Abstract
Objectives:
To identify the interrelations between the trajectories of social isolation and dementia in older adults.
Methods:
Data came from the National Health and Aging Trends Study 2011–2018 surveys. Group-based dual trajectory modeling was used to examine trajectories and their interrelations.
Results:
Four trajectories of social isolation—rarely isolated (62.2%), steady increase (13.5%), steady decrease (7.4%), and persistently isolated (16.9%) and dementia risk—persistently low risk (80.4%), increasing with early-onset (3.9%), increasing with late-onset (4.5%), and persistently high risk (11.2%) emerged. Two-thirds of the low-risk dementia group were in the rarely isolated group. The high-risk dementia group had the most overlap with the decreasing social isolation group (47%), followed by the persistently isolated group (28%).
Conclusions:
Social isolation and dementia mostly evolved in the same direction. However, the pattern of associations between these trajectories is intricate and may be reversed among long-term dementia survivors.
Keywords: dementia, social isolation, social relations, dual trajectories
Introduction
Over 43 million people worldwide lived with dementia in 2016, more than doubled from 1990 (Nichols et al., 2019). Care and support of patients with dementia have wide-ranging consequences for patients, families, health care, and society, especially given that there are no approved medicines that can cure dementia or slow it down. A modest one-year delay in the onset and progression of Alzheimer’s disease, the most common cause of dementia, could lead to 9.2 million fewer cases of the disease in 2050 (Brookmeyer, Johnson, Ziegler-Graham, & Arrighi, 2007). The impact of delayed onset has sparked an interest in identifying modifiable factors to delay or prevent the onset of dementia. One potentially modifiable factor involves social relationships. Deficiency in social relationships, reflected by both objective and subjective measures, has been linked to a range of adverse health outcomes (Smith & Victor, 2019). The health risks associated with deficient social relationships are particularly relevant to older adults due to life course transitions and loss of social network members in later life (Coyle & Dugan, 2012).
Social isolation is the lack of social contact, relationships, and social integration and is considered an objective measure of social relationships (Coyle & Dugan, 2012). Cacioppo and colleagues (Cacioppo & Hawkley, 2009) summarized the following mechanisms linking social isolation and cognitive decline based on a literature review: (a) increased inflammatory responses in the brain, (b) behavioral or neural plasticity, (c) reduction in social stimulation and engagement, (d) reduction in cognitive resources available for creative adaptations, and (e) increase in depression and reduction in physical activity. Although empirical evidence provides support for the association of social isolation and dementia (Evans, Martyr, Collins, Brayne, & Clare, 2018; Kuiper et al., 2016), results from individual studies are mixed (Crooks, Lubben, Petitti, Little, & Chiu, 2008; Griffin, Mezuk, Williams, Perrin, & Rybarczyk, 2018; Holwerda et al., 2014; Lara et al., 2019), sometimes within the same study involving multiple measures of social isolation (Amieva et al., 2010; Rafnsson, Orrell, d’Orsi, Hogervorst, & Steptoe, 2017; Sommerlad, Sabia, Singh-Manoux, Lewis, & Livingston, 2019). These heterogeneities could be partially due to differences in the measures of social relationships and the quality of methods (Evans et al., 2018).
Overall, existing evidence supports that deficiency in multiple aspects of social relationships is associated with cognitive decline (Evans et al., 2018; Kuiper et al., 2015), but their relationship is only partially understood. Most studies used conventional regression-based methods or growth curve modeling approaches that could only identify average associations of social isolation and cognitive impairment over heterogeneous subpopulations (Amieva et al., 2010; Crooks et al., 2008; Griffin et al., 2018; Holwerda et al., 2014; Lara et al., 2019; Rafnsson et al., 2017). Research suggests considerable heterogeneity in the rates and patterns of change in cognitive function in older adults (Haiqun Xie, Mayo, & Koski, 2011). Only a few studies considered this heterogeneity and examined how a deficiency in social relations and participation affects the trajectories of cognition in older adults (Béland, Zunzunegui, Alvarado, Otero, & Del Ser, 2005; Donovan et al., 2017; Elovainio et al., 2018; Park, Kwon, & Lee, 2017; Read, Comas-Herrera, & Grundy, 2020).
Another barrier to our understanding is that social isolation has mainly been considered as a risk factor and rarely examined as a consequence of cognitive impairment (Amieva et al., 2010; Crooks et al., 2008; Griffin et al., 2018; Holwerda et al., 2014; Lara et al., 2019; Rafnsson et al., 2017; Sommerlad et al., 2019). Moreover, most studies measured social isolation at one time only and did not examine changes in social networks and opportunities for social integration and how these changes relate to cognitive decline (Amieva et al., 2010; Crooks et al., 2008; Griffin et al., 2018; Holwerda et al., 2014; Lara et al., 2019; Rafnsson et al., 2017). One exception was a study based in a suburban city in Spain that found a widening gap in cognitive functioning over time between those with stable high and stable low levels of social integration, with the latter experiencing a faster decline in cognitive functioning (Béland et al., 2005). Donovan et al. (2017) found that loneliness at baseline predicted accelerated cognitive decline, and poor baseline cognition predicted greater loneliness over time using data from the U.S. Health and Retirement Study, pointing to the potential bidirectional relationship between aspects of social relations and cognitive function.
The complex and potentially bidirectional relationship between social relationships and cognitive functioning requires long-term cohort studies involving measures at multiple points. The objective of the present study was to examine the longitudinal relationship between social isolation and dementia. More specifically, we aimed to identify patterns of changes in social isolation and dementia and the interrelations between these trajectories in a nationally representative sample of older adults over eight years. We used a group-based dual trajectory modeling approach to identify the heterogeneous trajectories of change in dementia risk and social isolation and their interrelationships. Based on previous studies (Béland et al., 2005; Elovainio et al., 2018; Park et al., 2017; Haiqun Xie et al., 2011), we hypothesized that changes in social isolation and dementia would follow heterogeneous trajectories respectively, and there would be a significant association between the two sets of trajectories.
Methods
Data and participants
The National Health and Aging Trends Study (NHATS) is a nationally representative study of Medicare beneficiaries aged 65 and older (www.NHATS.org). The baseline NHATS interviews occurred in 2011 with older adults living in settings other than nursing homes. Annual follow-up interviews took place regardless of residential status. NHATS methodology included the use of proxy respondents familiar with the sample person if a respondent was unable to answer for themselves. The present study included data on the original NHATS sample from eight survey rounds (2011–2018). A total of 7,609 community-dwelling participants responded to the 2011 interview. The study sample completed an average of five annual surveys, and only 36% of the baseline participants completed the 2018 survey. The present study consisted of secondary analyses using publicly available datasets and was deemed activities not regulated as Human Subjects Research by the University of Michigan Institutional Review Board.
Measure of social isolation
Pohl et al. (Pohl, Cochrane, Schepp, & Woods, 2017) constructed a variable of social isolation using six NHATS items that address the four domains of network and integration in the general adult population: (a) marriage or partnership, (b) family and friends, (c) church participation, and (d) club participation (Berkman & Syme, 1979). One point was given for meeting each of the following isolation criteria: (1) no marriage or partner; unable to identify either (2) any family member or (3) a friend when asked “Looking back over the past year, who are the people you talked with most often about important things?”; (4) no in-person visit with friends or family not living with the respondent in the last month; (5) no attendance at religious services in the past month; and (6) no participation in clubs, classes, or other organized activities in the past month. The composite social isolation score ranged from 0 to 6, with a higher score indicating more isolation and deficiency of network contacts and integrating relationships. Following Pohl et al. (Pohl et al., 2017), we used the cut-off score of ≥ 4 to indicate social isolation.
Assessment of dementia
NHATS researchers classified participants into probable dementia, possible dementia, and no dementia based on respondent- or proxy- report of ever receiving a diagnosis of dementia or Alzheimer’s disease from a doctor, AD8 Dementia Screening Interview with proxies (Galvin, Roe, Xiong, & Morris, 2006), and cognitive tests (Kasper, Freedman, & Spillman, 2013). The cognitive tests assessed three domains of cognitive functioning: memory (immediate and delayed 10-word recall), orientation (today’s date and president-naming), and executive functioning (clock drawing test). These tests were administered to all self-respondents and to sample persons whose proxies agreed to have the tests done on the sample person. NHATS participants were classified as probable dementia if they (a) reported receiving a diagnosis of dementia or Alzheimer’s disease from a doctor; (b) a score of 2 or higher on the AD8 Dementia Screening Interview by proxies; or (c) scored ≤1.5 Standard Deviations (S.D.s) below the mean for self-respondents on at least two of the cognitive domains. Scores ≤1.5 SDs below the mean in one cognitive domain indicated possible dementia. In the present study, dementia was narrowly defined as probable dementia, whereas possible dementia and no dementia were collapsed into a single category (Moon, Badana, Hwang, Sears, & Haley, 2019). The narrow definition has a sensitivity of 65.7% against a diagnosis of dementia in The Aging, Demographics, and Memory Study (ADAMS) and high specificity against ADAMS-based criteria for normal (87.2%) or normal plus cognitive impairment without dementia (100%) (Kasper et al., 2013).
Measures of socio-demographic, health, and functioning status
Socio-demographic variables included age groups, sex (female, male), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other race), highest educational attainment (less than high school, high school or GED, some college but no degree, or college graduate), and family/household income. Health and functioning variables included chronic disease count and limitations in mobility (including going outside, getting around inside, and getting out of bed), activities of daily living (ADLs, including eating, bathing, toileting, and dressing), and instrumental activities of daily living (IADLs, including doing laundry, making hot meals, shopping for personal items, paying bills/banking, and keeping track of medications). Chronic disease count is the number of self-reported physician diagnosis of the following: heart disease, arthritis, osteoporosis, diabetes, lung disease, stroke, and cancer. A limitation in mobility, ADLs, and IADLs was defined as needing assistance carrying out the activity (must be for health or functioning related reasons for IADLs) or difficulty performing the activity alone. Another indicator of health is symptoms of depression and anxiety, as measured by the Patient Health Questionnaire for Depression and Anxiety (PHQ-4), a validated ultra-brief screening tool for both depression and anxiety symptoms (Kroenke, Spitzer, Williams, & Lowe, 2009). Participants were asked how often over the last month they “had little interest or pleasure in doing things,” “felt down, depressed or hopeless,” “felt nervous, anxious, or on edge,” and “been unable to stop or control worrying” on a 4-point Likert scale: not at all (0), several days (1), more than half the days (2), and nearly every day (3). PHQ-4 scores range from 0 to 12, with a higher score indicating more severe depression and anxiety symptoms.
Data analysis
Our analytical plan has two parts. Part I involves a group-based dual trajectory modeling approach to identify distinct trajectory patterns for social isolation and dementia and assess their interrelationships. The course or the evolution of an outcome over age or time is called a developmental trajectory (Nagin, 2005). Group-based trajectory modeling (GBTM) is an application of finite mixture modeling, which assumes that the population is composed of a mixture of distinct groups defined by their developmental trajectories (Nagin, 2005). GBTM aims to identify clusters of individuals, called trajectory groups, who have followed approximately the same developmental trajectory on an outcome of interest, using a finite set of different polynomial functions of age or time (Nagin, 2005; Nagin & Odgers, 2010). Estimation of model parameters involves maximum likelihood, performed using a general quasi-Newton procedure. The objective of GBTM is to estimate a set of parameters that maximizes the probability of Yi, a longitudinal sequence of measurements on individual i over T periods. These parameters, which depend on the specific form of the likelihood function, define the shapes of the trajectories and the probability of group membership. A separate set of parameters is estimated for each group. Therefore, the shapes of trajectories are free to vary (Nagin, 2005).
Drawing comparisons with more familiar forms of trajectory modeling, standard growth curve modeling assumes that the parameters only describe the average growth in the population and that the individual trajectories vary about this mean according to multivariate continuous distributions. In contrast, GBTM utilizes a multinomial modeling strategy, using a finite number of groups to approximate a continuous distribution (Nagin & Odgers, 2010). GBTM does not include random effects in each group’s trajectory model; instead, population variability is captured by differences in the shape and level of their trajectories. In other words, the growth curve models aim to identify the factors that account for individual variability, while GBTM concerns factors that distinguish group membership and how do groups differ in response to events (Nagin, 2005). An extended discussion of the methods and technical details is available in Nagin (2005). A detailed comparison of GBTM and other applications of trajectory modeling (e.g., growth mixture modeling) is available in Nagin & Odgers (2010).
Model outputs for the basic univariate GBTM include the number of trajectory groups, trajectory shapes, and the proportion of the population belonging to each trajectory group. Individuals are assigned to each trajectory group based on the highest posterior probability of group membership, the probability that an individual belongs to each of the trajectory groups. The univariate GBTM has been extended to a dual trajectory modeling that analyzes the interrelationship of two sets of trajectories (Nagin, Jones, Passos, & Tremblay, 2018). In addition to the standard model outputs, the dual model provides estimates of conditional and joint probabilities that link the two sets of trajectories and help identify the association between the two outcomes (Nagin et al., 2018).
Fitting the dual trajectory model involved two stages. First, we estimated the univariate GBTM for dementia and social isolation separately and identified the best univariate trajectory models for each. The optional trajectory group number was determined based on a combination of factors, including (1) the change in Bayesian Information Criteria (BIC), (2) classification quality, reflected by the average posterior probability of group assignment (≥ 0.7), (3) parsimony, and (4) interpretability of groups (Nagin, 2005). For each trajectory model, we started with 2-group models, testing zero-order, linear, and quadratic specifications for each group. We added more groups until we obtained the best fitting model. Second, a dual trajectory model for both dementia and social isolation was jointly estimated using starting parameters from the final univariate models. Although the number of optional trajectory groups in the dual model usually is consistent with those identified from the univariate models, we fitted several joint models by varying the number of groups for each variable from three to five and retained the model with the lowest absolute BIC value. In all GBTM models, we estimated social isolation and dementia as a function of time only, without any other predictors, to avoid contamination of group classification with an element of circularity (Nagin, 2005). The Proc Traj Stata plugin (Jones & Nagin, 2013) was used to fit the trajectory models, with the probability of Yi specified as the binary logit distribution.
Part II of the analytical plan involved merging results from the dual trajectory model with the main dataset and conducting descriptive, bivariate, and multivariable analyses on the merged dataset to identify correlates of dementia and social isolation trajectories. Descriptive analysis was used to characterize the sample stratified by the trajectory groups and performed global tests of association. A series of one-sided Fisher’s exact tests were conducted to test the association of two trajectory groups using Holm-adjusted p-values (Holm, 1979). We also used multinomial logistic regression to model dementia trajectory groups as a function of social isolation trajectories with adjustment for baseline demographics. All analyses, including the dual trajectory modeling, were conducted using Stata 15.1 (Stata Corp., College Station, TX).
Sensitivity analysis
We checked the robustness of our findings against the broad definition of dementia (i.e., combing possible and probable dementia into the dementia group). Because attrition occurred during the eight years, we also conducted a sensitivity analysis to examine the influence of attrition on our findings.
Results
Social isolation trajectories
In the dual model, a logit model with four trajectory groups for social isolation was the best fit based on the criteria described earlier, with an average posterior probability of group assignment ranging from .7 to .8 across groups. The four trajectory groups were: rarely isolated (62.2%), steady increase in social isolation (13.5%), steady decrease in social isolation (7.4%), and persistently isolated (16.9%) (Figure 1).
Figure 1.
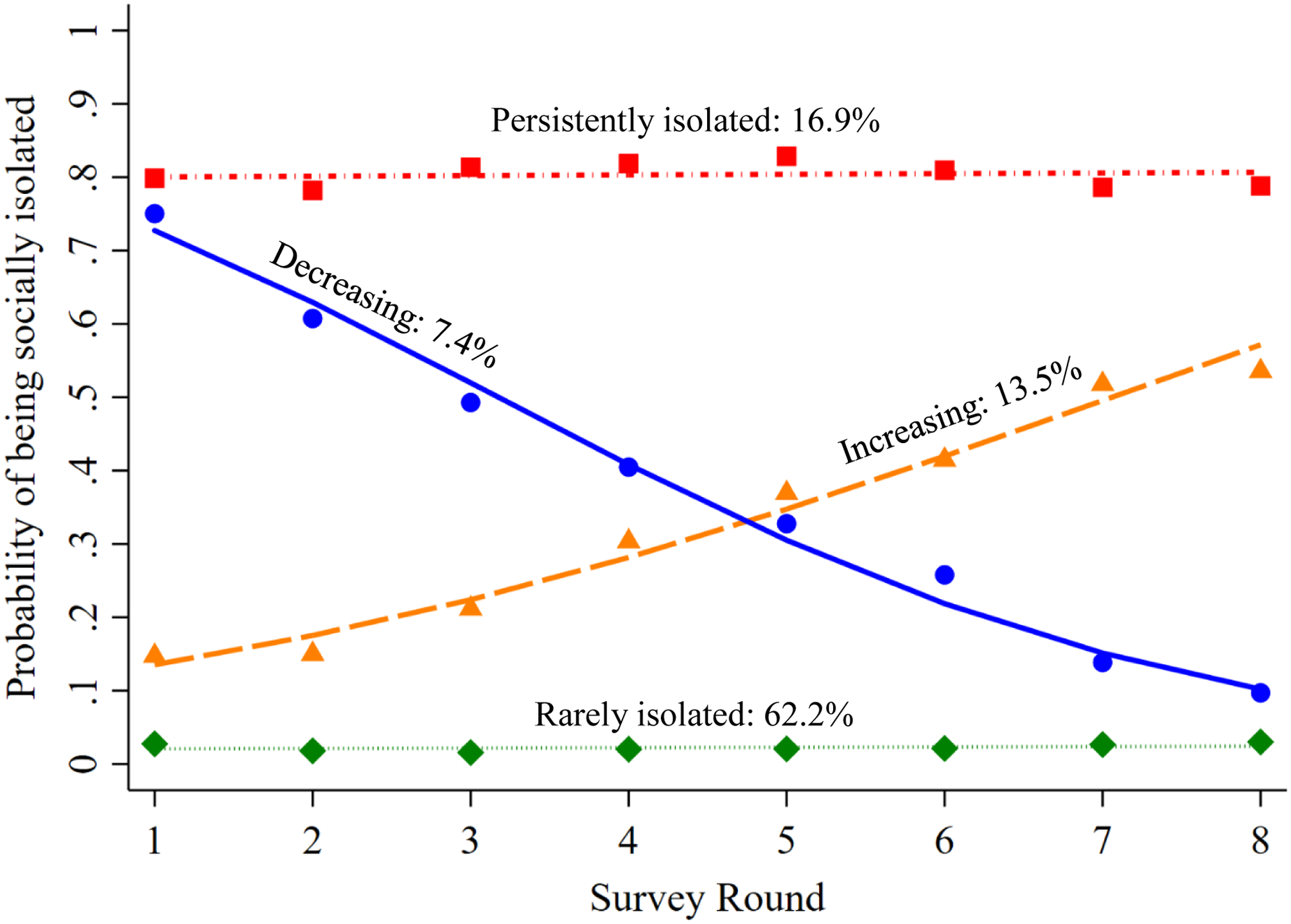
Social isolation trajectories over eight years, jointly estimated with dementia. It shows social isolation trajectories with an estimated probability of being socially isolated at each study round and the weighted proportions of the study population following each trajectory.
Participants who were 75 or older, women, racial and ethnic minorities, and those with lower education were significantly over-represented among all social isolation trajectory groups other than the rarely isolated. The rarely isolated group had the highest mean household income, the lowest number of chronic diseases and functional limitations, and the lowest levels of depression/anxiety symptoms as measured by the PHQ-4 (Kroenke et al., 2009) (Table 1).
Table 1.
Baseline sample characteristics by social isolation group (N=7,609)
| Social isolation trajectory groups | Global Test | |||||
|---|---|---|---|---|---|---|
| Rarely isolated (N=4,253) | Increasing (N=1,151) | Decreasing (N=770) | Persistently isolated (N=1,435) | Test Statistic | p | |
| Age groups (%) | F(10.61, 594.36) = 46.4 | <.001 | ||||
| 65–69 years | 33.6 (32.1, 35.2) | 16.7 (13.8, 20.2) | 13.0 (9.5, 17.5) | 22.5 (19.6, 25.8) | ||
| 70–74 years | 27.6 (26.6, 28.8) | 20.3 (17.4, 23.6) | 10.7 (8.6, 13.2) | 25.0 (22.3, 27.8) | ||
| 75–79 years | 18.7 (17.5, 20.0) | 23.8 (21.1, 26.8) | 15.7 (12.5, 19.5) | 18.0 (16.4, 19.8) | ||
| 80–84 years | 12.2 (11.4, 13.1) | 20.8 (18.7, 23.2) | 20.4 (17.0, 24.1) | 16.3 (14.5, 18.4) | ||
| 85–89 years | 6.0 (5.4, 6.7) | 12.5 (10.7, 14.4) | 23.2 (19.6, 27.3) | 11.7 (9.9, 13.9) | ||
| 90 years or over | 1.8 (1.5, 2.2) | 5.8 (4.7, 7.2) | 17.1 (14.3, 20.2) | 6.4 (5.3, 7.7) | ||
| Sex (%) | F(2.97, 166.49) = 3.0 | .032 | ||||
| Female | 55.2 (53.3, 57.2) | 59.3 (55.9, 62.6) | 61.1 (56.4, 65.5) | 57.7 (54.4, 60.8) | ||
| Male | 44.8 (42.8, 46.7) | 40.7 (37.4, 44.1) | 38.9 (34.5, 43.6) | 42.3 (39.2, 45.6) | ||
| Race/ethnicity (%) | F(6.44, 360.46) = 12.8 | <.001 | ||||
| White, non-Hispanic | 84.4 (82.8, 85.9) | 77.0 (73.7, 79.9) | 72.4 (67.2, 77.1) | 72.7 (69.1, 76.0) | ||
| Black, non-Hispanic | 6.6 (5.9, 7.5) | 9.3 (7.9, 10.9) | 11.9 (10.0, 14.1) | 10.9 (9.6, 12.5) | ||
| Other race, non-Hispanic | 3.8 (3.0, 4.8) | 4.7 (2.9, 7.4) | 6.8 (4.6, 9.9) | 6.5 (4.9, 8.6) | ||
| Hispanic | 5.1 (4.4, 6.0) | 9.1 (6.9, 11.9) | 8.9 (5.7, 13.6) | 9.8 (7.8, 12.3) | ||
| Education (%) | F(7.82, 438.11) = 64.8 | <.001 | ||||
| Less than high school | 13.9 (12.5, 15.5) | 29.3 (26.1, 32.8) | 40.9 (36.7, 45.2) | 36.6 (33.2, 40.2) | ||
| High school | 26.9 (25.2, 28.7) | 29.3 (26.7, 32.1) | 28.4 (24.8, 32.2) | 28.4 (25.6, 31.3) | ||
| Some college, no degree | 22.8 (21.6, 24.2) | 19.6 (16.9, 22.5) | 15.4 (12.5, 18.7) | 20.1 (17.3, 23.1) | ||
| College graduate | 36.3 (33.5, 39.1) | 21.8 (18.7, 25.1) | 15.4 (12.1, 19.4) | 14.9 (12.9, 17.2) | ||
| Family income in 2011 ($) (mean) | 71,312 (63,492, 79,131) | 42,004 (34,806, 49,203) | 28,556 (24,570, 32,543) | 34,881 (24,173, 45,590) | F(3, 54) = 32.6 | <.001 |
| Chronic disease count (mean) | 2.24 (2.18, 2.29) | 2.58 (2.49, 2.67) | 2.89 (2.75, 3.02) | 2.55 (2.46, 2.64) | F(3, 54) = 36.9 | <.001 |
| Activities of daily living limitations (mean) | .27 (.24, .29) | .62 (.55, .69) | 1.69 (1.56, 1.82) | .68 (.61, .75) | F(3, 54) = 190.8 | <.001 |
| Instrumental activities of daily living (mean) | .42 (.38, .45) | 1.04 (.95, 1.13) | 2.37 (2.24, 2.50) | 1.12 (1.02, 1.22) | F(3, 54) = 321.3 | <.001 |
| Mobility limitations (mean) | .36 (.34, .39) | .74 (.67, .80) | 1.53 (1.43, 1.64) | .80 (.72, .87) | F(3, 54) = 228.9 | <.001 |
| PHQ-4 score (mean) | 1.44 (1.36, 1.52) | 2.38 (2.18, 2.57) | 3.60 (3.31, 3.90) | 2.55 (2.36, 2.74) | F(3, 54) = 109.4 | <.001 |
| Proxy-respondent | 1.1 (.08, .01) | 4.5 (3.4, 5.9) | 40.4 (35.5, 45.5) | 9.5 (7.8, 11.5) | F(2.80, 156.62) = 449.3 | <.001 |
| # of surveys completed | 5.1 (5.0, 5.2) | 5.6 (5.4, 5.8) | 4.0 (3.7, 4.2) | 4.6 (4.4, 4.7) | F(3, 54) = 65.8 | <.001 |
| Estimated proportion in the population (%) | 62.2 (60.7, 63.8) | 13.5 (12.7, 14.4) | 7.4 (6.8, 8.0) | 16.9 (15.8, 18.0) | ||
Note. Weighted estimates were presented applying NHATS complex survey design features using Taylor linearization for variance estimation.
Dementia trajectories
In the dual model, a logit model with four trajectory groups for dementia was the best fit for the data based on criteria described earlier, with an average posterior probability of group assignment ranging from .7 to .9 across groups. The four trajectory groups were: persistently low risk (80.4%), increasing with early-onset (3.9%), increasing with late-onset (4.5%), and persistently high risk (11.2%) (Figure 2).
Figure 2.
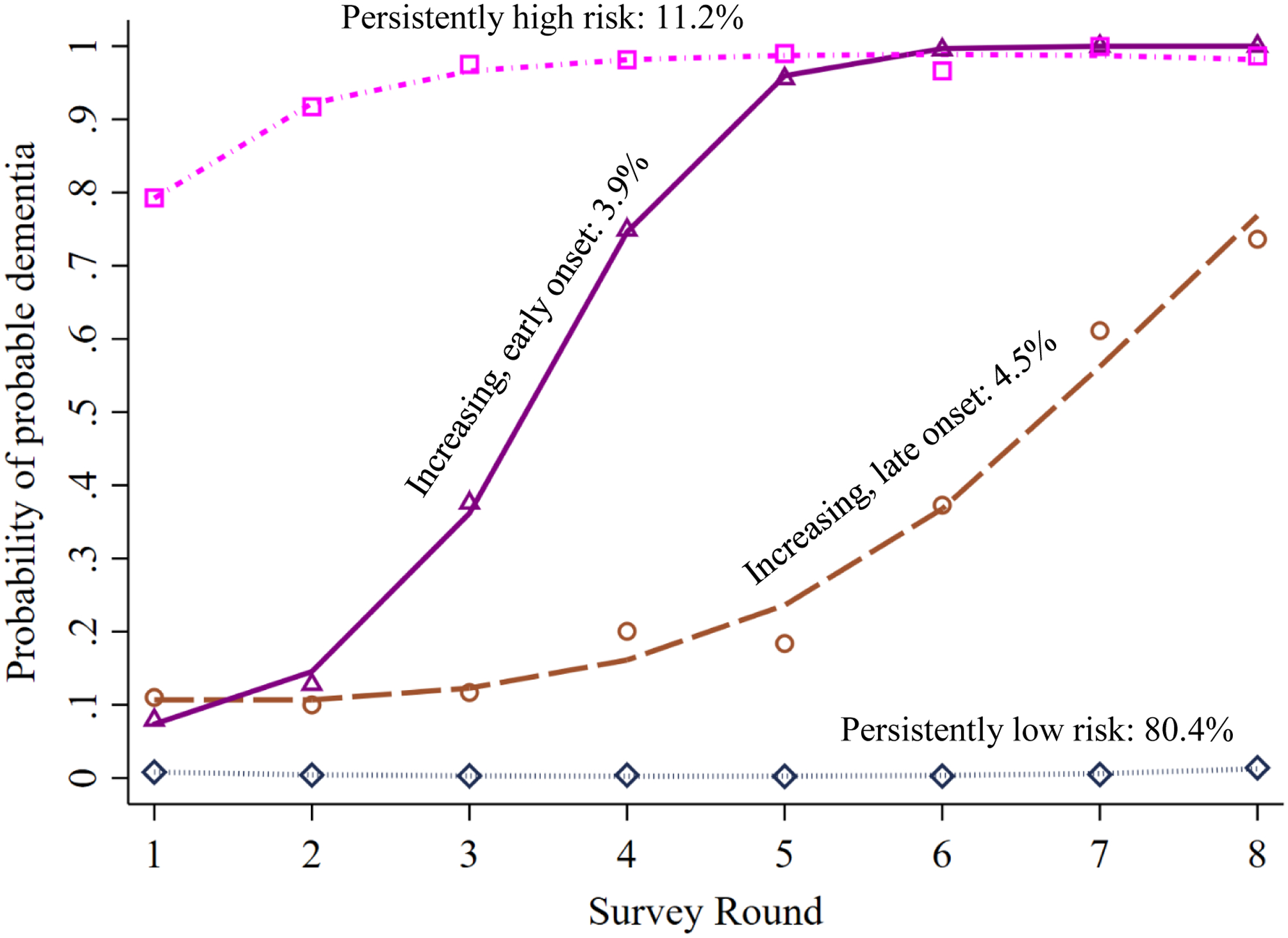
Dementia trajectories over eight years, jointly estimated with social isolation. It shows dementia trajectories with an estimated probability of having probable dementia at each study round and the weighted proportions of the study population following each trajectory.
Participants who were 75 or older, women, racial and ethnic minorities, and those with lower education were significantly over-represented among all dementia trajectory groups other than the persistently low trajectory. The persistently low group had the highest mean household income, the lowest number of chronic diseases and functional limitations, and the lowest levels of depression/anxiety symptoms as measured by the PHQ-4 (Kroenke et al., 2009) (Table 2).
Table 2.
Baseline sample characteristics by dementia group (N=7,609)
| Dementia trajectory groups | Global Test | |||||
|---|---|---|---|---|---|---|
| Persistently low (N=5,674) | Late onset (N=420) | Early onset (N=366) | Persistently high (N=1,149) | Test statistic | p | |
| Age groups (%) | F(9.98, 558.77) = 63.3078 | <.001 | ||||
| 65–69 years | 32.2 (31.1, 33.4) | 12.7 (8.7, 18.2) | 11.2 (7.0, 17.5) | 9.1 (6.6, 12.3) | ||
| 70–74 years | 27.6 (26.7, 28.5) | 24.4 (19.5, 30.0) | 15.0 (11.5, 19.2) | 9.7 (7.4, 12.5) | ||
| 75–79 years | 18.6 (17.6, 19.7) | 23.4 (19.2, 28.3) | 22.4 (17.7, 27.9) | 19.3 (16.4, 22.5) | ||
| 80–84 years | 12.7 (12.0, 13.5) | 20.3 (16.6, 24.6) | 25.7 (21.3, 30.6) | 22.9 (20.2, 25.7) | ||
| 85–89 years | 6.6 (6.0, 7.2) | 14.6 (11.7, 18.0) | 15.0 (11.3, 19.7) | 22.9 (20.5, 25.5) | ||
| 90 years or over | 2.3 (2.0, 2.6) | 4.6 (3.4, 6.2) | 10.7 (7.8, 14.4) | 16.2 (14.0, 18.7) | ||
| Sex (%) | F(2.76, 154.52) = 4.0548 | .010 | ||||
| Female | 55.7 (54.0, 57.3) | 59.1 (53.2, 64.7) | 61.7 (56.3, 66.8) | 60.7 (57.6, 63.7) | ||
| Male | 44.3 (42.7, 46.0) | 40.9 (35.3, 46.8) | 38.3 (33.2, 43.7) | 39.3 (36.3, 42.4) | ||
| Race/ethnicity (%) | F(5.94, 332.56) = 13.2744 | <.001 | ||||
| White, non-Hispanic | 82.8 (81.0, 84.4) | 70.0 (65.2, 74.4) | 74.5 (69.9, 78.5) | 70.7 (66.3, 74.8) | ||
| Black, non-Hispanic | 7.4 (6.6, 8.2) | 12.9 (10.4, 15.9) | 8.8 (6.5, 11.7) | 11.3 (9.6, 13.2) | ||
| Other race, non-Hispanic | 4.3 (3.4, 5.4) | 5.3 (3.0, 9.3) | 4.8 (2.4, 9.1) | 6.8 (4.7, 9.7) | ||
| Hispanic | 5.6 (4.7, 6.6) | 11.8 (8.8, 15.7) | 12.0 (8.8, 16.2) | 11.2 (8.7, 14.3) | ||
| Education (%) | F(7.25, 406.06) = 41.0472 | <.001 | ||||
| Less than high school | 17.3 (15.8, 18.9) | 42.3 (36.2, 48.6) | 30.7 (26.1, 35.6) | 43.0 (38.4, 47.8) | ||
| High school | 27.7 (26.1), 29.4) | 25.4 (21.2, 30.2) | 27.3 (23.0, 31.9) | 28.0 (24.7, 31.6) | ||
| Some college, no degree | 23.0 (21.6, 24.3) | 16.8 (12.5, 22.3) | 17.9 (13.0, 24.0) | 13.1 (10.5, 16.2) | ||
| College graduate | 32.1 (29.5, 34.7) | 15.4 (11.7, 20.1) | 24.2 (18.8, 30.6) | 15.9 (13.4, 18.8) | ||
| Family income in 2011 ($) (mean) | 64,186 (57,303, 71,069) | 28,403 (25,662, 31,144) | 44,946 (35,835, 54,058) | 30,401 (25,967, 34,836) | F(3, 54) = 35.7 | <.001 |
| Chronic disease count (mean) | 2.31 (2.26, 2.36) | 2.63 (2.50, 2.76) | 2.52 (2.33, 2.70) | 2.77 (2.66, 2.88) | F(3, 54) = 18.8 | <.001 |
| Activities of daily living limitations (mean) | .32 (.30, .34) | .59 (.49, .70) | .69 (.55, .83) | 1.62 (1.51, 1.73) | F(3, 54) = 187.8 | <.001 |
| Instrumental activities of daily living (mean) | .51 (.47, .54) | 1.00 (.85, 1.15) | 1.08 (.91, 1.25) | 2.42 (2.32, 2.53) | F(3, 54) = 322.7 | <.001 |
| Mobility limitations (mean) | .43 (.40, .45) | .76 (.64, .89) | .76 (.65, .88) | 1.51 (1.42, 1.60) | F(3, 54) = 215.0 | <.001 |
| PHQ-4 score (mean) | 1.61 (1.54, 1.68) | 2.67 (2.28, 3.05) | 2.14 (1.83, 2.45) | 3.74 (3.48, 4.00) | F(3, 54) = 88.4 | <.001 |
| Proxy-respondent (unweighted %) | 1.6 (1.3, 2.0) | 7.1 (4.7, 10.7) | 2.3 (1.0, 5.5) | 37.0 (33.7, 40.4) | F(2.89, 161.61) = 537.7 | <.001 |
| # of surveys completed | 5.0 (4.9, 5.1) | 6.8 (6.5, 7.0) | 6.1 (5.8, 6.3) | 3.7 (3.5, 3.9) | F(3, 54) = 187.2 | <.001 |
| Sample size | 5,674 | 420 | 366 | 1,149 | ||
| Estimated proportion in the population (%) | 80.4 (79.3, 81.5) | 4.5 (4.1, 5.0) | 3.9 (3.4, 4.4) | 11.2 (10.4, 12.0) | ||
Note. Weighted estimates were presented applying NHATS complex survey design features using Taylor linearization for variance estimation.
Relationship between social isolation and dementia trajectories
Table 3 reports the different representations of the linkage between dementia and social isolation trajectory groups through conditional probabilities and joint probabilities. Panel A shows the probability of membership in each of the social isolation trajectories, conditional upon membership in each of the dementia trajectory groups. Because the probabilities are conditional upon membership in a given dementia trajectory group, each column of probabilities in panel A sums to 1. Panel B reports the probability of membership in each of the dementia trajectories conditional upon membership in each of the social isolation trajectory groups. Each column of probabilities in panel B also sums to 1. Panel C is the joint probability of membership in a specific social isolation trajectory and a specific dementia trajectory. This panel includes all the possible combinations of social isolation and dementia trajectory groups; thus, the 16 joint probabilities sum to 1. These results showed a strong interrelationship between the developmental trajectories for social isolation and dementia. Panel A shows that older adults with persistently low dementia risk were least likely to be members of the three trajectories with a higher social isolation risk. Both increasing dementia groups were composed mainly of individuals from the increasing social isolation group (40–43%) and persistently isolated group (24–29%), which shows that social isolation and dementia evolve in the same direction for these groups. The persistently high-risk dementia group had a similar trajectory shape to the persistently isolated group (Figures 1 & 2), but the overlap between these two groups is only moderate (28%). Instead, individuals in the high-risk dementia group were mostly those from the decreasing social isolation group (47%).
Table 3.
The interrelationship of social isolation and dementia over eight years
| A. Probability of social isolation k conditional on dementia group j (πk|j) | ||||
|---|---|---|---|---|
| Dementia trajectory group | ||||
| Social isolation trajectory group | Persistently low | Increasing, late-onset | Increasing, early-onset | Persistently high |
| Rarely isolated | .68 | .23 | .20 | .07 |
| Increasing | .14 | .40 | .43 | .17 |
| Decreasing | .05 | .08 | .14 | .47 |
| Persistently isolated | .13 | .29 | .24 | .28 |
| B. Probability of dementia group j conditional on social isolation k (πj|k) | ||||
| Social isolation trajectory group | ||||
| Dementia trajectory group | Rarely isolated | Increasing | Decreasing | Persistently isolated |
| Persistently low | .90 | .49 | .27 | .49 |
| Increasing, late-onset | .05 | .23 | .08 | .18 |
| Increasing, early-onset | .03 | .16 | .09 | .10 |
| Persistently high | .02 | .13 | .57 | .22 |
| C. Joint probability of dementia group j and social isolation group k (πjk) | ||||
| Social isolation trajectory group | ||||
| Dementia trajectory group | Rarely isolated | Increasing | Decreasing | Persistently isolated |
| Persistently low | .45 | .10 | .03 | .09 |
| Increasing, late-onset | .03 | .05 | .01 | .03 |
| Increasing, early-onset | .02 | .03 | .01 | .02 |
| Persistently high | .01 | .03 | .07 | .04 |
Notes. Each column in panel A and B adds to 1. Conditional probability is based on the group assignment generated by the maximum likelihood procedure and had small differences from the proportions based on group assignments by their highest posterior probabilities of group memberships. All probabilities in panel B add to 1.
Fisher’s exact tests of pairwise comparisons showed significant differences in the proportions of social isolation groups between the three moderate- to high-risk dementia groups (late-onset, early-onset, and persistently high) and the persistently low-risk group. All pairwise comparisons of the proportions, which were based on group assignments by the highest posterior probabilities of group membership, were statistically significant using Holm’s adjusted p-values (Supplemental Digital Content Table S1). These pairwise comparisons were consistent with the results of adjusted multinomial logistic regression predicting dementia trajectory groups as a function of social isolation trajectories (Supplemental Digital Content Table S2). Due to the small numbers in some of the comparison groups, however, results from logistic regression showed signs of low precision and should be used as reference only.
Sensitivity analysis
We tested a dual model with the same model specification but using the broad definition of dementia (i.e., possible and probable dementia combined). Compared to findings using the narrow definition, the shape of dementia trajectories from the broad definition was almost identical with a small difference in group sizes such that a more substantial proportion of participants was on the persistently high and the two increasing trajectories. The conditional probabilities involving the broad definition revealed patterns of interrelations between dementia and social isolation trajectories very similar to those from the narrow definition (Table S3). In this regard, our findings were robust against different definitions of dementia. Attrition was higher in the persistently high-risk dementia trajectory than the persistently low-risk group. We examined the effect of attrition by adding an indicator of having dropped out by the end of the study period to the multinomial logistic regression model. The estimated regression coefficients for social isolation trajectories did not change substantially, with less than half a point change for most, and all remained significant. Thus, higher attrition rates in those with high disease burden did not attenuate the interrelations between dementia and social isolation trajectories identified from our main analysis.
Discussion
The present study used a group-based dual trajectory modeling approach to identify distinct trajectory patterns for social isolation and dementia and assess their interrelations in a nationally presentative community-based sample of older adults. Changes in social isolation among community-dwelling older adults followed four distinct trajectories over eight years. Most community-living older adults were rarely isolated (62%), though nearly 17% were persistently isolated. A non-trivial group experienced a steady increase in social isolation (14%), while a small group (7%) experienced a steady decrease in social isolation. The development of dementia also followed four trajectories: 80% of respondents had a persistently low risk of probable dementia over the study period, while 11% had persistently high risk. The remaining two groups (each about 4% of respondents) had increasing risk over time, with one group experiencing a rapid increase during the first survey years, while the other had a low initial risk that increased rapidly in the final survey years. The trajectory groups with the highest dementia and social isolation burden both had an overrepresentation of those over 75 years of age, women, racial and ethnic minority groups, and those in lower socioeconomic status. The linkage probabilities and pairwise comparisons of proportions showed that there was a fair amount of temporal correspondence between the trajectories of social isolation and dementia. For the most part, the two sets of trajectories were positively related: less social isolation corresponded to maintaining a low risk for dementia, and increasing social isolation corresponded to a steady increase in dementia risk. However, nearly half of those within the persistently high-risk dementia group belonged to the decreasing social isolation group.
Our findings are overall in line with syntheses of longitudinal studies that documented a significant association between social isolation and cognitive impairment or cognitive decline (Kuiper et al., 2016; Penninkilampi, Casey, Singh, & Brodaty, 2018). Our study results also echo reports from studies showing that changes in social relations and cognitive function are interconnected (Béland et al., 2005; Donovan et al., 2017; Elovainio et al., 2018; Kelly et al., 2017; Park et al., 2017). For example, one study found that individuals on the high cognitive performance trajectory over 21 years had more frequent social contacts and were more likely to be married than those on the low cognitive performance trajectory (Elovainio et al., 2018). Findings from limited experimental studies also lend support to our findings (Myhre, Mehl, & Glisky, 2017; Pitkala, Routasalo, Kautiainen, Sintonen, & Tilvis, 2011). For example, Pitkala et al. (2011) tested the effectiveness of a socially stimulating group intervention aimed at enhancing interaction and friendship among lonely older adults in daycare centers in Finland. In their study, the intervention group had a significantly more significant improvement in cognitive functioning at the three-month follow-up.
The mechanisms underlying the substantial overlap between the persistently high-risk dementia group and the decreasing social isolation group are unclear. One possible explanation is the confounding effects of age and proxy interviews. Persons aged 80+ and proxy interviews were overrepresented in both the high-risk dementia group and the decreasing social isolation groups. Social contacts may grow for persons of advanced age due to increased demand on the social network for assistance. Moreover, proxy respondents may systematically report more social contacts than self-respondents. However, the decreasing social isolation group remained a strong predictor of group membership in the high dementia risk group in multivariable multinomial logistic regression. This finding suggests that the confounding effects of age and proxy interviews could not explain away the substantial overlap between the two trajectory groups. A plausible but untested explanation for the substantial overlap between the two trajectory groups involves the impact of dementia on social networks. As the disease progresses, people require more help from their social networks. This demand for help may have inadvertently increased the patients’ contact with their network members. Previous studies have found that older adults with dementia have more extensive caregiving networks than older adults without dementia (Kasper, Freedman, Spillman, & Wolff, 2015; Spillman, Freedman, Kasper, & Wolff, 2019). The progression of the disease requires more hours of care over time, that accounts for more family and non-family caregivers. The expanded care network may reduce objective social isolation through increased social contacts with family caregivers (which counts toward family contact) and non-family caregivers (who may count as friends). Our findings point to a possible non-linear and directional relationship. While social isolation may be associated with an increased dementia risk during the early phases of the disease, social isolation may decrease as the disease progresses and requires more intensive caregiving. This potential curvilinear relationship between social isolation and dementia may be an explanation for the mixed findings from studies that examined the average association of social isolation and cognitive impairment. Nevertheless, the directionality underlying the overlap needs further investigation, particularly considering a recent study that showed a significant association between higher or increased social isolation and subsequent memory decline but no significant association between memory levels and subsequent change in social isolation (Read et al., 2020).
This study has several limitations. First, social isolation and cognition were measured concurrently, and their trajectories modeled jointly. Though there were apparent changes in dementia and social isolation risk over time, their interconnectedness does not prove a causal relationship. As such, our findings cannot be interpreted as causality. Time-varying covariates, such as residence status, were not incorporated in the dual trajectory modeling due to methodological challenges. As such, the mechanisms underlying the interconnectedness of dementia and social isolation patterns over time remains unclear. Second, although we used a social isolation measure that captured different dimensions of social relations, this measure did not assess the frequency of social contacts and social participation. Using an index of various aspects of social networks and social integration also did not allow us to decipher the specific aspect most strongly associated with changes in dementia risk (Béland et al., 2005). Third, like most large-scale population surveys, the battery of neuropsychological tests in NHATS is more limited than a diagnostic evaluation for dementia in a clinical setting. The narrow definition of dementia used in our study had a high specificity but moderate sensitivity, which may underestimate the burden of dementia and the size of the three trajectory groups with elevated dementia risk (i.e., persistently high, early-onset, and late-onset). Finally, the higher drop-out rates in the high-risk dementia group likely violate the assumption that probabilities of group membership and attrition are independent. When this assumption is violated, estimates of trajectory group sizes can be biased, typically leading to an underestimate of the group with the highest disease burden (Haviland, Jones, & Nagin, 2011). As such, group sizes of persistently high-risk dementia and persistently isolated trajectories are likely underestimated in our study. Nevertheless, sensitivity analysis showed that the relationship between dementia and social isolation trajectories were unaffected by the difference in attrition rates across trajectory groups.
Conclusion
Changes in dementia risk and social isolation among community-dwelling older adults followed four distinct trajectories, respectively, over eight years, and these trajectories were interconnected. For the most part, social isolation and dementia evolved in the same direction for older adults over the eight years observed in our data. However, the pattern of associations between these trajectories is intricate and may be reversed among long-term dementia survivors. Further studies need to clarify the directionality of this complicated relationship and underlying mechanisms. Reducing social isolation, particularly among those who are persistently isolated and those with increasing isolation, may delay the onset of dementia.
Supplementary Material
Funding & Acknowledgements
Xiaoling Xiang was supported by a grant from the National Institutes of Health, University of Michigan Older Americans Independence Center Research Education Core (Grant number: 5 P30 AG024824-13). Donovan Maust was supported by a grant from the National Institute on Aging (R01AG056407).
Footnotes
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication.
References
- Amieva H, Stoykova R, Matharan F, Helmer C, Antonucci TC, & Dartigues JF (2010). What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosomatic medicine, 72(9), 905–911. 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- Béland F, Zunzunegui MV, Alvarado B, Otero A, & del Ser T (2005). Trajectories of cognitive decline and social relations. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(6), P320–P330. 10.1093/geronb/60.6.P320 [DOI] [PubMed] [Google Scholar]
- Berkman LF, & Syme SL (1979). Social networks, host resistance, and mortality: a nine-year follow-up-study of Alameda County residents. American Journal of Epidemiology. 109(2), 186–204 [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, & Arrighi HM (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association, 3(3), S168 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, & Hawkley LC (2009). Perceived social isolation and cognition. Trends in Cognitive Sciences, 13(10), 447–454. 10.1016/j.tics.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CE, & Dugan E (2012). Social isolation, loneliness and health among older adults. Journal of Aging and Health, 24(8). 10.1177/0898264312460275 [DOI] [PubMed] [Google Scholar]
- Crooks VC, Lubben J, Petitti DB, Little D, & Chiu V (2008). Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health, 98(7), 1221–1227. 10.2105/AJPH.2007.115923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, & Glymour MM (2017). Loneliness, depression and cognitive function in older U.S. adults. International Journal of Geriatric Psychiatry, 32(5), 564–573. 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovainio M, Sommerlad A, Hakulinen C, Pulkki-Råback L, Virtanen M, Kivimäki M, & Singh-Manoux A (2018). Structural social relations and cognitive ageing trajectories: Evidence from the Whitehall II cohort study. International Journal of Epidemiology, 47(3), 701–708. 10.1093/ije/dyx209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IEM, Martyr A, Collins R, Brayne C, & Clare L (2018). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–S144. 10.3233/jad-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Xiong C, & Morris JC (2006). Validity and reliability of the AD8 informant interview in dementia. Neurology, 67(11), 1942–1948. 10.1212/01.wnl.0000247042.15547.eb [DOI] [PubMed] [Google Scholar]
- Griffin SC, Mezuk B, Williams AB, Perrin PB, & Rybarczyk BD (2018). Isolation, not loneliness or cynical hostility, predicts cognitive decline in older Americans. Journal of Aging and Health. 10.1177/0898264318800587 [DOI] [PubMed] [Google Scholar]
- Haviland AM, Jones BL, & Nagin DS (2011). Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods and Research, 40(2), 367–390. 10.1177/0049124111400041 [DOI] [Google Scholar]
- Holm S (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 65–70. [Google Scholar]
- Holwerda TJ, Deeg DJ, Beekman ATF, van Tilburg TG, Stek ML, Jonker C, & Schoevers RA (2014). Feelings of loneliness, but not social isolation, predict dementia onset: Results from the Amsterdam Study of the Elderly (AMSTEL). Journal of Neurology, Neurosurgery and Psychiatry, 85(2), 135–142. 10.1136/jnnp-2012-302755 [DOI] [PubMed] [Google Scholar]
- Jones BL, & Nagin DS (2013). A note on a Stata plugin for estimating group-based trajectory models. Sociological Methods & Research, 42(4), 608–13. [Google Scholar]
- Kasper JD, Freedman Vicki A., and Spillman B (2013). Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Technical Paper, 5 https://www.nhats.org/scripts/documents/DementiaTechnicalPaperJuly_2_4_2013_10_23_15.pdf. [Google Scholar]
- Kasper JD, Freedman VA, Spillman BC, & Wolff JL (2015). The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Affairs, 34(10), 1642–1649. 10.1377/hlthaff.2015.0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Duff H, Kelly S, McHugh Power JEM, Brennan S, Lawlor BA, & Loughrey DG (2017). The impact ofsocial activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, & Lowe B (2009). An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics, 50(6), 613–621. 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, & Smidt N (2015). Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Research Reviews, 22, 39–57. 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Zuidema SU, Burgerhof JGM, Stolk RP, Oude Voshaar RC, & Smidt N (2016). Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology, 45(4), 1169–1206. 10.1093/ije/dyw089 [DOI] [PubMed] [Google Scholar]
- Lara E, Caballero FF, Rico‐Uribe LA, Olaya B, Haro JM, Ayuso‐Mateos JL, & Miret M (2019). Are loneliness and social isolation associated with cognitive decline? International Journal of Geriatric Psychiatry, 34(11), 1613–1622. 10.1002/gps.5174 [DOI] [PubMed] [Google Scholar]
- Moon H, Badana ANS, Hwang SY, Sears JS, & Haley WE (2019). Dementia Prevalence in Older Adults: Variation by Race/Ethnicity and Immigrant Status. The American Journal of Geriatric Psychiatry, 27(3), 241–250. 10.1016/j.jagp.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Myhre JW, Mehl MR, & Glisky EL (2017). Cognitive benefits of online social networking for healthy older adults. The Journals of Gerontology - Series B, 72(5), 752–760. 10.1093/geronb/gbw025 [DOI] [PubMed] [Google Scholar]
- Nagin DS (2005). Group-based modeling of development. Harvard University Press. [Google Scholar]
- Nagin DS, Jones BL, Passos VL, & Tremblay RE (2018). Group-based multi-trajectory modeling. Statistical Methods in Medical Research, 27(7), 2015–2023. 10.1177/0962280216673085 [DOI] [PubMed] [Google Scholar]
- Nagin DS, & Odgers CL (2010). Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology, 6, 109–138. [DOI] [PubMed] [Google Scholar]
- Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, … & Awasthi A (2019). Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology, 18(1), 88–106. 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kwon E, & Lee H (2017). Life course trajectories of later-life cognitive functions: Does social engagement in old age matter? International Journal of Environmental Research and Public Health, 14(4), 383 10.3390/ijerph14040393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninkilampi R, Casey AN, Singh MF, & Brodaty H (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 1–15. 10.3233/JAD-180439 [DOI] [PubMed] [Google Scholar]
- Pitkala KH, Routasalo P, Kautiainen H, Sintonen H, & Tilvis RS (2011). Effects of socially stimulating group intervention on lonely, older people’s cognition: A randomized, controlled trial. The American Journal of Geriatric Psychiatry, 19(7), 654–663. 10.1097/JGP.0b013e3181f7d8b0 [DOI] [PubMed] [Google Scholar]
- Pohl JS, Cochrane BB, Schepp KG, & Woods NF (2017). Measuring social isolation in the National Health and Aging Trends Study. Research in Gerontological Nursing, 10(6), 277–287. 10.3928/19404921-20171002-01 [DOI] [PubMed] [Google Scholar]
- Rafnsson SB, Orrell M, d’Orsi E, Hogervorst E, & Steptoe A (2017). Loneliness, social integration, and incident dementia over 6 years: Prospective findings from the English longitudinal study of ageing. The Journals of Gerontology: Series B. 10.1093/geronb/gbx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S, Comas-Herrera A, & Grundy E (2020). Social Isolation and Memory Decline in Later-life. The Journals of Gerontology: Series B, 75(2), 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, & Victor C (2019). Typologies of loneliness, living alone and social isolation, and their associations with physical and mental health. Ageing and Society, 39(8), 1709–1730. 10.1017/S0144686X18000132 [DOI] [Google Scholar]
- Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, & Livingston G (2019). Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLOS Medicine, 16(8). 10.1371/journal.pmed.1002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman BC, Freedman VA, Kasper JD, & Wolff JL (2019). Change Over Time in Caregiving Networks for Older Adults With and Without Dementia. The Journals of Gerontology: Series B. 10.1093/geronb/gbz065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Mayo N, & Koski L (2011). Identifying and characterizing trajectories of cognitive change in older persons with mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 31(2), 165–172. 10.1159/000323568 [DOI] [PubMed] [Google Scholar]
- Xie H, McHugo GJ, He X, & Drake RE (2010). Using the group-based dual trajectory model to analyze two related longitudinal outcomes. Journal of Drug Issues, 40(1), 45–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


