Abstract
Personalized medicine is among the most exciting innovations in recent clinical research, offering the opportunity for tailored screening and management at the individual level. Biomarker-enriched clinical trials have shown increased efficiency and informativeness in cancer research due to the selective exclusion of patients unlikely to benefit.
In acute stress situations, clinically significant decisions are often made in time-sensitive manners and providers may be pressed to make decisions based on abbreviated clinical assessments. Up to 30% of trauma survivors admitted to the Emergency Department (ED) will develop long-lasting posttraumatic stress psychopathologies. The long-term impact of those survivors with posttraumatic stress sequelae are significant, impacting both long-term psychological and physiological recovery. An accurate prognostic model of who will develop posttraumatic stress symptoms does not exist yet. Additionally, no scalable and cost-effective method that can be easily integrated into routine care exists, even though especially the acute care setting provides a critical window of opportunity for prevention in the so-called golden hours when preventive measures are most effective. In this review, we aim to discuss emerging machine learning (ML) applications that are promising for precisely risk stratification and targeted treatments in the acute care setting.
The aim of this review is to present examples of digital health innovations and to discuss the potential of these new approaches for treatment selection and prevention of posttraumatic sequelae in the acute care setting. The application of artificial intelligence-based solutions have already had great success in other areas and are rapidly approaching the field of psychological care as well. New ways of algorithm-based risk predicting, and the use of digital phenotypes provide a high potential for predicting future risk of PTSD in acute care settings and to go new steps in precision psychiatry.
Keywords: machine learning, artificial intelligence, posttraumatic stress sequelae, digital phenotyping, digital health, risk stratification, individualized treatment selection, emergency medicine
Introduction
The Emergency Department (ED) is the frontline of the health care system, and often the earliest point of entry in the health system for patients treated for diverse and potentially life-threatening acute traumatic events. ED clinicians are tasked with delivering high-quality care while working in demanding clinical environments that may be crowded and highly stressful (Asplin et al., 2003). There are approximately 40 million ED visits annually in the U.S. after potential trauma exposure (McLean et al., 2019) leading to not just significant physical morbidity but also adverse mental health effects as 30% of ED patients exposed to trauma report moderate-to-high symptom severity of post-traumatic stress disorder (PTSD) one year after discharge (Lowe et al., 2020). The psychological sequelae of life-threatening events are not limited to the traumatic domain. Patient populations surviving acute medical emergencies are also at risk for long-term adverse psychological outcomes including patients with cardiovascular events. Acute coronary syndrome (ACS) describes a spectrum of cardiovascular diseases including acute myocardial infarction or unstable angina (Fanaroff et al., 2015). As shown in a meta-analysis of 2,383 patients they found that 1 out of 8 patients with suspected ACS develop PTSD (Edmondson et al., 2012). The development of PTSD after an ACS or stroke is associated with negative health outcomes, for instance, higher rates of recurrence of cardiovascular diseases (Musey Jr et al., 2020; Schultebraucks et al., 2020c).
It is well-known that PTSD is a particularly complex disorder with up to 636,120 different possible manifestations (Galatzer-Levy and Bryant, 2013). Numerous biological factors contribute to recent theory development of PTSD (Heim et al., 2018). Several studies have identified diverse risk factors early after the traumatic event (Galatzer-Levy et al., 2013; Galatzer-Levy et al., 2014; Galatzer-Levy et al., 2017; Karstoft et al., 2015; Papini et al., 2018; Segman et al., 2005; Shalev et al., 1998; Yehuda et al., 1998), such as biological factors (Heim et al., 2018; Hinrichs et al., 2019; Mellon et al., 2018; Michopoulos et al., 2019; Morris et al., 2016; Ressler, 2018), e.g., stress response and threat perception (Heim et al., 2018; Mellon et al., 2018; Morris et al., 2016; Ressler, 2018; Schultebraucks et al., 2019; Van Zuiden et al., 2012), psychophysiological arousal (Hinrichs et al., 2019; Shalev et al., 1998), inflammation, and immune response (Mellon et al., 2018; Michopoulos et al., 2019; Michopoulos et al., 2017), and psychosocial risk factors (Shalev et al., 2017) for PTSD. Other discussed risk factors for PTSD involve the acute care environment in which patients are treated. Recent work has found that factors of the ED environment, such as hourly ED occupancy rates have been shown to increase PTSD risk after ACS (Edmondson et al., 2014; Edmondson et al., 2013). Given the rich diversity and heterogeneity of risk factors, typically, these factors are difficult to integrate into a unified quantitative regression model due to high-dimensionality and potential multicollinearity of candidate predictors (Huys et al., 2016).
A reliable and precise predictive model is a facilitator for evidence-based treatment allocation as it will allow the timely targeted intervention (Roberts et al., 2010; Shalev and Barbano, 2019) within the “golden hours” (Carmi et al., 2016; Vermetten et al., 2014; Zohar et al., 2011) early after the traumatic events, such as an early exposure intervention in the ED hours after the traumatic event took place (Rothbaum et al., 2012; Rothbaum et al., 2014), early internet-based intervention (Mouthaan et al., 2013) and pharmacological interventions (Carmi et al., 2016; Vermetten et al., 2014; Yehuda et al., 2015). In this early phase, several critical pathogenic processes take place such as the neuroendocrine stress sensitization and manifold neurobiological alterations (Heim et al., 2018) that can become targets for early preventive interventions before the biological responses have become more permanent changes (Shalev and Barbano, 2019). In consequence, the ED provides an important window to proactively plan risk-based follow-up care for trauma survivors at an early stage, where patients are still in contact with the healthcare system.
Despite the evidence for the presence of significant psychological and physical morbidity and mortality associated with posttraumatic stress symptoms following acute medical/traumatic events, screening is currently limited in the ED setting. Several longitudinal cohort studies identified risk factors in the ED acute care setting by using clinical screening (Lowe et al., 2020; McLean et al., 2019; van der Mei et al., 2020) only 7% of EDs nationwide regularly screen for symptoms of PTSD (Love and Zatzick, 2014). The acute care setting in the ED is characterized by a high pace and acute care demand therefore time-consuming screenings are often not feasible. Therefore, automatable computational methods that provide precise risk prediction at scale are indicated for informing prevention PTSD after traumatic events in the acute care setting. The review aims to discuss recent advancements in algorithm-based risk stratification and digital phenotyping and their benefits for precision psychiatry in the acute care setting.
Automatable methods integrated into clinical care
Machine learning (ML) approaches for data-driven exploratory analysis offers unique opportunities for knowledge discovery and predictive modeling (Schultebraucks and Galatzer‐Levy, 2019). In past years, a large number of early factors immediately after trauma have been examined as candidate predictors for PTSD susceptibility and multiple independent studies have shown that early factors comprise significant predictive signals to discriminate clinically meaningful trajectories of symptom development or long-term risk (Galatzer-Levy et al., 2013; Galatzer-Levy et al., 2014; Galatzer-Levy et al., 2017; Karstoft et al., 2015; Papini et al., 2018; Segman et al., 2005; Shalev et al., 1998; Yehuda et al., 1998).
However, no clinical prediction model is yet available to be used directly in the acute care setting without additional clinical screening or diagnostic interviews in an already clinical and resource stretched setting (van der Mei et al., 2020). This lack significantly impedes the feasibility of clinical risk prediction models under conditions of ED overcrowding, large-scale emergency events, disasters, and epidemic outbreaks. The SARS-CoV-2 pandemic and future similar situations are a paradigm case illustrating the critical need to support highly charged EDs through computational methods to identify the possible risks of long-term mental health care needs.
In response to this fact, a vital public mental health funding objective of the NIMH is to enable “practical, scalable, and sustainable mental health screening and triage, and providing interventions at scale“ (Gordon and Borja, 2020). The National Institute of Mental Health (NIMH) has funded a large multi-site consortium to collect data to build generalized models of posttraumatic stress courses (McLean et al., 2019; NIMH, 2016). Similarly, military agencies are engaged in large initiatives to identify and predict risk for PTSD in soldiers deployed to warzones (Dean et al., 2019; Schultebraucks et al., 2020a; van der Wal et al., 2019). The use of ML-algorithms for the improvement of prognosis directly implemented into the ED is a new and emerging field aiming to prevent trauma-related stress pathology at first contact with the health system. Schultebraucks et al. (2020b) demonstrated that readily available data such as vital signs and lab tests abstracted from electronic medical records yield probabilistic information to discriminate patients with high non-remitting PTSD symptoms vs. patients with low symptoms (resilience). The authors recruited participants in two Level-1 Emergency Trauma Centers in the U.S. The model development sample was collected at Grady Memorial Hospital, Atlanta, Georgia (N=377), and the external validation sample was collected at Bellevue Hospital Center, New York, New York (N=221). The predictive model achieved high accuracy to discriminate PTSD trajectories of non-remitting symptoms vs. resilience using data from electronic medical records including biological markers from blood sampling along with four items from a validated stress questionnaire (discovery dataset, N=377, f1-score=0.85, AUC=0.85), and equivalent accuracy on the independent test set (Validation dataset, N=211, f1-score=0.86, AUC=0.86) (Schultebraucks et al., 2020b). These findings support the feasibility to develop and validate clinical prediction models using readily available data collected directly in the ED and show that important predictors of adverse mental health effects after trauma exposure can be used by utilizing routinely collectible data (Schultebraucks et al., 2020b). Since this algorithm is based on psychometric data of a four-item stress questionnaire it is not yet fully automated. However, it is very promising to further explore whether routinely collected data from electronic medical records alone is sufficient to predict PTSD risk with high discriminatory accuracy and there is a large-scale project funded by the National Institute of Health that promises to provide further evidence to test the potential of this research avenue (McLean et al., 2019).
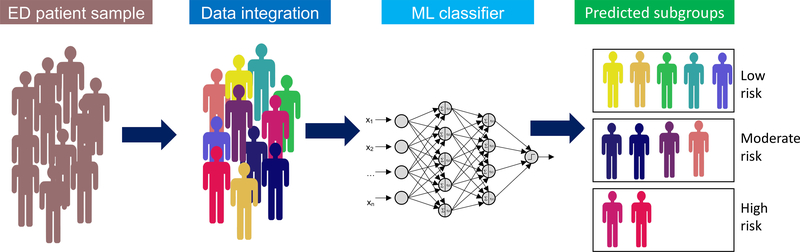
This builds on a larger vision of how computational psychiatry can lead to new directions to prognosticate who is at risk at the early onset directly after the traumatic event took place when the patients are still in contact with the health care systems and follow-up care can be planned (Fig. 1). Eventually, this may open new ways for precision psychiatry at scale and directly implemented into the electronic medical system of the acute care setting. These initially promising findings will need to be extended to the broad clinical population of ED patients from routine practice and to other types of acute emergencies such as ACS or stroke to demonstrate the full potential as an automated clinical readout of posttraumatic risk.
Figure 1.
Schematic depiction of the automated algorithm-based risk stratification in the ED for predicting high, moderate, and low risk patients.
In general, large hospital systems are currently actively working to identify novel automatable methods that can be integrated into the standard of care to improve patient outcomes and to decrease the long-term costs to the hospital system (Horwitz et al., 2019). It is important for future research to leverage such big data in the hundreds of thousands or millions of patients who are routinely cared for in the ED so that the predictive models can develop their full potential.
Digital health
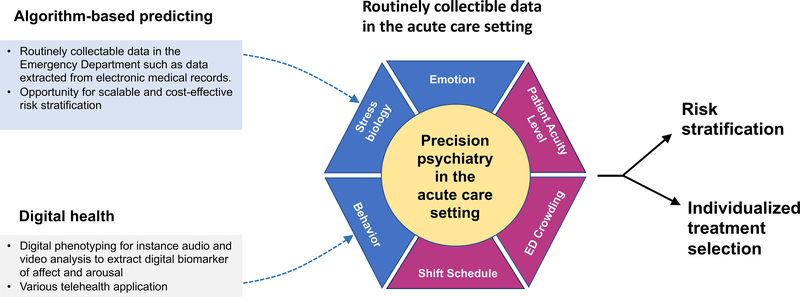
In addition to algorithm-based prediction models directly integrated directly into health systems such as large hospital EDs, new and flexible modalities to use artificial intelligence (AI) and ML offer novel opportunities to collect behavioral data and to of maladaptive stress responses. By using deep learning to integrate multi-model information from digital devices such as smartphones or smartwatches, new horizons emerged to identify transdiagnostic markers to remotely identify and monitor individuals at risk (Carmi et al., 2020). Digital phenotyping refers to new computerized methods for objectively measuring behavior and emotions via human-computer interaction or by passively extracting predictive features of facial expression, voice, and speech content using computerized processing of audio and video data in situ from wearables, smartphones, and other personal digital devices (Insel, 2017a; Onnela and Rauch, 2016). Digital phenotyping capitalizes on innovations in computational psychiatry to unlock the diagnostic and prognostic potential of digitally captured biomarkers (Fig.2). The clinical value of digital biomarkers is to enable self-monitoring of psychopathology signs and enables low threshold remote diagnostic screening that may reduce barriers to successful intervention. This facilitates efficient allocation of prevention measures and is of high relevance in circumstances of crisis such as infectious disease, pandemics, or other natural catastrophes.
Figure 2.
Shematic picture of the conceptual model for algorithm-based prediction and digital health. Integration of routinely collectible data provides informative data about diverse domains relevant to the acute care setting encompassing behavior and emotion through digital phenotyping, markers of stress biology obtained from diagnostic blood draws in the ED and contextual information about the index event as well as the ED environment. Togehter, the integration of multiple sources of information yields promise to unlock the potential of precision psychiatry for improving risk stratification and individualized treatment selection.
There are in general two broader types of applications: (1) digital phenotyping technologies to measure behavior by using AI technology to identify and predict psychological functioning and (2) digital technology for communication and provision of clinical care, such as telehealth applications for remote patient evaluations by clinicians and clinical monitoring or automated passive clinical monitoring and intelligent algorithm-based “chatbots” (Carmi et al., 2020; Marzano et al., 2015; Vaidyam et al., 2020).
AI-based digital phenotyping is using real-world data that is rapidly evolving in volume, velocity, and veracity as sensors of wearables have become more precise and very widespread. In the area of digital phenotyping, there are in particular two types of different data sources, active (Smart Active Monitoring – SAM) and passive data sources (Continuous Passive Monitoring – CPM). Passive data include behavioral patterns that are identified through the usage of the mobile device. Examples are the use of GPS, Glonass or Galileo data to monitor behavioral activation and avoidance (Glenn and Monteith, 2014; Torous et al., 2015), to use smartphones or wearables to monitor sleep and physiological states (Onnela et al., 2018) but also to use keystroke activity, taps and swipes to passively assess cognitive functions via smartphone apps (Dagum, 2018). Active data sources include information such as facial, speech, and voice data (Insel, 2017b). Facial expressions convey important information about the emotional and mental states of a person with decades of neuropsychological research showing that emotional expression and valence contain probabilistic information of diverse forms of psychopathology (Gaebel et al., 1992; Gehricke and Shapiro, 2000; Renneberg et al., 2005). Speech and voice are additional channels conveying probabilistic information about mental health (Cannizzaro et al., 2004; Cohn et al., 2009; France et al., 2000; Leff and Abberton, 1981). Further, digital approaches can also be used to collect detailed information by monitoring patients in their daily lives repeatedly over time, i.e., Ambulatory Assessment and Ecological Momentary Assessment (Doherty et al., 2014; Stone and Shiffman, 1994) for assessing behavioral and cognitive processes in their natural settings by using smartphone diaries or measuring physiological function, and physical behavior via accelerometers, or GPS trackers (Reichert et al., 2020; Trull and Ebner-Priemer, 2013).
Digital communication also offers the opportunity to administer clinical intervention via telehealth applications. These approaches are using AI and natural language processing to perform assessments as well as clinical interventions (Carmi et al., 2020). However, even when telehealth promises to allow broader and faster mobilization of clinical resources, it is difficult to perform complex and deep conversions. COVID-19 in particular has shown that new ways of not only risk stratification but also remote patient care must take place. However, digital health procedures such as remote cognitive behavioral therapy or “chatbots” (Marzano et al., 2015) still require broader evidence-based validation, e.g., through the use of randomized controlled trials or at least pragmatic clinical trials (Chang et al., 2020).
Digital phenotyping and digital communication might have crucial relevance for monitoring, diagnostic, and identification of behavioral patterns to allow low-threshold and timely targeted prevention strategies and optimized allocation of relevant resources and interventions (Insel, 2017a). These uprising trends are promising especially for PTSD research to identify reliable and ecologically valid signatures of stress pathology and prognostic markers of clinical functioning and to develop novel patient-oriented outcomes that complement the traditional quality of life measures by digital augmentation. Powerful open-source methodologies for face detection and speech recognition have made pre-trained neural nets available for academic research. A recent study by Schultebraucks et al. (2020e) used digital phenotypes to classify PTSD and depression in trauma survivors who were admitted to the ED after a traumatic event. A brief video-recorded semi-structured interview was used to objectively capture voice, speech, head movement, gaze, pupil dilation, and facial landmark features of emotion in trauma-survivors who were admitted to a Level 1 trauma center. The video-recording was decomposed into different modalities using separate neural nets to extract predictive information from independent verbal and non-verbal information channels. Facial recognition software was applied to identify emotional expressions and combined with deep learning-based analysis of voice prosody and speech content. Subsequently, the candidate predictive features were combined to systematically discover predictive signatures across multiple modalities spanning facial, voice, speech content along with head and eye movement. Similar to human beings that process emotional information in human interaction in an automated way using both verbal and non-verbal cues, the idea in this digital phenotyping approach is to combine multiple information channels. The aim was to define multi-model signatures extracted from free-speech video recordings that can be used as digital biomarkers in an lightweight and potentially automatble assessment. The benefit of deep learning is that a model trained on one dataset can easily “transferred” to another dataset and the existing model can be further adapted using the information of the new data. This is called “transfer learning” (Pratt, 1993) and the key drive of the great success of deep learning for tasks such as voice or speech recognition. This form of transfer learning can overcome some of the limitations of deep learning in clinical research such as the traditional “small” sample size of high-quality clinical research data. For example, while face recognition requires large amounts of independent samples to train a deep neural net from scratch, this task can be successfully performed on publicly available data outside of the clinical research context. Once the neural net has been successfully trained to detect faces and facial expressions in a non-clinical context, it can then be transferred to a clinical sample and adapted to perform specialized tasks such as the discrimination of clinically informative signatures in facial expression.
The potential of digital phenotyping based on deep learning has been explored by using face, voice and speech content to classify PTSD and depression (Schultebraucks et al., 2020e). The same approach has also been used to explain variance in predicting cognitive functioning (Schultebraucks et al., 2020d). A big advantage of this approach is its flexibility by making use of transfer learning and the possibility to adapt existing pre-trained neural nets to specific clinical tasks.
These results show that digital phenotyping might be an alternative to clinician-administered interviews or subjective self-reports — with high potential to develop an objective, flexible, economical, and ecologically valid digital biomarker.
To identify digital biomarkers of affect and arousal that are digital proxies for depression, PTSD, or cognitive functioning is of timely relevance. The recent events of the COVID-19 pandemic have only underlined the pressing need to rethink traditional approaches and to offer new opportunities for remote diagnosis and monitoring of psychiatric disorders. A digital biomarker approach has the potential to deliver a sensitive and objective measure and therefore low-threshold prevention. In consequence, time-to-treatment may be reduced, and interventions can be targeted more precisely to individual needs. The field of psychiatry has not yet benefitted from the new methods of digital phenotyping in the same way as fields such as cancer research where it is successfully used to detect differential recovery trends after surgery (Panda et al., 2020). Early application to schizophrenia (Henson et al., 2020), bipolar disorder, substance abuse, and risk of suicide (Huckvale et al., 2019) are very promising and show that the best of precision psychiatry is yet to come as researchers will continue to leverage digital methods to unlock clinical actionable targets for prevention methods in other fields of psychiatry as well.
Challenges of the application of ML
One reason why the field of psychiatry has not yet benefitted from the new methods of digital phenotyping may be suspected in the relative complexity of clinical phenotypes in psychiatry. Advances in psychiatric classification for research are promising for digital phenotyping in psychiatry (Insel et al., 2010; Insel, 2017a). Yet, the complexity of clinical phenotypes in psychiatry, often spanning multiple levels of explanations including individual factors (e.g. genetic and biological factors, or personality traits), social factors (e.g. environmental and cultural influences) and complex interactions thereof, comes at cost of leading to complex models that can be very demanding to interpret and comprehend.
As with all methodology used in clinical research, ML has to ensure that the results are robust and reproducible, the design is rigorously planned and the analytical approach is transparent (Nosek et al., 2018). While the flexibility of ML is a big advantage to identify novel diagnostic and prognostic information and to develop accurate and robust digital biomarkers, the associated algorithmic complexity is a significant challenge for the field. The use of open-source software can facilitate the critical appraisal of the strength and weaknesses and is important to allow the independent assessment including the examination of hidden pitfalls and sources of algorithmic biases (Obermeyer et al., 2019). However, there remain limitations of model transparency that simply reflect the mathematical sophistication required for understanding deep learning. These methods often approximate non-linear associations of multiple variables using mathematical equations whose functional form is not pre-specified beforehand but empirically discovered in a data-driven way. Where well-understood prior findings are available, e.g. empirical effect sizes of univariate linear relationships between a predictor and the outcome-of-interest, this information can be of high value to design ML models and to increase the transparency and credibility of the results, it is desirable, also for ML approaches, to make use of hypotheses that are based on clinical insights and prior knowledge. However, this is not always possible with high-dimensional data and exploratory data analysis is therefore also of high importance (Tukey, 1980). For ML, cross-validation, bootstrapping, and external validation sets must be used to ensure generalizability and replicability (Efron and Gong, 1983). Given these precautions to prevent overfitting the ML models to particularities in the data (Cawley and Talbot, 2010), the advantage of flexible ML methods can be used to study complex interactions of multiple factors that go well beyond testing univariate linear associations. Importantly, there are existing solutions aiming to better understand complex ML models, such as SHapley Additive exPlanation (Lundberg and Lee, 2017). These approaches can help to increase the confidence in ML models by approximating complex mathematical functions by simpler ones (i.e., better understandable proxy models) that are approximately correct but offer increased human interpretability. For instance, the influence of a particular variable on the predictions of a particular model can be studied post hoc by systematically changing the values of the variable and then observing the change in the model output. Using large computational power, these can be repeated for each variable and for all permutations of dropping variables from a complex model to better understand each variables unique contribution to the prediction. In clinical care, where human understanding is important for shared decision-making of patients and care providers, it will remain an important challenge for ML approaches to make use of such interpretable ML approaches (Roscher et al., 2020) so that patients can be informed on which clinical variables the predicted outcome is derived from.
Conclusion
Computational and informatics-driven approaches have previously yielded clinically actionable results in many health contexts, from improving the diagnosis of stroke (Petrone, 2017), congenital disease recognition through facial recognition (Gurovich et al., 2018), and the more precise allocation of preventive surgery in breast cancer (Bahl et al., 2017). In the context of psychiatric and behavioral pathology, new computational and AI-based approaches are starting to translate into the field of stress pathology. The unprecedented popularity of digital devices will allow the application of these new approaches to identify novel digital and biological markers for timely and precisely targeted psychological interventions, remote monitoring, and telehealth counseling.
Especially, the acute care setting provides an important opportunity and setting for automated risk stratification and individualized treatment allocation as part of precision psychiatry. The use of algorithm-based prediction models using routinely collectible data that can be automatically abstracted from electronic medical records will bear a high clinical potential for targeted and actionable clinical insight at the point of care. This approach to computational psychiatry and medicine might lower the threshold for the implementation of precision psychiatry at scale and to make the research available to clinicians at the time when prognostic information is most relevant for planning preventive measures using a risk-based approach. Despite the pace of recent advancements, some challenges remain. In particular, secure data protection, data-sharing for research purposes under adequate privacy protection, and the fair use of available datasets need to be realized on a large scale. Clinically heterogeneous, population-based datasets need to become available more easily to continue the facilitation of research innovation in precision psychiatry. Ultimately, digital phenotyping approaches will greatly impact and improve the precision and cost of diagnostic and prognostic screening and will provide a risk-based approach to treatment selection and preventive care in psychiatry.
Footnotes
Conflicts of interest
None of the authors has any conflict of interest to declare.
Contributor Information
Katharina Schultebraucks, Department of Emergency Medicine, Columbia University Irving Medical Center, New York, New York; Data Science Institute, Columbia University, New York, NY, USA.
Bernard P. Chang, Department of Emergency Medicine, Columbia University Irving Medical Center, New York, New York.
References
- Asplin BR, Magid DJ, Rhodes KV, Solberg LI, Lurie N, Camargo CA Jr, 2003. A conceptual model of emergency department crowding. Annals of emergency medicine 42, 173–180. [DOI] [PubMed] [Google Scholar]
- Bahl M, Barzilay R, Yedidia AB, Locascio NJ, Yu L, Lehman CD, 2017. High-risk breast lesions: a machine learning model to predict pathologic upgrade and reduce unnecessary surgical excision. Radiology 286, 810–818. [DOI] [PubMed] [Google Scholar]
- Cannizzaro M, Harel B, Reilly N, Chappell P, Snyder PJJB, cognition, 2004. Voice acoustical measurement of the severity of major depression. 56, 30–35. [DOI] [PubMed] [Google Scholar]
- Carmi L, Fostick L, Burshtein S, Cwikel-Hamzany S, Zohar J, 2016. PTSD treatment in light of DSM-5 and the “golden hours” concept. CNS spectrums 21, 279–282. [DOI] [PubMed] [Google Scholar]
- Carmi L, Schultebraucks K, Galatzer-Levy I, 2020. Identification, Prediction, and Intervention Via Remote Digital Technology: Digital Phenotyping & Deployment of Clinical Interventions Following Terror and Mass Casualty Events, in: Vermetten E, Frankova I, Carmi L, Chaban O, Zohar J (Eds.), Management of Terrorism Induced Stress – Guideline for the Golden Hours. IOS Press BV, Amsterdam, pp. 175–181. [Google Scholar]
- Cawley GC, Talbot NL, 2010. On over-fitting in model selection and subsequent selection bias in performance evaluation. Journal of Machine Learning Research 11, 2079–2107. [Google Scholar]
- Chang BP, Kessler RC, Pincus HA, Nock MK, 2020. Digital approaches for mental health in the age of covid-19. Bmj 369. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Kruez TS, Matthews I, Yang Y, Nguyen MH, Padilla MT, Zhou F, De la Torre F, 2009. Detecting depression from facial actions and vocal prosody, Affective Computing and Intelligent Interaction and Workshops, 2009. ACII 2009. 3rd International Conference on. IEEE, pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagum P, 2018. Digital biomarkers of cognitive function. npj Digital Medicine 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean KR, Hammamieh R, Mellon SH, Abu-Amara D, Flory JD, Guffanti G, Wang K, Daigle BJ, Gautam A, Lee I, 2019. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Molecular psychiatry, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty ST, Lemieux CJ, Canally C, 2014. Tracking human activity and well-being in natural environments using wearable sensors and experience sampling. Social Science & Medicine 106, 83–92. [DOI] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Wasson LT, Giglio JF, Davidson KW, Whang W, 2014. A test of the diathesis-stress model in the emergency department: who develops PTSD after an acute coronary syndrome? Journal of Psychiatric Research 53, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y, 2012. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PloS one 7, e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Shimbo D, Ye S, Wyer P, Davidson KW, 2013. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA internal medicine 173, 472–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Gong GJTAS, 1983. A leisurely look at the bootstrap, the jackknife, and cross-validation. 37, 36–48. [Google Scholar]
- Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK, 2015. Does this patient with chest pain have acute coronary syndrome?: the rational clinical examination systematic review. Jama 314, 1955–1965. [DOI] [PubMed] [Google Scholar]
- France DJ, Shiavi RG, Silverman S, Silverman M, Wilkes M.J.I.t.o.B.E., 2000. Acoustical properties of speech as indicators of depression and suicidal risk. 47, 829–837. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Wölwer W.J.E.a.o.p., neuroscience c., 1992. Facial expression and emotional face recognition in schizophrenia and depression. 242, 46–52. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, Shalev AY, 2013. Early PTSD Symptom Trajectories: Persistence, Recovery, and Response to Treatment: Results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLOS ONE 8, e70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bryant RA, 2013. 636,120 Ways to Have Posttraumatic Stress Disorder. Perspectives on psychological science : a journal of the Association for Psychological Science 8, 651–662. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Karstoft KI, Statnikov A, Shalev AY, 2014. Quantitative forecasting of PTSD from early trauma responses: A machine learning application. Journal of psychiatric research 59, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ma S, Statnikov A, Yehuda R, Shalev AY, 2017. Utilization of machine learning for prediction of post-traumatic stress: a re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Translational psychiatry 7, e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke J-G, Shapiro DJPR, 2000. Reduced facial expression and social context in major depression: discrepancies between facial muscle activity and self-reported emotion. 95, 157–167. [DOI] [PubMed] [Google Scholar]
- Glenn T, Monteith S, 2014. New measures of mental state and behavior based on data collected from sensors, smartphones, and the Internet. Current psychiatry reports 16, 523. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Borja SE, 2020. The COVID-19 Pandemic: Setting the Mental Health Research Agenda. Biological psychiatry, 10.1016/j.biopsych.2020.1005.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurovich Y, Hanani Y, Bar O, Fleischer N, Gelbman D, Basel-Salmon L, Krawitz P, Kamphausen SB, Zenker M, Bird LM, 2018. DeepGestalt-Identifying Rare Genetic Syndromes Using Deep Learning. arXiv preprint arXiv:1801.07637. [DOI] [PubMed] [Google Scholar]
- Heim C, Schultebraucks K, Marmar CR, Nemeroff CB, 2018. Neurobiological Pathways Involved in Fear, Stress, and PTSD. Post‐traumatic stress disorder, 331. [Google Scholar]
- Henson P, Barnett I, Keshavan M, Torous J, 2020. Towards clinically actionable digital phenotyping targets in schizophrenia. npj Schizophrenia 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs R, van Rooij SJ, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, Rothbaum AO, Stevens JS, Galatzer-Levy I, Rothbaum BO, 2019. Increased Skin Conductance Response in the Immediate Aftermath of Trauma Predicts PTSD Risk. Chronic Stress 3, 2470547019844441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz LI, Kuznetsova M, Jones SA, 2019. Creating a Learning Health System through Rapid-Cycle, Randomized Testing. The New England journal of medicine 381, 1175. [DOI] [PubMed] [Google Scholar]
- Huckvale K, Venkatesh S, Christensen H, 2019. Toward clinical digital phenotyping: a timely opportunity to consider purpose, quality, and safety. NPJ Digital Medicine 2, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Maia TV, Frank MJ, 2016. Computational psychiatry as a bridge from neuroscience to clinical applications. Nature neuroscience 19, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2017a. Digital Phenotyping: Technology for a New Science of Behavior. Jama 318, 1215–1216. [DOI] [PubMed] [Google Scholar]
- Insel TRJJ, 2017b. Digital phenotyping: technology for a new science of behavior. 318, 1215–1216. [DOI] [PubMed] [Google Scholar]
- Karstoft K-I, Galatzer-Levy IR, Statnikov A, Li Z, Shalev AY, 2015. Bridging a translational gap: using machine learning to improve the prediction of PTSD. BMC psychiatry 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff J, Abberton EJPM, 1981. Voice pitch measurements in schizophrenia and depression. 11, 849–852. [DOI] [PubMed] [Google Scholar]
- Love J, Zatzick D, 2014. Screening and Intervention for Comorbid Substance Disorders, PTSD, Depression, and Suicide: A Trauma Center Survey. Psychiatr Serv 65, 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Ratanatharathorn A, Lai BS, van der Mei W, Barbano AC, Bryant RA, Delahanty DL, Matsuoka YJ, Olff M, Schnyder U, Laska E, Koenen KC, Shalev AY, Kessler RC, 2020. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: pooled results from the International Consortium to predict PTSD. Psychological Medicine, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg SM, Lee S-I, 2017. A unified approach to interpreting model predictions, Advances in Neural Information Processing Systems, pp. 4765–4774. [Google Scholar]
- Marzano L, Bardill A, Fields B, Herd K, Veale D, Grey N, Moran P, 2015. The application of mHealth to mental health: opportunities and challenges. The Lancet Psychiatry 2, 942–948. [DOI] [PubMed] [Google Scholar]
- McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, Clifford GD, Zeng D, An X, Linnstaedt S, Beaudoin F, House S, Bollen KA, Musey P, Hendry P, Jones CW, Lewandowski C, Swor R, Datner E, Mohiuddin K, Stevens JS, Storrow A, Kurz MC, McGrath ME, Fermann GJ, Hudak LA, Gentile N, Chang AM, Peak DA, Pascual JL, Seamon MJ, Sergot P, Peacock WF, Diercks D, Sanchez LD, Rathlev N, Domeier R, Haran JP, Pearson C, Murty VP, Insel TR, Dagum P, Onnela J-P, Bruce SE, Gaynes BN, Joormann J, Miller MW, Pietrzak RH, Buysse DJ, Pizzagalli DA, Rauch SL, Harte SE, Young LJ, Barch DM, Lebois LAM, van Rooij SJH, Luna B, Smoller JW, Dougherty RF, Pace TWW, Binder E, Sheridan JF, Elliott JM, Basu A, Fromer M, Parlikar T, Zaslavsky AM, Kessler R, 2019. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Gautam A, Hammamieh R, Jett M, Wolkowitz OM, 2018. Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biological psychiatry 83, 866–875. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, Rothbaum BO, Ressler KJ, Nemeroff CB, 2019. Association of Prospective Risk for Chronic PTSD Symptoms With Low TNFα and IFNγ Concentrations in the Immediate Aftermath of Trauma Exposure. American Journal of Psychiatry, appi. ajp. 2019.19010039. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T, 2017. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, Rao U, 2016. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical psychology review 49, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthaan J, Sijbrandij M, de Vries GJ, Reitsma JB, van de Schoot R, Goslings JC, Luitse JS, Bakker FC, Gersons BP, Olff M, 2013. Internet-based early intervention to prevent posttraumatic stress disorder in injury patients: randomized controlled trial. Journal of medical Internet research 15, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musey PI Jr, Schultebraucks K, Chang BP, 2020. Stressing out about the heart: a narrative review of the role of psychological stress in acute cardiovascular events. Academic Emergency Medicine 27, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH, 2016. NIMH-Funded Study to Track the Effects of Trauma, https://www.nimh.nih.gov.
- Nosek BA, Ebersole CR, DeHaven AC, Mellor D.T.J.P.o.t.N.A.o.S., 2018. The preregistration revolution. 115, 2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer Z, Powers B, Vogeli C, Mullainathan S, 2019. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366, 447–453. [DOI] [PubMed] [Google Scholar]
- Onnela J-P, Keshavan M, Staples P, Barnett I, Torous J, 2018. 150. Automated Longitudinal Latent Interval Estimation With Applications to Sleep. Biological Psychiatry 83, S61. [Google Scholar]
- Onnela J-P, Rauch SL, 2016. Harnessing Smartphone-Based Digital Phenotyping to Enhance Behavioral and Mental Health. Neuropsychopharmacology 41, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda N, Solsky I, Huang EJ, Lipsitz S, Pradarelli JC, Delisle M, Cusack JC, Gadd MA, Lubitz CC, Mullen JT, Qadan M, Smith BL, Specht M, Stephen AE, Tanabe KK, Gawande AA, Onnela J-P, Haynes AB, 2020. Using Smartphones to Capture Novel Recovery Metrics After Cancer Surgery. JAMA Surgery 155, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini S, Pisner D, Shumake J, Powers MB, Beevers CG, Rainey EE, Smits JA, Warren AM, 2018. Ensemble machine learning prediction of posttraumatic stress disorder screening status after emergency room hospitalization. Journal of anxiety disorders 60, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone J, 2017. FDA approves stroke-detecting AI software. Nat. Biotechnol 35, 604–605. [DOI] [PubMed] [Google Scholar]
- Pratt LY, 1993. Discriminability-based transfer between neural networks, Advances in neural information processing systems, pp. 204–211. [Google Scholar]
- Reichert M, Braun U, Lautenbach S, Zipf A, Ebner-Priemer U, Tost H, Meyer-Lindenberg A, 2020. Studying the impact of built environments on human mental health in everyday life: Methodological developments, state-of-the-art and technological frontiers. Current opinion in psychology 32, 158–164. [DOI] [PubMed] [Google Scholar]
- Renneberg B, Heyn K, Gebhard R, Bachmann S.J.J.o.b.t., psychiatry e., 2005. Facial expression of emotions in borderline personality disorder and depression. 36, 183–196. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, 2018. Molecular Signatures of Stress and Posttraumatic Stress Disorder: An Overview. Biological psychiatry 83, 792–794. [DOI] [PubMed] [Google Scholar]
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI, 2010. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database of Systematic Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscher R, Bohn B, Duarte MF, Garcke J, 2020. Explainable machine learning for scientific insights and discoveries. IEEE Access 8, 42200–42216. [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, Lang D, Houry D, 2012. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biological psychiatry 72, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser ME, Davis MJS, Kerley MKA, Rothbaum MAO, Mercer MKB, Price M, Houry D, Ressler KJ, 2014. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. The Journal of clinical psychiatry 75, 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultebraucks K, Galatzer‐Levy IR, 2019. Machine Learning for Prediction of Posttraumatic Stress and Resilience Following Trauma: An Overview of Basic Concepts and Recent Advances. Journal of traumatic stress. [DOI] [PubMed] [Google Scholar]
- Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, Gautam A, Guffanti G, Hammamieh R, Misganaw B, 2020a. Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Molecular Psychiatry, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultebraucks K, Rombold-Bruehl F, Wingenfeld K, Hellmann-Regen J, Otte C, Roepke S, 2019. Heightened biological stress response during exposure to a trauma film predicts an increase in intrusive memories. Journal of abnormal psychology 128, 645. [DOI] [PubMed] [Google Scholar]
- Schultebraucks K, Shalev AY, Michopoulos V, Grudzen CR, Shin S-M, Stevens JS, Maples-Keller JL, Jovanovic T, Bonanno GA, Rothbaum BO, Marmar CR, Nemeroff CB, Ressler KJ, Galatzer-Levy IR, 2020b. A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor. Nature Medicine. [DOI] [PubMed] [Google Scholar]
- Schultebraucks K, Wen T, Kronish IM, Willey J, Chang BP, 2020c. Post-traumatic Stress Disorder Following Acute Stroke. Current Emergency and Hospital Medicine Reports, 1–8. [Google Scholar]
- Schultebraucks K, Yadav V, Galatzer-Levy I, 2020d. Utilization of Machine Learning-Based Computer Vision and Voice Analysis to Derive Digital Biomarkers of Cognitive Functioning in Trauma Survivors. Digital Biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultebraucks K, Yadav Y, Shalev AY, Bonanno GA, Galatzer-Levy I, 2020e. Deep learning-based classification of posttraumatic stress disorder and depression following trauma utilizing visual and auditory markers of arousal and mood. Psychological Medicine, 1–11. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY, 2005. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Molecular psychiatry 10, 500–513, 425. [DOI] [PubMed] [Google Scholar]
- Shalev A, Liberzon I, Marmar C, 2017. Post-traumatic stress disorder. New England Journal of Medicine 376, 2459–2469. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Barbano AC, 2019. PTSD: Risk Assessment and Early Management. Psychiatric Annals 49, 299–306. [Google Scholar]
- Shalev AY, Sahar T, Freedman S, et al. , 1998. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry 55, 553–559. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, 1994. Ecological momentary assessment (EMA) in behavorial medicine. Annals of Behavioral Medicine. [Google Scholar]
- Torous J, Staples P, Onnela J-P, 2015. Realizing the Potential of Mobile Mental Health: New Methods for New Data in Psychiatry. Current Psychiatry Reports 17, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer U, 2013. Ambulatory assessment. Annual review of clinical psychology 9, 151–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JWJTAS, 1980. We need both exploratory and confirmatory. 34, 23–25. [Google Scholar]
- Vaidyam AN, Linggonegoro D, Torous J, 2020. Changes to the Psychiatric Chatbot Landscape: A Systematic Review of Conversational Agents in Serious Mental Illness: Changements du paysage psychiatrique des chatbots: une revue systématique des agents conversationnels dans la maladie mentale sérieuse. The Canadian Journal of Psychiatry, 0706743720966429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mei WF, Barbano AC, Ratanatharathorn A, Bryant RA, Delahanty DL, deRoon-Cassini TA, Lai BS, Lowe SR, Matsuoka YJ, Olff M, Qi W, Schnyder U, Seedat S, Kessler RC, Koenen KC, Shalev AY, Errera-Ankri Y, Freedman S, Frijling J, Goslings CJ, Luitse J, McFarlane A, Silove D, Moergeli H, Mouthaan J, Nishi D, O’Donnell M, Rusch M, Sijbrandij M, Suliman S, van Zuiden M, International Consortium to Predict, P., 2020. Evaluating a screener to quantify PTSD risk using emergency care information: a proof of concept study. BMC Emergency Medicine 20, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal SJ, Gorter R, Reijnen A, Geuze E, Vermetten E, 2019. Cohort Profile: The prospective research in stress-related military operations (PRISMO) study in the Dutch armed forces. BMJ open 9, e026670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, Kavelaars A, Heijnen CJ, 2012. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biological psychiatry 71, 309–316. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Zhohar J, Krugers HJ, 2014. Pharmacotherapy in the aftermath of trauma; opportunities in the ‘golden hours’. Current psychiatry reports 16, 455. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, Hobfoll SE, Koenen KC, Neylan TC, Hyman SE, 2015. Post-traumatic stress disorder. Nature Reviews Disease Primers 1, 15057. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane A, Shalev A, 1998. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological psychiatry 44, 1305–1313. [DOI] [PubMed] [Google Scholar]
- Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H, 2011. New insights into secondary prevention in post-traumatic stress disorder. Dialogues in clinical neuroscience 13, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]