Abstract
Glycosylation is a well-regulated cell and microenvironment specific post-translational modification. Several glycosyltransferases and glycosidases orchestrate the addition of defined glycan structures on the proteins and lipids. Recent advances and systemic approaches in glycomics have significantly contributed to a better understanding of instrumental roles of glycans in health and diseases. Emerging research evidence recognized aberrantly glycosylated proteins as the modulators of the malignant phenotype of cancer cells. The Cancer Genome Atlas has identified alterations in the expressions of glycosylation-specific genes that are correlated with cancer progression. However, the mechanistic basis remains poorly explored. Recent researches have shown that specific changes in the glycan structures are associated with ‘stemness’ and epithelial-to-mesenchymal transition of cancer cells. Moreover, epigenetic changes in the glycosylation pattern make the tumor cells capable of escaping immunosurveillance mechanisms. The deciphering roles of glycans in cancer emphasize that glycans can serve as a source for the development of novel clinical biomarkers. The ability of glycans in intervening various stages of tumor progression and the biosynthetic pathways involved in glycan structures constitute a promising target for cancer therapy. Advances in the knowledge of innovative strategies for identifying the mechanisms of glycan-binding proteins are hoped to hold great potential in cancer therapy. This review discusses the fundamental role of glycans in regulating tumorigenesis and tumor progression and provides insights into the influence of glycans in the current tactics of targeted therapies in the clinical setting.
Keywords: Cancer, glycans, glycosyltransferases, biomarker, vaccines, glycoconjugate drugs
1. Introduction
The diversity of the monosaccharide building blocks and the multiple ways of assembling these building blocks to an oligosaccharide within the cell makes glycosylation a complex cellular event. Unlike the biosynthesis of proteins or nucleic acids, oligosaccharides are assembled through a systematic enzyme-catalyzed non-template driven machinery that generates glycans with diversity and heterogenicity [1]. Among the four major classes of biomolecules (nucleic acids, proteins, carbohydrates, and lipids), carbohydrates are the least understood biomolecule for their pathobiological functions. However, the past two decades of research provide remarkable progress in understanding the biology and pathology of glycosylation [2].
In addition to their metabolic role, oligosaccharides are often added to the proteins or lipids to form glycoconjugates such as glycoproteins, proteoglycans, and glycosphingolipids in the endoplasmic reticulum (ER) and Golgi apparatus. The formation of these glycan structures on proteins or lipids provides a vital stabilizing force for proteins within their microenvironment, thereby modulating their biological functions. Glycosyltransferases and glycosidases mainly orchestrate the sequential events of glycosylation. Functionally, the glycan biosynthetic ability largely depends upon the bioavailability and abundance of glycosylation enzymes, substrates, and sugar donors [3]. The direct precursors used in the biosynthesis of glycoproteins are nucleoside diphosphate or monophosphate sugars. Most of the cell surface and secreted proteins are co-translationally translocated and pre-manufactured in the lumen of ER. The partially glycosylated proteins make their way to the multiple stacks of the Golgi apparatus for further processing or trimming. The fully glycosylated proteins are then being distributed to various destinations. In the secretory pathway, two significant glycosylation types are known according to the nature of linkages between oligosaccharides: N-glycosylation and O-glycosylation. This glycosylation machinery allows biological tuning of proteins for selective functions such as protein folding, intracellular trafficking, mediating cell-cell and cell-matrix interactions, altering half-life of proteins, cell adhesion, host-pathogen recognition, etc. [4] (Figure 1).
Figure 1: Cellular regulation of glycan expression and function.
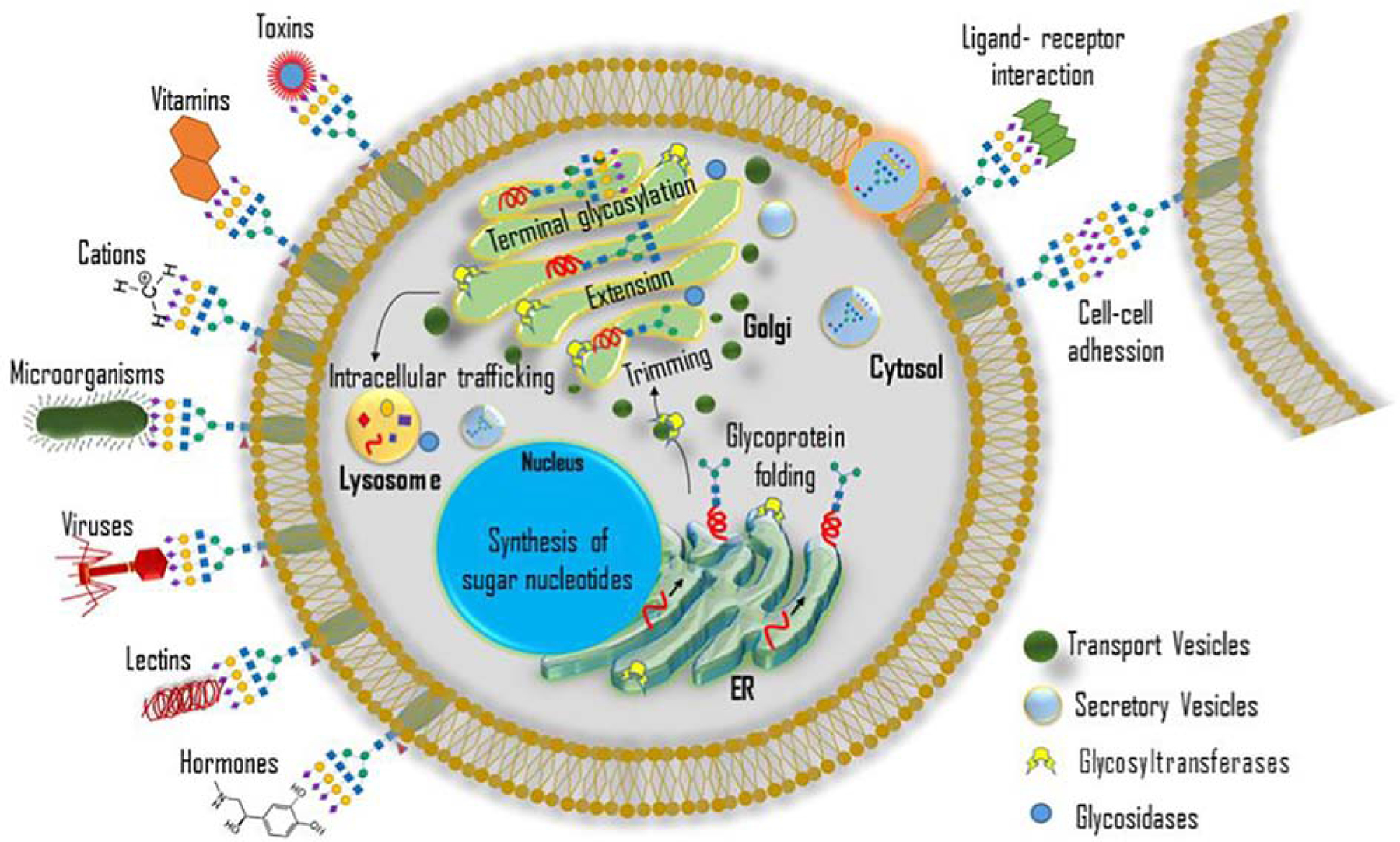
Initiation and maturation of glycoproteins occur in the ER-Golgi complex. Nucleotide sugar donors transport to the ER, and the folding of glycoproteins takes place in the ER. Trimming, extension, and terminal glycosylation occur in the Golgi apparatus. Glycans participate in the nascent protein folding and intracellular trafficking. The secretory vesicles transport the glycoproteins to the cell surface. Glycoproteins possess specific functions on cell surfaces such as cell-cell adhesion, ligand-receptor interaction, protecting from pathogens, providing receptors for toxins, etc.
The cellular functions of a glycan mainly depend upon the structure and function of the carrier protein on which it expresses. Cumulative evidence shows that alteration in the glycosylation patterns of the carrier proteins including incomplete synthesis of glycan structures, enhanced expressions of a complex branched N-glycans, expressions of truncated O-glycans (Tn and Sialyl Tn antigen), overexpression of ‘core’ fucosylation, altered expressions of sialylated glycans, etc. facilitate the acquisition of cellular features necessary for the malignant transformation of cells [5]. Such protein-specific, site-specific, and cell-specific tumor-associated glycans modifications increase molecular heterogeneity within the cell populations and the microenvironment. Variations in glycosylation seem to affect cell growth and survival directly and facilitate tumor-associated immune response and metastasis. Since cancer is a ‘microevolutionary’ process where there is the survival of only the fittest cells, the specific glycan changes may also be selected, and a limited subset of changes may be associated with tumor progression and metastasis [6]. The possibility of distinguishing the glycosylation pattern of proteins between healthy and cancer patients underscores glycobiology as a promising research area for cancer biomarker identification. This review provides a brief understanding of the fundamental concepts of glycobiology in health and disease. We discuss how altered glycosylation machinery is associated with cancer progression and metastasis. We also provide insights into the influence of aberrant glycosylation in the development of cancer biomarkers and targeted therapeutic interventions.
2. Glycosylation
The glycan ‘decorations’ found on the surface of cells and extracellular molecules are involved in almost all vital molecular processes ranging from intracellular trafficking, protein quality control, protein clearance, cell-cell interaction, cell-matrix adhesion, to various signal transduction cascades. Eight major glycosylation pathways have been recognized in mammals, of which two will be highlighted here because they house most of the glycosylation machinery associated with disease progression. The primary mechanisms by which glycans can be linked to proteins or lipids are N-glycosylation and O-glycosylation, where glycans are added sequentially to the amide group of an asparagine (Asn) residue and the hydroxyl oxygen of serine/threonine (Ser/Thr) residues respectively [7]. N-glycosylation encompasses a covalent linkage of various branched sugars, whereas O-glycosylation includes initial attachment of several monosaccharides, including galactose, mannose, fucose, and N-acetylgalactosamine (GalNAc). Most of the cases, both N- and O-linked glycans are capped at the terminal position with sialic acids (Figure 2).
Figure 2: Schema of N- and O-linked glycans.
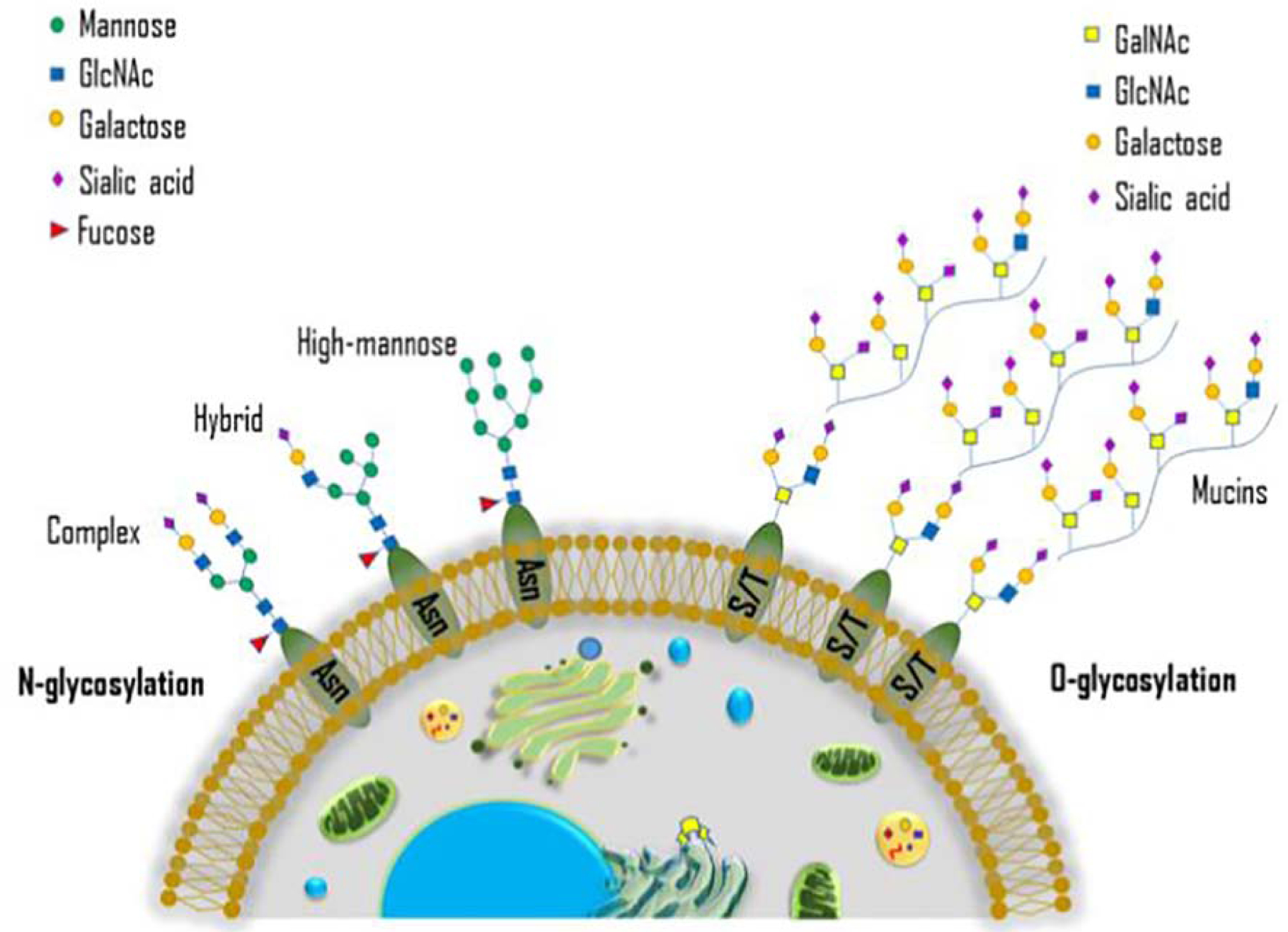
The conserved N-glycosylation and O-glycosylation core structure on the cell surface.
Since glycan synthesis is non-template driven, particular intracellular and extracellular stimuli can induce specific changes in the glycosylation machinery that contributes to the development of various diseases. The analysis of disease-associated glycosylation changes is crucial for a better understanding of the underlying mechanisms of disease progression. Here, we discuss N- and O-linked glycosylation, Sialylation and Fucosylation, and their role in cancer.
2.1. N-linked glycosylation
N-Linked glycosylation is an essential post-translational modification of proteins that commences with significant roles in deciding protein folding state and its oligomerization. N-glycans’ synthesis depends on specific cell types and tissues to generate complex glycans composed of N-acetylglucosamine (GlcNAc) and mannose residue (Man3GlcNAc2). N-glycans are classified into three major subtypes based on their branching of side-chain with variations like high mannose N-glycans (elongated by mannose residues), complex N-glycans (addition of GlcNAc further extends the chain in the Golgi complex), and hybrid N-glycans (addition of galactose or fucose residues along with mannose in the Golgi complex) [8].
The N-glycosylation is hugely conserved and is catalyzed by an enzyme Oligosaccharyltransferase (OST) complexes located in the ER membrane. OST functions in incorporating glycans to Asparagine residues into nascent polypeptides in the ER lumen. Mammalian OST complex contains several subunits, including ribophorins, Tusc3/N33, MagT1, DAD1, and STT3. Among these, STT3 (STT3A and STT3B) plays a vital role in the N-glycosylation machinery in mammals. These OST complexes are distinctive from each other; however, STT3A and STT3B are moderately overlapping with dolichol-linked oligosaccharides (DLO) glycans and their acceptor sites [9]. STT3A is specifically involved in the co-translational modification of nascent polypeptide, whereas STT3B is responsible for post-translational change of polypeptide sequon in the ER lumen. Inhibition of the STT3A subunit in the OST complex enhances the unfolded protein response in the mammalian cells results in pathological complications like cancer. The role of STT3B isoform during post-translational modification of polypeptide is to glycosylate the skipped glycosylation site in the polypeptide, which seems limited by the folding of the nascent protein in the ER lumen. Due to its high specificity to the substrate, OST inhibition will affect the glycosylation of protein, which perturbs the proper protein folding in the cells, which is further extended to various pathological conditions [10].
N-glycans have been known as molecular insulators that modulate the density of an intracellular protein crowded environment. The biosynthesis of N-glycans begins with the transfer of GlcNAc-P from UDP-GlcNAc to the lipid-like precursor dolichol phosphate (Dol-P) to generate dolichol pyrophosphate N-acetylglucosamine (Dol-P-P-GlcNAc) in the cytoplasmic face of the ER membrane. Sugars are added to Asn-X-Ser/Thr sequon in a protein, which is then translocated to the ER lumen. The remodeling of protein-bound N-glycan occurs in the ER lumen catalyzed by glycosidases and glycosyltransferases. The modified proteins further transferred to the Golgi apparatus, and additional modifications occur with the help of glycosyltransferases and sialyltransferases to form complex N-glycans [11,12].
2.2. O-linked Glycosylation
The modifications of serine (Ser) or threonine (Thr) amino acid residues on proteins by the addition of GalNAc residues are termed as O-glycosylation, which commonly occurs in high molecular weight proteins in the Golgi complex. O-linked glycans’ biosynthesis is considered a more straightforward and heterogeneous process than N- glycans because it does not require consensus sequences or dolichol precursors for synthesis [13]. The synthesis of O-linked glycans begins with the addition of sugars in the reducing N-terminal residues such as O-N-acetylgalactosamine, glucose, mannose, fucose, and sialic acid in the Cis-Golgi complex. The synthesis of O-Linked glycans is typically initiated by N-acetylgalactosaminyltransferase that catalyzes addition of first GalNAc residue to the hydroxyl group of Ser and Thr amino acids in the wholly folded proteins. The glycosyltransferases are responsible for the extension of linear and branched glycan chains by adding monosaccharides with the help of uridine and guanine triphosphate. Further elongation and termination of O-glycans are executed by a multitude of glycosyltransferases in the Golgi complex. The Golgi specific glycosyltransferases possess a short cytoplasmic amino N-terminal type II transmembrane domain, membrane anchor region, and a C- terminal catalytic domain. The O-linked glycans have a wide variety of biological functions, including hematopoiesis, inflammatory response, and involved in blood group antigens [14]. Of note, O-glycosylated mucins are predominantly expressed in a variety of tumor cell types, which facilitates the cell-cell interaction, cell-matrix interactions through the induction of various oncogenic signaling molecules during cancer progression.
Schachter and colleagues were the first to demonstrate the enzyme activity of GalNActransferase from submaxillary glands of porcine and bovine species [15]. The structures of O-glycans generated by the action of GalNActransferases may include eight types of core structures [16]. Briefly, peptides with GalNAc residue are converted to core 1 structure (Galβ1–3GalNAcα-Ser/Thr, also known as T antigen) by β1–3 galactosyltransferase or T synthase/C1GalT-1. The subcellular localization of C1GalT-1 is present in almost all cell types. A Core 1 synthase specific molecular chaperone is present in the ER called COSMC, which is required to synthesize functionally active C1GalT-1 in the Golgi. Loss of active C1GalT-1 may result in the deregulated expression of Tn and sialylated T-antigens. Tn antigens are considered a prognostic marker because of their appearance only in cancer tissue, not in healthy tissue. T antigens and galectin-3 are positively associated with colon cancer [17] and breast cancer metastasis [18]. One of the most significant mucin structures is Core 1, which has the antigenic properties [19]. The core 2 enzyme-C2GnT (β1–6 N-acetylglucosaminyltransferase) converts core 1 O-glycan to core 2 without the elongation, sialylation, or fucosylation process. C2GnT enzymes highly controlled mucin synthesis by regulating the expression pattern of glycosyltransferases and localization in the cells. C2GnT is a critical determinant of the branched O-linked glycans, whereas the upregulation of C2GnT levels resulted in invasiveness and metastasis of multiple cancers, including prostate cancer and endometrial carcinoma [20, 21]. Core 3 enzyme β1–3 N-acetylglucosaminyltransferase (C3GnT) present only in mucous epithelia of salivary glands, respiratory and gastrointestinal tracts. The C3GnT glycans are subsequently involved in the branching and extension of O-glycosylation. The core 3 O-glycan synthesis is highly regulated by GlcNAc-T activity, may utilize GalNAcα- Ser/Thr as their reaction substrate as similar to core 1 Gal-T. Therefore, Core 3 O-glycan synthesis may depend on the core 1 Gal T or core 3 Gal T on the cell types or glycoproteins. The enzymatic action of Core 3 GalT involved in the inhibition of Core 2 GlcNAcT to be active; meanwhile, Core 2 GlcNAcT needs a galactose residue in β1–3 linkage to the core GalNAc. Also, core 3 O-glycans act as a constituent for forming biantennary core 4 structure. The increased Core 3- and Core 4- GlcNAcT activities observed in the mucin secreting tissues include salivary glands, bronchi, and colon. Core 4 enzyme involved in the synthesis of branched poly-N-acetyl lactosamine structures. Core 5 enzyme α3-GalNAc transferases have been found in human meconium and adenocarcinoma. Core 6 β6-GlcNAc- transferases are specifically expressed in the human gut and mucins from the ovarian cyst. The biological role of Core 7 and 8 remains unclear. Core 7 enzyme was reported in bovine submaxillary mucins, and core 8 was found in human respiratory mucins [22, 23]. Several reports suggest that alterations in O-glycan synthesizing enzymes expression cause tumor development, progression, invasion, and metastasis, which is discussed in detail later sections. Major types of glycans and their key glycosyltransferase are summarized in Table 1.
Table 1:
Major types of glycans
| O-glycans | |
|---|---|
| Corel | core 1 β3-Gal-transferase (C1GnT) or core-1 synthase |
| Core 2 | β1–6 N- acetylglucosaminyltransferase (C2GnT) or C2GnT2 or C2GnT3 |
| Core 3 | β1–3 N-acetylglucosaminyltransferase (C3GnT) |
| Core 4 | β1–6 N-acetylglucosaminyltransferase (C2GnT-2) |
| Core 5 | Core 5 enzyme α3-GalNAc transferases |
| Core 6 | Core 6 β6-GlcNAc-transferase |
| Glycan Type | Key enzymes involved |
|---|---|
| N-glycans | |
| High mannose | Golgi α-mannosidase |
| Branched | GlcNAc-transferase (MGAT5) |
| Bisected | GlcNAc-transferase (MGAT3) |
| Hybrid | β1,4 galactosyltransferase |
| Complex N-glycans | β1,4 galactosyltransferase, α2,3 sialyltransferase, α2,6 sialyltransferase |
2.3. Sialylation
The terminal addition of sialic acid to the oligosaccharide and glycoconjugates catalyzed by Golgi localized sialyltransferases is known as sialylation. Sialic acid, a nine-carbon sugar, strategically decorates terminal position of glycans and function as a linkage molecule between the cell and the surrounding matrix. The addition of sialic acid to both N- and O-linked glycans at their GalNAc or galactose (Gal) units gives sialylated glycans with extensive structural diversity [24]. This bondage between α-linked sialic acid and distinct hydroxyl group on N- and O-linked glycans are elaborated through a family of twenty sialyltransferases that are stereoselective in action. The structure, function, and mechanism of action of sialyltransferases have been reviewed in detail previously [25, 26, 27].
Physiologically, sialic acid confers a negative charge to the sialylated glycoproteins and glycolipids that contribute to glycoconjugates’ biophysical and physiological functions. Pathologically, the differences in sialic acid expression have been associated with several disease conditions. Distinct changes in the sialylation pattern have been identified in many transformed tumor cells. We have reported that the sialyl Tn (STn) antigen overexpression has been correlated with increased malignant potential of pancreatic cancer cells [28, 29]. The predominance of sialoglycoforms over neutral glycoforms has been reported in the bladder tumor. Of interest, MUC16-STn glycoforms were found in advanced bladder tumors with a worse prognosis [30]. The detection of STn antigen on MUC16 can be used as a diagnostic tool to discriminate endometriosis from ovarian cancer [31]. Conversely, studies have shown that inhibition of sialyltransferases with inhibitors reduces cancer metastasis [32, 33].
2.4. Fucosylation
The process of transferring fucose from GDP-fucose to their substrates catalyzed by fucosyltransferases is referred to as fucosylation. Studies have shown that the de novo pathway synthesizes 90 % of the GDP-fucose in the cytosol under ordinary circumstances where GDP mannose is converted to GDP-fucose catalyzed by GDP-mannose 4,6-dehydratase and GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase [34]. In the salvage pathway, L-fucose in the cytosol is converted to GDP-fucose catalyzed by GDP-fucose phosphorylase and fucose kinase. Most of the secretory glycoproteins and membrane-bound glycoproteins on the cell surface are undergoing fucosylation. Generally, fucosylation is known as the process involved in the synthesis of terminal glycan structures; therefore, transferring fucose residue to the glycan is considered the end of the glycosylation process [35]. According to the location of fucose, fucosylation is categorized into core fucosylation and terminal fucosylation. Fucosyltransferases (FUT) are the major enzymes that catalyze fucosylation. Thirteen types of FUTs have been identified so far, among which FUT1–11 exists in the Golgi apparatus known to catalyze N-linked fucosylation. O-linked fucosylation is catalyzed by O-fucosyltransferases that exist in the ER. FUT8 participates in terminal fucosylation machinery, and the remaining FUTs catalyze core fucosylation machinery [36].
Physiologically, fucosylated glycoproteins play a pivotal role in determining ABO blood groups, maintaining a healthy microbiome, regulating cognitive processes, etc. The most well-known fucosylated glycans are ABO blood group antigens [37]. Pathologically, abnormal fucosylation is associated with various cancers. FUT8 has been reported as a responsible factor for the synthesis of cancer-associated N-glycan structures [38]. It was found that the amount of a biantennary galactosylated structure with one core fucosylation (A2G2F) was around 2.9% high in glioblastoma tissue as compared to healthy brain tissue [39]. Increased fucosylation of N-glycans was identified as a biomarker for the detection of colorectal carcinogenesis [40]. FUT8 was discovered as a prognostic marker in patients with stage II and III colorectal cancer [41]. A positive correlation between high levels of FUT6 and colorectal cancer progression has been verified. Further, the fucosylation of CD44 triggered PI3K/AKT/mTOR signaling pathway that promotes colorectal cancer progression [42]. Fucosylated carbohydrate epitope CD15 and sialylated CD15 was recognized as the determinant factors in the blood-brain barrier disruption and the transmigration of circulating cancer cells into brain parenchyma to promote brain metastasis [43]. Ganglioside fucosyl-GM1 (FucGM1) is a tumor-associated antigen expressed in a high percentage in small cell lung carcinoma. A human IgG1 antibody BMS-986012 that binds, specifically to FucGM1, was found highly effective in inhibiting tumor progression in a preclinical study [44]. It was reported that post-translational modification of E-cadherin by less core fucosylation activates EMT in lung cancer cells. E-cadherin core fucosylation in 95C lung cancer cells inhibited cancer cell migration, whereas knockdown of FUT8 in 95D lung cancer cells enhanced cancer cell migration [45]. It was demonstrated that inhibition of FUT8 expression significantly affected the breast cancer cell migration by impacting the core fucosylation of E-cadherin, which affect the downstream FAK/integrin pathway [46]. It was shown that clusterin that bears terminal fucosylated glycans produced by luminal breast cancer cells interact with the lectin receptor in myeloid cells and play a role in tumor progression [47]. Downregulation of FUT1 leads to the perinuclear localization of lysosome-associated membrane protein-1 and −2 correlated with increased autophagic flux in breast cancer cells [48]. Altogether, these studies demonstrate that aberrant fucosylation is associated with increased proliferation, migration, invasion, and metastasis of cancer cells, which raise the scope for further exploration in the field.
3. Glycosylation in cancer: signaling, tumor progression, and metastasis
Aberrant glycosylation due to cellular and metabolic changes leads to abnormal expressions of membrane-localized glycans that trigger malignant transformation of cells. The principle mechanisms underlying tumor-associated alterations in the carbohydrate structures are increased branching of complex and hybrid N-glycans, increased levels of sialyl lewis antigens, truncated O-glycan expression, and complex core fucosylation. Moreover, glycans dictate proteolysis and directly mediate oncogenic signal transduction, ligand-receptor interaction, cell-cell, and cell-matrix adhesion in cancer cells. Here, we discuss the glycosylation-mediated promotion of cancer hallmarks, including tumor cell-cell interaction, cell-matrix interaction, oncogenic signaling cascades, and metastasis.
3.1. Altered glycosylation in tumor cell-cell interaction
Cell-cell communications in epithelial cells are acquired by forming stable connections between adjacent cells in a well-organized manner. Two types of cell-cell communications are documented in mammals, which include tight junctions and adherens junctions. Tight junctions are responsible for cell-cell communications through the barrier, and adherens junctions function as adhesion regulators between adjacent cells. The calcium-dependent transmembrane protein E-cadherin actively regulates cell-cell adhesion, cell motility, and cell growth differentiation by forming a cadherin-catenin complex between the neighboring cells.
A disturbance in the cadherin-catenin complex leads to the modulation of cell-cell interaction and cellular integrity in cancer cells. An increasing body of evidence suggests that glycosylation affects the stability of cadherins and cadherin-mediated cell-cell adhesion. For example, the upregulated expressions of GnT-V that regulate β 1,6-GlcNAc branching alter function of N-cadherin, which resulted in the loss of cellular adherence and tumor invasion in human fibrosarcoma cells [49]. In contrast, removing specific N-glycans from E-cadherin was reported to increase the interaction of the cadherin-catenin complex and thereby stabilized cell-cell adhesion [50]. Similarly, the reduction of E-cadherin N-glycosylation promoted the stabilization of adherent junctions in healthy and cancer cells. It was shown that increased interaction of such hypo glycosylated E-cadherin with protein phosphatase 2A (PP2A) promotes tight junction assembly in cancer cells [51]. In addition to this, hyper-glycosylation of E-cadherin was found to mediate the destabilization of adherens junction proteins; and dysregulated N-glycosylation of E-cadherin stabilizes protein on adherens junctions as well as tight junctions that cause disturbances in intracellular adhesion in oral cancer [52]. β-catenin is a critical element of the Wnt canonical signaling pathway in various cancers, that is directly involved in the intercellular junction integrity in association with E-cadherin. Increased O-GlcNAcylation of β-catenin enhances overexpression of E-cadherin, and its nuclear translocation triggers the invasion and metastasis in colorectal cancer [53]. Intercellular adhesion molecule 1 (ICAM-1) and activated leukocyte cell adhesion molecule (ALCAM) are responsible for aggregation and sphere formation of tumor cells. Silencing of MAN1A1 (Golgi mannosidase) inhibited the tumor aggregate formation by affecting the N-glycosylation of ALCAM resulted in the reduction of cell adhesion and motility in ovarian cancer cells [54]. A recent study demonstrates that treatment with tunicamycin inhibited the N-glycosylation, which resulted in the increased dissociation of the desmosome complex, thereby inhibits cell-cell adhesion in primary keratinocyte cells [55].
The biological functions of E-cadherin is inhibited by the modification of N-glycan structure β1,6-GlcNAc at the Asn-554 site [56]. Earlier studies revealed that α−1,6 fucosyltransferase inhibition improves the E-cadherin function as cell-cell adhesion, thereby reducing the tumor invasion in lung cancer [57]. Also, knockdown of FUT8 suppresses the calcium-dependent E-cadherin mediated cell-cell adhesion in pancreatic acinar cell carcinoma [58]. In addition to this, FUT8 deficient MCF-7 cells showed decreased cell migration and invasion by suppressing the fucosylated modulation of E-cadherin followed by inhibition of integrin-mediated FAK signaling with the decreased localization of β-catenin to the nucleus [59].
Aberrant expression of N-acetylgalactosaminyltransferase 3 (GALNT3) increases the O-glycosylation of MUC1, which resulted in enhanced stabilization of E-cadherin and β-catenin complex and thereby promotes cell proliferation and migration in ovarian cancer cells. Further, inhibition of GALNT3 destabilizes the MUC1, which results in the suppression of cell proliferation and invasion [60]. Another study showed that binding of MUC1 with β-catenin enhances the destabilization of adherent junctions, cell invasion, and disturbs the cytoskeletal architecture of tumor cell invasion in breast cancer cells [61]. It has been reported that overexpression of CD82 decreased the expression of β-galactoside α 2, 3-sialyltransferase (ST3GAL4), thereby inhibits sialyl lewis antigen and also diminished the adhesion of tumor cells to the blood vessel that reduced metastasis [62]. Along with this, overexpression of ST3Gal IV was found to increase the levels of sialyl lewisx antigen with the decreased levels of α−2, 6 sialic acids. It promoted the migratory properties of PDAC cells [63]. Dysregulation of cell adhesion molecule E-cadherin and integrins influences the cancer cell invasion and metastasis. A previous study reported that overexpression of ST3Gal III alters the sialylation on the α2β1 integrin that resulted in diminished cell-cell aggregation and increased invasiveness in pancreatic cancer [64]. MUC16, a transmembrane protein, facilitates cell-cell contact in epithelial cells through its association with the actin cytoskeleton in the extracellular matrix (ECM). The genetic ablation of MUC16 perturbs the binding of actin cytoskeleton to the cytosolic domain of MUC16, which increases the migration and invasion in epithelial cells with altered expression of ZO-1 [65].
In summary, altered glycosylation affects the adherent junction complex with the simultaneous dissociation of tight junction proteins, which regulates integrin and Wnt/β-catenin signaling mediated invasion and metastasis of cancer cells (Figure 3).
Figure 3: Role of glycosylation in cell-cell and cell-matrix interaction.
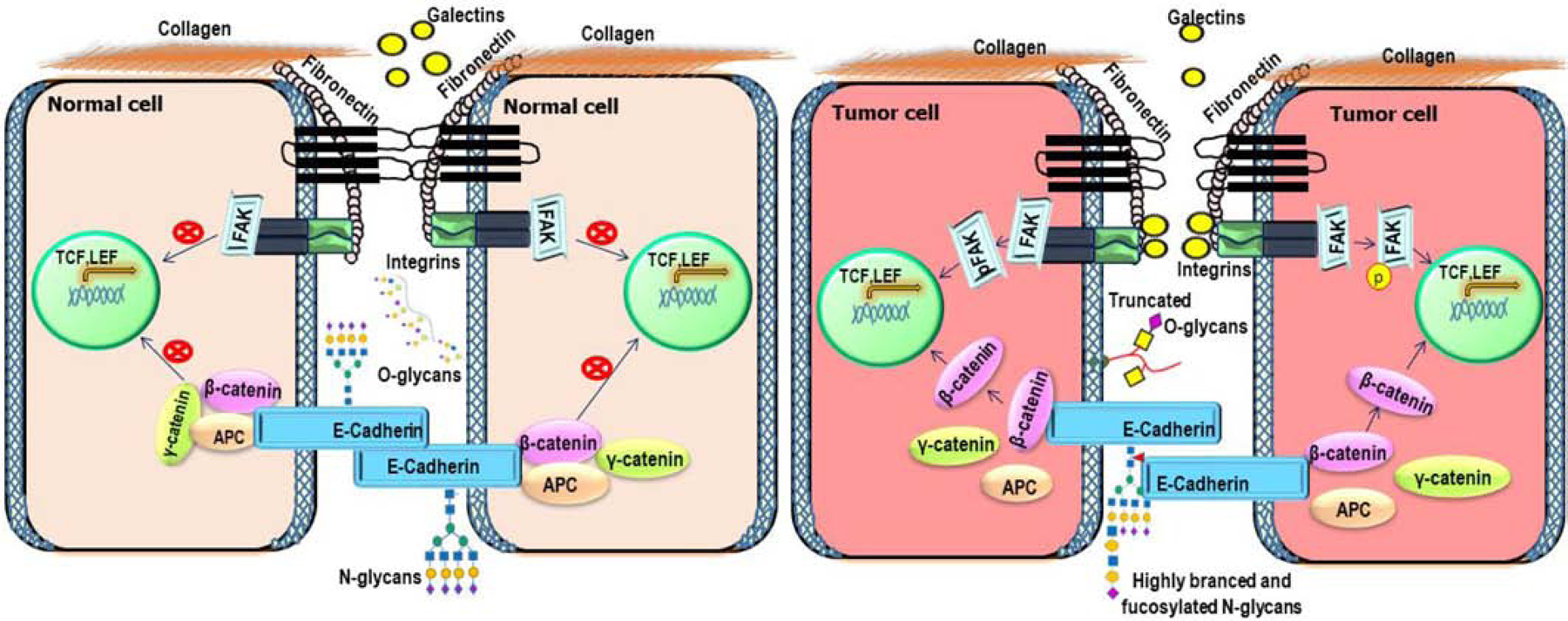
In healthy cells, E-cadherin functions as a critical hemophilic cell-cell adhesion molecule. In tumor cells, aberrantly glycosylated tight junction and adherence proteins activate Wnt and FAK signaling cascades that promote transcription of targeted genes responsible for tumor cell migration and proliferation. Heavily glycosylated integrins interact with ECM proteins with the help of galectins that trigger various signaling pathways to promote migration, invasion, and proliferation in tumor cells.
3.2. Altered glycosylation in cancer cell-matrix interaction
Atypical glycosylation influences the cell-matrix interaction by modifying the functions of anchoring proteins and extracellular basement membrane proteins, including integrins, laminin, fibronectin, and collagen in the ECM. The transmembrane protein Integrins regulate cell-cell and cell-matrix interaction to maintain cytoskeleton architecture and promote cell growth and proliferation through direct association with ECM proteins. Alterations in anchoring protein glycosylation would result in various pathological conditions like muscular dystrophy [66], cardiovascular diseases [67], neurodegenerative diseases [68], and cancer [69, 70].
An earlier study has reported that the genetic deletion of N-acetylglucosaminyltransferase III (GnT-III) inhibited the formation of a β−1,6 branch of complex N-glycans that affects the expression of integrins, which further resulted in the inhibition of invasion and metastasis in mouse melanoma cells [71]. Moreover, β−1,6 branched sialylated complex-type N-glycans alters the function of α5β3 integrins in tumor cells. Highly N-glycosylated α5β3 integrin alters the cell adhesion properties of cancer cells in association with vitronectin, a substrate for α5β3 integrin that proceeds the invasion of melanoma cells [72]. Overexpression of ST6Gal-I enhances the α−2, 6 sialylations on β1 integrin and talin that increases the expression of collagen IV, and promotes tumor invasion and motility in colon cancer cells [73]. Further, caveolin-1 triggers ST6Gal-I expression that enhances α−2, 6 sialylations of α5β1 integrin, promoting the adhesion of epithelial cells to the fibronectin in hepatocellular carcinoma [74, 75].
Previously, we have reported that overexpression of core 3 synthase alters the expression of α2β1 integrin through the modified glycosylation in MUC1, which further down-regulated the expression of phospho-FAK and its downstream targets, thereby decreases the tumor growth and metastasis in pancreatic ductal adenocarcinoma [76]. Inhibition of O-glycosylation and N-glycosylation by chemical inhibitors such as benzyl-α-GalNAc and tunicamycin enhanced the cellular adhesion to fibronectin and galectin-3 through the integrin-dependent manner in the ECM [77]. Attachment of β1,6-GlcNAc-branched N-glycans on the β4 integrin increases its cross-linking with gelatin-3 that subsequently enhances PI3K/Akt signaling mediated cell migration, invasion, and tumor growth. Whereas, the deletion of N-acetylglucosaminyltransferase III (GnT-III) inhibits bisecting of N-glycans that suppressed the β4 integrin/AKT mediated cancer cell invasion and motility [78]. Recent studies have shown that α−2,6 sialylation on the surface of α5β1 and α2β1 integrins reduced the binding of MDA-MB-231 breast cancer cells to the basement membrane protein fibronectin and Collagen IV [79]. Collectively, modified glycans on the integrins and recruitment of galectins disturb the matrix proteins in the extracellular space through FAK/Integrin signaling in tumorigenesis.
3.3. Altered glycosylation in oncogenic signaling cascades
Aberrant and modified glycosylation causes detrimental metabolic and cellular signaling, promoting cancer progression, and its exact molecular mechanism remains unclear. Numerous cellular factors impact tumorigenesis, including altered glycan expression, synthesizing enzymes and its localization, mutations in the tumor suppressor gene, and its instability. All such anomalies are leading to the activation of oncogenic signaling cascades such as Wnt/β-catenin, Hippo signaling, phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), Janus kinase/signal transducer, and activator of transcription (JAK/STAT), Transforming growth factor-beta (TGFβ/Smad), and Notch signaling. Altered glycosylation patterns on the cell surface molecules, transmembrane proteins, and growth factors cause tumor cell proliferation, invasion, and metastasis through the activation of these signaling cascades and its downstream targets [80].
Functional dysregulation and genetic mutations in the Wnt signaling components have a significant impact on various cancer cells. Glycosylation has been shown to affect the function of Wnt signaling components. Overexpression of MUC-13, a transmembrane glycoprotein, destabilizes the β-catenin/APC/Dshvelled complex by phosphorylating β-catenin at Ser552 and Ser675 and enhances its nuclear translocation. Nuclear binding of β-catenin to TCF/LEF transcription factors upregulated the expression of c-myc, Axin-2, and E-cadherin, enhancing tumor growth in hepatocellular carcinoma [81]. Overexpression of O-glycosylated MUC5AC increases the cell proliferation, invasion, and metastasis properties with significant upregulation of β-catenin and its downstream molecules in the gastric cancer cells. In contrast, the silencing of MUC5AC downregulated the expression of β-catenin, resulted in decreased cancer progression [82]. Moreover, knockdown of MUC16 resulted in the destabilization of the E-cadherin/β-catenin complex. It reduces the cell adhesion with the simultaneous increase in phosphorylated EGFR, Akt, and ERK expression to promote tumor cell invasion and metastasis in ovarian cancer cells [83]. It was shown that the N-glycosylation of Wnt ligand and its receptors and E-cadherin promoted the expression and nuclear translocation of β-catenin/γ-catenin that upregulated the transcriptional activity of dolichyl-phosphate N-acetylglucosamine phosphotransferase 1 (DPAGT1), which resulted in tumor progression and metastasis. Genetic deletion of DPAGT1 reduced the glycosylation of E-Cadherin and downregulated the canonical Wnt signaling pathways and inhibited tumor cell invasion and metastasis [84, 85]. Altogether, the evidence mentioned above clearly demonstrates that the altered pattern of glycosylation in the Wnt signaling components modulates the upregulation of β-catenin and its nuclear localization.
Human epidermal growth factor receptors (EGFR) are tyrosine kinase family members (ErbB1, ErbB2, ErbB3, and ErbB4) regulates the oncogenic signal transduction and cancer progression [86]. Homo or heterodimerization of receptors induced by ligand binding triggers downstream signaling through PI3K/Akt, JAK/STAT, and MAPK pathways. EGFR has 11 typical and 4 atypical N-glycosylation consensus sequences [87]. The N-glycans in the extracellular region of ErbB receptors could directly modulate their biological activity and intracellular transport. Bisecting GlcNAc was reported to inhibit EGFR and integrin signaling through the MAPK pathway [88]. The silencing of β1,4-N-acetylgalactosaminyltransferase III (B4GALNT3) was found to inhibit EGF mediated phosphorylation of EGFR by modifying the N-glycan structure. Further, it downregulated the phosphorylation of AKT and ERK due to the degradation of EGFR and thereby suppressed colorectal cancer stem cells [89]. Also, inhibition of N-glycosylation by tunicamycin (a well-known N-glycan inhibitor) decreased ALK’s phosphorylation and expression of its downstream target genes AKT, ERK, and STAT3 in melanoma cancer cells, which resulted in enhanced apoptosis [90]. Increased GalNAc-type O-glycosylation on the EGFR by abundantly expressed Core 1 β1,3-galactosyltransferase (C1GALT1) was found to promote tumor invasion and progression. In contrast, inhibition of C1GALT1 by the specific inhibitor Itraconazole reduced the elongation and binding of O-glycans to the EGFR, resulting in reduced tumor malignancy [91]. Aberrant glycosylation of EGFR by Ley carbohydrate enhanced the phosphorylation of EGFR with the concomitant upregulation of phosphorylated AKT that induced tumor cell migration and invasion in oral cancer cells; whereas the absence of Ley glycosylation of EGFR significantly reduced the cancer cell migration [92]. Therefore, impaired glycosylation mediated oncogenic EGFR signaling and its downstream pathways substantially contribute to the tumor progression and metastasis in various cancers.
TGF-β is a pleiotropic cytokine that contributes to various cellular functions such as tissue development, ECM regulation, proliferation, apoptosis, and tumorigenesis. Binding of TGF-β ligand to its receptors on the cell membrane triggers subsequent downstream signaling activation through Smad dependent or Smad independent manner in various pathological conditions, including cancer. The glycosylation of TGF-β and other cell surface proteins is of central importance in regulating signal transduction during cancer progression. Interestingly, cell surface proteoglycans such as endoglin and beta glycan act as co-receptors for TGF-β ligand, facilitating the binding of TGF-β to the receptor complex [93]. Several studies have highlighted the positive correlation of O-glycosylated mucins and TGF-β oncogenic signaling cascade. The interaction of highly glycosylated MUC-1 CT with TGF-β to promote tumor invasion of pancreatic cancer cells has been reported [94]. Clinical studies revealed that overexpression of TGF-β is correlated with MUC1 expression in association with CD10 in stromal cells of colorectal cancer tissues [95]. It was also reported that overexpression of N-acetylgalactosaminyltransferase14 (GALNT14) catalyzes the first step in the mucin-type O-glycosylation, significantly increased EMT markers to promote tumor progression and metastasis [96]. Conversely, a recent study reported that the fucosylation of TGF-β receptors enhanced the phosphorylation of Smad3 and promoted its nuclear translocation to promote tumor metastasis [97]. Taken together, the modulation of TGF-β and its receptors glycosylation significantly affect the oncogenic properties of cancer cells.
Notch signaling family consists of Notch ligands (Delta/serrate/Dsl) and Notch receptors (notch1–4) in mammalian cells. Notch receptors are predominantly O-glycosylated rather than N-glycosylation by glycosyltransferases. Changes in the glycosylation of Notch associated receptors induce oncogenic signaling in cancer progression [98]. Mammalian Notch 1 was shown to possess N-glycans based on its sensitivity towards N-glycosidases. It has been shown that silencing of ST6Gal-I decreased the expression of Notch1 and its downstream molecules jagged1, Hes1, MMP-2, MMP-9 and thereby decreased the cell proliferation, invasion, and metastasis in non-small cell lung carcinoma [99]. An increasing amount of evidence has reported aberrant expression of Notch-modifying glycosyltransferases in various types of cancers. Silencing of protein O-Glucosyltransferase 1 resulted in decreased Notch activation in human myeloid leukemia U937 cells [100]. Alternatively, overexpression of fucosyltransferases and protein O-Glucosyltransferase 1 might facilitate overexpression of Notch on the cell surface. However, further studies are required to understand how glycosylation affects Notch functions during tumor progression. Hence, glycosylation of cell surface components and their receptors is of great importance in directing oncogenic signaling pathways during tumorigenesis (Figure 4). Therefore, targeting the aberrant glycoforms of glycoproteins and glycolipids could reduce oncogenic potential of glycans in cancer.
Figure 4: Role of glycosylation in oncogenic signaling pathways.
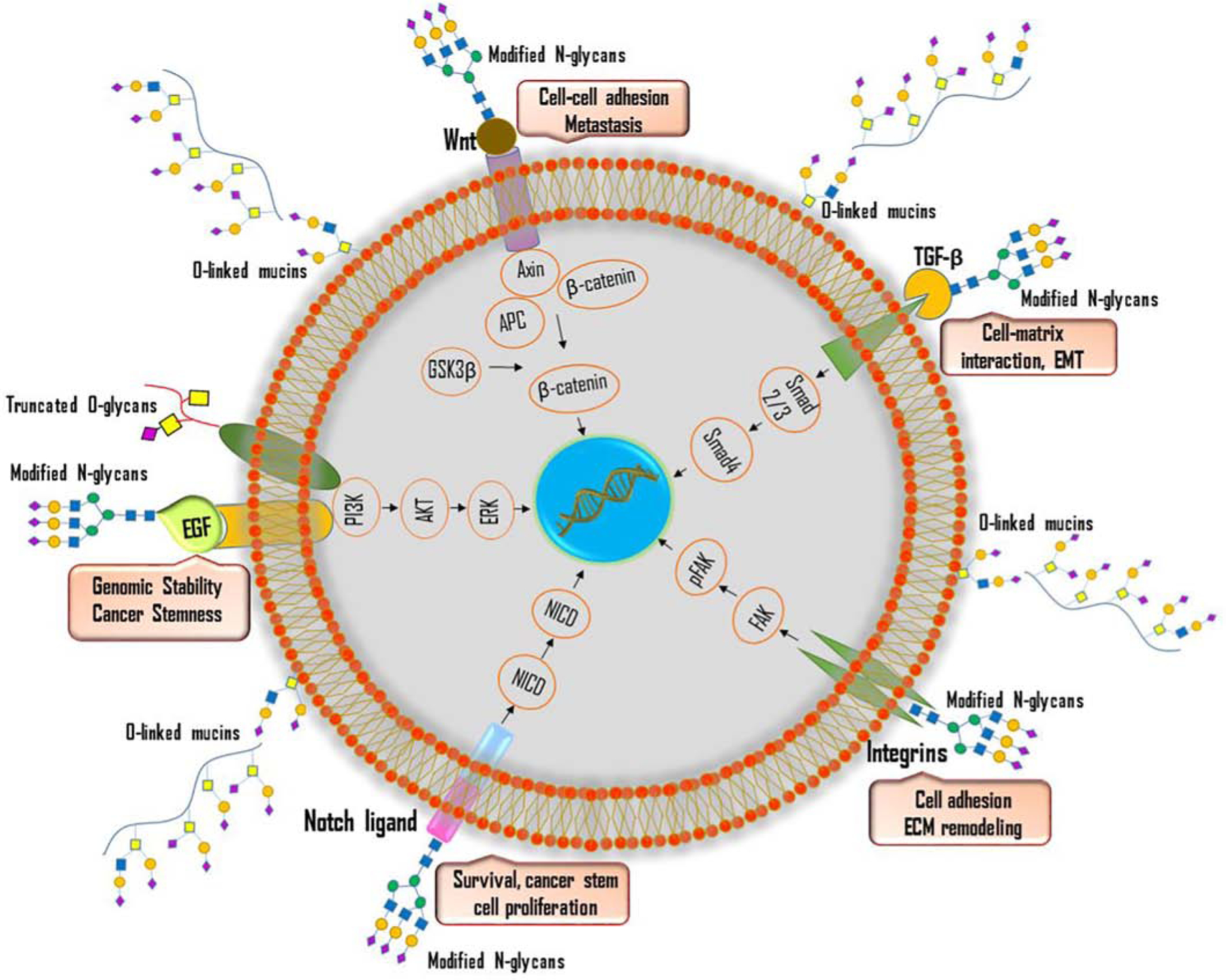
Aberrant glycosylation of cell surface receptors, growth factors, and transmembrane proteins activates various intracellular signaling pathways such as Wnt/β-catenin, Notch, TGFβ/Smad, PI3K/Akt, Integrin-mediated FAK signaling in tumor cells. Aberrant glycosylation of Wnt ligand promotes nuclear translocation of β-catenin and enhance cell-cell adhesion and metastasis. The presence of modified N-glycans on TGF-β ligand modulates cell-matrix interaction and promote EMT. Altered glycosylation of Notch ligand promotes cancer cell survival and stem cell proliferation. Aberrant glycosylation of integrins activates FAK signaling pathway and promotes ECM remodeling. Altered glycosylation of EGF receptors promotes genomic stability and cancer stemness.
3.4. Altered glycosylation in tumor metastasis
Malignant transformation and neoplastic progression are the consequences of alterations in several genes and protein expressions. Cumulative evidence shows that changes in protein glycosylation machinery accompany acquisition of cellular features essential for the invasion of tumor cells to distant regions. Remodeling glycans that have been implicated during tumor progression and metastasis is predominantly the consequence of mutation or modifications in the branching enzyme glycosyltransferases [101]. Altered glycosyltransferases in tumor metastasis have been reported in various cancer types such as colon cancer, pancreatic cancer, breast cancer, oral squamous carcinoma, skin cancer, and hepatocellular carcinoma. N-acetylglucosaminyltransferase, encoded by the MGAT gene that modulates the structure of complex N-glycans, can be associated with cancer progression and metastasis. It has been reported that modification in the branching of α−1, 6 mannose to β1–6-linked N-acetylglucosamine by N-acetylglucosaminyltransferase V (GnT-V) in the growth factors and cell surface receptors increases the metastasis in cancer tissues by regulating Src, EGFR, and TGF-β family oncogenes [102, 103]. Previous reports show that N-acetylglucosaminyltransferase V mediates the glycosylation of EGFR in gastric cancer cells. Inhibition of N-acetylglucosaminyltransferase V resulted in decreased ErBb1, ErBb2, and ErBb4 with increased E-cadherin and a concomitant decrease in vimentin expression in gastric cancer [104]. In contrast, N-acetylglucosaminyltransferase III (GnT-III) catalyzes the synthesis of bisected N-glycans, thereby suppresses tumor metastasis by modulating the turnover of E-cadherin on the cell surface and suppressing β-catenin mediated cell-cell adhesion [105].
The Golgi resident transmembrane protein N-acetylgalactosaminyltransferases that add GalNAc moieties to the nascent polypeptides at the ser/thr residues are known as one of the active participants in the malignant transformation of cancer cells. Altered localization of GALNT1 increases the O-glycosylation in liver cancer, promoting tumor tissue invasion and growth in association with the increased O-glycosylation of MMP-14 [106]. Localization GALNT1 is regulated by Late Endosomal/Lysosomal Adaptor, MAPK, and mTOR Activator 5 (LAMTOR5) through Src and c-Jun initiates the abnormal O-glycosylation of MUC1 and osteopontin with enhanced accumulation of Tn antigens in metastatic breast adenocarcinoma cells [107]. Aberrant O-glycosylation of mucins catalyzed by GALNT3 activates MUC1-PI3K/AKT axis with the upregulation of NFκB promoted tumorigenesis and metastasis in colon cancer cells [108]. Conversely, GALNT3 deficient PDAC cells showed upregulated expression ErbB family proteins with increased cell proliferation, motility, and metastasis by inhibiting E-cadherin expression in pancreatic cancer cells [109]. We have recently reported that the aberrant expression of MUC1 downregulates the appearance of the tumor suppressor polypeptide N-Acetylgalactosaminyltransferase 5 (GLANT5) by direct binding to the regulatory elements in the GALNT5 gene that promote pancreatic cancer progression and metastasis [110]. Core 1 β1, 3-galactosyltransferase (C1GALT1) is one of the critical enzymes involved in synthesizing O-glycans, which transfers galactose residue to the T-antigen favored by COSMC. We have reported that hypermethylation of COSMC resulted in the expression of truncated O-glycan structures Tn and STn antigen in pancreatic cancer that drives cancer-specific phenotype characterized by increased proliferation, compromised adhesion, and invasiveness in cancer cells [29]. Further, we explored the molecular mechanisms behind malignant potential of truncated O-glycans during tumor progression and metastasis. The genetic deletion of COSMC in pancreatic ductal adenocarcinoma cells resulted in the increased expression of mesenchymal markers with a concomitant reduction in the appearance of epithelial markers. Re-expression of COSMC in knockout cells reversed the induction of EMT. Also, we found that the aberrant expression of truncated O-glycans, maintaining stemness features of cancer cells that promote metastasis through the induction of EMT [28]. In contrast, modification of O-glycans on FGFR2 by C1GALT1 increases the tumor invasion and metastasis in colon cancer cells [111]. Also, overexpression of C1GALT1 increases the O-glycosylation of galectin-4, which mediated the activation of EGFR and its downstream molecules Akt/Gsk3β, upregulated expression of vimentin, and twist1 and promoted tumor metastasis in prostate cancer cells [112]. Further, the upregulated expression of C1GALT1 increases the O-glycosylation of MUC1 in esophagus squamous cell carcinoma patients [113] and increases the metastasis by modifying O-glycans on integrin β1 in hepatocellular carcinoma [114]. Altogether, these findings indicate C1galT1 mediated tumor promotion or tumor suppression is tissue / cell specific and context dependent, which needs to be further investigated. MUC1, MUC4, and MUC16 possess increased expression of these tumor-associated antigens Tn, STn, Lex, and SLea, which promote tumor metastasis by facilitating ligand for different selectins and galectins [115]. Furthermore, the aberrant O-glycosylation of the ectodomain of MUC1 increased the synthesis of sLex and sLea epitopes, which is correlated with invasion and metastasis in colon cancer cells [116, 117].
Overexpression of α−1, 6-fucosyltransferase (FUT8) induces EMT like process by increasing the core fucosylation of E-Cadherin with the upregulated expression of phospho-Src Y416 and β-catenin with the concomitant decrease in N-cadherin in lung cancer cells [118]. Fucosyltransferase IV (FUT4) upregulated the expression of snail and E-cadherin in association with NF-κB and PI3K/Akt-GSK-3β signaling to acquire mesenchymal phenotype that increases the metastasis by the enhanced secretion of MMP-9 in breast cancer cells [119]. TGF-β is known to be actively involved in tumor invasion and metastasis in several cancers. Herein, a recent study proposed that fucosylation of TGF-β receptor by FUT3 and FUT6 upregulated the expression of TGF-β/Smad signaling molecules leading to EMT in colorectal cancer cells [120]. In addition to this, the upregulated expression of FUT8 increased the fucosylation of the TGF-β receptor and its downstream signaling, which promoted the breast cancer tissue invasion and metastasis [121]. Alpha 1, 2 fucosyltransferases, FUT1 and FUT2 catalyzes the synthesis of alpha 1, 2-linked fucose of Lewisy and Globo H on the glycans. Overexpression of FUT1 and FUT2 increases the cancer stem cell potency, which is one of the critical factors for the EMT process. Upregulation of FUT1 and FUT2 increased N-cadherin expression and vimentin with the concomitant downregulation of E-cadherin, which enhances the tumor metastasis in breast cancer cells [122]. A recent computational biology-based study revealed that FUT8 mediated core fucosylation increased the tumor invasiveness and metastasis by modulating the L1 Cell Adhesion Molecule (L1CAM) [123]. Similarly, the dysregulation of sialyltransferase expression promoted tumorigenesis and metastasis in various cancers [124]. α−2,6, sialylation of N-glycans, catalyzed by the enzyme β-Galactoside α2,6-sialyltransferase 1 (ST6GAL1), is associated with tumor metastasis. Overexpression of ST6GAL1 induced TGF-β mediated activation of EMT in cancer cells, whereas the silencing of ST6GAL1 inhibited the TGF-β expression, thereby decreasing cancer progression [125]. Taken together, aberrant expression of glycosyltransferases affects the various oncogenic signaling molecules which trigger the tumor invasion and metastasis through the activation of EMT (Figure 5). The primary functions of different types of glycans in cancer are summed up in Table 2.
Figure 5: Role of altered glycosylation in tumor metastasis.

Altered expressions of glycosyltransferases, sialyltransferases, and fucosyltransferases induce aberrant glycosylation of TGF-β and Wnt ligands that promote EMT in a cancerous cell. Increased expression of mesenchymal markers facilitates the metastasis of tumor cells to distant organs.
Table 2:
Functions of glycans in cancer
| Glycan Type | Type of Cancer | Functions in cancer | Ref. |
|---|---|---|---|
| Branched N-glycan | Gastric cancer, | Cell-cell adhesion and metastasis | [126, 127] |
| Bisected N-glycan | Breast cancer, Ovarian cancer | Inhibits migration, proliferation, and metastasis | [128, 129] |
| KL-6 | Breast cancer | Tumor growth, metastasis | [130] |
| T-antigen | Pancreas | Tumor growth | [131] |
| Tn Antigen | Colon, Pancreas | Migration and tumor growth, metastasis | [132, 133] |
| Sialyl Tn Antigen | Pancreas, Gastric cancer | EMT, metastasis | [28, 29, 134] |
| Lewisb | Breast cancer | Invasion | [135] |
| Sialyl Lewisa | Non-small cell lung cancer | Metastasis | [136] |
| Lewisx | Lymphoblastic leukemia, Renal cell carcinoma | Cell adhesion, invasion, and metastasis | [157, 138] |
| Lewisy | Breast cancer, Ovarian cancer | Invasion, tumor growth | [139, 140, 141] |
| Sialyl Lewisx | Breast cancer, Leukemia | Metastasis, Cell adhesion | [142, 143] |
4. Glycans as biomarkers in cancer
Despite the advancement in targeted cancer therapy, only a small percentage of patients show beneficial with most of the treatments emphasize the importance of identification and development of reliable targets and therapies. Since glycosylation is a well-established hallmark of cancer, understanding the changes of glycosylation in a neoplastic cell may be a rationale for identifying innovative therapeutic strategies. The gene expression and enzymatic activity of glycosyltransferase are often altered in various pathophysiological conditions, including cancer. In a cancer cell, activities of glycosyltransferases are controlled by multiple factors; and the glycosylation results in several functional changes of glycoproteins that confer unique characteristic features of cancer cells. The aberrant expression of glycosylation enzymes also causes cancer cells to produce glycoproteins with specific cancer-associated anomalies in glycan structures. In this context, aberrant glycosylation of membrane-bound or secreted proteins associated with neoplastic transformation could be considered essential biomarkers for cancer detection. Tremendous research efforts have been devoted to characterizing the aberrant protein glycosylation associated with cancer progression. In this section, we discuss the clinical importance of glycans as biomarkers and therapeutic targets.
4.1. Glycosyltransferases as a prognosis marker for cancer
The glycosylation pattern seems to be highly sensitive to the neoplastic transformation of cells. Changes in the addition of saccharide structures to the healthy cellular proteins result in the formation of neoglycoforms. It is possible that these aberrantly glycosylated proteins can be released from the cells and may reach the bloodstream, and therefore, could serve as markers for cancer detection. Although multiple factors such as nucleotide sugars, nucleotide sugar transporters, acceptor substrates, endogenous lectins, and chaperones contribute to aberrant glycosylation in cancer, one critical mechanism appears to be the differential expressions of glycosylation enzymes-glycosyltransferases and glycosidases [144]. N-acetylgalactosaminyltransferase genes (GALNTs) and proteins (GalNAcTs) are known as unforeseen actors in neoplastic milieus. Each isoform of this family has substrate specificity and expression profile. Many of them are absent in healthy tissues and only found in cancer cells, making them the most reliable biomarkers for disease progression [145]. For example, GALNT1 mRNA expression was seen as 11.2-fold higher than healthy cells, suggesting that overexpression of GALNT1 could be a potential marker for human bladder cancer [146]. It has been reported that ppGalNAc-T3 is very restricted in healthy tissues, but present in oral squamous cell carcinoma [147]. Similarly, ppGalNAc-T6 is absent in healthy breast tissue; however, its presence has been verified in cell lines derived from mammary gland adenocarcinomas [148]. Studies have also confirmed the most significant specificity of ppGalNAc-T6 in breast cancer [149, 150]. Likewise, both GalNAc-T6 and -T3 expressions were found significantly correlated with tumor differentiation in a cohort of 70 clinicopathologically characterized pancreatic cancer cases; however, only GalNAcT6 was found to function as a predictable independent marker [151]. In another study, GalNAc-T3 was reported as an independent prognostic marker for high-grade tumors and poor prognosis in patients with renal cell carcinomas, but GalNAc-T6 was not [152]. Overexpression of GalNAc-T3 was characterized in both undifferentiated and differentiated early-stage gastric cancer, which also has a role in invasion and metastasis. Therefore, it could be used as a potential prognostic marker for early-stage gastric cancer [153]. In a cohort study, intra-tumor GALNT4 expression was identified as an independent potential prognostic marker for overall and relapse-free survival of patients with renal cell carcinoma [154]. Berois et al. [155] have reported that expression of GALNT9 was correlated with improved overall survival in both-low risk and high-risk category of patients with neuroblastoma and; also associated with improved clinical outcomes in low-risk patients. Therefore, GALNT9 may be considered as a potential prognostic marker for personalized therapy in patients with neuroblastoma. GALNT11 genotype was identified as a pre-therapeutic marker for a promising outcome in chronic lymphocytic leukemia [156]. In a cohort of 103 patients with pancreatic ductal adenocarcinoma (PDAC) who had received surgical resection, GALNT14 genotypes were correlated with postoperative prognosis. The genotype analysis revealed that 19.4% of patients possessed GALNT14 “TT” genotype, 60.2% included the “TG” genotype and 20.4% of patients contained “GG” genotypes. Sensitivity analysis studies have concluded that GALNT14 “GG” is associated with favorable overall survival outcomes in patients with resected PDAC, and, therefore, it could serve as a potential prognostic marker [157].
Another important family of enzymes that may be used as cancer biomarkers is O-linked-N-acetylglucosamine transferase (OGT) and O-GlcNAcase (OGA), which add or remove O-GlcNAc moieties, respectively [158]. Several studies have shown that O-GlcNAcylation that is dynamically regulated by OGT and OGA plays a critical role in cancer progression. O-GlcNAcylation machinery and OGT of colon and lung cancer tissues were significantly elevated compared with corresponding healthy tissues. O-GlcNAcylation increased the anchorage-independent growth of lung and colon cancer cells. Therefore, the study concluded that O-GlcNAcylation and OGT could be considered a valuable prognostic marker for lung and colon cancer [159]. Yet another study showed that OGT promotes proliferation and metastasis in colon cancer cells by altering O-GlcNAcylation machinery. Knockdown of OGT significantly inhibited cell migration, invasion, and proliferation of colon cancer cells indicated that OGT might be an important therapeutic target against colon cancer progression [160]. Human breast cancers with elevated levels of OGT and lower OGA levels significantly correlated independently with poor patient outcomes. The changes in O-GlcNAcylation are mediated through a HIF-1/GLUT1-dependent mechanism. Therefore, the study concluded that OGT is positioned as a prognostic marker, and targeting OGT may have potential therapeutic benefits in breast cancer [161]. Several other studies also suggested the prognostic importance of upregulated OGT and downregulated OGA in breast cancer progression [162, 163, 164]. Higher OGT levels and lower OGA/OGT ration in tumor cells compared to healthy cells indicate that OGT is an important prognostic marker in prostate cancer. Examination of OGT expression using the Oncomine™ database has revealed that OGT mRNA expression pattern in human prostate carcinoma is significantly higher than with adjacent healthy tissues in a cohort study of more than 200 patients [165]. Similarly, Kamigaito et al. [166] have proposed increasing OGT levels as a prognostic marker for prostate cancer, as they observed a close connection between levels of OGT and poor patient prognosis in a cohort of 56 patients. Yet another family of glycosyltransferases that are abnormally expressed in cancers is sialyltransferases. Among the sialyltransferases, the most studied one is ST6GAL1, which has been reported to be upregulated in several cancers. Schultz and colleagues illustrated that ST6Gal-I augmented tumor-initiating potential as evidenced by the inhibition of tumor formation upon knockdown of ST6Gal-I, and conditional overexpression ST6Gal-I promoted tumorigenesis in ovarian and pancreatic cancer cells [167].
Additionally, ST6GAL1 was shown to protect ovarian and pancreatic tumor cells from hypoxic stress by enhancing HIF-1α signaling [168]. Another study has reported that modifications of the O-glycosylation pattern by ST6GalNAc I are adequate to enhance breast cancer malignancy [169]. Several other studies also reported the prognostic utility and clinical relevance of ST6GAL-I. Radiometric assay of enzymatic activity of ST6GAL-I and ST3GAL-III in 55 gastric cancer patients and normal mucosa revealed that ST6GAL-I enzyme activity was enhanced within the tumor tissue and mainly localized to epithelial cells [170]. Further, enzyme activities of ST3Gal-I and ST3Gal-II were significantly increased in carcinoma specimens of colorectal cancer compared with normal mucosa. The expression of ST6GalNAc-II was significantly increased in patients with metastasis to the lymph node. Moreover, Thomsen-Friedenreich (TF) antigen expression was found in colon cancer cells after transfection with ST6GalNAc-II, whereas TF remained the same after the transfection of cells with either ST3Gal-I or ST3Gal-II. These results suggest that ST6GalNAc-II is crucial as a prognostic factor in colorectal cancer [171]. Apart from these, FUTs are also considered essential biomarkers for detecting several cancers as changes in protein fucosylation emerged as crucial features in cancer. A recent study has identified that high level of FUT3 has been associated with reduced overall survival of patients with triple-negative breast cancer [172]. Similarly, another research group has reported that a high level of FUT4 is significantly correlated with conventional cancer biomarker CA15.3 and can serve as a novel biomarker for the diagnosis and prognosis of breast cancer [173]. FUT8 was significantly associated with poor survival in non-small cell lung cancer [174]. Yet another study has identified the biological significance of FUT2 as a novel biomarker for the detection of lung adenocarcinoma [175].
4.2. Antibody / Lectin based detection of altered glycans in cancer
It is estimated that almost half of all mammalian proteins are glycosylated, where 300 to 500 genes are involved in protein glycosylation machinery [176]. Protein glycosylation is a complex phenomenon that is accomplished through several factors, including glycosyltransferases involved in trimming and addition of sugar moieties, nutrient resources, and expression of metabolic enzymes accountable for the synthesis and interconversion of glycan structures, etc. [177, 178]. Interestingly, each glycoprotein can be synthesized in different glycoforms based on the glycosylation site difference, even under normal conditions. Though there is significant heterogeneity in glycosylation pattern, the different glycoforms under normal physiological conditions are stable and reproducible. However, under the malignant transformation of cells, the balance between glycan moieties is disturbed, which results in either under-expression or overexpression of glycoproteins with upregulation or downregulation of glycosylation enzymes. Hence, glycoproteins are commonly used serological biomarkers for cancer diagnosis [80]. Some of the clinically monitored glycoprotein biomarkers in cancer patients are prostate-specific antigen (PSA) for prostate cancer; Carbohydrate antigen 125, MUC16 for ovarian cancer; α-fetoprotein (AFP) for liver cancer; CA27–29, MUC1 for breast cancer; CA15–5 and HER2 for breast cancer; hCG for testicular cancer, etc. [179, 180, 181, 182]. Although varying levels of these biomarkers are widely used for cancer detection, the specificity of these glycoproteins is a big concern. However, research evidence has shown that isoforms of several such glycoproteins show more specificity, enabling the detection of cancer easier. For example, elevated serum level of PSA is a well-known marker for prostate cancer detection; however, it is not prostate cancer-specific. Studies have proven that isoforms of PSA, such as [−2] proenzyme PSA exhibit more specificity over PSA to detect prostate cancer [183]. Table 3 describes the glycan-based diagnostic biomarkers with their clinical relevance including the Area under the ROC Curve (AUC), sensitivity, and specificity.
Table 3.
Glycan / Glycoprotein-based serum biomarkers for cancer diagnostics.
| Biomarker | Cancer | AUC; Sensitivity; Specificity | Clinical use | Ref. |
|---|---|---|---|---|
| PSA, Pro2 PSA | Prostate | 0.56; 85; 21 | Screening and diagnosis | [184, 185] |
| AFP | Liver | 0.9368; 95; 95 | Diagnosis, screening, monitoring therapy | [186, 187] |
| HER2 | Breast | 0.671; 37.5; 97.1 | Targeted therapy | [181, 188] |
| CA125/MUC16 | Ovarian | 0.851; 90.4; 86.4 | Diagnosis, detecting recurrence | [180, 189] |
| CA19–9 | Pancreas Ovarian | 0.98; 100; 90.3 0.93; 0.90; 0.83 |
Diagnosis and monitoring therapy | [190, 191, 192] |
| CA 15–3/MUC1 | Breast | 0.721; 31.9; 95 | Monitoring therapy | [182, 193] |
| CA27–29 | Breast | 0.645; 43.881; 78.000 | Detecting early recurrence | [194, 195] |
| Carcinoembryonic antigen | Colon | 0.789; 64.5; 89.2 | Detecting recurrence | [192, 196] |
| Thyroglobulin | Thyroid | 0.76; 69; 83 | Prognosis | [197, 198] |
| Human chorionic gonadotropin | Ovarian, Testicular | 0.65; 89; 75 | Prognosis, Detecting recurrence, monitoring therapy | [199, 200] |
| Human epididymis protein 4 | Ovarian | 0.955; 87.1; 100 | Detecting recurrence, monitoring therapy | [201, 202] |
| Chromogranin A | Neuroendocrine | 0.8962; 0.73; 0.95 | Diagnosis, prognosis | [203, 204] |
| EGFR | Non-small cell lung cancer | 0.89; 0.591; 0.954 | Prognosis, Selecting a treatment | [205, 206] |
| Fibrin/Fibrinogen | Bladder | 0.60; 58; 58 | Monitoring | [207, 208] |
| Estrogen receptor | Breast | 0.6131; 52.38; 70.24 | Prognosis, monitoring response to therapy | [209, 210, 211] |
| Plasminogen Activator Inhibitor-1 | Breast | 67.32; 53.44; 81.82 | Prognosis, monitoring response to therapy | [212, 213] |
The methods or various techniques used to detect glycan-based biomarkers on patient’s samples are clinically more critical. One such common approaches for the quantification of serum glycoproteins is the use of monoclonal antibodies against the given glycoprotein. Several anti-glycan monoclonal antibodies have been used in clinical applications. For example, Dinutuximab is a chimeric monoclonal antibody developed against di-sialoganglioside GD2 that is often overexpressed on neuroblastoma with limited expression on healthy neuronal cells [214]. Hu3S193 and BR96 are monoclonal antibodies generated against the Lewis Y glycan antigen and used in clinical trials for treating lung, breast, and colorectal carcinoma [215, 216, 217]. FG88 is another monoclonal antibody that recognizes Lec Lex-related glycans that are often overexpressed on a wide variety of tumor cells [218]. However, the use of monoclonal antibodies to detect glycoproteins has some limitations, such as this method could not see the changes in glycosylation machinery of the target glycoprotein.
Alterations in the neoglycoforms can be detected using lectin-proteins that specifically bind to the structural epitope on cancer-related protein glycoforms. For example, it was reported that Maackia amurensis agglutinin (MAA) lectins differentially bind to free serum PSA and can distinguish blood samples between prostate cancer and benign prostate hypertrophy where conventional PSA test fail to do [219]. The specificity of lectins towards neoglycoforms provides an added advantage for detecting glycoproteins in biological samples, as the low abundant glycoproteins in body fluids could not be detected using conventional assay methods. The use of various lectins has directed to the identification of several presumed neoglycan biomarkers with clinical importance. Research evidence shows that multiple lectins bind differently to cell lines derived from tumor sites and the metastatic site. Phytohaemagglutinin (PHA)-L lectin and other non-legume lectins, Sambucus nigra, Maackia amurensis, and Phaseolus vulgaris agglutinins, and wheat germ agglutinin (WGA) have been compared for their binding efficiency towards carbohydrate antigens on melanoma cells. This study demonstrated that expression of branched and sialylated complex type N-oligosaccharides had been consistently associated with the acquisition of metastatic potential of cells; and, lectins can be used to detect metastatic lesion, which is of clinically valuable [220]. In another study, Griffonia simplicifolia lectin-I (GS-I) and Vicia vilosa agglutinin (VVA) has been used to detect tumor-associated carbohydrate antigens expressions in breast cancer patients [221]. PHA is often used to distinguish hepatocellular carcinoma from other liver diseases [222]. All these studies suggest the potential clinical use of lectins as prognostic tools for cancer diagnosis and the detection of metastasis, and to some extent, to analyze the stage of disease progression.
5. Glycans as therapeutic targets in cancer
Decades of research on carbohydrates helped scientists develop glycan-based therapeutics, a promising approach in the treatment modalities for cancer. It is believed that the introduction of glycans themselves as therapeutics in the clinic is a significant breakthrough in the fight against cancer. Several recent reviews are available on the topic of glycan based therapeutics against cancer. Recently, Ferreira et al. have discussed glycan-based therapeutic opportunities in detail with particular reference to immunotherapy, antibody-based therapy, glycan mimetics, and nanovehicles [223]. This section briefly discusses some of the recent glycan-based therapeutics, including vaccine design, development of glycoconjugate drugs, glycan-based targeted nanotherapies, and glycosylation inhibitors that have clinical relevance.
5.1. Glycan-based vaccines
During cancer development, the characteristic change in glycosylation machinery pertains to an increase in the branched heavily glycosylated structures often accompanied by the differential glycosylation of mucin core proteins as MUC1 and MUC16 [224]. Tumor-associated carbohydrate antigens (TACA) have been considered a principal target for cancer vaccination development. The covalent linking of carbohydrates to an immunologically active protein exceptionally enhance their immunogenicity. Since the linker molecule that is used to couple carbohydrate to protein can impact the immunological properties, it is essential to use immunologically inactive linkers for the synthesis of the conjugate [225]. Based on the number of TACA used to synthesize a conjugate, glycoconjugate vaccines can be classified as mono-epitopic vaccines, mono-epitopic cluster vaccines, multi-epitopic vaccines containing single type TACA, cluster of one type of TACA, and multiple kinds of TACA respectively. Among these, mono-epitopic vaccines are highly explored and extensively used in clinical trials [226]. The synthesis of the Globo H hexasaccharide vaccine conjugated to keyhole limpet hemocyanin for prostate cancer patients is considered a revolutionary move in developing glycan-based cancer therapeutics [227, 228]. The synthetic Globo H-keyhole limpet hemocyanin (KLH) conjugates plus the immunologic adjuvant QS-21 was found as a well-tolerated vaccine for breast cancer patients in phase 1 clinical trial [229]. A phase 1 clinical trial of Thomsen-Friedenreich (TF)-KLH-QS21 vaccine showed a promising effect with higher titers of IgM and IgG antibodies in patients with relapsed prostate cancer [230]. Since multiple TACA is associated with tumor progression, scientists have tried the possibilities of designing multi-epitopic cancer vaccines to target different cell populations. Therefore, the same research group has later modified the vaccine containing five different carbohydrate antigens, Globo-H, TF, GM2, TF, STn, and Tn-to maleimide-modified carrier protein KLH that are specifically associated with prostate and breast cancer progression [231]. In a parallel study, another research group has synthesized a three-component vaccine composed of toll-like receptor 2 (TLR2) agonist, T-helper epitope, and a tumor-associated glycopeptide, which has been observed to elicit high titers of IgG antibodies that specifically recognize TACA [232]. Interestingly, the Sialyl-Tn antigen, a carbohydrate associated with MUC1, was identified as an ideal candidate to incorporate in the vaccine, as it is not expressed on healthy tissues. The vaccine synthesized by coupling synthetic STn antigen to KLH protein has successfully reached phase 3 clinical trial for breast cancer [233]. However, unfortunately, none of the vaccines has met the success rate of preventing disease progression and overall survival in clinical trials.
Studies demonstrated that semi-synthetic conjugate vaccines hold some inherent drawbacks, such as heterogenicity and imprecise structures acquired by carbohydrate-protein conjugation methods, resulting in conjugates with the difference in physical, chemical and immunological properties which may affect the reproducibility of the immune response. Therefore, researchers have focused on fully-synthetic conjugate vaccines that possess well-defined structures with no immunosuppression that enhance the immunogenicity of vaccines. Toyokuni and colleagues [234] have reported a synthetic carbohydrate with a robust immune response without using a carrier protein or linker molecule for the first time. Then, a glycopeptide containing a cluster of three Le(y)-serine epitopes were found as superior in action to the one containing a single Le(y) epitope [235]. Several synthetic carbohydrate vaccines have been developed with minimal structural features and useful T cell-dependent immune response. A synthetic vaccine using Tn antigen, YAF peptide, and lipopeptide Pam3Cys has been synthesized and found was effective in immune response for cancer treatment [236]. Another study provided evidence that covalent coupling of a TLR2 agonist, peptide T-helper epitope, and a TCGA gives a compound that exhibits high titers of IgG antibodies against breast cancer [237]. A MUC1 glycopeptide and Pam(3) Cys lipopeptide prepared by solid-phase synthesis provides an oligovalent synthetic anti-cancer vaccine with a more immunogenic response by click chemistry [238]. Several other synthetic glycan-based vaccines were also highly effective with a more immunogenic response to kill cancer cells [239, 240, 241, 242, 243]. Immunization with several of these synthetic vaccines resulted in a reduction of tumor size in vivo. However, more efforts are still warranted to analyze its clinical relevance.
5.2. Glycoconjugate drugs
Chemotherapeutic drugs face significant challenges, including increased toxicity and poor selectivity, to better clinical outcomes. Several efforts have been made to overcome this, and one such method is the glycoconjugation of drugs. For the first time, Pohl et al. [244] have synthesized sugar conjugated drugs in 1995. The glycoconjugate drug (glufosfamide) exhibited 4.5-fold decreased toxicity in rats. Later, glufosfamide had a good effect in phase II clinical trial in 20 patients with refractory solid tumors [245]. There are several other informative examples of such drug modifications with carbohydrate moiety to improve the efficacy of chemotherapeutic drugs.. For instance, Adriamycin’s cardiotoxicity and multidrug resistance have been shown significantly downregulated upon conjugation with 2-amino-2-deoxy-d-glucose and succinic acid [246]. Similarly, galactose and lactose conjugation to Geldanamycin, HSP90 inhibitor, resulted in a forty-fold increase in its anti-cancer activity [247]. Many other anti-cancer drugs such as Paclitaxel, Azomycin, Ketoprofen, Cadalene, Docetaxel, Chlorambucil, etc. have also been reported promising effects in cancer therapeutics upon conjugation with monosaccharides [248, 249, 250, 251, 252].
5.3. Inhibitors of glycosylation
Small molecule inhibitors of glycosylation machinery are being exploited for therapeutic benefit against cancer. Mechanisms of action of most of the glycosylation inhibitors are either by interfering with metabolism of precursors or by interfering with intracellular activities. Since most of the inhibitors are small molecules that can be easily taken up by various cell types, it provides an excellent opportunity for the researchers to design drugs to treat multiple diseases correlated with aberrant glycosylation. Several of such glycosylation inhibitors are being explored in clinical trials as a promising therapeutic strategy against cancer progression. For example, the use of Uproleselan (GMI-1271) and E-Selectin Antagonist along with chemotherapies was shown to result in well-tolerated, high remission rates and promising survival outcomes in patients with Acute Myeloid Leukemia in a phase 1/2 clinical trial [253]. Galectin inhibitor GR-MD-02 is being used in combination with chemotherapeutic drug Pembrolizumab against melanoma, non-small cell lung cancer, and squamous cell carcinoma head and neck [254]. Similarly, another galectin inhibitor GM-CT-01, and chemotherapeutic drug 5-Fluorouracil is in phase2 clinical trial for colorectal cancer [255]. GM-CT-01 with Fluorouracil also in phase 1 clinical trials for lung, breast, prostate, head, and neck cancers [256]. The fucosylation inhibitor SGN-2FF is currently in phase 1 clinical trial for several cancers, including breast, urinary bladder, renal, gastric, colorectal, squamous cell carcinoma, head and neck, and non-small cell lung cancer [257]. Several other preclinical studies have also highlighted glycosylation inhibitors’ potential to prevent cancer progression, which was reviewed in detail previously [258, 259, 260].
5.4. Glycan based nanotherapies
Glycan based polymers such as hydrogels, micelles, and nanoparticles have been considered as effective delivery vehicles of chemotherapeutics. Puranik et al. [261] have demonstrated that nanoscale hydrogels have desired physicochemical properties and possess feasible biocompatibility for oral delivery of hydrophobic chemotherapeutics. Recent research approaches led to the development of carbohydrate-based nanomaterials, especially for the controlled release of chemotherapeutics. Our recent study demonstrates that the use of polymeric nanogel significantly enhanced efficacy and systemic administration of a combination of cisplatin and gemcitabine in the treatment of pancreatic cancer. We found that encapsulation of cisplatin in polymeric nanogel with cross-linked ionic cores enhances cisplatin accumulation at the tumor site and improves its safety profile. To attain the targeted delivery, we have decorated nanogel encapsulated cisplatin with the TKH2 monoclonal antibody, which shows high specificity towards the STn antigen expressed explicitly on cancer cells. We found that combined simultaneous treatment with Gemcitabine and TKH2-functionalized cisplatin loaded nanogels significantly attenuated tumor growth both in vitro and in vivo with no detectable toxicity [262]. Another research group has developed a biocompatible nanoparticle for the targeted delivery of 5-fluorouracil and Paclitaxel to SLeA-expressing gastric cancer cells with minimal affinity for the healthy cells [263]. The need for controlled and targeted drug action has prompted researchers to use several polysaccharides such as chitosan, chondroitin sulfate, alginate, hyaluronic acid (HA) to synthesize nanoparticles as they provide unique physiochemical characteristics [264]. Using carbohydrate-based polymers enhances the solubility of low soluble or insoluble drugs, maximizes therapeutic efficacy, and minimizes side effects by affecting absorption, distribution, and metabolism of therapeutic drugs. Hence, carbohydrate-based polymers have been broadly considered promising materials in synthesizing effective nano-carriers for anti-cancer drug regimens [265].
Taken together, aberrant protein glycosylation in cancer provides scientists with additional opportunities to identify novel biomarkers. The presence of glycan structures on various biomolecules exhibits pathological importance, and therefore glycan-based therapies are gaining more attention in the therapeutic interventions against cancer progression (Figure 6).
Figure 6: Glycans as biomarkers for cancer therapy.
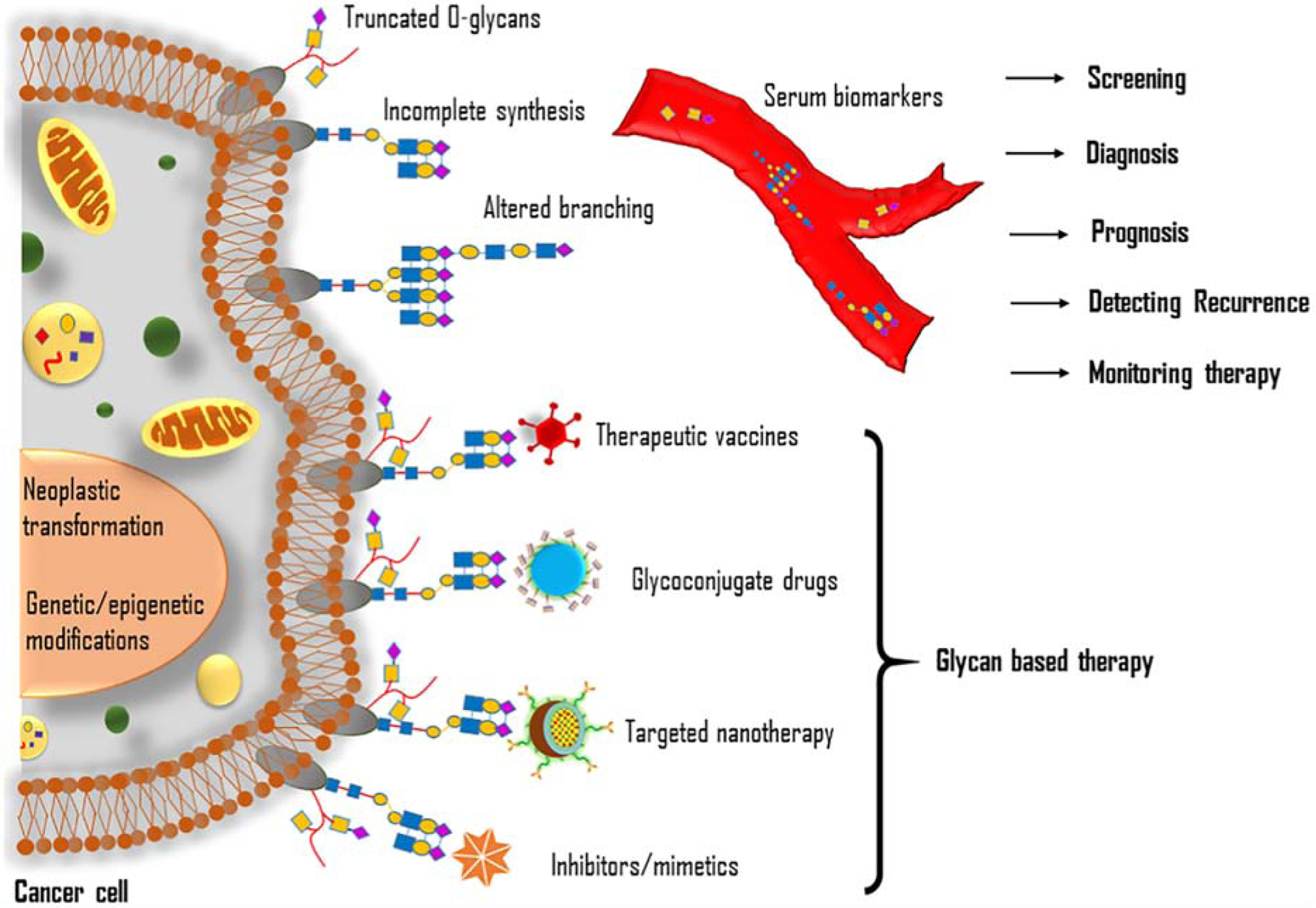
Genetic and epigenetic modifications of glycan biosynthesis result in the characteristic pattern of cancer-associated glycosylation, such as expression of truncated O-glycans, incomplete synthesis, and fucosylated altered branching of N-glycans. Tumor-associated glycoproteins in the serum are used as essential biomarkers for screening, diagnosis, prognosis, recurrence, and monitoring therapy. Glycoconjugate drugs, glycan-based vaccines, glycosylation specific inhibitors/mimetic, and targeted nanotherapies are used as glycan-based therapeutic strategies against cancer.
6. Conclusion
Cancer is a devastating disease that comprehends numerous cellular modifications. Most of the cancer-associated deaths are due to the lack of early diagnosis and effective targeted therapies. Current treatment regimens mostly rely upon conventional chemotherapy with a few targeted therapies. However, emerging evidence highlighted that altered glycans are key drivers throughout tumorigenesis and tumor progression. Glycoproteins that are found in cells and their glycan structures are commonly modified upon neo-transformation. Though glycosylation is complex and challenging, research efforts have been commenced to target altered glycosylation in cancer. Since altered glycans are abundant in tumor cells and specific glycan structures are uniquely expressed on tumor cells, these may serve as valuable targets for disease diagnosis, prognosis, and therapy. Glycosylation research encounters several challenges, such as identifying particular biological consequences of expression patterns and levels of glycosyltransferases/glycosidases; identification of glycan structures at specific sites, and its abundance in a protein. For example, the biomarkers CA125 and CA19.9 has been identified three decades ago; however, the specific sites and their abundance in proteins remain elusive. Apart from these, the development of effective synthetic tools for studying the effect of glycosylation on structure and functions of biomolecules is highly challenging because multiple isoforms of glycosyltransferases can encode a single glycan epitope. While the development of many glycan based therapeutics have failed to get a significant result against tumor progression, several research attempts have been made to tackle major problems associated with glycosylation machinery. Some of the emerging therapies possess promising outcomes. Further investigations of cancer-specific glycans are hoped to develop more useful prognostic, diagnostic, and therapeutic targets in the fight against cancer.
Funding
This study was supported by the National Institutes of Health (R01 CA208108) and Eppley Institute for Research in Cancer, Fred & Pamela Buffett Cancer Center start-up fund to PR.
Abbreviations:
- ECM
Extracellular matrix
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-to-mesenchymal transition
- ER
Endoplasmic reticulum
- FAK
Focal adhesion kinase
- FUT
Fucosyltransferase
- GalNAc
N-acetylgalactosamine
- GALNT
N-acetylgalactosaminyltransferase
- GlcNAc
N-acetylglucosamine
- OGA
O-GlcNAcase
- OGT
Peptide-N-acetylglucosaminyltransferase
- OST
Oligosaccharyltransferase
- PDAC
Pancreatic ductal adenocarcinoma
- PSA
Prostate-specific antigen
- TACA
Tumor-associated carbohydrate antigens
- TGFβ
Transforming growth factor β
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflicts of interest.
References
- 1.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006, 7(7):537–51. Epub 2006 Jun 6. Review. [DOI] [PubMed] [Google Scholar]; Erratum in: Nat Rev Genet. 2006, 7(8):660. doi: 10.1038/nrg1894. [DOI] [Google Scholar]
- 2.Varki A Biological roles of glycans. Glycobiology. 2017, 27(1):3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nairn AV, York WS, Harris K, Hall EM, Pierce JM, Moremen KW. Regulation of glycan structures in animal tissues: transcript profiling of glycan-related genes. J Biol Chem. 2008, 283(25):17298–313. doi: 10.1074/jbc.M801964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemka A, Corcionivoschi N, Bourke B. Defense and adaptation: the complex inter-relationship between Campylobacter jejuni and mucus. Front Cell Infect Microbiol. 2012, 2:15. doi: 10.3389/fcimb.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mereiter S, Balmaña M, Campos D, Gomes J, Reis CA. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell. 2019, 36(1):6–16. doi: 10.1016/j.ccell.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014, 4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019,15(6):346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011, 21(5):576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Cherepanova NA, Venev SV, Leszyk JD, Shaffer SA, Gilmore R. Quantitative glycoproteomics reveals new classes of STT3A- and STT3B-dependent N-glycosylation sites. J Cell Biol. 2019, 218(8):2782–2796. doi: 10.1083/jcb.201904004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009, 136(2):272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001, 291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 12.Li ST, Lu TT, Xu XX, et al. Reconstitution of the lipid-linked oligosaccharide pathway for assembly of high-mannose N-glycans. Nat Commun. 2019, 10(1):1813. doi: 10.1038/s41467-019-09752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Tol W, Wessels H, Lefeber DJ. O-glycosylation disorders pave the road for understanding the complex human O-glycosylation machinery. Curr Opin Struct Biol. 2019, 56:107–118. doi: 10.1016/j.sbi.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998, 33(3):151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 15.Schachter H, Brockhausen I. The biosynthesis of branched O-glycans. Symp Soc Exp Biol. 1989, 43:1–26. [PubMed] [Google Scholar]
- 16.Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans In: Varki A, Cummings RD, Esko JD, et al. , eds. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 17.Sindrewicz P, Lian LY, Yu LG. Interaction of the Oncofetal Thomsen-Friedenreich Antigen with Galectins in Cancer Progression and Metastasis. Front Oncol. 2016, 6:79. doi: 10.3389/fonc.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curigliano G, Bagnardi V, Ghioni M, et al. Expression of tumor-associated antigens in breast cancer subtypes. Breast. 2020, 49:202–209. doi: 10.1016/j.breast.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Sáez N, Peregrina JM, Corzana F. Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem Soc Rev. 2017, 46(23):7154–7175. doi: 10.1039/c6cs00858e. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Yoneyama MS, Hatakeyama S, et al. Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol Med Rep. 2013, 7(2):359–364. doi: 10.3892/mmr.2012.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto T, Suzuki A, Asaka R, et al. Immunohistochemical expression of core 2 β1,6-N-acetylglucosaminyl transferase 1 (C2GnT1) in endometrioid-type endometrial carcinoma: a novel potential prognostic factor. Histopathology. 2013, 62(7):986–993. doi: 10.1111/his.12107. [DOI] [PubMed] [Google Scholar]
- 22.Schachter H, Brockhausen I. The biosynthesis of branched O-glycans. Symp. Soc. Exp. Biol 1989;43:1–26. [PubMed] [Google Scholar]
- 23.Martensson S, Levery SB, Fang TT, Bendiak B. Neutral core oligosaccharides of bovine submaxillary mucin--use of lead tetraacetate in the cold for establishing branch positions. Eur J Biochem. 1998, 258(2):603–622. doi: 10.1046/j.1432-1327.1998.2580603.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem Biol. 2010, 5(2):163–76. doi: 10.1021/cb900266r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol. 2017, 147(2):149–174. doi: 10.1007/s00418-016-1520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology. 2011, 21(6):716–26. doi: 10.1093/glycob/cwq189. [DOI] [PubMed] [Google Scholar]
- 27.Huang RB, Cheng D, Liao SM, Lu B, Wang QY, Xie NZ, Troy Ii FA, Zhou GP. The Intrinsic Relationship Between Structure and Function of the Sialyltransferase ST8Sia Family Members. Curr Top Med Chem. 2017, 17(21):2359–2369. doi: 10.2174/1568026617666170414150730. [DOI] [PubMed] [Google Scholar]
- 28.Thomas D, Sagar S, Caffrey T, Grandgenett PM, Radhakrishnan P. Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells. J Cell Mol Med. 2019, 23(10):6885–6896. doi: 10.1111/jcmm.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci U S A. 2014, 111(39):E4066–75. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotton S, Azevedo R, Gaiteiro C, Ferreira D, Lima L, Peixoto A, Fernandes E, Neves M, Neves D, Amaro T, Cruz R, Tavares A, Rangel M, Silva AMN, Santos LL, Ferreira JA. Targeted O-glycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol Oncol. 2017, 11(8):895–912. doi: 10.1002/1878-0261.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akita K, Yoshida S, Ikehara Y, Shirakawa S, Toda M, Inoue M, Kitawaki J, Nakanishi H, Narimatsu H, Nakada H. Different levels of sialyl-Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. Int J Gynecol Cancer. 2012, 22(4):531–8. doi: 10.1097/IGC.0b013e3182473292 [DOI] [PubMed] [Google Scholar]
- 32.Chen JY, Tang YA, Huang SM, Juan HF, Wu LW, Sun YC, Wang SC, Wu KW, Balraj G, Chang TT, Li WS, Cheng HC, Wang YC. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011, 71(2):473–83. doi: 10.1158/0008-5472.CAN-10-1303 [DOI] [PubMed] [Google Scholar]
- 33.Chiang CH, Wang CH, Chang HC, More SV, Li WS, Hung WC. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J Cell Physiol. 2010, 223(2):492–9. doi: 10.1002/jcp.22068. [DOI] [PubMed] [Google Scholar]
- 34.Tonetti M, Sturla L, Bisso A, Benatti U, De Flora A. Synthesis of GDP-L-fucose by the human FX protein. J Biol Chem. 1996, 271(44):27274–27279. doi: 10.1074/jbc.271.44.27274. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Hsu HC, Mountz JD, Allen JG. Unmasking Fucosylation: from Cell Adhesion to Immune System Regulation and Diseases. Cell Chem Biol. 2018, 25(5):499–512. doi: 10.1016/j.chembiol.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Holdener BC, Haltiwanger RS. Protein O-fucosylation: structure and function. Curr Opin Struct Biol. 2019, 56:78–86. doi: 10.1016/j.sbi.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharberg EA, Olsen C, Bugert P. An update on the H blood group system. Immunohematology. 2019, 35(2):67–68. [PubMed] [Google Scholar]
- 38.Veillon L, Fakih C, Abou-El-Hassan H, Kobeissy F, Mechref Y. Glycosylation Changes in Brain Cancer. ACS Chem Neurosci. 2018, 9(1):51–72. doi: 10.1021/acschemneuro.7b00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchiya N, Yamanaka R, Yajima N, et al. Isolation and characterization of an N-linked oligosaccharide that is increased in glioblastoma tissue and cell lines. Int J Oncol. 2005, 27(5):1231–1239. [PubMed] [Google Scholar]
- 40.Holst S, Wuhrer M, Rombouts Y. Glycosylation characteristics of colorectal cancer. Adv Cancer Res. 2015, 126:203–256. doi: 10.1016/bs.acr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Noda M, Okayama H, Kofunato Y, et al. Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer. PLoS One. 2018, 13(7):e0200315 Published 2018 Jul 5. doi: 10.1371/journal.pone.0200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan S, Liu Y, Liu Q, et al. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway [published correction appears in Biochim Biophys Acta Mol Cell Res. 2019 Aug;1866(8):1353]. Biochim Biophys Acta Mol Cell Res. 2019, 1866(5):750–760. doi: 10.1016/j.bbamcr.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Jassam SA, Maherally Z, Ashkan K, Pilkington GJ, Fillmore HL. Fucosyltransferase 4 and 7 mediates adhesion of non-small cell lung cancer cells to brain-derived endothelial cells and results in modification of the blood-brain-barrier: in vitro investigation of CD15 and CD15s in lung-to-brain metastasis. J Neurooncol. 2019, 143(3):405–415. doi: 10.1007/s11060-019-03188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponath P, Menezes D, Pan C, et al. A Novel, Fully Human Anti-fucosyl-GM1 Antibody Demonstrates Potent In Vitro and In Vivo Antitumor Activity in Preclinical Models of Small Cell Lung Cancer. Clin Cancer Res. 2018, 24(20):5178–5189. doi: 10.1158/1078-0432.CCR-18-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao K, Chen ZY, Gautam S, Deng NH, Zhou Y, Wu XZ. Posttranslational modification of E-cadherin by core fucosylation regulates Src activation and induces epithelial-mesenchymal transition-like process in lung cancer cells. Glycobiology. 2016, 26(2):142–154. doi: 10.1093/glycob/cwv089. [DOI] [PubMed] [Google Scholar]
- 46.Liu D, Gao Z, Yue L. Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway. Cancer Biomark. 2019, 25(4):303–311. doi: 10.3233/CBM-190209. [DOI] [PubMed] [Google Scholar]
- 47.Merlotti A, Malizia AL, Michea P, et al. Aberrant fucosylation enables breast cancer clusterin to interact with dendritic cell-specific ICAM-grabbing non-integrin (DC-SIGN). Oncoimmunology. 2019, 8(9):e1629257. doi: 10.1080/2162402X.2019.1629257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan KP, Ho MY, Cho HC, Yu J, Hung JT, Yu AL. Fucosylation of LAMP-1 and LAMP-2 by FUT1 correlates with lysosomal positioning and autophagic flux of breast cancer cells. Cell Death Dis. 2016, 7(8):e2347. doi: 10.1038/cddis.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo HB, Lee I, Kamar M, Pierce M. N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell-cell adhesion and intracellular signaling pathways. J Biol Chem. 2003, 278(52):52412–52424. doi: 10.1074/jbc.M308837200. [DOI] [PubMed] [Google Scholar]
- 50.Liwosz A, Lei T, Kukuruzinska MA. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem. 2006, 281(32):23138–23149. doi: 10.1074/jbc.M512621200. [DOI] [PubMed] [Google Scholar]
- 51.Nita-Lazar M, Rebustini I, Walker J, Kukuruzinska MA. Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions. Exp Cell Res. 2010, 316(11):1871–1884. doi: 10.1016/j.yexcr.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009, 69(14):5673–5680. doi: 10.1158/0008-5472.CAN-08-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harosh-Davidovich SB, Khalaila I. O-GlcNAcylation affects β-catenin and E-cadherin expression, cell motility and tumorigenicity of colorectal cancer. Exp Cell Res. 2018, 364(1):42–49. doi: 10.1016/j.yexcr.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Hamester F, Legler K, Wichert B, et al. Prognostic relevance of the Golgi mannosidase MAN1A1 in ovarian cancer: impact of N-glycosylation on tumour cell aggregation. Br J Cancer. 2019, 121(11):944–953. doi: 10.1038/s41416-019-0607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin SP, Chung JH. Inhibition of N-glycosylation by tunicamycin attenuates cell-cell adhesion via impaired desmosome formation in normal human epidermal keratinocytes. Biosci Rep. 2018, 38(6):BSR20171641 Published 2018 Nov 28. doi: 10.1042/BSR20171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvalho S, Catarino TA, Dias AM, et al. Preventing E-cadherin aberrant N-glycosylation at Asn-554 improves its critical function in gastric cancer. Oncogene. 2016, 35(13):1619–1631. doi: 10.1038/onc.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng F, Shi BZ, Yuan YF, Wu XZ. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res. 2004, 14(5):423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- 58.Osumi D, Takahashi M, Miyoshi E, et al. Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 2009, 100(5):888–895. doi: 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu D, Gao Z, Yue L. Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway. Cancer Biomark. 2019, 25(4):303–311. doi: 10.3233/CBM-190209. [DOI] [PubMed] [Google Scholar]
- 60.Wang ZQ, Bachvarova M, Morin C, et al. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: possible implications in abnormal mucin O-glycosylation. Oncotarget. 2014, 5(2):544–560. doi: 10.18632/oncotarget.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003, 22(9):1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 62.Yoshihama N, Yamaguchi K, Chigita S, et al. A Novel Function of CD82/KAI1 in Sialyl Lewis Antigen-Mediated Adhesion of Cancer Cells: Evidence for an Anti-Metastasis Effect by Down-Regulation of Sialyl Lewis Antigens. PLoS One. 2015, 10(4):e0124743. doi: 10.1371/journal.pone.0124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pérez-Garay M, Arteta B, Llop E, et al. α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int J Biochem Cell Biol. 2013, 45(8):1748–1757. doi: 10.1016/j.biocel.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 64.Bassagañas S, Carvalho S, Dias AM, et al. Pancreatic cancer cell glycosylation regulates cell adhesion and invasion through the modulation of α2β1 integrin and E-cadherin function. PLoS One. 2014, 9(5):e98595. doi: 10.1371/journal.pone.0098595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One. 2014, 9(6):e100393. doi: 10.1371/journal.pone.0100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muntoni F, Brockington M, Blake DJ, Torelli S, Brown SC. Defective glycosylation in muscular dystrophy. Lancet. 2002, 360(9343):1419–1421. doi: 10.1016/S0140-6736(02)11397-3. [DOI] [PubMed] [Google Scholar]
- 67.Menni C, Gudelj I, Macdonald-Dunlop E, et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated With Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ Res. 2018, 122(11):1555–1564. doi: 10.1161/CIRCRESAHA.117.312174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schedin-Weiss S, Winblad B, Tjernberg LO. The role of protein glycosylation in Alzheimer disease. FEBS J. 2014, 281(1):46–62. doi: 10.1111/febs.12590. [DOI] [PubMed] [Google Scholar]
- 69.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015, 10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Läubli H, Borsig L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front Immunol. 2019, 10:2120. doi: 10.3389/fimmu.2019.02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci U S A. 1995, 92(19):8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pocheć E, Bubka M, Rydlewska M, Janik M, Pokrywka M, Lityńska A. Aberrant glycosylation of αvβ3 integrin is associated with melanoma progression. Anticancer Res. 2015, 35(4):2093–2103. [PubMed] [Google Scholar]
- 73.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005, 65(11):4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 74.Yu S, Zhang L, Li N, et al. Caveolin-1 up-regulates ST6Gal-I to promote the adhesive capability of mouse hepatocarcinoma cells to fibronectin via FAK-mediated adhesion signaling. Biochem Biophys Res Commun. 2012, 427(3):506–512. doi: 10.1016/j.bbrc.2012.09.086. [DOI] [PubMed] [Google Scholar]
- 75.Yu S, Fan J, Liu L, Zhang L, Wang S, Zhang J. Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett. 2013, 587(6):782–787. doi: 10.1016/j.febslet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Radhakrishnan P, Grandgenett PM, Mohr AM, et al. Expression of core 3 synthase in human pancreatic cancer cells suppresses tumor growth and metastasis. Int J Cancer. 2013, 133(12):2824–2833. doi: 10.1002/ijc.28322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki O, Abe M, Hashimoto Y. Sialylation and glycosylation modulate cell adhesion and invasion to extracellular matrix in human malignant lymphoma: Dependency on integrin and the Rho GTPase family. Int J Oncol. 2015, 47(6):2091–2099. doi: 10.3892/ijo.2015.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kariya Y, Oyama M, Hashimoto Y, Gu J, Kariya Y. β4-Integrin/PI3K Signaling Promotes Tumor Progression through the Galectin-3-N-Glycan Complex. Mol Cancer Res. 2018, 16(6):1024–1034. doi: 10.1158/1541-7786.MCR-17-0365. [DOI] [PubMed] [Google Scholar]
- 79.Yuan Y, Wu L, Shen S, Wu S, Burdick MM. Effect of alpha 2,6 sialylation on integrin-mediated adhesion of breast cancer cells to fibronectin and collagen IV. Life Sci. 2016, 149:138–145. doi: 10.1016/j.lfs.2016.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016, 7(23):35478–35489. doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai Y, Liu L, Zeng T, et al. Overexpression of MUC13, a Poor Prognostic Predictor, Promotes Cell Growth by Activating Wnt Signaling in Hepatocellular Carcinoma. Am J Pathol. 2018, 188(2):378–391. doi: 10.1016/j.ajpath.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 82.Lahdaoui F, Messager M, Vincent A, et al. Depletion of MUC5B mucin in gastrointestinal cancer cells alters their tumorigenic properties: implication of the Wnt/β-catenin pathway. Biochem J. 2017, 474(22):3733–3746. Published 2017 Nov 1. doi: 10.1042/BCJ20170348. [DOI] [PubMed] [Google Scholar]
- 83.Comamala M, Pinard M, Thériault C, et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer. 2011, 104(6):989–999. doi: 10.1038/bjc.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sengupta PK, Bouchie MP, Kukuruzinska MA. N-glycosylation gene DPAGT1 is a target of the Wnt/beta-catenin signaling pathway. J Biol Chem. 2010, 285(41):31164–31173. doi: 10.1074/jbc.M110.149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sengupta PK, Bouchie MP, Nita-Lazar M, Yang HY, Kukuruzinska MA. Coordinate regulation of N-glycosylation gene DPAGT1, canonical Wnt signaling and E-cadherin adhesion. J Cell Sci. 2013, 126(Pt 2):484–496. doi: 10.1242/jcs.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014, 79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi M, Hasegawa Y, Gao C, Kuroki Y, Taniguchi N. N-glycans of growth factor receptors: their role in receptor function and disease implications. Clin Sci (Lond). 2016, 130(20):1781–1792. doi: 10.1042/CS20160273. [DOI] [PubMed] [Google Scholar]
- 88.Gu J, Zhao Y, Isaji T, et al. Beta1,4-N-Acetylglucosaminyltransferase III downregulates neurite outgrowth induced by costimulation of epidermal growth factor and integrins through the Ras/ERK signaling pathway in PC12 cells. Glycobiology. 2004, 14(2):177–186. doi: 10.1093/glycob/cwh016. [DOI] [PubMed] [Google Scholar]
- 89.Che MI, Huang J, Hung JS, et al. β1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget. 2014, 5(11):3673–3684. doi: 10.18632/oncotarget.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Grosso F, De Mariano M, Passoni L, Luksch R, Tonini GP, Longo L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer. 2011, 11:525. doi: 10.1186/1471-2407-11-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin MC, Chien PH, Wu HY, et al. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene. 2018, 37(43):5780–5793. doi: 10.1038/s41388-018-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin WL, Lin YS, Shi GY, Chang CF, Wu HL. Lewisy promotes migration of oral cancer cells by glycosylation of epidermal growth factor receptor. PLoS One. 2015, 10(3):e0120162. doi: 10.1371/journal.pone.0120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gumienny T, Padgett RW. The other side of TGF-beta superfamily signal regulation: thinking outside the cell. Trends Endocrinol Metab. 2002, 13(7):295–299. doi: 10.1016/s1043-2760(02)00615-x. [DOI] [PubMed] [Google Scholar]
- 94.Grover P, Nath S, Nye MD, Zhou R, Ahmad M, Mukherjee P. SMAD4-independent activation of TGF-β signaling by MUC1 in a human pancreatic cancer cell line. Oncotarget. 2018, 9(6):6897–6910. doi: 10.18632/oncotarget.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khanh do T, Mekata E, Mukaisho K, et al. Transmembrane mucin MUC1 overexpression and its association with CD10+ myeloid cells, transforming growth factor-β1 expression, and tumor budding grade in colorectal cancer. Cancer Sci. 2013, 104(7):958–964. doi: 10.1111/cas.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huanna T, Tao Z, Xiangfei W, et al. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol Carcinog. 2015, 54(10):1159–1171. doi: 10.1002/mc.22186. [DOI] [PubMed] [Google Scholar]
- 97.Park S, Lim JM, Chun JN, et al. Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non-small cell lung cancer. Int J Oncol. 2020, 56(2):559–567. doi: 10.3892/ijo.2019.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pakkiriswami S, Couto A, Nagarajan U, Georgiou M. Glycosylated Notch and Cancer. Front Oncol. 2016, 6:37. doi: 10.3389/fonc.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan Q, Chen X, Han Y, et al. Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int J Cancer. 2018, 143(9):2319–2330. doi: 10.1002/ijc.31737. [DOI] [PubMed] [Google Scholar]
- 100.Ma W, Du J, Chu Q, et al. hCLP46 regulates U937 cell proliferation via Notch signaling pathway. Biochem Biophys Res Commun. 2011, 408(1):84–88. doi: 10.1016/j.bbrc.2011.03.124. [DOI] [PubMed] [Google Scholar]
- 101.Josic D, Martinovic T, Pavelic K. Glycosylation and metastases. Electrophoresis. 2019, 40(1):140–150. doi: 10.1002/elps.201800238. [DOI] [PubMed] [Google Scholar]
- 102.Murata K, Miyoshi E, Kameyama M, et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res. 2000, 6(5):1772–1777. [PubMed] [Google Scholar]
- 103.Nagae M, Kizuka Y, Mihara E, et al. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat Commun. 2018, 9(1):3380. doi: 10.1038/s41467-018-05931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang B, Sun L, Cao J, et al. Downregulation of the GnT-V gene inhibits metastasis and invasion of BGC823 gastric cancer cells. Oncol Rep. 2013, 29(6):2392–2400. doi: 10.3892/or.2013.2373. [DOI] [PubMed] [Google Scholar]
- 105.Xu Q, Isaji T, Lu Y, et al. Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines. J Biol Chem. 2012, 287(20):16563–16574. doi: 10.1074/jbc.M111.262154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen AT, Chia J, Ros M, Hui KM, Saltel F, Bard F. Organelle Specific O-Glycosylation Drives MMP14 Activation, Tumor Growth, and Metastasis. Cancer Cell 2017, 32(5):639–653.e6. doi: 10.1016/j.ccell.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Fang R, Xu F, Shi H, et al. LAMTOR5 raises abnormal initiation of O-glycosylation in breast cancer metastasis via modulating GALNT1 activity. Oncogene. 2020, 39(11):2290–2304. doi: 10.1038/s41388-019-1146-2. [DOI] [PubMed] [Google Scholar]
- 108.Liu B, Pan S, Xiao Y, Liu Q, Xu J, Jia L. LINC01296/miR-26a/GALNT3 axis contributes to colorectal cancer progression by regulating O-glycosylated MUC1 via PI3K/AKT pathway [published correction appears in J Exp Clin Cancer Res. 2019 Apr 1;38(1):142] [published correction appears in J Exp Clin Cancer Res. 2019 Aug 21;38(1):367]. J Exp Clin Cancer Res. 2018, 37(1):316. doi: 10.1186/s13046-018-0994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chugh S, Meza J, Sheinin YM, Ponnusamy MP, Batra SK. Loss of N-acetylgalactosaminyltransferase 3 in poorly differentiated pancreatic cancer: augmented aggressiveness and aberrant ErbB family glycosylation. Br J Cancer. 2016, 114(12):1376–1386. doi: 10.1038/bjc.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caffrey T, Sagar S, Thomas D, Lewallen ME, Hollingsworth MA, Radhakrishnan P. The glycoprotein mucin-1 negatively regulates GalNAc transferase 5 expression in pancreatic cancer. FEBS Lett. 2019, 593(19):2751–2761. doi: 10.1002/1873-3468.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hung JS, Huang J, Lin YC, et al. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2. Oncotarget. 2014, 5(8):2096–2106. doi: 10.18632/oncotarget.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai CH, Tzeng SF, Chao TK, et al. Metastatic Progression of Prostate Cancer Is Mediated by Autonomous Binding of Galectin-4-O-Glycan to Cancer Cells [published correction appears in Cancer Res. 77(10 ):2772]. Cancer Res. 2016, 76(19):5756–5767. doi: 10.1158/0008-5472.CAN-16-0641. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Liao X, Ye Q, Huang L. Clinic implication of MUC1 O-glycosylation and C1GALT1 in esophagus squamous cell carcinoma. Sci China Life Sci. 2018, 61(11):1389–1395. doi: 10.1007/s11427-017-9345-7. [DOI] [PubMed] [Google Scholar]
- 114.Liu CH, Hu RH, Huang MJ, et al. C1GALT1 promotes invasive phenotypes of hepatocellular carcinoma cells by modulating integrin β1 glycosylation and activity. PLoS One. 2014, 9(8):e94995. doi: 10.1371/journal.pone.0094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baldus SE, Mönig SP, Hanisch FG, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002, 40(5):440–449. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 116.Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997, 272(39):24198–24202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 117.Syrkina MS, Maslakova AA, Potashnikova DM, Veiko VP, Vassetzky YS, Rubtsov MA. Dual Role of the Extracellular Domain of Human Mucin MUC1 in Metastasis. J Cell Biochem. 2017, 118(11):4002–4011. doi: 10.1002/jcb.26056. [DOI] [PubMed] [Google Scholar]
- 118.Shao K, Chen ZY, Gautam S, Deng NH, Zhou Y, Wu XZ. Posttranslational modification of E-cadherin by core fucosylation regulates Src activation and induces epithelial-mesenchymal transition-like process in lung cancer cells. Glycobiology. 2016, 26(2):142–154. doi: 10.1093/glycob/cwv089. [DOI] [PubMed] [Google Scholar]
- 119.Yang X, Liu S, Yan Q. Role of fucosyltransferase IV in epithelial-mesenchymal transition in breast cancer cells. Cell Death Dis. 2013, 4(7):e735. doi: 10.1038/cddis.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirakawa M, Takimoto R, Tamura F, et al. Fucosylated TGF-β receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br J Cancer. 2014, 110(1):156–163. doi: 10.1038/bjc.2013.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tu CF, Wu MY, Lin YC, Kannagi R, Yang RB. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017, 19(1):111. doi: 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lai TY, Chen IJ, Lin RJ, et al. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells. Cell Death Discov. 2019, 5:74. doi: 10.1038/s41420-019-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agrawal P, Fontanals-Cirera B, Sokolova E, et al. A Systems Biology Approach Identifies FUT8 as a Driver of Melanoma Metastasis. Cancer Cell. 2017, 31(6):804–819.e7. doi: 10.1016/j.ccell.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vajaria BN, Patel KR, Begum R, Patel PS. Sialylation: an Avenue to Target Cancer Cells. Pathol Oncol Res. 2016, 22(3):443–447. doi: 10.1007/s12253-015-0033-6. [DOI] [PubMed] [Google Scholar]
- 125.Lu J, Isaji T, Im S, et al. β-Galactoside α2,6-sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J Biol Chem. 2014, 289(50):34627–34641. doi: 10.1074/jbc.M114.593392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carvalho S, Oliveira T, Bartels MF, Miyoshi E, Pierce M, Taniguchi N, Carneiro F, Seruca R, Reis CA, Strahl S, Pinho SS. O-mannosylation and N-glycosylation: two coordinated mechanisms regulating the tumour suppressor functions of E-cadherin in cancer. Oncotarget. 2016. October 4;7(40):65231–65246. doi: 10.18632/oncotarget.11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Gu J, Taniguchi N. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J. 2008. May;275(9):1939–48. doi: 10.1111/j.1742-4658.2008.06346.x. [DOI] [PubMed] [Google Scholar]
- 128.Cheng L, Cao L, Wu Y, Xie W, Li J, Guan F, Tan Z. Bisecting N-Acetylglucosamine on EGFR Inhibits Malignant Phenotype of Breast Cancer via Down-Regulation of EGFR/Erk Signaling. Front Oncol. 2020. June 16;10:929. doi: 10.3389/fonc.2020.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J, Xu J, Li L, Ianni A, Kumari P, Liu S, Sun P, Braun T, Tan X, Xiang R, Yue S. MGAT3-mediated glycosylation of tetraspanin CD82 at asparagine 157 suppresses ovarian cancer metastasis by inhibiting the integrin signaling pathway. Theranostics. 2020. May 16;10(14):6467–6482. doi: 10.7150/thno.43865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogawa Y, Ishikawa T, Ikeda K, Nakata B, Sawada T, Ogisawa K, Kato Y, Hirakawa K. Evaluation of serum KL-6, a mucin-like glycoprotein, as a tumor marker for breast cancer. Clin Cancer Res. 2000. October;6(10):4069–72. [PubMed] [Google Scholar]
- 131.Tevethia MJ, Bonneau RH, Griffith JW, Mylin L. A simian virus 40 large T-antigen segment containing amino acids 1 to 127 and expressed under the control of the rat elastase-1 promoter produces pancreatic acinar carcinomas in transgenic mice. J Virol. 1997. November;71(11):8157–66. doi: 10.1128/JVI.71.11.8157-8166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cornelissen LAM, Blanas A, Zaal A, van der Horst JC, Kruijssen LJW, O’Toole T, van Kooyk Y, van Vliet SJ. Tn Antigen Expression Contributes to an Immune Suppressive Microenvironment and Drives Tumor Growth in Colorectal Cancer. Front Oncol. 2020. August 18;10:1622. doi: 10.3389/fonc.2020.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hofmann BT, Schlüter L, Lange P, Mercanoglu B, Ewald F, Fölster A, Picksak AS, Harder S, El Gammal AT, Grupp K, Güngör C, Drenckhan A, Schlüter H, Wagener C, Izbicki JR, Jücker M, Bockhorn M, Wolters-Eisfeld G. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol Cancer. 2015. May 29;14:109. doi: 10.1186/s12943-015-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tamura F, Sato Y, Hirakawa M, Yoshida M, Ono M, Osuga T, Okagawa Y, Uemura N, Arihara Y, Murase K, Kawano Y, Iyama S, Takada K, Hayashi T, Sato T, Miyanishi K, Kobune M, Takimoto R, Kato J. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric Cancer. 2016. January;19(1):85–97. doi: 10.1007/s10120-014-0454-z. [DOI] [PubMed] [Google Scholar]
- 135.Madjd Z, Parsons T, Watson NF, Spendlove I, Ellis I, Durrant LG. High expression of Lewis y/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res. 2005;7(5):R780–7. doi: 10.1186/bcr1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferreira IG, Carrascal M, Mineiro AG, Bugalho A, Borralho P, Silva Z, Dall’olio F, Videira PA. Carcinoembryonic antigen is a sialyl Lewis x/a carrier and an E selectin ligand in non small cell lung cancer. Int J Oncol. 2019. November;55(5):1033–1048. doi: 10.3892/ijo.2019.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yi L, Hu Q, Zhou J, Liu Z, Li H. Alternative splicing of Ikaros regulates the FUT4/LeX-α5β1 integrin-FAK axis in acute lymphoblastic leukemia. Biochem Biophys Res Commun. 2019. February 26;510(1):128–134. doi: 10.1016/j.bbrc.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 138.Kobayashi M, Morita T. Significant expression patterns of lewis X-related antigens as a prognostic predictor of low-stage renal cell carcinomas. Anticancer Res. 2010. February;30(2):593–9. [PubMed] [Google Scholar]
- 139.Isla Larrain M, Demichelis S, Crespo M, Lacunza E, Barbera A, Cretón A, Terrier F, Segal-Eiras A, Croce MV. Breast cancer humoral immune response: involvement of Lewis y through the detection of circulating immune complexes and association with Mucin 1 (MUC1). J Exp Clin Cancer Res. 2009. August 28;28(1):121. doi: 10.1186/1756-9966-28-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, Hoskins WJ, Welt S, Lloyd KO. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer. 1996. February 8;65(4):406–12. doi: . [DOI] [PubMed] [Google Scholar]
- 141.Liu J, Liu Q, Wang Y, Liu M, Qi Y, Gao J, Lin B. Co expression of Lewis y antigen and CD147 in epithelial ovarian cancer is correlated with malignant progression and poor prognosis. Int J Mol Med. 2019. April;43(4):1687–1698. doi: 10.3892/ijmm.2019.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luna A, Rabassa ME, Isla Larrain M, Cabaleiro P, Zwenger A, Canzoneri R, Segal-Eiras A, Abba MC, Croce MV. Breast cancer cutaneous metastases are associated to uMUC1 and sialyl Lewis x and to highly malignant primary tumors. Pathol Res Pract. 2020. April;216(4):152859. doi: 10.1016/j.prp.2020.152859. [DOI] [PubMed] [Google Scholar]
- 143.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993. January 15;53(2):354–61. [PubMed] [Google Scholar]
- 144.Ashkani J, Naidoo KJ. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci Rep. 2016, 6:26451. doi: 10.1038/srep26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanisch FG. O-glycosylation of the mucin type. Biol Chem. 2001, 382(2):143–9. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 146.Ding MX, Wang HF, Wang JS, Zhan H, Zuo YG, Yang DL, Liu JY, Wang W, Ke CX, Yan RP. ppGalNAc T1 as a potential novel marker for human bladder cancer. Asian Pac J Cancer Prev. 2012, 13(11):5653–7. doi: 10.7314/apjcp.2012.13.11.5653. [DOI] [PubMed] [Google Scholar]
- 147.Harada Y, Izumi H, Noguchi H, et al. Strong expression of polypeptide N-acetylgalactosaminyltransferase 3 independently predicts shortened disease-free survival in patients with early-stage oral squamous cell carcinoma [published correction appears in Tumour Biol. 2015, 36(12):10003–4]. Tumour Biol. 2016, 37(1):1357–1368. doi: 10.1007/s13277-015-3928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berois N, Mazal D, Ubillos L, et al. UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem. 2006, 54(3):317–328. doi: 10.1369/jhc.5A6783.2005. [DOI] [PubMed] [Google Scholar]
- 149.Freire T, Berois N, Sóñora C, Varangot M, Barrios E, Osinaga E. UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer. 2006, 119(6):1383–8. doi: 10.1002/ijc.21959. [DOI] [PubMed] [Google Scholar]
- 150.Brooks SA, Carter TM, Bennett EP, Clausen H, Mandel U. Immunolocalisation of members of the polypeptide N-acetylgalactosaminyl transferase (ppGalNAc-T) family is consistent with biologically relevant altered cell surface glycosylation in breast cancer. Acta Histochem. 2007, 109(4):273–84. doi: 10.1016/j.acthis.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 151.Li Z, Yamada S, Inenaga S, Imamura T, Wu Y, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Sasaguri Y. Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br J Cancer. 2011, 104(12):1882–9. doi: 10.1038/bjc.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kitada S, Yamada S, Kuma A, Ouchi S, Tasaki T, Nabeshima A, Noguchi H, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Matsumoto T, Sasaguri Y. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer. 2013, 109(2):472–81. doi: 10.1038/bjc.2013.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ishikawa M, Kitayama J, Nariko H, Kohno K, Nagawa H. The expression pattern of UDP-N-acetyl-alpha-d-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 in early gastric carcinoma. J Surg Oncol. 2004, 86(1):28–33. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, Liu W, Xu L, Liu H, Zhang W, Zhu Y, Xu J, Gu J. GALNT4 predicts clinical outcome in patients with clear cell renal cell carcinoma. J Urol. 2014, 192(5):1534–41. doi: 10.1016/j.juro.2014.04.084. [DOI] [PubMed] [Google Scholar]
- 155.Berois N, Gattolliat CH, Barrios E, Capandeguy L, Douc-Rasy S, Valteau-Couanet D, Bénard J, Osinaga E. GALNT9 gene expression is a prognostic marker in neuroblastoma patients. Clin Chem. 2013, 59(1):225–33. doi: 10.1373/clinchem.2012.192328. [DOI] [PubMed] [Google Scholar]
- 156.Libisch MG, Casás M, Chiribao M, et al. GALNT11 as a new molecular marker in chronic lymphocytic leukemia. Gene. 2014;533(1):270–279. doi: 10.1016/j.gene.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 157.Chiang CC, Yeh CT, Hwang TL, Chu YD, Lim SN, Chen CW, Kuo CJ, Le PH, Chen TH, Lin WR. The GALNT14 Genotype Predicts Postoperative Outcome of Pancreatic Ductal Adenocarcinoma. J Clin Med. 2019, 8(12). pii: E2225. doi: 10.3390/jcm8122225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011, 80:825–58. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011, 1812(4):514–9. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 160.Xu D, Wang W, Bian T, Yang W, Shao M, Yang H. Increased expression of O-GlcNAc transferase (OGT) is a biomarker for poor prognosis and allows tumorigenesis and invasion in colon cancer. Int J Clin Exp Pathol. 2019, 12(4):1305–1314). [PMC free article] [PubMed] [Google Scholar]
- 161.Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014, 54(5):820–31. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010, 29(19):2831–42. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 163.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010, 70(15):6344–51. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 164.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013, 13(14):2088–99. doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- 165.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012, 287(14):11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kamigaito T, Okaneya T, Kawakubo M, Shimojo H, Nishizawa O, Nakayama J. Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis. 2014, 17(1):18–22. doi: 10.1038/pcan.2013.56. [DOI] [PubMed] [Google Scholar]
- 167.Schultz MJ, Holdbrooks AT, Chakraborty A, Grizzle WE, Landen CN, Buchsbaum DJ, Conner MG, Arend RC, Yoon KJ, Klug CA, Bullard DC, Kesterson RA, Oliver PG, O’Connor AK, Yoder BK, Bellis SL. The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype. Cancer Res. 2016, 76(13):3978–88. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jones RB, Dorsett KA, Hjelmeland AB, Bellis SL. The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling. J Biol Chem. 2018, 293(15):5659–5667. doi: 10.1074/jbc.RA117.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Julien S, Adriaenssens E, Ottenberg K, Furlan A, Courtand G, Vercoutter-Edouart AS, Hanisch FG, Delannoy P, Le Bourhis X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology. 2006, 16(1):54–64. [DOI] [PubMed] [Google Scholar]
- 170.Gretschel S, Haensch W, Schlag PM, Kemmner W. Clinical relevance of sialyltransferases ST6GAL-I and ST3GAL-III in gastric cancer. Oncology. 2003, 65(2):139–45.doi: 10.1159/000072339. [DOI] [PubMed] [Google Scholar]
- 171.Schneider F, Kemmner W, Haensch W, Franke G, Gretschel S, Karsten U, Schlag PM. Overexpression of sialyltransferase CMP-sialic acid:Galbeta1,3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001, 61(11):4605–11. [PubMed] [Google Scholar]
- 172.do Nascimento JCF, Beltrão EIC, Rocha CRC. High FUT3 expression is a marker of lower overall survival of breast cancer patients. Glycoconj J. 2020. April;37(2):263–275. doi: 10.1007/s10719-020-09914-2. [DOI] [PubMed] [Google Scholar]
- 173.Yan X, Lin Y, Liu S, Aziz F, Yan Q. Fucosyltransferase IV (FUT4) as an effective biomarker for the diagnosis of breast cancer. Biomed Pharmacother. 2015. March;70:299–304. doi: 10.1016/j.biopha.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 174.Honma R, Kinoshita I, Miyoshi E, Tomaru U, Matsuno Y, Shimizu Y, Takeuchi S, Kobayashi Y, Kaga K, Taniguchi N, Dosaka-Akita H. Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers. Oncology. 2015;88(5):298–308. doi: 10.1159/000369495. [DOI] [PubMed] [Google Scholar]
- 175.Zhou W, Ma H, Deng G, Tang L, Lu J, Chen X. Clinical significance and biological function of fucosyltransferase 2 in lung adenocarcinoma. Oncotarget. 2017. October 19;8(57):97246–97259. doi: 10.18632/oncotarget.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schachter H, Freeze HH. Glycosylation diseases: quo vadis? Biochim Biophys Acta. 2009, 1792(9):925–30. doi: 10.1016/j.bbadis.2008.11.002; [DOI] [PMC free article] [PubMed] [Google Scholar]; Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009, 5(10):1087–104. doi: 10.1039/b907931a [DOI] [PubMed] [Google Scholar]
- 177.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002, 12(4):43R–56R. doi: 10.1093/glycob/12.4.43r [DOI] [PubMed] [Google Scholar]
- 178.Gabius HJ, Siebert HC, André S, Jiménez-Barbero J, Rüdiger H. Chemical biology of the sugar code. Chembiochem. 2004, 5(6): 740–64. doi: 10.1002/cbic.200300753 [DOI] [PubMed] [Google Scholar]
- 179.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979, 17(2):159–63 [PubMed] [Google Scholar]
- 180.Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981, 68(5):1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.American Society of Clinical Oncology Tumor Markers Expert Panel. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001, 19(6):1865–78. [DOI] [PubMed] [Google Scholar]; Erratum in: J Clin Oncol 2001, 19(21):4185–8. [Google Scholar]; J Clin Oncol 2002, 20(8):2213. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [Google Scholar]
- 182.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984, 3(3):223–32. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 183.Sokoll LJ, Sanda MG, Feng Z, Kagan J, Mizrahi IA, Broyles DL, Partin AW, Srivastava S, Thompson IM, Wei JT, Zhang Z, Chan DW. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [−2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010, 19(5):1193–200. doi: 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979, 17(2):159–63. [PubMed] [Google Scholar]
- 185.Oto J, Fernández-Pardo Á, Royo M, Hervás D, Martos L, Vera-Donoso CD, Martínez M, Heeb MJ, España F, Medina P, Navarro S. A predictive model for prostate cancer incorporating PSA molecular forms and age. Sci Rep. 2020. February 12;10(1):2463. doi: 10.1038/s41598-020-58836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kelly SL, Bird TG. The Evolution of the Use of Serum Alpha-fetoprotein in Clinical Liver Cancer Surveillance. J Immunobiol. 2016, 1(4). doi: 10.4172/24761966.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, Cao X, Han M, Du H, Ye Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2020. February 13;15(2):e0228857. doi: 10.1371/journal.pone.0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ha JH, Seong MK, Kim EK, Lee JK, Seol H, Lee JY, Byeon J, Sohn YJ, Koh JS, Park IC, Noh WC, Kim HA. Serial Serum HER2 Measurements for the Detection of Breast Cancer Recurrence in HER2-Positive Patients. J Breast Cancer. 2014. March;17(1):33–9. doi: 10.4048/jbc.2014.17.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Han C, Bellone S, Siegel ER, Altwerger G, Menderes G, Bonazzoli E, Egawa-Takata T, Pettinella F, Bianchi A, Riccio F, Zammataro L, Yadav G, Marto JA, Penet MF, Levine DA, Drapkin R, Patel A, Litkouhi B, Ratner E, Silasi DA, Huang GS, Azodi M, Schwartz PE, Santin AD. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol Oncol. 2018. June;149(3):585–591. doi: 10.1016/j.ygyno.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sturgeon C Practice guidelines for tumor marker use in the clinic. Clin Chem. 2002, 48(8):1151–9. [PubMed] [Google Scholar]
- 191.Azizian A, Rühlmann F, Krause T, Bernhardt M, Jo P, König A, Kleiß M, Leha A, Ghadimi M, Gaedcke J. CA19–9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020. January 28;10(1):1332. doi: 10.1038/s41598-020-57930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guo J, Yu J, Song X, Mi H. Serum CA125, CA199 and CEA Combined Detection for Epithelial Ovarian Cancer Diagnosis: A Meta-analysis. Open Med (Wars). 2017. May 7;12:131–137. doi: 10.1515/med-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zaleski M, Kobilay M, Schroeder L, Debald M, Semaan A, Hettwer K, Uhlig S, Kuhn W, Hartmann G, Holdenrieder S. Improved sensitivity for detection of breast cancer by combination of miR-34a and tumor markers CA 15–3 or CEA. Oncotarget. 2018. April 27;9(32):22523–22536. doi: 10.18632/oncotarget.25077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Beveridge RA. Review of clinical studies of CA 27.29 in breast cancer management. Int J Biol Markers. 1999, 14(1):36–9. [DOI] [PubMed] [Google Scholar]
- 195.Hao Y, Ren G, Yang W, Zheng W, Wu Y, Li W, Li X, Li Y, Guo X. Combination diagnosis with elastography strain ratio and molecular markers effectively improves the diagnosis rate of small breast cancer and lymph node metastasis. Quant Imaging Med Surg. 2020. March;10(3):678–691. doi: 10.21037/qims.2020.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19–9 for colorectal carcinoma. Int J Clin Exp Pathol. 2015. August 1;8(8):9404–9. [PMC free article] [PubMed] [Google Scholar]
- 197.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005, 5(11):845–56. [DOI] [PubMed] [Google Scholar]
- 198.Sohn YM, Kim MJ, Kim EK, Kwak JY. Diagnostic performance of thyroglobulin value in indeterminate range in fine needle aspiration washout fluid from lymph nodes of thyroid cancer. Yonsei Med J. 2012. January;53(1):126–31. doi: 10.3349/ymj.2012.53.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bagshawe KD, Wass M, Searle F. Markers in gynaecological cancer. Arch Gynecol. 1980, 229(4):303–10. doi: 10.1007/BF02108581. [DOI] [PubMed] [Google Scholar]
- 200.Bastani A, Asghary A, Heidari MH, Karimi-Busheri F. Evaluation of the sensitivity and specificity of serum level of prostasin, CA125, LDH, AFP, and hCG+β in epithelial ovarian cancer patients. Eur J Gynaecol Oncol. 2017;38(3):418–424. [PubMed] [Google Scholar]
- 201.Chang X, Ye X, Dong L, Cheng H, Cheng Y, Zhu L, Liao Q, Zhao Y, Tian L, Fu T, Chen J, Cui H. Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma. Int J Gynecol Cancer. 2011, 21(5):852–8. doi: 10.1097/IGC.0b013e31821a3726. [DOI] [PubMed] [Google Scholar]
- 202.Fan Q, Luo G, Yi T, Wang Q, Wang D, Zhang G, Jiang X, Guo X. Diagnostic value of urinary-to-serum human epididymis protein 4 ratio in ovarian cancer. Biomed Rep. 2017. July;7(1):67–72. doi: 10.3892/br.2017.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gut P, Czarnywojtek A, Fischbach J, Bączyk M, Ziemnicka K, Wrotkowska E, Gryczyńska M, Ruchała M. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016, 12(1):1–9. doi: 10.5114/aoms.2016.57577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang X, Yang Y, Li Z, Cheng C, Yang T, Wang C, Liu L, Liu S. Diagnostic value of circulating chromogranin a for neuroendocrine tumors: a systematic review and meta-analysis. PLoS One. 2015. April 20;10(4):e0124884. doi: 10.1371/journal.pone.0124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li WY, Zhao TT, Xu HM, Wang ZN, Xu YY, Han Y, Song YX, Wu JH, Xu H, Yin SC, Liu XY, Miao ZF. The role of EGFR mutation as a prognostic factor in survival after diagnosis of brain metastasis in non-small cell lung cancer: a systematic review and meta-analysis. BMC Cancer. 2019, 19(1):145. doi: 10.1186/s12885-019-5331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep. 2014. September 9;4:6269. doi: 10.1038/srep06269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li X, Shu K, Zhou J, Yu Q, Cui S, Liu J, Zhou R, Ding D. Preoperative Plasma Fibrinogen and D-dimer as Prognostic Biomarkers for Non-Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer. 2020, 18(1):11–19.e1.doi: 10.1016/j.clgc.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 208.Ma C, Lu B, Diao C, Zhao K, Wang X, Ma B, Lu B, Sun E. Preoperative neutrophil-lymphocyte ratio and fibrinogen level in patients distinguish between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Onco Targets Ther. 2016. August 8;9:4917–22. doi: 10.2147/OTT.S107445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Semaan SM, Wang X, Marshall AG, Sang QX. Identification of Potential Glycoprotein Biomarkers in Estrogen Receptor Positive (ER+) and Negative (ER) Human Breast Cancer Tissues by LC-LTQ/FT-ICR Mass Spectrometry. J Cancer. 2012;3:269–84. doi: 10.7150/jca.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Samaan NA, Buzdar AU, Aldinger KA, Schultz PN, Yang KP, Romsdahl MM, Martin R. Estrogen receptor: a prognostic factor in breast cancer. Cancer. 1981, 47(3):554–60. doi: . [DOI] [PubMed] [Google Scholar]
- 211.Moi SH, Lee YC, Chuang LY, Yuan SF, Ou-Yang F, Hou MF, Yang CH, Chang HW. Cumulative receiver operating characteristics for analyzing interaction between tissue visfatin and clinicopathologic factors in breast cancer progression. Cancer Cell Int. 2018. February 9;18:19. doi: 10.1186/s12935-018-0517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014, 16(4):428. doi: 10.1186/s13058-014-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jevrić M, Matić IZ, Krivokuća A, Đorđić Crnogorac M, Besu I, Damjanović A, Branković-Magić M, Milovanović Z, Gavrilović D, Susnjar S, Kisić Tepavčević D, Stanojković T. Association of uPA and PAI-1 tumor levels and 4G/5G variants of PAI-1 gene with disease outcome in luminal HER2-negative node-negative breast cancer patients treated with adjuvant endocrine therapy. BMC Cancer. 2019. January 15;19(1):71. doi: 10.1186/s12885-018-5255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015, 19(3):349–62. doi: 10.1517/14728222.2014.986459; [DOI] [PubMed] [Google Scholar]; Sait S, Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther. 2017, 17(10):889–904. doi: 10.1080/14737140.2017.1364995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, Young L, Healey D, Onetto N, Slichenmyer W. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999, 17(2):478–84. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 216.Scott AM, Geleick D, Rubira M, Clarke K, Nice EC, Smyth FE, Stockert E, Richards EC, Carr FJ, Harris WJ, Armour KL, Rood J, Kypridis A, Kronina V, Murphy R, Lee FT, Liu Z, Kitamura K, Ritter G, Laughton K, Hoffman E, Burgess AW, Old LJ. Construction, production, and characterization of humanized anti-Lewis Y monoclonal antibody 3S193 for targeted immunotherapy of solid tumors. Cancer Res. 2000, 60(12):3254–61. [PubMed] [Google Scholar]
- 217.Farrugia W, Scott AM, Ramsland PA. A possible role for metallic ions in the carbohydrate cluster recognition displayed by a Lewis Y specific antibody. PLoS One. 2009,4(11):e7777. doi: 10.1371/journal.pone.0007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chua JX, Vankemmelbeke M, McIntosh RS, Clarke PA, Moss R, Parsons T, Spendlove I, Zaitoun AM, Madhusudan S, Durrant LG. Monoclonal Antibodies Targeting LecLex-Related Glycans with Potent Antitumor Activity. Clin Cancer Res. 2015, 21(13):2963–74. doi: 10.1158/1078-0432.CCR-14-3030. [DOI] [PubMed] [Google Scholar]
- 219.Ohyama C, Hosono M, Nitta K, Oh-eda M, Yoshikawa K, Habuchi T, Arai Y, Fukuda M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology. 2004, 14(8):671–9. doi: 10.1093/glycob/cwh071. [DOI] [PubMed] [Google Scholar]
- 220.Lityńska A, Przybyło M, Pocheć E, Hoja-Łukowicz D, Ciołczyk D, Laidler P, Gil D. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Res. 2001, 11(3):205–12. doi: 10.1097/00008390-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 221.Korourian S, Siegel E, Kieber-Emmons T, Monzavi-Karbassi B. Expression analysis of carbohydrate antigens in ductal carcinoma in situ of the breast by lectin histochemistry. BMC Cancer. 2008, 8:136. doi: 10.1186/1471-2407-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Blomme B, Van Steenkiste C, Callewaert N, Van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J Hepatol. 2009, 50(3):592–603. doi: 10.1016/j.jhep.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 223.Ferreira JA. Esophageal, gastric and colorectal cancers: Looking beyond classical serological biomarkers towards glycoproteomics-assisted precision oncology. Theranostics. 2020. March 31;10(11):4903–4928. doi: 10.7150/thno.42480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004, 4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 225.Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry. 2004, 10(14):3517–24. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 226.Franco A Glycoconjugates as vaccines for cancer immunotherapy: clinical trials and future directions. Anticancer Agents Med Chem. 2008, 8(1):86–91. doi: 10.2174/187152008783330888. [DOI] [PubMed] [Google Scholar]
- 227.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci U S A. 1999, 96(10):5710–5. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Danishefsky SJ, Shue YK, Chang MN, Wong CH. Development of Globo-H cancer vaccine. Acc Chem Res. 2015, 48(3):643–52. doi: 10.1021/ar5004187. [DOI] [PubMed] [Google Scholar]
- 229.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bornmann WG, Spassova M, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc Natl Acad Sci U S A. 2001, 98(6):3270–5. Erratum in: [DOI] [PMC free article] [PubMed] [Google Scholar]; Proc Natl Acad Sci U S A 2001, 98(24):14186. doi: 10.1073/pnas.051626298. [DOI] [Google Scholar]
- 230.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, Danishefsky S, Livingston P, Scher HI. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005, 54(7):694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhu J, Wan Q, Lee D, Yang G, Spassova MK, Ouerfelli O, Ragupathi G, Damani P, Livingston PO, Danishefsky SJ. From synthesis to biologics: preclinical data on a chemistry derived anticancer vaccine. J Am Chem Soc. 2009, 131(26):9298–303. doi: 10.1021/ja901415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007, 3(10):663–7. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Holmberg LA, Sandmaier BM. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004, 3(6):655–63. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 234.Toyokuni T, Dean B, Cai SP, Boivin D, Hakomori S, Singhal AK. Synthetic Vaccines - Synthesis of a Dimeric Tn Antigen-Lipopeptide Conjugate That Elicits Immune-Responses against Tn-Expressing Glycoproteins. J Am Chem Soc. 1994, 116:395–396. doi: 10.1021/ja00080a055 [DOI] [Google Scholar]
- 235.Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO. Toward optimized carbohydrate-based anticancer vaccines: epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewis(y) conjugates in mice. Proc Natl Acad Sci U S A. 2001, 98(6):3264–9. doi: 10.1073/pnas.051623598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Buskas T, Ingale S, Boons GJ. Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lapidated glycopeptide containing the tumor-associated tn antigen. Angew Chem Int Ed Engl. 2005, 44(37):5985–8. doi: 10.1002/anie.200501818 [DOI] [PubMed] [Google Scholar]
- 237.Ingale S, Wolfert MA, Buskas T, Boons GJ. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. Chembiochem. 2009. February 13;10(3):455–63. doi: 10.1002/cbic.200800596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cai H, Huang ZH, Shi L, Zhao YF, Kunz H, Li YM. Towards a fully synthetic MUC1-based anticancer vaccine: efficient conjugation of glycopeptides with mono-, di-, and tetravalent lipopeptides using click chemistry. Chemistry. 2011, 17(23):6396–406. doi: 10.1002/chem.201100217 [DOI] [PubMed] [Google Scholar]
- 239.Abdel-Aal AB, El-Naggar D, Zaman M, Batzloff M, Toth I. Design of fully synthetic, self-adjuvanting vaccine incorporating the tumor-associated carbohydrate Tn antigen and lipoamino acid-based Toll-like receptor 2 ligand. J Med Chem. 2012, 55(15):6968–74. doi: 10.1021/jm300822g [DOI] [PubMed] [Google Scholar]
- 240.Cai H, Sun ZY, Huang ZH, Shi L, Zhao YF, Kunz H, Li YM. Fully synthetic self-adjuvanting thioether-conjugated glycopeptide-lipopeptide antitumor vaccines for the induction of complement-dependent cytotoxicity against tumor cells. Chemistry. 2013, 19(6):1962–70. doi: 10.1002/chem.201203709 [DOI] [PubMed] [Google Scholar]
- 241.Zhou Z, Mondal M, Liao G, Guo Z. Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org Biomol Chem. 2014, 12(20):3238–45. doi: 10.1039/c4ob00390j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhou Z, Liao G, Mandal SS, Suryawanshi S, Guo Z. A Fully Synthetic Self-Adjuvanting Globo H-Based Vaccine Elicited Strong T Cell-Mediated Antitumor Immunity. Chem Sci. 2015, 6(12):7112–7121. doi: 10.1039/C5SC01402F; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhou Z, Mandal SS, Liao G, Guo J, Guo Z. Synthesis and Evaluation of GM2-Monophosphoryl Lipid A Conjugate as a Fully Synthetic Self-Adjuvant Cancer Vaccine. Sci Rep. 2017, 7(1):11403. doi: 10.1038/s41598-017-11500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pohl J, Bertram B, Hilgard P, Nowrousian MR, Stüben J, Wiessler M. D-19575—a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother Pharmacol. 1995, 35(5):364–70. doi: 10.1007/s002800050248. [DOI] [PubMed] [Google Scholar]
- 245.Briasoulis E, Judson I, Pavlidis N, Beale P, Wanders J, Groot Y, Veerman G, Schuessler M, Niebch G, Siamopoulos K, Tzamakou E, Rammou D, Wolf L, Walker R, Hanauske A. Phase I trial of 6-hour infusion of glufosfamide, a new alkylating agent with potentially enhanced selectivity for tumors that overexpress transmembrane glucose transporters: a study of the European Organization for Research and Treatment of Cancer Early Clinical Studies Group. J Clin Oncol. 2000, 18(20):3535–44. doi: 10.1200/JCO.2000.18.20.3535. [DOI] [PubMed] [Google Scholar]
- 246.Cao J, Cui S, Li S, Du C, Tian J, Wan S, Qian Z, Gu Y, Chen WR, Wang G. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013. February 15;73(4):1362–73. doi: 10.1158/0008-5472.CAN-12-2072. [DOI] [PubMed] [Google Scholar]
- 247.Cheng H, Cao X, Xian M, Fang L, Cai TB, Ji JJ, Tunac JB, Sun D, Wang PG. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J Med Chem. 2005. January 27;48(2):645–52. doi: 10.1021/jm049693a. [DOI] [PubMed] [Google Scholar]
- 248.Fu Y, Li S, Zu Y, Yang G, Yang Z, Luo M, Jiang S, Wink M, Efferth T. Medicinal chemistry of paclitaxel and its analogues. Curr Med Chem. 2009, 16(30):3966–85. doi: 10.2174/092986709789352277. [DOI] [PubMed] [Google Scholar]
- 249.Goff RD, Thorson JS. Assessment of chemoselective neoglycosylation methods using chlorambucil as a model. J Med Chem. 2010, 53(22):8129–39. doi: 10.1021/jm101024j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kumar P, Shustov G, Liang H, Khlebnikov V, Zheng W, Yang XH, Cheeseman C, Wiebe LI. Design, synthesis, and preliminary biological evaluation of 6-O-glucose-azomycin adducts for diagnosis and therapy of hypoxic tumors. J Med Chem. 2012, 55(13):6033–46. doi: 10.1021/jm2017336. [DOI] [PubMed] [Google Scholar]
- 251.Patra M, Awuah SG, Lippard SJ. Chemical Approach to Positional Isomers of Glucose-Platinum Conjugates Reveals Specific Cancer Targeting through Glucose-Transporter-Mediated Uptake in Vitro and in Vivo. J Am Chem Soc. 2016, 138(38):12541–51. doi: 10.1021/jacs.6b06937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gynther M, Ropponen J, Laine K, Leppänen J, Haapakoski P, Peura L, Järvinen T, Rautio J. Glucose promoiety enables glucose transporter mediated brain uptake of ketoprofen and indomethacin prodrugs in rats. J Med Chem. 2009, 52(10):3348–53. doi: 10.1021/jm8015409. [DOI] [PubMed] [Google Scholar]
- 253.DeAngelo DJ, Jonas BA, Liesveld JL, et al. Results of a phase I study of GMI-1271, a novel E-selectin antagonist in combination with induction chemotherapy in relapsed/refractory AML: a novel well-tolerated regimen with a high remission rate. Haematologica. 2016; 101: 43–44; [Google Scholar]; Clinicaltrials.gov identifier (NCT number): NCT03616470.
- 254.Clinicaltrials.gov identifier (NCT number): NCT02575404.
- 255.Clinicaltrials.gov identifier (NCT number): NCT00110721.
- 256.Clinicaltrials.gov identifier (NCT number): NCT00110721.
- 257.Clinicaltrials.gov identifier (NCT number): NCT02952989.
- 258.Kilcoyne M, Joshi L. Carbohydrates in therapeutics. Cardiovasc Hematol Agents Med Chem. 2007. July;5(3):186–97. doi: 10.2174/187152507781058663. [DOI] [PubMed] [Google Scholar]
- 259.Brown JR, Crawford BE, Esko JD. Glycan antagonists and inhibitors: a fount for drug discovery. Crit Rev Biochem Mol Biol. 2007. Nov-Dec;42(6):481–515. doi: 10.1080/10409230701751611. [DOI] [PubMed] [Google Scholar]
- 260.Costa AF, Campos D, Reis CA, Gomes C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer. 2020. September;6(9):757–766. doi: 10.1016/j.trecan.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 261.Puranik AS, Pao LP, White VM, Peppas NA. Synthesis and characterization of pH-responsive nanoscale hydrogels for oral delivery of hydrophobic therapeutics. Eur J Pharm Biopharm. 2016, 108:196–213. doi: 10.1016/j.ejpb.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 262.Soni KS, Thomas D, Caffrey T, Mehla K, Lei F, O’Connell KA, Sagar S, Lele SM, Hollingsworth MA, Radhakrishnan P, Bronich TK. A Polymeric Nanogel-Based Treatment Regimen for Enhanced Efficacy and Sequential Administration of Synergistic Drug Combination in Pancreatic Cancer. J Pharmacol Exp Ther. 2019, 370(3):894–901. doi: 10.1124/jpet.118.255372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fernandes E, Ferreira JA, Andreia P, Luís L, Barroso S, Sarmento B, Santos LL. New trends in guided nanotherapies for digestive cancers: A systematic review. J Control Release. 2015. July 10;209:288–307. doi: 10.1016/j.jconrel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 264.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010, 1:149–73. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Posocco B, Dreussi E, de Santa J, et al. Polysaccharides for the Delivery of Antitumor Drugs. Materials (Basel). 2015, 8(5):2569–2615. doi: 10.3390/ma8052569. [DOI] [Google Scholar]


