Abstract
Background:
Prenatal exposure to metals may play an important role in fetal growth. However, the epidemiologic evidence for certain metals is sparse, and most of the existing research has focused on evaluating single metals in highly exposed target populations.
Objectives:
We evaluated associations of cadmium, lead, manganese, selenium, and total mercury exposures during pregnancy with fetal growth using data from mother-infant pairs participating in the National Children’s Study.
Methods:
Prenatal metal exposures were measured using maternal blood collected from 6 to 32 weeks of gestation. Birth outcomes, including gestational age, birthweight, birth length, head circumference, and ponderal index, were ascertained through physical measurement at birth or abstraction from medical records. Regression coefficients and their 95% confidence intervals were estimated from multivariable linear regression models in the overall study population as well as among male and female infants. We further evaluated pairwise metal-metal interactions.
Results:
Sex-specific associations were observed for lead, with inverse associations for birthweight, birth length, head circumference, and gestational age observed only among female infants. Sex-specific associations were also observed for selenium, with a positive association for birthweight observed among male infants; selenium was also positively associated with ponderal index and inversely associated with birth length among female infants. Overall, total mercury was inversely associated with birthweight and ponderal index, and the association with birthweight was stronger among female infants. No significant associations were observed with cadmium and manganese. In the metal-metal interaction analyses, we found evidence of a synergistic interaction between lead and total mercury and antagonistic interaction between selenium and total mercury with selected birth outcomes.
Conclusions:
Our findings suggest that prenatal exposure to metals may be related to birth outcomes, and infant sex may modify these associations.
Keywords: Metal mixtures, prenatal exposure, birth outcomes, fetal growth, sex
Introduction
Growth in fetal and early infant life may influence disease outcomes later in life (1, 2). Specifically, low birthweight, an indicator of poor fetal growth, has been associated with cardiovascular disease (3–8), high blood pressure (9–11), impaired glucose tolerance (12), and diabetes mellitus (10, 13) in adult life. Additionally, intrauterine growth restriction and infants born small for gestational age are more susceptible to several adverse health outcomes such as cardiovascular disease and neurodevelopmental dysfunction (14–16). Significant risk factors for growth restriction in infants include maternal malnutrition (17), older maternal age (18, 19), socioeconomic factors (e.g., lower levels of educational attainment and unmarried marital status) (20, 21), maternal smoking (20, 22, 23), maternal alcohol use (24, 25), short interpregnancy interval (26, 27), inadequate gestational weight gain (28, 29), and placental or umbilical cord abnormalities (18, 30, 31). Notably, an increasing number of studies have suggested that prenatal exposures to metals may play an important role in fetal growth (32–44).
Pregnancy, gestation, and early development are unique health states, and previous work suggests they may be critical periods for susceptibility to the effects of metals due to hemodynamics, hormone changes, and immature immune systems (45, 46). Moreover, metals such as lead, mercury, and manganese readily pass the placental barrier and thus may affect fetal development (47, 48). Previous epidemiologic studies have provided suggestive evidence for the associations between toxic metal exposures and fetal growth. Cadmium, lead, and mercury are inversely associated with birthweight, birth length, and head circumference in observational studies (32–40). Epidemiologic research evaluating essential metals is limited but suggests positive associations of selenium with birthweight and birth length (41, 42). Additionally, there seems to be an optimal range of exposure to some essential metals; for instance, both low and high levels of manganese are associated with lower birthweight and smaller head circumference (43, 44). However, the existing epidemiologic literature is sparse, and the associations of metals with birth outcomes are still equivocal. Furthermore, prior epidemiologic research has focused on evaluating exposures to metals singly (rather than assessing joint effects) and in predominately highly exposed target populations.
There are known sex differences in metal exposure patterns, gastrointestinal absorption, and fetal growth (49, 50). Additionally, previous epidemiologic studies suggest the sex-specific effects of metals on fetal growth (40, 51). In this study, we evaluated the association between prenatal exposure to five metals (cadmium, lead, mercury, manganese, and selenium) and fetal growth overall and by infant sex in a cohort of mother-infant pairs from the National Children’s Study (NCS). We aimed to identify metals that may affect fetal growth and potential joint effects of metals in a general population sample with relatively low-level exposure. This study could help inform environmental health policies and provide insight into clinical blood metal screening during pregnancy.
Methods
Study Population
Women enrolled in the Initial Vanguard Study (IVS) of the NCS were included in the current analysis. The IVS was conducted between January 2009–September 2010 in seven primary sampling units using a geographically-based probability sample design. Recruitment information is detailed elsewhere (52). The following eligibility criteria were required for participation in the study: 1) household screening performed during or before the woman’s pregnancy; 2) current pregnancy or high probability of pregnancy; and, 3) ability to grant informed consent. A total of 618 mother-infant pairs were enrolled in the study. Two pregnancy visits were conducted for women enrolled during the first trimester: one during the first trimester (T1-first) and the other during the third trimester (T3-prior). For women enrolled during the third trimester, only one visit was conducted (T3-first). Each pregnancy visit consisted of a comprehensive interview, self-administered questionnaires, a physical examination, and biospecimen and environmental sample collections.
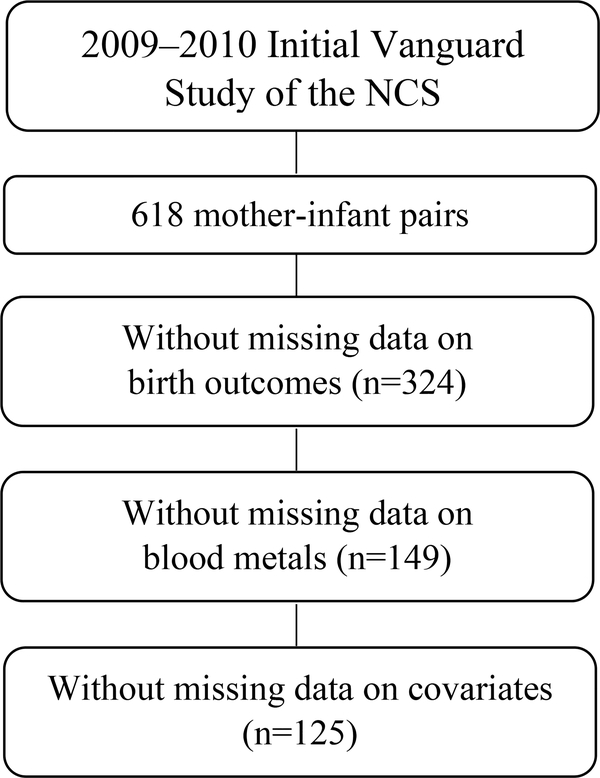
Since the purpose of the IVS was to test study procedures and feasibility, many changes were made throughout the 2-year study period. Specifically, changes in the data collection protocols led to only a subset of participants with all the needed variables for the current analysis. Among the 618 mother-infant pairs, 125 dyads that were singleton births without missing data were included in the present study to evaluate the associations between birth outcomes and maternal blood metal concentrations (Figure 1).
Figure 1.
Inclusion of National Children’s Study participants for analytical sample size.
Assessment of Exposures
Among the 125 dyads, venous blood collected between 6 and 32 weeks of gestation in ethylenediaminetetraacetic acid (EDTA) vacutainer tubes was used to measure whole blood metal concentrations. Blood cadmium, lead, manganese, selenium, and total mercury were measured using high-performance liquid chromatography coupled to inductively-coupled-plasma dynamic-reaction-cell mass spectrometry (ICP-DRC-MS). The EDTA tubes were prescreened for trace amounts of these metals before blood collection. The limit of detection (LOD) for each blood metal is as follows: cadmium (0.16 μg/L), lead (0.25 μg/dL), manganese (1.06 μg/L), selenium (30 μg/L), and total mercury (0.16 μg/L) (53). Blood manganese and selenium were >LOD in all participant samples; however, blood total mercury, cadmium, and lead were not detected in 3.2%, 18.4%, and 28.0% of samples, respectively. For values <LOD, we imputed the LOD divided by the square root of two. The substitution method of has been demonstrated to perform well when the censoring levels are below 50% (54).
Assessment of Birth Outcomes
The NCS protocol included a physical examination of infants at birth. Birth length (cm) and head circumference (cm) were measured twice, and the average of the two readings was used. For offspring without measures of birth length and head circumference (n=6), we used values available from medical records abstracted by the NCS using Community Health Information Architecture (CHITA) instruments. The Pearson correlation coefficients comparing measures between the physical examinations and medical records were 0.47 and 0.65 for birth length (n=118) and head circumference (n=118), respectively. Birthweight and gestational age were abstracted from medical records by the NCS using the same instruments. Gestational age was determined based on ultrasound (n=35), last menstrual period (n=46), and postnatal examination (n=9); other sources used to define gestational age included progress notes and gestational age by dates (n=9). There were 26 infants missing the information on the source used to define gestational age. Ponderal index, an indicator of infant adiposity as well as a measure of in utero growth restriction (55, 56), was derived by dividing birthweight in kilograms by cubed birth length in meters (kg/m3).
Assessment of Covariates
Self-reported maternal characteristics, including age, race/ethnicity, educational attainment, family income, smoking status during pregnancy, alcohol use during pregnancy, and the number of previous live births, were obtained from the first pregnancy interview (T1-first and T3-first). Self-reported weight and height before pregnancy were also recorded, and pre-pregnancy body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Infant sex was ascertained from medical records.
Statistical Analyses
Selected demographic, maternal, and infant characteristics were summarized among the 125 mother-infant dyads. The median, interquartile range (IQR), minimum, and maximum of each blood metal concentration were presented overall and by infant sex. Mann-Whitney U test statistics were used to assess whether the distributions of blood metals differed between male and female infants. Correlations among metals were assessed using Spearman’s rank correlation coefficients. To compare blood metal concentrations between pregnant women in the NCS and the U.S. general population, we examined data from the 2003–2012 survey cycles of the National Health and Nutrition Examination Survey (NHANES). A urine-based pregnancy test determined pregnancy status for NHANES participants.
Linear regression models were used to assess the effect of prenatal metal exposures on birth outcomes. Given the small sample size and weak correlations among the blood metals, we considered linear regression models to be a rigorous and parsimonious analytical approach for these data, over newer mixture methods such as Bayesian kernel machine regression models (BKMR) or weighted quantile sum regression. We first performed separate multivariable linear regression models (single-metal models) to estimate a regression coefficient and 95% confidence interval (CI) for each metal-outcome association of interest. Since linear association trends were generally suggested from preliminary analyses with BKMR (Supplemental Figure 1), blood metals were log2-transformed and modeled as continuous variables. The regression coefficients were interpreted as the change in birth outcomes per doubling of blood metal concentrations. Additionally, a cross-product term was included in the model to assess interaction by infant sex, and the corresponding Wald test statistic was interpreted as the P for interaction.
Subsequently, a single multivariable linear regression model was performed, including all five metals simultaneously in the model (mutually-adjusted model). Multicollinearity between metals was not detected based on the variance inflation factor.
Pairwise metal-metal interactions were also evaluated by including a cross-product term between the two log2-transformed metals in the model. Analyses were conducted in the overall study sample as well as by infant sex.
Potential confounders were selected based on a priori knowledge for associations with both metal exposures and birth outcomes (41, 45, 57–59). All models included the following covariates: maternal age (<20, 20–25, 26–30, 30–35, 35+ years), race/ethnicity (white, African American, other race), maternal educational attainment (<high school, high school graduate, >high school), family income (≤$29999, $30000–$74999, ≥$75000), smoking status during pregnancy (yes, no), number of previous livebirths, continuous pre-pregnancy BMI (kg/m2), and infant sex (male, female). Infant sex was considered as a confounder in the overall analyses since there are potential sex differences in placental immune response and metabolic function that may result in sex-specific placental transfer efficiency of metals (59–61). Since the prevalence of reported alcohol consumption during pregnancy was low (4%) and its inclusion as a covariate did not appreciably change the effect estimates, it was omitted from multivariable models. Gestational age was not included as a covariate a priori due to its potential role as a mediator of the associations of interest (62). All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC). P values <0.05 were considered statistically significant.
Five sensitivity analyses were conducted. For the first sensitivity analysis, we additionally included gestational age as a covariate in our models for comparative purposes since gestational age may play a role as a mediator or a confounder for the hypothesized associations between metals and infant birth outcomes. Second, we included gestational age at the time of blood sampling in the models to evaluate the potential impact on our effect estimates (n=121) since blood metal assessment may be influenced by a change in metabolism and blood volume increases throughout pregnancy. Third, given the potential differences in etiologies of preterm births and small size at birth, a sensitivity analysis was conducted by restricting the analytical sample to mother-infant pairs with gestational age ≥ 37 weeks (62). Fourth, since infant growth might not be linear, a sensitivity analysis was performed using gestational age- and sex-standardized z-scores for birth outcomes (i.e., birthweight, birth length, and head circumference) based on Fenton’s growth chart of 2013 (63). For the last sensitivity analysis, we evaluated potential residual confounding effects from gestational diabetes and preeclampsia on the associations of prenatal metal exposures with birth outcomes (N=122).
Results
Table 1 summarizes the selected characteristics of the 125 mother-infant dyads included in the present study. Per the disclosure policies of the NCS, cells with <10 participants have been suppressed. The majority of women included in the analyses were white, aged > 26 years, and had more than high school education. Supplemental Table 1 shows the selected maternal and infant characteristics among the 132 mother-infant pairs without missing data on birth outcomes and covariates who were not part of the analytical sample and the 125 pairs included in the current analyses. Although women included in the present analyses had higher educational attainment, the distributions of other covariates and infant birth outcomes between these two samples are not appreciably different from each other.
Table 1.
Selected characteristics of 125 mother-infant dyads in the National Children’s Study.
| Characteristics | Overall N (%) |
|---|---|
| Race/ethnicity [N (%)] | |
| White | 101 (80.8) |
| African American or Black | 7 (5.6) |
| Other | 17 (13.6) |
| Maternal age [N (%)] | |
| <20 years | 11 (8.8) |
| 20–25 years | 32 (25.6) |
| 26–30 years | 35 (28.0) |
| 30–35 years | 36 (28.8) |
| 36+ years | 11 (8.8) |
| Education level [N (%)] | |
| < High school | 8 (6.4) |
| High school graduate | 53 (42.4) |
| > High school | 64 (51.2) |
| Family income [N (%)] | |
| ≤ $29,999 | 29 (23.2) |
| $30,000 – $74,999 | 50 (40.0) |
| ≥ $75,000 | 46 (36.8) |
| Number of previous livebirths [N (%)] | |
| 0 | 43 (34.4) |
| 1 | 48 (38.4) |
| ≥ 2 | 34 (27.2) |
| Smoking status during pregnancy [n (%)] | |
| Yes | 9 (7.2) |
| No | 116 (92.8) |
| Pre-pregnancy BMIa [N (%)] | |
| Normal/Underweight | 74 (59.2) |
| Overweight | 51 (40.8) |
| Infant sex [N (%)] | |
| Male | 68 (54.4) |
| Female | 57 (45.6) |
| Infant birth outcomes (mean ± SD) | |
| Gestational age, weeks | 38.58 ± 1.81 |
| Birthweight, kg | 3340 ± 574 |
| Birth length, cm | 49.87 ± 2.88 |
| Head circumference, cm | 34.61 ± 1.81 |
| Ponderal index, kg/m3 | 26.89 ± 3.92 |
IQR: interquartile range.
The distributions of the blood metals overall and by infant sex are summarized in Table 2. Maternal blood metal concentrations were not appreciably different for male and female infants; however, male infants had slightly higher prenatal exposure to manganese than female infants (Table 2). Spearman’s rank correlation coefficients are summarized in Supplemental Table 2; generally, weak correlations were observed between the blood metal concentrations (rS: −0.18–0.21). The only significant negative correlation coefficients were observed between selenium and lead (rS: −0.18). Maternal blood concentrations of the five metals in the current study are comparable to those of pregnant women who participated in the NHANES (Supplemental Table 3), although pregnant participants in the NCS had slightly lower blood concentrations of lead. When restricting NHANES participants to only the 2009–2010 survey cycle, blood lead levels were comparable between NCS and NHANES.
Table 2.
Maternal blood metal concentrations
| Median | IQR | Min | Max | |
|---|---|---|---|---|
| Cadmium (μg/L) | ||||
| Overall | 0.24 | 0.17 | 0.11 | 1.80 |
| Male infants | 0.26 | 0.17 | 0.11 | 1.80 |
| Female infants | 0.22 | 0.24 | 0.11 | 1.40 |
| P-valuea | 0.25 | |||
| Lead (μg/dL) | ||||
| Overall | 0.34 | 0.32 | 0.18 | 2.86 |
| Male infants | 0.35 | 0.33 | 0.18 | 2.86 |
| Female infants | 0.33 | 0.31 | 0.18 | 0.85 |
| P-valuea | 0.54 | |||
| Manganese (μg/L) | ||||
| Overall | 10.54 | 3.80 | 4.79 | 20.72 |
| Male infants | 10.64 | 3.45 | 4.84 | 20.72 |
| Female infants | 9.67 | 4.62 | 4.79 | 18.23 |
| P-valuea | 0.02 | |||
| Selenium (μg/L) | ||||
| Overall | 187.76 | 27.69 | 137.75 | 264.74 |
| Male infants | 188.12 | 27.88 | 137.75 | 243.71 |
| Female infants | 187.43 | 27.74 | 147.46 | 264.74 |
| P-valuea | 0.69 | |||
| Total Mercury (μg/L) | ||||
| Overall | 0.58 | 0.66 | 0.11 | 5.32 |
| Male infants | 0.64 | 0.61 | 0.11 | 5.32 |
| Female infants | 0.50 | 0.65 | 0.11 | 4.17 |
| P-valuea | 0.21 | |||
Mann-Whitney U test comparing male and female infants.
Results from the single-metal models are summarized in Table 3 (see Supplemental Table 4 for unadjusted results). Maternal blood lead concentration was inversely associated with gestational age (β = −0.98; 95% CI = −1.67, −0.30; P-value for interaction <0.01), birthweight (β = −381; 95% CI = −583, −178; P-value for interaction <0.01), birth length (β = −1.44; 95% CI = −2.45, −0.42; P-value for interaction <0.01), and head circumference (β = −1.10; 95% CI = −1.70, −0.50; P-value for interaction <0.01), with effects limited to female infants. Maternal blood selenium concentration was positively associated with birthweight among male infants (β = 786; 95% CI = 26, 1545; P-value for interaction = 0.13). Maternal blood selenium concentration was also inversely associated with birth length (β = −4.86; 95% CI = −9.36, −0.36; P-value for interaction = 0.04) and positively related to ponderal index (β = −7.29; 95% CI = −1.06, −13.53; P-value for interaction = 0.38) among female infants. Although the confidence intervals marginally crossed zero (null hypothesis of no difference), suggestive inverse associations were observed for maternal blood total mercury concentration with birthweight (β = −88; 95% CI = −1.77, 0) and ponderal index (β = −0.53; 95% CI = −1.14, 0.09) in the overall study sample; the association with birthweight was stronger among female infants (β = −127; 95% CI = −247, −9; P-value for interaction = 0.33). Maternal blood cadmium and manganese concentrations were not associated with birth outcomes.
Table 3.
Adjusted expected change (95% confidence interval) for birth outcomes per doubling of blood metal concentrations among 125 mother-infants pairs overall and by infant sex (male N=68; female N=57)
| Blood metals | Gestational age (weeks)a | Birthweight (g)a | Birth length (cm)a | Head circumference (cm)a | Ponderal index (kg/m3)a |
|---|---|---|---|---|---|
| Cadmium (μg/L) | |||||
| Overall | 0.32 (−0.09, 0.74) | 47 (−78, 172) | 0.27 (−0.35, 0.89) | 0.29 (−0.08, 0.65) | 0.24 (−0.63, 1.11) |
| Male infants | 0.21 (−0.36, 0.78) | 60 (−112, 232) | 0.39 (−0.46, 1.24) | 0.14 (−0.36, 0.65) | −0.08 (−1.27, 1.11) |
| Female infants | 0.42 (−0.12, 0.97) | 35 (−129, 200) | 0.15 (−0.66, 0.97) | 0.42 (−0.07, 0.90) | 0.53 (−0.61, 1.67) |
| P-value for interaction | 0.58 | 0.83 | 0.68 | 0.41 | 0.44 |
| Lead (μg/dL) | |||||
| Overall | −0.13 (−0.53, 0.28) | −94 (−214, 25) | −0.08 (−0.68, 0.52) | −0.29 (−0.65, 0.06) | −0.73 (−1.56, 0.10) |
| Male infants | 0.26 (−0.20, 0.73) | 34 (−103, 172) | 0.53 (−0.16, 1.22) | 0.07 (−0.33, 0.48) | −0.57 (−1.56, 0.41) |
| Female infants | −0.98 (−1.67, −0.30)* | −381 (−583, −178)* | −1.44 (−2.45, −0.42)* | −1.10 (−1.70, −0.50)* | −1.07 (−2.53, 0.39) |
| P-value for interaction | < 0.01* | < 0.01* | < 0.01* | < 0.01* | 0.58 |
| Manganese (μg/L) | |||||
| Overall | −0.29 (−1.07, 0.48) | −53 (−286, 180) | 0.08 (−1.08, 1.23) | 0.08 (−1.08, 1.23) | −0.56 (−2.18, 1.05) |
| Male infants | −0.45 (−1.57, 0.66) | −158 (−492, 176) | −0.20 (−1.86, 1.47) | −0.11 (−1.10, 0.88) | −0.78 (−3.10, 1.54) |
| Female infants | −0.14 (−1.23, 0.94) | 47 (−279, 373) | 0.34 (−1.29, 1.96) | −0.40 (−1.36, 0.57) | −0.36 (−2.62, 1.91) |
| P-value for interaction | 0.70 | 0.39 | 0.66 | 0.68 | 0.80 |
| Selenium (μg/L) | |||||
| Overall | 0.89 (−1.09, 2.87) | 412 (−181, 1004) | −1.26 (−4.21, 1.69) | 0.60 (−1.16, 2.36) | 5.17 (1.13, 9.20)* |
| Male infants | 1.51 (−1.04, 4.06) | 786 (26, 1545)* | 1.22 (−2.53, 4.98) | 0.84 (−1.44, 3.11) | 3.69 (−1.51, 8.89) |
| Female infants | 0.01 (−3.06, 3.07) | −129 (−1039, 781) | −4.86 (−9.36, −0.36)* | 0.26 (−2.47, 2.99) | 7.29 (1.06, 13.53)* |
| P-value for interaction | 0.46 | 0.13 | 0.04* | 0.75 | 0.38 |
| Total mercury (μg/L) | |||||
| Overall | 0.14 (−0.16, 0.44) | −88 (−177, 0) | 0.03 (−0.42, 0.48) | 0.05 (−0.22, 0.31) | −0.53 (−1.14, 0.09) |
| Male infants | 0.16 (−0.25, 0.57) | −48 (−168, 73) | 0.30 (−0.31, 0.91) | 0.02 (−0.34, 0.38) | −0.59 (−1.43, 0.26) |
| Female infants | 0.12 (−0.28, 0.52) | −127 (−247, −9)* | −0.23 (−0.83, 0.37) | 0.07 (−0.29, 0.43) | −0.47 (−1.30, 0.36) |
| P-value for interaction | 0.55 | 0.33 | 0.20 | 0.84 | 0.84 |
P-value <0.05.
Models adjusted for maternal age (<20, 20–25, 26–30, 30–35, 35+ years), race/ethnicity (white, African American or black, other race), education (< high school, high school graduate, >high school), income (≤$29999, $30000–$74999, ≥$75000), smoking status during pregnancy (yes, no), number of prior livebirths, continuous body mass index (kg/m2), and infant sex (male, female).
In mutually-adjusted models that included all five metals, findings were not appreciably different from those observed in the single-metal models, suggesting the observed associations from the single-metal models were not likely the result of confounding effects of other correlated metals (Supplemental Table 5).
In the analyses exploring metal-metal interactions, we detected significant synergistic interactions between selenium and lead for birth length in the overall study sample (Interaction β = 5.57; P-value for interaction = 0.03) and among male infants (Interaction β=8.95; P-value for interaction=0.01). A significant synergistic interaction between selenium and total mercury for birth length was also observed among male infants (Interaction β = 10.08; P-value for interaction <0.01). Further, a significant synergistic interaction between selenium and manganese for ponderal index was observed among female infants (Interaction β = 24.01; P-value for interaction <0.01), while an antagonistic interaction was observed among male infants (Interaction β = −17.04; P-value for interaction <0.01). Additionally, we detected a significant synergistic interaction between lead and total mercury for head circumference in the overall study sample (Interaction β = 0.37; P-value for interaction = 0.03) and, significant antagonistic interactions between selenium and total mercury for ponderal index in the overall study sample (Interaction β = −4.25; P-value for interaction = 0.02) and among female infants (Interaction β = −6.31; P-value for interaction = 0.02). Lastly, a significant antagonistic interaction between manganese and cadmium for ponderal index was observed in the overall study sample (Interaction β = −1.98; P-value for interaction = 0.02), and a significant antagonistic interaction between manganese and lead for gestational age was observed among female infants (Interaction β = −2.09; P-value for interaction = 0.04). No additional significant metal-metal interactions were observed (data not shown).
In sensitivity analyses where gestational age was included as a covariate, the associations between maternal blood lead and birth outcomes among female infants were attenuated, and the associations of selenium with birthweight among male infants and with ponderal index in the overall study sample were also weaker. In contrast, the magnitude of the associations observed for total mercury in the overall study sample and among male infants became stronger (Supplemental Table 6). Still, it is important to consider gestational age as a potential mediator for the relationships between metal exposures and birth outcomes. In sensitivity analyses adjusting for gestational age at the time of blood sampling, the effect estimates of blood metals on birth outcomes were of a similar direction and magnitude (Supplemental Table 7). Additionally, in the sensitivity analysis restricted to mother-infant pairs with gestational age ≥ 37 weeks (N=117), findings were not appreciably different from the overall analyses presented (Supplemental Table 8); however, we observed an inverse association between maternal blood manganese concentration and gestational age among male infants which was not observed in the main analyses. In the sensitivity analysis using z-scores of birth outcomes (i.e., birthweight, birth length, and head circumference), we observed similar results with consistent directionality and significance to the analyses using the original measures (data not shown). The sensitivity analysis, including gestational diabetes and preeclampsia as covariates, were also consistent, suggesting no important confounding by these variables (data not shown).
Discussion
We assessed the effects of five metal exposures (i.e., cadmium, lead, manganese, selenium, and total mercury) measured in maternal blood collected during pregnancy on birth outcomes among a sample of 125 mother-infant pairs in the NCS. Sex-specific associations were observed for lead and selenium, with inverse associations generally seen with selected birth outcomes among female infants. Additionally, inverse associations of total mercury with selected birth outcomes were found in the overall study sample. Metal-metal interaction analyses revealed synergistic interactions between selenium and lead, selenium and total mercury, selenium and manganese, as well as lead and total mercury with selected birth outcomes. We also detected antagonistic interaction between lead and total mercury, selenium and manganese, manganese and cadmium, as well as manganese and lead for selected birth outcomes.
Evidence of sex-specific associations of lead with adverse birth outcomes was observed, with effects generally limited to female infants. The observed inverse associations suggest that female infants may be more susceptible to lead toxicity prenatally. The existing literature generally supports inverse associations between prenatal lead exposure and decreased birthweight (38, 64–71), birth length (69, 70, 72, 73), and head circumference (65, 70, 72, 73). At the same time, studies on gestational age are relatively limited and report null associations (36, 71, 74, 75). Existing epidemiologic research evaluating sex differences in susceptibility to lead exposure is scarce. In a cohort study conducted in New Hampshire of 989 mother-infant pairs, postpartum toenail lead concentrations were inversely associated with birthweight and head circumference only among female infants (51). In contrast, a study of 1,009 mother-infant pairs in Shanghai found a positive association between cord blood lead concentration and birthweight among male infants and an inverse association with ponderal index among female infants (40). A study of 138 mother-infant dyads in Michigan reported neither significant associations in the overall study population nor sex-specific relationships between maternal tooth lead concentration measured during second and third trimesters and z-scores of birthweight (74). Furthermore, given that studies have demonstrated sex-specific effects of perinatal lead exposure on DNA methylation (76–78), future research investigating the sex-differential effects of lead toxicity on adverse birth outcomes is needed.
In this study, we observed a positive association between selenium and birthweight among male infants. Selenium was also inversely associated with birth length and positively related to ponderal index among female infants. Most previous studies evaluating the effect of selenium on birthweight showed a positive association with selenium measured in maternal serum (79), maternal blood (41), and cord blood (37, 80). To our knowledge, only two studies, one among 271 newborns in Baltimore and the other among 250 Saudi Arabian infants, evaluated the cross-sectional association of cord serum and placenta selenium with ponderal index, and null results were reported (37, 81). Previous research on birth length has been mixed, with one study reporting a borderline significant decrease in birth length with higher placenta selenium levels (81), one study demonstrating a positive association with cord blood selenium levels (41), and the others finding null results (32, 82–85). Inconsistent findings may be due to different study populations, timing and type of exposure biomarkers evaluated, exposure levels, or masking of interaction effects due to other environmental exposures. To our knowledge, no prior work evaluated the heterogeneity of the associations between selenium and birth outcomes by infant sex.
Our result linking higher mercury exposure with decreased birthweight is consistent with several prior studies. Inverse associations have been observed with mercury levels measured in maternal blood during pregnancy (35), cord blood (86, 87), placenta (88), and diet (89). Specifically, a study of 334 Japanese mother-infant pairs showed significant inverse associations between birthweight and both first trimester and second trimester blood mercury levels (35). To date, only three studies explored the association between mercury exposure and ponderal index; one observed an inverse association with cord blood levels of mercury (37), while two found no significant associations (85, 90). Although ponderal index was correlated with birthweight in our study population (Pearson’s r = 0.51), infants with higher ponderal index may still have lower birthweight when they also have shorter birth length. Given the limited evidence, more studies on ponderal index and mercury are warranted to confirm our findings and ultimately make meaningful clinical conclusions.
In the current study, no association was observed for cadmium on birth outcomes. Previous studies on cadmium have generally reported inverse associations with birthweight, birth length, and head circumference (e.g., (65, 84, 86, 90–93)). The observed null findings for cadmium may be due to the small sample size and low cadmium exposure levels in the present study. For example, a study of 319 mother-infant pairs in North Carolina found that elevated maternal blood cadmium concentrations were associated with decreased birthweight (93); blood cadmium concentrations were higher (mean = 4.54 ng/g) compared to our study sample.
A statistically significant synergistic interaction between lead and total mercury with head circumference was observed in the analyses evaluating metal-metal interactions. The interaction between lead and mercury with infant growth is not well investigated, and the biologic mechanisms underlying this interaction are uncertain. To date, no studies evaluated the combined effect of lead and mercury on head circumference, and only two studies reported the combined effect on decreased birthweight (94, 95). In a study of 248 mother-infant pairs in Flanders, Belgium, an association of co-exposure to lead, mercury, thallium, cadmium, manganese, and perfluorooctane sulfonate with decreased birthweight was reported among female infants (95). Another study of 542 mother-infant pairs in Korea found that combined prenatal exposure to lead and mercury was associated with decreased birth weight (94). However, a principal component analysis was utilized by both studies; therefore, interaction effects cannot be directly ascertained from these analyses.
We also observed antagonistic interaction between selenium and total mercury with ponderal index that was consistent with findings from a study conducted in Baltimore of 271 infants (37). That study reported an inverse association between cord blood methylmercury concentrations and ponderal index only among infants with lower levels of cord blood selenium concentrations. It has been suggested that selenium may provide protective effects against mercury-mediated cell apoptosis that can further affect birth size (96). However, our metal-metal interaction analyses also suggested significant synergistic interactions between selenium and two toxic metals (i.e., total mercury and lead) with birth length, and opposite interaction effects between selenium and manganese with ponderal index were observed among male and female infants. Future large prospective cohort studies are needed to consider potential effect modification of selenium on the relationship between metals and infant growth.
Although manganese showed no significant effects on birth outcomes in this study, antagonistic interactions between manganese and two toxic metals (i.e., lead and cadmium) were observed. This finding contrasts with an experimental study that suggested synergistic toxicity between manganese and lead or cadmium (97). However, a recent study in Northern Puerto Rico found no interaction between manganese and lead with birth outcomes based on BKMR (98), and a study of 275 U.S. mother-infant pairs reported an antagonistic interaction between a mixture of essential metals (i.e., manganese, zinc, copper, and magnesium) and cadmium with birthweight in male infants (38).
The potential biological mechanism underlying metal exposures with birth outcomes generally involves oxidative stress, a factor that has been linked to growth restriction (99–101). Previous studies have shown that toxic metals such as lead, cadmium, and mercury are associated with increased oxidative stress (102, 103). In contrast, selenium serves as a component of antioxidative enzymes that can further protect the organism from oxidative stress (104). Toxic metals are also potent endocrine disruptors that disturb the mechanism involved in energy storage or adipogenesis (105). Furthermore, in vitro experiments and human studies have shown that toxic metals such as mercury, lead, and cadmium could disturb placental enzyme activity, oxygen consumption, and interfere with placental transport of essential elements, including calcium and zinc, that are needed for fetal growth (106). Our analyses suggest that effects of lead and selenium on selected birth outcomes differed by sex. Although the biological mechanisms underlying the observed sex-specific associations are still unclear, previous studies have suggested that sex-specific structural and functional differences in the placenta may impact how a fetus responds to environmental chemical exposures (107, 108). Further, given the sexual dimorphism of growth hormone and insulin-like growth factor (IGF) (109), environmental chemicals may trigger sex-specific interference of the IGF-axis.
This study contributes to a burgeoning body of evidence linking prenatal metal exposures with birth outcomes. However, we acknowledge several limitations of the present study. First, the statistical power to detect associations may be insufficient, particularly for sex-specific effects, potentially explaining some results with large effect sizes that were not statistically significant. Second, while our hypotheses were pre-specified, our results may be subject to multiple hypothesis testing considerations, resulting in the possibility of an increased type I error. Third, although an average of two measurements of birth length and head circumference was used for the analyses, we cannot rule out the possibility of misclassification given the moderate correlations between measurements obtained from physical examination versus medical records. Misclassification of birth outcomes in this study could have biased the results either towards or away from the null. Fourth, the majority of participants were white; thus, the results of this study may not be generalizable to the general population of the United States. However, while the NCS participants were not recruited to be a representative sample, the exposure levels are comparable to those observed from a more diverse and nationally representative sample in the NHANES. Fifth, blood metal concentrations were measured between 6 and 32 weeks of gestation; it is unknown to what extent exposure levels were similar across trimesters in the current study population, and the potential misclassification of blood metal concentrations might obscure trimester-specific associations. A previous study reported high correlations between first trimester and third trimester blood metal concentrations of cadmium, lead, and mercury but not manganese (110). Finally, this study may be limited by uncontrolled confounding, such as dietary behaviors and supplement intake during pregnancy, genetic factors, other socioeconomic status-related variables, or co-exposure to other environmental chemicals. For instance, iron is similar to manganese with respect to their chemical properties, and previous studies have shown high correlations and potential metabolic interaction between iron and manganese (111, 112). Thus, positive confounding may be present if iron is positively associated with birth outcomes and vice versa.
In summary, findings from the current study support associations between prenatal exposures to lead, total mercury, and selenium with birth outcomes. Associations for lead and selenium appear to be sex-specific and more apparent in female infants. The data also suggest a synergistic interaction between lead and total mercury on head circumference and an antagonistic interaction between selenium and total mercury on ponderal index. Future prospective studies are needed to confirm our findings and further investigate the impact of prenatal or early life exposure to metal mixtures on children’s growth and health effects later in life. These efforts can ultimately inform health policy decision making addressing metal contamination in the environment and blood metal concentrations in pregnancy.
Supplementary Material
Highlights.
Prenatal exposure to metals may play an important role in fetal growth.
Lead was inversely related to birthweight, birth length, head circumference, and gestational age among female infants.
Selenium was positively associated with ponderal index and inversely associated with birth length among female infants.
Selenium was positively related to birthweight among male infants.
Infant sex may modify associations between prenatal metal exposures and birth outcomes.
Acknowledgments:
This manuscript was prepared using National Children’s Study Research Materials obtained from the NCS Vanguard Data and Sample Archive and Access System and does not necessarily reflect the opinions or views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health or the United States Environmental Protection Agency. This work was supported by grants from the National Institutes of Health (R01 ES024423 and P30 ES027792).
Footnotes
Competing financial interests: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJP. The developmental origins of adult disease. Eur J Epidemiol 2003;18(8):733–6. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80. [DOI] [PubMed] [Google Scholar]
- 4.RichEdwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315(7105):396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, adult risk factors and incident coronary heart disease: The Caerphilly study. Public Health. 1996;110(3):139–43. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s - Findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–8. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322(7292):949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanetti D, Tikkanen E, Gustafsson S, Priest JR, Burgess S, Ingelsson E. Birthweight, Type 2 Diabetes Mellitus, and Cardiovascular Disease: Addressing the Barker Hypothesis With Mendelian Randomization. Circ Genom Precis Med 2018;11(6):e002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens 1996;14(8):935–41. [PubMed] [Google Scholar]
- 10.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993;36(1):62–7. [DOI] [PubMed] [Google Scholar]
- 11.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 2000;18(7):815–31. [DOI] [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, et al. Fetal and Infant Growth and Impaired Glucose-Tolerance at Age 64. BMJ. 1991;303(6809):1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312(7028):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: A consensus statement of the international societies of pediatric endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92(3):804–10. [DOI] [PubMed] [Google Scholar]
- 15.Sharma D, Shastri S, Sharma P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin Med Insights Pediatr 2016;10:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundgren EM, Tuvemo T. Effects of being born small for gestational age on long-term intellectual performance. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22(3):477–88. [DOI] [PubMed] [Google Scholar]
- 17.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr 2004;134(9):2169–72. [DOI] [PubMed] [Google Scholar]
- 18.Muhammad T, Khattak AA, Shafiq ur R, Khan MA, Khan A, Khan MA. Maternal factors associated with intrauterine growth restriction. J Ayub Med Coll Abbottabad. 2010;22(4):64–9. [PubMed] [Google Scholar]
- 19.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol 2006;23(5):325–8. [DOI] [PubMed] [Google Scholar]
- 20.Phung H, Bauman A, Nguyen TV, Young L, Tran M, Hillman K. Risk factors for low birth weight in a socio-economically disadvantaged population: parity, marital status, ethnicity and cigarette smoking. Eur J Epidemiol 2003;18(3):235–43. [DOI] [PubMed] [Google Scholar]
- 21.Gage TB, Fang F, O’Neill E, Dirienzo G. Maternal education, birth weight, and infant mortality in the United States. Demography. 2013;50(2):615–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delpisheh A, Brabin L, Drummond S, Brabin BJ. Prenatal smoking exposure and asymmetric fetal growth restriction. Ann Hum Biol 2008;35(6):573–83. [DOI] [PubMed] [Google Scholar]
- 23.Reeves S, Bernstein I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstet Gynecol 2008;3(6):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandoli G, Coles CD, Kable JA, Wertelecki W, Yevtushok L, Zymak-Zakutnya N, et al. Patterns of Prenatal Alcohol Use That Predict Infant Growth and Development. Pediatrics. 2019;143(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foltran F, Gregori D, Franchin L, Verduci E, Giovannini M. Effect of alcohol consumption in prenatal life, childhood, and adolescence on child development. Nutr Rev 2011;69(11):642–59. [DOI] [PubMed] [Google Scholar]
- 26.Liauw J, Jacobsen GW, Larose TL, Hutcheon JA. Short interpregnancy interval and poor fetal growth: Evaluating the role of pregnancy intention. Paediatr Perinat Epidemiol 2019;33(1):O73–O85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes - A meta-analysis. Jama-Journal of the American Medical Association. 2006;295(15):1809–23. [DOI] [PubMed] [Google Scholar]
- 28.Hasan SMT, Khan MA, Ahmed T. Inadequate maternal weight gain in the third trimester increases the risk of intrauterine growth restriction in rural Bangladesh. PLoS One. 2019;14(2):e0212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr 1999;129(5):988–93. [DOI] [PubMed] [Google Scholar]
- 30.Schmid A, Jacquemyn Y, Loor JD. Intrauterine growth restriction associated with excessively long umbilical cord. Clinical Practice. 2013;3(2):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna U, Bhalerao S. Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India. 2011;61(5):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bank-Nielsen PI, Long MH, Bonefeld-Jorgensen EC. Pregnant Inuit Women’s Exposure to Metals and Association with Fetal Growth Outcomes: ACCEPT 2010–2015. Int J Environ Res Public Health. 2019;16(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Xu X, Chen A, Davuljigari CB, Zheng X, Kim SS, et al. Maternal urinary cadmium levels during pregnancy associated with risk of sex-dependent birth outcomes from an e-waste pollution site in China. Reprod Toxicol 2018;75:49–55. [DOI] [PubMed] [Google Scholar]
- 34.Guo JQ, Wu CH, Qi XJ, Jiang S, Liu Q, Zhang JM, et al. Adverse associations between maternal and neonatal cadmium exposure and birth outcomes. Sci Total Environ 2017;575:581–7. [DOI] [PubMed] [Google Scholar]
- 35.Vigeh M, Nishioka E, Ohtani K, Omori Y, Matsukawa T, Koda S, et al. Prenatal mercury exposure and birth weight. Reprod Toxicol 2018;76:78–83. [DOI] [PubMed] [Google Scholar]
- 36.Freire C, Amaya E, Gil F, Murcia M, S LL, Casas M, et al. Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int J Hyg Environ Health. 2019;222(3):468–78. [DOI] [PubMed] [Google Scholar]
- 37.Wells EM, Herbstman JB, Lin YH, Jarrett J, Verdon CP, Ward C, et al. Cord Blood Methylmercury and Fetal Growth Outcomes in Baltimore Newborns: Potential Confounding and Effect Modification by Omega-3 Fatty Acids, Selenium, and Sex. Environ Health Perspect. 2016;124(3):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo YW, McCullough LE, Tzeng JY, Darrah T, Vengosh A, Maguire RL, et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health. 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neda AN, Fahimeh S, Tahereh ZK, Leila F, Zahra N, Bahman C, et al. Lead Level in Umbilical Cord Blood and its Effects on Newborns Anthropometry. Journal of Clinical and Diagnostic Research. 2017;11(6):Sc01–Sc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Gao ZY, Yan J, Ying XL, Tong SL, Yan CH. Sex differences in the effects of prenatal lead exposure on birth outcomes. Environmental Pollution. 2017;225:193–200. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Chen W, Wang D, Jin Y, Chen X, Xu Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere. 2014;108:33–9. [DOI] [PubMed] [Google Scholar]
- 42.Tsuzuki S, Morimoto N, Hosokawa S, Matsushita T. Associations of Maternal and Neonatal Serum Trace Element Concentrations with Neonatal Birth Weight. PLoS One. 2013;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia W, Zhou YQ, Zheng TZ, Zhang B, Bassig BA, Li YY, et al. Maternal urinary manganese and risk of low birth weight: a case-control study. BMC Public Health. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eum JH, Cheong HK, Ha EH, Ha MN, Kim Y, Hong YC, et al. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environmental Health. 2014;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C, et al. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res 2014;160(3):437–44. [DOI] [PubMed] [Google Scholar]
- 46.Yoder SR, Thornburg LL, Bisognano JD. Hypertension in Pregnancy and Women of Childbearing Age. Am J Med 2009;122(10):890–5. [DOI] [PubMed] [Google Scholar]
- 47.Li AJ, Zhuang TF, Shi JB, Liang Y, Song MY. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. Journal of Environmental Sciences-China. 2019;80:99–106. [DOI] [PubMed] [Google Scholar]
- 48.Iyengar GV, Rapp A. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 1: physiology, function and sampling of placenta for elemental characterisation. Sci Total Environ 2001;280(1–3):195–206. [DOI] [PubMed] [Google Scholar]
- 49.Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1–2):3–12. [DOI] [PubMed] [Google Scholar]
- 50.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender Medicine. 2007;4(1):19–30. [DOI] [PubMed] [Google Scholar]
- 51.Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, et al. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ Epidemiol 2019;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanford JB, Brenner R, Fetterer D, Palmer L, Schoendorf KC, Study USNCs. Impact of preconception enrollment on birth enrollment and timing of exposure assessment in the initial vanguard cohort of the U.S. National Children’s Study. BMC Med Res Methodol 2015;15:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The Center for Disease Control and Prevention (CDC). Cadmium, Lead, Manganese, Mercury, and Selenium Laboratory Procedure Manual [updated January, 2018; cited 2020 May 14]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PBCD_I_met.pdf.
- 54.Verbovšek T A comparison of parameters below the limit of detection in geochemical analyses by substitution methods = Primerjava ocenitev parametrov pod mejo določljivosti pri geokemičnih analizah z metodo nadomeščanja. RMZ - Materials and Geoenvironment. 2011;58:393–404. [Google Scholar]
- 55.Yagel S, Zacut D, Igelstein S, Palti Z, Hurwitz A, Rosenn B. In utero ponderal index as a prognostic factor in the evaluation of intrauterine growth retardation. American journal of obstetrics and gynecology. 1987;157(2):415–9. [DOI] [PubMed] [Google Scholar]
- 56.Vintzileos AM, Lodeiro JG, Feinstein SJ, Campbell WA, Weinbaum PJ, Nochimson DJ. Value of fetal ponderal index in predicting growth retardation. Obstet Gynecol 1986;67(4):584–8. [PubMed] [Google Scholar]
- 57.Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int J Environ Res Public Health. 2017;14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang K, Li H, Zhang B, Zheng T, Li Y, Zhou A, et al. Prenatal cadmium exposure and preterm low birth weight in China. J Expo Sci Environ Epidemiol 2017;27(5):491–6. [DOI] [PubMed] [Google Scholar]
- 59.Li XY, Li AJ, Zhang WJ, Liu XW, Liang Y, Yao XL, et al. A pilot study of mothers and infants reveals fetal sex differences in the placental transfer efficiency of heavy metals. Ecotoxicol Environ Saf 2019;186. [DOI] [PubMed] [Google Scholar]
- 60.Clifton VL. Sex and the Human Placenta: Mediating Differential Strategies of Fetal Growth and Survival. Placenta. 2010;31:S33–S9. [DOI] [PubMed] [Google Scholar]
- 61.Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011;214(2):79–101. [DOI] [PubMed] [Google Scholar]
- 62.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol 2011;174(9):1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodosthenous RS, Burris HH, Svensson K, Amarasiriwardena CJ, Cantoral A, Schnaas L, et al. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environ Int 2017;99:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: a prospective birth cohort study. Bjog-an International Journal of Obstetrics and Gynaecology. 2015;122(3):322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berkowitz Z, Price-Green P, Bove FJ, Kaye WE. Lead exposure and birth outcomes in five communities in Shoshone County, Idaho. Int J Hyg Environ Health. 2006;209(2):123–32. [DOI] [PubMed] [Google Scholar]
- 67.Zhu MT, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal Low-Level Lead Exposure and Fetal Growth. Environ Health Perspect 2010;118(10):1471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B, Xia W, Li YY, Bassig BA, Zhou AF, Wang YJ, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in China. Reprod Toxicol 2015;57:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie X, Ding GD, Cui C, Chen LM, Gao Y, Zhou YJ, et al. The effects of low-level prenatal lead exposure on birth outcomes. Environmental Pollution. 2013;175:30–4. [DOI] [PubMed] [Google Scholar]
- 70.Osman K, Akesson A, Berglund M, Bremme K, Schutz A, Ask K, et al. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33(2):131–8. [DOI] [PubMed] [Google Scholar]
- 71.Mikelson CK, Troisi J, LaLonde A, Symes SJK, Thurston SW, DiRe LM, et al. Placental concentrations of essential, toxic, and understudied metals and relationships with birth outcomes in Chattanooga, TN. Environ Res 2019;168:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang M, Xu C, Lin N, Liu K, Zhang Y, Yu X, et al. Lead, mercury, and cadmium in umbilical cord serum and birth outcomes in Chinese fish consumers. Chemosphere. 2016;148:270–5. [DOI] [PubMed] [Google Scholar]
- 73.Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57(5):482–8. [DOI] [PubMed] [Google Scholar]
- 74.Cassidy-Bushrow AE, Wu KH, Sitarik AR, Park SK, Bielak LF, Austin C, et al. In utero metal exposures measured in deciduous teeth and birth outcomes in a racially-diverse urban cohort. Environ Res 2019;171:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, Huo X, Li Y, Wu K, Liu J, Huang J, et al. Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in China. Sci Total Environ 2010;408(16):3113–7. [DOI] [PubMed] [Google Scholar]
- 76.Mohanty AF, Farin FM, Bammler TK, MacDonald JW, Afsharinejad Z, Burbacher TM, et al. Infant sex-specific placental cadmium and DNA methylation associations. Environ Res 2015;138:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kippler M, Engstrom K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, et al. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics. 2013;8(5):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sen A, Heredia N, Senut MC, Hess M, Land S, Qu W, et al. Early life lead exposure causes gender-specific changes in the DNA methylation profile of DNA extracted from dried blood spots. Epigenomics. 2015;7(3):379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bogden JD, Kemp FW, Chen XH, Stagnaro-Green A, Stein TP, Scholl TO. Low-normal serum selenium early in human pregnancy predicts lower birth weight. Nutr Res 2006;26(10):497–502. [Google Scholar]
- 80.Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G, et al. Mercury (Hg) and oxidative stress status in healthy mothers and its effect on birth anthropometric measures. Int J Hyg Environ Health. 2014;217(4–5):567–85. [DOI] [PubMed] [Google Scholar]
- 81.Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G, et al. Interaction between cadmium (Cd), selenium (Se) and oxidative stress biomarkers in healthy mothers and its impact on birth anthropometric measures. Int J Hyg Environ Health. 2015;218(1):66–90. [DOI] [PubMed] [Google Scholar]
- 82.Lozano M, Murcia M, Soler-Blasco R, Iniguez C, Irizar A, Lertxundi A, et al. Prenatal Se concentrations and anthropometry at birth in the INMA study (Spain). Environmental Research. 2020;181. [DOI] [PubMed] [Google Scholar]
- 83.Cabrera-Rodriguez R, Luzardo OP, Gonzalez-Antuna A, Boada LD, Almeida-Gonzalez M, Camacho M, et al. Occurrence of 44 elements in human cord blood and their association with growth indicators in newborns. Environ Int 2018;116:43–51. [DOI] [PubMed] [Google Scholar]
- 84.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering. 2010;45(11):1468–74. [DOI] [PubMed] [Google Scholar]
- 85.Callan AC, Hinwood AL, Heyworth J, Phi DT, Odland JO. Sex specific influence on the relationship between maternal exposures to persistent chemicals and birth outcomes. Int J Hyg Environ Health. 2016;219(8):734–41. [DOI] [PubMed] [Google Scholar]
- 86.Tatsuta N, Kurokawa N, Nakai K, Suzuki K, Iwai-Shimada M, Murata K, et al. Effects of intrauterine exposures to polychlorinated biphenyls, methylmercury, and lead on birth weight in Japanese male and female newborns. Environ Health Prev Med 2017;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramon R, Ballester F, Aguinagalde X, Amurrio A, Vioque J, Lacasana M, et al. Fish consumption during pregnancy, prenatal mercury exposure, and anthropometric measures at birth in a prospective mother-infant cohort study in Spain. Am J Clin Nutr. 2009;90(4):1047–55. [DOI] [PubMed] [Google Scholar]
- 88.Kosik-Bogacka D, Lanocha-Arendarczyk N, Kot K, Malinowski W, Szymanski S, Sipak-Szmigiel O, et al. Concentrations of mercury (Hg) and selenium (Se) in afterbirth and their relations with various factors. Environ Geochem Health. 2018;40(4):1683–95. [DOI] [PubMed] [Google Scholar]
- 89.Vejrup K, Brantster AL, Knutsen HK, Magnus P, Alexander J, Kvalem HE, et al. Prenatal mercury exposure and infant birth weight in the Norwegian Mother and Child Cohort Study. Public Health Nutr 2014;17(9):2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Saleh I, Shinwari N, Mashhour A, Rabah A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int J Hyg Environ Health. 2014;217(2–3):205–18. [DOI] [PubMed] [Google Scholar]
- 91.Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, et al. Maternal Cadmium Exposure during Pregnancy and Size at Birth: A Prospective Cohort Study. Environ Health Persp 2012;120(2):284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnston JE, Valentiner E, Maxson P, Miranda ML, Fry RC. Maternal Cadmium Levels during Pregnancy Associated with Lower Birth Weight in Infants in a North Carolina Cohort. PLoS One. 2014;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vidal AC, Semenova V, Darrah T, Vengosh A, Huang Z, King K, et al. Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol Toxicol 2015;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee S, Hong YC, Park H, Kim Y, Ha M, Ha E. Combined effects of multiple prenatal exposure to pollutants on birth weight: The Mothers and Children’s Environmental Health (MOCEH) study. Environ Res 2020;181:108832. [DOI] [PubMed] [Google Scholar]
- 95.Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, et al. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. Int J Environ Res Public Health. 2016;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi S, Kishi R, Saijo Y, Ito Y, Oba K, Araki A, et al. Association of blood mercury levels during pregnancy with infant birth size by blood selenium levels in the Japan Environment and Children’s Study: A prospective birth cohort. Environ Int 2019;125:418–29. [DOI] [PubMed] [Google Scholar]
- 97.Lu CL, Svoboda KR, Lenz KA, Pattison C, Ma HB. Toxicity interactions between manganese (Mn) and lead (Pb) or cadmium (Cd) in a model organism the nematode C. elegans. Environmental Science and Pollution Research. 2018;25(16):15378–89. [DOI] [PubMed] [Google Scholar]
- 98.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, et al. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ Int 2020;138:105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol 2017;77(5). [DOI] [PubMed] [Google Scholar]
- 100.Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest 2007;64(4):187–92. [DOI] [PubMed] [Google Scholar]
- 101.Ozsurekci Y, Aykac K. Oxidative Stress Related Diseases in Newborns. Oxid Med Cell Longev 2016;2016:2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011;2011:457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 2011;256(3):405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tapiero H, Townsend DM, Tew KD. The antioxidant role of selenium and seleno-compounds. Biomedicine & Pharmacotherapy. 2003;57(3–4):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dyer CA. Heavy Metals as Endocrine-Disrupting Chemicals In: Gore AC, editor. Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice. Totowa, NJ: Humana Press; 2007. p. 111–33. [Google Scholar]
- 106.Gundacker C, Hengstschlager M. The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr. 2012;162(9–10):201–6. [DOI] [PubMed] [Google Scholar]
- 107.Rosenfeld CS. Sex-Specific Placental Responses in Fetal Development. Endocrinology. 2015;156(10):3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ 2013;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geary MPP, Pringle PJ, Rodeck CH, Kingdom JCP, Hindmarsh PC. Sexual dimorphism in the growth hormone and insulin-like growth factor axis at birth. J Clin Endocrinol Metab 2003;88(8):3708–14. [DOI] [PubMed] [Google Scholar]
- 110.Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere. 2016;163:270–82. [DOI] [PubMed] [Google Scholar]
- 111.Mehra R, Thakur AS. Relationship between lead, cadmium, zinc, manganese and iron in hair of environmentally exposed subjects. Arabian Journal of Chemistry. 2016;9:S1214–S7. [Google Scholar]
- 112.Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res 1996;55(1–2):39–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.