Abstract
Rationale & Objective:
Current dietary guidelines recommend that chronic kidney disease (CKD) patients restrict individual nutrients, such as sodium, potassium, phosphorus and protein. This approach can be difficult for patients to implement and ignores important nutrient interactions. Dietary patterns are an alternative method to intervene on diet. Our objective was to define the associations of four healthy dietary patterns with risk of CKD progression and all-cause mortality among people with CKD.
Study Design:
Prospective cohort study.
Setting & Participants:
2,403 participants aged 21–74 years with an estimated glomerular filtration rate (eGFR) of 20–70 mL/min/1.73 m2 and dietary data in the Chronic Renal Insufficiency Cohort (CRIC) study.
Exposures:
Healthy Eating Index-2015 (HEI-2015), Alternative Healthy Eating Index-2010 (AHEI-2010), alternate Mediterranean diet (aMed), and Dietary Approaches to Stop Hypertension (DASH) diet scores were calculated from food frequency questionnaires.
Outcomes:
1) CKD progression defined as ≥50% eGFR decline, kidney transplantation, or dialysis and 2) all-cause mortality.
Analytical Approach:
Cox proportional hazards regression models adjusted for demographic, lifestyle, and clinical covariates to estimate hazard ratios (HR) and 95% confidence intervals (CI).
Results:
There were 855 cases of CKD progression and 773 deaths over a maximum of 14 years. Compared with participants with the lowest adherence, the most highly adherent tertile of AHEI-2010, aMed, and DASH had lower adjusted risk of CKD progression with the strongest results for aMed (HR: 0.75, 95% CI: 0.62–0.90). Compared with participants with the lowest adherence, the highest adherence tertiles for all scores had lower adjusted risk of all-cause mortality for each index (24–31% lower risk).
Limitations:
Self-reported dietary intake.
Conclusions:
Greater adherence to several healthy dietary patterns is associated with a lower risk of CKD progression and all-cause mortality among people with CKD. Guidance to adopt healthy dietary patterns can be considered as a strategy for managing CKD.
Index Words:
dietary patterns, nutrition, kidney disease, renal, mortality
PLAIN-LANGUAGE SUMMARY
Few studies have been conducted to assess how healthy dietary patterns are associated with chronic kidney disease (CKD) progression among people who have CKD. Therefore, we conducted an analysis among 2,403 people with CKD and examined the associations between their diet quality and risk of CKD progression and all-cause mortality. We found that following a healthy dietary pattern was associated with lower risk of CKD progression and lower risk of death. These results may inform clinicians to recommend patients with CKD to follow overall healthy dietary patterns that are rich in fruits, vegetables, nuts, legumes, and whole grains, and low in red/processed meats, added sugars, and sodium.
INTRODUCTION
Chronic kidney disease (CKD) affects about 15% of adults in the U.S. and is a growing public health problem (1). CKD can be costly and burdensome, especially if it progresses to end-stage renal disease (ESRD) and is associated with a higher risk of death (2).
Healthy dietary patterns, which are generally characterized by high consumption of fruits, vegetables, nuts, legumes, fish, low-fat dairy, and whole grains and low in red and processed meats, sodium, and added sugar, may reduce risk of incident CKD in general populations (3–7). However, there is less evidence on whether adherence to a healthy dietary pattern during early stages of CKD is associated with lower risk of CKD progression or mortality (8). Clinical guidelines and clinicians have historically recommended that patients with CKD stages 1–4 should reduce the amount of sodium and protein in their diet and, at more advanced stages of CKD, should limit potassium and phosphorous intake (9). However, the optimal daily intake of these nutrients is largely theoretical and there is limited empirical evidence for the recommendations’ effectiveness (10). Additionally, nutrient-based dietary restrictions are difficult to implement and may result in patients consuming less healthy diets (11–13). Consuming a healthy dietary pattern that emphasizes a combination of food groups may be easier for patients to follow and be effective in preventing adverse health outcomes.
In 2019, the public review draft of the KDOQI Clinical Practice Guidelines for Nutrition in Chronic Kidney Disease suggested future research should focus on implementing dietary patterns in clinical trials for CKD patients and should examine multiple dietary patterns with CKD progression in a large cohort of established CKD over a long duration (14).
To address these gaps, we examined associations of four measures of high-quality dietary patterns with CKD progression and all-cause mortality in the Chronic Renal Insufficiency Cohort (CRIC), a large, prospective cohort of adults with CKD in the U.S.
METHODS
Study Population
The CRIC Study is an ongoing multicenter, prospective cohort study of people with CKD (15, 16). In brief, 3,939 men and women aged 21–74 years with an estimated glomerular filtration rate (eGFR) 20–70 ml/min/1.73 m2 based on the Modification of Diet in Renal Disease (MDRD) study equation were recruited between 2003 and 2008 from seven U.S. clinical centers. Participants were ineligible if they were institutionalized, pregnant, or had certain severe chronic conditions (15). Participants are followed every six months, with annual in-person visits and interim six-month telephone calls. The study protocol was approved by institutional review boards of all participating centers. All participants provided informed consent.
We included 2,403 participants in our study. Participants were excluded if they did not fill out the diet questionnaire at baseline (n=983), had extreme self-reported energy intakes [women: <500 or >3,500 kcal/d; men: <700 or >4,500 kcal/d (n=27)], did not have sufficient data to calculate all dietary pattern scores (n=419), or were missing covariates of interest (n=107). Compared with participants included in our analysis, participants excluded were more likely to be male, non-white, have a lower education and lower income, have diabetes, hypertension, a history of CVD, and worse kidney measures (Table S1). Dietary scores between the two groups were comparable.
Diet Assessment
Diet was assessed using the National Cancer Institute 124-item Diet History Questionnaire (DHQ) at baseline, year 2, and year 4. The DHQ has been validated previously (17). Participants were asked to self-report frequency and portion size of foods and beverages consumed over the preceding 12 months. Nutrient intakes were estimated using Diet*Calc software. To leverage the repeated assessment of dietary intake for better precision, we used a cumulative average approach to calculate food and nutrient intakes. If participants were censored (e.g. had a CKD progression event, died, or lost to follow-up) before year 2, their baseline diet was used (47% of participants). If they were censored between years 2 and 4, we used the average of baseline and year 2 dietary intake (20% of participants) and if they were censored after year 4, we used the average of baseline, year 2, and year 4 dietary intake (33% of participants) (18).
The four dietary scores were calculated using responses from the DHQ. The Healthy Eating Index-2015 (HEI-2015), Alternative Healthy Eating Index-2010 (AHEI-2010), alternate Mediterranean diet (aMed), and DASH scores are commonly used dietary indices to assess diet quality and have been defined previously (19–22). The HEI-2015 score ranges from 0 to 100, consists of 13 components, and was created to assess adherence to the 2015–2020 U.S. Dietary Guidelines for Americans (Table S2) (19). The AHEI-2010 score ranges from 0 to 110, consists of 11 components, and was designed to incorporate foods and nutrients that were associated with total chronic disease based on previous literature (20). The aMed ranges from 0 to 9 and includes nine components to assess adherence to a Mediterranean-style diet in a U.S. population (21). The DASH score ranges from 8 to 40, includes 8 components, and was created to reflect the DASH diet that was tested in 2 randomized feeding trials (22–24).
Outcomes
Our primary outcome was CKD progression, which was defined as a 50% or greater decline in eGFR from baseline or ESRD (long-term dialysis therapy or kidney transplantation). Time to eGFR halving was imputed assuming a linear decline in kidney function between annual visits (25, 26). Information on dialysis and kidney transplantation was obtained during follow-up visits and telephone interviews and confirmed by dialysis unit or hospital chart review. Ascertainment of ESRD was supplemented by data from the U.S. Renal Data System.
Our secondary outcome was all-cause mortality. Deaths were ascertained from reports by next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. For the present study, follow-up data was available through January 2018, allowing for a maximum duration of 14 years. Participant follow-up was censored at time of death, loss to follow-up, or end of the follow-up period.
As a sensitivity analysis, we used a composite of CKD progression or death since death is a competing risk for CKD progression.
Assessment of Covariates
Sociodemographic information, medical history, and medication use were obtained at baseline through self-reported questionnaires. Physical activity was measured using the Multi-Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey, which summarizes physical activity into metabolic equivalent task (METs) per week (27). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Weight and height were measured using standard protocols (16). GFR was estimated using a CRIC-specific equation that includes age, sex, race, cystatin C, and creatinine (25). A 24-hour urine sample was used to measure protein excretion. High-density lipoprotein (HDL) cholesterol was measured using the enzymatic colorimetric method. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL, a non-fasting plasma glucose ≥200 mg/dL, or self-reported use of anti-diabetes mellitus medication. Hypertension was defined as mean systolic/diastolic blood pressures ≥140/90 mmHg or self-reported use of antihypertensive medications. Blood pressure was based on three seated measurements that were obtained by trained staff after five minutes of rest. Participants were asked to self-report whether they had a history of cardiovascular disease (CVD).
Statistical Analysis
Descriptive statistics of baseline characteristics were summarized by tertiles of each dietary score and compared using χ2 tests and ANOVA. Pearson’s correlation coefficients were calculated to assess the correlation between dietary scores. We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the associations between dietary scores and outcomes. We adjusted for total energy intake, clinical site, age, sex, race, education level, income level, eGFR (CRIC equation), 24-hour urinary protein, health behaviors, including smoking status, physical activity, and alcohol status (for HEI-2015 and DASH only since alcohol was not included in these scores), and clinical covariates, i.e. BMI, diabetes mellitus, hypertension, CVD, HDL cholesterol, and angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) use.
We used the median value of the dietary score within each tertile to calculate a p-value for linear trend test. To visually evaluate potential non-linearity of the associations, we created restricted cubic spline models with 3 knots placed at the median of each tertile of each score. We explored potential interactions between dietary scores and sex, age, race, diabetes, BMI, and eGFR (<45 and ≥45 ml/min/1.73 m2) on outcomes using the likelihood ratio test. As a sensitivity analysis, we repeated the models using baseline dietary intake rather than the cumulative average. We also conducted a sensitivity analysis excluding participants who had a CKD progression event in the first two years of follow-up to address potential reverse causation. In post hoc analyses, we examined the association between individual components of the dietary scores and CKD progression and all-cause mortality. We also conducted mediation analyses by examining change in effect estimates after additionally adjusting for C-reactive protein (CRP), fibroblast growth factor-23 (FGF-23), serum bicarbonate, blood urea nitrogen, and uric acid to explore their roles in the diet and CKD progression association. All analyses were performed using Stata (version 14.0; StataCorp, College Station, Texas). P-values < 0.05 were considered statistically significant.
RESULTS
Baseline Characteristics
Participants who had higher dietary scores, indicating healthier diet quality, were generally more likely to be older, female, a college graduate, have a higher income level, be a current drinker, have diabetes, higher eGFR, lower urinary protein, higher HDL cholesterol, and were less likely to smoke and have hypertension compared with participants in the lowest tertile of dietary scores (Table 1). Trends in baseline characteristics were similar across tertiles of adherence of all four dietary scores (Tables S3–6). The correlation between scores ranged from 0.63 (HEI-2015 and AHEI-2010) to 0.80 (HEI-2015 and DASH) (Table S7).
Table 1.
Baseline characteristics of CRIC participants in lowest and highest tertiles of dietary scoresa
| HEI-2015 | AHEI-2010 | aMed | DASH | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Tertile 1: 55b | Tertile 3: 79 | Tertile 1: 34 | Tertile 3: 58 | Tertile 1: 2 | Tertile 3: 6.5 | Tertile 1: 19 | Tertile 3: 29 |
| n | 801 | 801 | 801 | 801 | 870 | 682 | 912 | 795 |
| Age, years | 55 ± 12 | 60 ± 10 | 56 ± 12 | 59 ± 10 | 55 ± 12 | 56 ± 12 | 55 ± 12 | 60 ± 10 |
| Female, % | 39 | 57 | 43 | 53 | 48 | 49 | 37 | 59 |
| Non-white, % | 42 | 47 | 51 | 40 | 45 | 45 | 54 | 41 |
| ≥College graduate, % | 31 | 50 | 29 | 51 | 30 | 49 | 30 | 49 |
| Income ≥$50,000, % | 34 | 41 | 29 | 44 | 33 | 43 | 33 | 40 |
| Current smoker, % | 21 | 5 | 18 | 6 | 19 | 5 | 21 | 5 |
| Current drinker, % | 22 | 24 | 18 | 28 | 21 | 28 | 24 | 19 |
| Physical activity, METs/wk | 201 ± 130 | 200 ± 125 | 196 ± 130 | 205 ± 124 | 201 ± 130 | 196 ± 130 | 204 ± 135 | 198 ± 118 |
| BMI, kg/m2 | 32 ±8 | 31 ±7 | 32 ±8 | 31 ±8 | 32 ±8 | 32 ±8 | 32 ±8 | 32 ±8 |
| Diabetes, % | 40 | 43 | 38 | 47 | 41 | 44 | 37 | 49 |
| Hypertension, % | 85 | 80 | 87 | 80 | 84 | 82 | 85 | 79 |
| Systolic BP, mmHg | 126 ± 21 | 126 ±21 | 127 ± 21 | 125 ± 21 | 127 ± 21 | 126 ± 21 | 127 ± 21 | 125 ± 20 |
| Diastolic BP, mmHg | 72 ± 12 | 70 ± 12 | 72 ± 12 | 70 ± 12 | 71 ± 12 | 71 ± 12 | 73 ± 13 | 69 ± 11 |
| History of CVD, % | 30 | 30 | 31 | 29 | 31 | 30 | 30 | 30 |
| eGFR, mL/min/1.73 m2 | 45 ± 17 | 48 ± 17 | 44 ± 16 | 49 ± 18 | 45 ± 17 | 44 ± 16 | 45 ± 17 | 48 ± 17 |
| Urinary protein, g/24 hr | 1.1 ± 2.4 | 0.7 ± 1.6 | 1.0 ± 2.3 | 0.8 ± 1.8 | 1.1 ± 2.4 | 1.0 ± 2.3 | 1.1 ± 2.3 | 0.6 ± 1.4 |
| HDL cholesterol, mg/dL | 46 ± 15 | 50 ± 15 | 47 ± 15 | 50 ± 16 | 46 ± 15 | 47 ± 15 | 46 ± 15 | 51 ± 17 |
| FGF23, RU/mL | 243 ± 471 | 189 ± 301 | 256 ± 569 | 186 ± 274 | 257 ± 546 | 178 ± 298 | 246 ± 541 | 189 ± 292 |
| ACEi or ARB use, % | 66 | 66 | 66 | 67 | 66 | 67 | 67 | 64 |
| Total energy intake, kcal/d | 1,927 ± 864 | 1,682 ± 670 | 1,681 ± 747 | 1,957 ± 811 | 1,927 ± 865 | 1,681 ± 747 | 1,905 ± 836 | 1,741 ± 728 |
| Protein intake, g/d | 71 ±36 | 68 ±30 | 60 ±29 | 81 ±38 | 61 ±31 | 82 ±37 | 70 ±35 | 70 ±33 |
| Sodium intake, mg/d | 2,999 ± 1,493 | 2,656 ± 1,140 | 2,473 ± 1,175 | 3,224 ± 1,486 | 2,521 ± 1,237 | 3,275 ± 1,388 | 2,922 ± 1,415 | 2,788 ± 1,268 |
| Potassium intake, mg/d | 2,748 ± 1,288 | 3,224 ± 1,288 | 2,495 ± 1,088 | 3,543 ± 1,412 | 2,488 ± 1,126 | 3,649 ± 1,351 | 2,723 ± 1,240 | 3,311 ± 1,313 |
| Phosphorus intake, mg/d | 1,136 ± 539 | 1,140 ± 478 | 971 ± 441 | 1,323 ± 545 | 1,002 ± 480 | 1,339 ± 543 | 1,094 ± 505 | 1,199 ± 520 |
| Dietary acid load, mEq/d | 2.9 ± 17 | −10.4 ± 14 | −1.3 ± 16 | −6.6 ± 19 | 0.2± 16 | −8.0 ± 18 | −3.5 ± 17 | −10.1 ± 15 |
Values for categorical variables are given as percentage; for continuous variables, mean ± standard deviation. ACEi, angiotensin-converting enzyme inhibitor; AHEI, Alternative Healthy Eating Index; aMed, alternate Mediterranean diet; ARB; angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23; g, grams; HDL, high-density lipoprotein; HEI, Healthy Eating Index; kcal, kilocalories; m, meters; MET, metabolic equivalent task, mg/dL, milligrams per deciliter; mmHg, millimeters of mercury; RU/mL, reference units per milliliter.
Median score of tertile.
Dietary Patterns and CKD Progression
Over a median (interquartile range) follow-up time of 7 (3.0–11.5) years, there were 855 CKD progression events (647 were ESRD events). There were no significant associations between HEI-2015 and CKD progression (Table 2). Participants in the highest tertile of AHEI-2010 had a 17% lower risk of CKD progression (HR: 0.83, 95% CI: 0.69–0.99) compared with participants in tertile 1 (P-trend=0.04). Participants in tertile 3 of aMed score had a 25% lower risk of CKD progression compared with participants in tertile 1 (HR: 0.75, 95% CI: 0.62–0.90) (P-trend=0.002). Hazard ratios for all covariates in the model are reported in Table S8. Higher DASH scores were significantly associated with lower risk of CKD progression, comparing participants in tertile 3 with participants in tertile 1 (HR: 0.83, 95% CI: 0.69–0.99) (P-trend=0.04).
Table 2.
Risk of chronic kidney disease progression, all-cause mortality, and composite of chronic kidney disease progression or all-cause mortality by tertile of each dietary scorea.
| Tertile 1 | Tertile 2 | Tertile 3 | PD | |
|---|---|---|---|---|
| No. events (IRC per 1000 p-y) | 301 (59.1) | 295 (52.6) | 259 (41.9) | |
| HEI-2015 | 1 (ref.) | 1.00 (0.85–1.18) | 0.91 (0.77–1.09) | 0.3 |
| No. events (IR per 1000 p-y) | 307 (59.5) | 294 (51.6) | 254 (42.2) | |
| AHEI-2010 | 1 (ref.) | 1.01 (0.85–1.19) | 0.83 (0.69–0.99) | 0.04 |
| No. events (IR per 1000 p-y) | 325 (58.1) | 323 (55.2) | 207 (38.1) | |
| aMed | 1 (ref.) | 1.10 (0.94–1.29) | 0.75 (0.62–0.90) | 0.002 |
| No. events (IR per 1000 p-y) | 347 (58.7) | 272 (55.3) | 236 (39.0) | |
| DASH | 1 (ref.) | 0.92 (0.78–1.09) | 0.83 (0.69–0.99) | 0.04 |
| All-Cause Mortality | ||||
| Tertile 1 | Tertile 2 | Tertile 3 | P | |
| No. events (IR per 1000 p-y) | 287 (35.8) | 257 (30.8) | 229 (26.3) | |
| HEI-2015 | 1 (ref.) | 0.83 (0.69–0.98) | 0.76 (0.63–0.92) | 0.004 |
| No. events (IR per 1000 p-y) | 293 (36.1) | 257 (30.7) | 223 (26.1) | |
| AHEI-2010 | 1 (ref.) | 0.84 (0.70–0.99) | 0.73 (0.60–0.88) | 0.001 |
| No. events (IR per 1000 p-y) | 312 (35.7) | 274 (30.9) | 187 (25.1) | |
| aMed | 1 (ref.) | 0.85 (0.72–1.01) | 0.69 (0.57–0.84) | <0.001 |
| No. events (IR per 1000 p-y) | 325 (35.1) | 224 (30.5) | 224 (26.5) | |
| DASH | 1 (ref.) | 0.78 (0.66–0.93) | 0.75 (0.62–0.90) | 0.002 |
| Composite of CKD Progression or All-Cause Mortality | ||||
| Tertile 1 | Tertile 2 | Tertile 3 | P | |
| No. events (IR per 1000 p-y) | 418 (82.1) | 406 (72.4) | 377 (61.0) | |
| HEI-2015 | 1 (ref.) | 0.96 (0.84–1.11) | 0.91 (0.79–1.06) | 0.2 |
| No. events (IR per 1000 p-y) | 433 (83.9) | 404 (70.9) | 364 (60.5) | |
| AHEI-2010 | 1 (ref.) | 0.94 (0.81–1.08) | 0.82 (0.71–0.96) | 0.01 |
| No. events (IR per 1000 p-y) | 462 (82.6) | 430 (73.5) | 309 (56.9) | |
| aMed | 1 (ref.) | 1.00 (0.87–1.15) | 0.77 (0.66–0.89) | <0.001 |
| No. events (IR per 1000 p-y) | 486 (82.3) | 363 (73.9) | 352 (58.1) | |
| DASH | 1 (ref.) | 0.87 (0.76–1.01) | 0.83 (0.72–0.97) | 0.01 |
Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) were adjusted for total energy intake, clinical site, age, sex, race, education, income level, estimated glomerular filtration rate, urinary protein, smoking status, physical activity, and alcohol status (for HEI-2015 and DASH scores), body mass index, diabetes mellitus, hypertension, cardiovascular disease, high-density lipoprotein cholesterol, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. AHEI, Alternative Healthy Eating Index; aMed, alternate Mediterranean diet; CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; HEI; Healthy Eating Index; HR, hazard ratio; IR, incidence rate; no, number; p-y, person-years; ref, reference.
P refers to p-value for a test of linear trend. Trend was tested using the median value within each tertile.
Crude incidence rate per 1,000 person-years.
Dietary Patterns and All-Cause Mortality
Over a median (interquartile range) follow-up time of 12 (8.4–13.2) years, there were 773 deaths. Participants in the highest tertile of HEI-2015 score had a 24% lower risk of all-cause mortality compared with tertile 1 (HR: 0.76, 95% CI: 0.63–0.92) (P-trend=0.004) (Table 2). For the AHEI-2010 score, participants in tertile 3 had a 27% (HR: 0.73, 95% CI: 0.60–0.88) (P-trend=0.001) lower risk of all-cause mortality compared with participants in tertile 1. There were similar inverse associations for aMed (HR: 0.69, 95% CI: 0.57–0.84) (P-trend<0.001) and DASH (HR: 0.75, 95% CI: 0.62–0.90) (P-trend=0.002).
Dietary Patterns and Composite Outcome of CKD Progression or Death
Using the composite outcome of CKD progression or death, estimates were similar to our results for CKD progression (Table 2). Participants in tertile 3 of AHEI-2010 (HR: 0.82, 95% CI: 0.71–0.96), aMed (HR: 0.77, 95% CI: 0.66–0.89), and DASH (HR: 0.83, 95% CI: 0.72–0.97) had a significantly lower likelihood of the composite outcome compared with participants in tertile 1.
Sensitivity Analyses
When we examined the individual components of the aMed score, we found that participants who consumed more vegetables (HR for T3 vs. T1: 0.80, 95% CI: 0.64–1.00) and nuts (HR for T3 vs. T1: 0.75, 95% CI: 0.62–0.90) had a lower likelihood of CKD progression (Figure 1).
Figure 1. Risk of chronic kidney disease progression by component of aMed score (comparing tertile 3 to tertile 1).
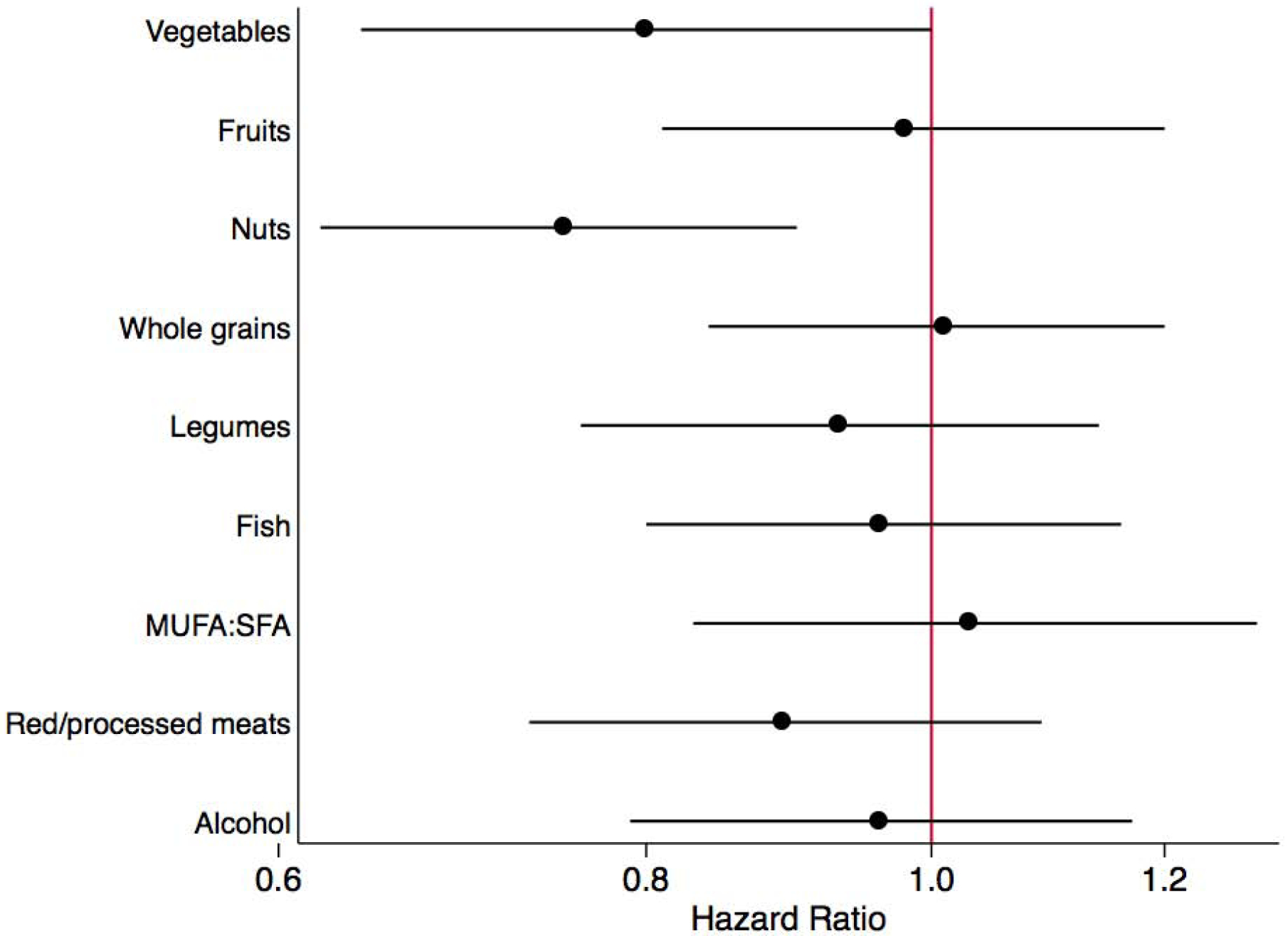
Risk of chronic kidney disease progression by component of aMed score (comparing tertile 3 to tertile 1)a.
a Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) on a logarithmic scale. Hazard ratios can be interpreted as the likelihood of chronic kidney disease progression comparing participants in tertile 3 to participants in tertile 1 for the given component of aMed score. Models were adjusted for total energy intake, clinical site, age, sex, race, education, income level, estimated glomerular filtration rate, urinary protein, smoking status, physical activity, body mass index, diabetes mellitus, hypertension, cardiovascular disease, high-density lipoprotein cholesterol, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, and all other components of the aMed score.
aMed, alternate Mediterranean diet; MUFA, monounsaturated fatty acids; SFA, saturated fatty acids.
When we individually adjusted for CRP, FGF-23, serum bicarbonate, blood urea nitrogen, and uric acid in addition to our main model for CKD progression, we did not find that any of these factors were strong mediators (Table S9).
There was a non-significant inverse linear trend between HEI-2015 and CKD progression (Figure S1). For the other scores, there was an inverse J-shape, but associations were only significant for values greater than the median. We did not find any consistent significant interactions by sex, age, race, diabetes, BMI, or eGFR. Our results were similar and slightly attenuated when we examined the associations using only the baseline diet instead of the cumulative average. When we excluded participants with a CKD progression event in the first 2 years of follow-up, the associations persisted.
DISCUSSION
In this prospective analysis of 2,403 individuals with CKD, we observed an inverse association between healthy dietary scores and risk of CKD progression and all-cause mortality. Higher diet quality based on each of the four dietary patterns evaluated was consistently associated with lower risk of death. Our findings were generally consistent across subgroups and in sensitivity analyses.
The association between aMed and CKD progression was the strongest, e.g. tertile 3 compared with tertile 1 was associated with a 25% reduced risk of CKD progression, compared with 17% for AHEI-2010, 17% for DASH, and 9% for HEI-2015. The strong association in aMed may be due to the individual components included in the score. We found that the vegetables and nuts components were independently associated with lower risk of CKD progression. aMed is the only score that includes its own component for nuts. Therefore, nuts were weighted more heavily in this index, which may be the reason why it had the strongest association. Previous studies have found a significant inverse association between a Mediterranean-style dietary pattern and incident CKD (3, 4) but not with CKD progression (28). An analysis in the PREDIMED (Prevención con Dieta Mediterránea) Study did not find differences in kidney function between participants who followed a Mediterranean-style diet compared with participants who followed a control low-fat diet after 1 year of follow-up (28).
Our results of an association between healthy dietary patterns and lower risk of CKD progression were in line with a previous study that found lower adherence to the DASH score was associated with increased risk of ESRD (relative hazard for Q1 vs. Q5: 1.7 (95% CI: 1.1–2.7) among people with CKD and hypertension in the NHANES study over a median follow-up time of 7.8 years (29). However, our results are contrary to a previous meta-analysis of three studies in 2 cohorts, which did not find an association between healthy dietary patterns and risk of ESRD (adjusted relative risk: 1.04, 95% CI: 0.68–1.40) (30). This may have been due to the relatively few number of ESRD cases recorded (1,027 events out of 10,071 participants), relatively short follow-up time (maximum of 7 years), or crude scoring criteria for healthy dietary scores.
Few randomized clinical trials have tested the effect of a healthy dietary pattern on kidney function among people with CKD. A review that included 17 randomized or quasi-randomized clinical trials of 1,639 people with CKD did not find food-based dietary interventions (e.g. DASH diet, Mediterranean diet, American Heart Association diet) to have an effect on ESRD, CVD, or all-cause mortality (31). However, healthy dietary interventions were associated with lower systolic and diastolic blood pressures and low-density lipoprotein cholesterol. In the review of diet interventions, the quality of evidence was very low for ESRD and mortality due to the short follow-up time of the studies and, consequently, the limited number of events. Previous randomized intervention studies have demonstrated that high consumption of fruits and vegetables among people with stage 2 and stage 4 CKD reduced markers of kidney injury and was comparable to the group that received oral sodium bicarbonate in regards to metabolic acidosis (32, 33). More trials are warranted to establish a causal association between healthy dietary interventions and kidney function among CKD patients.
Earlier studies have found an association between healthy dietary patterns and greater survival among people with CKD. In a meta-analysis of seven cohort studies, a healthy dietary pattern (rich in vegetables, fruits, legumes, whole grains, and fiber and low in red meat, sodium, and refined sugars) was associated with lower risk of mortality (adjusted relative risk: 0.73, 95% CI: 0.63–0.83) (30). Our results were consistent with these previous findings as all four of our dietary scores were inversely associated with all-cause mortality and similar in magnitude (HRs: 0.69 to 0.76) to the pooled estimate reported in the meta-analysis. The stronger inverse associations observed for death vs. CKD progression may be due to the beneficial impact of healthy diets on multiple chronic disease outcomes (CKD, CVD, cancer, diabetes) which can all increase mortality risk.
There may be several plausible biological mechanisms to explain the association between dietary patterns and CKD progression. Previous literature has suggested that a high dietary acid load may increase renal injury and CKD progression by elevating ammonium concentrations, causing complement activation or by stimulating endothelin-1 and aldosterone production, leading to fibrosis (34–36). Animal protein increases dietary acid load by producing acid after ingestion while fruits and vegetables decrease dietary net acid load because they are base-producing (35). Because healthy dietary patterns are characterized by high amounts of fruits and vegetables and low amounts of red and processed meats, they tend to have low dietary acid loads (37). Furthermore, fruits and vegetables contain numerous phytochemicals that may reduce oxidative stress and inflammation, and also deliver fiber, which can impact the gastrointestinal microbiota (38). A healthy low-fat diet that is rich in fiber has been found to be associated with increased microbiota diversity compared with moderate-to-low fiber diets and high-fat diets (39, 40). Adherence to a Mediterranean-style diet has been found to be associated with higher levels of short chain fatty acids, a marker of healthy microbiota from bacterial fermentation of complex carbohydrates, and higher proportions of beneficial microbiota (41, 42). In advanced CKD, uremia, accumulation of metabolites such as uric acid, and inadequate fiber may alter the biochemical environment, leading to dysbiosis, which may increase uremic toxins such as trimethylamine-N-oxide, indoxyl sulfate, and p-cresyl sulfate (42). These alterations in the gut are associated with increased CKD progression and complications.
In our study, we found that nuts were associated with lower risk of CKD progression. Nuts are a rich source of dietary magnesium, protein, phytate, and unsaturated fatty acids. Dietary magnesium may improve renal function by preventing endothelial dysfunction and inflammation (43, 44). Plant sources of protein including nuts and legumes have been found to be associated with lower serum concentrations of fibroblast growth factor-23 and higher serum bicarbonate levels, improving kidney function (45). Nuts and also deliver phytate, which improves phosphorus metabolism by lowering the rate of intestinal phosphorus absorption (46). Furthermore, nuts have important prebiotic properties due to high fiber content and polyphenols, which form bioactive metabolites when metabolized by the gut (42).
Our study had limitations. First, diet was self-reported by FFQs, which might have resulted in measurement error (47). To increase precision, we used a cumulative average of all available FFQs (18). Participants excluded from our study had less healthy baseline characteristics compared with included participants. Therefore, our results might underestimate the true association as our study population consisted of healthier participants. Second, the dietary scores that we examined are commonly used indices to assess diet quality among the general population. However, they may not be the optimal dietary patterns or scores for people with CKD, who still might benefit from restriction of certain nutrients. Our research suggests that diets high in fruits, vegetables, nuts, and legumes may be beneficial for kidney function but more randomized clinical trials intervening on dietary patterns are warranted to determine the optimal dietary pattern for people with CKD. Further evidence is needed to determine whether clinicians should recommend dietary patterns in lieu of or in addition to nutrient restrictions. Due to the observational nature of this study, there is likely to be residual confounding. However, we adjusted for known confounders, which were rigorously measured by trained staff. Our study also had several strengths. First, the cohort was very diverse, with white, black, and Hispanic men and women from seven sites, allowing for greater generalizability to people with CKD in the U.S. Second, the follow-up time was longer than previous studies, with a maximum of 14 years. Third, we had rigorous follow-up with ascertainment of CKD progression, incorporating both in-person visit data and linkage to the national registry for ESRD.
In summary, our study found that adherence to several healthy dietary patterns among people with CKD was associated with lower risk of CKD progression and even more strongly associated with lower risk of all-cause mortality. Our findings support a shift of nutritional advice for CKD patients from managing single nutrients to considering an overall food-based dietary pattern for better health outcomes. Future kidney guidelines should consider adopting a patterns approach to dietary recommendations.
Supplementary Material
Figure S1. Restricted cubic spline plot of adjusted hazard ratios for CKD progression by dietary score.
Table S1. Baseline characteristics of participants included in the present study and excluded from the present study
Table S2. Criteria for scoring HEI-2015, AHEI-2010, aMed, and DASH scores.
Table S3. Baseline characteristics of CRIC participants by tertile of HEI-2015 score.
Table S4. Baseline characteristics of CRIC participants by tertile of AHEI-2010 score.
Table S5. Baseline characteristics of CRIC participants by tertile of aMed score.
Table S6. Baseline characteristics of CRIC participants by tertile of DASH score.
Table S7. Correlation coefficients between dietary scores.
Table S8. Hazard ratios of covariates in Model 3 for aMed and CKD progression and all-cause mortality.
Table S9. Hazard ratios of CKD progression adjusting for C-reactive protein, fibroblast growth factor-23, serum bicarbonate, blood urea nitrogen, and uric acid levels
Acknowledgements:
The authors thank the staff and participants of the CRIC Study for their important contributions.
Support: Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA) National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago CTSA grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical & Translational Science Institute grant UL1 RR-024131. A.C.R. is funded by NIDDK award K23DK094829. S.E.R. receives salary support from NIDDK grants R01 HL127028 and 1UC4 DK101108. J.P.L. is funded by NIDDK awards K24DK092290 and R01-DK072231-91. EAH is supported by a grant from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (training grant T32 HL007024). DCC is supported, in part, by grant 1K24 HL148181 from NHLBI. KTM is supported, in part, by grant P20GM109036 from the National Institute of General Medical Sciences. CMR is supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782) and a grant from the National Heart, Lung, and Blood Institute (R21 HL143089). The funders had no role in the study design; collection, analysis, and interpretation of these data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Material Descriptive Text for Online Delivery
CRIC Study Investigators: The CRIC Study Investigators not already named in the author list include Alan S. Go, MD (Kaiser Permanente Division of Research), Panduranga S. Rao, MD (University of Michigan), and Raymond R. Townsend, MD (University of Pennsylvania).
Financial Disclosure: Dr. Scialla has received consulting fees from Tricida and modest research support for clinical trial event committees from GlaxoSmithKline and Sanofi. The remaining authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: Disclaimer: Some of the data reported here have been supplied by the U.S. Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Peer Review: Received November 25, 2019. Evaluated by 3 external peer reviewers, with editorial input from a Statistics/Methods Editor and an Acting Editor-in-Chief (Editorial Board Member Jeffrey Perl MD, FRCP(C), SM). Accepted in revised form April 23, 2020. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3S1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 3.Hu EA, Steffen LM, Grams ME, Crews DC, Coresh J, Appel LJ, et al. Dietary patterns and risk of incident chronic kidney disease: the Atherosclerosis Risk in Communities study. Am J Clin Nutr. 2019;110(3):713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, et al. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol. 2014;9(11):1868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, et al. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am J Kidney Dis. 2016;68(6):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis. 2011;57(2):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach KE, Kelly JT, Palmer SC, Khalesi S, Strippoli GFM, Campbell KL. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin J Am Soc Nephrol. 2019;14(10):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu EA, Rebholz CM. Can Dietary Patterns Modify Risk for CKD? Clin J Am Soc Nephrol. 2019;14(10):1419–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013(3):1–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Jules DE, Goldfarb DS, Sevick MA. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J Ren Nutr. 2016;26(5):282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Fouque D. Nutritional Management of Chronic Kidney Disease. N Engl J Med. 2017;377(18):1765–76. [DOI] [PubMed] [Google Scholar]
- 12.Elliott JO, Ortman C, Almaani S, Lee YH, Jordan K. Understanding the associations between modifying factors, individual health beliefs, and hemodialysis patients’ adherence to a low-phosphorus diet. J Ren Nutr. 2015;25(2):111–20. [DOI] [PubMed] [Google Scholar]
- 13.Thomas LK, Sargent RG, Michels PC, Richter DL, Valois RF, Moore CG. Identification of the factors associated with compliance to therapeutic diets in older adults with end stage renal disease. J Ren Nutr. 2001;11(2):80–9. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation and Academy of Nutrition and Dietetics. Clinical Practice Guideline for Nutrition in Chronic Kidney Disease: 2019 Update. October 2019. [in press]. [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–53. [DOI] [PubMed] [Google Scholar]
- 16.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 19.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 22.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 23.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Lopez A, Bullo M, Martinez-Gonzalez MA, Guasch-Ferre M, Ros E, Basora J, et al. Effects of Mediterranean diets on kidney function: a report from the PREDIMED trial. Am J Kidney Dis. 2012;60(3):380–9. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee T, Crews DC, Tuot DS, Pavkov ME, Burrows NR, Stack AG, et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019;95(6):1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly JT, Palmer SC, Wai SN, Ruospo M, Carrero JJ, Campbell KL, et al. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin J Am Soc Nephrol. 2017;12(2):272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer SC, Maggo JK, Campbell KL, Craig JC, Johnson DW, Sutanto B, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev. 2017;4:CD011998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8(3):371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Rios-Burrows N, et al. High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol. 2015;26(7):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scialla JJ, Appel LJ, Astor BC, Miller ER 3rd, Beddhu S, Woodward M, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda E, Ai M, Kuriyama R, Yoshida M, Shiigai T. Dietary acid intake and kidney disease progression in the elderly. Am J Nephrol. 2014;39(2):145–52. [DOI] [PubMed] [Google Scholar]
- 37.Scialla JJ, Anderson CA. Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis. 2013;20(2):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit Rev Food Sci Nutr. 2018;58(8):1260–70. [DOI] [PubMed] [Google Scholar]
- 39.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. [DOI] [PubMed] [Google Scholar]
- 40.Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12(3):169–81. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol. 2018;9:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, et al. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CA, Appel LJ, et al. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am J Nephrol. 2015;42(6):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudonck KJ, Mitchell MW, Nemet L, Keresztes L, Nyska A, Shinar D, et al. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol. 2009;37(3):280–92. [DOI] [PubMed] [Google Scholar]
- 45.Scialla JJ, Appel LJ, Wolf M, Yang W, Zhang X, Sozio SM, et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Ren Nutr. 2012;22(4):379–88 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(3):519–30. [DOI] [PubMed] [Google Scholar]
- 47.Paul DR, Rhodes DG, Kramer M, Baer DJ, Rumpler WV. Validation of a food frequency questionnaire by direct measurement of habitual ad libitum food intake. Am J Epidemiol. 2005;162(8):806–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Restricted cubic spline plot of adjusted hazard ratios for CKD progression by dietary score.
Table S1. Baseline characteristics of participants included in the present study and excluded from the present study
Table S2. Criteria for scoring HEI-2015, AHEI-2010, aMed, and DASH scores.
Table S3. Baseline characteristics of CRIC participants by tertile of HEI-2015 score.
Table S4. Baseline characteristics of CRIC participants by tertile of AHEI-2010 score.
Table S5. Baseline characteristics of CRIC participants by tertile of aMed score.
Table S6. Baseline characteristics of CRIC participants by tertile of DASH score.
Table S7. Correlation coefficients between dietary scores.
Table S8. Hazard ratios of covariates in Model 3 for aMed and CKD progression and all-cause mortality.
Table S9. Hazard ratios of CKD progression adjusting for C-reactive protein, fibroblast growth factor-23, serum bicarbonate, blood urea nitrogen, and uric acid levels


