Abstract
Arsenic exposure is well established to impair the function of zinc finger proteins, including PARP-1. Previous studies from our lab show that early developing T cells in the thymus are very sensitive to arsenite (As+3)-induced genotoxicity mediated through PARP-1 inhibition. Additionally, it has been shown that uranium (in the form of uranyl acetate, UA) also suppresses PARP-1 activity in HEK cells. However, very little is known about whether the As+3 metabolite, monomethylarsonous acid (MMA+3), also inhibits PARP-1 activity and if this is modified by combined exposures with other metals, such as uranium. In the present study, we found that MMA+3 significantly suppressed PARP-1 function, whereas UA at high concentrations significantly increased PARP-1 activity. To evaluate whether the effects on PARP-1 activity were mediated through oxidative stress, we measured the induction of hemoxygenase-1 (Hmox-1) expression by qPCR. MMA+3, but not UA, significantly induced oxidative stress; however, the inhibition of PARP-1 produced by MMA+3 was not reversed by the addition of the antioxidant, Tempol. Further evaluation revealed minimal interactive effects of MMA+3 and UA on PARP-1 function. Collectively, our results show that contrary to As+3, the suppressive effects of MMA+3 on PARP-1 were not substantially driven by oxidative stress in mouse thymus cells. Results for this study provide important insights into the effects of MMA+3 and uranium exposures on PARP-1 function, which is essential for future studies focused on understanding the effects of complex environmentally relevant metal mixtures.
Keywords: Poly-ADP-ribose polymerase-1 (PARP-1), Metals, Arsenic, Monomethylarsonous acid (MMA+3), Uranium, Immunotoxicity, T cells
Introduction
Poly-ADP-ribose polymerase-1 (PARP-1) is a zinc finger protein that has important roles in DNA repair and apoptosis in most mammalian cells and tissues (Sousa et al., 2012). The strongest expression of PARP-1 in cynomolgus monkeys was detected in the pituitary, ovaries, male adrenal glands, and the thymus (Ferreira et al., 2020). PARP-1 deficiency is associated with alterations in T cells and increased autoimmune diseases (Selvaraj et al., 2009). The thymus is an essential tissue for T cell development (Takaba and Takayanagi, 2017). In the thymus, greater than 95% of all T cells undergoing differentiation are deleted during processes known as positive and negative selection (Surh and Sprent, 1994; Daley et al., 2017) and during these selection processes, T cells in the thymus have a very high rate of cell proliferation. Humans exposed to arsenic in utero or in early life are known to have thymic involution and insufficiency (Ahmed et al., 2012).
PARP inhibitors are currently being explored as agents to treat cancer (Plummer, 2006). Although PARP-1 has been best studied, there are six isoforms of PARP (Hassa and Hottiger, 2008). Many of the roles of PARPs are not well understood and are independent of DNA repair. Recent studies have shown that PARP-1 has important signaling properties pertaining to cell survival and may play important roles in necroptosis and autophagy (Aredia and Scovassi, 2014).
Previous studies from our labs have shown that PARP-1 is sensitive to inhibition by arsenite (As+3) in a variety of mouse and human cell types (Qin et al., 2008b; Ding et al., 2009; Zhou et al., 2009; Qin et al., 2012; Zhou et al., 2015; Huestis et al., 2016; Zhou et al., 2016; Zhou et al., 2019). We have found that the mouse thymus is a particularly sensitive target of As+3 for PARP inhibition. In the mouse thymus, we have examined the inhibition of PARP by As+3 in cells exposed in vivo and in vitro (Xu et al., 2016a; Xu et al., 2016c). We are also interested in the modulation of PARP activity by other metals and mixed metal exposures.
In the present study we have compared the action of monomethylarsonous acid (MMA+3) and uranium (in the form of uranyl acetate, UA) on mouse thymic PARP activity. The potential for combined exposure to uranium and arsenic results from more than 4,000 abandoned uranium mines remaining in the United States, associated with more than 10,000 individual exposures sites, with many of these having arsenic as a co-contaminant in the waste (EPA, 2007). We performed these studies in thymus because uranium mine tailings are rich in these two metals and populations have expressed concerns that the combination of uranium and arsenic exposures may lead to immune alterations (Wan et al., 2006; Erdei et al., 2019; Greene et al., 2019).
Methods
Chemicals and Reagents
UA, UO2(OCOCH3)2.2H2O; U238 – 99.9%, U235 – 0.1% (>98% purity, CAS #541-09-3, Cat. No. 22400) was purchased from Electron Microscopy Sciences (Hatfield, PA). MMA+3, CH5AsO2 (≥95% purity, CAS 25400-23-1, Cat. No. M565100) was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) was purchased from Sigma Aldrich (St. Louis, MO). A list of additional reagents used in this study is presented in Supplementary Materials.
Primary mouse thymus cell isolation
All experiments were performed in accordance with protocols approved by the Institutional Animal Use and Care Committee at the University of New Mexico Health Sciences Center (UNM HSC). Male wildtype C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and allowed to acclimate in the animal resource facility at UNM HSC for 1 week prior to use in experimental procedures.
Thymus cells were isolated as previously described (Xu et al., 2016a; Xu et al., 2016c). In brief, thymi were harvested from 12-14-week-old mice and were maintained on ice in Hank’s balanced salt solution until preparation of cell suspensions. Single cell suspensions were prepared by homogenizing each thymus between the frosted end of two sterilized microscope slides (Fisher Scientific, Pittsburgh, Pennsylvania) in a dish containing complete mouse media (RPMI-1640 with 10% FBS, 2 mM L-glutamine, 100 mg/ml streptomycin/100 units/ml penicillin). Thymus cells from three mice were pooled and cell viability and counts were determined by acridine orange/propidium iodide (AO/PI) staining using a Nexcelom Cellometer 2000.
Assessment of PARP-1 activity
Thymus cells were exposed in vitro to MMA+3, UA, or MMA+3 + UA for 18 h and PARP-1 activity was measured using the PARP/Apoptosis ELISA kit (R&D Systems, Minneapolis, MN) as described by the kit manual and elsewhere (Sun et al., 2014; Xu et al., 2016c). In brief, thymus cell extracts were prepared according to the manufacturer’s protocol and total protein amounts were quantified using the BCA Protein Assay (Thermo Scientific, Rockford, Illinois). PARP-1 activity was measured in 200 ng of total protein and was determined by activating PARP-1 in each sample using a combination of damaged DNA and nicotinamide adenine dinucleotide which results in the PARylation and subsequent binding histone proteins coated on the wells of the ELISA plate. The amount of PAR was used as an indication of the level of PARP activity in a sample and was measured using an anti-PAR monoclonal antibody, followed by HRP conjugated secondary antibody, and incubation with a colorimetric substrate (TACSSapphire). Reactions were stopped with 0.2 M HCI and absorbance was measured at 450 nm using a SpectraMax 340PC microplate reader (Molecular Devices, San Jose, CA). The level of PARP-1 activity was quantified using an exponential standard curve generated from serial dilutions of PARP-1 standard provided in the kit. For all PARP assays, 1 μM etoposide (provided in the PARP/Apoptosis ELISA kit) was used as positive control according to manufacturer’s recommendations.
RNA isolation and qPCR.
RNA isolation was performed using the QIAshredder and RNeasy Kit according to manufacturer instructions (Qiagen, Germantown, MD). The quantity and quality of isolated RNA was determined using an Agilent Nanodrop spectrophotometer (Santa Clara, California). cDNA was prepared using 1 μg of total RNA with the High Capacity Reverse Transcription Kit (ThermoFisher Scientific, Waltham, MA). cDNA was diluted 1:10 in RNAse/DNase free water and utilized for qPCR. qPCR reactions (10 μl) were setup in triplicate, technical replicates for each sample using TaqMan Gene Expression Master Mix with TaqMan gene expression probes for Gapdh (Mm99999915_g1) and Hmox-1 (Mm00516005_m1) (ThermoFisher Scientific, Waltham, MA) and analyzed using a BioRad CFX384 Touch Real-Time PCR Detection System (BioRad, Hercules, CA). Gene expression was calculated with the comparative CT method using Gapdh as the endogenous control.
Statistics
Data analysis was performed using Sigma Plot version 12.5 (Systat Software). Differences between control (unexposed) and exposure groups were assessed using one-way analysis of variance followed by a Dunnett’s t-test. Comparisons of single exposure and combination exposure groups were determined using a one-way ANOVA followed by a Tukey’s multiple comparisons post hoc test. Statistically significant changes were defined as p<0.05. For all experiments, samples were prepared in triplicate for each exposure group and were analyzed using triplicate technical replicates in each assay. Two to three independent experiments were performed with consistent results attained.
RESULTS
Differential effects of MMA+3 vs. UA on PARP-1 activity in mouse thymus cells
To determine whether MMA+3 produces inhibitory effects on PARP-1, we exposed primary mouse thymus cells in vitro to increasing concentrations of MMA+3 (i.e. 0, 50, and 500 nM) for 18 h. Following exposure, PARP-1 activity was assessed in thymus cell extracts using the mouse PARP/Apoptosis ELISA kit from R&D Systems. A dose-dependent suppression of PARP-1 activity was found with concentrations of MMA+3 as low as 50 nM (~20% reduction) and was almost completely inhibited (~88% reduction) by 500 nM MMA+3 (Fig. 1A).
Figure 1.
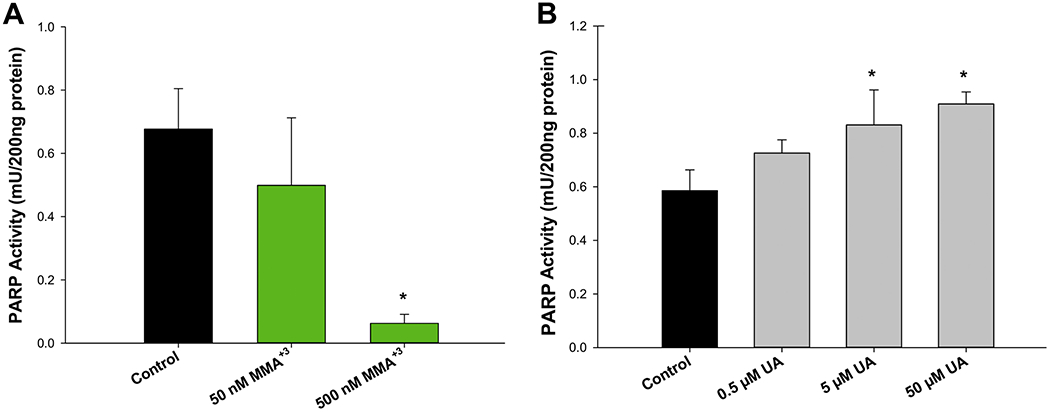
MMA+3 and UA produce opposing effects on PARP-1 activity in mouse thymus cells. Mouse thymus cells were exposed in vitro to MMA+3or UA for 18 h and PARP-1 activity was measured using the mouse PARP/Apoptosis ELISA kit from R&D Systems. (A) Inhibition of PARP-1 activity caused by exposure to 0, 50, 100, and 500 nM MMA+3. (B) Increased PARP-1 activity produced by exposure to 0, 0.5, 5, and 50 μM UA UA. Data are presented as mean ± SD. *Statistically significant difference (p<0.05) in one-way ANOVA followed by a Dunnett’s t-test compared to the control group.
To determine the effects of UA on PARP-1 activity, mouse thymus cells were exposed to 0.5, 5, and 50 μM for 18 h and PARP-1 activity was assessed by ELISA. In contrast to MMA+3, UA exposures produced a dose-dependent increase in PARP-1 activity (Fig. 1B). PARP-1 activity was significantly elevated with both the 5 and 50 μM UA treatments (Fig. 1B), which was not significantly mediated by changes in PARP protein expression (Supplementary Fig. S1). Collectedly, these results suggest that MMA+3 and UA produce differential and perhaps opposing effects on PARP-1 activity in mouse thymus cells.
MMA+3, but not UA produces oxidative stress in mouse thymus cells, as indicated by the induction of Hmox-1 expression
Oxidative stress is well known to play an essential role in the PARP-1 inhibition caused by As+3; however, the extent that the PARP-1 inhibition produced by MMA+3 has not been determined. To determine if the inhibition of PARP-1 activity produced by MMA+3 is mediated by oxidative stress we performed qPCR to measure the induction of Hmox-1, a well-established marker of oxidative stress, following exposure of thymus cells to 0, 100, 200, 300, 400, and 500 nM MMA+3 (Fig. 2A). MMA+3 exposure significantly increased Hmox-1 expression in a dose-dependent manner. MMA+3 concentrations greater than 300 nM were found to increase Hmox-1 expression ~6 fold greater than the control group (Fig. 2A).
Figure 2.
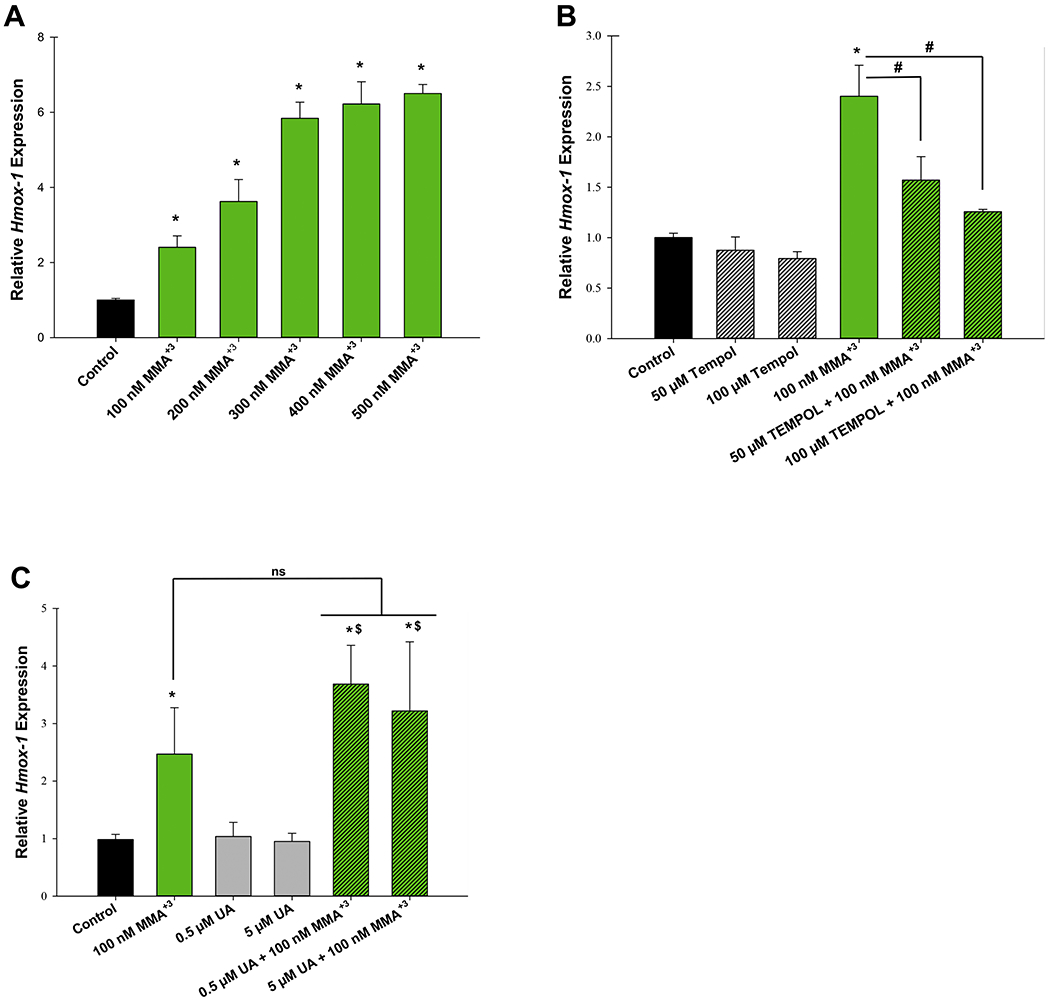
MMA+3, but not UA induces oxidative stress in mouse thymus cells. Mouse thymus cells were exposed in vitro to MMA+3 or UA for 4 h and Hmox-1 expression was measured by qPCR. (A) Dose dependent increase of Hmox-1 expression. (B) The antioxidant, Tempol significantly reverses MMA+3-induced Hmox-1 expression. (C) Exposure to UA at 0.5 and 5 μM does not induce Hmox-1 expression. Data are presented as mean ± SD. *Statistically significant difference (p<0.05) in one-way ANOVA followed by a Dunnett’s t-test compared to the control group. Panel A: one-way ANOVA followed by a Dunnett’s t-test; panels B and C: one-way ANOVA followed by a Tukey’s multiple comparisons post hoc test. *Statistically significant difference (p<0.05) compared to the control group. #Statistically significant difference (p<0.05) compared to 100 nM MMA+3. $Statistically significant difference (p<0.05) compared to individual UA doses, ns, no statistically significant difference compared to 100 nM MMA+3.
To verify that the induction of Hmox-1 was produced by oxidative stress, we co-treated mouse thymus cells with 100 nM MMA+3 and the antioxidant, Tempol and subsequently measured levels of Hmox-1 by qPCR. We found that addition of 50 and 100 μM Tempol significantly reversed the induction of Hmox-1 expression produced by 100 nM MMA+3 to levels comparable to the untreated control group (Fig. 2B). UA did not induce Hmox-1 expression, nor did it significantly modify the increase of Hmox-1 expression induced by MMA+3 (Fig. 2C).
MMA+3-induced suppression of PARP-1 activity is not significantly mediated by oxidative stress
To determine whether in suppression of PARP-1 activity produced by MMA+3 exposures was mediated via oxidative stress, we co-treated thymus cells with 100 or 500 nM MMA+3 + 100 μM Tempol for 18 h and evaluated using the mouse PARP/Apoptosis ELISA kit from R&D Systems. A significant inhibition of PARP-1 activity was found with 500 nM MMA+3, but this was not significantly modified by co-treatment with 100 μM Tempol (Fig. 3). These results suggest that the inhibition of PARP-1 activity induced by MMA+3 was not substantially mediated by oxidative stress.
Figure 3.
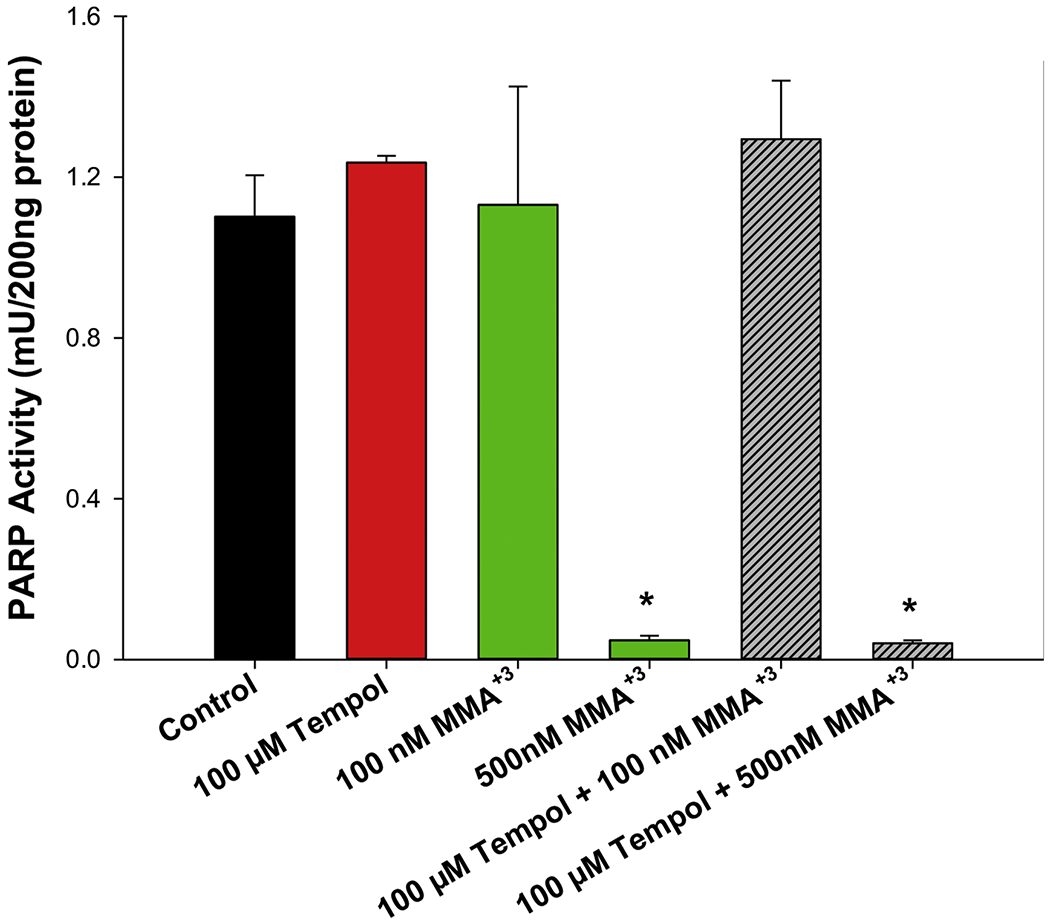
MMA+3-induced inhibition of PARP-1 activity is not reversed by the antioxidant, Tempol. Mouse thymus cells were exposed in vitro to 100 nM MMA+3 +/− 50 or 100 μM Tempol for 18 h and PARP-1 activity was measured using the mouse PARP/Apoptosis ELISA kit from R&D Systems. Data are presented as mean ± SD. *Statistically significant difference compared to the control group (p<0.05) in one-way ANOVA followed by a Tukey’s multiple comparisons post hoc test.
compared to the control group.
MMA+3 and UA produce minimal interactive effects on PARP-1 activity
Based on the differential effects of MMA+3 and UA on PARP-1 activity, we sought to determine whether the two metals would produce interactive effects on PARP activity. PARP-1 activity was determined following exposure to 100 nM MMA+3 or 0.5 and 5 μM UA, alone or in combination for 18 h. No substantial interactive effects of MMA+3 and UA were found; however, there was a modest improvement of PARP-1 activity levels with combined exposures to 100 nM MMA+3 + 0.5 and 5 μM UA, when compared to MMA+3 exposure alone (Fig. 4). No statistically significant differences were found between the individual UA exposures (5 or 50 μM) and the combined treatments with 100 nM MMA+3 alone (Fig. 4). Interestingly, the effects of UA appear to outweigh the reduction of PARP-1 activity produced by MMA+3, but only to the level of the individual UA exposures (Fig. 4). However, UA did not significantly modulate the strong inhibitory effect on PARP activity produced by high level exposure to 500 nM MMA+3 (Supplementary Fig. S2).
Figure 4.
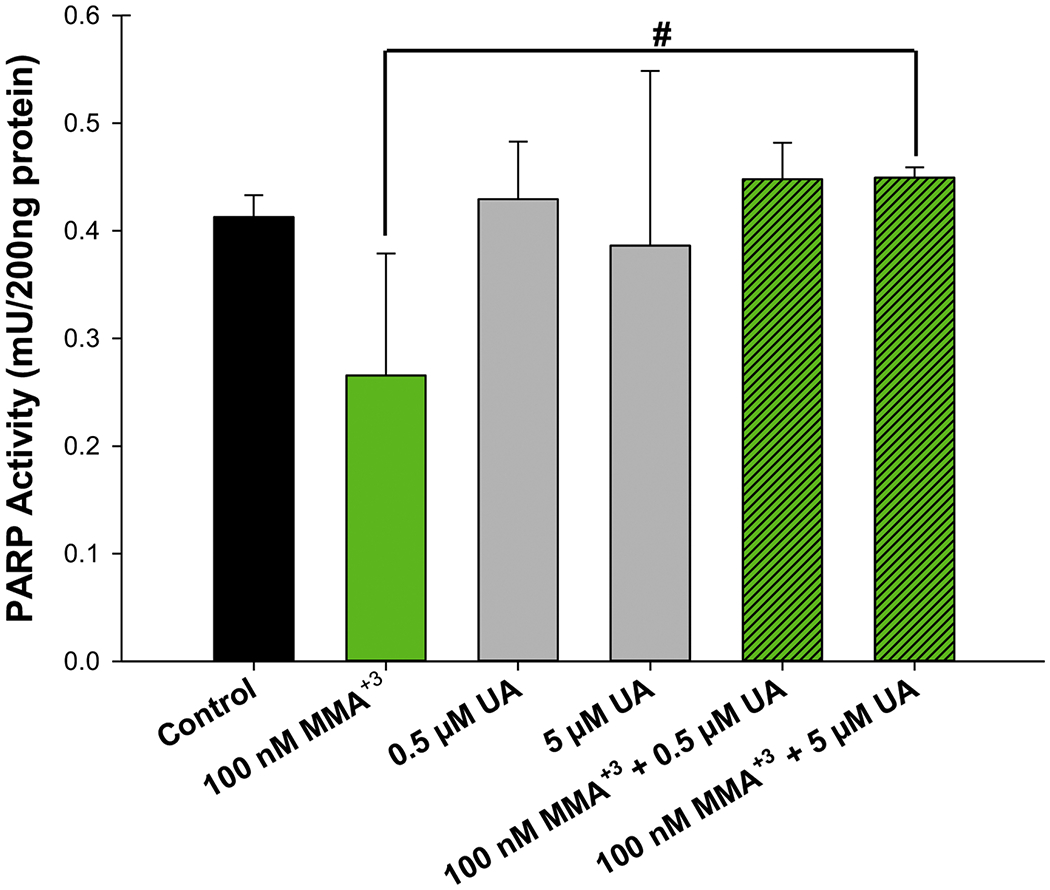
MMA+3 and UA do not produce substantial interactive effects on PARP-1 activity in mouse thymus cells. Mouse thymus cells were exposed in vitro to 100 nM MMA+3 +/− 0.5 or 5 μM UA for 18 h and PARP-1 activity was measured using the mouse PARP/Apoptosis ELISA kit from R&D Systems. Data are presented as mean ± SD. Statistically significant difference (p<0.05) compared to control (*) or 100 nM MMA+3 (#) in one-way ANOVA followed by a Tukey’ multiple comparisons post hoc test.
DISCUSSION
Uranium, arsenic, and other metals are commonly found in mill tailings surrounding abandoned uranium and hard rock mines in New Mexico and elsewhere (EPA, 2007; Hoover et al., 2017; Lewis et al., 2017). Our studies show that humans living near these mines have elevated exposures to metals, in particular uranium and mercury and have increased prevalence of markers for autoimmune diseases in their blood (Ong et al., 2014; Erdei et al., 2019). Metals exert complex effects on zinc finger proteins exemplified by PARP (Hartwig et al., 2002a; Hartwig et al., 2002b). Previous studies by our group have found that metal exposures cause DNA damage and inhibition of DNA repair associated with the suppression of PARP activity in a variety of in vitro models (Qin et al., 2012; Sun et al., 2014; Zhou et al., 2015; Zhou et al., 2016; Zhou et al., 2019).
We are interested in how metals alter PARP activity in the mouse thymus, as the development of T cells which are involved in systemic immune responses have been found to be particularly sensitive to PARP inhibition by arsenic (Xu et al., 2016c; Xu et al., 2017b; Xu et al., 2018). Clinically, it is known that in utero and early childhood arsenic exposures cause thymic atrophy and severe immune consequences (Ahmed et al., 2012; Ahmed et al., 2014). From our mouse studies, it appears that immature CD3+ double negative (DN, CD4−, CD8−) cells are the most sensitive targets in the thymus (Xu et al., 2017a). The reason for this sensitivity appears to be two-fold: 1) thymic DN cells do not express the mrp and mdr transporters needed to export As+3 and MMA+3 and 2) MMA+3 uptake and retention in DN cells is very high.
Previously, we found that drinking water exposure of mice to As+3 produced a dose-dependent increase in DNA fragmentation in the thymus, which was associated with PARP inhibition (Xu et al., 2017b). As+3 produced transient oxidative stress in thymus cells treated in vitro, with a peak at 4 hr, as measured by dihyrdro-ethidium (DHE) flow cytometry and Hmox-1 mRNA induction (Xu et al., 2016c). Phosphorylation of γH2AX was also transient and maximal 4 hrs after As+3 exposure of thymus cells in vitro. Thus, there is strong evidence that both in vivo and in vitro exposures to As+3 inhibit PARP activity and thereby allow for the persistence of DNA damage. As+3 binds to PARP-1 zinc finger C3H1 and C4 domains to release zinc (Zhou et al., 2011; Sun et al., 2014; Zhou et al., 2014; Huestis et al., 2016). As+3 binding to the PARP zinc finger make it more vulnerable for the redox sensitive cysteines in zinc finger to be oxidized by reactive oxidative species (ROS) (Shi et al., 2004; Qin et al., 2008a; Xu et al., 2016d). Thus, there is interest in examining the role of oxidative stress and subsequent ROS in the action of metals on thymic PARP.
In the present studies, we have further evaluated the effects of MMA+3 on PARP activity in the absence or presence of UA, a commercially available depleted uranium model compound. MMA+3 was examined because we have found that following oral As+3 exposures in mice, MMA+3 is the predominant form or arsenic found intracellularly in the thymus (Xu et al., 2016b). Drinking water exposure of mice to 500 ppb (μg/L) As+3 resulted in a significant accumulation of MMA+3 in the thymus, reaching concentrations as high as 408 nM (Xu et al., 2016b). Intriguingly, there was only minimal amounts of As+3 detected in the thymus (Xu et al., 2016b). These findings suggest that the thymus receives significant MMA+3 exposure and that the previously observed toxicity to developing T cells at this site may be driven primarily by MMA+3 and not As+3. As such, in the present study, we focused on doses of MMA+3 exposure (i.e., 100 and 500 nM) that were achieved physiologically following oral exposures to environmentally relevant levels of As+3.
We have previously found that MMA+3 produces significant genotoxicity to early DN and DP following in vitro exposures of mouse thymus cells for 18 hr (Xu et al., 2017b). While the oxidative stress produced by MMA+3 was inhibited by Tempol, we did not find that Tempol prevented MMA+3-induced PARP inhibition. These findings are expected since MMA+3 binding with PARP and inhibiting its activity is oxidative stress independent (Qin et al., 2008a). Tempol treatment does not impact arsenic binding (i.e. no change on PARP activity), but reduces overall oxidative level in cells. This result is similar to our previous finding with As+3, where Tempol produced a small protection against As+3-induced DNA damage, but not PARP inhibition (Xu et al., 2016d).
For uranium, a previous report for human skin cells treated in vitro with As+3 after UV radiation found that UA exacerbated H2O2-induced PARP-1 inhibition, but on its own, UA at high concentrations actually increased PARP-1 activity (Cooper et al, 2016). These results are in agreement with our findings for the thymus where we found a dose-dependent increase in PARP activity produced by UA. Although the form of uranium used in the present study, UA is depleted of radioactivity, it is not completely devoid. As such it is possible that these low levels of radioactivity may be producing DNA damage and subsequently increasing PARP activity. In support, Cooper et al., 2016 found that increasing UA doses increased the presence of DNA strand breaks as measured by the phosphorylation of γH2AX.
In conclusion, our results suggest that there are important differences between the action of MMA+3 and As+3 on PARP in the thymus and other tissues. While the binding of As+3 to PARP-1 has been well studied, there are other isoforms of PARP that are present in the thymus that also need to be evaluated. The binding of arsenicals and zinc release from PARP isoforms has also not been well-studied, nor do we have a complete understanding of the effects of oxidative stress on PARP activity. It is also important to understand the effects of other environmentally prevalent metals, such as uranium, copper, mercury, and others on PARP. We also know that these metals are present in various inorganic and organic forms in nature that haven’t been well-characterized. To understand the effects of environmental metals, we need to study complex mixtures at environmentally and physiologically relevant exposure levels.
Supplementary Material
Highlights.
MMA+3 inhibits PARP-1 activity in mouse thymus cells.
UA, at high concentrations, increases PARP-1 activity in mouse thymus cells.
MMA+3 effects on PARP-1 were not substantially mediated by oxidative stress.
MMA+3 + UA exposures produced minimal interactive effects on PARP-1 activity.
Acknowledgments
This work was funded by the National Institute of Environmental Health Sciences, UNM METALS Superfund Research Program [grant number P42 ES025589] and the National Cancer Institute, UNM Comprehensive Cancer Center [grant number P30 CA118100].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Supplementary data
References
- Ahmed S, Ahsan KB, Kippler M, Mily A, Wagatsuma Y, Hoque AM, Ngom PT, El Arifeen S, Raqib R, Vahter M, 2012. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicological sciences : an official journal of the Society of Toxicology 129, 305–314. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Moore SE, Kippler M, Gardner R, Hawlader MD, Wagatsuma Y, Raqib R, Vahter M, 2014. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicological sciences : an official journal of the Society of Toxicology 141, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aredia F, Scovassi AI, 2014. Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol 92, 157–163. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG, 2016. Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium. Toxicol Appl Pharmacol 291, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley SR, Teh C, Hu DY, Strasser A, Gray DHD, 2017. Cell death and thymic tolerance. Immunol Rev 277, 9–20. [DOI] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ, 2009. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem 284, 6809–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, U., 2007. Technical report on technologically enhanced naturally occurring radioactive materials from uranium mining: volume 1: mining and reclamation background. Washington, DC: , US Environmental Protection Agency, Office of Radiation and Indoor Air, Radiation Protection Division 2008 Contract No.: EPA 402-R-08-005, https://www.epa.gov/sites/production/files/2015-05/documents/402-r-08-005-v2.pdf, pp [Google Scholar]
- Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, Rubin RL, 2019. Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo Nation. J Autoimmun 99, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MT, Berger L, Rouleau M, Poirier GG, 2020. Assessment of PARP-1 Distribution in Tissues of Cynomolgus Monkeys. J Histochem Cytochem 68, 413–435. [DOI] [PubMed] [Google Scholar]
- Greene AD, Kendziorski JA, Buckholz JM, Niu L, Xie C, Pinney SM, Burns KA, 2019. Elevated serum chemokines are independently associated with both endometriosis and uranium exposure. Reprod Toxicol 84, 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A, Asmuss M, Blessing H, Hoffmann S, Jahnke G, Khandelwal S, Pelzer A, Bürkle A, 2002a. Interference by toxic metal ions with zinc-dependent proteins involved in maintaining genomic stability. Food Chem Toxicol 40, 1179–1184. [DOI] [PubMed] [Google Scholar]
- Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Bürkle A, 2002b. Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ Health Perspect 110 Suppl 5, 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO, 2008. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci 13, 3046–3082. [DOI] [PubMed] [Google Scholar]
- Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J, 2017. Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA. Expo Health 9, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis J, Zhou X, Chen L, Feng C, Hudson LG, Liu KJ, 2016. Kinetics and thermodynamics of zinc(II) and arsenic(III) binding to XPA and PARP-1 zinc finger peptides. J Inorg Biochem 163, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Hoover J, MacKenzie D, 2017. Mining and Environmental Health Disparities in Native American Communities. Curr Environ Health Rep 4, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J, Erdei E, Rubin RL, Miller C, Ducheneaux C, O’Leary M, Pacheco B, Mahler M, Henderson PN, Pollard KM, Lewis JL, 2014. Mercury, autoimmunity, and environmental factors on cheyenne river sioux tribal lands. Autoimmune Dis 2014, 325461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer ER, 2006. Inhibition of poly(ADP-ribose) polymerase in cancer. Curr Opin Pharmacol 6, 364–368. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ, 2008a. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol 21, 1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Timmins GS, Liu KJ, 2008b. Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol Appl Pharmacol 232, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Liu W, Li YN, Sun X, Hai CX, Hudson LG, Liu KJ, 2012. Poly(ADP-ribose) polymerase-1 inhibition by arsenite promotes the survival of cells with unrepaired DNA lesions induced by UV exposure. Toxicological sciences : an official journal of the Society of Toxicology 127, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Soundarapandian MM, Chechneva O, Williams AJ, Sidorov MK, Soulika AM, Pleasure DE, Deng W, 2009. PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis. J Biol Chem 284, 26070–26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Ding W, Wang S, Cooper KL, Liu S, Chen Y, Shi X, Liu KJ, 2004. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol 17, 871–878. [DOI] [PubMed] [Google Scholar]
- Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JA, Larsen AK, Saffi J, 2012. PARPs and the DNA damage response. Carcinogenesis 33, 1433–1440. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhou X, Du L, Liu W, Liu Y, Hudson LG, Liu KJ, 2014. Arsenite binding-induced zinc loss from PARP-1 is equivalent to zinc deficiency in reducing PARP-1 activity, leading to inhibition of DNA repair. Toxicol Appl Pharmacol 274, 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J, 1994. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372, 100–103. [DOI] [PubMed] [Google Scholar]
- Takaba H, Takayanagi H, 2017. The Mechanisms of T Cell Selection in the Thymus. Trends Immunol 38, 805–816. [DOI] [PubMed] [Google Scholar]
- Wan B, Fleming JT, Schultz TW, Sayler GS, 2006. In vitro immune toxicity of depleted uranium: effects on murine macrophages, CD4+ T cells, and gene expression profiles. Environ Health Perspect 114, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Lauer FT, Liu KJ, Hudson LG, Burchiel SW, 2016a. Editor’s Highlight: Interactive Genotoxicity Induced by Environmentally Relevant Concentrations of Benzo(a)Pyrene Metabolites and Arsenite in Mouse Thymus Cells. Toxicological sciences : an official journal of the Society of Toxicology 154, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, McClain S, Medina S, Lauer FT, Douillet C, Liu KJ, Hudson LG, Stýblo M, Burchiel SW, 2016b. Differential sensitivities of bone marrow, spleen and thymus to genotoxicity induced by environmentally relevant concentrations of arsenite. Toxicol Lett 262, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Medina S, Lauer FT, Douillet C, Liu KJ, Hudson LG, Stýblo M, Aleksunes LM, Burchiel SW, 2017a. Efflux Transporters Regulate Arsenite-Induced Genotoxicity in Double Negative and Double Positive T Cells. Toxicological sciences : an official journal of the Society of Toxicology 158, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Medina S, Lauer FT, Douillet C, Liu KJ, Styblo M, Burchiel SW, 2017b. Genotoxicity induced by monomethylarsonous acid (MMA(+3)) in mouse thymic developing T cells. Toxicol Lett 279, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Burchiel SW, 2018. Toxicity of environmentally-relevant concentrations of arsenic on developing T lymphocyte. Environ Toxicol Pharmacol 62, 107–113. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhou X, Wen X, Lauer FT, Liu KJ, Hudson LG, Aleksunes LM, Burchiel SW, 2016c. Environmentally Relevant Concentrations of Arsenite Induce Dose-Dependent Differential Genotoxicity Through Poly(ADP-Ribose) Polymerase Inhibition and Oxidative Stress in Mouse Thymus Cells. Toxicol Sci. 149, 31–41. doi: 10.1093/toxsci/kfv1211 Epub 2015 Oct 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhou X, Wen X, Lauer FT, Liu KJ, Hudson LG, Aleksunes LM, Burchiel SW, 2016d. Environmentally Relevant Concentrations of Arsenite Induce Dose-Dependent Differential Genotoxicity Through Poly(ADP-Ribose) Polymerase Inhibition and Oxidative Stress in Mouse Thymus Cells. Toxicological sciences : an official journal of the Society of Toxicology 149, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF, Liu JP, Chowbay B, 2009. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41, 89–295. [DOI] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Huestis J, Xu H, Burchiel SW, Hudson LG, Liu KJ, 2016. S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite. Oncotarget 7, 80482–80492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG, 2015. Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding. J Biol Chem 290, 18361–18369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ding X, Shen J, Yang D, Hudson LG, Liu KJ, 2019. Peroxynitrite contributes to arsenic-induced PARP-1 inhibition through ROS/RNS generation. Toxicol Appl Pharmacol 378, 114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG, 2011. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem 286, 22855–22863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Mobarak C, Gandolfi AJ, Burchiel SW, Hudson LG, Liu KJ, 2014. Differential binding of monomethylarsonous acid compared to arsenite and arsenic trioxide with zinc finger peptides and proteins. Chem Res Toxicol 27, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


