Lai Z, Grandhi R, Lewis AM, Newton LM, Eastridge BJ, Schwacha MG (2018): Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock. 10.1097/SHK.0000000000001211. [DOI] [PubMed] [Google Scholar]
- Nir TM, Lam HY, Ananworanich J, Boban J, Brew BJ, Cysique L, Fouche JP, Kuhn T, Porges ES, Law M, Paul RH, Thames A, Woods AJ, Valcour VG, Thompson PM, Cohen RA, Stein DJ, Jahanshad N (2019): Effects of Diffusion MRI Model and Harmonization on the Consistency of Findings in an International Multi-cohort HIV Neuroimaging Study. In: . Computational Diffusion MRI. Springer International Publishing. pp 203–215. [Google Scholar]
- Nir TM, Jahanshad N, Villalon-Reina JE, Isaev D, Zavaliangos-Petropulu A, Zhan L, Leow AD, Jack CR Jr, Weiner MW, Thompson PM, Alzheimer’s Diseaase Neuroimaginng, Initiative (2017): Fractional anisotropy derived from the diffusion tensor distribution function boosts power to detect Alzheimer’s disease deficits. Magn Reson Med 78:2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, Babikian T, Dennis E, Ellis-Blied MU, Giza CC, Marion SD, Mink R, Johnson J, Babbitt C, Thompson P, Asarnow RF (2019): Functional brain hyper-activations are linked to an electrophysiological measure of slow inter-hemispheric transfer time after pediatric moderate/severe traumatic brain injury. J Neurotrauma. http://dx.doi.Org/10.1089/neu.2019.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, Brunner JF, Indredavik Evensen KA, Finnanger TG, Vik A, Skandsen T, Landrø NI, Håberg AK (2015): Altered Cognitive Control Activations after Moderate-to-Severe Traumatic Brain Injury and Their Relationship to Injury Severity and Everyday-Life Function. Cereb Cortex 25:2170–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, Ferenc Brunner J, Evensen KAI, Garzon B, Landrø NI, Håberg AK (2013): The functional topography and temporal dynamics of overlapping and distinct brain activations for adaptive task control and stable task-set maintenance during performance of an fMRI-adapted clinical continuous performance test. J Cogn Neurosci 25:903–919. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Sagar S, Schachar RJ, Ewing-Cobbs L, Chapman SB, Dennis M, Saunders AE, Yang TT, Levin HS, Max JE (2014): Neuropsychological performance of youth with secondary attention-deficit/hyperactivity disorder 6- and 12-months after traumatic brain injury. J Int Neuropsychol Soc 20:971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palk A, Illes J, Thompson PM, Stein DJ (2019): Ethical Issues in Global Imaging Genetics Collaborations. Neuroimage In Review. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB (2005): The plastic human brain cortex. Annu Rev Neurosci 28:377–401. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A, Schönberger M (2012): Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology 26:304–313. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE (2013): Control-related systems in the human brain. Curr Opin Neurobiol 23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ (2006): Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging 23:816–826. [DOI] [PubMed] [Google Scholar]
- Rabinowitz AR, Levin HS (2014): Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 37:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt DA, Tournier J-D, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A (2017): Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SP, Zhang N, Kirkwood MW, Taylor HG, Stancin T, Brown TM, Wade SL (2018): Online Family Problem Solving for Pediatric Traumatic Brain Injury: Influences of Parental Marital Status and Participation on Adolescent Outcomes. J Head Trauma Rehabil 33:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Rao S, Rincon S, Caruso P, Ptak T, Raja AS, Prabhakar AM, Harvey HB (2016): Assessment of Pediatric Neurotrauma Imaging Appropriateness at a Level I Pediatric Trauma Center. J Am Coll Radiol 13:788–793. [DOI] [PubMed] [Google Scholar]
- Resnik DB (2009): Re-consenting human subjects: ethical, legal and practical issues. J Med Ethics 35:656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ (2000): Developmental neuroimaging of children using magnetic resonance techniques. Ment Retard Dev Disabil Res Rev 6:68–80. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Mathias JL, Rose SE (2016): Relationship Between Diffusion Tensor Imaging (DTI) Findings and Cognition Following Pediatric TBI: A Meta-Analytic Review. Dev Neuropsychol 41:176–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Hall ED (2000): Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma 17:1155–1169. [DOI] [PubMed] [Google Scholar]
- Rosema S, Crowe L, Anderson V (2012): Social function in children and adolescents after traumatic brain injury: a systematic review 1989-2011. J Neurotrauma 29:1277–1291. [DOI] [PubMed] [Google Scholar]
- Rosenbaum SB, Lipton ML (2012): Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging Behav 6:255–282. [DOI] [PubMed] [Google Scholar]
- Rose SR, Auble BA (2012): Endocrine changes after pediatric traumatic brain injury. Pituitary 15:267–275. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M (2006): Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp 27:973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell KL, Kutchko KM, Fowler SC, Berman NEJ, Levant B (2011): Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: assessment of sex differences. J Neurosci Methods 199:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Beauchamp MH, Anderson VA (2015): The emergence of age-dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Hum Brain Mapp 36:1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen V, Leemans A, Tax CMW (2018): Fast and accurate Slicewise OutLIer Detection (SOLID) with informed model estimation for diffusion MRI data. Neuroimage. http://dx.doi.Org/10.1016/j.neuroimage.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Richards JE, Almli CR (2012): Age-specific MRI templates for pediatric neuroimaging. Dev Neuropsychol 37:379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Hopson R, Prabhakaran K, Behr M, Qiu H, Mentch FD, Chiavacci R, Sleiman PMA, Gur RC, Hakonarson H, Gur RE (2016): The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage 124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, Mentch FD, Sleiman P, Verma R, Davatzikos C, Hakonarson H, Gur RC, Gur RE (2014): Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS (2017): Functional Magnetic Resonance Imaging of Cognitive Control following Traumatic Brain Injury. Front Neurol 8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W (2010): White matter development during adolescence as shown by diffusion MRI. Brain Cogn 72:16–25. [DOI] [PubMed] [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, Gauthier S, Pruessner JC (2016): Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage 129:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, O’Brien TJ (2017): Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav Brain Res 319:48–62. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M (2010): Pediatric traumatic brain injury: rehabilitation and transition to home and school. Appl Neuropsychol 17:116–122. [DOI] [PubMed] [Google Scholar]
- Sheeler CA (2016): The Influence of Systemic Immune Response and Sleep Modulation on the Secondary Effects of Traumatic Brain Injury in the Rodent Model. J Neurosci 36:7341–7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores DR, Everett AD (2018): Children as Biomarker Orphans: Progress in the Field of Pediatric Biomarkers. J Pediatr 193:14–20.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills MR, Libby AM, Orton HD (2005): Prehospital and in-hospital mortality: a comparison of intentional and unintentional traumatic brain injuries in Colorado children. Arch Pediatr Adolesc Med 159:665–670. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE (2004): Sex differences in injury severity and outcome measures after traumatic brain injury. Arch Phys Med Rehabil 85:376–379. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Michael Brady J, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. http://dx.doi.Org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Elam JS, Gaffrey MS, Harms MP, Hodge C, Kandala S, Kastman EK, Nichols TE, Schlaggar BL, Smith SM, Thomas KM, Yacoub E, Van Essen DC, Barch DM (2018): The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5-21 year olds. Neuroimage 183:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002): Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 44:4–16. [DOI] [PubMed] [Google Scholar]
- Sta Maria NS, Sargolzaei S, Prins ML, Dennis EL, Asarnow RF, Hovda DA, Harris NG, Giza CC (2019): Bridging the gap: Mechanisms of plasticity and repair after pediatric TBI. Exp Neurol 318:78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Salorio CF, Barber AD, Risen SR, Mostofsky SH, Suskauer SJ (2018): Preliminary findings of altered functional connectivity of the default mode network linked to functional outcomes one year after pediatric traumatic brain injury. Dev Neurorehabil 21:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Pasternak O, Kubicki M, Rathi Y, Zhang T, Wang J, Li H, Woodberry KA, Xu L, Qian Z, Zhu A, Whitfield-Gabrieli S, Keshavan MS, Niznikiewicz M, Stone WS, McCarley RW, Shenton ME, Wang J, Seidman LJ (2019): Altered Cellular White Matter But Not Extracellular Free Water on Diffusion MRI in Individuals at Clinical High Risk for Psychosis. Am J Psychiatry:appiajp201918091044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, Xu L (2017): Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Jahanshad N, Ching CRK, Salminen L, Thomopoulos SI, Bright J, Baune BT, Bertolin S, Bralten J, Bruin WB, Bülow R, Chen J, Chye Y, Dannlowski U, de Kovel CGF, Donohoe G, Eyler L, Faraone SV, Favre P, Filippi CA, Frodl T, Garijo D, Gil Y, Grabe HJ, Grasby KL, Hajek T, Han LKM, Hatton SN, Hilbert K, Ho TC, Holleran L, Homuth G, Hosten N, Houenou J, Ivanov I, Jia T, Kelly S, Klein M, Kwon JS, Laansma MA, Leerssen J, Lueken U, Nunes A, O’Neill J, Opel N, Piras F, Piras F, Postema M, Pozzi E, Shatokhina N, Soriano-Mas C, Spalletta G, Sun D, Teumer A, Tilot AK, Tozzi L, van der Merwe C, Van Someren E, van Wingen G, Völzke H, Walton E, Wang L, Winkler AM, Wittfeld K, Wright MJ, Yun J-Y, Zhang G, Zhang-James Y, Adhikari BM, Agartz I, Aghajani M, Aleman A, Althoff RR, Altmann A, Andreassen OA, Baron DA, Bartnik-Olson BL, Bas-Hoogendam JM, Baskin-Sommers A, Bearden CE, Berner LA, Boedhoe PSW, Brouwer RM, Buitelaar J, Caeyenberghs K, Cecil CAM, Cohen RA, Cole J, Conrod PJ, De Brito SA, de Zwarte SMC, Dennis EL, Desrivieres S, Dima D, Ehrlich S, Esopenko C, Fairchild G, Fisher S, Fouche J-P, Francks C, Frangou S, Franke B, Garavan H, Glahn DC, Groenewold NA, Gurholt TP, Gutman BA, Hahn T, Harding I, Hernaus D, Hibar DP, Hillary F, Hoogman M, Pol HEH, Jalbrzikowski M, Karkashadze GA, Klapwijk E, Knickmeyer RC, Kochunov P, Koerte IK, Kong X, Liew S-L, Lin AP, Logue MW, Luders E, Macciardi F, Mackey S, Mayer AR, McDonald CR, McMahon AB, Medland SE, Modinos G, Morey RA, Mueller SC, Mukherjee P, Namazova-Baranova L, Nir TM, Olsen A, Paschou P, Pine D, Pizzagalli F, Renteria ME, Rohrer JD, Sämann PG, Schmaal L, Schumann G, Shiroishi MS, Sisodiya SM, Smit DJA, Sønderby IE, Stein DJ, Stein JL, Tahmasian M, Tate DF, Turner J, van den Heuvel OA, van der Wee N, van der Werf YD, van Erp TGM, van Haren N, van Rooij D, van Velzen LS, Veer I, Veltman DJ, Villalon-Reina JE, Walter H, Whelan CD, Wilde EA, Zarei M, Zelman V (2019): ENIGMA and Global Neuroscience: A Decade of Large-Scale Studies of the Brain in Health and Disease across more than 40 Countries. PsyArXiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, McIntosh AR, Levine B (2011): Prefrontal Compensatory Engagement in TBI is due to Altered Functional Engagement Of Existing Networks and not Functional Reorganization. Front Syst Neurosci 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I, Waller L, Lett T, Erk S, Walter H (2019): ENIGMA task-based fMRI: A workgroup studying the genetic basis of task-evoked brain activity. In: .
- Verhelst H, Linden CV, De Pauw T, Vingerhoets G, Caeyenberghs K (2018): Impaired rich club and increased local connectivity in children with traumatic brain injury: Local support for the rich? Human Brain Mapping. http://dx.doi.Org/10.1002/hbm.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, Pérez-Stable EJ, Riley WT, Bloch MH, Conway K, Deeds BG, Dowling GJ, Grant S, Howlett KD, Matochik JA, Morgan GD, Murray MM, Noronha A, Spong CY, Wargo EM, Warren KR, Weiss SRB (2018): The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci 32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Michaud L, Brown TM (2006): Putting the pieces together: preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. J Head Trauma Rehabil 21:57–67. [DOI] [PubMed] [Google Scholar]
- Wallace EJ, Mathias JL, Ward L (2018): Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: a meta-analysis. Brain Imaging Behav 12:1607–1621. [DOI] [PubMed] [Google Scholar]
- Walz NC, Cecil KM, Wade SL, Michaud LJ (2008): Late proton magnetic resonance spectroscopy following traumatic brain injury during early childhood: relationship with neurobehavioral outcomes. J Neurotrauma 25:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein R, Tyndall JA, Manley GT (2018): An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn 18:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CG, DeMaster D, Ewing-Cobbs L (2019): Graph theory analysis of DTI tractography in children with traumatic injury. Neuroimage Clin 21:101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R, Minnes P, Phillips M (2009): Predicting social and functional outcomes for individuals sustaining paediatric traumatic brain injury. Dev Neurorehabil 12:12–23. [DOI] [PubMed] [Google Scholar]
- Westfall DR, West JD, Bailey JN, Arnold TW, Kersey PA, Saykin AJ, McDonald BC (2015): Increased brain activation during working memory processing after pediatric mild traumatic brain injury (mTBI). J Pediatr Rehabil Med 8:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Bouix S, Tate DF, Lin AP, Newsome MR, Taylor BA, Stone JR, Montier J, Gandy SE, Biekman B, Shenton ME, York G (2015): Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav 9:367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Dennis EL, Tate DF (2019a): The ENIGMA Brain Injury Working Group: Approach, Challenges, and Potential Benefits. Brain Imaging Behav Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Pedroza C, Roberson GS, Bachevalier J, Levin HS (2005): Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma 22:333–344. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Ware AL, Li X, Wu TC, McCauley SR, Barnes A, Newsome MR, Biekman BD, Hunter JV, Chu ZD, Levin HS (2019b): Orthopedic Injured versus Uninjured Comparison Groups for Neuroimaging Research in Mild Traumatic Brain Injury. J Neurotrauma 36:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO (2009): Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Myseros JS, Schmithorst VJ, Ball WS Jr (2003): Functional magnetic resonance imaging in pediatrics. Neuropediatrics 34:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R (2015): Progesterone in traumatic brain injury. The New England journal of medicine. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, McCauley SR, Schnelle KP, Vasquez AC, Chu Z, Hanten G, Hunter JV, Levin HS (2010): Longitudinal Changes in the Corpus Callosum following Pediatric Traumatic Brain Injury. Dev Neurosci. http://www.karger.com/Article/Pdf/317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guo Y, Wen D, Yang L, Chen Y, Zhang G, Fan Z (2016): Bone Fracture Enhances Trauma Brain Injury. Scand J Immunol 83:26–32. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Beauchamp M, Craig W, Doan Q, Zemek R, Bjornson B, Gravel J, Mikrogianakis A, Goodyear B, Abdeen N, Beaulieu C, Dehaes M, Deschenes S, Harris A, Lebel C, Lamont R, Williamson T, Barlow KM, Bernier F, Brooks BL, Emery C, Freedman SB, Kowalski K, Mrklas K, Tomfohr-Madsen L, Schneider KJ, Pediatric Emergency Research Canada (PERC) (2017): Advancing Concussion Assessment in Pediatrics (A-CAP): a prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: protocol study. BMJ Open 7:e017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Gerhardt CA, Bigler ED, Abildskov T, Dennis M, Rubin KH, Stancin T, Taylor HG, Vannatta K (2013): Peer relationships of children with traumatic brain injury. J Int Neuropsychol Soc 19:518–527. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N (2004): Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc 10:412–426. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL (2010): The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology 24:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Treble-Barna A, Sohlberg MM, Harn B, Wade SL (2017): Changes in Structural Connectivity Following a Cognitive Intervention in Children With Traumatic Brain Injury. Neurorehabil Neural Repair 31:190–201. [DOI] [PubMed] [Google Scholar]
- Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AIR, Mukherjee P, Yuh EL (2013): Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma 30:1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliangos-Petropulu A, Nir TM, Thomopoulos SI, Jahanshad N, Reid RI, Bernstein MA, Borowski B, Jack CR Jr, Weiner MW, Thompson PM (2018): Ranking diffusion tensor measures of brain aging and Alzheimer’s disease. In: . 14th International Symposium on Medical Information Processing and Analysis. International Society for Optics and Photonics. Vol. 10975, p 109750O. [Google Scholar]
- Zhu AH, Moyer DC, Nir TM, Thompson PM, Jahanshad N (2019): Challenges and Opportunities in dMRI Data Harmonization. In: . Computational Diffusion MRI. Springer International Publishing. pp 157–172. [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW (2010): Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences 107:18191–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.