Abstract
Objective
To examine the association between age at menarche and risk of vasomotor menopausal symptoms (VMS) and whether midlife body mass index (BMI) modified the association.
Design
A pooled analysis of six cohort studies.
Setting
The International collaboration on the Life course Approach to reproductive health and Chronic disease Events (InterLACE).
Population
18,555 women from the UK, USA, and Australia.
Methods
VMS frequency data (never, rarely, sometimes, and often) were harmonised from two studies (n=13,602); severity data (never, mild, moderate, and severe) from the other four studies (n=4,953). Multinominal logistic regression models were used to estimate relative risk ratios (RRRs) and 95%CIs adjusted for confounders and incorporated study as random effects.
Main Outcome Measures
Hot flushes and night sweats.
Results
Frequency data showed that early menarche ≤11 years was associated with an increased risk of ‘often’ hot flushes (RRR 1.48, 1.24–1.76) and night sweats (RRR 1.59, 1.49–1.70) compared with menarche at ≥14 years. Severity data showed similar results, but appeared less conclusive, with RRRs of 1.16 (0.94–1.42) and 1.27 (1.01–1.58) for ‘severe’ hot flushes and night sweats, respectively. BMI significantly modified the association as the risk associated with early menarche and ‘often’ VMS was stronger among women who were overweight or obese than those of normal-weight, while this gradient across BMI categories was not as strong with the risk of ‘severe’ VMS.
Conclusions
Early age at menarche is a risk factor for VMS, particularly for frequent VMS, but midlife BMI may play an important role in modifying this risk.
Keywords: Age at menarche, body mass index, hot flushes, night sweats, vasomotor menopausal symptoms
Tweetable abstract
Overweight and obesity exacerbate the risk of vasomotor symptoms associated with early menarche.
INTRODUCTION
The timing of menarche, defined as the first menstrual period, is an indicator of the onset of reproductive capacity and has significant health implications. The International collaboration on the Life course Approach to reproductive health and Chronic disease Events (InterLACE) recently reported that the average age at menarche across 20 studies ranged from 12.5 to 13.6 years, and the pooled analyses showed a significant increase for earlier age at menarche for women born more recently and substantial variations across racial/ethnic groups.1 There are known biological links between childhood obesity and earlier onset of puberty that is often defined by age at menarche in girls.2 Increasing prevalence of childhood obesity may be contributing to the decline in age at menarche.
Women with early menarche are at higher risk of premature and early menopause,3 but the association with menopausal symptoms is unclear. Vasomotor menopausal symptoms (VMS), including hot flushes and night sweats, are the most bothersome symptoms during the menopausal transition.4 Race/ethnicity, low socioeconomic position, obesity, and smoking are key risk factors for VMS.5 For instance, the rate of reporting VMS was higher among African American but lower among Japanese and Chinese compared with Caucasian women.6 Reproductive history, such as age at menarche, has also been examined for links with VMS; however, the results have been conflicting. The Midlife Women’s Health Study found that menarche at ≤10 years was associated with higher odds of hot flushes compared with menarche at 11–12 years, though this association was attenuated after adjusting for covariates.7 In contrast, two studies found that women with earlier menarche were less likely to experience hot flushes.8,9 A few studies have reported no associations.10–14
Early menarche15–17 and VMS18–20 have been associated with an increased risk of cardiovascular diseases, type 2 diabetes, and breast cancer in later life. Adult obesity may be part of the mechanism linking early menarche with these disease risks.21 As obesity is also a key risk factor for VMS, it may play an important role in the association between age at menarche and VMS. Therefore, this study pooled data from over 18,000 women to examine the association between age at menarche and risk of frequent/severe VMS and to investigate whether body mass index (BMI) modified the association.
METHODS
Study populations
InterLACE consortium has pooled individual-level data on women’s health from over 20 observational studies. More detailed descriptions of the InterLACE collaboration and the harmonisation protocols have been published previously.22,23 The InterLACE project is funded by the Australian National Health and Medical Research Council project grant (APP1027196). The present study used data from six studies that collected information on age at menarche and frequency or severity of VMS: Australian Longitudinal Study on Women’s Health (ALSWH), Healthy Ageing of Women Study (HOW), MRC National Survey of Health and Development (NSHD), National Child Development Study (NCDS), Study of Women’s Health Across the Nation (SWAN), and Seattle Midlife Women’s Health Study (SMWHS). Patient involvement is not relevant to this study.
The SWAN and SMWHS had different recruitment criteria regarding menopausal stage. SWAN recruited pre-/perimenopausal women (at least one menstrual period in the previous three months), who were not taking hormones and who had not undergone surgical removal of the uterus and/or both ovaries, with a median age of 46 years at baseline. SMWHS also recruited women without surgical removal of uterus or ovaries, but in their early 40s (median age 41 years), which was too young to experience VMS. Thus, we used the follow-up data collected at around age 50 years as our analytic baseline. The two birth cohorts (NSHD and NCDS) first collected information on women’s health at age 47 and 50 years respectively, so data collected at midlife were used. For ALSWH, data from the baseline survey were used in the analyses (median age of 48 years), while HOW recruited women at age 55 years. A total of 24,596 women were included at analytic baseline. Women who had missing data on age at menarche (n=4,001), frequency/severity of VMS (n=207), BMI (n=1,089), and other covariates (listed below; n=744) were excluded, leaving 18,555 women for the complete case analyses (Table 1). Women excluded from the analyses were more likely to report frequent/severe VMS and more likely to be a current smoker, less educated, postmenopausal, and having had a hysterectomy/oophorectomy, compared with included women.
Table 1.
Characteristics of the six studies in the InterLACE consortium whose data were used for this study
| Study | Country | N | Age at analytical baseline Median (Q1, Q3) | Age at menarche |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | ≤11 years n (%) | 12 years n (%) | 13 years n (%) | ≥14 years n (%) | ||||
| Australian Longitudinal Study on Women’s Health (ALSWH) | Australia | 10,523 | 48 (46, 49) | 12.9 (1.6) | 1976 (18.8) | 2274 (21.6) | 2944 (28.0) | 3229 (31.6) |
| Healthy Ageing of Women Study (HOW) | Australia | 462 | 55 (53, 57) | 13.0 (1.6) | 91 (19.7) | 74 (16.0) | 128 (27.7) | 169 (36.6) |
| MRC Survey of Health and Development (NSHD) | UK | 1,041 | 47a | 12.7 (1.2) | 177 (17.0) | 296 (28.4) | 358 (34.4) | 210 (20.2) |
| National Child Development Study (NCDS) | UK | 3,261 | 50a | 12.7 (1.2) | 532 (16.3) | 808 (24.8) | 1080 (33.1) | 841 (25.8) |
| Study of Women’s Health Across the Nation (SWAN) | USA | 3,079 | 46 (44, 48) | 12.5 (1.6) | 737 (23.9) | 810 (26.3) | 832 (27.0) | 700 (22.7) |
| Seattle Midlife Women’s Health Study (SMWHS) | USA | 189 | 50 (46, 53)b | 12.6 (1.4) | 32 (16.9) | 60 (31.7) | 60 (31.7) | 37 (19.6) |
| Overall | 18,555 | 48 (46, 50) | 12.8 (1.5) | 3545 (19.1) | 4322 (23.3) | 5402 (29.1) | 5286 (28.5) | |
Abbreviation: Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
NSHD (1946 British Birth Cohort) and NCDS (1958 British Birth Cohort) first collected information on women’s health in 1993 (aged 47) and 2008 (aged 50), respectively, so we used age 47 and 50 years as the baseline age for the analyses.
SMWHS first recruited women at early 40s (median age 41 years, interquartile range: 38–44), which was too young to experience menopausal symptoms. Thus, we used the data collected at the follow-up survey around 50 years.
Main outcome and exposure variables
In each study, the frequency or severity of hot flushes and night sweats were collected from the menopausal symptom checklists. For example, women in ALSWH were asked how frequently they have had the symptoms in the last 12 months (response categories: never, rarely, sometimes, and often). SWAN asked the frequency in the last 2 weeks (not at all, 1–5 days, 6–8 days, 9–13 days, and every day); we categorised 9–13 days and every day as often, 6–8 days as sometimes, and 1–5 days as rarely. However, women in NSHD and NCDS were asked how severely they have been bothered by the symptoms in the last 12 months. SMWHS and HOW also asked the severity of symptoms but over much shorter periods (in the last 24 hours and at the moment, respectively). The severity response categories were generally similar across studies as not at all, a little, quite a bit, and quite a lot/extremely. In our analyses, the frequency data were harmonised from ALSWH and SWAN and categorised as never, rarely, sometimes, and often (n=13,602); the severity data were harmonised from the other four studies and categorised as never, mild, moderate, and severe (n=4,953).
Age at menarche was collected retrospectively in midlife (except the two birth cohorts) ranged 8–20 years, and was categorised as ≤11 (defined as early menarche3), 12, 13, and ≥14 years.
BMI and other covariates
Weight and height were collected at analytical baseline. BMI was calculated as weight divided by height squared (kg/m2) and was categorised as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30). As only 1.5% of women were underweight, they were combined into the normal weight group (<25 kg/m2). Other covariates included: birth year (1940–49 and 1950–59), race/ethnicity/region (Caucasian-Australian, Caucasian-European, Caucasian-American, Asian, African American/Black, and Other), education level (≤10, 11–12, and >12 years), and smoking status (never, past, and current smoker). Menopausal status at the time of reporting VMS was categorised as unknown due to surgery (hysterectomy/oophorectomy), unknown due to hormone use (before the menopause), premenopause (regular menstrual cycles in the last 3 and 12 months), perimenopause (menses in the last 3 months and changes/irregularity in menstrual patterns in the last 12 months; or no menses in the past 3 months but menses in the preceding 11 months), and postmenopause (amenorrhea for at least 12 months). Menopausal hormone therapy (MHT) was categorised as current and non-current user. Certain medications or conditions, such as type 2 diabetes, are linked with VMS like symptoms or worse symptoms. Physician-diagnosed type 2 diabetes reported before or at analytic baseline was defined as a diabetes case.
Statistical analyses
We performed multinominal logistic regression models to examine the association of age at menarche with four categories of each outcome for the frequency and severity of VMS, which estimated the probabilities relative to the reference group. The exponentiated coefficients from multinomial logistic regression provided an estimate of relative risk ratios (RRRs) and 95% confident intervals (CIs).24 No VMS was the referent for the outcome and menarche at ≥14 years as the referent for the exposure. We also treated age at menarche as a continuous variable in the analyses. We first conducted study-specific analyses in studies with sufficient data, and then performed pooled analyses separately for the frequency (n=13,602) and severity (n=4,953) of VMS.
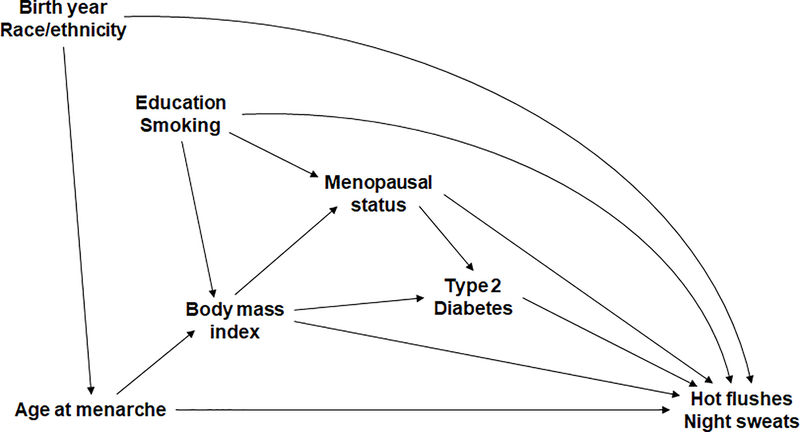
Based on the directed acyclic graph (DAG; Figure 1), birth year and race/ethnicity were considered as confounders that influenced both the exposure and outcome. Other factors that were a priori believed to be associated with VMS were also included in the DAG, including BMI, education, smoking status, menopausal status, and type 2 diabetes. BMI was considered as the key intermediate variable on the causal path from exposure to outcome. Thus, the main results were only adjusted for birth year and race/ethnicity/region (Model 1) and then adjusted for BMI (Model 2) before examining effect modifications. Other factors were adjusted for in the sensitivity analyses described below.
Figure 1.
Directed acyclic graph based on the association between age at menarche, body mass index, and risk of hot flushes and night sweats
To investigate whether BMI modified the association between age at menarche and risk of VMS, we assessed the effect of statistical interaction between age at menarche and BMI, and also estimated their joint effects. A new variable with the combination of age at menarche (≤11, 12, 13, and ≥14 years) and BMI (normal weight, overweight, and obese) was created. The joint associations were adjusted for confounders (Model 1).
Multiple sensitivity analyses were conducted to examine the robustness of the results. First, we adjusted for confounders listed in Model 1 plus factors that only affected VMS, including education level, smoking status, menopausal status, MHT use, type 2 diabetes (Model 3), as well as BMI (Model 4), which produced results comparable with previous studies that adjusted for these factors. However, this may lead to over-adjustment and makes the findings difficult to interpret. To better understand the influence of menopausal stage, we further stratified the analyses by women who had hysterectomy/oophorectomy, natural menopause, and those who were pre-/perimenopausal. Second, this cohort was relatively early in the menopausal transition (median age of 48 years), and the women may not yet have experienced VMS. Thus, we conducted a sensitivity analysis using only women with VMS and used the lowest category of frequency/severity as referent, rather than compared with those without VMS. Third, to minimise the influence of concurrent hot flushes and night sweats, we examined the association with hot flushes alone (any hot flushes but never or rarely/mild night sweats), night sweats alone (any night sweats but never or rarely/mild hot flushes), and both symptoms (same degree of both symptoms, i.e., both never or both often/severe). The Glimmix procedure in SAS 9.4 was used for multinomial logistic regression, with the study ID included as a random effect.
RESULTS
Participant characteristics
A total of 18,555 women with a median age of 48 years (interquartile range 46–50 years) were included in this study (Table 1). The mean age at menarche was 12.8 years (SD 1.5), with 19.1% of the women experiencing early menarche ≤11 years. Across studies, over 60% of women were premenopausal (34%) or perimenopausal (28%), and 8% were postmenopausal; 17% had unknown menopausal status due to surgery and 12% due to hormone use (Table S1). Half of the women were either overweight (29%) or obese (21%), 18% were current smokers, and 3% had type 2 diabetes. There were significant differences in mean age at menarche across birth year and racial/ethnic/regional groups (Table 2). All participant characteristics also showed significant differences across the hot flushes groups.
Table 2.
Participant characteristics according to women’s age at menarche (N=18,555)
| Age at menarche |
|||||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | ≤11 years n (%) | 12 years n (%) | 13 years n (%) | ≥14 years n (%) | p-value | |
| Birth year | <0.0001 | ||||||
| 1940–1949 | 10585 | 12.8 (1.5) | 2030 (19.2) | 2396 (22.6) | 2993 (28.3) | 3166 (29.9) | |
| 1950–1959 | 7970 | 12.7 (1.5) | 1515 (19.0) | 1926 (24.2) | 2409 (30.2) | 2120 (26.6) | |
| Race/ethnicity/region | <0.0001 | ||||||
| Caucasian- Australian | 8762 | 12.9 (1.6) | 1636 (18.7) | 1880 (21.5) | 2474 (28.2) | 2772 (31.6) | |
| Caucasian- European | 6059 | 12.8 (1.3) | 1055 (17.4) | 1452 (24.0) | 1905 (31.4) | 1647 (27.2) | |
| Caucasian- American | 169 | 12.5 (1.5) | 376 (22.2) | 482 (28.5) | 496 (29.3) | 340 (20.1) | |
| Asian | 787 | 12.8 (1.6) | 136 (17.3) | 211 (26.8) | 229 (29.1) | 211 (26.8) | |
| African American/Black | 861 | 12.5 (1.9) | 245 (28.5) | 212 (24.6) | 207 (24.0) | 197 (22.9) | |
| Other | 392 | 12.7 (1.7) | 97 (24.7) | 85 (21.7) | 91 (23.2) | 119 (30.4) | |
Abbreviation: SD, standard deviation.
Age at menarche and risk of frequent and severe VMS
Overall, almost 50% of the women experienced some degree of hot flushes, and nearly 40% experienced night sweats. Four studies (ALSWH, SWAN, NSHD, and NCDS) had sufficient data to conduct the study-specific analyses (Table S2). There was evidence suggesting that women with earlier age at menarche were more likely to experience ‘often’ or ‘severe’ VMS, but the strength of the effect varied across studies and the confidence intervals were wide due to small sample size.
We then performed pooled analyses separately for the frequency and severity of VMS. The frequency data showed that early menarche was associated with increasing frequency of hot flushes compared with menarche at ≥14 years, with adjusted relative risk ratios (RRRs; 95%CI) of 1.20 (1.05–1.37), 1.15 (1.01–1.32), and 1.48 (1.24–1.76) for ‘rarely’, ‘sometimes’, and ‘often’ hot flushes, respectively (Table 3; Model 1). Similar results were seen for the frequency of night sweats, with adjusted RRRs (95%CI) of 1.06 (0.89–1.26), 1.10 (1.06–1.15), and 1.59 (1.49–1.70), respectively. Earlier age at menarche was also associated with higher risk of ‘often’ hot flushes and night sweats, with adjusted RRRs of 1.10 (1.09–1.10) and 1.12 (1.10–1.13) per 1-year decrease in age at menarche, respectively. After adjustment for BMI, the association remained, but the effect of early menarche was attenuated considerably (Model 2). Severity data showed similar results, but appeared less conclusive as the effect estimates were smaller and with wider confidence intervals. Early menarche was associated with ‘severe’ hot flushes (1.16, 0.94–1.42) and night sweats (1.27, 1.01–1.58) (Model 1).
Table 3.
Association between age at menarche and risk of hot flushes and night sweats
|
Hot flushes n (%) |
Hot flushes Model 1: RRR (95% CI)a |
Hot flushes
Model 2: RRR (95% CI)b |
|||||||||
| Age at menarche | n | Never | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe |
| Frequency (ALSWH + SWAN) | 13602 | 7693 (56.6) | 2273 (16.7) | 2433 (17.9) | 1203 (8.8) | ||||||
| ≤11 years | 2713 | 1455 (53.6) | 497 (18.3) | 489 (18.0) | 272 (10.0) | 1.20 (1.05–1.37) | 1.15 (1.01–1.32) | 1.48 (1.24–1.76) | 1.12 (1.10–1.13) | 1.02 (0.98–1.07) | 1.30 (1.25–1.35) |
| 12 years | 3084 | 1795 (58.2) | 497 (16.1) | 503 (16.3) | 289 (9.4) | 0.98 (0.86–1.12) | 0.95 (0.84–1.09) | 1.28 (1.07–1.52) | 0.95 (0.93–0.97) | 0.90 (0.84–0.96) | 1.20 (1.14–1.26) |
| 13 years | 3776 | 2166 (57.4) | 619 (16.4) | 671 (17.8) | 320 (8.5) | 1.01 (0.89–1.14) | 0.99 (0.87–1.12) | 1.12 (0.95–1.32) | 0.99 (0.91–1.08) | 0.96 (0.92–1.00) | 1.08 (1.07–1.09) |
| ≥14 years | 4029 | 2277 (56.5) | 660 (16.4) | 770 (19.1) | 322 (8.0) | Ref | Ref | Ref | Ref | Ref | Ref |
| Per 1-year decrease | 1.03 (1.02–1.05) | 1.03 (1.01–1.05) | 1.10 (1.09–1.10) | 1.02 (1.00–1.03) | 1.00 (0.99–1.02) | 1.07 (1.07–1.07) | |||||
| Severity (NSHD + NCDS + HOW + SMWHS) | 4953 | 2182 (44.1) | 503 (10.2) | 1534 (31.0) | 734 (14.8) | ||||||
| ≤11 years | 832 | 355 (42.7) | 90 (10.8) | 270 (32.5) | 117 (14.1) | 1.18 (0.89–1.56) | 1.18 (0.94–1.47) | 1.16 (0.94–1.42) | 1.17 (0.90–1.52) | 1.13 (0.91–1.40) | 1.07 (0.87–1.31) |
| 12 years | 1238 | 542 (43.8) | 124 (10.0) | 376 (30.4) | 196 (15.8) | 1.16 (0.79–1.70) | 1.05 (0.97–1.14) | 1.26 (1.18–1.34) | 1.16 (0.77–1.73) | 1.03 (0.94–1.13) | 1.20 (1.13–1.28) |
| 13 years | 1626 | 715 (44.0) | 156 (9.6) | 505 (31.1) | 250 (15.4) | 1.06 (0.82–1.37) | 1.06 (0.98–1.14) | 1.19 (1.11–1.28) | 1.06 (0.81–1.38) | 1.04 (0.96–1.13) | 1.16 (1.08–1.25) |
| ≥14 years | 1257 | 570 (45.4) | 113 (10.6) | 383 (30.5) | 171 (13.6) | Ref | Ref | Ref | Ref | Ref | Ref |
| Per 1-year decrease | 1.04 (1.00–1.07) | 1.04 (0.99–1.10) | 1.05 (1.02–1.09) | 1.03 (1.01–1.06) | 1.03 (0.98–1.08) | 1.03 (1.00–1.07) | |||||
|
Night sweats n (%) |
Night sweats Model 1: RRR (95% CI)a |
Night sweats
Model 2: RRR (95% CI)b |
|||||||||
| Age at menarche | n | Never | Rarely/Mild | Sometimes/Moderate | Often /Severe | Rarely/Mild | Sometimes/Moderate | Often /Severe | Rarely/Mild | Sometimes/Moderate | Often/Severe |
| Frequency (ALSWH + SWAN) | 13602 | 8624 (63.4) | 2231 (16.4) | 1830 (13.4) | 917 (6.7) | ||||||
| ≤11 years | 2713 | 1659 (61.2) | 462 (17.0) | 367 (13.5) | 225 (8.3) | 1.06 (0.89–1.26) | 1.10 (1.06–1.15) | 1.59 (1.49–1.70) | 1.03 (0.86–1.23) | 1.03 (0.99–1.07) | 1.46 (1.39–1.54) |
| 12 years | 3084 | 1971 (63.9) | 497 (16.1) | 407 (13.2) | 209 (6.8) | 0.98 (0.97–0.99) | 1.03 (0.97–1.09) | 1.26 (1.19–1.34) | 0.97 (0.96–0.98) | 1.00 (0.94–1.06) | 1.22 (1.14–1.30) |
| 13 years | 3776 | 2426 (64.3) | 624 (16.5) | 478 (12.7) | 248 (6.6) | 1.02 (0.94–1.10) | 0.93 (0.85–1.02) | 1.17 (1.16–1.18) | 1.01 (0.94–1.10) | 0.92 (0.84–1.00) | 1.15 (1.14–1.16) |
| ≥14 years | 4029 | 2568 (63.7) | 648 (16.1) | 578 (14.4) | 235 (5.8) | Ref | Ref | Ref | Ref | Ref | Ref |
| Per 1-year decrease | 1.02 (1.00–1.04) | 1.02 (1.02–1.03) | 1.12 (1.10–1.13) | 1.01 (0.98–1.04) | 1.01 (0.97–1.04) | 1.09 (1.05–1.14) | |||||
| Severity (NSHD + NCDS + HOW + SMWHS) | 4953 | 2743 (55.4) | 397 (8.0) | 1276 (25.8) | 537 (10.8) | ||||||
| ≤11 years | 832 | 464 (55.8) | 58 (7.0) | 218 (26.2) | 92 (11.1) | 0.86 (0.67–1.11) | 1.10 (1.01–1.20) | 1.27 (1.01–1.58) | 0.88 (0.68–1.14) | 1.13 (1.03–1.24) | 1.27 (1.03–1.56) |
| 12 years | 1238 | 662 (53.5) | 102 (8.2) | 320 (25.9) | 154 (12.4) | 1.22 (1.01–1.47) | 1.12 (0.89–1.40) | 1.47 (1.27–1.71) | 1.24 (1.03–1.49) | 1.14 (0.91–1.43) | 1.47 (1.28–1.69) |
| 13 years | 1626 | 905 (55.7) | 124 (7.6) | 423 (26.0) | 174 (10.7) | 1.02 (0.84–1.24) | 1.06 (1.01–1.12) | 1.19 (1.15–1.23) | 1.03 (0.84–1.25) | 1.07 (1.02–1.13) | 1.19 (1.15–1.23) |
| ≥14 years | 1257 | 712 (56.6) | 113 (9.0) | 315 (25.1) | 117 (9.3) | Ref | Ref | Ref | Ref | Ref | Ref |
| Per 1-year decrease | 0.99 (0.94–1.03) | 1.04 (1.00–1.08) | 1.10 (1.05–1.16) | 0.99 (0.95–1.04) | 1.04 (1.01–1.09) | 1.10 (1.05–1.15) | |||||
Data are presented as n (%) or relative risk ratio (RRR) and 95% confidence intervals (95% CI) using generalised linear mix models with multinomial distribution and generalised logit link. The Glimmix procedure in SAS was used to incorporate study as a random effect into the analyses. No symptoms was used as the reference group for the outcome.
Model 1 was adjusted for birth year and race/ethnicity/region.
Model 2 was additionally adjusted for body mass index.
Joint effect of age at menarche and BMI
Women with early menarche had a much higher prevalence of obesity in midlife than those with menarche at ≥14 years (29.4% vs 19.6%). There was a significant interaction between age at menarche and BMI on the risk of VMS (p<0.0001), indicating potential effect modification by BMI. The frequency data showed that the association of early menarche with ‘often’ hot flushes was stronger among women who were overweight (2.36, 2.17–2.57) or obese (2.87, 2.79–2.95) than those of normal-weight (1.10, 0.95–1.28) (Table 4). Similarly, the risk associated with early menarche and ‘often’ night sweats was higher among women who were overweight (2.04, 1.80–2.30) or obese (2.42, 2.36–2.47) than women with normal-weight (1.24, 1.20–1.28). The effect modification by BMI was more apparent for hot flushes than night sweats (graphically shown in Figure S1). However, this gradient across BMI categories was not as strong for the association of early menarche and the risk of ‘severe’ VMS, especially for night sweats.
Table 4.
Joint association between age at menarche and midlife BMI with risk of hot flushes and night sweats
|
Frequency of hot flushes (n=13,602)b
Model 1: RRR (95% CI)c |
Severity of hot flushes (n=4,953)b
Model 1: RRR (95% CI)c |
|||||||||
| Age at menarche & midlife BMIa | n | Never | Rarely | Sometimes | Often | n | Never | Mild | Moderate | Severe |
| ≤11 years & normal weight | 991 | Ref | 1.03 (0.91–1.16) | 0.96 (0.93–0.99) | 1.10 (0.95–1.28) | 289 | Ref | 1.56 (0.94–2.59) | 1.19 (0.97–1.46) | 1.09 (0.79–1.51) |
| 12 years & normal weight | 1470 | Ref | 0.87 (0.75–1.01) | 0.96 (0.95–0.97) | 1.29 (1.24–1.34) | 557 | Ref | 1.06 (0.71–1.59) | 0.92 (0.86–0.98) | 1.21 (1.16–1.27) |
| 13 years & normal weight | 2009 | Ref | 0.95 (0.84–1.07) | 0.96 (0.90–1.03) | 1.22 (1.12–1.32) | 800 | Ref | 1.23 (0.94–1.61) | 1.02 (0.93–1.13) | 1.37 (1.02–1.84) |
| ≥14 years & normal weight | 2358 | Ref | Ref | Ref | Ref | 700 | Ref | Ref | Ref | Ref |
| ≤11 years & overweight | 814 | Ref | 1.31 (1.17–1.47) | 1.32 (1.31–1.33) | 2.36 (2.17–2.57) | 285 | Ref | 1.46 (0.92–2.32) | 1.13 (0.79–1.62) | 1.61 (1.25–2.07) |
| 12 years & overweight | 916 | Ref | 1.13 (1.12–1.13) | 1.26 (1.22–1.30) | 1.78 (1.56–2.03) | 394 | Ref | 1.42 (1.01–2.01) | 1.22 (1.17–1.28) | 1.72 (1.45–2.04) |
| 13 years & overweight | 1045 | Ref | 1.12 (1.06–1.18) | 1.53 (1.48–1.59) | 1.72 (1.67–1.76) | 507 | Ref | 1.31 (1.11–1.55) | 1.16 (0.99–1.35) | 1.40 (1.20–1.64) |
| ≥14 years & overweight | 1070 | Ref | 1.11 (0.99–1.26) | 1.48 (1.39–1.59) | 1.76 (1.63–1.89) | 380 | Ref | 1.37 (0.99–1.90) | 1.13 (0.94–1.37) | 1.37 (1.12–1.67) |
| ≤11 years & obese | 908 | Ref | 1.66 (1.51–1.82) | 2.39 (2.25–2.54) | 2.87 (2.79–2.95) | 258 | Ref | 0.95 (0.75–1.22) | 1.37 (1.02–1.82) | 1.55 (1.03–2.31) |
| 12 years & obese | 698 | Ref | 1.41 (1.23–1.61) | 1.64 (1.42–1.89) | 2.61 (2.07–3.28) | 287 | Ref | 1.75 (1.56–1.98) | 1.33 (1.11–1.58) | 2.02 (1.46–2.80) |
| 13 years & obese | 722 | Ref | 1.44 (1.35–1.54) | 1.62 (1.60–1.65) | 1.93 (1.66–2.23) | 319 | Ref | 0.95 (0.82–1.11) | 1.22 (1.15–1.29) | 1.74 (1.63–1.86) |
| ≥14 years & obese | 601 | Ref | 1.29 (1.25–1.33) | 1.90 (1.87–1.93) | 1.96 (1.89–2.03) | 177 | Ref | 1.18 (0.65–2.15) | 1.03 (0.92–1.15) | 1.86 (1.64–2.12) |
|
Frequency of night sweats (n=13,602)bModel 1: RRR (95% CI)c |
Severity of night sweats (n=4,953)bModel 1: RRR (95% CI)c |
|||||||||
| Age at menarche & midlife BMIa | n | Never | Rarely | Sometimes | Often | n | Never | Mild | Moderate | Severe |
| ≤11 years & normal weight | 991 | Ref | 0.91 (0.60–1.36) | 1.14 (1.01–1.30) | 1.24 (1.20–1.28) | 289 | Ref | 0.93 (0.48–1.79) | 0.94 (0.81–1.10) | 1.44 (1.16–1.79) |
| 12 years & normal weight | 1470 | Ref | 0.87 (0.76–0.98) | 1.10 (1.08–1.11) | 1.30 (1.15–1.49) | 557 | Ref | 1.10 (0.80–1.53) | 0.99 (0.69–1.43) | 1.66 (1.35–2.04) |
| 13 years & normal weight | 2009 | Ref | 1.03 (1.02–1.05) | 0.93 (0.88–0.98) | 1.13 (1.07–1.20) | 800 | Ref | 1.20 (0.71–2.04) | 1.02 (0.85–1.23) | 1.55 (1.24–1.94) |
| ≥14 years & normal weight | 2358 | Ref | Ref | Ref | Ref | 700 | Ref | Ref | Ref | Ref |
| ≤11 years & overweight | 814 | Ref | 0.92 (0.74–1.14) | 1.17 (1.15–1.18) | 2.04 (1.80–2.30) | 285 | Ref | 1.24 (0.65–2.38) | 1.18 (0.91–1.53) | 1.56 (1.37–1.78) |
| 12 years & overweight | 916 | Ref | 1.02 (0.94–1.10) | 1.30 (1.29–1.32) | 1.50 (1.46–1.53) | 394 | Ref | 1.62 (1.52–1.72) | 1.01 (0.73–1.42) | 2.12 (1.54–2.92) |
| 13 years & overweight | 1045 | Ref | 0.91 (0.70–1.19) | 1.27 (1.19–1.36) | 1.54 (1.53–1.55) | 507 | Ref | 0.96 (0.91–1.02) | 0.83 (0.74–0.93) | 1.14 (1.04–1.26) |
| ≥14 years & overweight | 1070 | Ref | 0.95 (0.91–0.99) | 1.26 (1.23–1.30) | 1.29 (1.21–1.38) | 380 | Ref | 1.32 (0.98–1.77) | 0.78 (0.57–1.08) | 1.47 (1.03–2.10) |
| ≤11 years & obese | 908 | Ref | 1.33 (1.10–1.60) | 1.63 (1.51–1.75) | 2.42 (2.36–2.47) | 258 | Ref | 0.61 (0.23–1.62) | 0.83 (0.67–1.03) | 1.46 (0.77–2.78) |
| 12 years & obese | 698 | Ref | 1.11 (1.01–1.22) | 1.32 (1.12–1.55) | 1.76 (1.57–1.97) | 287 | Ref | 1.27 (0.88–1.84) | 0.98 (0.73–1.32) | 1.37 (1.03–1.83) |
| 13 years & obese | 722 | Ref | 1.04 (0.80–1.36) | 1.31 (1.27–1.35) | 1.72 (1.47–2.03) | 319 | Ref | 1.03 (0.75–1.42) | 0.96 (0.92–1.00) | 1.50 (1.45–1.55) |
| ≥14 years & obese | 601 | Ref | 0.98 (0.77–1.24) | 1.77 (1.51–2.06) | 1.53 (1.33–1.76) | 177 | Ref | 0.78 (0.51–1.20) | 0.70 (0.65–0.76) | 1.22 (1.18–1.26) |
Data are presented as relative risk ratio (RRR) and 95% confidence intervals (95% CI) using generalised linear mix models with multinomial distribution and generalised logit link. The Glimmix procedure in SAS was used to incorporate study as a random effect into the analyses.
normal weight: BMI <25 kg/m2; overweight: BMI 25–29.9 kg/m2; obese: BMI ≥30 kg/m2.
Frequency data were collected from ALSWH and SWAN (n=13,602), while severity data were collected from NSHD, NCDS, HOW, and SMWHS (n=4,953).
Model 1 was adjusted for birth year and race/ethnicity/region.
Sensitivity analyses
For the frequency of VMS, the association with early menarche was robust in multiple sensitivity analyses. The increased risk of frequent VMS was attenuated slightly after adjusting for factors that significantly affected VMS, including menopausal status (Table S3). The association remained even after adjusting for BMI. Similar results were obtained in the analyses restricted to women who had undergone a hysterectomy/oophorectomy, and for those who were postmenopausal and even those who were pre-/perimenopausal (Table S4). When we excluded women without VMS and compared the risk with the lowest category of frequency, the association with increased risk of ‘often’ VMS remained (Table S5). Furthermore, hot flushes and night sweats were moderately correlated (Kendall’s Tau-b=0.61, 95%CI 0.60–0.62) (Table S6). A sensitivity analysis, in which the association was examined with hot flushes alone, night sweats alone, and both symptoms, yielded similar findings (Table S7). Women with early menarche had a higher risk of often experiencing both symptoms (1.71, 1.48–1.96) than hot flushes alone (1.28, 1.23–1.33) or night sweats alone (1.31, 0.82–2.11). However, for the severity of VMS, the results were less robust as the effect sizes were smaller, and some estimates were not statistically significant.
DISCUSSION
Main findings
To our knowledge, this is the first pooled study to provide robust evidence for the association between earlier age at menarche and higher risk of frequent VMS, but the association with increased risk of severe VMS was less conclusive. Women with early menarche had an increased risk of frequent hot flushes and night sweats compared with those with menarche at ≥14 years, and the risk was higher for experiencing both symptoms than hot flushes alone or night sweats alone. However, midlife BMI modified the association. The increased risk of frequent VMS associated with early menarche was greater among women who were overweight or obese than those who were of normal-weight.
Strengths and limitations
The large sample size of the pooled data ensured adequate power for more precise estimates and sufficient variation in the distributions of age at menarche and VMS to examine the graded associations. However, some limitations need to be considered in interpreting the findings. First, the main limitation was the different assessments of VMS across studies. As frequency and severity measure distinct concepts, we performed all the analyses separately for the frequency and severity of VMS. Future studies will benefit from the COMMA initiative (Core Outcome set in Menopause) currently under development that aims to achieve consensus on standardised measures for menopause, including VMS. Second, previous research has found a negative association between BMI and VMS in late menopause.25 In the stratification analysis by menopausal status, we found that the association between early menarche and frequent VMS remained in all menopausal stages, but the association with severe VMS was not apparent in postmenopausal women, including those who had undergone a hysterectomy/oophorectomy. Further studies are needed to confirm these findings for postmenopausal women. Third, we found variability in the strength of the associations across studies, though the effect estimates were consistently in the same direction, partly due to differences in study designs and distributions of menopausal status.26 Fourth, most of the women reported their age at menarche retrospectively (except the two birth cohorts), which may have led to some degree of recall bias. The NSHD had information on age at menarche reported prospectively and retrospectively and found that validity was improved by categorising the age as early (≤11 years), normal (12–13 years), and late (≥14 years),27 which is the same categorisation used in this study. Fifth, women who were excluded from the analyses due to missing data were more likely to report frequent/severe VMS compared with those who were included, which may have led to an underestimation of VMS. However, as there was sufficient variation in the distributions of age at menarche and VMS, we do not expect the relationship observed to change substantively. Last, we were unable to distinguish between the potential confounding and mediating effect of body size, due to lack of repeated measures of body size before the onset of puberty and in adulthood in most studies. The relationship between early menarche and body size is likely to be bi-directional.28 Rapid growth in early childhood precedes a faster tempo of childhood maturation and early puberty onset,29 but in turn early onset of puberty can lead to subsequent rapid weight gain during adolescence and early adulthood as well as shorter adult stature.2
Interpretation
This study confirms earlier findings from the Midlife Women’s Health Study that suggested women with early menarche ≤10 years were more likely to report a history of hot flushes and to experience current hot flushes compared with those with menarche at 11–12 years, but this association was largely attenuated after adjusting for covariates including BMI and menopausal status.7 A similar pattern of increasing odds of hot flushes with declining age at menarche was also identified. However, two studies found that earlier menarche was associated with decreased prevalence of hot flushes (<12 vs ≥12 years) or reduced odds of hot flushes (<13 vs ≥13 years),8,9 and other studies reported no association.10–14 To date, only ALSWH has examined the association with night sweats, in which ‘sometimes’ and ‘often’ categories were combined, but did not find an association.10 There are a number of possible reasons why the results have been inconsistent, including that study participants had different racial, cultural, and socioeconomic backgrounds and that the measure of VMS varied from a single question to a more detailed history. Most studies dichotomised the response categories for frequency or severity of VMS to have sufficient numbers of women within each symptom category (e.g., combined moderate and severe as presence), with only one study examining the graded association.13 Another potential reason is that the studies used different cut-off ages for early menarche or treated age at menarche as a continuous variable.13,14
This study adds to the body of knowledge on menopausal symptoms in which midlife BMI was taken into consideration, allowing for a more thorough understanding of the role of body size in the relationship between early menarche and VMS. We found the association between early menarche and VMS was stronger among women with overweight or obese BMI. Previously, the InterLACE study showed that the association between early menarche and incident type 2 diabetes was only observed in women who were overweight or obese.15 These findings suggest that being overweight or obese exacerbates the risk associated with early menarche. On the other hand, it may point to a cumulative effect on adverse health outcomes of exposure to excess body weight in childhood.
Obesity is associated with increased heat production, and adipose tissue provides an insulating barrier that impedes heat loss, which during the menopausal transition reduces the ability to respond to changes in core temperature (thermoregulatory dysfunction) and results in VMS,30,31 particularly for hot flushes.32 The North American Menopause Society recommends that weight loss may be beneficial for alleviating VMS, but additional studies to confirm the effect are warranted.33 Early monitoring of women with early menarche and encouraging them to maintain healthy weight in adult life may have important implications for the prevention of VMS and other adverse outcomes.
There might be other non-adiposity-related mechanisms linking early menarche and VMS, such as hormone imbalance31 and psychological problems.34 Some oestrogen-related polymorphisms have been associated with age at menarche, age at menopause, and timing of onset of menopausal transition.35 These polymorphisms involved in oestrogen synthesis and metabolism may also influence symptoms during the menopausal transition.36 Further research is needed to investigate possible biological, genetic, and psychosocial mechanisms.
CONCLUSION
This pooled study provided robust evidence for early menarche as a risk factor for VMS, but midlife BMI may play an important role in modifying this risk. Early age at menarche has been linked with many adverse health outcomes in later life and therefore represents a potential target for early preventive interventions, especially weight management in adulthood.
Supplementary Material
ACKNOWLEDGEMENTS
The data on which this research is based were drawn from eight observational studies. The research included data from the Australian Longitudinal Study on Women’s Health (ALSWH), the University of Newcastle, Australia, and the University of Queensland, Australia. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. Healthy Ageing of Women Study (HOW) was supported by the Queensland University of Technology Early Career Research Grant and the JSPS Grant-in-aid for Scientific Research. MRC National Survey of Health Development (NSHD) has core funding from the UK Medical Research Council (MC UU 12019/1). National Child Development Study (NCDS) is funded by the UK Economic and Social Research Council. Seattle Midlife Women’s Health Study (SMWHS) was supported in part by grants from the National Institute of Nursing Research, P50-NU02323, P30-NR04001, and R01-NR0414.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994 – 2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016 – present; Winifred Rossi 2012 – 2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 – present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA-Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
All studies would like to thank the participants for volunteering their time to be involved in the respective studies. The findings and views in this paper are not necessarily those of the original studies or their respective funding agencies.
FUNDING
InterLACE project is funded by the Australian National Health and Medical Research Council project grant (APP1027196). GDM is supported by the Australian National Health and Medical Research Council Principal Research Fellowship (APP1121844).
DISCLOSURE OF INTEREST
EBG reports grants from the National Institute on Aging during the conduct of the study; SLC reports grants from the National Institutes of Health during the conduct of the study; NEA reports grants from the National Institute on Aging during the conduct of the study and grants from the National Institutes of Health outside the submitted work. All other authors report no conflicts of interest. Completed disclosure of interest forms are available to view online as supporting information.
Footnotes
DETAILS OF ETHICS APPROVAL
Each study in the InterLACE has been undertaken with ethical approval from the Institutional Review Board or Human Research Ethics Committee at each participating institution, and all participants provided consent for that study.
REFERENCES
- 1.InterLACE Study Team. Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum Reprod 2019; 34: 881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab 2009; 20: 237–42. [DOI] [PubMed] [Google Scholar]
- 3.Mishra GD, Pandeya N, Dobson AJ, Chung HF, Anderson D, Kuh D, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 2017; 32: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaunitz AM, Manson JE. Management of Menopausal Symptoms. Obstet Gynecol 2015; 126: 859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am 2011; 38: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health 2006; 96: 1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallicchio L, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Risk factors for hot flashes among women undergoing the menopausal transition: baseline results from the Midlife Women’s Health Study. Menopause 2015; 22: 1098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delavar MA, Hajiahmadi M. Factors Affecting the Age in Normal Menopause and frequency of Menopausal Symptoms in Northern Iran. Iran Red Crescent Med J 2011; 13: 192–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingl PJ, Hulka BS, Harlow SD. Risk factors for menopausal hot flashes. Obstet Gynecol 1994; 84: 29–34. [PubMed] [Google Scholar]
- 10.Herber-Gast GC, Mishra GD, van der Schouw YT, Brown WJ, Dobson AJ. Risk factors for night sweats and hot flushes in midlife: results from a prospective cohort study. Menopause 2013; 20: 953–9. [DOI] [PubMed] [Google Scholar]
- 11.Staropoli CA, Flaws JA, Bush TL, Moulton AW. Predictors of menopausal hot flashes. J Womens Health 1998; 7: 1149–55. [DOI] [PubMed] [Google Scholar]
- 12.Leidy LE. Symptoms of menopause in relation to the timing of reproductive events and past menstrual experience. Am J Hum Biol 1996; 8: 761–9. [DOI] [PubMed] [Google Scholar]
- 13.Gjelsvik B, Rosvold EO, Straand J, Dalen I, Hunskaar S. Symptom prevalence during menopause and factors associated with symptoms and menopausal age. Results from the Norwegian Hordaland Women’s Cohort study. Maturitas 2011; 70: 383–90. [DOI] [PubMed] [Google Scholar]
- 14.Nakano K, Pinnow E, Flaws JA, Sorkin JD, Gallicchio L. Reproductive history and hot flashes in perimenopausal women. J Womens Health (Larchmt) 2012; 21: 433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandeya N, Huxley RR, Chung HF, Dobson AJ, Kuh D, Hardy R, et al. Female reproductive history and risk of type 2 diabetes: A prospective analysis of 126 721 women. Diabetes Obes Metab 2018; 20: 2103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luijken J, van der Schouw YT, Mensink D, Onland-Moret NC. Association between age at menarche and cardiovascular disease: A systematic review on risk and potential mechanisms. Maturitas 2017; 104: 96–116. [DOI] [PubMed] [Google Scholar]
- 17.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012; 13: 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PLoS One 2016; 11: e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray KE, Katon JG, LeBlanc ES, Woods NF, Bastian LA, Reiber GE, et al. Vasomotor symptom characteristics: are they risk factors for incident diabetes? Menopause 2018; 25: 520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT, Mortimer JE, Crandall CJ, Pan K, Manson JE, Nelson R, et al. Persistent vasomotor symptoms and breast cancer in the Women’s Health Initiative. Menopause 2018; 26: 578–87. [DOI] [PubMed] [Google Scholar]
- 21.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 2013; 37: 1036–43. [DOI] [PubMed] [Google Scholar]
- 22.Mishra GD, Chung HF, Pandeya N, Dobson AJ, Jones L, Avis NE, et al. The InterLACE study: Design, data harmonisation and characteristics across 20 studies on women’s health. Maturitas 2016; 92: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra GD, Anderson D, Schoenaker DA, Adami HO, Avis NE, Brown D, et al. InterLACE: A New International Collaboration for a Life Course Approach to Women’s Reproductive Health and Chronic Disease Events. Maturitas 2013; 74: 235–40. [DOI] [PubMed] [Google Scholar]
- 24.Borooah VK. Logit and probit: Ordered and multinomial models, vol. 138 Thousand Oaks: Sage; 2002. [Google Scholar]
- 25.Gold EB, Crawford SL, Shelton JF, Tepper PG, Crandall CJ, Greendale GA, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women’s Health Across the Nation (SWAN). Menopause 2017; 24: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung HF, Pandeya N, Dobson AJ, Kuh D, Brunner EJ, Crawford SL, et al. The role of sleep difficulties in the vasomotor menopausal symptoms and depressed mood relationships: an international pooled analysis of eight studies in the InterLACE consortium. Psychol Med 2018; 48: 2550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth MEJ, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 2006; 60: 993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep 2015; 5: 11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth ME. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol 2002; 31: 405–12. [DOI] [PubMed] [Google Scholar]
- 30.Savastano DM, Gorbach AM, Eden HS, Brady SM, Reynolds JC, Yanovski JA. Adiposity and human regional body temperature. Am J Clin Nutr 2009; 90: 1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, et al. Menopausal hot flushes and night sweats: where are we now? Climacteric 2011; 14: 515–28. [DOI] [PubMed] [Google Scholar]
- 32.Saccomani S, Lui-Filho JF, Juliato CR, Gabiatti JR, Pedro AO, Costa-Paiva L. Does obesity increase the risk of hot flashes among midlife women?: a population-based study. Menopause 2017; 24: 1065–70. [DOI] [PubMed] [Google Scholar]
- 33.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015; 22: 1155–72; quiz 73–4. [DOI] [PubMed] [Google Scholar]
- 34.Terauchi M, Hiramitsu S, Akiyoshi M, Owa Y, Kato K, Obayashi S, et al. Associations among depression, anxiety and somatic symptoms in peri- and postmenopausal women. J Obstet Gynaecol Res 2013; 39: 1007–13. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell ES, Farin FM, Stapleton PL, Tsai JM, Tao EY, Smith-DiJulio K, et al. Association of estrogen-related polymorphisms with age at menarche, age at final menstrual period, and stages of the menopausal transition. Menopause 2008; 15: 105–11. [DOI] [PubMed] [Google Scholar]
- 36.Woods NF, Mitchell ES, Tao Y, Viernes HM, Stapleton PL, Farin FM. Polymorphisms in the estrogen synthesis and metabolism pathways and symptoms during the menopausal transition: observations from the Seattle Midlife Women’s Health Study. Menopause 2006; 13: 902–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.