Abstract
The management of persistent postsurgical pain and neuropathic pain remains a challenge in the clinic. Local anesthetics have been widely used as simple and effective treatment for these two disorders, but the duration of their analgesic effect is short. We here reported a new poly lactic-co-glycolic acid (PLGA)-coated ropivacaine that was continuously released in vitro for at least 6 days. Peri-sciatic nerve injection of the PLGA-coated ropivacaine attenuated paw incision-induced mechanical allodynia and heat hyperalgesia during the incisional pain period, and spared nerve injury-induced mechanical and cold allodynia for at least 7 days post-injection. This effect was dose-dependent. Peri-sciatic nerve injection of the PLGA-coated ropivacaine did not produce detectable inflammation, tissue irritation, or damage in the sciatic nerve and surrounding muscles at the injected site, dorsal root ganglion, spinal cord or brain cortex, although the scores for grasping reflex were mildly and transiently reduced in the higher dosage-treated groups.
Perspective:
Given that PLGA is an FDA-approved medical material, and that ropivacaine is used currently in clinical practice, the injectable PLGA-coated ropivacaine represents a new and highly promising avenue in the management of postsurgical pain and neuropathic pain.
Keywords: Microparticles, Neuropathic pain, PLGA, Postsurgical pain, Ropivacaine, Long-lasting analgesia
Introduction
Despite great efforts in research on the control of persistent postsurgical pain during past decades, management of this disorder remains a challenge in a large number of patients59. Systemic administration of analgesic drugs (e.g., opioids) may cause severe side effects, especially when given repeatedly8. Local anesthetics (LA; e.g., ropivacaine (RVC)) have been used widely as simple and effective treatment for persistent pain with absent or reduced adverse effects39. However, their analgesic effect lasts only several hours after single injection41. Given that postsurgical pain may persist for several days, months, and in some cases, even more than one year60, prolonged, continuous infusions of LA through a catheter implanted near the target nerve tissue are often required, although long-tern catheter use is limited due to adverse effects including tissue damage and infection16, 48. A sustained release strategy has also been employed using polymeric microparticles 5 μm to 500 μm in diameter as implanted carriers. However, clinical application as a non- or minimally invasive approach to postsurgical pain management has not been achieved, as these microparticles cannot be injected directly21, 30, 31. Therefore, there is a compelling need for new LA formulations to produce a prolonged analgesic effect55.
Previous research has also focused on encapsulating LA within nanocarriers to prolong analgesia and decrease toxicity. Various nano-sized drug delivery systems for LA have been developed, including liposomes, hydrogel and polymeric nanoparticles, nanostructured lipid carriers, etc.13, 18, 27, 38. However, these formulations have limitations related to efficacy, toxicity and tissue reactions42. For example, peri-sciatic nerve injection of Exparel™ (DepoFoam bupivacaine), a liposomal local anesthetic formulation, produced long-lasting nerve blockade, but also led to significant inflammation and myotoxicity32. Under some circumstances, nanoparticles themselves enhanced local myotoxicity and augmented inflammatory responses at the nerve20, 33. In addition, due to nanoparticles’ fast diffusion rate, nano-formulations often could not meet the need for prolonged duration of analgesic effect.
In the present study, we have developed an injectable LA formulation using a degradable polymer poly (lactic-co-glycolic acid) (PLGA) with average size of 1.7 μm in diameter as a drug delivery carrier. This carrier is small enough for minimally invasive injection, but large enough to stay at injection sites as a drug depot for prolonged release. RVC was used as a model of anesthetic drug because it is one of the safest LA in the market and widely used in the management of pain. We used RVC-loaded PLGA particles to realize sustained-release RVC (SRR) and to observe the effect of SRR on pain after hind paw incision or spared nerve injury (SNI), two commonly used rodent models of postsurgical pain and neuropathic pain, respectively.
Methods
Preparation and characterization of RVC·HCl microparticles
Materials
PLGA was purchased from Absorbable Polymers International. Poly (vinyl alcohol) (PVA, 87~89 % hydrolyzed, MW 13,000~23,000), dichloromethane (DCM) and RVC hydrochloride (RVC·HCl) were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of RVC·HCl-loaded PLGA microparticles
RVC·HCl-loaded PLGA microparticles were prepared using a water-in-oil-in-water (w/o/w) double emulsion, solvent evaporation technique. Briefly, 100 mg of 50:50 PLGA was dissolved in 5 ml of DCM. 20 mg/ml of RVC·HCl was prepared using 0.5% PVA. 1 ml of the RVC·HCl was mixed with PLGA/DCM solution using a probe sonicator (Q700 Sonicator, Qsonica, Llc, USA) for 30 sec at 30% amplitude to form the first emulsion. This emulsion was then rapidly added to 20 ml of 0.5% (w/v) PVA solution by stirring at 10,000 rpm for 30 sec using a homogenizer (Bio-Gen PRO200, Pro Scientific) to form the second emulsion. The mixture was stirred overnight to allow the DCM solvent to evaporate. The particles were collected by washing 3 times with distilled water using a centrifugal filter device (Amicon® Ultra 15 mL Centrifugal Filter, Millipore).
RVC·HCl loading on PLGA microparticles
To determine the encapsulation efficiency (EE) and drug loading capacity (DLC) of RVC·HCl, 10 mg samples of RVC·HCl microparticles were dissolved in 0.2 ml acetonitrile and 0.8 ml distilled water. This mixture was agitated using a vortex mixer and centrifuged at 13,000 rpm for 5 min to remove PLGA precipitates. The RVC·HCl content in the supernatant was analyzed by High-Pressure Liquid Chromatography (HPLC) (Agilent 1200 series, USA) with an Agilent Eclipse XDB-C18 column (150 mm × 4.6 mm, 5 μm) using a mobile phase consisting of acetonitrile/water (20:80) containing 0.1% trifluoroacetic acid and UV detection at 260 nm. EE and DLC of RVC·HCl microparticles were calculated according to the following formula: EE (%) = (weight of RVC·HCl loaded into PLGA microparticles)/ (weight of RVC·HCl in the system) ×100%. DLC (%) = (weight of entrapped drug/weight of all materials in the system) × 100%.
Ropivacaine nanoparticle preparation
The nanoparticles encapsulated with a payload of RVC were formulated via the double-emulsion solvent evaporation technique. In brief, copolymer PLGA-PEG was dissolved in DCM. RVC solution (0.5 ml) was added drop-wise into 1 ml of PLGA-PEG solution and emulsified by probe sonification to form the first emulsion. The emulsified mixture was then added into 3 ml of aqueous solution containing 1% PVA, followed by probe sonification to form the double emulsion. The final emulsion solution was poured into 15 ml of water and stirred for 3 h to allow the DCM solvent to evaporate and the particles to harden. The remaining organic solvent and free molecules were removed by washing the particle solution three times using an Amicon Ultra-4 centrifugal filter (MWCO 100kDa; Millipore). The nanoparticle size and zeta potential were determined using a ZetaPALS dynamic light-scattering (DLS) detector (15-mW laser, incident beam of 676 nm; Brookhaven Instruments Corporation). The particle size was characterized as 200 nm using DLS.
Microparticles size and surface morphology analysis
Microparticles’ sizes and zeta potentials were measured using dynamic light scattering technique (DLS) on Zetasizer Nano ZS (Malvern, Southborough, MA). Briefly, the particles were suspended in deionized water at a concentration of 1mg/ml. The mean diameter of the hydrodynamic volume size was confirmed by cumulative analysis. The zeta potential was also measured based on the electrophoretic mobility of the microparticles in aqueous solution, and was confirmed with folded capillary cells. Microparticles’ morphologies were assessed by Scanning Electron Microscopy (SEM, LEO 1530 VP). Air-dried microparticles were placed on adhesive carbon tabs mounted on SEM specimen stubs. The specimen stubs were coated with ~5 nm of carbon before examination in the SEM.
In vitro drug release study
Drug release tests were carried out at constant body temperature (37°C). Briefly, RVC·HCl loaded microparticles (10 mg) were added to 1 ml of 10 mM PBS and incubated at 37°C by shaking in a Thermomixer (Eppendorf, Germany) at 300 rpm. At predetermined time intervals, the sample was centrifuged at 2,000 rpm for 3 min. The supernatant was collected, and the medium was replaced with 1ml of fresh PBS. The amount of RVC·HCl released into each medium was quantified by HPLC analysis. All experiments were performed in triplicate (n = 3 repeats).
Animal experiments
Animal preparation
Male Sprague-Dawley rats weighing 200–250g were obtained from Charles River Laboratories (Wilmington, MA, US). All rats were housed in an animal facility that was kept in a standard 12-h light/dark cycle, with standard laboratory water and food pellets available ad libitum. Animal experiments were conducted with the approval of the Animal Care and Use Committee at Rutgers-New Jersey Medical School, and consistent with the ethical guidelines of the US National Institutes of Health and the International Association for the Study of Pain. To minimize intra- and inter-individual variability of behavioral outcome measures, animals were trained for 1–2 days before behavioral testing was performed. The experimenters were blinded to treatment condition during behavioral testing.
Peri-sciatic nerve injection
Peri-sciatic nerve injections were carried out with a 25-gauge needle attached to a 1 ml syringe under isoflurane-oxygen anesthesia as described50. The needle was inserted percutaneously from the posterior aspect of the upper leg and advanced anterolaterally toward the greater trochanter. Once bone was contacted, the drug was injected with total volume of 0.2 ml.
Incisional pain model
The incisional surgery was carried out with minor modification as described7. Rats were anesthetized with 2% isoflurane delivered via a nose cone. The plantar aspect of the left hind paw was prepared in a sterile manner with a 10% povidone-iodine solution. A 1-cm longitudinal incision was made with a number 11 blade through skin and fascia of the plantar aspect of the foot, starting 0.5 cm from the proximal edge of the heel and extending toward the toes. The muscle was elevated and separated bluntly and longitudinally. After hemostasis with gentle pressure, the skin was sutured with 5–0 nylon thread.
Spared nerve injury (SNI) model
The SNI surgery was carried out with minor modification as described14. Rats were anesthetized with 2% isoflurane delivered via a nose cone. A 1 cm skin incision was applied in the longitudinal direction proximal to the posterior knee. The muscle layers were separated to expose the sciatic nerve. The trifurcation of the sciatic nerve was identified, and the common peroneal and tibial branches exposed and ligated with 5–0 silk suture. Nerve segments distal to the ligature approximately 2 mm in length was transected from both branches. Special care was taken to avoid damaging the sural nerve. Sham surgery was carried out in the same manner as described above, except for nerve ligation and transection.
Behavioral analysis
Mechanical allodynia was tested by measuring paw withdrawal thresholds in response to mechanical stimuli using the up–down testing paradigm as described5, 10, 24, 26, 63, 64. Briefly, rats were placed in Plexiglas chambers on an elevated mesh screen. Calibrated von Frey filaments in log increments of force (0.69, 1.20, 2.04, 3.63, 5.50, 8.51, 15.14, and 26.00 g) were applied to the center (for incision) or lateral (for SNI) aspect of the plantar surface of the rats’ left and right hind paws. The 2.04-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next larger von Frey hair was used. The test was terminated when (i) a negative response was obtained with the 26.00-g hairor (ii) three stimuli were applied after the first positive response. Paw withdrawal threshold was determined by converting the pattern of positive and negative responses to the von Frey filament stimulation to a 50% threshold value with a formula provided by Dixon9.
Heat hyperalgesia was tested by measuring paw withdrawal latencies to noxious heat with a Model 336 Analgesic Meter (IITC Inc./Life Science Instruments, Woodland Hills, CA, USA) as described previously40, 64. Rats were placed in a Plexiglas chamber on a glass plate. Radiant heat was applied by aiming a beam of light through a hole in the light box through the glass plate to the center of the plantar surface of each hind paw. When the animal lifted its foot, the light beam was turned off. The length of time between the start of the light beam and the foot lift was defined as the paw withdrawal latency. Each trial was repeated five times at 5-min intervals for each side. A cut-off time of 20 s was used to avoid tissue damage to the hind paw.
Cold allodynia was examined by measuring paw withdrawal latencies to noxious cold with a cold plate, which was set at 0°C as described40, 64. The length of time between the placement of the hind paw on the plate and the animal lifting its hind paw, with or without paw licking and flinching, was defined as the paw withdrawal latency. Each trial was repeated three times at 10-min intervals for the paw on the ipsilateral side. A cut off time of 60 s was used to avoid paw tissue damage.
Locomotor function test
Tests of locomotor function, including placing, grasping and righting reflexes, were performed before and after incision surgery or SNI according to the previously described protocol37, 46, 64. (1) Placing reflex: The rat was held with hind limbs slightly lower than the forelimbs, and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. Whether the hind paws were placed on the table surface reflexively was recorded; (2) Grasping reflex: The rat was placed on a wire grid and whether the hind paws grasped the wire on contact was recorded; (3) Righting reflex: The rat’s back was placed on a flat surface and whether it immediately assumed the normal upright position was recorded. Scores for placing, grasping, and righting reflexes were based on counts of each normal reflex exhibited in five trials. In addition, the animal’s general behaviors, including spontaneous activity (e.g. walking and running), were observed.
Morphological/biochemical experiments
Electron microscopy
Rats (n =3 per group) from the vehicle-, PLGA-, 0.25% free RVC- and 0.25% SRR- treated groups were anesthetized with isoflurane (for harvesting of the sciatic nerve) and perfused with 300 mL of 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer (pH 7.4). After perfusion, sciatic nerves were dissected and postfixed at 4˚C for 3 hours. After dehydration by gradient ethanol elution, samples were cleared by propylene, then infiltrated with resin and embeded in molds. Three ultra-thin sections (50 nm in thickness) from each rat were collected by grouping every tenth section and counterstained with uranyl acetate and lead citrate. The sections were examined with a Hitachi H-7500 transmission electron microscope (Hitachi, Ltd. Japan). Images from three randomized fields per section were taken. A total of 27 images per group were examined.
Immunohistochemistry
Immunohistochemistry was performed as described previously47. Three rats from each treated group as indicated above (four groups; N = 3 rats/group) were anesthetized with isoflurane and perfused with 300 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). After perfusion, sciatic nerves or adjacent muscles were dissected, postfixed at 4°C for 4 hours and cryoprotected in 30% sucrose overnight. The transverse or longitudinal sections were cut on a cryostat at a thickness of 20 μm and collected from each tissue by grouping every third sections. The sections were first blocked for 1 hour at 25°C in 0.01M PBS containing 5% goat serum and 0.3% Triton X-100 and then incubated overnight at 4°C with mouse anti-myelin basic protein (MBP; RRID: AB_10120129; catalog number: SMI-99P; lot number: 808401. 1:500; BioLegend, San Diego, CA, USA)17 or rabbit anti-CD68 (RRID: AB_10975465; catalog number: ab125212; lot number: 43780. 1:800; Abcam, Cambridge, MA, USA)56. The sections were finally incubated with goat anti–mouse or anti-rabbit antibody conjugated to Cy2 or Cy3 (1:500; Jackson ImmunoResearch, West Grove, PA, USA) for 2 hours at room temperature. Immunofluorescence-labeled images were randomly taken using a Leica DMI4000 fluorescence microscope with a DFC365FX camera (Leica, Germany). At least 5 sections per rat (15 sections/group) were examined.
TdT-mediated dUTP nick end labeling (TUNEL) and cresyl violet histochemical staining
After rats (3 rats/group) from four treatment groups as described above were anesthetized with isoflurane, sciatic nerve, lumbar dorsal root ganglions (DRGs), lumbar spinal cord and brain cortex were harvested and post-fixed in 4% paraformaldehyde. The tissues were cryoprotected in 30% sucrose overnight. Two sets of the sections at thickness of 20 μm were collected from each tissue by grouping every third sections. TUNEL histochemical staining was performed on one set of sections using an in situ cell death detection kit (Roche Molecular Biochemical, IN). Briefly, the sections were incubated with proteinase K solution (20 μg/ml) for 20 min at room temperature, and then with a TUNEL reaction mixture composed of terminal deoxynucleotidyl transferase (TdT) at 37°C in a humidified chamber for 60 min. TdT enzyme-incorporated fluorescein was detected with converter-alkaline phosphatase (AP), consisting of sheep anti-fluorescein antibody conjugated with AP. The signal was detected using nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate as color substrate. Another set of sections was stained with cresyl violet. The sections were rinsed in distilled water and incubated for 30 min in a solution of 0.2% cresyl violet (cresyl violet acetate; Sigma, St. Louis, MO) in acetate buffer, then washed in distilled water, dehydrated through a graded series of ethanol, and cover-slipped. The images were randomly taken using a Nikon microscope (Nikon Eclipse 80i, Japan). At least 5 sections per rat (15 sections/group/test) were examined.
Western blot analysis
Protein extraction and Western blot analysis were carried out as described25. Briefly, the ipsilateral L4/5 spinal cords on day 12 post-SNI or sham surgery were harvested and homogenized in lysis buffer. After centrifugation at 4°C for 15 min at 1,000 g, the supernatant was collected. After protein concentration was measured, equal amounts of total protein from each sample were loaded22. The samples were heated at 99°C for 5 min and loaded onto a 4 to 15% stacking/7.5% separating SDS–polyacrylamide gel (Bio-Rad Laboratories). The proteins were then electrophoretically transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories). After being blocked with 3% nonfat milk in the Tris-buffered saline containing 0.1% Tween 20 for 2 hours, the membranes were then incubated at 4°C overnight with the rabbit anti–phospho-ERK1/2 (RRID: AB_2315112; catalog number: 4370; lot number: 24. 1:1,000; Cell Signaling Technology, USA), rabbit anti-ERK1/2 (RRID: AB_390779; catalog number: 4695; lot number: 25. 1:1000, rabbit, Cell Signaling Technology, USA)23, mouse anti-GFAP (RRID: AB_561049; catalog number: 3670; lot number: 6. 1:1,000; Cell Signaling Technology, USA) or goat anti-GAPDH (RRID: 641107; catalog number: sc-20357; lot number: A0714. 1:3,000; Santa Cruz Biotechnology, USA)54. The proteins were detected by horseradish peroxidase–conjugated anti-rabbit, anti-mouse or anti-goat secondary antibody (1:3,000, Bio-Rad Laboratories, USA), and visualized by chemiluminescence reagent (enhanced chemiluminescence, Bio-Rad Laboratories). Images were generated using ChemiDoc XRS System with Image Lab software (Bio-Rad Laboratories, USA). Band intensities were quantified with densitometry by System with Image Lab software (Bio-Rad Laboratories, USA). Band intensities were normalized to the loading control GAPDH, which has been demonstrated to be stable even after peripheral nerve injury insult25, 64.
Plasma samples analysis
Plasma concentrations of RPV were determined by high-performance liquid chromatograph (HPLC; Agilent Technologies 1200 series, California, USA) based on the method reported previously2. In brief, blood was withdrawn from a tail vein into heparinized tubes 1 d before 0.25% free RVC or 0.25% SRR injection and 10 min, 20 min, 40 min, 2 h, 4 h, 1 d, 3 d, 5 d, 7 d and 14 d after 0.25% free RVC or 0.25% SRR injection. Plasma (50 μl) was separated by centrifugation and added to 150 μl acetonitrile to extract the RVC; this was followed by sonication and centrifugation. The supernatant of each sample was analyzed using HPLC equipped with a C18 column (5 μm, 4.6 ×150 mm) under 220 nm UV wavelength. The mobile phase consisted of water with 0.1% TFA (solvent A), acetonitrile with 0.1% TFA (solvent B), and mixed A/B (v/v) at ratio of 30:70, which were used at a flow rate of 1 ml/min. The column thermostat was set at 25°C, and injection volume was set to 10 μl. The concentration of RVC was calculated according to its concentration standard curve (y=30014x+1872.1, R2=0.9927). The experiments was repeated three times in each group (N = 3 repeats/group).
Statistical analysis
All data were collected using random sampling, and given as means ± SEM. The data were analyzed with two-tailed, paired/unpaired Student’s t test and a one-way or two-way ANOVA. When ANOVA showed a significant difference, pairwise comparisons between means were tested by the post hoc Tukey method (SigmaStat, San Jose, CA, USA). Locomotor function data were analyzed using Wilcoxon rank-sum test. Significance was set at P < 0.05.
Results
Preparation and characterization of RVC·HCl microparticles
In this study, we fabricated the RVC·HCl-encapsulated PLGA microparticles using water-in-oil-in-water double-emulsion solvent evaporation techniques (Figure 1a). The mean diameters of RVC·HCl microparticles were found as 1.7 ± 0.2 μm (Figure 1b). The RVC·HCl microparticles had a smooth morphology and spherical shape with some deviations and nominal aggregation (Figure 1c). In vitro release of RVC·HCl microparticles showed that the drug payload was released from the particle in a sustained release fashion (Figure 1d). About 25.6 % of total RVC·HCl was rapidly released over the first 8 h, followed by a sustained release after 12 h. This lasting release of RVC·HCl from the microparticles extended over 6 days, reaching a maximum value of 95.7% thereafter. Consistently, the release rates were high over the first 8 h, then gradually reduced after 12 h, and subsequently maintained at persistently low levels for at least 6 d (Figure 1e).
Figure 1.

(a) Structure model of ropivacaine (RVC)·HCl microparticles. (b) Size distribution of PLGA microparticles containing RVC·HCl by dynamic light scattering. (c) Representative SEM image of the RVC·HCl microparticles. Scale bar: 2 μm. (d) In vitro cumulative release of RVC·HCl from PLGA microparticles. (e) In vitro release rate (mg/h) of RVC·HCl from PLGA microparticles. Data are expressed as the mean ± S.E.M of 3 biological repeats in d and e.
Effect of peri-sciatic nerve injection of SRR on postsurgical pain
We examined whether SRR had long-term analgesic effect when applies to the ipsilateral sciatic nerve after surgery. A unilateral hind paw plantar incision was performed in male rats immediately after peri-sciatic nerve injection of SRR (0.25%, 0.125% and 0.06%; dissolved in PBS), free RVC (dissolved in PBS), PLGA alone (dissolved in PBS) or vehicle (PBS). The RVC concentration in each formulation was characterized by HPLC per fixed volume of formulation. Consistent with previous reports53, paw incision led to persistent mechanical and heat pain hypersensitivities on the ipsilateral (but not contralateral) side, which occurred at 2 h post-incision, peaked around 4 h-1 d post-incision, and lasted for 5–6 days post-incision, in the vehicle-treated group (Figures 2a and 2b). As expected, the injection of PLGA solution alone did not significantly affect incision-induced mechanical allodynia and heat hyperalgesia during the observation period (Figures 2a and 2b). The injection of 0.25% free RVC produced an analgesic effect on incisional pain only within 12 h post-injection (Figures 2a and 2b). The injection of SRR at the same dose significantly blocked mechanical allodynia and heat hyperalgesia during the 5–6 day incisional pain period (Figures 2a and 2b). None of 0.25% SRR, PLGA, free RVC and vehicle altered basal paw withdrawal responses to mechanical and heat stimuli on the contralateral side (Figures 2c and 2d).
Figure 2.
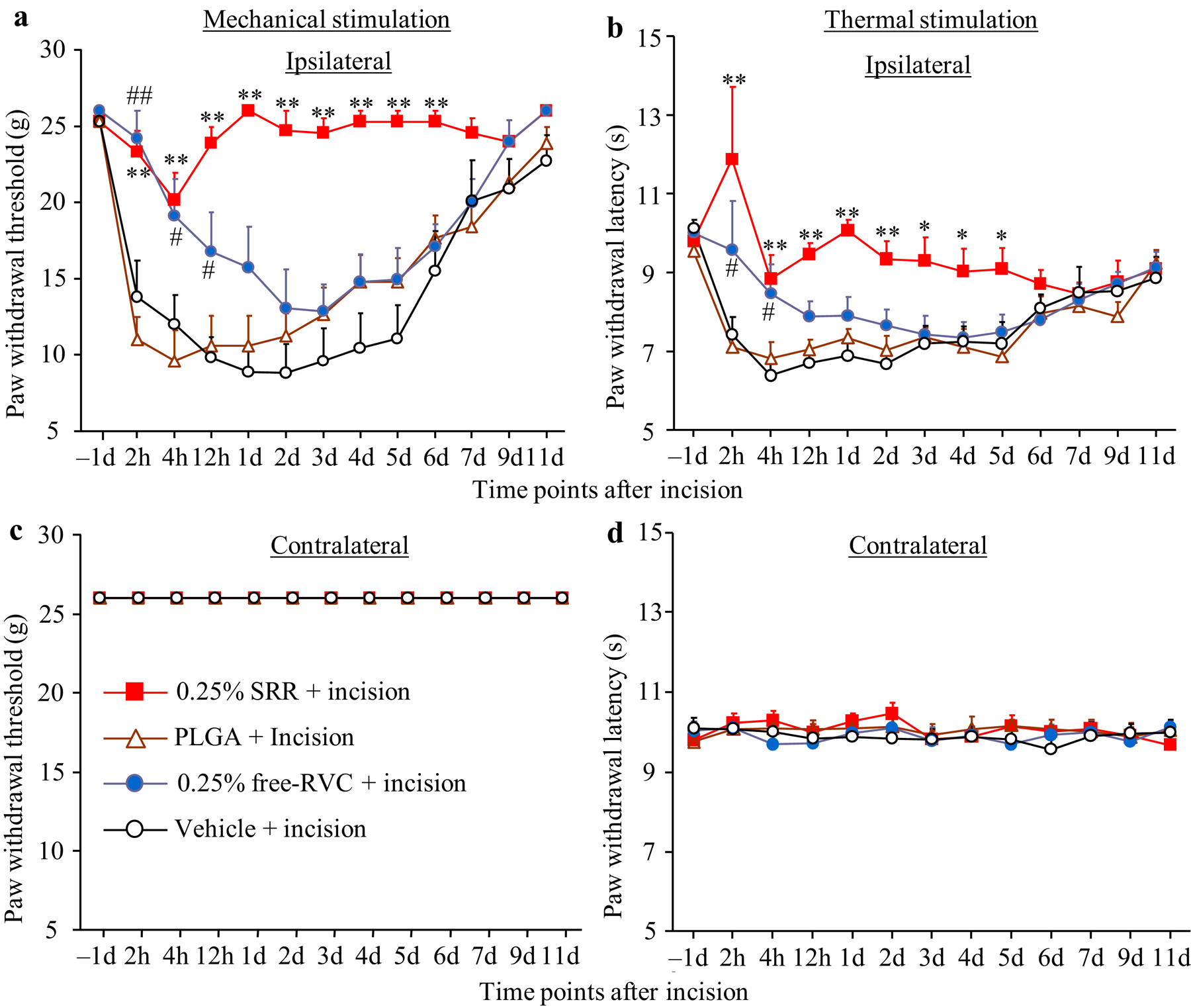
Effect of peri-sciatic nerve injection of 0.25% SRR, 0.25% free ropivacaine (RVC), PLGA, or vehicle on paw withdrawal responses to mechanical (a and c) and heat (b and d) stimuli on the ipsilateral (a and b) and contralateral (c and d) sides at the different time points as indicated after incision. Data are expressed as the mean ± S.E.M of 8 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test. *p < 0.05 or **p < 0.01 comparison between the SRR-treated incisional group and the vehicle-treated incisional group at the corresponding time point. #p < 0.05 or ##p < 0.01 comparison between the free ropivacaine-treated incisional group and the vehicle-treated incisional group at the corresponding time point.
The analgesic effect of SRR is dose-dependent. The injection of 0.125% SRR produced a similar analgesic effect on incision-induced mechanical allodynia as the injection of 0.25% SRR, but SRR at the former dose led to an analgesic effect on incision-induced heat hyperalgesia only within 2 days post-incision (Figures 3a and 3b). The injection of 0.06% SRR did not produce any significant analgesic effects on incision-induced mechanical allodynia and heat hyperalgesia (Figures 3a and 3b). As expected, neither SRR at doses used nor vehicle changed basal paw withdrawal responses to mechanical and heat stimuli on the contralateral side (Figures 3c and 3d).
Figure 3.

Dose-dependent effect of SRR on incisional pain. The injection of 0.25% SRR showed stronger analgesic effect than the injection of 0.125% SRR on both the duration and intensity of incision-induced mechanical allodynia (a) and heat hyperalgesia (b) on the ipsilateral side. The injection of 0.06% SRR did not produce significant analgesic effect on incision-induced mechanical allodynia (a) and heat hyperalgesia (b) on the ipsilateral side. None of the doses of SRR altered basal responses to mechanical (c) and thermal (d) stimuli on the contralateral side. Data are expressed as the mean ± S.E.M of 8 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test. *P < 0.05 or **P < 0.01 comparison between the 0.25% SRR-treated incisional group and the vehicle-treated incisional group at the corresponding time point. ##P < 0.05 or ##P < 0.01 comparison between the 0.125% SRR-treated incisional group and the vehicle-treated incisional group at the corresponding time point.
To further examine whether the long-lasting effect of SRR was related to the size of the microparticle, we manufactured different sizes of PLGA microparticles, which were then loaded with distinct concentrations of RVC-HCI. Microparticles larger than 2 μm in size could not be injected through 25-gauge syringe when the concentration of SRR exceeded 0.125%, a concentration required in clinical practice (Table 1). As expected, microparticles smaller than 2 μm in size could be injected through 25-gauge syringes, except when loaded with the highest concentration of 1.2% SRR (stock solution) (Table 1). We also generated nanoparticle-coated RVC. Peri-sciatic nerve injection of 0.25% nanoparticle-coated RVC relieved incision-induced heat hyperalgesia, but not incision-induced mechanical allodynia (Figures 4a and 4b). This analgesic effect occurred at 4 h post-incision and lasted only for 2 d post-incision (Figure 3b). Neither nanoparticle-coated RVC, nor free RVC, nor vehicle altered basal paw withdrawal response on the contralateral side (Figures 4c and 4d).
Table 1.
Microparticle sizes and drug concentrations for 25-gauge syringe
| Drug Concentrations | 12 ± 2.4 mg/μl | 5 mg/ml | 2.5 mg/ml | 1.25 mg/ml | 0.6 mg/ml |
|---|---|---|---|---|---|
| Microparticle size | (stock solution) | ||||
| < 2 μm | Not injectable | Injectable | Injectable | Injectable | Injectable |
| > 2 μm | Not injectable | Not injectable | Not injectable | Not injectable | Injectable |
Figure 4.
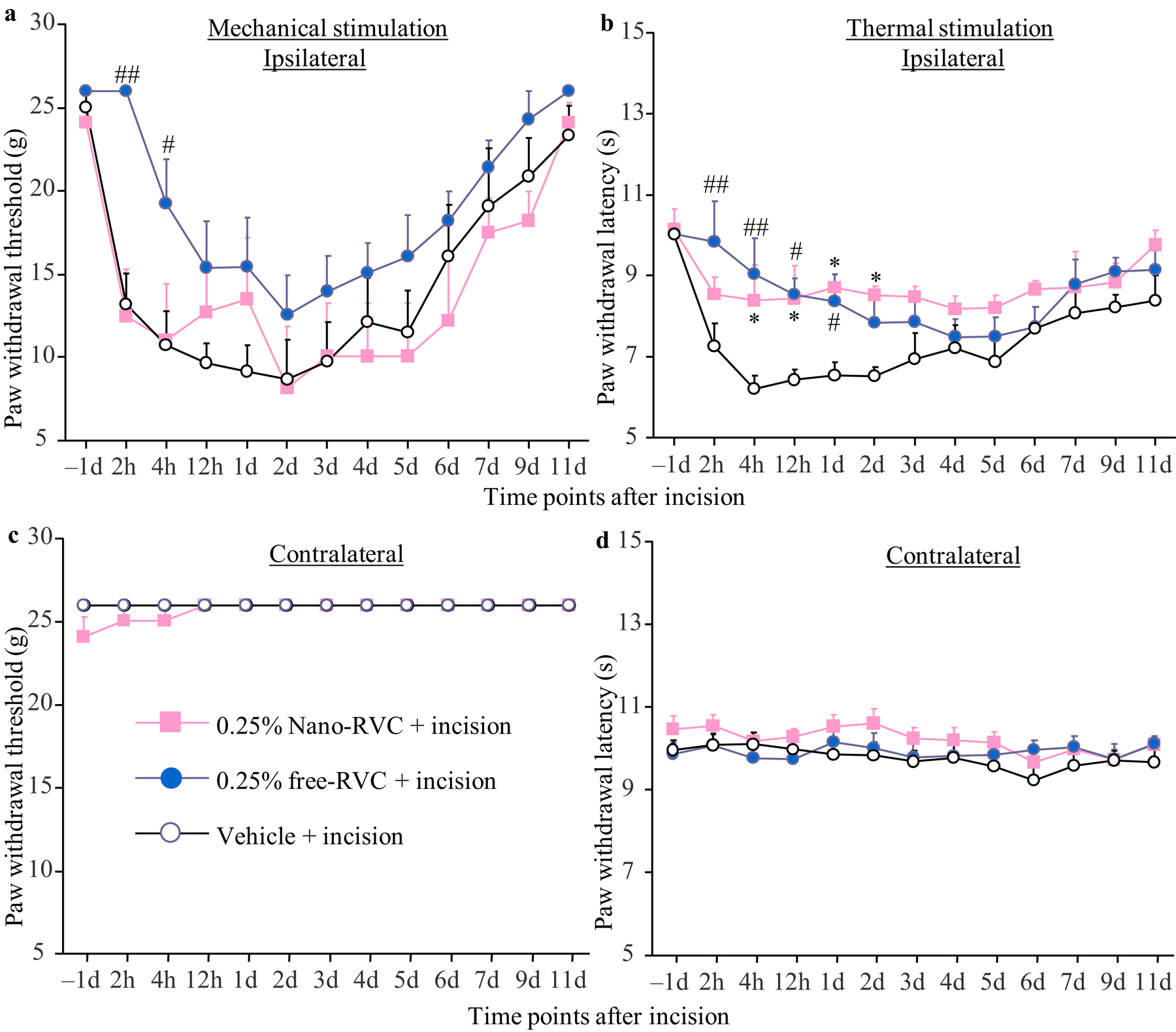
Effect of peri-sciatic nerve injection of 0.25% nanoparticle (Nano)-coated ropivacaine (RVC), 0.25% free ropivacaine, or vehicle on paw withdrawal responses to mechanical (a and c) and heat (b and d) stimuli on the ipsilateral (a and b) and contralateral (c and d) sides at different time points as indicated after incision. Data are expressed as the mean ± S.E.M of 6 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test. *P < 0.05 comparison between the 0.25% nanoparticle-coated ropivacaine-treated incisional group and the vehicle-treated incisional group at the corresponding time point. #P < 0.05 or ##P < 0.01 comparison between the 0.25% free ropivacaine-treated incisional group and the vehicle-treated incisional group at the corresponding time point.
Effect of peri-sciatic nerve injection of SRR on neuropathic pain
We also examined whether SRR had a long-term analgesic effect on SNI-induced neuropathic pain when injected on the ipsilateral peri-sciatic nerve. SRR at different concentrations (0.25%, 0.125%, and 0.06%) or vehicle was injected on day 7 post-SNI. Consistent with a previous report44, mechanical allodynia and cold allodynia peaked on day 7 post-SNI. and were maintained for at least 2 weeks after SNI on the ipsilateral (but not contralateral) side of the vehicle-treated SNI group (Figures 5a–c). Post-injection of SRR alleviated SNI-induced mechanical allodynia and cold allodynia in a dose-dependent manner (Figures 5a and 5c). The analgesic effects lasted for up to 12 days post-injection on SNI-induced mechanical allodynia, and for up to 7 days post-injection on cold allodynia at the dose of 0.25% SRR (Figures 5a and 5c). SRR at the dose of 0.125% led to analgesic effects on mechanical allodynia for up to 7 days post-injection and on cold allodynia for up to 3 days post-injection (Figures 5a and 5c). There were no significant differences in SNI-induced decreases in paw withdrawal thresholds and latencies between the 0.06% SRR-treated SNI group and the vehicle-treated SNI group (Figures 5a and 5c). As expected, injection of SRR at three doses did not change basal responses on the contralateral side (Figure 5b). To further define that peri-sciatic nerve injection of SRR produced local effect, vehicle, 0.25% free RVC or 0.25% SRR were injected into peri-sciatic nerve on the contralateral side on day 7 after SNI or sham surgery (Figures 5d–h). As expected, contralateral injection of none of these solutions affected SNI-induced mechanical allodynia, heat hyperalgesia and cold allodynia on the ipsilateral side (Figures 5d–f). Contralateral injection of neither 0.25% SRR nor 0.25% free RVC altered basal paw withdrawal thresholds in response to mechanical stimulation on the contralateral side during the observation period (Figure 5g), as basal mechanical responses had reached the ceiling effect. However, contralateral injections of 0.25% SRR and 0.25% free RVC significantly increased basal paw withdrawal latencies in response to heat stimulation over 9 days and 4 hours, respectively, after peri-nerve injection on the contralateral side (Figure 5h). Finally, we monitored the plasma concentration of RVC before and after peri-sciatic nerve injection of 0.25% SRR or 0.25% free RVC. Consistent with the previous reports4, 57, RVC was detected in blood plasma over 4 hours after peri-nerve injection, but was undetectable after 1 day (Figure 5i). There was no marked difference in the level of plasma RVC between 0.25% SRR- and 0.25% free RVC-treated groups (Figure 5i).
Figure 5.
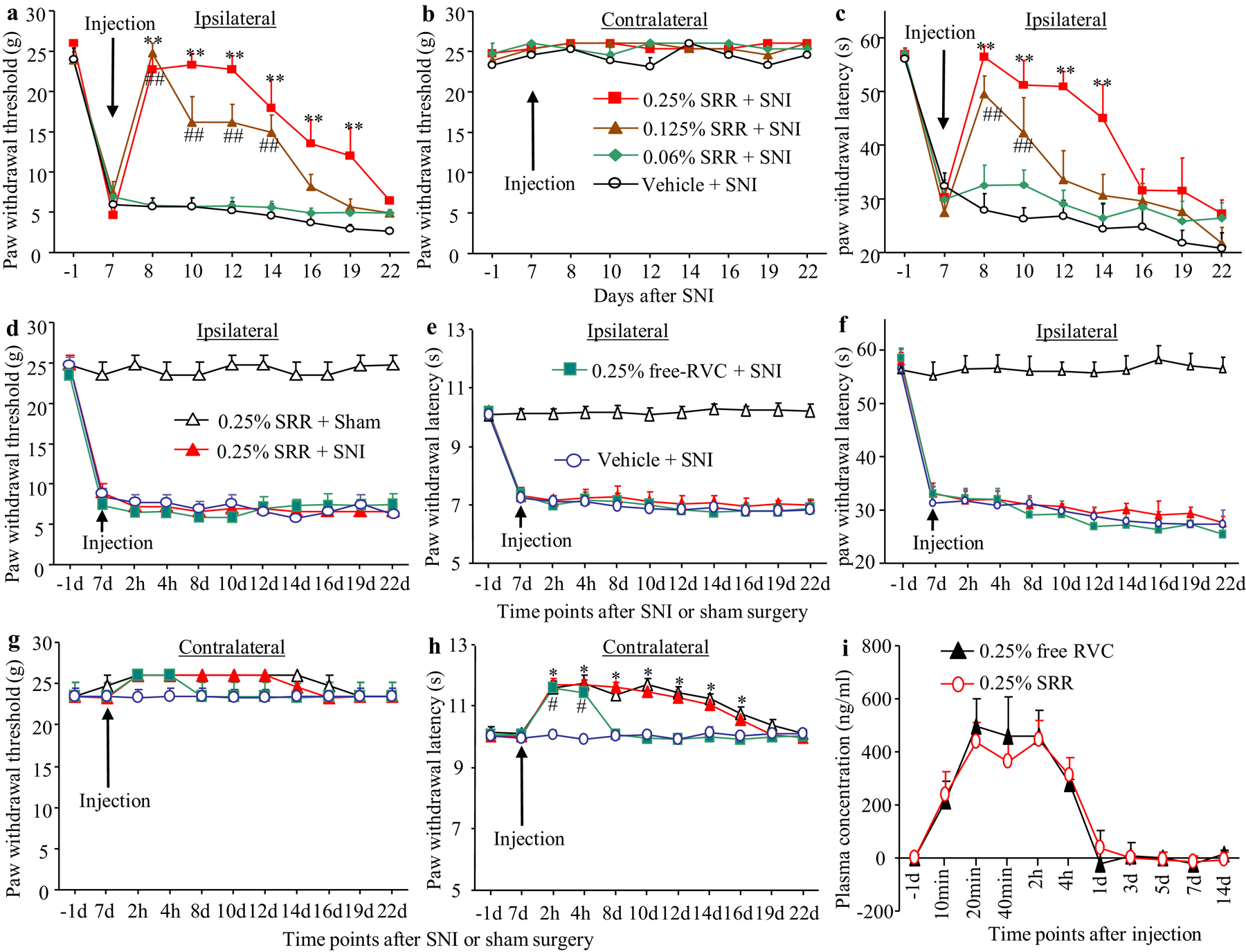
Behavioral responses and mean plasma concentrations of ropivacaine (RVC) after peri-sciatic nerve injection of SRR in rats with spare nerve injury (SNI) or sham surgery. (a-c) Effect of ipsilateral peri-sciatic nerve injection of 0.06% SRR, 0.125% SRR, 0.25% SRR or vehicle on paw withdrawal responses to mechanical (a and b) and cold (c) stimuli on the ipsilateral (a and c) and contralateral (b) sides at different days as indicated after SNI. Data are expressed as the mean ± S.E.M of 8 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test. **p < 0.01 comparison between the 0.25% SRR-treated SNI group and the vehicle-treated SNI group at the corresponding time point. ##p < 0.01 comparison between the 0.125% SRR-treated SNI group and the vehicle-treated SNI group at the corresponding time point. (d-h) Effect of contralateral peri-sciatic nerve injection of 0.25% SRR, 0.25% free RVC or vehicle on paw withdrawal responses to mechanical (d and g), heat (e and h) and cold (f) stimuli on the ipsilateral (d and e) and contralateral (g and h) sides at different days as indicated after SNI or sham surgery. Data are expressed as the mean ± S.E.M of 8 rats per group. Two-way ANOVA with repeated measures followed by post hoc Tukey test. *p < 0.05 comparison between the 0.25% SRR-treated SNI group and the vehicle-treated SNI group at the corresponding time point. #p < 0.05 comparison between the 0.25% free RVC-treated SNI group and the vehicle-treated SNI group at the corresponding time point. (i) Mean plasma concentrations of RVC at different time points as indicated after peri-scatic nerve injection of 0.25% SRR or 0.25% free RVC. Data are expressed as the mean ± S.E.M of 3 biological repeats per group.
We further examined whether SRR affected SNI-induced dorsal horn central sensitization as indicated by increases in phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) and glial fibrillary acidic protein (GFAP) in the dorsal horn. In line with previous studies25, the levels of p-ERK1/2 (but not total ERK 1/2) and GFAP were significantly increased in the ipsilateral L4/5 dorsal horn of the vehicle-treated SNI rats, but not in the vehicle-treated sham rats, on day 12 post-SNI or sham surgery (that is, 5 d after SRR or vehicle injection) (Figures 6a and 6b). These increases were dramatically blocked in the 0.25% SRR-treated SNI rats (Figures 6a and 6b). Injection of 0.25% SRR did not affect basal expression of p-ERK1/2, ERK1/2 and GFAP in dorsal horn of sham rats (Figures 6a and 6b).
Figure 6.
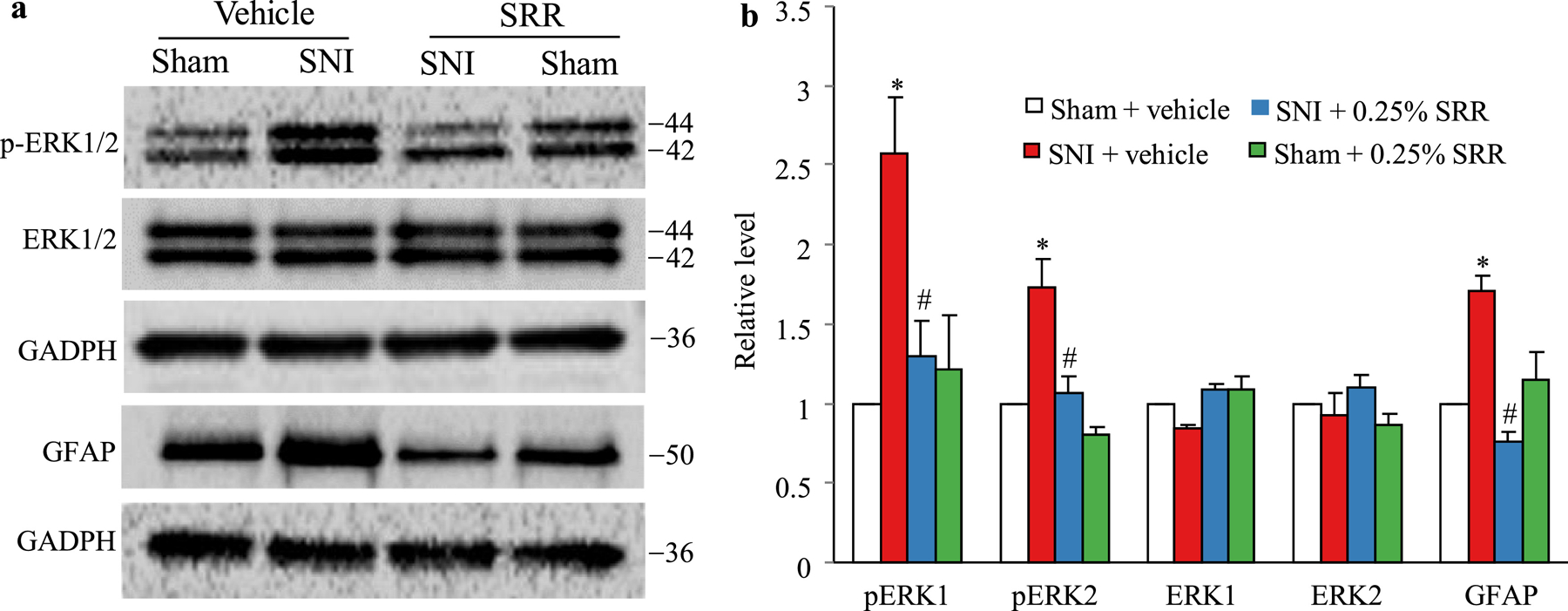
Effect of peri-sciatic nerve injection of 0.25% SRR or vehicle on spare nerve injury (SNI)-induced increases in the levels of phosphorylation of extracellular signal–regulated kinase 1/2 (p-ERK1/2) and glial fibrillary acidic protein (GFAP) in the ipsilateral L4/5 spinal cord on day 12 post-SNI or sham surgery (that is, 5 d after SRR or vehicle injection). Representative Western blots (a) and a summary of densitometric analysis (b) are shown. Data are expressed as the mean ± S.E.M of 3 biological repeats (6 rats) per group. One-way ANOVA with repeated measures followed by post hoc Tukey test. *P < 0.05 comparison between the vehicle-treated SNI group and the corresponding vehicle-treated sham group. #P < 0.05 comparison between the 0.25% SRR-treated SNI group and the corresponding vehicle-treated SNI group.
Safety of SRR
We finally examined the safety of SRR. Locomotor function tests showed that only the scores for grasping reflex were reduced at 2 h post-incision in the 0.25% SRR-, 0.125% SRR- and 0.25% free RVC-treated incisional groups, and 4 h post-incision in the 0.25% SRR-treated group (Table 2). However, these reductions were limited to the ipsilateral hind paw, and had no statistical significance compared to the vehicle-treated incisional group. The grasping reflex at the remaining time points after incision, and placing and righting reflexes at any time points after incision were normal in all four treatment groups (Table 2). In addition, SRR- or vehicle-injected SNI rats displayed normal placing, grasping and righting reflexes on day 8 (1 d post-injection) and 22 (15 d post-injection) after SNI surgery (Table 3). Additionally, except for the first 2 h post-incision in the 0.25% SRR-, 0.125% SRR- and 0.25% free RVC-treated incisional groups, and 4 h post-incision in the 0.25% SRR-treated group, we did not observe any marked difference in general behaviors, including the gait and spontaneous activity among the treated groups. None of the treated rats exhibited the injected residuals at the injection site. Gross examination of surgical sites showed normal wound healing. No prolonged healing or muscle atrophy were found. The hematoxylin and eosin (H&E) staining showed dense myelinated nerve fibers and fully developed axons in all four treatment groups on day 11 after peri-nerve injection (Figures 7a–d). The uniformity and thickness of myelin sheaths were similar among these four treatment groups. No swollen and/or shriveled axons were detected in any treated groups (Figures 7a–d). To further examine whether 0.25% SRR or 0.25% RVC injection damaged myelinated nerve fibers of sciatic nerves, we observed the expression pattern of myelin basic protein (MBP), a major constituent of the myelin sheath of oligodendrocytes and Schwann cells52, in the sciatic nerve at the injected site on day 11 after peri-nerve injection. The densities and distribution patterns of MBP-like immunoreactivity were similar among these four treatment groups (Figures 7e–h). Under electron microscopy, the fibers were observed to be myelinated, and the myelin sheaths were dense, uniform and arranged as concentric rings in all four treatment groups on day 11 after peri-nerve injection (Figures 7i–l). Specific features characteristic of the degeneration of myelin sheaths, including thinner lamellae, loose lamellae, split myelin lamellae with discontinuities, or vacuole-like inclusions in the cytoplasm of Schwann cells were not observed in these four groups (Figures 7i–l). Although a very slight separation of myelin sheath layers appears in a few sections in each group, there was no significant difference among four groups. To exclude the possibility that long-lasting release of local SRR caused neurotoxicity in peripheral and central nervous systems, we examined neuronal damages in the lumbar DRG, lumbar spinal cord dorsal horn and brain cortex using TUNEL histochemistry. The thymus was used as a positive control since, under normal conditions, it expresses many apoptosis-positive cells15. As shown in Figure 8a, many thymus cells were positive for TUNEL. In contrast, no TUNEL-positive cells were observed in the ipsilateral fourth lumbar DRG, fourth lumbar spinal dorsal horn or brain cortex on day 15 after peri-sciatic nerve injection of 0.25% SRR (Figures 8b–d). To determine whether long-lasting release of local SRR produced inflammation of tissue at the injected site, we examined the expression of CD68, a marker of microphages and monocytes49, in adjacent muscles at the injected site. Since complete Freund’s adjuvant (CFA) produced persistent inflammation37, we used the CFA-injected muscles as a positive control. As expected, the muscles injected with 20 μl of 50% CFA displayed abundant expression of CD68 (Figures 8e). However, there was no expression of CD68 in the adjacent muscles on day 15 after peri-sciatic nerve injection of either 0.25% SRR or 0.25% free RVC (Figures 8f and 8g).
Table 2.
Mean (± SD) changes in locomotor function at the different time points after SRR, free RVC or vehicle injection
| 2 h | 4 h | 12 h | 11 d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Placing | Grasping | Righting | Placing | Grasping | Righting | Placing | Grasping | Righting | Placing | Grasping | Righting |
| 0.25% SRR + Incision | 5 (0) | 3.75 (0.82) | 5 (0) | 5 (0) | 4.75 (0.25) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| 0.25% SRR + Incision | 5 (0) | 3.75 (0.82) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| 0.06% SRR + Incision | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| PLGA + Incision | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| 0.25% RVC + Incision | 5 (0) | 4.38 (0.63) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| Vehicle + Incision | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
N = 5/group; 5 trials; mean (± SD). PLGA: poly(lactic-co-glycolic acid. RVC: ropivacaine. SRR: sustained-release RVC.
Table 3.
Mean (± SD) changes in locomotor function at the different time points after SRR or vehicle injection
| 7 d (before SRR or vehicle injection) | 8 d | 22 d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Placing | Grasping | Righting | Placing | Grasping | Righting | Placing | Grasping | Righting |
| 0.25% SRR + SNI | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| 0.125% SRR + SNI | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| 0.06% SRR + SNI | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
| Vehicle + SNI | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) | 5 (0) |
N = 5/group; 5 trials; mean (± SD). SRR: sustained-release ropivacaine.
Figure 7.
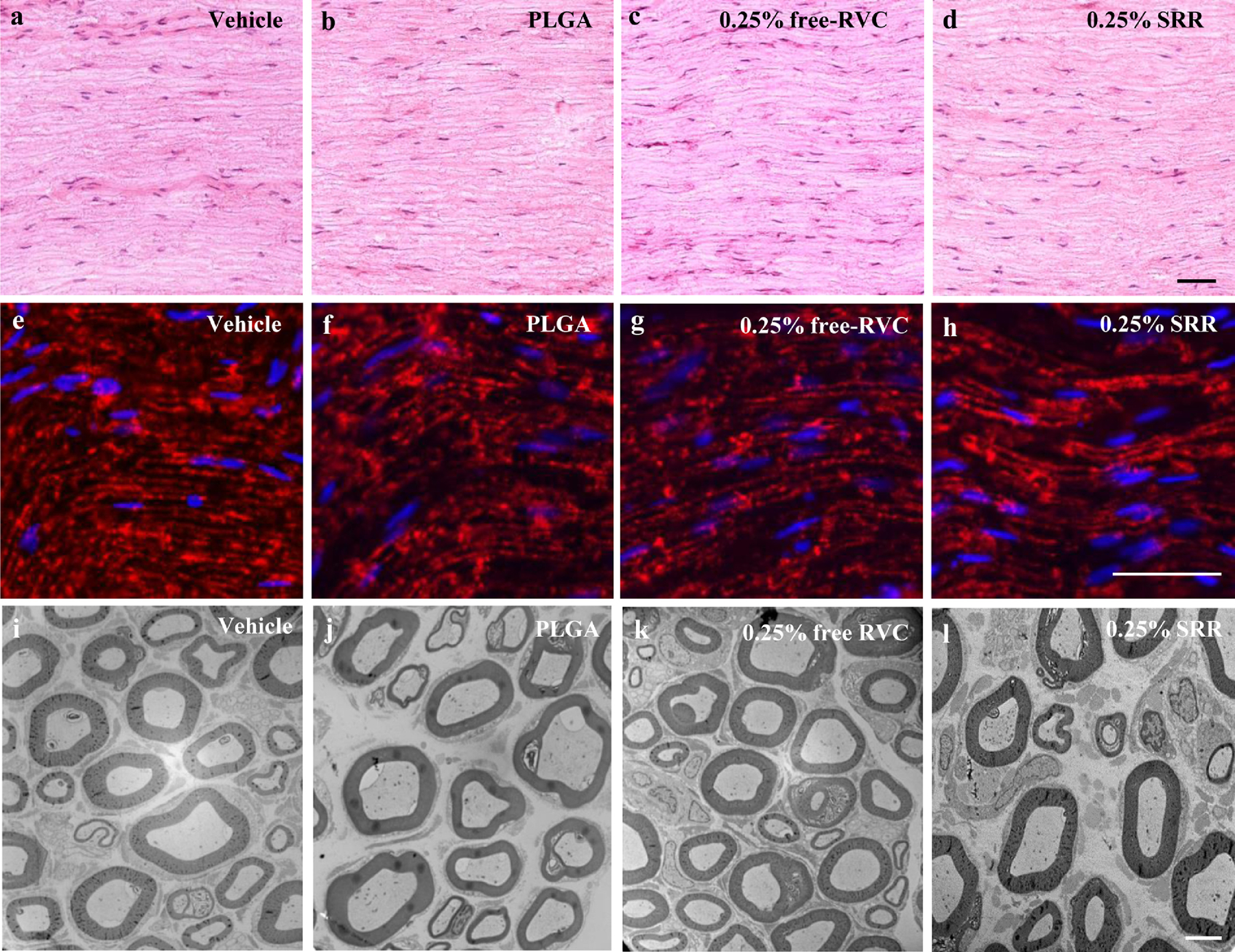
(a-d) Representative hematoxylin and eosin stained images of sciatic nerves at the injected site on day 11 after the ipsilateral peri-sciatic nerve injection of vehicle (a), PLGA (b), 0.25 free RVC (c) or 0.25% SRR (d). n = 3 rats (15 sections)/group. Scale bar: 10 μm. (e-h) Representative images of MBP-like immunoreactivities in sciatic nerves at the injected site on day 11 after the ipsilateral peri-sciatic nerve injection of vehicle (e), PLGA (f), 0.25 free RVC (g) or 0.25% SRR (h). n = 3 rats (15 sections)/group. Scale bar: 50 μm. (i-l) Representative ultrastructure images of sciatic nerves at the injected site on day 11 after the ipsilateral peri-sciatic nerve injection of vehicle (i), PLGA (j), 0.25 free RVC (k) or 0.25% SRR (l). n = 3 rats (9 ultra-sections/27 images)/group. Scale bar: 6 μm.
Figure 8.
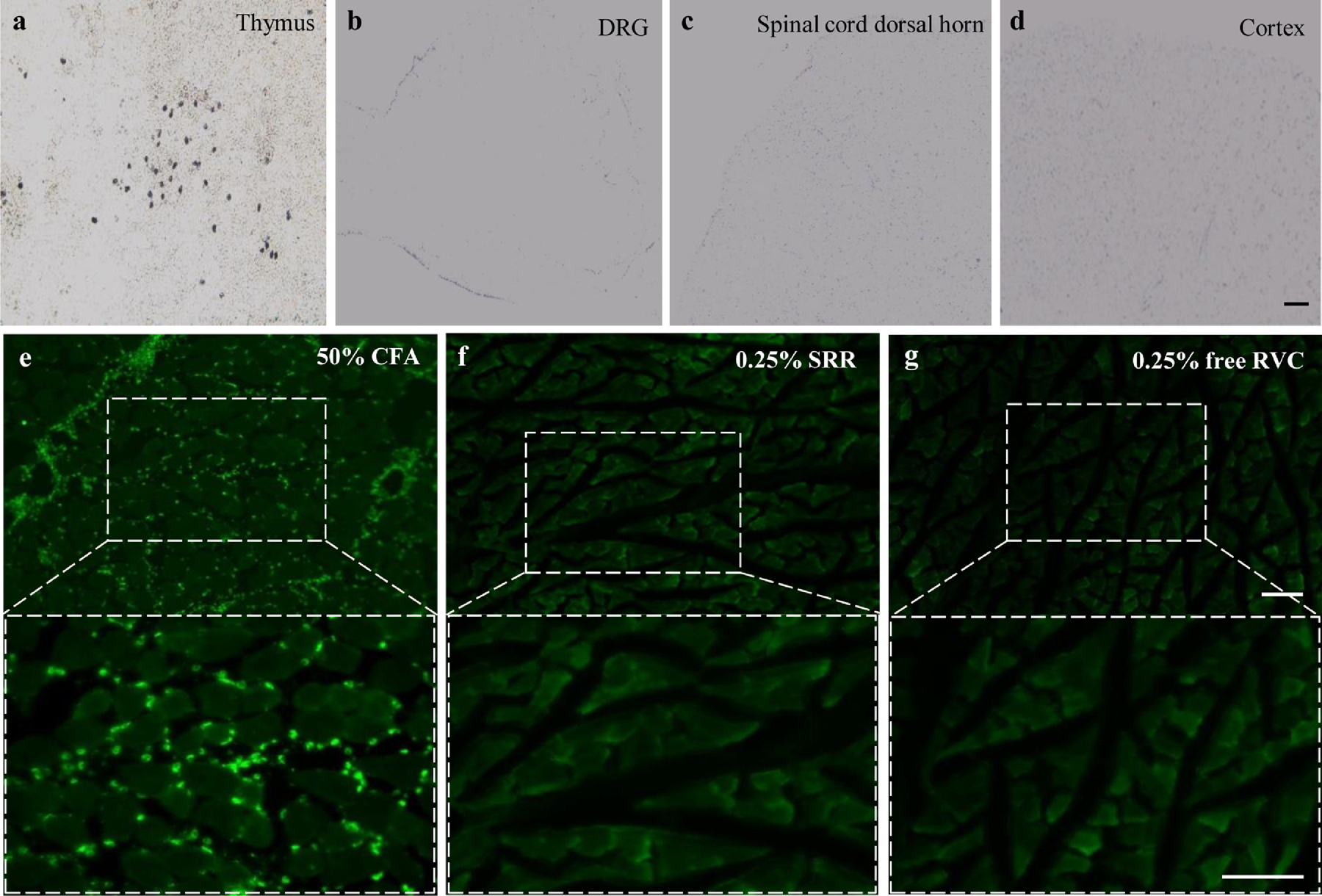
(a-d) Representative photographs showing TUNEL-positive cells in the thymus (a), the fourth lumbar dorsal root ganglion (b), the fourth lumbar spinal cord dorsal horn (c) and brain cortex (d) on the ipsilateral side on day 15 after the peri-sciatic nerve injection of 0.25% SRR. n = 3 rats (15 sections)/group. Scale bar: 100 μm. (e-g) Representative images of CD68-like immunoreactivities in the muscles on day 15 after 50% complete Freund’s adjuvant (CFA) injection (e) and at the injected site after peri-sciatic nerve injection of 0.25% SRR (f) or 0.25% free RVC (g). n = 3 rats (15 sections)/group. Scale bar: 10 μm.
Discussion
The present study demonstrated that injectable PLGA-coated RVC produced prolonged analgesic effects on postsurgical pain and neuropathic pain after single-dose local administration without detectable inflammation, tissue irritation or pathological nerve damage in the targeted nerve and adjacent muscles. Given that PLGA is an FDA-approved biomedical material, and that RVC is used currently in clinical practice, the injectable PLGA-coated RVC is a new and highly promising prolonged LA in the management of persistent pain.
PLGA has been one of the most attractive biodegradable polymers used to fabricate devices for controlled delivery of small drug molecules, therapeutic proteins, nucleic acid-based therapeutics and other macromolecules12, 45. PLGA is biocompatible and can degrade in the body via hydrolysis of its ester linkages in the presence of water. Importantly, the by-products of the hydrolysis process are its original monomers, lactic acid and glycolic acid, which can be further eliminated by the normal metabolic pathways under normal physiological conditions. As such, PLGA has been widely used as an FDA approved polymer for drug delivery and medical device fabrication29. In this study, we fabricated the RVC·HCl encapsulated PLGA microparticles with average size of 1.7 μm in diameter and found that the in vitro lasting release of RVC·HCl from these microparticles extended over 6 days. We also tried different sizes of microparticle and found out that microparticles larger than 2 μm in size could not be injected through 25-gauge syringes when the concentration of SRR exceeded the 0.125% that is required in clinical practice. Interestingly, the previous studies generated PLGA-coated bupivacaine microspheres in the range of 45–105 μm in size (containing 5, 10, 20, or 40 mg bupivacaine-free base; 0.4 ml) for injection around the sciatic nerve with a 21-gauge needle, or for subcutaneous injection with a 25-gauge needle35, 36. It is unclear how microspheres larger than 45 μm in size containing the higher concentration of bupivacaine (e.g., 10%) were injected subcutaneously with a 25-gauge needle.
Current LA hardly covers the period of persistent post-operative pain, which usually lasts for several days, and neuropathic pain by one injection. Repeated LA administration or catheter implantation followed by continuous LA infusion may lead to adverse effects such as infection or tissue damage, and can also present an inconvenience for patients39. In addition, although the PLGA-coated bupivacaine microspheres injected around the sciatic nerve or subcutaneously as mentioned above significantly reduced postoperative pain for up to 4 days35, 36, the toxicity of bupivacaine limited its clinical application. To this end, we examined whether SRR had a long-term analgesic effect on postoperative pain and neuropathic pain when applied to the ipsilateral sciatic nerve. As expected, a single injection of 0.25% SRR led to a dose-dependent analgesic effect on the development of incisional pain, and on the maintenance of SNI-induced neuropathic pain for at least 6 days. Peripheral nerve injury causes central sensitization indicated by the increases in the levels of p-ERK1/2 and GFAP in the spinal dorsal horn19, 58. Our results indicate that peri-sciatic nerve injection of SRR also attenuated SNI-induced increases in the amounts of p-ERK1/2 and GFAP in the dorsal horn. These findings are consistent with a previous observation that peri-sciatic nerve injection of the sodium channel blocker saxitoxin plus the glucocorticoid agonist dexamethasone decreased the SNI-induced activation of astrocytes in the lumbar dorsal horn of the spinal cord44. Our findings indicate that administration of local SRR has a long-lasting anti-nociceptive effect on neuropathic pain. Although previous studies have reported that local anesthetics attenuated the development of neuropathic pain when applied to the injured nerve at the time of nerve injury61, 62, our study may have more clinical application in patients seeking medical care after nerve injury-induced pain.
Due to the potential for neurotoxicity with administration of LA’s, especially at high dosage and when long-lasting effect is desired43, and given that exposure to drug delivery materials may also result in tissue reaction and toxicity33, we examined the safety of SRR by observing the locomotor function, the morphology of sciatic nerve and surrounding muscle at the injected site, and cell damage in peripheral and central nervous systems. In our study, only transient abnormal grasping reflex was observed, which may be attributed to the effect of RVC at higher dosage on motor nerve function59. Normal nerve fibers, fully developed axons stained via H&E, and similar expression of MBP and ultrastructure of nerve fibers in sciatic nerves were seen among all groups. There was no detectable cell damage in the DRG, dorsal horn and brain cortex after local injection of SRR. In addition, gross inflammation was absent in sciatic nerve and adjacent muscles at the injection site. Although prolonged nerve block may alter the process of wound healing and result in muscle atrophy in patients6, 51, neither muscle atrophy nor the variation in the process of wound healing were observed among all treatment groups. Consistent with previous reports4, 57, RVC in blood plasma was transiently detected after local injection of SRR. We found that this detectable level of systemic RVC was too low to affect SNI-induced pain hypersensitivity and basal behavioral responses. Whether this detectable level of systemic RVC affects heart function remains to be further examined. Taken together, our findings suggest that the PLGA-coated RVC is biologically safe and well tolerated by the body.
Several controlled-release LAs have been developed in recent years41. Exparel™ is the only one that has been applied in clinical practice3, 34. Exparel™ provides prolonged postsurgical analgesia for up to 3 days with a single-dose local administration at the surgical site in patients3, 34. However, its 3-day analgesic effect still did not completely cover the total period of postsurgical pain. Rats receiving injections with 0.6 ml of Exparel™ (1.31% bupivacaine HCl) at the sciatic nerve exhibited maximal sensory blockage for up to 240 min, which was only slightly longer than 1.31% free-bupivacaine HCl (210 min)32. Compared to Exparel™, SRR used in the present study produced much longer-lasting analgesic effect for up to at least 6 days with a single-dose administered via local peri-sciatic nerve injection. Although our findings were not exactly comparable to the previous study32, we may conclude that SRR has the potential for a longer analgesic duration than Exparel™. The analgesic dosages of SRR used in the present study did not exceed clinical dose and concentration of RVC. No evidence of active inflammation, tissue irritation and pathological nerve changes was detected in the sciatic nerve and adjacent muscles at the injected site, indicating good tolerability and safety of the in-situ application of this drug. In addition, the PLGA-coated RVC can be drained into powder, making it easier to transport and providing a long preservation time (up to years), whereas Exparel™ has the limitation of short preservation time (up to 30 days) according to its prescribing information. In addition, FDA-approved PLGA along with its by-products are biodegradable under proper conditions, and can be well tolerated by the body.
In conclusion, although present findings are based on male rat models, our research demonstrated that injectable PLGA-coated RVC can have the exact characteristics desired by physicians for application into their clinical practice: easy to use, achieving long-lasting analgesia and providing a high margin of safety with minimization of complications and side-effects. Given that females have shown greater prevalence of chronic pain conditions compared with males1, 11, 28, the effect of the injectable PLGA-coated RVC on incisional/neuropathic pain in female rats will be further examined. In addition, gross and histological safety in large numbers of rodents and non-rodent species remains to be further confirmed before clinical trials can be undertaken. Even so, our findings provide a new and highly promising avenue in the management of post-surgical pain and neuropathic pain.
Highlights:
PLGA-coated ropivacaine is continuously released in vitro for at least 6 days.
PLGA-coated ropivacaine abolishes postoperative pain.
PLGA-coated ropivacaine leads to a long-lasting analgesia in neuropathic pain.
PLGA-coated ropivacaine does not produce marked toxicity and inflammation.
Disclosures:
This work was supported by Rutgers New Jersey Medical School start-up fund to YXT and by New Jersey Health Foundation (PC102-17, ISFP 18-19, and PC25-18), and American Heart Association (19AIREA34380849) to XX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The authors declare no competing interests.
References
- 1.Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Sex differences in neuropathic pain intensity in diabetes. J Neurol Sci. 388:103–106, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Adams HA, Biscoping J, Ludolf K, Borgmann A, Bachmann MB, Hempelmann G. [The quantitative analysis of amide local anesthetics using high pressure liquid chromatography and ultraviolet detection (HPLC/UV)]. Regional-Anaesthesie. 12:53–57, 1989 [PubMed] [Google Scholar]
- 3.Aggarwal N. Local anesthetics systemic toxicity association with exparel (bupivacaine liposome)- a pharmacovigilance evaluation. Expert Opin Drug Saf. 17:581–587, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Al-Musawi A, Matar K, Kombian S, Andersson L. Blood concentration of prilocaine and lidocaine after the use of topical anesthesia (Oraqix((R)) ) in lacerated wounds. Dental traumatology : official publication of International Association for Dental Traumatology. 32:502–506, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Angeby Moller K, Klein S, Seeliger F, Finn A, Stenfors C, Svensson CI. Monosodium iodoacetate-induced monoarthritis develops differently in knee versus ankle joint in rats. Neurobiology of pain. 6:100036–100035, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvidsson I, Arvidsson H, Eriksson E, Jansson E. Prevention of quadriceps wasting after immobilization: an evaluation of the effect of electrical stimulation. Orthopedics 9:1519–1528, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 64:493–501, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Carter GT, Duong V, Ho S, Ngo KC, Greer CL, Weeks DL. Side effects of commonly prescribed analgesic medications. Phys Med Rehabil Clin N Am. 25:457–470, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Zhang J, Sun L, Zhang Y, Gan WB, Tang P, Yang G. Long-term imaging of dorsal root ganglia in awake behaving mice. Nature communications. 10:3087–3098, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow LH, Chen YH, Lai CF, Lin TY, Chen YJ, Kao JH, Huang EY. Sex Difference of Angiotensin IV-, LVV-Hemorphin 7-, and Oxytocin-Induced Antiallodynia at the Spinal Level in Mice With Neuropathic Pain. Anesthesia and analgesia. 126:2093–2101, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 161:505–522, 2012 [DOI] [PubMed] [Google Scholar]
- 13.de Araujo DR, da Silva DC, Barbosa RM, Franz-Montan M, Cereda CM, Padula C, Santi P, de Paula E. Strategies for delivering local anesthetics to the skin: focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert opinion on drug delivery. 10:1551–1563, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 87:149–158, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Denis G, Humblet C, Verlaet M, Boniver J, Defresne MP. p53, Bax and Bcl-2 in vivo expression in the murine thymus after apoptogenic treatments. Anticancer research. 18:3315–3321, 1998 [PubMed] [Google Scholar]
- 16.Fredrickson MJ, Ball CM, Dalgleish AJ. A prospective randomized comparison of ultrasound guidance versus neurostimulation for interscalene catheter placement. Regional anesthesia and pain medicine. 34:590–594, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:843–850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillo R, de Melo NF, de Araujo DR, de Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. Journal of drug targeting. 18:688–699, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Kerstman E, Ahn S, Battu S, Tariq S, Grabois M. Neuropathic pain. Handb Clin Neurol. 110:175–187, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. Journal of biomedical materials research. 59:450–459, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NG, Berde CB, Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 104:415–421, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Li R, Shen Y. An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life sciences. 92:747–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Ma A, Liu K. Geniposide alleviates lipopolysaccharide-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy. Artificial cells, nanomedicine, and biotechnology. 47:1524–1532, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz BM, Bekker A, Zhang W, Tao YX. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain. 156:711–721, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Mao Y, Liang L, Wu S, Yuan J, Mo K, Cai W, Mao Q, Cao J, Bekker A, Zhang W, Tao YX. The transcription factor C/EBPbeta in the dorsal root ganglion contributes to peripheral nerve trauma-induced nociceptive hypersensitivity. Sci Signal 10:eaam5345–5358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liaw WJ, Zhu XG, Yaster M, Johns RA, Gauda EB, Tao YX. Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Molecular pain. 4:45–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma P, Li T, Xing H, Wang S, Sun Y, Sheng X, Wang K. Local anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: In vitro and in vivo evaluation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 89:689–695, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Machelska H, Celik MO. Recent advances in understanding neuropathic pain: glia, sex differences, and epigenetics. F1000Res. 5:2743–2753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel). 3:1377–1397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masters DB, Berde CB, Dutta S, Turek T, Langer R. Sustained local anesthetic release from bioerodible polymer matrices: a potential method for prolonged regional anesthesia. Pharm Res. 10:1527–1532, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Masters DB, Berde CB, Dutta SK, Griggs CT, Hu D, Kupsky W, Langer R. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 79:340–346, 1993 [DOI] [PubMed] [Google Scholar]
- 32.McAlvin JB, Padera RF, Shankarappa SA, Reznor G, Kwon AH, Chiang HH, Yang J, Kohane DS. Multivesicular liposomal bupivacaine at the sciatic nerve. Biomaterials. 35:4557–4564, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAlvin JB, Reznor G, Shankarappa SA, Stefanescu CF, Kohane DS. Local toxicity from local anesthetic polymeric microparticles. Anesthesia and analgesia. 116:794–803, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo DJ. Local Infiltration Analgesia With Liposomal Bupivacaine Improves Pain Scores and Reduces Opioid Use After Total Knee Arthroplasty: Results of a Randomized Controlled Trial. J Arthroplasty. 33:90–96, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Ohri R, Blaskovich P, Wang JC, Pham L, Nichols G, Hildebrand W, Costa D, Scarborough N, Herman C, Strichartz G. Prolonged nerve block by microencapsulated bupivacaine prevents acute postoperative pain in rats. Regional anesthesia and pain medicine. 37:607–615, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Ohri R, Wang JC, Blaskovich PD, Pham LN, Costa DS, Nichols GA, Hildebrand WP, Scarborough NL, Herman CJ, Strichartz GR. Inhibition by local bupivacaine-releasing microspheres of acute postoperative pain from hairy skin incision. Anesthesia and analgesia. 117:717–730, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29:3206–3219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puglia C, Sarpietro MG, Bonina F, Castelli F, Zammataro M, Chiechio S. Development, characterization, and in vitro and in vivo evaluation of benzocaine- and lidocaine-loaded nanostructrured lipid carriers. Journal of pharmaceutical sciences. 100:1892–1899, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 33:160–171, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Renn CL, Carozzi VA, Rhee P, Gallop D, Dorsey SG, Cavaletti G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Molecular pain. 7:29–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santamaria CM, Woodruff A, Yang R, Kohane DS. Drug delivery systems for prolonged duration local anesthesia. Mater Today (Kidlington). 20:22–31, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute medicine & surgery. 4:152–160, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selander D. Neurotoxicity of local anesthetics: animal data. Regional anesthesia. 18:461–468, 1993 [PubMed] [Google Scholar]
- 44.Shankarappa SA, Tsui JH, Kim KN, Reznor G, Dohlman JC, Langer R, Kohane DS. Prolonged nerve blockade delays the onset of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 109:17555–17560, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 28:5–24, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Singh OV, Yaster M, Xu JT, Guan Y, Guan X, Dharmarajan AM, Raja SN, Zeitlin PL, Tao YX. Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury. Proteomics. 9:1241–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L, Zhao JY, Gu X, Liang L, Wu S, Mo K, Feng J, Guo W, Zhang J, Bekker A, Zhao X, Nestler EJ, Tao YX. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain. 158:1153–1165, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang YZ, Ni JX, An JX. Complex regional pain syndrome type I following discTRODE radiofrequency treated with continuous lumbar sympathetic trunk block using patient-controlled analgesia. Pain medicine. 14:309–310, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Tedesco S, Bolego C, Toniolo A, Nassi A, Fadini GP, Locati M, Cignarella A. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology. 220:545–554, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 82:1013–1025, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Todkar M. Sciatic nerve block causing heel ulcer after total knee replacement in 36 patients. Acta orthopaedica Belgica. 71:724–725, 2005 [PubMed] [Google Scholar]
- 52.Vellosillo L, Munoz MP, Paino CL. Adipose tissue-derived stromal cells (ADSC) express oligodendrocyte and myelin markers, but they do not function as oligodendrocytes. Histochem Cell Biol. 148:503–515, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Wang PK, Cao J, Wang H, Liang L, Zhang J, Lutz BM, Shieh KR, Bekker A, Tao YX. Short-Term Sleep Disturbance-Induced Stress Does not Affect Basal Pain Perception, but Does Delay Postsurgical Pain Recovery. J Pain. 16:1186–1199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Shan Q, Meng Y, Pan J, Yi S. Mrpl10 and Tbp Are Suitable Reference Genes for Peripheral Nerve Crush Injury. International journal of molecular sciences. 18: 263–273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiniger CF, Golovanevski M, Sokolsky-Papkov M, Domb AJ. Review of prolonged local anesthetic action. Expert opinion on drug delivery. 7:737–752, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Wes PD, Easton A, Corradi J, Barten DM, Devidze N, DeCarr LB, Truong A, He A, Barrezueta NX, Polson C, Bourin C, Flynn ME, Keenan S, Lidge R, Meredith J, Natale J, Sankaranarayanan S, Cadelina GW, Albright CF, Cacace AM. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PloS one. 9:e106050–106073, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wildsmith JA. Interactions between mivacurium and prilocaine. British journal of anaesthesia. 79:262, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 152:S2–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 377:2215–2225, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Wylde V, Dennis J, Beswick AD, Bruce J, Eccleston C, Howells N, Peters TJ, Gooberman-Hill R. Systematic review of management of chronic pain after surgery. Br J Surg. 104:1293–1306, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 116:243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yatziv SL, Devor M. Suppression of neuropathic pain by selective silencing of dorsal root ganglion ectopia using nonblocking concentrations of lidocaine. Pain. 160:2105–2114, 2019 [DOI] [PubMed] [Google Scholar]
- 63.Zhang ZJ, Guo JS, Li SS, Wu XB, Cao DL, Jiang BC, Jing PB, Bai XQ, Li CH, Wu ZH, Lu Y, Gao YJ. TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. The Journal of experimental medicine. 215:3019–3037, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, Gao YJ, Hoffman PN, Cui H, Li M, Dong X, Tao YX. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nature neuroscience. 16:1024–1031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


