Abstract
OBJECTIVES
To assess whether no enhancement on pre-treatment MRI can rule out malignancy of additional US mass(es) initially assessed as BI-RADS 3 or 4 in women with newly diagnosed breast cancer.
METHODS
This retrospective study included consecutive women from 2010–2018 with: newly diagnosed breast cancer; at least one additional breast mass (distinct from index cancer) assigned a BI-RADS 3 or 4 on US; and a bilateral contrast-enhanced breast MRI performed within 90 days of US. All malignant masses were pathologically proven; benign masses were pathologically proven or defined as showing at least two years of imaging stability. Incidence of malignant masses and NPV were calculated on a per-patient level using proportions and exact 95% CIs.
RESULTS
In 230 patients with 309 additional masses, 140/309 (45%) masses did not enhance while 169/309 (55%) enhanced on MRI. Of the 140 masses seen in 105 women (mean age, 54 years; range 28–82) with no enhancement on MRI, all had adequate follow-up and 140/140 (100%) were benign, of which 89/140 (63.6%) were pathologically proven and 51/140 (36.4%) demonstrated at least two years of imaging stability. Pre-treatment MRI demonstrating no enhancement of US mass correlate(s) had an NPV of 100% (95% CI: 96.7–100.0%).
CONCLUSIONS
All BI-RADS 3 and 4 US masses with a non-enhancing correlate on pre-treatment MRI were benign. The incorporation of MRI, when ordered by the referring physician, may decrease unnecessary follow-up imaging and/or biopsy if the initial US BI-RADS assessment and management recommendation were to be retrospectively updated.
Keywords: Magnetic resonance imaging, Ultrasound, breast neoplasm, retrospective studies, follow-up studies
Introduction
The BI-RADS lexicon has been practice-changing by facilitating a standardized approach to breast imaging reporting; however, while an initial BI-RADS assessment is made based on mammography and US, women with a new diagnosis of breast cancer often undergo a pre-treatment breast MRI. MRI is increasingly used to define the extent of disease and to screen the contralateral breast and several studies have been published reporting its high negative predictive value of equivocal breast findings [1–3]. Prior studies have also demonstrated that MRI is more accurate than US for breast cancer diagnosis [4; 5]. Nevertheless, there is a lack of consensus in the clinical setting regarding a retrospective change to the initial BI-RADS assessment and management recommendations after a pre-treatment MRI shows no enhancement of a correlate to a US BI-RADS 3 or 4 mass (BI-RADS 4A) [6–8].
Currently, in the United States, the BI-RADS assessment and management recommendation based on US tend to be upheld and followed even after a negative MRI [9]. Therefore, some patients undergo unnecessary biopsy and/or short interval follow-up imaging of BI-RADS 3 or 4 US masses despite an MRI demonstrating no enhancement of US correlate. These additional procedures also lead to a delay in treatment for some patients. In the era of personalized medicine, there is no doubt that MRI is more accurate than US for breast cancer diagnosis. Breast MRI has been reported to have a high sensitivity and NPV of up to 100% [1; 10; 11], and it can provide high-resolution 3D morphological imaging as well as depict neo-angiogenesis as a cancer-specific feature, which is present in lesions as small as 3 mm [12].
While several studies have reported on the diagnostic accuracy of breast MRI in enhancing masses [13; 14], it remains unclear whether breast MRI can classify US masses that were initially characterized as BI-RADS 3 or 4 as benign if there is no contrast enhancement. Thus, the purpose of our study was to assess whether no enhancement on pre-treatment MRI can rule out malignancy of additional US mass(es) initially assessed as BI-RADS 3 or 4 in women newly diagnosed breast cancer.
Materials and Methods
The institutional review board approved this Health Insurance Portability and Accountability Act–compliant retrospective study and waived the need for written informed consent. For this study, there is no patient overlap in any prior published studies.
Study Population
We retrospectively reviewed our electrònic hospital information system for consecutive women who were 18 years or older and who met the following inclusion criteria between 2010 and 2018: newly diagnosed operable breast cancer, at least one additional breast mass that was distinct from the index cancer and assigned a BI-RADS 3 or 4 on US, and a bilateral contrast-enhanced breast MRI performed within 90 days after US. Women were excluded from the study if they had: MRI performed after initiation of treatment (surgery or chemotherapy) or MRI performed for suspected locally recurrent disease or metastatic disease.
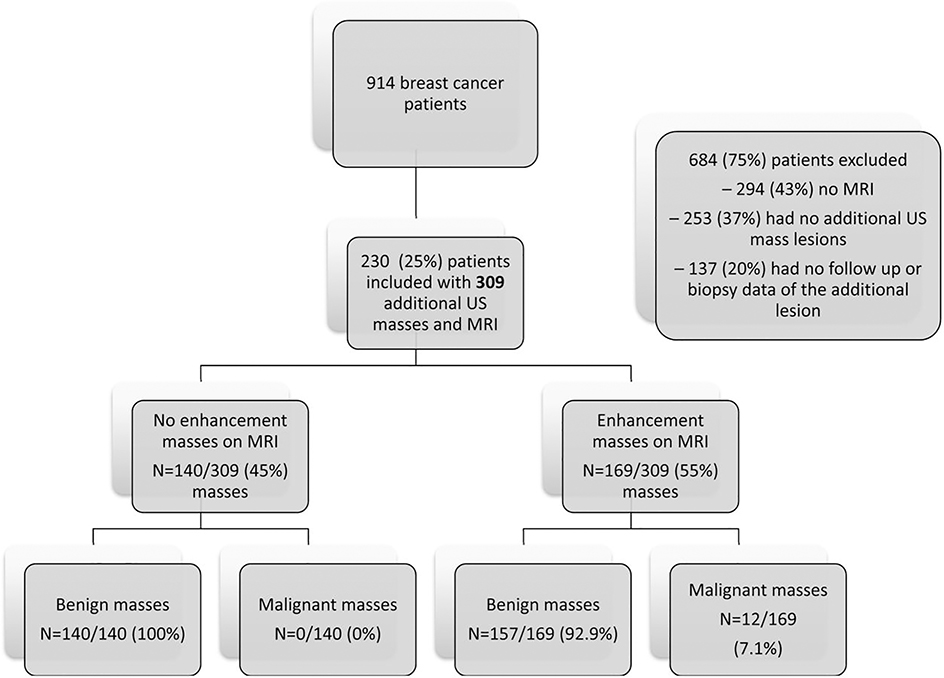
Figure 1 shows the patient inclusion flowchart. 915 breast cancer patients were reviewed and 684 patients were excluded for the following reasons: 294 (43%) had no MRI, 253 (37%) had no additional lesions on MRI, and 137 (20%) had no follow up or biopsy data of the additional lesion. Thus, the final study population comprised 105 women with 140 masses that did not enhance on MRI.
Figure 1.
Flowchart of the initial cohort of US masses with MRI and associated enhancement on MRI.
Breast MRI Acquisition
All breast MRI studies were performed for evaluating extent of disease. 127/230 (55%) breast MRI studies were performed at our institution while 103/230 (45%) were performed at outside institutions. At our institution, breast MRI studies were performed on either a 1.5 or 3.0 Tesla System (Discovery 750, GE Medical Systems) with a dedicated 8- or 16-channel breast coil (see Data Supplement for full protocol details). Axial T1-weighted fat-suppressed images were acquired before and at three time points after the intravenous administration of a gadolinium-based contrast agent (Gadavist; Bayer Healthcare Pharmaceuticals Inc.), which was administered at a concentration of 0.1 mmol gadobutrol per kg body weight at a rate of 2 ml/sec. The temporal resolution of steady-state DCE-MRI was ~90 seconds.
At outside institutions, breast MRI studies were performed on either a 1.5 or 3 Tesla MRI System [i.e. GE, Philips or Siemens MRI Machine Models] with a dedicated 7-, 8- or 16-channel breast coil. All outside MRI studies were performed at sites accredited by the American College of Radiology (ACR), which includes yearly review of each sites MRI protocol to ensure it meets the minimum requirements [15]. The outside studies were acquired in either a sagittal or axial plane. All protocols included bilateral T1-weighted fat-suppressed images before and at three time points after the intravenous administration of a gadolinium-based contrast agent with a minimum in-plane spatial resolution of 1 mm and a maximum slice thickness of 3 mm. All studies included bilateral T1-weighted images as well as T2-weighted images with or without fat-suppression. Because of differences in hardware, the sequence acquisition parameters varied slightly between outside institutions.
Image Analysis
Two fellowship-trained breast radiologists (DA and MAM) with 4 and 6 years of experience in breast imaging, respectively, retrospectively reviewed all imaging in consensus in random order while being blinded to final pathology. All breast MRI were analyzed and additional breast masses reassessed according to the ACR BI-RADS lexicon 5th edition [16]. For MRI, the presence or absence of enhancement was recorded, also along with T2-weighted hyperintensity relative to the fibroglandular tissue in the breast.
Reference Standard
All malignant masses were pathologically proven and benign masses were either pathologically proven or defined as showing 2 years of imaging stability. In cases of high-risk pathology on percutaneous biopsy for which excision was recommended, final surgical pathology was used as the reference standard.
Statistical Analysis
Continuous variables were summarized using mean and standard deviation or median and range where appropriate. Values were compared using the two-tailed Student’s t-test or the Mann–Whitney U test, respectively, with significance defined as p-value < 0.05. Categorical variables were summarized using frequencies and percentages and the Fisher’s exact test was used to test for statistical significance defined as p-value < 0.05. Finally, the incidence of malignant masses and the NPV were calculated on a per-patient level using proportions and exact 95% CIs. Statistical analysis was performed using IBM SPSS, version 25.0 (IBM).
Results
Lesion characteristics
The distribution of clinical and demographic factors by the presence or absence of enhancement on MRI of the US correlate (Table 1) was examined.
Table 1:
Characteristics of All Patients and Masses with Ultrasound and MRI
| MRI Enhancement | ||||
|---|---|---|---|---|
| No | Yes | P value | All | |
| Number of masses | 140 | 169 | ||
| - | 309 | |||
| Age, mean (range) | 54.6 (28–82) | 50.0 (21–76) | 0.0003* | 52 (21–82) |
| Size, mean ± SD, cm | 0.85 ± 0.43 | 1.01 ± 0.51 | 0.002* | 0.94 ± 0.48 |
| US BI-RADS, n (%) | ||||
| 3 | 118 (84.3%) | 120 (71.0%) | 0.007*** | 238 (77.0%) |
| 4 | 22 (15.7%) | 49 (29.0%) | 71 (23.0 %) | |
| Biopsy, n (%) | ||||
| Yes | 89 (63.6%) | 140 (82.8%) | 0.0001*** | 229 (74.1%) |
| No | 51 (36.4%) | 29 (17.2%) | 80 (25.9%) | |
| Malignant, n (%) | ||||
| No | 140 (100%) | 157 (92.9%) | 0.001*** | 297 (96.1%) |
| Yes | 0 | 12 (7.1%) | 12 (3.9%) | |
*Two-tailed student t-test
**Mann-Whitney U test
***Two-sided Fisher exact test
In total, 101 women with 140 masses were included in the study (mean age, 54 years; range 28–82 years). The mean time between initial US assessment and subsequent MRI was 23.4 days (range, 0–90 days). Of the 101 women, 82 (81%) had 1 mass and 19 (19%) had 2 or more masses. Of the women who had more than 1 mass, 16/101 (16%) had 2 masses; 4/101 (4%) had 3 masses, 2/101 (2%) had 4 masses, 0/101 (0%) had 5 masses, and 1/101 (1%) had 6 masses.
Imaging characteristics
Of the 140 masses, 118/140 (84.3%) masses were BI-RADS 3 and 22/140 (15.7%) were BI-RADS 4. The mean size of the masses, defined as the longest single diameter, was 0.85 cm (range, 0.3–3.4 cm). Of the 140 masses, all had adequate follow-up, with 89/140 (63.6%) undergoing biopsy per the patient or referring physician’s preference and 51/140 (36.4%) having at least two years of imaging stability. For BI-RADS 3 masses, 58/118 (49.2%) were pathologically proven as benign and 60/118 (50.8%) demonstrated at least two years of imaging stability; for BI-RADS 3 masses, 21/22 were pathologically proven as benign and 1/22 demonstrated at least two years of imaging stability. All breast MRI were analyzed and additional breast masses reassessed according to the ACR BI-RADS lexicon 5th edition. See Table 1 for additional information. It was noted that MRI enhancing lesions were more prevalent in younger women (p = 0.0003), larger in size (p = 0.002), and more likely to have a higher US BI-RADS score (p = 0.007).
All masses that did not enhance on MRI were benign either on biopsy (89/140 [63.6%]) or showed 2 years of imaging stability (51/140 [36.4%]). US masses with no enhancement on MRI had an NPV of 100% (95% CI: 96.7–100.0%).
Of all the masses that did not enhance on MRI, 92/140 (66%) were T2 hyperintense; 45/140 (32%) were T2 isointense and 3/140 (2%) did not have a T2 sequence.
Representative imaging features separated by the presence or absence of enhancement on MRI are described in Table 2, as is the specific biopsy pathology. Figure 2 and Figure 3 are representative cases of BI-RADS 3 and 4 masses, respectively, that did not enhance on MRI.
Table 2:
Characteristics, Distribution, and Frequency of All Lesions on MRI
| No enhancement on MRI | n = 140 (%) | |
| MRI morphology | ||
| Oval/round | 137 (97.9) | |
| Irregular | 4 (2.1) | |
| MRI margins | ||
| Circumscribed | 117 (83.6) | |
| Non-circumscribed | 23 (16.4) | |
| MRI T2 Hyperintense | ||
| Yes | 92 (65.7) | |
| No | 48 (34.3) | |
| Biopsy pathology (n=89) | ||
| Adenosis Focal fibrosis Fibrocystic changes Usual ductal hyperplasia Pseudoangiomatous stromal hyperplasia |
19 (21.3) 17 (19.1) 15 (16.9) 9 (10.1) 1 (1.1) |
|
| Fibroadenoma | 26 (29.2) | |
| Papilloma | 1 (1.1) | |
| Lobular carcinoma in situ | 1 (1.1) | |
| Enhancement on MRI | n=169 (%) | |
| MRI morphology | ||
| Oval/round | 155 (92) | |
| Irregular | 14 (8) | |
| MRI margins | ||
| Circumscribed | 131 (78) | |
| Non-circumscribed | 38 (22) | |
| MRI T2 Hyperintense | ||
| Yes | 120 (71) | |
| No | 49 (29 | |
| MRI enhancement | ||
| Initial phase | Fast | 6 (3) |
| Slow | 163 (97) | |
| Internal enhancement | Homogeneous | 140 (83) |
| Heterogeneous | 29 (17 | |
| Biopsy pathology (n=140) | ||
| Adenosis | 12 (8.6) | |
| Focal fibrosis | 12 (8.6) | |
| Usual ductal hyperplasia | 11 (7.9) | |
| Pseudo-angiomatous stromal hyperplasia | 5 (3.6) | |
| Fibroadenoma | 53 (37.9) | |
| Benign Papilloma | 19 (13.6) | |
| Columnar cell changes | 1 (0.7) | |
| Lymph node | 1 (0.7) | |
| Atypical Papilloma | 3 (2.1) | |
| Phyllodes tumor | 1 (0.7) | |
| Lobular carcinoma in situ | 3 (2.1) | |
| Atypical ductal hyperplasia | 7 (5) | |
| Carcinoma in situ | 6 (4.2) | |
| Invasive ductal carcinoma | 6 (4.2) | |
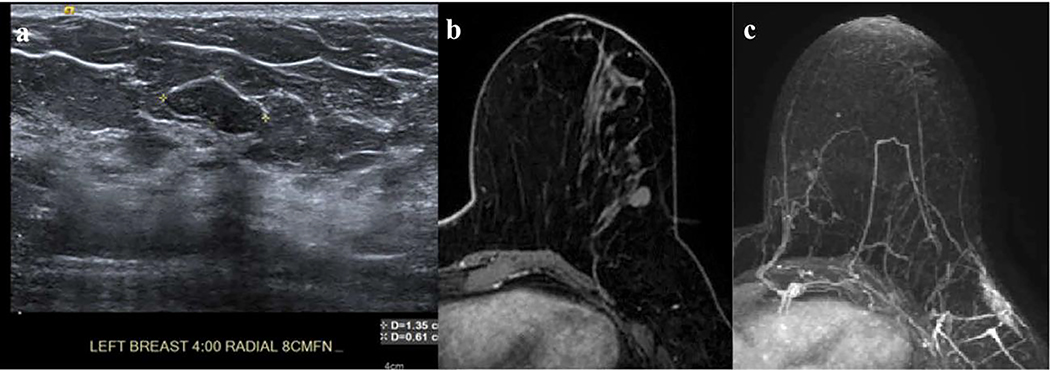
Figure 2.
(a) In the left breast, 4:00 axis, 8 cm from the nipple, there is a 1.35 cm oval, circumscribed, hypoechoic, avascular mass that was given a BI-RADS 3 assessment for which imaging follow-up was recommended; (b) T1-weighted fat-suppressed first post-contrast axial and (c) Maximum intensity projection (MIP) MRI demonstrates a corresponding mass to the US that does not enhance. This mass was followed for 2 years.
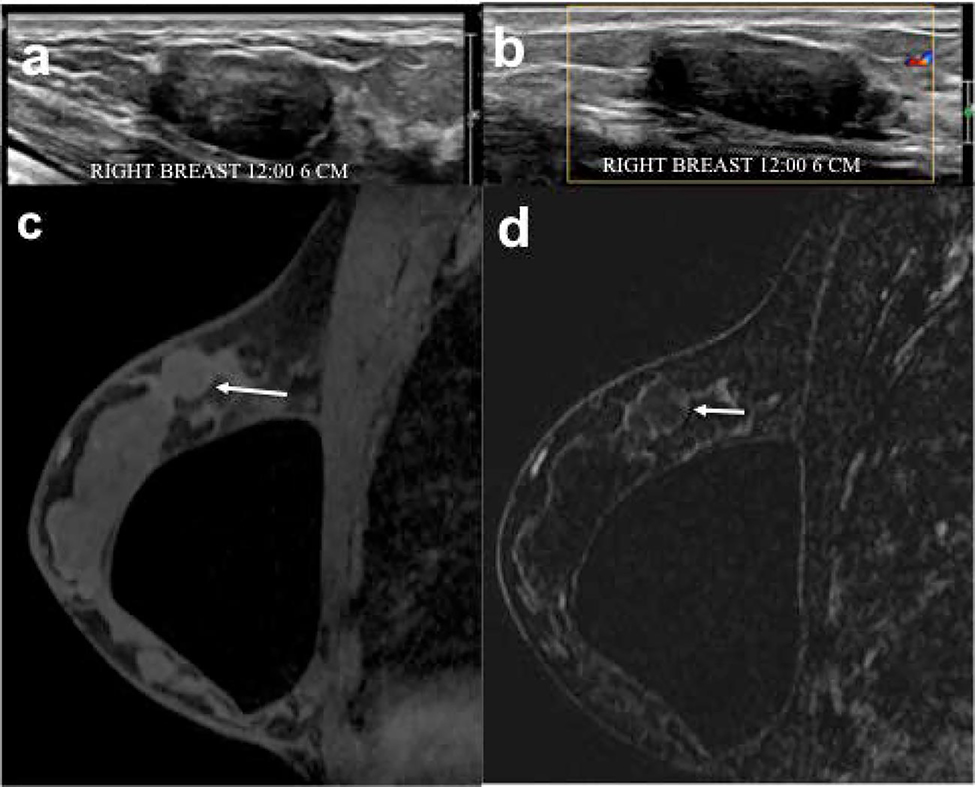
Figure 3.
(a, b) In the right breast, 12:00 axis, 6 cm from the nipple, there is a 3.4 cm oval, not-circumscribed, hypoechoic, avascular mass imaged in the US radial image that was given a BI-RADS 4 assessment for which biopsy was recommended. (c) T1-weighted fat-suppressed first post-contrast axial MRI and (d) subtracted image demonstrate a corresponding mass (white arrow) to the US that does not enhance. This mass underwent US-guided biopsy that yielded fibroadenoma benign pathology.
Of note, a total of 169 enhancing masses were found in 137 women (mean age 51, range 21–76), with a mean size 10 mm (range 3–35 mm). In 140/169 cases (83%) a biopsy was performed and 29/169 (17%) demonstrated 2-years of imaging stability. Of the enhancing masses, 141/169 (83.4%) were benign, 16/169 (9.5 %) were high-risk and 12/169 (7%) were malignant.
Discussion
The BI-RADS lexicon is standardized across imaging modalities but does not address the possibility of updating management recommendation when complimentary imaging is subsequently performed. Thus, we conducted this study to determine whether no enhancement on pre-treatment MRI, when ordered by the referring physician, can rule out malignancy of at least one additional US mass initially assessed as BI-RADS 3 or 4 in women newly diagnosed breast cancer. Our study found that 100% of additional US breast masses that were initially assessed as BI-RADS 3 or 4 and did not enhance on a subsequent breast MRI were benign. Our results suggest that if the subsequent MRI results are retrospectively incorporated with the initial US BI-RADS assessment and management recommendations, this would lead to change in management recommendations, increased accuracy, improved patient care, and decreased healthcare costs. Our study does not suggest that MRI should be performed for all US BI-RADS 3 or 4 cases. However, if an MRI is performed (either for high risk screening or to define extent of disease within 90 days of US), then it could be leveraged to retrospectively assess the US BI-RADS 3 or 4 masses, and subsequently, and if there is no enhancement, then the US BI-RADS assessment should be updated to BI-RADS 2, thus obviating unnecessary imaging follow-up (cost, time, patient anxiety) and/or biopsy (cost, time, patient morbidity and anxiety).
Our findings are in line with prior work demonstrating that for uncertain findings, contrast-enhanced MRI has a high sensitivity and NPV [1; 2; 17-19]. Bennani-Baiti et al. [1] conducted a systematic review and meta-analysis on the performance of DCE-MRI for the diagnosis of breast cancer in non-calcified equivocal breast findings. Histopathological sampling or imaging follow-up of at least 12 months served as the reference standard. They found that in 14 studies comprising 2,316 lesions, DCE-MRI had a sensitivity of 99%, specificity of 89%, PPV of 56%, and NPV of 100%. Spick et al. [17] reported that in 302 women undergoing 3T breast MRI for further workup of conventional and clinical breast findings, with histopathology or follow-up ≥ two years serving as the reference standard, breast MRI had a sensitivity of 96.4%, specificity of 92.4%, PPV of 72.6%, and NPV of 99.2%. Similar results were also reported by Giess et al. [2] in a retrospective study with 294 asymptomatic women who underwent breast MRI for equivocal mammographic findings. For 296 breast MRI examinations, breast MRI had a sensitivity of 92.5%, specificity of 62.4%, NPV of 97.8%, and PPV of 31.9%. In addition, 44/294 (15%) patients also had incidental lesions on breast MRI. Taskin et al. reported that MRI identified lesions in 414/986 problem-solving MRI examinations, with a sensitivity of 91.7%, specificity of 69%, NPV of 98.7%, and PPV of 24%.
Breast cancer that does not enhance on MRI though uncommon have been reported [20–23]. In 2006, Schnall et al. [24] reported that in 995 lesions in 854 women with available pathology data, the absence of enhancement was associated with an NPV of 88% for malignancy. In 2010, Shimauchi et al. [23] evaluated false negatives on MRI, which was defined as no enhancement at the site of biopsy proven cancer. Of the 7/220 cancers (3.2%) that did not enhance, 3/7 (42.9%) were invasive ductal carcinoma and 4/7 (57.1%) were ductal carcinoma in situ. False negatives were attributed again to diffuse background parenchymal enhancement or small tumor size. In 2012, Dorrius et al. [3] showed that breast MRI can provide a sufficient NPV to safely rule out malignancy in mammographic non-calcified BI-RADS 3 lesions with a sensitivity of 100%, specificity of 82.5%, a PPV of 54.2% and a NPV of 100% [3], suggesting that breast MRI can obviate the need for some invasive diagnostic procedures. Finally, Baum et al. [18] developed a classification system for contrast-enhanced breast MRI based on the ACR BI-RADS mammographic categories and evaluated it in 522 patients (1031 breasts). This classification system yielded a sensitivity of 92%, specificity of 92%, PPV of 92%, and NPV of 92%. In 787 breasts without focal enhancement, only 2 were revealed by histopathology to be malignant (one ductal carcinoma in situ and one invasive ductal carcinoma).
Currently, MRI is being used as a complementary imaging modality when masses are detected on US to guide further management [25]. Despite BI-RADS guidelines that state a subsequent imaging test can change the initial assessment, radiologists are sometimes hesitant to change the assessment or management recommendations retrospectively; therefore, perhaps more guidance on this topic in the next BI-RADS edition could be helpful. Our findings show that leveraging the strength of complimentary imaging modalities even when they are not performed on the same day and retrospectively changing the initial BI-RADS assessment would improve overall patient care by serving to minimize unnecessary biopsies. Further, our results suggest that it may be prudent to do a pre-treatment breast MRI in all patients with a new diagnosis of breast cancer not only to evaluate the extent of disease but also to inform how to manage additional lesions found on US.
In terms of reducing biopsies, Zuley et al. [26] have retrospectively assessed if Contrast Enhanced Digital Mammography (CEDM) can accurately reduce biopsy rates for soft tissue BI-RADS 4A or 4B lesions. In their study, which included 49 benign lesions, 2 high-risk lesions, and 9 cancerous lesions, CEDM increased true positive rates from 0.74 under digital mammography/digital breast tomography, and 0.89 with US, to 0.90 with CEDM. For an expected cancer rate of 10%, CEDM had a PPV of 20.5% (95% CI: 16%–27%) and an NPV of 98.3% (95% CI: 96%–100%). The authors concluded that adding CEDM for the evaluation of low-moderate suspicion soft tissue breast lesions substantially reduced the biopsy rate of benign lesions without compromising cancer detection. In liver and kidney imaging, if there is no corresponding enhancement on MRI of an indeterminate mass on US, it is considered benign. Whereas there is a good body of evidence on the management of US-detected lesions that demonstrate enhancement on MRI, the data is relatively scarce for non-enhancing lesions.
We acknowledge several limitations to our study. Firstly, this was a retrospective study performed at a tertiary cancer center. Secondly, there were significantly more US masses categorized as BI-RADS 3 (84.3%) compared to BI-RADS 4 (15.7%). Thirdly, no co-registration was performed between US masses and MRI correlates. Finally, our study only included women with a new diagnosis of breast cancer where additional US masses are often identified on the initial diagnostic work-up. Future studies should include women without breast cancer.
The NPV of breast masses that were initially assigned a BI-RADS 3 or 4 on US with a non-enhancing correlate on pre-treatment MRI was 100%. The incorporation of MRI, when ordered independently by the referring physicians may decrease unnecessary follow-up imaging and/or biopsy if the initial US BI-RADS assessment and management recommendation were to be retrospectively updated.
Supplementary Material
Key Points.
Of 309 BI-RADS 3 or 4 US masses with a corresponding mass on MRI, 140/309 (45%) demonstrated no enhancement whereas 169/309 (55%) demonstrated enhancement
All masses classified as BI-RADS 3 or 4 on US without enhancement on MRI were benign
MRI can rule out malignancy in non-enhancing US masses with an NPV of 100%
Acknowledgements
We thank Joanne Chin, MFA, ELS, who provided editorial support for this manuscript.
Funding information
This work was supported in part by the NIH/NCI P30 Cancer Center Support Grant (P30 CA008748), the Susan G. Komen Foundation, and the Breast Cancer Research Foundation.
Abbreviations
- ACR
American College of Radiology
- CEDM
Contrast Enhanced Digital Mammography
Footnotes
3. Guarantor:
The scientific guarantor of this publication is Elizabeth J Sutton, MD.
4. Conflict of Interest:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
5. Statistics and Biometry:
One of the authors (Peter Gibbs, PhD) has significant statistical expertise.
6. Informed Consent:
Written informed consent was waived by the Institutional Review Board.
7. Ethical Approval:
Institutional Review Board approval was obtained.
8. Study subjects or cohorts overlap:
None.
9. Methodology
Methodology:
• retrospective
• observational
• performed at one institution
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bennani-Baiti B, Bennani-Baiti N, Baltzer PA (2016) Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS One 11:e0160346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giess CS, Chikarmane SA, Sippo DA, Birdwell RL (2017) Clinical Utility of Breast MRI in the Diagnosis of Malignancy After Inconclusive or Equivocal Mammographic Diagnostic Evaluation. AJR Am J Roentgenol 208:1378–1385 [DOI] [PubMed] [Google Scholar]
- 3.Dorrius MD, Pijnappel RM, Sijens PE, van der Weide MC, Oudkerk M (2012) The negative predictive value of breast Magnetic Resonance Imaging in noncalcified BIRADS 3 lesions. Eur J Radiol 81:209–213 [DOI] [PubMed] [Google Scholar]
- 4.Riedl CC, Luft N, Bernhart C et al. (2015) Triple-Modality Screening Trial for Familial Breast Cancer Underlines the Importance of Magnetic Resonance Imaging and Questions the Role of Mammography and Ultrasound Regardless of Patient Mutation Status, Age, and Breast Density. 33:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggertson L (2004) MRIs more accurate than mammograms but expensive. CMAJ : Canadian Medical Association Journal 171:840–840 [Google Scholar]
- 6.Lee KA, Talati N, Oudsema R, Steinberger S, Margolies LR (2018) BI-RADS 3: Current and Future Use of Probably Benign. Curr Radiol Rep 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C (2012) Positive predictive value of BI-RADS MR imaging. Radiology 264:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal G, Su MY, Nalcioglu O, Feig SA, Chen JH (2009) Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 115:1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AY, Ichikawa L, Lee JM et al. (2016) Concordance of BI-RADS Assessments and Management Recommendations for Breast MRI in Community Practice. AJR Am J Roentgenol 206:211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heywang SH, Hahn D, Schmidt H et al. (1986) MR imaging of the breast using gadolinium-DTPA. J Comput Assist Tomogr 10:199–204 [DOI] [PubMed] [Google Scholar]
- 11.Kuhl C (2007) The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 244:356–378 [DOI] [PubMed] [Google Scholar]
- 12.Marino MA, Helbich T, Baltzer P, Pinker-Domenig K (2018) Multiparametric MRI of the breast: A review. J Magn Reson Imaging 47:301–315 [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Jang M, Kim SM, Yun B, Jang JY, Ahn HS (2018) Preoperative magnetic resonance imaging characteristics of oval circumscribed fast enhancing lesions in patients with newly diagnosed breast cancer. Medicine (Baltimore) 97:e0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhl CK, Klaschik S, Mielcarek P, Gieseke J, Wardelmann E, Schild HH (1999) Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging 9:187–196 [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology (2017) Breast Magnetic Resonance Imaging (MRI) Accreditation Program Requirements, [Incomplete para]
- 16.Morris EA, Comstock CE, al. LCHe (2013) ACR BI-RADS® Magnetic Resonance Imaging In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, [Incomplete para] [Google Scholar]
- 17.Spick C, Szolar DHM, Preidler KW et al. (2018) 3 Tesla breast MR imaging as a problem-solving tool: Diagnostic performance and incidental lesions. PLoS One 13:e0190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum F, Fischer U, Vosshenrich R, Grabbe E (2002) Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol 12:1087–1092 [DOI] [PubMed] [Google Scholar]
- 19.Taskin F, Polat Y, Erdogdu IH, Turkdogan FT, Ozturk VS, Ozbas S (2018) Problem-solving breast MRI: useful or a source of new problems? Diagn Interv Radiol 24:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman J, Merler E, Abernathy C, Williams G (1971) Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133:275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heywang SH, Wolf A, Pruss E, Hilbertz T, Eiermann W, Permanetter W (1989) MR imaging of the breast with Gd-DTPA: use and limitations. Radiology 171:95–103 [DOI] [PubMed] [Google Scholar]
- 22.Kaiser WA (1994) False-positive results in dynamic MR mammography. Causes, frequency, and methods to avoid. Magn Reson Imaging Clin N Am 2:539–555 [PubMed] [Google Scholar]
- 23.Shimauchi A, Jansen SA, Abe H, Jaskowiak N, Schmidt RA, Newstead GM (2010) Breast cancers not detected at MRI: review of false-negative lesions. AJR Am J Roentgenol 194:1674–1679 [DOI] [PubMed] [Google Scholar]
- 24.Schnall MD, Blume J, Bluemke DA et al. (2006) Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology 238:42–53 [DOI] [PubMed] [Google Scholar]
- 25.Spick C, Szolar DHM, Preidler KW, Tillich M, Reittner P, Baltzer PA (2015) Breast MRI used as a problem-solving tool reliably excludes malignancy. Eur J Radiol 84:61–64 [DOI] [PubMed] [Google Scholar]
- 26.Zuley ML, Bandos AI, Abrams GS et al. (2019) Contrast Enhanced Digital Mammography (CEDM) Helps to Safely Reduce Benign Breast Biopsies for Low to Moderately Suspicious Soft Tissue Lesions. Acad Radiol. 10.1016/j.acra.2019.07.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.